Emission enhancement in a luminescent polychlorinated diphenylpyridylmethyl radical through coordination to silver(I)†
Takuto
Mibu
*ab,
Ryota
Matsuoka
 ab,
Masanari
Nagasaka
ab,
Masanari
Nagasaka
 a and
Tetsuro
Kusamoto
a and
Tetsuro
Kusamoto
 *abcd
*abcd
aInstitute for Molecular Science, Okazaki, Aichi 444-8585, Japan. E-mail: t-mibu@sagami.or.jp; kusamoto.tetsuro.es@osaka-u.ac.jp
bDivision of Frontier Materials Science, Department of Materials Engineering Science, Graduate School of Engineering Science, Osaka University, Toyonaka, Osaka 560-8531, Japan
cSpintronics Research Network Division, Institute for Open and Transdisciplinary Research Initiatives, Osaka University, Toyonaka, Osaka 560-8531, Japan
dFOREST, JST, Honcho 4-1-8, Kawaguchi, Saitama 332-0012, Japan
First published on 17th December 2024
Abstract
A luminescent silver(I) complex containing a luminescent radical ligand was prepared for the first time. Coordination to AgI enhanced and red-shifted the radical-centered emission. This study demonstrates similar effects in the luminescence of the radical by complexation with group 11 d10-metal ions.
Introduction
Luminescent radicals exhibit fluorescence based on the transition from the lowest doublet excited state (D1) to the doublet ground state (D0).1 This enables a range of unusual photophysical characteristics including the absence of heavy-atom effects2,3 and efficient carrier-to-photon conversion in electroluminescence.4 Furthermore, the luminescence of assemblies of luminescent radicals responds to a magnetic field through a mechanism unique to open-shell molecular systems.5 Overall, luminescent radicals appear promising for use in the next generation of photonic and spintronic devices.6Coordination with metal ions is an attractive way to modulate the emission properties of organic radicals. For example, complexes comprising AuI ligated with (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical (PyBTM) and its analogues have shown longer emission wavelengths and higher photoluminescence quantum yields than the radical ligands alone,7–9 and a PyBTM-AuI complex has recently been utilized as an X-ray scintillator.10 In addition, several metal–organic frameworks11 and supramolecular cages12 with distinctive emission characteristics have been developed using luminescent radical ligands. The emission characteristics of these radical complexes depend on the choice of metal ion, e.g., AuI, ZnII, and LaIII enhance the emission,2,7 whereas CuII, FeIII, and IrIII weaken or quench the emission.2,13 Despite the increasing number of examples of luminescent radical–metal complexes, the range of metal ions employed remains limited, and more importantly, differences and similarities in the effects of different metal ions on the emission of radicals have rarely been discussed. Further investigation is thus necessary to comprehensively understand the effect of different metal ions on the luminescence of radicals.
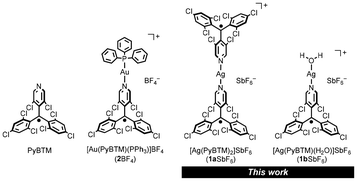
This study investigated the complexation of PyBTM with AgI ions and the luminescence of the resulting complex in solution, to elucidate the effect of the metal ion on the electronic structure and emission characteristics of this class of radical complexes (Chart 1). Single-crystal X-ray diffraction (SCXRD) and titration studies revealed that two PyBTM molecules coordinate to one AgI ion in the crystalline state, whereas PyBTM formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with AgI in solution. The 1
1 complex with AgI in solution. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex exhibited enhanced and redshifted photoluminescence compared with PyBTM alone in solution. Comparison of the synthesized 1
1 complex exhibited enhanced and redshifted photoluminescence compared with PyBTM alone in solution. Comparison of the synthesized 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AgI complex with some well-studied AuI complexes (e.g., [AuI(PyBTM)(PPh3)]BF4, labelled 2BF4
1 AgI complex with some well-studied AuI complexes (e.g., [AuI(PyBTM)(PPh3)]BF4, labelled 2BF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in Chart 1) revealed commonalities between the effects of AgI and AuI (i.e., group 11 d10) metal ions on the radical's luminescence.
7 in Chart 1) revealed commonalities between the effects of AgI and AuI (i.e., group 11 d10) metal ions on the radical's luminescence.
Results and discussion
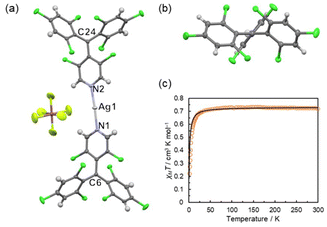
Details of the synthesis and characterization of the silver complex [AgI(PyBTM)2]SbF6 (1aSbF6) is described in the Experimental section. Slow vapor diffusion of diisopropyl ether into a mixed solution of PyBTM and AgSbF6 in dichloromethane at room temperature afforded crystals of 1aSbF6. A SCXRD analysis revealed 1a+ to have a linear two-coordinate geometry around the AgI ion, with the two PyBTM molecules coordinating to the AgI ion through their pyridyl nitrogen atoms with a N1–Ag1–N2 bond angle of 176.0(1)° (Fig. 1a). Trigonal planar geometries around the central carbon atoms (C6 and C24) of the PyBTM units indicate their sp2 hybridization and radical character (Fig. 1b). The shortest intermolecular Ag⋯Ag distance of 8.5341(5) Å indicates no significant interaction between the AgI ions. The shortest intermolecular distance between the radical centers (C6 and C24) is 7.212(5) Å, much shorter than the intramolecular C6⋯C24 distance of 12.880(7) Å. Several intermolecular Cl⋯Cl contacts were also detected (Fig. S1†).The magnetic properties of 1aSbF6 were investigated using a SQUID magnetometer (Fig. 1c and Fig. S3†). The temperature dependence of χMT (χM is molar magnetic susceptibility) was analyzed by the Curie–Weiss law to afford a Curie constant (C) of 0.732 cm3 K mol−1 and a Weiss temperature (θ) of −1.5 K (Fig. 1c). The C value is close to that expected for two non-interacting S = 1/2 spins (0.75 cm3 K mol−1), confirming the radical character of the two PyBTM ligands in 1aSbF6. The negative θ value indicates weak antiferromagnetic interactions dominant between the spin centers, which would be mediated via intermolecular atomic contacts.
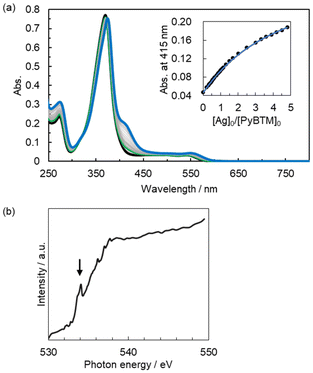
1aSbF6 in dichloromethane demonstrated concentration-dependent UV-vis absorption characteristics (Fig. S4†). The absorption spectrum of a dilute solution (4.93 μM) was almost identical to that of PyBTM in dichloromethane, whereas a concentrated solution (149 μM) showed absorption similar to that of the gold(I) complex with ligated PyBTM, 2BF4.7 The result suggests the presence of an equilibrium between the dissociation and association of PyBTM from and to the AgI ion in solution. The equilibrium behaviour was further investigated by titration studies (Fig. 2a). The incremental addition of AgSbF6 to PyBTM in dichloromethane caused a new absorption band to appear around 420 nm and the lowest-energy absorption band to shift to a longer wavelength. Two isosbestic points were observed at 373 and 320 nm, suggesting that only one UV-vis detectable species was in equilibrium with PyBTM under the tested conditions. Two possible chemical species, both AgI-PyBTM complexes, formed in this titration: one with a ligand–metal stoichiometry of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (i.e., 1a+) and another with a 1
1 (i.e., 1a+) and another with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (i.e., [Ag(PyBTM)L]+, where L represents a ligand other than PyBTM). Simulations suggest that the selective formation of the 2
1 stoichiometry (i.e., [Ag(PyBTM)L]+, where L represents a ligand other than PyBTM). Simulations suggest that the selective formation of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AgI complex requires an unfeasibly strong positive cooperativity between the first and second binding of the ligand to an AgI ion (Fig. S6†).14 Furthermore, no additional change in the absorption spectral shape was observed upon the addition of a large excess of AgI ions. We thus tentatively conclude that the 1
1 AgI complex requires an unfeasibly strong positive cooperativity between the first and second binding of the ligand to an AgI ion (Fig. S6†).14 Furthermore, no additional change in the absorption spectral shape was observed upon the addition of a large excess of AgI ions. We thus tentatively conclude that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AgI complex formed as the major product in this titration experiment. The titration plots show a good fit with a 1
1 AgI complex formed as the major product in this titration experiment. The titration plots show a good fit with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, giving an association constant (K) of (8.7 ± 0.4) × 103 M−1 (Fig. 2a and Fig. S5†).
1 binding model, giving an association constant (K) of (8.7 ± 0.4) × 103 M−1 (Fig. 2a and Fig. S5†).
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AgI–PyBTM complex [Ag(PyBTM)L]+ is expected to possess an auxiliary ligand L, which is likely to be H2O according to soft X-ray absorption spectroscopy. Fig. 2b shows an oxygen K-edge X-ray absorption spectrum of a dichloromethane solution containing [Ag(PyBTM)L]+ and PyBTM in a molar ratio of 99
1 AgI–PyBTM complex [Ag(PyBTM)L]+ is expected to possess an auxiliary ligand L, which is likely to be H2O according to soft X-ray absorption spectroscopy. Fig. 2b shows an oxygen K-edge X-ray absorption spectrum of a dichloromethane solution containing [Ag(PyBTM)L]+ and PyBTM in a molar ratio of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (soln_99). The molar ratio was determined based on the association constant K (further details are given in ESI†). A distinct pre-edge peak assignable to H2O was detected around 534 eV. It was not observed from either the dichloromethane solvent or AgSbF6 in dichloromethane (Fig. S7†). The results suggest that the peak is attributable to the H2O molecule coordinating to the AgI ion in [Ag(PyBTM)(H2O)]+ (1b+) rather than the very small amount of free H2O molecules that were under the detection limit of X-ray absorption spectroscopy.
1 (soln_99). The molar ratio was determined based on the association constant K (further details are given in ESI†). A distinct pre-edge peak assignable to H2O was detected around 534 eV. It was not observed from either the dichloromethane solvent or AgSbF6 in dichloromethane (Fig. S7†). The results suggest that the peak is attributable to the H2O molecule coordinating to the AgI ion in [Ag(PyBTM)(H2O)]+ (1b+) rather than the very small amount of free H2O molecules that were under the detection limit of X-ray absorption spectroscopy.
ESR spectroscopy for soln_99 and PyBTM in CH2Cl2 was performed to characterize the spin density distribution of 1b+. The measured ESR spectrum of PyBTM in CH2Cl2 at 175 K matched the simulated spectrum involving hyperfine splitting due to six 1H, one 14N, and one 13C atoms, indicating the delocalization of the unpaired electron over the triarylmethyl skeleton (Fig. S8†). This result is consistent with the previous report.15 On the other hand, the spectrum of soln_99 at 175 K showed a different hyperfine structure, which was reproduced by simulation considering hyperfine coupling with six 1H, one 14N, one α-13C, and one 107Ag/109Ag atoms (Fig. S9†). This indicates the coordination of PyBTM to AgI and partial distribution of the spin density on the AgI ion in 1b+.
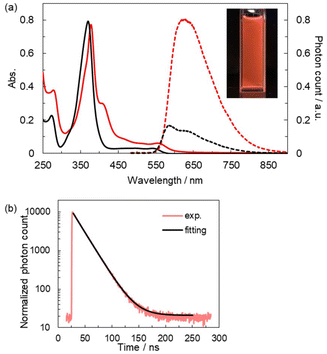
Absorption and emission spectroscopic investigations of soln_99 enabled us to characterize 1bSbF6 as the first luminescent open-shell AgI complex (Fig. 3a). soln_99 displayed absorption peaks around 420 and 620 nm in the visible region. The similarity of the absorption spectra of soln_99 and 2BF4 suggests the coordination of PyBTM to AgI in 1bSbF6.7soln_99 exhibited distinct photoluminescence with a broad and structureless emission band at λem = 620 nm. The excitation spectrum of soln_99 was similar to its absorption spectrum, indicating the same origin of the light absorption and emission (Fig. S10†). Importantly, the decay profile of this emission fitted well with a mono-exponential function with a lifetime (τ) of 19.0 ns (Fig. 3b), which differed from that of PyBTM (τ = 6.4 ns).15 These results confirm that the emission originated mostly from a single substance, namely 1bSbF6.
Table 1 summarizes the photophysical characteristics of 1bSbF6 and the related compounds, PyBTM15 and 2BF4, in dichloromethane. The emission maximum wavelength of 1bSbF6 (λem = 620 nm) was bathochromically shifted with respect to that of PyBTM (585 nm). In addition, its absolute photoluminescence quantum yield (ϕ = 0.12) was about five times that of PyBTM (ϕ = 0.025). The radiative and non-radiative rate constants (kr and knr, respectively) estimated from ϕ and τ suggest that the increased ϕ of 1SbF6 was due to both increasing kr and decreasing knr. These coordination-induced changes in the emission characteristics are qualitatively similar to those previously observed in 2BF4.
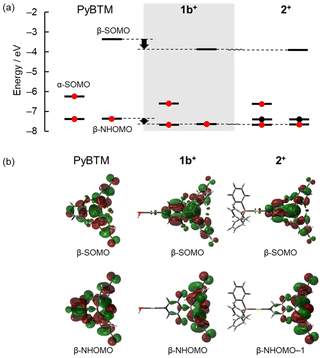
Density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations also suggested that AgI and AuI ions similarly affect the electronic structure and excited state characteristics of PyBTM. Fig. 4 shows the frontier orbital energies of PyBTM, 1b+, and 2+, along with the respective frontier orbitals mainly involved in their lowest-energy excitation. The lowest-energy excitations of all three compounds were attributed to the transitions of β-spin electrons between orbitals mainly distributed on the PyBTM moiety. This suggests that photoexcitation and the resulting emission were centred on the PyBTM moiety. The frontier β-orbital energies of 1b+ and 2+ are lower than those of PyBTM, with the energy lowering of β-singly occupied molecular orbital (β-SOMO) being especially remarkable (Fig. 4a). In addition, the distributions of the frontier orbitals in 1b+ and 2+ are similar to each other but different from those in PyBTM, whose molecular orbitals are more evenly distributed on the three aryl rings (Fig. 4b).
