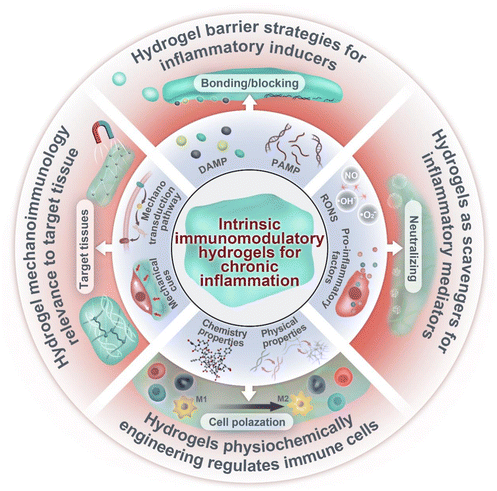
Intrinsic immunomodulatory hydrogels for chronic inflammation
Yuna
Qian†
ab,
Jiayi
Ding†
ac,
Rui
Zhao†
a,
Yang
Song
d,
Jiyoung
Yoo
e,
Huiyeon
Moon
e,
Seyoung
Koo
*f,
Jong Seung
Kim
 *f and
Jianliang
Shen
*f and
Jianliang
Shen
 *ab
*ab
aNational Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China. E-mail: sjl1@wmu.edu.cn
bZhejiang Engineering Research Center for Tissue Repair Materials, Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou, 325001, China. E-mail: shenjl@wiucas.ac.cn
cZhejiang Key Laboratory of Imaging and Interventional Medicine, Institute of Imaging Diagnosis and Minimally Invasive Intervention, The Fifth Affiliated Hospital of Wenzhou Medical University, Lishui, 323000, China
dWest China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, 610065, China
eDepartment of Chemistry, Korea University, Seoul 02841, Korea
fDepartment of Chemical and Molecular Engineering, Hanyang University ERICA, Ansan, Gyeonggi-do 15588, Korea. E-mail: jongskim@korea.ac.kr; sykoo@hanyang.ac.kr
First published on 5th November 2024
Abstract
The immune system plays a pivotal role in maintaining physiological homeostasis and influencing disease processes. Dysregulated immune responses drive chronic inflammation, which in turn results in a range of diseases that are among the leading causes of death globally. Traditional immune interventions, which aim to regulate either insufficient or excessive inflammation, frequently entail lifelong comorbidities and the risk of severe side effects. In this context, intrinsic immunomodulatory hydrogels, designed to precisely control the local immune microenvironment, have recently attracted increasing attention. In particular, these advanced hydrogels not only function as delivery mechanisms but also actively engage in immune modulation, optimizing interactions with the immune system for enhanced tissue repair, thereby providing a sophisticated strategy for managing chronic inflammation. In this tutorial review, we outline key elements of chronic inflammation and subsequently explore the strategic design principles of intrinsic immunomodulatory hydrogels based on these elements. Finally, we examine the challenges and prospects of such immunomodulatory hydrogels, which are expected to inspire further preclinical research and clinical translation in addressing chronic inflammation.
Key learning pointsThe immune system can be targeted to improve tissue repair by lowering inflammatory or tissue-damaging responses. Intrinsic immunomodulatory hydrogels provide well-orchestrated biomaterial–immune system interactions with simple fabrication and minimal toxic side effects. Strategies for the design of immunomodulatory hydrogels with intrinsic features such as chemical composition as well as physicochemical and mechano-properties. Challenges, perspectives, and potential future directions of intrinsic immunomodulatory hydrogels. |
1. Introduction
The immune system and inflammatory processes are involved in a wide variety of health problems that significantly contribute to global morbidity and mortality.1 It is well-established that chronic inflammation is a predominant factor responsible for over 50% of deaths worldwide due to inflammation-related diseases.2 These diseases include ischemic heart disease, cerebrovascular accidents (strokes), cancer,3 diabetes mellitus,4 chronic renal insufficiency, non-alcoholic fatty liver disease (NAFLD) as well as autoimmune5 and neurodegenerative conditions. Therefore, the targeted regulation of the immune system is considered a promising therapeutic strategy for a broad spectrum of diseases.Immunomodulation therapy is widely used to manage inflammation. However, systemic immunomodulatory treatments pose the potential risk of life-threatening side effects, including increased susceptibility to infections, organ toxicity, and gastrointestinal issues.6,7 This has spurred a paradigm shift towards innovative biomaterial-based strategies, with hydrogels emerging as a state-of-the-art modality for targeted and localized immunomodulation.8,9 Hydrogels, which are three-dimensional polymeric networks characterized by their high water content, closely mimic the structural and functional attributes of native tissues and organs.10 This engineered biomaterial can be applied directly to the affected area, providing a localized therapeutic effect and reducing the risk of systemic side effects. It has attracted significant interest in the field of bioengineering and regenerative medicine as its physical and chemical properties can be adjusted to match the requirements of different therapeutic agents and target tissues.11,12 The ease of application and reduced frequency of dosing are expected to improve patient compliance and overall treatment outcomes. More importantly, the inherent characteristics of hydrogels confer upon them intrinsic immunomodulatory potential through their chemical composition, physicochemical properties, and mechanobiological interactions.13 By carefully designing and tailoring these intrinsic properties, hydrogels can be engineered to modulate immune responses in a controlled manner, which is essential for the success of regenerative medicine strategies and the development of effective therapeutic interventions.
In this review, we discuss the recent advances in designing intrinsic immunomodulatory hydrogels for chronic inflammation (Fig. 1). We will start with an essential introduction of key elements and stages of chronic inflammation and then discuss the design of immunomodulatory hydrogels according to these elements. Notably, this review mainly focuses on immunomodulatory hydrogels based on their intrinsic properties, such as barrier strategies in hydrogels for inflammatory inducers, hydrogels as scavengers of mediators, the impact of physiochemically engineered hydrogels on immune cells, and the relevance of hydrogel mechanoimmunology for the target tissue. The use of hydrogels as drug delivery platforms, incorporating various bioactive molecules, such as small molecular agents, nucleic acid, growth factors and cells,14–16 has been extensively reviewed in prior literature and thus will not be discussed in detail in this review. Last but not least, we discuss the future directions and challenges for the clinical translation of immunomodulation hydrogels. We hope that this review can provide a comprehensive framework for future research endeavors and the potential therapeutic applications of these biomaterials in the management of chronic inflammatory diseases.
2. A brief review of chronic inflammation
The immune system plays a critical role in the continuous surveillance of the body to detect tissue damage, pathogen invasion and foreign substances.17,18 Physiological inflammation is characterized by the temporally restricted upregulation of inflammatory processes, which are triggered by the detection of a threat and resolved once the threat is eliminated.19 During the early stages of inflammation, inflammatory inducers, both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), are recognized by stromal and tissue-resident immune cells, which activates the cascade of inflammatory mediator production.20 These inflammatory mediators enhance vascular permeability, thus facilitating the influx of circulating immune cells for the purpose of eliminating detrimental agents.21 Subsequently, the tissue repair mechanism is activated to supplant the inflammatory reaction, thereby restoring the homeostasis of the target tissue.22However, influenced by systemic or localized factors, the highly-programmed inflammatory response has the potential to transition from short- to long state, leading the breakdown of immune tolerance.23 These factors include autoimmune diseases, bacterial and viral infections, obesity, intestinal dysbiosis, diet, xenobiotic exposure, and psychological stress,1 all of which have been reported to disrupt normal immune function and increase susceptibility to chronic disease,24 encompassing metabolic disorders, neurodegenerative diseases, autoimmune diseases and cancer. Chronic inflammation is characterized by the persistent activation of immune components that differ from those involved in acute inflammation. This section offers an overview of key elements of chronic inflammation: (I) Inflammatory inducers: DAMP and PAMP, (II) sensors: immune cells, (III) mediators: vasoactive amines/peptides, chemokines, cytokines, proteases oxygen and nitrogen free radicals (RONs), (IV) target tissues: mechanical signals (Fig. 2).
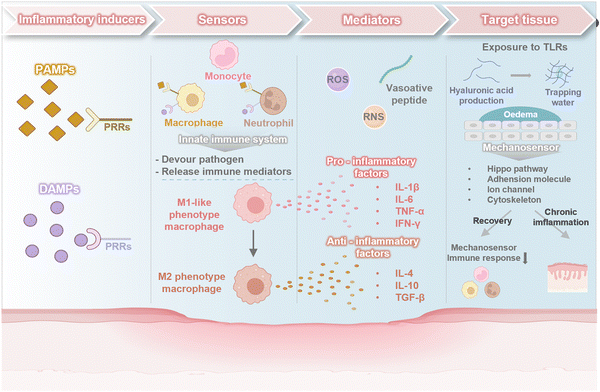 | ||
| Fig. 2 An overview of key elements of chronic inflammation: (I) inflammatory inducers, (II) sensors, (III) mediators, and (IV) target tissues. Image was drawn using Biorender. | ||
2.1 Inflammatory inducers
This includes both DAMPs and PAMPs, which serve as the initiators of the inflammatory cascade. PAMPs25 are molecular features that are evolutionarily conserved and shared among various species of bacteria, parasites, and viruses and signal the presence of a foreign invader. Similarly, DAMPs are molecules released extracellularly by dying, damaged, or stressed cells in response to physical, chemical or metabolic noxious stimuli.26 In particular, the inflammatory responses are primarily initiated during infections through PAMPs, while DAMPs can also activate the inflammatory response as endogenous counterparts to PAMPs. In contrast, chronic inflammation is typically initiated by DAMPs without an acute infectious insult or the activation of PAMPs, which is known as dysregulated “sterile inflammation”.27The stromal and tissue-resident immune cells recognize DAMPs and PAMPs by their pattern recognition receptors (PRRs). The classical PRRs28 include not only membrane bound Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) but also two cytoplasmic NOD-like receptors (NLRs), retinoic acid inducible gene I (RIG-I)-like receptors (RLRs) and several intracellular DNA sensors. Recently, numerous new non-PRR DAMP receptors have expanded our understanding, such as the receptor for advanced glycation end products (RAGE), several G-protein-coupled receptors (GPCRs)29 and ion channels. Once activated, these receptors initiate a cascade of innate immune signaling pathways and even activate adaptive immune cells directly to induce inflammation.
2.2 Sensors
Immune cell is the central sensor of inflammatory response. Initially, inflammation is orchestrated by innate immune cells, such as monocytes, macrophages and neutrophils.30,31 As soon as a tissue injury occurs, circulating neutrophils and tissue-resident macrophages are activated by DAMPs and PAMPs in minutes, devour pathogens and release inflammatory mediators.32 Subsequently, circulating monocytes are recruited and infiltrated in the followed 24–72 hours post-injury. These monocytes can mature into macrophages with high phagocytic activity, enabling them to engulf dying neutrophils and pathogens, remove cellular debris, and release soluble mediators.33 Macrophages display considerable functional plasticity in response to their inflammatory environment.34 During the early stage of inflammation, macrophages move toward an M1-like phenotype (pro-inflammatory) and secrete pro-inflammatory cytokines. As the threat is cleared, macrophages are transformed into M2 phenotype (anti-inflammatory), which can resolve inflammation and promote angiogenesis and tissue repair.35,36 As the first line of defense against infection, neutrophils and macrophages are crucial innate responders. Historically, it was believed that these innate immune cells cannot induce immunological memory. However, the emerging concept as “trained immunity” or “innate immune memory”37,38 show that innate immune cells can also induce immunological memory through epigenetic and metabolic reprogramming, enabling them to react differently to subsequent challenges based on prior stimulations.The innate immune system presents antigens to T and B cells to induce adaptive responses. T cells commonly referred to as T-helper (Th)1, Th2, Th17, and regulatory T (Treg) cells, based on cytokines present in the inflammatory microenvironment.39 During early inflammation, CD4+ TH1 cells infiltrate the injury site and secrete pro-inflammatory cytokines. In the subsequent stages of inflammation, Treg cells and TH2 cells increase and produce anti-inflammatory factors like TGF-β and IL-10 to resolve inflammation.40 In addition, inflammatory antigens can be transported via the lymphatic system to the adjacent (draining) lymph nodes, where they can engage and activate B cells to produce antibodies.41
In acute inflammation, granulocytes are the main infiltrating inflammatory cells, while in chronic inflammation, macrophages and lymphocytes are the main infiltrating immune cells.3 Thus, the switch from pro-inflammatory phenotype to anti-inflammatory phenotype of macrophages is crucial for resolving inflammation, and this transitional process is disrupted in chronic inflammation. Numerous molecules have been recognized as signaling factors in the phenotypic shift, such as cytokines generated by TH2 cells and efferocytosis by neutrophils. The metabolic status of the inflammatory microenvironment also plays a critical role.42 Redox profiling, which impacts various signaling pathways, results in distinct molecular profiles that distinguish acute from chronic inflammation. For example, M1 macrophages rely on aerobic glycolysis (also known as the Warburg effect) for ATP production, leading to increased lactate production from pyruvate. Lactic acids43 are known to promote the progression of inflammation in the chronic inflammatory microenvironment by activating the Akt/mTOR/HIF-1α and signaling axis and regulating histone lactylation in chronic inflammation. In contrast, M2 macrophages increase oxidative metabolism and lipoxygenase to produce metabolite itaconate and pro-resolving mediators (SPMs), which suppress the induction of multiple inflammatory genes. Chronic inflammation is distinguished by the simultaneous occurrence of tissue destruction and repair, indicating the possibility that different cells within the chronically inflamed area may exist in different metabolic states.44 Such insights and observations pose significant challenges for a comprehensive understanding of chronic inflammation, while simultaneously offering promising opportunities for essential research in this field.
2.3 Mediators
Inflammatory responses are driven by a complex network of mediators to45 perpetuate and amplify the inflammatory cascade. These mediators include vasoactive amines, peptides, chemokines, cytokines, proteases, and reactive oxygen and nitrogen species (RONS). Vasoactive amines and peptides directly or indirectly cause vasodilation and increase the permeability of the vasculature.46 This results in a massive influx of plasma containing chemotactic factors and cytokines47,48 to the inflammation site. These soluble mediators play a crucial role in facilitating the directed migration of leukocytes and influencing the activity of infiltrating49 immune cells. Cytokines serve as the primary signaling molecules, which are classified into pro-inflammatory factors (IL-1β, IL-6, TNF-α and IFN-γ) and anti-inflammatory factors (IL-4, IL-10 and TGF-β). Pro-inflammatory factors are involved in various aspects of the inflammatory response, including the activation of multiple inflammatory signaling pathways (e.g., NF-κB,50 JAKs-STAT pathways51). These pathways, in turn, lead to the activation of endothelium, neutrophils, lymphocytes and white blood cells. They have also been reported to participate in the production of other inflammatory mediators, such as proteases52 and RONS,53 which are inflammatory mediators that degrade ECM and cause oxidative stress.Chronic inflammation is primarily characterized by the persistent presence of inflammatory mediators and ongoing tissue damage. A critical aspect in resolving inflammation is maintaining a balance between various pro- and anti-inflammatory mediators, such as redox molecules, matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP) levels. The disruption of any of the balance can result in uncontrolled, overactive chronic inflammation. Chronic inflammation, characterized by its low-grade and persistent nature (as the name suggests), can ultimately lead to tissue and organ damage over time through the induction of oxidative stress. New studies have found that inflammation resolution is a programmed active process.29 Researchers have showed that inflammation resolution is regulated by a superfamily of lipid signaling molecules as specialized SPMs.54 These molecules act as “stop signals” to end inflammation and promote healing. The use of lipidomics and metabolomics has revealed that levels of SPMs are lower in chronic inflammation compared to acute inflammation. These findings may offer new standard biomarkers and therapeutic targets for treating chronic inflammation.55
2.4 Target tissues
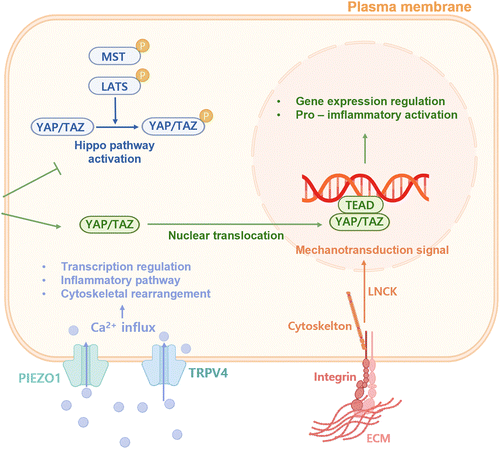
Inflammation is regulated by signals from the target tissue microenvironment, where not only biochemical signals but also the intrinsic mechanical cues and forces of the target tissues influence immune cell functions.56 Indeed, cells in target tissues are subjected to a variety of mechanical forces, such as matrix stiffness,57 tension, compression, shear stress58 and hydrostatic pressure. In many pathological conditions, including inflammatory states, cancer, and aging process, these mechanical signals are altered.59 It is now understood that alterations in tissue mechanics occur before the activation of immune responses. Upon exposure to TLR ligands, such as lipopolysaccharide (LPS), epithelial and stromal cells, markedly enhance the production of high molecular weight hyaluronic acid, leading to water retention and tissue swelling (i.e., oedema) within minutes to hours.56 Immune cells are able to detect these dynamic mechanical changes as they move through the tissue, helping them recognize danger and initiating immune reactions.Many innate immune cells exhibit adhesion and contact-dependency, making them acutely responsive to mechanical stimuli.60 They share conserved pathways to sense and respond to such mechanical stimuli, with key mechanosensors including the classical Hippo pathway, adhesion molecules (e.g., integrins), ion channels (e.g., transient receptor potential vanilloid type 4 (TRPV4) and members of PIEZO family) and elements of the cytoskeleton (Fig. 3). Research has demonstrated that stiff substrates can activate the PIEZO1, which in turn induces Ca2+ influx that enhances NF-κB activation and F-actin assembly in macrophages exposed to LPS and IFN-γ, resulting in a more pro-inflammatory phenotype.61 Similarly, stiff substrates promote nuclear localization of the secondary mediators of force transduction, YAP and TAZ,62 further driving the pro-inflammatory macrophage activation, while inhibiting the anti-inflammatory macrophage polarization induced by IL-4 and IL-10. Additionally, exposure to cyclic hydrostatic pressure can also drive macrophages towards a pro-inflammatory state.63 Besides, cyclic stretch can trigger the transition of macrophages to a pro-inflammatory phenotype and enhance BMP9 secretion through YAP/TAZ mechano-sensing, consequently promoting MSC osteogenesis.64 These data indicate that various mechanical stimuli can affect the signaling and metabolism of innate immune cells, thereby modulating their activation, function and inflammatory response. Additionally, adaptive immune cells demonstrate a profound sensitivity to mechanical signals. For example, an increase in tissue stiffness from 4 kPa (similar to that of a quiescent lymph node) to 40 kPa (resembling a swollen lymph node) was found to enhance T cell proliferation, activation and metabolic activity, concurrently reducing the antigen threshold required for effector responses.65 A recent research also showed that the unique mechanical characteristics of biomaterial implants can impact the B cell response to muscle injury.66
In short, tissue-level mechanical stresses present in pathological conditions, such as oedema during inflammation, can trigger mechanosensors within immune cells, enhancing their sensitivity to boost immune effector functions. With the recovery of diseases, tissues restore its homeostasis, leading to the deactivation of mechanosensors and an increased threshold for danger signaling, which in turn results in decreased immune activation. However, chronic inflammation causes fibrotic and mechanically stiffer over time, further exacerbating inflammation, resulting in a vicious circle. Further investigation into how tissue mechanics regulate immune responses is expected to offer new insights that can be directly applied to boost or resolve chronic inflammation and provide new ideas in the design and application of novel immunomodulatory hydrogels to promote tissue regeneration.
3. Design of hydrogels for immunomodulation
The design of hydrogels for immunomodulation is a complex strategy that focuses on addressing dysregulated stages of chronic inflammation. This entails the fabrication of biomaterials capable of interacting with the immune system in a deliberate manner, either by facilitating anti-inflammatory reactions or by inhibiting excessive inflammation. In this context, we explore the design of immunomodulatory hydrogels with inherent properties for modulating the four key elements of chronic inflammation.3.1. Hydrogels barrier strategies for inflammation inducer
Inflammatory inducers, including DAMPs and PAMPs, can promote the recruitment of immune cells and cause inflammatory response. The hydrogel can serve as a barrier, isolating the damaged spot from the surrounding tissues and protecting against pathogen infections to block DAMP or PAMP-triggered immune response.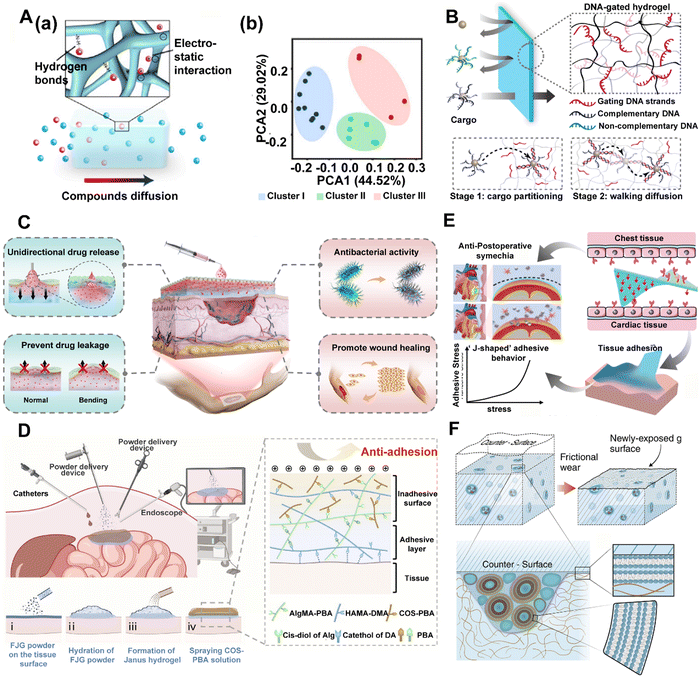 | ||
| Fig. 4 Hydrogels can be designed as barriers to DAMP propagation. (A) (a) Schematic of NPV-ECM selective permeability. (b) Principal-component analysis (PCA) for clustering molecules into 3 non-overlapping clusters. Compounds in different clusters have certain characteristics in the molecular structure. Reproduced with permission from ref. 67 Copyright 2023 Springer Nature. (B) Schematic representation of a selective transport process facilitated by transient DNA–DNA interactions. A DNA-gated hydrogel selectively transports cargo-bearing DNA sequences that are complementary to the gating DNA strands, provided that the DNA–DNA interaction is attractive enough to facilitate cargo partitioning into the hydrogel (Stage 1), yet sufficiently transient to allow the walking diffusion of cargo within the hydrogel (Stage 2). Reproduced with permission from ref. 68 Copyright © 2021, American Chemical Society. (C) Unidirectional drug delivery and wound care capability of a functional hydrogel bandage with Janus wettability. Reproduced with permission from ref. 69 Copyright © 2024, American Chemical Society. (D) Sprayable fast Janus gel (FJG) creates Janus viscous hydrogel barrier powder in situ to prevent postoperative adhesion of various organs such as the heart, liver and intestine. Reproduced with permission. Reproduced with permission from ref. 70 Copyright 2023 PNAS. (E) The Janus CPAMC/PCA hydrogel can quickly adhere to the wet heart tissue and enable on-demand stimuli-triggered detachment and simultaneously prevent post-tissue synechia. Reproduced with permission from ref. 71 Copyright © 2022, The Author(s). (F) As the surface of the hydrogel, incorporating lipids as vesicles in microreservoirs, wears away because of friction, additional microreservoirs of lipid are exposed. This enables boundary layers of lipids to form on the surfaces, leading to friction reduction via the hydration lubrication mechanism at the slip plane between the highly hydrated lipid headgroups. Reproduced with permission. Reproduced with permission from ref. 72 Copyright 2020 Science. | ||
In chronic wounds, the application of a hydrogel barrier provides an optimal moist environment, enhancing cell growth and regeneration while absorbing exudate that mitigates inflammation and facilitates the healing process. Recently, inspired by natural phenomena such as the lotus leaf, Janus materials with asymmetric wetting properties have opened new avenues for unidirectional liquid transport.69,73,74 These materials harness the power of surface tension imbalances to drive fluids in a specific direction, leading to their broad application in various fields, including oil–water separation, sweat collection, and wound exudate management (Fig. 4C).69 Furthermore, the implementation of a programmable oxygenation device with the capacity for controlled oxygen generation and unidirectional transmission offers a direct means of regulating the hypoxic microenvironment to expedite the healing of chronic wounds.75
Hydrogels with barrier function have also been widely used for other chronic inflammation-related conditions such as IBD, postoperative adhesions non-invasive cardiac repair, and joint retrograde diseases.76 Thermosensitive polymer hydrogel has been used as a protective physical barrier for the colonic mucosa to isolate ulcer sites from the external environment and block out the pro-inflammatory factors.77 Adhesive sequelae often emerge following surgical interventions, representing a common complication associated with nearly all forms of surgical procedures. Hydrogel barriers with Janus-adhesive properties have been identified as a promising solution for physically isolating adjacent tissues. The in situ creation of a Janus-adhesive hydrogel barrier through the application of a sprayable fast-Janus-gelation (FJG)70 powder has been proposed (Fig. 4D). This approach has proven effective in preventing postoperative adhesions across various organs such as the heart, liver, and intestine, and its feasibility and effectiveness have been confirmed in minimally invasive surgery, as validated through large animal studies. In addition to the repair of gastrointestinal inflammation and abdominal wall defect, more specifically challenging Janus cardiac patches for myocardial infarction (MI) repair have also been developed.71,78In vitro and in vivo experiments demonstrated that these Janus hydrogels have promising effects on fibrosis reduction and heart function recovery by reducing oxidative damage and inflammatory response without secondary injury and tissue synechia (Fig. 4E). Besides, inspired by the boundary layer of joint lipids, a dynamic lubrication barrier hydrogel72,79 has also been designed, incorporating a low concentration of lipids (phosphatidylcholine) (Fig. 4F). Taken together, these hydrogels provide a method for sustained barrier in applications for tissue engineering and highlight their great potential for clinical translation.
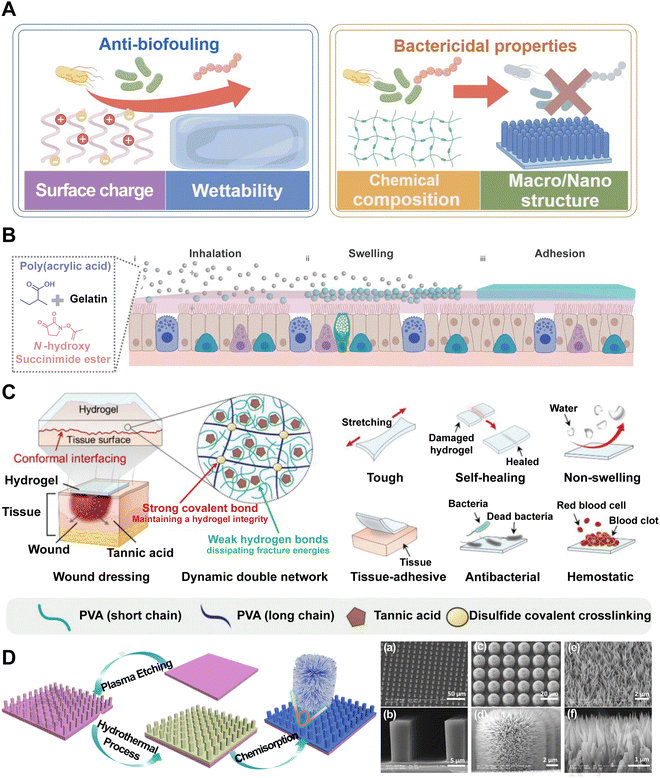 | ||
| Fig. 5 Hydrogels can be designed as barriers for PAMP propagation. (A) Classical PAMP barrier hydrogels can be divided as anti-biofouling and bactericidal properties. Image was drawn using FigDraw. (B) Dry SHIELD particles (grey spheres) are inhaled and they swell (blue spheres) once they are in contact with the mucus layer (pink layer). Finally, it forms a layer of the hydrogel (blue layer) and adheres to the mucus layer. Reproduced with permission from ref. 79 Copyright 2023 Springer Nature. (C) Schematics of a DN hydrogel wound dressing patch and its multifunctionality. Reproduced with permission from ref. 81 Copyright © 2023 The Authors. Advanced Science published by Wiley-VCH GmbH. (D) Schematic of the fabrication procedure of lotus leaf-like structures and SEM images of the microscale structure patterned silicon surface (MS). Reproduced with permission from ref. 82 Copyright © 2020 Published by Elsevier B.V. | ||
The hydrophilic surface of the hydrogel and its hydration layer can serve as a barrier that reduces bacterial adhesion through steric and electrostatic repulsion, thereby inhibiting the formation of bacterial biofilms. A recent work reports an inhalable bioadhesive hydrogel as a physical barrier for enhanced lung defense (named SHIELD).79 This hydrogel can be conveniently delivered via the inhalation of a dry powder formulation, composed of poly(acrylic acid) grafted with N-hydroxysuccinimide ester (PAAc-NHS ester) and gelatin (Fig. 5B). The powder is then swollen to form a hydrogel layer that adheres to the mucus layer, providing a protective barrier against SARS-CoV-2 and other respiratory pathogens. This innovative approach supplements the use of face masks and offers effective and additional protection to pulmonary infection. Currently, hydrophilic polymers such as poly(ethylene glycol) and zwitterionic polymers are often regarded as “gold standards” in the realm of device coatings designed to mitigate biofouling. However, in the quest for novel anti-biofouling materials, researchers have developed a combinatorial library of polyacrylamide-based copolymer hydrogels and employed a high-throughput screening method to assess their anti-biofouling capabilities. Surprisingly, certain copolymer compositions were found to have superior anti-biofouling properties over current gold standard materials. Machine learning techniques were then utilized to discern the key molecular characteristics that contribute to their enhanced performance.83 This novel methodology enables the discovery of new PAMP barrier hydrogels. Through the meticulous selection of monomers and crosslinkers, hydrogels can be tailored to exhibit desired bactericidal properties through electrostatic interactions that enhance the binding between polymers and anionic bacterial membranes, such as antibacterial peptide self-assembled supramolecular hydrogels, certain chitosan-based hydrogels and amphoteric ion hydrogel. For example, hydrogels comprised of thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) and redox-responsive polyferrocenylsilane macromolecules have demonstrated pronounced antibacterial efficacy while maintaining a high level of biocompatibility.84 Advances in multifunctional hydrogels have been achieved in the design of PAMA barrier. Researchers have crafted a non-swellable hydrogel bioadhesive that is mechanically durable and capable of seamlessly interfacing with biological tissues. Tannic acid (TA) with abundant hydroxyl groups was integrated into a double-network (DN) hydrogel, enabling a dynamic network for effective energy dissipation. Notably, TA is known to exhibit antibacterial properties due to its astringent and antioxidative characteristics. These properties enable TA to complex with bacterial enzymes, thereby imbuing the hydrogel with inherent antibacterial capabilities that effectively safeguard against infections (Fig. 5C).81 Moreover, the study of bacterial interactions with surface topographies has revealed that combining nano and microstructures has a highly effective, non-biocide bactericidal effect on the physical mechanism.85 The deposition of bacteria onto certain surface geometries, particularly pillar structures, can exert substantial stretching forces on the bacterial cell membrane. When the mechanical strain surpasses the elasticity of the cell membrane, it will lead to membrane rupture and bacterial lysis. This “deformation-stretching-torn apart effect” is independent of the surface chemistry of the materials and can be precisely modulated through the design of the physical characteristics, including radius, height, and density.86 Ren et al. have reported that the nano–macro structure of lotus leaf, celebrated for its superhydrophobic properties, not only repels bacteria effectively but also exerts a bactericidal effect through a cell-rupturing mechanism, underscoring the potential of surface topography in controlling bacterial adhesion and viability (Fig. 5D).82 These hydrogels, possessing inherent antimicrobial properties, have been developed in recent years, offering a new class of effective antibacterial agents that demonstrate a reduced or potentially eliminated side effect profile in comparison to conventional treatments.
