Open Access Article
Open Access ArticleTetranuclear and dinuclear Cu(II) complexes with risedronate as anti-Trypanosoma cruzi and anti-Leishmania mexicana agents. Synthesis, crystal structures, and biological evaluation†
Miguel Á.
Romero-Solano
 a,
Eya Caridad
Rodríguez-Pupo
a,
Eya Caridad
Rodríguez-Pupo
 b,
Ignacio
Martinez
b,
Ignacio
Martinez
 b,
Berenice
Prestegui-Martel
b,
Berenice
Prestegui-Martel
 b,
Alberto
Martínez-Muñoz
b,
Alberto
Martínez-Muñoz
 c,
Bertha
Espinoza
c,
Bertha
Espinoza
 *b,
Diego
Martínez-Otero
*b,
Diego
Martínez-Otero
 d,
Víctor
López-Guerrero
d,
Víctor
López-Guerrero
 a,
Alma K.
Esteban Covarrubias
a,
Alma K.
Esteban Covarrubias
 a and
Alejandro
Dorazco-González
a and
Alejandro
Dorazco-González
 *a
*a
aInstitute of Chemistry, National Autonomous University of Mexico, Ciudad Universitaria, Mexico City, 04510, Mexico. E-mail: adg@unam.mx
bDepartamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México City 04510, Mexico. E-mail: besgu@iibiomedicas.unam.mx
cFS Scientia Pharma S.A de C.V. Fray Diego de La Magdalena 630, Jardín Vista Hermosa, 78270, San Luis Potosí, S.L.P., Mexico
dCentro Conjunto de Investigación en Química Sustentable, UAEM-UNAM, Instituto de Química, Universidad Nacional Autónoma de México, Carretera Toluca-Atlacomulco Km 14.5, C. P. 50200 Toluca, Estado de México, México
First published on 14th February 2025
Abstract
The development of new biometal-based complexes containing antiparasitic bioactive ligands is a central field of coordination chemistry that impacts bioinorganic, medicinal, and biological chemistry. Herein, two novel water-soluble polynuclear Cu(II)-complexes with formula [Cu4(4,4′-dmbp)4(μ3-HRis)2Cl2], 1 and [Cu2(5,5′-dmbp)2(μ2-HRis)(H2O)Cl] 2, (4,4′-dmbp = 4,4′-dimethyl-2,2′-bipyridine, 5,5′-dmbp = 5,5′-dimethyl-2,2′-bipyridine, HRis = risedronate), were synthesized by self-assembly solution reactions between the corresponding precursor complexes [Cu2(dmbp)Cl2] and the drug Na2Ris in aqueous media. Both Cu(II)–Ris complexes 1–2 were structurally described by single-crystal X-ray diffraction, characterized by spectroscopic tools (IR-ATR, HRMS, UV-Vis, EPR) and studied as antiparasitic agents against Trypanosoma cruzi causative agent of Chagas disease and Leishmania mexicana that is the etiological agent of cutaneous leishmaniasis. X-ray structural analysis reveals that 1 is an uncommon tetranuclear Cu4 complex where four crystallographically independent units [Cu(4,4′dmbp)]2+ are double-bridged coordinated by two tetra-deprotonated ligands HRis containing the protonated pyridine ring. All Cu(II) centers show a distorted square-based pyramid geometry, and they are coordinated by two bisphosphonate fragments from HRis in a coordination environment μ3 for each one. Crystal analysis of 2 displays a dinuclear Cu2 complex where one ligand HRis coordinated to two different units [Cu(4,4′dmb)]2+ in a μ2 mode, both Cu(II) present distorted square-based pyramid geometry. In general, complexes 1–2 are hydrostable in the millimolar concentration range and present low citotoxicity (<22% on the growth of cancer cell lines and healthy COS-7 cells) similar to the commercial drug, monosodium salt of risedronic acid (NaRis). Complexes 1–2 and NaRis were evaluated in vitro against T. cruzi epimastigotes and L. mexicana promastigotes. Results demonstrated that these Cu-Ris complexes improved the percentages of growth inhibition for T. cruzi. This inhibition ranged from 62–70% at concentrations of 5.0 mM after 24 h and 48 h incubation, compared to those observed for free, which has an inhibition of ∼38%. Under the same concentration at 24 h incubation, complex 1 has a significantly greater inhibition effect against L. mexicana (63%) compared to free NaRis (50%). Reduction in parasite metabolisms and morphological changes included membrane damage, vacuolization, reduction of size and loss of flagellum were also observed. Importantly cytotoxicity to VERO cells was minor with the Cu(II)–Ris complexes compared with NaRis. The molecular docking analysis showed significant affinity towards the enzyme farnesyl diphosphate synthase from T. cruzi (TcFPPS), including simulations of the complexes Cu(II) at the TcFPPS binding site.
Introduction
Chagas disease and leishmaniasis are diseases caused by the protozoan parasites Trypanosoma cruzi (T. cruzi) and Leishmania spp. that affect millions of humans around the world with high mortality and currently, they are associated with the drawbacks of used drugs such as severe side reactions and the emergence of resistance.1–4 These medical conditions are considered neglected tropical diseases and a global challenge by the World Health Organization (WHO)5 because cause serious public health problems in countries from Asia, Africa and Latin America.6 It is known that these parasitic diseases can also affect non-endemic regions such as Europe and North America due to population mobility.7,8In 2022, the WHO estimated that there are ∼7 million people infected with the parasite T. cruzi and around 70 million presenting a high risk of acquiring it.9 The estimated global incidence of leishmaniasis is ∼12 million people6 and a population at risk of around 350 million.10,11 Infected flies transmit Leishmania spp. to human populations.12Leishmania mexicana (L. mexicana) is responsible for the endemic disease in México and Central America.13
While the need for novel efficient drugs with a low grade of toxicity is evident, currently the pharmacologic treatments are partially effective against the target parasites, and the vast majority produce considerable side reactions as well as, these available drugs for Chagas and leishmaniasis treatment are not recommended in persons with hepatic/renal impairment among other medical conditions.2,14,15 Additionally, they induce the rise of resistant parasites.1
For Chagas disease (American Trypanosomiasis), only two commercial drugs are available, a 2-nitroimidazole-based, benznidazole and nitrofuran-based drug, nifurtimox have been used now for over 50 years.9,14,16 Meanwhile, for leishmaniasis some Sb(V)-based complexes such as stibogluconate17 and meglumine antimonate18 have been clinically used in the first-line of the infection for decades.19 Besides, alternative commercial antiprotozoal drugs such as liposomal amphotericin,20 pentamidine21 and paromomycin.22
In general, these pharmaceutics are considerably toxic, require prolonged treatment, and are not very effective in the chronic stage of diseases mentioned.12,21,23 For these reasons, the search for new drugs that have significant activity against these parasites without presenting serious side effects is a relevant field of research and an ongoing challenge. However, the creation of such drugs is not a trivial task and clearly, needs elaborate molecular strategy and synthetic approaches.
Typical searching efforts consist of finding novel bioactive compounds from natural sources.24,25 Another emerging molecular strategy is the development of synthetic metal complexes bearing a ligand with proven antiparasitic activity (bioactive ligand).19,26–29
Recent reports in the context of medicinal inorganic chemistry have displayed that transition-metal coordination complexes may have multiple mechanisms of action by combining bioactive antiparasitic ligands (e.g. clotrimazole, ketoconazol, N-acylhydrazones, aminoquinoxalines, α-aminoacidates, polypiridyl ligands, nitrofuryl-derivatives, (tio)semicarbazones, thiols, purines and glycosylated pyridines) with metal centers such as Ru(II),18,30 Fe(III),31 V(IV),32–34 Cu(II),35,36 Pd(II),37–39 Pt(II)40,41 and Au(I),42,43 leading to synergistic/additive effects against T. cruzi and Leishmania spp.
On the other hand, bisphosphonate compounds are analogs of pyrophosphate (P2O74−) anion where the central O atom is substituted by a C atom leading a rigid P–C–P backbone. This chemical change generates compounds more stable to hydrolysis and a great variety of functional groups on the C atom can be achieved. Among these compounds, risedronate (Ris; 1-hydroxy-2-(3-pyridinyl)-ethylidene-bisphosphonate) is a worldwide prescribed drug for osteoporosis and bone cancer therapy.44–46 Interestingly, Ris can inhibit the proliferation of T. cruzi and Leishmania spp., both in vitro and in vivo without presenting toxicity to host cells.44,47,48 In biochemical terms, Ris is able to inhibit the farnesyl pyrophosphate synthase (FPPS) which is an essential enzyme for trypanosomatid parasites and Leishmania promastigotes.3,47,49,50 However, antiparasitic studies of transition-metal complexes with Ris still remain largely unexplored, probably due to metal-Ris complexes being typically limited to high-dimensional metal–organic networks, which are practically insoluble in aqueous media.51,52 The literature features only one report of a series of metal complexes containing Ris with activity against Chagas disease.53 These complexes ([M(Ris)2], M = Ni(II), Cu(II), Mn(II), Co(II)) were synthesized under strongly acidic conditions to achieve mononuclear species. One of the advantages of these metal complexes with Ris is that an improvement can be achieved in the pharmacokinetics of the complex in comparison with the free sodium salt of Ris because the coordination to a metal center modifying its solubility and lipophilicity.53
To the best of our knowledge, Cu(II)–Ris metal complexes have never been studied as anti-Leishmania agents. Taking this into account, we assumed that a potential drug for Chagas or leishmaniasis can be achieved by metal complexes containing a commercial drug already authorized by regulatory agencies (Ris) with a biometal such as Cu(II)35 and an ancillary chelating ligand to get control of the coordination and geometry of the metal atom.
The results obtained for two novel hydrostable polynuclear Ris–Cu(II) complexes bearing two different dimethyl-2,2′-bipys including synthesis, X-ray structural analysis, spectroscopic studies, antiproliferative effect on T. cruzi epimastigotes and L. mexicana promastigotes, cytotoxic activity and molecular docking calculations are summarized below. In this work, biological results are compared to those obtained with NaRis for T. cruzi and L. mexicana.
