Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and X-ray structure analysis of cytotoxic 2-picolylamino-type HfIV-bis-chelated complexes†
Tiankun
Zhao‡
 *ab,
Qi
Zhang‡
a,
Jialiu
Zhao‡
a,
Dongyu
Mei
a,
Jing
Ma
a,
Isabel
Correia
*ab,
Qi
Zhang‡
a,
Jialiu
Zhao‡
a,
Dongyu
Mei
a,
Jing
Ma
a,
Isabel
Correia
 b,
Zhongduo
Yang
a,
Sa-Hyun
Kim
c and
Thomas
Huhn
b,
Zhongduo
Yang
a,
Sa-Hyun
Kim
c and
Thomas
Huhn
 *d
*d
aSchool of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, 730050, China. E-mail: tiankun.zhao@tecnico.ulisboa.pt; zhaotiankun2006@163.com
bCentro de Química Estrutural and Departamento de Engenharia Química, Institute of Molecular Sciences, Instituto Superior Técnico, Avenida Rovisco Pais 1, 1049-001 Lisboa, Portugal
cDepartment of Clinical Laboratory Science, Kyungpook National University, Daegu, 27136, South Korea
dFachbereich Chemie, Universität Konstanz, Universitätsstr. 10, D-78457 Konstanz, Germany
First published on 18th March 2025
Abstract
Eight novel heteroleptic HfIV complexes containing differently substituted 2-picolylamino-bis-phenolate and 2,6-dipicolinic acid (Dipic) groups as chelating ligands were synthesized and characterized with yields higher than 80%. These [ONON] type HfIV complexes have good aqueous stability and potent anti-tumor activity against HeLa S3 (human cervical adenocarcinoma) and Hep G2 (human derived hepatoma) cells. In particular, the complexes demonstrated selective inhibitory activity against Hep G2 cells. The IC50 value of the most cytotoxic complex [L1HfIVDipic4-Cl] (0.9 ± 0.4 μM) was ten-fold higher than that of cisplatin (11.2 ± 2.1 μM) on Hep G2 cells, being the most cytotoxic anti-tumor HfIV complex to date. Furthermore, [L1HfIVDipic4-Cl] could inhibit tumor cell migration, induce reactive oxygen species generation (particularly HO˙), loss of mitochondrial membrane potential and almost exclusive early apoptosis in HeLa S3 cells. [L1HfIVDipic4-Cl] exhibited rapid cellular uptake by HeLa S3 cells, and when in aqueous media, these HfIV complexes slowly hydrolyzed, releasing non-toxic phenolato ligands as the product of hydrolysis. Overall, these rare earth complexes, particularly [L1HfIVDipic4-Cl], show promising potential as novel anticancer agents with significant efficacy against human liver cancer cells and favorable selectivity profiles for further therapeutic development.
Introduction
Titanocene dichloride ([Cp2TiIVCl2], TDC) and budotitane (bis-(1-phenyl-1,3-butanedione)TiIV) were the first group 4 metal complexes with anti-tumor activity reported in the 1980s.1,2 Both entered clinical trials but failed due to limited aqueous stability and undefined mechanisms of action. Still, they opened a new chapter in medicinal chemistry as a large number of their derivatives have been synthesized and evaluated for anti-tumor efficacy over the last decades.3 In 2007, Tshuva et al. reported a di-amino-phenolato TiIV bis-alkoxyl complex that exhibited significantly improved aqueous stability and stronger anti-tumor activity than TDC,4 thus laying the foundation for a new generation of anti-tumor TiIV complexes.5,6 Following studies, by us7,8 and others,9,10 it was shown that the aqueous stability can be further improved by replacing the labile alkoxy groups on TiIV with additional chelators. These can either be part of the phenolato backbone (N-hydroxyethyl), resulting in homoleptic complexes, or achieved by introducing an additional chelator (2,6-dipicolinic acid, Dipic), resulting in heteroleptic TiIV complexes. These heteroleptic TiIV complexes have significantly enhanced kinetic stability and anti-tumor activity. The anti-tumor active species formed by hydrolysis,11 the in vivo anti-tumor efficacy12 and the mechanism of action were subsequently investigated.13,14In addition to their role as potential anti-tumor candidates, the above heteroleptic Ti-complexes have recently found application in tumor sensing and as tumor targeting drugs.15 A 45Ti complex stabilized by the above heteroleptic ligand system has recently been used as a PET (positron emission tomography) tracer to visualize tumor tissue. Conjugation of a Dipic-based heteroleptic 45Ti complex with tumor targeting PSMA (prostate specific membrane antigen) has combined chemotherapeutic effects with diagnostic features.16
Recently, we reported a post-functionalization protocol for [(Salan)TiIV(Dipic)]via a pallidum-catalyzed Sonogashira reaction,17 and an environmentally benign synthesis of heteroleptic bis-chelated TiIV complexes in green solvents within minutes.18 Since SAR (structure–activity relationship) data revealed that the phenolato “part” is crucial for the anti-tumor activity of Salan-type TiIV complexes,19 we further expanded the complex library to three types of phenolato ligands: [ONNO] (Salan) type, [ONON] type with a 2-picolylamino “side bridge” and [ONOO] type with an N-(2-hydroxyethyl) “side arm”. Among them, the [ONON] type TiIV complexes showed the strongest anti-tumor activity and low toxicity on primary cells.13 Despite the above achievements for anti-tumor TiIV complexes, the field for the other two group 4 metals, Zr (Zirconium) and Hf (Hafnium), is surprisingly underdeveloped. In particular, to the best of our knowledge, for anti-tumor HfIV complexes, only 10 related reports have been found.