The barrier function of a hydrogel serves not only as a physical barrier to protect the site of inflammation from external contaminants but also as a modulator of the local microenvironment. As research in this field continues to advance, the development of more sophisticated hydrogel systems with tailored barrier properties will likely play an increasingly important role in the treatment of various conditions associated with tissue damage and inflammation.
3.2. Hydrogels as scavengers for inflammation mediator
Activated inflammatory cells release various inflammation mediators, including RONS, inflammatory factors and cytokines, which are crucial in the amplification and perpetuation of inflammation in the local microenvironment. In order to counteract the harmful consequences of chronic inflammation, hydrogels can be engineered to selectively target specific features to scavenge inflammation mediators for RONS-neutralization and cytokines-absorption.RONS-scavenging hydrogels are emerging as prospective candidates for therapeutic interventions due to their excellent biocompatibility, 3D structural matrix, and potential for functional modification. Strategies to endow standard hydrogels with antioxidant characteristics involve the attachment of organic groups capable of adsorbing RONS through proton or electron transfer mechanisms as well as the integration of linkers susceptible to cleavage by RONS within the polymer framework (Fig. 6A). A photoenhanced glycyrrhizic acid hydrogel, which incorporates a reductive diglucuronic unit, was developed to promote rapid diabetic wound healing by regulating the oxidative stress microenvironment (Fig. 6B).91 Polyphenols, including tannic acid (TA), dopamine (DA), curcumin, and EGCG, are organic compounds known for their ability to directly scavenge ROS. The ROS scavenging mechanism is largely due to the presence of hydroxyl groups attached to the benzene rings. These hydroxyl groups possess the unique ability to donate either a hydrogen atom or a single electron to ROS, thereby effectively stabilizing these otherwise highly reactive species. Thus, the structure of hydrogels can be modified to include multiple phenol groups in order to control the oxidative environments.92 For example, a hydrogel crosslinked by multiple hydrogen bonds and loaded with TA and kartogenin was synthesized through the polymerization addition reaction for the purpose of treating osteoarthritis (Fig. 6C).93 In another study, DA-based bioadhesive hydrogel has also been proposed for sutureless ocular tissue repair, which can prevent the accumulation of ROS and decrease the inflammatory response and fibrosis resulting from suture surgical trauma (Fig. 6D).94 In order to develop an ROS-scavenging hydrogel that can selectively release therapeutic reagents to lesions, polymers containing ROS-labile linkers, such as boronic acid, thioether, thioketal, and disulfide bond, would be more suitable. However, it is important to acknowledge the limitations of using organic polymers as RONS scavengers for long-term treatment as the functional groups may be depleted during RONS scavenging. Therefore, the combination of RONS-scavenging hydrogels with other drugs or therapeutic molecules could be explored to enhance the treatment of chronic inflammation with a wider range of functionalities.
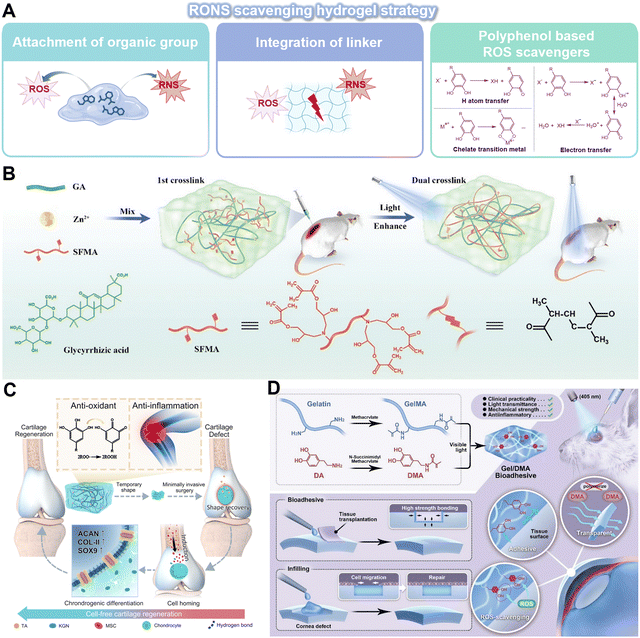 | ||
| Fig. 6 Innovative hydrogels for RONS scavenging strategy. (A) An overview of the RONS scavenging hydrogel strategy. (I) Attachment of organic groups capable of absorbing RONS. (II) Integration of the linker labile to RONS. (III) Encapsulation of the polyphenol-based ROS scavenger. Image was drawn using BioRender. (B) A photo-enhanced glycyrrhizic acid hybrid hydrogel as an immunoregulatory scaffold for diabetic wound repair. Reproduced with permission from ref. 91 Copyright 2022 Wiley-VCH GmbH. (C) PTK hydrogel with multiple hydrogen-bonds crosslinked network features ultra-durable mechanical properties, adequate adhesiveness with cartilage tissues, anti-inflammation and antioxidant capabilities, and the fast shape memory property endows the hydrogel with potential for minimally-invasive surgery. Reproduced with permission from ref. 93 Copyright 2023 Springer Nature. (D) Synthesis and application of a Gel/DMA bioadhesive for sutureless ocular tissue repair. Reproduced with permission from ref. 94 Copyright 2023 Wiley-VCH GmbH. | ||
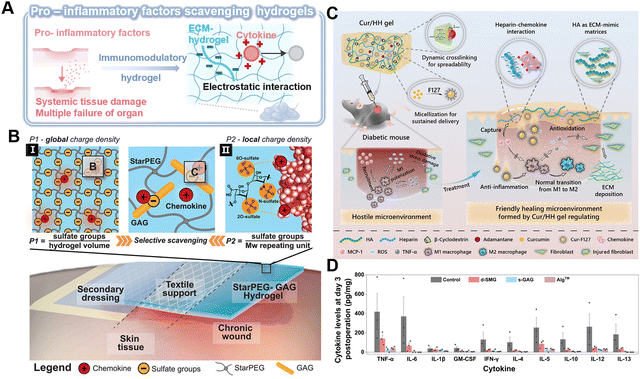 | ||
| Fig. 7 Immunomodulatory hydrogel as a platform for pro-inflammatory scavenging. (A) Immunomodulatory hydrogel can be used as a platform for neutralizing proinflammatory factors through electrostatic interaction, thereby eliminating the level of cytokines or blocking their biological activity. Image was drawn using BioRender. (B) Schematic of the electrostatic interaction between the starPEG-GAG hydrogel with chemokine. The scavenging effectiveness can be determined via (I) global charge density and (II) local charge density. Reproduced with permission from ref. 98 Copyright 2021 Wiley-VCH GmbH. (C) Schematic of the Cur/HH Gel with multifunctional properties and its therapeutic effect in diabetic chronic wounds over time. Reproduced with permission from ref. 102 Copyright 2022 American Chemical Society. (D) Cytokine levels in wound tissue on day 3 (n = 3 biologically independent samples in each group). Reproduced with permission from ref. 103 Copyright 2023 Springer Nature. | ||
Currently, the majority of research on GAG-based hydrogels is still confined to common GAG categories. Therefore, more GAGs with good biocompatibility and regenerative properties are warranted. A natural bioadhesive has been developed from snail mucin gel, which is composed of positively charged proteins and polyanionic GAGs.103 The abundant heparan-like GAGs, serving as the primary active component, can capture inflammatory cytokines, such as TNF-α, IL-6, and IL-5, and effectively modulate the inflammatory response within the wound microenvironment (Fig. 7D).103 Besides, non-anticoagulant heparin analogs, including polyglycerol sulfate and polyphosphonates, which act as scavengers of pro-inflammatory factors, have the potential to mitigate chronic inflammation.
In fact, the function of inflammation mediators is extraordinarily complex. Based on electrostatic interactions, negatively charged polysaccharides will inevitably deplete some positively charged soluble proteins and other molecules that facilitate inflammation resolution, such as IL-10 and SPM. How to minimize this side effect is an urgent issue that needs to be addressed.
3.3. The physiochemical engineering of hydrogels regulates inflammation sensors
Hydrogels can be designed with tailored physical and chemical characteristics to provide specific immune microenvironments that influence the behavior of inflammation sensors (immune cells). By carefully selecting the composition (natural or synthetic architectures), crosslinking density (gel porosity), and other characteristics (such as their chiral structure) of hydrogels, researchers can selectively suppress or activate immune responses, offering a sophisticated approach to treating chronic inflammation.Natural materials. Natural materials, such as decellularized extracell matrix (dECM), glycosaminoglycan, and proteins, can be used in regenerative medicine to modulate the chronic inflammation microenvironment. They are rich in functional groups such as hydroxyl, carboxyl, carboxylic acid, and amino groups, providing ample molecular modification space for the design and construction of 3D cross-linked networks. This results in naturally sourced hydrogels with diverse tunable functionalities, including biocompatibility, biodegradability, mechanical properties, self-healing capability, and adhesiveness.
By removing cells from tissue, dECM maintains its inherent physicochemical cues and biological signals, which can bind to specific immune cell receptors to enhance adhesion and modulate immune cell activity. Many immune cells including macrophages,104–106 mast cells107, and T cells105,108,109 have been implicated in the immune response triggered by dECM (Table 1).104–108,110,111 In general, the dECM hydrogel guides the host immune response away from a classical TH1-proinflammatory profile.105,108 However, the immunological effects of dECM may vary depending on factors such as the specific organ or tissue type, presence of remaining cellular elements, antigenicity, and tissue structural architecture (Fig. 8A). For example, macrophages exposed to epithelial cells types such as small intestinal submucosa (SIS), urinary bladder matrix (UBM), brain ECM (bECM), esophageal ECM (eECM), and colonic ECM (coECM) demonstrate an M2-like phenotype (iNOS−/Fizz1+/CD206+) that promotes tissue remodeling and reduces inflammation. On the other hand, when macrophages are exposed to dermal ECM, they predominantly exhibit an M1-like, pro-inflammatory phenotype (iNOS+/Fizz1−/CD206−) (Fig. 8B).104
| Cell type | dECM type | Function | Ref. |
|---|---|---|---|
| Macrophages | Small intestinal submucosa (SIS), urinary bladder matrix (UBM), brain ECM (bECM), esophageal ECM (eECM), and colonic ECM (coECM) | dECM promoted a M2-like phenotype (iNOS−/Fizz1+/CD206+) that promotes tissue remodeling and reduces inflammation | 104 |
| Dermal ECM | Macrophages exhibited an M1-like, pro-inflammatory phenotype (iNOS+/Fizz1−/CD206−) | 104 | |
| Decellularized porcine or human cadaveric myocardium | Biomaterials induced the infiltration of macrophages polarized towards an M2 phenotype | 105 | |
| Decellularized (DC)-Wharton's jelly (WJ) | Macrophages altered their morphologies and polarization states from the resting M0 phenotype to the hybrid M1/M2 phenotype | 105 | |
| Bladders-ECM breakdown products | Bladders-ECM breakdown products raise anti-inflammatory cytokines from macrophages and reduce pro-inflammatory cytokines | 106 | |
| Brain-derived ECM | Brain-derived ECM increased the synthesis of TNF-α, nitric oxide, and arginase in primary rat macrophages | 111 | |
| Decellularized muscle scaffolds | Scaffolds polarized the macrophage response in vivo toward an M2 phenotype | 108 | |
| T-helper cells | Decellularized porcine or human cadaveric myocardium | Biomaterials induced the infiltration of the T-helper cells polarized towards a TH2 phenotype | 105 |
| Decellularized muscle scaffolds | Scaffolds down-regulated T-cell xeno responses and TH1 effector cytokines in vitro | 108 | |
| Mast cells | Decellularized porcine dermis | Mast cells displayed distinct metabolic activity and increased the IgE receptors linked to mast cell maturation/activation | 107 |
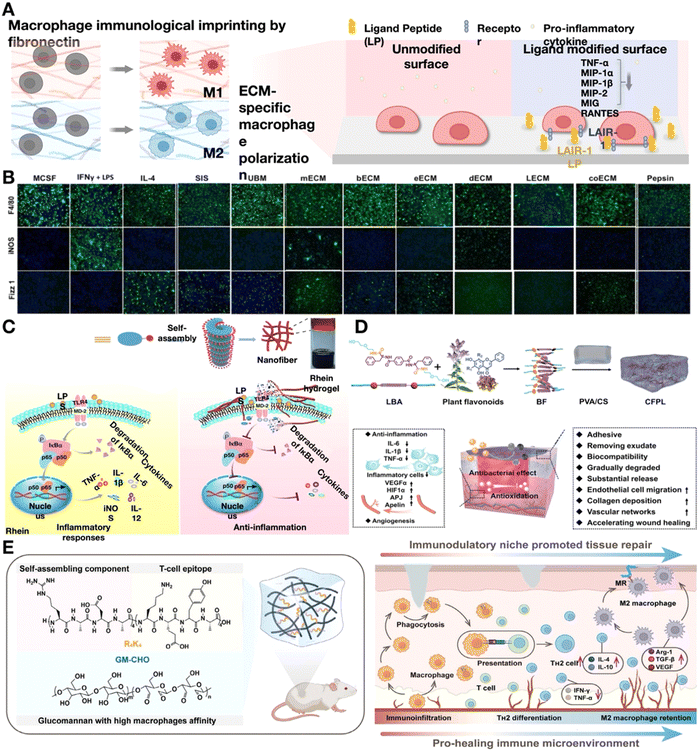 | ||
| Fig. 8 Natural hydrogels play an anti-inflammatory role. (A) Schematic showing ECM-specific macrophage polarization and the influence of ligand-receptor interaction, which can reduce the production of pro-inflammatory cytokines. Image was drawn using BioRender. (B) F4/80 (pan macrophage), iNOS (M1 marker), and Fizz1 (M2 marker) immunofluorescence images of macrophages cultivated on polystyrene with pepsin or cytokines, or different tissues of the extracellular matrix (ECM). Reproduced with permission. Reproduced with permission from ref. 104 Copyright 2016 Wiley-VCH GmbH. (C) Schematic diagram of rhein hydrogel synthesis and the anti-inflammatory mechanism. They bind to MD-2, the TLR4 receptor subunit, intensely, obstructing TLR4's active site and further preventing substrate access, which inhibits NF-κB activation. Reproduced with permission from ref. 112 Copyright 2019 Springer Nature. (D) Diagrammatic representation of the steps involved in making a hydrogel dressing functionalized with flavonoids and how CFPL chiral hydrogel helps rats repair chronic wounds. Reproduced with permission. Reproduced with permission from ref. 113 Copyright 2022 Wiley-VCH GmbH. (E) Diagram showing the design and synthesis of glucomannan-R4K4 peptide hydrogel (GRKgel) and the interaction between macrophages and T cells to speed up the healing process of injured tissue. Reproduced with permission. Reproduced with permission from ref. 114 Copyright 2022 Wiley-VCH GmbH. | ||
Alternatively, more defined and purified naturally-derived proteins can be used in immunomodulation hydrogels, such as collagen, gelatin and fibronectin. Collagens (in particular collagen type II and III) are natural binding ligands for leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1)115, which can reduce the expression of pro-inflammatory cytokines such as TNF-α, CXCL-9, CCL-3 and CXCL-2.116 During T/B cell development and activation, the levels of LAIR-1 change considerably117 and can then be immunomodulated by collagen in the hydrogel. Collagen-based matrices such as gelatin is widely used. It is believed that more RGD sites become visible when collagen I is denatured to create gelatin, which provides adhesion sites and signals the overexpression of αvβ3 integrin in B cells, which is essential to B-cell differentiation.118 Hyaluronic acid (HA), as the only representative of non-sulfated GAGs, is another significant component of the ECM, which is covalently linked to the core protein within proteoglycans and is capable of communicating with cell surface receptors. HA binds to the TLR to initiate the signaling pathways and activate both innate and adaptive immune responses. High-molecular-weight HA (HWHA) promotes anti-inflammatory macrophages polarization, leading to the expression of arginase-1 (Arg-1), IL-10, and MRC-1.119 Considering the influence of HWHA on the regulation of immune cell behaviors, it has been widely used to construct functional hydrogels aimed at immunomodulation in chronic inflammation.
