 Open Access Article
Open Access ArticleCorrelation of the catalytic performance with Ruδ+ species on Ru/Nb2O5 in furfural aqueous reductive conversion†
Yulong
Deng
abc,
Binyu
Zhang
abc,
Huiru
Wu
abc,
Zhuo
He
abc,
Xiaorui
Du
 abc,
Jiayi
Ou
abc,
Tianyu
Ren
abc,
Jiayi
Ou
abc,
Tianyu
Ren
 bc,
Haiyong
Wang
bc,
Haiyong
Wang
 abc,
Yuhe
Liao
abc,
Qiying
Liu
*d,
Chenguang
Wang
abc,
Yuhe
Liao
abc,
Qiying
Liu
*d,
Chenguang
Wang
 *abc and
Yanbin
Cui
*abc and
Yanbin
Cui
 *abc
*abc
aSchool of Energy Science and Engineering, University of Science and Technology of China, Hefei 230026, PR China
bCAS Key Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, PR China. E-mail: wangcg@ms.giec.ac.cn; cuiyb@ms.giec.ac.cn
cGuangdong Provincial Key Laboratory of Renewable Energy, Guangzhou 510640, P.R. China
dJiangsu Co-Innovation Centre of Efficient Processing and Utilization of Forest Resources, International Innovation Centre for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing 210037, PR China. E-mail: liuqy@njfu.edu.cn
First published on 2nd December 2024
Abstract
Ru/Nb2O5 is effective for furfural aqueous reductive conversion. Systematic characterization, kinetic studies and in situ DRIFT tests demonstrated that the Ruδ+ species abundance is highly correlated with the adsorption behavior of the substrate, key intermediate and product (furfural, 2-cyclopentenone, and cyclopentanone), which governs the FFR conversion rate and cyclopentanoid product selectivity.
1. Introduction
Furfural (FFR) is one of the 12 most important biomass-derived platform compounds, which can be industrially prepared from hemicellulose in large quantities.1 Reductive conversion of FFR has been extensively investigated to prepare diverse value-added chemicals, among which cyclopentanoid compounds (such as cyclopentanone (CPO) and cyclopentanol (CPOL)) are of significant interest due to their wide range of applications across various industrial sectors, such as fungicides, pharmaceuticals, rubber chemicals, and flavour and fragrance chemicals.2,3 Since the first study reported by Hronec and co-workers on the aqueous phase conversion of FFR to CPO using carbon-supported precious metal catalysts,4 numerous studies have been conducted to optimize catalyst design and streamline production processes.5,6The reaction proceeds through a series of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O hydrogenation, ring-opening, isomerization, dehydration and C
O hydrogenation, ring-opening, isomerization, dehydration and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenation steps. Therefore, to achieve high selectivity for cyclopentanoid products, it is essential to develop a catalyst that preferentially hydrogenates the C
C hydrogenation steps. Therefore, to achieve high selectivity for cyclopentanoid products, it is essential to develop a catalyst that preferentially hydrogenates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond over the C
O bond over the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and excels in acid-catalysed ring rearrangement. Ru as an oxophilic metal with excellent H2 dissociation and deoxygenation activity is one of the most widely applied metal catalysts for aqueous reductive conversion of biomass-derived carbonyl compounds.7–9 Support matrices such as metal oxides can act as promoters by anchoring metal species, introducing defects, interacting with supported metal and stabilizing particular oxidation states of the active metal.10 Nb2O5 is widely applied as a stable support in catalytic processes involving the synergy of Brønsted and Lewis acid sites, making Ru/Nb2O5 a versatile catalyst with outstanding performance for hydrodeoxygenation (HDO) of a variety of substrates, such as phenols,11 alkenes,12 ethers,13 and particularly unsaturated aldehydes.14
C bond and excels in acid-catalysed ring rearrangement. Ru as an oxophilic metal with excellent H2 dissociation and deoxygenation activity is one of the most widely applied metal catalysts for aqueous reductive conversion of biomass-derived carbonyl compounds.7–9 Support matrices such as metal oxides can act as promoters by anchoring metal species, introducing defects, interacting with supported metal and stabilizing particular oxidation states of the active metal.10 Nb2O5 is widely applied as a stable support in catalytic processes involving the synergy of Brønsted and Lewis acid sites, making Ru/Nb2O5 a versatile catalyst with outstanding performance for hydrodeoxygenation (HDO) of a variety of substrates, such as phenols,11 alkenes,12 ethers,13 and particularly unsaturated aldehydes.14
The electronic structure is a key factor determining the intrinsic activity of the catalyst because it affects the stability of the transition state during the reaction and determines the interaction strength between the catalyst and reactants. Numerous studies reported that electron-deficient Mδ+ species on the catalyst surface is responsible for the high selectivity toward FAOL conversion of furfural.15,16 The Nb2O5 support could boost the HDO performance of Ru by forming a Ru–RuOx–Nb2O5 interface through tunable electron transfer between the support and metal,17,18 which is critical in manipulating substrate adsorption behaviour. Moreover, the desorption behaviour of CPO can also be tuned on electron-deficient metal species derived from heteroatom doping, which further governs the distribution of over-hydrogenation products.15 However, the impact of the electronic structure of various Ru species on cyclopentanoid compounds from FFR aqueous reductive conversion has been elusive, and the correlative analysis between the abundance of reactive Ruδ+ species and catalytic performance is scarcely investigated.
In this contribution, a series of Ru/Nb2O5 catalysts with varied reduction temperatures were employed as model catalysts to investigate the metal–support interaction in FFR reductive conversion. Systematic characterization was applied to investigate the physicochemical properties of the as-prepared catalysts, and the abundance of partially oxidized Ruδ+ species was analysed by XPS and H2-TPR on activated catalysts. The kinetic study profiled the transition from the substrate to key intermediates, and to final products, providing a comprehensive perspective to investigate the relationship between the Ruδ+ species and catalytic performance. The role of Ruδ+ species in FFR conversion and product selectivity was further elucidated by interpreting the adsorptive behaviour of FFR, furfural alcohol (FAOL), 2-cyclopentenone (CPEO), and CPO using in situ DRIFTS.
2. Results and discussion
2.1 FFR aqueous reductive conversion using various Ru/MOx catalysts
Different types of Ru/MOx catalysts using various supports (MoO3, TiO2, Al2O3, ZrO2, Nb2O5) with consistent Ru loading were prepared, to assess the impact of metal oxide supports on product distribution in FFR conversion (Table 1). The carbon balance of Ru supported on MoO3, Al2O3 and ZrO2 was lower than 75%, indicating that significant furfural loss takes place due to coking. Ru/MoO3 and Ru/Al2O3 also resulted in the formation of a considerable amount of tetrahydrofurfuryl alcohol (THFAOL) with a respective yield of 31.6% and 10.0%, associated with excessive hydrodeoxygenation of the furan ring. Considerable accumulation of the FAOL intermediate (27.6%) was detected for Ru/ZrO2, which is associated with limited acidic strength that is less effective in promoting the Piancatelli rearrangement for further conversion.19,20 In comparison, Ru/Nb2O5 showed a full conversion of FFR and a much higher carbon balance (90.2%), and the overall yield of and selectivity to cyclopentanoid products reached up to 87.4% and 96.9%, respectively.| Entry | Catalyst | Conversion of FFR (%) | Yield (C-mol%) | Carbon balance of the product (C-mol%) | |||
|---|---|---|---|---|---|---|---|
| THFAOL | FAOL | CPO | CPOL | ||||
| Catalyst preparation conditions: calcined at 500 °C and reduced at 450 °C. Reaction conditions: 0.20 g furfural, 0.04 g catalyst, 20 mL of H2O, p (initial, H2) = 4.0 MPa, reaction temperature is 160 °C, heating rate = 5 °C min−1, stirring speed = 800 rpm, reaction time = 6 h. | |||||||
| 1 | 5% Ru/MoO3 | >99.9 | 10.0 | — | 0.2 | 60.5 | 70.7 |
| 2 | 5% Ru/Al2O3 | >99.9 | 31.6 | — | 0.8 | 42.5 | 74.9 |
| 3 | 5% Ru/ZrO2 | >99.9 | — | 27.6 | 0.2 | 43.4 | 71.2 |
| 4 | 5% Ru/TiO2 | 25.9 | — | 2.0 | 23.7 | — | 25.7 |
| 5 | 5% Ru/Nb2O5 | >99.9 | — | 2.8 | 21.1 | 66.3 | 90.2 |
| 6 | Pristine Nb2O5 | 1.9 | — | — | — | — | — |
| 7 | Nb2O5-H(450) | 3.1 | — | — | — | — | — |
Due to its satisfactory catalytic performance, Ru/Nb2O5 was selected as the model catalyst for further investigation via material manipulation techniques. The reaction temperature and pressure were optimized for Ru/Nb2O5 catalysed FFR conversion (Fig. S1†). At low reaction temperatures, considerable FAOL accumulation was observed due to the low efficiency in initiating the acid-driven ring rearrangement.21 However, carbon balance dropped sharply at elevated reaction temperatures, associated with extensive self-polymerization of FFR and formation of humins under such conditions.22,23 Low initial hydrogen pressure was shown to be ineffective in hydrogenating the key intermediates, leaving considerable amounts of FAOL and CPEO unconverted, while over hydrogenation of the furan ring occurred and generated the THFAOL by-product when the hydrogen pressure was beyond 5 MPa. Overall, 4 MPa and 160 °C were selected due to the satisfactory conversion, carbon balance and cyclopentanoid product selectivity.