Results and discussion
Synthesis and crystal structures
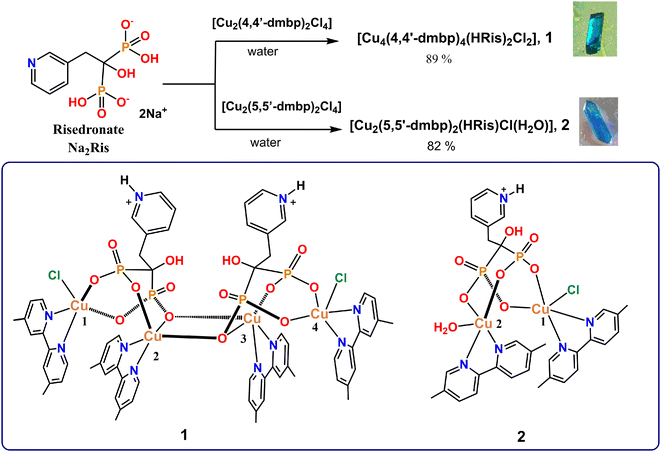
Ris-based transition-metal complexes are often supramolecular metal–organic frameworks because the bisphosphonate fragment is a potent complexing agent with multiple coordination sites. These metal organophosphate compounds have been used in magnetism,52,54 fluorescent properties,55 structural topologies56,57 and antibacterial studies.51 However, antiparasitic studies in aqueous media are practically unexplored.In order to further investigate this chemistry of Cu(II) with the commercial drug Ris and explore for potential anti-Chagas/Leishmania applications of such compounds, we synthesized two novel Cu(II)–Ris complexes 1–2 based on the reaction of Cu(II)-2,2′-bipyridine compounds: [Cu2(4,4′-dmbp)2Cl4] or [Cu2(5,5′-dmbp)2Cl4] (4,4′-dmbp = 4,4′-dimethyl-2,2′-bipyridine and 5,5′-dmbp = 5,5′-dimethyl-2,2′-bipyridine) with Na2Ris in water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. We chose bipyridines as co-ligands to (1) control the structure around the Cu(II), (2) favor square pyramid geometry58 and (3) avoid the formation of infinite networks which are insoluble in physiological media. Crystals of 1–2 were grown directly from the reaction mixture by slow evaporation after 1 week and were characterized by single crystal structure determination. The synthesis path and chemical structures of 1–2 are shown in Scheme 1.
1 molar ratio. We chose bipyridines as co-ligands to (1) control the structure around the Cu(II), (2) favor square pyramid geometry58 and (3) avoid the formation of infinite networks which are insoluble in physiological media. Crystals of 1–2 were grown directly from the reaction mixture by slow evaporation after 1 week and were characterized by single crystal structure determination. The synthesis path and chemical structures of 1–2 are shown in Scheme 1.
Tables S1–S5 in the ESI† contain crystallographic data, select distances, and angles around metal centers in addition to H-bonds within the crystal packing of these complexes.
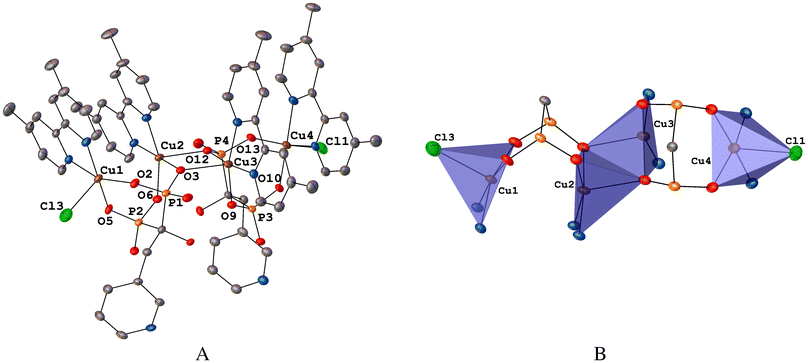 | ||
| Fig. 1 (A) ORTEP diagram at 40% probability of 1. Hydrogen atoms and solvent molecules were omitted for clarity. (B) Combinated ORTEP/polyhedral representation of the Cu4 core from 1. | ||
On the other hand, the four Cu(II) atoms inside 1 are crystallographically independent. In geometric terms, the four Cu(II) atoms form the vertices of a parallelogram which are linked through a double bisphosphonate bridge (Fig. 1B) with internal distances Cu1⋯Cu2, Cu2⋯Cu3 and Cu3⋯Cu4 varying from 3.2073(6) to 3.4509(7) Å, see Table 1. In this line, similar geometric parallelogram array was reported to a tetranuclear complex with formula [Cu4(μ4-PO4)2-(μ2-CO3)].60
| Complex | Atoms | Distance (Å) |
|---|---|---|
| 1 | Cu1⋯Cu2 | 3.4509(7) |
| Cu2⋯Cu3 | 3.2073(6) | |
| Cu3⋯Cu4 | 3.4077(7) | |
| Cu1⋯Cu4 | 8.9777(5) | |
| 2 | Cu1⋯Cu2 | 3.4695(4) |
The short internuclear Cu⋯Cu distances in 1 strongly suggest that the tetranuclear Cu4 core should present magnetic interactions similar to other tetranuclear Cu(II)-complexes built with phosphate anions in bridge μ3-phosphonate/phosphate coordination mode.59–61
In general, all Cu(II) atoms have a coordination number CN = 5 and distorted square pyramid geometries (Fig. 1B). Their Addison trigonality index (τ) are less than 0.19, (Table S6†) which is consistent with this type of coordination geometry.62 The value of τ is zero for an ideal square pyramid with C4v symmetry, while τ = 1 for an ideal trigonal bipyramid with D3h symmetry (τ = (α − β)/60, where α and β correspond to the two largest angles).62
In the axial positions, Cu1 and Cu4 have occupational disorders between Cl atoms and water molecules. For Cu1 atom, the occupational ratio is 27/73 (Cl3/H2O) while for Cu4 atom is 40/60 (Cl1/H2O). For these Cu atoms, τ values were estimated considering the coordinated Cl atoms. The four Cu(II) centers are slightly above the mean basal plane as is shown in the polyhedral representations in Fig. 1B.
The two terminal Cu1 and Cu4 atoms present a similar [CuN2O2Cl] coordination sphere where two N atoms of 4,4′-dmbp and two O atoms of different O–P fragments of the bisphosphonate (P1/P2 for Cu1 and P3/P4 for Cu4) are coordinated in the basal position, and a Cl− atom (Cu1–Cl3 and Cu4–Cl1) is positioned in the apical site.
The central Cu2 and Cu3 atoms are double-bridged by two O atoms (μ2-O3 and μ2-O12) from two different bisphosphonate fragments forming a tight four-membered ring {Cu2–μ2(O3)–μ2(O12)–Cu3} where all atoms are practically coplanar (Fig. S2†) with angles Cu2–O–Cu3, ∢ = 97.46°–98.80° and O3–Cu–O12, ∢ = 81.81°–81.92°.
Both internal Cu(II) atoms contain a [CuN2O3] coordination sphere where two N atoms of 4,4′-dmbp and two O of different O–P fragments (P1 and P4) are coordinated in the basal positions and the apical position is occupied by an O atom of one of the bridging bisphosphonate moieties with bond lengths, Cu2⋯O12–P4, 2.258(2) and Cu3⋯O3–P1, 2.236(2) Å, significantly longer than the basal plane ones, which generates a square-based pyramid elongated over the apical position.63
The 4,4′-dmbp ligands participate in intramolecular slightly offset π–π stacking interactions with distances centroid⋯centroid in the range 3.728(7) Å–3.804(5) Å (Fig. S3†) and dihedral angles of ∼5°. The crystalline net is stabilized by multiple O–H⋯O and N–H⋯O hydrogen bonds formed by central C-OH groups, pyridinium fragments and the phosphonate groups (Table S4†).
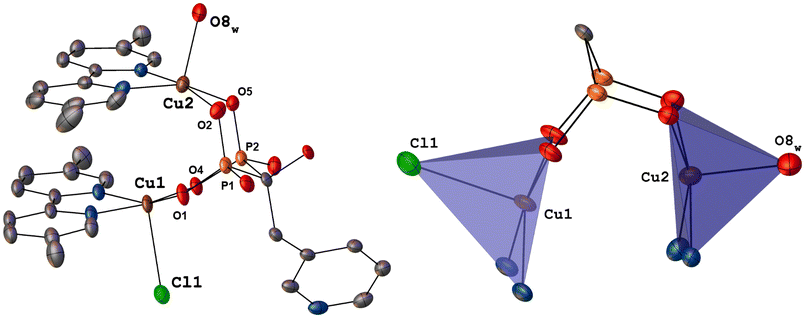 | ||
| Fig. 2 (A) ORTEP diagram at 40% probability of 2. Hydrogen atoms and solvent molecules were omitted for clarity. (B) Combinated ORTEP/polyhedral representation of the Cu2 core from 2. | ||
For both Cu(II) atoms, 5,5′-dmbp provides a bidentate N2 coordination mode and the other two positions of the basal plane are occupied by two O atoms of a bridging bisphosphonate fragment. The apical sites are occupied by one Cl atom (Cu1–Cl1, 2.5341(7) Å) and one water molecule (Cu2–O8, 2.066(5) Å). The Cu1⋯Cu2 distance, 3.4695(4) Å, varies marginally from that found in 1 (Table 1).
In this instance, 5,5′-dmbp also forms offset π–π interactions with a separation of centroid⋯centroid ranging from 3.649(7) Å–3.683(4) Å, Fig. S4.† Furthermore, intermolecular O–H⋯O hydrogen bonds between C-OH groups grow the crystal packing (Table S5†).
