Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Persistence of Ce3+ species on the surface of ceria during redox cycling: a modulated chemical excitation investigation†
C.
Hachemi
 a,
H.
Dib
b,
M.
Debbichi
a,
H.
Dib
b,
M.
Debbichi
 c,
M.
Badawi
c,
M.
Badawi
 d,
C.
Eads
ef,
M.
Ibrahim
b,
S.
Loridant
d,
C.
Eads
ef,
M.
Ibrahim
b,
S.
Loridant
 a,
J.
Knudsen
ef,
H.
Kaper
a,
J.
Knudsen
ef,
H.
Kaper
 b and
L.
Cardenas
b and
L.
Cardenas
 *a
*a
aUniv. Lyon, Université Claude Bernard-Lyon 1, CNRS, IRCELYON-UMR 5256, 2 av. A. Einstein, F-69626 Villeurbanne Cedex, France. E-mail: luis.cardenas@ircelyon.univ-lyon1.fr
bSaint Gobain Recherche Provence, 550, Ave Alphonse Jauffret, 84306 Cavaillon, France
cUniversité de Monastir, Faculté des Sciences de Monastir, Laboratoire de la matière condensée et nanosciences, LR11ES40, 5019 Monastir, Tunisia
dUniversité de Lorraine, CNRS, L2CM, F-54000 Nancy, France
eDivision of Synchrotron Radiation Research, Department of Physics, Lund University, Lund, Sweden
fMAX IV Laboratory, Lund University, Lund, Sweden
First published on 16th May 2025
Abstract
Operando resonant photoelectron spectroscopy (RPES) combined with modulated chemical excitation revealed the dynamic evolution of Ce3+/Ce4+ redox states at the surface of CeO2 during the CO oxidation reaction. Using alternating CO and O2 pulses as chemically modulated signals, we monitored the surface states in the valence band region, unveiling the evolution of electronic structure during the catalytic process. The analysis with different gas flow ratios revealed that under CO-rich conditions (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ≥ 1), only partial conversion from Ce3+ to Ce4+ occurred. In contrast, complete Ce3+ to Ce4+ conversion was achieved when pulsing O2 into O2-rich environments. Furthermore, we find that intermediate oxygen species, such as peroxo and OH, impact the conversion of Ce3+ and Ce4+. These oxygenated species coexist between 330 °C and 360 °C in pure O2, while above 390 °C only OH groups remain stable on the ceria surface.
O2 ≥ 1), only partial conversion from Ce3+ to Ce4+ occurred. In contrast, complete Ce3+ to Ce4+ conversion was achieved when pulsing O2 into O2-rich environments. Furthermore, we find that intermediate oxygen species, such as peroxo and OH, impact the conversion of Ce3+ and Ce4+. These oxygenated species coexist between 330 °C and 360 °C in pure O2, while above 390 °C only OH groups remain stable on the ceria surface.
Introduction
Ceria-based catalysts are of pivotal importance in many chemical processes, such as automotive exhaust treatment,1 water gas shift,2 and CO oxidation reactions.3 The driving force of ceria as an oxygen-storage catalyst stems from its Ce3+ species, which act as an electron reservoir to enhance catalytic performance. Although it is widely accepted that Ce3+ is essential in triggering these reactions, the catalytic processes initiated when these species interact with reactants are poorly understood. In particular, knowledge about the nature of active intermediate species formed under reaction conditions remains limited.The excess electron from Ce3+ serves as a reactive center, facilitating the activation of oxygen-containing molecules in the gas phase.4,5 For instance, the dissociation process of O2 has been described as electrophilic, where oxygen species sequentially accept electrons from the catalyst surface until complete reduction, following the path:6
| O2 → (O2)s → (O2−)s → (O22−)s → 2(O−)s → 2(O2−)s → 2(O2−)Lattice |
In the Mars–van Krevelen (MvK) mechanism, gas-phase oxygen interactions play a fundamental role in maintaining the catalytic cycle. Initially, CO reacts with lattice oxygens (O2−), creating oxygen vacancies in the CeO2 lattice. These vacancies are subsequently replenished when gas-phase O2 interacts with CeO2. However, other pathways that entail the formation of intermediate oxygen species during the CO oxidation reaction may also play a major role.
These oxygen intermediates may serve as potential active species that influence the Ce3+/Ce4+ ratio7–9via oxygen vacancy occupation. However, detecting these species remains a significant challenge in heterogeneous catalysis, primarily due to their low concentrations and short lifetimes.10 To address this challenge, we propose an effective detection approach using temporal analysis methods, such as periodic modifications of the feed-gas composition. This methodology enables the catalyst surface to cycle through different activity phases during the catalytic reaction.
In this study, the surface of CeO2 was brought to oscillate between two rich phases, oxidized (Ce4+) and reduced (Ce3+) by temporally changing the feed-gas composition between pure O2 and CO. Using repeated gas pulses, we monitored the Ce3+ in the valence band while simultaneously detecting O2 (m/z = 32) and CO (m/z = 28) by mass spectrometry (MS). As a selective reducing agent, CO converts Ce4+ to Ce3+, which we detected through the Ce 4f electronic states in the valence band. We conducted synchronized measurements of Ce 4f and CO mass spectra to monitor the cyclical formation and depletion of Ce 4f during alternating exposure to O2 (oxidizing) and CO (reducing) gases. This approach of studying catalyst response to an external chemical variable excitation is usually referred to as modulation excitation spectroscopy and has so far been used in APXPS for core-level analysis.11–13
Modulation excitation spectroscopy (MES) is an analytical technique that applies periodic chemical stimuli to study dynamic processes in catalytic materials.14 The technique identifies specific spectroscopic features as reference events that can be tracked through repeated measurement cycles. After reaching a transient state, these cycles are averaged to enhance data quality. This approach enables systematic observation of active species responding to the excitation. The data can be further analyzed using phase-sensitive detection (PSD), described by:15
Here, we developed a novel surface-sensitive operando technique that combines modulated chemical excitation with resonant photoelectron spectroscopy (RPES) to track transient catalytic species in the valence band region. This combined approach, supported by DFT calculations, enables the identification of both transient and stationary activated oxygen and ceria species on the CeO2 surface as well as their relationship with the electronic structure. For the first time, we directly observed the oscillation of the Ce 4f states in the valence band as modulated chemical excitation drives the reversible conversion between Ce4+ and Ce3+, highlighting the persistence of Ce3+ species in O2-rich conditions.
Experimental methods
CeO2 preparation and standard characterization
Pure cerium oxide nanoparticles were synthesized by the decomposition of cerium nitrate, as reported previously.16 In brief, Ce(NO3)·6H2O (AcrosOrganic, 99+%) was dissolved in isopropanol at room temperature. After complete dissolution, the solvent was removed by rotational evaporation at 44 °C until nearly complete dryness. The resulting solid was calcined following a controlled temperature program: from room temperature to 180 °C for 2 h, then sequentially to 200 °C (3 h), 220 °C (3 h), and finally to 500 °C (3 h). The obtained powder was ground into a fine powder using a mortar.X-Ray diffraction (XRD) patterns of powdered samples were collected using a Bruker D8 Advance A25 diffractometer equipped with a Cu anode (Cu Kα: 0.154184 nm) (Fig. S1, ESI†). Raman spectroscopy measurements were performed using a Horiba labRAM HR spectrometer with laser excitation at 532 nm. The Raman spectra were acquired using a 300 lines per mm−1 grating at an incident nominal power of 1 mW (Fig. S2, ESI†).