2.2 Structural and textural characterization of Ru/Nb2O5 catalysts
A series of Ru/Nb2O5 catalysts were prepared by tuning the reduction temperatures following an identical calcination protocol. As shown in Fig. S2,† the SEM images of all Ru/Nb2O5 catalysts present similar morphological features, and the TEM images show that the Ru nanoparticle size is around 5 nm, with the d-spacing of 0.201 nm of the Ru nanoparticles in the catalysts corresponding to the Ru(101) plane. The amount and dispersion of Ru were measured by ICP-OES and H2 pulse chemisorption (Table S1†). The actual Ru loading of all Ru/Nb2O5 catalysts matches well with their nominal Ru loading, indicating negligible Ru leaching or Nb2O5 loss during the material synthesis process. No significant difference in metal dispersion was found among Ru/Nb2O5 catalysts with different reduction temperature treatments, indicating the excellent stabilization capability of Nb2O5 as a metal support.No characteristic XRD patterns of Ru or RuO2 phases are detected for all catalysts, indicating that Ru particles are highly dispersed on the support with their sizes below the limit of phase detection (Fig. 1a). The sharp peaks for all samples indicate the high degree of crystallinity of the Nb2O5 support. Catalysts reduced at temperatures below 650 °C share multiple sharp diffraction peaks of different planes of Nb2O5 (PDF#72-1484) and Nb16.8O42 (PDF#71-0336). In Ru/Nb2O5-H(850), the XRD pattern exhibits new diffraction peaks at 26.0°, 35.2°, and 52.1°, corresponding to the presence of a newly formed NbO2 phase (PDF#71-0020). The BET test indicates that the crystallized support showed a very limited porous structure (Table S2†). In Fig. 1b, the Raman spectra show a prominent vibrational peak centred at 130 cm−1, attributed to the stretching vibration between the Nb–Nb bond.24,25 The Raman vibrational peak at 267 cm−1 and 627 cm−1 is assigned to the bending vibration and bridging vibration mode of the Nb–O–Nb bond, respectively.26,27 The vibrational peak at ∼997 cm−1 is attributed to the symmetric stretching vibration mode of the Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond between edge-sharing NbO6 octahedra, which contribute to Lewis acidic sites (LASs) on the surface.28,29Fig. 1c displays the Py-FTIR spectra of Ru/Nb2O5 catalysts after desorption at 150 °C, showing characteristic bands for Lewis acidic sites (1450 cm−1, 1610 cm−1) and minor bands (1490 cm−1) for both Brønsted and Lewis acidic sites.30 For all tested Ru/Nb2O5 catalysts, LASs present the dominant acid species and their density remains essentially consistent. The LA abundant sites in Nb2O5 promote cyclopentanoid selectivity by suppressing hydrogenation to THFAOL, as reported in previous research.31 H2-TPR shows a dominant H2 uptake peak (β peak) at 193 °C, and the reduction peak slightly shifts to a higher temperature as the metal loading increases, which is associated with the broader size distribution. The γ reduction peak centred at 814 °C is attributed to the removal of surface oxygen (Fig. 1d).32,33
O bond between edge-sharing NbO6 octahedra, which contribute to Lewis acidic sites (LASs) on the surface.28,29Fig. 1c displays the Py-FTIR spectra of Ru/Nb2O5 catalysts after desorption at 150 °C, showing characteristic bands for Lewis acidic sites (1450 cm−1, 1610 cm−1) and minor bands (1490 cm−1) for both Brønsted and Lewis acidic sites.30 For all tested Ru/Nb2O5 catalysts, LASs present the dominant acid species and their density remains essentially consistent. The LA abundant sites in Nb2O5 promote cyclopentanoid selectivity by suppressing hydrogenation to THFAOL, as reported in previous research.31 H2-TPR shows a dominant H2 uptake peak (β peak) at 193 °C, and the reduction peak slightly shifts to a higher temperature as the metal loading increases, which is associated with the broader size distribution. The γ reduction peak centred at 814 °C is attributed to the removal of surface oxygen (Fig. 1d).32,33
The surface electronic structure/chemical states of Ru and Nb were determined by X-ray photoelectron spectroscopy (XPS). In Fig. 1e, the deconvoluted peaks at ∼280.0 eV and ∼280.8 eV are assigned to the Ru0 3d5/2 state and Ruδ+ 3d5/2 state, respectively.34 With the increase of reduction temperature, the proportion of Ru0 in total ruthenium species increases along with a shift to a lower binding energy, which is associated with intensified electron transfer from the support to metallic Ru sites.35,36 Detailed Nb 3d spectra of these Ru/Nb2O5 catalysts present a broad Nb 3d5/2 peak between 205 and 209 eV (Fig. 1f), which can be deconvoluted to two peaks at ∼207.1 and ∼207.4 eV, respectively, corresponding to Nb4+ and Nb5+ states.37 The ratio of the Nb4+ peak increases gradually with reduction temperature. As a result of the intensified electron transfer effect, the Ruδ+/(Ru0 + Ruδ+) ratio decreased from 0.41 to 0.08, with the reduction temperature increasing from 250 °C to 850 °C (Table S3†).
