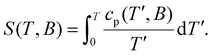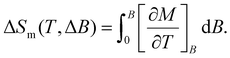Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dodecanuclear [NiII8Ln4] clusters and rings of corner-sharing {NiII2Ln2} cubanes (Ln = Dy, Gd, Y); magnetic and magnetothermal properties†
Thomais G. Tziotzia,
Eleftheria Agapakib,
Emmanouil K. Charkiolakisc,
David Graciac,
Marc Ubach I. Cerverac,
Scott J. Dalgarno d,
Marco Evangelisti
d,
Marco Evangelisti *c,
Euan K. Brechin
*c,
Euan K. Brechin *b and
Constantinos J. Milios
*b and
Constantinos J. Milios *a
*a
aDepartment of Chemistry, The University of Crete, Voutes, 71003, Herakleion, Greece. E-mail: komil@uoc.gr
bEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, Scotland EH9 3FJ, UK. E-mail: E.Brechin@ed.ac.uk
cInstituto de Nanociencia y Materiales de Aragón (INMA), CSIC – Universidad de Zaragoza, 50009 Zaragoza, Spain. E-mail: evange@unizar.es
dInstitute of Chemical Sciences, Heriot-Watt University, Riccarton, Edinburgh, Scotland EH14 4AS, UK
First published on 13th February 2025
Abstract
The solvothermal reaction of NiCl2·6H2O with 1,3,5-tri(2-hydroxyethyl)-1,3,5-triazacyclohexane, LH3, and Ln(OAc)3·4H2O in the presence of salicylaldehyde in MeOH leads to the formation of the dodecanuclear isostructural species [NiII8Ln4(L′)8(OAc)4(OH)8(H2O)4]·xH2O (Ln = Dy, x = 6 (1); Gd, x = 24 (2); Y, x = 10 (3); L′ = the dianion of the Schiff-base resulting from the reaction of salicylaldehyde and ethanolamine). The metallic core of the clusters describes a ring of corner-sharing {Ni2Ln2} cubanes assembled on a central, planar rectangular {Ln4} unit. Magnetization studies conducted on complexes 1–3 demonstrate the presence of dominant ferromagnetic exchange at low temperatures, which is largely attributed to the Ni⋯Ni interactions. The significant magnetocaloric effect observed in the Gd analogue has been assessed through heat capacity and magnetization measurements.
Introduction
The varied physical properties displayed by polymetallic complexes continues to attract the interest of the scientific community, since such species find application in various fields of science and technology, including luminescence, ferroelectricity, molecular imaging and applied bioinorganic chemistry.1 Paramagnetic 3d–4f complexes displaying large spin ground-states resulting from ferro- or ferrimagnetic exchange are of particular interest in areas ranging from information storage to cryogenic refrigeration. For instance, certain 3d–4f clusters have been found to function as single-molecule magnets (SMMs), i.e. molecules that can retain their magnetization once magnetized in the absence of an external magnetic field.2,3 A pertinent example is the recently reported metallacrown complex {Dy[15-MCCu-5]}, which displays the largest effective energy barrier (U = 625 cm−1) for the re-orientation of the magnetization for any reported d–f SMM.4 In addition, heterometallic 3d–4f clusters – particularly those based on GdIII – have been employed as molecular coolants, since they can display an enhanced magnetocaloric effect at cryogenic temperatures.5 A fine example being a {Ni21Gd20} cage with an S = 91 spin ground-state and a low-field magnetic entropy of 14.1 J kg−1 K−1 for ΔB = 1 T at 1.1 K.6We recently started exploring the use of the 1,3,5-tri(2-hydroxyethyl)-1,3,5-triazacyclohexane ligand, LH3 (Scheme 1), in manganese chemistry, which led to the synthesis of a mixed-valent {MnIII12MnII6} wheel, a {MnIII12MnII4} double square wheel and a {MnIII10} contorted wheel,7 thus establishing the versatility of the LH3 ligand towards the formation of polynuclear species. Given the tendency of the LH3 ligand to promote the formation of mixed-valent species, we investigated its use in the MII/LnIII (M = Ni, Co) reaction system. Herein we report the synthesis and characterisation of three dodecanuclear heterometallic [NiII8LnIII4(L′)8(OAc)4(OH)8(H2O)4]·xH2O (Ln = Dy, x = 6 (1); Gd, x = 24 (2); Y, x = 10 (3)) complexes formed upon the decomposition of the LH3 ligand under the solvothermal conditions and the in situ formation of the dianion of the Schiff-base between salicylaldehyde and ethanolamine, L′.
 | ||
| Scheme 1 The ligands discussed in the text; LH3 (left), L′H2 (middle) and salicylaldehyde (right). Colour code: C = grey, N = blue, O = red, H = white. | ||
Experimental
General methods
All chemicals were obtained from commercial suppliers (Sigma-Aldrich) and were used without further purification/treatment.Synthesis
LH3 was prepared as previously described.8[NiII8Gd4(OH)8(OAc)4(L′)8(H2O)4]·24H2O (2·24H2O) was prepared in a similar manner to 1 using Gd(OAc)3·4H2O instead of Dy(OAc)3·4H2O. Elemental analysis (%) calcd for C80H110Gd4N8Ni8O41 (2·5H2O): C 32.70, H 3.77, N 3.81; found: C 32.59, H 3.61, N 3.72.
Crystallography
Using Olex2,9 single crystal X-ray structures were solved with the SHELXT10 structure solution program using Intrinsic Phasing and refined with the SHELXL11 refinement package using least squares minimisation. Crystal data for 1 (CCDC 2410260†): C80H112Dy4N8Ni8O42 (M = 2977.45 g mol−1), monoclinic, space group C2/c, a = 20.9513(16) Å, b = 19.9583(16) Å, c = 25.322(2) Å, β = 101.165(4)°, V = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388.1(14) Å3, Z = 4, T = 195(2) K, Bruker Apex II diffractometer, μ(MoKα) = 4.331 mm−1, Dcalc = 1.835 g cm−3, 126
388.1(14) Å3, Z = 4, T = 195(2) K, Bruker Apex II diffractometer, μ(MoKα) = 4.331 mm−1, Dcalc = 1.835 g cm−3, 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 reflections measured (4.622° ≤ 2Θ ≤ 56.668°), 12
728 reflections measured (4.622° ≤ 2Θ ≤ 56.668°), 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 unique (Rint = 0.0578, Rsigma = 0.0304) which were used in all calculations. The final R1 was 0.0402 (I > 2σ(I)) and wR2 was 0.1076 (all data). Crystal data for 2 (CCDC 2410261†): C80H148Gd4N8Ni8O60 (M = 3280.74 g mol−1), monoclinic, space group C2/c, a = 20.9663(10) Å, b = 20.0465(9), c = 26.2609(13) Å, V = 10
917 unique (Rint = 0.0578, Rsigma = 0.0304) which were used in all calculations. The final R1 was 0.0402 (I > 2σ(I)) and wR2 was 0.1076 (all data). Crystal data for 2 (CCDC 2410261†): C80H148Gd4N8Ni8O60 (M = 3280.74 g mol−1), monoclinic, space group C2/c, a = 20.9663(10) Å, b = 20.0465(9), c = 26.2609(13) Å, V = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830.3(9) Å3, Z = 4, T = 210(2) K, Bruker Apex II diffractometer, μ(MoKα) = 3.874 mm−1, Dcalc = 2.012 g cm−3, 314
830.3(9) Å3, Z = 4, T = 210(2) K, Bruker Apex II diffractometer, μ(MoKα) = 3.874 mm−1, Dcalc = 2.012 g cm−3, 314![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 reflections measured (4.36° ≤ 2Θ ≤ 58.306°), 14
922 reflections measured (4.36° ≤ 2Θ ≤ 58.306°), 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 593 unique (Rint = 0.0573, Rsigma = 0.0195) which were used in all calculations. The final R1 was 0.0514 (I > 2σ(I)) and wR2 was 0.1225 (all data). Crystal data for 3 (CCDC 2410262†): C80H120N8Ni8O46Y4 (M = 2755.15 g mol−1), monoclinic, space group C2/c, a = 20.9054(7) Å, b = 19.9035(6) c = 26.4714(8) Å, V = 10
593 unique (Rint = 0.0573, Rsigma = 0.0195) which were used in all calculations. The final R1 was 0.0514 (I > 2σ(I)) and wR2 was 0.1225 (all data). Crystal data for 3 (CCDC 2410262†): C80H120N8Ni8O46Y4 (M = 2755.15 g mol−1), monoclinic, space group C2/c, a = 20.9054(7) Å, b = 19.9035(6) c = 26.4714(8) Å, V = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798.1(6) Å3, Z = 4, T = 220(2) K, Bruker Apex II diffractometer, μ(MoKα) = 3.571 mm−1, Dcalc = 1.695 g cm−3, 85
798.1(6) Å3, Z = 4, T = 220(2) K, Bruker Apex II diffractometer, μ(MoKα) = 3.571 mm−1, Dcalc = 1.695 g cm−3, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 reflections measured (4.522° ≤ 2Θ ≤ 57.514°), 13
906 reflections measured (4.522° ≤ 2Θ ≤ 57.514°), 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988 unique (Rint = 0.0857, Rsigma = 0.0701) which were used in all calculations. The final R1 was 0.0528 (I > 2σ(I)) and wR2 was 0.1348 (all data).
988 unique (Rint = 0.0857, Rsigma = 0.0701) which were used in all calculations. The final R1 was 0.0528 (I > 2σ(I)) and wR2 was 0.1348 (all data).
