 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The overestimated capability of fluid shear to induce secondary nucleation: an urgent call for diligently executed control experiments†
Lorijn
De Vrieze
 and
Simon
Kuhn
and
Simon
Kuhn
 *
*
Department of Chemical Engineering, KU Leuven, 3001 Leuven, Belgium. E-mail: simon.kuhn@kuleuven.be
First published on 9th June 2025
Abstract
Many examples from the literature emphasize the important role of fluid shear in secondary nucleation. Moreover, they confidently state that fluid shear alone is surely capable of inducing secondary crystal formation. In this article, inspired by the most representative works in the field, four sets of experiments were designed to specifically isolate this fluid shear-induced secondary nucleation phenomenon. Great care was taken to meticulously conduct control experiments (avoiding attrition, but also initial breeding and primary nucleation), thus ensuring the envisioned isolation of secondary nucleation from fluid shear. Contrary to current conception in the community, no fluid shear-induced secondary nucleation could be observed in the conducted experiments, suggesting that its occurrence is much rarer than currently perceived. Based on these results, we also strongly encourage crystallization scientists to review their experimental procedures (control experiments) as to guarantee that secondary nucleation from fluid shear is indeed the acting nucleation phenomenon in their studies.
The relation between fluid shear and secondary nucleation has always been an intricate one. Ever since the term secondary nucleation was defined in the context of solution crystallization,1 research papers keep stressing the large influence that stirring and agitation has on it.2–4 The presence of prior crystals indisputably catalyzes the formation of new ones, but the underlying mechanisms and the role of agitation therein still remain topics of debate.1,5–7 What is clear, however, is that both fluid shear and attrition might play a crucial role. Admittedly, the existence of crystal attrition or breakage caused by mechanical impact cannot be denied and is thus by far the most dominant secondary nucleation mechanism in stirred crystallizers.3,8–12 Nevertheless, several of these pioneering works firmly proclaim that the motion of fluid relative to the crystal in itself suffices to cause secondary nucleation;13–15 a belief that persists up to today.16,17
The first work that experimentally demonstrated the existence of secondary nucleation purely from fluid shear was conducted by Powers.18 By attaching a single large crystal onto a glass rod and rotating it around its axis, he was able to apply fluid shear while avoiding the dominant mechanism of attrition. Furthermore, the presence of this rotating seed crystal led to significantly higher nucleation rates, characteristic to secondary nucleation.7,19 With these findings in mind, he postulated that next to a seed crystal a complex boundary layer has to exist, made up of “formless aggregates of solute molecules which have not yet attained a regular crystal lattice”. The fluid shear acting on the spinning seed crystal is apparently sufficient to sweep these aggregates into the bulk solution and thus cause the formation of new (secondary) crystals. The concept of an intriguing crystal-solution boundary layer as origin of new crystals is even now a frequently recurring theme in several secondary nucleation papers.20–26 Boundary layer removal simply remains the only plausible explanation for the observed occurrence of secondary nucleation originating from fluid shear alone.
Incentivized by these initial works, several others also aimed at isolating fluid shear-induced secondary nucleation. To ensure the sole presence of fluid shear as acting secondary nucleation mechanism, a “seed-on-a-stick” (tethered crystal) approach is typically adopted.27,28 Analogously to Powers' concept, the crystalline seed is immobilized upon an inert stationary rod and subjected to fluid shear after introduction into the solution. This way, the formation of the first new crystals cannot originate from crystal breakage as mechanical impact on the seed crystal has been eliminated. Ample examples of such works have been published: Denk and Botsaris;29 Sung and Youngquist;30 Estrin and Youngquist;31 Jagannathan and Estrin;32 Wang and Yang;33 Wang and Estrin;34 Youngquist;35 Wang and Estrin;36 Qian and Botsaris;37 Buhse and Kondepudi;38 Tai and Chang;39 Yousuf and Frawley;40 Cashmore and Sefcik.41 In line with the initial papers, these works unanimously conclude that fluid shear alone is capable of inducing secondary nucleation. Moreover, the large variation in applied crystallizing systems (solute and solvent used), experimental conditions (fluid shear values and supersaturations), and experimental setups (ranging from continuously stirred tanks to short-pulsed fluid jets) suggests fluid shear-induced secondary nucleation to be a truly universal and easily provoked phenomenon.
While the importance of avoiding attrition is frequently reiterated in literature, securing the absence of two other interfering nucleation phenomena receives only little attention: (i) initial breeding and (ii) primary nucleation (see S1†).
The inevitable preparation of seed crystals (drying, handling, gluing) in secondary nucleation experiments results in small crystalline particles on the seed crystal surface.5,20 Once introduced into solution, these fines can dislodge from the crystal surface and grow to larger sizes. This initial breeding phenomenon occurs on a much shorter timescale compared to secondary nucleation, and is commonly avoided by washing the seed crystals.42 Even so, several authors noticed that the time to eliminate all the crystalline debris is incredibly long.22,28 If one wants to avoid the initial burst of crystals from initial breeding, a carefully designed washing procedure thus needs to be in place.43
Unlike secondary nucleation, primary nucleation describes how crystals form in absence of seed crystals and thus directly from the liquid solution.44 By definition, the rate of crystal formation from secondary nucleation should be significantly higher than the one from primary nucleation, which might explain the tendency in literature to forget checking the absence of the latter. However, primary nucleation is inherently stochastic45–47 and heavily dependent on present supersaturation and fluid shear values,48–51 dictating the need for numerous setup-specific control experiments. The trend in literature that fluid shear-induced secondary nucleation is more prominent at high fluid shear values and high supersaturation highlights the importance of these control experiments once more.52,53 The common usage of a seed-on-a-stick also implicates the introduction of a stagnant object in flow for secondary nucleation experiments. To mimic the locally enhanced fluid shear values around the stagnant object, one should also introduce an object of the same shape in the primary nucleation control experiments.
The objective of this work is to isolate the so-perceived universal phenomenon of secondary nucleation induced by solely fluid shear, while strictly adhering to a rigorous set of control experiments. In particular, the absence of attrition, initial breeding, and primary nucleation (mimicking locally enhanced fluid shear values caused by introducing a stagnant object in flow) needs to be ensured. Hereafter, four experimental setups will be discussed, all designed with this specific goal in mind. An overview of the performed experiments is provided in Fig. 1.
 | ||
| Fig. 1 Graphical overview of the four central experiments in this work. Where the first two experiments stress the importance of eliminating initial breeding, the last two highlight the vital need for primary nucleation control experiments. More experimental details can be found in S2–S8.† | ||
Experiment I – rotating seed crystal (initial breeding)
As mentioned before, the experiment from Powers prompted others to believe that fluid shear suffices to cause secondary nucleation, making it a key experiment to repeat if one wants to demonstrate or refute the existence of such a phenomenon.18 For this reason, Lal and Strickland-Constable already repeated Powers' measurements in 1969 and came to the surprising conclusion that no fluid shear-induced secondary nucleation could be observed in their case.54 They stressed the importance of a “pretreatment step” in which the seed crystal should be thoroughly washed: something that is missing in Powers' experiment. Subsequently, they also attributed the perceived occurrence of secondary nucleation in Powers' experiment to the lack of such pretreatment step and thus initial breeding.Nonetheless, Lal and Strickland-Constable themselves also suffered from practical limitations caused by their applied crystallizing system of MgSO4·7H2O. Their rotating seed crystal was only 2 mm in size while dendrite formation limited the maximum achievable ΔT to just 4 °C. Nevertheless, higher fluid shear values (larger crystal sizes) and supersaturation, while primary nucleation is still absent, are generally believed to increase the odds of detecting fluid shear-induced secondary nucleation.52,53
Here, to settle the discussion, a large KH2PO4 seed crystal (1.0 cm in size) is rotated at similar RPM values and at a higher supersaturation (no dendrite formation detected). A more detailed description of the followed experimental procedure, control experiments, and followed washing procedure are documented in S3–S5.†Table 1 then shows the measured induction times obtained using either the washed KH2PO4 crystal (secondary nucleation), or a 3D printed object in the same shape as the used crystals (primary nucleation).
| Trial number | Secondary nucleation | Primary nucleation |
|---|---|---|
| Induction time [min] | Induction time [min] | |
| Run 1 | 29 | 25 |
| 32 | 20 | |
| 14 | ||
| Run 2 | 74 | 42 |
| 85 | 47 | |
| 10 | ||
| Run 3 | 65 | 23 |
| 8 | 20 | |
| 48 | ||
| Run 4 | 13 | 34 |
| 10 | 32 | |
| 22 | ||
| Mean induction time | 34.17 ± 17.35 | 30.38 ± 8.51 |
No clear difference could be detected between both cases, hinting at an absence of fluid shear-induced secondary nucleation in line with the observations from Lal and Strickland-Constable.54 The presence of a seed crystal should have been able to strongly increase nucleation rates by provoking secondary nucleation from fluid shear. Rather, these results stress the need for a pretreatment (washing) step to counter the occurrence of initial breeding.
Experiment II – seed-on-a-stick (initial breeding)
While the concept of initial breeding was still new at the time of Powers and Strickland-Constable,18,22 it is now common practice to wash seed crystals before secondary nucleation experiments. To evaluate the consistency of current washing procedures, the more recent methods from Yousuf and Frawley40 are also being reproduced here. Using a seed-on-a-stick approach and polythermal experiments, they demonstrated a clear distinction between the onset of attrition-free secondary nucleation and primary nucleation. In their work, it was opted to wash the seed crystals in anti-solvent. The full experimental procedure followed to detect the onset of crystal formation while temperature was reduced, has been adopted from their publication (see S6†).Since the focus of this experiment is on the washing step, three different approaches are investigated. Either (i) a solvent washed seed crystal is used, (ii) an anti-solvent washed seed crystal,40 or (iii) an unwashed seed crystal. The results are shown in Fig. 2.
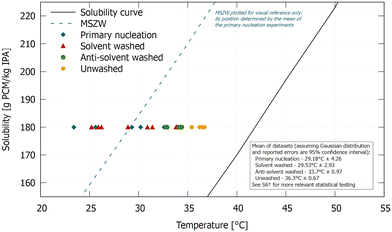 | ||
| Fig. 2 Impact of the selected washing procedure on the onset of nucleation. Only solvent washed is capable of fully eliminating initial breeding. | ||
Initial breeding is clearly taking place in presence of the unwashed seed crystal, as the distinct difference in nucleation onset between the unwashed seed crystal and solvent washed case indicates. Moreover, a smaller but significant difference is visible when comparing the anti-solvent washed seed crystal with the solvent washed one. This indicates that not all crystalline debris are removed by anti-solvent washing, rendering it unsuitable as an elimination procedure for initial breeding. Hence, the observed attrition-free secondary nucleation in the paper from Yousuf and Frawley40 should be ascribed to initial breeding instead.
Accordingly, it does not only matter whether the seed crystal is washed, it is also paramount that it is washed in a correct manner and no damage is inflicted on it after the washing step. It was shown that even the simple action of holding a washed seed crystal with a pair of tweezers suffices to create the crystalline debris needed for initial breeding.43 The washing procedure presented here takes these findings into account, which is demonstrated in Fig. 2, as the solvent washed seed crystal and primary nucleation case fully overlap. Since the presented washing procedure seems the most plausible candidate for full elimination of initial breeding, it will also be applied in the remaining experiments.
Experiment III – seed-on-a-stick (primary nucleation)
With initial breeding now eliminated in the best way possible, the focus of the following experiments shifts towards the primary nucleation control experiments. The previously discussed setup from Yousuf and Frawley40 has therefore been slightly altered (experimental details in S7†). Since fluid shear-induced secondary nucleation is closely entangled with the concept of the crystal-solution boundary layer, several aspects from literature are incorporated in this continuously spinning seed-on-a-stick experiment to increase the odds of detecting the intended phenomenon.Firstly, NaClO3 in water is now employed as crystallizing system because of its use in the landmark papers from Qian and Botsaris,37,52 in which they proposed a theoretical description of fluid shear-induced secondary nucleation from experimental findings. Secondly, higher fluid shear values are induced by locating the seed crystal as close as possible to the stirrer of the crystallizer. Lastly, a polythermal method is selected, based on the frequently re-occurring observation in literature that a temperature lowering step is often required for the formation of secondary crystals. Any aggregates dislodged from the crystal-solution boundary layer thus end up in a solution with increasing supersaturation, analogously to a temperature decrease.
The results from this polythermal seed-on-a-stick experiment can be seen in Fig. 3. By definition, one expects attrition-free secondary nucleation to occur faster than primary nucleation. Here, however, there is no difference between the primary and secondary nucleation experiments, again indicating an absence of the latter. While the primary nucleation experiments have a broad distribution because of their stochastic nature, this should not be the case for secondary nucleation. Although some broadening could be explained by crystal growth dispersion,55–58 the observed wide distribution hints more at primary nucleation.
 | ||
| Fig. 3 The overlap between the secondary and primary nucleation control experiments shows the absence of secondary nucleation by fluid shear. | ||
Apart from locally increasing fluid shear values, the presence of a stagnant object might also enhance heterogeneous primary nucleation by presenting a foreign surface (templating). Accordingly, experiments without any introduced object were also performed and no difference could be detected with the primary nucleation control experiments, proving that the surface of the stagnant object does not induce heterogeneous primary nucleation.