Coordination complexes of rare earth metals, especially those of hafnium, have been mainly used in luminescent materials20,21 and as catalysts for polymerization reactions.22–25 Regarding bioactive HfIV complexes, early reports showed that hafnocene dichloride did not exhibit anti-tumor activity.26 A folate–HfIV conjugate showed anti-bacterial and anti-fungal activity.27 In contrast, McGowan et al. reported that the IC50 of a tris-diphenyl β-diketonato HfIV complex was in the same range as that of cisplatin against HT-29 (human colorectal adenocarcinoma) and MCF-7 (Michigan Cancer Foundation-7, human breast adenocarcinoma). However, its aqueous stability was not investigated,28 probably for the same reasons that budotitane failed earlier. Recently, Choi et al. reported that a Hf-MOF (metal organic framework) containing a folate moiety could generate ROS (reactive oxygen species), deregulate tumor-cell proliferation, as well as increase apoptosis.29 We recently reported four Salan-type HfIV complexes containing Dipic derivatives as the second chelator.30 The complex with Dipic substituted with Cl at position 4 showed rapid cellular uptake and induced almost exclusively apoptosis in HeLa S3 cells (human cervical adenocarcinoma). Furthermore, an oxo-bridged dimeric HfIV–HfIV complex was identified as a novel anti-tumor compound.31 In contrast to Hf-based nanomaterials, which have been intensively studied,32,33 molecular HfIV complexes are rather scarce, with only four representatives of HfIV bis-chelates known to date. We report herein the synthesis and characterization of eight new HfIV bis-chelates with tripodal tetradentate [ONON]-type ligands, H2L1–4 bearing a 2-picolylamino side bridge (Scheme 1). Their in vitro anti-tumor activity and mechanism of cellular uptake, ROS production and apoptosis are also investigated and presented.
 | ||
| Scheme 1 Synthesis of 2-picolylamino [ONON]-type HfIV bis-chelates [L1–4HfIVDipic] and [L1–4HfIVDipic4-Cl]. | ||
Results and discussion
Synthesis and molecular structure
The SAR (structure–activity relationship) from our previous report on phenolato TiIV bis-chelates indicated that the aqueous stability and anti-tumor activity of the complexes were dominated by the substitution pattern on the phenolato moiety.13 When the 2 position of the phenyl (phenolato) was occupied by sterically demanding groups, such as t-butyl, the stability was significantly enhanced. While cytotoxicity was maintained for 2-picolylamino and 2-hydroxyethylamino TiIV complexes, it disappeared for Salan TiIV complexes bearing the ethylenediamino linker. Less bulky substitutions such as those with methyl were important for both cytotoxicity and stability. Lighter halogen atoms, such as Cl and F, increased the cytotoxicity, whereas Br decreased it. Additionally, our previous work with HfIV complexes showed that the Cl atom on the Dipic co-ligand was also important, since it enhanced the compound's polarity and provided the necessary solubility in polar solvents.30Based on the above observations, ligands H2L1–4 containing t-butyl and methyl at the 2, 3 and 4 positions (phenyl) were synthesized, and Dipic4-Cl was also employed as a second chelator. It is worth mentioning that 2-picolylamino ligands bearing halogens could not be converted into the corresponding HfIV complexes and were therefore excluded from this study. [L1–4HfIVDipic4-H,Cl] complexes were easily synthesized from Hf(OnBu)4 in toluene, following our previous report,30 and in good yields (>80%). The HfIV complexes of H2L2 and H2L3 containing t-butyl in the ortho position to the OH group could be purified directly by flash chromatography on silica gel, whereas the HfIV complexes of H2L1 and H2L4 are kinetically less stable and decompose on silica gel. For these, purification relied on filtration of insoluble matter and washing with toluene to remove unreacted ligands. The enhanced stability with increasing bulkiness at the ortho position is a direct consequence of steric shielding of the O–Ti bond.19 All ligands and HfIV complexes were characterized by the usual analytical techniques of 1H and 13C NMR, FTIR, HRMS (ESI-TOF), elemental analysis and UV-vis absorption spectroscopy and details are given in the ESI.† These confirm the molecular structure of the compounds and their purity. The HPLC analyses (see the ESI†) show, for all complexes, the presence of one peak, corresponding to >99%, corroborating their high purity.
Single crystals suitable for X-ray diffraction analysis of [L2HfIVDipic] and [L2HfIVDipic4-Cl] were obtained by slow crystallization in a mixed solvent consisting of dichloromethane and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −20 °C. The solid state ORTEP diagrams of the molecular structures are depicted in Fig. 1. The main crystallographic data and selected bond distances and angles are given in the ESI.†[L2HfIVDipic] is triclinic and [L2HfIVDipic4-Cl] is monoclinic and they crystallized in P
1) at −20 °C. The solid state ORTEP diagrams of the molecular structures are depicted in Fig. 1. The main crystallographic data and selected bond distances and angles are given in the ESI.†[L2HfIVDipic] is triclinic and [L2HfIVDipic4-Cl] is monoclinic and they crystallized in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and P21/n space groups, respectively. The [L2HfIVDipic] unit cell also contains one molecule of hexane and one molecule of H2O. Similar to the reported [ONNO]-type (Salan) [L5HfIVDipic] (L5 = 6,6′-((ethane-1,2-diylbis(methylazanediyl))bis(methylene))bis(2,4-dimethylphenol)),30 the tripodal [ONON]-type ligand occupies one face of a pseudo-octahedron with Dipic occupying the remaining cis-positions, forming a hepta-coordination sphere around the HfIV centre. The bond angles of O(1)–Hf–O(2) (161.31° and 162.93°) and O(3)–Hf–O(4) (137.06° and 137.91°) of [L2HfIVDipic] and [L2HfIVDipic4-Cl] are all smaller than those of [L5HfIVDipic] (168.33° and 138.52°), due to the tripodal arrangement of L2. However, the 2-picolylamino side bridge does not significantly affect the N(1)–Hf–N(2) angle (Table 1). Finally, the dihedral angle of the phenyl moieties in [L5HfIVDipic] (78.44°) is much larger than in [L2HfIVDipic] and [L2HfIVDipic4-Cl] (49.3° and 48.7°) (Fig. S3†). This rather “flat” arrangement of the phenyl rings reflects to some extent the spatial flexibility of the 2-picolylamino HfIV complexes.