Magnetometry
Magnetization data were collected on MPMS-XL and MPMS3 SQUID magnetometers equipped with 5 and 7 T magnets, respectively. Diamagnetic corrections were applied using Pascal's constants.Heat capacity
Heat capacity measurements were carried out on a PPMS equipped with a 9 T magnet and a 3He cryostat, using a thin pressed pellet (ca. 1 mg) of a polycrystalline sample of 2, thermalized by ca. 0.2 mg of Apiezon N grease, whose contribution was subtracted using a phenomenological expression.Powder XRD measurements
Powder XRD measurements were collected on freshly prepared samples of 1–3 on a PANanalytical X'Pert Pro MPD diffractometer.Infra-red spectroscopy
FTIR-ATR (Fourier-transform infrared-attenuated total reflectance) spectra were recorded on a PerkinElmer FTIR Spectrum BX spectrometer.Energy-dispersive X-ray spectroscopy
EDS measurements were performed on a JEOL Scanning Electron Microscope.Results and discussion
The initial system investigated was the simple synthetic triad of NiII/DyIII/LH3 in the absence of any co-ligands. However, after many experiments in which all synthetic parameters were varied (i.e. reaction times, presence/absence of base, bench/solvothermal conditions, metal salts and metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratios) no crystalline material was obtained. We employed salicylaldehyde as a co-ligand in the reaction system, since it is well-known that it can serve both as a chelate ligand (blocking polymerisation and the formation of aggregates) as well as a bridging ligand via the deprotonation of the hydroxyl group. The 1
ligand ratios) no crystalline material was obtained. We employed salicylaldehyde as a co-ligand in the reaction system, since it is well-known that it can serve both as a chelate ligand (blocking polymerisation and the formation of aggregates) as well as a bridging ligand via the deprotonation of the hydroxyl group. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvothermal reaction of LH3 with NiCl2·6H2O, Dy(OAc)3·4H2O and salicylaldehyde in basic methanolic solution produced olive-green crystals of [NiII8DyIII4(L′)8(OAc)4(OH)8(H2O)4]·6H2O (1·6H2O) in good yields. The crystal structure of the complex (vide infra) revealed the presence of the dianion of the Schiff-base ligand that forms from the reaction between salicylaldehyde and ethanolamine, the latter originating from the dissociation of the LH3 ligand to its components under the high-temperature and high-pressure conditions of the autoclave. Repeating the same reaction under ambient conditions led to the formation of a green solution, which yielded a crude oil upon standing. The gadolinium and yttrium analogues of complex 1 were formed following analogous synthetic routes (in the case of the yttrium analogue YCl3 was used as the yttrium salt) and are isostructural with 1. Despite the fact that the Schiff-base ligand produced in situ, L′H2, has been extensively employed for the formation of metallic clusters,12 this is the first time that the formation of such large 3d–4f species is observed.
1 solvothermal reaction of LH3 with NiCl2·6H2O, Dy(OAc)3·4H2O and salicylaldehyde in basic methanolic solution produced olive-green crystals of [NiII8DyIII4(L′)8(OAc)4(OH)8(H2O)4]·6H2O (1·6H2O) in good yields. The crystal structure of the complex (vide infra) revealed the presence of the dianion of the Schiff-base ligand that forms from the reaction between salicylaldehyde and ethanolamine, the latter originating from the dissociation of the LH3 ligand to its components under the high-temperature and high-pressure conditions of the autoclave. Repeating the same reaction under ambient conditions led to the formation of a green solution, which yielded a crude oil upon standing. The gadolinium and yttrium analogues of complex 1 were formed following analogous synthetic routes (in the case of the yttrium analogue YCl3 was used as the yttrium salt) and are isostructural with 1. Despite the fact that the Schiff-base ligand produced in situ, L′H2, has been extensively employed for the formation of metallic clusters,12 this is the first time that the formation of such large 3d–4f species is observed.
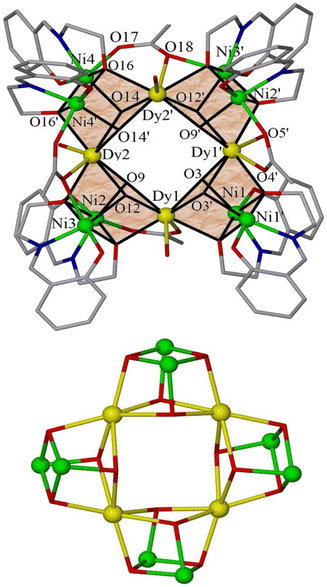
Since complexes 1–3 are isostructural, we limit discussion to the crystal structure of 1. Complex 1 crystallises in the monoclinic space group C2/c (Fig. 1). Its metallic core describes a central, planar rectangular {Dy4} unit of dimension ∼3.9 × 3.7 Å, serving as a base for the assembly of four {Ni2Dy2(OH)2(OR)2} cubes in a circular fashion, with each cube sharing two Dy corners (Dy1, Dy2 and symmetry equivalents, s.e.) with two neighboring cubes. All cubes are distorted with: (i) edge dimensions ranging from ∼2.06 to 2.37 Å, (ii) Dy–O(H)–Dy angles in the ∼109.7–112.8° range, (iii) Ni–OR–Ni angles of ∼97°, (iv) Ni–OR/H–Dy angles in the ∼91.0–104.5° range and (v) Ni⋯Ni distances in the ∼3.10–3.15 Å range.
All eight L′ ligands are found in an η3:η1:η1:μ3 coordination mode, bridging two Ni and one Dy centres within each cube, while eight μ3-OH− bridges (O3, O9, O12, O14, and s.e., BVS values = 0.77–0.88) complete the formation of the cubes. Neighbouring cubes are linked to each other via one η2:η1:μ3 acetate, bridging two Ni and one Dy centres. All eight Ni ions are six-coordinate with a {O5N} coordination sphere, adopting octahedral geometry. The only exceptions to this are Ni1 and Ni3 which adopt severely distorted octahedral geometries as evidenced by: (i) the presence of a large bonding distance (Ni1–O4 = 2.43 Å and Ni3–O18′ = 2.38 Å) and (ii) the deviations of the O2′–Ni1–O4 and O7–Ni3–O18′ angles of ∼136° and 135°, respectively, from the theoretical value of 180° (Fig. 2). The coordination environment of the 4f ions is completed by the presence of a terminal H2O molecule, with all DyIII cations being eight-coordinate and square-antiprismatic (SHAPE analysis, Table S1†).13
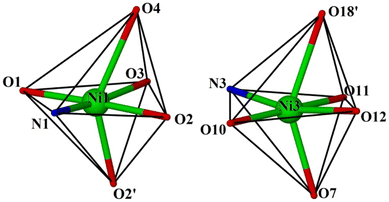 | ||
| Fig. 2 The severely distorted octahedral geometry for Ni1 and Ni3 (and s.e.). Colour code: NiII = green, O = red, N = blue. | ||
In the extended structure, molecules of 1 pack in a “brickwork” like manner, forming sheets in the ab plane, with the water solvate molecules occupying the void space between the clusters (Fig. 3). There exists an intricate inter- and intramolecular hydrogen-bonded network mediated by the eight water molecules.
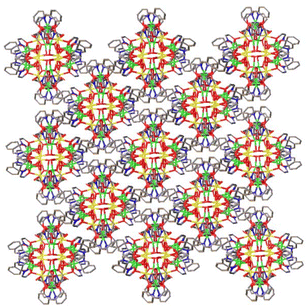 | ||
| Fig. 3 The crystal packing of 1 in the ab plane. Solvate molecules and H-atoms are omitted. Colour code: DyIII = yellow, NiII = green, O = red, N = blue. | ||
Magnetic properties
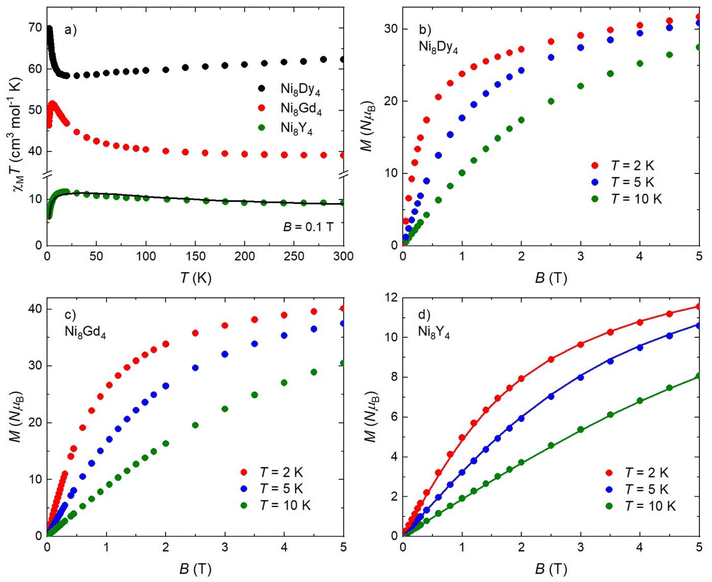
The direct current molar magnetic susceptibility, χM, of freshly prepared polycrystalline samples of 1–3 were measured in an applied field, B, of 0.1 T, over the 2–300 K temperature, T, range. The purity of all samples was established by means of PXRD comparison with the simulated data from the single-crystal structure (Fig. S1†), as well as from EDS measurements (Fig. S2†). The experimental results are presented in the form of the χMT product vs. T in Fig. 4a.The experimental room-temperature χMT values of 62.3 cm3 K mol−1 for 1, 39.1 cm3 K mol−1 for 2 and 9.3 cm3 K mol−1 for 3, respectively, are close to the theoretical values expected for eight independent NiII ions (gNi = 2.0) and four DyIII ions (s = 5/2, L = 5, J = 15/2, gJ = 4/3) of 64.7 cm3 K mol−1 (for 1), eight independent NiII ions (gNi = 2.0) and four GdIII ions (gGd = 2.0) of 39.5 cm3 K mol−1 (for 2), and eight independent NiII ions (gNi = 2.0) of 8.0 cm3 K mol−1 (for 3). For 1, the χMT value decreases slowly upon cooling from room temperature down to ∼60 K, below which it steeply increases reaching 69.9 cm3 K mol−1 at T = 2 K, indicating the presence of ferromagnetic exchange interactions. For 2, χMT slightly increases upon cooling down to ∼70 K, while upon further cooling it increases rapidly to reach the maximum value of 51.6 cm3 K mol−1 at T = 5.5 K, before it drops to 46.4 cm3 K mol−1 at the lowest temperature of 2 K, suggesting dominant ferromagnetic interactions. The abrupt drop at the lowest T may be attributed to ZFS and/or intermolecular interactions. For 3, the χMT product decreases slowly upon cooling until ∼50 K, below which it slightly increases reaching a maximum value of 11.6 cm3 K mol−1 at T = 18 K, denoting dominant ferromagnetic exchange. Upon further cooling, it decreases steeply reaching 6.3 cm3 K mol−1 at T = 2 K. This is attributed to the presence of antiferromagnetic exchange and/or crystal field effects. For 1, ac susceptibility measurements (not shown here) in the temperature range 2–30 K, at zero-applied field, reveal no evidence of slow relaxation of the magnetic moment. Low-temperature variable-temperature and variable-field magnetization, M, data were measured in the temperature range 2–7 K, in magnetic fields up to 5.0 T for complexes 1 and 3, and up to 7.0 T for complex 2 (Fig. 4b–d and S3†). At the lowest temperature and highest field measured, M reaches a value of 31.7μB and 11.6μB for 1 and 3, respectively, while for the Gd analogue 2 a value of 40.1μB is obtained.
The magnetic properties of 3 can be well accounted for by a simple theoretical model of dimeric {Ni2} units, 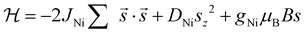 . The best fit of the χMT vs. T (Fig. 4a) and M vs. B (Fig. 4d) data affords DNi/kB = 11.5 K, gNi = 2.0 and JNi/kB = 29.4 K, denoting a relatively strong ferromagnetic Ni⋯Ni exchange interaction. For 1 and 2, the diagonalization of the corresponding Hamiltonians and the calculation of observables are not feasible due to the large dimensions of the associated Hilbert spaces.
. The best fit of the χMT vs. T (Fig. 4a) and M vs. B (Fig. 4d) data affords DNi/kB = 11.5 K, gNi = 2.0 and JNi/kB = 29.4 K, denoting a relatively strong ferromagnetic Ni⋯Ni exchange interaction. For 1 and 2, the diagonalization of the corresponding Hamiltonians and the calculation of observables are not feasible due to the large dimensions of the associated Hilbert spaces.
Magnetothermal properties and MCE
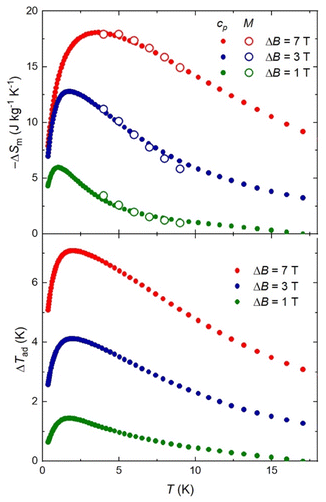
The magnetocaloric effect (MCE), that is, the change of magnetic entropy ΔSm and adiabatic temperature ΔTad in response to a change of the applied magnetic field ΔB, can be significantly large in selected molecular clusters. Given that GdIII is the typical ion of choice due to the large s = 7/2 and negligible anisotropy, we pursued magnetocaloric studies of complex 2. The heat capacity cp of a sample of 2 was measured over the 0.3–30 K temperature range in selected applied fields B = 0, 1, 3 and 7 T (Fig. 5). A strong field dependence is particularly visible at temperatures below ∼10 K, while the nonmagnetic lattice contribution tends to dominate at the larger temperatures. The analysis that is systematically carried out for this class of molecular materials, consists of fitting the high-field, low-temperature cp data to Debye's law, i.e., clatt = a × T3, where the coefficient a typically has values of the order of 10−2 K−3.14,15 However, the experimental cp data of 2 are approximately one order of magnitude larger than the expected clatt below 1 K (Fig. 5), making characterization unfeasible.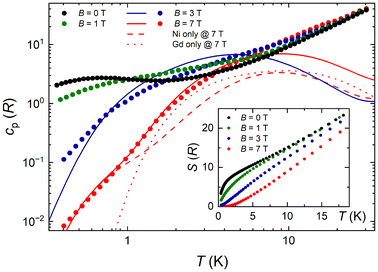 | ||
| Fig. 5 Molar heat capacity cp and entropy S (inset) versus temperature for selected applied field values for 2. Symbols are experimental data, while lines are theoretical simulations (see main text). | ||
Exchange interactions involving GdIII ions lose dominance against the Zeeman term of the Hamiltonian when increasing the magnetic field. The model proposed to evaluate cp collected at the higher field values neglects all exchange interactions but Ni⋯Ni from the dimeric {Ni}2 units, which we assume to be the same as in 3. The Schottky curves were calculated for four dimeric {Ni}2 units and four free GdIII ions per molecular unit, shown in Fig. 5 as the dashed and dotted curves, respectively. The sum of both contributions leads to the solid line that fits the experimental cp data at B = 7 T. Exchange correlations involving GdIII ions become dominant for fields lower than 3 T, breaking the agreement between the experiment and model.
Next, we calculate the temperature dependence of the entropy, S (inset of Fig. 5), for any B value from the experimental cp, using
The zero-field applied entropy increases sharply on increasing T at low temperatures, reaching values of 7R = 16.3 J kg−1 K−1 near 1–2 K. The available magnetic entropy content, i.e., Sm = [8 × ln(2 × 1 + 1) + 4 × ln(2 × 3.5 + 1)]R = 17.1R = 39.7 J kg−1 K−1, is not attained at low temperatures due to the relatively strong Ni⋯Ni interactions. At high temperatures, all experimental curves at different fields tend to overlap because of the nonmagnetic lattice contribution.
To evaluate the MCE, ΔSm and ΔTad are obtained as differences between entropy curves, for magnetic field changes ΔB = B − 0. ΔSm is also calculated from the magnetization curves of Fig. 4 using the Maxwell relation,
The ΔSm results from both calculations overlap, proving the validity of our approach (Fig. 6). The magnetic entropy change reaches 18.1 J kg−1 K−1, which corresponds to ∼46% of the available entropy at 3.7 K for the largest applied field of 7 T. The adiabatic temperature change shows a maximum of 7.1 K at T = 2 K for ΔB = 7 T. Complex 2 joins a growing family of heterometallic Ni–Gd clusters displaying a relatively large −ΔSm value (Table 1).
| Formula | −ΔSm J kg−1 K −1 | T (K) | ΔB (T) | Ref. |
|---|---|---|---|---|
| [Ni6Gd6(OH)2(O3PCH2Ph)6(O2CtBu)16(MeCO2H)2]·4MeCN | 26.5 | 3.0 | 7 | 16 |
| [Gd36Ni12(CH3COO)18(μ3-OH)84(μ4-O)6(H2O)54(NO3)Cl2](NO3)6Cl9·30H2O | 36.3 | 3.0 | 7 | 17 |
| [Gd42Ni10(μ3-OH)68(CO3)12(CH3COO)30(H2O)70]·(ClO4)24·80H2O | 38.2 | 2.0 | 7 | 18 |
| [NiII8Gd4(OH)8(OAc)4(L′)8(H2O)4]·24H2O | 18.1 | 3.7 | 7 | This work |
| [Ni2Gd(L)6](NO3) | 13.7 | 4.0 | 7 | 19 |
| [NiGd(HL)2(NO3)3] | 5.7 | 7.0 | 7 | 20 |
| [Gd2Ni2(NO3)6(H2O)1.5(CH3CN)2(L)2]·CH3CN | 18.5 | 3.0 | 5 | 21 |
| [Ni(H2O)6][Gd(oda)3]·3H2O | 31.6 | 2.0 | 9 | 22 |
| [Ni64Gd96(μ3-OH)156(IDA)66(DMPA)12(CH3COO)48(NO3)24(H2O)64]Cl24·35CH3OH·120H2O | 42.8 | 3.0 | 7 | 23 |
| [Ni4Gd4(μ2-OH)2(μ3-OH)4(μ-OOCCH3)8(LH2)4]·(OH)2·H2O | 22.6 | 3.0 | 5 | 24 |
| [Na2Gd78Ni64(IDA)58(OAc)2(SiO4)6(Cl)4(μ2-OH)4(μ4-O)4(μ3-O)38(μ3-OH)126(H2O)82]·(Cl)4(H2O)37 | 40.6 | 3.0 | 7 | 25 |
| [Ni36Gd102(OH)132(mmt)18(dmpa)18(H2dmpa)24(CH3COO)84(SO4)18(NO3)18(H2O)30]·Br6(NO3)6·(H2O)130·(CH3OH)60 | 41.3 | 2.0 | 7 | 26 |
| [Ni5Gd8(μ3-OH)7(μ-OH2)(O3P-tBu)6(O2C-tBu)15(MeCN)] | 30.6 | 3.0 | 7 | 27 |
| [Gd152Ni14Cl24(CO3)42(OAc)48(H2O)138(OH)308]·Cl20·(H2HEIDA)10·20H2O | 52.7 | 3.5 | 7 | 28 |
| [Gd22Ni22(DTA)21(SO4)4(OH)48(OCH3)4](NO3)6·(H2O)50 | 46.0 | 2.0 | 7 | 29 |
| {[Gd2Ni(L)(C2O4)2(H2O)2]·H2O}n | 38.0 | 2.0 | 7 | 30 |
Conclusions
In conclusion, in this work we report three isostructural dodecanuclear {Ni8Ln4} (Ln = Dy, Gd and Y) complexes, upon the in situ formation of the dianionic form of the Schiff-base ligand resulting from the reaction of salicylaldehyde and ethanolamine. All three clusters feature an aesthetically pleasing ring-like assembly of four {Ni2Ln2(OH)2(OR)2} cubes arranged in a circular fashion around a planar {Ln4} rectangle. The study of the magnetic properties revealed dominant ferromagnetic interactions in the Y analogue (complex 3), while the Gd analogue (complex 2) displays a significant magnetocaloric effect.Author contributions
TGT synthesized and characterized the complexes. SJD solved the single-crystal XRD data. EA, EKC, DG and ME measured the magnetic/heat capacity data and analysed the MCE parameters. MU and ME simulated the magnetic/heat capacity data. EKB and CJM conceived the idea. All authors contributed to writing the manuscript.Data availability
Crystallographic data for compounds 1–3 have been deposited at the CCDC under numbers 2410260–2410262.† Further data supporting this manuscript have been included as part of the ESI.† All data presented in this article can be retrieved from DIGITAL.CSIC at https://doi.org/10.20350/digitalCSIC/17066.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received support from EU (MSCA-DN MolCal, 101119865), MICIU/AEI/10.13039/501100011033/ and ERDF/EU (PID2021-124734OB-C21, CEX2023-001286-S), Gobierno de Aragón (E11-23R, E12-23R). CJM and TGT would like to thank the National Recovery and Resilience Plan Greece 2.0 (Award Number TAEDR-0535821), funded by the European Union – NextGenerationEU.References
- See for example: J. Long, J. Rouquette, J.-M. Thibaud, R. A. S. Ferreira, L. D. Carlos, B. Donnadieu, V. Vieru, L. F. Chibotaru, L. Konczewicz, J. Haines, Y. Guari and J. Larionova, Angew. Chem., Int. Ed., 2015, 54, 2236–2240 CrossRef CAS PubMed; A. J. Amoroso and S. A. J. Pope, Chem. Soc. Rev., 2015, 44, 4723–4742 RSC; C. Zhang, C. Chen, H. Dong, J. R. Shen, H. Dau and J. Zhao, Science, 2015, 348, 690–693 CrossRef PubMed.
- For representative reviews on SMMs see: D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268–297 CrossRef CAS PubMed; G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66–71 CrossRef; C. J. Milios and R. E. P. Winpenny, Struct. Bonding, 2015, 164, 1–109 Search PubMed; L. Sorace, C. Benelli and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3092–3104 RSC; X.-Y. Wang, C. Avendañoa and K. R. Dunbar, Chem. Soc. Rev., 2011, 40, 3213–3238 RSC; R. A. Layfield, Organometallics, 2014, 33, 1084–1099 CrossRef; L. K. Thompson and L. N. Dawe, Coord. Chem. Rev., 2015, 289, 13–31 CrossRef; S. Demir, I.-R. Jeon, J. R. Long and T. D. Harris, Coord. Chem. Rev., 2015, 289, 149–176 CrossRef; P. Happ, C. Plenk and E. Rentschler, Coord. Chem. Rev., 2015, 289, 238–260 CrossRef; G. A. Craig and M. Murrie, Chem. Soc. Rev., 2015, 44, 2135–2147 RSC; A. Chakraborty, J. Goura, P. Kalita, A. Swain, G. Rajaraman and V. Chandrasekhar, Dalton Trans., 2018, 47, 8841–8864 RSC; A. Zabala-Lekuona, J. M. Seco and E. Colacio, Coord. Chem. Rev., 2021, 441, 213984 CrossRef.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400–1403 CrossRef CAS PubMed; C. A. Gould, K. R. McClain, D. Reta, J. G. C. Kragskow, D. A. Marchiori, E. Lachman, E.-S. Choi, J. G. Analytis, R. D. Britt, N. F. Chilton, B. G. Harvey and J. R. Long, Science, 2022, 375, 198–202 CrossRef PubMed.
- J. Wang, Q.-W. Li, S.-G. Wu, Y.-C. Chen, R.-C. Wan, G.-Z. Huang, Y. Liu, J.-L. Liu, D. Reta, M. J. Giansiracusa, Z.-X. Wang, N. F. Chilton and M.-L. Tong, Angew. Chem., Int. Ed., 2020, 60, 5299–5306 CrossRef PubMed.
- See for example: J. W. Sharples, D. Collison, E. J. L. McInnes, J. Schnack, E. Palacios and M. Evangelisti, Nat. Commun., 2014, 5, 5321 CrossRef CAS PubMed; G. Lorusso, O. Roubeau and M. Evangelisti, Angew. Chem., Int. Ed., 2016, 55, 3360–3363 CrossRef; G. Lorusso, J. W. Sharples, E. Palacios, O. Roubeau, E. K. Brechin, R. Sessoli, A. Rossin, F. Tuna, E. J. L. McInnes, D. Collison and M. Evangelisti, Adv. Mater., 2013, 25, 4653–4656 CrossRef; G. Lorusso, M. Jenkins, P. González-Monje, A. Arauzo, J. Sesé, D. Ruiz-Molina, O. Roubeau and M. Evangelisti, Adv. Mater., 2013, 25, 2984–2988 CrossRef PubMed; M. Evangelisti, Molecule-Based Magnetic Coolers: Measurement, Design and Application, in Molecular Magnets: Physics and Applications, ed. J. Bartolomé, F. Luis and J. F. Fernández, Springer Verlag, Berlin Heidelberg, 2014, pp. 365–387 Search PubMed.
- W.-P. Chen, J. Singleton, L. Qin, A. Camón, L. Engelhardt, F. Luis, R. E. P. Winpenny and Y.-Z. Zheng, Nat. Commun., 2018, 9, 2107 CrossRef.
- T. G. Tziotzi, M. Coletta, M. Gray, C. L. Campbell, S. J. Dalgarno, G. Lorusso, M. Evangelisti, E. K. Brechin and C. J. Milios, Inorg. Chem. Front., 2021, 8, 1804–1809 RSC; M. Coletta, T. G. Tziotzi, M. Gray, G. S. Nichol, M. K. Singh, C. J. Milios and E. K. Brechin, Chem. Commun., 2021, 57, 4122–4125 RSC.
- M. V. Baker, D. H. Brown, B. W. Skelton and A. H. White, J. Chem. Soc., Dalton Trans., 1999, 1483–1490 RSC.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A Complete Structure Solution, Refinement and Analysis Program, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- G. M. Sheldrick, SHELXT – Integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3 CrossRef PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- See for example: C. Boskovic, A. Sieber, G. Chaboussant, H. U. Gudel, J. Ensling, W. Wernsdorfer, A. Neels, G. Labat, H. Stoeckli-Evans and S. Janssen, Inorg. Chem., 2004, 43, 5053 CrossRef CAS PubMed; C. Boskovic, R. Bircher, P. L. W. Tregenna-Piggott, H. U. Gudel, C. Paulsen, W. Wernsdorfer, A.-L. Barra, E. Khatsko, A. Neels and H. Stoeckli-Evans, J. Am. Chem. Soc., 2003, 125, 14046 CrossRef; A. K. Jami, S. P. Behera, S. Mondal and V. Baskar, Inorg. Chem. Commun., 2022, 143, 109784 CrossRef; D. Basak, J. van Leusen, T. Gupta, P. Kögerler and D. Ray, Dalton Trans., 2020, 49, 7576 RSC; C. Boskovic, H. U. Gudel, G. Labat, A. Neels, W. Wernsdorfer, B. Moubaraki and K. S. Murray, Inorg. Chem., 2005, 44, 3181 CrossRef; D. Basak, E. R. Martí, M. Murrie, I. Nemec and D. Ray, Dalton Trans., 2021, 50, 9574 RSC; O. Stetsiuk, N. Plyuta, N. Avarvari, E. Goreshnik, V. Kokozay, S. Petrusenko and A. Ozarowski, Cryst. Growth Des., 2020, 20, 1491 CrossRef; I. C. Lazzarini, L. Carrella, E. Rentschler and P. Albores, Polyhedron, 2012, 31, 779 CrossRef; Y.-G. Li, L. Lecren, W. Wernsdorfer and R. Clérac, Inorg. Chem. Commun., 2004, 7, 1281 CrossRef; H. Oshio, N. Hoshino, T. Ito and M. Nakano, J. Am. Chem. Soc., 2004, 126, 8805 CrossRef PubMed; N. Hoshino, T. Ito, M. Nihei and H. Oshio, Inorg. Chem. Commun., 2003, 6, 377 CrossRef; D. S. Nesterov, E. N. Chygorin, V. N. Kokozay, V. V. Bon, R. Boča, Y. N. Kozlov, L. S. Shul'pina, J. Jezierska, A. Ozarowski, A. J. L. Pombeiro and G. B. Shul'pin, Inorg. Chem., 2012, 51, 9110 CrossRef PubMed.
- M. Llunell, D. Casanova, J. Girera, P. Alemany and S. Alvarez, SHAPE, version 2.0, Barcelona, Spain, 2010 Search PubMed.
- T. G. Tziotzi, D. Gracia, S. J. Dalgarno, J. Schnack, M. Evangelisti, E. K. Brechin and C. J. Milios, J. Am. Chem. Soc., 2023, 145, 7743–7747 CrossRef CAS PubMed.
- E. Agapaki, E. K. Charkiolakis, G. S. Nichol, D. Gracia, M. Evangelisti and E. K. Brechin, Front. Chem., 2024, 12, 1494609 CrossRef CAS PubMed.
- Y. Zheng, M. Evangelisti and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2011, 50, 3692–3695 CrossRef CAS PubMed.
- J. Peng, Q. Zhang, X. Kong, Y. Ren, L. Long, R. Huang, L. Zheng and Z. Zheng, Angew. Chem., Int. Ed., 2011, 50, 10649–10652 CrossRef CAS PubMed.
- J.-B. Peng, Q.-C. Zhang, X.-J. Kong, Y.-Z. Zheng, Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2012, 134, 3314–3317 CrossRef CAS PubMed.
- A. Upadhyay, N. Komatireddy, A. Ghirri, F. Tuna, S. K. Langley, A. K. Srivastava, E. C. Sañudo, B. Moubaraki, K. S. Murray, E. J. L. McInnes, M. Affronte and M. Shanmugam, Dalton Trans., 2014, 43, 259–266 RSC.
- N. Ahmed, C. Das, S. Vaidya, A. K. Srivastava, S. K. Langley, K. S. Murray and M. Shanmugam, Dalton Trans., 2014, 43, 17375–17384 RSC.
- C. Meseguer, S. Titos-Padilla, M. M. Hänninen, R. Navarrete, A. J. Mota, M. Evangelisti, J. Ruiz and E. Colacio, Inorg. Chem., 2014, 53, 12092–12099 CrossRef CAS PubMed.
- J. Qiu, L. Wang, Y. Chen, Z. Zhang, Q. Li and M. Tong, Chem. – Eur. J., 2016, 22, 802–808 CrossRef CAS.
- W. Chen, P. Liao, Y. Yu, Z. Zheng, X. Chen and Y. Zheng, Angew. Chem., Int. Ed., 2016, 55, 9375–9379 CrossRef CAS PubMed.
- P. Kalita, J. Goura, J. M. Herrera, E. Colacio and V. Chandrasekhar, ACS Omega, 2018, 3, 5202–5211 CrossRef CAS PubMed.
- Q. Lin, J. Li, X. Luo, C. Cui, Y. Song and Y. Xu, Inorg. Chem., 2018, 57, 4799–4802 CrossRef CAS PubMed.
- W.-P. Chen, P.-Q. Liao, P.-B. Jin, L. Zhang, B.-K. Ling, S.-C. Wang, Y.-T. Chan, X.-M. Chen and Y.-Z. Zheng, J. Am. Chem. Soc., 2020, 142, 4663–4670 CrossRef CAS PubMed.
- Y. Zhai, W. Chen, M. Evangelisti, Z. Fu and Y. Zheng, Aggregate, 2024, 5, e520 CrossRef CAS.
- N. Xu, W. Chen, Y.-S. Ding and Z. Zheng, J. Am. Chem. Soc., 2024, 146, 9506–9511 CrossRef CAS PubMed.
- Z. Lu, C. Duan and J. Wang, J. Mol. Struct., 2024, 1312, 138622 CrossRef CAS.
- Q. Wang, L. Xu, J. Wang, Y. Yu, X. Bai, C. Jiang, Q. Chen and Y. Xu, Dalton Trans., 2025, 54, 2930–2936 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns (Fig. S1), EDS spectra (Fig. S2), SHAPE calculations (Table S1). CCDC 2410260–2410262. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00175g |
| This journal is © The Royal Society of Chemistry 2025 |