HRMS, IR-ATR, UV-Vis and EPR characterization
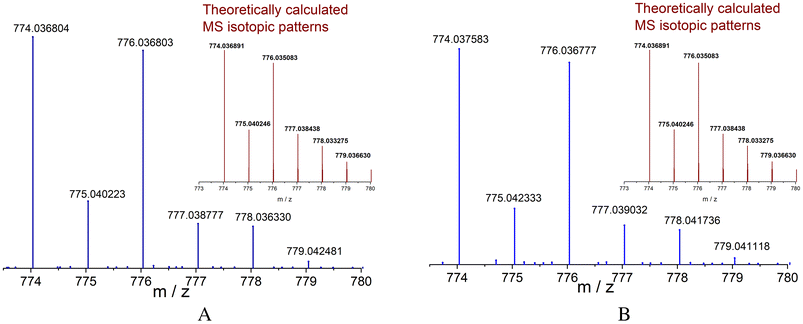
Cu(II)–Ris complexes 1–2 were isolated as pure crystalline samples according to high-resolution electrospray mass spectrometry (ESI-HRMS), elemental analysis (C, H, N), IR-ATR, UV-Vis and EPR.One charged state for 1 at 774.036804 m/z and for 2 at 774.037583 m/z corresponding to monocationic dinuclear species [Cu2(4,4′-dmbp)2(HRis)]+ and [Cu2(5,5′-dmbp)2(HRis)], respectively, are clearly observed by a positive scan. The signals in both complexes, separated by 1.0 unit, were isotopically resolved and matched very well the theoretical isotopically distribution (Fig. 3A and B); in addition, the multiplicities fit well with the presence of two Cu(II) atoms for each analysis.
The compiled infrared spectra of the salt NaRis and their related Cu(II)–Ris complexes 1–2 are illustrated in Fig. S5.† In the IR spectrum of NaRis, the stretching vibration band assigned to the hydroxyl group of the quaternary carbon (C-OH) appears at 3336 cm−1.65 In cases of complexes 1 and 2, this signal is strongly widened and shifted at 3372 and 3375 cm−1, respectively. This shift can be attributed to the presence of intra/inter-molecular hydrogen bonds of type O–H⋯O between two different hydroxyl groups as observed in their crystal structures (see supra). Additionally, crystal structures of 1–2 are species with a high degree of hydration, thus, the broad bands centered at approximately 3380 cm−1 can be assigned to overlapped stretching frequencies of –OH groups from multiple water molecules of crystallization with the C-OH group from Ris.63
In the 1100–1250 cm−1 range, symmetric and asymmetric stretching bands of phosphonate {C-PO(O−)2} groups from Ris are observed.53,65 The IR spectra of 1–2 are grossly similar in this region when allowance is made for the different coordinated ligands (Ris and dimethyl-2,2′-bipys). The most significant difference is a shifted (Δν = 8–22 cm−1) to higher wavenumbers of P–O stretching bands of complexes 1–2 in comparison to NaRis which is consistent with coordination of phosphonate groups.53
Crystal structures of 1 and 2 show a high degree of hydration around the polynuclear cores which suggest that these complexes are hydrostable in solid-state. Considering that the hydrostability in solution is a key physicochemical property of biological interest in design of drugs, we verified the chemical stability of 1 and 2 in neutral water.
Complexes 1 and 2 can be dissolved in pure water in the millimolar concentration range (<10 mM) and, in general, these aqueous solutions are very stable for a couple of days, which was determined from their UV-Vis spectra corresponding to the fresh solutions, and measurements at different hours up to 48 h as is shown in Fig. S6 and S7.†
For both complexes, the absorption maxima in the visible region (λmax = 646 nm for 1 and λmax = 643 nm for 2) practically do not present changes in this period of time.
On the other hand, the lipophilicity of metallodrugs is an important parameter involved in the cellular uptake;66 thus, the partition coefficients between n-octanol and water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w) were estimated for complexes 1–2 (see Fig S8 and ESI†).
Po/w) were estimated for complexes 1–2 (see Fig S8 and ESI†).
Both complexes showed greater affinity for the aqueous phase (complex 1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w = −1.31 ± 0.12 and complex 2, log
Po/w = −1.31 ± 0.12 and complex 2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w = −1.26 ± 0.08), these values are not unexpected, due to the ionic character of the complexes and the high hydration energy of the Cl− counterions (ΔG° = −345 kJ mol−1).67
Po/w = −1.26 ± 0.08), these values are not unexpected, due to the ionic character of the complexes and the high hydration energy of the Cl− counterions (ΔG° = −345 kJ mol−1).67
Taking into account the short distances between Cu(II) atoms inside crystal structures of 1–2, we recorded the EPR spectra in water frozen at 77 K (Fig. S9†).
EPR spectra are similar, exhibiting the characteristic signals of an S = 1 effective spin system that contains two signals centered at 300 mT corresponding to ΔMS = ±1 transitions.68 These signals have a perpendicular component (D⊥) with a separation of ∼50 mT and a parallel component (D∥) with separation of ∼100 mT. For both complexes the signal due to the “forbidden” half-field ΔMS = ±2 transition can be observed centered at 150 mT which confirmed the formation of dimer Cu2 cores.
For complex 1g∥ = 2.2400 and g⊥ = 2.0946 and for complex 2g∥ = 2.2325 and g⊥ = 2.0915.
On the half-field signal and the low-field parallel component of the spectra, a seven-line hyperfine structure with an intensity ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be observed. Also, the hyperfine structure can be observed (insets, Fig. S9†) in 120–280 mT region of the spectra taken at higher gain. This seven-line hyperfine structure corresponds to the coupling of an unpaired spin with two Cu(II) centers with I = 3/2. Interestingly, there are very few examples in the literature of dinuclear Cu(II) complexes with similar magnetic properties.68,69
1 can be observed. Also, the hyperfine structure can be observed (insets, Fig. S9†) in 120–280 mT region of the spectra taken at higher gain. This seven-line hyperfine structure corresponds to the coupling of an unpaired spin with two Cu(II) centers with I = 3/2. Interestingly, there are very few examples in the literature of dinuclear Cu(II) complexes with similar magnetic properties.68,69
Bioassays on cancer cellular lines
It has been noted that some characteristics of the metabolism of trypanosomatids and cancer cells share some similarities, namely inefficient mitochondria systems that promote a high rate of aerobic glycolysis, among others.70 There have also been studies that have shown that metal–ligand complexes containing polypyridyl DNA-intercalating ligands have activity against L. mexicana and T. cruzi.71 Therefore, it is probable that antineoplastic moieties can also have anti-trypanosomatid activity. More recently, heteroleptic oxovanadium(IV) complexes containing polypyridyl ligands and Schiff bases have been evaluated against T. cruzi and against three cancer cell lines.72Next, we have evaluated complexes 1–2 inhibitory activity on six cancer cell lines (glioblastoma, prostate, leukemia, colon, breast and lung) and on healthy COS-7 cells, Table 2 shows the growth inhibition studies on these cancer cell lines and on COS-7 at 1.0 μM concentration. Interestingly for our proposals, complex 2 presents a slightly lower inhibition to healthy cells COS-7 (7.4%) compared to the value of inhibition (9.59%) shown by the commercial drug NaRis.
| Compound [1.0 μM] | % of growth inhibition | ||||||
|---|---|---|---|---|---|---|---|
| U-251 | PC-3 | K-562 | HCT-15 | MCF-7 | SKLU-1 | COS-7 | |
| U-251 = human glioblastoma, PC-3 = human prostate, K-562 = leukemia, HCT-15 = human colon, MCF-7 = human breast, SKLU-1 = human lung, COS-7 = monkey liver (non-cancerous). NC: non-cytotoxic. | |||||||
| NaRis | NC | 14.94 | 1.56 | NC | NC | 0.34 | 9.59 |
| 1 | 3.45 | NC | 10.12 | NC | 14.15 | 14.42 | 22.00 |
| 2 | NC | NC | 2.48 | 5.54 | 1.55 | NC | 7.44 |
Bioassays on T. cruzi and L. mexicana
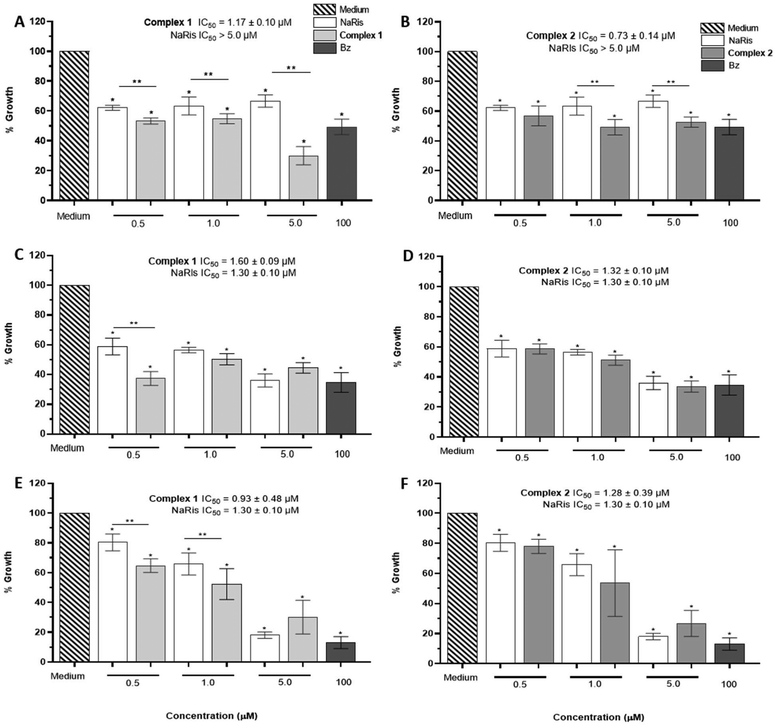
The growth inhibition of epimastigotes of T. cruzi was evaluated with complexes 1–2 and compared with NaRis. As shown in Fig. 4, T. cruzi proliferation was reduced with all three compounds compared to untreated control parasites cultured in LIT medium at the first 24 h of exposure (Fig. 4A and B). In general, complexes 1 and 2 were more active against this form of parasite than the free NaRis at the evaluated concentrations of 0.5, 1.0, and 5.0 μM. Complex 1 at 5.0 μM produced the maximum inhibitory effect. Next, the IC50 values were determined: 1 and 2 had an IC50 of 1.17 ± 0.09 μM and 0.73 ± 0.14 μM, respectively, resulting in lower concentrations than NaRis control which had an IC50 greater than 5.0 μM.In addition, after 48 h of incubation with the compounds, there was also a general decrease in proliferation. Significative differences between NaRis and 1 were found at 0.5 μM. The IC50 of the three compounds were similar 1.30 ± 0.10 μM (NaRis), 1.60 ± 0.09 μM (complex 1) and 1.32 ± 0.10 μM (complex 2) (Fig. 4C and D).
After 72 h of exposure, a progressive reduction in the growth of the treated parasites existed, achieving the best effect for the three compounds at the highest tested concentration of 5.0 μM (Fig. 4E and F).
There were significant differences between the 0.5 and 1.0 μM activities of 1 and NaRis, nevertheless, IC50 values of the three compounds (NaRis: 1.30 ± 0.10 μM, complex 1: 0.93 ± 0.48 μM, complex 2: 1.28 ± 0.39 μM) were similar. It is worth mentioning that when comparing the commercially available drug to treat Chagas disease (benznidazole) there was an inhibition percentage of more than 50% but using a concentration of 100 μM, which makes both complexes 1 and 2 very effective at a lower concentration.
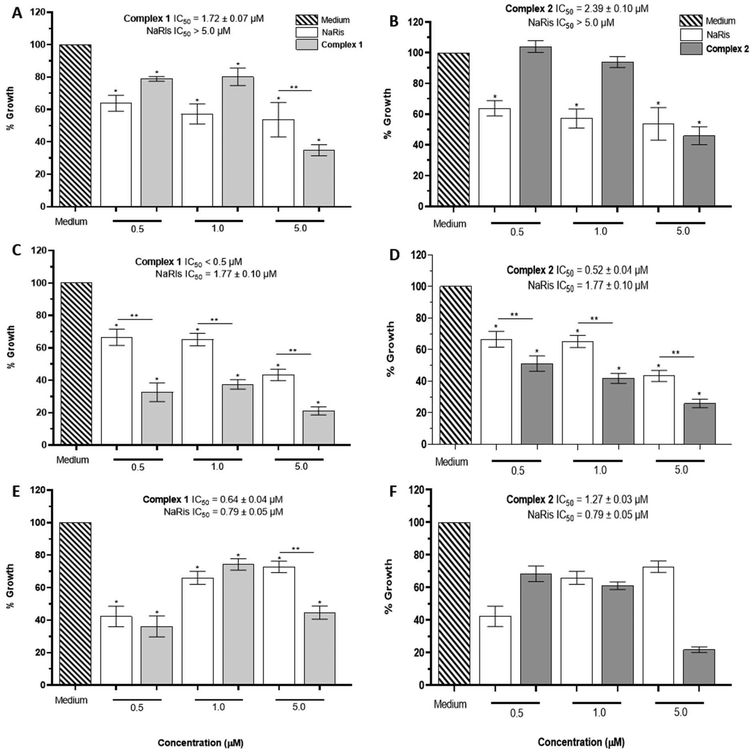
In the case of promastigotes from L. mexicana, compounds 1 and 2 at concentrations 0.5, 1.0, and 5.0 μM at the three different times mentioned before were also tested. In this case, at 24 h, the inhibition was better with NaRis at 0.5, 1.0, and 5.0 μM, except for 1 at 5.0 μM with 65.17 ± 3.43% of growth inhibition. In general, only at the highest concentration tested were the Cu(II) compounds more effective. The calculated IC50 were complex 1, 1.72 ± 0.07 μM and complex 2, 2.39 ± 0.10 μM, these concentrations are lower than the IC50 for the drug NaRis (Fig. 5A and B).
Interestingly, after 48 h of exposure to both Cu(II)-compounds, it was observed that they were more effective than NaRis at all concentrations used. In the case of L. mexicana the mean inhibitory concentration turned out to be less than 0.50 μM for complex 1 and 0.52 ± 0.04 μM for complex 2, while the IC50 of NaRis was 1.77 ± 0.10 μM (Fig. 5C and D). When analyzing the results in the maximum time evaluated (72 h), the growth percentage values varied irregularly concerning the concentration of the compounds. Only a more significant inhibition was observed at the highest concentrations with the Cu(II) compounds compared with NaRis. However, the calculated IC50 was similar for the three compounds (Fig. 5E and F).
On the other hand, we evaluated the metabolic activity of the parasites after incubation with NaRis and complexes 1 and 2 at 48 h using the MTT assay. Complexes 1 and 2 decreased the percentage of metabolic activity gradually with the increase in the concentration of the compound (data not shown), obtaining for L. mexicana IC50 of 5.31 ± 0.03 μM and 3.97 ± 0.18 μM for complexes 1 and 2, respectively. With T. cruzi the IC50 value for complex 1 was like that obtained with L. mexicana. For NaRis the IC50 were not reached at the maximum concentration testes (7.6 and 7.0 μM) (Table 3).
| Compound | CC50 (μM), (VERO) | IC50 (μM), T. cruzi Qro | IC50 (μM), L. mexicana M379 |
|---|---|---|---|
| CC50 cytotoxic concentration is the concentration that causes 50% of cell death IC50 is the concentration that inhibits 50% metabolic activity. The values represent the average of 2 experiments ± standard deviation, evaluated by the MTT method after 48 h of incubation with the compounds. ND nor done. | |||
| NaRis | 0.61 ± 0.06 | >7.6 | >7.0 |
| 1 | 1.45 ± 0.04 | 5.27 ± 0.12 | 5.31 ± 0.03 |
| 2 | 0.67 ± 0.03 | ND | 3.97 ± 0.18 |
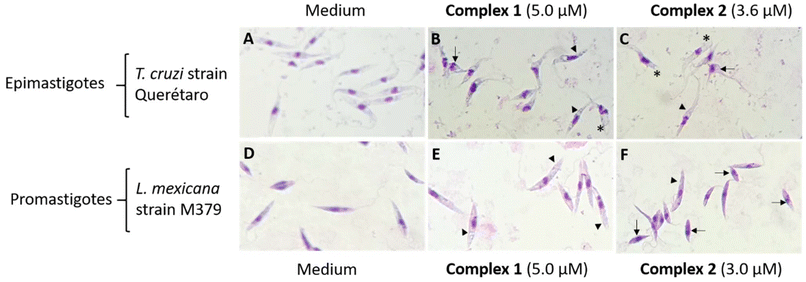
To evaluate the morphological damage on parasites, different concentrations of the compounds 1–2 were tested (data not shown), including the IC50 calculated by the MTT assays. Parasites were compared with those incubated with medium (Fig. 6A–D). For T. cruzi epimastigote the observed cellular alterations were cell membrane damage, vacuolization, loss of flagellum, and smaller size with complex 1 (5.0 μM) and complex 2 (3.6 μM), (Fig. 6B and C). For L. mexicana, similar damages were observed for complex 1 (5.0 μM) and complex 2 (3.0 μM) (Fig. 6E and F).
A previous work has reported the use of a related risedronate complex with formula [Cu(Ris)2], however, in that work the percentage of growth inhibition obtained on T. cruzi epimastigotes turned out to be only of 16% with a concentration at 25 μM.53
Metal-free Ris has been previously tested in several parasitological studies which have demonstrated intracellular inhibition for Toxoplasma gondii of 70% with an IC50 of 95 μM after 19 h of treatment.73
In this line, Garzoni used Ris to inhibit the proliferation of epimastigotes of the Y and EP strains of T. cruzi, at concentrations of 25 μM to 400 μM, obtaining IC50 values of 30.3 μM and 26.4 μM, respectively.74
Therefore, the results of the present work are promising because using lower concentrations was possible to inhibit the growth, metabolic activity and produce cell damage of T. cruzi and L. mexicana.
Taking into account that the lowest inhibitory concentration of parasite proliferation and the shortest exposure time to the compound are sought, it can be said that both Cu(II)-complexes 1–2 are more efficient than drug NaRis.
Molecular docking
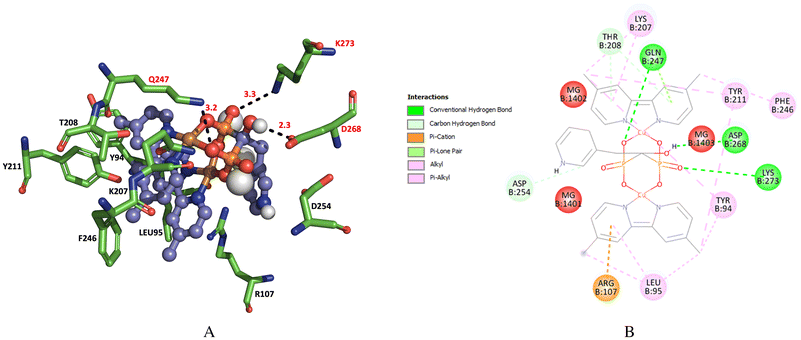
The farnesyl diphosphate synthase of T. cruzi (TcFPPS) is a homodimeric Mg(II) dependent enzyme (Fig. S10†), for molecular docking we use the crystal reported in the protein data bank with accession number 1YHM, we analyze the interactions of Cu(II)–Ris complexes 1 and 2 with the enzyme. It has been reported to have two highly conserved aspartate-rich motifs named the first aspartate-rich motif (FARM) and second aspartate-rich motif (SARM) located at opposite sides of the active site.44,75,76 The interaction with amino acid residue ARG 107 (R107), GLN 247 (Q247), LYS 264 (K264) and LYS 207 (K207) stabilizes the binding between the enzyme and Ris (Fig. S11 and S12†) with a binding energy of ΔG = −8.1 kcal mol−1.When we carried out the analysis of the interactions between enzyme and complex 1, we found that the amino acids LYS 51 (K51), LYS 207 (K207), GLN 247 (Q247), LYS 264 (K264) and LYS 362 (K362) are stabilizing of the binding in the complex 1-protein (Fig. 7A and B) with an estimated energy of ΔG = −8.3 kcal mol−1.
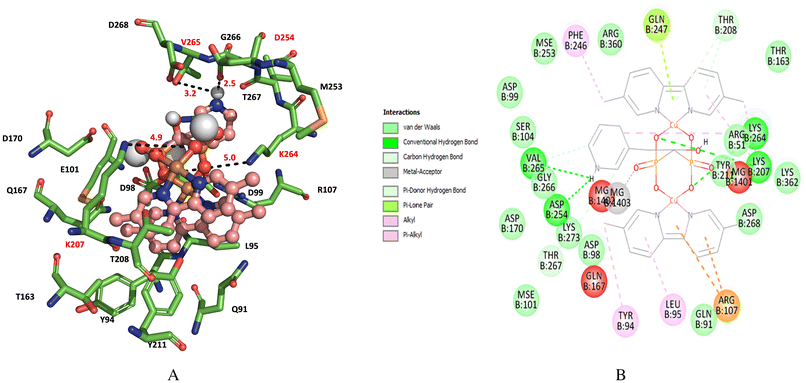
Finally, we find for complex 2 that the interaction with amino acid residue LYS 207 (K207), ASP 254 (D254), LYS 264 (K264), VAL 265 (V265) are stabilizing of the binding between the enzyme and complex 2 (Fig. 8A and B) and with calculated energy of ΔG = −8.5 kcal mol−1, which is slightly more negative than the energy found with complex 1.
Unlike NaRis, complexes 1 and 2 present interactions with the homoallylic region of the catalytic site (R51, Y49 interaction) conferring greater stability.
It is well-known that the FPPS enzyme catalyzes two consecutive condensation reactions, one of which takes place on a DMAPP allyl substrate and the other on the IPP homoallylic substrate, the end product of which is FPP.
The inhibition of this condensation involves the blocking of the synthesis of FPP, which is an interesting therapeutic target, and it has also been shown that several compounds derived from the phosphonate group have activity against several trypanosomatid organisms,47,48,77 including the effect of the study carried out by Bouzahzah in 2005, where they showed that NaRis significantly increases the survival of mice that were infected with T. cruzi.78
Inhibition of these allylic and homoallylic sites are responsible for the inhibition of FPPS activity. According to our studies, we observed that comparing risedronate with compound 1, the latter interacts with the homoallylic region of the enzyme, which could be interpreted as a better inhibitor of FPSS activity, we also analyzed the favorable values of entropy, as previously reported, these values are very similar to those found by Aripirala.75
These study reports that drug NaRis and complex 1 have interactions with all three Mg(II) atoms in their phosphate groups, while complex 2 interacts with only one Mg(II).
We also report the amino acids that are key to the interactions between each of the tested compounds and the TcFPPS enzyme, thus providing new knowledge about the interaction of new phosphonate derivatives with their target. Aripirala also reported that IPP binds to TcFPPS through Arg51, Arg 108 and Arg360 residues, while in our study we found that compound 1 also binds through its interaction with Arg51.75
Conclusions
We have synthesized two novel water-soluble polynuclear Cu(II) compounds with the bioactive drug risedronate, a tetranuclear complex [Cu4(4,4′-dmbp)4(μ3-HRis)2Cl2], 1 and a dinuclear complex [Cu2(5,5′-dmbp)2(μ2-HRis)(H2O)Cl], 2.These complexes were described structurally by single-crystal X-ray diffraction, characterized by several analytical tools (EPR, HRMS, UV-Vis, IR-ATR) and tested as anti-Trypanosoma cruzi and anti-Leishmania mexicana agents.
Cu(II)–Ris complexes 1–2 may be readily prepared through the reaction of CuCl2, a dimethyl-2,2′-bipyridine and disodium salt of risedronate in aqueous media.
Complex 1 possesses a tetranuclear Cu4 core containing two double-bridged bisphosphonate moieties from two different risedronate ligands in a coordination environment of μ3,η4-bisphosphonate, while complex 2 involves a dinuclear Cu2 core built by a μ2-η4-bisphosphonate. Such coordination modes of bisphosphonate moieties still are rare in literature.
Complexes 1–2 are hydrostable in the millimolar concentration range and present low cytotoxicity (<22% in vitro on the growth of cancer cell lines and on healthy COS-7 cells) similar to commercial drug NaRis.
The antiparasitic results demonstrated that Cu(II)–Ris complexes 1–2 have higher activity against epimastigote form of T. cruzi and the promastigote form of L. mexicana compared to NaRis in the low micromolar range, furthermore, these Cu(II)-compounds presented moderated selectivity index using VERO cells as mammalian cell model.
Importantly these complexes affected morphological damage that affects the viability of the parasites. The use of bioinformatics tools such as molecular docking provides a basis for the search for new trypanocidal agents, since these models provide information about the biological activity of new Cu(II)–Ris complexes.
Overall, these results further highlight the usefulness of water-soluble polynuclear Cu(II)-bipyridine complexes bearing the ligand risedronate as promising candidates for further anti-T. cruzi and anti-L. mexicana drug development. Studies of the effect of these compounds in infection in vitro and in vivo should be done in the future to have more information on these promising novel polynuclear Cu(II)–Ris compounds.
Experimental section
Materials and methods
General conditions, chemical reagents and equipment are described in the ESI.†Chemical synthesis of Cu(II)–Ris complexes 1–2
For elemental analysis, the crystalline sample was dried in vacuo at r.t for 24 h. Found: C, 43.56; H, 4.67; N, 8.14%. Calc. for [Cu4C62H64Cl2N10O14P4]·4(H2O): C, 43.95; H, 4.28; N, 8.27%. IR-ATR (cm−1): 3372m ν(C-OH), 3056w ν(Caromatic–H), 1615s ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1222w νas(PO2−), 1131m νs(PO2−), 1023s ρ(C–H). m.p: 215 °C. ESI(+)-HRMS (m/z): calculated for [Cu2C31H32N5O7P2]+ corresponding to monocationic species [Cu2(4,4′-dmbp)2(HRis)]+, 774.036891. Found: 774.037583. UV-Vis: λmax (nm) in H2O: 646 (ε = 175 M−1 cm−1). EPR (H2O, 77 K): g⊥ = 2.0946 and g∥ = 2.2400.
N), 1222w νas(PO2−), 1131m νs(PO2−), 1023s ρ(C–H). m.p: 215 °C. ESI(+)-HRMS (m/z): calculated for [Cu2C31H32N5O7P2]+ corresponding to monocationic species [Cu2(4,4′-dmbp)2(HRis)]+, 774.036891. Found: 774.037583. UV-Vis: λmax (nm) in H2O: 646 (ε = 175 M−1 cm−1). EPR (H2O, 77 K): g⊥ = 2.0946 and g∥ = 2.2400.
For elemental analysis, the crystalline sample was dried in vacuo at r.t for 24 h. Found: C, 44.91; H, 4.13; N, 8.45%. Calc. for [C31H34ClCu2N5O8P2]: C, 44.78; H, 4.21; N, 8.40%. IR-ATR (cm−1): 3375m ν(C-OH), 3045w ν(Caromatic–H), 1476s ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1230w νas(PO2−), 1137m νs(PO2−), 1045s ρ(C–H). m.p. = 206 °C.
N), 1230w νas(PO2−), 1137m νs(PO2−), 1045s ρ(C–H). m.p. = 206 °C.
ESI(+)-HRMS (m/z): calculated for [C31H32Cu2N5O7P2]+ corresponding to monocationic species [Cu2(5,5′-dmbp)2(HRis)]+, 774.036891. Found: 774.036804. UV-Vis: λmax (nm) in H2O: 643 (ε = 140 M−1 cm−1). EPR (H2O, 77 K): g⊥ = 2.0915 and g∥ = 2.2325.
In both cases, the highly disordered water molecules were modeled using the SQUEEZE tool implemented in PLATON.83 The disorder moieties in complexes 1 and 2 were modeled using RIGU, SIMU, SAME and EADP instructions described in SHELXL.80
In complex 1, in the axial position of Cu1 and Cu4 presented occupational disorder between Cl atoms and water molecules, for the Cu1 atom, the occupational ratio is 27/73 while for the Cu4 atom it is 40/60 between the Cl atoms and the water molecules respectively.
Because the two pyridinium fragments are protonated, it is necessary that the occupation of the Cl− ions is equal to 2, for this reason, the occupation of the Cl atoms is modeled in partial occupation of 73% for Cl2 and 60% for Cl4. However, the exact positions of the Cl− anions cannot be determined with certainty due to the possible positional disorder between Cl−/H2O of the highly disordered solvent that was modeled with the PLATON SQUEEZE tool. One of the pyridine moieties presents a positional disorder that was modeled in 2 positions in ratio 50/50.
In 2, the axial position in the square-based pyramid geometry of the Cu(II) atom labeled as Cu2, presents occupational disorder in a 50/50 relationship with a Cl− atom and a water molecule, the charge of the Cl− atom with 50% occupation is balanced by a hydrogen atom with 50% occupation that protonates one of the oxygen atoms of the phosphate group. For the case of the occluded solvent molecules in the crystal of compound 2, only two CH3CN molecules with occupancy of 71 and 52% respectively, as well as three water molecules with partial occupations of 33, 24 and 13% respectively, could be partially modeled. The molecular graphics were prepared using Olex2.84 Crystallographic data for the two crystal structures have been deposited with the Cambridge Crystallographic Data Centre, no. CCDC 2329846 and 2396933.† X-ray crystallographic data in CIF format are available in ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cell per mL, were seeded in 96 well micro-titer plates (Costar) by triplicates and incubated at 37 °C for 24 h in a 5% CO2 atmosphere to allow for cell attachment. After incubation, 100 μL of a solution of the compound obtained by diluting the stocks were added to each well. After 48 h, adherent cell culture was fixed in situ by the addition of 50 μL of cold 50% aqueous trichloroacetic acid (TCA), and incubated for 60 min at 4 °C. The supernatant was discarded, and the plates were washed with water and air-dried. Cultures fixed with TCA were stained by the addition of 0.4% SRB. Free SRB solution was removed by washing with 1% aqueous acetic acid. Protein bounded dye was extracted with 10 mM of non-buffered Tris base and the optical densities (515 nm) were measured on Microplate Reader Synergy (Bio-Tex Instruments)
000 cell per mL, were seeded in 96 well micro-titer plates (Costar) by triplicates and incubated at 37 °C for 24 h in a 5% CO2 atmosphere to allow for cell attachment. After incubation, 100 μL of a solution of the compound obtained by diluting the stocks were added to each well. After 48 h, adherent cell culture was fixed in situ by the addition of 50 μL of cold 50% aqueous trichloroacetic acid (TCA), and incubated for 60 min at 4 °C. The supernatant was discarded, and the plates were washed with water and air-dried. Cultures fixed with TCA were stained by the addition of 0.4% SRB. Free SRB solution was removed by washing with 1% aqueous acetic acid. Protein bounded dye was extracted with 10 mM of non-buffered Tris base and the optical densities (515 nm) were measured on Microplate Reader Synergy (Bio-Tex Instruments)
Samples were tested in duplicate in two independent assays, and the percentage of reductase activity was compared to total activity in the non-treated control group.
Data availability
Crystallographic data for compounds 1 and 2 have been deposited at the Cambridge Crystallographic Data Centre under CCDC 2329846 (complex 1) and 2396933 (complex 2).†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank M. Sc. Eréndira García Rios, M. Sc. Lucero Mayra Ríos Ruiz, M. Sc. Lucía del Carmen Márquez Alonso, M. Sc. Lizbeth Triana Cruz, M. Sc. Teresa Ramírez-Apam, Dra. Beatriz Quiroz-García, Dra. Adriana Romo Pérez, M. Sc. Virginia Gómez Vidales, Chem. María de la Paz Orta Pérez, Dra. Isabel Chávez Uribe and M. Sc. Elizabeth Huerta Salazar for technical assistance. We thank CONACYT PRONACES-160671 and PAPIIT-UNAM IN220023 for financial support. M. A. R.-S. and V. G.-L., A.K. E.-C. are grateful to CONAHCYT for scholarship 1315102, 1269866 and 2038145, respectively. E. C. R.-P. thanks to CONAHCyT for postdoctoral fellowship.References
- A. F. Francisco, S. Jayawardhana, F. Olmo, M. D. Lewis, S. R. Wilkinson, M. C. Taylor and J. M. Kelly, Challenges in Chagas Disease Drug Development, Molecules, 2020, 25, 1–14 CrossRef.
- S. S. Santos, R. V. de Araújo, J. Giarolla, O. El Seoud and E. I. Ferreira, Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: a review, Int. J. Antimicrob. Agents, 2020, 55, 105906 CrossRef CAS PubMed.
- J. C. B. Santos, J. A. De Melo, S. Maheshwari, W. M. T. Q. De Medeiros, J. W. D. F. Oliveira, C. J. Moreno, L. M. Amzel, S. B. Gabelli and M. S. Silva, Bisphosphonate-based molecules as potential new antiparasitic drugs, Molecules, 2020, 25, 2602 CrossRef CAS PubMed.
- A. Cavalli and M. L. Bolognesi, Neglected tropical diseases: Multi-target-directed ligands in the search for novel lead candidates against Trypanosoma and Leishmania, J. Med. Chem., 2009, 52, 7339–7359 CrossRef CAS PubMed.
- World Health Organization (WHO). Neglected Tropical Diseases. https://www.who.int/neglected_diseases/diseases/en (accessed December 14, 2024).
- S. Aripirala, D. González-Pacanowska, E. Oldfield, M. Kaiser, L. M. Amzel and S. B. Gabelli, Structural and thermodynamic basis of the inhibition of Leishmania major farnesyl diphosphate synthase by nitrogen-containing bisphosphonates, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2014, 70, 802–810 CrossRef CAS PubMed.
- A. Requena-Méndez, E. Aldasoro, E. de Lazzari, E. Sicuri, M. Brown, D. A. J. Moore, J. Gascon and J. Muñoz, Prevalence of Chagas Disease in Latin-American Migrants Living in Europe: A Systematic Review and Meta-analysis, PLoS Neglected Trop. Dis., 2015, 9, e0003540 CrossRef PubMed.
- C. Bern, L. A. Messenger, J. D. Whitman and J. H. Maguire, Chagas disease in the United States: A public health approach, Clin. Microbiol. Rev., 2019, 33, e00023–e00019 CrossRef PubMed.
- A. S. de Sousa, D. Vermeij, A. N. Ramos and A. O. Luquetti, Chagas disease, Lancet, 2024, 403, 203–218 CrossRef PubMed.
- A. Monroy-Ostria and G. Sanchez-Tejeda, in The Epidemiology and Ecology of Leishmaniasis, InTech, 2017, ch. 9, pp. 153–165 Search PubMed.
- P. Díaz-Garrido, R. E. Cárdenas-Guerra, I. Martínez, S. Poggio, K. Rodríguez-Hernández, L. Rivera-Santiago, J. Ortega-López, S. Sánchez-Esquivel and B. Espinoza, Differential activity on trypanosomatid parasites of a novel recombinant defensin type 1 from the insect Triatoma (Meccus) pallidipennis, Insect Biochem. Mol. Biol., 2021, 139, 103673 CrossRef.
- R. Arenas, E. Torres-Guerrero, M. R. Quintanilla-Cedillo and J. Ruiz-Esmenjaud, Leishmaniasis: A review, F1000Research, 2017, 6, 1–15 Search PubMed.
- M. Berzunza-Cruz, Á. Rodríguez-Moreno, G. Gutiérrez-Granados, C. González-Salazar, C. R. Stephens, M. Hidalgo-Mihart, C. F. Marina, E. A. Rebollar-Téllez, D. Bailón-Martínez, C. D. Balcells, C. N. Ibarra-Cerdeña, V. Sánchez-Cordero and I. Becker, Leishmania (L.) mexicana Infected Bats in Mexico: Novel Potential Reservoirs, PLoS Neglected Trop. Dis., 2015, 9, e0003438 CrossRef PubMed.
- F. Lascano, F. García Bournissen and J. Altcheh, Review of pharmacological options for the treatment of Chagas disease, Br. J. Clin. Pharmacol., 2022, 88, 383–402 CrossRef CAS.
- S. Patterson and S. Wyllie, Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects, Trends Parasitol., 2014, 30, 289–298 CrossRef CAS PubMed.
- P. García-Huertas and N. Cardona-Castro, Advances in the treatment of Chagas disease: Promising new drugs, plants and targets, Biomed. Pharmacother., 2021, 142, 112020 CrossRef.
- S. L. Croft and P. Olliaro, Leishmaniasis chemotherapy-challenges and opportunities, Clin. Microbiol. Infect., 2011, 17, 1478–1483 CrossRef CAS PubMed.
- M. Fandzloch, T. Jędrzejewski, L. Dobrzańska, G. M. Esteban-Parra, J. Wiśniewska, A. Paneth, P. Paneth and J. Sitkowski, New organometallic ruthenium(II) complexes with purine analogs - a wide perspective on their biological application, Dalton Trans., 2021, 50, 5557–5573 RSC.
- Y. C. Ong, S. Roy, P. C. Andrews and G. Gasser, Metal Compounds against Neglected Tropical Diseases, Chem. Rev., 2019, 119, 730–796 CrossRef CAS PubMed.
- M. Tiphine, V. Letscher-Bru and R. Herbrecht, Amphotericin B and its new formulations: Pharmacologic characteristics, clinical efficacy, and tolerability, Transplant Infect. Dis., 1999, 1, 273–283 CrossRef CAS PubMed.
- M. Piccica, F. Lagi, A. Bartoloni and L. Zammarchi, Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: A systematic review, J. Travel Med., 2021, 28, 1–13 CrossRef.
- A. Jhingran, B. Chawla, S. Saxena, M. P. Barrett and R. Madhubala, Paromomycin: Uptake and resistance in Leishmania donovani, Mol. Biochem. Parasitol., 2009, 164, 111–117 CrossRef CAS.
- I. Y. Zaghloul and M. Al-Jasser, Effect of renal impairment on the pharmacokinetics of antimony in hamsters, Ann. Trop. Med. Parasitol., 2004, 98, 793–800 CrossRef CAS.
- K. D. Rodríguez-Hernández, I. Martínez, L. T. Agredano-Moreno, L. F. Jiménez-García, R. Reyes-Chilpa and B. Espinoza, Coumarins isolated from Calophyllum brasiliense produce ultrastructural alterations and affect in vitro infectivity of Trypanosoma cruzi, Phytomedicine, 2019, 61, 152827 CrossRef.
- K. D. Rodríguez-Hernández, I. Martínez, R. Reyes-Chilpa and B. Espinoza, Mammea type coumarins isolated from Calophyllum brasiliense induced apoptotic cell death of Trypanosoma cruzi through mitochondrial dysfunction, ROS production and cell cycle alterations, Bioorg. Chem., 2020, 100, 103894 CrossRef.
- M. B. Camarada, C. Echeverria and R. Ramirez-Tagle, Medicinal organometallic compounds with anti-chagasic activity, MedChemComm, 2016, 7, 1307–1315 RSC.
- D. Gambino and L. Otero, Metal Compounds in the Development of Antiparasitic Agents: Rational Design from Basic Chemistry to the Clinic, Met. Ions Life Sci., 2019, 19, 331–357 CAS.
- J. C. Carrero, B. Espinoza, L. Huerta, M. Silva-Miranda, S. L. Guzmán-Gutierrez, A. Dorazco-González, R. Reyes-Chilpa, C. Espitia and S. Sánchez, Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases, Pharmaceuticals, 2024, 17, 957 CrossRef CAS.
- M. Navarro, C. Gabbiani, L. Messori and D. Gambino, Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives, Drug Discovery Today, 2010, 15, 1070–1078 CrossRef CAS.
- C. F. N. Da Silva, P. B. H. Chrispim, B. Possato, G. B. Portapilla, T. N. Rohrabaugh, L. C. B. Ramos, R. Santana Da Silva, S. De Albuquerque, C. Turro and S. Nikolaou, Anticancer and antitrypanosomal activities of trinuclear ruthenium compounds with orthometalated phenazine ligands, Dalton Trans., 2020, 49, 16440–16452 RSC.
- F. Figueirôa Moreira, J. de Araujo Portes, N. F. Barros Azeredo, C. Fernandes, A. Horn Jr., C. P. Santiago, B. B. Segat, G. F. Caramori, L. M. P. Madureira, D. R. S. Candela, M. M. Marques, J. A. L. C. Resende, W. De Souza, R. A. DaMatta and S. H. Seabra, Development of new dinuclear Fe(III) coordination compounds with in vitro nanomolar antitrypanosomal activity, Dalton Trans., 2021, 50, 12242–12264 RSC.
- J. Benítez, A. Cavalcanti De Queiroz, I. Correia, M. A. Alves, M. S. Alexandre-Moreira, E. J. Barreiro, L. M. Lima, J. Varela, M. González, H. Cerecetto, V. Moreno, J. Costa Pessoa and D. Gambino, New oxidovanadium(IV) N-acylhydrazone complexes: Promising antileishmanial and antitrypanosomal agents, Eur. J. Med. Chem., 2013, 62, 20–27 CrossRef PubMed.
- J. Benítez, L. Guggeri, I. Tomaz, G. Arrambide, M. Navarro, J. Costa Pessoa, B. Garat and D. Gambino, Design of vanadium mixed-ligand complexes as potential anti-protozoa agents, J. Inorg. Biochem., 2009, 103, 609–616 CrossRef PubMed.
- M. Fernández, J. Varela, I. Correia, E. Birriel, J. Castiglioni, V. Moreno, J. Costa Pessoa, H. Cerecetto, M. González and D. Gambino, A new series of heteroleptic oxidovanadium(IV) compounds with phenanthroline-derived co-ligands: Selective Trypanosoma cruzi growth inhibitors, Dalton Trans., 2013, 42, 11900–11911 RSC.
- L. Becco, A. Rodríguez, M. E. Bravo, M. J. Prieto, L. Ruiz-Azuara, B. Garat, V. Moreno and D. Gambino, New achievements on biological aspects of copper complexes Casiopeínas®: Interaction with DNA and proteins and anti-Trypanosoma cruzi activity, J. Inorg. Biochem., 2012, 109, 49–56 CrossRef CAS.
- A. Reddy, L. S. Sangenito, A. D. A. Guedes, M. H. Branquinha, K. Kavanagh, J. McGinley, A. L. S. Dos Santos and T. Velasco-Torrijos, Glycosylated metal chelators as anti-parasitic agents with tunable selectivity, Dalton Trans., 2017, 46, 5297–5307 RSC.
- D. Benítez, M. L. Lavaggi, D. Gambino, M. H. Torre, H. Cerecetto and M. González, Effect of complexation of 3-aminoquinoxaline-2-carbonitrile 1,4-dioxides with palladium and copper on their anti-T. cruzi activity, Med. Chem. Res., 2012, 21, 1439–1444 CrossRef.
- M. Vieites, P. Smircich, B. Parajón-Costa, J. Rodríguez, V. Galaz, C. Olea-Azar, L. Otero, G. Aguirre, H. Cerecetto, M. González, A. Gómez-Barrio, B. Garat and D. Gambino, Potent in vitro anti-Trypanosoma cruzi activity of pyridine-2-thiol N-oxide metal complexes having an inhibitory effect on parasite-specific fumarate reductase, J. Biol. Inorg. Chem., 2008, 13, 723–735 CrossRef CAS.
- E. Rodríguez Arce, E. Putzu, M. Lapier, J. D. Maya, C. Olea-Azar, G. A. Echeverría, O. E. Piro, A. Medeiros, F. Sardi, M. Comini, G. Risi, G. Salinas, I. Correia, J. C. Pessoa, L. Otero and D. Gambino, New heterobimetallic ferrocenyl derivatives are promising antitrypanosomal agents, Dalton Trans., 2019, 48, 7644–7658 RSC.
- M. Vieites, L. Otero, D. Santos, C. Olea-Azar, E. Norambuena, G. Aguirre, H. Cerecetto, M. González, U. Kemmerling, A. Morello, J. D. Maya and D. Gambino, Platinum-based complexes of bioactive 3-(5-nitrofuryl)acroleine thiosemicarbazones showing anti-Trypanosoma cruzi activity, J. Inorg. Biochem., 2009, 103, 411–418 CrossRef CAS.
- M. Vieites, L. Otero, D. Santos, J. Toloza, R. Figueroa, E. Norambuena, C. Olea-Azar, G. Aguirre, H. Cerecetto, M. González, A. Morello, J. D. Maya, B. Garat and D. Gambino, Platinum(II) metal complexes as potential anti-Trypanosoma cruzi agents, J. Inorg. Biochem., 2008, 102, 1033–1043 CrossRef CAS.
- M. Vieites, P. Smircich, L. Guggeri, E. Marchán, A. Gómez-Barrio, M. Navarro, B. Garat and D. Gambino, Synthesis and characterization of a pyridine-2-thiol N-oxide gold(I) complex with potent antiproliferative effect against Trypanosoma cruzi and Leishmania sp. insight into its mechanism of action, J. Inorg. Biochem., 2009, 103, 1300–1306 CrossRef CAS.
- I. S. Oliveira, M. S. A. Garcia, N. M. Cassani, A. L. C. Oliveira, L. C. F. Freitas, V. K. S. Bertolini, J. Castro, G. Clauss, J. Honorato, F. R. Gadelha, D. C. Miguel, A. C. G. Jardim and C. Abbehausen, Exploring antiviral and antiparasitic activity of gold N-heterocyclic carbenes with thiolate ligands, Dalton Trans., 2024, 47, 18963–18973 RSC.
- A. Montalvetti, B. N. Bailey, M. B. Martin, G. W. Severin, E. Oldfield and R. Docampo, Bisphosphonates Are Potent Inhibitors of Trypanosoma cruzi Farnesyl Pyrophosphate Synthase, J. Biol. Chem., 2001, 276, 33930–33937 CrossRef CAS PubMed.
- H. Sahana, D. K. Khajuria, R. Razdan, D. R. Mahapatra, M. R. Bhat, S. Suresh, R. R. Rao and L. Mariappan, Improvement in bone properties by using risedronate adsorbed hydroxyapatite novel nanoparticle based formulation in a rat model of osteoporosis, J. Biomed. Nanotechnol., 2013, 9, 193–201 CrossRef CAS.
- G. B. Deacon, C. M. Forsyth, N. B. Greenhill, P. C. Junk and J. Wang, Coordination Polymers of Increasing Complexity Derived from Alkali Metal Cations and (4-Amino-1-hydroxybutylidine)-1,1-bisphosphonic Acid (Alendronic Acid): The Competitive Influences of Coordination and Supramolecular Interactions, Cryst. Growth Des., 2015, 15, 4646–4662 CrossRef CAS.
- M. B. Martin, J. S. Grimley, J. C. Lewis, H. T. Heath, B. N. Bailey, H. Kendrick, V. Yardley, A. Caldera, R. Lira, J. A. Urbina, S. N. J. Moreno, R. Docampo, S. L. Croft and E. Oldfield, Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: A potential route to chemotherapy, J. Med. Chem., 2001, 44, 909–916 CrossRef CAS PubMed.
- M. B. Martin, A. Burzynska, P. Kafarski, S. L. Croft, E. Oldfield, J. M. Sanders, H. Kendrick, K. De Luca-Fradley, J. C. Lewis, J. S. Grimley, E. M. Van Brussel, J. R. Olsen and G. A. Meints, Activity of bisphosphonates against Trypanosoma brucei rhodesiense, J. Med. Chem., 2002, 45, 2904–2914 CrossRef CAS PubMed.
- T. Galaka, B. N. Falcone, C. Li, S. H. Szajnman, S. N. J. Moreno, R. Docampo and J. B. Rodriguez, Synthesis and biological evaluation of 1-alkylaminomethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii, Bioorg. Med. Chem., 2019, 27, 3663–3673 CrossRef CAS PubMed.
- A. Ortiz-Gómez, C. Jiménez, A. M. Estévez, J. Carrero-Lérida, L. M. Ruiz-Pérez and D. González-Pacanowska, Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overexpression confers resistance to risedronate, Eukaryotic Cell, 2006, 5, 1057–1064 CrossRef.
- D. Thakre, S. R. Ali, S. Mehta, N. Alam, M. Ibrahim, D. Sarma, A. Mondal, M. De and A. Banerjee, Polyoxovanadates with Ethylidene-Pyridine Functionalized Bisphosphonate Ligands: Synthesis, Structure, Spectroscopic Characterization, Magnetic, and Antibacterial Studies, Cryst. Growth Des., 2021, 21, 4285–4298 CrossRef CAS.
- C. Li, C. Q. Jiao, Z. G. Sun, K. Chen, C. L. Wang, Y. Y. Zhu, J. Zhu, Y. Zhao, M. J. Zheng, S. H. Sun, W. Chu and H. Tian, Synthesis, structures and surface photovoltage properties of four novel metal phosphonates with a 3D supramolecular structure, CrystEngComm, 2012, 14, 5479–5486 RSC.
- B. Demoro, F. Caruso, M. Rossi, D. Benítez, M. Gonzalez, H. Cerecetto, B. Parajón-Costa, J. Castiglioni, M. Galizzi, R. Docampo, L. Otero and D. Gambino, Risedronate metal complexes potentially active against Chagas disease, J. Inorg. Biochem., 2010, 104, 1252–1258 CrossRef CAS PubMed.
- A. A. Vannathan, D. Thakre, S. R. Ali, M. De, A. Banerjee and S. S. Mal, Investigations into the supercapacitor activity of bisphosphonate-polyoxovanadate compounds, J. Solid State Chem., 2021, 304, 122566 CrossRef CAS.
- K. R. Ma, J. Z. Yin, H. Y. Hu and Y. H. Kan, Syntheses, Structures and Fluorescent Properties of Two Zn(II)-Diphosphonate Coordination Polymers, J. Cluster Sci., 2021, 32, 875–886 CrossRef CAS.
- K. R. Ma, L. Cao, M. H. Cong, Y. H. Kan and R. Q. Li, Three pyridyl modified Cu(II)/Cd(II)-diphosphonates: Syntheses, crystal structures and properties, J. Mol. Struct., 2017, 1139, 67–77 CrossRef CAS.
- A. Banerjee, F. S. Raad, N. Vankova, B. S. Bassil, T. Heine and U. Kortz, Polyoxomolybdodiphosphonates: Examples incorporating ethylidenepyridines, Inorg. Chem., 2011, 50, 11667–11675 CrossRef CAS PubMed.
- S. Martínez-Vargas, A. Dorazco-González, S. Hernández-Ortega, R. A. Toscano, J. E. Barquera-Lozada and J. Valdés-Martínez, Interaction between aromatic rings as organizing tools and semi-coordination in Cu(II) compounds, CrystEngComm, 2017, 19, 4595–4604 RSC.
- S. Youngme, P. Phuengphai, N. Chaichit, G. A. Van Albada, O. Roubeau and J. Reedijk, An unprecedented tetranuclear Cu(II) cluster, exclusively bridged by two μ3,η3-hydrogenphosphate anions: Synthesis, structure, and magnetic properties, Inorg. Chim. Acta, 2005, 358, 849–853 CrossRef CAS.
- R. P. Doyle, P. E. Kruger, B. Moubaraki, K. S. Murray and M. Nieuwenhuyzen, Synthesis and structural and magnetic characterisation of tetranuclear Cu(II) complexes possessing novel [Cu4(μ4-PO4)2-(μ2-CO3)] butterfly cores that exhibit supramolecular isomerism, Dalton Trans., 2003, 4, 4230–4237 RSC.
- T. J. Greenfield, T. Takemoto, J. Cano, F. Lloret, M. Julve, J. Zubieta and R. P. Doyle, A methylenediphosphonate bridged copper(II) tetramer: Synthesis, structural, thermal, and magnetic characterization of [Cu4(H2O)2(phen)4(μ-P2O6CH2)2]·21H2O, Polyhedron, 2019, 169, 162–168 CrossRef CAS.
- A. W. Addison and T. N. Rao, Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[l,7-bis(N-methylbenzimidazol-2′-yl)- 2,6-dithiaheptane]copper(II) Perchlorate, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- A. Dorazco-González, S. Martinez-Vargas, S. Hernández-Ortega and J. Valdés-Martínez, Directed self-assembly of mono and dinuclear copper(II) isophthalates into 1D polymeric structures. Design and an unusual cocrystallization, CrystEngComm, 2013, 15, 5961–5968 RSC.
- Q. J. Niu, Y. Q. Zheng, J. X. Zhou, H. L. Zhu, Q. Huang and W. Xu, Mono-, di-, and trinuclear phosphonate oxygen-bridged copper(II) complexes: syntheses, structures, and properties, J. Coord. Chem., 2016, 69, 1447–1462 CrossRef CAS.
- N. Redman-Furey, M. Dicks, A. Bigalow-Kern, R. T. Cambron, G. Lubey, C. Lester and D. Vaughn, Structural and analytical characterization of three hydrates and an anhydrate form of risedronate, J. Pharm. Sci., 2005, 94, 893–911 CrossRef CAS PubMed.
- G. Canil, S. Braccini, T. Marzo, L. Marchetti, A. Pratesi, T. Biver, T. Funaioli, F. Chiellini, J. D. Hoeschele and C. Gabbiani, Photocytotoxic Pt(IV) complexes as prospective anticancer agents, Dalton Trans., 2019, 48, 10933–10944 RSC.
- J. Valdes-García, J. Zamora-Moreno, C. Pinzón-Vanegas, A. O. Viviano-Posadas, D. Martínez-Otero, J. Barroso-Flores, B. Ortiz-Lopez, V. F. Ortiz-Navarrete and A. Dorazco-González, Selective Luminescent Chemosensing of Chloride Based on a Cyclometalated Platinum(II) Complex in Water: Crystal Structures, Spectroscopic Studies, Extraction, and Bioimaging, Inorg. Chem., 2023, 62, 6629–6641 CrossRef PubMed.
- L. A. Sharma, O. I. Singh, A. K. M. Singh, R. K. H. Singh, R. M. Kadam, M. K. Bhide, A. R. Dhobale and M. D. Sastry, Molecular magnetic properties of two-copper(II) containing complexes [Cu(II) (1-phenylamidino-O-n-butylurea) en (H2O)]22+ and [Cu(II) sulphato-mono (1-phenylamidino-O-methylurea)] 2: An EPR study, Spectrochim. Acta, Part A, 2004, 60, 1593–1600 CrossRef PubMed.
- S. Pramodini Devi, R. K. Hemakumar Singh and R. M. Kadam, Synthesis and spectroscopic studies on copper(II) binuclear complexes of 1-phenylamidino-O-alkylurea (alkyl = n-propyl, n- and iso-butyl) with 1,3-diaminopropane or ethylenediamine, Inorg. Chem., 2006, 45, 2193–2198 CrossRef CAS PubMed.
- K. E. Kinnamon, E. A. Steck and D. S. Rane, Activity of Antitumor Drugs Against African Trypanosomes, Antimicrob. Agents Chemother., 1979, 15, 157–160 CrossRef CAS PubMed.
- G. Lowe, A. S. Droz, T. Vilaivan, G. W. Weaver, L. Tweedale, J. M. Pratt, P. Rock, V. Yardley and S. L. Croft, Cytotoxicity of (2,2′:6′,2′′-Terpyridine)platinum(II) complexes to Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei, J. Med. Chem., 1999, 42, 999–1006 CrossRef CAS PubMed.
- G. Scalese, M. F. Mosquillo, S. Rostán, J. Castiglioni, I. Alho, L. Pérez, I. Correia, F. Marques, J. Costa Pessoa and D. Gambino, Heteroleptic oxidovanadium(IV) complexes of 2-hydroxynaphtylaldimine and polypyridyl ligands against Trypanosoma cruzi and prostate cancer cells, J. Inorg. Biochem., 2017, 175, 154–166 CrossRef CAS PubMed.
- C. O. Rodrigues, D. A. Scott, B. N. Bailey, W. De Souza, M. Benchimol, B. Moreno, J. A. Urbina, E. Oldfield and S. N. J. Moreno, Vacuolar proton pyrophosphatase activity and pyrophosphate (PPi) in Toxoplasma gondii as possible chemotherapeutic targets, Biochem. J., 2000, 349, 737–745 CrossRef CAS.
- L. R. Garzoni, A. Caldera, M. D. N. L. Meirelles, S. L. De Castro, R. Docampo, G. A. Meints, E. Oldfield and J. A. Urbina, Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi, Int. J. Antimicrob. Agents, 2004, 23, 273–285 CrossRef CAS PubMed.
- S. Aripirala, S. H. Szajnman, J. Jakoncic, J. B. Rodriguez, R. Docampo, S. B. Gabelli and L. M. Amzel, Design, synthesis, calorimetry, and crystallographic analysis of 2-alkylaminoethyl-1,1-bisphosphonates as inhibitors of trypanosoma cruzi farnesyl diphosphate synthase, J. Med. Chem., 2012, 55, 6445–6454 CrossRef CAS PubMed.
- S. B. Gabelli, J. S. McLellan, A. Montalvetti, E. Oldfield, R. Docampo and L. M. Amzel, Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: Implications for drug design, Proteins: Struct., Funct., Genet., 2006, 62, 80–88 CrossRef CAS PubMed.
- S. H. Szajnman, G. E. García Liñares, Z. H. Li, C. Jiang, M. Galizzi, E. J. Bontempi, M. Ferella, S. N. J. Moreno, R. Docampo and J. B. Rodriguez, Synthesis and biological evaluation of 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase, Bioorg. Med. Chem., 2008, 16, 3283–3290 CrossRef CAS PubMed.
- B. Bouzahzah, L. A. Jelicks, S. A. Morris, L. M. Weiss and H. B. Tanowitz, Risedronate in the treatment of Murine Chagas’ disease, Parasitol. Res., 2005, 96, 184–187 CrossRef PubMed.
- O. González Q, A. M. Atria, E. Spodine, J. Manzur and M. T. Garland, Structure of Dimeric Dichloro(4,4′-dimethyl-2,2′-bipyridine)copper(II) Hemihydrate, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1993, 49, 1589–1591 CrossRef.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- G. M. Sheldrick, SHELXT - Integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A: Found. Crystallogr., 2015, 71, 3–8 CrossRef PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, ShelXle: A Qt graphical user interface for SHELXL, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
- A. L. Spek, PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- D. Villarreal, C. Barnabé, D. Sereno and M. Tibayrenc, Lack of correlation between in vitro susceptibility to Benznidazole and phylogenetic diversity of Trypanosoma cruzi, the agent of Chagas disease, Exp. Parasitol., 2004, 108, 24–31 CrossRef CAS PubMed.
- E. Grela, A. Ząbek and A. Grabowiecka, Interferences in the optimization of the MTT assay for viability estimation of Proteus mirabilis, Avicenna J. Med. Biotechnol., 2015, 7, 159–167 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General information, X-ray crystallographic data, IR-ATR and EPR spectra for 1–2, crystal packing diagrams of 1–2 and molecular docking depictions. CCDC 2329846 and 2396933. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03516j |
| This journal is © The Royal Society of Chemistry 2025 |