Biomedical applications related to self-assembling natural drug hydrogels that can function as carriers without requiring structural alteration are interesting. The potential of rhein, a naturally occurring herbal product, to form hydrogels through direct self-assembly via noncovalent interactions has been investigated (Fig. 8C).112 These hydrogels facilitate the nuclear translocation of p65 to the NF-κB signaling pathway in lipopolysaccharide-induced BV2 microglia, leading to the significant dephosphorylation of IκBα. Furthermore, rhein hydrogels have a minimally cytotoxic and long-lasting effect on neuroinflammation.112 Noncovalent interactions between supramolecular hydrogelators and flavonoids sharing a common skeleton are utilized to generate co-assemblies of multiple flavonoids (Fig. 8D).113 The chain-to-chain noncovalent interactions aid in the transmission of chirality from these co-assemblies to poly(vinyl alcohol) and chitosan, enhancing their mechanical strength and ability to absorb water. The utilization of hydrogels contributes to the amelioration of chronic wound healing by reducing the release of inflammatory mediators by the immune cells.113
Following macrophage polarization, the T-cell response initiated within the injured tissue environment is instrumental in dictating the extent of inflammation. Evidence abounds, highlighting the indispensable role of various T-cell subsets, including regulatory T cells (Tregs), TH2, γδT, and TH17 cells in wound repair and tissue regeneration.120 However, research on restoring immune homeostasis in regenerative medicine through biomaterials that induce regenerative T-cell responses is limited. Wang et al.114 developed a glycopeptide hydrogel (GRKgel) that activates a reparative TH2 immune response by mediating macrophage-T cell crosstalk to further induce T-cell immune response. The hydrogel incorporated an oxidized glucomannan (GM) grafted with auto-assembling peptide RADA16 (R4K4) through dynamically bonded imine linkages to create an immunomodulatory niche. This peptide can be engulfed and presented by macrophages to naïve T cells, inducing antigen-specific TH2 immune response in vivo without any cytokines or drugs (Fig. 8E).114 This study underscores the pivotal role of crosstalk between immune cells in activating reparative T cell immunity, presenting a promising strategy for the regulation of endogenous chronic inflammation that is free from the constraints of drugs and cytokines.
Synthetic materials. Synthetic materials, such as PCL, PLGA and PEG, are produced through artificial processes, which enhances their consistency in batch production, cost-effectiveness, and adjustable physicochemical properties.121 Compared to naturally occurring scaffolds, synthetic materials generally exhibit greater mechanical strength and precise control over tunability.122 However, the implantation of bulk synthetic biomaterials into soft tissue often elicits a foreign body reaction, characterized by the prolonged presence of macrophages,122 which impedes the biological function and integration of medical devices.121 Protein adsorption on the surface of biomaterials leads to the activation of the coagulation cascade, recruitment and activation of leukocytes, production of pro-inflammatory cytokines, activation of the complement system, and formation of a provisional matrix. These components of the inflammatory cascade are closely associated with the physicochemical surface properties of the biomaterial.121–123
Synthetic materials elicit a robust pro-inflammatory immune response involving various immune cell types, including neutrophils,124 macrophages,125 B cells126 and dendritic cells (DCs).121,127 The presence of synthetic materials (PE, PEG) exacerbates neutrophil recruitment in the injury site, resulting in prolonged neutrophil infiltration (Fig. 9A and B; green cluster) and a reduction in type-2 macrophage markers (Fig. 9C).124 When compared to tissue formation on Matrigel (MTG), tissue assembly on synthetic PEG hydrogels produced 3D planar neural organoids with increased neuronal diversity (Fig. 9D). Following stimulation with LPS, planar neural organoids exhibited higher levels of TNF-α and IL-6 in comparison to neural tissues grown on Matrigel.128
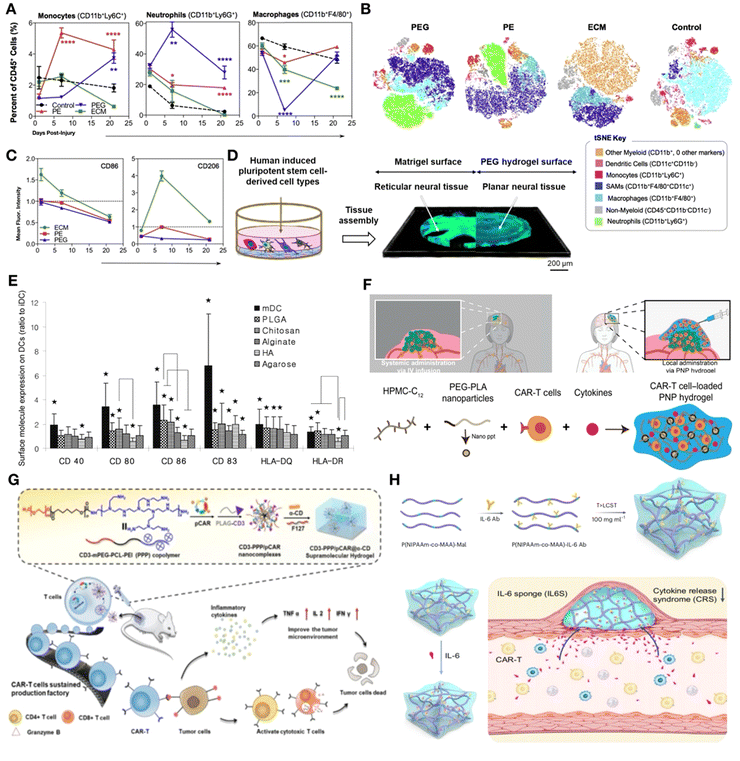 | ||
| Fig. 9 Synthetic materials induce stronger pro-inflammatory immunity. (A) Flow cytometry of cells extracted from the wound scaffold immunological microenvironment. The synthetic material had a higher level of neutrophil infiltration. (B) tSNE clustering of the scaffold immune microenvironment at 3 weeks post-injury. (C) Flow cytometry analysis of CD86 and CD206 expression on scaffold-associated macrophages at day 1, 7, and 21 post-injury displayed as fold change over saline-treated control. Reproduced with permission. (A)–(C) Reproduced with permission from ref. 124 Copyright 2018 Elsevier Ltd. (D) Schematic representation showing the culture of neural tissues on Matrigel or PEG hydrogels surfaces. Reproduced with permission. Reproduced with permission from ref. 128 Copyright 2024 Elsevier Ltd. (E) Allostimulatory capacities for DCs treated with various biomaterial films in the Mixed Lymphocyte Reaction (MLR). Reproduced with permission. Reproduced with permission from ref. 129 Copyright 2012 Elsevier Ltd. (F) Dodecyl-modified hydroxypropyl methylcellulose (HPMC) and degradable block-copolymer nanoparticles self-assemble to form PNP hydrogels that coencapsulate CAR-T cells and stimulatory cytokines. Reproduced with permission from ref. 130 Copyright 2024 Elsevier Ltd. (G) Diagram showing the in situ programming of CAR-T cells in a tumor microenvironment using the gene-loaded supramolecular hydrogel system's infiltrative capabilities against a solid tumor. Reproduced with permission from ref. 131 Copyright 2012 Elsevier Ltd. (H) (Top) Diagrammatic representation of the IL6S preparation process. (Bottom) CAR-T-cell-induced cytokine-release storm and associated side effects are inhibited by IL6S. Reproduced with permission from ref. 132 Copyright 2023 Springer Nature Ltd. | ||
In addition, research comparing polymer biomaterials derived from synthetic and natural sources has allowed for the identification of unique macrophage subgroups that distinguish between regenerative and fibrotic reactions. These works suggest that innate and adaptive immune cells may interact with one another.125 The association between B cells, antibody production, and synthetic materials has been established in previous studies. Following the implantation of various biomaterial classes (ceramic, polymer, and hydrogel), colony stimulating factor-1 receptor (CSF1R) expression is significantly increased, leading to the activation of the NALP3 inflammasome in dendritic cells (DCs) as a result of particulate engulfment. It was observed that both PLGA and PS particles markedly enhanced the release of IL-1β within early time intervals (30 min to 24 h).126,127 After a 24-hour exposure period, the maturation of DCs was found to be impacted to varying degrees by several biomaterial films. More precisely, PLGA or chitosan films stimulated DC maturation, resulting in increased expression of CD80, CD86, CD83, HLA-DQ, and CD44, as well as higher levels of DC allo-stimulatory potential and cytokine production (Fig. 9E). Alginate films induced an increase in pro-inflammatory cytokine production and a decrease in CD44 expression. In contrast, hyaluronic acid films have been shown to exert suppressive effects on the phenotype of dendritic cells, thus modulating their activation and function.129
On the one hand, various strategies have been employed to reduce the pro-inflammatory response of synthetic materials. One such method involves the application of natural material coatings on the surface of synthetic scaffolds to modulate inflammation. The phenotypes of macrophages and their phagocytic activities can be altered by incorporating a dECM coating onto synthetic materials such as polypropylene. Furthermore, the ongoing exploration of synthetic polymers stands to reap significant benefits from the cutting-edge design of polymers, construction of extensive combinatorial libraries, and direct assessment of their physiological immune responses.121
On the other hand, the pro-inflammatory response of synthetic materials can also be utilized for the activation of immune cells, particularly T cells, in cancer immunotherapy.133–136 CAR-T cell therapy has encountered significant challenges in treating solid tumors, largely due to physiological barriers and the immunosuppressive microenvironment of the solid tumor. Researchers have developed an injectable hydrogel carrier composed of hydroxypropyl methylcellulose and PEG–PLA polymer-nanoparticle (PNP)130 hydrogels to encapsulate CAR-T cells and stimulatory cytokines temporarily. By direct injecting near the tumor site, these hydrogels enable the establishment of a transient inflammatory microenvironment in vivo, which significantly promotes the expansion and activation of CAR-T cells, thereby effectively targeting and eliminating cancer cells. (Fig. 9F).130 In addition to CAR-T delivery, immunomodulatory hydrogels promote the anticancer functions of T cells in a multilevel manner through cell-material interactions, affecting recruitment, engagement, and reinvigoration (RER-T). Yang et al. designed an RER-T system based on an in situ-formed β-cyclodextrin-decorated alginate (ALG-βCD) hydrogel, which is capable of trapping the chemokine CCL25 to recruit a subset of T cells with robust antitumor activity, specifically the CCR9 + CD8+ T cells. By integrating the systemic administration of CAR-encoding nanocarriers with immunomodulatory hydrogels, the recruited T cells can undergo the in vivo production of CAR-T cells, thereby eliminating a significant amount of ex vivo culture and amplification costs, making the approach highly attractive (Fig. 9G).131 Furthermore, immunomodulatory hydrogels as inflammatory cytokine sponge are capable of effectively mitigating the cytokine-release storm and associated adverse effects induced by CAR-T cells (Fig. 9H).132
Technologies used for chemistry descriptors. Previous studies have shown that changes in biomaterial chemistry can impact macrophage behavior.137 However, most studies have not identified the specific compositions that control the diverse actions of individual cells. Given the complexity of immune cell phenotypic identification, scientists are seeking an alternative approach that would be simpler, less resource-intensive, and more widely adoptable. Proteome analysis has been utilized to elucidate the tissue-specific functions of dECM materials, revealing distinct compositions of matrisome proteins in each dECM tissue type that can significantly impact resident cells.138–140 Comprehensive differential proteomic studies were carried out on four porcine dECMs such as liver (LdECM), heart (HdECM), skin (SdECM), and cornea (CdECM). Particularly, it was shown that the composition of matrisome proteins varies in each type of decellularized tissue. These differences can have a substantial impact on the resident cells in particular tissues. Additionally, depending on the multipotency of MSCs, microarray analyses of human bone marrow MSCs printed with different dECM bioinks were carried out to highlight the effect of compositional differences in a tissue-specific way at the cellular level. Genes were found to express differently in different tissues based on whole transcriptome analysis.140 Another work has demonstrated that cellular proteins are more challenging to eliminate compared to DNA and continue to represent a significant portion of the proteome within decellularized scaffolds.138
Machine learning algorithms, such as least absolute shrinkage and selection operator (LASSO), random forest, support vector machines (SVMs), and multilayer perceptrons (MLPs), can be used to develop polymer structure-cell response models from the data obtained from high-throughput research. These models allow for the discovery of the kinds of chemical characteristics that either promote or hinder macrophage attachment and polarization, making it possible to anticipate the immune-instructive traits of novel materials that have not yet been produced.141 An extensive library of simple polymer chemistries is undergoing unbiased high-throughput screening to identify compounds that can direct macrophage attachment and polarization. Machine-learning models have been developed to predict the composite variable class, which is the logarithm of the M2/M1 ratio multiplied by the cell attachment index, because both polarization and cell attachment were equally significant (Fig. 10A). The appearance of alkoxy molecular fragments, propyloxy, 2,3-dimethylpropyloxy and ethylene glycol fragments are related with the induction of the most anti-inflammatory macrophage phenotype with a high cell attachment (Fig. 10B). A quick and easy imaging-based method based on machine learning algorithms was also developed, which allows the automatic identification of various macrophage functional phenotypes based on their cell size and morphology. Fluorescence microscopy was utilized to evaluate the morphology of various cell types that had been stained with DAPI or phalloidin, respectively, for the nucleus and actin dispersion. With an average accuracy of 90%, they were able to distinguish M1 and M2 phenotypes from naïve macrophages and monocytes by merely examining their shape (Fig. 10C).142 Comprehending how surface chemistry impacts protein adsorption is essential for creating innovative bioinert materials. Machine learning can not only characterize the immunomodulatory capacity of materials but also predict different material components with immune effects to guide the preparation of immunomodulatory hydrogels. A study has demonstrated how a quantifiable relationship between surface chemistry and protein adsorption may be produced by computational modeling with machine learning methods. For novel surface chemistries, the model can accurately forecast the amount of protein adsorbed on SAMs (within the models’ applicability region). The predictions based on machine learning are therefore shown to help determine surfaces that perform optimally for synthesis and manufacturing.143 These models highlight the importance of steric and electronic factors in macrophage response, underscoring the potential of integrating machine learning with high-throughput experiments to determine the materials chemistry as a major driver of immune cell polarization and the underlying molecular mechanisms.141 However, the accuracy of machine learning is limited by the quality and quantity of data, selection of relevant features, and complexity of immune responses. While these approaches provide critical insights into new polymers with immune-modulatory properties, their applicability to the intricate in vivo environment and clinical practice requires further scrutiny.
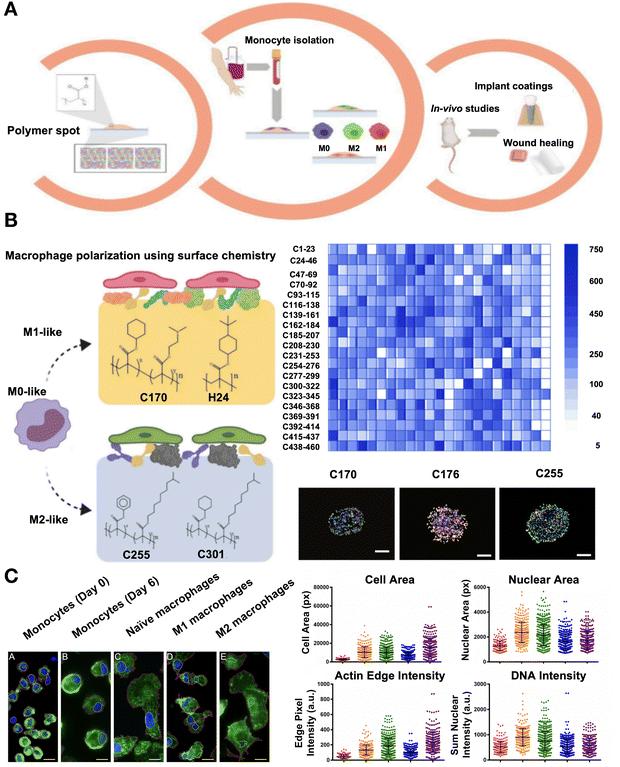 | ||
| Fig. 10 Technologies including machine learning used for chemistry descriptors such as phenotypic identification of macrophages and prediction of anti-inflammatory materials. (A) Diagram of the in vitro and in vivo high-throughput screening method used to find hit polymers that transform macrophage phenotype to a pro- or anti-inflammatory status. (B) Screening a vast library of polymers to identify the ability to encourage the differentiation of macrophages into pro- or anti-inflammatory phenotypes. Adherent cell count on a co-polymer array on average. Fluorescent images of co-polymers that produce a similar number of M0 (M1 and M2) bias cells, or M1 (CD176 and CD170) and M2 (CD255) bias. (A)-(B) Reproduced with permission from ref. 141 Copyright 2020 Elsevier. (C) Macrophages and monocytes stained by immunofluorescence. An analysis of the main morphometric indicators, including nuclear area, cell area, sum nuclear DNA intensity normalized to nuclear area, and sum edge actin intensity adjusted to cell area. Reproduced with permission from ref. 142 Copyright 2017 Springer Nature. | ||
Hydrogels offer a versatile platform for immune cells’ modulation, with natural and synthetic materials each presenting unique advantages and challenges. The key to success lies in understanding the complex interplay between biomaterials and immune cells and in the development of strategies to harness this interplay for therapeutic purposes.
