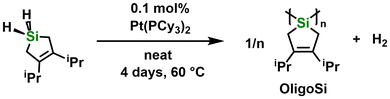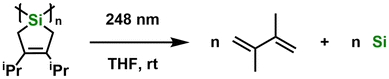Open Access Article
Open Access ArticleTowards the photodeposition of SixGey-type materials via oligomers of cyclogermapentenes and cyclosilapentenes†
William
Medroa del Pino
 a,
Andres
Forero Pico
a,
Andres
Forero Pico
 b,
Abhishek V.
Muralidharan
b,
Abhishek V.
Muralidharan
 a,
Manisha
Gupta
a,
Manisha
Gupta
 b and
Eric
Rivard
b and
Eric
Rivard
 *a
*a
aDepartment of Chemistry, University of Alberta, 11227 Saskatchewan Dr, Edmonton, Alberta T6G 2G2, Canada. E-mail: erivard@ualberta.ca
bDepartment of Electrical Engineering, University of Alberta, 9211 116 St., Edmonton, Alberta T6G 2H5, Canada. E-mail: mgupta1@ualberta.ca
First published on 9th January 2025
Abstract
This article provides an alternative pathway towards cyclosilapentenes (e.g., SiH2-iPr and SpiroSi) involving the use of Rieke magnesium to activate the requisite dienes for synthesis. Subsequent metal-mediated dehydrocoupling of cyclosilapentene SiH2-iPr and mixtures with another cyclogermapentene gives oligomers with backbone Si–Si (number average molecular weight, Mn = 1.0 kDa) and Si–Ge (Mn = 1.4 kDa) linkages, respectively. UV-irradiation (248 nm) of the abovementioned oligotetrelenes and the molecular spirosilane SpiroSi were examined in solution; while evidence for SixGey-type materials was noted through Raman and EDX spectroscopy, the presence of substantial amorphous carbon as a contaminant was also observed. (TD)-DFT computational studies on the spirosilane SpiroSi provide some insight into the lack of efficient SixGey photodeposition noted in this study.
Introduction
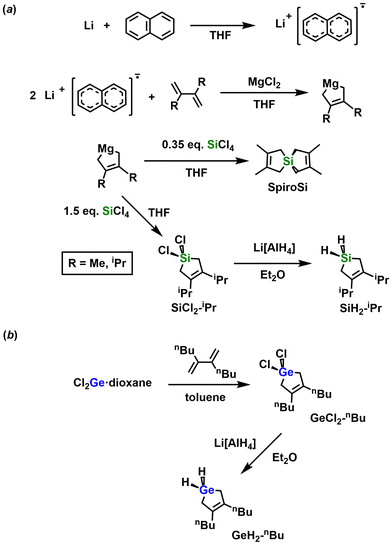
Silicon is the most widely used element in the production of electronic devices due to its low bandgap (1.1 eV) and its large-scale production.1 Additionally, Si forms stable oxides when exposed to air, which enables its use in the fabrication of photolithographic masks.1a,b Silicon germanide (SixGey) is also a useful semiconductor due to the bandgap tuning that is possible through variation in the Si/Ge ratio. Likewise, SixGey exhibits exceptional thermoelectric properties.2a These features, alongside its resistance to extreme temperatures (up to 1000 °C) and radiation has prompted the use of SixGey in the fabrication of thermoelectrics, photodetectors, photodiodes, transistors, and photovoltaic cells.2Despite all of the described advantages, standard routes towards Si and SixGey (e.g., chemical vapor deposition, CVD) make use of toxic and sometimes corrosive chlorinated precursors, such as HSiCl3. Furthermore, the need of high temperatures alongside sophisticated instrumentation makes these syntheses difficult to access for many.3 Therefore, the development of more efficient techniques to deposit films of SixGey is highly desirable.
In previous work, we demonstrated the photopatterning of crystalline Ge films via the light-induced elimination of diene from poly(cyclogermapentene)s at room temperature.4 Building on this approach, the present study shows the partially-successful photodeposition of Si and SixGey-type materials via UV-light irradiation of new tetrelene oligomers and a molecular spirosilane, albeit with carbon contamination. (TD)-DFT computations and stability assays on the photoextruded dienes are also discussed.
Results and discussion
Synthesis of Si and SixGey precursors
The initial step towards the attempted room temperature photodeposition of elemental Si and SixGey alloys involved the synthesis of the cyclic silanes 1,1-dihydro-3,4-di-iso-propylsilacyclopent-3-ene (SiH2-iPr) and silaspiro-[4.4]-2,3,7,8-tetramethyl-2,7-nonadiene (SpiroSi) through a modified Rieke magnesium approach (Scheme 1a).5 Characterization of both silanes by 1H, 13C{1H}, and 29Si{1H} NMR spectroscopy showed the expected resonances for the assigned structures (Fig. S1–S6†). Additionally, high-resolution mass spectrometry (HR-MS) confirmed the presence of the molecular ion (M+) in each product. Finally, FT-IR analysis of SiH2-iPr supported the formation of a cyclic silane with an intense Si–H vibration centered at 2144 cm−1 (Fig. S22†).6The synthesis of 1,1-dihydro-3,4-dibutylsilacyclopent-3-ene (GeH2-nBu) was achieved via the cycloaddition of the dibutylated diene (diene-nBu), H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(nBu)–C(nBu)
C(nBu)–C(nBu)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, with Cl2Ge·dioxane to first afford 1,1-dichloro-3,4-dibutylgermacyclopent-3-ene (GeCl2-nBu). Halogen/hydride metathesis between GeCl2-nBu and Li[AlH4] gave the expected germane GeH2-nBu in 81% yield (Scheme 1b). The identities of diene-nBu, GeCl2-nBu, and GeH2-nBu were confirmed by 1H and 13C{1H} NMR spectroscopies (Fig. S7–S12†), elemental analyses, and HR-MS.
CH2, with Cl2Ge·dioxane to first afford 1,1-dichloro-3,4-dibutylgermacyclopent-3-ene (GeCl2-nBu). Halogen/hydride metathesis between GeCl2-nBu and Li[AlH4] gave the expected germane GeH2-nBu in 81% yield (Scheme 1b). The identities of diene-nBu, GeCl2-nBu, and GeH2-nBu were confirmed by 1H and 13C{1H} NMR spectroscopies (Fig. S7–S12†), elemental analyses, and HR-MS.
The next step of this study involved the attempted dehydrocoupling polymerization of the silacyclopentene, SiH2-iPr, using the known transition metal-based (pre-)catalysts [Rh(COD)Cl]2, [RhCl(PPh3)3], and Cp2Zr(pyr)(Me3SiCCSiMe3) (COD = cyclooctadiene; pyr = pyridine; Cp = η5-C5H5).7 Unexpectedly, analysis of the final reaction mixtures by 1H NMR spectroscopy showed only partial monomer conversion with [Rh(COD)Cl]2 or no reaction with [RhCl(PPh3)3] or Cp2Zr(pyr)(Me3SiCCSiMe3). Despite changes in the catalyst loading from 0.1 to 2 mol%, the solvent employed (THF or toluene), the temperature of the reaction mixture (−30 °C, room temperature, or 70 °C), and time (1 to 7 days), no improvements were observed. Nevertheless, use of 0.1 mol% of Pt(PCy3)2 (Cy = cyclohexyl) as a dehydrocoupling catalyst8 and heating to 60 °C without solvent led to observable bubbling (presumably H2 formation) and the progressive coloration of the reaction mixture to light-yellow. Purification of the product by precipitation into MeCN (−30 °C) afforded a light-yellow semi-solid, OligoSi, in a yield of 58% (Scheme 2).
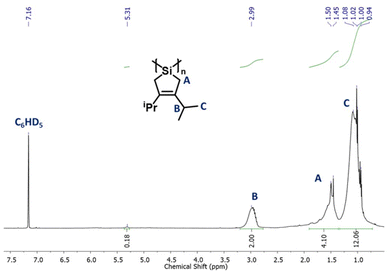
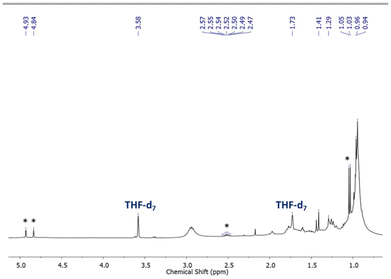
Characterization of OligoSi by Raman spectroscopy showed bands corresponding to the expected Si–Si, Si–C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretches at 505, 880, and 1617 cm−1, respectively.6 Si–H stretches, expected at around 2100 cm−1, were not observed (Fig. S23†). The Raman spectral data suggest the successful oligomerization to give a product with backbone Si–Si linkages and retention of the cyclosilapentene side units. Moreover, the absence of discernable Si–H units suggests the formation of linear chains of moderate molecular weight and/or generation of cyclic oligomers. 1H NMR spectroscopic analysis of OligoSi showed broadened alkyl signals and the disappearance of the hydride peak from the monomer SiH2-iPr; there is also a broad spectral feature around 5.3 ppm, assigned to Si–H end-groups (Fig. 1).9
C stretches at 505, 880, and 1617 cm−1, respectively.6 Si–H stretches, expected at around 2100 cm−1, were not observed (Fig. S23†). The Raman spectral data suggest the successful oligomerization to give a product with backbone Si–Si linkages and retention of the cyclosilapentene side units. Moreover, the absence of discernable Si–H units suggests the formation of linear chains of moderate molecular weight and/or generation of cyclic oligomers. 1H NMR spectroscopic analysis of OligoSi showed broadened alkyl signals and the disappearance of the hydride peak from the monomer SiH2-iPr; there is also a broad spectral feature around 5.3 ppm, assigned to Si–H end-groups (Fig. 1).9
Integration of the 1H NMR alkyl signals and the Si–H peak in from OligoSi suggested the presence of ca. 10–11 repeating units, or a number average molecular weight (Mn) of 1.6 to 1.8 kDa. Likewise, gel permeation chromatography (GPC) in THF was also performed giving Mn = 1.0 kDa and a polydispersity index (PDI) of 2.3 (Fig. S34†). This analysis indicates the formation of oligomers composed of 6 repeating units (on average), which correlates moderately well with the 1H NMR end-group analysis, given the GPC instrumentation used. Thermogravimetric analysis (TGA) of OligoSi revealed an experimental weight loss of 60% upon heating to 500 °C; notably, this value is higher than the overall expected weight loss expected if only Si metal were to remain (83%) (Fig. S26†). Multiple TGA measurements using different samples of OligoSi were attempted, showing similar weight loss in each case.
Dehydrocoupling of GeH2-nBu was also accomplished using 6 mol% of Pt(PCy3)2 in benzene at room temperature with continuous stirring under inert atmosphere for 18 hours. As with the synthesis of OligoSi, bubbling and the progressive coloration of the reaction mixture to light-yellow were observed. Purification of the product by precipitation from a concentrated solution in THF into cold MeCN (−30 °C) afforded OligoGe as a light-yellow semi-solid. Characterization of OligoGe by 1H NMR spectroscopy showed the expected signal broadening and the disappearance of the GeH2 resonance from GeH2-nBu; in place, a broad resonance spanning 4.54 to 4.82 ppm was present, likely due to Ge–H end groups (Fig. S14†). Moreover, Raman spectroscopic analysis of this product supported the successful dehydrocoupling polymerization of GeH2-nBu as a Ge–Ge stretch was present at 300 cm−1 along with a small band corresponding to a Ge–H stretch (end groups) at 2021 cm−1 (Fig. S24†).6 As with OligoSi, the molecular weight of OligoGe was estimated by 1H NMR end-group analysis and GPC, with the latter method giving Mn = 1.4 kDa and a PDI of 1.2, indicating the formation of oligomers with ca. 6 repeat units on average (Fig. S35†). TGA of OligoGe showed weight loss to a final remaining value of 35% upon heating to 400 °C, which is close in value to the expected remaining weight for the formation of elemental Ge (32%; Fig. S27†).
The successful dehydrocoupling of both GeH2-nBu and SiH2-iPr enabled the latter co-oligomerization of both monomers to form a possible Si/Ge photolithographic precursor to SixGey. Note: different heterocyclic side groups (nBu and iPr) were used for each monomer to enable determination of Si/Ge element ratios in the final oligomer by 1H NMR spectroscopy. Upon addition of 2 mol% of Pt(PCy3)2 to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of GeH2-nBu and SiH2-iPr at 80 °C (in toluene) immediate bubbling and the progressive color change of the reaction mixture to light-orange were observed. After stirring for 18 h, thickening of the solution was noted until a light-orange viscous mixture remained. Purification of the product was accomplished by precipitation from THF into cold MeCN (−30 °C) to give OligoSiGe as an orange semi-solid (Scheme 3). Thermogravimetric analysis (TGA) of OligoSiGe under argon (Fig. S28†) showed a weight loss value of 68% upon heating to 400 °C; for comparison, the expected weight loss if only Si and Ge remained in a 1
1 mixture of GeH2-nBu and SiH2-iPr at 80 °C (in toluene) immediate bubbling and the progressive color change of the reaction mixture to light-orange were observed. After stirring for 18 h, thickening of the solution was noted until a light-orange viscous mixture remained. Purification of the product was accomplished by precipitation from THF into cold MeCN (−30 °C) to give OligoSiGe as an orange semi-solid (Scheme 3). Thermogravimetric analysis (TGA) of OligoSiGe under argon (Fig. S28†) showed a weight loss value of 68% upon heating to 400 °C; for comparison, the expected weight loss if only Si and Ge remained in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is 75%.
1 ratio is 75%.
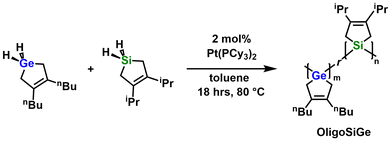 | ||
| Scheme 3 Synthesis of oligo(3,4-dibutylgermacyclopentene-r-3,4-di-iso-propylsilacyclopentene) (OligoSiGe). | ||
The 1H NMR spectrum of OligoSiGe confirms the successful dehydrocoupling co-polymerization of GeH2-nBu and SiH2-iPr, as evidenced by the appearance of broad alkyl signals alongside the disappearance of the Si–H and Ge–H peaks from the monomers at 4.10 and 4.12 ppm, respectively. Likewise, the formation of new broad set of signals from 4.27 to 5.35 ppm suggested the presence of new silicon/germanium hydride end-groups (Fig. 2). Integration of the alkyl signals revealed an iPr/nBu subunit ratio of 51/49, which matches closely with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silane/germane monomer feed ratio used in the dehydrocoupling oligomerization. The Raman spectrum of OligoSiGe further supported the formation of this co-oligomer with discernable Ge–Ge, Si–Si, Si–Ge, Si–C, C
1 silane/germane monomer feed ratio used in the dehydrocoupling oligomerization. The Raman spectrum of OligoSiGe further supported the formation of this co-oligomer with discernable Ge–Ge, Si–Si, Si–Ge, Si–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, Ge–H, and Si–H stretches at 304, 463, 563, 878, 1622, 1997, and 2116 cm−1, respectively (Fig. S25†).6 End-group analysis of the alkyl and hydride 1H NMR resonances in OligoSiGe gave an overall degree of polymerization of approximately 13 (Mn = 2.8 kDa). GPC analysis of OligoSiGe in THF was then performed, giving a number average molecular weight of 1.4 kDa, suggesting 7 repeating units per chain, and a PDI of 1.2 (Fig. S36†).
C, Ge–H, and Si–H stretches at 304, 463, 563, 878, 1622, 1997, and 2116 cm−1, respectively (Fig. S25†).6 End-group analysis of the alkyl and hydride 1H NMR resonances in OligoSiGe gave an overall degree of polymerization of approximately 13 (Mn = 2.8 kDa). GPC analysis of OligoSiGe in THF was then performed, giving a number average molecular weight of 1.4 kDa, suggesting 7 repeating units per chain, and a PDI of 1.2 (Fig. S36†).
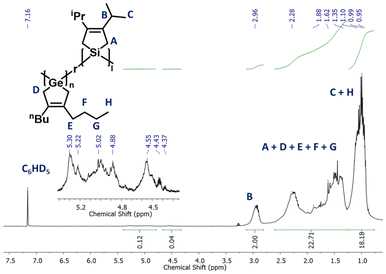 | ||
| Fig. 2 1H NMR spectrum of oligo(3,4-dibutylgermacyclopentene-r-3,4-di-iso-propylsilacyclopentene) (OligoSiGe) in C6D6. | ||
The above data point to the presence of low molecular weight oligomers for OligoSi, OligoGe, and OligoSiGe. These experimental results align with a related study by Tanaka and co-workers, who used Pt(COD)2 as a pre-catalyst to synthesize silacyclopentane oligomers with a Mn value of 0.8 kDa, equivalent to 9 repeating units.10
Photodeposition attempts
In order to determine the light absorption properties of the new Si/Ge oligomers reported here, UV-Vis spectra were recorded (Fig. S30–S33†). The UV-Vis spectrum of OligoSi in THF indicated no clear maxima in the range of 220–800 nm, but an absorption onset was observed at 310 nm. The spectrum of OligoGe gave an absorption maximum at 290 nm with a red-shifted onset of absorption at 336 nm. The UV-Vis spectrum of OligoSiGe in THF showed a similar absorption profile as OligoSi, with no discernible maxima and an absorption onset at 316 nm. Finally, the UV-Vis spectrum of the spirocyclic silane SpiroSi gave an absorption onset of 304 nm. These findings suggest that OligoSi, OligoSiGe, and SpiroSi are susceptible to irradiation by UV light with wavelengths below ca. 300 nm.Photodeposition involving OligoSi was then attempted with irradiation from a 248 nm laser into a solution of the oligosilane in THF-d8 at room temperature, using a custom-built4 quartz reactor (Scheme 4).
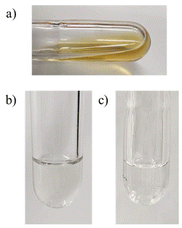
As the photolysis of OligoSi proceeded, the color of the solution changed from colorless to light-yellow, and a dark solid started to form on the inner walls of the quartz reactor (Fig. 3). Unfortunately, the mass of the dark precipitate that also formed on the bottom of the reactor was too low for an accurate yield determination (<0.1 mg). 1H NMR spectroscopic analysis of the reaction mixture after exposing OligoSi to UV light (in THF-d8) showed oligosilane starting material along with small signals corresponding to 2,3-di-iso-propyl-1,3-butadiene (5%), confirming that the partial reverse cycloaddition of diene from OligoSi had occurred (Fig. 4). Surprisingly, analysis of the deposited inner grey-layer of the quartz reactor by Raman spectroscopy showed intense bands at 1585, 1326, and 1173 cm−1, corresponding to amorphous carbon, and a weak signal at 493 cm−1 that could be assigned to Si–Si stretches (Fig. S38†).6,11
 | ||
| Fig. 3 Photograph of the deposited powder after photolysis of oligo(3,4-di-iso-propylsilacyclopentene) (OligoSi) in THF. | ||
The deposited layer on the quartz reactor from the photolysis of OligoSi was also characterized by scanning electron microscopy (SEM), revealing irregular morphologies (Fig. S39†). Moreover, energy dispersive X-ray (EDX) analysis of this thin film indicated a high atomic carbon content (44.36%), further supporting the formation of amorphous carbon-based material during photolysis (Fig. S40†). It should be noted that detection of Si by EDX is unreliable in this case as the grey thin film was deposited on a quartz substrate. Finally, EDX analysis often presents background C contamination in our instrument, however, the carbon content observed for the thin film produced by the photolysis of OligoSi is significantly higher than what would be expected from background carbon alone.
Photodeposition of SixGey from OligoSiGe was also attempted using similar conditions as those employed for the photolysis experiments with OligoSi. As observed in previous experiments, a thin-layer of a dark-grey precipitate formed on the inner wall of the quartz reactor after the UV-light irradiation of OligoSiGe in THF at 248 nm (Fig. 5). 1H NMR characterization of the remaining solution in THF-d8 after photolysis revealed broad signals corresponding to unreacted OligoSiGe. Nonetheless, low intensity peaks belonging to the expected dienes H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–C(R)
C(R)–C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (R = nBu and iPr; nBu = 8% and iPr = 4%) were also observed (Fig. S17†).
CH2 (R = nBu and iPr; nBu = 8% and iPr = 4%) were also observed (Fig. S17†).
 | ||
| Fig. 5 Photograph of the deposited SixGey film (and carbon) after photolysis of oligo(3,4-dibutylgermacyclopentene-r-3,4-di-iso-propylsilacyclopentene) (OligoSiGe) in THF-d8. | ||
The Raman spectrum of the deposited insoluble material from the photolysis of OligoSiGe shows a vibration at 298 cm−1, due to Ge–Ge stretches, and a less intense/broader band between 410–506 cm−1. This latter band likely corresponds to a combination of Si–Ge and Si–Si stretches, typically observed around 400 and 500 cm−1, respectively.6 These results indicate the successful deposition of a solid with some local SixGey structure. Finally, as observed in the photolysis of OligoSi, intense bands at 1173, 1327, and 1586 cm−1 were noted (Fig. 6), which match the Raman spectrum of amorphous carbon,11 pointing to the partial decomposition of the organic by-products formed during the photolysis.
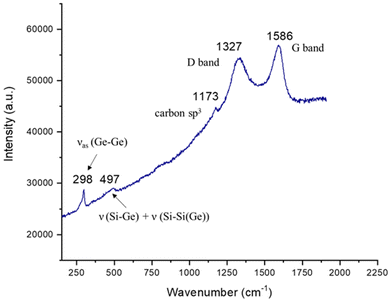 | ||
| Fig. 6 Raman spectrum of the dark-grey film obtained from the photolysis of oligo(3,4-dibutylgermacyclopentene-r-3,4-di-iso-propylsilacyclopentene) (OligoSiGe). | ||
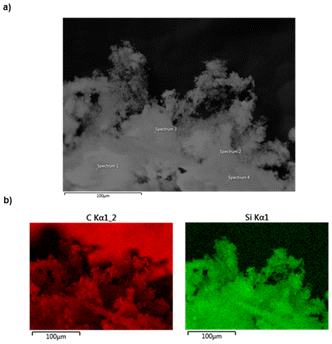
SEM and EDX data were also collected on the fine dark-grey powder obtained during the photolysis reaction of OligoSiGe. The SEM image of the precipitate, deposited onto carbon tape prior to analysis, revealed the formation of granules (Fig. S43†); EDX mapping of the material indicated presence of Ge and Si at 14.47 and 8.50 atomic%, respectively (Fig. S44†). These results suggest the formation of a bulk material with a Si/Ge ratio of approximately Si0.37Ge0.63.
To gain further insight into the results obtained from the photodeposition attempts of OligoSi and OligoSiGe, photolysis experiments using SpiroSi were also conducted in cyclohexane-d12 under a nitrogen atmosphere at room temperature. Similar to the photolysis of OligoSi, a fine layer of a grey solid was formed on the inner surface of the quartz reactor and the initially colorless solution adopted a deep yellow color after completion of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 light pulses (Fig. 7). The photolysis of SpiroSi was also attempted in THF-d8 to probe the influence of a more polar solvent on the reaction outcome. As before, coloration to deep yellow and the formation of a small amount of a black precipitate were observed. These results indicate that formation of the carbon contaminant is not influenced substantially by the solvent employed. Analysis of the reaction mixture using 1H NMR spectroscopy indicated a significant amount of unreacted SpiroSi. Additionally, signals corresponding to 2,3-dimethyl-1,3-butadiene (diene-Me) were also detected at 5.05, 4.94, and 1.89 ppm, suggesting the partially successful reverse-cycloaddition of diene from the SpiroSi (Fig. S18†).
000 light pulses (Fig. 7). The photolysis of SpiroSi was also attempted in THF-d8 to probe the influence of a more polar solvent on the reaction outcome. As before, coloration to deep yellow and the formation of a small amount of a black precipitate were observed. These results indicate that formation of the carbon contaminant is not influenced substantially by the solvent employed. Analysis of the reaction mixture using 1H NMR spectroscopy indicated a significant amount of unreacted SpiroSi. Additionally, signals corresponding to 2,3-dimethyl-1,3-butadiene (diene-Me) were also detected at 5.05, 4.94, and 1.89 ppm, suggesting the partially successful reverse-cycloaddition of diene from the SpiroSi (Fig. S18†).
 | ||
| Fig. 7 Photograph of the reaction mixture after photolysis of silaspiro-[4.4]-2,3,7,8-tetramethyl-2,7-nonadiene (SpiroSi) in cyclohexane-d12. | ||
SEM analysis of the recovered dark-grey precipitate obtained from the photolysis of SpiroSi showed an uneven solid (Fig. 8a). EDX characterization revealed a high carbon content (73.57%) and only a small atomic percentage of Si (6.02%) (Fig. 8b). These findings suggest that irradiation of SpiroSi with UV light leads to the decomposition of the organic fraction(s) leading to amorphous carbon, as observed with the photolysis of OligoSi and OligoSiGe.
Photostability studies involving dienes
The presence of amorphous carbon after the photolysis of OligoSi, OligoSiGe, and SpiroSi led an evaluation of the stability of the corresponding diene by-products under UV-light. In this regard, solutions of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–C(R)
C(R)–C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (R = Me, iPr, nBu) in dry THF were irradiated with a KrF laser at 248 nm, following the same conditions as employed in the abovementioned photolysis experiments. The colorless solution of the diene-iPr changed rapidly to a light-yellow tone once irradiation began, as observed with OligoSi and SpiroSi. Additionally, traces of a dark-grey precipitate formed on the inner wall of the quartz reactor, albeit in lower quantities than observed with the Ge and Si oligomers (Fig. 9a). In contrast, solutions of diene-Me and diene-nBu in THF showed no visible change after exposure to UV light (Fig. 9b and c).
CH2 (R = Me, iPr, nBu) in dry THF were irradiated with a KrF laser at 248 nm, following the same conditions as employed in the abovementioned photolysis experiments. The colorless solution of the diene-iPr changed rapidly to a light-yellow tone once irradiation began, as observed with OligoSi and SpiroSi. Additionally, traces of a dark-grey precipitate formed on the inner wall of the quartz reactor, albeit in lower quantities than observed with the Ge and Si oligomers (Fig. 9a). In contrast, solutions of diene-Me and diene-nBu in THF showed no visible change after exposure to UV light (Fig. 9b and c).
1H NMR spectroscopic analysis of the THF-d8 solutions of diene-iPr, diene-Me, and diene-nBu after photolysis revealed the formation of some unknown signals, along with unreacted butadienes (Fig. S19–S21†). It is salient to mention here prior work by Traetteberg and co-workers, who observed the rearrangement of the bulky diene 2,3-di-tert-buyl-1,3-butadiene into an unsymmetric cyclobutene upon irradiation with a high-pressure Hg lamp (356 nm) at room temperature;12 note, the authors did not report the formation of insoluble precipitates during their photolysis experiments.
The photolysis of diene-iPr, diene-Me, and diene-nBu alongside the study conducted by Traetteberg show the susceptibility of alkylated-dienes towards UV-light. However, the generation of the amorphous carbon in our abovementioned studies suggests further degradation of any possible diene-rearrangement product(s) had transpired. Of note, our photolysis studies involving the free dienes OligoSi, SpiroSi, and OligoSiGe were conducted at room temperature. However, during irradiation the reaction mixtures undergo significant heating (to ca. 50 °C) due to the intense energy supplied by the laser, requiring a cooling period of five minutes after every 2500 pulses to return to room temperature. In contrast, Traetteberg and co-workers conducted their photolysis studies at room temperature; also, it is possible that their use of a lower wavelength (356 nm) light source might have prevented further degradation of their soluble products. Moreover, Traetteberg12 employed a Hg lamp for their irradiation experiments, while our study used more intense and a higher energy (248 nm) excimer KrF laser. Finally, our previous work involving the photopatterning of elemental Ge from poly(cyclogermapentene)s, employing diene-iPr, diene-nhexyl, and diene-Me as organic moieties, did not show presence of carbon contamination in the Raman spectra of the deposited films.4 One possible explanation for this is the more rapid decomposition of the Ge polymers in this prior work, leading to formation of Ge films that block further UV-light from reaching the dienes formed; thus, preventing the generation of amorphous carbon.
TD-DFT computations on SpiroSi
To gain a better understanding of the UV-vis transition(s) available when SpiroSi is exposed to UV-light, time-dependent density functional theory (TD-DFT) computations at the B3LYP/cc-pVDZ13 level, as well as standard DFT calculations, were performed (Fig. 10). The computed UV-vis spectrum (Fig. S49†) for SpiroSi reveals an intense absorption at 185 nm, driven by an electronic transition between the HOMO (C–C π) and the LUMO (C–C π*). Nonetheless, a minor intensity transition that could potentially induce the reverse cycloaddition of diene from Si is computed at 176 nm, corresponding to the excitation between the HOMO−2 (of Si–C σ-character) and the LUMO (C–C π*) (Table S1†). Unfortunately, the oscillator strength for this transition is only 0.0002, making the process exceedingly inefficient (Table S1†). Thus, the experimentally employed excitation wavelength (248 nm) is likely responsible for the low yield of photoextruded products (diene and insoluble solids) noted.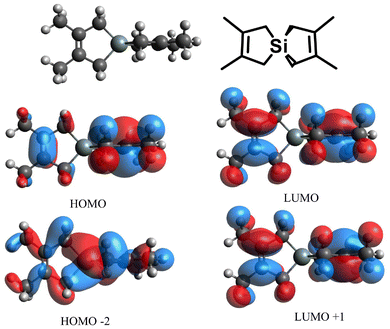 | ||
Fig. 10 Geometry optimization of Si{CH2C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)CH2}2 (SpiroSi) in the gas phase, and the computed frontier molecular orbitals at the B3LYP/cc-pVDZ level of theory. C(Me)CH2}2 (SpiroSi) in the gas phase, and the computed frontier molecular orbitals at the B3LYP/cc-pVDZ level of theory. | ||
The abovementioned computational studies on SpiroSi alongside the experimental data from the photolysis of SpiroSi, OligoSi, and OligoSiGe suggest that some photodeposition of SixGey-type materials could be possible via the reverse cycloaddition of diene. However, the formation of carbon contaminants during irradiation and the extremely low photodeposition reaction rate limit the applicability of this method to date. In this context, computations on SpiroSi, along with previous studies done by Traetteberg, as well as the experimental irradiation assays on diene-Me, diene-nBu, and diene-iPr, offer hints to overcome some of these challenges. First, the temperature at which the photolysis experiments is conducted could be key to preventing the formation of amorphous carbon from diene degradation. Thus, future experiments should employ a cooling bath (ca. 0 °C) during the photolysis. Unfortunately, our current laser set-up does not allow such temperature control. Secondly, a higher-energy/lower wavelength UV-light source14 is likely required to enhance the rate of SixGey-type material deposition. In this regard, there are no suitable organic solvents in the literature with a UV absorption cut-off below 190 nm, indicating that further experiments following our methodology would need to be attempted without solvent (i.e., in the bulk/film state).
Conclusions
The present work introduces an alternative synthesis of cyclosilapentadienes (SiH2-iPr and SpiroSi). Dehydrocoupling oligomerization of SiH2-iPr was possible to give the oligosilane OligoSi; co-oligomerization of SiH2-iPr and GeH2-nBu afforded OligoSiGe. Efforts to photodeposit Si and SixGey from SpiroSi, OligoSi, and OligoSiGe were explored with a 248 nm laser in THF. While evidence for the expected loss of diene was noted (albeit in low yield), the final SixGey-type products were contaminated with amorphous carbon. Future work will involve monomer modification to encourage more efficient photoextusion of diene from Si-containing heterocyclic precursors and extension of this element-extrusion process to other areas of the Periodic Table. Overall, this work represents a new chapter in the use of inorganic polymers as precursors to bulk semiconducting materials under mild, non-CVD, conditions.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. R. thanks NSERC of Canada for Discovery and Accelerator Grants, and M. G. would like to acknowledge CFI JELF for funding. The authors would like to thank the following undergraduates for their experimental contributions to the manuscript: Long Dao and Mauricio Toshio Ito Tohara (synthesis of diene-iPr, diene-nBu, and SiH2-iPr).References
- (a) E. E. Haller, Mater. Sci. Semicond. Process., 2006, 9, 408 CrossRef CAS; (b) Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, ed. N. Farahi, D. Y. N. Truong, H. Kleinke, X. Zhang, X. Zhang and J. Reedijk, Elsevier, 2016 Search PubMed; (c) P. Geng, W. Li, X. Zhang, Y. Deng and H. Kou, J. Phys. D: Appl. Phys., 2017, 50, 40LT02 CrossRef; (d) Handbook of Silicon Based MEMS Materials and Technologies, ed. M. Tilli, M. Paulasto-Krockel, T. Motooka and V. Lindroos, Elsevier, 2020 Search PubMed; (e) A. V. Shah, R. Platz and H. Keppner, Sol. Energy Mater. Sol. Cells, 1995, 38, 501 CrossRef CAS; (f) Semiconductors for Room Temperature Nuclear Detector Applications, ed. A. C. Beer, R. K. Willardson and E. R. Weber, Elsevier, 1995 Search PubMed.
- (a) B. Cook, Energies, 2022, 15, 2957 CrossRef CAS; (b) E. M. T. Fadaly, A. Dijkstra, J. R. Suckert, D. Ziss, M. A. J. van Tilburg, C. Mao, Y. Ren, V. T. van Lange, K. Korzun, S. Kölling, M. A. Verheijen, D. Busse, C. Rödl, J. Furthmüller, F. Bechstedt, J. Stangl, J. J. Finley, S. Botti, J. E. M. Haverkort and E. P. A. M. Bakkers, Nature, 2020, 580, 205 CrossRef CAS PubMed; (c) D. J. Paul, Semicond. Sci. Technol., 2004, 19, 75 CrossRef; (d) M. Arienzo, S. S. Iyer, B. S. Meyerson, G. L. Patton and J. M. C. Stork, Appl. Surf. Sci., 1991, 48, 377 CrossRef; (e) D. L. Harame, S. J. Koester, G. Freeman, P. Cottrel, K. Rim, G. Dehlinger, D. Ahlgren, J. S. Dunn, D. Greenberg, A. Joseph, F. Anderson, J.-S. Rieh, S. A. S. T. Onge, D. Coolbaugh, V. Ramachandran, J. D. Cressler and S. Subbanna, Appl. Surf. Sci., 2004, 224, 9 CrossRef CAS.
- (a) Silicon–Germanium (SiGe) Nanostructures, ed. Y. Shiraiki and N. Usami, Woodhead Publishing, 2011 Search PubMed; (b) M. Gerwig, U. Böhme and M. Friebel, Chem. – Eur. J., 2024, 33, e202400013 Search PubMed; (c) D.-S. Byeon, C. Cho, D. Yoon, Y. Choi, K. Lee, S. Baik and D. H. Ko, Coatings, 2021, 11, 568 CrossRef CAS; (d) A. A. Lovtsus, A. S. Segal, A. P. Sid'ko, R. A. Talalaev, P. Storck and L. Kadinski, J. Cryst. Growth, 2006, 287, 446 CrossRef CAS.
- W. Medroa del Pino, A. A. Forero Pico, M. Gupta and E. Rivard, Chem. Commun., 2023, 59, 6849 Search PubMed.
- H. Xiong and R. D. Rieke, J. Org. Chem., 1989, 54, 3247 CrossRef CAS.
- (a) S. Tasleem, A. Sabah, M. Tahir, A. Sabir, A. Shabbir and M. Nazir, ACS Omega, 2022, 7, 3940 CrossRef CAS PubMed; (b) C. X. Cui and M. Kertesz, Macromolecules, 1992, 25, 1103 CrossRef CAS; (c) P. Vora, S. A. Solin and P. John, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 29, 3423 CrossRef CAS; (d) S. A. Mala, L. Tsybeskov, D. J. Lockwood, X. Wu and J.-M. Baribeau, J. Appl. Phys., 2014, 116, 014305 CrossRef; (e) H. Li, I. S. Butler and J. F. Harrod, Appl. Spectrosc., 1993, 47, 1571 CrossRef CAS.
- (a) B. J. Grimmond and J. Y. Corey, Organometallics, 1999, 18, 2223 CrossRef CAS; (b) F. Fang, Q. Jiang and R. S. Klausen, J. Am. Chem. Soc., 2022, 144, 7834 CrossRef CAS PubMed; (c) H.-G. Woo, J. F. Walzer and T. D. Tilley, J. Am. Chem. Soc., 1992, 114, 7047 CrossRef CAS; (d) U. Rosenthal, Organometallics, 2020, 39, 4403 CrossRef CAS.
- M. Garçon, C. Bakewell, G. A. Sackman, A. J. P. White, R. I. Cooper, A. J. Edwards and M. R. Crimmin, Nature, 2019, 574, 390 CrossRef PubMed.
- A. A. Omaña, R. K. Green, R. Kobayashi, Y. He, E. R. Antoniuk, M. J. Ferguson, Y. Zhou, J. G. C. Veinot, T. Iwamoto, A. Brown and E. Rivard, Angew. Chem., Int. Ed., 2021, 60, 228 CrossRef PubMed.
- B. P. S. Chauhan, T. Shimizu and M. Tanaka, Chem. Lett., 1997, 26, 785 CrossRef.
- S. Katz, A. Pevzner, V. Shepelev, S. Marx, H. Rotter, T. Amitay-Rosen and I. Nir, MRS Adv., 2022, 7, 245 CrossRef CAS.
- H. Hopf, H. Lipka and M. Traetteberg, Angew. Chem., Int. Ed. Engl., 1994, 33, 204 CrossRef.
- (a) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed; (b) T. H. Dunning Jr., J. Chem. Phys., 1989, 90, 1007 CrossRef; (c) A. K. Wilson, D. E. Woon, K. A. Peterson and T. H. Dunning Jr., J. Chem. Phys., 1999, 110, 7667 CrossRef CAS; (d) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; (e) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed; (f) P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623 CrossRef CAS; (g) S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS.
- (a) N. A. Shepelin, Z. P. Tehrani, N. Ohannessian, C. W. Schneider, D. Pergolesi and T. Lippert, Chem. Soc. Rev., 2023, 52, 2294 RSC; (b) K. An, H.-N. Lee, K. H. Cho, S.-W. Lee, D. J. Hwang and K.-T. Kang, Micromachines, 2020, 11, 88 CrossRef PubMed; (c) Z. Zhao, G. Jose, P. Steenson, N. Bamiedakis, R. V. Penty, I. H. White and A. Jha, J. Phys. D: Appl. Phys., 2011, 44, 095501 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental and computational details. See DOI: https://doi.org/10.1039/d4dt03446e |
| This journal is © The Royal Society of Chemistry 2025 |