and P21/n space groups, respectively. The [L2HfIVDipic] unit cell also contains one molecule of hexane and one molecule of H2O. Similar to the reported [ONNO]-type (Salan) [L5HfIVDipic] (L5 = 6,6′-((ethane-1,2-diylbis(methylazanediyl))bis(methylene))bis(2,4-dimethylphenol)),30 the tripodal [ONON]-type ligand occupies one face of a pseudo-octahedron with Dipic occupying the remaining cis-positions, forming a hepta-coordination sphere around the HfIV centre. The bond angles of O(1)–Hf–O(2) (161.31° and 162.93°) and O(3)–Hf–O(4) (137.06° and 137.91°) of [L2HfIVDipic] and [L2HfIVDipic4-Cl] are all smaller than those of [L5HfIVDipic] (168.33° and 138.52°), due to the tripodal arrangement of L2. However, the 2-picolylamino side bridge does not significantly affect the N(1)–Hf–N(2) angle (Table 1). Finally, the dihedral angle of the phenyl moieties in [L5HfIVDipic] (78.44°) is much larger than in [L2HfIVDipic] and [L2HfIVDipic4-Cl] (49.3° and 48.7°) (Fig. S3†). This rather “flat” arrangement of the phenyl rings reflects to some extent the spatial flexibility of the 2-picolylamino HfIV complexes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30
| [L2HfIVDipic] | [L2HfIVDipic4-Cl] | [L5HfIVDipic] | |
|---|---|---|---|
| O(1)–Hf | 1.980(19) | 1.998(3) | 1.985(2) |
| O(2)–Hf | 1.983(19) | 1.969(3) | 1.988(2) |
| O(3)–Hf | 2.133(19) | 2.156(3) | 2.121(2) |
| O(4)–Hf | 2.149(19) | 2.116(3) | 2.135(2) |
| N(1)–Hf | 2.398(2) | 2.402(4) | 2.432(3) |
| N(2)–Hf | 2.394(2) | 2.427(4) | 2.420(3) |
| N(3)–Hf | 2.292(2) | 2.282(4) | 2.283(3) |
| O(1)–Hf–O(2) | 161.31(8) | 162.93(14) | 168.33(9) |
| O(3)–Hf–O(4) | 137.06(7) | 137.91(12) | 138.52(9) |
| N(1)–Hf–N(2) | 71.11(8) | 70.51(13) | 72.67(9) |
Additionally, PXRD (powder X-ray diffraction) was performed on [L2HfIVDipic] and [L2HfIVDipic4-Cl], and the obtained spectra are consistent with the simulated ones (Fig. S4†), suggesting a pure phase for the two Hf-based crystals.
Stability and hydrolysis
The aqueous stability of the synthesized HfIV complexes was investigated in a mixed solvent of 1/10 (v/v) of H2O/THF (H2O calculated to be 1 × 105 equiv.) by time-resolved UV-vis spectroscopy at 37 °C. As shown in Table 2, [L1HfIVDipic], [L1HfIVDipic4-Cl], [L4HfIVDipic] and [L4HfIVDipic4-Cl] decomposed rather slowly with t1/2 (half-hydrolyzation time) calculated to be 20 h, 10 h, 20 h and 15 h, respectively. The stability characteristics of these [ONON]-type HfIV complexes are similar to those of the Salan HfIV bis-chelates, as evidenced by the complexes of L2 and L3 with at least one t-butyl ortho to the phenolato oxygen, which remained stable (see the ESI, Fig. S9–S16†) in the time frame studied. Moreover, the products of hydrolysis of [L1HfIVDipic4-H,Cl] and [L4HfIVDipic4-H,Cl] complexes were isolated from scaled-up reactions at 50 °C and characterized by HRMS (high resolution mass spectrometry) to be H2L1 and H2L4, respectively (Fig. S17 and S18 in the ESI†).| Entrya | Complex | t 1/2 (h) | λ max (nm) |
|---|---|---|---|
| a Hydrolysis was followed by time-resolved UV-vis spectroscopy after mixing 3 mL of THF solution of HfIV complex (13.9 mmol) with 0.3 mL of H2O (0.3 mL, 1 × 105 equiv.) at 37 °C. b No decomposition after 2 weeks. c λ max of each HfIV complex was determined by UV-vis spectroscopy. | |||
| 1 | [L1HfIVDipic] | 20 | 343 |
| 2 | [L2HfIVDipic] | Stableb | 288 |
| 3 | [L3HfIVDipic] | Stableb | 343 |
| 4 | [L4HfIVDipic] | 20 | 343 |
| 5 | [L1HfIVDipic4-Cl] | 10 | 343 |
| 6 | [L2HfIVDipic4-Cl] | Stableb | 295 |
| 7 | [L3HfIVDipic4-Cl] | Stableb | 343 |
| 8 | [L4HfIVDipic4-Cl] | 15 | 343 |
Cytotoxicity assay
The anti-tumor activity of the [L1–4HfIVDipic4-H,Cl] complexes and respective ligands was examined by the MTT (methylthiazolyldiphenyl-tetra-zolium bromide) assay on HeLa S3 (human cervical carcinoma) and Hep G2 (human derived hepatoma) cells with cisplatin as the reference. The IC50 value for each sample was calculated as the average of the data obtained from three experiments on different days. In each replicate, all concentrations were repeated five times.As shown in Table 3, [L1HfIVDipic] (entry 1) showed reduced inhibitory activity against the two tumor cell lines compared to cisplatin (entry 15). However, the anti-tumor activity of [L2HfIVDipic] (entry 2) disappeared completely. Interestingly, [L3HfIVDipic] and [L4HfIVDipic] demonstrated high selectivity against Hep G2 cells (entries 3 and 4). The introduction of Cl into the Dipic co-ligand significantly increased the anti-tumor activity, and [L1–4HfIVDipic4-Cl] all showed anti-tumor activity in the low micromolar range against the two tumor cell lines, in some cases higher than cisplatin (entries 5–8). Furthermore, all Dipic4-Cl complexes are more cytotoxic to Hep G2 than to HeLa S3 cancer cells, and among them [L1HfIVDipic4-Cl] is the most cytotoxic complex (IC50: 2.2 ± 0.1 μM on HeLa S3; 0.9 ± 0.4 μM on Hep G2). Its IC50 value is comparable to the cytotoxic Salan type, [L5HfIVDipic4-Cl]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (entry 9), and to the structurally related TiIV complex, [L2TiIVDipic].13 The ligands H2L1–4, Dipic and Dipic4-Cl were tested separately for cytotoxicity, and except for H2L1 and H2L4, which have partial activity on HeLa S3 cells in the concentration range of 1 × 10−7 μM to 1 × 10−5 μM, the others were all non-toxic (entries 10–14).