Porosity. The porosity of hydrogels encompasses two aspects: mesh size and pore size (Fig. 11A). Mesh size refers to the distance between molecular cross-links within a material and is a critical parameter that affects the permeability of molecules, such as the inward diffusion of oxygen and nutrients as well as the outward diffusion of drugs and immunomodulatory factors. The pore size refers to the internal pore diameter of the hydrogel that is formed either by compacting the discrete hydrogel to create a granular hydrogel or through phase separation. These pores mimic the percolating interstitial voids found in natural tissues. The presence of pores in hydrogels facilitates cell infiltration, promoting integration with the surrounding tissue. This integration leads to angiogenesis and vasculogenesis within the hydrogel.144
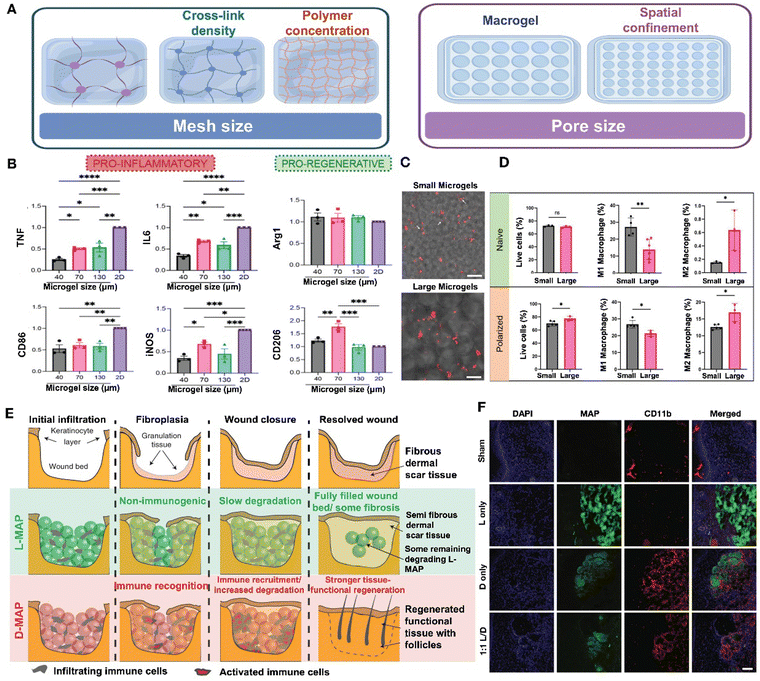 | ||
| Fig. 11 Effect of material porosity and chirality on immune regulation. (A) The porosity of hydrogels encompasses two aspects: mesh size and pore size. Image was drawn using FigDraw. (B) The expression level in the production of the pro-inflammatory cytokines and markers as well as pro-regenerative markers between 2D culture and different sizes of microgels. Reproduced with permission from ref. 145 Copyright 2023 Wiley-VCH GmbH. (C) Trapped in small microgel scaffolds, macrophages (red) have an extended cell shape (white arrows). (D) M1 polarization is higher in the smaller microgel scaffolds, while M2 polarization is higher in large microgel scaffolds. (C) and (D) Reproduced with permission from ref. 146 Copyright 2023 Wiley-VCH GmbH. (E) The wound healing process of L-MAP and D-MAP. (F) Confocal immunofluorescence pictures of labeled myeloid cells (CD11b+) in mice's healed wounds when indicated. Reproduced with permission. (E) and (F) Reproduced with permission from ref. 147 Copyright 2020 Springer Nature. | ||
Porosity is a critical physical parameter that affects immune response. The higher porosity of hydrogel enhances cell–material interactions and promotes a more effective immune response and tissue regeneration.148 Generally, natural hydrogels formed from collagen or fibrin typically possess a micrometer-scale mesh structure, which allows cells to migrate without disrupting or degrading the hydrogel. In contrast, synthetic hydrogels crosslinked covalently often exhibit a nanometer-scale mesh size that restricts cell growth and migration.149 The mesh size of hydrogels, which is inherently linked to their mechanical stiffness, poses a challenge in discerning its specific impact on cell behavior. To address this, researchers have developed a hydrogel-on-glass substrate with precise thicknesses, allowing for the independent adjustment of both stiffness and mesh size. Utilizing this hydrogel system, they have successfully decoupled the effects of mesh size from stiffness, enabling a detailed examination of its influence on various aspects of cell behavior such as cell attachment, spreading, migration, formation of focal adhesions, and the intranuclear localization of YAP.150 These results demonstrate that the mesh size of the hydrogel has significant effects on the cell–substrate interaction. However, the underlying mechanisms by which mesh size modulates the activity of immune cells require further investigation.
Studies have shown that the pore size of granular hydrogels and biomaterials significantly influence macrophage polarization.145,146,151 In one particular study, HA-based annealed microgels with pore sizes of 40, 70, and 130 μm were generated and implanted subcutaneously in C57BL/6 mice for a period of 21 days. Under M1(LPS/IFN-γ) activation stimuli, all scaffold sizes led to a reduction in the inflammatory response, with the most significant reduction observed in the smallest pore size (Fig. 11B). These findings indicate that smaller spatial confinement can suppress the expression of pro-inflammatory markers and cytokines in M1 macrophages. Meanwhile, the highest expression of M2 macrophage markers (Arg1 + CD206+) was observed in 70 um scaffolds, suggesting that factors other than pore size may regulate the pro-regenerative response.145 In comparison to Oasis and Aquaphor, both microporous annealed particle (MAP) groups exhibited a higher prevalence of M2 polarization over M1 at both time points. By day 7, more than 77% of the macrophages in both MAP conditions displayed the M2 phenotype, with the 130 μm confinement scaffolds showing a decline in the M1 macrophages.151 Another study demonstrated that the size of microgels influences macrophage morphology; macrophages seeded on microgels with a diameter of approximately 48 μm adopted an elongated shape, while those on larger microgels with a diameter of approximately 146 μm retained a rounded shape (Fig. 11C). Additionally, smaller microgels maintained a higher proportion of M1 macrophages, whereas larger microgels predominantly housed M2 macrophages, as observed under both naïve and M1 polarization conditions (Fig. 11D).146 The population of M1 cells was found to increase in association with nonporous hydrogels, whereas pore diameters exceeding 20 μm led to a transition towards an M2 phenotype.152 The infiltration of T-lymphocyte and pro-inflammatory cytokine penetration are also controlled by the microarchitecture and physical characteristics of the hydrogel. The porosity of the hydrogel biomaterial plays a crucial role in regulating the diffusion of inflammatory T-cells within the implanted MSC-hydrogel constructs, thereby impacting the apoptosis of stem cells derived from human exfoliated deciduous teeth.153
Chirality. Chirality, the spatial arrangement of atoms in a molecule that results in non-superimposable mirror images or enantiomers, has been shown to impact the immune effects of hydrogels in various studies. Specifically, research has demonstrated that the chiral properties of L-phenylalanine gelators (LPFEG) and D-phenylalanine gelators (DPFEG) play a significant role in enhancing drug transdermal administration through enantioselective interactions with the stratum corneum. In this study, the tissue penetration of sodium aescinate, an anti-inflammatory drug, proved more effective when loaded in LPFEG-based hydrogels compared to DPFEG-based hydrogels. This enhanced delivery influenced the local immunological response by reducing the M1 macrophage polarization.154D-Chiral peptide MAP hydrogels (D-MAPs) induced linker-specific IgG responses and a biased IgG2b response, typically associated with a Th1 immune profile or a more inflammatory response. In contrast, L-chiral MAPs (L-MAPs) elicited a heightened IgG1 response, commonly indicative of a pro-reparative response.145 In some instances, D-MAPs can trigger an inflammatory response that aids tissue regeneration. For example, Griffin et al. observed that a D-MAP hydrogel, initially resistant to MMP degradation in vitro, underwent rapid degradation in vivo. This was attributed to a foreign body reaction, which not only accelerated degradation but also stimulated an adaptive immune response, ultimately promoting hair neogenesis and improving tensile strength (Fig. 11E and F).147
The development of immunomodulatory hydrogels should take into account the physical properties of immune modulation. However, the mechanisms of how physical stimuli are transduced into intracellular signaling pathways that subsequently influence immune cell activities is not fully understood and needs further exploration. Additionally, the specific physical properties of hydrogels that can modulate the immune responses in vivo also remains to be elucidated.
3.4. Mechanoimmunology of hydrogels for the target tissue
The dynamic immune environment is finely tuned by mechanical forces, which get converted to biochemical signals for cell migration, activation and regulation, known as “mechanoimmunology”.155 Though the acute and chronic stiffening of the target tissue in response to inflammatory signals has long been recognized, the field of mechanoimmunology still remains in its early stages. The recent development of novel methods in this field has ignited a resurgence of interest in mechanisms of mechanosensing within the immune system.Compared to static hydrogels, structurally dynamic hydrogels are superior in replicating the complex dynamics and functions of the natural extracellular matrix found in soft tissues like the brain, skin, liver, and adipose tissue, which exhibit viscoelastic properties, including stress relaxation, creep, and hysteresis.160 The viscoelastic properties of hydrogels can be adjusted through various sophisticated strategies.161,162 Natural ECM-based matrices, such as those derived from collagen, fibrin or Matrigel, are known for their significant stress relaxation properties, indicative of their viscoelastic nature,163 but offer limited capacity for independent tunability. In contrast, synthetic dynamic hydrogels are typically engineered through reversible interactions within polymer chains. This encompasses a variety of dynamic chemical cross-linking mechanisms, such as Schiff base reactions, boronate ester formation, and Diels-Alder reactions, as well as physical cross-linking strategies, including host–guest interactions, ionic interactions, and hydrogen bonding.161 Furthermore, with the incorporation of degradation-dependent light-sensitive (o-nitrobenzyl) or enzymatically sensitive groups, the mechanical properties of hydrogels can be modulated with spatiotemporal control. Employing these strategies, researchers can precisely tailor the mechanical and biochemical attributes of hydrogel scaffolds, including their stiffness, force relaxation kinetics, and ligand exposure. This precise control enables the observation and study of cellular responses to these dynamic alterations in the material's properties.
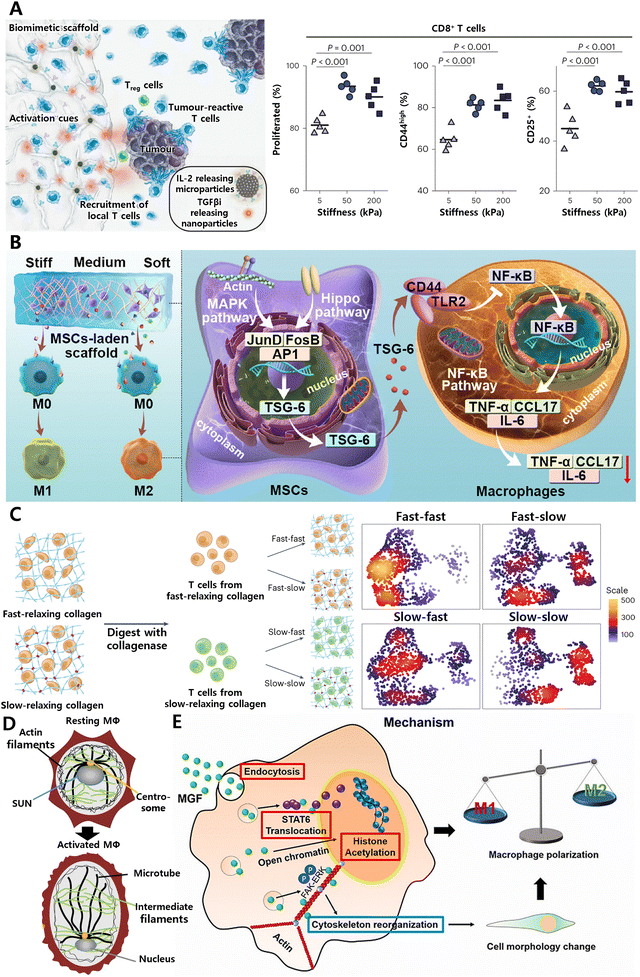 | ||
| Fig. 12 Tissue mechanical cues play crucial roles in determining immune cell behavior. (A) Systemic enhancement of antitumour immunity by peritumourally implanted immunomodulatory microporous scaffolds. T-cell activation was maximized when the mechanical stiffness of microporous hydrogel was ∼50 kPa. Reproduced with permission. Reproduced with permission from ref. 166 Copyright 2022 Springer Nature. (B) Matrix stiffness regulates the immunomodulatory effects of MSCs on macrophages via AP1/TSG-6 signaling pathways. Reproduced with permission from ref. 167 Copyright © 2022 Acta Materialia Inc. (C) Generation of functionally distinct T-cell populations obtained by altering the viscoelasticity of their extracellular matrix. Reproduced with permission from ref. 65 Copyright 2023 Springer Nature. (D) SUN1/2 proteins act as mechano-sensors to remodel the nucleus and chromatin for M1 macrophage polarization. Reproduced with permission from ref. 168 Copyright Springer 2023 Nature. (E) Mechano-growth factor (MGF) directs the transformation of macrophages by promoting STAT6 expression and histone H3 acetylation (AcH3). Reproduced with permission from ref. 169 Copyright 2021 Elsevier B.V. | ||
As cis-regulatory elements, mechanical cues extend beyond the plasma membrane and cytoskeleton, transmitting signals to the nucleus to regulate three-dimensional chromatin organization and gene expression. For example, in response to inflammatory signals, SUN1/2 proteins function as mechano-sensors to remodel the nucleus and chromatin for M1 macrophage polarization (Fig. 12D).168 The mechano-chemical cue, a short-peptide mechano-growth factor (MGF), enhances chromatin accessibility in macrophages by promoting histone H3 acetylation (AcH3), which can prime the macrophages involve the connective tissue injury repair (Fig. 12E).169 This biological mechanism can be harnessed to develop immunomodulatory hydrogels by incorporating MGF or SUN1/2 modulators into the scaffolds. Such hydrogels can actively modulate macrophage behavior and polarization, thereby directing specific immune responses at injury sites to improve repair and regeneration.
In a parallel approach, medical devices that generate mechanical stimulation can be utilized to engineer immune response, thus improving chronic wound healing. Magnetic materials, serving as localized mechanical signal generators, can precisely regulate macrophage polarization.170 Utilizing this principle, Andy Tay171 combined magneto-responsive hydrogels with cells and employed wireless magneto-induced dynamic mechanical stimulation to regulate the biofunctions of encapsulated cells, thereby improving diabetic wound closure (Fig. 13A). The concept of biomaterial-mediated mechanoregulation of cells for therapy may also be exploited for other tissue repair. Another research172 designed a specialized skin stretching device that adjusts strain to recruit macrophages (Fig. 13B). These macrophages undergo a transition to the M2 phenotype and secrete a range of growth factors that promote hair regeneration. Such studies highlight the critical axis of mechanical force-immune response-regeneration and demonstrate how organs initiate regeneration in response to mechanical stimuli.
