Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, characterization, and thermal properties of volatile 1,4-dialkyl-5-silatetrazolines: nitrogen-rich silicon heterocycles as possible CVD precursors for silicon nitride†
Vincent J. Flores and
Gregory S. Girolami
and
Gregory S. Girolami *
*
School of Chemical Sciences, University of Illinois at Urbana-Champaign, 600 S. Mathews Ave., Urbana, IL 61801, USA. E-mail: ggirolam@illinois.edu
First published on 12th March 2025
Abstract
We describe the synthesis, thermal properties, and volatilities of three 1,4-dialkyl-5-silatetrazolines of the form (L)Si(N4R2), where R = Et (2), iPr (3), or tBu (4) and L is the 1,4-diazabutenediyl chelate tBuNCHCHNtBu. The compounds were characterized by NMR and IR spectroscopy and X-ray crystallography. Volatility studies show that compound 2, the 1,4-diethyl-5-silatetrazoline compound, is the most volatile of the three new compounds, exhibiting a 1 Torr vapor pressure at 95 °C and subliming at 65 °C and 5 mTorr. These compounds are of interest as possible low temperature CVD precursors for the growth of silicon nitride (SiNx) thin films.
Introduction
Silicon nitride (SiNx) thin films have multiple applications in the manufacturing of microelectronic devices, ranging from gate dielectrics1–3 to encapsulation layers and diffusion barriers.4,5 These applications impose strict requirements on the growth process and the film quality, including good dielectric properties, high physical density, and low etch rate in hydrofluoric acid.2–5 In back-end processes, SiNx films must be deposited at temperatures below 400 °C to avoid device degradation caused by interfacial reactions, and the films must be grown conformally, which has become more difficult as the sizes of device features decrease and the aspect ratios of vias and trenches increase.6–8 The back-end applications for SiNx films include etch stop layers, conformal spacers, and seamless gapfill of trenches or vias, depending on the device integration scheme. Gapfill with SiO2 is already available and the development of a SiNx process at comparable temperatures is of interest because it will provide etch contrast vs. SiO2.Atomic layer deposition (ALD) has been used successfully to grow conformal films of SiNx at temperatures within the thermal budget.3,6,7,9 ALD typically affords films with excellent conformality, but also has disadvantages, such as typically requiring two separate precursors, each containing a component of the desired film composition, which are alternately passed over the growth surface with chamber purge steps in between each exposure. These factors result in slow deposition rates (often ∼1 Å per cycle), which lead to increased processing time and higher manufacturing costs.10,11 In comparison, chemical vapor deposition (CVD) can afford films much more quickly than ALD, and under the right conditions this method can afford highly conformal films.12 Furthermore, CVD allows for the use of single-source precursors tailored to the desired film, simplifying the deposition process. In addition, CVD has some capabilities that ALD lacks, such as the ability to afford superconformal films in which growth is faster at the bottom of a deep feature.12 This capability has become increasingly important as feature sizes on integrated circuits decrease with each new device generation.
Of the various CVD precursors for silicon nitride, some of the most interesting are single-source molecular silicon azides. Several such compounds have been studied as CVD/ALD precursors; however, some are explosive and therefore unsafe to work with, especially on a larger industrial scale.13–16 In addition, many of the silicon azides that have been used as CVD precursors also contain silicon–carbon bonds, which inevitably lead to carbon incorporation in the resulting films; for example, films grown from an alkyl-triazido silane, SiEt(N3)3, contain approximately 10% carbon.13
The design of new CVD precursors for the deposition of SiNx films presents an interesting challenge. The precursor must be thermally stable at and above room temperature, volatile enough to ensure efficient vapor-phase transport into the CVD chamber, but reactive enough to deposit the desired film within the 400 °C thermal budget. An ideal single-source CVD precursor for SiNx should contain only silicon–nitrogen bonds, so as to minimize the incorporation of heteroatoms such as carbon.
The isolation of the thermally stable N-heterocyclic silylene 1,3-di-tert-butyl-2,3-dihydro-1H-1,3,2-diazasilol-2-ylidene (1) by West and co-workers was followed by several reports of the reactions of this silylene toward a variety of inorganic, organic, and organometallic reagents.17–19 Of interest in the context of the current paper is that 1 reacts with several organic azides (see Fig. 1) to afford 1,4-disubstituted 5-silatetrazolines with bulky substituents (R = Ph, p-tolyl, adamantyl, CPh3, SiPh3, or a substituted 1,3,2-diazaboryl group).18,20 Except for the large substituents, which greatly reduce the volatility of these compounds, these nitrogen-rich silatetrazolines have many of the attributes needed for a good silicon nitride CVD precursor.
Here we report the synthesis of three new spirocyclic 1,4-dialkyl-5-silatetrazolines by treatment of silylene 1 with low molecular weight alkyl azides. The new compounds have been characterized by NMR and IR spectroscopy and X-ray crystallography. In addition, we have investigated their thermal properties to assess their suitability as CVD precursors for the deposition of SiNx films at low temperatures.
Results and discussion
Synthesis of 1,4-dialkyl-5-silatetrazoline compounds
Following the same method used to prepare silatetrazoline compounds (tBuNCHCHNtBu)Si(N4R2) with bulky R groups,18 we have found that treatment of silylene 1 with ethyl-, iso-propyl, or tert-butyl azide21,22 in pentane (Fig. 1) affords three new 1,4-dialkyl-5-silatetrazolines of the form (tBuNCHCHNtBu)Si(N4R2), where R = Et (2), iPr (3), or tBu (4). The mechanism of this reaction has previously been proposed to involve coordination of the first equiv. of the azide to the silylene, followed by loss of N2 to form an iminosilane, (tBuNCHCHNtBu)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR, which reacts with the second equivalent of the azide by a [3 + 2] cycloaddition to form the silatetrazoline product.23 Compounds 2 and 3 are best obtained by removing the solvent and subliming the product under vacuum (5 mTorr) at 65–75 °C; the isolated yields are 43% and 75%, respectively. Compound 4 precipitates from solution as a white solid with excellent purity and 30% yield. It sublimes at 115 °C under vacuum.
NR, which reacts with the second equivalent of the azide by a [3 + 2] cycloaddition to form the silatetrazoline product.23 Compounds 2 and 3 are best obtained by removing the solvent and subliming the product under vacuum (5 mTorr) at 65–75 °C; the isolated yields are 43% and 75%, respectively. Compound 4 precipitates from solution as a white solid with excellent purity and 30% yield. It sublimes at 115 °C under vacuum.
We did not attempt to prepare the methyl-substituted analog due to the explosive nature of the methyl azide starting material.24
Crystal structures of the new 1,4-dialkyl-5-silatetrazoline compounds
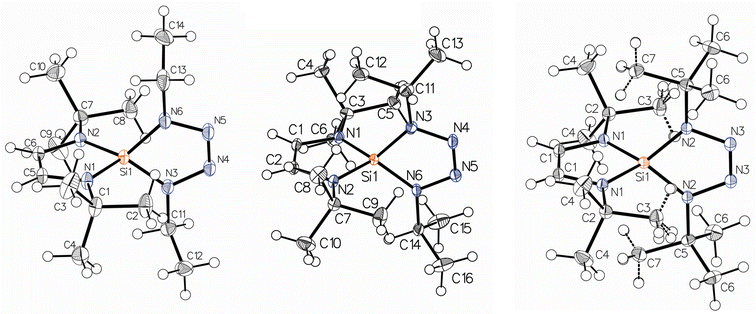
Crystal data for the new 1,4-dialkyl-5-silatetrazolines are listed in Table S1† and views of the molecules are given in Fig. 2. The ethyl compound 2 crystallizes in the space group Pna21, with one molecule (residing on a general position) per asymmetric unit. The iso-propyl compound 3 crystallizes in the space group P1, with 2 molecules of similar geometry per asymmetric unit. The tert-butyl compound 4 crystallizes in the space group Cmcm, with one-fourth molecule (mm2 site symmetry) per asymmetric unit.Selected bond distances and angles for compounds 2–4 are listed in Tables S2–S4.† In all compounds, the silicon atom is bound to one 1,4-di(tert-butyl)-1,4-diazabutenediyl (DAD) ligand and one 1,4-dialkyltetrazenediyl (TET) ligand. DAD ligands are potentially redox non-innocent: the backbone C–C unit is a double bond in the dianionic state and a single bond in the neutral state.25 The DAD ligands in 2–4 are clearly dianions, as shown by the backbone C–C bond lengths of 1.338(2) Å in 2, 1.332(4) Å in 3, and 1.343(3) Å in 4.
In all three compounds, the average Si–N distances of the DAD ligand are similar to the Si–N distances of the TET ligand: 1.725(2) Å vs. 1.729(1) Å for 2, 1.718(3) Å vs. 1.732(3) Å for 3, and 1.726(1) Å vs. 1.735(1) Å for 4. The N–Si–N angle between the two nitrogen atoms of the TET ligand is 85.62(7)°, 85.92(12)°, and 86.71(7)° for 2–4, respectively.
The Si–N distances between the silicon and nitrogen atoms in the TET ligands range from 1.729 to 1.735 Å. These bond distances are very similar to those of 1.735(2)–1.740(2) Å and 1.740(3)–1.746(3) Å reported for two other 5-silatetrazoline compounds, the 1,4-diphenyl-5-silatetrazoline molecule, (tBuNCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNtBu)Si(N4Ph2), and the 1,4-bis(triphenylsilyl)-5-silatetrazoline molecule, (tBuNCH
CHNtBu)Si(N4Ph2), and the 1,4-bis(triphenylsilyl)-5-silatetrazoline molecule, (tBuNCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNtBu)Si[N4(SiPh3)2].18 The N–Si–N angles in these two compounds are also very similar to those in compounds 2–4: 85.53(11)° for the 1,4-diphenyl compound and 88.72(13)° for the 1,4-bis(triphenylsilyl) compound.18
CHNtBu)Si[N4(SiPh3)2].18 The N–Si–N angles in these two compounds are also very similar to those in compounds 2–4: 85.53(11)° for the 1,4-diphenyl compound and 88.72(13)° for the 1,4-bis(triphenylsilyl) compound.18
Spectroscopic properties
In the 1H NMR spectra of 2–4, the DAD ligand shows singlets at δ 5.55, 5.57, and 5.59, respectively, for the backbone CH protons and at δ 1.19, 1.14, and 1.11, respectively, for the tert-butyl groups. The alkyl groups of the TET ligand appear as a quartet at δ 3.45 and a triplet at δ 1.25 for the ethyl groups in 2, a septet at δ 3.81 and a doublet at δ 1.37 for the iso-propyl groups in 3, and at δ 1.49 for the tert-butyl groups in 4. The latter resonances are significantly deshielded with respect to those for the tert-butyl groups of the DAD ligand. 13C{1H} NMR data for all three compounds can be found in the Experimental section.Comparisons of the IR spectra of 2–4 with that of the silylene compound 1 (which lacks the TET group) show some additional bands whose frequencies are largely independent of the alkyl group on the tetrazene ring. One of these bands appears at 1117 cm−1 and the other appears between 993 and 1007 cm−1; we tentatively assign these bands to N–N stretches within the tetrazene ring.
Air sensitivity, thermal properties, and volatility
Samples of 2–4 were exposed as solids to air for 1 day and subsequently analyzed by 1H NMR spectroscopy. The tert-butyl compound 4 showed little decomposition even after being exposed to air for 3 days (Fig. S15†). In contrast, all of the ethyl compound 2 and half of the iso-propyl compound 3 decompose over this period. For both 2 and 3, the principal decomposition product and only identifiable species is 1,4-di(tert-butyl)-1,4-diazabutadiene, which results from the hydrolysis and oxidation of the DAD ligand (Fig. S9†). For 2, there is no sign of the ethyl groups of the TET ligand or their possible decomposition product, ethylene; these species, if formed, evidently volatilized out of the sample during sample handling. For 3, several multiplets at δ 2.80–3.23 and several small doublets at δ 0.75–1.55 are due to the iso-propyl groups derived from the tetrazene ring (Fig. S12†), but the identities of the species responsible for these peaks are uncertain.Because nitrogen-rich compounds have the potential to be explosive, we conducted shock tests. No sparks, fires, fumes, or noises were observed when compounds 2–4 were shocked. This assessment was supported by TGA data for 2–4 and silylene 1 (Table 1). When the compounds are heated under N2 in a temperature ramp from room temperature to 600 °C, no anomalies appear in the TGA curves that would indicate a tendency to explode (Fig. 3). Instead, compounds 2–4 all evaporate smoothly without decomposition, as shown by the small residual masses of 0.3% (2), 0.4% (3), and 1.4% (4).
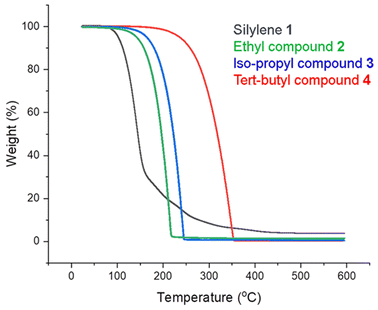 | ||
| Fig. 3 TGA plots for the 1,4-dialkyl-5-silatetrazoline compounds 2–4 under 1 atm of nitrogen at ramp rates of 10 °C min−1. | ||
The onset and peak temperatures for evaporation increase in the order 2 < 3 < 4 as expected from the increase in the size and molecular weight of the substituents on the TET ring. In contrast to the smooth volatilization seen for 2–4, silylene 1 shows a decrease in the slope of its TGA curve above 150 °C. This slope change could reflect adventitious oxidation of a portion of this highly air-sensitive compound during the loading of the sample into the TGA instrument, but could also indicate the onset of decomposition, for example by addition of C–H bonds to the silicon(II) center. The residual mass of 3.7% is small, however, indicating that most of 1 volatilizes rather than decomposes.
Isothermal TGA data were also collected for the ethyl compound 2 (Fig. S3†). After 18 mg of 2 was heated to 60 °C under 1 atm of N2 for 4 h, 95% of the sample remained, which was shown to be unreacted 2 by NMR spectroscopy. These results indicate that, even when heated over an extended period of time, compound 2 continues to volatilize smoothly without decomposition. A series of isothermal TGA experiments (Fig. S3†) at different set temperatures, analyzed by Price's method,26 showed that compound 2 has a room temperature vapor pressure of 5.11 ± 0.04 mTorr, a 1 Torr vapor pressure at 94.6 ± 0.2 °C, and an enthalpy of vaporization (ΔHvap) of 69.1 ± 0.2 kJ mol−1.
Conclusions
Three silicon 1,4-dialkyl-5-silatetrazoline compounds of the form (tBuNCHCHNtBu)Si(N4R2), where R = Et (2), iPr (3), or tBu (4), have been synthesized by treatment of silylene 1 with 2 equiv. of an alkyl azide. The R groups on the tetrazenediyl ligand are smaller than those in previously reported compounds, and as a result compounds 2–4 are appreciably volatile.The synthesis, thermal properties, and volatilities of these compounds suggest that they may serve as precursors for the low temperature CVD of SiNx films. Of the three new compounds reported here, the 1,4-diethyl-5-silatetrazoline compound 2 shows the most promise as a CVD precursor because it is the most volatile. It sublimes at 65 °C and 5 mTorr, and TGA studies show an onset of volatility at 179 °C under 1 atm of N2. Isothermal TGA studies show that 2 has a room temperature vapor pressure of 5 mTorr and a 1 Torr vapor pressure temperature of 95 °C.
Experimental section
All experiments were carried out under vacuum or under argon by using standard Schlenk techniques. Solvents were distilled under nitrogen from sodium/benzophenone immediately before use. The silylene compound 1 (1,3-di-tert-butyl-2,3-dihydro-1H-1,3,2-diazasilol-2-ylidene)27 and organic azides21,22 were prepared through literature routes. Microanalyses were performed by the University of Illinois Microanalytical Laboratory using combustion techniques. The microanalytical results for 2–4 are about 1% low in nitrogen, for reasons that are unclear. The spectroscopic results suggest that the isolated samples are pure.1H NMR data were collected using a Varian Unity Inoca spectrometer at 400 MHz and 13C NMR data were collected using a Bruker Avance III HD 500 NMR spectrometer; 1H and 13C NMR chemical shifts are reported in δ units (positive chemical shifts to higher frequencies) relative to tetramethylsilane (TMS). The IR spectra were recorded using a Nicolet Impact 410 instrument as Nujol mulls between KBr plates.
Caution: Catenated nitrogen compounds often present explosion hazards. Although we have not experienced any problems in the synthesis, characterization, sublimation, or handling of the compounds described here, their energetic properties have not been fully investigated and therefore they should be handled using standard safety precautions for explosive materials (safety glasses, face shield, blast shield, puncture resistant gloves, and ear protection).
[1,4-Di(tert-butyl)-1,4-diazabut-2-ene-1,4-diyl](1,4-diethyltetraaz-2-ene-1,4-diyl)silane, 6,9-di-tert-butyl-1,4-diethyl-1,2,3,4,6,9-hexaaza-5-silaspiro[4.4]nona-2,7-diene, 2
To a solution of ethyl azide (2.8 mmol) in pentane was added dropwise a solution of silylene 1 (0.275 g, 1.40 mmol) in pentane (20 mL). The solution was stirred at room temperature for 4 days. The colorless solution was evaporated to dryness under vacuum, resulting in a white microcrystalline solid. Yield: 0.340 g (78%). Crystals suitable for single-crystal XRD were grown from concentrated solutions of pentane at −20 °C. Anal. calcd for C14H30N6Si: C, 54.2; H, 9.74; N, 27.1. Found: C, 54.2; H, 9.76; N, 26.0. 1H NMR (C6D6, 20 °C): 5.59 (s, CH, 2 H), 3.45 (q, 3JHH = 7.2 Hz, 4 H, CH2), 1.25 (t, 3JHH = 7.3 Hz3, 6 H, CH3), 1.11 (s, 18 H, tBu). 13C{1H} NMR (C6D6, 20 °C): 109.58 (s, CH), 51.70 (s, CH2), 41.41 (s, C), 30.63 (s, CH3 of tBu), 15.62 (s, CH3 of Et). IR (cm−1): 1314 m, 1267 s, 1226 s, 1197 w, 1152 w, 1117 m, 1097 m, 1060 w, 1006 s, 750 s.[1,4-Di(tert-butyl)-1,4-diazabut-2-ene-1,4-diyl][1,4-di(iso-propyl)tetraaz-2-ene-1,4-diyl]silane, 6,9-di-tert-butyl-1,4-di(iso-propyl)-1,2,3,4,6,9-hexaaza-5-silaspiro[4.4]nona-2,7-diene, 3
To a solution of iso-propyl azide (3.22 mmol) in pentane was added dropwise a solution of silylene 1 (0.317 g, 1.61 mmol) in pentane (20 mL). The solution was allowed to stir at room temperature for 4 days. The colorless solution was evaporated to dryness under vacuum, resulting in a white microcrystalline solid. Yield: 0.412 g (75%). Crystals suitable for single-crystal XRD were grown from concentrated solutions of pentane at −20 °C. Anal. calcd for C16H34N6Si: C, 56.8; H, 10.1; N, 24.8. Found: C, 56.7; H, 10.2; N, 23.7. 1H NMR (C6D6, 20 °C): 5.57 (s, 2 H, CH of DAD), 3.81 (sep, 3JHH = 6.5 Hz, 2 H, CH of iPr), 1.37 (d, 3JHH = 6.6 Hz, 12 H, CH3 of iPr), 1.14 (s, 18 H, CH3 of tBu). 13C{1H} NMR (C6D6, 20 °C): 109.09 (s, CH of DAD), 51.83 (s, CH of iPr), 48.49 (s, C of tBu), 30.54 (s, CH3 of tBu), 22.96 (s, CH3 of iPr). IR (cm−1): 1267 m, 1226 m, 1117 m, 1096 w, 1063 w, 1007 m, 745 s.[1,4-Di(tert-butyl)-1,4-diazabut-2-ene-1,4-diyl][1,4-di(tert-butyl)tetraaz-2-ene-1,4-diyl]silane, 6,9-di-tert-butyl-1,4-di(tert-butyl)-1,2,3,4,6,9-hexaaza-5-silaspiro[4.4]nona-2,7-diene, 4
To a solution of tert-butyl azide (5.09 mmol) in pentane was added dropwise a solution of silylene 1 (0.500 g, 2.55 mmol) in pentane (15 mL). The solution was allowed to stir at room temperature for 4 days. The colorless solution was evaporated to dryness under vacuum, resulting in a white microcrystalline solid. Yield: 0.284 g (30%). Crystals suitable for single-crystal XRD were grown from concentrated solutions of pentane at −20 °C. Anal. calcd for C18H38N6Si: C, 59.0; H, 10.4; N, 22.9. Found: C, 58.6, H, 10.4; N, 22.2. 1H NMR (C6D6, 20 °C): 1.19 (s, 18 H, CH3 of DAD tBu), 1.49 (s, 18 H, CH3 of TET tBu), 5.55 (s, 2H, CH). 13C{1H} NMR (C6D6, 20 °C): 108.83 (s, CH), 54.45 (s, C of TET tBu), 52.05 (s, C of DAD tBu), 30.49 (s, CH3 of TET tBu), 30.11 (s, CH3 of DAD tBu). IR (cm−1): 1267 m, 1222 s, 1117 m, 1093 w, 1041 w, 993 m, 731 m.Thermogravimetric studies
TGA experiments were performed on Pt sample pans in a TA Instruments Q50 analyzer. Because the TGA instrument was not inside a glovebox, a sample preparation protocol was followed to limit the exposure of these air- and moisture-sensitive compounds to the atmosphere during the sample loading process. In an argon-filled glovebox, weighed 7–9 mg and 11–18 mg samples were loaded into sealable vials for ramp and isothermal experiments, respectively. At the TGA instrument, the sample was quickly transferred from the vial into the ceramic cup, and the cup was placed on the Pt sample pan and immediately loaded into the TGA instrument.For ramp experiments, the samples were heated under a flow of N2 to 600 °C at a rate of 10 °C min−1. The onset of volatilization was measured as the intersection of the tangents of the initial plateau and the point in the TGA curve at which the mass loss rate is the greatest. The residual masses of 0.3% (2), 0.4% (3), and 1.4% (4) for these compounds are very low, suggesting that these compounds volatilize smoothly with little decomposition.
For the isothermal time-study experiment, 18 mg of sample was heated under a flow of N2 to 60 °C and held at this temperature. In the stepwise isothermal experiment, 11 mg of sample was heated under a flow of N2 with dwell times of 10 minutes in 10 °C steps with a ramp rate between steps of 10 °C min−1.
General crystallographic procedures
All crystals were mounted on a Nylon loop with Krytox oil and immediately cooled to −173 °C in a cold nitrogen gas stream on the diffractometer. The measured intensities were reduced to structure factor amplitudes and their estimated standard deviations (esds) by correction for background, scan speed, and Lorentz and polarization effects. No corrections for crystal decay were necessary, but multi-scan absorption corrections were applied for 2 and 3. Systematically absent reflections were deleted and symmetry equivalent reflections were averaged to yield the set of unique data.All structures were solved by direct methods (SHELXS) and correct positions for all the non-hydrogen atoms were deduced from an E-map and subsequent least-squares refinement and difference Fourier calculations. The analytical approximations to the scattering factors were used and all structure factors were corrected for both real and imaginary components of anomalous dispersion. In the final cycle of least squares, independent anisotropic displacement factors were refined for the non-hydrogen major site atoms. Unless otherwise stated in the ESI,† hydrogen atoms were placed in idealized positions; the displacement parameters for all hydrogen atoms were set equal to 1.2 times Ueq for the attached carbon. A final analysis of variance between the observed and calculated structure factors showed no apparent errors. Final refinement parameters are given in Table S1.† Aspects of the refinement unique to each structure are reported in the ESI.†
Data availability
The data supporting this article have been included as part of the ESI.†Crystallographic data for compounds 2, 3, and 4 have been deposited at the Cambridge Crystallographic Data Centre under accession codes CCDC 2347929, 2347931, and 2347934.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Semiconductor Research Corporation (SRC) through grant number 2021-NM-3028. The authors thank Jeff Bielefeld, Scott Clendenning, Scott Semproni, and Charles Mokhtarzadeh of the Intel Corporation for their helpful comments and suggestions. The authors thank Dr Danielle Gray and Dr Toby Woods of the G. L. Clark Laboratory for collecting the X-ray diffraction data and the NMR laboratory and the microanalytical laboratory of the School of Chemical Sciences at the University of Illinois at Urbana-Champaign for assistance with data collection. The authors also thank Dr Roddel Remy for assistance with collecting TGA data in the Materials Research Laboratory Central Research Facilities, University of Illinois Urbana-Champaign.References
- G. R. Nowling, S. E. Babayan, V. Jakovic and R. F. Hicks, Remote Plasma-Enhanced Chemical Vapour Deposition of Silcion Nitride at Atmospheric Pressure, Plasma Sources Sci. Technol., 2002, 11, 97–103 CrossRef CAS.
- G. D. Wil, R. M. Wallace and J. M. Anthony, High-k Gate Dielectrics: Current Status and Materials Properties Considerations, J. Appl. Phys., 2001, 89, 5243–5275 CrossRef.
- X. Meng, Y. Byun, H. S. Kim, J. S. Lee, A. T. Lucero, L. Cheng and J. Kim, Atomic Layer Deposition of Silicon Nitride Thin Films: A Review of Recent Progress, Challenges, and Outlooks, Materials, 2016, 9, 1–20 CrossRef PubMed.
- C. Dussarrat, J. Girard, T. Kimura, N. Tamoki and Y. Sato, Methods for Producing Silicon Nitride Films and Silicon Oxynitride Films by Thermal Chemical Vapor Deposition, U.S. Patent 7192626B2, March 20, 2007 Search PubMed.
- F. L. Rily, Silicon Nitride and Related Materials, J. Am. Ceram. Soc., 2000, 83, 245–265 CrossRef.
- T. Faraz, M. Drunen, H. C. M. Knoops, A. Mallikarjunan, I. Buchanan, D. M. Hausmann, J. Henri and W. M. M. Kessels, Atomic Layer Deposition of Wet-Etch Resistant Silicon Nitride Using Di(sec-butylamino)silane and N2 Plasma on Planar and 3D Substrate Topographies, ACS Appl. Mater. Interfaces, 2017, 9, 1858–1869 CrossRef CAS PubMed.
- R. A. Ovanesyan, N. Leick, K. M. Kelchner, D. M. Hausmann and S. Agarwal, Atomic Layer Deposition of SiCxNy Using Si2Cl6 and CH3NH2 Plasma, Chem. Mater., 2017, 29, 6269–6278 CrossRef CAS.
- R. S. Iyer, S. M. Seutter, S. Tandon, E. A. C. Sanchez and S. Wang, Method for Silicon Nitride Chemical Vapor Deposition, US 7365029B2, April 29, 2008 Search PubMed.
- C. A. Murray, S. D. Elliott, D. Hausmann, J. Henri and A. LaVoie, Effect of Reaction Mechanism on Precursor Exposure Time in Atomic Layer Deposition of Silicon Oxide and Silicon Nitride, ACS Appl. Mater. Interfaces, 2014, 6, 10534–10541 CrossRef CAS PubMed.
- W.-J. Lee and Y.-H. Choa, Novel Plasma Enhanced Chemical Vapor Deposition of Highly Conformal SiN Films and their Barrier Properties, J. Vac. Sci. Technol., B, 2018, 36, 022201 CrossRef.
- M. Leskelä and M. Ritala, Atomic Layer Deposition (ALD): From Precursors to Thin Film Structures, Thin Solid Films, 2002, 409, 138–146 CrossRef.
- J. R. Abelson and G. S. Girolami, New Strategies for Conformal, Superconformal, and Ultrasmooth Films by Low Temperature Chemical Vapor Deposition, J. Vac. Sci. Technol., A, 2020, 38, 030802 CrossRef CAS.
- D. A. Roberts, A. K. Hochlberg, D. L. O'Meara, F. Rusnak and H. Hockenhull, The LPCVD of Silicon Nitride Films from Alkylazidosilanes, Mater. Res. Soc. Symp. Proc., 1991, 24, 515–520 Search PubMed.
- A. K. Hochberg, S. Beach, D. L. O'Meara and D. A. Roberts, Deposition of Silicon Nitride Films from Azidosilane Sources, U.S. Patent 4992299A, February 12, 1991 Search PubMed.
- M. Ishikawa, H. Machida and H. Sudo, Film Forming Material, Film Forming Method and Device, Japanese Psatent JP2011009479A, January 1, 2011 Search PubMed.
- A. LaVoie, M. J. Saly, R. D. Odedra and R. Kanjolia, Precursors for Plasma Activated Conformal Film Deposition, U.S. Patent US20130210241A1, August 15, 2013 Search PubMed.
- N. J. Hill and R. West, Recent Developments in the Chemistry of Stable Silylenes, J. Organomet. Chem., 2004, 689, 4165–4183 CrossRef CAS.
- N. J. Hill, D. F. Moser, I. A. Guzei and R. West, Reactions of Stable Silylenes with Organic Azides, Organometallics, 2005, 24, 3346–3349 CrossRef CAS.
- A. C. Tomasik, A. Mitra and R. West, Synthesis and Reactivity of Three New N-Heterocyclic Silylenes, Organometallics, 2009, 28, 378–381 CrossRef CAS.
- K. Yuvaraj and C. Jones, Synthesis and Reactivity of Boryl Substituted Silaimines, Dalton Trans., 2019, 48, 11961–11965 RSC.
- J. C. Bottaro, P. E. Penwell and R. J. Schmitt, Expedient Synthesis of t-Butyl Azide, Synth. Commun., 1997, 27, 1465–1467 CrossRef CAS.
- M. Swetha, P. V. Ramana and S. G. Shirodkar, Simple and Efficient Method for the Synthesis of Azides in Water-THF Solvent System, Org. Prep. Proced. Int., 2011, 43, 348–353 CrossRef CAS.
- R. West and M. Denk, Stable silylenes: Synthesis, Structure, Reactions, Pure Appl. Chem., 1996, 68, 785–788 CrossRef CAS.
- J. Chae and Methyl Azide, in Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons, 2008 Search PubMed.
- E. N. Nikolaevskaya, N. O. Druzhkov, M. A. Syroeshkin and M. P. Egorov, Chemistry of Diazadiene Type Ligands with Extra Coordination Groups. Prospects of Reactivity, Coord. Chem. Rev., 2020, 417, 213353 CrossRef CAS.
- D. M. Price, Vapor Pressure Determination by Thermogravimetry, Thermochim. Acta, 2001, 367–368, 253–262 CrossRef CAS.
- M. Haaf, A. Schmiedl, T. A. Schmedake, D. R. Powell, A. J. Millevolte, M. Denk and R. West, Synthesis and Reactivity of a Stable Silylene, J. Am. Chem. Soc., 1998, 120, 12714–12719 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal structure analyses, thermogravimetric analyses, and NMR spectra of 2–4. CCDC 2347929, 2347931 and 2347934. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03473b |
| This journal is © The Royal Society of Chemistry 2025 |