Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
How metal ions link in metal–organic frameworks: dots, rods, sheets, and 3D secondary building units exemplified by a Y(III) 4,4′-oxydibenzoate†‡
Cyrielle L. F.
Dazem
ab,
Niklas
Ruser
 a,
Erik
Svensson Grape
a,
Erik
Svensson Grape
 c,
A. Ken
Inge
c,
A. Ken
Inge
 c,
Davide M.
Proserpio
c,
Davide M.
Proserpio
 d,
Norbert
Stock
d,
Norbert
Stock
 *a and
Lars
Öhrström
*a and
Lars
Öhrström
 *e
*e
aInstitute of Inorganic Chemistry, Christian-Albrechts-University Kiel, Max-Eyth-Straße 2, 24118 Kiel, Germany
bInorganic Chemistry Department, University of Yaoundé 1, P.O. Box 812, Yaoundé, Cameroon
cDepartment of Materials and Environmental Chemistry, Stockholm University, 10691, Stockholm, Sweden
dDipartimento di Chimica, Università degli studi di Milano, Via Golgi 19, 20133 Milano, Italy
eChemistry and Biochemistry, Dept. of Chemistry and Chemical Engineering, Chalmers University of Technology, SE-41296 Gothenburg, Sweden. E-mail: ohrstrom@chalmers.se
First published on 10th March 2025
Abstract
In the field of metal–organic frameworks, the use of yttrium(III) cations and the formation of 3D inorganic building units are rather rare. Here we report an yttrium(III) metal–organic framework based on the V-shaped ditopic linker 4,4′-oxydibenzoate, oba2−: [Y16(μ-OH2)(μ3-OH)8(oba)20(dmf)4]·7H2O·7dmf, 1, which was solvothermally prepared, with single crystal X-ray diffraction revealing an unusual 3D metal secondary building unit. When activated at 200 °C, 1 desolvated to form compound 2, [Y16(μ-OH2)(μ3-OH)8(oba)20]·6H2O, retaining the same structure with a 3% shrinkage in unit cell volume.
While research into metal–organic frameworks, popularly known as MOFs,1 is increasingly engineering related,2–5 with devices already on the market6 the scientific rationale for synthesising new MOF materials is shifting. The quest for record-breaking surface area, porosity and selectivity is supplemented by a what-if approach using unusual linkers, and in the process, we will acquire new knowledge enabling record-breaking surface areas, porosities and selectivities.
One such what-if scenario involves changing the divergent ditopic linkers, which fit well into the blueprint of existing network topologies due to the perfect mapping of their propagation vectors of 180° onto high symmetry nets, for V-shaped linkers.7–20 One such linker is 4,4′-oxydibenzoic acid (H2oba) (Fig. 1) showing an average angle of 125° between the carboxylate carbons and the bridging oxygen (measured on 520 structures in the Cambridge Structural Database21).
The other fundamental component of a MOF is the metal secondary building unit, metal-SBU. This part is harder to control and may depend on exact synthesis conditions as well as the linker used.22 It can be as small as a single metal ion, be composed of multinuclear entities of varying geometries, or form infinite 1D units or 2D units. For simplicity, we can refer to the resulting MOFs as dot-, rod-, and sheet-MOFs.23
This is not simply semantics, as the dimensionality of the metal-SBUs has been implicated in the stability of a MOF,24–26 with sheet-MOFs suggested to be more stable than rod-MOFs.
While rod-MOFs are relatively common,27 sheet-MOFs are less so, and their 3D analogues, frame-MOFs (also called MOFs with three-dimensional inorganic connectivity28), are rare. Two examples are ZJNU-120, Me2NH2[Sm3(5,5′-(5-carboxy-1,3-phenylene)dinicotinate)3(OH)]·6dma28 (dma = N,N-dimethylacetamide), featuring a metal-SBU that can be described as a three-periodic (three-dimensional) srs-net, and [Pb(2,5-dichloroterephthalate)] with a metal SBU described by the pcu-h net.29 We here explore the unusual structure and synthesis of the yttrium(III) MOF [Y16(μ-OH2)(μ3-OH)8(oba)20(dmf)4]·7H2O·7dmf, 1 (Fig. 1), and its desolvated variety 2.
Solvothermal synthesis in a mixture of dimethylformamide, dmf, and water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 170 °C for 16 h gave [Y16(μ-OH2)(μ3-OH)8(oba)20(dmf)4]·7H2O·7dmf, 1, and subsequent heating at 200 °C overnight resulted in the activated form [Y16(μ-OH2)(μ3-OH)8 (oba)20]·6H2O, 2.
1) at 170 °C for 16 h gave [Y16(μ-OH2)(μ3-OH)8(oba)20(dmf)4]·7H2O·7dmf, 1, and subsequent heating at 200 °C overnight resulted in the activated form [Y16(μ-OH2)(μ3-OH)8 (oba)20]·6H2O, 2.
Single crystal X-ray diffraction of 1 revealed a large unit cell (space group I41, a = b = 28.1762(6) Å; c = 39.8472(8) Å; V = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634(2) Å3) with eight crystallographically independent yttrium(III) ions and ten independent oba2− linkers; see Fig. 2.
634(2) Å3) with eight crystallographically independent yttrium(III) ions and ten independent oba2− linkers; see Fig. 2.
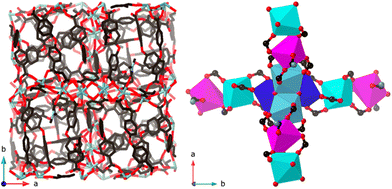 | ||
| Fig. 2 X-ray single crystal structure of [Y16(μ-OH2)(μ3-OH)8(oba)20(dmf)4]·7H2O·7dmf, 1. Left: unit cell (I41, a = b = 28.1762(6) Å; c = 39.8472(8) Å) view in the z-axis direction with hydrogens omitted for clarity. Right: part of one of the two independent dia-nets that form the 3D-metal SBUs with the same colour coding as in Fig. 1. | ||
We also note the unusual space group, I41, one of the Sohncke space groups that preserve chirality, despite there being no intrinsically chiral component in the building blocks. Such MOFs may find use in enantioselective catalysis and separation, or non-linear optics.
While the unit cell of 1 at 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634(2) Å3 is not exceptionally large, as MOFs with cell volumes over 400
634(2) Å3 is not exceptionally large, as MOFs with cell volumes over 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3 have been reported,30 it falls into a very small category where relatively short, single linkers create exceptional complexity. A classic example is MIL-101,31 where two simple SBUs, the trigonal prism [Cr3O(RCO2)6(X)3] (X = OH− or H2O) with a diameter of 9 Å and the terephthalate ditopic linker with length around 7.0 Å, give a MOF structure with a unit cell volume of 175
000 Å3 have been reported,30 it falls into a very small category where relatively short, single linkers create exceptional complexity. A classic example is MIL-101,31 where two simple SBUs, the trigonal prism [Cr3O(RCO2)6(X)3] (X = OH− or H2O) with a diameter of 9 Å and the terephthalate ditopic linker with length around 7.0 Å, give a MOF structure with a unit cell volume of 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3.
000 Å3.
The key to the large unit cell in MIL-101 is the network topology. MIL-101 has four kinds of six-connected nodes (vertices) and seven kinds of links (edges) and can be described by the topology mtn-e.32 This net, in turn, is probably the consequence of the metal-SBU, a flattened trigonal prism.
Also, in 1 the metal-SBU is the key. The central feature is a tetrahedral [Y4(μ3-OH)4] coordination entity. This entity is also found as a defining part of the dot-MOF [Y7(dcpb)4(μ3-OH)8(HCOO) (dmf)5(H2O)]·dmf·H2O (H3dcpb = 3,5-di(4′-carboxylphenyl)benzoic acid), Y-MOF1, as a part of a heptanuclear metal-SBU;33 in the rod-MOFs [Y6(OH)8(2,5-pyridinedicarboxylate)5(H2O)2];34 and [Ln5(μ2-O)(μ3-OH)5(tca)2(NO3) (CH3COO)(H2O)2]·NH(CH3)2·dma·(H2O)3, (H3tca = 4,4′,4′′-tricarboxy-triphenylamine);35 and finally in the sheet-MOF [Y5(OH)6(HCO2)3(CO3)2(C4O4)]·2.5H2O.36
In 1, however, each [Y4(μ3-OH)4]8+ entity connects in all four directions of the tetrahedron to a short [Y2(RCOO)9] chain connecting to the next [Y4(μ3-OH)4]8+ entity forming a dia-net. This accounts for four of the eight crystallographic yttrium ions. The other four assemble into an identical dia-net, related to the first by translation (type I interpenetration37, see Fig. 3, left), giving an overall topology of the metal-SBU as dia-c.
These 3D metal-SBUs, with cations joined by carboxylate groups and the μ3-OH of the tetrahedra, are then further bridged by the oba2− –C6H4–O–C6H4– organic SBU forming a fully connected non-interpenetrated MOF. As can be deduced from Fig. 1 there are nine of these in three triplets protruding from a [Y2(RCOO)9] entity between two [Y4(μ3-OH)4] tetrahedra, and one symmetrically independent oba2− linker from each tetrahedron, making a total of ten crystallographically independent oba2− bridges. This has been illustrated in Fig. 3, right.
In this way, the framework of 1 can be topologically described using the straight rod (STR) approach by adding mid-points between the metals and connecting the carbon atom of the COO bridge to the midpoint of the rod.38,39 The network analysis of the network in 1 is complex, as it involves two 6-connected vertices (the tetrahedral nodes of the two different metal-SBUs are 4-connected in the SBU and then bridges with two oba2− linkers) and six 5-connected vertices (2 connections within the metal-SBUs and 3 via the oba2− linkers), see Fig. 4.
Hardly surprising, this does not reduce to any of the high symmetry common nets, but to a chiral 56·62-c net, thus an 8-nodal net (see the ESI‡ for further information).
There are no apparent channels when looking at the structure of 1, and pore analysis using mercury reveals only 4% solvent-accessible volume, but 19% based on the contact surface (probe radius: 1.2 Å), yet these seem to be isolated pockets rather than channels (see the ESI‡).
Indeed, using a solvent mask routine during structure refinement (see the ESI‡) we detected three distinct pockets with volumes and electron densities consistent with a total of 6.5 dmf molecules and 5.5 water molecules per formula unit. Elemental analysis of 1 indicates seven dmf and seven H2O molecules per formula unit, while thermogravimetric analysis (TGA) results indicate the composition [Y16(μ-OH2)(μ3-OH)8(oba)20(dmf)4]·5H2O·6dmf, data that are consistent given the handling of the sample in air and variations in relative humidity. For the formula of 1, we use the elemental analysis results.§
TGA indicates the possibility of thermal stability up to 400 °C (Fig. S5‡) consistent with the idea that higher dimensionality of metal SBUs should be more stable.
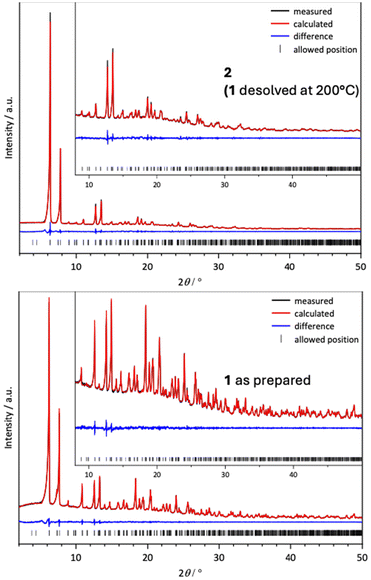
Despite the apparent lack of channels, 1 can be desolvated by heating to 200 °C overnight leading to compound 2 that we formulate, based on elemental analysis, as [Y16(μ-OH2)(μ3-OH)8(oba)20]·6H2O. The X-ray powder pattern of 2 was successfully fitted by a Pawley procedure (Rwp 2.96%) starting from the initial cell parameters and space group of 1. This resulted in a 3% smaller cell volume. As a control, we also conducted a Pawley fit on the powder data of 1, yielding similar fitting parameters (Rwp 2.19%); see the ESI‡ and Fig. 5.
At 298 K 2 shows a modest uptake of CO2 of 0.85 mmol g−1 at 100 kPa, in reasonable agreement with the void space in the structure if we use van Heerden and Barbour's 50% rule of thumb,42 which gives an estimate of 0.61 mmol g−1 uptake of CO2 (see the ESI‡). The PXRD pattern in Fig. S1‡ shows that 2 remains unchanged after the sorption experiment.
In conclusion, the use of V-shaped rather than divergent high-symmetry linkers can yield complex MOF structures, and potentially facilitate the formation of materials with 3D metal-SBUs, herein described as frame-MOFs. These highly interconnected motifs may yield materials with superior stability and lead to the discovery of previously unobserved network structures. Moreover, this study highlights the rarely found class of frame-MOFs, having metal SBUs periodic in three dimensions, 3D-SBUs. This has implications on our thinking of the whole class of metal–organic frameworks and coordination polymers—how they are constructed and synthesised.
Data availability
The data supporting this article have been included as part of the ESI.‡Crystallographic data for 1 have been deposited at the CCDC under 2240527‡. All other data presented in this study are available in the article.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. Ö thanks the Swedish Research Council and D. M. P. thanks the MUR for the grant PRIN2020 “Nature Inspired Crystal Engineering (NICE)” and Prof. Vladislav A. Blatov at the Samara Center for Theoretical Materials Science for providing the free ToposPro software (https://topospro.com). C. L. F. D. thanks DAAD, the Deutscher Akademischer Austauschdienst, for a stipend.References
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CrossRef CAS.
- To date and published in 2024, the top five Web of Science Categories for a search of “Metal-Organic Frameworks” are: Materials Science Multidisciplinary 1669; Chemistry Multidisciplinary 1544; Chemistry Physical 1443; Nanoscience Nanotechnology 920; Engineering Chemical 811.
- T. Jia, Y. F. Gu and F. T. Li, J. Environ. Chem. Eng., 2022, 10, 108300 CrossRef CAS.
- T. Lassitter, N. Hanikel, D. J. Coyle, M. I. Hossain, B. Lipinski, M. O'Brien, D. B. Hall, J. Hastings, J. Borja, T. O'Neil, S. E. Neumann, D. R. Moore, O. M. Yaghi and T. G. Glover, Chem. Eng. Sci., 2024, 285, 119430 CrossRef CAS.
- X. G. Liu, Y. Y. Shan, S. T. Zhang, Q. Q. Kong and H. Pang, Green Energy Environ., 2023, 8, 698–721 CrossRef CAS.
- Z. Chen, M. C. Wasson, R. J. Drout, L. Robison, K. B. Idrees, J. G. Knapp, F. A. Son, X. Zhang, W. Hierse, C. Kühn, S. Marx, B. Hernandez and O. K. Farha, Faraday Discuss., 2021, 225, 9–69 RSC.
- S. F. Du, X. Y. Yu, G. L. Liu, M. Zhou, E. S. M. El-Sayed, Z. F. Ju, K. Z. Su and D. Q. Yuan, Cryst. Growth Des., 2021, 21, 692–697 CrossRef CAS.
- C. M. Ngue, Y. H. Liu, M. Leung and K. L. Lu, Inorg. Chem., 2021, 60, 11458–11465 CrossRef CAS PubMed.
- T. Rabe, E. S. Grape, T. A. Engesser, A. K. Inge, J. Ströh, G. Kohlmeyer-Yilmaz, M. Wahiduzzaman, G. Maurin, F. D. Sönnichsen and N. Stock, Chem. – Eur. J., 2021, 27, 7696–7703 CrossRef CAS PubMed.
- T. Rabe, E. S. Grape, H. Rohr, H. Reinsch, S. Wöhlbrandt, A. Lieb, A. K. Inge and N. Stock, Inorg. Chem., 2021, 60, 8861–8869 CrossRef CAS PubMed.
- E. P. Asiwal, D. S. Shelar, C. S. Gujja, S. T. Manjare and S. D. Pawar, New J. Chem., 2022, 46, 12679–12685 RSC.
- J. J. Huang, D. Zhao and G. J. Yin, Chin. J. Struct. Chem., 2022, 41, 2203030–2203039 CAS.
- L. Yu, S. Ullah, K. Zhou, Q. B. Xia, H. Wang, S. Tu, J. J. Huang, H. L. Xia, X. Y. Liu, T. Thonhauser and J. Li, J. Am. Chem. Soc., 2022, 144, 3766–3770 CrossRef CAS PubMed.
- J. Gosch, V. Guiotto, F. Steinke, E. S. Grape, C. Atzori, K. Mertin, T. Otto, N. Ruser, C. Meier, D. M. Venturi, A. K. Inge, K. A. Lomachenko, V. Crocellà and N. Stock, Inorg. Chem., 2023, 62, 20929–20939 CrossRef CAS PubMed.
- J. Gosch, D. M. Venturi, E. S. Grape, C. Atzori, L. Donà, F. Steinke, T. Otto, T. Tjardts, B. Civalleri, K. A. Lomachenko, A. K. Inge, F. Costantino and N. Stock, Inorg. Chem., 2023, 62, 5176–5185 CrossRef CAS PubMed.
- M. B. Kim, A. J. Robinson, M. L. Sushko and P. K. Thallapally, J. Ind. Eng. Chem., 2023, 118, 181–186 CrossRef CAS.
- W. W. Zhong, F. D. Firuzabadi, Y. Hanifehpour, X. Zeng, Y. J. Feng, K. G. Liu, S. W. Joo, A. Morsali and P. Retailleau, Inorganics, 2023, 11, 11050184 CrossRef.
- M. Xiong, A. Mohanty, D. H. Liao, L. Lu, W. Zhang, J. Wang, M. Muddassir, S. Al-Sulaimi and Y. Pan, J. Mol. Struct., 2024, 1297, 136958 Search PubMed.
- A. M. Plonka, D. Banerjee, W. R. Woerner, Z. J. Zhang, J. Li and J. B. Parise, Chem. Commun., 2013, 49, 7055–7057 Search PubMed.
- D. Banerjee, S. K. Elsaidi and P. K. Thallapally, J. Mater. Chem. A, 2017, 5, 16611–16615 RSC.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B:Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- L. Öhrström and F. M. Amombo Noa, Metal–Organic Frameworks, American Chemical Society, 2021 Search PubMed.
- F. M. Amombo Noa, M. Abrahamsson, E. Ahlberg, O. Cheung, C. R. Göb, C. J. McKenzie and L. Öhrström, Chem, 2021, 7, 2491–2512 CAS.
- C. Healy, K. M. Patil, B. H. Wilson, L. Hermanspahn, N. C. Harvey-Reid, B. I. Howard, C. Kleinjan, J. Kolien, F. Payet, S. G. Telfer, P. E. Kruger and T. D. Bennett, Coord. Chem. Rev., 2020, 419, 20 Search PubMed.
- N. Hanikel, M. S. Prévot, F. Fathieh, E. A. Kapustin, H. Lyu, H. Wang, N. J. Diercks, T. G. Glover and O. M. Yaghi, ACS Cent. Sci., 2019, 5, 1699–1706 CrossRef CAS PubMed.
- P. Siman, C. A. Trickett, H. Furukawa and O. M. Yaghi, Chem. Commun., 2015, 51, 17463–17466 RSC.
- A. Schoedel, M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2016, 116, 12466–12535 CrossRef CAS PubMed.
- X. Wang, L. Yue, P. Zhou, L. Fan and Y. He, Inorg. Chem., 2021, 60, 17249–17257 CrossRef CAS PubMed.
- S. Khan, S. Naaz, S. Ahmad, R. M. Gomila, A. Chanthapally, A. Frontera and M. H. Mir, Chem. Commun., 2024, 60, 10370–10373 RSC.
- T. Li, M. T. Kozlowski, E. A. Doud, M. N. Blakely and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS PubMed.
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675–702 Search PubMed.
- P. Qu, M.-H. Zhang and J.-W. Zhang, Inorg. Chem. Commun., 2023, 151, 110623 Search PubMed.
- M. S. M. Abdelbaky, Z. Amghouz, E. Fernández-Zapico, S. García-Granda and J. R. García, J. Solid State Chem., 2015, 229, 197–207 Search PubMed.
- H. Wu, S. Zhang, M. Li, C. Qiao, L. Sun, Q. Wei, G. Xie, S. Chen and S. Gao, ChemistrySelect, 2016, 1, 3335–3342 CrossRef CAS.
- Y.-T. Huang, Y.-C. Lai and S.-L. Wang, Chem. – Eur. J., 2012, 18, 8614–8616 CrossRef CAS PubMed.
- V. A. Blatov, L. Carlucci, G. Ciani and D. M. Proserpio, CrystEngComm, 2004, 6, 377–395 RSC.
- L. S. Xie, E. V. Alexandrov, G. Skorupskii, D. M. Proserpio and M. Dincă, Chem. Sci., 2019, 10, 8558–8565 RSC.
- P. Tshuma, B. C. E. Makhubela, L. Öhrström, S. A. Bourne, N. Chatterjee, I. N. Beas, J. Darkwa and G. Mehlana, RSC Adv., 2020, 10, 3593–3605 RSC.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3586 Search PubMed.
- O. Delgado-Friedrichs and M. O'Keeffe, Acta Crystallogr., Sect. A:Found. Crystallogr., 2003, 59, 351–360 CrossRef PubMed.
- D. P. van Heerden and L. J. Barbour, Chem. Soc. Rev., 2021, 50, 735–749 RSC.
- A. A. Coelho, TOPAS and, TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++, J. Appl. Crystallogr., 2018, 51, 210–218, DOI:10.1107/S1600576718000183.
Footnotes |
| † Dedicated to Professor Omar M. Yaghi on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Crystallographic information files, details of synthesis, characterisation, and topology analysis. CCDC 2240527. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00271k |
| § The reported MOFs were characterised by single crystal diffraction (1) (CCDC 2240527†) PXRD, TGA, IR, and elemental analysis. Topologies were analysed with ToposPro40 and SYSTRE.41 Gas sorption analysis was run on a MICROTRAC BELSORP MINI X. Pawley fits for the powder patterns were conducted with TOPAS Academic.43 |
| This journal is © The Royal Society of Chemistry 2025 |


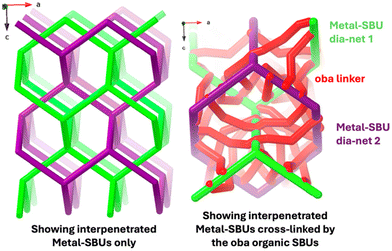
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–Y. Right: the bridging of the two metal-SBUs by the oba –C6H4–O–C6H4– organic SBU in red. For clarity some of the oba links have been omitted.
O–Y. Right: the bridging of the two metal-SBUs by the oba –C6H4–O–C6H4– organic SBU in red. For clarity some of the oba links have been omitted.