Nitrogen (N2) adsorption/desorption isotherms were measured at 77 K with a Micromeritics Tristar II 3020 instrument. Prior to each analysis, the samples were degassed under vacuum (<5 Torr) overnight at 350 °C (Fig. S3, ESI†). Thermogravimetric analyses (TGA-MS) were recorded on a temperature range between 30 and 1000 °C with a ramp of 5 °C min−1 using a LabSysEvo TGA by Setaram, coupled with a Pfeiffer Omnistar GSD 320 mass spectrometer (Fig. S4, ESI†).
Modulated chemical excitation spectroscopy
Modulated chemical excitation spectroscopy experiments were performed at the SPECIES beamline of the MAX IV laboratory.17,18 The measurements were conducted in a near-ambient pressure (NAP) cell (≈1 L) at a constant total pressure of 0.5 mbar using a SPECS Phoibos 150 NAP electron analyzer. To maximize the signal-to-noise ratio, the analyzer nozzle was positioned within a few millimeters of the sample surface.CeO2 powder was deposited on a silver foil by drop-casting after sonication in isopropanol to minimize charging effects.19 The sample was mounted on a grounded stainless steel holder with a K-type thermocouple spot-welder near the sample surface. The sample holder was maintained on a vertical manipulator with the sample perpendicular to the analyzer nozzle (Fig. S5, ESI†). Prior to modulated chemical excitation measurements, the sample was oxidized in pure O2 at 400 °C to remove surface carbon contamination.
Modulated chemical excitation spectroscopy measurements were collected at 1 Hz using a snapshot mode, where a fixed kinetic energy window was maintained without lens voltage scanning to ensure an adequate signal-to-noise ratio.20 CO and O2 gases were independently injected through mass flow controllers with a 40 s pulse length at constant flow rates, maintaining a total pressure in the NAP cell of 0.5 mbar. Modulated chemical excitation spectroscopy data were recorded at temperatures ranging from 330 to 420 °C (330, 360, 390, 400, and 420 °C) using a CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 flow ratio of 0.6
O2 flow ratio of 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 standard cubic centimeters per minute (sccm). Additional measurements at 420 °C were performed with CO
0.6 standard cubic centimeters per minute (sccm). Additional measurements at 420 °C were performed with CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratios of 1.2
O2 ratios of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 and 0.6
0.6 and 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 sccm. Gas composition was monitored using a quadrupole mass spectrometer (Hiden HAL/3F PIC), which sampled gases through the electron analyzer's aperture near the sample surface.
2.4 sccm. Gas composition was monitored using a quadrupole mass spectrometer (Hiden HAL/3F PIC), which sampled gases through the electron analyzer's aperture near the sample surface.
XPS spectra were acquired at photon energies of 1030 eV (Ce 3d, wide scan), 590 eV (O 1s), and 390 eV (C 1s) to optimize photoionization cross-sections and enhance surface sensitivity. For valence band RPES, the photon energy was fixed at 121.2 eV (Ce3+), 124.5 eV (Ce4+), and 115 eV (off-resonance). The pass energy was set to 100 eV for snapshot modes and 50 eV for standard spectrum mode. Energy calibration was performed using the quasi-metallic Ce 4f states, which cross the Fermi level in the Ce3+-rich species, and the f0 features in the Ce 3d spectra. The survey XPS spectrum of CeO2 (Fig. S6, ESI†) was collected both at room temperature (as-received) and at 230 °C under ultrahigh vacuum (UHV) conditions prior to modulated chemical excitation spectroscopy experiments. The spectrum revealed Ce and O as the predominant elements, with minimal carbon contamination, indicating high sample purity and well-controlled synthesis conditions. Core-level spectra were fitted using Voigt functions (L/G ratio 0.3) and Shirley backgrounds in IgorPro 9 (Wavemetrics) (see Table S1, ESI†).
DFT calculations
The Vienna Ab initio Simulation Package21,22 (VASP) was used to perform all the DFT calculations. The projector augmented wave (PAW) method and the Perdew–Burke–Ernzerhof (PBE) functional under the generalized gradient approximation (GGA) were used in the calculations.23,24 For Ce and O atoms, the (5s, 5p, 6s, 4f, 5d) and (2s, 2p) states, respectively, were treated as valence with a plane-wave cutoff energy of 520 eV. We adopt a Γ-centered 12 × 12 × 1 k-point grid for the integration in the reciprocal space.25 A vacuum layer of about 20 Å was used to avoid the interaction between the images of the CeO2(111) surface. All the calculations are spin-polarized. To treat the electron–electron interaction on Ce 4f orbitals, we have adopted the GGA+U approach formulated by Dudarev et al.26 by setting the effective Coulomb interaction Ueff to 5 eV, based on previous reports.27,28 The effect of van der Waals (vdW) interactions on the adsorption of O2 and OH on the CeO2(111) surface was included through the dispersion correction of the DFT-D3 method of Grimme.29 The convergence criteria for the total energy and the forces were set as 10−5 eV and 0.025 eV A−1, respectively.Results and discussion
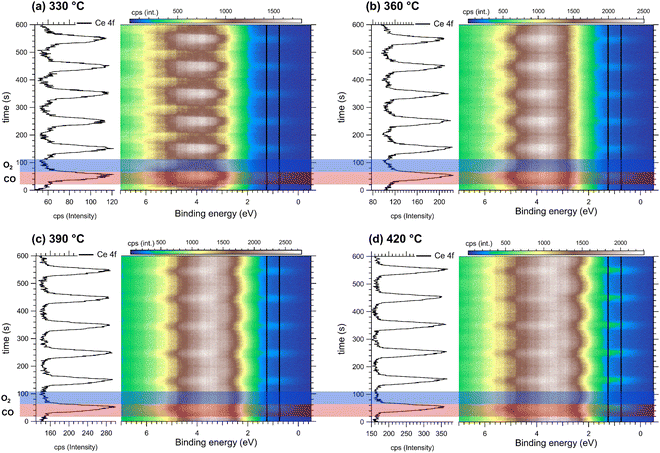
For modulated excitation spectroscopy experiments on CeO2, synchronization between reference events and gas pulses is crucial. Periodic O2 and CO injections increase the pressure in the NAP cell while switching ceria between Ce4+ and Ce3+-rich species, respectively. During CO pulses, the reduction of Ce4+ to Ce3+ is evidenced by the formation of localized Ce 4f states in the valence band (≈1 eV), characteristic of reduced Ce3+ species.30–33 The synchronization between CO pulses and Ce 4f events was established by simultaneously monitoring the CO mass signal (m/z = 28) using mass spectrometry (MS) (Fig. S7, ESI†) and detecting the Ce 4f state in the valence band using RPES (Fig. S8, ESI†). Using the Ce 4f signal as a reference, we monitored its evolution during alternating cycles of CO and O2 exposure.These modulated chemical excitations were monitored by RPES using two beam resonant energies: Ce3+ (121.2 eV) and Ce4+ (124.5 eV; see Fig. S9, ESI†). The resonant mode allows us to improve the signal detection level for Ce3+ down to 0.1% of atomic concentration.34 Fig. S10 (ESI†) demonstrates that RPES exhibits high selectivity to detect Ce3+ or Ce4+ species compared to conventional Ce 3d XPS measurements. The Ce 3d XPS spectra present multiplet structure and overlapping features, which make the analysis and determination of Ce3+ and Ce4+ species difficult.33 In contrast, RPES enables direct identification of Ce3+ through selective resonant excitation of the Ce 4f state, allowing tracking of changes in the electronic structure and oxidation state of CeO2.
(a) Tracking the Ce3+ event in a mixing flow ratio CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 1
O2 = 1
Fig. 1 shows the modulated chemical excitation RPES spectra of ceria measured at a photon energy of 121.2 eV, which selectively enhances Ce3+ excitation through resonant processes. RPES spectra were collected during alternating gas pulses, where red horizontal bands indicate CO exposure and blue bands indicate O2 exposure, with the sample maintained at (a) 330 °C, (b) 360 °C, (c) 390 °C, and (d) 420 °C. A CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 mixing ratio of 1
O2 mixing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was maintained throughout the measurements. The formation of a Ce 4f state at 1.0 eV during CO pulses indicates Ce3+ formation, while its subsequent decrease during O2 pulses demonstrates the conversion back to Ce4+. This is demonstrated by the black oscillation in the left panels, which shows the integrated intensity of the Ce 4f state measured along the vertical black profile centered at 1.0 eV (±0.5 eV integration window). This periodic oscillation reaches its maximum amplitude during each CO pulse (red band) and decreases to a minimum when CO is switched off and O2 is injected (blue band). Thus, the oscillation of the Ce 4f state clearly shows the transition between Ce3+ and Ce4+ species during periodic CO and O2 pulses. Here, the population rate of Ce 4f serves as an excellent indicator to monitor the reduced Ce3+ form during ceria's redox cycle (Fig. S8(d), ESI†). Similarly, the impact of CO and O2 pulses extends to deeper states of the valence band, particularly in the hybridized region where Ce 4f, Ce 5d, and O 2p orbitals coexist (between ≈2 eV and ≈6 eV). Although changes in this region suggest transitions between Ce3+ and Ce4+ species, the hybrid character of these states makes the identification of individual orbitals difficult.
1 was maintained throughout the measurements. The formation of a Ce 4f state at 1.0 eV during CO pulses indicates Ce3+ formation, while its subsequent decrease during O2 pulses demonstrates the conversion back to Ce4+. This is demonstrated by the black oscillation in the left panels, which shows the integrated intensity of the Ce 4f state measured along the vertical black profile centered at 1.0 eV (±0.5 eV integration window). This periodic oscillation reaches its maximum amplitude during each CO pulse (red band) and decreases to a minimum when CO is switched off and O2 is injected (blue band). Thus, the oscillation of the Ce 4f state clearly shows the transition between Ce3+ and Ce4+ species during periodic CO and O2 pulses. Here, the population rate of Ce 4f serves as an excellent indicator to monitor the reduced Ce3+ form during ceria's redox cycle (Fig. S8(d), ESI†). Similarly, the impact of CO and O2 pulses extends to deeper states of the valence band, particularly in the hybridized region where Ce 4f, Ce 5d, and O 2p orbitals coexist (between ≈2 eV and ≈6 eV). Although changes in this region suggest transitions between Ce3+ and Ce4+ species, the hybrid character of these states makes the identification of individual orbitals difficult.
Here, a key finding is the complete conversion between Ce3+ and Ce4+ species across a temperature range of 330 °C to 420 °C. This conversion is evident in the left panels, where the Ce 4f red oscillation reaches its maximum (Ce3+) during CO injection and its minimum (Ce4+) during O2 injection. Furthermore, the profile exhibits a gradual increase in intensity (cps) with temperature, suggesting an enhancement of the Ce3+ state. During CO injection, the concentration of the Ce3+ species reaches its maximum intensity (cps) at 420 °C (see also Fig. S8(d), ESI†). Although the redox cycle highlights complete conversion between Ce3+ and Ce4+ species, the influence of feed-gas composition on ceria's redox reaction kinetics requires further investigation. Therefore, we study how different CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 mixing ratios, under both CO-rich and O2-rich conditions, affect the rates of ceria oxidation (Ce4+) and reduction (Ce3+).
O2 mixing ratios, under both CO-rich and O2-rich conditions, affect the rates of ceria oxidation (Ce4+) and reduction (Ce3+).
(b) Detection of persistent Ce3+ species under O2-rich conditions
Here, modulated chemical excitation RPES experiments were carried out at various relative flowrates between reactants with the following mixing CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 flow ratios: 1.2
O2 flow ratios: 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 sccm (Fig. 2(a)), 0.6
0.6 sccm (Fig. 2(a)), 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 sccm (Fig. 2(b)), and 0.6
0.6 sccm (Fig. 2(b)), and 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 sccm (Fig. 2(c)). The periodic CO and O2 pulses were synchronized with RPES spectra using mass spectrometry signals from O2 (m/z = 32) and CO (m/z = 28) while RPES spectra were scanned. Using this synchronization protocol, we aligned the Ce 4f state in the valence band with the CO mass signal pulse (m/z = 28) (see Fig. S7 and S8, ESI†). The synchronized MS signals at the bottom of Fig. 2 (left panel) – CO (black-dotted), O2 (blue-dotted), and CO2 (brown-line) – reveal the correlation between gas reactivity and Ce3+ population states at different CO
2.4 sccm (Fig. 2(c)). The periodic CO and O2 pulses were synchronized with RPES spectra using mass spectrometry signals from O2 (m/z = 32) and CO (m/z = 28) while RPES spectra were scanned. Using this synchronization protocol, we aligned the Ce 4f state in the valence band with the CO mass signal pulse (m/z = 28) (see Fig. S7 and S8, ESI†). The synchronized MS signals at the bottom of Fig. 2 (left panel) – CO (black-dotted), O2 (blue-dotted), and CO2 (brown-line) – reveal the correlation between gas reactivity and Ce3+ population states at different CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratios. The MS data shows that alternating O2 and CO injections induce reversible transitions between Ce3+ and Ce4+ species throughout the reaction cycle.
O2 ratios. The MS data shows that alternating O2 and CO injections induce reversible transitions between Ce3+ and Ce4+ species throughout the reaction cycle.
(i) CO-rich (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 2) conditions: Fig. 2(a) shows the electronic structure evolution of CeO2 at 420 °C under alternating O2 (blue band) and CO (yellow band) pulses, applied every 40 s with a CO-rich flow mixing ratio of CO
O2 = 2) conditions: Fig. 2(a) shows the electronic structure evolution of CeO2 at 420 °C under alternating O2 (blue band) and CO (yellow band) pulses, applied every 40 s with a CO-rich flow mixing ratio of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 2. The corresponding MS data is shown at the bottom in the left panel of Fig. 2(d). The MS data reveal CO2 formation when CO and O2 reactants coexist (brown line (d)), confirming CO oxidation at 420 °C. Additionally, by varying the flow ratios, we identified the impact of reactant concentrations on CO2 formation, as clearly evidenced by the double-peak in the CO2 MS signal in Fig. 2(f). Here, the enhancement of the Ce 4f state at 1.0 eV correlates with CO pulses, as shown by the MS data and RPES spectra.