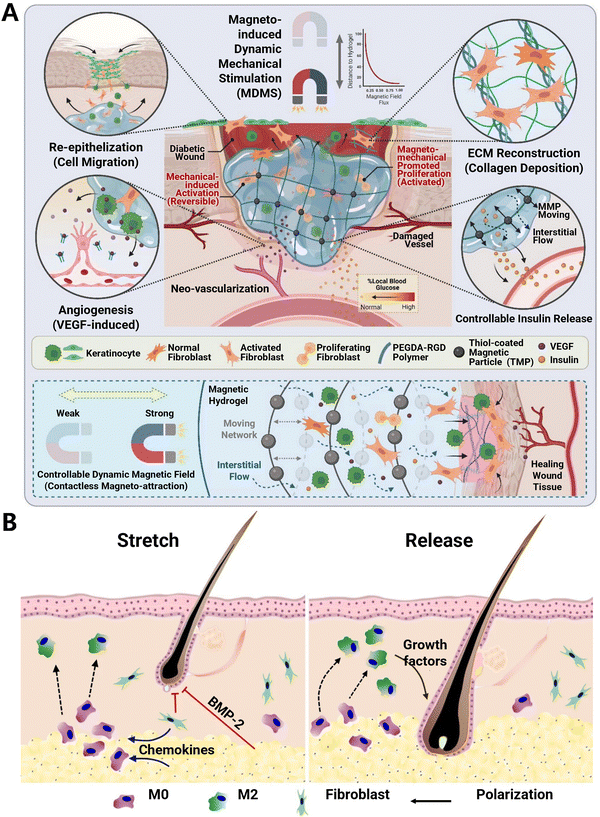 | ||
| Fig. 13 Medical devices that generate mechanical stimulation for enhancing regenerative medicine. (A) Schematic of magneto-responsive hydrogel-activated cell therapy for accelerated diabetic wound healing. Dynamic magnetic fields induce hydrogel deformation to mechanically activate the encapsulated cells. Reproduced with permission. Reproduced with permission from ref. 171 Copyright 2023 Wiley-VCH GmbH. (B) Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Mechanical stretch stimulates chemokine production to recruit macrophages, which undergo M2 polarization to facilitate anagen initiation. Stretch-induced hair regeneration could be halted by clodronate (chemical inhibitor to ablate macrophages) injection. Reproduced with permission from ref. 172 Copyright 2019 Springer Nature. | ||
Today, the important role of mechanical cues in cell signaling, which influence immune cell activation, regulation of biochemical signals, and cis-regulation of immune cell behaviors, is widely acknowledged. The integration of mechanochemical signaling into the development of materials for regenerative medicine and the establishment of more predictive in vitro model systems will provide novel inquiries and valuable insights. These in turn can be used to engineer the next generation of immunomodulatory hydrogels based on mechanobiology.
4. Conclusions and perspectives
Recent studies underscore the substantial potential of biomaterial-based immunoregulatory strategies in treating a variety of chronic inflammation-related diseases. Notably, over 30173 hydrogel-based products have received approval from regulatory agencies, including the FDA, indicating significant advancements in the translation of hydrogel research into clinical applications. The intra-articular administration of synthetic polyacrylamide-based hydrogels for the treatment of osteoarthritis is currently a subject of extensive research. Hyaluronic acid (HA)-based hydrogels have been widely used to promote the healing of various chronic wounds and serve as temporary dermal fillers for soft tissue augmentation. Furthermore, gelatin-based hydrogels are widely employed in the investigation of cardiac and renal diseases owing to their capacity to enhance interactions with natural bio-matrices. These hydrogels are engineered to engage with the immune system by modulating immune cell responses and creating a microenvironment conducive to tissue regeneration. This review outlines the current progress in materials engineering strategies for the rational design of intrinsic immunomodulatory hydrogels as well as the associated mechanisms. However, these design strategies for immunomodulatory hydrogels face significant challenges related to both mechanistic elucidation and clinical translation (Fig. 14).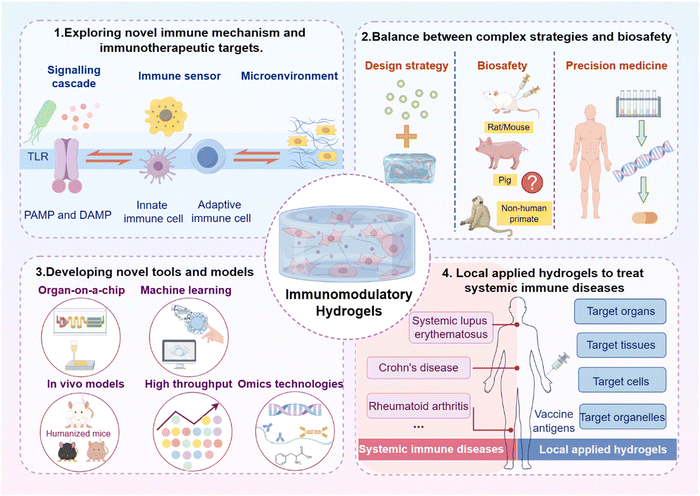 | ||
| Fig. 14 Future directions and challenges for the clinical translation of immunomodulation hydrogels. Image was drawn using FigDraw. | ||
(1) In-depth exploration of the immune mechanism of chronic inflammation and developing novel immunotherapeutic targets
A comprehensive dissection of the immune mechanisms that drive chronic inflammation is essential for the development of innovative immunotherapeutic interventions. This entails a meticulous examination of the complex interplay between immune cells, signaling molecules, and the tissue microenvironment in the context of persistent inflammation. Particular focus on macrophages is essential for elucidating immune-related disorders associated with chronic inflammation. Additionally, the identification of novel therapeutic targets within these pathways can pave the way for the design of more effective immunomodulatory hydrogels.(2) Enhancing tissue regeneration through immunomodulation involves a balance between complex strategies and biosafety
The immune system plays a dual role in tissue repair as it is crucial for clearing damaged cells and guarding against infection; however, overactive or unregulated immune responses hinder the healing process. Immunomodulation, and therefore the necessary kinetics of therapeutic intervention, may fluctuate based on the affected tissue. Further development of immunomodulatory hydrogels based on microenvironment response is a good choice, but it may be costly and require more regulatory approval. Thus, immunoregulatory strategies must be balanced between complex strategies and biosafety. The long-term effects and potential for adverse reactions must be carefully assessed. Additionally, precision medicine approaches, which tailor treatments to individual patient profiles, can help mitigate risks and enhance the safety and efficacy of immunomodulatory therapies.(3) Developing novel tools and models for investigating the inflammatory response to biomaterials
New tools and models are being developed to better understand and predict the inflammatory response to biomaterials. These include in vitro assays using immune-relevant cell lines, 3D cell culture models, and organ-on-a-chip systems that mimic the complexity of the human immune response.49 To determine the impact of the materials on the immune response, several immune cell phenotype can be assessed with machine learning methods already in operation. Future studies are anticipated to employ machine learning models to screen the immune properties of materials with various physical structures, such as chiral structures, to optimize material selection. In vivo models, such as animal studies and humanized mice, are also crucial for translating findings to clinical settings. Advanced imaging techniques and omics technologies (genomics, proteomics, metabolomics) are also instrumental in providing a comprehensive view of the immune response at the molecular level. These tools enable researchers to identify biomarkers of inflammation and to understand how different biomaterials modulate the immune system, guiding the design of immunomodulatory hydrogels.(4) Developing local applied hydrogels to treat autoimmune diseases and systemic degenerative diseases
There are no targeted effects from most immunomodulatory hydrogels; instead, they exert their effects in a non-specific, regional manner, thus modulating immunity without the ability to hone in on specific pathological sites. The clinical symptoms of some autoimmune disorders, such Crohn's disease and systemic lupus erythematosus, can be localized or systemic. For enhanced immunomodulatory effects, hydrogel materials that are capable of targeting diseased tissues are necessary.174 Future developments in targeted therapeutic hydrogels are anticipated to tackle various immune diseases by targeting different tissues, including the gut and nerves, targeting cells, such as macrophages and t-cells, and targeting organelles, involving mitochondria and the endoplasmic reticulum to achieve the accurate therapeutic function.In brief, immunomodulatory hydrogels have great promise in both basic research and clinical translation. In this review, we focus on the intrinsic immunomodulatory properties of hydrogels. Certainly, the design strategies should not be limited to the biological properties and functions summarized in this review. Although many processes are still long and challenging, we believe that immunomodulatory hydrogels are highly significant and valuable for further extensive and in-depth explorations for chronic disease theranostics in the future.
Data availability
No primary research results, software or code has been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Y. Q., J. D. and R. Z. contributed equally to this work. This work was financially supported by the National Natural Science Foundation of China (32371387, 32101066), Zhejiang Provincial Natural Science Foundation (LBZ24H110001), Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholar (LR23C100001), Wenzhou Institute, University of Chinese Academy of Science (WIUCASQD2021047), National Research Foundation of Korea (CRI project No. 2018R1A3B1052702 for J.S.K; 2022R1C1C2007637 for S.K.).References
- D. Furman, J. Campisi, E. Verdin, P. Carrera-Bastos, S. Targ, C. Franceschi, L. Ferrucci, D. W. Gilroy, A. Fasano, G. W. Miller, A. H. Miller, A. Mantovani, C. M. Weyand, N. Barzilai, J. J. Goronzy, T. A. Rando, R. B. Effros, A. Lucia, N. Kleinstreuer and G. M. Slavich, Nat. Med., 2019, 25, 1822–1832 CrossRef CAS PubMed
.
- G. C. O. D. Collaborators, Lancet, 2018, 392, 1736–1788 CrossRef
.
- H. Zhao, L. Wu, G. Yan, Y. Chen, M. Zhou, Y. Wu and Y. Li, Signal Transduction Targeted Ther., 2021, 6, 263 CrossRef CAS
.
- A. W. Armstrong, C. T. Harskamp and E. J. Armstrong, JAMA Dermatol., 2013, 149, 84–91 CrossRef PubMed
.
- M. D. Rosenblum, I. K. Gratz, J. S. Paw and A. K. Abbas, Sci. Transl. Med., 2012, 4, 125sr121 Search PubMed
.
- M. O. Dellacherie, B. R. Seo and D. J. Mooney, Nat. Rev. Mater., 2019, 4, 379–397 CrossRef
.
- X. B. Zhang, B. W. Yang, Q. Q. Ni and X. Y. Chen, Chem. Soc. Rev., 2023, 52, 2886–2910 RSC
.
- X. B. Ma, S. J. Li, Y. T. Liu, T. Zhang, P. Xue, Y. J. Kang, Z. J. Sun and Z. G. Xu, Chem. Soc. Rev., 2022, 51, 5136–5174 RSC
.
- Z. X. Tu, Y. L. Zhong, H. Z. Hu, D. Shao, R. N. Haag, M. Schirner, J. Lee, B. Sullenger and K. W. Leong, Nat. Rev. Mater., 2022, 7, 557–574 CrossRef CAS
.
- P. Mehta, M. Sharma and M. Devi, J. Mech. Behav. Biomed. Mater., 2023, 147, 106145 CrossRef CAS
.
- Y. Xiong, Q. Feng, L. Lu, K. K. Zha, T. Yu, Z. Lin, Y. Q. Hu, A. C. Panayi, V. Nosrati-Ziahmagi, X. Y. Chu, L. Chen, M. A. Shahbazi, B. B. Mi and G. H. Liu, Adv. Funct. Mater., 2023, 33, 000906948500001 Search PubMed
.
- Z. Zhang, C. L. He and X. S. Chen, Adv. Mater., 2024, 36, 001109915200001 Search PubMed
.
- R. Whitaker, B. Hernaez-Estrada, R. M. Hernandez, E. Santos-Vizcaino and K. L. Spiller, Chem. Rev., 2021, 121, 11305–11335 CrossRef CAS
.
- Z. Zhang, C. He and X. Chen, Adv. Mater., 2024, 36, e2308894 CrossRef PubMed
.
- X. Qi, E. Cai, Y. Xiang, C. Zhang, X. Ge, J. Wang, Y. Lan, H. Xu, R. Hu and J. Shen, Adv. Mater., 2023, 35, e2306632 CrossRef
.
- M. Kharaziha, A. Baidya and N. Annabi, Adv. Mater., 2021, 33, e2100176 CrossRef
.
- M. E. Kotas and R. Medzhitov, Cell, 2015, 160, 816–827 CrossRef CAS
.
- M. G. Netea, F. Balkwill, M. Chonchol, F. Cominelli, M. Y. Donath, E. J. Giamarellos-Bourboulis, D. Golenbock, M. S. Gresnigt, M. T. Heneka, H. M. Hoffman, R. Hotchkiss, L. A. B. Joosten, D. L. Kastner, M. Korte, E. Latz, P. Libby, T. Mandrup-Poulsen, A. Mantovani, K. H. G. Mills, K. L. Nowak, L. A. O'Neill, P. Pickkers, T. van der Poll, P. M. Ridker, J. Schalkwijk, D. A. Schwartz, B. Siegmund, C. J. Steer, H. Tilg, J. W. M. van der Meer, F. L. van de Veerdonk and C. A. Dinarello, Nat. Immun., 2017, 18, 826–831 CrossRef CAS
.
- J. N. Fullerton and D. W. Gilroy, Nat. Rev. Drug Discovery, 2016, 15, 551–567 CrossRef CAS PubMed
.
- D. L. Tang, R. Kang, C. B. Coyne, H. J. Zeh and M. T. Lotze, Immunol. Rev., 2012, 249, 158–175 CrossRef CAS PubMed
.
- A. Mantovani, C. A. Dinarello, M. Molgora and C. Garlanda, Immunity, 2019, 50, 778–795 CrossRef CAS PubMed
.
- C. D. Buckley, D. W. Gilroy, C. N. Serhan, B. Stockinger and P. P. Tak, Nat. Rev. Immunol., 2013, 13, 59–66 CrossRef CAS
.
- L. Ferrucci and E. Fabbri, Nat. Rev. Cardiol., 2018, 15, 505–522 CrossRef CAS PubMed
.
- B. K. Kennedy, S. L. Berger, A. Brunet, J. Campisi, A. M. Cuervo, E. S. Epel, C. Franceschi, G. J. Lithgow, R. I. Morimoto, J. E. Pessin, T. A. Rando, A. Richardson, E. E. Schadt, T. Wyss-Coray and F. Sierra, Cell, 2014, 159, 708–712 CrossRef PubMed
.
- S. Akira, S. Uematsu and O. Takeuchi, Cell, 2006, 124, 783–801 CrossRef CAS PubMed
.
- N. Yatim, S. Cullen and M. L. Albert, Nat. Rev. Immunol., 2017, 17, 262–275 CrossRef CAS
.
- T. Gong, L. Liu, W. Jiang and R. Zhou, Nat. Rev. Immunol., 2020, 20, 95–112 CrossRef CAS
.
- T. Kawai and S. Akira, Nat. Immun., 2010, 11, 373–384 CrossRef CAS
.
- G. Fredman and C. N. Serhan, Nat. Rev. Cardiol., 2024, 21, 808–823 CrossRef PubMed
.
- M. F. Neurath, Nat. Immun., 2019, 20, 970–979 CrossRef CAS
.
- O. Soehnlein, S. Steffens, A. Hidalgo and C. Weber, Nat. Rev. Immunol., 2017, 17, 248–261 CrossRef CAS PubMed
.
- B. McDonald and P. Kubes, Gastroenterology, 2016, 151, 1087–1095 CrossRef CAS PubMed
.
- Y. Zhao, W. Zou, J. Du and Y. Zhao, J. Cell. Physiol., 2018, 233, 6425–6439 CrossRef CAS PubMed
.
- S. A. Eming, T. A. Wynn and P. Martin, Science, 2017, 356, 1026–1030 CrossRef CAS
.
- T. Lazarov, S. Juarez-Carreño, N. Cox and F. Geissmann, Nature, 2023, 618, 698–707 CrossRef CAS
.
- Y. Oishi and I. Manabe, Int. Immunol., 2018, 30, 511–528 CrossRef CAS PubMed
.
- M. G. Netea, J. Domínguez-Andrés, L. B. Barreiro, T. Chavakis, M. Divangahi, E. Fuchs, L. A. B. Joosten, J. W. M. van der Meer, M. M. Mhlanga, W. J. M. Mulder, N. P. Riksen, A. Schlitzer, J. L. Schultze, C. Stabell Benn, J. C. Sun, R. J. Xavier and E. Latz, Nat. Rev. Immunol., 2020, 20, 375–388 CrossRef CAS PubMed
.