Experiment IV – seed-on-stirrer (primary nucleation)
Before it is possible to sweep away aggregates from a crystal-solution boundary layer and subsequently cause attrition-free secondary nucleation, sufficient aggregates first have to be present there (i.e. a fully developed boundary layer). For this reason, several literature works indicate or stress the need for a delay time between seed crystal introduction and secondary nucleation detection,41,53 required for the replenishment of these aggregates. Consequently, the constantly acting fluid shear in a continuously spinning experiment might not provide sufficient chances for this replenishment to occur. A pulsed spinning experiment, however, would allow for this and is therefore selected as fourth experiment here. The full experimental procedure can be found in S8.†Since no fluid shear-induced secondary nucleation has been observed so far, a more elaborate screening procedure is adopted. By slowly incrementing the solute concentration, one would expect to enter the secondary nucleation zone in Miers' phase diagram, as demonstrated in Fig. 4. Moreover, since the seed crystal is now glued directly on the stirrer and higher rotational speeds are employed, fluid shear values are even higher than before. What is observed, however, is a direct transition from the crystal growth only directly into the primary nucleation zone. Despite all measures to observe fluid shear-induced secondary nucleation, it still remains elusive.
 | ||
| Fig. 4 The adopted systematic procedure to find fluid shear-induced secondary nucleation. However, the envisioned zone could not be found. | ||
One could argue that compound specificity is at play in this study. Although the tendency of glycine molecules to form aggregates in bulk solution has been shown,59 the selected γ-glycine crystal is a notoriously slow growing crystal. In light of the hypothesized link between crystal growth and secondary nucleation28,55,57,60 (aggregates are crystal growth units), the chosen delay time between the pulses might thus be insufficient. For this reason, the same experiments were also conducted using KH2PO4, a fast growing crystal. Again, no fluid shear-induced secondary nucleation could be observed, the common theme throughout all the conducted experiments in this work.
Conclusion
To conclude, this article presented four experimental setups purposely designed to find fluid shear-induced secondary nucleation. However, in not a single experiment fluid shear was observed to induce attrition-free secondary nucleation.The in literature observed lack of control experiments for initial breeding and primary nucleation urged the reproduction of some crucial papers from the field. The first two experiments highlight the need for a rigorous seed crystal washing procedure, while the last two demonstrate the need for numerous primary nucleation control experiments. Moreover, the use of an inert object with the same shape as the seed crystal was applied for the first time, thus compensating the locally increased fluid shear values caused by a stagnant seed crystal. Overall, these findings should encourage scientists in the field to revisit their own control experiment procedures while also to be more thorough and detailed when reporting them.
The central point of this article is that, when adhering to all control experiments, the phenomenon of fluid shear-induced secondary nucleation turns elusive. It is thus safe to state that fluid shear has a less effective role in secondary nucleation than is currently believed. In particular, the presumed easily provoked and universal nature of fluid shear-induced secondary nucleation does not hold in the light of the current findings.
Even though this work seems to suggest that fluid shear-induced secondary nucleation does not exist, it still cannot serve as firm evidence of such a statement. Nevertheless, the controversial nature of these findings makes them an excellent starting point for a broader debate on secondary nucleation mechanisms, regardless whether fluid shear plays a major role in them.
Data availability
The data supporting this article are fully accessible on the KU Leuven Research Data Repository (RDR). See DOI: https://doi.org/10.48804/D7OQJW.Author contributions
L. D. V.: conceptualization, data curation, methodology, investigation, visualization, writing – original draft, writing – review & editing. S. K.: conceptualization, data curation, funding acquisition, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. D. V. thanks Research Foundation – Flanders (FWO) for a Fundamental PhD fellowship (Grant No. 11P4824N).References
- T. Melia and W. Moffitt, J. Colloid Sci., 1964, 19, 433–447 CrossRef CAS.
- N. W. Cayey and J. Estrin, Ind. Eng. Chem. Fundam., 1967, 6, 13–20 CrossRef CAS.
- J. N. Ness and E. T. White, AIChE Symp. Ser., 1976, 72, 64–73 CAS.
- P. Ayazi Shamlou, A. Jones and K. Djamarani, Chem. Eng. Sci., 1990, 45, 1405–1416 CrossRef.
- H. H. Ting and W. L. McCabe, Ind. Eng. Chem., 1934, 26, 1201–1207 CrossRef.
- S. G. Agrawal and A. H. J. Paterson, Chem. Eng. Commun., 2015, 202, 698–706 CrossRef CAS.
- A. S. Myerson, D. Erdemir and A. Y. Lee, Handbook of Industrial Crystallization, Cambridge University Press, 2019, pp. 1–528 Search PubMed.
- Crystallization Technology Handbook, ed. A. Mersmann, CRC Press, 2001 Search PubMed.
- C. Gahn and A. Mersmann, Chem. Eng. Sci., 1999, 54, 1273–1282 CrossRef CAS.
- C. Gahn and A. Mersmann, Chem. Eng. Sci., 1999, 54, 1283–1292 CrossRef CAS.
- S. Tait, E. T. White and J. D. Litster, Cryst. Growth Des., 2009, 9, 2198–2206 CrossRef CAS.
- J. Hoffmann, J. Flannigan, A. Cashmore, M. L. Briuglia, R. R. E. Steendam, C. J. J. Gerard, M. D. Haw, J. Sefcik and J. H. ter Horst, Faraday Discuss., 2022, 235, 109–131 RSC.
- T. W. Evans, A. F. Sarofim and G. Margolis, AIChE J., 1974, 20, 959–966 CrossRef CAS.
- G. D. Botsaris, in Industrial Crystallization, Springer, US, Boston, MA, 1976, pp. 3–22 Search PubMed.
- A. D. Randolph and M. A. Larson, Theory of Particulate Processes, Elsevier, 1988 Search PubMed.
- D. Zhang, X. Wang, J. Ulrich, W. Tang, S. Xu, Z. Li, S. Rohani and J. Gong, Cryst. Growth Des., 2019, 19, 3070–3084 CrossRef CAS.
- S. Xu, Z. Hou, X. Chuai and Y. Wang, Ind. Eng. Chem. Res., 2020, 59, 18335–18356 CrossRef CAS.
- H. E. C. Powers, Nature, 1956, 178, 139–140 CrossRef CAS.
- M. L. Briuglia, J. Sefcik and J. H. ter Horst, Cryst. Growth Des., 2019, 19, 421–429 CrossRef CAS.
- N. A. Clontz, R. T. Johnson, W. L. McCabe and R. W. Rousseau, Ind. Eng. Chem. Fundam., 1972, 11, 368–373 CrossRef CAS.
- C. A. Knight, J. Cryst. Growth, 1971, 11, 201–217 CrossRef CAS.
- R. E. A. Mason and R. F. Strickland-Constable, Trans. Faraday Soc., 1966, 62, 455 RSC.
- J. Anwar, S. Khan and L. Lindfors, Angew. Chem., Int. Ed., 2015, 54, 14681–14684 CrossRef CAS PubMed.
- L. Bosetti, B. Ahn and M. Mazzotti, Cryst. Growth Des., 2022, 22, 87–97 CrossRef CAS.
- B. Ahn, L. Bosetti and M. Mazzotti, Cryst. Growth Des., 2022, 22, 74–86 CrossRef CAS PubMed.
- B. Ahn, L. Bosetti and M. Mazzotti, Cryst. Growth Des., 2022, 22, 3625–3636 CrossRef CAS PubMed.
- T. L. Threlfall and S. J. Coles, CrystEngComm, 2016, 18, 369–378 RSC.
- S. J. Coles and T. L. Threlfall, CrystEngComm, 2014, 16, 4355 RSC.
- E. G. Denk and G. D. Botsaris, J. Cryst. Growth, 1972, 13-14, 493–499 CrossRef CAS.
- C. Y. Sung, J. Estrin and G. R. Youngquist, AIChE J., 1973, 19, 957–962 CrossRef CAS.
- J. Estrin, M. L. Wang and G. R. Youngquist, AIChE J., 1975, 21, 392–395 CrossRef CAS.
- R. Jagannathan, C. Sung, G. Youngquist and J. Estrin, AIChE Symp. Ser., 1980, 76, 90–97 CAS.
- M. Wang and H. Yang, Chem. Eng. Commun., 1981, 12, 241–251 CrossRef CAS.
- M. Wang, H. Huang and J. Estrin, AIChE J., 1981, 27, 312–315 CrossRef CAS.
- G. Youngquist, in Crystallization and Precipitation, Elsevier, 1987, pp. 45–51 Search PubMed.
- J. Wang and J. Estrin, Chem. Eng. Commun., 1996, 152–153, 275–286 CrossRef CAS.
- R.-Y. Qian and G. D. Botsaris, Chem. Eng. Sci., 1998, 53, 1745–1756 CrossRef CAS.
- T. Buhse, D. Durand, D. Kondepudi, J. Laudadio and S. Spilker, Phys. Rev. Lett., 2000, 84, 4405–4408 CrossRef CAS PubMed.
- C. Y. Tai, C.-D. Tai and M.-H. Chang, J. Taiwan Inst. Chem. Eng., 2009, 40, 439–442 CrossRef CAS.
- M. Yousuf and P. J. Frawley, Cryst. Growth Des., 2018, 18, 6843–6852 CrossRef CAS.
- A. Cashmore, K. Georgoulas, C. Boyle, M. Lee, M. D. Haw and J. Sefcik, Cryst. Growth Des., 2024, 24, 4975–4984 CrossRef CAS.
- C. Y. Tai, W. L. McCabe and R. W. Rousseau, AIChE J., 1975, 21, 351–358 CrossRef CAS.
- R. R. Steendam and P. J. Frawley, Cryst. Growth Des., 2019, 19, 3453–3460 CrossRef CAS.
- D. Kashchiev, Nucleation, Elsevier, 2000 Search PubMed.
- R. J. Davey, S. L. M. Schroeder and J. H. ter Horst, Angew. Chem., Int. Ed., 2013, 52, 2166–2179 CrossRef CAS PubMed.
- S. A. Kulkarni, S. S. Kadam, H. Meekes, A. I. Stankiewicz and J. H. ter Horst, Cryst. Growth Des., 2013, 13, 2435–2440 CrossRef CAS.
- G. M. Maggioni and M. Mazzotti, Faraday Discuss., 2015, 179, 359–382 RSC.
- C. Forsyth, P. A. Mulheran, C. Forsyth, M. D. Haw, I. S. Burns and J. Sefcik, Cryst. Growth Des., 2015, 15, 94–102 CrossRef CAS.
- F. Mura and A. Zaccone, Phys. Rev. E, 2016, 93, 042803 CrossRef PubMed.
- V. Nappo, R. Sullivan, R. Davey, S. Kuhn, A. Gavriilidis and L. Mazzei, Chem. Eng. Res. Des., 2018, 136, 48–56 CrossRef CAS.
- C. Devos, C. Xiouras, T. Van Gerven and S. Kuhn, Cryst. Growth Des., 2024, 24, 2713–2723 CrossRef CAS.
- R.-Y. Qian and G. D. Botsaris, Chem. Eng. Sci., 1997, 52, 3429–3440 CrossRef CAS.
- C. Y. Tai, J.-F. Wu and R. W. Rousseau, J. Cryst. Growth, 1992, 116, 294–306 CrossRef.
- D. Lal, R. Mason and R. Strickland-Constable, J. Cryst. Growth, 1969, 5, 1–8 CrossRef CAS.
- A. E. Flood, CrystEngComm, 2010, 12, 313–323 RSC.
- N. S. Tavare, Can. J. Chem. Eng., 1985, 63, 436–442 CrossRef CAS.
- P. Pantaraks and A. E. Flood, Cryst. Growth Des., 2005, 5, 365–371 CrossRef CAS.
- P. Pantaraks, M. Matsuoka and A. E. Flood, Cryst. Growth Des., 2008, 8, 1078–1078 CrossRef CAS.
- G. Zimbitas, A. Jawor-Baczynska, M. J. Vesga, N. Javid, B. D. Moore, J. Parkinson and J. Sefcik, Colloids Surf., A, 2019, 579, 123633 CrossRef CAS.
- J. Estrin and G. R. Youngquist, in Industrial Crystallization, Springer, US, Boston, MA, 1976, pp. 61–66 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: S1 overview control experiments in literature; S2 description experimental setup; S3 seed washing procedure; S4 solution preparation; S5 characteristics exp I; S6 characteristics exp II; S7 characteristics exp III; S8 characteristics exp IV. See DOI: https://doi.org/10.1039/d5ce00323g |
| This journal is © The Royal Society of Chemistry 2025 |