This series of experimental and calculated results indicates that coordination of PyBTM to an AgI ion has a qualitatively similar effect on its luminescence to its coordination to an AuI ion. In accordance with previous studies of several AuI complexes of PyBTM derivatives including 2+,7,8 inductive and electrostatic effects of the AgI ion stabilized the β-SOMO and broke the symmetry of the frontier orbital distribution of the PyBTM moiety. The former contributed to the red-shifting of the emission, and the latter contributed to the increase of oscillator strength of the D1 ← D0 transition (and thus the increase of the kr for emission), because this transition is forbidden for ideal three-fold symmetric triarylmethyl radicals. In addition, the reduced overlap of the empty and occupied orbitals might suppress vibronic coupling between the D1 and D0 states, thereby decreasing knr.17
The photoluminescence characteristics of 1b+ and 2+ are quantitatively slightly different, with 1bSbF6 having a shorter maximum wavelength of emission and higher ϕ than 2+ (Table 1).7 The higher ϕ is due to the smaller knr, which can be explained by the energy gap law.18 One reason for the blueshifting of the emission of 1bSbF6 relative to that of 2BF4 might be AgI complexes having longer metal–nitrogen bonds than the corresponding AuI complexes.19 The average AgI–N bond length in crystalline [AgI(PyBTM)2](SbF6) was 2.158 Å, larger compared with the AuI–N bond length of 2.101 Å in crystalline 2ClO4.7 DFT calculations also indicated longer metal–nitrogen bonds in 1b+ (2.178 Å) than in 2+ (2.150 Å). The more distal AgI ion and its consequently weaker electrostatic effects may stabilize the β-SOMO less than in AuI complexes, resulting in 1bSbF6 having a higher emission energy than 2BF4.
Conclusions
In conclusion, the complexation of PyBTM with AgI ions was investigated. While two PyBTM molecules coordinated to one AgI ion in the crystalline state, PyBTM formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with AgI in solution. The labile nature of the AgI–radical coordination bond can aid the construction of more elaborate molecular and supramolecular architectures including metal–organic frameworks and self-assembled cage-like supramolecules. In addition, facile exchange of the auxiliary ligands on an AgI ion could serve new photofunctions such as sensing. The 1
1 complex with AgI in solution. The labile nature of the AgI–radical coordination bond can aid the construction of more elaborate molecular and supramolecular architectures including metal–organic frameworks and self-assembled cage-like supramolecules. In addition, facile exchange of the auxiliary ligands on an AgI ion could serve new photofunctions such as sensing. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex exhibited enhanced redshifted photoluminescence compared with free PyBTM, as did the corresponding AuI complex. This study demonstrated the similarity in the effect of AgI and AuI ions on the electronic structure and excited states of the radical complexes, which would contribute to the rational design of luminescent radical complexes with controllable and predictable emission characteristics.
1 complex exhibited enhanced redshifted photoluminescence compared with free PyBTM, as did the corresponding AuI complex. This study demonstrated the similarity in the effect of AgI and AuI ions on the electronic structure and excited states of the radical complexes, which would contribute to the rational design of luminescent radical complexes with controllable and predictable emission characteristics.
Experimental
Materials and methods
Unless otherwise noted, solvents and reagents were purchased from TCI Co., Ltd, Wako Pure Chemical Industries, Ltd, or Kanto Chemical Co., Inc. and used without further purification. Dichloromethane was purified with AS ONE Ultimate Solvent System 4S.UV-Vis absorption spectra were recorded on a JASCO V-770 spectrophotometer. Steady-state emission spectra and fluorescence decay curves were measured using a measurement system with a picosecond diode laser with the emission wavelength of 375 nm (Advanced Laser Diode Systems PIL037X) as light source, a single grating spectrometer (Andor Kymera193i-B1), a multichannel CCD detector (Andor iDus DV420A-OE), and a photon counting detector (MPD SPD-050-CTE) operated with a time-correlated single photon counting (TCSPC) technique. Solvents used for the spectroscopic measurements were air-saturated. Absolute photoluminescence quantum yields were determined by a Hamamatsu Photonics Quantaurus-QY absolute PL quantum yield spectrometer (C11347-01). MALDI-TOF mass data were recorded by a Bruker microflex LRF system.
Soft X-ray absorption spectra were obtained using a transmission-type liquid cell at the soft X-ray beamline BL3U of the UVSOR-III Synchrotron.20 The liquid layer was sandwiched between two 100 nm thick Si3N4 membranes, and the thickness of the liquid layer was precisely controlled from 20 nm to 40 μm for obtaining the appropriate absorbance of soft X-rays. The liquid samples were introduced to the liquid cell using a syringe pump keeping the inert condition.
The data for single crystal X-ray diffraction (SCXRD) analysis was collected at 123 K on a ROD, Synergy Custom system (Rigaku Oxford Diffraction) equipped with mirror monochromated Mo-Kα radiation. A suitable single crystal was mounted on a looped film (micromount) with Paraton-N. Data were processed using CrysAlisPro 1.171.39.43c (Rigaku Oxford Diffraction). The structures were solved using ShelXT21 and the whole structure was refined against F2 with SHELXL-2018/3.22 Hydrogen atoms were located in idealized positions and were refined using a riding model with fixed thermal parameters. Crystal structure data (CIF, CCDC 2331661† for [Ag(PyBTM)2]SbF6) can be obtained free of charge from The Cambridge Crystallographic Data Centre. Powder X-ray diffraction measurements were performed at room temperature using a Rigaku MiniFlex600 diffractometer (Cu-Kα radiation, λ = 1.5406 Å) operating at 40 kV/15 mA with a Kβ foil filter.
The temperature dependence of the magnetic susceptibility was measured with a quantum design MPMS-7 SQUID magnetometer. Aluminium foil was used as a sample container, whose magnetic contribution was subtracted as background by measuring its own magnetic susceptibilities in every measurement. The diamagnetic correction χdia for [Ag(PyBTM)2](SbF6) was carried out with Pascal's constants (χdia = 5.35 × 10−4 cm3 mol−1).
DFT and TD-DFT calculations were carried out using the Gaussian 16 Revision C.01 program package.23 The TD-DFT calculations were performed after the geometry optimization in the electronic ground state at the UM06/SDD(Ag, Au);6-31G(d)(H, C, N, Cl, O, P) level under the SCRF (solvent = CH2Cl2) effect.
Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 (soln_99).
1 (soln_99).
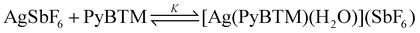
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio to obtain soln_99 suitable for absorption, emission, and ESR spectroscopy. Similarly, a dichloromethane solution containing 21.3 mM silver(I) hexafluoroantimonate and 10 mM PyBTM was prepared to obtain soln_99 suitable for Soft X-ray absorption spectroscopy.
1 v/v ratio to obtain soln_99 suitable for absorption, emission, and ESR spectroscopy. Similarly, a dichloromethane solution containing 21.3 mM silver(I) hexafluoroantimonate and 10 mM PyBTM was prepared to obtain soln_99 suitable for Soft X-ray absorption spectroscopy.
Author contributions
T.M.: conceptualization, project administration, investigation, formal analysis, visualization, and writing – original draft. R.M.: investigation, formal analysis, visualization, and writing – original draft. M.N.: methodology, resources, and investigation (X-ray absorption spectroscopy). T.K.: conceptualization, funding acquisition, project administration, and supervision. All the authors contributed equally to writing – review & editing.Data availability
Crystallographic data for 1aSbF6 has been deposited at the CCDC under 2331661.† The other data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present study was supported by the JSPS KAKENHI (Grant Numbers JP23H01980, JP23H04863, and JP23K04699) and the JST FOREST (Grant Number JPMJFR221Q). A part of this work was conducted in Institute for Molecular Science, supported by Advanced Research Infrastructure for Materials and Nanotechnology in Japan (JPMXP1222MS5001 and JPMXP1223MS5002) of the Ministry of Education, Culture, Sport, Science and Technology (MEXT), Japan. The soft X-ray absorption spectroscopy was measured at the BL3U of UVSOR Synchrotron Facility, Institute for Molecular Science (IMS Program 23IMS6620). The computation was performed using Research Centre for Computational Science, Okazaki, Japan (Project: 23-IMS-C244, 22-IMS-C191). This work was partly supported by the Spintronics Research Network of Japan (Spin-RNJ).References
-
(a) Z. Cui, A. Abdurahman, X. Ai and F. Li, CCS Chem., 2020, 2, 1129–1145 CrossRef CAS
; (b) A. Mizuno, R. Matsuoka, T. Mibu and T. Kusamoto, Chem. Rev., 2024, 124, 1034–1121 CrossRef CAS
.
- Y. Hattori, S. Kimura, T. Kusamoto, H. Maeda and H. Nishihara, Chem. Commun., 2018, 54, 615–618 RSC
.
- C. H. Liu, E. Hamzehpoor, Y. Sakai-Otsuka, T. Jadhav and D. F. Perepichka, Angew. Chem., Int. Ed., 2020, 59, 23030–23034 CrossRef CAS PubMed
.
-
(a) A. Obolda, X. Ai, M. Zhang and F. Li, ACS Appl. Mater. Interfaces, 2016, 8, 35472–35478 CrossRef CAS PubMed
; (b) X. Ai, E. W. Evans, S. Dong, A. J. Gillett, H. Guo, Y. Chen, T. J. H. Hele, R. H. Friend and F. Li, Nature, 2018, 563, 536–540 CrossRef CAS PubMed
.
-
(a) S. Kimura, T. Kusamoto, S. Kimura, K. Kato, Y. Teki and H. Nishihara, Angew. Chem., Int. Ed., 2018, 57, 12711–12715 CrossRef CAS
; (b) R. Matsuoka, S. Kimura, T. Miura, T. Ikoma and T. Kusamoto, J. Am. Chem. Soc., 2023, 145, 13615–13622 CrossRef CAS PubMed
; (c) A. Abdurahman, L. Shen, J. Wang, M. Niu, P. Li, Q. Peng, J. Wang and G. Lu, Light: Sci. Appl., 2023, 12, 272 CrossRef CAS PubMed
; (d) A. Mizuno, R. Matsuoka, S. Kimura, K. Ochiai and T. Kusamoto, J. Am. Chem. Soc., 2024, 146, 18470–18483 CrossRef CAS PubMed
.
-
(a) S. Gorgon, K. Lv, J. Grüne, B. H. Drummond, W. K. Myers, G. Londi, G. Ricci, D. Valverde, C. Tonnelé, P. Murto, A. S. Romanov, D. Casanova, V. Dyakonov, A. Sperlich, D. Beljonne, Y. Olivier, F. Li, R. H. Friend and E. W. Evans, Nature, 2023, 620, 538–544 CrossRef CAS
; (b) Y. R. Poh, D. Morozov, N. P. Kazmierczak, R. G. Hadt, G. Groenhof and J. Yuen-Zhou, J. Am. Chem. Soc., 2024, 146, 15549–15561 CrossRef CAS
.
- Y. Hattori, T. Kusamoto and H. Nishihara, Angew. Chem., Int. Ed., 2015, 54, 3731–3734 CrossRef CAS
.
- Y. Hattori, T. Kusamoto, T. Sato and H. Nishihara, Chem. Commun., 2016, 52, 13393–13396 RSC
.
-
(a) Y. Ogino, T. Kusamoto, Y. Hattori, M. Shimada, M. Tsuchiya, Y. Yamanoi, E. Nishibori, K. Sugimoto and H. Nishihara, Inorg. Chem., 2017, 56, 3909–3915 CrossRef CAS PubMed
; (b) Y. Hattori, R. Kitajima, R. Matsuoka, T. Kusamoto, H. Nishihara and K. Uchida, Chem. Commun., 2022, 58, 2560–2563 RSC
.
- J.-W. Yuan, Q.-C. Peng, J.-C. Fu, Q. Yang, Z.-Y. Gao, Z.-Y. Wang, K. Li, S.-Q. Zang and B. Z. Tang, J. Am. Chem. Soc., 2023, 145, 27095–27102 CrossRef CAS PubMed
.
-
(a) A. Datcu, N. Roques, V. Jubera, I. Imaz, D. Maspoch, J. Sutter, C. Rovira and J. Veciana, Chem. – Eur. J., 2011, 17, 3644–3656 CrossRef CAS PubMed
; (b) A. Datcu, N. Roques, V. Jubera, D. Maspoch, X. Fontrodona, K. Wurst, I. Imaz, G. Mouchaham, J. P. Sutter, C. Rovira and J. Veciana, Chem. – Eur. J., 2012, 18, 152–162 CrossRef CAS
; (c) F. Wang, J. Wang, S. F. Maehrlein, Y. Ma, F. Liu and X. Y. Zhu, J. Phys. Chem. Lett., 2020, 11, 762–766 CrossRef CAS
; (d) S. Kimura, M. Uejima, W. Ota, T. Sato, S. Kusaka, R. Matsuda, H. Nishihara and T. Kusamoto, J. Am. Chem. Soc., 2021, 143, 4329–4338 CrossRef CAS PubMed
; (e) S. Kimura, R. Matsuoka, S. Kimura, H. Nishihara and T. Kusamoto, J. Am. Chem. Soc., 2021, 143, 5610–5615 CrossRef CAS PubMed
.
- J. Shi, W. Xu, H. Yu, X. Wang, F. Jin, Q. Zhang, H. Zhang, Q. Peng, A. Abdurahman and M. Wang, J. Am. Chem. Soc., 2023, 145, 24081–24088 CrossRef CAS PubMed
.
-
(a) X. Liu, M. Wu, R. Zeng, G. Li, Q. Li, F. Li, A. Yuan and C. Shi, Inorg. Chem., 2022, 61, 20942–20948 CrossRef CAS PubMed
; (b) Y. Zhao, A. Abdurahman, Y. Zhang, P. Zheng, M. Zhang and F. Li, CCS Chem., 2022, 4, 722–731 CrossRef CAS
.
- W. C. Vosburgh and S. A. Cogswell, J. Am. Chem. Soc., 1943, 65, 2412–2413 CrossRef CAS
.
- Y. Hattori, T. Kusamoto and H. Nishihara, Angew. Chem., Int. Ed., 2014, 53, 11845–11848 CrossRef CAS PubMed
.
- S. Kimura, A. Tanushi, T. Kusamoto, S. Kochi, T. Sato and H. Nishihara, Chem. Sci., 2018, 9, 1996–2007 RSC
.
-
(a) Y. Kameoka, M. Uebe, A. Ito, T. Sato and K. Tanaka, Chem. Phys. Lett., 2014, 615, 44–49 CrossRef CAS
; (b) M. Uejima, T. Sato, D. Yokoyama, K. Tanaka and J.-W. Park, Phys. Chem. Chem. Phys., 2014, 16, 14244–14256 RSC
.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS
.
-
(a) J. C. Slater, J. Chem. Phys., 1964, 41, 3199–3204 CrossRef CAS
; (b) R. Hamze, S. Shi, S. C. Kapper, D. S. M. Ravinson, L. Estergreen, M.-C. Jung, A. C. Tadle, R. Haiges, P. I. Djurovich, J. L. Peltier, R. Jazzar, G. Bertrand, S. E. Bradforth and M. E. Thompson, J. Am. Chem. Soc., 2019, 141, 8616–8626 CrossRef CAS PubMed
.
-
(a) M. Nagasaka, H. Yuzawa and N. Kosugi, Anal. Sci., 2020, 36, 95–99 CrossRef CAS
; (b) M. Nagasaka and N. Kosugi, Chem. Lett., 2021, 50, 956–964 CrossRef CAS
.
- G. M. Sheldrick, SHELXT – Integrated space-group and crystal-structure determination, Acta Crystallogr. Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed
.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed
.
-
M. J. Frisch, et al., Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, X-ray crystallography, magnetic properties, photophysical properties, X-ray absorption spectroscopy, Titration analyses, and DFT calculations. CCDC 2331661. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03129f |
| This journal is © The Royal Society of Chemistry 2025 |