30 (entry 9), and to the structurally related TiIV complex, [L2TiIVDipic].13 The ligands H2L1–4, Dipic and Dipic4-Cl were tested separately for cytotoxicity, and except for H2L1 and H2L4, which have partial activity on HeLa S3 cells in the concentration range of 1 × 10−7 μM to 1 × 10−5 μM, the others were all non-toxic (entries 10–14).
| Entry | Complex | HeLa S3 | Hep G2 |
|---|---|---|---|
| a Partially active on HeLa S3 cells, see ESI.† | |||
| 1 | [L1HfIVDipic] | 15.1 ± 2.7 | 31.8 ± 9.8 |
| 2 | [L2HfIVDipic] | Non-toxic | Non-toxic |
| 3 | [L3HfIVDipic] | Non-toxic | 44.3 ± 0.1 |
| 4 | [L4HfIVDipic] | Non-toxic | 38.4 ± 18.6 |
| 5 | [L1HfIVDipic4-Cl] | 2.2 ± 0.1 | 0.9 ± 0.4 |
| 6 | [L2HfIVDipic4-Cl] | 14.4 ± 2.2 | 4.3 ± 1.5 |
| 7 | [L3HfIVDipic4-Cl] | 17.3 ± 5.1 | 3.9 ± 1.4 |
| 8 | [L4HfIVDipic4-Cl] | 7.3 ± 4.5 | 2.1 ± 0.6 |
| 9 | [L5HfIVDipic4-Cl] | 1.5 ± 0.3 | 6.7 ± 1.4 |
| 10 | H2L1 | Non-toxica | Non-toxic |
| 11 | H2L2 | Non-toxic | Non-toxic |
| 12 | H2L3 | Non-toxic | Non-toxic |
| 13 | H2L4 | Non-toxica | Non-toxic |
| 14 | Dipic and Dipic4-Cl | Non-toxic | Non-toxic |
| 15 | Cisplatin | 3.5 ± 0.4 | 11.2 ± 2.1 |
From the above results, we can conclude that the 2-picolylamino HfIV complexes containing Dipic4-Cl generally have higher anti-tumor activity than the reported Salan-type HfIV bis-chelates and cisplatin against Hep G2 cells, and that [L1HfIVDipic4-Cl] performs better than cisplatin in HeLa S3 cells.
Structure–activity relationship
The structure–activity (stability) relationship of the phenolato ([ONON] and [ONNO]-type) HfIV bis-chelates is summarized in Fig. 2. The HfIV bis-chelates containing Dipic as co-ligand generally exhibit low molecular polarity, resulting in poor solubility in DMSO. The use of Dipic4-Cl significantly enhances the molecular polarity leading to better solubility and good cytotoxicity. The HfIV complexes bearing phenolato(t-butyl) moieties demonstrated good aqueous stability but insufficient anti-tumor activity. The [ONNO] and [ONON]-type HfIV complexes containing non-bulky substituents, such as methyl at either the 2 or 3 positions of phenolato, generally showed good cytotoxicity. In comparison to the [ONNO]-type HfIV complexes, which selectively inhibit the growth of HeLa S3 cells, the [ONON]-type HfIV complexes are more prone to inhibit Hep G2 cells. Furthermore, neither methyl nor H at the phenolato-2 position has a significant effect on the aqueous stability.Apoptosis assay
Since [L1HfIVDipic4-Cl] was the complex with the lowest IC50 values, it was selected for studies on the mechanism of action. First, [L1HfIVDipic4-Cl] was subjected to apoptotic analysis using the Annexin V-FITC/PI apoptosis assay kit. HeLa S3 cells were used for direct comparison with the [ONNO]-type HfIV complex, [L5HfIVDipic4-Cl].30 Cells were treated with 1 × 10−2 μM, 1 μM and 1 × 102 μM of [L1HfIVDipic4-Cl] for 24 h and were analyzed by flow cytometry. As depicted in Fig. S8,†[L1HfIVDipic4-Cl] could induce 17.0%, 19.6% and 23.4% apoptosis in total at the three concentrations in HeLa S3 cells. In comparison to necrosis, apoptosis leads to significantly reduced side effects and is therefore preferred for anti-tumor drugs. In our study, [L1HfIVDipic4-Cl] induced only 1.3%, 1.6% and 1.3% necrosis of HeLa S3 cells at the tested concentrations (1.4% necrotic cells observed in the control group) (Fig. 3). Next, the apoptosis levels in Hep G2 cells were investigated using [L1HfIVDipic4-Cl], [L5HfIVDipic4-Cl] and [L2TiIVDipic] aiming to gain more insights into their cytotoxicity behavior (Fig. 3 and Fig. S7†). The results indicate that the three metal complexes could almost exclusively induce apoptosis in Hep G2 cells, with a percentage of apoptotic cells in dead cells of 26.9%, 17.1% and 32.3% at 1 × 102 μM, respectively (Fig. S8†). A closer look at all data also shows that [L1HfIVDipic4-Cl] and [L5HfIVDipic4-Cl] induce more early apoptosis than late apoptosis on both HeLa S3 and Hep G2 cells (detailed data are given in Table S15†), suggesting that they avoid the inflammatory responses associated with late apoptosis or necrosis. In comparison, [L2TiIVDipic] is prone to inducing late Hep G2 apoptosis (early/late apoptosis: 9.6%/22.7% at 1 × 102 μM). This has already been observed for [L5HfIVDipic4-Cl]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and [L2TiIVDipic]
30 and [L2TiIVDipic]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 on HeLa S3 cells, which at 1 × 102 μM induced 30.5%(Hf)/20.8%(Ti) and 15.5%(Hf)/62.3%(Ti) of early and late apoptosis, respectively. Overall, the ability to induce early apoptosis seems to be a hallmark of Hf complexes.
13 on HeLa S3 cells, which at 1 × 102 μM induced 30.5%(Hf)/20.8%(Ti) and 15.5%(Hf)/62.3%(Ti) of early and late apoptosis, respectively. Overall, the ability to induce early apoptosis seems to be a hallmark of Hf complexes.