O2 = 2. The corresponding MS data is shown at the bottom in the left panel of Fig. 2(d). The MS data reveal CO2 formation when CO and O2 reactants coexist (brown line (d)), confirming CO oxidation at 420 °C. Additionally, by varying the flow ratios, we identified the impact of reactant concentrations on CO2 formation, as clearly evidenced by the double-peak in the CO2 MS signal in Fig. 2(f). Here, the enhancement of the Ce 4f state at 1.0 eV correlates with CO pulses, as shown by the MS data and RPES spectra.
In contrast, during O2 pulses a residual spectral weight persists, as pointed out by the white arrow. To analyze the distinctive features of Ce 4f during each injection, we applied a normalization procedure (detailed in Fig. S11, ESI†). Fig. 2(d) shows the normalized data with two vertical line profiles at energies of 1.0 ± 0.5 eV (blue) and 1.7 ± 0.5 eV (red), with their corresponding intensities displayed in the left panel. The phase shift between these oscillations was used to track the Ce3+ features at 1.0 eV and 1.7 eV. During O2 pulsing (blue band), the oscillation at 1.7 eV reaches its maximum while the 1.0 eV signal diminishes, demonstrating a clear phase shift between these features. During CO pulses (yellow band), both oscillations reach their maxima simultaneously, showing in-phase behavior. Under pure O2 conditions (blue band), although the complete oxidation of Ce3+ to Ce4+ should theoretically eliminate the Ce 4f signal, a detectable Ce 4f feature nevertheless persists at 1.7 eV. This result suggests that some Ce3+ species persist despite the oxidizing nature of O2 pulses. This behavior continues throughout the entire 1300-second sequence of redox injection cycles (Fig. S9, ESI†), indicating a fundamental limitation in achieving complete conversion from Ce3+ to Ce4+ during the oxidation process.
RPES measurements at Ce3+ (121.2 eV) and Ce4+ (124.5 eV) threshold energies reveal the intricate interplay between O 2p–Ce 4f covalent and ionic interactions. For instance, RPES selectively probes Ce3+ ions through an intra-atomic process that significantly enhances the spectral intensity of pure f–f electrons, enabling the detection of these species at extremely low concentrations:
| Ce 4d104f1O 2p6 + hν → Ce 4d94f2O 2p6 → Ce 4d104f0O 2p6 + e− |
In contrast, for a Ce4+ ion, the process is inter-atomic, providing insights into the covalent interactions between cations and anions:35
| Ce 4d104f0O 2p6 + hν → Ce 4d94f1O 2p6 → Ce 4d104f0O 2p5 + e− |
Analysis of modulated chemical excitation RPES data and time-constant profiles under CO and O2 injections (Fig. S12(a) and (b), ESI†) reveals selective enhancement of Ce3+ (121.2 eV, top panel (a)) and Ce4+ (124.5 eV, bottom panel (a)) signals when using a CO-rich flow gas mixture (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 2). During O2 injection, the time-constant profile shifts to lower binding energies (blue line, upper panel of Fig. S12b, ESI†), appearing as a distinctive “V-shaped” electron density feature in the RPES spectrum (dashed blue line, upper panel of Fig. S12a, ESI†). The observed energy shift suggests a lattice contraction resulting from changes in oxygen stoichiometry.36 While this shift may reflect changes in the Ce3+ electron population, it can also be attributed to enhanced Ce4+–O 2p covalent bonding during O2 injection at this energy.35 RPES measurements at the Ce 4d → 4f photoabsorption threshold (121.2 eV) reveal electronic interactions more complex than simple Ce4+–O 2p orbital hybridization or pure f–f electron transitions.
O2 = 2). During O2 injection, the time-constant profile shifts to lower binding energies (blue line, upper panel of Fig. S12b, ESI†), appearing as a distinctive “V-shaped” electron density feature in the RPES spectrum (dashed blue line, upper panel of Fig. S12a, ESI†). The observed energy shift suggests a lattice contraction resulting from changes in oxygen stoichiometry.36 While this shift may reflect changes in the Ce3+ electron population, it can also be attributed to enhanced Ce4+–O 2p covalent bonding during O2 injection at this energy.35 RPES measurements at the Ce 4d → 4f photoabsorption threshold (121.2 eV) reveal electronic interactions more complex than simple Ce4+–O 2p orbital hybridization or pure f–f electron transitions.
Furthermore, the observation of distinct Ce3+ states at ≈1.7 eV and ≈1.0 eV suggests implications between Ce3+ species and catalytic properties across different crystallographic surfaces. Recent studies demonstrate that ceria's surface morphology fundamentally controls the formation and stability of single-atom catalysts (SACs).37,38 The exposed surface facet governs oxygen vacancy formation, which in turn determines substrate adsorption strength and catalytic activity.39 This surface-dependent behavior manifests differently across crystallographic surfaces. For instance, the CeO2(100) surface has been shown to be catalytically active and less stable than the (111) surface,40 making it more reactive and easily reduced under CO exposure. Similar surface-dependent behavior has been observed in H2 reactions with CeO2.41
Pd/CeO2(100) achieves exceptional selectivity (90.3%) for N alkylation reactions, performing 2.8 times better than Pd/CeO2(111).42 A study of Au SACs shows that (110) and (111) surfaces stabilize single Au atoms in various morphologies, while the (100) surface leads to Au nanoparticle formation.39 These differences in metal dispersion and oxygen vacancy distribution demonstrate how crystallographic structure controls both Ce3+ persistence and catalytic activity, making facet control a key strategy for catalyst optimization.
(ii) CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 1 flow ratio conditions: here, we used a flow of 0.6 sccm per gas, resulting in a mixing flow ratio CO
O2 = 1 flow ratio conditions: here, we used a flow of 0.6 sccm per gas, resulting in a mixing flow ratio CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 1 with a pulse length of 40 s. In Fig. 2(b), we observed the same layout describing the cycling between Ce3+ and Ce4+ rich-species as CO or O2 are injected.
O2 = 1 with a pulse length of 40 s. In Fig. 2(b), we observed the same layout describing the cycling between Ce3+ and Ce4+ rich-species as CO or O2 are injected.
To analyze the spectral weight at 1.7 eV (white arrow) during the O2 pulse, we applied the same analysis protocol to the normalized data in Fig. 2(e). Vertical line profiles were centered at the same energies as in Fig. 2(a): 1.0 ± 0.5 eV (blue) and 1.7 ± 0.5 eV (red). Here, both profiles exhibit behavior similar to the CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 2 conditions, where CO pulses generate Ce 4f states at 1.0 eV and 1.7 eV. The CO pulse drives the Ce4+ to Ce3+ conversion, evidenced by the formation of Ce 4f states. The Ce 4f character is observed at ≈1 eV and in the hybridized region between ≈3 eV and ≈6 eV,35 demonstrating significant f-orbital contribution. During O2 pulses, the phase shift is again observed between red and blue oscillations in the left panel, confirming the persistent reduced Ce3+ species. Additionally, a spectral weight between ≈2 eV and ≈6 eV in the normalized image allows us to speculate about the extension of Ce 4f in the hybridized region. These features highlight the persistence of the Ce3+ during O2 pulses.
O2 = 2 conditions, where CO pulses generate Ce 4f states at 1.0 eV and 1.7 eV. The CO pulse drives the Ce4+ to Ce3+ conversion, evidenced by the formation of Ce 4f states. The Ce 4f character is observed at ≈1 eV and in the hybridized region between ≈3 eV and ≈6 eV,35 demonstrating significant f-orbital contribution. During O2 pulses, the phase shift is again observed between red and blue oscillations in the left panel, confirming the persistent reduced Ce3+ species. Additionally, a spectral weight between ≈2 eV and ≈6 eV in the normalized image allows us to speculate about the extension of Ce 4f in the hybridized region. These features highlight the persistence of the Ce3+ during O2 pulses.
