X and Q-band EMR study of ultrasmall Zn1−xMnxFe2O4 spinel nanoparticles fabricated under nonhydrolytic conditions
Iwona
Rogalska
ab,
Bogumił
Cieniek
 a,
Anna
Tomaszewska
a,
Anna
Tomaszewska
 c,
Magdalena
Kulpa-Greszta
c,
Piotr
Krzemiński
c,
Magdalena
Kulpa-Greszta
c,
Piotr
Krzemiński
 a,
Bartosz
Zarychta
d,
Ireneusz
Stefaniuk
a,
Bartosz
Zarychta
d,
Ireneusz
Stefaniuk
 *a and
Robert
Pązik
*a and
Robert
Pązik
 *c
*c
aInstitute of Materials Engineering, College of Natural Sciences, University of Rzeszow, Pigonia 1, Rzeszow PL-5310, Poland. E-mail: istef@ur.edu.pl
bInternational Research Centre MagTop, Institute of Physics, Polish Academy of Sciences, Aleja Lotników 32/46, Warsaw PL-02668, Poland
cDepartment of Biotechnology, Institute of Biotechnology, College of Natural Sciences, University of Rzeszow, Pigonia 1, Rzeszow PL35-310, Poland. E-mail: rpazik@ur.edu.pl
dFaculty of Chemistry, University of Opole, Oleska 48, Opole PL-45052, Poland
First published on 16th December 2024
Abstract
In this work, we are showing the results of the X- and Q-band electron magnetic resonance measurements of ultra-small Zn1−xMnxFe2O4 nanoparticles (ca. 8 nm) with a very narrow size distribution. The chosen synthetic route allows for precise structural modifications with a broad concentration range (x = 0, 0.2, 0.5, 0.8, 1). The crystal structure was evaluated by means of X-ray diffraction, while cell parameters were calculated using Rietveld refinement. EMR spectral studies indicated that the prepared nanoparticles were superparamagnetic. The linewidth of EMR signal for any ferrite material generally originates from two sources: (a) magnetic dipole–dipole interactions among particles and (b) interparticle superexchange interactions between magnetic ions through oxygen ions. Observed effects are more complex interactions than in pure zinc and manganese ferrites. As a result of the study, a relationship was observed between the composition of the material and the magnetic properties with striking antiferromagnetism and ferrimagnetism change. Hence, by structural modification of materials, the magnetic character (FM–AFM–FiM) can be controlled.
1. Introduction
Ferrite-type compounds described with the general chemical formula AFe2O4, where metal cation A can be commonly represented by Zn2+, Mn2+, Co2+, Ni2+, Fe2+, etc. are a fairly broad subgroup of inorganic materials belonging to the larger spinel family depicted by the cubic crystal structure assigned to the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 227) space group.1–4 The unit cell is composed of two subnets. The one with A2+ cations has a Td point symmetry with tetrahedral coordination by O2− anions, while the second constitutes Fe3+ cations in 6-fold coordination that forms high-symmetry octahedral crystallographic sites Oh. In the general case, the ionic distribution is mixed, and it can be represented by [A1−δFe]a[AFe2−δ]bO4, where δ is called the inversion parameter that specifies the fraction of Fe3+ ions located at A sites. Consequently, if δ is 0 or 1 it represents the normal and inverse spinel, respectively. Interestingly, ferrites can show crystallographic site occupancy inversion between the positions of A2+ and Fe3+ cations. It leads to the formation of an inverted spinel with a net cationic disorder that can be strongly dependent on the synthetic route.5–8 This phenomenon is more pronounced for nanomaterials, where the degree of inversion can be higher than that in bulkier crystals. It is also worth noting that ferrite structures existing in bulk form only as normal spinel at the nanoscale can show a high cationic site disorder characteristic of inverted ferrites.9
m (no. 227) space group.1–4 The unit cell is composed of two subnets. The one with A2+ cations has a Td point symmetry with tetrahedral coordination by O2− anions, while the second constitutes Fe3+ cations in 6-fold coordination that forms high-symmetry octahedral crystallographic sites Oh. In the general case, the ionic distribution is mixed, and it can be represented by [A1−δFe]a[AFe2−δ]bO4, where δ is called the inversion parameter that specifies the fraction of Fe3+ ions located at A sites. Consequently, if δ is 0 or 1 it represents the normal and inverse spinel, respectively. Interestingly, ferrites can show crystallographic site occupancy inversion between the positions of A2+ and Fe3+ cations. It leads to the formation of an inverted spinel with a net cationic disorder that can be strongly dependent on the synthetic route.5–8 This phenomenon is more pronounced for nanomaterials, where the degree of inversion can be higher than that in bulkier crystals. It is also worth noting that ferrite structures existing in bulk form only as normal spinel at the nanoscale can show a high cationic site disorder characteristic of inverted ferrites.9
The most well known representative of the nanoferrite family is the magnetite Fe3O4 (FeFe2O4) where iron has a mixed Fe2+ and Fe3+ valency. However, unless it is surface-protected, magnetite is highly prone to oxidation into α-Fe2O3.10 This chemical transformation results in a drastic change in magnetic properties and can have a detrimental effect on certain bio-related applications due to a change of physical properties characteristic.11 To avoid that, one of the possibilities relies on the usage of more chemically stable AFe2O4 ferrites, for instance ZnFe2O4 or MnFe2O4. Both compounds are generally defined by the normal spinel structure.5,9 However, upon particle size reduction, some cationic disorder can be observed, especially at the nanoscale. The degree of inversion in ZnFe2O4 and MnFe2O4 was shown to depend quite strongly on the fabrication technique.6,7,12 What makes them particularly interesting is that they offer extraordinary particle size-dependent physicochemical properties that can be fine-tuned by chemical approaches.13–16 The range of applications is wide and includes electronic devices,17 telecommunications,18 hyperthermia,19 virus sensor detection,20 batteries,21 catalysis,22 contrast agents for magnetic imaging resonance (MRI),23,24 drug delivery platforms,25 gas sensing,26etc. Mixed zinc–manganese ferrites (Zn1−xMnxFe2O4) are of great interest, as their properties can be shaped by structural modification.16,25,27 A careful reader can find numerous reports on the synthetic routes towards ferrite compounds such as coprecipitation,28 hydrothermal,19 sol–gel,29 solid-state,30 rapid hot-injection,31 thermal decomposition,32etc.
Many antiferromagnetic materials have been reported to have a positive Curie–Weiss temperature, which is a sign of ferromagnetic interactions.33–36 This behaviour was explained by the coexistence of competing antiferromagnetic and ferromagnetic interactions. In fact, ZnFe2O4 is a model system for studying competing interactions. The normal (Zn)a(Fe2)bO4 is antiferromagnetic, but the exchange of only a few Zn–Fe cations between sites gives place to (Zn1-δFe)a[ZnFe2-δ]bO4. Recent evidence for such coexistence of ferrimagnetic clusters within an antiferromagnetic matrix37–39 allows for correlation of Zn–Fe exchange with the anomalous increase in positive Curie–Weiss temperature despite the antiferromagnetic character of sample.36 If the A ion is magnetic, AFe2O4 can be decomposed into two inequivalent magnetic sublattices, denoted as O (octahedral) and T (tetrahedral), respectively.40 The diamond-like T sublattice is not frustrated within the nearest neighbour (NN) interaction, whereas in the presence of antiferromagnetic (AFM) interactions within the sublattice, the pyrochlore-like O sublattice can host a large NN frustration.41 The ions within a sublattice often interact ferromagnetically (FM), and the ions between two sublattices interact antiferromagnetically.40,42,43 Some spinel compounds can cause the interaction of AFM between ions at O sites, which leads to geometric frustration.44,45 Consequently, the general magnetic behaviour depends on the strength of the exchange interactions between O–O (JOO), T–T (JTT) and O–T (JOT).44–46
All Fe3+ ions are coupled to each other via a superexchange pathway through A sites, and their interactions are very weak. The normal ZnFe2O4 spinel shows long-range antiferromagnetic ordering at TN = 9–11 K.47,48 Magnetization was found to increase with grain size reduction. This characteristic is generally associated with an increase in cation inversion and a decrease in grain size.43,49,50
Electron magnetic resonance spectroscopy (EMR) is a technique used to study chemical species with unpaired electrons. EMR spectroscopy also plays an important role in understanding organic and inorganic radicals, transition-metal complexes, and biomolecules. Among many experimental research methods used to study the magnetic properties of ferrite nanoparticles, EMR proves to be a very sensitive and provides information on local magnetic properties, the correlation between particle moments and the internal field distribution.51–55 The properties and range of EMR applications depend mainly on the frequency band used. The maximum geometrical size of the object that can be examined by the EMR method is directly proportional to the wavelength of the microwave. As the frequency of the microwave signal increases, the ability to detect closely located resonance lines increases. EMR is a universal technique that provides valuable information on a variety of paramagnetic systems. This is favoured by high measurement sensitivity, selectivity, the ability to measure a specific compound in the presence of many other unidentified compounds, relatively short measurement time, or uncomplicated sample preparation. The information extracted from EMR spectroscopy includes the identity and atomic oxidation state of atoms, properties of free radicals, the local environment around the unpaired electrons, and the hyperfine interaction of nuclei with unpaired electrons.
When Zn1−xMnxFe2O4 is subject to a strong magnetic field, an induced magnetic potential energy splits the spin state of unpaired electrons (Zeeman effect) accordingly. Afterwards, simultaneously applied microwave frequencies are absorbed by the unpaired electrons, which can cause the transition from one spin state to another. This mechanism can be affected by several interactions such as the hyperfine interaction and the zero-field interaction, etc. We focused on investigating the influence of the Zn/Mn content on the microstructure, magnetic properties, and EMR characteristics of Mn–Zn ferrite nanoparticles, without neglecting the effect of the contribution of Fe ions. It is also worth recalling that in 1947 Néel was interested in the so-called spinel ferrites of formula Fe2O3MO, where M is a 3d divalent metal cation (e.g. Fe2+). The magnetic properties of these materials, with spontaneous magnetization, were not understood in detail. Néel called them non-compensated antiferromagnets “ferrimagnets”.56
In this work, we conducted an in-depth characterisation of the chosen physical properties of ultrasmall (ca. 8 nm) Zn1−xMnFe2O4 nanoparticles with narrow size distribution fabricated by the non-hydrolytic approach. The main focus was on detailed EMR characteristics. We observed that the positive Curie–Weiss temperature obtained from macroscopic measurements of the inverse susceptibility, at temperatures well below the Curie temperature of the ferrimagnetic clusters, is assigned to the superparamagnetic behaviour of clusters of atoms with a net magnetic moment originated through A–B superexchange interactions. The linear thermal dependence of the inverse of susceptibility appears only at temperatures above the blocking temperatures of these superparamagnetic clusters. Thus, the apparent Curie–Weiss temperature was expected to be in the range of the blocking temperatures corresponding to the cluster size distribution. It is important to note that this temperature is not the critical one for a phase transition, but it corresponds to the temperature at which the relaxation time of the magnetic moment is similar to the measurement time.36 The EMR method makes it possible to measure the magnetic properties of materials in a way that other methods cannot. In some respects, the EMR can be more sensitive than SQUID because it responds only to magnetic ions with unpaired spin, whereas SQUID responds to both magnetic ions (seen in the EMR) and elements (atoms).
2. Experimental
2.1. Synthesis of Zn1−xMnxFe2O4 nanoparticles
The preparation procedure of the pure ZnFe2O4, MnFe2O4, and mixed Zn1−xMnxFe2O4 ferrites was based on the non-hydrolytic thermal decomposition technique in high boiling solvent i.e. benzyl alcohol (99% Thermo Scientific) as previously described by Bilecka et al.57 with our amendments. As a source of metal cations, the respective acetylacetonate complexes were chosen Zn(acac)2·H2O (99% Thermo Scientific), Mn(acac)2 (97% Thermo Scientific) as well as Fe(acac)3 (99% Thermo Scientific) where necessary were chosen. Since all ferrites were prepared using the same protocol, only the example of fabrication of Zn0.5Mn0.5Fe2O4 will be explained. In other cases, the recalculation of the main chemical quantities is mandatory to keep the adequate stoichiometry ratio for a given compound. The fabrication of Zn0.5Mn0.5Fe2O4 involved 1.25 mmol of Zn(acac)2·H2O, 1.25 mmol of Mn(acac)2, and 5 mmol of Fe(acac)3. Handling of all chemicals was carried out under the protective atmosphere of N2 using an acrylic glovebox (GS Glove Box Systemtechnik GMBH P10R250T2, Germany) equipped with automatic pressure gas control to protect them from possible deterioration. The metal complexes were dissolved in 70 ml of benzyl alcohol facilitated by sonication for 20 min in a cap-protected 100 ml two-neck glass flask. Since the zinc complex contains crystalline water before synthesis, the mixture temperature was raised to 120 °C for 1 hour to remove it. Subsequently, the flask was moved directly to the set-up containing the heating mantle, reflux column, and Pt-100 sensor connected to the automatic temperature controller (LTR 2500, Juchheim, Germany). The temperature was quickly increased to the boiling point of benzyl alcohol (205 °C) and left for 4 hours under constant reflux. After that, the reaction was stopped, and the flask with the final product was cooled to room temperature. The resulting black powder was separated and purified from the mother solution by washing/centrifugation cycles with ethanol (96% Chempur, Poland) and resuspended for long-term storage. The other ferrites were prepared by repeating the same procedure. Samples for TEM imaging were prepared in the form of suspensions of ethanol, while, for other experiments, ferrites were dried in a Petri dish at 60 °C for approximately 1 hour.2.2. Characterization of nanoparticle physicochemical properties
The powder X-ray diffraction technique was used to evaluate the structural properties of Zn1−xMnxFe2O4 nanomaterials with the help of the Bruker D8 Advance diffractometer. As an X-ray source, a Cu lamp (1.54 Å) was used, and to cut the Kα2 line Ni filter was mounted. X-ray patterns were collected in the 15–70° 2θ range with a 0.02° step while the integration time was set to 0.8 s. Zinc–manganese ferrite samples were ground before the experiment with the agate mortar and then placed in a special holder. All recorded reflections were compared with the reference standards of the Crystallography Open Database58 following card numbers 1010130 for ZnFe2O4, 2300583 Zn0.2Mn0.8Fe2O4, (c) Zn0.5Mn0.5Fe2O4, (d) Zn0.8Mn0.2Fe2O4, as well as 1528316 for MnFe2O4, respectively. Rietveld refinement was performed using the fundamental parameters approach implemented in MAUD software.59 A five-polynomial coefficient background, sample displacement, unit cell parameters, scale factor, Popa microstructure rules,60 and occupancy factors (only for (II) to (IV)) were sequentially refined. The presentation of the X-ray data and the analysis were performed using Origin Pro 2019 9.6 software (OriginLab, USA).A Tecnai Osiris X-FEG (FEI Company, USA) transmission electron microscope (TEM) operating at 200 kV was used for nanoparticle (NP) imaging and evaluation of particle size, distribution, and morphology. The sample preparation involved the sonication of ferrite-containing ethanol suspensions (ca. 25 μg ml−1) and placing a droplet of colloids on a 200 mesh carbon-coated copper grid (EM Resolutions, United Kingdom). The grids with the deposited materials were dried overnight under dust protection. Image analysis was performed in ImageJ freeware software (v. 1.8.0_1720). The concentration of particles in stock solution was determined using the Radwag MYA 5.4Y scale through three independent repetitions and final mass averaging.
Elemental analysis of mixed zinc manganese ferrites was performed using a Tescan Vega 3 scanning electron microscope (SEM) equipped with a Bruker Nano XFlash 6130 detector for energy-dispersive X-ray spectroscopy analysis (EDS). All samples for SEM-EDS analysis were prepared by placing a dry powder on a carbon tape attached to the aluminium-made holder. Data analysis was performed in a dedicated Bruker Esprit software (v. 2.1.1.1.17430) provided by the manufacturer.
Magnetic resonance measurements were performed on X-band (ν ≈ 9.4 GHz) and Q-band (ν ≈ 34 GHz) with modulation of the magnetic field at 100 kHz and 50 kHz, respectively, by a Bruker multifrequency and multiresonance FT-EPR ELEXSYS E580 spectrometer with X-band with a sensitivity of 7 × 109 spins per 0.1 mT having a resolution of 2.35 (micro) T or even better.61 Temperature dependence measurements were carried out at room temperature, and as a function of temperature in the X-band in the range from 95 K to 300 K using a Bruker liquid nitrogen gas-flow cryostat with 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131 VT digital controller, and in the Q-band in the range from 4 K to 300 K, using an ESR 900 liquid helium gas-flow cryostat from Oxford Instruments with Mercury iTC digital controller. Samples containing approximately 20 mg of sample powder were placed in 4 mm diameter quartz tubes.
131 VT digital controller, and in the Q-band in the range from 4 K to 300 K, using an ESR 900 liquid helium gas-flow cryostat from Oxford Instruments with Mercury iTC digital controller. Samples containing approximately 20 mg of sample powder were placed in 4 mm diameter quartz tubes.
3. Results and discussion
3.1. Physicochemical characterization of materials
The diffraction patterns for the zinc, manganese, and zinc–manganese nanoferrites were measured using the XRD technique and are presented in Fig. 1. In all cases, characteristic reflections were recorded that match with a reference standard card (COD database), namely 1010130 and 1528316 that were ascribed to the crystalline structure of ZnFe2O4 and MnFe2O4 cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 227) crystalline structure. No additional peaks were detected, which confirmed the structural purity of the obtained phases and the absence of impurities. The main feature observed for all compounds relies on a significant peak broadening. This effect was associated to the presence of a small crystallites with comparable full width at half maximum (FWHM) values (1.3 2θ, [220] plane) between samples. The average size of the crystals was calculated by using the well-known Scherrer method defined by the following equation:
m (no. 227) crystalline structure. No additional peaks were detected, which confirmed the structural purity of the obtained phases and the absence of impurities. The main feature observed for all compounds relies on a significant peak broadening. This effect was associated to the presence of a small crystallites with comparable full width at half maximum (FWHM) values (1.3 2θ, [220] plane) between samples. The average size of the crystals was calculated by using the well-known Scherrer method defined by the following equation: | (1) |
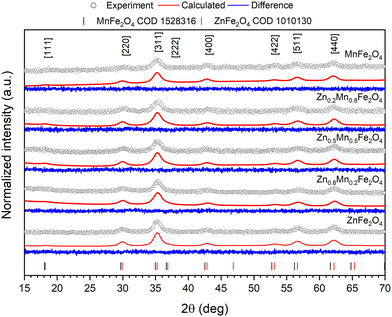 | ||
| Fig. 1 Graphical representation of the recorded X-ray patterns of pure and mixed zinc–manganese ferrites along with fitting results obtained through Rietveld refinement. | ||
| Crystal data | |||||
|---|---|---|---|---|---|
| Chemical Formula | ZnFe2O4 | Zn0.8Mn0.2Fe2O4 | Zn0.5Mn0.5Fe2O4 | Zn0.2Mn0.8Fe2O4 | MnFe2O4 |
| Crystal system, space group | Regular, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
||||
| a, (Å) | 8.4388 | 8.4259 | 8.4370 | 8.4355 | 8.4473 |
| V (Å3) | 600.95 | 598.20 | 600.57 | 600.25 | 602.77 |
| Z | 8 | ||||
| Radiation type | Cu Kα | ||||
| Refinement | |||||
| R | 0.0208 | 0.0216 | 0.0218 | 0.0210 | 0.0206 |
| wR | 0.0261 | 0.0269 | 0.0273 | 0.0261 | 0.0258 |
| S | 0.32 | 0.32 | 0.33 | 0.32 | 0.32 |
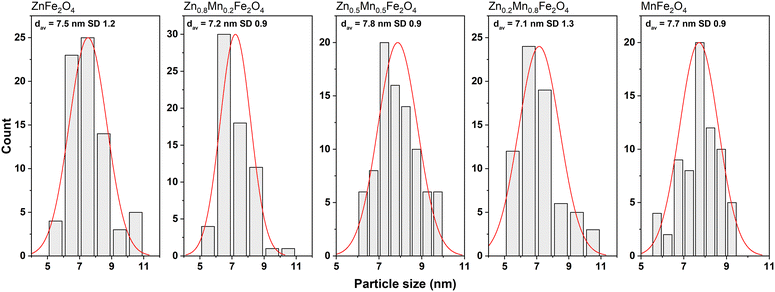
The size of the pure and mixed zinc–manganese ferrite particles, their distribution, and morphology were estimated using the TEM technique (Fig. 2 and 3). It should be noted that the fabricated nanomaterials did not differ in size, which was estimated to be 7.5 in all cases with a narrow distribution (monodisperse particles). This is beneficial since all of the observed effects will be associated only with that small particle size. The morphology of all ferrites is close to spherical with the presence of less regular particles with a tendency to form agglomerates. This behaviour is very typical for the synthesis of nanomaterials without the addition of any surface-blocking agents that will lead to a reduction of the surface energy responsible for agglomeration. Concerning particle size, good correspondence was found with the crystallite size estimated by the Scherrer formula, meaning that individual particles seen in TEM are represented as single crystallites.
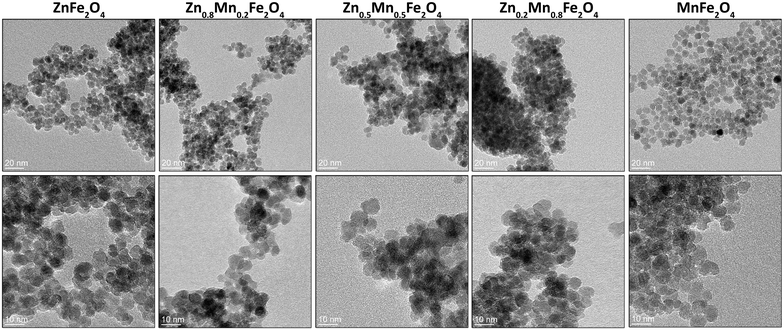 | ||
| Fig. 2 TEM images of the pure and mixed zinc–manganese ferrites obtained by the non-hydrolytic approach. | ||
SEM images as well as element maps of the Zn1−xMnxFe2O4 nanoferrites are shown in Fig. 4. It is evident that after manganese doping, the Mn2+ signal increases, while Zn2+ decreases significantly. It is, together with the XRD data, a straightforward proof of the Mn2+ incorporation into the structure of zinc ferrite for a broad concentration range. The content of the manganese ratio was calculated from the EDS data to be sufficiently close to the desired final composition, i.e., 19% for x = 0.2, 46% for x = 0.5, and 71% for the x = 0.8, respectively. Some deviation from the expected content of Mn2+ might be caused by the standard error of the EDS analysis (5%) and losses during substrate transfer.
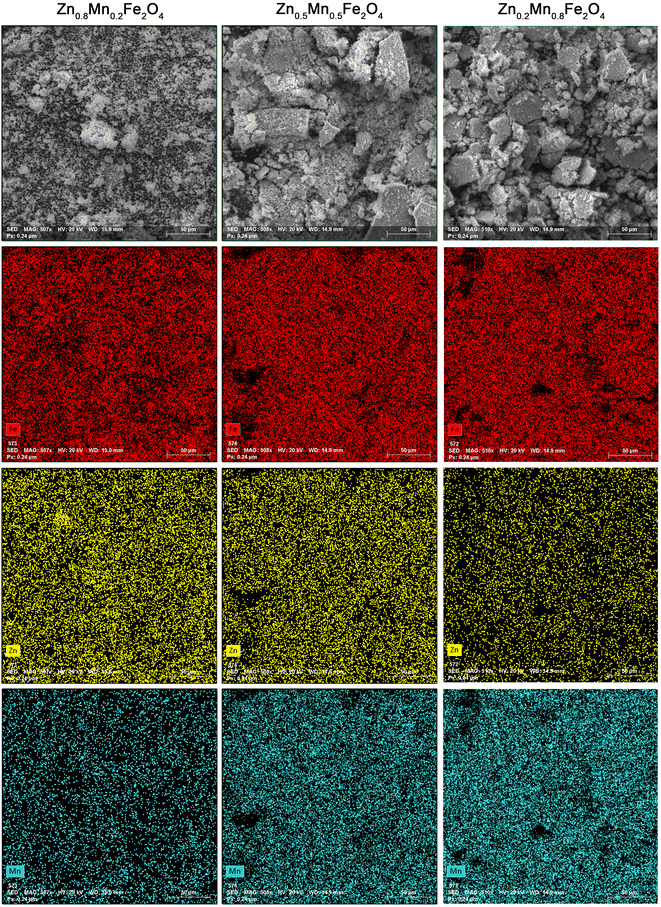 | ||
| Fig. 4 EDS and SEM elemental mapping of the Zn1−xMnxFe2O4 nanoparticles. EDS was performed covering a sample area of 250 μm × 250 μm in each case. | ||
The EMR characteristics of Zn1−xMnxFe2O4 ferrites samples were studied by electron magnetic resonance spectroscopy. The authors determined the resonance field (Bres) and resonance linewidth (Bpp) from measured EMR spectra to understand the homogeneity and crystalline anisotropy of Zn1−xMnxFe2O4 ferrites. Representative EMR spectra of Zn1−xMnxFe2O4 ferrites are plotted in Fig. 5.
Various magnetic parameters such as resonance linewidth (BPP), Lande's splitting factor (effective g-value) of Zn1−xMnxFe2O4 ferrite nanoparticles are listed in Table 2. The g-value is a function of the molecular motion, the symmetry of the ions, and the paramagnetic properties, which is also a constant of proportionality between the field and frequency. The effective g-value is calculated using the following relation:64
 | (2) |
| ZnFe2O4 | Zn0.8Mn0.2Fe2O4 | Zn0.5Mn0.5Fe2O4 | Zn0.2Mn0.8Fe2O4 | MnFe2O4 | |
|---|---|---|---|---|---|
| B pp – resonance linewidth, QCW – paramagnetic Curie temperature, C – Curie constant. | |||||
| Effective g-factor | 2.010 | 2.014 | 2.124 | 2.115 s | 2.253 |
| B pp (mT) | 33.60 | 56.03 | 113.46 | 133.67 | 108.40 |
| Q CW (K) | 89.1 | 75.6 | 5.6 | −112.0 | −183.6 |
| C | 1.57 × 10−10 | 3.88 × 10−10 | 8.32 × 10−10 | 14.58 × 10−10 | 71.43 × 10−10 |
The material studied Zn1−xMnxFe2O4 is a complex structure with various magnetic interactions. It is a kind of combination of two materials, MnFe2O4 and ZnFe2O4. A detailed analysis of the line parameters is planned in a separate article, including the calculation of the spin Hamiltonian. In the case of MnFe2O4, Fe-3d electrons occupy the crowded B-site while Mn-3d occupy the less electrons crowded A-site. From this anomalous behavior for the Fe 3d energy states position, electrons are not able to share the conduction mechanism (−8 eV away from Fermi level). The conduction mechanism in the spinel depends mainly on the super exchange between sites (A and B, for example between Mn and Fe cations through oxygen). Therefore, the Fe-3d electrons site for this cycle of conduction (Fe, O, and Mn) is missing. From the above anomalous explanation of density of states figures, it can be observed that a ferrimagnetic spin current between Fe-3d and Mn-3d through O-2p is yielded according to spin open or closed loop.42 Spin open loop:
(1) Fe–O spin exchange interaction in minority channel between −10 and −8 eV;
| Fe-3d↓ + O-2p → Fe-3d + O-2p↓, | (3) |
(2) O–O spin exchange interaction between minority and majority;
| O-2p↓(minority) + O-2p(majority) → O-2p↑, | (4) |
(3) O–Mn spin exchange interaction in majority channel between −2 and 0 eV.
| O-2p↑, Mn-3d → O-2p, Mn-3d↑, | (5) |
Spin closed loop: this p–d overlap is the origin of the ferrimagnetic superexchange interaction between tetra and octahedral sites in ferrites, which are characterized by high hardness, further confirming the p–d overlapping for oxygen, and that TM is too much strong.42 In the case of ZnFe2O4, the susceptibility χ is the sum of all magnetic contributions, paramagnetic (PM), diamagnetic (DM), AFM, and FM-like. It can be described as:
| χ = χPM + χDM + χAFM + χFM. | (6) |
On the other hand, at high fields, only the PM, DM, and AFM orderings vary with the field since the others are saturated.66 Therefore, FM-like contributions can be discarded at HF, and high-field susceptibility χHF can be described as:
| χHF = χDM + χPM + χAFM. | (7) |
Consequently, the ferri-, ferro-, and/or superparamagnetic contributions can be evaluated by calculating χHF and subtracting it from the entire magnetization curve.37 Theoretical calculations show the influence of inversion within a single unit cell, the smallest local inversion (δc) is either 0 or 1/8, the last corresponding to the exchange of a single Zn/Fe pair in the unit cell. Assuming that only a single Zn/Fe exchange can occur in a cell, around 40% of the unit cells suffer a single Zn/Fe cations exchange when the macroscopic δ is as small as 0.05. Unit cells with δc = 1/8 are FiM (40%) with a magnetic moment of 5.9μB,37 while for δc = 0 the cells are AFM (60%). This illustrates the dramatic effect that δ ≈ 0 can have on the magnetic and calorimetric properties.
Cobos et al.38 show for ZnFe2O4, that the magnetic entropy increment can be associated with the AFM to PM transition, and the small experimental value of 8.7 J mol−1 K−1 indicates that only 29% of the sample has evolved from AFM to the paramagnetic phase. In summary, instead of the expected 60%, only 29% of the sample appears to be AFM ordered.38
To briefly recap, the contribution of ZnFe2O4 gives a narrow line, while the line of MnFe2O4 is several times wider. The observed spectrum consists of a line from the Fe3+ ion (a line of about 150 mT), and a broad line that is a superposition of at least two components. In addition, a narrow line (about 350 mT) is also visible. As the concentration of Mn ions increases, the width of the resonance line also increases. The only exception is MnFe2O4, due to the fact that the effective g-factor comes from the superposition of the line. The spectrum of the Fe3+-coupled pair (Fe3+–O–Fe3+) in Fe2O3 is known to give a resonance line with an effective g-factor around 2.0. So, the effective g-value, 2.169, for the MnFe2O3 sample can be attributed to the superexchange interaction between the Fe3+ ions. A weak signal observed at about effective g-factor 4.2, in addition to the intense spectral line, can be attributed to an isolated Fe3+ in the orthorhombic field.67,68
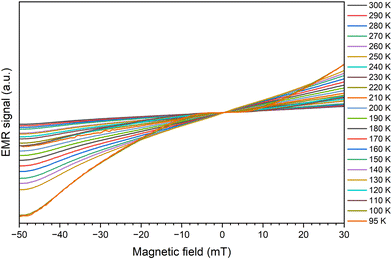
As the temperature increases, the signals gradually get sharper and more symmetrical, with a shift to a high field (see Fig. 6). Furthermore, with increasing temperature, a sharp decrease in the line width and an increase in the intensity of the EMR signal is observed (Fig. 9). This is because the thermal fluctuation of magnetic moments can reduce the extent of the anisotropic contributions to the free energy density and the effective magnetic field (the superparamagnetic effect).69 In this event, at elevated temperatures very narrow resonance spectra are observed, while at low temperatures the thermal fluctuations are gradually frozen out, and the magnetization direction is blocked, resulting in the broadening of the resonance line.
Broadening and shift to lower magnetic fields of magnetic resonance spectra with increasing temperature are typically observed for superparamagnetic (SPM) nanoparticles, and analogous cases can be found in a number of systems.69 However, for SPM particles, whose direction of magnetization fluctuates at a rate faster than the Larmor frequency, this results in a narrow resonance line because of an averaging effect of this fluctuation on the magnetocrystalline anisotropy. With a decrease in the temperature, the resonance line of the SPM particle broadens as the averaging effect of thermal fluctuations is reduced and the direction of magnetization is blocked, first in bigger and progressively in smaller particles. Thus, the narrow resonance line at room temperature, which progressively broadens with a reduction in temperature, once again confirms the superparamagnetic behavior of the prepared samples.68
In ferromagnetic materials, magnetic domains are in a fragile state of equilibrium, and the Bloch wall, which is a narrow transition region at the boundary between magnetic domains, moves with very low applied fields. In fact, LFMA is associated with the dynamics of magnetic domains in material.70 The existence of this absorption at room temperature is an indication of the ferromagnetic state of the material used to detect magnetic order. For bulk samples, the LFMA signal is used to determine the Curie temperature of ferromagnetic compounds.71,72 Above 28 nm, the existence of LFMA shows that these compounds are in a magnetic multi-domain state, and a flat response for compounds with smaller size shows that they are in a single-domain state. The absence of the LFMA signal in ferromagnetic compounds is a good indication of the superparamagnetic state in samples.73 By size reduction, the nanocrystalline passes from a particle with several magnetic domains to a monodomain particle; the latter is either in a single-domain state or in a superparamagnetic state. Low-field absorption cannot determine the intermediate state. Particles belonging to the single-domain state are characterized by the maximum magnetocrystalline anisotropy energy, consequently the direction of magnetization is ‘frozen’. This characteristic has an effect on the linewidth of resonant absorption; a comparative analysis can reveal the critical size of changes in magnetic states.
In the higher temperature range, the paramagnetic region, the line is relatively narrow and more regular. When the temperature is lower, we observe a widening of the line along with its deformation (the upper part), the centre moves along with the lower part of the line. The lower the temperature, the deformation increases. In the Q-band we observe a clear separation of the line (Fig. 7).
Increasing the content of manganese the value of the effective factor g increases, from 2.008 to 2.169 (for 100% Mn ions). A similar relation occurs for the width of the line. For ZnFe2O4, the line is the narrowest (33.6 mT). We observe an increase in line width to 130.6 mT for 80% manganese. Finally, for 100% Mn ion, the width is 108 mT, which is less than described above, due to the fact that we are facing two components of the resonance line that are offset from each other. Still, as a result of the summation, we will eventually get the widest recorded line. If the ZnFe2O4 component is missing, the line from the MnF2O4 component becomes narrower. The superposition of the two components of the line causes the relationship shown in Fig. 8 to change rapidly at temperatures around 120 K. This is particularly visible at contents of 50% or more manganese.
 | ||
| Fig. 8 Temperature dependence of the effective g-factor for Zn1−xMnxFe2O4 nanoparticles obtained in the X-band. | ||
We used the Curie–Weiss law to analyze the temperature dependences of the integral intensity, which is directly proportional to the magnetic susceptibility χ. A linear increase of χ−1 (T) at higher temperatures can be fitted to the Curie–Weiss law:
| (χ − χ0)−1 = (T − QCW)/C, | (8) |
Q CW is the paramagnetic Curie temperature, χ0 temperature independent coefficient to calculate the magnetic permeability, and the Pauli term for paramagnetism.74 Based on the theory, the inverse relationship of intensity (1/Int) is shown as a function of temperature for Zn1−xMnxFe2O4 nanoparticles samples (Fig. 9). Obtained values of Curie temperatures and Curie constants for all samples shown in Table 2.
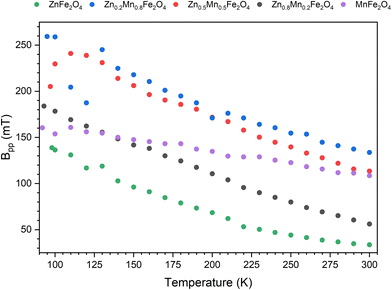 | ||
| Fig. 9 Temperature dependence of peak to peak line width for Zn1−xMnxFe2O4 nanoparticles obtained in X-band. | ||
Fig. 10(a–e) show the dependence of the inverse intensity as a function of temperature for Zn1−xMnxFe2O4 nanoparticles obtained in the X-band for EMR lines in a higher magnetic field (about 350 mT). Due to the antiferromagnetic character of the B–B and A–B superexchange interactions in spinel ferrites, the experimental finding of a positive value of the apparent Curie–Weiss temperature may be surprising. As Lotgering75 indicated, the measuring temperature should increase to 800 K to observe the straight line with negative Curie–Weiss temperature due to the antiferromagnetic A–B coupling, which gives rise to the ferrimagnetic clusters. On the other hand, the negative Curie–Weiss temperature associated with the B–B antiferromagnetic coupling could only be observed for δ = 0 (for example, with ideal ZnFe2O4 samples, in which there is no trace of ferrimagnetic clusters). However, since this ideal case is difficult to achieve, the previously reported anomalous sign for the Curie–Weiss temperature can be understood as a consequence of superparamagnetism associated with the almost unavoidable presence of a few ferrimagnetic clusters.36 We observed a non-linear increase in Qcw values for the increase in Zn content (Fig. 10f) as well as deviations from the designated Qcw from the literature. We identified the following reasons for these differences (1) only values for pure compounds are given in the literature; (2) particle size affects the Curie temperature76 that is especially manifested for nanoparticles with dimensions in the range of a few to several nanometers, thus observed changes in Qcw of several tens of K; (3) it is not insignificant that our measurements involve materials being a combination of Zn and Mn, so more complex magnetic interactions are to be expected than for single compounds.
Observed effects are more complex interactions than in pure zinc an manganese ferrites. As a result of the study, a relationship was observed between the composition of the material and the magnetic properties with striking antiferromagnetism to ferrimagnetism change (see Fig. 10f). Hence, by modifying the composition of materials, we have the possibility of controlling the magnetic properties (FM–AFM–FiM).
4. Conclusions
Ultra-small Zn1−xMnxFe2O4 nanoparticles with an average crystallite size of 8 nm were synthesized using a convenient non-hydrolytic route by thermal decomposition of zinc and manganese complexes in high-temperature boiling organic solvent. Fabricated materials did not differ in particle size and showed a narrow distribution while being close to spherical morphology. The visible tendency to form agglomerated structures is due to the lack of surface surface-blocking agents that, if necessary, can be used for applications where it is mandatory. The chosen synthetic approach allows for control of the chemical composition within a broad concentration range, which additionally has a significant effect on the final physicochemical properties as shown by EMR spectroscopy.EMR spectral studies indicated that the prepared nanoparticles were superparamagnetic. The linewidth of EMR signal for any ferrite material generally originated from two sources: (a) magnetic dipole–dipole interactions among particles and (b) interparticle superexchange interactions between magnetic ions through oxygen ions. The presented EMR lines are obtained from contributions from ZnFe2O4 and MnFe2O4. The contribution of ZnFe2O4 gives a narrow line, while the line of MnFe2O4 is several times wider. The observed spectrum is composed of a line originating from the Fe3+ ion (a line around 150 mT) and a broad line that is a superposition of at least two components. A narrow line (around 350 mT) was also analyzed. An increase in the width of the resonant line was observed, proportional to the increase in Mn concentration in the sample. The only exception is MnFe2O4, due to the fact that the effective g-factor comes from the superposition of the line.
In the higher temperature range, the paramagnetic region, the observed lines are relatively narrow and regular. With a decrease in the temperature, a broadening of the line is observed along with a deformation of the upper part, with the centre of the line moving along with the lower part. The lower the temperature, the greater the deformation becomes. Performing measurements in the Q-band allowed us to observe separated resonant lines. Both ZnFe2O4 and MnFe2O4 can be decomposed into two inequivalent magnetic sublattices, denoted as O and T, respectively. The T sublattice is not frustrated in the NN interaction, whereas in the presence of AFM interactions within the sublattice, the O sublattice can host a large NN frustration. While the ions within a sublattice often interact FM and the ions between two sublattices interact AFM, some spinel compounds can give rise to the interaction of AFM between ions at the O sites that leads to geometric frustration. As a result, the overall magnetic behavior depends on the strength of the exchange interaction between O–O (JOO), T–T (JTT) and O–T (JOT). We observe a non-linear increase in Qcw values with an increase of Zn content. Such complex behavior is caused by the size of the particles as well as chemical structural modification that leads to more complex magnetic interactions than that expected for pure compounds.
Author contributions
M. K.-G. and A. T. synthesis of particles, TEM imaging, R. P. XRD measurements, P. K. SEM and EDS characterization, B. Z. Rietveld refinement, I. R., B. C. EMR measurements, I. R., I. S. EMR measurements analysis and interpretations of EMR spectra, I. R., B. C., M. K.-G., A. T., R. P., I. S., P. K. writing manuscript, editing, and corrections. R. P. and I. S. idea and conceptualization. All authors read the manuscript and agreed with its content.Data availability
All data are accessible from the corresponding authors upon request.Conflicts of interest
We declare no conflict of interest.References
- B. Skołyszewska, W. Tokarz, K. Przybylski and Z. Kakol, Phys. C, 2003, 387, 290–294 CrossRef
.
- M. Sugimoto, J. Am. Ceram. Soc., 1999, 82, 269–280 CrossRef CAS
.
- K. E. Sickafus, J. M. Wills and N. W. Grimes, J. Am. Ceram. Soc., 1999, 82, 3279–3292 CrossRef CAS
.
- D. Dadarlat, C. Neamtu, M. Streza, R. Turcu, I. Craciunescu, D. Bica and L. Vekas, J. Nanopart. Res., 2008, 10, 1329–1336 CrossRef CAS
.
- H. S. C. O'Neill and A. Navrotsky, Am. Mineral., 1983, 68, 181–194 Search PubMed
.
- C. Simon, A. Blösser, M. Eckardt, H. Kurz, B. Weber, M. Zobel and R. Marschall, Z. Anorg. Allg. Chem., 2021, 647, 2061–2072 CrossRef CAS
.
- M. V. Nikolic, Z. Z. Vasiljevic, M. D. Lukovic, V. P. Pavlovic, J. B. Krstic, J. Vujancevic, N. Tadic, B. Vlahovic and V. B. Pavlovic, Int. J. Appl. Ceram. Technol., 2019, 16, 981–993 CrossRef CAS
.
- B. Antic, A. Kremenovic, A. S. Nikolic and M. Stoiljkovic, J. Phys. Chem. B, 2004, 108, 12646–12651 CrossRef CAS
.
- R. J. Hill, J. R. Craig and G. V. Gibbs, Phys. Chem. Miner., 1979, 4, 317–339 CrossRef CAS
.
- R. Pązik, E. Piasecka, M. Małecka, V. G. Kessler, B. Idzikowski, Z. Śniadecki and R. J. Wiglusz, RSC Adv., 2013, 3, 12230 RSC
.
- G. Xu, L. Li, Z. Shen, Z. Tao, Y. Zhang, H. Tian, X. Wei, G. Shen and G. Han, J. Alloys Compd., 2015, 629, 36–42 CrossRef CAS
.
- S. Wen, B. Chen, J. Zhang, W. Zhan, Z. He and L. Gao, Crystals, 2023, 13, 1509 CrossRef CAS
.
- A. G. Niculescu, C. Chircov and A. M. Grumezescu, Methods, 2022, 199, 16–27 CrossRef CAS PubMed
.
- M. D. Nguyen, H. V. Tran, S. Xu and T. R. Lee, Appl. Sci., 2021, 11, 11301 CrossRef CAS PubMed
.
- H. Gavilán, S. K. Avugadda, T. Fernández-Cabada, N. Soni, M. Cassani, B. T. Mai, R. Chantrell and T. Pellegrino, Chem. Soc. Rev., 2021, 50, 11614–11667 RSC
.
- S. J. Salih and W. M. Mahmood, Heliyon, 2023, 9, e16601 CrossRef CAS PubMed
.
- B. Sun, F. Chen, W. Yang, H. Shen and D. Xie, J. Magn. Magn. Mater., 2014, 349, 180–187 CrossRef CAS
.
- A. Goldman and A. M. Laing, J. Phys. (Paris), Colloq., 1976, 38, 297–301 Search PubMed
.
- P. T. Phong, P. H. Nam, D. H. Manh, D. K. Tung, I. J. Lee and N. X. Phuc, J. Electron. Mater., 2015, 44, 287–294 CrossRef CAS
.
- V. D. Krishna, K. Wu, A. M. Perez and J. P. Wang, Front. Microbiol., 2016, 7, 400 Search PubMed
.
- P. F. Teh, S. S. Pramana, Y. Sharma, Y. W. Ko and S. Madhavi, ACS Appl. Mater. Interfaces, 2013, 5, 5461–5467 CrossRef CAS PubMed
.
- A. Khazaei, A. Ranjbaran, F. Abbasi, M. Khazaei and A. R. Moosavi-Zare, RSC Adv., 2015, 5, 13643–13647 RSC
.
- C. Bárcena, A. K. Sra, G. S. Chaubey, C. Khemtong, J. P. Liu and J. Gao, Chem. Commun., 2008, 2224–2226 RSC
.
- N. Dogan, G. Caliskan and M. Irfan, J. Mater. Sci.: Mater. Electron., 2023, 34, 390 CrossRef CAS
.
- P. Thakur, D. Chahar, S. Taneja and N. Bhalla, Ceram. Int., 2020, 46, 15740–15763 CrossRef CAS PubMed
.
- Z. M. Abdulhamid, A. A. Dabbawala, T. Delclos, R. Straubinger, M. Rueping, K. Polychronopoulou and D. H. Anjum, Sci. Rep., 2023, 13, 19705 CrossRef CAS PubMed
.
- T. Sembiring, D. Siburian and M. Rianna, Mater. Sci. Energy Technol., 2023, 6, 124–129 CAS
.
- F. Ameen Ramiza, S. K. Ajmal, M. B. Khan, A. Nasim, Y. Jamil, K. Kashif and S. Amira, IOP Conf. Ser.:Mater. Sci. Eng., 2016, 146, 012029 Search PubMed
.
- S. J. Haralkar, R. H. Kadam, S. S. More, S. E. Shirsath, M. L. Mane, S. Patil and D. R. Mane, Mater. Res. Bull., 2013, 48, 1189–1196 CrossRef CAS
.
- S. F. Wang, Y. F. Hsu and C. H. Chen, J. Electroceram., 2014, 33, 172–179 CrossRef CAS
.
- M. Kulpa-Greszta, A. Tomaszewska, A. Dziedzic and R. Pązik, RSC Adv., 2021, 11, 20708–20719 RSC
.
- V. I. Popkov, V. P. Tolstoy and V. G. Semenov, J. Alloys Compd., 2020, 813, 152179 CrossRef CAS
.
- X. L. Wang, M. James, J. Horvat and S. X. Dou, Supercond. Sci. Technol., 2002, 15, 427–430 CrossRef CAS
.
- V. Tsurkan, J. Hemberger, A. Krimmel, H. A. Krug Von Nidda, P. Lunkenheimer, S. Weber, V. Zestrea and A. Loidl, Phys. Rev. B:Condens. Matter Mater. Phys., 2006, 73, 224442 CrossRef
.
- J. Hemberger, H. A. K. Von Nidda, V. Tsurkan and A. Loidl, Phys. Rev. Lett., 2007, 98, 147203 CrossRef CAS PubMed
.
- A. Hernando, M. Á. Cobos, J. A. Jiménez, I. Llorente, A. García-Escorial and P. de la Presa, Materials, 2022, 15, 4789 CrossRef CAS PubMed
.
- M. A. Cobos, P. De La Presa, I. Llorente, J. M. Alonso, A. Garciá-Escorial, P. Marín, A. Hernando and J. A. Jiménez, J. Phys. Chem. C, 2019, 123, 17472–17482 CrossRef CAS
.
- M. A. Cobos, A. Hernando, J. F. Marco, I. Puente-Orench, J. A. Jiménez, I. Llorente, A. García-Escorial and P. de la Presa, Materials, 2022, 15, 1198 CrossRef CAS PubMed
.
- M. Á. Cobos, P. de la Presa, I. Puente-Orench, I. Llorente, I. Morales, A. García-Escorial, A. Hernando and J. A. Jiménez, Ceram. Int., 2022, 48, 12048–12055 CrossRef CAS
.
- M. L. Néel, Ann. Phys., 1948, 12, 137–198 Search PubMed
.
-
C. Lacroix, P. Mendels and F. Mila, Introduction to Frustrated Magnetism, Springer, 2011 Search PubMed
.
- A. Elfalaky and S. Soliman, J. Alloys Compd., 2013, 580, 401–406 CrossRef CAS
.
- D. S. Mathew and R. S. Juang, Chem. Eng. J., 2007, 129, 51–65 CrossRef CAS
.
- T. A. Kaplan and N. Menyuk, Philos. Mag., 2007, 87, 3711–3785 CrossRef CAS
.
- C. Ederer and M. Komelj, Phys. Rev. B:Condens. Matter Mater. Phys., 2007, 76, 064409 CrossRef
.
- R. Nepal, M. Saghayezhian, J. Zhang and R. Jin, J. Magn. Magn. Mater., 2020, 497, 165955 CrossRef CAS
.
- F. J. Burghart, W. Potzel, G. M. Kalvius, E. Schreier, G. Grosse, D. R. Noakes, W. Schak, W. Kockelmann, S. J. Campbell, W. A. Kaczmarek, A. Martin and M. K. Krause, Phys. B, 2000, 289–290, 286–290 CrossRef
.
- J. C. Ho, H. H. Hamdeh, Y. Y. Chen, S. H. Lin, Y. D. Yao, R. J. Willey and S. A. Oliver, Phys. Rev. B:Condens. Matter Mater. Phys., 1995, 52, 10122–10126 CrossRef CAS PubMed
.
- J. F. Hochepied, P. Bonville and M. P. Pileni, J. Phys. Chem. B, 2000, 104, 905–912 CrossRef CAS
.
- M. R. Anantharaman, S. Jagatheesan, K. A. Malini, S. Sindhu, A. Narayanasamy, C. N. Chinnasamy, J. P. Jacobs, S. Reijne, K. Seshan, R. H. H. Smits and H. H. Brongersma, J. Magn. Magn. Mater., 1998, 189, 83–88 CrossRef CAS
.
- J. P. Jamet and A. P. Maiozemoff, Phys. Rev. B:Condens. Matter Mater. Phys., 1978, 8, 75–91 CrossRef
.
- F. Gazeau, J. C. Bacri, F. Gendron, R. Perzynski, Y. L. Raikher, V. I. Stepanov and E. Dubois, J. Magn. Magn. Mater., 1998, 186, 175–187 CrossRef CAS
.
- E. De Biasi, C. A. Ramos, R. D. Zysler and H. Romero, Phys. Rev. B:Condens. Matter Mater. Phys., 2002, 65, 1–8 CrossRef
.
- R. Berger, J. Kliava, J. C. Bissey and V. Baïetto, J. Appl. Phys., 2000, 87, 7389–7396 CrossRef CAS
.
- A. Mendoza, J. Prado and O. Almanza, Acta Phys. Pol., A, 2012, 121, 950–953 CrossRef CAS
.
- B. Barbara, C. R. Phys., 2019, 20, 631–649 CrossRef CAS
.
- I. Bilecka, I. Djerdj and M. Niederberger, Chem. Commun., 2008, 886–888 RSC
.
- A. Vaitkus, A. Merkys and S. Grazulis, J. Appl. Crystallogr., 2021, 54, 661–672 CrossRef CAS PubMed
.
- L. Lutterotti, Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, 268, 334–340 CrossRef CAS
.
- N. C. Popa, J. Appl. Crystallogr., 1998, 31, 176–180 CrossRef CAS
.
- A. V. Raut, S. A. Jadhav, D. R. Shengule and K. M. Jadhav, J. Sol-Gel Sci. Technol., 2016, 79, 1–11 CrossRef CAS
.
-
H. P. Klug and L. E. Alexander, X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, Wiley, New York, 2nd edn, 1974 Search PubMed
.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef
.
-
A. Lund and M. Shiotani, Applications of EPR in radiation research, Springer International Publishing, 2014 Search PubMed
.
- F. A. Ahmed and L. N. Singh, J. Mater. Sci. Surf. Eng., 2018, 6, 825–830 CAS
.
- C. Richter and B. A. Van Der Pluijm, Phys. Earth Planet. Inter., 1994, 82, 113–123 CrossRef
.
- K. Tanaka, K. Kamiya, T. Yoko, S. Tanabe, K. Hirao and N. Soga, J. Non-Cryst. Solids, 1989, 109, 289–294 CrossRef CAS
.
- K. V. P. M. Shafi, A. Gedanken, R. Prozorov and J. Balogh, Chem. Mater., 1998, 10, 3445–3450 CrossRef CAS
.
- X. Cao and L. Gu, Nanotechnology, 2005, 16, 180–185 CrossRef CAS PubMed
.
- R. Valenzuela, G. Alvarez, H. Montiel, M. P. Gutiérrez, M. E. Mata-Zamora, F. Barrón, A. Y. Sánchez, I. Betancourt and R. Zamorano, J. Magn. Magn. Mater., 2008, 320, 1961–1965 CrossRef CAS
.
- H. Gavi, B. D. Ngom, A. C. Beye, A. M. Strydom, V. V. Srinivasu, M. Chaker and N. Manyala, J. Magn. Magn. Mater., 2012, 324, 1172–1176 CrossRef CAS
.
- G. Alvarez, H. Montiel, J. F. Barron, M. P. Gutierrez and R. Zamorano, J. Magn. Magn. Mater., 2010, 322, 348–352 CrossRef CAS
.
-
M. Yahya, F. Hosni and A. H. Hamzaoui, in Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis, ed. T. Shabatina and V. Bochenkov, Intechopen, 2020, pp. 2–22 Search PubMed
.
- K. Kambe, J. Phys. Soc. Jpn., 1950, 5, 48–51 CrossRef CAS
.
- F. K. Lotgerlng, J. Phys. Chem. Solids, 1966, 27, 139–145 CrossRef
.
- N. S. Gajbhiye, G. Balaji and M. Ghafari, Phys. Status Solidi A, 2002, 189, 357–361 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |