High-efficiency oxygen evolution catalysts: composite hexagonal structure SrCo1−yNiyO3−δ/Sr9Ni7O21†
Xiaolong
Yang
,
Hongyuan
Song
 *,
Ruihang
Yao
,
Haorong
Wu
,
Kun
Dong
,
Liangwei
Chen
,
Bin
Liu
*,
Ruihang
Yao
,
Haorong
Wu
,
Kun
Dong
,
Liangwei
Chen
,
Bin
Liu
 ,
Zhenhua
Ge
and
Lan
Yu
*
,
Zhenhua
Ge
and
Lan
Yu
*
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, 650093, PR China. E-mail: songhongyuan0227@outlook.com; yulan000@hotmail.com
First published on 21st April 2025
Abstract
Transition metal perovskite oxides are regarded as ideal catalysts for the oxygen evolution reaction (OER) owing to the high tunability of the B-site active elements. Among them, SrCoO3 has been widely studied due to its high theoretical catalytic performance, but incomplete oxidation of the B-site elements results in poor intrinsic activity. Herein, we prepared a series of SrCo1−xNixO3−δ (x = 0–0.5) catalysts for the alkaline OER using a simple sol–gel method and optimized their catalytic performance by modulating the Co/Ni ratio and annealing temperature (950 °C and 1050 °C). Among the series, SrCo0.6Ni0.4O3−δ (annealed at 950 °C) with a composite hexagonal structure (SrCo1−yNiyO3−δ/Sr9Ni7O21, y = 0.1–0.2) exhibited the best OER activity, achieving an overpotential of 321 mV at 10 mA cm−2 (1 M KOH). The introduction of Ni ions and the presence of Sr9Ni7O21 not only enhance the Co4+/Co3+ and Ni3+/Ni2+ ratios but also promote the generation of more highly oxidative oxygen species (O22−/O−). Additionally, the abundant Ni3+ surface facilitates the formation of a highly active NiOOH phase during the OER, leading to a further reduction in the overpotential (after 1000 CV cycles, the overpotential on the nickel foam electrode decreased by 45 mV).
1. Introduction
The oxygen evolution reaction (OER) is a critical process in various energy conversion and storage systems, such as electrochemical water splitting and rechargeable metal–air batteries.1,2 Its complex intermediate steps involve multiple electron/proton transfers, leading to slow reaction kinetics.3 Consequently, it is essential to develop OER catalysts that exhibit both high performance and long-term stability. Noble metal-based oxides (IrO2, RuO2, etc.) show excellent OER activity, but their scarcity and high cost limit their large-scale practical application.4,5 Among transition metals, cobalt and nickel oxides exhibit water oxidation performance comparable to that of noble metal catalysts under alkaline conditions, making them ideal alternative materials.6,7Currently, transition metal-based OER catalysts mainly include three structural types: layered hydroxide, spinel and perovskite.8 Perovskite oxides have been widely studied due to their flexible composition and adjustable structure.9,10 Several OER activity descriptors have been developed based on the B-site active ions, such as the electron occupancy of the eg orbital,11 metal–oxygen bond covalency12, the p-band center of O relative to the Fermi level,13etc. Among the diverse perovskite oxides, cubic SrCoO3 is predicted to have the highest theoretical OER activity.14 Due to the incomplete oxidation of Co ions, only oxygen-deficient SrCoO3−δ with a hexagonal Sr6Co5O15 structure can be synthesized under normal pressure.15,16
Grimaud's research demonstrates that the surface-shared coordination and one-dimensional prism structure of hexagonal perovskites have important effects on the activity and stability of the OER.17 Wei et al. prepared an Fe-doped hexagonal Sr6(Co0.8Fe0.2)5O15 (SCF-H), which exhibits markedly better OER activity compared to cubic SrCo0.8Fe0.2O3−δ (SCF-C). Specifically, hexagonal SCF-H features a higher concentration of surface reactive oxygen species (O22−/O−), and its O p band center is closer to the Fermi level.18 Similarly, Lee et al. reported a phase transition mechanism from hexagonal BaNiO3 to Ba6Ni5O15 during the OER. The formation of Ni vacancies optimizes the oxidation state (Ni4+ → Ni3+/Ni2+), thereby enhancing both the OER activity and cycling stability.19 The above studies have convincingly confirmed that single-phase hexagonal perovskites have great potential in the field of OER catalysis. Composite structure engineering is an emerging strategy to enhance the catalytic performance of single-phase perovskites.20,21 Recently, Wang's team has developed a new 2D descriptor (dB, nB) for predicting the OER activity of perovskites and discovered an unreported hexagonal perovskite SrCo0.6Ni0.4O3−δ with high OER activity.22 However, SrCo0.6Ni0.4O3−δ is not a single-phase structure material, making it inappropriate to apply the activity descriptors designed for single-phase perovskites to understand the activity source of this composite catalyst. Furthermore, the formation of such a composite structure originates from the solubility limits of the dopant elements and demonstrates a significant simplicity advantage over alternative fabrication approaches.23,24
Here, we have successfully isolated two major hexagonal perovskite structures (SrCo1−yNiyO3−δ, y = 0.1–0.2 and Sr9Ni7O21) in SrCo0.6Ni0.4O3−δ. The relationship between the phase composition and surface states of the composite structure (SrCo1−yNiyO3−δ/Sr9Ni7O21) and the properties of the OER has been investigated. This composite catalyst exhibits superior OER activity compared to pure hexagonal SrCoO3−δ, SrCo0.9Ni0.1O3−δ and SrCo1−yNiyO3−δ/NiO composite structures. Ni doping and the presence of SNO not only improve the oxidation state of B-site active elements (Co/Ni) but also introduce a higher concentration of highly oxidative oxygen species (O22−/O−). SrCo1−yNiyO3−δ/Sr9Ni7O21 enhances NiOOH formation during the reaction, which reduces the overpotential by 45 mV after 1000 CV cycles. This study provides new insights for the design and development of efficient OER catalysts through multi-metallic synergy and composite structure design.
2. Experimental
2.1. Catalyst synthesis
A series of catalyst powders SrCo1−xNixO3−δ (x = 0–0.5) (SCNxO) were prepared at 950 °C and 1050 °C by using the sol–gel method: firstly, Sr(NO3)2, Co(NO3)2·6H2O and Ni(NO3)2·6H2O with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 − x
1 − x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x were completely dissolved in deionized water and stirred at 70 °C for 30 minutes to obtain a precursor solution. Ethylenediaminetetraacetic acid (EDTA) and citric acid (CA) were added as complexing agents, with metal ions
x were completely dissolved in deionized water and stirred at 70 °C for 30 minutes to obtain a precursor solution. Ethylenediaminetetraacetic acid (EDTA) and citric acid (CA) were added as complexing agents, with metal ions![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDTA
EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA = 1
CA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The pH of the solution was adjusted to 7–9 by dropwise adding ammonia solution. The temperature was subsequently raised to 120 °C, and the solution was continuously stirred until it formed a purplish-red gel due to water evaporation. This gel was transferred to a vacuum drying oven at 250 °C and dried for 5 h to obtain a black and fluffy precursor. After simple grinding, the precursor was annealed at 950 °C (SCNxO9) and 1050 °C (SCNxO10) for 6 h at a heating rate of 5 °C min−1, respectively. Finally, the black catalyst powders were obtained by fully grinding the product.
2. The pH of the solution was adjusted to 7–9 by dropwise adding ammonia solution. The temperature was subsequently raised to 120 °C, and the solution was continuously stirred until it formed a purplish-red gel due to water evaporation. This gel was transferred to a vacuum drying oven at 250 °C and dried for 5 h to obtain a black and fluffy precursor. After simple grinding, the precursor was annealed at 950 °C (SCNxO9) and 1050 °C (SCNxO10) for 6 h at a heating rate of 5 °C min−1, respectively. Finally, the black catalyst powders were obtained by fully grinding the product.
2.2. Characterization
The phase structures of SCNxO catalysts were characterized using an Anton Paar XRDynamic500 X-ray diffractometer, and the radiation source was Cu Kα (λ = 0.154 nm). The XRD patterns were Rietveld refined using GSAS-II software.25 The Raman tests were carried out on a Renishaw inVia Raman spectrometer with a 532 nm laser. The microstructure of the catalyst was studied by scanning electron microscopy (SEM, Tescan-VEGA3, Czechia). The surface elemental distribution of the catalyst was analyzed using an electron probe microanalyzer (EPMA, Shimadzu EPMA-1720H) equipped with energy-dispersive spectroscopy (EDS) and wavelength-dispersive spectroscopy (WDS). The surface chemical state of the catalyst was analyzed by X-ray photoelectron spectroscopy (XPS Thermo Scientific K-Alpha). TEM images were obtained using a FEI-Tecnai G2 F30 S-Twin transmission electron microscope with an operating voltage of 200 kV. The Micromeritics ASAP 2460 automatic specific surface and porosity analyzer was used to measure the specific surface area of the catalyst powders based on the Brunauer–Emmett–Teller (BET) method.2.3. Electrochemical measurements
The preparation process of the working electrode was as follows: 7 mg catalyst powders and 70 μL Nafion solution (20 wt%) were placed in a 2 mL ethanol solution (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 7
deionized water = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), followed by ultrasonic treatment for 1 h. The 10 μL catalyst ink drop prepared above was coated on a glassy carbon electrode (d = 5 mm) and dried in the air for 1 h. The mass load of the catalyst was approximately 0.173 mgoxide cm−2.
3), followed by ultrasonic treatment for 1 h. The 10 μL catalyst ink drop prepared above was coated on a glassy carbon electrode (d = 5 mm) and dried in the air for 1 h. The mass load of the catalyst was approximately 0.173 mgoxide cm−2.
Electrochemical measurements were performed in an O2-saturated 1 M KOH solution using a standard three-electrode configuration on an electrochemical workstation (CHI 760E). The Hg/HgO electrode and graphite rod were used as the reference electrode and the counter electrode, respectively. The working electrodes were subjected to 50 cyclic voltammetry (CV) tests in a potential range of 0–0.8 V (vs. Hg/HgO) to activate the catalysts. Then linear sweep voltammetry curves (LSV) of the OER were obtained at a scanning rate of 5 mV s−1. Electrochemical impedance spectroscopy (EIS) tests were performed from 10−5 to 10−1 Hz at an applied potential of 0.66 V (vs. Hg/HgO) with an amplitude of 5 mV. Double-layer capacitance (Cdl) tests were evaluated by CV measurements at scan rates of 20, 40, 60, 80, and 100 mV s−1 at 0.12–0.22 V (vs. Hg/HgO), and the electrochemically active surface area (ECSA) of the catalysts was obtained using the following formula: ECSA = Cdl/Cs, where specific capacitance Cs = 60 μF cm−2 (referred to the reported value of perovskite oxide in 1 M KOH solution). All measured potential data were manually calibrated with 95% IR to compensate for solution resistance and then were converted to the reversible hydrogen electrode (RHE) scale using the following formula: ERHE = EHg/HgO + 0.9122 V.
The stability tests were divided into two parts: one is accelerated durability testing (ADT), and the catalyst stability was evaluated by performing 1000 CV tests at a scan rate of 100 mV s−1 at 0–0.8 V (vs. Hg/HgO). The second method was durability testing by chronopotentiometry. The working electrodes were changed to Ni-foam coated with the catalyst: the ink was made by ultrasonic mixing of 5 mg catalyst powders, 50 μL Nafion solution and 1 mL ethanol, and 400 μL of the ink was coated on Ni-foam (1 × 1 × 0.1 cm). The mass load of the catalyst was about 1.9 mgoxide cm−2.
3. Results and discussion
3.1. Crystal structure and the microstructure
Fig. 1a shows the XRD patterns of SCNxO9 (x = 0–0.5) series catalysts. The initial SrCoO3−δ (SCO) exhibits a hexagonal structure indexed to PDF#40-1018 (SrCoO2.52),26 and Ni doping does not induce a phase transition. When the Ni content x reaches 0.2, SCxNO forms a composite structure consisting of SrCo1−yNiyO3−δ (y = 0.1–0.2) (SCNyO) and Sr9Ni7O21 (SNO, PDF#89-0819), with a minor presence of NiO (PDF#78-0423).27 SNO has a hexagonal structure similar to SCO (Fig. 1b) and NiO has a cubic structure with the space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. However, when the annealing temperature is increased to 1050 °C, these two phases appear only when the Ni content x exceeds 0.3 (Fig. S1†). Furthermore, the number of SNO characteristic peaks decreases significantly, while the intensity of NiO peaks increases markedly. The higher annealing temperature increases the solid solubility of Ni in SCO and the content of the NiO phase and inhibits the formation of the SNO phase. These phenomena are consistent with the study of the Sr–Ni–O system phase diagram: within the temperature range of 800 °C–1030 °C, only SNO and NiO can exist stably. When the temperature exceeds 1030 °C, SNO will decompose into SrO and NiO.28 Therefore, for x = 0.3–0.5, the content of SNO in SCNxO10 is significantly reduced, leading to the formation of a composite structure primarily consisting of SCNyO (y = 0.2–0.3) and NiO.
m. However, when the annealing temperature is increased to 1050 °C, these two phases appear only when the Ni content x exceeds 0.3 (Fig. S1†). Furthermore, the number of SNO characteristic peaks decreases significantly, while the intensity of NiO peaks increases markedly. The higher annealing temperature increases the solid solubility of Ni in SCO and the content of the NiO phase and inhibits the formation of the SNO phase. These phenomena are consistent with the study of the Sr–Ni–O system phase diagram: within the temperature range of 800 °C–1030 °C, only SNO and NiO can exist stably. When the temperature exceeds 1030 °C, SNO will decompose into SrO and NiO.28 Therefore, for x = 0.3–0.5, the content of SNO in SCNxO10 is significantly reduced, leading to the formation of a composite structure primarily consisting of SCNyO (y = 0.2–0.3) and NiO.
The phase structures of SCNxO9 have been further analyzed by Raman spectroscopy (Fig. 1c). The peaks at approximately 429 cm−1 and 629 cm−1 come from the B2g and Ag vibration modes of hexagonal SCO,29 and these exhibit a slight blue shift with the partial substitution of Co by Ni. SNO does not lead to the emergence of new Raman characteristic peaks due to the similar hexagonal structure. The new peak gradually appearing near 500 cm−1 arises from the stretching vibration of the Ni–O bond in NiO.30 The XRD pattern of SCN0.4O9 has been refined using the Rietveld method (Fig. 1d), which further confirmed the SCNyO/SNO composite structure. Fig. 1e and f display the TEM images of SCN0.4O9. The lattice spacings of 4.4 Å and 1.89 Å correspond to the (113) plane of SNO and the (102) plane of SCNyO, respectively. The lattice spacing of 2.41 Å corresponds to the (111) plane of NiO.
SEM images reveal the microstructure of SCNxO (x = 0–0.5) series catalysts, as shown in Fig. 2 and S2.† All samples consist of micron-scale granules. The surfaces of the SCNxO10 series are denser than those of the SCNxO9 series due to the higher annealing temperature. At the same annealing temperature, the increase of Ni doping leads to the decrease of catalyst particle size and the increase of surface pore number. This change exposes more active sites on the catalyst surface for an efficient OER. Furthermore, we measured the BET specific surface area of the SCNxO9 series catalysts (Fig. S3†), and the results are consistent with the SEM observations. SCN0.5O9 exhibits the largest BET specific surface area, approximately five times that of SCO9. EDS mappings of SCN0.4O9 show that the four elements Sr, Co, Ni and O are evenly distributed within individual particles (Fig. S4†). To further investigate the distribution of newly formed SNO and NiO, the elements on the surface of SCN0.4O9 particles were analyzed by WDS (Fig. S5†). The SNO phase and the NiO phase, represented by dark blue and red, respectively, are uniformly distributed on the catalyst powders’ surface, with no evident segregation.
 | ||
| Fig. 2 SEM images of SCNxO9 series catalysts: (a) x = 0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3, (e) x = 0.4, and (f) x = 0.5. | ||
3.2. OER catalytic performance
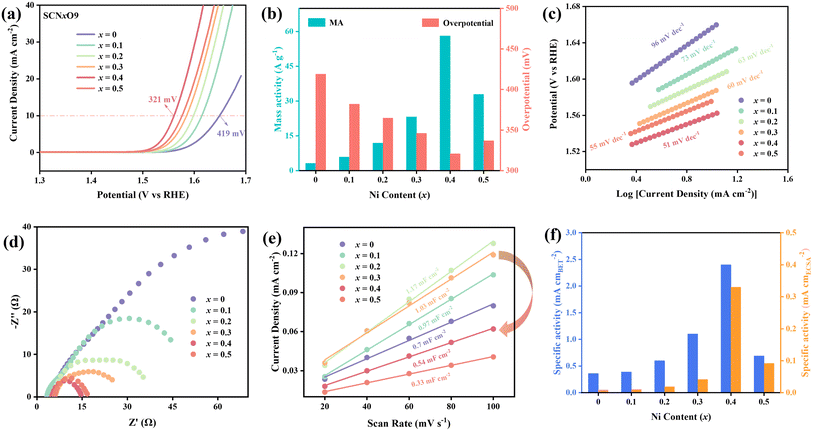
The LSV curves of the SCNxO9 (x = 0–0.5) series catalysts are shown in Fig. 3a. The overpotential of the catalysts at 10 mA cm−2 decreases with the increasing Ni doping amount. Among them, SCN0.4O9 with a composite structure (SCNyO/SNO) exhibits the best OER activity (321 mV), significantly better than the initial SCO (419 mV) and single-phase SCN0.1O9 (382 mV). This composite perovskite catalyst demonstrates comparable or even superior performance relative to recently reported Co and Ni based oxides (Table S1†). Furthermore, the mass activity of the SCNxO9 series catalysts was calculated (Fig. 3b). SCN0.4O9 showed 18.7 times higher mass activity than SCO, indicating its greater potential for commercial applications. The Tafel slope is an important tool for studying OER kinetics. As shown in Fig. 3c, the Ni-doped SCNxO9 series exhibits a reduced Tafel slope value, which indicates a faster kinetic process. Specifically, the rate-determining step (RDS) of the OER can be calculated using the formula (59 mV dec−1)/(n′ + α), where α is the symmetry/transfer coefficient (usually taken as 0.5) and n′ is the number of single electron transfer steps before the RDS.31,32 The Tafel slope of the initial SCO is 96 mV dec−1, close to the characteristic value of 120 mV dec−1, suggesting that its RDS primarily follows the first electron transfer process (* + OH− → OH* + e−); the Tafel slope of SCN0.4O9 is 51 mV dec−1, approaching the characteristic value of 40 mV dec−1, indicating that the second electron transfer process (OH* + OH− → O* + O2 + e−) dominates its RDS. Additionally, SCN0.4O9 shows the smallest surface charge transfer resistance (Rct) (Fig. 3d).The ECSA of the SCNxO9 series catalysts have been calculated through Cdl tests (Fig. 3e and S6, S7†). A lower amount of Ni doping (x = 0.1–0.3) results in an increase in ECSA. Surprisingly, the ECSA of SCN0.4O9, which exhibits the best OER activity in the series, is sharply reduced compared to SCN0.3O9. BET test results suggest that this phenomenon is not due to a reduction in the specific surface area. Relevant studies indicate that this may be because the Ni2+ species in the catalyst are non-conductive within the non-faradaic potential range, leading to inaccuracies in the Cdl tests.33,34 Therefore, the ECSA cannot accurately reflect the true number of active sites on the sample surface. However, both the BET area specific activity and the ECSA specific activity of the SCNxO9 series exhibit the same trend (Fig. 3f), with the SCN0.4O9 composite demonstrating the highest intrinsic OER activity in the series.
3.3. Relationship between surface states and OER activity
The XPS spectra of Sr, Co and Ni in the SCNxO9 (x = 0–0.5) series catalysts are shown in Fig. 4. The two main peaks in the Co 2p spectrum can be deconvoluted into two sets of doublets: the peaks at 780.1 eV and 795.1 eV come from Co3+ ions, while the peaks at 781.6 eV and 796.6 eV belong to Co4+ ions (Fig. 4a).35 As the Ni content x increases from 0 to 0.1, the Co4+/Co3+ ratio significantly increases, after which it remains relatively constant (Fig. 4d). This indicates that the Ni doping effect can enhance the average oxidation state of Co, which is beneficial for improving the intrinsic OER activity of Co-based oxides.36 The peak fitting results of Ni are shown in Fig. 4b. For x = 0.1–0.5, both Ni3+ ions (856 eV and 873.3 eV) and Ni2+ ions (854.5 eV and 871.8 eV) coexist.37 Two Ni-containing phases (SNO and NiO) are present in the catalysts. The increased Ni3+ ions originate from SNO, as NiO can only provide Ni2+ species. The composite SCN0.4O9 surface possesses the highest Ni3+/Ni2+ ratio (Fig. 4d), which means the highest proportion of SNO. Compared with Ni2+, the higher-valent Ni3+ has a stronger OH− adsorption capacity. Consequently, the enhancement of the Ni oxidation state can strengthen the adsorption of reaction intermediates on the sample surface.38 In the previous OER test, SCN0.4O9 exhibited the lowest overpotential and the highest specific activity among the series. The introduction of Ni ions and the presence of SNO in its composite structure optimize the electronic structure of surface active elements (Co/Ni), thereby enhancing the intrinsic activity. This plays a dominant role in enhancing the OER catalytic performance of the SCNxO9 series samples. The fitting results of the Sr 3d spectra reveal two types of Sr on the surface of the SCNxO9 series (Fig. 4c): the lattice Sr with lower binding energy and the surface Sr with higher binding energy (SrO or Sr(OH)2).39 Furthermore, compared with SCO, the composite SCN0.4O9 surface contains a higher concentration of highly oxidative oxygen species (O22−/O−) (Fig. 4e). Many studies have shown that highly oxidized oxygen (O22−/O−) plays an important role in the OER process.40,41To further determine the contribution of the SCNyO/SNO composite structure to OER activity, electrochemical tests were conducted on the SCNxO10 (x = 0–0.5) series catalysts (Fig. 5a). Although their OER activity improved with Ni doping, the extent of performance enhancement is not as significant as the SCNxO9 series. Fig. 5b presents the LSV curves for SCN0.4O9, SCN0.4O10, and pure NiO, and it has been found that the catalytic activity of the SCNyO/SNO composite structure is significantly better than that of SCN0.4O10 (372 mV) and pure NiO (410 mV).
3.4. Catalytic stability and surface reconstruction during the OER
The catalytic stability of the SCN0.4O9 composite sample on a Ni-foam substrate was assessed by accelerated durability testing (ADT) and chronopotentiometry (CP). As shown in Fig. S8,† the OER activity of pristine nickel foam is significantly lower than that of SCN0.4O9-loaded Ni-foam and SCN0.4O9-coated GC. Therefore, replacing GC with Ni-foam will not affect the reliability of the stability test results. Fig. 6a and b show the ADT results for SCO9 and SCN0.4O9, where both materials exhibit enhanced OER activity after ADT. The overpotential of SCO9 decreased by 22 mV at 10 mA cm−2, while the overpotential of the SCN0.4O9 composite reduced by up to 45 mV. Previous studies have indicated that this improvement may be attributed to surface reconstruction during the OER.42,43 The Raman spectra of SCN0.4O9 after ADT reveal two new peaks at 473 and 555 cm−1 (Fig. 6c), which are characteristic signals of NiOOH.44 This suggests that Ni on the catalyst surface gradually combines with OH− in the electrolyte to form a highly active NiOOH phase during the OER. The high ratio of Ni3+/Ni2+ on the surface of SCN0.4O9 facilitates this transformation more easily compared to other catalysts in the series.45 Additionally, this reconstruction is typically accompanied by the dissolution of surface A-site cations.46 As shown in Fig. 6d, the atomic fraction of Sr on the initial surface is 21.39%, which decreases to 18.51% after ADT. The relative intensity of WDS mapping of Sr also decreased significantly after ADT (Fig. S9†), further confirming the leaching of Sr ions. Sr-deficient surfaces facilitate exposure to more B-site active elements (Co/Ni), and the surface morphology of SCN0.4O9 does not change significantly before and after ADT. Fig. 6e displays the XRD patterns of SCN0.4O9 before and after ADT, with no additional impurity peaks appearing, indicating that the main structure remains intact. This composite structure (SCNyO/SNO) exhibits good structural stability and catalytic stability.The XPS spectra of SCN0.4O9 on the carbon paper before and after ADT have been acquired, as illustrated in Fig. S10.† The Sr 3d spectral peak exhibits a notable shift toward the lower binding energy region, accompanied by a slight reduction in intensity (Fig. S10a†). This indicates a reduction in the concentration of surface Sr species, consistent with the EDS and WDS results, indicating that Sr2+ ions on the sample surface are slightly leached during the OER cycle. Furthermore, the leaching process promotes the exposure of deeper Co sites, resulting in a significant increase in the Co signal intensity on the sample surfaces after ADT (Fig. S10b†). The Co 2p spectral peaks exhibit no significant shift after ADT, suggesting that the surface active Co sites possess good chemical stability. In the Ni 2p spectrum (Fig. S10c†), the proportion of Ni2+ ions is significantly reduced after ADT, while the proportion of Ni3+ ions shows a slight increase. This could be attributed to the formation and aggregation of the NiOOH active phase. The deconvolution of the O 1s spectrum indicates that the content of –OH species on the surface of SCN0.4O9 significantly increases after ADT (Fig. S10d†), further confirming the presence of NiOOH. The catalytic performance of SCN0.4O9 does not significantly deteriorate during 25 h operation, as shown in Fig. 6f. Based on stability studies, the surface B-site element of SCN0.4O9 is activated by the slight leaching of Sr ions, and the formation of highly active hydroxyl oxides further promotes OER performance.
4. Conclusion
In this study, we have successfully synthesized a series of SrCo1−xNixO3−δ (x = 0–0.5) (SCNxO) catalysts and systematically investigated the correlation between their phase composition and OER activity. The initial SCO and SCN0.1O9 (annealed at 950 °C) exhibit a hexagonal single-phase structure. For compositions with x = 0.2–0.5, incorporation of excess Ni ions leads to the formation of a SrCo1−yNiyO3−δ/Sr9Ni7O21 composite hexagonal structure. This composite structure increases the average oxidation states of Co and Ni on the surface and introduces more active oxygen species (O22−/O−). Notably, the SCN0.4O9 composite demonstrates the lowest overpotential of 321 mV and the smallest Tafel slope of 51 mV dec−1, significantly outperforming both SCO and pure-phase SCN0.1O9. The high Ni3+/Ni2+ ratio in SCN0.4O9 promotes the formation of a highly active NiOOH phase during the OER process, further enhancing its catalytic performance. Furthermore, due to the high-temperature decomposition of SNO, SCN0.4O10 (annealed at 1050 °C) transforms into a SCNyO/NiO composite structure and has poor OER activity.Author contributions
Xiaolong Yang: conceptualization, investigation, writing – original draft and writing – review & editing. Hongyuan Song: writing – review & editing, investigation, supervision, and conceptualization. Ruihang Yao: investigation. Haorong Wu: investigation. Kun Dong: investigation. Liangwei Chen: investigation. Bin Liu: investigation. Zhenhua Ge: investigation. Lan Yu: supervision, project administration, resources, visualization, and review & editing.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (no. 51962017 and 51462017).References
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, 73–78 CrossRef PubMed.
- C. Wei, R. R. Rao, J. Peng, B. Huang, I. E. L. Stephens, M. Risch, Z. J. Xu and Y. Shao-Horn, Adv. Mater., 2019, 31, 1806296 CrossRef.
- J. Song, C. Wei, Z.-F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC.
- E. Fabbri, A. Habereder, K. Waltar, R. Kötz and T. J. Schmidt, Catal. Sci. Technol., 2014, 4, 3800–3821 RSC.
- L. L. Li, H. N. Sun, Z. W. Hu, J. Zhou, Y. C. Huang, H. L. Huang, S. Z. Song, C. W. Pao, Y. C. Chang, A. C. Komarek, H. J. Lin, C. T. Chen, C. L. Dong, J. Q. Wang and L. J. Zhang, Adv. Funct. Mater., 2021, 31, 2104746 CrossRef CAS.
- X. Ren, T. Wu, Y. Sun, Y. Li, G. Xian, X. Liu, C. Shen, J. Gracia, H.-J. Gao, H. Yang and Z. J. Xu, Nat. Commun., 2021, 12, 22865 Search PubMed.
- J. S. Yoo, X. Rong, Y. Liu and A. M. Kolpak, ACS Catal., 2018, 8, 4628–4636 CrossRef CAS.
- F.-Y. Chen, Z.-Y. Wu, Z. Adler and H. Wang, Joule, 2021, 5, 1704–1731 CrossRef CAS.
- H. Song, Y. Pang, L. Dong, K. Dong, H. Wu, L. Chen, B. Liu, Z. Ge and L. Yu, J. Alloys Compd., 2025, 1010, 177709 CrossRef CAS.
- Y. Wang, L. Wang, K. Zhang, J. Xu, Q. Wu, Z. Xie, W. An, X. Liang and X. Zou, Chin. J. Catal., 2023, 50, 109–125 CrossRef CAS.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS.
- J. T. Mefford, X. Rong, A. M. Abakumov, W. G. Hardin, S. Dai, A. M. Kolpak, K. P. Johnston and K. J. Stevenson, Nat. Commun., 2016, 7, 11053 CrossRef CAS.
- A. Grimaud, K. J. May, C. E. Carlton, Y.-L. Lee, M. Risch, W. T. Hong, J. Zhou and Y. Shao-Horn, Nat. Commun., 2013, 4, 3439 Search PubMed.
- I. C. Man, H. Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- H. Song, B. Liu, J. Zeng, G. Huo, L. Chen, J. Wang and L. Yu, Dalton Trans., 2023, 52, 4398–4406 RSC.
- M. T. Azcondo, M. Orfila, M. Linares, R. Molina, J. Marugán, U. Amador, K. Boulahya, J. Á. Botas and R. Sanz, ACS Appl. Energy Mater., 2021, 4, 7870–7881 CrossRef CAS.
- A. Grimaud, C. E. Carlton, M. Risch, W. T. Hong, K. J. May and Y. Shao-Horn, J. Phys. Chem., 2013, 117, 25926–25932 CAS.
- L. Wei, J. Hu, H. Liu, W. Zhang, H. Zheng, S. Wu and K. Tang, Dalton Trans., 2022, 51, 7100–7108 RSC.
- J. G. Lee, J. Hwang, H. J. Hwang, O. S. Jeon, J. Jang, O. Kwon, Y. Lee, B. Han and Y.-G. Shul, J. Am. Chem. Soc., 2016, 138, 3541–3547 CrossRef CAS PubMed.
- F. Abdelghafar, X. Xu, D. Guan, Z. Lin, Z. Hu, M. Ni, H. Huang, T. Bhatelia, S. P. Jiang and Z. Shao, ACS Mater. Lett., 2024, 6, 2985–2994 CrossRef CAS.
- X. Xu, Y. Pan, L. Ge, Y. Chen, X. Mao, D. Guan, M. Li, Y. Zhong, Z. Hu, V. K. Peterson, M. Saunders, C.-T. Chen, H. Zhang, R. Ran, A. Du, H. Wang, S. P. Jiang, W. Zhou and Z. Shao, Small, 2021, 17, 2101573 CrossRef CAS PubMed.
- J. Wang, H. Xie, Y. Wang and R. Ouyang, J. Am. Chem. Soc., 2023, 145, 11457–11465 CrossRef CAS.
- H. Sun, X. Xu, G. Chen and Z. Shao, Carbon Energy, 2024, 6, e595 CrossRef CAS.
- X. Xu, Y. Pan, Y. Zhong, R. Ran and Z. Shao, Mater. Horiz., 2020, 7, 2519–2565 RSC.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- Y.-r. Hao, H. Xue, J. Sun, N. Guo, T. Song, H. Dong, Z. Zhao, J. Zhang, L. Wu and Q. Wang, Energy Environ. Sci., 2024, 17, 4044–4054 RSC.
- X. Liu, Z.-Y. Zhai, Z. Chen, L.-Z. Zhang, X.-F. Zhao, F.-Z. Si and J.-H. Li, Catalysts, 2018, 8, 310 CrossRef.
- M. Zinkevich, J. Solid State Chem., 2005, 178, 2818–2824 CrossRef CAS.
- A. Glamazda, K. Y. Choi, P. Lemmens, W. S. Choi, H. Jeen, T. L. Meyer and H. N. Lee, J. Appl. Phys., 2015, 118, 064901 CrossRef.
- J. Qiu, T. H. Nguyen, S. Kim, Y. J. Lee, M. T. Song, W. J. Huang, X. B. Chen, T. M. H. Nguyen and I. S. Yang, Spectrochim. Acta, Part A, 2022, 280, 121498 CrossRef CAS PubMed.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Sci. Rep., 2015, 5, 13801 CrossRef.
- D. Chen, J. Qiu, X. Chen, S. Chen, J. Zhang and Z. Peng, J. Phys. Chem. Lett., 2023, 14, 2148–2154 CrossRef CAS PubMed.
- S. S. Jeon, P. W. Kang, M. Klingenhof, H. Lee, F. Dionigi and P. Strasser, ACS Catal., 2023, 13, 1186–1196 CrossRef CAS.
- Q. Lu, ACS Nano, 2024, 18, 13973–13982 CrossRef CAS PubMed.
- X. Li, H. Wang, Z. M. Cui, Y. T. Li, S. Xin, J. S. Zhou, Y. W. Long, C. Q. Jin and J. B. Goodenough, Sci. Adv., 2019, 5, eaav6262 CrossRef CAS PubMed.
- R. Gottschall, R. Schollhorn, M. Muhler, N. Jansen, D. Walcher and P. Gutlich, Inorg. Chem., 1998, 37, 1513–1518 CrossRef CAS.
- Y. Wang, S. Tao, H. Lin, S. Han, W. Zhong, Y. Xie, J. Hu and S. Yang, RSC Adv., 2020, 10, 33475–33482 RSC.
- S. R. Ede, C. N. Collins, C. D. Posada, G. George, H. Wu, W. D. Ratcliff, Y. Lin, J. Wen, S. Han and Z. Luo, ACS Catal., 2021, 11, 4327–4337 CrossRef CAS PubMed.
- Y. A. Yi, Q. B. Wu, J. S. Li, W. T. Yao and C. H. Cui, ACS Appl. Mater. Interfaces, 2021, 13, 17439–17449 CrossRef CAS PubMed.
- K. Zhu, F. Shi, X. Zhu and W. Yang, Nano Energy, 2020, 73, 104761 CrossRef CAS.
- Y. Sun, R. Li, X. X. Chen, J. Wu, Y. Xie, X. Wang, K. K. Ma, L. Wang, Z. Zhang, Q. L. Liao, Z. Kang and Y. Zhang, Adv. Energy Mater., 2021, 11, 2003755 CrossRef CAS.
- Y. Sun, C. R. Wu, T. Y. Ding, J. Gu, J. W. Yan, J. Cheng and K. H. L. Zhang, Chem. Sci., 2023, 14, 5906–5911 RSC.
- Y. Yao, G. Zhao, X. Guo, P. Xiong, Z. Xu, L. Zhang, C. Chen, C. Xu, T.-S. Wu, Y.-L. Soo, Z. Cui, M. M.-J. Li and Y. Zhu, J. Am. Chem. Soc., 2024, 146, 15219–15229 CrossRef CAS PubMed.
- H. Y. Wang, Y. Y. Hsu, R. Chen, T. S. Chan, H. M. Chen and B. Liu, Adv. Energy Mater., 2015, 5, 1802912 Search PubMed.
- J. Li, F. Yang, M. Jiang, X. Y. Cai, Q. D. Hu and J. L. Zhang, Chem. Eng. J., 2023, 451, 138646 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00324e |
| This journal is © The Royal Society of Chemistry 2025 |