Open Access Article
Open Access Article[Cu(NHC)(OR)] (R = C(CF3)3) complexes for N–H and S–H bond activation and as pre-catalysts in the Chan–Evans–Lam reaction†
Tommaso
Ruggiero
,
Kristof
Van Hecke
 ,
Catherine S. J.
Cazin
,
Catherine S. J.
Cazin
 * and
Steven P.
Nolan
* and
Steven P.
Nolan
 *
*
Department of Chemistry and Center for Sustainable Chemistry, Ghent University, Krijgslaan 281 (S-3), 9000, Ghent, Belgium. E-mail: steven.nolan@ugent.be; catherine.cazin@ugent.be
First published on 17th December 2024
Abstract
The synthesis, isolation, and full characterization of a series of NHC–copper perfluoro–alkoxide complexes are reported. Their exceptional stability resides with the steric hindrance of the nonafluoro-tert-butyl alkoxide moiety, which exhibits a strong electron withdrawing effect. These new Cu(I) complexes are synthons that can permit the activation of acidic N–H and S–H bonds. The well-defined [Cu(IPr)(OC(CF3)3)] was investigated as a pre-catalyst in the Chan–Evans–Lam reaction.
Nonafluoro-tert-butyl alcohol (NFTBA) is a polar, strong H-bond donating solvent, and is the least explored member among the family of fluorinated alcohols that include 2,2,2-trifluoroethanol (TFE) and 1,1,1,3,3,3-hexafluoroisopropanol (HFIP). Its less frequent use can be reasoned from its higher cost and its rather unique physical/chemical properties: NFTBA has a pKa = 5.4 in water and its boiling point is 45 °C.1 These properties make it behave almost as a volatile carboxylic acid. It was recently used as a solvent for catalytic selective oxidation,2,3 as an additive for organocatalyzed reactions4 and it has been adopted as a valuable building block for 19F magnetic resonance imaging.5 It has been scarcely used as a reagent; one notable example is its role in C–O coupling reactions.6 It has been adopted as a ligand for metal centres such as aluminium,7,8 molybdenum and tungsten,9 palladium10 and copper,11,12 and some 21 metals from the Periodic Table (see ESI† for full references) for both complexes and salts, although the design of those NFTBA containing compounds has never incorporated other important and widely used families of ligands such as NHCs. NHCs have proven to be a particularly important ligand family, able to stabilize and protect metals prone to oxidation.13 Copper is one of the most earth abundant metals, but it also undergoes facile oxidation and thus, this reaction route accounts for several examples where, due to reactions with oxygen, decomposition of its complexes is observed.
The combination of NHC-type ligands with Cu–alkoxide species has proven to lead to significant advances in copper-mediated catalysis.14–18 The alkoxide moiety has been shown to be a useful fragment in conferring to the NHC–Cu complex the required stability to act as a synthon, allowing easy access to a plethora of other useful copper(I) complexes. One of the key representative compounds in this regards is [Cu(IPr)(OtBu)] (IPr = [1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene]), which can act as an excellent pre-catalyst in many catalytic transformations.19–24 Another important copper synthon is the remarkably stable copper(I) hydroxide complex [Cu(IPr)(OH)] which is capable of activating numerous X–H bonds, including all hybridizations of C–H (i.e., sp, sp2, sp3), N–H, P–H, O–H, S–H bonds.25 Despite numerous advantages, such as their excellent reactivity, these Cu–OR (where R is not H) complexes have some limitations. Firstly, their synthesis requires the use of a strong base and usually anaerobic conditions. Secondly, the handling of copper-alkoxide complexes generally necessitates anaerobic conditions and anhydrous solvents, as these are sensitive to both air and moisture. A notable exception is [Cu(IPr)(OH)], which displays remarkable stability.25
As a key complex, the very sensitive [Cu(IPr)(OtBu)] is a salient example. We thus reasoned that we could use the nonafluoro-tert-butoxide group's unique properties to generate more stable copper complexes and that this would enable a comparison with the stability and chemistry displayed by the recently reported [Cu(IPr)(O(C(H)(CF3)2))].26
The synthesis of the first NHC–copper–NFTBA complex was achieved by reacting [Cu(IPr)Cl] with 1.1 eq. of NFTBA in EtOH in the presence of 3 eq. of K2CO3 and conducting the reaction in air at 25 °C for 1 hour. The desired copper-alkoxide complex [Cu(IPr)(OC(CF3)3)] (1) was obtained in excellent yield using this simple procedure (Scheme 1a).
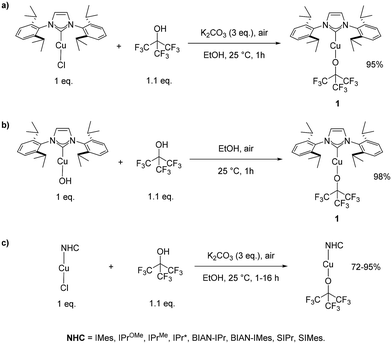 | ||
| Scheme 1 Syntheses of [Cu(IPr)(OC(CF3)3)] (1) using (a) [Cu(IPr)(Cl)], (b) [Cu(IPr)(OH)], (c) general synthesis of [Cu(IPr)(OC(CF3)3)]. | ||
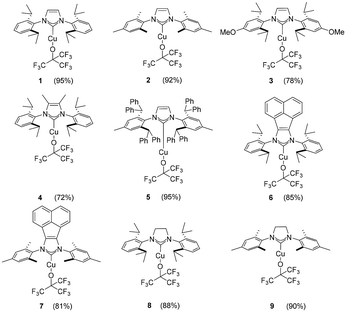
Complex 1 was also obtained in a slightly higher yield of 98%, using the [Cu(IPr)(OH)] complex as the copper source on a larger scale (500 mg of starting material instead of 100 mg, Scheme 1b). Once a simple protocol was established, we explored its compatibility and reactivity to support other Cu–NHC relatives (Scheme 1c). Nine NHC–Cu(I)–NFTBA complexes have been successfully synthesized in good to excellent yields using this simple protocol (Scheme 2).
Notably, lower yields were obtained in cases where the most electron donating NHCs (compounds 3 and 4) were used. In addition, the reactions with [Cu(BIAN-IPr)(Cl)] and [Cu(BIAN-IMes)(Cl)] required longer reaction time to reach completion, with respect to other (smaller) substrates, delivering products 6 and 7, respectively. We suspect the strong electron-withdrawing property of the fluoroalkyl groups creates a stable Cu–O bond in 1. Interestingly, the Cu–O bond length in [Cu(IPr)(OtBu)] is slightly shorter (1.8104(13) Å) than the one measured for [Cu(IPr)(OC(CF3)3)] (1) (1.8403(18) Å) (see Scheme 3).27 Next, to highlight the versatility of [Cu(IPr)(OC(CF3)3)] (1) as a synthon, its reactivity towards H–X activation was examined (Scheme 4).
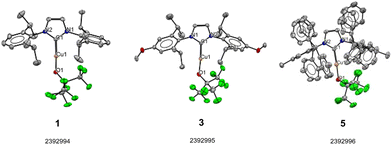 | ||
| Scheme 3 XRD molecular structures of compounds 1, 3 and 5. Thermal displacement ellipsoids shown at the 50% probability level. H-atoms are omitted for clarity. CCDC 2392994–2392996 contain the supplementary crystallographic data for this paper.† | ||
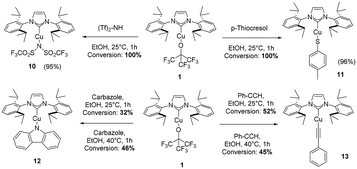
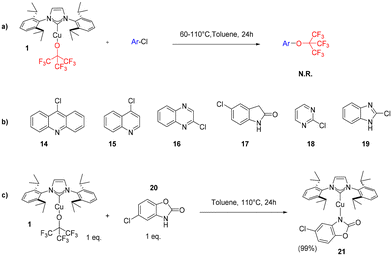
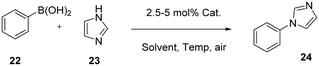
We initially tested the displacement/removal of the NFTBA moiety from 1 by simple protonolysis/ligand exchange with 1 equivalent of a protic ligand. As shown in Scheme 4, four ligands were able to displace the alkoxy fragment, leading to the formation of complexes 10–13. Gratifyingly, full conversion was achieved for the isolation of 10 and 11, due to high nucleophilicity of p-thiocresol and trifluoromethanesulfonimide. Unfortunately, the substitution with carbazole in EtOH at room temperature afforded only 32% conversion to 12, which was increased to 46% by heating the reaction mixture to 40 °C. The substitution of phenylacetylene also led to a 52% conversion, which decreased to 45% at 40 °C, probably due to the intrinsic instability of 13 in solution at more elevated temperatures than 25 °C. The lower reactivity of 1 highlights its increased stability given by steric hindrance of three CF3 groups and the overall stronger electronegativity effect which pulls electron density away from the oxygen atom. Synthon 1 was unreactive with pyrazole, 1,2,4-triazole, 1,2,4,5-tetrafluorobenzene, nitromethane, phenylboronic acid and phenylboronic acid pinacol ester under the same conditions presented for [Cu(IPr)(HFIP)] (see ESI†).26 Similarly to the work of Dhiman, the reactivity of 1 as a nucleophile was investigated by carrying out stoichiometric reactions with hetero-aryl chlorides, to form aryl fluoroalkoxides by nucleophilic substitution via heating the reaction of 1 with hetero-aryls 14–19 at 60 and 110 °C, in toluene (Scheme 5a).28 In the reaction between 1 and 19, decomposition of the former was observed, whereas for the reactions with reactants 14–18, 1 remained unreactive and intact. Employing chlorzoxazone (20) as reagent led to 99% yield of 21 (Scheme 5c). This affords the first cyclic carbamate copper complex in the literature. The single-crystal XRD structure of 21 is provided in the ESI (CCDC 2392997†). Finally, we investigated the catalytic activity of 1 and other NHC–Cu–NFTBA complexes. Our first attempt was to replace the air- and moisture-sensitive [Cu(IPr)(OtBu)] as a pre-catalyst with our thermally, air- and moisture-stable [Cu(IPr)(OC(CF3)3)]. [Cu(IPr)(OtBu)] has been widely used as a precursor to copper hydride species formed with the aid of silanes such as (EtO)3SiH or PMHS, as H sources. The copper hydride species is the main catalyst for a wide array of many catalytic reactions such as reduction of α,β-unsaturated carbonyl compounds,29 the hydrogenation of alkenes and alkynes,30 and the hydrosilylation of hindered and functionalized ketones.31 The copper hydride species are quite unstable, hence their highly reactive nature towards several substrate classes.27 Thus, we explored reactions of [Cu(IPr)(OC(CF3)3)] (1) with (EtO)3SiH or PMHS (Table 1). 1 was found completely unreactive under these conditions, showcasing its outstanding stability (see ESI† for reaction conditions). We next explored the possible catalytic activity of the new complexes in the Chan–Evans–Lam (CEL) reaction, employing 1 as pre-catalyst. There are many examples of copper species involved in CEL systems, mostly employing Cu(II) complexes, with very few examples of NHC-containing complexes.32 In most recent examples, the catalyst loading required for CEL coupling is between 5 and 10 mol%,33 (with only two exceptions in literature presenting 0.1–1 mol% catalyst loading34,35) so we began an exploration of this catalytic transformation using 5 mol% as catalyst loading involving phenylboronic acid and imidazole as the limiting reagent (Table 1). The initial comparison of entries 1 and 4 with 5 and 6 shows that a smaller amount of solvent increases the yields, and by comparing entries 1 and 5 with 4 and 6 it is found that a higher level of oxygen is a detrimental factor for maximum efficiency. After screening several solvents (Table 1, entries 6, 9, 10 and 11), methanol was found to be the best, affording 94% of the desired product. The use of base is essential for the reaction to proceed (Table 1, entry 14), with Na2CO3 affording the highest yield of 24 (Table 1, entries 6, 12 and 13). Lowering the catalyst loading to 2.5 mol% resulted in negligible change in the isolated yield (Table 1, entries 20 and 26).
| Entry | Mol% (cat.) | Solvent (mL) | T (°C) | Time (h) | Base | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 27 (0.50 mmol, 2 eq.), 28 (0.25 mmol, 1 eq.), Na2CO3 (0.50 mmol, 2 eq.), Cat. (5 mol%), MeOH (1.0 mL), 60 °C, 20 h, under air with an air-filled balloon above the vial. b NMR yield were calculated with 3-methoxy-benzene as internal standard. Average of two runs. c Reaction carried in closed vial without the air-filled balloon. d Isolated yield. e [Cu(IPr)Cl] used as catalyst. f Oxygen-filled balloon above the vial. | ||||||
| 1 | 5 | MeOH (3) | 60 | 20 | Na2CO3 | 58c |
| 2 | 5 | MeOH (3) | 60 | 5 | Na2CO3 | 57c |
| 3 | 5e | MeOH (3) | 60 | 5 | Na2CO3 | 52c |
| 4 | 5 | MeOH (3) | 60 | 20 | Na2CO3 | 68 |
| 5 | 5 | MeOH (1) | 60 | 20 | Na2CO3 | 78c |
| 6 | 5 | MeOH (1) | 60 | 20 | Na 2 CO 3 | 94 (92) |
| 7 | 5 | MeOH (1) | 60 | 10 | Na2CO3 | 85 |
| 8 | 0 | MeOH (1) | 60 | 20 | Na2CO3 | 0 |
| 9 | 5 | EtOH (1) | 60 | 20 | Na2CO3 | 82 |
| 10 | 5 | IPrOH (1) | 60 | 20 | Na2CO3 | 80 |
| 11 | 5 | Acetone (1) | 60 | 20 | Na2CO3 | 0 |
| 12 | 5 | MeOH (1) | 60 | 20 | K2CO3 | 90 |
| 13 | 5 | MeOH (1) | 60 | 20 | K3PO4 | 89 |
| 14 | 5 | MeOH (1) | 60 | 20 | No base | 0 |
| 15 | 5 | MeOH (1) | RT | 20 | Na2CO3 | 28 |
| 16 | 5e | MeOH (1) | RT | 20 | Na2CO3 | 22 |
| 17 | 5 | MeOH (1) | 40 | 20 | Na2CO3 | 83 |
| 18 | 5 | MeOH (1) | 50 | 20 | Na2CO3 | 88 |
| 19 | 5 | MeOH (1) | 60 | 5 | Na2CO3 | 77 |
| 20 | 2.5 | MeOH (1) | 60 | 20 | Na2CO3 | 90 |
| 21 | 2.5e | MeOH (1) | 60 | 20 | Na2CO3 | 82 |
| 22 | 2.5e | MeOH (1) | 60 | 7 | Na2CO3 | 79 |
| 23 | 5f | MeOH (1) | 60 | 20 | Na2CO3 | 94 |
| 24 | 5f | MeOH (1) | 60 | 5 | Na2CO3 | 88 |
| 25 | 5f | MeOH (1) | 60 | 7 | Na2CO3 | 93 |
| 26 | 2.5f | MeOH (1) | 60 | 7 | Na2CO3 | 90 |
| 27 | 2.5e,f | MeOH (1) | 60 | 7 | Na2CO3 | 85 |
Lastly, the amount of oxygen was increased in the reactor by affixing a balloon of pure oxygen gas to the vial. This increased oxygen concentration did not lead to higher conversion to product, but the reaction kinetics are greatly accelerated. Indeed, by comparing entries 6 and 25, the reaction time can be decreased to 7 hours leading to an excellent yield. We next examined the activity of [Cu(IPr)(Cl)] for the sake of comparison. Under these conditions, the activity of [Cu(IPr)(Cl)] does not significantly differ from that displayed by 1.
A proposed mechanism for CEL coupling using 1 as pre-catalyst is shown in Scheme 6 and is supported by previous mechanistic studies carried out under similar reaction conditions.36,37 [Cu(IPr)(OMe)] (A) is believed to be the active catalytic species which undergoes aerobic oxidation forming a Cu(II) species (B). The two methoxide ligands on B are displaced with the nucleophilic amine by ligand exchange and with the phenylboronic acid via transmetalation, forming D. The final Cu(III) species (E) is formed by a second aerobic oxidation that subsequently undergoes reductive elimination, yielding the coupling product and the starting catalytic species A.
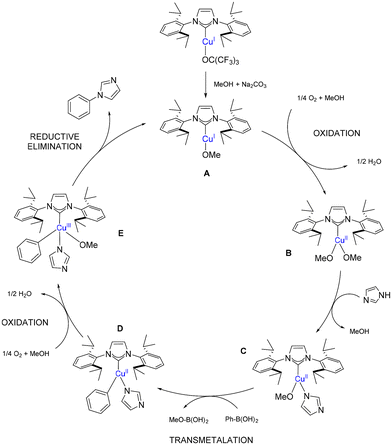 | ||
| Scheme 6 Proposed mechanism for the Chan–Evans–Lam reaction using [Cu(IPr)(OC(CF3)3)] (1) as pre-catalyst. | ||
The active species in the CEL reactions remains a matter of debate, however, the present study encourages us to explore the CEL reaction in more detail with very simple catalyst. Such studies are presently ongoing in our laboratories.
In summary, we have developed a simple and efficient protocol to isolate air-stable NHC-containing copper perfluorinated alkoxide compounds. We have shown that complex 1 exhibits chemical versatility as an air-stable synthon in reactions involving N–H and S–H bond activations. Additionally, we have shown that 1 is an effective pre-catalyst for an early example of the Chan–Evans–Lam coupling reaction. Further studies involving this and related congeners in stoichiometric and catalytic reactions are ongoing in our laboratories.
Data availability
All data included and leading to conclusions presented in this manuscript are included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge support from the Special Research Fund (BOF/24J/2023/084 to K. V. H.) of Ghent University and The Research Foundation – Flanders (FWO) (G0A6823N and G0C5423N). Umicore AG is also thanked for generous gifts of materials.References
- B. L. Dyatkin, E. P. Mochalina and I. L. Knunyants, Tetrahedron, 1965, 21, 2991–2995 CrossRef CAS.
- S.-C. Chan, A. Palone, M. Bietti and M. Costas, Angew. Chem., 2024, 136, e202402858 CrossRef.
- M. Galeotti, L. Vicens, M. Salamone, M. Costas and M. Bietti, J. Am. Chem. Soc., 2022, 144, 7391–7401 CrossRef CAS PubMed.
- D. Niedek, S. Schuler, C. Eschmann, R. Wende, A. Seitz, F. Keul and P. Schreiner, Synthesis, 2016, 49, 371–382 CrossRef.
- T. Wu, A. Li, K. Chen, X. Peng, J. Zhang, M. Jiang, S. Chen, X. Zheng, X. Zhou and Z.-X. Jiang, Chem. Commun., 2021, 57, 7743–7757 RSC.
- H. Meng, L. Wen, Z. Xu, Y. Li, J. Hao and Y. Zhao, Org. Lett., 2019, 21, 5206–5210 CrossRef CAS.
- I. Krossing, Chemistry, 2001, 7, 490–502 CrossRef CAS.
- A. Kraft, N. Trapp, D. Himmel, H. Böhrer, P. Schlüter, H. Scherer and I. Krossing, Chem. – Eur. J., 2012, 18, 9371–9380 CrossRef CAS PubMed.
- G. S. Quiñones, G. Hägele and K. Seppelt, Chem. – Eur. J., 2004, 10, 4755–4762 CrossRef.
- J. Dhankhar, E. González-Fernández, C.-C. Dong, T. K. Mukhopadhyay, A. Linden and I. Čorić, J. Am. Chem. Soc., 2020, 142, 19040–19046 CrossRef CAS PubMed.
- A. P. Purdy, C. F. George and G. A. Brewer, Inorg. Chem., 1992, 31, 2633–2638 CrossRef CAS.
- S. E. N. Brazeau, E. E. Norwine, S. F. Hannigan, N. Orth, I. Ivanović-Burmazović, D. Rukser, F. Biebl, B. Grimm-Lebsanft, G. Praedel, M. Teubner, M. Rübhausen, P. Liebhäuser, T. Rösener, J. Stanek, A. Hoffmann, S. Herres-Pawlis and L. H. Doerrer, Dalton Trans., 2019, 48, 6899–6909 RSC.
- S. Díez-González and S. P. Nolan, Coord. Chem. Rev., 2007, 251, 874–883 CrossRef.
- A. A. Danopoulos, T. Simler and P. Braunstein, Chem. Rev., 2019, 119, 3730–3961 CrossRef CAS.
- J. Plotzitzka and C. Kleeberg, Inorg. Chem., 2016, 55, 4813–4823 CrossRef CAS.
- H. Jang, A. R. Zhugralin, Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7859–7871 CrossRef CAS PubMed.
- D. S. Laitar, P. Müller and J. P. Sadighi, J. Am. Chem. Soc., 2005, 127, 17196–17197 CrossRef CAS PubMed.
- C. Kleeberg and C. Borner, Organometallics, 2018, 37, 4136–4146 CrossRef CAS.
- T. Ohishi, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2008, 47, 5792–5795 CrossRef CAS PubMed.
- W. Xie and S. Chang, Angew. Chem., Int. Ed., 2016, 55, 1876–1880 CrossRef CAS.
- L. Zhang, Z. Li, M. Takimoto and Z. Hou, Chem. Rec., 2020, 20, 494–512 CrossRef CAS PubMed.
- S. Bagherzadeh and N. P. Mankad, J. Am. Chem. Soc., 2015, 137, 10898–10901 CrossRef CAS PubMed.
- L. E. English, T. M. H. Downie, C. L. Lyall, M. F. Mahon, C. L. McMullin, S. E. Neale, C. M. Saunders and D. J. Liptrot, Chem. Commun., 2023, 59, 1074–1077 RSC.
- T. M. H. Downie, J. W. Hall, T. P. C. Finn, D. J. Liptrot, J. P. Lowe, M. F. Mahon, C. L. McMullin and M. K. Whittlesey, Chem. Commun., 2020, 56, 13359–13362 RSC.
- G. C. Fortman, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2010, 29, 3966–3972 CrossRef CAS.
- S. Ostrowska, P. Arnaut, D. J. Liptrot, C. S. J. Cazin and S. P. Nolan, Chem. Commun., 2023, 59, 9126–9129 RSC.
- N. P. Mankad, D. S. Laitar and J. P. Sadighi, Organometallics, 2004, 23, 3369–3371 CrossRef CAS.
- A. K. Dhiman, R. Kumar and U. Sharma, Synthesis, 2021, 53, 4124–4130 CrossRef CAS.
- V. Jurkauskas, J. P. Sadighi and S. L. Buchwald, Org. Lett., 2003, 5, 2417–2420 CrossRef CAS PubMed.
- K. Semba, T. Fujihara, T. Xu, J. Terao and Y. Tsuji, Adv. Synth. Catal., 2012, 354, 1542–1550 CrossRef CAS.
- S. Díez-González, H. Kaur, F. K. Zinn, E. D. Stevens and S. P. Nolan, J. Org. Chem., 2005, 70, 4784–4796 CrossRef.
- J. Chen, J. Li and Z. Dong, Adv. Synth. Catal., 2020, 362, 3311–3331 CrossRef CAS.
- P. S. Devi, S. Saranya and G. Anilkumar, Catal. Sci. Technol., 2024, 14, 2320–2351 RSC.
- M. Guo, B. Chen, K. Chen, S. Guo, F.-S. Liu, C. Xu and H.-G. Yao, Tetrahedron Lett., 2022, 107, 154074 CrossRef CAS.
- M. Zhang, Z. Xu and D. Shi, Tetrahedron, 2021, 79, 131861 CrossRef CAS.
- A. E. King, T. C. Brunold and S. S. Stahl, J. Am. Chem. Soc., 2009, 131, 5044–5045 CrossRef CAS PubMed.
- S.-Y. Moon, J. Nam, K. Rathwell and W.-S. Kim, Org. Lett., 2014, 16, 338–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and protocols. CCDC 2392994–2392997. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03322a |
| This journal is © The Royal Society of Chemistry 2025 |