- B. Priem, M. M. T. van Leent, A. J. P. Teunissen, A. M. Sofias, V. P. Mourits, L. Willemsen, E. D. Klein, R. S. Oosterwijk, A. E. Meerwaldt, J. Munitz, G. Prévot, A. Vera Verschuur, S. A. Nauta, E. M. van Leeuwen, E. L. Fisher, K. A. M. de Jong, Y. Zhao, Y. C. Toner, G. Soultanidis, C. Calcagno, P. H. H. Bomans, H. Friedrich, N. Sommerdijk, T. Reiner, R. Duivenvoorden, E. Zupančič, J. S. Di Martino, E. Kluza, M. Rashidian, H. L. Ploegh, R. M. Dijkhuizen, S. Hak, C. Pérez-Medina, J. J. Bravo-Cordero, M. P. J. de Winther, L. A. B. Joosten, A. van Elsas, Z. A. Fayad, A. Rialdi, D. Torre, E. Guccione, J. Ochando, M. G. Netea, A. W. Griffioen and W. J. M. Mulder, Cell, 2020, 183, 786–801 CrossRef CAS PubMed
.
- D. Burzyn, W. Kuswanto, D. Kolodin, J. L. Shadrach, M. Cerletti, Y. Jang, E. Sefik, T. G. Tan, A. J. Wagers, C. Benoist and D. Mathis, Cell, 2013, 155, 1282–1295 CrossRef CAS PubMed
.
- R. L. Gieseck, 3rd, M. S. Wilson and T. A. Wynn, Nat. Rev. Immunol., 2018, 18, 62–76 CrossRef PubMed
.
- T. Kurosaki, K. Kometani and W. Ise, Nat. Rev. Immunol., 2015, 15, 149–159 CrossRef CAS
.
- J. Jung, H. Zeng and T. Horng, Nat. Cell Biol., 2019, 21, 85–93 CrossRef CAS
.
- M. Certo, C. H. Tsai, V. Pucino, P. C. Ho and C. Mauro, Nat. Rev. Immunol., 2021, 21, 151–161 CrossRef CAS PubMed
.
- V. Falanga, R. R. Isseroff, A. M. Soulika, M. Romanelli, D. Margolis, S. Kapp, M. Granick and K. Harding, Nat. Rev. Dis. Primers., 2022, 8, 50 CrossRef PubMed
.
- M. Bäck, A. Yurdagul, Jr., I. Tabas, K. Oorni and P. T. Kovanen, Nat. Rev. Cardiol., 2019, 16, 389–406 Search PubMed
.
- C. Oschatz, C. Maas, B. Lecher, T. Jansen, J. Björkqvist, T. Tradler, R. Sedlmeier, P. Burfeind, S. Cichon, S. Hammerschmidt, W. Müller-Esterl, W. A. Wuillemin, G. Nilsson and T. Renné, Immunity, 2011, 34, 258–268 CrossRef CAS
.
- K. Chen, Z. Bao, P. Tang, W. Gong, T. Yoshimura and J. M. Wang, Cell. Mol. Immunol., 2018, 15, 324–334 CrossRef CAS
.
- P. Lacy, Front. Immunol., 2015, 6, 190 Search PubMed
.
- E. A. Gosselin, H. B. Eppler, J. S. Bromberg and C. M. Jewell, Nat. Mater., 2018, 17, 484–498 CrossRef CAS PubMed
.
- M. Karin, Nature, 2006, 441, 431–436 CrossRef CAS
.
- S. C. Jordan, J. Choi, I. Kim, G. Wu, M. Toyoda, B. Shin and A. Vo, Transplantation, 2017, 101, 32–44 CrossRef CAS PubMed
.
- Y. Zayani, N. El Golli, W. Zidi, I. Guizani, S. Boussairi, S. Aloui, I. Ayadi, B. Ftouhi, M. Feki, N. Ben Romdhane, H. Ben Sliman, M. Allal-Elasmi and N. Kaabachi, Cytokine, 2016, 86, 47–52 CrossRef CAS PubMed
.
- J. Tejero, S. Shiva and M. T. Gladwin, Physiol. Res., 2019, 99, 311–379 CAS
.
- C. N. Serhan and N. Chiang, Rheum. Dis. Clin. North Am., 2004, 30, 69–95 CrossRef PubMed
.
- C. N. Serhan and B. D. Levy, J. Clin. Invest., 2018, 128, 2657–2669 CrossRef
.
- H. Du, J. M. Bartleson, S. Butenko, V. Alonso, W. F. Liu, D. A. Winer and M. J. Butte, Nat. Rev. Immunol., 2023, 23, 174–188 CrossRef CAS
.
- V. Vogel, Annu. Rev. Physiol., 2018, 80, 353–387 CrossRef CAS
.
- T. K. Hsiai, S. K. Cho, P. K. Wong, M. Ing, A. Salazar, A. Sevanian, M. Navab, L. L. Demer and C. M. Ho, FASEB J., 2003, 17, 1648–1657 CrossRef CAS
.
- V. Mittelheisser, V. Gensbittel, L. Bonati, W. Li, L. Tang and J. G. Goetz, Nat. Nanotechnol., 2024, 19, 281–297 CrossRef CAS PubMed
.
- V. S. Meli, P. K. Veerasubramanian, T. L. Downing, W. Wang and W. F. Liu, Sci. Signaling, 2023, 16, eadc9656 CrossRef CAS PubMed
.
- S. Feske, H. Wulff and E. Y. Skolnik, Annu. Rev. Immunol., 2015, 33, 291–353 CrossRef CAS PubMed
.
- X. Zhou, W. Li, S. Wang, P. Zhang, Q. Wang, J. Xiao, C. Zhang, X. Zheng, X. Xu, S. Xue, L. Hui, H. Ji, B. Wei and H. Wang, Cell Rep., 2019, 27, 1176–1189 CrossRef CAS
.
- A. G. Solis, P. Bielecki, H. R. Steach, L. Sharma, C. C. Harman, S. Yun, M. R. de Zoete, J. N. Warnock, S. F. To, A. G. York and M. Mack, Nature, 2019, 573, 69–74 CrossRef CAS
.
- L. Dong, Y. Song, Y. Zhang, W. Zhao, C. Wang, H. Lin, M. K. Al-Ani, W. Liu, R. Xue and L. Yang, J. Cell. Physiol., 2021, 236, 6376–6390 CrossRef CAS PubMed
.
- K. Adu-Berchie, Y. Liu, D. K. Y. Zhang, B. R. Freedman, J. M. Brockman, K. H. Vining, B. A. Nerger, A. Garmilla and D. J. Mooney, Nat. Biomed. Eng., 2023, 7, 1374–1391 CrossRef CAS
.
- E. M. Moore, D. R. Maestas, Jr., C. C. Cherry, J. A. Garcia, H. Y. Comeau, L. Davenport Huyer, S. H. Kelly, A. N. Peña, R. L. Blosser, G. D. Rosson and J. H. Elisseeff, Sci. Adv., 2021, 7, eabj5830 CrossRef CAS PubMed
.
- P. F. Chen, K. F. Pan, N. Song, Y. Yang, C. H. Gu, P. Y. Zhong, L. Li, M. B. Li, Y. Zhang, Z. Q. Dai, L. Q. Shangguan, C. Y. Lei, Z. M. Liu, J. F. Zhang, R. K. Tang, C. Liu, S. W. Fan and X. F. Lin, Matter, 2023, 6, 397–428 CrossRef CAS
.
- Y. Gu, M. E. Distler, H. F. Cheng, C. Huang and C. A. Mirkin, J. Am. Chem. Soc., 2021, 143, 17200–17208 CrossRef CAS PubMed
.
- L. Wang, Y. Luo, Y. Song, X. He, T. Xu and X. Zhang, ACS Nano, 2024, 18, 3468–3479 CrossRef CAS PubMed
.
- Y. B. Jia, J. T. Feng, Z. Feng, J. Y. Liu, Y. S. Yang, X. K. Li, M. Lei, H. Guo, Z. Wei, Y. Lv and F. Xu, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2219024120 CrossRef CAS
.
- Y. He, Q. Li, P. Chen, Q. Duan, J. Zhan, X. Cai, L. Wang, H. Hou and X. Qiu, Nat. Commun., 2022, 13, 7666 CrossRef CAS PubMed
.
- W. F. Lin, M. Kluzek, N. Iuster, E. Shimoni, N. Kampf, R. Goldberg and J. Klein, Science, 2020, 370, 335 CrossRef CAS
.
- B. Dai, K. Li, L. X. Shi, X. Z. Wan, X. Liu, F. L. Zhang, L. Jiang and S. T. Wang, Adv. Mater., 2019, 31, 1904113 CrossRef CAS
.
- F. Bao, G. Pei, Z. Wu, H. Zhuang, Z. Zhang, Z. Huan, C. Wu and J. Chang, Adv. Funct. Mater., 2020, 30, 2005422 CrossRef CAS
.
- J. M. Yang, X. Jin, W. G. Liu and W. Wang, Adv. Mater., 2023, 35, 001107092300001 Search PubMed
.
- J. Ouyang, B. Deng, B. Zou, Y. Li, Q. Bu, Y. Tian, M. Chen, W. Chen, N. Kong, T. Chen and W. Tao, J. Am. Chem. Soc., 2023, 145, 12193–12205 CrossRef CAS
.
- Z. Y. Guo, Y. Bai, Z. Z. Zhang, H. Mei, J. Li, Y. J. Pu, N. Zhao, W. X. Gao, F. Wu, B. He and J. Xie, J. Mater. Chem. B, 2021, 9, 3874–3884 RSC
.
- X. Jiang, T. Feng, B. An, S. Ren, J. Meng, K. Li, S. Liu, H. Wu, H. Zhang and C. Zhong, Adv. Mater., 2022, 34, e2201411 CrossRef PubMed
.
- X. Mei, J. L. Li, Z. Z. Wang, D. S. Zhu, K. Huang, S. Q. Hu, K. D. Popowski and K. Cheng, Nat. Mater., 2023, 22, 903 CAS
.
- J. Ouyang, X. Ji, X. Zhang, C. Feng, Z. Tang, N. Kong, A. Xie, J. Wang, X. Sui, L. Deng, Y. Liu, J. S. Kim, Y. Cao and W. Tao, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 28667–28677 CrossRef CAS PubMed
.
- J. Park, T. Y. Kim, Y. Kim, S. An, K. S. Kim, M. Kang, S. A. Kim, J. Kim, J. Lee, S. W. Cho and J. Seo, Adv. Sci., 2023, 10, 2303651 CrossRef CAS
.
- R. J. Jiang, L. W. Hao, L. J. Song, L. M. Tian, Y. Fan, J. Zhao, C. Z. Liu, W. H. Ming and L. Q. Ren, Chem. Eng. J., 2020, 398, 125609 CrossRef CAS
.
- D. Chan, J. C. Chien, E. Axpe, L. Blankemeier, S. W. Baker, S. Swaminathan, V. A. Piunova, D. Y. Zubarev, C. L. Maikawa, A. K. Grosskopf, J. L. Mann, H. T. Soh and E. A. Appel, Adv. Mater., 2022, 34, e2109764 CrossRef PubMed
.
- Z. Hao, X. Li, R. Zhang and L. Zhang, Adv. Healthcare Mater., 2024, e2400513, DOI:10.1002/adhm.202400513
.
- W. Li, E. S. Thian, M. Wang, Z. Wang and L. Ren, Adv. Sci., 2021, 8, e2100368 CrossRef
.
- D. P. Linklater, V. A. Baulin, S. Juodkazis, R. J. Crawford, P. Stoodley and E. P. Ivanova, Nat. Rev. Microbiol., 2021, 19, 8–22 CrossRef CAS
.
- W. Y. Chen and D. L. Li, Front. Chem., 2020, 8, 732 CrossRef CAS
.
- H. Sies, Redox Biol., 2015, 4, 180–183 CrossRef CAS PubMed
.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119, 4881–4985 CrossRef CAS PubMed
.
- F. Zhang, Y. Kang, L. Feng, G. Xi, W. Chen, N. Kong, W. Tao, T. Luan, S. Koo and X. Ji, Mater. Horiz., 2023, 10, 5474–5483 RSC
.
- Y. Qian, Y. Zheng, J. Jin, X. Wu, K. Xu, M. Dai, Q. Niu, H. Zheng, X. He and J. Shen, Adv. Mater., 2022, 34, e2200521 CrossRef PubMed
.
- J. H. Zhang, Y. Fu, P. Yang, X. H. Liu, Y. W. Li and Z. P. Gu, Adv. Mater. Interfaces, 2020, 7, 2000632 CrossRef CAS
.
- Y. Yang, X. Zhao, S. Wang, Y. Zhang, A. Yang, Y. Cheng and X. Chen, Nat. Commun., 2023, 14, 7771 CrossRef CAS PubMed
.
- Y. A. Qian, K. J. Xu, L. L. Shen, M. L. Dai, Z. L. Zhao, Y. J. Zheng, H. O. Wang, H. L. Xie, X. Wu, D. C. Xiao, Q. X. Zheng, J. Y. Zhang, Y. Song, J. L. Shen and W. Chen, Adv. Funct. Mater., 2023, 33, 001042347600001 Search PubMed
.
- Y. P. Liang, J. H. He and B. L. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed
.
- T. L. Wu, C. Y. Cui, C. C. Fan, Z. Y. Xu, Y. Liu and W. G. Liu, Bioact. Mater., 2021, 6, 2820–2828 CAS
.
- D. L. Shi, A. R. Sheng and L. L. Chi, Front. Mol. Biosci., 2021, 8, 639666 CrossRef CAS
.
- L. Schirmer, P. Atallah, U. Freudenberg and C. Werner, Adv. Sci., 2021, 8, e2100293 CrossRef
.
- I. Capila and R. J. Linhardt, Angew. Chem., Int. Ed., 2002, 41, 391–412 CrossRef PubMed
.
- N. Lohmann, L. Schirmer, P. Atallah, E. Wandel, R. A. Ferrer, C. Werner, J. C. Simon, S. Franz and U. Freudenberg, Sci. Transl. Med., 2017, 9, 28424334 Search PubMed
.
- L. Schirmer, P. Atallah, U. Freudenberg and C. Werner, Adv. Sci., 2021, 8, e2100293 CrossRef
.
- X. Zhang, J. Feng, W. Feng, B. Xu, K. Zhang, G. Ma, Y. Li, M. Yang and F. J. Xu, ACS Appl. Mater. Interfaces, 2022, 14, 31737–31750 CrossRef CAS
.
- T. Deng, D. Gao, X. Song, Z. Zhou, L. Zhou, M. Tao, Z. Jiang, L. Yang, L. Luo, A. Zhou, L. Hu, H. Qin and M. Wu, Nat. Commun., 2023, 14, 396 CrossRef CAS PubMed
.
- J. L. Dziki, D. S. Wang, C. Pineda, B. M. Sicari, T. Rausch and S. F. Badylak, J. Biomed. Mater. Res. A, 2017, 105, 138–147 CrossRef CAS PubMed
.
- R. M. Wang, T. D. Johnson, J. He, Z. Rong, M. Wong, V. Nigam, A. Behfar, Y. Xu and K. L. Christman, Biomaterials, 2017, 129, 98–110 CrossRef CAS
.
- H. Q. Nguyen, C. Y. Kao, C. P. Chiang, Y. H. Hung and C. M. Lo, Gels, 2022, 8, 828424 Search PubMed
.
- E. W. Ozpinar, A. L. Frey, G. K. Arthur, C. Mora-Navarro, A. Biehl, D. B. Snider, G. Cruse and D. O. Freytes, Tissue Eng., Part A, 2021, 27, 1008–1022 CrossRef CAS
.
- J. M. Fishman, M. W. Lowdell, L. Urbani, T. Ansari, A. J. Burns, M. Turmaine, J. North, P. Sibbons, A. M. Seifalian, K. J. Wood, M. A. Birchall and P. De Coppi, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 14360–14365 CrossRef CAS PubMed
.