Inhibition of cell migration
Tumor migration or invasion is a major issue that needs to be addressed in cancer therapy, since it is responsible for metastasis, which is the primary cause of cancer-related mortality. In order to preliminarily examine the metastasis inhibition ability of the new HfIV complexes, a wound healing assay of [L1HfIVDipic4-Cl] and the previously reported [L5HfIVDipic4-Cl] on HeLa S3 cells was performed. In brief, cells were incubated with each complex for 24 h, and the WCR (wound closure rate) was used for describing the metastasis inhibition activity (see eqn (S1)†). As depicted in Fig. 4a, the WCR of [L1HfIVDipic4-Cl] and [L5HfIVDipic4-Cl] were both concentration dependent, since at concentrations of 2 μM and 4 μM, the WCR values were 8.3% and 5.4% for [L1HfIVDipic4-Cl], and 9.5% and 7.6% for [L5HfIVDipic4-Cl], respectively (Fig. 4b; the detailed experimental procedure can be found in the ESI†), while for the control it was 14.6% at 24 h. Therefore, the results show that both complexes reduce the cellular migration of HeLa S3 cells.Cellular uptake
Next, we performed a cellular uptake investigation of [L1HfIVDipic4-Cl] using HeLa S3 cells, following the procedure used in our previous report.30 A total of 3 × 10−9 mol of [L1HfIVDipic4-Cl] (2.26 × 10−3 mg) was added to HeLa S3 cells, and samples taken at 10 min, 30 min, 1 h, 2 h, 24 h and 48 h were subjected to ICP-MS (inductively coupled plasma mass spectrometry) analysis for cellular Hf content. The percentages of cellular Hf in relation to total Hf were 3.55%, 4.86%, 5.97%, 7.09%, 8.22% and 8.59%, at each time point. Taking the cellular Hf amount at 48 h (46 ng) as the maximum uptake, 41.30% of the maximum Hf uptake was achieved after 10 min and 69.60% after 30 min (Fig. 5a). In comparison with [L5HfIVDipic4-Cl], which showed 61.90% (10 min) and 76.20% (1 h) of the maximum Hf uptake (42 ng, 48 h),30 it can be concluded that the initial Hf uptake of [L1HfIVDipic4-Cl] is slower, but is similar or higher after one hour (Fig. 5b).Intracellular ROS assessments
Oxidative stress and accumulation of ROS (reactive oxygen species) including ˙O2− (superoxide anion), HO˙ (hydroxyl radical), 1O2 (singlet oxygen) and H2O2 (hydrogen peroxide) are closely related to tumor formation and mitosis.34 ROS at higher levels can lead to cell death, including apoptosis, autophagy, necroptosis or ferroptosis, with multiple cellular targets being involved.35 In this study, the ROS production induced by [L1HfIVDipic4-Cl] and [L5HfIVDipic4-Cl] in HeLa S3 cells was examined with the 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCFDA probe)36 (three repeats). H2DCFDA is not fluorescent, but after reaction with ROS, it can be transformed into DCF (2′,7′-dichlorofluorescein), which shows green emission and can be detected by fluorescence microscopy. One representative experiment is depicted in Fig. 6. We found that both [L1HfIVDipic4-Cl] and [L5HfIVDipic4-Cl] could induce ROS generation at the two tested concentrations (1 μM and 2 μM). Moreover, the fluorescence in the case of [L1HfIVDipic4-Cl] was much stronger in comparison with [L5HfIVDipic4-Cl], indicating a higher level of ROS production with the former. | ||
| Fig. 6 Fluorescence microscopic images of intracellular ROS levels in HeLa S3 cells analyzed by the H2DCFDA probe. The green spots are cells that are stained with DCF. | ||
In order to find what types of ROS are generated in tumor cells, and the cellular effects for which they are responsible, an in vitro ROS investigation was conducted on HeLa S3 cells by flow cytometry. HeLa S3 cells were incubated with [L1HfIVDipic4-Cl] followed by different ROS scavengers, namely NaN3 (1O2 scavenger), Tiron (disodium 4,5-dihydroxybenzene-1,3-disulfonate, ˙O2− scavenger), D-mannitol (HO˙ scavenger) and Nap (sodium pyruvate, H2O2 scavenger). As depicted in Fig. 7, the fluorescence intensity of DCF was slightly reduced (<40%) in the NaN3, Tiron and Nap groups when compared to the control (only with [L1HfIVDipic4-Cl]), indicating that 1O2, ˙O2− and H2O2 are not the main ROS species formed. Obvious quenching of the DCF fluorescence (<80%) was observed in the D-mannitol group, suggesting that HO˙ accounted for the majority of [L1HfIVDipic4-Cl]-induced ROS.
Metal ions such as Cu, Fe, Mn and Ru can induce ROS generation through the Fenton reaction, the Haber–Weiss reaction, and mitochondrial dysfunction.37 However, reports on how molecular Hf complexes induce ROS generation are scarce since Hf cannot easily undergo redox cycling, and is Fenton inactive. Hf loses all the outermost s and d electrons, leaving ionic sites with almost empty orbitals that are not able to catalyze oxygen-related reactions.38 Hf-based materials such as nanoparticles of HfO2 and Hf-based MOFs are RT (radio therapy) sensitive, and HfIV can absorb X-ray energy and convert it to high-energy electrons, which subsequently interact with H2O to generate HO˙.39,40 It has been shown that mitochondrial ETC (electron transport chain) activity can lead to the production of ROS, including superoxide and hydroxyl radicals. In the process, the mitochondrial ETC transfers electrons to O2, and O2 is reduced to ˙O2− anions, which are ultimately converted to hydroxyl radicals (HO˙).41 This mechanism may explain how ROS are generated by [L1HfIVDipic4-Cl]. Obviously, mitochondrial-related investigations are required to prove such a hypothesis.
