 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-resolution leak-out spectroscopy of HHe2+
Thomas
Salomon
 a,
Carlo
Baddeliyanage
a,
Carla
Schladt
a,
Irén
Simkó
b,
Attila G.
Császár
a,
Carlo
Baddeliyanage
a,
Carla
Schladt
a,
Irén
Simkó
b,
Attila G.
Császár
 c,
Weslley G. D. P.
Silva
c,
Weslley G. D. P.
Silva
 a,
Stephan
Schlemmer
a,
Stephan
Schlemmer
 a and
Oskar
Asvany
a and
Oskar
Asvany
 *a
*a
aI. Physikalisches Institut, Universität zu Köln, Zülpicher Str. 77, D-50937, Köln, Germany. E-mail: asvany@ph1.uni-koeln.de
bNew York University, Simons Center for Computational Physical Chemistry, 24 Waverly Place, New York, New York, 10003, USA
cInstitute of Chemistry, ELTE Eötvös Loránd University, H-1117, Budapest, Pázmány Péter sétány 1/A, Hungary
First published on 4th February 2025
Abstract
Applying a novel and universal action spectroscopic technique, called leak-out spectroscopy, this paper revisits the ν3 proton shuttle motion of the symmetric linear molecule He–H+–He. For this, a 4 K cryogenic ion trap apparatus has been combined with a high-resolution quantum cascade laser operating around 1300 cm−1. Seven rovibrational lines of this fundamental three-nucleus-four-electron system are recorded, demonstrating the suitability of the leak-out method for such fundamental hydrogen–helium cations.
1. Introduction
A proton solvated by helium atoms, HHen+, is an interesting molecular system. The first ion in this series, the H2-like and strongly bound HHe+ cation, has been known in the laboratory since 1925,1 has been investigated by high-resolution spectroscopy,2–7 and was detected in space quite recently.8 The next ion within this series, He–H+–He (ref. 9–16), is a linear three-nucleus-four-electron system. It is fairly strongly bound with a dissociation energy of D0 = 3931 ± 20 cm−1 (ref. 14), whereas all additional He atoms in higher order complexes (n = 3–6) are loosely bound to the central proton by less than 200 cm−1. The species n = 3–6 have been investigated by low resolution vibrational predissociation spectroscopy,17 in which the antisymmetric stretch (ν3) and bend (ν2) fundamentals of the He–H+–He core were observed around 1300 cm−1 and 850 cm−1, respectively. More recently, the ν3 mode of “naked” He–H+–He has been investigated in high resolution, with three rovibrational lines detected.18 This particular mode, ν3, is the proton shuttle motion and can be considered as a molecular realization of a Hertzian dipole, with its infrared (IR) intensity computed harmonically to be as large as 2661 km mol−1.13The invention of the leak-out-spectroscopy method (LOS19) has boosted ion-trap-based spectroscopy of molecular ions, as it is a universal and close to background-free technique, with many recent applications to astrophysically relevant cations.19–27 In brief, LOS exploits the fact that the vibrational energy of a laser-excited ion can be converted into kinetic energy in a collision with a suitable neutral molecule or atom. These accelerated ions may then escape the ion trap and can be counted in a detector. By counting the “leaked-out” ions as a function of the laser wavelength, a spectrum is generated. In the current work, we revisit the ν3 mode of He–H+–He with the LOS method, using the very He atoms in the ion trap as the neutral collision partner required for LOS. The high-quality results obtained for HHe2+ encourage us to investigate further fundamental hydrogen–helium cations with LOS.
2. Experimental methods
The experiments of this study have been carried out in the cryogenic 22-pole ion trapping instrument COLTRAP.28 In brief, a pulse of several ten thousand HHe+ ions was generated in an ion source by electron impact ionization of an H2–He mixture, selected in a quadrupole mass spectrometer for a mass range 2–6 u (exact selection was considered not necessary), and then injected into the 22-pole trap.29 The trap was held at a temperature of T = 4 K and was constantly filled with He gas (∼1015 cm−3). During the trapping time of typically several 100 ms, the cold He gas fulfilled two roles: primarily, it enabled HHe2+ ions to be formed by 3-body collisions, and secondarily, it served as the collision partner required for the leak-out of the HHe2+ ions. For LOS, the ion ensemble was irradiated with narrow-bandwidth 7.5 μm IR radiation, which passed through the ion trap with a measured power on the order of 40 mW. The ions leaking out from the trap during the trapping time were selected in a second quadrupole for mass 9 u, and counted in a high-efficiency ion counter. The light source was a quantum cascade laser (Daylight Solutions) operating in the range of 1284–1355 cm−1, whose frequency was measured by a wavemeter (Bristol model 621 A-XIR).3. Results and discussion
The high resolution LOS spectrum obtained for the proton-shuttle motion ν3 of HHe2+ is shown in Fig. 1. Similar to CO2, only levels with even rotational quantum number J are allowed in the ground state owing to nuclear spin statistics. An analysis of the observed line widths (∼60 MHz including an estimated laser linewidth of ∼30 MHz) yields a kinetic temperature of about Tkin ≈ 30 K, and the simulation of the intensity distribution seen in Fig. 1, obtained with PGOPHER,30 corresponds to a rotational temperature of Trot = 35 K. While much lower temperatures could be obtained by optimizing some experimental settings (e.g. by better mass selection and storing less ions), the high temperature of the spectrum shown in Fig. 1 serendipitously permitted us to record as many as seven rovibrational lines, given in Table 1. The lines show excellent agreement, with a difference of only 0.26 cm−1, with accurate variationally calculated values (“first principles”, also listed in Table 1), obtained with the same methodology as used in ref. 16. This constant difference is solely due to the vibrational contribution, whereas the rotational structure in the ground and vibrationally excited states is matched extremely well. Performing a least-squares fit of the observed lines using a linear rotor Hamiltonian as implemented in PGOPHER, the spectroscopic parameters shown in Table 2 are obtained. As can be seen in Table 2, there is a large change of the rotational constant B upon vibrational excitation whose value decreases by about 10% in comparison to the ground state. This difference is caused by the shallow and anharmonic potential energy surface of HHe2+ that results in the asymmetric appearance of the ν3 band depicted in Fig. 1.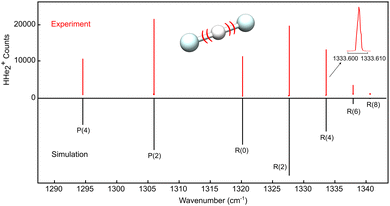 | ||
| Fig. 1 LOS spectrum of the ν3 antisymmetric stretching band of HHe2+. The upper part shows the experimental spectrum, obtained by individually targeting the transitions, as shown for the zoom of the line R(4). The lower part is a stick spectrum simulated at a temperature of 35 K using the fitted parameters from Table 2. | ||
| (v3,J′) | ← | (v3,J′′) | Experiment | First principles | |
|---|---|---|---|---|---|
| P(4) | (1,3) | ← | (0,4) | 1294.5488(4) | 1294.28 |
| P(2) | (1,1) | ← | (0,2) | 1305.9777(4) | 1305.72 |
| R(0) | (1,1) | ← | (0,0) | 1320.1882(4) | 1319.93 |
| R(2) | (1,3) | ← | (0,2) | 1327.6915(4) | 1327.43 |
| R(4) | (1,5) | ← | (0,4) | 1333.6069(4) | 1333.35 |
| R(6) | (1,7) | ← | (0,6) | 1337.9292(4) | 1337.67 |
| R(8) | (1,9) | ← | (0,8) | 1340.6515(4) | 1340.39 |
| This work | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental | First principles | Ab initio, ref. 18 | Ab initio, ref. 13 | Ab initio, ref. 15 | |||||
| v 3 = 0 | v 3 = 1 | v 3 = 0 | v 3 = 1 | v 3 = 0 | v 3 = 1 | v 3 = 0 | v 3 = 1 | v 3 = 1 | |
| ν 3 | 1315.8444(2) | 1315.58 | 1306.2 | 1345.2 | 1318.6 | ||||
| B | 2.36877(6) | 2.17208(6) | 2.3693 | 2.1726 | 2.3616 | 2.1491 | 2.3622 | 2.1506 | |
| D | 0.000056(2) | 0.000052(2) | 0.00005 | 0.00005 | 0.000046 | 0.00004 | |||
4. Conclusion and outlook
In conclusion, the exceptional sensitivity of LOS and its application to the ν3 proton shuttle motion of HHe2+ allowed us to detect seven of its rovibrational lines, four of which are reported here for the first time. In comparison to our former work,18 a much better determination of the spectroscopic parameters of HHe2+ (see Table 2) could be achieved here. Our novel results will certainly facilitate astronomical searches for HHe2+, preferentially in the infrared region using the recently launched James Webb Space Telescope. After this successful demonstration, we plan to measure the bending mode ν2 or the combination band ν1 + ν3 of HHe2+, with calculated band origins of ν2 = 874.9 cm−1 and ν1 + ν3 = 2057.9 cm−1 (ref. 16), respectively. An equally attractive target is the fundamental H2 stretch in linear H2He+, or the D2 stretch in D2He+. The wavenumber of the latter is predicted to be at 1318 cm−1 (ref. 31), which we could confirm by recording its first rovibrational fingerprints, using the same quantum cascade laser as in this work.Data availability
All the important data (transition frequencies) are contained in Table 1.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by an ERC Advanced Grant (MissIons: 101020583), and by the Deutsche Forschungsgemeinschaft (DFG) via the Collaborative Research Centre SFB 1601 (project ID: 500700252, sub-project C4) and via SCHL 341/15-1 (“Cologne Center for Terahertz Spectroscopy”). W. G. D. P. S. thanks the Alexander von Humboldt Foundation for support through a postdoctoral fellowship. A. G. C received support from NKFIH (grant no. K138233). I. S. gratefully acknowledges a postdoctoral fellowship from the Simons Foundation, through the Simons Center for Computational Physical Chemistry at NYU.Notes and references
- T. R. Hogness and E. G. Lunn, Phys. Rev., 1925, 26, 44–55 CrossRef CAS.
- D. E. Tolliver, G. A. Kyrala and W. H. Wing, Phys. Rev. Lett., 1979, 43, 1719–1722 CrossRef CAS.
- P. Bernath and T. Amano, Phys. Rev. Lett., 1982, 48, 20–22 CrossRef CAS.
- M. W. Crofton, R. S. Altman, N. N. Haese and T. Oka, J. Chem. Phys., 1989, 91, 5882–5886 CrossRef CAS.
- A. J. Perry, J. N. Hodges, C. R. Markus, G. S. Kocheril and B. J. McCall, J. Chem. Phys., 2014, 141, 101101 CrossRef PubMed.
- F. Matsushima, T. Oka and K. Takagi, Phys. Rev. Lett., 1997, 78, 1664–1666 CrossRef CAS.
- D.-J. Liu, W.-C. Ho and T. Oka, J. Chem. Phys., 1987, 87, 2442 CrossRef CAS.
- R. Güsten, H. Wiesemeyer, D. Neufeld, K. M. Menten, U. U. Graf, K. Jacobs, B. Klein, O. Ricken, C. Risacher and J. Stutzki, Nature, 2019, 568, 357–359 CrossRef.
- N. G. Adams, D. K. Bohme and E. E. Ferguson, J. Chem. Phys., 1970, 52, 5101–5105 CrossRef CAS.
- T. M. Kojima, N. Kobayashi and Y. Kaneko, Z. Phys. D: At., Mol. Clusters, 1992, 23, 181–185 CrossRef CAS.
- L. Lundberg, P. Bartl, C. Leidlmair, P. Scheier and M. Gatchell, Molecules, 2020, 25, 1066 CrossRef CAS PubMed.
- B. Balta and F. Gianturco, Chem. Phys., 2000, 254, 203–213 CrossRef CAS.
- C. J. Stephan and R. C. Fortenberry, Mon. Not. R. Astron. Soc., 2017, 469, 339–346 CrossRef CAS.
- A. G. Császár, T. Szidarovszky, O. Asvany and S. Schlemmer, Mol. Phys., 2019, 117, 1559–1583 CrossRef.
- D. Kędziera, G. Rauhut and A. G. Császár, Phys. Chem. Chem. Phys., 2022, 24, 12176–12195 RSC.
- I. Simkó, C. Schran, F. Brieuc, C. Fábri, O. Asvany, S. Schlemmer, D. Marx and A. G. Császár, Angew. Chem., Int. Ed., 2023, 62, e202306744 CrossRef PubMed.
- O. Asvany, S. Schlemmer, T. Szidarovszky and A. G. Császár, J. Phys. Chem. Lett., 2019, 10, 5325–5330 CrossRef CAS PubMed.
- M. Töpfer, A. Jensen, K. Nagamori, H. Kohguchi, T. Szidarovszky, A. G. Császár, S. Schlemmer and O. Asvany, Phys. Chem. Chem. Phys., 2020, 22, 22885–22888 RSC.
- P. C. Schmid, O. Asvany, T. Salomon, S. Thorwirth and S. Schlemmer, J. Phys. Chem. A, 2022, 126, 8111 CrossRef CAS PubMed.
- O. Asvany, S. Thorwirth, P. C. Schmid, T. Salomon and S. Schlemmer, Phys. Chem. Chem. Phys., 2023, 25, 19740–19749 RSC.
- M. Bast, J. Böing, T. Salomon, S. Thorwirth, O. Asvany, M. Schäfer and S. Schlemmer, J. Mol. Spectrosc., 2023, 398, 111840 Search PubMed.
- S. Schlemmer, E. Plaar, D. Gupta, W. G. D. P. Silva, T. Salomon and O. Asvany, Mol. Phys., 2024, 122, e2241567 CrossRef.
- W. G. D. P. Silva, J. Cernicharo, S. Schlemmer, N. Marcelino, J. C. Loison, M. Agúndez, D. Gupta, V. Wakelam, S. Thorwirth, C. Cabezas, B. Tercero, J. L. Doménech, R. Fuentetaja, W. J. Kim, P. de Vicente and O. Asvany, Astron. Astrophys., 2023, 676, L1 Search PubMed.
- W. G. D. P. Silva, D. Gupta, E. Plaar, J. L. Doménech, S. Schlemmer and O. Asvany, Mol. Phys., 2024, 122, e2296613 CrossRef.
- D. Gupta, W. G. D. P. Silva, J. L. Doménech, E. Plaar, S. Thorwirth, S. Schlemmer and O. Asvany, Faraday Discuss., 2023, 245, 298–308 RSC.
- K. Steenbakkers, T. van Boxtel, G. C. Groenenboom, O. Asvany, B. Redlich, S. Schlemmer and S. Brünken, Phys. Chem. Chem. Phys., 2024, 26, 2692–2703 RSC.
- W. G. D. P. Silva, L. Bonah, P. C. Schmid, S. Schlemmer and O. Asvany, J. Chem. Phys., 2024, 160, 071101 CrossRef CAS PubMed.
- O. Asvany, S. Brünken, L. Kluge and S. Schlemmer, Appl. Phys. B: Lasers Opt., 2014, 114, 203–211 CrossRef CAS.
- O. Asvany, F. Bielau, D. Moratschke, J. Krause and S. Schlemmer, Rev. Sci. Instrum., 2010, 81, 076102 CrossRef PubMed.
- C. M. Western, J. Quant. Spectrosc. Radiat. Transfer, 2017, 186, 221–242 CrossRef CAS.
- O. Asvany, S. Schlemmer, A. van der Avoird, T. Szidarovszky and A. G. Császár, J. Mol. Spectrosc., 2021, 377, 111423 CrossRef CAS.
| This journal is © the Owner Societies 2025 |
