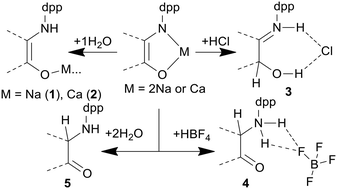
During the partial hydrolysis of metal complexes with the mono-imino-acenaphthen-1-olate (R-mian) dianion, the nitrogen atom is protonated at the first stage, while the metal–ligand bond is preserved. Further hydrolysis leads to products whose structure depends on the reaction conditions. Hydrolysis by an excess of water leads to the formation of the products with a reduced C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N fragment, (amino ketones, R-mianH2). The complexes based on the dianion of (E)-2-(2,6-diisopropylphenylimino)acenaphthylen-1(2H)-one (dpp-mian) are hydrolyzed by a HBF4 aqueous solution to give an ammonium salt containing a reduced C
N fragment, (amino ketones, R-mianH2). The complexes based on the dianion of (E)-2-(2,6-diisopropylphenylimino)acenaphthylen-1(2H)-one (dpp-mian) are hydrolyzed by a HBF4 aqueous solution to give an ammonium salt containing a reduced C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N fragment with the proton bound to the ammonium nitrogen atom [(dpp-mainH3)1+BF4]. When these compounds interact with an aqueous solution of HCl, the carbonyl group C
N fragment with the proton bound to the ammonium nitrogen atom [(dpp-mainH3)1+BF4]. When these compounds interact with an aqueous solution of HCl, the carbonyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is involved, while the acid proton coordinates to the nitrogen atom [(dpp-mainH3)1+Cl]. MP2 calculations explain the formation of different products. Hydrolysis of the metal complexes, bearing a R-mian radical-anion, by water produces a stoichiometric mixture of R-mianH2 and R-mian as a result of the R-mianH disproportionation. The reaction of R-mian with lithium aluminum hydride leads to the reduction of both the C
O is involved, while the acid proton coordinates to the nitrogen atom [(dpp-mainH3)1+Cl]. MP2 calculations explain the formation of different products. Hydrolysis of the metal complexes, bearing a R-mian radical-anion, by water produces a stoichiometric mixture of R-mianH2 and R-mian as a result of the R-mianH disproportionation. The reaction of R-mian with lithium aluminum hydride leads to the reduction of both the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O fragments.
O fragments.