2.3 Kinetic study of FFR reductive conversion over tailored Ru/Nb2O5 catalysts
The batch study showed that the FFR conversion drops as the catalyst reduction temperature increases, while the CPO selectivity ascends from 23% for 1% Ru/Nb2O5-H(250) to 98% for 1% Ru/Nb2O5-H(850) (Fig. S3†). In contrast, the CPO selectivity decreases as the metal loading increases to 3% with a full conversion of FFR obtained, indicating that reactive Ru species can be simultaneously tuned by reduction temperature and Ru loading. Such notable results promote us to further investigate the changes of FFR conversion and product selectivity with the reaction time (Fig. 2). Among the tested catalysts, 1% Ru/Nb2O5-H(250) showed an efficient conversion toward FAOL, CPEO and CPO in a sequential order, and high FFR conversion (99%) and high CPO selectivity (93%) were achieved at 300 min. After that, the selectivity to CPO dropped sharply as the reaction proceeded, due to the concurrent formation of CPOL. For the 1% Ru/Nb2O5-H(450) catalyst, it took a longer reaction time for CPO generation, achieving a high selectivity of 90% toward CPO after 10 h, with a very low level of CPOL found. The other two catalysts reduced at higher temperatures (650 °C and 850 °C) displayed a slower rate for FFR conversion and CPO formation, with negligible CPOL detected over the whole reaction span (Fig. 2), indicating that over hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of CPO was suppressed due to the retarded conversion of the substrate and intermediates.
O bond of CPO was suppressed due to the retarded conversion of the substrate and intermediates.
The apparent reaction rate constants for FFR conversion of various Ru/Nb2O5 catalysts were calculated using the pseudo-first-order model (Table S4†), assuming that hydrogen is in significant excess.36,37 The calculated reaction rate constant decreases as the reduction temperature of Ru/Nb2O5 catalysts increases (Fig. 3a). Considering the similar properties of the Ru/Nb2O5 catalysts, such as porosity, surface acidity, geometric features and dispersion, we infer that the main factor attributed to the different catalytic performances is the electronic structures of reactive Ru species. XPS results showed that electron transfer from the Nb2O5 carrier to the Ru metal active site was promoted, leading to a decreased ratio of Ruδ+ among Ru species. It has been extensively reported that electron-deficient metal sites are critical for furfural adsorption, and partially oxidized Ruδ+ sites were also reported as reactive sites dominating furfural conversion.15,38
To quantitatively interpret the role between active sites and catalytic activity, the activated catalysts were subjected to H2-TPR analysis, and hydrogen uptake was used to determine the abundance of reactive Ruδ+ species in Ru/Nb2O5 catalysts (Fig. 3b, Table S5†). No β reduction peak was found in the activated Ru/Nb2O5 catalysts, indicating that the dominant species in these reduced catalysts is metallic Ru. By contrast, a distinct α reduction peak can be observed at around 100 °C for all reduced Ru/Nb2O5 catalysts, which is attributed to the reduction of the reactive Ruδ+ species on the surface that can be easily oxidized upon air exposure.39 The hydrogen consumption of the α reduction peak decreased gradually with the increase of reduction temperature, indicating that the abundance of Ruδ+ species on the catalyst surface was negatively affected by reduction temperature.
This is consistent with the observed trend presented by XPS analysis. Notably, a good correlation between reactive Ruδ+ species on the surface of the Ru/Nb2O5 series catalysts and the apparent reaction rate constant k for FFR conversion was found, with the corresponding fitting curve's correlation coefficient R2 = 0.975 (Fig. 3c). The H2-TPR hydrogen consumption increases as the reduction temperature of the catalyst decreases, and the conversion rate of FFR by the catalyst increases accordingly.