Mitochondrial membrane potential
Most of the production and conversion of O2 and ATP takes place in the mitochondria, through oxidative phosphorylation, and the generated ROS can cause MMP (mitochondrial membrane potential) decrease and even mitochondrial dysfunction. Hence, MMP has been identified as an indicator of mitochondrial dysfunction and early cell apoptosis.42 In this study, the MMP change of HeLa S3 cells treated with the two HfIV complexes was investigated with the mitochondrial fluorescent cyanine tracer, JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide).43 JC-1 demonstrates red fluorescence when the mitochondrion is healthy and the MMP is unchanged. However, JC-1 can dissociate from the mitochondrial membrane with the fluorescence changing from red to green when the MMP decreases. In this study, HeLa S3 cells were incubated with the HfIV complexes (at 1 μM and 2 μM) for 24 h and subsequently treated with the JC-1 staining solution. As depicted in Fig. 8, the MMP decreased in a concentration-dependent manner after treating HeLa S3 cells with [L1HfIVDipic4-Cl] and [L5HfIVDipic4-Cl]. Stronger green fluorescence could be observed for cells treated with [L1HfIVDipic4-Cl] compared to [L5HfIVDipic4-Cl], indicating that [L1HfIVDipic4-Cl] could induce more ROS generation in HeLa S3 cells and most probably is more prone to inducing mitochondria dysfunction. This is in agreement with the study made with the H2DCFDA probe.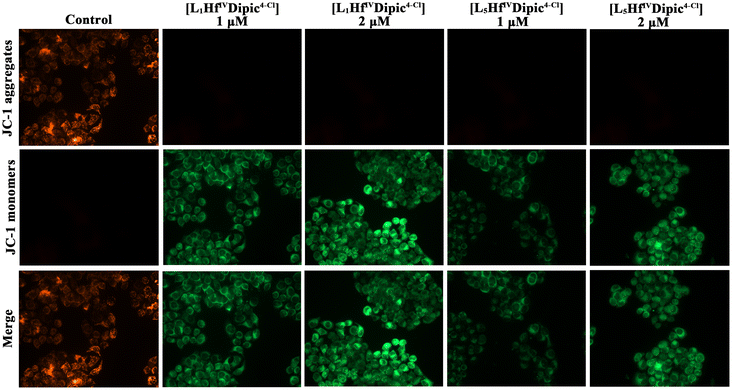 | ||
| Fig. 8 Staining of mitochondrial membrane potential in HeLa S3 cells after 24 h of treatment with different concentrations (1 μM and 2 μM) of complexes [L1HfIVDipic4-Cl] and [L5HfIVDipic4-Cl]. | ||
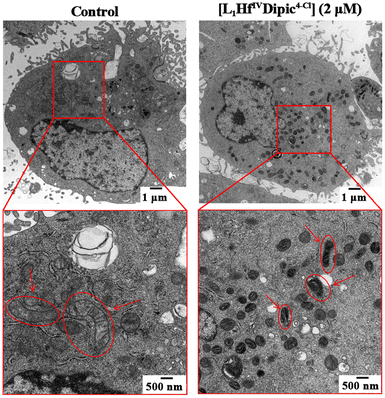
Moreover, excessive ROS accumulation in mitochondria leads to structural changes that compromise their function.41 As shown in Fig. 9, a transmission electron microscopy (TEM) analysis revealed that HeLa S3 cells treated with [L1HfIVDipic4-Cl] exhibited pronounced mitochondrial damage, including atrophy and disrupted cristae. Typical apoptotic-related hyper-chromatin could be observed. In the control group, mitochondria maintained their normal tubular architecture—an intact outer membrane, clearly defined cristae, and no sign of edema or atrophy.
Conclusions
In conclusion, eight novel [ONON]-type HfIV bis-chelates containing tripodal-tetradentate L1–4 and a common 2-picolylamino co-ligand were synthesized in good yields and high purity. The complexes were purified by flash chromatography or by extraction with organic solvents. Single-crystal X-ray diffraction studies indicate that these [ONON]-type HfIV complexes have more flexible coordination spheres than the [ONNO] (Salan)-type HfIV complexes. Similarly to the Salan-type HfIV complexes, the [ONON]-type complexes remained stable in aqueous media when t-butyl groups are located at the ortho-position of the phenolato moiety. Most [ONON]-type HfIV complexes showed a selective inhibitory effect against Hep G2 cells. Among them, the four complexes ([L1–4HfIVDipic4-Cl]) containing the chloro-substituted Dipic co-ligand exhibit the strongest cytotoxicity. The SAR suggests that both ligands of the heteroleptic complex are crucial for bioactivity; i.e. the substitution on the Dipic can be used to adjust the molecular polarity, leading to enhanced solubility in polar solvents and higher cytotoxicity, and methyl groups on the phenolate also increase the activity. In comparison with the Salan-type HfIV complexes, the cellular uptake of [ONON]-type HfIV complexes is initially slower, but the total Hf uptake is higher. In addition, the ROS production of [L1HfIVDpic4-Cl] is dose dependent and it outperformed [L5HfIVDpic4-Cl] (Salan type). [L1HfIVDpic4-Cl] selectively produces hydroxyl radicals (HO˙) as the major ROS. Furthermore, [L1,5HfIVDpic4-Cl] can cause mitochondrial dysfunction and MMP decrease, which may account for the ROS generation. Cell apoptosis (HeLa S3 and Hep G2) studies have provided some insight into the unexplored features of anti-tumor HfIV complexes. An interesting finding is that both families of HfIV complexes ([ONON] and [ONNO]) induce mostly early apoptosis, which is preferred because it causes less inflammation. Further investigations such as molecular modification, targeted therapy and detailed molecular mechanisms are currently underway in our laboratory.Author contributions
Tiankun Zhao: supervision, conceptualization. Qi Zhang: methodology, writing – original draft. Jialiu Zhao: investigation, validation. Dongyu Mei: investigation, validation. Jing Ma: data curation. Isabel Correia: data curation, writing – review & editing. Zhongduo Yang: writing – review & editing. Sa-Hyun Kim: resources, formal analysis. Thomas Huhn: supervision, conceptualization.Data availability
CCDC 2390816 ([L2HfIVDipic]) and 2390817 ([L2HfIVDipic4-Cl]).† Supplementary data associated with this article can be found in the online version.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful to the Gansu College Teacher Innovation Project (2025A-035); the Gansu Technological Innovation Guidance Plan (24CXJA005); the National Natural Science Foundation of China (No. 22267011); and the project of the Bureau of Science and Technology of Lanzhou (2023-RC-43) for the funding of this project. The support provided by China Scholarship Council (CSC) during a visit of T. Zhao to University of Lisbon is acknowledged. I. C. thanks Fundação para a Ciência e a Tecnologia (FCT) through projects UIDB/00100/2020 (https://doi.org/10.54499/UIDB/00100/2020), UIDP/00100/2020 (https://doi.org/10.54499/UIDP/00100/2020), and LA/P/0056/2020 (https://doi.org/10.54499/LA/P/0056/2020). The authors thank Dr Xianggao Meng from Central China Normal University for useful discussion on the X-ray analysis.References
- H. Köpf and P. Köpf-Maier, Titanocene Dichloride—The First Metallocene with Cancerostatic Activity, Angew. Chem., Int. Ed. Engl., 1979, 18(6), 477–478 Search PubMed.
- H. J. Keller, B. Keppler and D. Schmähl, Antitumor Activity of cis-Dihalogenobis(1-phenyl-1,3-butanedionato) Titanium(IV) Compounds. A New Class of Antineoplastic Agents, J. Cancer Res. Clin. Oncol., 1983, 105(1), 109–110 CAS.
- F. Caruso and M. Rossi, Antitumor Titanium Compounds, Mini-Rev. Med. Chem., 2004, 4(1), 49–60 CAS.
- M. Shavit, D. Peri, C. M. Manna, J. S. Alexander and E. Y. Tshuva, Active Cytotoxic Reagents Based on Non-metallocene Non-diketonato Well-Defined C2-Symmetrical Titanium Complexes of Tetradentate Bis(phenolato) Ligands, J. Am. Chem. Soc., 2007, 129(40), 12098–12099 CAS.
- T. A. Immel, U. Groth and T. Huhn, Cytotoxic Titanium Salan Complexes: Surprising Interaction of Salan and Alkoxy Ligands, Chem. – Eur. J., 2010, 16(9), 2775–2789 Search PubMed.
- H. Glasner and E. Y. Tshuva, C 1-Symmetrical Titanium(IV) Complexes of Salan Ligands with Differently Substituted Aromatic Rings: Enhanced Cytotoxic Activity, Inorg. Chem., 2014, 53(6), 3170–3176 CAS.
- M. Grützke, T. Zhao, T. A. Immel and T. Huhn, Heptacoordinate Heteroleptic Salan (ONNO) and Thiosalan (OSSO) Titanium(IV) Complexes: Investigation of Stability and Cytotoxicity, Inorg. Chem., 2015, 54(14), 6697–6706 Search PubMed.
- T. A. Immel, M. Grützke, A.-K. Späte, U. Groth, P. Öhlschläger and T. Huhn, Synthesis and X-ray structure analysis of a heptacoordinate titanium(IV)-bis-chelate with enhanced in vivo antitumor efficacy, Chem. Commun., 2012, 48(46), 5790–5792 CAS.
- G. Nahari and E. Y. Tshuva, Synthesis of asymmetrical diaminobis(alkoxo)-bisphenol compounds and their C1-symmetrical mono-ligated titanium(IV) complexes as highly stable highly active antitumor compounds, Dalton Trans., 2021, 50(19), 6423–6426 CAS.
- R. Manne, M. Miller, A. Duthie, M. F. C. Guedes da Silva, E. Y. Tshuva and T. S. Basu Baul, Cytotoxic homoleptic Ti(IV) compounds of ONO-type ligands: synthesis, structures and anti-cancer activity, Dalton Trans., 2019, 48(1), 304–314 RSC.
- T. Zhao, M. Grützke, K. H. Götz, T. Druzhenko and T. Huhn, Synthesis and X-ray structure analysis of cytotoxic heptacoordinate sulfonamide salan titanium(IV)-bis-chelates, Dalton Trans., 2015, 44(37), 16475–16485 CAS.
- G. Nahari, O. Braitbard, L. Larush, J. Hochman and E. Y. Tshuva, Effective Oral Administration of Antitumorigenic Nanoformulated Titanium Complex, ChemMedChem, 2021, 16(1), 108–112 CrossRef CAS PubMed.
- S. Li, X. Zhang, T. Zhao, N. Liu, Y. Zhang, P. Wang, Z. Yang and T. Huhn, Synthesis, in vitro antitumor evaluation and structure activity relationship of heptacoordinated amino-bis(Phenolato) Ti(IV) complexes stabilized by 2,6-dipicolinic acid, J. Biol. Inorg. Chem., 2024, 29(3), 315–330 Search PubMed.
- Z. Shpilt, N. Melamed-Book and E. Y. Tshuva, An anticancer Ti(IV) complex increases mitochondrial reactive oxygen species levels in relation with hypoxia and endoplasmic-reticulum stress: A distinct non DNA-related mechanism, J. Inorg. Biochem., 2023, 243, 112197 CrossRef CAS PubMed.
- G. W. Severin, C. H. Nielsen, A. I. Jensen, J. Fonslet, A. Kjær and F. Zhuravlev, Bringing Radiotracing to Titanium-Based Antineoplastics: Solid Phase Radiosynthesis, PET and ex Vivo Evaluation of Antitumor Agent [45Ti](salan)Ti(dipic), J. Med. Chem., 2015, 58(18), 7591–7595 CrossRef CAS PubMed.
- K. Pedersen, C. Baun, K. Nielsen, H. Thisgaard, A. Jensen and F. Zhuravlev, Design, Synthesis, Computational, and Preclinical Evaluation of natTi/45Ti-Labeled Urea-Based Glutamate PSMA Ligand, Molecules, 2020, 25, 1104 Search PubMed.
- T. Zhao, P. Wang, M. Ji, S. Li, M. Yang and X. Pu, Post-Synthetic Modification Research of Salan Titanium bis-Chelates via Sonogashira Reaction, Acta Chim. Sin., 2021, 79(11), 1385–1393 Search PubMed.
- T. Zhao, P. Wang, N. Liu, S. Li, M. Yang and Z. Yang, Facile synthesis of [ONON] type titanium(IV) bis-chelated complexes in alcoholic solvents and evaluation of anti-tumor activity, J. Inorg. Biochem., 2022, 235, 111925 CAS.
- T. Zhao, P. Wang, X. Zhang, N. Liu, W. Zhao, Y. Zhang, P. Yuan, S. Li, M. Yang, Z. Yang and T. Huhn, Anti-tumoral Titanium(IV) Complexes Stabilized with Phenolato Ligands and Structure-Activity Relationship, Curr. Top. Med. Chem., 2023, 23(19), 1835–1849 Search PubMed.
- B. Yu, Z.-H. Zhu, W.-W. Qin, H.-L. Wang, Y.-L. Li, F.-P. Liang and H.-H. Zou, Enhancement of Luminescence, Multiple-Sensing, and Differentiated Live-Cell-Imaging Properties of High-Nuclear Lanthanide Nanoclusters via the Zn(II)-Chelate-Controlled Dual Antenna Effect, ACS Mater. Lett., 2024, 6(8), 3312–3326 CAS.
- S. Garon, E. K. C. Lau, S.-L. Chew, S. T. Lee and M. E. Thompson, Metal (IV) tetras (8-hydroxyquinoline)(M = Zr, Hf) used as electroluminescent material and electron-transport layer in OLEDs, J. Soc. Inf. Disp., 2005, 13(5), 405–409 Search PubMed.
- A. J. Chmura, M. G. Davidson, M. D. Jones, M. D. Lunn, M. F. Mahon, A. F. Johnson, P. Khunkamchoo, S. L. Roberts and S. S. F. Wong, Group 4 Complexes with Aminebisphenolate Ligands and Their Application for the Ring Opening Polymerization of Cyclic Esters, Macromolecules, 2006, 39(21), 7250–7257 CAS.
- S. L. Hancock, M. F. Mahon, G. Kociok-Kőhn and M. D. Jones, Homopiperazine and Piperazine Complexes of ZrIV and HfIV and Their Application to the Ring-Opening Polymerisation of Lactide, Eur. J. Inorg. Chem., 2011, 2011(29), 4596–4602 CAS.
- M. Mandal, V. Ramkumar and D. Chakraborty, Salen complexes of zirconium and hafnium: synthesis, structural characterization and polymerization studies, Polym. Chem., 2019, 10(25), 3444–3460 Search PubMed.
- K. Press, V. Venditto, I. Goldberg and M. Kol, Zirconium and hafnium Salalen complexes in isospecific polymerisation of propylene, Dalton Trans., 2013, 42(25), 9096–9103 CAS.
- P. Köpf-Maier, B. Hesse and H. Köpf, Tumor Inhibition by Metallocenes: Effect of Titanocene, Zirconocene, and Hafnocene Dichlorides on Ehrlich Ascites Tumor in Mice, J. Cancer Res. Clin. Oncol., 1980, 96(1), 43–51 Search PubMed.
- A. A. El-Habeeb, Novel Gallium(III), Germanium(IV), and Hafnium(IV) Folate Complexes and Their Spectroscopic, Thermal Decomposition, Morphological, and Biological Characteristics, Bioinorg. Chem. Appl., 2020, 2020, 6678688 Search PubMed.
- R. M. Lord, J. J. Mannion, A. J. Hebden, A. E. Nako, B. D. Crossley, M. W. McMullon, F. D. Janeway, R. M. Phillips and P. C. McGowan, Mechanistic and Cytotoxicity Studies of Group IV β-Diketonate Complexes, ChemMedChem, 2014, 9(6), 1136–1139 CAS.
- E. Choi, M. Landry, N. Pennock, M. Neufeld, K. Weinfurter, A. Goforth, J. Walker and C. Sun, Nanoscale Hafnium Metal–Organic Frameworks Enhance Radiotherapeutic Effects by Upregulation of Type I Interferon and TLR7 Expression, Adv. Healthcare Mater., 2023, 12, 2202830 CAS.
- T. Zhao, P. Wang, N. Liu, W. Zhao, M. Yang, S. Li, Z. Yang, B. Sun and T. Huhn, Synthesis and X-ray structure analysis of cytotoxic heptacoordinated Salan hafnium(IV) complexes stabilized with 2,6-dipicolinic acid, J. Inorg. Biochem., 2023, 240, 112094 Search PubMed.
- W. Zhao, P. Yuan, Q. Zhang, N. Liu, Y. Wang, P. Wang, T. Huhn, Z. Yang and T. Zhao, Novel bimetallic oxido-bridged phenolato hafniumIV complex with enhanced anti-tumor activity and aqueous stability, Results Chem., 2023, 6, 101161 CAS.
- J. Wang, J. Pan, Y. Tang, J. Chen, X. Fei, W. Xue and X. Liu, Advances of hafnium based nanomaterials for cancer theranostics, Front. Chem., 2023, 11, 1283924 CAS.
- S. Ding, L. Chen, J. Liao, Q. Huo, Q. Wang, G. Tian and W. Yin, Harnessing Hafnium-Based Nanomaterials for Cancer Diagnosis and Therapy, Small, 2023, 19(32), 2300341 CAS.
- G. de la Riva Alberto, F. López Mendoza Javier and G. Agüero-Chapin, Known Hepatoprotectors Act as Antioxidants and Immune Stimulators in Stressed Mice: Perspectives in Animal Health Care, Curr. Pharm. Des., 2018, 24(40), 4825–4837 Search PubMed.
- M. Azmanova and A. Pitto-Barry, Oxidative Stress in Cancer Therapy: Friend or Enemy?, ChemBioChem, 2022, 23(10), e202100641 CAS.
- M. Oparka, J. Walczak, D. Malinska, L. M. P. E. Van Oppen, J. Szczepanowska, W. J. H. Koopman and M. R. Wieckowski, Quantifying ROS levels using CM-H2DCFDA and HyPer, Methods, 2016, 109, 3–11 CAS.
- Z.-H. Zhu, L. Zhang, S. Jia, Z. Ni, Y.-L. Li, H.-H. Zou, Y. Yang, Y. Hu, D. Ding, B. Z. Tang and G. Feng, Nanoscale Metal-Organic Framework Leveraging Water, Oxygen, and Hydron Peroxide to Generate Reactive Oxygen Species for Cancer Therapy, Adv. Funct. Mater., 2025, 2419548 Search PubMed.
- B. Ji, J. Gou, Y. Zheng, X. Pu, Y. Wang, P. Kidkhunthod and Y. Tang, Coordination Chemistry of Large-Sized Yttrium Single-Atom Catalysts for Oxygen Reduction Reaction, Adv. Mater., 2023, 35(24), 2300381 CAS.
- Y. Chen, S. Liu, P. Gao, M. Shi, W. Pan, N. Li and B. Tang, NIR-II light-assisted radiotherapy based on ultrasmall HfO2-embedded porous carbon nanooctahedra for overcoming tumor radioresistance, Mater. Today Nano, 2022, 20, 100253 CAS.
- Y. Zhao, C. Liang, Z. Mei, H. Yang, B. Wang, C. Xie, Y. Xu and J. Tian, Oxygen-Enriched MOF-Hemoglobin X-ray Nanosensitizer for Enhanced Cancer Radio–Radiodynamic Therapy, ACS Mater. Lett., 2023, 5(12), 3237–3247 CrossRef CAS.
- F. R. Palma, B. N. Gantner, M. J. Sakiyama, C. Kayzuka, S. Shukla, R. Lacchini, B. Cunniff and M. G. Bonini, ROS production by mitochondria: function or dysfunction?, Oncogene, 2024, 43(5), 295–303 CrossRef CAS PubMed.
- K. Facecchia, L. A. Fochesato, S. D. Ray, S. J. Stohs and S. Pandey, Oxidative Toxicity in Neurodegenerative Diseases: Role of Mitochondrial Dysfunction and Therapeutic Strategies, J. Toxicol., 2011, 2011, 683728 Search PubMed.
- A. Perelman, C. Wachtel, M. Cohen, S. Haupt, H. Shapiro and A. Tzur, JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry, Cell Death Dis., 2012, 3(11), e430 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2390816 and 2390817. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02859g |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |