Open Access Article
Open Access ArticleRecent progress in calcium-catalyzed polyester synthesis†
Jesús
Naranjo
 ,
José A.
Castro-Osma
,
José A.
Castro-Osma
 ,
Felipe
de la Cruz-Martínez
,
Felipe
de la Cruz-Martínez
 * and
Agustín
Lara-Sánchez
* and
Agustín
Lara-Sánchez
 *
*
Universidad de Castilla-La Mancha, Departamento de Química Inorgánica, Orgánica y Bioquímica-Centro de Innovación en Química Avanzada (ORFEO-CINQA), Facultad de Ciencias y Tecnologías Químicas and Instituto Regional de Investigación Científica Aplicada-IRICA, 13071-Ciudad Real, Spain. E-mail: Felipe.Cruz@uclm.es; Agustin.Lara@uclm.es
First published on 4th March 2025
Abstract
The rapid depletion of fossil fuels and, more importantly, the environmental challenges associated with their use, have driven efforts to transition toward biomass-derived platform molecules with the aim of producing more biodegradable and functional materials. In this context, the catalytic ring-opening polymerization (ROP) and ring-opening copolymerization (ROCOP) of cyclic monomers have emerged as effective synthetic routes for the controlled production of polyesters with defined properties. Among the catalysts developed for these processes, those based on calcium are particularly appealing due to the abundance, low cost and biocompatibility of this metal. This Frontier article summarizes recent advancements in the use of calcium-based catalysts for the synthesis of polyesters via ROP and ROCOP of bio-sourced cyclic substrates.
1. Introduction
Over the past few decades, bio-sourced polyesters have attracted significant attention from both industrial and academic sectors as a promising alternative to traditional petroleum-derived plastics.1 Apart from the undeniable advantage of low environmental impact owing to their high biodegradability, some of them, such as polylactide (PLA), have demonstrated considerable potential in applications ranging from biomedical devices to bulk packaging.1,2 Nevertheless, despite the expansion in the production of these polymers, materials derived from fossil fuels continue to dominate manufacturing practices. In fact, only 0.5% of the over 400 million tonnes of plastic produced annually come from bio-based feedstocks.3 This stark contrast highlights the need for further research in bioplastic production to reduce the dependence on fossil fuels, especially in terms of thoroughly investigating feedstocks and optimizing synthesis processes.Among synthetic procedures for bio-derived polyesters, ring-opening polymerization (ROP) of cyclic esters has proven to be the most efficient method for preparing polymeric materials with well-defined properties, specifically controlled molecular weight and narrow polydispersity index (Scheme 1a).4 Many commercially valuable polymers, such as PLA, poly(caprolactone) and poly(ethylene glycol) (PEG), are synthesized through this technique. Recently, the scientific community has increasingly focused on polyester production via ring-opening copolymerization (ROCOP) of cyclic anhydrides and epoxides (Scheme 1b). Unlike the ROP method, this approach offers a versatile platform for synthesizing polymers with varied properties due to the structural diversity of the monomers used and the potential for post-synthetic functionalization. Additionally, the ready availability of naturally occurring substrates (e.g. terpenes or fatty acids) makes this technique ideal for creating custom bio-derived polyesters.5 Furthermore, both ROP and ROCOP processes allow the synthesis of new enantiomerically enriched materials from racemic monomers with improved physical and mechanical properties relative to those of atactic polymers.4e
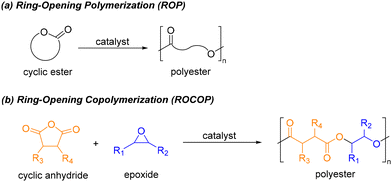 | ||
| Scheme 1 Synthesis of polyester materials via (a) ROP of cyclic esters, (b) ROCOP of cyclic anhydrides and epoxides. | ||
Significant research has focused on the catalytic study of these polymerization processes, particularly on the design of well-defined catalysts that demonstrate high activity and selectivity for polyester production, while allowing precise control over polymer architecture.4–6 In addition to the inherent challenges faced in any catalytic process, such as ensuring sustainability and managing the costs associated with catalyst use, one key issue is the need to direct research towards the development of biocompatible catalysts. Since complete removal of catalyst residues from the polymer is often impractical, it is highly advantageous to use non-toxic or low-toxicity metals for biomedical or food packaging applications.7 Thus, the development of safe and biocompatible catalytic systems has become a priority in the field of polyester synthesis.
In this context, calcium-based catalysts are particularly attractive due to the metal's abundance, low cost and biocompatibility. Moreover, its Lewis-acidity, combined with calcium's large atomic and corresponding decrease in bond strength, contribute to the sharp reactivity of its organometallic complexes. As a result of an improved understanding of organometallic structures and chemistry, significant advances have been produced in the field of calcium-based homogeneous catalysis over recent decades.8,9 For instance, calcium compounds have shown to be very efficient in a variety of hydroelementation processes,8,10 as well as in applications such as hydrogenation reactions,8,11 dehydrocoupling,8c enantioselective and Lewis-acid catalysis,8c cyclic carbonate formation,12 and even alkylation processes through C–H bond activation.13 Finally, in the realm of catalytic polymerization, it is worth highlighting the well-established activity demonstrated by a wide range of calcium compounds in polyolefin synthesis.8 Nonetheless, the growing demand for sustainable alternatives to fossil-derived plastics has increasingly directed research toward synthesizing new biodegradable materials from renewable and readily accessible substrates, particularly through the aforementioned ring-opening polymerization pathways, in which different calcium complexes have shown important catalytic activity.
This Frontier article provides a comprehensive overview of recent advances in the field of calcium-catalyzed polyester synthesis and emphasizes the importance to contribute to this area. In this context, we examine recent developments in the ROP of cyclic esters and ROCOP of cyclic anhydrides and epoxides (Scheme 1), with a particular focus on the catalyst employed. Details of operating conditions, including solvent and temperature, as well as catalytic outcomes such as turnover frequency (TOF) values and molecular weight and polydispersity index of the resulting polymers, are compiled in the ESI in Tables S1 and S2.†
2. ROP of cyclic esters
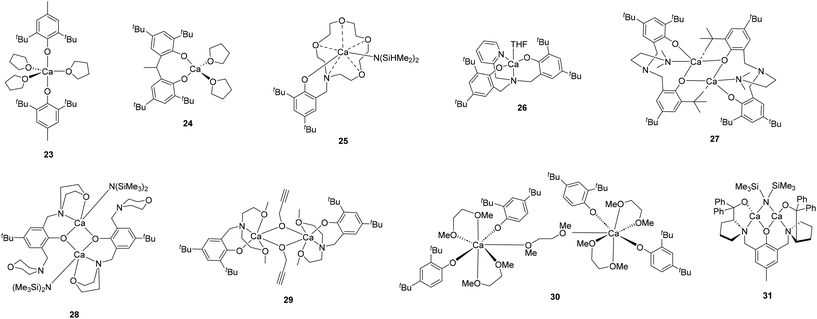
A lot of research has been devoted in the last twenty years on calcium-catalyzed ROP of cyclic esters such as lactide (LA), ε-caprolactone (ε-CL) and other cyclic precursors as trimethylene carbonate (TMC). The earliest documented studies employed simple precursors such as calcium hydride and calcium acetylacetonate to produce LA-based block copolymers.14 Notably, these processes required elevated temperatures and extended reaction times. Furthermore, although reasonable molecular weights were achieved, no data were provided on polydispersity values or on the control over polymerization. Similar results were achieved when employed calcium bis(trimethylsilyl)amide as catalyst precursor for LA and ε-CL polymerization. In this case, improved operating and catalytic conditions were required, including room temperature and shorter reaction times, likely due to the increased solubility of this compound in the reaction medium.15,16 This advancement rapidly prompted the development of new calcium silylamide complexes supported by well-defined ligands, aiming to enhance polymer properties while maintaining the favorable catalytic conditions previously established. Fig. 1–6 provides a summary of calcium catalysts reported for the ROP of cyclic esters. | ||
| Fig. 2 Calcium catalysts based on N,N- and N,O-bidentate ligands described for polyester synthesis via ROP. | ||
The most extensively studied calcium complexes as catalysts for this type of catalytic process are those supported by bi- or multidentate ligands with N and O donor atoms. Notably, the first calcium complexes designed for this purpose featured Schiff base-type multidentate ancillary chelating agents, such as salen or iminophenolate ligands. Metal compounds bearing this type of ligands have been extensively utilized as catalysts for ROP processes due to their ability to stabilize the metal centre and enable controlled polymerization.17 In this context, Darensbourg and co-workers developed a series of salen-based calcium catalysts and studied the influence of different substituents on their catalytic activity for the ROP of TMC. In the presence of tetrabutylammonium azide as a cocatalyst, complex 1, bearing a salen ligand with tert-butyl groups in the 3,5-positions of the phenolate rings and an ethylene backbone for the diamine (Fig. 1), showed to be the most active initiator achieving a TOF value of 1286 h−1 in a melt polymerization performed at 86 °C. More importantly, complex 1 was found to be more active than its corresponding magnesium, zinc and aluminum analogues with a TOF value up to four times higher than that observed for the zinc catalyst.18 A few years later, the same research group reported the synthesis of various tridentate Schiff base calcium derivatives, which demonstrated excellent catalytic activity for the polymerization of TMC or LA. Among them, bis(trimethylsilyl)amide derivative 2 proved to be most active catalyst to produce high molecular weight polyesters with narrow polydispersities. Furthermore, this catalyst was very effective at producing diblock copolymers from both monomers19 and, more recently, it has also been employed in combination with benzyl alcohol for the efficient homopolymerization of polypentadecalactone (PDL) under bulk conditions.20
The calcium compounds developed by Carpentier's group also exhibited higher catalytic efficiency compared to their aluminum analogues. Thus, heteroleptic amido complex 3, featuring a phenoxy-iminophenolate ligand framework (Fig. 1), led to the controlled polymerization of racemic LA at room temperature, whilst aluminum derivative required a higher temperature to achieve comparable catalytic results.21 In contrast, it is worth mentioning at this point that compound 4 (Fig. 1), a complex structurally similar to those mentioned above, was less active than its magnesium counterpart under the same reaction conditions. Despite this, in combination with 2-propanol, this compound proved to be an efficient initiator for the controlled ROP of LA at ambient temperature.22
The other monometallic species described for the polymerization of cyclic esters display an homoleptic structure lacking the amido group as a potential initiator (compounds 5–7, Fig. 1). Consequently, most of these complexes did not exhibit catalytic activity by themselves and required the presence of equimolar quantities of an alcohol as co-initiator to enable the polymerization process. For instance, complexes 5 and 6 synthesized by Lin and co-workers showed to be, in combination with benzyl alcohol, efficient catalysts for the ROP of L-LA, yielding polymers with expected molecular weights and narrow polydispersity indexes.23 In another study, the same group reported the preparation of a series of homoleptic calcium derivatives supported by NNO-tridentade ketiminate ligands. Complex 7 exhibited a good catalytic performance to produce polylactide in high yields but, as expected, it also needed benzyl alcohol to initiate the process.24
Finally, in the field of bimetallic species supported by this type of ligands, it is worth highlighting the calcium compounds developed by Lin and co-workers for the ROP of LA (complexes 8 and 9, Fig. 1). These compounds exhibited great catalytic efficiency in the presence of various alcohols as co-initiators, achieving polylactide materials with high molecular weights and low polydispersity values.24,25
Some calcium complexes based on N,N- or N,O-type bidentate ligands have also been shown to be active for this polymerization process (Fig. 2). For instance, β-diketiminate species 10 proved to be an effective initiator for the ROP of rac-LA, yielding polylactides with expected molecular weights and narrow polydispersities.26 The structurally similar complex 11 exhibited catalytic activity comparable but resulted in polylactide with poorer molecular weight control. Additionally, this compound efficiently catalyzed the production of polycaprolactone at ambient temperature, achieving a TOF value of 8000 h−1.27 More recently, several studies have highlighted the potential of amidinate calcium compounds as catalysts for the ROP of cyclic esters. Cui and co-workers developed a large family of heteroleptic silylamido calcium derivatives and investigated the influence of the amidinate moiety on their catalytic activity in the ROP of rac-LA. Complex 12 demonstrated the highest activity when combined with phenol as a chain transfer agent, producing polylactides with the expected molecular weights.28 In subsequent studies, calcium derivative 13 reported by Trifonov's group exhibited significantly higher catalytic performance than complex 12. Specifically, compound 13, bearing an aminophosphine pendant donor group, catalyzed the quantitative production of high molecular weight polycaprolactone in just a few seconds, achieving an impressive TOF value of 360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1.29
000 h−1.29
On the other hand, Cui's group also reported the use of guanidinate calcium species for the ROP of rac-LA. In combination with phenol as co-initiator, dimeric compound 14 was able to catalyze the controlled production of polylactide at room temperature.28 Calcium derivative 15, bearing the radical anionic bisamidoacenaphthylene moiety, also proved to be moderately active in this catalytic process. Notably, in the absence of any co-catalyst, this complex enabled the formation of polylactide in just fifteen minutes.30 Finally, in the realm of this type of complex, calcium compounds 16–18 featuring pyrrolyl ligands, should also be mentioned (Fig. 2). Despite the absence of initiating groups in their structure, it is noteworthy that species 16 and 17 demonstrated high efficiency for the ROP of ε-CL at ambient temperature and without the need for additives.31,32 Moreover, analysis of the NMR spectrum of low-molecular weight polycaprolactone obtained by catalyst 16 revealed signals corresponding to a terminal iminopyrrolyl group, suggesting that the reaction mechanism of these species involves an initial attack by the pyrrolyl nitrogen atom on the monomer.31 Interestingly, dimeric calcium complex 18 exhibited lower catalytic performance than the latter species, despite containing bis(trimethylsilyl)amide as an initiator in its structure.32
Some research groups have also focused on designing calcium complexes supported by N,O-bidentate ligands for use in the ROP of cyclic esters.33,34 Thus, for instance, complex 19 demonstrated catalytic activity for both the ROP of rac-LA and ε-CL at room temperature. This catalyst led to the formation of high molecular weight polyesters but resulted in broadened polydispersity values (Đ = 2.06–2.34). The addition of small amounts of isopropanol substantially improved these values, albeit at the cost of significantly reducing the molecular weight of the obtained polymers.33 Trimeric calcium compound 20, which contains isopropoxide ligands as initiating groups, also exhibited moderate activity in the ROP of the same cyclic esters. In this case, the catalyst provided better control over the polymerization, as it resulted in lower molecular weights but also achieved lower polydispersity values.34a Finally, it should be mentioned calcium derivatives 21 and 22 reported by Lukoyanov and co-workers. Despite exhibiting activity both in solution and under melt conditions, these compounds proved to be considerably less active than the previously mentioned catalysts in the ROP of L-LA. This performance could be improved by increasing the reaction temperature from 20 to 180 °C; however, this enhancement came at the cost of a decrease in molecular weight, which the authors attribute to the lower thermal stability of these compounds. In this case, polymerization is initiated by a formation of a radical cycloadduct between the calcium derivatives and a L-LA molecule.34b
Fig. 3 summarizes calcium catalysts based on phenolate groups that have not been included in the previous categories. Compound 23, with 2,6-di-tert-butyl-4-methylphenol as the ligand, effectively catalyzed the bulk polymerization of L-LA at room temperature in the presence of benzyl alcohol as co-initiator. Notably, its catalytic activity was higher than that of the magnesium analogue but remained lower than that of the sodium and lithium alkali metal derivatives. Moreover, this complex was also more efficient than calcium species 24 featuring 2,2′-ethylidenebis(4,6-di-tert-butylphenol) as the chelating ligand, with its superiority being particularly pronounced in THF solution.35 Carpentier's group also investigated the catalytic efficiency of various heteroleptic silylamido and alkoxide calcium derivatives supported by amino ether phenolate ligands in the polymerization of L-LA. The authors observed that the enhanced electron-donating ability of the ligands favored increased catalytic activity. Complex 25, containing a crown ether side arm, proved to be the most effective catalyst to produce polylactide with a TOF value of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 h−1. In addition, the influence of the alkaline earth metal centre on the activity was also evaluated, revealing an increase upon substituting calcium by strontium and barium, i.e., with the size and electropositive character of the metal.36 More recently, the calcium amino-bis(phenolato) complex 26 described by Kozak and co-workers has also demonstrated high efficiency for rac-LA polymerization under solvent free conditions. The end group analysis of the polymers revealed, in this case, the presence of cyclic polylactide in the low-mass region. Additionally, a comparative study varying the metal centre showed, as mentioned earlier, higher activity for alkaline analogues (sodium, lithium and potassium).37
100 h−1. In addition, the influence of the alkaline earth metal centre on the activity was also evaluated, revealing an increase upon substituting calcium by strontium and barium, i.e., with the size and electropositive character of the metal.36 More recently, the calcium amino-bis(phenolato) complex 26 described by Kozak and co-workers has also demonstrated high efficiency for rac-LA polymerization under solvent free conditions. The end group analysis of the polymers revealed, in this case, the presence of cyclic polylactide in the low-mass region. Additionally, a comparative study varying the metal centre showed, as mentioned earlier, higher activity for alkaline analogues (sodium, lithium and potassium).37
Dimeric species based on phenolate moieties 27–31 (Fig. 3) have also proved to be active for the ROP of cyclic esters. Thus, for instance, calcium amino-bis(phenolate) complex 27 described by Bochmann and co-workers displayed good catalytic performance in the ROP of ε-CL even in the absence of an external initiator, achieving high molecular weight polymers with low polydispersities. Interestingly, this compound showed a higher activity than the magnesium and zinc derivatives supported by identical ligands, which the authors attribute to a decrease in the Lewis acidity of the metal centre.38 On the other hand, dinuclear compound 28 described by Carpentier's group achieved a TOF value of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 h−1, enabling the quantitative production of polylactide in just one minute. However, and in contrast to complex 27, this catalyst required high quantities of isopropanol as co-initiator to achieve this performance.39 Additionally, the same group reported the synthesis of the alkoxide dinuclear species 29, which catalyzed polylactide production at room temperature without the need for any co-catalyst.36 In contrast, dinuclear complex 30, in which the calcium centres are bridged by an 1,2-dimethoxyethane moiety, demonstrated poor efficacy on its own and required the addition of benzyl alcohol or benzylamine to give controlled polymerizations.40 Finally, it should be mentioned bis(trimethylsilyl)amide compound 31 recently reported by Garden and co-workers (Fig. 3). This complex proved to be more efficient than its corresponding zinc and magnesium homobimetallic counterparts for the ROP of rac-LA. More importantly, the incorporation of zinc in the Zn/Ca heterobimetallic species notably enhanced the catalytic activity. This enhancement is attributed to the calcium Lewis acidic, which favored the monomer coordination and increase the nucleophilicity of the bonds at the zinc metal centre.41
200 h−1, enabling the quantitative production of polylactide in just one minute. However, and in contrast to complex 27, this catalyst required high quantities of isopropanol as co-initiator to achieve this performance.39 Additionally, the same group reported the synthesis of the alkoxide dinuclear species 29, which catalyzed polylactide production at room temperature without the need for any co-catalyst.36 In contrast, dinuclear complex 30, in which the calcium centres are bridged by an 1,2-dimethoxyethane moiety, demonstrated poor efficacy on its own and required the addition of benzyl alcohol or benzylamine to give controlled polymerizations.40 Finally, it should be mentioned bis(trimethylsilyl)amide compound 31 recently reported by Garden and co-workers (Fig. 3). This complex proved to be more efficient than its corresponding zinc and magnesium homobimetallic counterparts for the ROP of rac-LA. More importantly, the incorporation of zinc in the Zn/Ca heterobimetallic species notably enhanced the catalytic activity. This enhancement is attributed to the calcium Lewis acidic, which favored the monomer coordination and increase the nucleophilicity of the bonds at the zinc metal centre.41
Another type of complex that has proved to be active in ROP processes are those supported by phosphorus-containing ligands, such as imino-phosphine and PPP-pincer-type ligands (Fig. 4). Compound 32, for instance, demonstrated relatively good control in the ROP of ε-CL and L-LA at room temperature, achieving polyesters with expected molecular weights and moderate dispersity data.42 Enhanced catalytic performances and, in some cases, good iso-selectivities were observed for the calcium complexes described by Panda and co-workers (compounds 33–37, Fig. 4). Phosphinoselenoic amide complex 33 catalyzed the quantitative formation of polycaprolactone at room temperature in just fifteen minutes. Nevertheless, in line with observations from other research groups when comparing alkaline earth metal catalysts, this compound was less active than its strontium and barium analogues.43 Structurally similar species 34 and 35 also exhibited this trend for the ROP of rac-LA, showing lower activities than their corresponding strontium and barium derivatives. Interestingly, the increase in the size of the chalcogen atom in complex 35, from sulfur to selenium, resulted in enhanced catalytic activity. It is also noteworthy that both catalysts proved to be highly iso-selective initiators for the ROP of rac-LA, achieving Pi values of 0.87 and 0.81 for the sulfide and selenide complexes, respectively.44 This iso-selectivity could be further enhanced by compound 36, in which the calcium centre is supported by a 2-picolylamino-diphenylphosphane selenide ligand. Remarkably, a Pi value of 0.89 was achieved enabling the production of high molecular weight polylactide in just one hour at ambient temperature.45 In a more recent study, this group reported the synthesis of calcium compound 37 featuring a bis-aminophosphine borane ligand (Fig. 4). This complex showed high catalytic performance and effective selectivity control in the ROP of rac-LA, producing polylactide with molecular weights of up to 128.7 kDa.46
Finally, calcium complex 38, supported by a PPP-type pincer ligand, deserves special mention. This complex was able to catalyze the rapid production of high molecular weight polycaprolactone in just 30 seconds without any additives, achieving a TOF value of nearly 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The high polydispersity value obtained could be mitigated by the addition of isopropanol to the reaction mixture, but this came at the cost of a considerable reduction in the molecular weight of the polymer. Compound 38 also proved to be active for the ROP of L-, D- and rac-LA under mild reaction conditions, producing polyesters with expected molecular weights and narrow polydispersity values. However, while L- and D-LA were polymerized almost quantitatively, rac-LA exhibited lower reactivity, yielding only 24% yield of atactic polylactide. On the other hand, a comparative study between complex 38 and its magnesium and zinc analogues revealed similar activity between the earth-alkaline compounds, but a different behavior compared to the zinc species. Specifically, compound 38 exhibited higher activity for the ROP of ε-CL but lower activity for polylactide production. According to the authors, the higher stability of the calcium adduct compared to the zinc analogue could explain the difference in reactivity in the latter case.47
000 h−1. The high polydispersity value obtained could be mitigated by the addition of isopropanol to the reaction mixture, but this came at the cost of a considerable reduction in the molecular weight of the polymer. Compound 38 also proved to be active for the ROP of L-, D- and rac-LA under mild reaction conditions, producing polyesters with expected molecular weights and narrow polydispersity values. However, while L- and D-LA were polymerized almost quantitatively, rac-LA exhibited lower reactivity, yielding only 24% yield of atactic polylactide. On the other hand, a comparative study between complex 38 and its magnesium and zinc analogues revealed similar activity between the earth-alkaline compounds, but a different behavior compared to the zinc species. Specifically, compound 38 exhibited higher activity for the ROP of ε-CL but lower activity for polylactide production. According to the authors, the higher stability of the calcium adduct compared to the zinc analogue could explain the difference in reactivity in the latter case.47
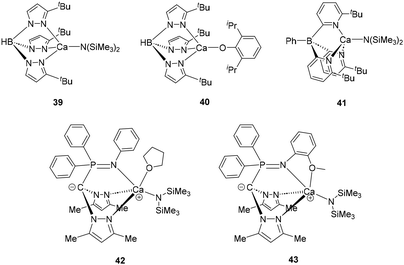
Although a significant number of scorpionate-based catalysts have been described for ROP processes,48 calcium complexes within category remain notably scarce (Fig. 5). Nonetheless, most of these complexes have demonstrated high catalytic activity. For instance, the tris-pyrazolyl borate calcium species 39 and 40 reported by Chisholm's group exhibited high reactivity and remarkable stereoselectivity in polylactide production. Additionally, the authors observed that the incorporation of bulky substituents in the ligand is essential to confer single-site living polymerization behaviour and to enhance stereoselectivity during the ring-opening process.49 On the other hand, compound 41, in which the boron atom of the ligand is bonded to a phenyl ring, proved to be considerably less active than its homologous 39 for the ROP of L-LA, even in the presence of benzyl alcohol as an co-initiator.50 Finally, in this group of catalysts, it should be mentioned zwitterionic calcium complexes 42 and 43 developed by Cui and colleagues. These compounds, featuring heteroscorpionate iminophosphine ligands, catalyzed the ROP of rac-LA in a controlled manner to give atactic polylactide at room temperature. Interestingly, at low temperature, complex 42 yielded a heterotactic sequence enriched polylactide, while derivative 43 produced a polymer enriched with isotactic sequences. In the latter case, the coordination of the side arm seems to be the key factor determining the iso-selectivity observed. As the temperature decreases, the swing rate of this arm slows down, allowing the complex to remain in a specific state long enough to enable the continuous insertion of monomers with the same chirality. Notably, catalyst 43 produced a polylactide molecular weight of 181.5 kDa, the highest reported to date for a calcium-based complex.51
Finally, to conclude the section on the ROP of cyclic esters, it is worth highlighting the use of compounds 44–46, which have not been included in the previous sections (Fig. 6). For instance, Okuda and co-workers investigated the catalytic performance in the ROP of meso-LA of a series of calcium derivatives featuring a chiral tetradentate polyether ligand. Among them, bis(trimethylsilyl)amide complex 44 exhibited the highest syndiotacticity (Ps = 0.73). However, the observed stereoselectivity does not appear to be a consequence of the chiral backbone in the ligand, as the use of an achiral triglyme calcium complex led to similar results. Compound 44 was also used to catalyze the polymerization of rac- and L-LA, achieving full conversion in less than one hour at room temperature.52 In the field of organometallic species, compounds 45 and 46 supported by pentadienyl ligands also exhibited a good performance (Fig. 6). The borohydride derivative 45 efficiently catalyzed the ROP of ε-CL and L-LA and, more importantly, provided relatively good control over the polymerization under the operating conditions, namely ambient temperature and absence of a co-catalyst.42 Compound 46, recognized as the first enantiomerically pure open metallocene of calcium to be isolated, was also an active catalyst for the ROP of rac-LA. In the absence of additives, this complex produced heterotactically enriched polylactides with narrow polydispersities. Interestingly, the analogous strontium metallocene showed a similar activity, indicating that the ionic radii had no significant influence on the rate of the polymerization process.53
3. ROCOP of epoxides and cyclic anhydrides
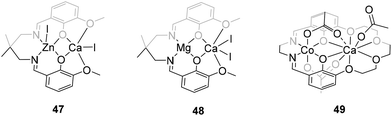
Although ROCOP processes of epoxides and cyclic anhydrides have been extensively studied with a variety of first-row transition and main group metal catalysts,5 the use of calcium species for this transformation remains almost unexplored. Until very recently, the first successful uses of this metal in ROCOP processes had not been reported. In 2021, Williams’ group developed a series of heterobimetallic complexes supported by Schiff base ligands and incorporating metals from Groups 1, 2, and 12. These complexes were tested as catalysts for polyester synthesis via copolymerization of phthalic anhydride (PA) and cyclohexene oxide (CHO). Calcium iodide species 47 and 48, featuring Zn(II)/Ca(II) and Mg(II)/Ca(II) combinations, respectively (Fig. 7), exhibited good catalytic activity and excellent selectivity for polyester bond formation without the need for additives and under relatively mild reaction conditions. Moreover, polymerization reactions displayed precise control, yielding polyesters with molecular weights closely matching the theoretical values and displaying low polydispersity. Nonetheless, these compounds were less active than the corresponding Zn(II)/Zn(II) homodinuclear species, demonstrating that the catalytic synergy is not solely determined by the nature of the metal centres.54Building on this, the same group developed a series of heterobimetallic complexes featuring a macrocyclic Schiff base ligand, Co(II) and an s-block element as metal centre, along with bridging acetate ligands. These complexes were conveniently designed to explore the influence of different metal centres on their catalytic performance in various polymerization processes. Among them, compound 49, containing the Co(II)/Ca(II) combination (Fig. 7), proved to be active for the ROCOP of propylene oxide (PO) and PA, allowing the highly selective and controlled production of polyesters with narrow polydispersity values. Interestingly, analysis of the data and trends for the catalysts revealed an increase in activity as the Lewis acidity decreased. Thus, complex 49 exhibited lower catalytic performance compared to systems based on alkali metals such as sodium and potassium. According to the authors, this data can be explained by the fact that the least Lewis acidic s-block metals possess slightly destabilized, and therefore more reactive, carboxylate intermediates, compared to those with more Lewis acidic metals.55
More recently, our research group has reported the first exclusively calcium-based complexes for ROCOP processes. Compound 50, featuring diethylamino groups on the heteroscorpionate ligand, showed to be the most active catalyst (Fig. 8a). This complex exhibited good performance and excellent selectivity towards polyester formation during the copolymerization of CHO and PA under mild reaction conditions and without the need for a cocatalyst. Notably, the use of THF as a solvent significantly improved the catalytic activity even at reduced temperatures. This enhancement is attributed to the fragmentation of the dimeric species in this coordinating solvent, leading to the formation of the catalytically active mononuclear species responsible for the process. Compound 50 was further investigated as catalyst for the copolymerization of a variety of bio-sourced epoxides and cyclic anhydrides (Fig. 8b), resulting in the highly selective production of renewable polyester materials with narrow molecular weight distributions. Some of these polymers, reported for the first time in this study, also possess potential for functionalization, enabling access to tailor-made materials with specific properties and applications.56
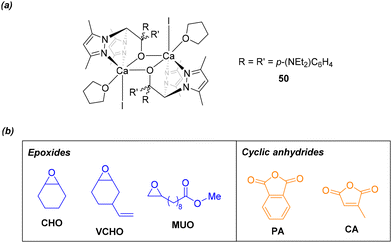 | ||
| Fig. 8 (a) Heteroscorpionate calcium-based catalyst for the ROCOP of epoxides and cyclic anhydrides, (b) substrates utilized for the ROCOP reactions in this study. | ||
4. Conclusions and outlook
As is widely known, climatic challenges have driven the scientific community to transition from a linear to a circular economy by using reusable materials and sustainable synthetic procedures. In this context, the development of efficient catalytic systems based on abundant and low-cost metals is essential. This Frontier article reviews the recent advancements in the calcium-based catalysts for polyester production via ROP and ROCOP of bio-derived cyclic monomers. Moreover, this work highlights not only the efficacy of the catalysts in terms of activity and selectivity but also their ability to control the polymerization process.As deduced from the discussion above, significant advances have been made in the last twenty years in the field of calcium-catalyzed polyester synthesis via ROP of cyclic esters. A wide variety of calcium compounds featuring different ligands as supports, have proven to be active in these processes, achieving in some cases TOF values comparable to those of other metal-based catalysts, namely zinc and aluminum. In particular, calcium catalysts containing N,N- and N,O-bidentate ligands have exhibited remarkable catalytic activity in the ROP of ε-CL, while complexes supported by multidentate ligands with phenolate groups have been particularly active in the polymerization of LA. Many of these catalysts also demonstrated their ability to control the polymerization reaction, as evidenced by the reported molecular weight and polydispersity values. These findings are consistent with results obtained for other metal complexes featuring similar ligands, which, as previously noted, contribute to stabilize the metal centre and enable controlled polymerization. Additionally, most of the active calcium compounds share the presence of a silylamide group in their structure, playing a crucial role in facilitating the initiation of the process. However, in most cases, the use of an alcohol as co-initiator is typically required to achieve better control over the polymerization. This underscores the need for further research into the design of more active calcium species that avoid the use of co-initiators while still ensuring proper control over the polymerization process. Beyond improving activity and control, another key challenge that needs to be addressed is achieving stereocontrol, particularly in the polymerization of rac-LA. The reported data indicate that very few calcium catalysts achieve a high level of iso-selectivity, which is essential for producing polymers with desired stereochemical properties. In this sense, it should be noted that high Pi values have been achieved using calcium catalysts supported by iminophosphine-type ligands.
In contrast, the use of calcium in the ROCOP processes of epoxides and cyclic anhydrides is still in its early stages and there are challenges ahead. The limited reports described to date confirm the great potential of this metal for such catalytic process, but also the need to further investigate the design of active species that can effectively control this polymerization process. Note that ROCOP approach enables to the preparation of a variety of structurally versatile polymeric materials due to the wide range of available monomers and the possibility for post-polymerization modifications and, therefore, the search for efficient catalysts in this process is crucial for the development of tailor-made polymers.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.††Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the Ministerio de Ciencia, Innovación y Universidades/Agencia Estatal de Investigación, Spain and Fondo Europeo de Desarrollo Regional (FEDER), UE MICIU/AEI/10.13039/501100011033 (Grants PID2020-117788RB-I00, RED2022-134287-T, PID2023-147240OB-I00), Junta de Comunidades de Castilla-La Mancha through FEDER (Grants SBPLY/21/180501/000132, SBPLY/23/180225/000094) and Universidad de Castilla-La Mancha (Grant 2021-GRIN-31240).References
- (a) Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed; (b) R. Chinthapalli, P. Skoczinski, M. Carus, W. Baltus, D. de Guzman, H. Käb, A. Raschka and J. Ravenstijn, Ind. Biotechnol., 2019, 15, 23–241 Search PubMed; (c) J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed; (d) V. Sessini, S. Ghosh and M. E. G. Mosquera, in Biopolymers: Synthesis, Properties, and Emerging Applications, ed. V. Sessini, S. Ghosh and M. E. G. Mosquera, Elsevier, Amsterdam, 2023; pp. 1–17 Search PubMed.
- (a) T. A. Swetha, A. Bora, K. Mohanrasu, P. Balaji, R. Raja, K. Ponnuchamy, G. Muthusamy and A. Arun, Int. J. Biol. Macromol., 2023, 234, 123715 CrossRef CAS PubMed; (b) H. Ramezani Dana and F. Ebrahimi, Polym. Eng. Sci., 2023, 63, 22–43 Search PubMed; (c) N. G. Khouri, J. O. Bahú, C. Blanco-Llamero, P. Severino, V. O. C. Concha and E. B. Souto, J. Mol. Struct., 2024, 1309, 138243 CrossRef CAS.
- European Bioplastics (2023): Bioplastics market development update 2023, https://www.european-bioplastics.org/bioplastics-market-development-update-2023-2/ (accessed November 2024).
- (a) C. Jérôme and P. Lecomte, Adv. Drug Delivery Rev., 2008, 60, 1056–1076 CrossRef; (b) A. Duda and A. Kowalski, in Handbook of Ring Opening polymerisation, ed. P. Dubois, O. Coulembier and J.-M. Raquez, Wiley-VCH Verlag, Weinheim, 2009; pp. 1–51 Search PubMed; (c) P. Lecomte and C. Jérôme, in Synthetic Biodegradable Polymers, ed. N. Rieger, A. Künkel, G. W. Coates, R. Reichardt, E. Dinjus and A. T. Zevaco, Springer-Verlag, Berlin and Heidelberg, 2012, pp. 173–217 Search PubMed; (d) A. Tardy, J. Nicolas, D. Gigmes, C. Lefay and Y. Guillaneuf, Chem. Rev., 2017, 117, 1319–1406 CrossRef CAS PubMed; (e) X. Xie, Z. Huo, E. Jang and R. Tong, Commun. Chem., 2023, 6, 202 CrossRef CAS PubMed.
- (a) S. Paul, Y. Zhu, C. Romain, R. Brooks, P. Saini and C. K. Williams, Chem. Commun., 2015, 51, 6459–6479 RSC; (b) J. M. Longo, M. J. Sanford and G. W. Coates, Chem. Rev., 2016, 116, 15167–15197 CrossRef CAS; (c) F. de la Cruz-Martínez, M. Martínez de Sarasa Buchaca, J. A. Castro-Osma and A. Lara-Sánchez, in Biopolymers: Synthesis, Properties, and Emerging Applications, ed. V. Sessini, S. Ghosh and M. E. G. Mosquera, Elsevier, Amsterdam, 2023, pp. 347–383 Search PubMed.
- (a) J. C. Wu, T. L. Yu, C. T. Chen and C. C. Lin, Coord. Chem. Rev., 2006, 250, 602–626 CrossRef CAS; (b) A. P. Dove, ACS Macro Lett., 2012, 1, 1409–1412 CrossRef CAS; (c) S. Pappuru and D. Chakraborty, Eur. Polym. J., 2019, 212, 109276 CrossRef.
- (a) W. N. Ottou, H. Sardon, D. Mecerreyes, J. Vignolle and D. Taton, Prog. Polym. Sci., 2016, 56, 64–115 CrossRef CAS; (b) G. X. De Hoe, T. Şucu and M. P. Shaver, Acc. Chem. Res., 2022, 55, 1514–1523 CrossRef CAS PubMed.
- (a) S. Harder, Chem. Rev., 2010, 110, 3852–3876 CrossRef CAS PubMed; (b) S. Harder, Alkaline-Earth Metal compounds: oddities and applications, Springer, Berlin, 2013 CrossRef; (c) S. Harder, Early Main Group Metal catalysis: Concepts and Reactions, Wiley-VCH Verlag, Weinheim, 2020 CrossRef.
- D. Mukherjee, in Comprehensive Coordination Chemistry III, ed. E. C. Constable, G. Parkin and L. Que Jr, Elsevier, Amsterdam, 2021, pp. 106–138 Search PubMed.
- M. Magre, M. Szewczyk and M. Rueping, Chem. Rev., 2022, 122, 8261–8312 CrossRef CAS.
- (a) X. Shi, C. Hou, L. Zhao, P. Deng and J. Cheng, Chem. Commun., 2020, 56, 5162–5165 Search PubMed; (b) Z.-W. Qu, H. Zhu and S. Grimme, Chem. – Eur. J., 2023, 29, e202202602 Search PubMed; (c) Y. Liang, I. Efremenko, Y. Diskin-Posner, L. Avram and D. Milstein, Angew. Chem., Int. Ed., 2024, 63, e202401702 CrossRef CAS.
- (a) L. Longwitz, J. Steinbauer, A. Spannenberg and T. Werner, ACS Catal., 2018, 8, 665–672 CrossRef CAS; (b) Y. Hu, J. Steinbauer, V. Stefanow, A. Spannenberg and T. Werner, ACS Sustainable Chem. Eng., 2019, 7, 13257–13269 CrossRef CAS; (c) C. Terazzi, K. Laatz, J. von Langermann and T. Werner, ACS Sustainable Chem. Eng., 2022, 10, 13335–13342 Search PubMed.
- (a) S. Brand, H. Elsen, J. Langer, S. Grams and S. Harder, Angew. Chem., Int. Ed., 2019, 58, 15496–15503 Search PubMed; (b) X. Zheng, I. del Rosal, X. Xu, Y. Yao, L. Maron and X. Xu, Inorg. Chem., 2021, 60, 5114–5121 Search PubMed.
- (a) S. M. Li, I. Rashkov, J. L. Espartero, N. Manolova and M. Vert, Macromolecules, 1996, 29, 57–62 CrossRef CAS; (b) P. Dobrzynski, J. Kasperczyk and M. Bero, Macromolecules, 1999, 32, 4735–4737 CrossRef CAS.
- Z. Zhong, P. J. Dijkstra, C. Birg, M. Westerhausen and J. Feijen, Macromolecules, 2001, 34, 3863–3868 CrossRef CAS.
- M. P. Coles, Coord. Chem. Rev., 2015, 297, 24–39 Search PubMed.
- O. Santoro, X. Zhang and C. Redshaw, Catalysts, 2020, 10, 800 CrossRef CAS.
- D. J. Darensbourg, W. Choi, P. Ganguly and C. P. Richers, Macromolecules, 2006, 39, 4374–4379 CrossRef CAS.
- D. J. Darensbourg, W. Choi, O. Karroonnirun and N. Bhuvanesh, Macromolecules, 2008, 41, 3493–3502 Search PubMed.
- M. Bouyahyi and R. Duchateau, Macromolecules, 2014, 47, 517–524 Search PubMed.
- M. Bouyhayi, Y. Sarazin, O. L. Casagrande Jr and J.-F. Carpentier, Appl. Organomet. Chem., 2012, 26, 681–688 CrossRef.
- W. Yi and H. Ma, Inorg. Chem., 2013, 52, 11821–11835 CrossRef CAS PubMed.
- M.-W. Hsiao, G.-S. Wu, B.-H. Huang and C.-C. Lin, Inorg. Chem. Commun., 2013, 36, 90–95 CrossRef CAS.
- M.-W. Hsiao and C.-C. Lin, Dalton Trans., 2013, 42, 2041–2051 RSC.
- H.-Y. Chen, H.-Y. Tang and C.-C. Lin, Polymer, 2007, 48, 2257–2262 CrossRef CAS.
- X. Xu, Y. Chen, G. Zou, Z. Ma and G. Li, J. Organomet. Chem., 2010, 695, 1155–1162 CrossRef CAS.
- R. A. Collins, J. Unruangsri and P. Mountford, Dalton Trans., 2013, 42, 759–769 RSC.
- N. Liu, B. Liu and D. Cui, Dalton Trans., 2018, 47, 12623–12632 RSC.
- A. O. Tolpygin, A. V. Cherkasov, G. K. Fukin, T. A. Kovylina, K. A. Lyssenko and A. A. Trifonov, Eur. J. Inorg. Chem., 2019, 4289–4296 CrossRef CAS.
- A. G. Morozov, M. V. Moskalev, D. A. Razborov and I. L. Fedushkin, J. Organomet. Chem., 2020, 927, 121535 CrossRef CAS.
- R. K. Kottalanka, A. Harinath, S. Rej and T. K. Panda, Dalton Trans., 2015, 44, 19865–19879 RSC.
- S.-M. Ho, C.-S. Hsiao, A. Datta, C.-H. Hung, L.-C. Chang, T.-Y. Lee and J.-H. Huang, Inorg. Chem., 2009, 48, 8004–8011 CrossRef CAS.
- N. Y. Rad'kova, T. A. Kovylina, A. S. Shavyrin, A. V. Cherkasov, G. K. Fukin, K. A. Lyssenko and A. A. Trifonov, New J. Chem., 2020, 44, 7811–7822 RSC.
- (a) S.-M. Ho, C.-S. Hsiao, A. Datta, C.-H. Hung, L.-C. Chang, T.-Y. Lee and J.-H. Huang, Inorg. Chem., 2009, 48, 8004–8011 CrossRef CAS PubMed; (b) A. N. Lukoyanov, Y. V. Zvereva, D. A. Parshina, A. V. Cherkasov and S. Y. Ketkov, Eur. J. Inorg. Chem., 2022, e202200348 CrossRef CAS.
- H.-Y. Chen, L. Mialon, K. A. Abboud and S. A. Miller, Organometallics, 2012, 31, 5252–5261 CrossRef CAS.
- B. Liu, T. Rosinel, J.-P. Guégan, J.-F. Carpentier and Y. Sarazin, Chem. – Eur. J., 2012, 18, 6289–6301 CrossRef CAS.
- K. Devaine-Pressing, F. J. Oldenburg, J. P. Menzel, M. Springer, L. N. Dawe and C. M. Kozak, Dalton Trans., 2020, 49, 1531–1544 RSC.
- Y. Sarazin, R. H. Howard, D. L. Hughes, S. M. Humphrey and M. Bochmann, Dalton Trans., 2006, 340–350 RSC.
- V. Poirier, T. Roisnel, J.-F. Carpentier and Y. Sarazin, Dalton Trans., 2009, 9820–9827 RSC.
- L. Clark, G. B. Deacon, C. M. Forsyth, P. C. Junk, P. Mountford, J. P. Townley and J. Wang, Dalton Trans., 2013, 42, 9294–9312 RSC.
- W. Gruszka, H. Sha, A. Buchard and J. A. Garden, Catal. Sci. Technol., 2022, 12, 1070–1079 RSC.
- M. Kuzdrowska, L. Annunziata, S. Marks, M. Schmid, C. G. Jaffredo, P. W. Roesky, S. M. Guillaume and L. Maron, Dalton Trans., 2013, 42, 9352–9360 RSC.
- R. K. Kottalanka, H. Adimulam, J. Bhattacharjee, H. V. Babu and T. K. Panda, Dalton Trans., 2014, 43, 8757–8766 RSC.
- J. Bhattacharjee, A. Harinath, H. P. Nayek, A. Sarkar and T. K. Panda, Chem. – Eur. J., 2017, 23, 9319–9331 CrossRef CAS PubMed.
- A. Harinath, J. Bhattacharjee, A. Sarkar, H. P. Nayek and T. K. Panda, Inorg. Chem., 2018, 57, 2503–2516 CrossRef CAS PubMed.
- J. Bhattacharjee, A. Harinath, A. Sarkar and T. K. Panda, Chem. – Asian J., 2020, 15, 860–866 Search PubMed.
- M. Lamberti, A. Botta and M. Mazzeo, Appl. Organomet. Chem., 2014, 28, 140–145 CrossRef CAS.
- C. Pettinari, R. Pettinari and F. Marchetti, Adv. Organomet. Chem., 2016, 65, 175–260 CrossRef CAS.
- M. H. Chisholm, J. C. Gallucci and K. Phomphrai, Inorg. Chem., 2004, 43, 6717–6725 CrossRef CAS PubMed.
- J. Qian and R. J. Comito, Inorg. Chem., 2022, 61, 10852–10862 CrossRef CAS PubMed.
- N. Liu, D. Liu, B. Liu, H. Zhang and D. Cui, Polym. Chem., 2021, 12, 1518–1525 RSC.
- J. P. Davin, J.-C. Buffet, T. P. Spaniol and J. Okuda, Dalton Trans., 2012, 41, 12612–12618 RSC.
- A. C. Fecker, M. Freytag, P. G. Jones, N. Zhao, G. Zi and M. D. Walter, Dalton Trans., 2015, 44, 16325–16331 RSC.
- A. C. Deacy, C. B. Durr, R. W. F. Kerr and C. K. Williams, Catal. Sci. Technol., 2021, 11, 3109–3118 RSC.
- F. Fiorentini, W. T. Diment, A. C. Deacy, R. W. F. Kerr, S. Faulkner and C. K. Williams, Nat. Commun., 2023, 14, 4783 CrossRef CAS PubMed.
- E. Francés-Poveda, M. Martínez de Sarasa Buchaca, C. Moya-López, I. J. Vitorica-Yrezabal, I. López-Solera, J. A. Castro-Osma, F. de la Cruz-Martínez and A. Lara-Sánchez, Polym. Chem., 2024, 15, 3238–3245 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Catalytic results of calcium catalysts 1–43 are compiled in Tables S1 and S2. See DOI: https://doi.org/10.1039/d4dt03469d |
| This journal is © The Royal Society of Chemistry 2025 |