2.4 Adsorptive behaviour of the substrate, intermediates, and product over Ru/Nb2O5
Time-resolved in situ DRIFTS was used to study the adsorption behaviours of the substrate, key intermediates and product on different Ru/Nb2O5 catalysts (Fig. 4). For FFR, there were two possible adsorption patterns of the carbonyl group on Ru nanoparticles: direct adsorption by carbonyl oxygen atoms via η1(O) or bi-coordination adsorption through both carbon and oxygen atoms via η2(C–O).40 The wavenumber of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O infrared vibration was found at 1710 cm−1 using a control adsorbent, indicating that FFR molecules solely undergo physical adsorption on these surfaces (Fig. S4†).41Fig. 4 presents a signal peak at 1716 cm−1 that is assigned to the C
O infrared vibration was found at 1710 cm−1 using a control adsorbent, indicating that FFR molecules solely undergo physical adsorption on these surfaces (Fig. S4†).41Fig. 4 presents a signal peak at 1716 cm−1 that is assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of gas-phase furfural at the early stage of the adsorption process. As the adsorption proceeds, the wavenumber assigned to C
O stretching vibration of gas-phase furfural at the early stage of the adsorption process. As the adsorption proceeds, the wavenumber assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration is notably redshifted to around 1673 cm−1 due to weakened adsorbed C–O stretching,42 whereas the position of the C
O vibration is notably redshifted to around 1673 cm−1 due to weakened adsorbed C–O stretching,42 whereas the position of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the furan ring (1400 cm−1) group is invariant (Fig. S5†). This suggests that the FFR is perpendicularly adsorbed and forms an η1(O) surface intermediate.43,44
C stretching in the furan ring (1400 cm−1) group is invariant (Fig. S5†). This suggests that the FFR is perpendicularly adsorbed and forms an η1(O) surface intermediate.43,44
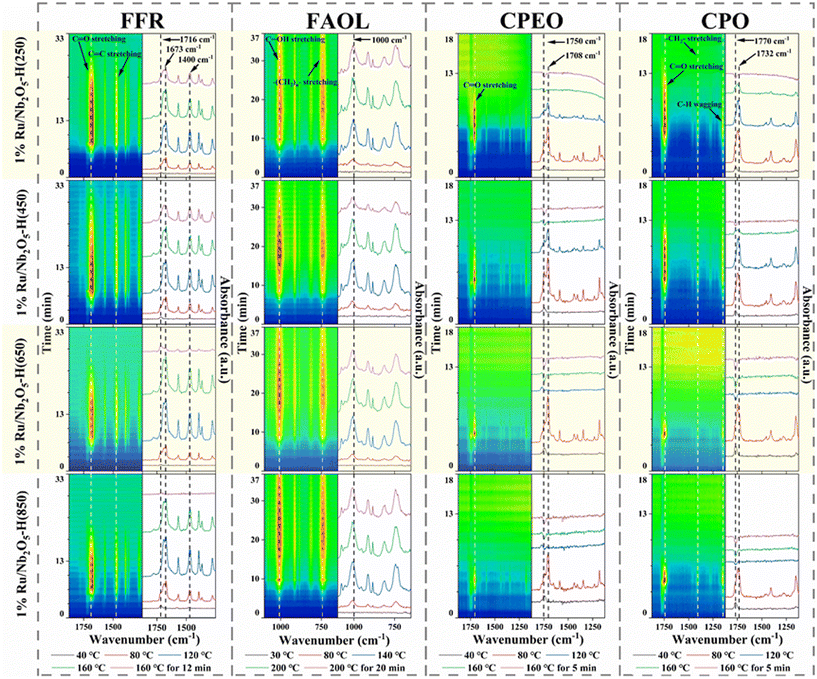 | ||
| Fig. 4 Time-resolved in situ DRIFT spectra for FFR, FAOL, CPEO, and CPO adsorption over various 1% Ru/Nb2O5 catalysts reduced at varied temperatures. Note: FAOL was subjected to programmed heating to 200 °C, for detailed conditions see Fig. S5–S8.† | ||
Notably, the retention time of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal upon heating treatment decreases from 13 min (Ru/Nb2O5-H(250)) to 3.5 min (Ru/Nb2O5-H(850)), indicating weaker FFR adsorption with increasing reduction temperature due to decreasing active Ruδ+ sites. Also, the intensity of the characteristic C
O signal upon heating treatment decreases from 13 min (Ru/Nb2O5-H(250)) to 3.5 min (Ru/Nb2O5-H(850)), indicating weaker FFR adsorption with increasing reduction temperature due to decreasing active Ruδ+ sites. Also, the intensity of the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal after consistent heating treatment displayed a decreasing trend as the catalyst reduction temperature increases (Table S6†). In fact, a good linear fitting relationship was found between the H2-TPR uptake and the adsorption intensity of the C
O signal after consistent heating treatment displayed a decreasing trend as the catalyst reduction temperature increases (Table S6†). In fact, a good linear fitting relationship was found between the H2-TPR uptake and the adsorption intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of FFR on the catalyst surface (Fig. 3d). These results indicate that the presence of active Ruδ+ sites is highly related to the substrate adsorption orientation and adsorption strength. Kim et al. reported that the oxygen atoms of the C
O group of FFR on the catalyst surface (Fig. 3d). These results indicate that the presence of active Ruδ+ sites is highly related to the substrate adsorption orientation and adsorption strength. Kim et al. reported that the oxygen atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of these carbonyl containing compounds preferentially adsorbed at the coordinatively unsaturated Ruδ+ sites in an η1(O)-aldehyde configuration,40 which is consistent with the result presented in our study. In comparison, the adsorption behaviour of FAOL onto different Ru/Nb2O5 catalysts showed no obvious difference. A strong signal assigned to –OH group adsorption centred at ∼1000 cm−1 was detected, corresponding to the adsorption configuration of η1(O)-alcohol hydroxyl of FAOL (Fig. S6†).16 It has been reported that the main sites for the adsorption of η1(O)-alcohol hydroxyl groups in FAOL are Lewis acid sites.24,45 This result is consistent with the Py-FTIR result that showed similar abundance and distribution of LA sites among the catalysts.
