Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Homoleptic coordination polymers and complexes of transition metals with 2-(1,2,4-1H-triazol-3-yl) pyridine and tuning towards white-light emission†
Marcel T. Seufferta,
Alexander E. Sedykh a,
Thomas C. Schäfera,
Jonathan Beckera and
Klaus Müller-Buschbaum
a,
Thomas C. Schäfera,
Jonathan Beckera and
Klaus Müller-Buschbaum *ab
*ab
aInstitute of Inorganic and Analytical Chemistry, Justus Liebig University Giessen, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: kmbac@uni-giessen.de
bCenter for Materials Research (LAMA), Justus Liebig University Giessen, Heinrich-Buff-Ring 16, 35392 Giessen, Germany
First published on 19th February 2025
Abstract
Twelve coordination compounds ranging from polymers to complexes based on divalent ions of the 3d-transition metals Mn to Zn, and Cd together with the ligand 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) were synthesised and fully characterised. The main products are homoleptic, one-dimensional coordination polymers 1∞[M(pt)2] (M = Mn–Zn, and Cd, pt = 3-(pyridin-2-yl)-1,2,4-triazolate), 1∞[Cu(pt)2]·0.5Py, besides complexes [MX2(Hpt)2] from MnCl2, FeCl2, CoCl2, CoBr2, ZnCl2, and Hpt = 2-(1,2,4-1H-triazol-3-yl)pyridine. In addition to these series, single crystalline by-products of the reactions were identified and their structures determined. The obtained products were investigated with single-crystal (SCXRD) and powder X-ray diffraction (PXRD), including temperature-dependent PXRD, physisorption experiments, UV-Vis, IR, and photoluminescence spectroscopy (PL), simultaneous thermal analysis, and elemental analysis. Based on 1∞[Zn(pt)2], it was possible to generate a white light-emitting compound by addition of Eu3+ and Tb3+. It follows the RGB concept with blue ligand-based emission of the coordination polymer and red/green emission of lanthanide ions and shows excitation dependent tuneable character of emission colour from blue to practically perfect white.
Introduction
Coordination compounds and complexes have been under extensive chemical investigation and have multiple applications e.g., functioning as catalysts1 as well as for medical and biochemical purposes.2 This includes e.g., various zinc-based coordination compounds acting as anticancer agents,3 or complexes based on the tridentate Schiff base ligand, 4-(2-((1E,2E)-1-(2-(p-tolyl)hydrazineylidene)-propan-2-ylidene)-hydrazineeyl) with anticancer or antimicrobial activities depending on contained metals.4 Complexes of Fe(III), and Co(II) with Isatin-hydrazone derivates were reported showing anti-inflammatory properties.5 Besides, coordination complexes can be utilised as sensor materials for various analytes by luminescence monitoring. For example, the formation of a complex and thereon fluorescence quenching of a 2-pyridinecarbonyl substituted 8-aminoquinoline derivative was reported being applicable to quantify Cu2+ in water and food.6 A complex based on Pd(II) and a hydrazone derived Schiff base was found to be capable of ratiometric detection of F−.7 The compound [(ppy)2Ir(IPP)]PF6 (ppy = 2-phenylpyridine, IPP = 1-(1H-imidazo[4,5-f][1,10]phenatrolin-2-yl)phenol) was reported being applicable as a “turn-off” fluorescence sensor for Ni2+.8 This shows the numerous potential applications, coordination complexes possess.By usage of multidentate ligands and depending on the ligand structure, it is possible to obtain polymeric coordination compounds, namely coordination polymers (CPs) and metal–organic frameworks (MOFs). These groups of coordination compounds were intensively studied over the recent years.9–12,13 The properties of CPs and MOFs depend on their structure and containing building blocks – often metal ions and ligands – leading to various possible applications, such as electrochemical properties14 resulting in energy storage implementations,11,15 catalytic utilisation,16,17 medical purposes,9,12,18,19 and environmental protection applications.10,16,20
One of the most fascinating properties of manifold MOFs is photoluminescence, leading to applications like sensing,21 bioimaging,9,18,22 optoelectronic devices,23 and aesthetical or anti-counterfeiting applications.24,25 Investigated photoluminescent coordination compounds often contain multiple chromophores, leading to the possibility of tuning the emission colour. This can be achieved e.g., by functionalisation of MOFs with lanthanide ions.26,27,28–31 White light emission is a frequently desired property of alike compounds, attained by utilising additive colour mixing according to the RGB concept.25,28,32 Thereby, white light emitting CPs and MOFs as solid-state light sources are a more environment-friendly alternative to e.g. mercury containing fluorescent lights.33,34 This makes them promising candidates for lighting technologies or anti-counterfeiting applications.35 As a matrix material for such luminescent compounds, transition metal coordination polymers can be exploited, as it was shown for ZIF-8,29,31 MOF-5,26 or 3∞{[Zn3(bdc)3(EtOH)2]·(EtOH)0.6} (bdc = benzene dicaboxylate).30 Moreover, by intercalation of Eu3+ and Tb3+ to MIL-124, a white light emitter was created, which potentially can be utilised in luminescent devices like displays, or for lighting.33 Furthermore, a coordination polymer gel obtained by reaction of europium acetate and terbium acetate with 5,5′,5′′-((benzene-1,3,5-tricarbonyl)tris(azanedi-yl))triisophthalic acid featuring white light emission was published, which is applicable as invisible anti-counterfeit ink.36 By coating of UV-LEDs with a composite comprising carbon dots, and the MOF [Eu1.22Tb0.78(1,4-phda)3(H2O)](H2O) it was possible to generate a white LED.37
There are several examples of CPs containing transition metals and 1,2,4-1H-triazole (TzH), or rather 1,2,4-triazolate (Tz), as ligand.38 It was shown, that those compounds can exhibit guest-sensitive luminescence.39 Therefore, also derivates of triazoles are of increasing scientific interest as potential ligands for CPs with photoluminescent properties. A ligand, which was recently used for this purpose, is 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt)40–42,43–47 or derivates of it.46,48,49 Numerous compounds based on these ligands in combination with transition metals were reported, such as coordination polymers, like the isotypic, homoleptic compounds 1∞[Ni(pt)2]·1.5H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 1∞[Cu(pt)2]·H2O,41 as well as the heteroleptic polymers 1∞[Cu2I(pt)2],50 and 1∞[Cu(pt)(OAc)].51 They all exhibit a one-dimensional polymeric structure with η2-μ2-coordination mode of the deprotonated pt ligand. Also, compounds containing derivates of Hpt were published, such as 3∞[Cu(H-2py-trz-pba)2] (H-2py-trz-pba = 4-(3-(Pyridin-2-yl)-4H-1,2,4-triazol-4-yl)benzoate),52 [Mn2(bpt)2(SCN)2(H2O)2] (containing the Hpt derivate bpt = 3,5-bis(2-pyridyl)-4H-1,2,4-triazole),46 and 1∞[Zn(5-nipa)(L22)(H2O)] (5-nipa = 5-nitroisophthalate, L22 = 2,2′-(4H-1,2,4-triazole-3,4-diyl)dipyridine).49
40 and 1∞[Cu(pt)2]·H2O,41 as well as the heteroleptic polymers 1∞[Cu2I(pt)2],50 and 1∞[Cu(pt)(OAc)].51 They all exhibit a one-dimensional polymeric structure with η2-μ2-coordination mode of the deprotonated pt ligand. Also, compounds containing derivates of Hpt were published, such as 3∞[Cu(H-2py-trz-pba)2] (H-2py-trz-pba = 4-(3-(Pyridin-2-yl)-4H-1,2,4-triazol-4-yl)benzoate),52 [Mn2(bpt)2(SCN)2(H2O)2] (containing the Hpt derivate bpt = 3,5-bis(2-pyridyl)-4H-1,2,4-triazole),46 and 1∞[Zn(5-nipa)(L22)(H2O)] (5-nipa = 5-nitroisophthalate, L22 = 2,2′-(4H-1,2,4-triazole-3,4-diyl)dipyridine).49
Various reported compounds also feature additional properties, like [Fe(Hpt)3](BF4)2·H2O, which shows a temperature-dependent spin crossover of Fe(II).43 Also, the d10 transition metal ion Cu(I) forms complexes with Hpt derivates, such as [Cu(bptzH)(PPh3)2](ClO4) (bptzH = 2-(3-tert-butyl-1,2,4-1H-triazol-5-yl)pyridine), [Cu(fptzH)(PPh3)2](ClO4) (fptzH = 2-(3-trifluoromethyl-1,2,4-1H-triazol-5-yl)pyridine), and [Cu(bptzH)(dppe)](ClO4) (dppe = 1,2-bi(diphenylphosphino)ethane), which all show intense, luminescence in the solid state with emission colours from blue to green.53 This shows the potential of coordination compounds based on Hpt and its derivates with regard to optical properties.
In this work, we extend the number of coordination compounds based on Hpt and present the structures and properties of new compounds with 3-(pyridin-2-yl)-1,2,4-triazolate (pt) as ligand, namely the polymers 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Ni (4), Cu (5), Zn (6), Cd (7)), and 1∞[Cu(pt)2]·0.5Py (8), besides the complexes [MX2(Hpt)2] (MX2 = MnCl2 (9), FeCl2 (10), CoCl2 (11), CoBr2 (12)) containing 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt). Depending on the contained transition metal ion, the synthesised CPs can exhibit photophysical properties and – in case of a zinc-containing CP – can even be used as blue component of a RGB-type white light-emitter.
The obtained compounds were investigated with regard to structure, photophysical properties and thermal properties, which is described hereafter.
Results and discussion
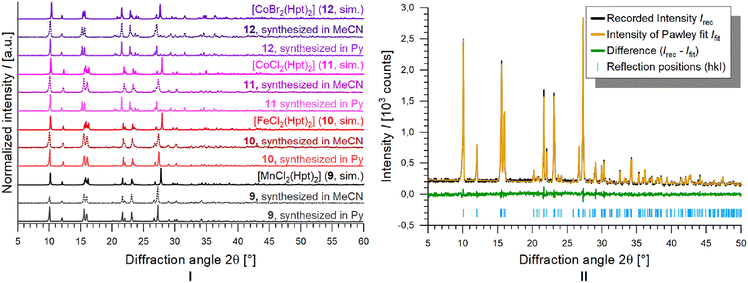
The compounds 1∞[Zn(pt)2] (6), 1∞[Cu(pt)2]·0.5Py (8), [MnCl2(Hpt)2] (9), [FeCl2(Hpt)2] (10), [CoCl2(Hpt)2] (11), [CoBr2(Hpt)2] (12), and [ZnCl2(Hpt)2] (13) were obtained as single crystals suitable for SCXRD. Therefore, the crystal structures were determined for these compounds based on SCXRD data, isostructural or even isotypic character of further elements of the series of compounds were determined by PXRD.Syntheses of and crystal structure investigations on 1∞[M(pt)2] (1–7)
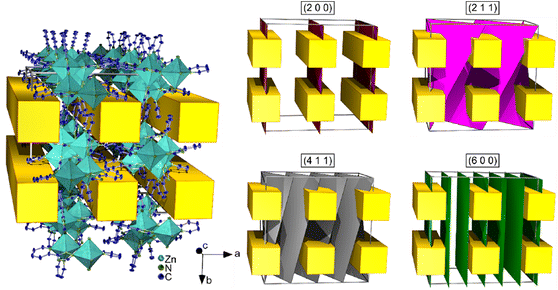
Based on single crystal X-ray data, the crystal structure of this series of compounds was determined for the homoleptic, one-dimensional coordination polymer (CP) 1∞[Zn(pt)2] (6). Crystals of 6 suitable for single crystal X-ray diffraction investigations were obtained by reaction of water-free ZnCl2, 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt), and benzylamine in a mixture of toluene and pyridine. CP 6 crystallises in the tetragonal crystal system in the space group I41/acd. The CP contains distorted, zinc-based octahedra, in which Zn2+ is coordinated by four deprotonated, 3-(pyridin-2-yl)-1,2,4-triazolate (pt) ligand molecules. Two η2-pt ligands coordinate via N1 of the pyridine ring and N2 of the triazole ring. The other two η1 ligand molecules coordinate via N3 of the triazole ring to Zn. Thereby, each pt ligand coordinates to two zinc ions. This results in octahedra which are interconnected by two neighbouring nitrogen atoms of the triazole rings (here N2 and N3). The consequential, one-dimensional polymeric strands screw about the crystallographic c axis and are stacked within the unit cell, forming square-shaped pore channels.Detailed crystallographic data is listed in Table S1.† A depiction of the crystal structure is given in Fig. 1. Considering weak C–H⋯N interactions,54 each pt ligand establishes two hydrogen bonds to ligand molecules of a neighbouring polymeric strand. This results in a supermolecular three-dimensional polymeric structure (Fig. S1†).
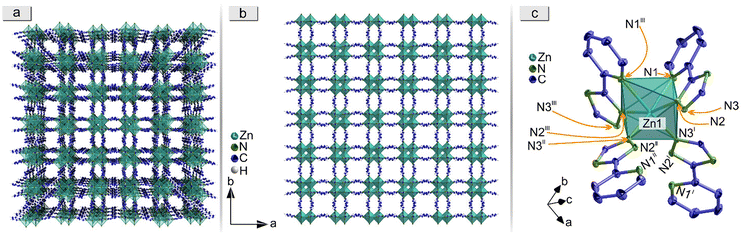 | ||
| Fig. 1 Depiction of the crystal structure of 1∞[Zn(pt)2] (6). Presented are: A three-dimensional depiction of the square-shaped pore channels along the crystallographic c axis (a), an excerpt of the crystal structure along the crystallographic c axis in parallel projection (b), and the extended coordination of Zn2+ (c, hydrogen atoms are omitted). Polyhedra around Zn are highlighted dark cyan. Displayed thermal ellipsoids correspond to 50% probability level of the atoms. For clarity, only one of the two disorders of the ligand molecule is depicted. Crystallographic data is summarised in Table S1.† Symmetry operations: I = 1/4 + y, 1/4 − x, −1/4 + z; II = x, −y, 1/2 − z; III = 1/4 − y, 1/4 − x, 1/4 − z. | ||
The interatomic Zn–N distances in CP 6 were determined to be within the range of 207(5) pm to 232(4) pm (Table S2†). These distances match with published values for e.g. 3∞[Zn(H-2py-trz-ia)]·5.25H2O (H-2py-trz-ia = 5-(3-pyridyl-1,2,4-triazol-4-yl)iso-phthalate), which contains fivefold coordinated Zn2+ interconnected three-dimensionally by H-2py-z-ia, and for which corresponding distances in a range of 212–221 pm are reported.47
The distances in 6 are also in good agreement with distances in 1∞[Zn(5-nipa)(L22)(H2O)] (5-nipa = 5-nitro-isophthalate, L22 = 2,2′-(4H-1,2,4-triazole-3,4-diyl)dipyridine),49 which contains octahedrally coordinated Zn2+ and for which Zn–N distances of 214–235 pm are reported.
By comparison of the reported sum formulas, CP 6 is closely related to reported compounds 1∞[Ni(pt)2]·1.5H2O,40 and 1∞[Cu(pt)2]·H2O,41 which also feature octahedral coordination of the metal ions by η2-μ2-coordinating pt ligands and one-dimensional polymeric structures. Within these two CPs, polymeric zig-zag chains with two different orientations within the unit cell are formed, rather than screwing strands as observed for 6. This leads to a denser packing within the unit cell for the already reported compounds in comparison to 6. For the single crystal structure of CP 6, a residual electron density of 215 electrons per unit cell located in the square-shaped channels were found, indicating solvent molecules being intercalated there.
PXRD investigations on 1∞[M(pt)2]
Bulk materials of 1∞[M(pt)2] (1–7) were obtained by solvothermally reacting stoichiometric amounts of the metal acetate (hydrates) M(OAc)2·xH2O, M = Mn–Zn, Cd, and the ligand Hpt in pyridine (see Experimental section). Subsequent to washing with fresh solvent and drying in vacuum, the homoleptic compounds 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Ni (4), Cu (5), Zn (6), Cd (7)) are obtained, which are isotypic, and can be synthesised phase-pure, as proven by powder diffractograms (Fig. 2). The isotypic character of the CPs 1–7 was confirmed by Pawley refinements (see Fig. S4, and Table S15†). By these, the cell constants of 1–7 were determined to the values listed in Table S27.†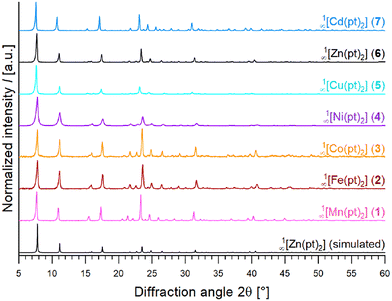 | ||
| Fig. 2 PXRD patterns of 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Ni (4), Cu (5), Zn (6), Cd (7)), in comparison to a pattern of 6 simulated from SCXRD data. | ||
It is noteworthy, that CP 5 (1∞[Cu(pt)2]) differs from the already reported compound 1∞[Cu(pt)2]·H2O41 although having the same polymer sum formula. Indeed, 5 is isotypic to 1∞[Zn(pt)2] (6) as proven by PXRD and Pawley refinement utilizing SCXRD data of 6 (see Fig. 2, Fig. S4, and Table S15†). Hence, 5 also crystallises in the tetragonal crystal system in a body-centred Bravais lattice and exhibits polymeric strands screwing about crystallographic c axis, as described above. Contrary, 1∞[Cu(pt)2]·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 contains polymeric zig-zag chains with two different orientations within the unit cell crystallizing in a primitive Bravais lattice.41
41 contains polymeric zig-zag chains with two different orientations within the unit cell crystallizing in a primitive Bravais lattice.41
Temperature-dependent PXRD investigations on 1∞[M(pt)2]
The effect of intercalated solvents is further noticeable. Differences in reflection intensities between recorded patterns and the simulated pattern of 1∞[Zn(pt)2] (6), which are observable within Fig. 2, can be explained by solvent molecules being intercalated within the square-shaped pore channels. An influence on reflection intensities is a known effect of guests being present in pore systems.55 Furthermore, different constituting metal ions and the tendency of these CPs to crystallise with a needle-shaped habitus leading to preferred orientation effects with regard to PXRD influence the reflection intensities, as well.To investigate the differences and changes of reflection intensities more intensively, temperature-dependent PXRD recordings were performed. When analysing respective patterns of 1∞[Zn(pt)2] (6) (Fig. 3), the intensity of the (2 0 0) reflection at approx. 7.8° in 2θ shows the largest detectable variation in intensity in dependence of temperature T and increases upon heating (between 30 °C and 405 °C). Since related lattice planes intersect the square-shaped pore channels (Fig. 4), the intensity of this reflection is possibly influenced by guest molecules intercalated in the pores. With increasing temperature, the differences in intensities between recorded and simulated patterns of 6 diminish with solvent molecules leaving the pores. The powder pattern of 6 can be identified up to a temperature of 430 °C, while the intensities of observed reflections start to decrease above 405 °C, as shown for the four most intense reflections in Fig. S6.† Additionally, the broad reflections that are assigned to the formation of ZnCN2, begin to emerge. At 455 °C, the coordination polymer is decomposed completely, and the majority phase is identified as ZnCN2 (Fig. 3).
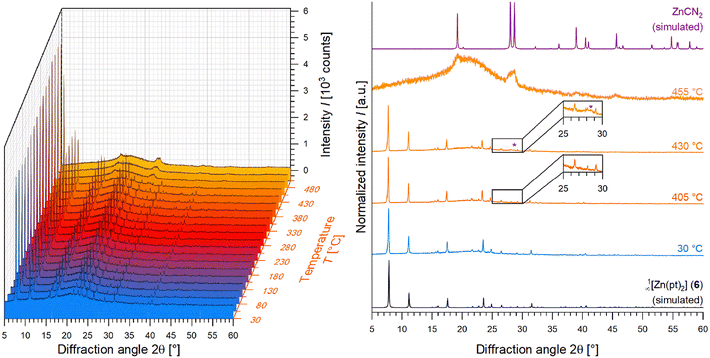 | ||
| Fig. 3 Temperature-dependent powder X-ray diffractograms of 1∞[Zn(pt)2] (6) reaching from 30 °C to 505 °C in steps of 25 °C (left) besides recorded diffractograms of 6 at 30 °C, 405 °C and 430 °C in comparison to diffractograms CP 6, and ZnCN2,61 both simulated from respective SCXRD data (right). | ||
Analogous temperature-dependent PXRD investigations were performed for 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Ni (4), Cu (5), Cd (7)) leading to comparable results.
For 1∞[Mn(pt)2] (1), the formation of unknown phase(s) is observable. 1∞[Fe(pt)2] (2) converts to a high pressure phase of magnetite,56 followed by transition to magnetite57 at 480 °C and finally to hematite58 at 505 °C. 1∞[Co(pt)2] (3) and 1∞[Cu(pt)2] (5) decompose to amorphous compounds. Temperature-dependent PXRD investigations on 1∞[Ni(pt)2] (4) show the formation of Ni3C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 above 280 °C. In analogy to the high-temperature behaviour of 1∞[Zn(pt)2] (6) described above, CP 7 (1∞[Cd(pt)2]) decomposes to CdCN2
59 above 280 °C. In analogy to the high-temperature behaviour of 1∞[Zn(pt)2] (6) described above, CP 7 (1∞[Cd(pt)2]) decomposes to CdCN2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 above 405 °C, at first, which subsequently converts to CdO
48 above 405 °C, at first, which subsequently converts to CdO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 at 505 °C. Temperature-dependent PXRD investigation results are summarised in Table 1. Corresponding PXRD patterns are given within the ESI (Fig. S6–S18†), besides Fig. 3. This shows that the solvents can be removed from square-shaped pore channels by thermal treatment.
60 at 505 °C. Temperature-dependent PXRD investigation results are summarised in Table 1. Corresponding PXRD patterns are given within the ESI (Fig. S6–S18†), besides Fig. 3. This shows that the solvents can be removed from square-shaped pore channels by thermal treatment.
| Compound | Sum formula | Tst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [°C] a [°C] |
Tmax [°C] | Tdec [°C] | Majority phase at Tdec |
|---|---|---|---|---|---|
a The temperature stability was evaluated by investigation of reflection intensities in temperature-dependent PXRD. As soon as a drop in intensity of the reflections is observed, the beginning decomposition of the crystalline structure is assumed.b See also Fig. S7–S18.†c See Fig. 3, and Fig. S6.†d High-pressure crystal structure of magnetite at 250 kPa. It converts to magnetite57 at 455 °C and finally to hematite58 at 505 °C.e Transition to CdO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 is observed at 505 °C. 60 is observed at 505 °C. |
|||||
| 1 | ∞1[Mn(pt)2]b | 330 | 430 | 455 | Unknown phase |
| 2 | 1∞[Fe(pt)2]b | 305 | 430 | 455 | Fe3O4 (magnetited)56 |
| 3 | 1∞[Co(pt)2]b | 455 | 455 | 480 | Amorphous |
| 4 | 1∞[Ni(pt)2]b | 280 | 480 | 505 | Ni3C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
| 5 | 1∞[Cu(pt)2]b | 305 | 330 | 330 | Amorphous |
| 6 | 1∞[Zn(pt)2]c | 405 | 430 | 455 | ZnCN2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 61 |
| 7 | 1∞[Cd(pt)2]b | 405 | 430 | 455 | CdCN2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e62 e62 |
Crystal structure of the one-dimensional, homoleptic coordination polymer 1∞[Cu(pt)2]·0.5Py (8)
A structure which is closely related to CP 6, was found for the coordination polymer 1∞[Cu(pt)2]·0.5Py (8, Fig. 5). This compound crystallises in the orthorhombic crystal system in the space group Ibca. The same structural motif of 1∞[Zn(pt)2] (6) is present in CP 8. It contains distorted, copper-ion-based octahedra, in which Cu2+ is coordinated by four deprotonated pt molecules, two η2- and two η1-pt, in analogy to CP 6.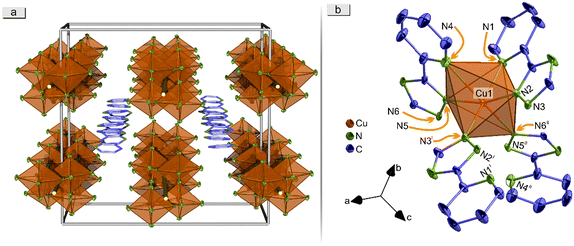 | ||
| Fig. 5 Depiction of the crystal structure of 1∞[Cu(pt)2]·0.5Py (8). Presented are: An enlarged 3D illustration of the rectangular channels within the elemental cell with depiction of both disorders of trapped pyridine molecules (a; for simplification, only Cu atoms and coordinating N atoms of the CP are depicted and pyridine molecules are shown in wire stick representation), as well as the extended coordination of Cu2+ (b; hydrogen atoms are omitted). Polyhedra around Cu are highlighted in orange. Displayed thermal ellipsoids correspond to 50% probability level of the atoms. Crystallographic data is summarised in Table S3.† Symmetry operations: I = 1 − x, 3/2 − y, z; II = 3/2 − x, y, 1 − z. | ||
Solvent molecules (pyridine) are intercalated in the rectangular pore channels in a crystallographically ordered manner, resulting in a change of the crystal system from tetragonal to orthorhombic in comparison to 1∞[Zn(pt)2] (6).
Excerpts of the crystal structure of CP 8 are given in Fig. 5 and Fig. S2.† Detailed crystallographic information is given in Table S3.† Interatomic Cu–N distances ranging from 198(3) pm to 260(2) pm (Table S4†) coincide with distances reported for the one-dimensional coordination polymer 1∞[Cu(pt)2]·H2O, which consist of pt-linked, Cu(II)-based octahedra. Interatomic Cu–N distances for this compound are ranging from 197 pm to 254 pm.41
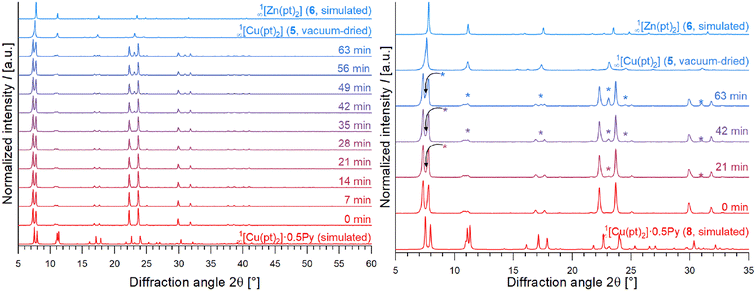
PXRD investigations on 1∞[Cu(pt)2]·0.5Py (8)
As mentioned above, the CPs 1∞[M(pt)2] can be obtained as bulk material by solvothermal reaction of metal acetates and Hpt in pyridine. However, due to the porosity of these substances, solvent molecules are intercalated. Accordingly, drying is essential to achieve 1∞[Cu(pt)2] (5). The product initially obtained by reaction of Cu(OAc)2·H2O and Hpt in pyridine (without washing and vacuum drying), contained both, single crystals of 1∞[Cu(pt)2]·0.5Py (8) and reflections of 8 in the powder diffractogram, only. This means, the as-synthesised, still damp product of the reaction of Cu(OAc)2·H2O and Hpt contains 1∞[Cu(pt)2]·0.5Py (8) as majority phase instead of 5. However, CP 8 is only stable in the presence of pyridine and converts completely into 1∞[Cu(pt)2] (5) upon drying, which was proven by comparison of recorded PXRD patterns after several minutes of air-drying and complete vacuum-drying (Fig. 6, reflections corresponding to 5 are marked with*). The pattern of a freshly synthesised, still damp product shows reflections of CP 8, only. Upon drying, the reflections of CP 5 begin to appear. The vacuum-dried product exhibits reflections of 5, only (Fig. 6). This shows the direct effect of crystallographically ordered solvent molecules within the structure, leading to the reduction of the symmetry as described above.Photophysical properties of selected compounds
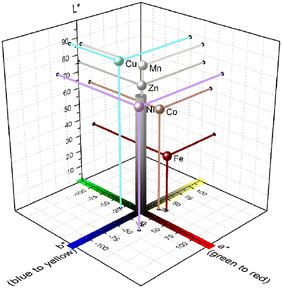 | ||
| Fig. 7 L*a*b* scatter diagram with inserted L*a*b chromaticity coordinates of the coordination polymers 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Ni (4), Cu (5), Zn (6)). Corresponding reflectance spectra are given in Fig. S20.† Calculated chromaticity coordinates are listed in Table S16.† | ||
Considering potential luminescence application, these two candidates are therefore appropriate due to their white appearance and, hence, low absorption of visible light. Upon UV excitation, 6 exhibits blue luminescence. Since both, incoming UV and emitted light, are not absorbed by Zn2+ ion due to the d10 electron configuration, this emission is considered as ligand-based.
The pure ligand exhibits a broad band emission in the UV/blue region upon UV excitation (λem max = 360 nm, λexc = 324 nm, Fig. S22, and S24†), while the emission band of CP 6 is located at λem max = 337 nm for excitation at 306 and 311 nm. At higher excitation wavelengths (λexc = 324 nm; 346 nm) λem max = 360 nm is located at 395 nm (Fig. S23 and S25†).
Comparable photoluminescence behaviour was reported for other d10 transition metal compounds, e.g. for [Zn6(H2O)3(dmf)6(ur)2(tdc)6]·4H2O (ur = urotropin, tdc = 2,5-thiophenedicarboxylate), which exhibits a broad emission at 470 nm (λexc = 380 nm) assigned to intra-ligand π* → π or LMCT transitions,63 or [Zn3(btc)2(tpt)(H2O)2]·4H2O (btc = 3,5-benzenetricarboxylate, tpt = tris(4-pyridyl)triazine) featuring a broad emission around 380 nm (λexc = 280 nm) assigned to the π* → π transition of the N-donor ligand tpt.64 Likewise, [Cu(bptzH)(PPh3)2](ClO4), exhibits blue luminescence (λem = 456 nm) upon UV excitation.53 The observed luminescence of the coordination polymer (see lifetime determination following) is therefore considered ligand based and may be explained with π* → π of higher energy levels (compared to pure ligand Hpt) of the deprotonated ligand pt− and/or LMCT.63,65
The photoluminescence properties render 1∞[Zn(pt)2] a suitable candidate to function as the blue component of an RGB-type white light emitter. To generate a suchlike luminescent compound, an impregnation of the square-shaped pore channels of CP 6 with Eu3+ ions as red emitter, and Tb3+ ions as green emitter, was performed. For this purpose, an activated sample of CP 6 was dispersed and stirred in a solution containing equal amounts of Eu(NO3)3·6H2O and Tb(NO3)3·6H2O for eight days followed by centrifugation, decantation and vacuum drying of the solid product.
Three different solvents, namely N,N-dimethylformamide (DMF), acetonitrile (MeCN), and methanol (MeOH), were evaluated (For detailed information, see Experimental section.). Exemplarily, emission and excitation spectra, besides the chromaticity diagram of the product of impregnation in DMF – which is hereafter to be referred to as EuTb@1∞[Zn(pt)2] – are shown in Fig. 8, and Fig. 9, respectively.
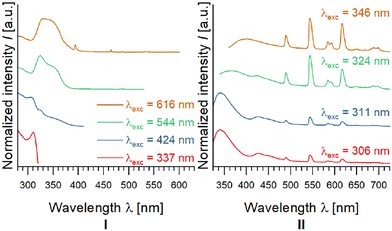 | ||
| Fig. 8 Excitation (left) and emission spectra (right) of 1∞[Zn(pt)2] impregnated with Eu3+ and Tb3+ ions for eight days using DMF as solvent. | ||
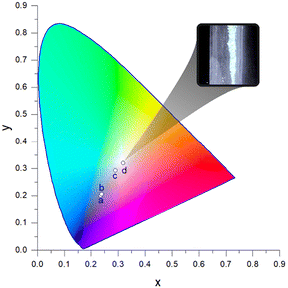 | ||
| Fig. 9 CIE chromaticity diagram of 1∞[Zn(pt)2] (CP 6) impregnated with Eu and Tb in DMF as solvent with inserted chromaticity coordinates xy at different excitation wavelengths. a: λexc = 306 nm, b: λexc = 311 nm, c: λexc = 324 nm, d: λexc = 346 nm. Accordingly, the lanthanide luminescence results from a sensitisation effect of the direct surrounding of the respective lanthanide ions, also known as antenna effect.66–68 | ||
For excitation at λexc = 306 nm, 311 nm, and 324 nm, the emission spectra feature broad bands in the UV/blue region at wavelengths of 337 nm and 424 nm. Besides these, characteristic 4f–4f-transitions of the two lanthanide ions, Eu3+ and Tb3+, are detected. As shown by the excitation spectra, the emission at 337 nm is initiated by excitation at 311 nm. The excitation spectrum for the emission band at λem = 424 nm features a maximum at λexc = 306 nm with a shoulder at 324 nm. The excitation spectra of most intense transitions of Tb3+ (544 nm) and Eu3 (617 nm) exhibit a maximum at λexc = 324 nm with a shoulder at 346 nm. A comparison between the emission spectra of pure Hpt, 1∞[Zn(pt)2] (6), and EuTb@1∞[Zn(pt)2] (Fig. S26†) shows, that the blue luminescence of EuTb@1∞[Zn(pt)2] corresponds to the luminescence observed for CP 6.
However, the observed band at 424 nm for λexc = 306, 311, and 324 nm is not present within emission spectra of non-impregnated, pure 1∞[Zn(pt)2]. This indicates a different source of this band. One possible explanation is, that this band arises due to formation of exciplexes between the lanthanide species inside the pore system and the coordination polymer. Interestingly, the emission band at 424 nm vanishes at λexc = 346 nm and a new emission band is observed at λem max = 395 nm, which coincides with the spectrum for non-impregnated CP 6 at the same excitation wavelength. For short excitation wavelengths, the blue CP-based emission exceeds the lanthanide-based luminescence, wherefore the resulting overall emission colour is neon blue.
With rising excitation wavelength, the lanthanide transitions become more dominant in the emission spectrum (Fig. 8) resulting in a shift of the chromaticity. Hence, a variation in intensity ratios of the observed bands in dependence of the excitation wavelength, was determined for EuTb@1∞[Zn(pt)2] synthesised in DMF. This results in variable chromaticity (Fig. 8 and 9). In fact, the emitted light of EuTb@1∞[Zn(pt)2] impregnated in DMF, when excited at λexc = 346 nm, exhibits chromaticity coordinates according to CIE of xy = (0.32|0.32). It therefore reaches practically perfect white chromaticity (xy = (0.333…|0.333…), Fig. 9). Thus, the concept of an excitation dependent red-shift of emission was utilised for an emission tuning towards RGB white light emission.
For the products of impregnations in methanol (Fig. S27†) and acetonitrile (Fig. S28†), qualitatively the same emission and excitation bands are observed, resulting in an analogous dependency of emission colour on excitation wavelength (Fig. S29, and S30†) Therefore, involved radiative processes are considered as independent from the chosen solvent.
As a result, the overall luminescence occurs due to excitation of the ligand followed by light emission of the CP (blue), as well as energy transfer to the lanthanides and subsequent characteristic Tb3+ (green) and Eu3+ (red) emissions. The respective solvent utilised for impregnation does not participate in the radiative luminescence processes. Corresponding chromaticities of the products from impregnation in acetonitrile and methanol at λexc = 346 nm are less ideal and given in Fig. S29 and S30.†
A comparison of scanning electron microscopy images before and after impregnation of 6 with Eu3+ and Tb3+shows, that the habitus of CP 6 crystallites does not change upon impregnation (see Fig. S38†). Lanthanide contents between 1.7 and 2.3 wt% were determined by inductively coupled mass spectrometry (ICP-MS, for details, see Table S28†).
In summary, the coordination polymer 1∞[Zn(pt)2] (6) being a blue light emitter, is capable of forming an RGB emitter by impregnation with Eu and Tb ions. Thereby, due to dependency of band intensities on excitation wavelength, the emission colour is tuneable from neon blue to white light (Fig. 9).
In addition to the qualitative photoluminescence characterisations, the luminescence lifetimes of the white light emitter EuTb@1∞[Zn(pt)2] were determined. For the emission band at λem = 395 nm excited at λexc = 340 nm of pure 1∞[Zn(pt)2] (without lanthanide impregnation), the luminescence intensity decay can be described by a multi-exponential fit function leading to determination of the lifetimes τ1 = 1.00(2) ns, and τ2 = 3.10(3) ns. An average luminescence lifetime69,70 of τav = 1.95(3) ns was calculated. For the pure ligand, the lifetimes t1 = 1.16(1) ns, and t2 = 2.89(3) resulting in τav = 1.58(2) ns were determined, which are of the same magnitude as lifetimes of pure CP 6 (For detailed description of the calculation, see ESI.†).
For EuTb@1∞[Zn(pt)2], τav for λem = 395 nm (λexc = 340 nm) was determined as 2.11(3) ns. Hence, the luminescence lifetime is slightly elongated for 1∞[Zn(pt)2] (6) impregnated with lanthanides in comparison to non-impregnated CP 6. This can be explained by rigidification due to coordination of pt to Zn2+, which results in reduced vibrational freedom of the ligand and, hence, reduced rate of non-radiative depopulation of excited states71 The recorded luminescence intensity decays of lanthanides within EuTb@1∞[Zn(pt)2] had to be fitted with a multi-exponential function, as well, in order to obtain a good quality of fit. Thereby, for Eu3+ emission (λem = 698 nm, λexc = 346 nm), two luminescence lifetimes were found (τ1 = 0.48(3) ms, τ2 = 1.30(3) ms), resulting in τav = 1.04(2) ms. In case of Tb3+ emission (λem = 544 nm, λexc = 346 nm), lifetimes of τ1 = 0.40(2) ms and τ2 = 1.160(6) ms were determined resulting in τav = 1.028(6) ms. These values in the μs–ms region all coincide with the range, in which lifetimes of the respective lanthanide ions are expected.67,68,72 The discovery of two luminescence lifetimes for luminescent constituents indicates e.g. occupation of different positions within the pores resulting in varied chemical surroundings and, therefore, different non-radiative depopulation rates. In principle, the formation of lanthanide-containing side phases could also lead to detection of multiple luminescence lifetimes. However, PXRD patterns of EuTb@1∞[Zn(pt)2] impregnated in different solvents show reflections of the coordination polymer, only (Fig. S19†). Variations in reflection intensities further substantiate the assumption of lanthanide species intercalated in the pores of 6.
An influence of the solvents utilised for impregnation on luminescence lifetimes by non-radiative processes can be assumed, as variations in τav are observed when using e.g. MeOH instead of DMF. For EuTb@1∞[Zn(pt)2] prepared in methanol, an average lifetime of the CP emission is determined to τav = 1.27(2) ns, which is 59% of the value for the composite prepared in DMF (2.14(2) ns). This can be explained by non-radiative depopulation processes involving the O–H group of methanol.73 For Eu3+ (τav = 0.86(2) ms) and Tb3+ based emission (τav = 0.927(5) ms), the influence on lifetimes is less pronounced, resulting in the described overall changes of the chromaticity. All determined fitting parameters and calculated average luminescence lifetimes are summarised in Table S20.† Furthermore, the quantum yield was determined for the white light-emission of EuTb@1∞[Zn(pt)2] prepared in DMF. With an excitation wavelength of λexc = 346 nm, a quantum yield of 24(3) % was reached.
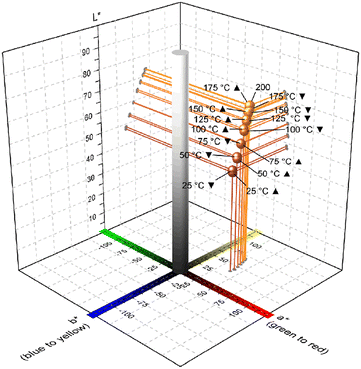 | ||
| Fig. 10 L*a*b* scatter diagram with inserted L*a*b* chromaticity coordinates of 1∞[Fe(pt)2] (CP 2) at different measurement temperatures. Recorded points during heating up are marked with ▲. Accordingly, corresponding points recorded with decreasing temperature are marked with ▼. Corresponding L*a*b* chromaticity values are listed in Table S17.† | ||
Furthermore, high absorbance in the blue/green region (overlapping bands at approx. 400 and 500 nm) caused most likely by MLCT was detected, leading to the blood-red colour of CP 2. Analogously, d–d transitions at λ > 800 nm are observed (Fig. S20†). This indicates the presence of both, high-spin and low-spin configuration of FeII.73–78 Upon increasing temperature, the absorbance at 350–600 nm decreases.
On the contrary, the intensity of the d–d transition band increases, indicating a continuous spin crossover (SCO) from low-spin to high-spin. Since the intensity decrease is more pronounced for absorbance in the green region, the colour of the compound is shifted from blood red to orange/yellow (Fig. 10, Fig. S21, and Table S17†). Such thermochromic behaviour is known for Fe(II) complexes with octahedral FeN6 coordination.73–78
Investigations on the formation and crystal structures of complexes [MX2(Hpt)2]
In addition to the homoleptic coordination polymers, several heteroleptic complex compounds were obtained and characterised.Single crystals of the heteroleptic complexes [MX2(Hpt)2] (MX2 = MnCl2 (9), FeCl2 (10), CoCl2 (11), CoBr2 (12)) were obtained by solvothermal reaction of divalent transition metal halides and an excess of Hpt in pyridine without addition of a base, wherefore the ligand remains neutral within the compounds, different from the deprotonated form in 1–8. Single crystals of [ZnCl2(Hpt)2] (13) were found within the product of the single crystal preparation of 1∞[Zn(pt)2] (6).
The isotypic complexes [MX2(Hpt)2] (MX2 = MnCl2 (9), FeCl2 (10), CoCl2 (11), CoBr2 (12), ZnCl2 (13)) crystallise in the triclinic crystal system in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The respective metal ions are coordinated trans in a distorted octahedral geometry by two halido ligands and two η2-Hpt molecules binding via N1 of the pyridine ring and N4 of the triazole ring.
. The respective metal ions are coordinated trans in a distorted octahedral geometry by two halido ligands and two η2-Hpt molecules binding via N1 of the pyridine ring and N4 of the triazole ring.
The complexes are interconnected by N3–H3⋯Cl1 hydrogen bonds.
A depiction of the crystal structure is exemplarily shown for [MnCl2(Hpt)2] (9) in Fig. 11. Crystallographic data for 9–13 is given in Tables S5, S7, S9, S11, and S13.† Interatomic distances and angles are listed in Tables S6, S8, S10, S12, and S14.† Mn–N distances for 9 were determined to 223.7(2) pm, and 225.2(2) pm, which are comparable to distances reported for e.g. [Mn4(H3bpa)2(mpt)4(N3)2]·2H2O (219–238 pm, H3bpa = N,N′-bis(picolinamide)azine). This compound contains the Hpt derivative mpt = 3-methyl-5-(2-pyridyl)-1H-1,2,4-triazolate and exhibits three crystallographically independent octahedrally coordinated Mn2+ ions.46 Analogously, for the two-dimensional coordination polymer [Mn(N3)(pytr)(H2O)]n. (pytr = pt), which contains octahedrally coordinated Mn interconnected by N−3, interatomic Mn–N distances to the η2-pt ligand of 213–223 pm are reported.79
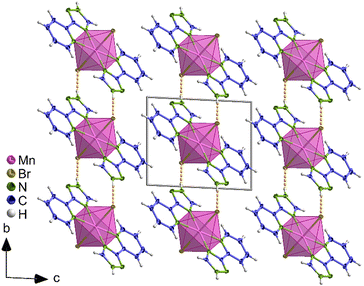 | ||
| Fig. 11 Depiction of the crystal structure of [MnCl2(Hpt)2] (9) along the crystallographic a axis. The octahedral coordination of Mn2+ is highlighted in pink. Displayed thermal ellipsoids correspond to 50% probability level of the atoms. N–H⋯Cl hydrogen bonds are shown in dashed orange lines. Crystallographic data is summarised in Table S5.† Symmetry operations: I = 1 − x, 1 − y, 1 − z. | ||
In case of [FeCl2(Hpt)2] (10), the Fe–N distances of 218.48(8) pm and 220.20(8) pm coincide with the 213–223 pm in [Fe(Hpt)3](BF4)2·H2O,43 which contains Fe2+ octahedrally coordinated by three η2-Hpt ligands.43 For the complexes [CoCl2(Hpt)2] (11), and [CoBr2(Hpt)2] (12), respectively, the distances 213.6(6) pm and 214.6(2) pm for 11, and 213.4(2) pm and 218.8(2) pm for 12, are in good agreement to distances in [Co(Hpt)2(H2O)2](NO3)2 (215 and 216 pm), which contains Co ions coordinated trans in an octahedral geometry by two aquo ligands and two η2-Hpt molecules.45
The Zn–N distances in [ZnCl2(Hpt)2] (13), 214.0(2) pm and 215.7(1) pm, are in the range of Zn–pt distances observed for [ZnL(pt)]·0.5MeOH·1.5H2O (200–225 pm; with L = tris(5-methyl-3-phenylpyrazolyl)borate), which features a fivefold coordinated Zn2+.44 Alternatively, to the synthesis described above, the complexes 9–13 can be synthesised by choosing acetonitrile instead of pyridine as a non-basic solvent. Due to a lower solubility of the ligand, sublimation of excess Hpt is necessary after the reaction for the products’ purification.
Additionally, synthesis utilising water-free chlorides or corresponding hydrates is also feasible (see Experimental section and Fig. 12).
Analogous reactions of FeBr2 and Hpt in either pyridine, or acetonitrile result in phase mixtures containing 1∞[Fe(pt)2] (2) and [FeBr2(Hpt)2], which is isotypic to [FeCl2(Hpt)2] (10). Reactions of FeI2 also result in phase mixtures containing 1∞[Fe(pt)2] (2) and [FeI2(Hpt)2], which again is isotypic to [FeCl2(Hpt)2] (10). Therefore, the existence of [FeBr2(Hpt)2], and [FeI2(Hpt)2], both being isotypic to 10, was proven by PXRD (see ESI†).
Accordingly, reactions of ZnCl2 and Hpt in either pyridine, or acetonitrile result in phase mixtures containing 1∞[Zn(pt)2] (6). In case of the product synthesised in acetonitrile, [ZnCl2(Hpt)2] (13) is also contained within the product, as shown by PXRD.
Infrared spectroscopy
The synthesised coordination polymers 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Ni (4), Zn (6), Cd (7)), as well as the complexes [MX2(Hpt)2] (MX2 = MnCl2 (9), FeCl2 (10), CoCl2 (11), CoBr2 (12)) were also characterised by infrared spectroscopy. For 1–7, respective spectra (Fig. S31†) feature bands being characteristic for functional groups of the ligand, as well as the solvents being present within the square-shaped pore channels. The broad band around 3400 nm can be assigned to both, ν(O–H) of intercalated water and ν(N–H) due to established C–H⋯N hydrogen bonds. For 9, 10, 11, and 12, bands of the neutral ligand are observable, as well (Fig. S32†). Wavenumbers of observed bands, as well as assignments of those are listed in Tables S22, and S23.†Composition and thermal analyses
Elemental analysis of the synthesised coordination polymers 1∞[M(pt)2] (1–7) confirm the assumption of intercalated solvent molecules (pyridine and water). For CPs 1–7, sum formulas considering amounts of solvents were calculated (Table S24†). Elemental analyses of the complexes [MnCl2(Hpt)2] (9), [FeCl2(Hpt)2] (10), [CoCl2(Hpt)2] (11), and [CoBr2(Hpt)2] (12) synthesised in pyridine confirm the phase purity already assumed from PXRD results, since calculated and determined elemental percentages are in good agreement (Table S25†). The same applies for complexes 9, 10, 11, and 12 synthesised in acetonitrile (Table S25†).By simultaneous thermal analysis, 1∞[Mn(pt)2] (1), 1∞[Co(pt)2] (3), 1∞[Ni(pt)2] (4), and 1∞[Zn(pt)2] (6) were further investigated. In all cases, the relative mass decreases by 3–7% for temperatures up to approx. 130 °C followed by a plateau. This is considered to be an effect of solvent molecules escaping the solids. Upon further heating, subsequent to the plateau, a significant decrease in relative mass is detected for all investigated CPs, coinciding with a detection of water and carbon dioxide and/or nitrous oxide by mass spectrometry. This indicates an oxidation of the corresponding CP. Thereby, Hpt is combusted, leading to detection of H2O, and CO2/N2O (Fig. S33–S36†). An overview of onsets and relative mass losses determined by STA is given within ESI (Table S26†). The obtained results are in good agreement with the high temperature behaviour of these CPs observed by temperature-dependent PXRD (Fig. 3, and Fig. S6–S18†).
Physisorption experiments on 1∞[M(pt)2] (M = Fe (2), Co (3), Zn (6))
To investigate the inner surface area of the CPs 2, 3, and 6, they were activated at 300 °C and 10−3 mbar for seven hours to remove solvent molecules from the pores. The samples were further degassed at 10−6 mbar. Subsequently, the inner surface area was determined by recording gas sorption isotherms of N2 at 77 K (Fig. S37†).The determined surface areas for BET and Langmuir are listed in Table 2. The determined, high C constants (>150) are considered to be an effect of filling narrow micropores,80 which is consistent with the square-shaped pore channels observed by SCXRD.
| CP | SABET [m2 g−1] | C constant | SAL [m2 g−1] |
|---|---|---|---|
| 1∞[Fe(pt)2] (2) | 217 | 181 | 286 |
| 1∞[Co(pt)2] (3) | 181 | 868 | 208 |
| 1∞[Zn(pt)2] (6) | 160 | 247 | 210 |
Identification of additional compounds
Besides the coordination compounds described above, a series of additional compounds were identified. Since, in most of the cases, those are considered as intermediates or byproducts, the description of synthesis conditions and single crystal characterizations are discussed in the ESI.† This includes synthesis and SCXRD characterization of the homoleptic complex [Zr(pt)4] (14), the dinuclear complexes [Fe2Cl2(pt)2(Py)4] (15) and [Fe2Br2Cl2(pt)2(Py)4] (16), the two-dimensional CP 2∞[Cu2Cl(pt)] (17), the one-dimensional CPs 1∞[MnCl(bpt)] (bpt = 3,5-bis(2-pyridyl)-1,2,4-triazolate, 18) and 1∞[Co2Cl3(pt)(H2O)2]·4CyOH (CyOH = cyclohexanol, 19), an finally the two-dimensional coordination polymer 2∞[Co2Br2(pt)2] (20).Experimental
Synthesis procedure
Within this work, solvothermal syntheses were performed according to the following procedure: solid components were mortared and transferred into Duran® glass ampoules. Liquid components and solvents were added, and the resulting mixture was frozen in liquid nitrogen. The ampoules were evacuated, and the mixtures were allowed to thaw again. This freeze–pump–thaw procedure was repeated, followed by freezing the mixture, evacuating the ampoule, and sealing it. The reaction mixtures within the sealed ampoules were homogenised by ultrasonication for three minutes. Afterwards, the ampoules were placed in resistance ovens with corundum tube and heated for several days. For single crystal X-ray diffraction (SCXRD) measurements, the ampoules were opened, and a small amount of the resulting products was isolated. In case of bulk syntheses, the ampoules were opened, the solids were transformed into centrifugation tubes and were washed by repeated centrifugation, decantation, and suspension in fresh solvent. The purified solids were dried for one hour under vacuum (10−3 mbar). In case of usage of air-sensitive compounds (e.g. anhydrous metal halides), mortaring and tranferring the mixtures to ampoules were carried out under inert atmosphere.Materials
2-(1,2,4-1H-Triazol-3-yl)pyridine (98%, BLD Pharmatech GmbH), europium(III) nitrate hexahydrate (Eu(NO3)3·6H2O, 99.9% (REO), abcr GmbH), terbium(III) nitrate hexahydrate (Tb(NO3)3·6H2O, 99.9% (REO), abcr GmbH), Mn(OAc)2·4H2O (99%, abcr GmbH), Fe(OAc)2 (anhydrous, 97%, abcr GmbH), Co(OAc)2·4H2O (98%, abcr GmbH), Ni(OAc)2·4H2O (98+%, Alfa Aesar), Cu(OAc)2·H2O (p.a., Riedel-de Haën AG), Zn(OAc)2·2H2O (p.a., Merck), Cd(OAc)2·2H2O (98%, abcr GmbH), MnCl2 (99.99%, Alfa Aesar), FeCl2 (anhydrous, 99.5%, Alfa Aesar), FeBr2 (anhydrous, 98%, abcr GmbH), FeI2 (ultra-dry, 99.99%, abcr GmbH), CoCl2 (99+%, abcr GmbH), CoBr2 (anhydrous, 97%, Alfa Aesar), CuCl2 (anhydrous, 98%, abcr GmbH), ZnCl2 (anhydrous, 98+%, Alfa Aesar), ZrCl4 (anhydrous, 99.5+%, Alfa Aesar), benzylamine (99.5+%, dry, AcroSeal™, Acros Organics), benzene (99.0%, dry, AcroSeal™, Acros Organics), acetonitrile (99.9%, extra dry, AcroSeal™, Acros Organics) and pyridine (99%, VWR Chemicals) were used as purchased without further purification. When dry pyridine is specified, it was dried by stirring over 20 g L−1 KOH for several days and distilled under inert atmosphere.Bulk synthesis of one-dimensional coordination polymers 1∞[M(pt)2] (1–7)
Larger quantities of the coordination polymers 1∞[M(pt)2] (M = Mn (1), Fe (2), Co (3), Cu (5), Zn (6), Cd (7)) were obtained by solvothermally combining 500 μmol (123 mg) Mn(OAc)2·4H2O, (87.0 mg) Fe(OAc)2, (125 mg) Co(OAc)2·4H2O, (99.8) Cu(OAc)2·H2O, (114 mg) Zn(OAc)2·2H2O, or (125 mg) Cd(OAc)2·2H2O with 1.00 mmol (146 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine in 4 mL pyridine following the general synthesis principle mentioned above. In case of 1∞[Ni(pt)2] (4), 1.00 mmol (249 mg) Ni(OAc)2·4H2O and 2.00 mmol (292 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine were used. The reaction mixtures were heated to 150 °C in 4 h, followed by heating to 180 °C with 2 °C h−1. This temperature was held constant for 120 h. Subsequently, the reaction mixtures were allowed to cool down to 150 °C with 2 °C h−1, and to ambient temperature within 4 h. Obtained products were purified by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min), decantation of the liquid phase, addition of 4 mL fresh pyridine and ultrasonication for 10 min. Subsequent to a final centrifugation (12000 rpm, 10 min) and solvent decantation, the products were vacuum-dried at 10−3 mbar. The yields were 49% for 1, 52% for 2, 77% for 3, 61% for 4, 65% for 5, 42% for 6, and 58% for 7, based on the corresponding metal acetate(s) and the sum formulae considering solvent molecules, as listed in Table S24.†
000 rpm, 10 min), decantation of the liquid phase, addition of 4 mL fresh pyridine and ultrasonication for 10 min. Subsequent to a final centrifugation (12000 rpm, 10 min) and solvent decantation, the products were vacuum-dried at 10−3 mbar. The yields were 49% for 1, 52% for 2, 77% for 3, 61% for 4, 65% for 5, 42% for 6, and 58% for 7, based on the corresponding metal acetate(s) and the sum formulae considering solvent molecules, as listed in Table S24.†
1∞[Mn(pt)2] (1): IR (KBr): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3419 (br), 3030 (multiple, m), 1604 (s), 1513 (m), 1472(s), 1449 (s), 1417 (s), 1384 (w), 1350 (m), 1271 (m), 1250 (m), 1203 (m), 1145 (m), 1112 (s), 1048 (m), 1029 (s), 1007 (m), 876 (m), 805 (m), 755 (m), 676 (m), 636 (m), 483 (m), 407 cm−1 (m). Anal. calcd for 1∞[Mn(pt)2]·0.34Py·0.21H2O = C15.70H12.12MnN8.34O0.21: C 50.19, H 3.25, N 31.07; found: C 50.20, H 2.99, N 30.98.
= 3419 (br), 3030 (multiple, m), 1604 (s), 1513 (m), 1472(s), 1449 (s), 1417 (s), 1384 (w), 1350 (m), 1271 (m), 1250 (m), 1203 (m), 1145 (m), 1112 (s), 1048 (m), 1029 (s), 1007 (m), 876 (m), 805 (m), 755 (m), 676 (m), 636 (m), 483 (m), 407 cm−1 (m). Anal. calcd for 1∞[Mn(pt)2]·0.34Py·0.21H2O = C15.70H12.12MnN8.34O0.21: C 50.19, H 3.25, N 31.07; found: C 50.20, H 2.99, N 30.98.
1∞[Fe(pt)2] (2): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3423 (br), 3030 (multiple, m), 1605 (s), 1516 (m), 1471(s), 1448 (s), 1417 (s), 1384 (w), 1357 (m), 1274 (m), 1252 (m), 1201 (m), 1149 (m), 1115 (s), 1050 (m), 1031 (s), 1009 (m), 874 (m), 803 (m), 757 (m), 676 (m), 636 (m), 490 (m), 409 cm−1 (m). Anal. calcd for 1∞[Fe(pt)2]·0.34Py·0.21H2O = C15.7H12.12FeN8.34O0.21: C 50.04, H 3.24, N 31.00; found: C 50.02, H 2.96, N 30.91.
= 3423 (br), 3030 (multiple, m), 1605 (s), 1516 (m), 1471(s), 1448 (s), 1417 (s), 1384 (w), 1357 (m), 1274 (m), 1252 (m), 1201 (m), 1149 (m), 1115 (s), 1050 (m), 1031 (s), 1009 (m), 874 (m), 803 (m), 757 (m), 676 (m), 636 (m), 490 (m), 409 cm−1 (m). Anal. calcd for 1∞[Fe(pt)2]·0.34Py·0.21H2O = C15.7H12.12FeN8.34O0.21: C 50.04, H 3.24, N 31.00; found: C 50.02, H 2.96, N 30.91.
1∞[Co(pt)2] (3): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3445 (br), 3034 (multiple, m), 1608 (s), 1516 (m), 1473(s), 1449 (s), 1419 (s), 1384 (w), 1360 (m), 1264 (m), 1252 (m), 1204 (m), 1149 (m), 1118 (s), 1050 (m), 1033 (s), 1009 (m), 874 (m), 805 (m), 757 (m), 674 (m), 638 (m), 496 (m), 411 cm−1 (m). Anal. calcd for 1∞[Co(pt)2]·0.34Py·0.21H2O = C15.7H12.12CoN8.34O0.21: C: 49.65, H 3.22, N 30.74; found: C 49.82, H 2.93, N 30.96.
= 3445 (br), 3034 (multiple, m), 1608 (s), 1516 (m), 1473(s), 1449 (s), 1419 (s), 1384 (w), 1360 (m), 1264 (m), 1252 (m), 1204 (m), 1149 (m), 1118 (s), 1050 (m), 1033 (s), 1009 (m), 874 (m), 805 (m), 757 (m), 674 (m), 638 (m), 496 (m), 411 cm−1 (m). Anal. calcd for 1∞[Co(pt)2]·0.34Py·0.21H2O = C15.7H12.12CoN8.34O0.21: C: 49.65, H 3.22, N 30.74; found: C 49.82, H 2.93, N 30.96.
1∞[Ni(pt)2] (4): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3423 (br), 3037 (multiple, w), 1608 (s), 1519 (m), 1473 (s), 1451 (s), 1418 (s), 1391 (w), 1367 (m), 1267 (m), 1254 (m), 1206 (m), 1150 (m), 1126 (s), 1051 (m), 1034 (s), 1012 (m), 871 (m), 803 (m), 757 (m), 676 (m), 639 (m), 502 (m), 415 cm−1 (m). Anal. calcd for 1∞[Ni(pt)2]·0.11Py·0.63H2O = C14.55H11.81N8.11NiO0.63: C 47.38, H 3.23, N 30.78; found: C 47.37, H 3.20, N 30.88.
= 3423 (br), 3037 (multiple, w), 1608 (s), 1519 (m), 1473 (s), 1451 (s), 1418 (s), 1391 (w), 1367 (m), 1267 (m), 1254 (m), 1206 (m), 1150 (m), 1126 (s), 1051 (m), 1034 (s), 1012 (m), 871 (m), 803 (m), 757 (m), 676 (m), 639 (m), 502 (m), 415 cm−1 (m). Anal. calcd for 1∞[Ni(pt)2]·0.11Py·0.63H2O = C14.55H11.81N8.11NiO0.63: C 47.38, H 3.23, N 30.78; found: C 47.37, H 3.20, N 30.88.
1∞[Cu(pt)2] (5): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3441 (br), 3031 (multiple, m), 1612 (s), 1598 (s), 1573 (m), 1517 (w), 1471 (s), 1452 (s), 1420 (s), 1369 (m), 1352 (m), 1286 (m), 1268 (m), 1252 (m), 1208 (w), 1137 (m), 1121 (s), 1095 (m), 1042 (m), 1022 (m), 998 (m), 870 (m), 801 (m), 752 (m), 676 (m), 723(s), 702 (m), 677 (m), 665 (m), 642 (m), 628 (m) 490 (w), 408 cm−1 (w). Anal. calcd for 1∞[Cu(pt)2]·0.05Py·0.63H2O = C14.25H11.51N8.05CuO0.63: C 46.38, 3.14, N 30.55; found: C 46.07, H 2.78, N 29.64.
= 3441 (br), 3031 (multiple, m), 1612 (s), 1598 (s), 1573 (m), 1517 (w), 1471 (s), 1452 (s), 1420 (s), 1369 (m), 1352 (m), 1286 (m), 1268 (m), 1252 (m), 1208 (w), 1137 (m), 1121 (s), 1095 (m), 1042 (m), 1022 (m), 998 (m), 870 (m), 801 (m), 752 (m), 676 (m), 723(s), 702 (m), 677 (m), 665 (m), 642 (m), 628 (m) 490 (w), 408 cm−1 (w). Anal. calcd for 1∞[Cu(pt)2]·0.05Py·0.63H2O = C14.25H11.51N8.05CuO0.63: C 46.38, 3.14, N 30.55; found: C 46.07, H 2.78, N 29.64.
1∞[Zn(pt)2] (6): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3421 (br), 3035 (multiple, m), 1605 (s), 1517 (m), 1476 (s), 1451 (s), 1438 (s), 1421 (s), 1391 (w), 1363 (m), 1265 (m), 1252 (m), 1208 (m), 1149 (m), 1125 (s), 1050 (m), 1035 (s), 1007 (m), 876 (m), 806 (m), 755 (m), 674 (m), 635 (m), 493 (m), 408 cm−1 (m). Anal. calcd for 1∞[Zn(pt)2]·0.39Py·0.63H2O = C16.05H13.31N8.41O0.63Zn: C 48.15, H 3.34, N 29.55; found: C 48.31, H 2.96, N 29.53.
= 3421 (br), 3035 (multiple, m), 1605 (s), 1517 (m), 1476 (s), 1451 (s), 1438 (s), 1421 (s), 1391 (w), 1363 (m), 1265 (m), 1252 (m), 1208 (m), 1149 (m), 1125 (s), 1050 (m), 1035 (s), 1007 (m), 876 (m), 806 (m), 755 (m), 674 (m), 635 (m), 493 (m), 408 cm−1 (m). Anal. calcd for 1∞[Zn(pt)2]·0.39Py·0.63H2O = C16.05H13.31N8.41O0.63Zn: C 48.15, H 3.34, N 29.55; found: C 48.31, H 2.96, N 29.53.
1∞[Cd(pt)2] (7): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3421 (br), 3035 (multiple, m), 1605 (s), 1517 (m), 1476 (s), 1451 (s), 1438 (s), 1421 (s), 1391 (w), 1363 (m), 1265 (m), 1252 (m), 1208 (m), 1149 (m), 1125 (s), 1050 (m), 1035 (s), 1007 (m), 876 (m), 806 (m), 755 (m), 674 (m), 635 (m), 493 (m), 408 cm−1 (m). Anal. calcd for 1∞[Cd(pt)2]·0.24Py·0.63H2O = C15.20H12.46N8.24CdO0.63: C 42.15, H 2.90, N 26.66; found: C 41.97, H 2.53, N 26.26.
= 3421 (br), 3035 (multiple, m), 1605 (s), 1517 (m), 1476 (s), 1451 (s), 1438 (s), 1421 (s), 1391 (w), 1363 (m), 1265 (m), 1252 (m), 1208 (m), 1149 (m), 1125 (s), 1050 (m), 1035 (s), 1007 (m), 876 (m), 806 (m), 755 (m), 674 (m), 635 (m), 493 (m), 408 cm−1 (m). Anal. calcd for 1∞[Cd(pt)2]·0.24Py·0.63H2O = C15.20H12.46N8.24CdO0.63: C 42.15, H 2.90, N 26.66; found: C 41.97, H 2.53, N 26.26.
Synthesis of single crystals of 1∞[Zn(pt)2] (6)
Single crystals suitable for single crystal X-ray diffraction (SCXRD) of CP 6 were obtained by reaction of 55 μmol (7.5 mg) of water-free ZnCl2, 0.15 mmol (22 mg) of 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt), and 2 droplets of benzylamine according to the procedure described above. A mixture of 0.9 mL water-free toluene and 0.1 mL water-free pyridine was used as solvent. The reaction mixture was heated to 180 °C with 2 °C h−1 and held constant at this temperature for 480 h. Subsequently the mixture was allowed to cool down to ambient temperature with 2 °C h−1.Synthesis of single crystals of 1∞[Cu(pt)2]·0.5Py (8)
Single crystals suitable for SCXRD of CP 8 were obtained by reaction of 0.46 mmol (91 mg) Cu(OAc)2·H2O and 1.00 mmol (146 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) in 4 mL pyridine following the general synthesis principle mentioned above. The reaction mixture was heated to 150 °C in 4 h, followed by heating to 180 °C with 2 °C h−1. This temperature was held constant for 120 h. Subsequently, the reaction mixture was allowed to cool down to 150 °C with 2 °C h−1, and to ambient temperature within 4 h. It is noteworthy that this synthesis is analogous to the bulk synthesis of 1∞[M(pt)2] (1–7) but without vacuum-drying of the obtained product. Consequently, CP 8 is only existent when still damp. Upon drying, the obtained product converts to 5.Impregnation of 1∞[Zn(pt)2] (6) with Eu and Tb
1∞[Zn(pt)2] (6) was activated at 300 °C for seven hours at 10−3 mbar. Subsequently, three samples of 15 mg (42 μmol) of the activated polymer were each dispersed in a solution of 18 mg (40 μmol) EuNO3·6H2O and 18 mg (40 μmol) Tb(NO3)3·6H2O in 4 mL DMF, MeCN or MeOH. The resulting suspensions were homogenised by ultrasonication for ten minutes and were magnetically stirred for eight days. Afterwards, the solids were separated from the liquid phase by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min) and decantation of the solution. The obtained solids were dried in vacuum (10−3 mbar) for one hour.
000 rpm, 10 min) and decantation of the solution. The obtained solids were dried in vacuum (10−3 mbar) for one hour.
Bulk syntheses of complexes [MX2(Hpt)2] (MX2 = MnCl2 (9) and FeCl2 (10))
1.00 mmol (126 mg) MnCl2 or (127 mg) FeCl2 were reacted with 2.40 mmol (351 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) in 0.5 mL pyridine following the general synthesis principle mentioned above under inert atmosphere. The reaction mixture was heated to 150 °C within four hours, followed by heating up to 200 °C with 2 °C h−1. This temperature was held constant for 120 h. Subsequently, the mixture was allowed to cool down to 150 °C with 2 °C h−1 and then to room temperature within four hours. The liquid phase was removed manually under inert atmosphere, and the obtained solid was dried in vacuum (10−3 mbar). The yield was 73% for 9 and 77% for 10, based on the corresponding metal halide.[MnCl2(Hpt)] (9): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3450 (br), 3079 (s), 2994 (s), 2891 (s), 1816 (m), 1657 (m), 1603 (s), 1569 (m), 1537 (m), 1503 (s), 1477 (s), 1448 (s), 1422 (s), 1340 (s), 1289 (s), 1272 (s), 1188 (m), 1149 (m), 1133 (m), 1098 (m), 1085 (m), 1051 (s), 1016 (m), 993 (s), 974 (m), 912 (m), 846 (s), 797 (s), 749 (s), 724 (s), 714 (s), 639 (s), 479 (m), 412 cm−1 (m). Anal. calcd for [MnCl2(Hpt)2] = C14H12Cl2MnN8: C 40.32, H 2.30, N 26.80; found: C 40.06, H 2.70, N 26.19.
= 3450 (br), 3079 (s), 2994 (s), 2891 (s), 1816 (m), 1657 (m), 1603 (s), 1569 (m), 1537 (m), 1503 (s), 1477 (s), 1448 (s), 1422 (s), 1340 (s), 1289 (s), 1272 (s), 1188 (m), 1149 (m), 1133 (m), 1098 (m), 1085 (m), 1051 (s), 1016 (m), 993 (s), 974 (m), 912 (m), 846 (s), 797 (s), 749 (s), 724 (s), 714 (s), 639 (s), 479 (m), 412 cm−1 (m). Anal. calcd for [MnCl2(Hpt)2] = C14H12Cl2MnN8: C 40.32, H 2.30, N 26.80; found: C 40.06, H 2.70, N 26.19.
[FeCl2(Hpt)] (10): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3442 (br), 3085 (s), 3006 (s), 2901 (s), 1809 (w), 1639 (m), 1605 (s), 1569 (w), 1540 (m), 1503 (s), 1482 (m), 1448 (m), 1425 (s), 1341 (s), 1288 (s), 1271 (s), 1190 (m), 1150 (m), 1136 (m), 1098 (w), 1085 (m), 1051 (m), 1016 (m), 994 (s), 976 (m), 908 (m), 836 (s), 797 (m), 751 (s), 724 (s), 715 (m), 639 (s), 486 (m), 415 cm−1 (m). Anal. calcd for [FeCl2(Hpt)2] = C14H12Cl2FeN8: C 40.13, 2.89, N 26.74; found: C 40.87, H 2.80, N 26.43.
= 3442 (br), 3085 (s), 3006 (s), 2901 (s), 1809 (w), 1639 (m), 1605 (s), 1569 (w), 1540 (m), 1503 (s), 1482 (m), 1448 (m), 1425 (s), 1341 (s), 1288 (s), 1271 (s), 1190 (m), 1150 (m), 1136 (m), 1098 (w), 1085 (m), 1051 (m), 1016 (m), 994 (s), 976 (m), 908 (m), 836 (s), 797 (m), 751 (s), 724 (s), 715 (m), 639 (s), 486 (m), 415 cm−1 (m). Anal. calcd for [FeCl2(Hpt)2] = C14H12Cl2FeN8: C 40.13, 2.89, N 26.74; found: C 40.87, H 2.80, N 26.43.
Synthesis of single crystals of [MnCl2(Hpt)2] (9) and [FeCl2(Hpt)2] (10)
Crystals suitable for SCXRD of the isotypic complexes 9 and 10 were obtained by solvothermal reaction of 0.20 mmol (25 mg) MnCl2 or FeCl2 and 0.48 mmol (70 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) in 0.3 mL pyridine following the procedure described above. The reaction mixture was heated to 150 °C within 4 h, followed by heating to 200 °C with 2 °C h−1. This temperature was held for 120 h. Subsequently the mixture was allowed to cool down to 150 °C with 2 °C h−1, and to ambient temperature within 4 h.Bulk syntheses of [MX2(Hpt)2] (MX2 = CoCl2 (11) and CoBr2 (12))
0.40 mmol (52 mg) CoCl2, or (88 mg) CoBr2 were reacted with 1.00 mmol (146 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) in 1 mL acetonitrile following the general synthesis principle mentioned above under inert atmosphere. The reaction mixture was heated up to 115 °C within four hours. This temperature was held constant for 144 h followed by cooling down to room temperature within four hours. The liquid phase was manually removed under inert atmosphere and the obtained solid was vacuum-dried at 10−3 mbar. Excess ligand was separated from the product by sublimation at 150 °C for five days. The yield was 74% for 11 and 87% for 12, based on the corresponding metal halide.[CoCl2(Hpt)] (11): ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 3085 (s), 2997 (s), 2892 (s), 1807 (w), 1657 (m), 1608 (s), 1569 (w), 1542 (w), 1504 (s), 1479 (m), 1449 (m), 1426 (m), 1344 (m), 1289 (m), 1271 (m), 1193 (w), 1152 (w), 1139 (m), 1099 (w), 1088 (w), 1051 (m), 1018 (m), 996 (m), 976 (m), 907 (w), 844 (s), 797 (m), 752 (m), 724 (s), 717 (m), 639 (m), 490 (w), 419 cm−1 (w). Anal. calcd for C14H12Cl2CoN8: C 39.83, H 2.87, N 26.54; found: C 40.29, H 2.57, N 26.47.
= 3085 (s), 2997 (s), 2892 (s), 1807 (w), 1657 (m), 1608 (s), 1569 (w), 1542 (w), 1504 (s), 1479 (m), 1449 (m), 1426 (m), 1344 (m), 1289 (m), 1271 (m), 1193 (w), 1152 (w), 1139 (m), 1099 (w), 1088 (w), 1051 (m), 1018 (m), 996 (m), 976 (m), 907 (w), 844 (s), 797 (m), 752 (m), 724 (s), 717 (m), 639 (m), 490 (w), 419 cm−1 (w). Anal. calcd for C14H12Cl2CoN8: C 39.83, H 2.87, N 26.54; found: C 40.29, H 2.57, N 26.47.
[CoBr2(Hpt)] (12): 3432 (br), 3085 (s), 3011 (s), 2898 (s), 1788 (w), 1635 (w), 1606 (s), 1569 (m), 1542 (w), 1501 (s), 1477 (s), 1448 (s), 1423 (s), 1341 (m), 1285 (s), 1265 (s), 1212 (m), 1190 (m), 1149 (m), 1137 (m), 1118 (m), 1098 (m), 1086 (m), 1067 (w), 1051 (m), 1031 (w), 1017 (m), 1006 (m), 996 (m), 974 (m), 923 (w), 899 (w), 894 (w), 874 (w), 817 (s), 797 (m), 752 (s), 724 (s), 717 (m), 676 (w), 635 (m), 490 (w), 419 cm−1 (w) (Recorded for a product synthesised in pyridine). Anal. calcd for C14H12Br2CoN8: C 32.90, H 2.37, N 21.93; found: C 32.40, H 2.23, N 20.96.
Synthesis of single crystals of [CoCl2(Hpt)2] (11) and [CoBr2(Hpt)2] (12)
Crystals suitable for SCXRD of the isotypic complexes 13 and 14 were obtained by solvothermal reaction of 0.50 mmol (65 mg) CoCl2 or (0.11 g) CoBr2 and 1.20 mmol (176 mg) 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) in 0.3 mL pyridine following the procedure described above. The reaction mixture was thermally treated in analogy to the synthesis of 9 and 10.Synthesis of single crystals of [ZnCl2(Hpt)2] (13)
Single crystals of 13 were found within the solid product, in which crystals of 1∞[Zn(pt)2] (6) were found, as well (see above).Conclusions
Within this work, coordination compounds based on divalent 3d-transition metal ions and the ligand 2-(1,2,4-1H-triazol-3-yl)pyridine (Hpt) were synthesised. Altogether, 12 fully characterised compounds and the structures of eight more that were obtained as side products are reported. The deprotonated ligand is able to interconnect transition metal ions (here Mn2+, Fe2+; Co2+, Ni2+, Cu2+, Zn2+, Cd2+) to form homoleptic one-dimensional coordination polymers (CPs) with a generalised sum formula of 1∞[M(pt)2], which are stable to temperatures up to 300–400 °C, depending on the metal ion. Considering C–H⋯N interactions between the polymeric strands, the CPs can be described supermolecular three-dimensional structures exhibiting square-shaped channel pores, in which solvent molecules are intercalated from the syntheses, as shown by temperature-dependent PXRD, STA and elemental analyses.Besides colourful products containing Fe, Co, Ni, and Cu, the polymers containing Mn and Zn are colourless solids. Especially for 1∞[Zn(pt)2], this appearance – in combination with UV absorption and ligand-based blue light emission of the CP – allows creation of a white light-emitter according to the RGB concept by activation of the CP and subsequent impregnation with Eu3+ and Tb3+. The colour of the emitted light can be tuned from neon blue to practically perfect white light by choosing an appropriate excitation wavelength. Furthermore, the coordination polymer 1∞[Fe(pt)2] shows thermochromic behaviour based on continuous spin crossover. In addition to the homoleptic coordination polymers, several mononuclear complexes [MX2(Hpt)2] and numerous crystalline by-products were found and their structures determined. They illuminate a product rich chemistry of the ligand Hpt with transition metal ions.
Data availability
Single crystal structures determined within the scope of this work were uploaded to the CCDC database.CCDC 2347927 (6), 2347920 (8), 2347949 (9), 2347948 (10), 2347938 (11), 2347937 (12), 2347952 (13), 2347953 (14), 2347947 (15), 2347946 (16), 2347951 (17), 2347925 (18), 2347917 (19), and 2347950 (20).In addition, the ESI† (62 pages) contains detailed description of analytical methods used, tables with crystallographic data, selected interatomic distances and angles, UV-Vis spectra, photoluminescence spectra, IR spectra, powder X-ray diffraction plots, simultaneous thermal analysis graphs, sorption isotherms, and additional experiments and single crystal structures description. Besides references cited in the main paper, the authors have cited additional references within the ESI.†41,43,46,56–60,62,69,70,81
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Lisa-Marie Wagner for performing single crystal X-ray diffraction experiments. Furthermore, we gratefully acknowledge Chemisches Labor Dr. Graser KG for the opportunity of performing ICP-MS measurements.References
- (a) J. Malinowski, D. Zych, D. Jacewicz, B. Gawdzik and J. Drzeżdżon, Int. J. Mol. Sci., 2020, 21, 5443 CrossRef CAS PubMed; (b) R. Kumar, A. Thakur, Sachin, D. Chandra, A. Kumar Dhiman, P. Kumar Verma and U. Sharma, Coord. Chem. Rev., 2024, 499, 215453 CrossRef CAS; (c) S. Tai, E. J. Dover, S. V. Marchi and J. D. Carrick, J. Org. Chem., 2015, 80, 6275–6282 CrossRef CAS PubMed; (d) J. Halpern, Adv. Chem., 1974, 70, 1–24 Search PubMed.
- R. H. Elattar, S. F. El-Malla, A. H. Kamal and F. R. Mansour, Coord. Chem. Rev., 2024, 501, 215568 CrossRef CAS.
- M. Pellei, F. Del Bello, M. Porchia and C. Santini, Coord. Chem. Rev., 2021, 445, 214088 CrossRef CAS.
- M. S. A. Mansour, A. T. Abdelkarim, A. A. El-Sherif and W. H. Mahmoud, BMC Chem., 2024, 18, 150 CrossRef CAS PubMed.
- M. Albqmi, N. A. Elkanzi, A. M. Ali and A. Abdou, J. Mol. Struct., 2025, 1319, 139494 CrossRef CAS.
- Y. Senpradit, S. Wacharasindhu and M. Sukwattanasinitt, Spectrochim. Acta, Part A, 2025, 326, 125128 CrossRef CAS PubMed.
- I. Bala, K. Singh, Kiran, R. Kataria and J. Sindhu, Inorg. Chim. Acta, 2025, 574, 122411 CrossRef CAS.
- Y. Jiao, Y. Li, H. Tang, S. Xian, Y. He, L. Wang and J. Zhang, J. Photochem. Photobiol., A, 2025, 458, 115940 CrossRef CAS.
- X. Cao, N. Feng, Q. Huang and Y. Liu, ACS Appl. Bio Mater., 2024, 7, 7965–7986 CrossRef CAS PubMed.
- S. Dilshad, M. Muslim, A. Ahmed, A. Ali, S. Firdaus, M. J. Alam, S. Ahmad, M. Ahmad and A. Ahmad, J. Mol. Struct., 2024, 1301, 137350 CrossRef CAS.
- M. N. Khrizanforov, A. A. Zagidullin, R. P. Shekurov, F. F. Akhmatkhanova, I. A. Bezkishko, V. V. Ermolaev and V. A. Miluykov, Comments Inorg. Chem., 2024, 44, 98–142 CrossRef CAS.
- Y. Xiong, Q. Feng, L. Lu, X. Qiu, S. Knoedler, A. C. Panayi, D. Jiang, Y. Rinkevich, Z. Lin, B. Mi, G. Liu and Y. Zhao, Adv. Mater., 2024, 36, e2302587 CrossRef PubMed.
- (a) S. J. Lee and S. G. Telfer, Angew. Chem., Int. Ed., 2023, 62, e202306341 CrossRef CAS PubMed; (b) C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366 RSC; (c) D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC.
- H. Liang, K. Otsubo, Y. Wakabayashi, H. Sagayama, S. Kawaguchi and H. Kitagawa, Angew. Chem., Int. Ed., 2024, e202400162 CAS.
- M. Zhang, R. Tan, M. Wang, Z. Zhang, C. J. Low and Y. Lai, Battery Energy, 2024, 3, 20230050 CrossRef.
- H. Zhou, H. Wang, C. Yue, L. He, H. Li, H. Zhang, S. Yang and T. Ma, Appl. Catal., B, 2024, 344, 123605 CrossRef CAS.
- (a) Z. Ma, X. Song, Z. Li, Y. Ren, J. Wang and Y. Liang, Dalton Trans., 2024, 53, 3797–3807 RSC; (b) M. Kacem and M. Dib, Inorg. Chem. Commun., 2023, 158, 111561 CrossRef CAS; (c) Z. Liu, D. Sun, C. Wang, B. You, B. Li, J. Han, S. Jiang, C. Zhang and S. He, Coord. Chem. Rev., 2024, 502, 215612 CrossRef CAS.
- P. Hajivand, J. C. Jansen, E. Pardo, D. Armentano, T. F. Mastropietro and A. Azadmehr, Coord. Chem. Rev., 2024, 501, 215558 CrossRef CAS.
- S. Huang, Y. Ye, C. Jiang, R. Wang, W. Hu, S. Raza, J. Ouyang, Y. Pan and J. Liu, React. Funct. Polym., 2023, 193, 105743 CrossRef CAS.
- W. Li, X. Liu, X. Yu, B. Zhang, C. Ji, Z. Shi, L. Zhang and Y. Liu, Inorg. Chem., 2023, 62, 18248–18256 CrossRef CAS PubMed.
- (a) W. Liu, H. Cui, J. Zhou, X. Chen, H. Yang and J. Wang, J. Mol. Struct., 2024, 1302, 137468 CrossRef CAS; (b) Y. Qiao, C. Sun, J. Jian, T. Zhou, X. Xue, J. Shi, L. Zhao and G. Liao, Inorg. Chem., 2024, 63, 2060–2071 CrossRef CAS PubMed; (c) J.-X. Wang, T.-T. Wu, M.-Q. Tuo, H.-B. Pan, J. Ge, P.-P. Huang, J.-H. Gao, M. Jiang and J.-F. Lu, Polyhedron, 2024, 252, 116884 CrossRef CAS; (d) Y.-F. Ma, X.-L. Liu, X.-Y. Lu, M.-L. Zhang, Y.-X. Ren and X.-G. Yang, Spectrochim. Acta, Part A, 2024, 309, 123803 CrossRef CAS PubMed.
- A. J. Amoroso and S. J. A. Pope, Chem. Soc. Rev., 2015, 44, 4723–4742 RSC.
- (a) Z. Gao, B. Xu, T. Zhang, Z. Liu, W. Zhang, X. Sun, Y. Liu, X. Wang, Z. Wang, Y. Yan, F. Hu, X. Meng and Y. S. Zhao, Angew. Chem., Int. Ed., 2020, 59, 19060–19064 CrossRef CAS PubMed; (b) C. Sun, R. Xi and H. Fei, Acc. Chem. Res., 2023, 56, 452–461 CrossRef CAS PubMed.
- (a) C. Chiatti, I. Kousis, C. Fabiani and A. L. Pisello, Renewable Energy, 2022, 196, 28–39 CrossRef CAS; (b) X. Zhang, D. Liu, J. Yu, X. Li, H. Zheng, Y. Zhou, C. Huang, Y. Zhu, C. Jiao and Z. Sun, Dalton Trans., 2023, 52, 8558–8566 RSC.
- M. T. Seuffert, S. Wintzheimer, M. Oppmann, T. Granath, J. Prieschl, A. Alrefai, H.-J. Holdt, K. Müller-Buschbaum and K. Mandel, J. Mater. Chem. C, 2020, 8, 16010–16017 RSC.
- P. Falcaro and S. Furukawa, Angew. Chem., Int. Ed., 2012, 51, 8431–8433 CrossRef CAS PubMed.
- (a) P. R. Matthes, C. J. Höller, M. Mai, J. Heck, S. J. Sedlmaier, S. Schmiechen, C. Feldmann, W. Schnick and K. Müller-Buschbaum, J. Mater. Chem., 2012, 22, 10179 RSC; (b) Y. Shu, Q. Ye, T. Dai, Q. Xu and X. Hu, ACS Sens., 2021, 6, 641–658 CrossRef CAS PubMed; (c) X. Chen and Z. Jiang, Inorg. Chim. Acta, 2024, 561, 121852 CrossRef CAS.
- S. S. Mondal, K. Behrens, P. R. Matthes, F. Schönfeld, J. Nitsch, A. Steffen, P.-A. Primus, M. U. Kumke, K. Müller-Buschbaum and H.-J. Holdt, J. Mater. Chem. C, 2015, 3, 4623–4631 RSC.
- Y.-B. Hao, Z.-S. Shao, C. Cheng, X.-Y. Xie, J. Zhang, W.-J. Song and H.-S. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 31755–31762 CrossRef CAS PubMed.
- H. P. Toledo-Jaldin, C. Pinzón-Vanegas, A. Blanco-Flores, J. Zamora-Moreno, L. D. Rosales-Vázquez, A. R. Vilchis-Nestor, I. A. Reyes-Domínguez, M. Á. Romero-Solano and A. Dorazco-González, Environ. Pollut., 2024, 343, 123195 CrossRef CAS PubMed.
- X. Zuo, P. Liu, Q. Sun, J. Huang, Y. Zhang, X. Zhu, R. Chen and X. Meng, New J. Chem., 2024, 48, 1642–1649 RSC.
- (a) S. Yang, Z. Zhou, Z. Zhao, J. Liu, Y. Sun, Y. Zhang, E. Gao, M. Zhu and S. Wu, Microchem. J., 2024, 196, 109660 CrossRef CAS; (b) A. E. Psalti, D. Andriotou, S. A. Diamantis, A. Chatz-Giachia, A. Pournara, M. J. Manos, A. Hatzidimitriou and T. Lazarides, Inorg. Chem., 2022, 61, 11959–11972 CrossRef CAS PubMed; (c) Q. Tang, S. Liu, Y. Liu, D. He, J. Miao, X. Wang, Y. Ji and Z. Zheng, Inorg. Chem., 2014, 53, 289–293 CrossRef CAS PubMed; (d) J. Xue, Y. Wang, G. Yang and Y. Wang, J. Rare Earths, 2024, 42, 446–454 CrossRef CAS.
- X.-Y. Xu and B. Yan, Dalton Trans., 2015, 44, 1178–1185 RSC.
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS PubMed.
- W. Cao, Y. Tang, Y. Cui and G. Qian, Small Struct., 2020, 1, 2000019 CrossRef.
- N. Alam, S. Mondal, S. S. Hossain, S. Sahoo and D. Sarma, ACS Appl. Eng. Mater., 2023, 1, 1201–1212 CrossRef CAS.
- J. Othong, J. Boonmak, V. Promarak, F. Kielar and S. Youngme, ACS Appl. Mater. Interfaces, 2019, 11, 44421–44429 CrossRef CAS PubMed.
- (a) V. Raman, M. Y. Borzehandani, M. A. M. Latif, M. I. M. Tahir, M. B. Abdul Rahman and Y. Sulaiman, Polyhedron, 2024, 253, 116890 CrossRef CAS; (b) F. A. Brede, J. Heine, G. Sextl and K. Müller-Buschbaum, Chem. – Eur. J., 2016, 22, 2708–2718 CrossRef CAS PubMed; (c) M. Smida, J. Lhoste, V. Pimenta, A. Hémon-Ribaud, L. Jouffret, M. Leblanc, M. Dammak, J.-M. Grenèche and V. Maisonneuve, Dalton Trans., 2013, 42, 15748–15755 RSC; (d) Y.-G. Huang, B. Mu, P. M. Schoenecker, C. G. Carson, J. R. Karra, Y. Cai and K. S. Walton, Angew. Chem., Int. Ed., 2011, 50, 436–440 CrossRef CAS PubMed; (e) G. Wei, Y.-F. Shen, Y.-R. Li and X.-C. Huang, Inorg. Chem., 2010, 49, 9191–9199 CrossRef CAS PubMed; (f) W. Ouellette, B. S. Hudson and J. Zubieta, Inorg. Chem., 2007, 46, 4887–4904 CrossRef CAS PubMed; (g) W. Ouellette, J. R. Galan-Mascarós, K. R. Dunbar and J. Zubieta, Inorg. Chem., 2006, 45, 1909–1911 CrossRef CAS PubMed; (h) J. Kröber, I. Bkouche-Waksman, C. Pascard, M. Thomann and O. Kahn, Inorg. Chim. Acta, 1995, 230, 159–163 CrossRef.
- (a) J. Liu, L. Y. Yang and F. Luo, J. Solid State Chem., 2021, 301, 122369 CrossRef CAS; (b) J.-Y. Zou, L. Li, S.-Y. You, H.-M. Cui, Y.-W. Liu, K.-H. Chen, Y.-H. Chen, J.-Z. Cui and S.-W. Zhang, Dyes Pigm., 2018, 159, 429–438 CrossRef CAS.
- C.-H. Li, Y.-L. Li, W. Li and Y.-F. Kuang, Chin. J. Struct. Chem., 2021, 40, 363 CAS.
- C.-H. Li, W. Li, Y.-L. Li and Y.-Q. Yang, Chin. J. Struct. Chem., 2012, 1089 CAS.
- (a) J.-J. Zhang, N.-F. Yang, C.-H. Zhang and B.-H. Li, Chin. J. Inorg. Chem., 2010, 26, 533–536 CAS; (b) S. Sarkar, T. Mukherjee, S. Sen, E. Zangrando and P. Chattopadhyay, J. Mol. Struct., 2010, 980, 117–123 CrossRef CAS.
- A. F. Stassen, M. de Vos, P. J. van Koningsbruggen, F. Renz, J. Ensling, H. Kooijman, A. L. Spek, J. G. Haasnoot, P. Gütlich and J. Reedijk, Eur. J. Inorg. Chem., 2000, 2000, 2231–2237 CrossRef.
- B. L. Dick and S. M. Cohen, Inorg. Chem., 2018, 57, 9538–9543 CrossRef CAS PubMed.
- C.-C. Du, J.-Z. Fan, J.-P. Li and D.-Z. Wang, Chin. J. Inorg. Chem., 2017, 33, 1685 CAS.
- L. Cheng, W.-X. Zhang, B.-H. Ye, J.-B. Lin and X.-M. Chen, Inorg. Chem., 2007, 46, 1135–1143 CrossRef CAS PubMed.
- J. Bergmann, K. Stein, M. Kobalz, M. Handke, M. Lange, J. Möllmer, F. Heinke, O. Oeckler, R. Gläser, R. Staudt and H. Krautscheid, Microporous Mesoporous Mater., 2015, 216, 56–63 CrossRef CAS.
- (a) Q.-L. Liu, L.-J. Yang, Y.-H. Luo, W. Wang, Y. Ling and B.-W. Sun, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2016, 46, 1725–1734 CrossRef CAS; (b) X. Bao, J.-L. Liu, J.-D. Leng, Z. Lin, M.-L. Tong, M. Nihei and H. Oshio, Chem. – Eur. J., 2010, 16, 7973–7978 CrossRef CAS PubMed; (c) G.-B. Jiang, X.-N. Zeng, Y.-S. Fang, M.-H. Zeng and S. W. Ng, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, m2319 CrossRef CAS.
- M.-Z. Li, J.-H. Guo and J. Chen, Z. Anorg. Allg. Chem., 2015, 641, 2581–2586 CrossRef CAS.
- J.-J. Yan, C.-H. Li, W. Li, Z.-T. Yang, L. Li and H.-B. Liu, Jiegou Huaxue, 2019, 38, 1823 CAS.
- C. Li, H. Gao, L. Li, W. Wei and Y. Tan, Inorg. Chem. Commun., 2020, 111, 107613 CrossRef CAS.
- A. V. Kuttatheyil, M. Handke, J. Bergmann, D. Lässig, J. Lincke, J. Haase, M. Bertmer and H. Krautscheid, Chemistry, 2015, 21, 1118–1124 CrossRef CAS PubMed.
- J.-L. Chen, X.-F. Cao, J.-Y. Wang, L.-H. He, Z.-Y. Liu, H.-R. Wen and Z.-N. Chen, Inorg. Chem., 2013, 52, 9727–9740 CrossRef CAS PubMed.
- A. L. Webber, J. R. Yates, M. Zilka, S. Sturniolo, A.-C. Uldry, E. K. Corlett, C. J. Pickard, M. Pérez-Torralba, M. Angeles Garcia, D. Santa Maria, R. M. Claramunt and S. P. Brown, J. Phys. Chem. A, 2020, 124, 560–572 CrossRef CAS PubMed.
- (a) W. Guo, J. Liu, P. G. Weidler, J. Liu, T. Neumann, D. Danilov, W. Wenzel, C. Feldmann and C. Wöll, Phys. Chem. Chem. Phys., 2014, 16, 17918–17923 RSC; (b) S. Lee, H.-B. Bürgi, S. A. Alshmimri and O. M. Yaghi, J. Am. Chem. Soc., 2018, 140, 8958–8964 CrossRef CAS PubMed; (c) K. Schlichte, T. Kratzke and S. Kaskel, Microporous Mesoporous Mater., 2004, 73, 81–88 CrossRef CAS; (d) A. A. Yakovenko, Z. Wei, M. Wriedt, J.-R. Li, G. J. Halder and H.-C. Zhou, Cryst. Growth Des., 2014, 14, 5397–5407 CrossRef CAS.
- H.-K. Mao, T. Takahashi, W. A. Bassett, G. L. Kinsland and L. Merrill, J. Geophys. Res., 1974, 79, 1165–1170 CrossRef CAS.
- M. E. Fleet, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37, 917–920 CrossRef.
- L. Pauling and S. B. Hendricks, J. Am. Chem. Soc., 1925, 47, 781–790 CrossRef CAS.
- S. Nagakura, J. Phys. Soc. Jpn., 1958, 13, 1005–1014 CrossRef CAS.
- P. Scherrer, Z. Kristallogr. – Cryst. Mater., 1922, 57, 186–189 CrossRef CAS.
- M. Becker and M. Jansen, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2001, C57, 347–348 CrossRef CAS PubMed.
- G. Baldinozzi, B. Malinowska, M. Rakib and G. Durand, J. Mater. Chem., 2002, 12, 268–272 RSC.
- S. A. Sapchenko, D. G. Samsonenko and V. P. Fedin, Polyhedron, 2013, 55, 179–183 CrossRef CAS.
- Y. Meng, N. Zhang, J. Li, Y. Xu, Q. Yang, Y. Yuan, X. Zhang, J. Wu and L. Zhao, Spectrochim. Acta, Part A, 2022, 266, 120419 CrossRef CAS PubMed.
- E. San Sebastian, A. Rodríguez-Diéguez, J. M. Seco and J. Cepeda, Eur. J. Inorg. Chem., 2018, 2018, 2155–2174 CrossRef CAS.
- R. C. Leif, L. M. Vallarino, M. C. Becker and S. Yang, Cytometry, Part A, 2006, 69, 767–778 CrossRef PubMed.
- J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
- A. de Bettencourt-Dias, Luminescence of lanthanide ions in coordination compounds and nanomaterials, Wiley, Chichester, England, 2014, pp. 40–44 Search PubMed.
- A. Sillen and Y. Engelborghs, Photochem. Photobiol., 1998, 67, 475–486 CrossRef CAS.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- M. Pamei and A. Puzari, Nano-Struct. Nano-Objects, 2019, 19, 100364 CrossRef CAS.
- (a) Lanthanide probes in life, chemical and earth sciences: Theory and practice, ed. J.-C. G. Bünzli and G. R. Choppin, Elsevier, Amsterdam, 1989 Search PubMed; (b) J. Maillard, K. Klehs, C. Rumble, E. Vauthey, M. Heilemann and A. Fürstenberg, Chem. Sci., 2020, 12, 1352–1362 RSC.
- Y. Bayeh, P. Osuský, N. J. Yutronkie, R. Gyepes, A. Sergawie, P. Hrobárik, R. Clérac and M. Thomas, Polyhedron, 2022, 227, 116136 CrossRef CAS.
- A. E. Thorarinsdottir, A. I. Gaudette and T. D. Harris, Chem. Sci., 2017, 8, 2448–2456 RSC.
- J. England, G. J. P. Britovsek, N. Rabadia and A. J. P. White, Inorg. Chem., 2007, 46, 3752–3767 CrossRef CAS PubMed.
- V. Balland, F. Banse, E. Anxolabéhère-Mallart, M. Nierlich and J.-J. Girerd, Eur. J. Inorg. Chem., 2003, 2003, 2529–2535 CrossRef.
- Anorganische Chemie. Prinzipien von Struktur und Reaktivität, ed. J. Huheey, R. Keiter and E. Keiter, De Gruyter, Inc., Berlin, 5th edn, 2014 Search PubMed.
- Photochemistry and Photophysics of Coordination Compounds I, ed. V. Balzani and S. Campagna, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, vol. 280 Search PubMed.
- X.-L. Xu, P.-F. Yao, Da.-Ye Zhu, H.-Ye Li, H.-Fu Liu and Fu.-P. Huang, Chin. J. Struct. Chem., 2017, 36, 958 CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- (a) BRUKER AXS Inc., APEX4, SAINT and SADABS, Madison, WI, USA, 2021 Search PubMed; (b) BRUKER AXS Inc., XPREP – Reciprocal space exploration (Version 2014/2), Madison, WI, USA, 2014 Search PubMed; (c) G. M. Sheldrick, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef PubMed; (d) G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed; (e) C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed; (f) W. T. Pennington, J. Appl. Crystallogr., 1999, 32, 1028–1029 CrossRef CAS; (g) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed; (h) A. L. Spek, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2020, 76, 1–11 CrossRef CAS PubMed; (i) A. L. Spek, Inorg. Chim. Acta, 2018, 470, 232–237 CrossRef CAS; (j) A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed; (k) A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS; (l) A. A. Coelho, J. Appl. Crystallogr., 2018, 51, 210–218 CrossRef CAS; (m) M. S. Wrighton, D. S. Ginley and D. L. Morse, J. Phys. Chem., 1974, 78, 2229–2233 CrossRef CAS; (n) A. E. Sedykh, M. Becker, M. T. Seuffert, D. Heuler, M. Maxeiner, D. G. Kurth, C. E. Housecroft, E. C. Constable and K. Müller-Buschbaum, ChemPhotoChem, 2023, 7, e202200244 CrossRef CAS; (o) F. Billes, H. Endrédi and G. Keresztury, J. Mol. Struct.:THEOCHEM, 2000, 530, 183–200 CrossRef CAS; (p) G. A. Foulds, G. C. Percy and D. A. Thornton, Spectrochim. Acta, Part A, 1978, 34, 1231–1234 CrossRef; (q) K. I. Hadjiivanov, D. A. Panayotov, M. Y. Mihaylov, E. Z. Ivanova, K. K. Chakarova, S. M. Andonova and N. L. Drenchev, Chem. Rev., 2021, 121, 1286–1424 CrossRef CAS PubMed; (r) C. S. Stan, P. Horlescu, M. Popa, A. Coroaba and L. E. Ursu, New J. Chem., 2016, 40, 6505–6512 RSC; (s) C. S. Stan, C. Peptu, N. Marcotte, P. Horlescu and D. Sutiman, Inorg. Chim. Acta, 2015, 429, 160–167 CrossRef CAS; (t) M. K. Trivedi and R. M. Tallapragada, J. Mol. Pharm. Org. Process Res., 2015, 3, 1000128 Search PubMed; (u) K. B. Wiberg, V. A. Walters, K. N. Wong and S. D. Colson, J. Phys. Chem., 1984, 88, 6067–6075 CrossRef CAS; (v) R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef; (w) R. Borjas Nevarez, S. M. Balasekaran, E. Kim, P. Weck and F. Poineau, Acta Crystallogr., Sect. C: Struct. Chem., 2018, 74, 307–311 CrossRef CAS PubMed; (x) G. Bartlett and I. Langmuir, J. Am. Chem. Soc., 1921, 43, 84–91 CrossRef CAS; (y) M. Topper, M. L. McLaughlin and F. R. Fronczek, CSD Commun., 2007 Search PubMed; (z) R. Balamurugan, M. Palaniandavar and R. S. Gopalan, Inorg. Chem., 2001, 40, 2246–2255 CrossRef CAS PubMed; (a a) L. Jin, J. Ying, Y. Zhang, C. Sun, A. Tian and X. Wang, New J. Chem., 2022, 46, 8422–8432 RSC; (a b) R. Wartchow, CSD Commun., 2019 Search PubMed; (a c) D. Hass, O. Bechstein and M. Hentschel, Z. Anorg. Allg. Chem., 1989, 576, 131–138 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Analytical methods as well as 50 figures and 39 tables showing the crystallographic data, powder X-ray diffractograms, photoluminescence spectra, luminescence intensity decays and corresponding lifetimes, absorption spectra, IR spectra, thermal properties, and physisorption properties. CCDC 2347927, 2347920, 2347949, 2347948, 2347938, 2347937, 2347952, 2347953, 2347947, 2347946, 2347951, 2347925, 2347917 and 2347950. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03149k |
| This journal is © The Royal Society of Chemistry 2025 |