(iii) O2-rich (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 0.25) conditions: modulated chemical excitation RPES experiments were performed with alternating gas flows of O2 = 2.4 sccm and CO = 0.6 sccm, creating O2-rich conditions. Analysis of Fig. 2(c) shows the reversible switching between Ce3+
O2 = 0.25) conditions: modulated chemical excitation RPES experiments were performed with alternating gas flows of O2 = 2.4 sccm and CO = 0.6 sccm, creating O2-rich conditions. Analysis of Fig. 2(c) shows the reversible switching between Ce3+ ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) Ce4+ species during O2 (blue band) and CO (yellow band) pulses. The Ce 4f state formation at ≈1 eV during CO pulses is less intense compared to conditions with CO
Ce4+ species during O2 (blue band) and CO (yellow band) pulses. The Ce 4f state formation at ≈1 eV during CO pulses is less intense compared to conditions with CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratios of 2 and 1, which can be attributed to the abundance of remaining O2.
O2 ratios of 2 and 1, which can be attributed to the abundance of remaining O2.
Under O2 pulsing, the Ce 4f state disappears, indicating a complete conversion of Ce3+ to Ce4+. The normalized data in Fig. 2(f) confirm this through the in-phase behavior of blue and red oscillations as shown in the left panel. Both oscillations reach their maximum during CO pulsing while becoming undetectable at 1.7 eV during O2 injections. Additionally, the Ce 4f density of states is absent between ≈2 and ≈6 eV, confirming the complete conversion of Ce3+ species to Ce4+ under O2-rich conditions.
The MS signals (Fig. 2, bottom panels d, e, f) show the CO2 formation across all reactant ratios. The time intervals between CO2 peaks vary with the CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio: with intervals of 40, 22, and 11 seconds observed for ratios of 2, 1, and 0.25, respectively. As the CO
O2 ratio: with intervals of 40, 22, and 11 seconds observed for ratios of 2, 1, and 0.25, respectively. As the CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio decreases to 0.25, the two CO2 peaks gradually merge into a near-single peak, indicating a decline in the CO2 production. Under O2-rich conditions, the extended O2 residence time promotes the formation of active oxygen and Ce4+ species at the surface of ceria. Consequently, the sustained CO2 production in these conditions, despite low Ce3+ concentration, suggests synergistic activity between activated oxygen (Ce4+–O 2p) and Ce3+ species.
O2 ratio decreases to 0.25, the two CO2 peaks gradually merge into a near-single peak, indicating a decline in the CO2 production. Under O2-rich conditions, the extended O2 residence time promotes the formation of active oxygen and Ce4+ species at the surface of ceria. Consequently, the sustained CO2 production in these conditions, despite low Ce3+ concentration, suggests synergistic activity between activated oxygen (Ce4+–O 2p) and Ce3+ species.
A key objective of this study is to characterize surface oxygen species and their binding interactions with Ce3+ and Ce4+ sites. Using NAP-XPS and NAP-RPES under pure O2 steady-state conditions, we investigate oxygen speciation at the ceria surface and its interaction with Ce3+ cations.
(c) Identification of surface-active oxygen species on CeO2
Here, we combine NAP-XPS and NAP-RPES under steady-state O2 gas flow to identify oxygen species formed at the ceria surface and to investigate their interactions with Ce3+ species. Fig. 3(a) shows the O 1s core level spectra obtained at 590 eV of photon energy, with ceria exposed to 0.5 mbar of O2 at constant temperatures of 330 °C, 360 °C, 390 °C, 400 °C and 420 °C, respectively. The O 1s spectra show contributions attributed to ceria lattice oxygen (529.5 eV),43 hydroxyls (531.0 eV)44,45 peroxide-like oxygen groups,46,47 (532.7 eV), and O2 gas phase (gray band).48 The fitting at 330 °C revealed 26% of lattice oxygen, 42% of hydroxyl groups, and 32% of peroxide species. However, as shown in Fig. 3(b) a substantial evolution of all species is observed between 330 °C and 420 °C, with a marked decrease of OH and peroxo groups between 360 °C and 390 °C, while the concentration of lattice oxygen species gradually increases. From 400 °C, the peroxo contribution disappears while hydroxyl species reach a concentration of 37% constant between 400 °C and 420 °C. Thus, above 400 °C only ceria lattice oxygen and OH species remain on the CeO2 surface (see Table S1 for more details, ESI†).
The NAP-XPS results were then contrasted with the NAP-RPES valence band analysis. Fig. 3(c) shows the NAP-RPES spectra measured at 121.2 eV (Ce3+) using the same experimental temperature and gas pressure conditions as those of the NAP-XPS analysis. Here, NAP-RPES was unable to detect Ce3+ species (≈1 eV) despite its extreme sensitivity (down to 0.1%).34 The absence of Ce3+ can be attributed to their short lifetime, which renders them undetectable within the steady-state regime.10 However, the valence band clearly shows the formation of a shoulder at ≈6.5 eV (dashed line), which has been attributed to OH groups by XPS, RPES, and DFT calculations.44,45,49 Thus, this state (dashed line) confirms the attribution of OH contribution detected here by NAP-XPS. Therefore, our steady-state NAP-RPES and NAP-XPS measurements are mutually consistent, revealing that the redox cycle involves the OH species at all temperatures, while peroxo participates only between 330 °C and 390 °C. In the following section, we used DFT calculations to support the findings observed in the valence band.
(d) DFT modeling of CeO2 surface
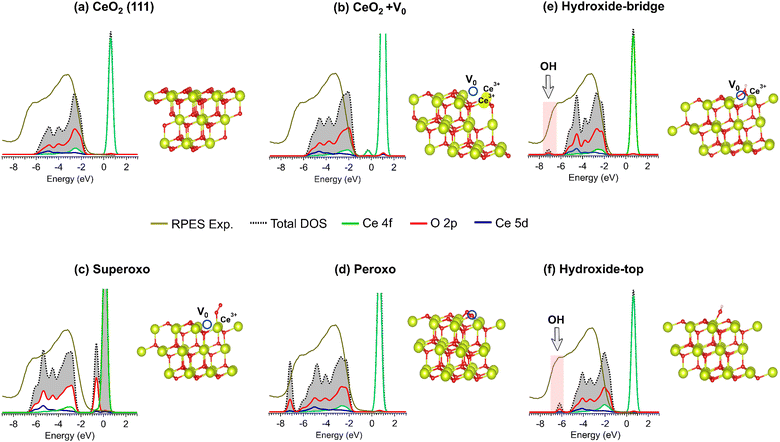
DFT calculations were performed to evaluate the impact of superoxo, peroxo, and OH species on the density of states (DOS) at the ceria surface. Our work focused on the CeO2(111) surface due to its high catalytic activity and low surface energy compared to other surfaces. We further verified its stability by calculating surface energies, which provide insights into the stabilities of surfaces. A lower surface energy corresponds to a more stable surface. The calculated surface energies for the (111) and (100) surfaces are 0.421 and 0.795 eV Å−2, respectively, indicating that the (111) face is indeed the more stable surface. Fig. S16 (ESI†) shows an oxygen vacancy (Vo) on the (100) surface with the corresponding PDOS.
Fig. 4 shows the structural models of (a) pristine CeO2(111), (b) a defective CeO2 surface with oxygen vacancies (V0), and the corresponding DOS after adsorption of (c) superoxo, (d) peroxo, and hydroxide species in (e) bridge and (f) top configurations, where the DOS is compared with RPES experimental results. A Gaussian broadening of 0.2 eV was applied to all projected density of states (PDOS) to match the bandwidth observed in the experimental RPES data.
On CeO2(111) and CeO2(111) + Vo, we find that the total DOS (dashed line) in the occupied region is mainly formed by hybridized covalent O 2p and Ce 4f.35 For CeO2(111) in the stoichiometric form (a), Ce 4f state is absent in the intragap region, while for CeO2(111) + Vo (b) the emergence of localized Ce 4f occurs as expected.50 Ce 4f state is obtained at a Vo concentration corresponding to 1/12 atoms established in the CeO2(111) 2 × 2 unit cell with 12 atoms of cerium and 24 of oxygen. Likewise, the calculated bandgaps were 2.25 eV and 0.89 eV for CeO2(111) and CeO2(111) + Vo, respectively, being both indirect gaps.30
In Fig. 4(c)–(f), we considered the three most common adsorbed oxygen species on ceria corresponding to: (c) superoxide (O2−), (d) peroxide (O22−), and (e) hydroxyl species (OH). The method, geometrical parameters, Bader charges, magnetic moment, and gap energies are available in Table S2 (ESI†). Here, Fig. 4(c) reveals the surface chemistry of superoxo (O2−) species adsorbed on CeO2(111) + Vo. The formation of an oxygen vacancy results in two electrons localizing on adjacent Ce atoms. Upon oxygen adsorption at this site, one electron transfers to form a superoxo species. This electron transfer mechanism leads to a final electronic structure containing a single Ce3+ center, characterized by a Ce 4f state and an electronic gap of 1.45 eV. Previous studies have reported that the stability of superoxo species from 200 °C is compromised.51,52 In addition, it has been suggested that superoxo species can easily transform into peroxide species at high temperatures. Moreover, the RPES data do not support the presence of superoxo, as the experimental spectrum shows a state at approximately 6.5 eV that is not predicted by the DFT calculations, while the Ce 4f state predicted by DFT is not observed in the RPES data. These findings, together with the instability of superoxo species at temperatures above 330 °C, exclude the presence of superoxo on ceria under such conditions.
Fig. 4(d): DFT on O22−/CeO2(111) + Vo. Here, peroxide species replace Vo and induce a new extra state at ≈−7.0 eV attributed to O 2p orbitals, while the Ce 4f state is absent and a wide gap of 1.87 eV is formed. The depopulation of the Ce 4f occupied state is due to the substitution of the Vo site by the peroxo groups. The stability of this group and the O 2p orbitals formation is consistent with RPES spectrum, and the peroxo evolution observed by NAP-XPS, which allows us to speculate that peroxo species can be active species between 330 °C and 360 °C.
Fig. 4(e): DFT on OH/CeO2(111). The calculations were performed following two OH adsorption models. (i) Bridge: OH molecule adsorbs at the oxygen vacancy site (Vo) on the CeO2(111) (e). (ii) Top: in this configuration, the OH molecule is adsorbed on top of a Ce4+ cation without oxygen vacancy (f). Both structures exhibit different energy gaps (Eg), with 2.34 eV for the bridge and 1.72 eV for the on-top configuration, respectively. Likewise, the O 2p state localized at ≈6.6 eV fits well with the state observed in RPES (see arrow in Fig. 4e and f). However, the stability of both OH configurations is strongly temperature dependent.53 As evidenced in Fig. 3, the evolution of OH contribution decreases between 330 °C and 360 °C, while it remains constant between 400 and 420 °C. Thus, although NAP-XPS cannot distinguish between the top and bridging electron configurations, this reveals that OH groups are formed and dehydroxylate at different temperature ranges, which may provide valuable information about their stability.
Finally, DFT and NAP-XPS/RPES experiments show that between 330 °C and 390 °C, both peroxo and OH species coexist, and above 390 °C, only the OH species remain on the surface. Therefore, it is clear that the activation of O2 to form a particular oxygen species is a temperature-dependent process with high affinity here to form OH species at the surface of CeO2.
Conclusion
Through combined modulated chemical excitation investigation and DFT calculations, we characterized the dynamics of oxygen species on the ceria surface. Our findings revealed distinct temperature-dependent behavior: Ce3+ species persist at 420 °C under CO-rich and CO/O2 = 1 conditions, while O2-rich environments promote complete Ce3+ to Ce4+ conversion. We identified temperature-specific surface species, with OH and peroxo groups coexisting between 330–390 °C, and exclusively OH species above 390 °C.Our modulated chemical excitation RPES approach provided valuable insights into how changes in gas composition affect the electronic structure of the CeO2 surface. This methodology opens up new avenues for studying catalyst behavior under dynamic conditions. Future research should focus on more complex systems, particularly Pt/CeO2, which demonstrates promising CO oxidation activity at low temperatures.
Abbreviations
| RPES | Resonant photoelectron spectroscopy |
| NAP | Near-ambient pressure |
| XPS | X-Ray photoelectron spectroscopy |
| MvK | Mars–van Krevelen |
| MS | Mass spectrometry |
| APCell | Ambient pressure cell |
| MES | Modulation excitation spectroscopy |
Author contributions
L. C.: conceptualization, data curation, formal analysis, visualization, supervision, project administration, methodology, investigation, funding acquisition, writing & editing the original draft. H. K., H. D. & M. I.: synthesis of catalysts & editing the original draft. J. K. & C. E.: data curation, editing the original draft. C. H.: data curation & editing the original draft. M. D. & M. B.: DFT calculations & editing the original draft. S. L.: data curation & editing the original draft.Data availability
The supporting data in this work are presented in the paper and/or the ESI.† Source data are available from the corresponding author upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge MAX IV Laboratory for time on Beamline SPECIES under Proposal 20220419. Research conducted at MAX IV, a Swedish national user facility, is supported by the Swedish Research Council under contract 2018-07152, the Swedish Governmental Agency for Innovation Systems under contract 2018-04969, and Formas under contract 2019-02496. We thank the following funding sources for this project: The LABEX iMUST of the University of Lyon (PINCELL: ANR-10-LABX-0064) and ANR-NACELL (Project-ANR-22-CE42-0011), created within the “Plan France 2030” set up by the French government and managed by the French National Research Agency. C. H. acknowledges financial support by a PhD fellowship from the French Ministry of Education. J. K. acknowledges financial support from the Swedish Research Council, grant number 2022-04363, and from the Crafoord Foundation. This work was granted access to the HPC resources of TGCC under the allocation 2023-A0140810433 made by GENCI. H. D. and H. K. also gratefully acknowledge the French National Agency for Research (ANR, Menihr Project, ref. ANR-21-CE08-0028).References
- J. Kašpar, P. Fornasiero and M. Graziani, Use of CeO2-based oxides in the three-way catalysis, Catal. Today, 1999, 50, 285–298 CrossRef
.
- Q. Fu, H. Saltsburg and M. Flytzani-Stephanopoulos, Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts, Science, 2003, 301, 935–938 CrossRef CAS PubMed
.
- F. C. Meunier, L. Cardenas, H. Kaper, B. Šmíd, M. Vorokhta, R. Grosjean, D. Aubert, K. Dembélé and T. Lunkenbein, Synergy between Metallic and Oxidized Pt Sites Unravelled during Room Temperature CO Oxidation on Pt/Ceria, Angew. Chem., Int. Ed., 2021, 60, 3799–3805 CrossRef CAS PubMed
.
- O. V. Safonova, A. Guda, Y. Rusalev, R. Kopelent, G. Smolentsev, W. Y. Teoh, J. A. van Bokhoven and M. Nachtegaal, Elucidating the Oxygen Activation Mechanism on Ceria-Supported Copper-Oxo Species Using Time-Resolved X-ray Absorption Spectroscopy, ACS Catal., 2020, 10, 4692–4701 CrossRef CAS
.
- C. Yang, X. Yu, S. Heißler, P. G. Weidler, A. Nefedov, Y. Wang, C. Wöll, T. Kropp, J. Paier and J. Sauer, O2 Activation on Ceria Catalysts—The Importance of Substrate Crystallographic Orientation, Angew. Chem., Int. Ed., 2017, 56, 16399–16404 CrossRef CAS
.
- G. Panov, K. Dubkov and E. Starokon, Active oxygen in selective oxidation catalysis, Catal. Today, 2006, 117, 148–155 CrossRef CAS
.
- G. Preda, A. Migani, K. M. Neyman, S. T. Bromley, F. Illas and G. Pacchioni, Formation of Superoxide Anions on Ceria Nanoparticles by Interaction of Molecular Oxygen with Ce3+ Sites, J. Phys. Chem. C, 2011, 115, 5817–5822 CrossRef CAS
.
- C. Li, K. Domen, K. Maruya and T. Onishi, Dioxygen adsorption on well-outgassed and partially reduced cerium oxide studied by FT-IR, J. Am. Chem. Soc., 1989, 111, 7683–7687 CrossRef CAS
.
- C. Li, K. Domen, K.-I. Maruya and T. Onishi, Oxygen exchange reactions over cerium oxide: An FT-IR study, J. Catal., 1990, 123, 436–442 CrossRef CAS
.
- R. Kopelent, J. A. van Bokhoven, J. Szlachetko, J. Edebeli, C. Paun, M. Nachtegaal and O. V. Safonova, Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts, Angew. Chem., Int. Ed., 2015, 54, 8728–8731 CrossRef CAS PubMed
.
- A. Shavorskiy, E. Kokkonen, E. Redekop, G. D’Acunto, J. Schnadt and J. Knudsen, Time-Resolved APXPS with Chemical Potential Perturbations: Recent Developments at the MAX IV Laboratory, Synchrotron Radiat. News, 2022, 35, 4–10 CrossRef
.
- J. Knudsen, T. Gallo, V. Boix, M. D. Strømsheim, G. D’Acunto, C. Goodwin, H. Wallander, S. Zhu, M. Soldemo, P. Lömker, F. Cavalca, M. Scardamaglia, D. Degerman, A. Nilsson, P. Amann, A. Shavorskiy and J. Schnadt, Stroboscopic operando spectroscopy of the dynamics in heterogeneous catalysis by event-averaging, Nat. Commun., 2021, 12, 6117 CrossRef CAS PubMed
.
- M. Roger, L. Artiglia, A. Boucly, F. Buttignol, M. Agote-Arán, J. A. van Bokhoven, O. Kröcher and D. Ferri, Improving time-resolution and sensitivity of in situ X-ray photoelectron spectroscopy of a powder catalyst by modulated excitation, Chem. Sci., 2023, 14, 7482–7491, 10.1039/D3SC01274C
.
- J. Weyel, L. Schumacher, M. Ziemba, M. Pfeiffer and C. Hess, Modulation Excitation Spectroscopy: A Powerful Tool to Elucidate Active Species and Sites in Catalytic Reactions, Acc. Chem. Res., 2024, 57, 2643–2652 CrossRef CAS PubMed
.
-
A. Urakawa, D. Ferri and R. J. G. Nuguid, in Springer Handbook of Advanced Catalyst Characterization, ed. I. E. Wachs and M. A. Bañares, Springer International Publishing, Cham, 2023, pp. 967–977 Search PubMed
.
- S. Gatla, D. Aubert, G. Agostini, O. Mathon, S. Pascarelli, T. Lunkenbein, M. G. Willinger and H. Kaper, Room-Temperature CO Oxidation Catalyst: Low-Temperature Metal–Support Interaction between Platinum Nanoparticles and Nanosized Ceria, ACS Catal., 2016, 6, 6151–6155 CrossRef CAS
.
- S. Urpelainen, C. Såthe, W. Grizolli, M. Agåker, A. R. Head, M. Andersson, S.-W. Huang, B. N. Jensen, E. Wallén, H. Tarawneh, R. Sankari, R. Nyholm, M. Lindberg, P. Sjöblom, N. Johansson, B. N. Reinecke, M. A. Arman, L. R. Merte, J. Knudsen, J. Schnadt, J. N. Andersen and F. Hennies, The SPECIES beamline at the MAX IV Laboratory: a facility for soft X-ray RIXS and APXPS, J. Synchrotron Radiat., 2017, 24, 344–353 CrossRef CAS PubMed
.
- E. Kokkonen, F. Lopes da Silva, M.-H. Mikkelã, N. Johansson, S.-W. Huang, J.-M. Lee, M. Andersson, A. Bartalesi, B. N. Reinecke, K. Handrup, H. Tarawneh, R. Sankari, J. Knudsen, J. Schnadt, C. Såthe and S. Urpelainen, Upgrade of the SPECIES beamline at the MAX IV Laboratory, J. Synchrotron Radiat., 2021, 28, 588–601 CrossRef CAS PubMed
.
- C. Maheu, L. Cardenas, E. Puzenat, P. Afanasiev and C. Geantet, UPS and UV spectroscopies combined to position the energy levels of TiO2 anatase and rutile nanopowders, Phys. Chem. Chem. Phys., 2018, 20, 25629–25637 RSC
.
- S. Neppl and O. Gessner, Time-resolved X-ray photoelectron spectroscopy techniques for the study of interfacial charge dynamics, J. Electron Spectrosc. Relat. Phenom., 2015, 200, 64–77 CrossRef CAS
.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS
.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B:Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed
.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B:Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- H. J. Monkhorst and J. D. Pack, Special points for Brillouin-zone integrations, Phys. Rev. B, 1976, 13, 5188–5192 CrossRef
.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA + U study, Phys. Rev. B:Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS
.
- C. W. M. Castleton, J. Kullgren and K. Hermansson, Tuning LDA + U for electron localization and structure at oxygen vacancies in ceria, J. Chem. Phys., 2007, 127, 244704 CrossRef CAS PubMed
.
- J. Fan, C. Li, J. Zhao, Y. Shan and H. Xu, The Enhancement of Surface Reactivity on CeO2(111) Mediated by Subsurface Oxygen Vacancies, J. Phys. Chem. C, 2016, 120, 27917–27924 CrossRef CAS
.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed
.
- A. Pedrielli, P. De Vera, P. E. Trevisanutto, N. M. Pugno, R. Garcia-Molina, I. Abril, S. Taioli and M. Dapor, Electronic excitation spectra of cerium oxides: from ab initio dielectric response functions to Monte Carlo electron transport simulations, Phys. Chem. Chem. Phys., 2021, 23, 19173–19187 RSC
.
- A. Pfau and K. D. Schierbaum, The electronic structure of stoichiometric and reduced CeO2 surfaces: an XPS, UPS and HREELS study, Surf. Sci., 1994, 321, 71–80 CrossRef CAS
.
- T. Skála, F. Šutara, K. C. Prince and V. Matolín, Cerium oxide stoichiometry alteration via Sn deposition: Influence of temperature, J. Electron Spectrosc. Relat. Phenom., 2009, 169, 20–25 CrossRef
.
- L. Cardenas, C. Molinet-Chinaglia and S. Loridant, Unraveling Ce3+ detection at the surface of ceria nanopowders by UPS analysis, Phys. Chem. Chem. Phys., 2022, 24, 22815–22822 RSC
.
- Y. Lykhach, S. M. Kozlov, T. Skála, A. Tovt, V. Stetsovych, N. Tsud, F. Dvořák, V. Johánek, A. Neitzel, J. Mysliveček, S. Fabris, V. Matolín, K. M. Neyman and J. Libuda, Counting electrons on supported nanoparticles, Nat. Mater., 2016, 15, 284–288 CrossRef CAS PubMed
.
- T. Duchoň, M. Aulická, E. F. Schwier, H. Iwasawa, C. Zhao, Y. Xu, K. Veltruská, K. Shimada and V. Matolín, Covalent versus localized nature of 4f electrons in ceria: Resonant angle-resolved photoemission spectroscopy and density functional theory, Phys. Rev. B, 2017, 95, 165124 CrossRef
.
- J. L. F. Da Silva, Stability of the Ce2O3 phases: A DFT+U investigation, Phys. Rev. B:Condens. Matter Mater. Phys., 2007, 76, 193108 CrossRef
.
- L. Zhang, R. Chen, Y. Tu, X. Gong, X. Cao, Q. Xu, Y. Li, B. Ye, Y. Ye and J. Zhu, Revealing the Crystal Facet Effect of Ceria in Pd/CeO2 Catalysts toward the Selective Oxidation of Benzyl Alcohol, ACS Catal., 2023, 13, 2202–2213 CrossRef CAS
.
- M. Ziemba, C. Schilling, M. V. Ganduglia-Pirovano and C. Hess, Toward an Atomic-Level Understanding of Ceria-Based Catalysts: When Experiment and Theory Go Hand in Hand, Acc. Chem. Res., 2021, 54, 2884–2893 CrossRef CAS PubMed
.
- X. Zhou, A. Mavridis, M. A. Isaacs, C. Drivas, C. D’Agostino and C. M. A. Parlett, Impact of Ceria Support Morphology on Au Single-Atom Catalysts for Benzyl Alcohol Selective Oxidation, ChemCatChem, 2024, 16, e202301673 CrossRef CAS
.
- J. P. Y. Tan, H. R. Tan, C. Boothroyd, Y. L. Foo, C. B. He and M. Lin, Three-Dimensional Structure of CeO2 Nanocrystals, J. Phys. Chem. C, 2011, 115, 3544–3551 CrossRef CAS
.
- O. Matz and M. Calatayud, Breaking H2 with CeO2: Effect of Surface Termination, ACS Omega, 2018, 3, 16063–16073 CrossRef CAS PubMed
.
- B. Hu, K. Sun, Z. Zhuang, Z. Chen, S. Liu, W.-C. Cheong, C. Chen, M. Hu, X. Cao, J. Ma, R. Tu, X. Zheng, H. Xiao, X. Chen, Y. Cui, Q. Peng, C. Chen and Y. Li, Distinct Crystal-Facet-Dependent Behaviors for Single-Atom Palladium-On-Ceria Catalysts: Enhanced Stabilization and Catalytic Properties, Adv. Mater., 2022, 34, 2107721 CrossRef CAS PubMed
.
- D. J. Morgan, Photoelectron spectroscopy of ceria: Reduction, quantification and the myth of the vacancy peak in XPS analysis, Surf. Interface Anal., 2023, 55, 845–850 CrossRef CAS
.
- Y. Lykhach, V. Johánek, H. A. Aleksandrov, S. M. Kozlov, M. Happel, T. Skála, P. St. Petkov, N. Tsud, G. N. Vayssilov, K. C. Prince, K. M. Neyman, V. Matolín and J. Libuda, Water Chemistry on Model Ceria and Pt/Ceria Catalysts, J. Phys. Chem. C, 2012, 116, 12103–12113 CrossRef CAS
.
- V. Matolín, I. Matolínová, F. Dvořák, V. Johánek, J. Mysliveček, K. C. Prince, T. Skála, O. Stetsovych, N. Tsud, M. Václavů and B. Šmíd, Water interaction with CeO2(111)/Cu(111) model catalyst surface, Catal. Today, 2012, 181, 124–132 CrossRef
.
- A. I. Stadnichenko, V. V. Muravev, S. V. Koscheev, V. I. Zaikovskii, H. A. Aleksandrov, K. M. Neyman and A. I. Boronin, Study of active surface centers of Pt/CeO2 catalysts prepared using radio-frequency plasma sputtering technique, Surf. Sci., 2019, 679, 273–283 CrossRef CAS
.
- Y. Zhu, J. Wang, S. B. Patel, C. Li, A. R. Head, J. A. Boscoboinik and G. Zhou, Tuning the surface reactivity of oxides by peroxide species, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2215189120 CrossRef CAS PubMed
.
- T. G. Avval, S. Chatterjee, G. T. Hodges, S. Bahr, P. Dietrich, M. Meyer, A. Thißen and M. R. Linford, Oxygen gas, O2(g), by near-ambient pressure XPS, Surf. Sci. Spectra, 2019, 26, 014021 CrossRef
.
- P. A. Thiel and T. E. Madey, The interaction of water with solid surfaces: Fundamental aspects, Surf. Sci. Rep., 1987, 7, 211–385 CrossRef CAS
.
- R. Gillen, S. J. Clark and J. Robertson, Nature of the electronic band gap in lanthanide oxides, Phys. Rev. B:Condens. Matter Mater. Phys., 2013, 87, 125116 CrossRef
.
- C. Li, K. Domen, K. Maruya and T. Onishi, Dioxygen adsorption on well-outgassed and partially reduced cerium oxide studied by FT-IR, J. Am. Chem. Soc., 1989, 111, 7683–7687 CrossRef CAS
.
- Y. Zhao, B.-T. Teng, X.-D. Wen, Y. Zhao, Q.-P. Chen, L.-H. Zhao and M.-F. Luo, Superoxide and Peroxide Species on CeO2(111), and Their Oxidation Roles, J. Phys. Chem. C, 2012, 116, 15986–15991 CrossRef CAS
.
- K. K. Chakarova, V. R. Zdravkova, B. S. Karapenchev, D. D. Nihtianova, E. Z. Ivanova, H. A. Aleksandrov, I. Z. Koleva, D. A. Panayotov, M. Y. Mihaylov, G. N. Vayssilov and K. I. Hadjiivanov, Evolution of Ce4+ Lewis acidity during dehydroxylation of ceria nanoparticles with different morphology: An integrated FTIR, DFT and HRTEM study, J. Catal., 2024, 433, 115463 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp01283j |
| This journal is © the Owner Societies 2025 |