O groups of these carbonyl containing compounds preferentially adsorbed at the coordinatively unsaturated Ruδ+ sites in an η1(O)-aldehyde configuration,40 which is consistent with the result presented in our study. In comparison, the adsorption behaviour of FAOL onto different Ru/Nb2O5 catalysts showed no obvious difference. A strong signal assigned to –OH group adsorption centred at ∼1000 cm−1 was detected, corresponding to the adsorption configuration of η1(O)-alcohol hydroxyl of FAOL (Fig. S6†).16 It has been reported that the main sites for the adsorption of η1(O)-alcohol hydroxyl groups in FAOL are Lewis acid sites.24,45 This result is consistent with the Py-FTIR result that showed similar abundance and distribution of LA sites among the catalysts.
Similar adsorption tests were conducted for other carbonyl containing compounds CPEO and CPO (Fig. S7 and S8†). Both molecules show a band at around 1750 cm−1 attributed to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O),46 and the peak shows a red-shift (30 cm−1) with significant improvement of relative intensity, indicating chemically adsorbed carbonyl groups onto Ruδ+ sites. As the catalyst reduction temperature increases, the C
O),46 and the peak shows a red-shift (30 cm−1) with significant improvement of relative intensity, indicating chemically adsorbed carbonyl groups onto Ruδ+ sites. As the catalyst reduction temperature increases, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal intensity follows the same decreasing trend as that of FFR, correlating with the H2-TPR uptake well (Fig. 3d). The decreasing CPO adsorption strength reveals that high CPO selectivity can be obtained by avoiding over-hydrogenation using a catalyst produced at elevated reduction temperature. The adsorptive behaviour of these different types of carbonyl containing intermediates and the target product affords a panoramic vision regarding FFR conversion and final product selectivity in aqueous reductive conversion processes.
O signal intensity follows the same decreasing trend as that of FFR, correlating with the H2-TPR uptake well (Fig. 3d). The decreasing CPO adsorption strength reveals that high CPO selectivity can be obtained by avoiding over-hydrogenation using a catalyst produced at elevated reduction temperature. The adsorptive behaviour of these different types of carbonyl containing intermediates and the target product affords a panoramic vision regarding FFR conversion and final product selectivity in aqueous reductive conversion processes.
3. Conclusions
In this contribution, we investigated selective production of cyclopentanoid products from FFR using Ru/Nb2O5 model catalysts, with the interaction between the Ru site and the Nb2O5 support regulated through reduction temperature variation. The FFR conversion rate constant decreased as the reduction temperature increased, accompanied with increased CPO selectivity. Through various catalyst characterization techniques, it was confirmed that surface Ruδ+ species were inversely related to reduction temperature due to intensified electron transfer from the support to metal. The adsorption mode and strength of FFR, FAOL, CPEO, and CPO onto the surface of Ru/Nb2O5 catalysts were detected with in situ DRIFTS, revealing that carbonyl containing compounds (FFR, CPEO, CPO) preferentially interact with Ruδ+ sites through an η1(O) configuration, and a positive correlation between adsorption intensity and Ruδ+ abundance was noted. We propose that in these Ru/Nb2O5 catalysts, the Ruδ+ sites coordinatively bind and activate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the substrate, and the conversion rate of FFR and over-hydrogenation of CPO can be tuned through reduction temperature-dependent Ru0 and Ruδ+ distribution, thereby manipulating substrate conversion and product selectivity.
O group in the substrate, and the conversion rate of FFR and over-hydrogenation of CPO can be tuned through reduction temperature-dependent Ru0 and Ruδ+ distribution, thereby manipulating substrate conversion and product selectivity.
Data availability
The data that support the findings of this study are included within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the International Science and Technology Innovation Cooperation Program of the National Key Research and Development Plan of China (2021YFE0114400) and the Guangdong Basic and Applied Basic Research Foundation of China (2023A1515110745). The authors would also like to thank the Analysis and Test Centre at Guangzhou Institute of Energy Conversion for their support in spectrometry analysis.References
- X. Li, P. Jia and T. Wang, ACS Catal., 2016, 6, 7621–7640 CrossRef CAS.
- S. Kotha and Y. Tangella, Synlett, 2020, 31, 1976–2012 CrossRef CAS.
- V. B. Kurteva and C. A. M. Afonso, Chem. Rev., 2009, 109, 6809–6857 CrossRef CAS.
- M. Hronec and K. Fulajtarova, Catal. Commun., 2012, 24, 100–104 CrossRef CAS.
- G.-S. Zhang, M.-M. Zhu, Q. Zhang, Y.-M. Liu, H.-Y. He and Y. Cao, Green Chem., 2016, 18, 2155–2164 RSC.
- P. Pan, W.-Y. Xu, T.-J. Pu, X.-D. Wang, X.-J. Pei, F. Tang and Y.-S. Feng, ChemistrySelect, 2019, 4, 5845–5852 CrossRef CAS.
- R. Fang, H. Liu, R. Luque and Y. Li, Green Chem., 2015, 17, 4183–4188 RSC.
- P. Panagiotopoulou, N. Martin and D. G. Vlachos, ChemSusChem, 2015, 8, 2046–2054 CrossRef CAS.
- F. Liu, Q. Y. Liu, J. M. Xu, L. Li, Y. T. Cui, R. Lang, L. Li, Y. Su, S. Miao, H. Sun, B. T. Qiao, A. Q. Wang, F. Jérôme and T. Zhang, Green Chem., 2018, 20, 1770–1776 RSC.
- Z. Gao, L. Yang, G. L. Fan and F. Li, ChemCatChem, 2016, 8, 3769–3779 CrossRef CAS.
- R. Huang, O. Kwon, C. Lin and R. J. Gorte, J. Catal., 2021, 398, 102–108 CrossRef CAS.
- T. Uchijima, Catal. Today, 1996, 28, 105–117 CrossRef CAS.
- H. Ali, T. Vandevyvere, J. Lauwaert, S. K. Kansal, M. K. K. Sabbe, S. Saravanamurugan and J. W. W. Thybaut, Catal. Sci. Technol., 2023, 13, 1140–1153 RSC.
- J. Fontana, C. Vignado, E. Jordao, F. C. A. Figueiredo and W. A. Carvalho, Catal. Today, 2011, 172, 27–33 CrossRef CAS.
- W. C. Wang, H. K. Zhang, Y. D. Wang, F. Y. Zhou, Z. Y. Xiang, W. B. Zhu and H. L. Wang, J. Energy Chem., 2024, 92, 43–51 CrossRef CAS.
- N. Karanwal, R. G. Kurniawan, S. K. Kwak and J. Kim, Chem. Eng. J., 2024, 498, 155603 CrossRef CAS.
- Y. Xin, L. Dong, Y. Guo, X. Liu, Y. Hu and Y. Wang, J. Catal., 2019, 375, 202–212 CrossRef CAS.
- R. Tu, W. Lv, Y. Sun, Y. J. Wu, Y. W. Wu, X. D. Fan, E. C. Jiang, Q. Lu and X. W. Xu, Chem. Eng. J., 2023, 453, 139718 CrossRef CAS.
- Y. Yang, Z. Du, Y. Huang, F. Lu, F. Wang, J. Gao and J. Xu, Green Chem., 2013, 15, 1932–1940 RSC.
- C. A. M. R. van Slagmaat, J. Noordijk, L. G. Monsegue, S. Mogensen, D. Hadavi, P. Han, P. J. L. M. Quaedflieg, G. K. M. Verzijl, P. L. Alsters and S. M. A. De Wildeman, Green Chem., 2021, 23, 7100–7114 RSC.
- V. Ranaware, R. G. Kurniawan, D. Verma, S. K. Kwak, B. C. Ryu, J. W. Kang and J. Kim, Appl. Catal., B, 2022, 318, 121838 CrossRef CAS.
- S. Karnjanakom, A. Bayu, P. Maneechakr, C. Samart, S. Kongparakul and G. Guan, ACS Sustainable Chem. Eng., 2021, 9, 14170–14179 CrossRef CAS.
- X. Hu, C. Lievens, A. Larcher and C.-Z. Li, Bioresour. Technol., 2011, 102, 10104–10113 CrossRef CAS PubMed.
- U. Balachandran and N. G. Eror, J. Mater. Sci. Lett., 1982, 1, 374–376 CrossRef CAS.
- T. Ikeya and M. Senna, J. Non-Cryst. Solids, 1988, 105, 243–250 CrossRef CAS.
- F. D. Hardcastle and I. E. Wachs, Solid State Ionics, 1991, 45, 201–213 CrossRef CAS.
- M. Ristic, S. Popovic and S. Music, Mater. Lett., 2004, 58, 2658–2663 CrossRef CAS.
- Y. Wang, S. Aghamohammadi, D. Li, K. Li and R. Farrauto, Appl. Catal., B, 2019, 244, 438–447 CrossRef CAS.
- F. B. Passos, D. A. G. Aranda, R. R. Soares and M. Schmal, Catal. Today, 1998, 43, 3–9 CrossRef CAS.
- M. H. Brijaldo, H. A. Rojas, Y. Xing, F. B. Passos and J. J. Martínez, Catalysts, 2024, 14, 493 CrossRef CAS.
- Z. J. Yu, H. L. Tian, K. Sun, Y. W. Shao, L. J. Zhang, S. Zhang, P. G. Duan, Q. Liu, S. L. Niu, D. H. Dong and X. Hu, Mol. Catal., 2020, 496, 111187 CrossRef CAS.
- F. B. Noronha, D. A. G. Aranda, A. P. Ordine and M. Schmal, Catal. Today, 2000, 57, 275–282 CrossRef CAS.
- K. A. Resende, F. B. Noronha and C. E. Hori, Renewable Energy, 2020, 149, 198–207 CrossRef CAS.
- W. J. Guo, T. Tong, X. H. Liu, Y. Guo and Y. Q. Wang, ChemCatChem, 2019, 11, 4130–4138 CrossRef CAS.
- J. Y. Shen, A. Adnot and S. Kaliaguine, Appl. Surf. Sci., 1991, 51, 47–60 CrossRef CAS.
- D. J. Morgan, Surf. Interface Anal., 2015, 47, 1072–1079 CrossRef CAS.
- T. A. Sasaki and Y. Baba, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 791–797 CrossRef CAS.
- Z. Zhu and G. Xu, Nanomaterials, 2024, 14(9), 788 CrossRef CAS PubMed.
- Y. Ma, B. Liu, M. Jing, R. Zhang, J. Chen, Y. Zhang and J. Li, Chem. Eng. J., 2016, 287, 155–161 CrossRef CAS.
- R. Insyani, A. F. Barus, R. Gunawan, J. Park, G. T. Jaya, H. S. Cahyadi, M. G. Sibi, S. K. Kwak, D. Verma and J. Kim, Appl. Catal., B, 2021, 291, 120120 CrossRef CAS.
- S. Li, Y. Fan, C. Wu, C. Zhuang, Y. Wang, X. Li, J. Zhao and Z. Zheng, ACS Appl. Mater. Interfaces, 2021, 13, 8507–8517 CrossRef CAS.
- H. Daly, H. G. Manyar, R. Morgan, J. M. Thompson, J. J. Delgado, R. Burch and C. Hardacre, ACS Catal., 2014, 4, 2470–2478 CrossRef CAS.
- Y. An, Q. Wu, L. Niu, C. Zhang, Q. Liu, G. Bian and G. Bai, J. Catal., 2024, 429, 115271 CrossRef CAS.
- J. Yu, Y. Yang, L. Chen, Z. Li, W. Liu, E. Xu, Y. Zhang, S. Hong, X. Zhang and M. Wei, Appl. Catal., B, 2020, 277, 119273 CrossRef CAS.
- C. Verrier, S. Moebs-Sanchez, Y. Queneau and F. Popowycz, Org. Biomol. Chem., 2018, 16, 676–687 RSC.
- E. Lemmon, M. McLinden, D. Friend, P. Linstrom and W. Mallard, Thermophysical Properties of Fluid Systems, Isobaric Properties for Water, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy01272k |
| This journal is © The Royal Society of Chemistry 2025 |