- K. Sadtler, K. Estrellas, B. W. Allen, M. T. Wolf, H. Fan, A. J. Tam, C. H. Patel, B. S. Luber, H. Wang, K. R. Wagner, J. D. Powell, F. Housseau, D. M. Pardoll and J. H. Elisseeff, Science, 2016, 352, 366–370 CrossRef CAS PubMed
.
- M. Dubus, L. Scomazzon, J. Chevrier, A. Montanede, A. Baldit, C. Terryn, F. Quilès, C. Thomachot-Schneider, S. C. Gangloff, N. Bouland, F. Gindraux, H. Rammal, C. Mauprivez and H. Kerdjoudj, Front. Bioeng. Biotechnol., 2022, 10, 828424 CrossRef CAS PubMed
.
- F. W. Meng, P. F. Slivka, C. L. Dearth and S. F. Badylak, Biomaterials, 2015, 46, 131–140 CrossRef CAS PubMed
.
- J. Zheng, R. Fan, H. Wu, H. Yao, Y. Yan, J. Liu, L. Ran, Z. Sun, L. Yi, L. Dang, P. Gan, P. Zheng, T. Yang, Y. Zhang, T. Tang and Y. Wang, Nat. Commun., 2019, 10, 1604 CrossRef CAS
.
- M. Sun, S. Peng, C. Zhao, J. Huang, J. Xia, D. Ye, X. Dou, W. Hou and C. Feng, Adv. Funct. Mater., 2022, 32, 2204291 CrossRef CAS
.
- J. R. Wang, X. Liu, Y. P. Wang, Y. S. Zhang, R. Gao, Y. Gao, P. X. Kong, Y. N. Huangfu, C. N. Zhang, Z. J. Feng, P. S. Huang, P. X. Yang and W. W. Wang, Adv. Funct. Mater., 2024, 34, 001134841000001 Search PubMed
.
- R. J. Lebbink, N. Raynal, T. de Ruiter, D. G. Bihan, R. W. Farndale and L. Meyaard, Matrix Biol., 2009, 28, 202–210 CrossRef CAS PubMed
.
- Y. K. Kim, S. H. Chu, J. Y. Hsieh, C. M. Kamoku, A. J. Tenner, W. F. Liu and S. W. Wang, Adv. Healthcare Mater., 2017, 6, 29083540 Search PubMed
.
- N. Guo, K. Zhang, X. Gao, M. Lv, J. Luan, Z. Hu, A. Li and X. Gou, Curr. Res. Transl. Med., 2020, 68, 119–124 Search PubMed
.
- A. Purwada and A. Singh, Nat. Protoc., 2017, 12, 168–182 CrossRef CAS PubMed
.
- J. E. Rayahin, J. S. Buhrman, Y. Zhang, T. J. Koh and R. A. Gemeinhart, ACS Biomater. Sci. Eng., 2015, 1, 481–493 CrossRef CAS PubMed
.
- A. Schnell, D. R. Littman and V. K. Kuchroo, Nat. Immun., 2023, 24, 19–29 CrossRef CAS PubMed
.
- S. S. Soni and C. B. Rodell, Acta Biomater., 2021, 133, 139–152 CrossRef CAS
.
- B. G. Keselowsky and J. S. Lewis, Semin. Immunol., 2017, 29, 33–40 CrossRef CAS PubMed
.
- A. Slezak, K. Chang, S. Hossainy, A. Mansurov, S. J. Rowan, J. A. Hubbell and M. O. Guler, Chem. Soc. Rev., 2024, 53, 1789–1822 RSC
.
- K. Sadtler, M. T. Wolf, S. Ganguly, C. A. Moad, L. Chung, S. Majumdar, F. Housseau, D. M. Pardoll and J. H. Elisseeff, Biomaterials, 2019, 192, 405–415 CrossRef CAS PubMed
.
- T. R. Kyriakides, H.-J. Kim, C. Zheng, L. Harkins, W. Tao and E. Deschenes, Biomed. Mater., 2022, 17, 022007 CrossRef CAS PubMed
.
- J. C. Doloff, O. Veiseh, A. J. Vegas, H. H. Tam, S. Farah, M. Ma, J. Li, A. Bader, A. Chiu, A. Sadraei, S. Aresta-Dasilva, M. Griffin, S. Jhunjhunwala, M. Webber, S. Siebert, K. Tang, M. Chen, E. Langan, N. Dholokia, R. Thakrar, M. Qi, J. Oberholzer, D. L. Greiner, R. Langer and D. G. Anderson, Nat. Mater., 2017, 16, 671–680 CrossRef CAS PubMed
.
- F. A. Sharp, D. Ruane, B. Claass, E. Creagh, J. Harris, P. Malyala, M. Singh, D. T. O'Hagan, V. Pétrilli, J. Tschopp, L. A. J. O’Neill and E. C. Lavelle, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 870–875 CrossRef CAS PubMed
.
- J. Majumder, E. E. Torr, E. A. Aisenbrey, C. S. Lebakken, P. F. Favreau, W. D. Richards, Y. Yin, Q. Chang and W. L. Murphy, J. Tissue Eng., 2024, 15, 20417314241230633 CrossRef PubMed
.
- J. Park and J. E. Babensee, Acta Biomater., 2012, 8, 3606–3617 CrossRef CAS PubMed
.
- A. K. Grosskopf, L. Labanieh, D. D. Klysz, G. A. Roth, P. Xu, O. Adebowale, E. C. Gale, C. K. Jons, J. H. Klich, J. Yan, C. L. Maikawa, S. Correa, B. S. Ou, A. I. d'Aquino, J. R. Cochran, O. Chaudhuri, C. L. Mackall and E. A. Appel, Sci. Adv., 2022, 8, eabn8264 CrossRef CAS PubMed
.
- C. Zhu, L. Ke, X. Ao, Y. Chen, H. Cheng, H. Xin, X. Xu, X. J. Loh, Z. Li, H. Lyu, Q. Wang, D. Zhang, Y. Ping, C. Wu and Y. L. Wu, Adv. Mater., 2024, 36, e2310078 CrossRef PubMed
.
- X. Li, N. Gong, F. Tian, S. Zhang, Y. Zhang, Y. Wang, G. Qing, Y. Wang, F. Li, Y. Xu, L. Zhang, J. Wang, Q. Ni, Y. Gan, C. Gu, H. Jiang, X. Huang, X. Shi, T. Zhang, Y. Wu and X. J. Liang, Nat. Biomed. Eng., 2023, 7, 1129–1141 CrossRef CAS PubMed
.
- Y. Yi, M. Yu, C. Feng, H. Hao, W. Zeng, C. Lin, H. Chen, F. Lv, D. Zhu, X. Ji, L. Mei, M. Wu and W. Tao, Matter, 2022, 5, 2285–2305 CrossRef CAS
.
- J. Ouyang, A. Xie, J. Zhou, R. Liu, L. Wang, H. Liu, N. Kong and W. Tao, Chem. Soc. Rev., 2022, 51, 4996–5041 RSC
.
- C. Feng, J. Ouyang, Z. Tang, N. Kong, Y. Liu, L. Fu, X. Ji, T. Xie, O. C. Farokhzad and W. Tao, Matter, 2020, 3, 127–144 CrossRef
.
- D. Wang, C. Feng, Z. Xiao, C. Huang, Z. Chen, W. Fang, X. Ma, X. Wang, L. Luo, K. Hu and W. Tao, Nano Today, 2022, 47, 101673 CrossRef CAS
.
- H. M. Rostam, S. Singh, N. E. Vrana, M. R. Alexander and A. M. Ghaemmaghami, Biomater. Sci., 2015, 3, 424–441 RSC
.
- Q. Li, B. E. Uygun, S. Geerts, S. Ozer, M. Scalf, S. E. Gilpin, H. C. Ott, M. L. Yarmush, L. M. Smith, N. V. Welham and B. L. Frey, Biomaterials, 2016, 75, 37–46 CrossRef CAS PubMed
.
- E. A. Calle, R. C. Hill, K. L. Leiby, A. V. Le, A. L. Gard, J. A. Madri, K. C. Hansen and L. E. Niklason, Acta Biomater., 2016, 46, 91–100 CrossRef CAS PubMed
.
- W. Han, N. K. Singh, J. J. Kim, H. Kim, B. S. Kim, J. Y. Park, J. Jang and D. W. Cho, Biomaterials, 2019, 224, 119496 CrossRef CAS PubMed
.
- H. M. Rostam, L. E. Fisher, A. L. Hook, L. Burroughs, J. C. Luckett, G. P. Figueredo, C. Mbadugha, A. C. K. Teo, A. Latif, L. Kämmerling, M. Day, K. Lawler, D. Barrett, S. Elsheikh, M. Ilyas, D. A. Winkler, M. R. Alexander and A. M. Ghaemmaghami, Matter, 2020, 2, 1564–1581 CrossRef
.
- H. M. Rostam, P. M. Reynolds, M. R. Alexander, N. Gadegaard and A. M. Ghaemmaghami, Sci. Rep., 2017, 7, 3521 CrossRef PubMed
.
- T. C. Le, M. Penna, D. A. Winkler and I. Yarovsky, Sci. Rep., 2019, 9, 265 CrossRef PubMed
.
- A. E. Widener, A. Roberts and E. A. Phelps, Adv. Healthcare Mater., 2023, e2303005, DOI:10.1002/adhm.202303005
.
- Y. Liu, A. Suarez-Arnedo, L. Riley, T. Miley, J. Xia and T. Segura, Adv. Healthcare Mater., 2023, 12, e2300823 CrossRef PubMed
.
- J. M. Lowen, G. C. Bond, K. H. Griffin, N. K. Shimamoto, V. L. Thai and J. K. Leach, Adv. Healthcare Mater., 2023, 12, e2202239 CrossRef PubMed
.
- D. R. Griffin, M. M. Archang, C. H. Kuan, W. M. Weaver, J. S. Weinstein, A. C. Feng, A. Ruccia, E. Sideris, V. Ragkousis, J. Koh, M. V. Plikus, D. Di Carlo, T. Segura and P. O. Scumpia, Nat. Mater., 2020, 20, 560–569 CrossRef PubMed
.
- J. Ouyang, W. Chen and W. Tao, Matter, 2022, 5, 2471–2473 CrossRef CAS
.
- U. Blache, E. M. Ford, B. Ha, L. Rijns, O. Chaudhuri, P. Y. W. Dankers, A. M. Kloxin, J. G. Snedeker and E. Gentleman, Nat. Rev. Methods Primers, 2022, 2, 98 CrossRef CAS PubMed
.
- J. Xia, Z. Y. Liu, Z. Y. Han, Y. Yuan, Y. Shao, X. Q. Feng and D. A. Weitz, Acta Biomater., 2022, 141, 178–189 CrossRef CAS PubMed
.
- L. J. Pruett, C. H. Jenkins, N. S. Singh, K. J. Catallo and D. R. Griffin, Adv. Funct. Mater., 2021, 31, 2104337 CrossRef CAS
.
- P. T. Coburn, A. C. Herbay, M. Berrini and N. Y. K. Li-Jessen, J. Biomed. Mater. Res. A, 2021, 109, 1337–1352 CrossRef CAS PubMed
.
- S. Ansari, C. Chen, M. M. Hasani-Sadrabadi, B. Yu, H. H. Zadeh, B. M. Wu and A. Moshaverinia, Acta Biomater., 2017, 60, 181–189 CrossRef CAS PubMed
.
- X. Wang, C. Cui, X. Meng, C. Han, B. Wu, X. Dou, C. Zhao, Y. Zhang, K. Li and C. Feng, Adv. Sci., 2023, 11, 2303495 CrossRef
.
- M. Mukhopadhyay, Nat. Med., 2023, 20, 35 CAS
.
- U. Blache, E. M. Ford, B. Ha, L. Rijns, O. Chaudhuri, P. Y. W. Dankers, A. M. Kloxin, J. G. Snedeker and E. Gentleman, Nat. Rev. Methods Primers, 2022, 2, 98 CrossRef CAS PubMed
.
- R. Tang, L. Yang, L. Shen, X. Ma, Y. Gao, Y. Liu, Z. Bai and X. Wang, Front. Chem., 2022, 10, 771027 CrossRef CAS PubMed
.
- R. K. Das, V. Gocheva, R. Hammink, O. F. Zouani and A. E. Rowan, Nat. Mater., 2016, 15, 318–325 CrossRef CAS
.
- A. Faroni, V. L. Workman, A. Saiani and A. J. Reid, Adv. Healthcare Mater., 2019, 8, e1900410 CrossRef
.
- F. J. Vernerey, S. L. Sridhar, A. Muralidharan and S. J. Bryant, Chem. Rev., 2021, 121, 11085–11148 CrossRef CAS PubMed
.
- K. Y. Zhang, Q. Feng, Z. W. Fang, L. Gu and L. M. Bian, Chem. Rev., 2021, 121, 11149–11193 CrossRef CAS PubMed
.
- Y. Kang, H. Zhang, L. Chen, J. Dong, B. Yao, X. Yuan, D. Qin, A. V. Yaremenko, C. Liu, C. Feng, X. Ji and W. Tao, Innovation, 2022, 3, 100327 CAS
.
- A. G. Clark, A. Maitra, C. Jacques, M. Bergert, C. Pérez-González, A. Simon, L. Lederer, A. Diz-Muñoz, X. Trepat, R. Voituriez and D. M. Vignjevic, Nat. Mater., 2022, 21, 1200 CrossRef CAS
.
- K. P. Meng, F. S. Majedi, T. J. Thauland and M. J. Butte, J. Exp. Med., 2020, 217, 32484502 CrossRef PubMed
.
- F. S. Majedi, M. M. Hasani-Sadrabadi, T. J. Thauland, S. Li, L. S. Bouchard and M. J. Butte, Biomaterials, 2020, 252, 120058 CrossRef CAS PubMed
.
- F. S. Majedi, M. M. Hasani-Sadrabadi, T. J. Thauland, S. G. Keswani, S. Li, L. S. Bouchard and M. J. Butte, Nat. Biomed. Eng., 2023, 7, 56–71 CrossRef CAS PubMed
.
- Z. Zhuang, Y. Zhang, X. Yang, T. Yu, Y. Zhang, K. Sun, Y. Zhang, F. Cheng, L. Zhang and H. Wang, Acta Biomater., 2022, 149, 69–81 CrossRef CAS PubMed
.
- S. Jiao, C. Li, F. Guo, J. Zhang, H. Zhang, Z. Cao, W. Wang, W. Bu, M. Lin, J. Lü and Z. Zhou, Nat. Commun., 2023, 14, 6416 CrossRef CAS PubMed
.
- Y. Song, L. Li, W. Zhao, Y. Qian, L. Dong, Y. Fang, L. Yang and Y. Fan, Bioact. Mater., 2021, 6, 2983–2998 CAS
.
- J. Jiang, F. Wang, W. Huang, J. Sun, Y. Ye, J. Ou, M. Liu, J. Gao, S. Wang, D. Fu, B. Chen, L. Liu, F. Peng and Y. Tu, Exploration, 2023, 3, 20220147 CrossRef CAS
.
- Y. Shou, Z. Le, H. S. Cheng, Q. Liu, Y. Z. Ng, D. L. Becker, X. Li, L. Liu, C. Xue, N. J. Y. Yeo, R. Tan, J. Low, A. R. K. Kumar, K. Z. Wu, H. Li, C. Cheung, C. T. Lim, N. S. Tan, Y. Chen, Z. Liu and A. Tay, Adv. Mater., 2023, 35, e2304638 CrossRef
.
- S. Y. Chu, C. H. Chou, H. D. Huang, M. H. Yen, H. C. Hong, P. H. Chao, Y. H. Wang, P. Y. Chen, S. X. Nian, Y. R. Chen, L. Y. Liou, Y. C. Liu, H. M. Chen, F. M. Lin, Y. T. Chang, C. C. Chen and O. K. Lee, Nat. Commun., 2019, 10, 1524 CrossRef
.
- A. Mandal, J. R. Clegg, A. C. Anselmo and S. Mitragotri, Bioeng. Transl. Med., 2020, 5, e10158 CrossRef CAS
.
- H. Wang and D. J. Mooney, Nat. Mater., 2018, 17, 761–772 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |