Well-defined Pt(0) heterogeneous hydrosilylation catalysts supported by a surface bound phosphenium ligand†
Damien B.
Culver
 *a,
Marco
Mais
*a,
Marco
Mais
 a,
Min-Chul
Kang
a,
Lin
Zhou
ab and
Frédéric A.
Perras
a,
Min-Chul
Kang
a,
Lin
Zhou
ab and
Frédéric A.
Perras
 ac
ac
aDivision of Chemical and Biological Sciences, Ames National Laboratory, Ames, IA 50011, USA. E-mail: culver@ameslab.gov
bDepartment of Materials Science and Engineering, Iowa State University, IA 50011, USA
cDepartment of Chemistry, Iowa State University, Ames, IA 50011, USA
First published on 17th April 2025
Abstract
Single atom, low valent transition metals are important for heterogeneous catalysis but are challenging to generate and stabilize in a well-defined manner. Herein, we explored the functionalization of silica with well-defined N-heterocyclic phosphenium (NHP) ions to heterogenize low-valent metals. The surface electrostatically bound [NHP]+ ions coordinate to Pt(0) precursors, resulting in well-defined, chemisorbed [(NHP)Pt(0)Ln]+ sites. The resulting materials catalyze the hydrosilylation of alkynes and exhibit activities and selectivities that rival the current industry standard homogeneous catalysts. The catalysts leach Pt, limiting their recyclability; however, recycling studies support that the high regioselectivities arise from heterogeneous sites and Pt particles do not form on the surface. We suspect that this phosphenium-based immobilization strategy will result in stable, tunable, low valent heterogeneous transition metal catalysts in a wider array of catalytic reactions.
Introduction
Platinum group-containing heterogeneous catalysts are important for hydrogenation, alkane dehydrogenation, oxidation, and other reactions.1–5 Stabilizing low-valent platinum group metals using oxide supports, however, remains a challenge. For instance, traditional nanoparticle-based catalysts are prone to sintering, which leads to a decrease in activity over time. These catalysts also show poor metal utilization, with only a small percentage of the precious metal often being catalytically active. Single atom catalysts (SACs) have shown promise in improving the homogeneity of active site structures and activity; however, they often suffer from poor stability and tunability and require low metal loadings to reduce sintering.6–12 Surface organometallic chemistry (SOMC) is a related field13 that utilizes molecular and solid-state techniques to anchor molecular species onto solid supports with well-defined structures.14–20 Traditionally, the metal is chemisorbed onto the surface of an oxide via protonolysis, requiring reactive metal-X species (X = alkyl, amide, alkoxide), with a metal oxidation state greater than zero (Fig. 1A). Pt group metals in the +2 oxidation state have been chemisorbed onto oxide surfaces for applications such as cross-coupling,21 hydrogenation,22 copolymerization of acrylate monomers and ethylene,23–25 and olefin isomerization.26 Well-defined surface organometallic Pt(II/IV) species, however, decompose to form low valent nanoparticles under reducing conditions for heterogeneous alkane dehydrogenation,27 polyolefin upcycling,28 and others.29 To date, no reports have been disclosed regarding the stabilization of well-defined, formally Pt(0), monometallic surface species. | ||
| Fig. 1 (A) Traditional SOMC chemisorption reaction. (B) NHC tethered to silica for the support of a metal. (C) This work: support of metal catalysts via surface bound phosphenium ions. | ||
There is a long history of the use of dative ligands, such as N-heterocyclic carbenes (NHCs), for supporting low valent metals for catalysis in molecular systems.30,31 In heterogeneous systems, NHCs have been applied as a tether to covalently affix metals to solid supports such as graphene, polymers, and silica (Fig. 1B).21,32,33 The syntheses of these materials are often challenging and require many steps, adding barriers to their adoption in industrial processes. Complexity is further increased by the fact that NHCs are often unstable and require in situ formation with a base in the presence of the metal precursor.
N-heterocyclic phosphenium ([NHP]+) ions are isolobal with NHCs and coordinate to transition metals in low oxidation states,34–40 including Pt(0).41–43 In this work, an [NHP]+ ion was formed on the surface of an oxide and utilized as a ligand to immobilize Pt(0) complexes (Fig. 1C). The well-defined (NHP)+–Pt(0) complexes were applied to heterogeneous alkyne hydrosilylation catalysis and compared to other leading catalysts, including the current industrial standard.
Results and discussion
Conley and co-workers reported that iPr3Si+ and Al(OC(CF3)3)3 functionalized SiO2-700 ([iPr3Si][ASO])44 and a related material ([iPr3Si][SZO]) abstracted chloride ions from metal chloride salts to generate well-defined Pd(II) and Ir(I) catalysts, and chlorosilanes as by-products.25,45 Similarly, molecular phosphenium ions have been synthesized by abstraction of halides from NHPX precursors with Me3SiOTf and related silicon-based electrophiles.40 We thus decided to adapt Conley's grafting strategy to generate electrostatically bound surface [NHP]+ ligands. To this end, we reacted DippNHPCl (Dipp = 2,6-diisopropylphenyl) with [iPr3Si][ASO] to produce the surface bound phosphenium ion [DippNHP][ASO] (1) and iPr3SiCl (Fig. 2A).To determine whether the phosphenium ion was indeed generated in 1 as expected, we utilized solid-state 31P NMR spectroscopy. The 31P NMR chemical shift is known to be sensitive to the formation of phosphenium ions.35,36,46–48 The 31P solid-state NMR (SSNMR) spectrum of 1 (Fig. 2B) contains an isotropic chemical shift at 273 ppm, which is comparable to the chemical shift of analogous [MesNHP][BarF4] (31P chemical shift = 257 ppm) reported by Baker and co-workers, and distinctly different from the value of 154 ppm measured for the DippNHPCl precursor, supporting the structural assignment.49 The 31P SSNMR spectrum of 1 contains a number of spinning sidebands due to the large chemical shift anisotropy (CSA, see the ESI for details†) that results from the low coordinate P cation. In solution, [NHP]+ ions are known to coordinate Pt(0); therefore, we hypothesized that 1 will support Pt(0) and effectively chemisorb Pt(0) single atoms onto the surface of silica.
1 reacts with (Ph3P)2Pt(C2H4) to generate [(DippNHP)Pt(PPh3)2][ASO] (2) and ethylene (Fig. 2A). The 31P SSNMR spectrum (Fig. 2C and D) of 2 contains signals at 294 (1JPt–P = 6400 Hz) and 45 (1JPt–P = 4200 Hz) ppm, assigned to the [DippNHP]+ and PPh3 ligands bound to Pt, respectively. The 31P chemical shifts and 1JPt–P couplings are similar to the molecular analogue [(MesNHP)Pt(PPh3)2][OTf] (31P chemical shift = 289.0 (1JPt–P = 6498 Hz) and 43.9 (1JPt–P = 4237 Hz) ppm), supporting the structural assignment.411 also reacts with Karstedt's catalyst (Pt(dvtms)n, dvtms = 1,3-divinyltetramethyldisiloxane) to produce [(DippNHP)Pt(dvtms)][ASO] (3) (Fig. 2E), which is analogous to the highly active and selective (NHC)Pt(dvtms) hydrosilylation catalysts reported by Markó and co-workers.30,50 The 31P SSNMR spectrum of 3 exhibits overlapping signals of unreacted [DippNHP]+ and [(DippNHP)Pt(dvtms)]+ at 277 ppm, close to the value of 272 ppm measured for a triflate analogue (see the ESI†). All attempts at acquiring 195Pt NMR spectra either directly or via indirect detection through 31P,51 were unsuccessful due to short relaxation times. Accordingly, even attempts at performing 31P CPMAS NMR were also unsuccessful. Pt and P ICP-OES measurements of 3 indicate that 84% of the [DippNHP]+ ligands react with Pt, supporting the presence of unreacted [DippNHP]+ (see Table S3 for details†). Solution NMR analyses of the reaction of 2 with PMe3 and 3 with PPh3 resulted in the desorption of 1.9 (±2) equivalents of PPh3 and 1.07 (±7) equivalents of dvtms per Pt, respectively, further supporting the assigned Pt coordination environments for 2 and 3 as (dippNHP)Pt(PPh3)2 and (dippNHP)Pt(dvtms), respectively.
One of the most important applications of low valent Pt is catalytic hydrosilylation of olefins and alkynes.52–56 Pt catalyzed hydrosilylation is challenging to perform with heterogeneous catalysts due to poor reactivities, selectivities, and metal leaching into the solution.53,55,57–59 There are limited examples of well-defined heterogeneous Pt,60–63 Rh,64–66 and other67 hydrosilylation catalysts generated using SOMC techniques; moreover, most reported systems have poor activity, selectivity, or stability.
Catalysts 2 and 3 were subjected to standard 1-octyne hydrosilylation conditions (Table 1)30,52–55 and compared to homogeneous catalysts for activity and product regioselectivity.68 Catalyst 2 is modestly active but highly regioselective for the β-(E) isomer (β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α = 11) at 0.4 mol% loading of Pt when compared to Markó and co-workers’ (IPr)Pt(dvtms) (Pt = 0.005%, 22 h, β-(E)
α = 11) at 0.4 mol% loading of Pt when compared to Markó and co-workers’ (IPr)Pt(dvtms) (Pt = 0.005%, 22 h, β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α = 11.5) catalyst69 and the (PPh3)2Pt(C2H4) precursor (β-(E)
α = 11.5) catalyst69 and the (PPh3)2Pt(C2H4) precursor (β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α = 4.8, see the ESI†), while selectivity and reactivity are considerably reduced at a lower Pt loading of 0.04%. The modest catalytic activity of 2 is likely due to the inhibition of the catalyst by PPh3.
α = 4.8, see the ESI†), while selectivity and reactivity are considerably reduced at a lower Pt loading of 0.04%. The modest catalytic activity of 2 is likely due to the inhibition of the catalyst by PPh3.
| Material | Pt loading (mol %) | Timeb (h) | 1-octyne conv. (%) | PhMe2SiH conv. (%) | Combined yieldc (%) | Product TONc | Product TOFc | β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αc,d αc,d |
|---|---|---|---|---|---|---|---|---|
a Typical catalysis conditions: catalyst + 0.5 M 1-octyne/PhMe2SiH in toluene at 80 °C (see the ESI for more details†). Average of 2 runs. Errors are provided in parentheses.
b Time to achieve the indicated conversions of 1-octyne or PhMe2SiH.
c Combined yield, TON (mol products mol Pt−1), and TOF (mol products mol Pt−1 h−1) of the β-(E) and α-hydrosilylation products as determined by GC-FID with an internal standard of cyclooctane. The TOF was determined at the time indicated in the table and should be considered a minimum.
d Ratio of the β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α product isomers.
e Same relative mass of the catalyst utilized for 2 at 0.43% Pt.
f Same mass of the catalyst used for 3 at 0.058% Pt. NR = no reaction and NA = not applicable. α product isomers.
e Same relative mass of the catalyst utilized for 2 at 0.43% Pt.
f Same mass of the catalyst used for 3 at 0.058% Pt. NR = no reaction and NA = not applicable.
|
||||||||
| 2 | 0.43 (4) | 20 | >99.9 | 90.4 (6) | 88 (1) | 205 (2) | 10 | 11 (1) |
| 2 | 0.043 (4) | 48 | 23 (1) | 21 (2) | 22 (3) | 510 (60) | 11 | 6.6 (2) |
| 3 | 0.058 (2) | 0.17 | >99.9 | 91.5 (2) | 84 (1) | 1460 (10) | 8600 | 6.96 (4) |
| 3 | 0.0058 (2) | 2 | 97 (3) | 99.5 (5) | 86 (2) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (300) 900 (300) |
7450 | 6.20 (3) |
| 4 | 0.12 (1)e | 20 | >99.9 | 89.2 (1) | 80 (1) | 646 (5) | 32 | 4.6 (2) |
| 5 | 0.011 (2)f | 0.5 | 94.3 (1) | 98.3 (2) | 85.3 (4) | 7920 (40) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
5.30 (1) |
Catalyst 3 exhibits comparable activities and selectivities to (NHC)Pt catalysts in solution.30,69–71 This makes 3 more active than most other heterogeneous alkyne and alkene hydrosilylation catalysts synthesized using SOMC techniques62,64–66,72 or nanoparticles73,74 under similar conditions. The regioselectivity of 3 is nevertheless lower than that reported for analogous (IPr)Pt(dvtms), suggesting that the regioselectivity may not be entirely controlled by the steric bulk of the NHP ligand, as was reported in the case of NHCs. Further studies are being conducted to determine the root cause for the reduced selectivity.
DippNHPCl reacts with ∼50% of the [iPr3Si][ASO] sites (based on Al loading)44 in the synthesis of 1; therefore, it is feasible that the unreacted [iPr3Si]+ reacts with Pt(0) to form Pt → Si Lewis pairs or formally oxidize the Pt(0) to [Pt(II)(Si(iPr)3)]+ sites that may catalyze the hydrosilylation reaction. To assess these possibilities, (Ph3P)2Pt(C2H4) and Karstedt's catalyst were absorbed onto [iPr3Si][ASO] (without the [NHP]+ ligand) utilizing the same conditions for the synthesis of 2 and 3, yielding 4 and 5, respectively. SSNMR and ICP analyses indicate that both precursors absorb onto the surface, albeit in ∼5× lower concentrations than 2 and 3. 4 and 5 both catalyze the hydrosilylation of 1-octyne with dimethylphenylsilane under similar conditions to 2 and 3 with slightly lower product yields, which may be attributed to the reduced Pt loadings; however, both catalysts produce silanes with lower regioselectivities that are comparable to the molecular precursors, indicating that the surface species are most likely physisorbed precursor molecules that desorb during catalysis.
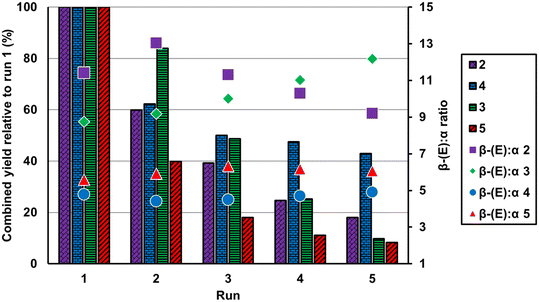
A significant benefit of stable heterogeneous catalysts is their recyclability. Owing to the chemisorption of Pt in 2 and 3, we hypothesized that they will be more recyclable than 4 and 5. To verify this, we subjected catalysts 2, 3, 4 and 5 to recycling experiments. These experiments involved recovering the solid catalyst after the reaction reached partial conversion (∼40–70%). The catalysts were recovered by simple filtration, washed with toluene and used in additional reactions with fresh substrates. A summary of normalized recycling data is provided in Fig. 3 (see the ESI for full details†).
2 (0.58% Pt) was run for 2 hours and showed a steady loss in activity over 5 reactions, with an initial drop of 40% of the initial activity. 3 (0.058% Pt) showed better recyclability than 2 with only a 16% drop in the first recycling of the catalyst; however, the activity of 3 further declined for subsequent recycling attempts. Although the activities of 2 and 3 decrease with recycling, the regioselectivities remain similar throughout the 5 runs and the catalysts do not turn dark in color, supporting that the major active site is the heterogeneous [(DippNHP)Pt]+ species and that particles do not form during the reaction.
Utilizing 4 and 5, we observed initial drops in activity of 38 and 60%, respectively, after the first recycling of the catalysts. Subsequent recycling of 4 only resulted in a slow decline in activity of approximately 5%, suggesting that a stable or rebounding surface Pt species may form. In contrast to this, the activity of 5 declined by ∼50% after each recycling step, making it the poorest recycling catalyst in the study. Pt leaching may lead to active homogeneous catalyst species that may contribute to the catalytic activities and attribute to the poor recyclability. Indeed, hot filtrations of the catalytic reactions revealed that active species do form in solution (see the ESI for details†). The leaching was highest for 2 and 4. In 3 and 5, the reductions in activity slowed in subsequent recycling steps, suggesting a reduction in Pt leaching. The limited recyclability and hot filtration study of 3 suggest that the catalyst is primarily heterogeneous.
Conclusions
In summary, silica was functionalized with well-defined phosphenium ions. The phosphenium ions coordinate to Pt(0), resulting in chemisorbed Pt(0) sites with well-defined structures analogous to molecular complexes. The chemisorbed Pt(0) sites heterogeneously catalyze the hydrosilylation of alkynes with activities and selectivities comparable to those of molecular catalysts. Stable solid catalysts can be further recycled and reused for a number of independent reactions due to their heterogeneous nature. Further investigation utilizing similar strategies from this study is underway and anticipated to discover more stable catalysts for hydrosilylation and other catalytic reactions.Author contributions
All authors contributed to the project design. D. B. C. performed the laboratory syntheses and material characterization studies. D. B. C. and M. M. performed the SSNMR experiments under the direction of F. A. P. M.-C. K. performed the electron microscopy experiments under the direction of L. Z. The manuscript was written through contributions by all authors. All authors have given approval to the final version of this manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported through Ames National Laboratory's Laboratory Directed Research and Development (LDRD) Program. Solid-state NMR work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Science, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science program. Ames National Laboratory is operated for the U.S. Department of Energy by Iowa State University under Contract No. DEAC02-07CH11358. The purchase of the AVIII-600 NMR spectrometer used to obtain some of the results included in this publication was supported by the National Science Foundation under Grant No. MRI 1040098. The purchase of the 400 MR NMR spectrometer used to obtain some of the results included in this publication was supported by the National Science Foundation under Grant No. CHE 0946687. The purchase of the NEO-400 NMR spectrometer used to obtain some of the results included in this publication was supported by the National Science Foundation under the award CHE MRI 1726173. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.References
- L. Liu and A. Corma, Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles, Chem. Rev., 2018, 118(10), 4981–5079, DOI:10.1021/acs.chemrev.7b00776.
- Q. Liu and Z. Zhang, Platinum Single-Atom Catalysts: A Comparative Review towards Effective Characterization, Catal. Sci. Technol., 2019, 9(18), 4821–4834, 10.1039/C9CY01028A.
- Y. Xu, M. Cao and Q. Zhang, Recent Advances and Perspective on Heterogeneous Catalysis Using Metals and Oxide Nanocrystals, Mater. Chem. Front., 2021, 5(1), 151–222, 10.1039/D0QM00549E.
- C. Pei, S. Chen, D. Fu, Z.-J. Zhao and J. Gong, Structured Catalysts and Catalytic Processes: Transport and Reaction Perspectives, Chem. Rev., 2024, 124(6), 2955–3012, DOI:10.1021/acs.chemrev.3c00081.
- H. Sharma, A. Bisht, N. Sethulakshmi and S. Sharma, Catalysis by Substituted Platinum (Ionic Pt) Catalysts, Int. J. Hydrogen Energy, 2024, 51, 748–768, DOI:10.1016/j.ijhydene.2023.08.343.
- S. Ji, Y. Chen, X. Wang, Z. Zhang, D. Wang and Y. Li, Chemical Synthesis of Single Atomic Site Catalysts, Chem. Rev., 2020, 120(21), 11900–11955, DOI:10.1021/acs.chemrev.9b00818.
- S. K. Kaiser, Z. Chen, D. Faust Akl, S. Mitchell and J. Pérez-Ramírez, Single-Atom Catalysts across the Periodic Table, Chem. Rev., 2020, 120(21), 11703–11809, DOI:10.1021/acs.chemrev.0c00576.
- J. Li, M. F. Stephanopoulos and Y. Xia, Introduction: Heterogeneous Single-Atom Catalysis, Chem. Rev., 2020, 120(21), 11699–11702, DOI:10.1021/acs.chemrev.0c01097.
- S. Mitchell and J. Pérez-Ramírez, Single Atom Catalysis: A Decade of Stunning Progress and the Promise for a Bright Future, Nat. Commun., 2020, 11(1), 4302, DOI:10.1038/s41467-020-18182-5.
- Z. Zhang, H. Li, D. Wu, L. Zhang, J. Li, J. Xu, S. Lin, A. K. Datye and H. Xiong, Coordination Structure at Work: Atomically Dispersed Heterogeneous Catalysts, Coord. Chem. Rev., 2022, 460, 214469, DOI:10.1016/j.ccr.2022.214469.
- K. Endo, M. Saruyama and T. Teranishi, Location-Selective Immobilisation of Single-Atom Catalysts on the Surface or within the Interior of Ionic Nanocrystals Using Coordination Chemistry, Nat. Commun., 2023, 14(1), 4241, DOI:10.1038/s41467-023-40003-8.
- J. Fang, Q. Chen, Z. Li, J. Mao and Y. Li, The Synthesis of Single-Atom Catalysts for Heterogeneous Catalysis, Chem. Commun., 2023, 59(20), 2854–2868, 10.1039/D2CC06406E.
- M. K. Samantaray, V. D’Elia, E. Pump, L. Falivene, M. Harb, S. Ould-Chikh, L. Cavallo and J.-M. Basset, The Comparison between Single Atom Catalysis and Surface Organometallic Catalysis, Chem. Rev., 2020, 120(2), 734–813, DOI:10.1021/acs.chemrev.9b00238.
- C. Copéret, A. Comas-Vives, M. P. Conley, D. P. Estes, A. Fedorov, V. Mougel, H. Nagae, F. Núñez-Zarur and P. A. Zhizhko, Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities, Chem. Rev., 2016, 116(2), 323–421, DOI:10.1021/acs.chemrev.5b00373.
- J. D. A. Pelletier and J.-M. Basset, Catalysis by Design: Well-Defined Single-Site Heterogeneous Catalysts, Acc. Chem. Res., 2016, 49(4), 664–677, DOI:10.1021/acs.accounts.5b00518.
- M. K. Samantaray, E. Pump, A. Bendjeriou-Sedjerari, V. D'Elia, J. D. A. Pelletier, M. Guidotti, R. Psaro and J.-M. Basset, Surface Organometallic Chemistry in Heterogeneous Catalysis, Chem. Soc. Rev., 2018, 47(22), 8403–8437, 10.1039/C8CS00356D.
- R. J. Witzke, A. Chapovetsky, M. P. Conley, D. M. Kaphan and M. Delferro, Nontraditional Catalyst Supports in Surface Organometallic Chemistry, ACS Catal., 2020, 10(20), 11822–11840, DOI:10.1021/acscatal.0c03350.
- M. K. Samantaray, S. K. Mishra, A. Saidi and J.-M. Basset, Surface Organometallic Chemistry: A Sustainable Approach in Modern Catalysis, J. Organomet. Chem., 2021, 945, 121864, DOI:10.1016/j.jorganchem.2021.121864.
- C. Copéret and M. D. Korzyński, 6.04 – Surface Organometallic and Coordination Chemistry Approach to Formation of Single Site Heterogeneous Catalysts, in Comprehensive Inorganic Chemistry III (Third Edition), ed. J. Reedijk and K. R. Poeppelmeier, Elsevier, Oxford, 2023, pp. 67–85. DOI:10.1016/B978-0-12-823144-9.00003-0.
- K. K. Samudrala and M. P. Conley, Effects of Surface Acidity on the Structure of Organometallics Supported on Oxide Surfaces, Chem. Commun., 2023, 59(28), 4115–4127, 10.1039/D3CC00047H.
- W. J. Sommer and M. Weck, Supported N-Heterocyclic Carbene Complexes in Catalysis, Coord. Chem. Rev., 2007, 251(5), 860–873, DOI:10.1016/j.ccr.2006.07.004.
- M. P. Conley, R. M. Drost, M. Baffert, D. Gajan, C. Elsevier, W. T. Franks, H. Oschkinat, L. Veyre, A. Zagdoun, A. Rossini, M. Lelli, A. Lesage, G. Casano, O. Ouari, P. Tordo, L. Emsley, C. Copéret and C. Thieuleux, A Well-Defined Pd Hybrid Material for the Z-Selective Semihydrogenation of Alkynes Characterized at the Molecular Level by DNP SENS, Chem. – Eur. J., 2013, 19(37), 12234–12238, DOI:10.1002/chem.201302484.
- H. Tafazolian, D. B. Culver and M. P. Conley, A Well-Defined Ni(II) α-Diimine Catalyst Supported on Sulfated Zirconia for Polymerization Catalysis, Organometallics, 2017, 36(13), 2385–2388, DOI:10.1021/acs.organomet.7b00402.
- D. B. Culver, H. Tafazolian and M. P. Conley, A Bulky Pd(II) α-Diimine Catalyst Supported on Sulfated Zirconia for the Polymerization of Ethylene and Copolymerization of Ethylene and Methyl Acrylate, Organometallics, 2018, 37(6), 1001–1006, DOI:10.1021/acs.organomet.8b00016.
- J. Gao, R. W. Dorn, G. P. Laurent, F. A. Perras, A. J. Rossini and M. P. Conley, A Heterogeneous Palladium Catalyst for the Polymerization of Olefins Prepared by Halide Abstraction Using Surface R3Si+ Species, Angew. Chem., Int. Ed., 2022, 61(20), e202117279, DOI:10.1002/anie.202117279.
- A. S. Chang, M. A. Kascoutas, Q. P. Valentine, K. I. How, R. M. Thomas and A. K. Cook, Alkene Isomerization Using a Heterogeneous Nickel-Hydride Catalyst, J. Am. Chem. Soc., 2024, 146(22), 15596–15608, DOI:10.1021/jacs.4c04719.
- K. Searles, K. W. Chan, J. A. Mendes Burak, D. Zemlyanov, O. Safonova and C. Copéret, Highly Productive Propane Dehydrogenation Catalyst Using Silica-Supported Ga–Pt Nanoparticles Generated from Single-Sites, J. Am. Chem. Soc., 2018, 140(37), 11674–11679, DOI:10.1021/jacs.8b05378.
- K. E. McCullough, I. L. Peczak, R. M. Kennedy, Y.-Y. Wang, J. Lin, X. Wu, A. L. Paterson, F. A. Perras, J. Hall, A. J. Kropf, R. A. Hackler, Y. Shin, J. Niklas, O. G. Poluektov, J. Wen, W. Huang, A. D. Sadow, K. R. Poeppelmeier, M. Delferro and M. S. Ferrandon, Synthesis of Platinum Nanoparticles on Strontium Titanate Nanocuboids via Surface Organometallic Grafting for the Catalytic Hydrogenolysis of Plastic Waste, J. Mater. Chem. A, 2023, 11(3), 1216–1231, 10.1039/D2TA08133D.
- K. Lian, Y. Yue, J.-M. Basset, X. Liu, L. Chen, C. Ozsoy-Keskinbora, X. Bao and H. Zhu, Surface Organometallic Chemistry as a Versatile Strategy for Synthesizing Supported Bimetallic Cluster Catalysts, J. Phys. Chem. C, 2022, 126(39), 16663–16671, DOI:10.1021/acs.jpcc.2c04869.
- S. Dierick and I. E. Markó, NHC Platinum(0) Complexes: Unique Catalysts for the Hydrosilylation of Alkenes and Alkynes, in N-Heterocyclic Carbenes, John Wiley & Sons, Ltd, 2014, pp. 111–150. DOI:10.1002/9783527671229.ch05.
- M. S. Khan, A. Haque, M. K. Al-Suti and P. R. Raithby, Recent Advances in the Application of Group-10 Transition Metal Based Catalysts in C–H Activation and Functionalization, J. Organomet. Chem., 2015, 793, 114–133, DOI:10.1016/j.jorganchem.2015.03.023.
- K. V. S. Ranganath, S. Onitsuka, A. K. Kumar and J. Inanaga, Recent Progress of N-Heterocyclic Carbenes in Heterogeneous Catalysis, Catal. Sci. Technol., 2013, 3(9), 2161–2181, 10.1039/C3CY00118K.
- W. Wang, L. Cui, P. Sun, L. Shi, C. Yue and F. Li, Reusable N-Heterocyclic Carbene Complex Catalysts and Beyond: A Perspective on Recycling Strategies, Chem. Rev., 2018, 118(19), 9843–9929, DOI:10.1021/acs.chemrev.8b00057.
- L. Rosenberg, Metal Complexes of Planar PR2 Ligands: Examining the Carbene Analogy, Coord. Chem. Rev., 2012, 256(5), 606–626, DOI:10.1016/j.ccr.2011.12.014.
- M. Zafar, V. Subramaniyan, F. Tibika and Y. Tulchinsky, Cationic Ligands – from Monodentate to Pincer Systems, Chem. Commun., 2024, 60(73), 9871–9906, 10.1039/D4CC01489H.
- D. Gudat, Recent Developments in the Chemistry of N-Heterocyclic Phosphines, in Phosphorus Heterocycles II, ed. R. K. Bansal, Springer, Berlin, Heidelberg, 2010, pp. 63–102. DOI:10.1007/7081_2009_5.
- H. Nakazawa, Transition Metal Complexes Bearing a Phosphenium Ligand, in Advances in Organometallic Chemistry, Academic Press, 2004, vol. 50, pp. 107–143. DOI:10.1016/S0065-3055(03)50002-8.
- H. Nakazawa and K. Miyoshi, Synthesis and Property of Phosphenium Complexes Containing Double Bond Character between a Transition Metal and a Phosphorus Atom, J. Synth. Org. Chem., Jpn., 2001, 59(1), 52–61, DOI:10.5059/yukigoseikyokaishi.59.52.
- H. Nakazawa, The Chemistry of Transition Metal Complexes Containing a Phosphenium Ligand, J. Organomet. Chem., 2000, 611(1), 349–363, DOI:10.1016/S0022-328X(00)00499-X.
- D. Gudat, Cationic Low Coordinated Phosphorus Compounds as Ligands: Recent Developments, Coord. Chem. Rev., 1997, 163, 71–106, DOI:10.1016/S0010-8545(97)00010-6.
- N. J. Hardman, M. B. Abrams, M. A. Pribisko, T. M. Gilbert, R. L. Martin, G. J. Kubas and R. T. Baker, Molecular and Electronic Structure of Platinum Bis(N-Arylamino)Phosphenium Complexes Including [Pt(Phosphane)(Phosphenium)(N-Heterocyclic Carbene)], Angew. Chem., Int. Ed., 2004, 43(15), 1955–1958, DOI:10.1002/anie.200352326.
- C. A. Caputo, M. C. Jennings, H. M. Tuononen and N. D. Jones, Phospha-Fischer Carbenes: Synthesis, Structure, Bonding, and Reactions of Pd(0)− and Pt(0)−Phosphenium Complexes, Organometallics, 2009, 28(4), 990–1000, DOI:10.1021/om800973v.
- B. Pan, Z. Xu, M. W. Bezpalko, B. M. Foxman and C. M. Thomas, N-Heterocyclic, Phosphenium Ligands as Sterically and Electronically-Tunable Isolobal Analogues of Nitrosyls, Inorg. Chem., 2012, 51(7), 4170–4179, DOI:10.1021/ic202581v.
- D. B. Culver, A. Venkatesh, W. Huynh, A. J. Rossini and M. P. Conley, Al(ORF)3 (RF = C(CF3)3) Activated Silica: A Well-Defined Weakly Coordinating Surface Anion, Chem. Sci., 2020, 11(6), 1510–1517, 10.1039/C9SC05904K.
- J. Rodriguez and M. P. Conley, A Heterogeneous Iridium Catalyst for the Hydroboration of Pyridines, Org. Lett., 2022, 24(25), 4680–4683, DOI:10.1021/acs.orglett.2c01859.
- A. H. Cowley and R. A. Kemp, Synthesis and Reaction Chemistry of Stable Two-Coordinate Phosphorus Cations (Phosphenium Ions), Chem. Rev., 1985, 85(5), 367–382, DOI:10.1021/cr00069a002.
- C. Chuit and C. Reyé, Hypercoordinate PII, PIII, and PIV Phosphorus Derivatives with Intramolecular Coordination by Donor Groups, Eur. J. Inorg. Chem., 1998, 1998(12), 1847–1857, DOI:10.1002/(SICI)1099-0682(199812)1998:12<1847::AID-EJIC1847>3.0.CO;2-K.
- D. Gudat, Diazaphospholenes: N-Heterocyclic Phosphines between Molecules and Lewis Pairs, Acc. Chem. Res., 2010, 43(10), 1307–1316, DOI:10.1021/ar100041j.
- M. B. Abrams, B. L. Scott and R. T. Baker, Sterically Tunable Phosphenium Cations: Synthesis and Characterization of Bis(Arylamino)Phosphenium Ions, Phosphinophosphenium Adducts, and the First Well-Defined Rhodium Phosphenium Complexes, Organometallics, 2000, 19(24), 4944–4956, DOI:10.1021/om0005351.
- I. E. Markó, S. Stérin, O. Buisine, G. Mignani, P. Branlard, B. Tinant and J.-P. Declercq, Selective and Efficient Platinum(0)-Carbene Complexes As Hydrosilylation Catalysts, Science, 2002, 298(5591), 204–206, DOI:10.1126/science.1073338.
- B. A. Atterberry, E. J. Wimmer, S. Klostermann, W. Frey, J. Kästner, D. P. Estes and A. J. Rossini, Structural Characterization of Surface Immobilized Platinum Hydrides by Sensitivity-Enhanced 195Pt Solid State NMR Spectroscopy and DFT Calculations, Chem. Sci., 2025, 16(3), 1271–1287, 10.1039/D4SC06450J.
- L. N. Lewis, J. Stein, Y. Gao, R. E. Colborn and G. Hutchins, Platinum Catalysts Used in the Silicones Industry, Platinum Met. Rev., 1997, 41(2), 66–75 CrossRef CAS.
- D. Troegel and J. Stohrer, Recent Advances and Actual Challenges in Late Transition Metal Catalyzed Hydrosilylation of Olefins from an Industrial Point of View, Coord. Chem. Rev., 2011, 255(13), 1440–1459, DOI:10.1016/j.ccr.2010.12.025.
- Y. Nakajima and S. Shimada, Hydrosilylation Reaction of Olefins: Recent Advances and Perspectives, RSC Adv., 2015, 5(26), 20603–20616, 10.1039/C4RA17281G.
- T. K. Meister, K. Riener, P. Gigler, J. Stohrer, W. A. Herrmann and F. E. Kühn, Platinum Catalysis Revisited—Unraveling Principles of Catalytic Olefin Hydrosilylation, ACS Catal., 2016, 6(2), 1274–1284, DOI:10.1021/acscatal.5b02624.
- R. Y. Lukin, A. M. Kuchkaev, A. V. Sukhov, G. E. Bekmukhamedov and D. G. Yakhvarov, Platinum-Catalyzed Hydrosilylation in Polymer Chemistry, Polymers, 2020, 12(10), 2174, DOI:10.3390/polym12102174.
- H. Yang, Z. Zhou, C. Tang and F. Chen, Recent Advances in Heterogeneous Hydrosilylation of Unsaturated Carbon-Carbon Bonds, Chin. Chem. Lett., 2024, 35(6), 109257, DOI:10.1016/j.cclet.2023.109257.
- S. Shimada, Recent Advances of Group 10 Transition Metal Hydrosilylation Catalysts, in Perspectives of Hydrosilylation Reactions, ed. B. Marciniec and H. Maciejewski, Springer Nature Switzerland, Cham, 2023, pp. 13–93. DOI:10.1007/3418_2023_99.
- M. Pagliaro, R. Ciriminna, V. Pandarus and F. Béland, Platinum-Based Heterogeneously Catalyzed Hydrosilylation, Eur. J. Org. Chem., 2013, 6227–6235, DOI:10.1002/ejoc.201300290.
- L. Chen, G. E. Sterbinsky and S. L. Tait, Synthesis of Platinum Single-Site Centers through Metal-Ligand Self-Assembly on Powdered Metal Oxide Supports, J. Catal., 2018, 365, 303–312, DOI:10.1016/j.jcat.2018.07.004.
- L. Chen, I. S. Ali, G. E. Sterbinsky, J. T. L. Gamler, S. E. Skrabalak and S. L. Tait, Alkene Hydrosilylation on Oxide-Supported Pt-Ligand Single-Site Catalysts, ChemCatChem, 2019, 11(12), 2843–2854, DOI:10.1002/cctc.201900530.
- A. Mollar-Cuni, P. Borja, S. Martin, G. Guisado-Barrios and J. A. Mata, A Platinum Molecular Complex Immobilised on the Surface of Graphene as Active Catalyst in Alkyne Hydrosilylation, Eur. J. Inorg. Chem., 2020, 2020(45), 4254–4262, DOI:10.1002/ejic.202000356.
- E. L. Bruske, N. D. Maldonado, M. S. Jeletic and A. R. Fout, Latency and Immobilization in Silicone Resin by (NHC)Pt(Dvtms) Complexes through a Partially Self-Consuming Ligand, Organometallics, 2024, 43(2), 134–140, DOI:10.1021/acs.organomet.3c00456.
- B. Sánchez-Page, M. V. Jiménez, J. J. Pérez-Torrente, V. Passarelli, J. Blasco, G. Subias, M. Granda and P. Álvarez, Hybrid Catalysts Comprised of Graphene Modified with Rhodium-Based N-Heterocyclic Carbenes for Alkyne Hydrosilylation, ACS Appl. Nano Mater., 2020, 3(2), 1640–1655, DOI:10.1021/acsanm.9b02398.
- P. K. R. Panyam, B. Atwi, F. Ziegler, W. Frey, M. Nowakowski, M. Bauer and M. R. Buchmeiser, Rh(I)/(III)-N-Heterocyclic Carbene Complexes: Effect of Steric Confinement Upon Immobilization on Regio- and Stereoselectivity in the Hydrosilylation of Alkynes, Chem. – Eur. J., 2021, 27(68), 17220–17229, DOI:10.1002/chem.202103099.
- H. Acikalin, P. K. R. Panyam, A. W. Shaikh, D. Wang, S. R. Kousik, P. Atanasova and M. R. Buchmeiser, Hydrosilylation of Alkynes Under Continuous Flow Using Polyurethane-Based Monolithic Supports with Tailored Mesoporosity, Macromol. Chem. Phys., 2023, 224(1), 2200234, DOI:10.1002/macp.202200234.
- U. Kanbur, R. J. Witzke, J. Xu, M. S. Ferrandon, T. A. Goetjen, A. J. Kropf, F. A. Perras, C. Liu, T. D. Tilley, D. M. Kaphan and M. Delferro, Supported Electrophilic Organoruthenium Catalyst for the Hydrosilylation of Olefins, ACS Catal., 2023, 13383–13394, DOI:10.1021/acscatal.3c03399.
- B. Karstedt, Platinum Complexes of Unsaturated Siloxanes and Platinum Containing Organopolysiloxanes.US3775452A, 1973. https://patents.google.com/patent/US3775452A/en (accessed 2023-11-30).
- G. De Bo, G. Berthon-Gelloz, B. Tinant and I. E. Markó, Hydrosilylation of Alkynes Mediated by N-Heterocyclic Carbene Platinum(0) Complexes, Organometallics, 2006, 25(8), 1881–1890, DOI:10.1021/om050866j.
- G. Berthon-Gelloz, J.-M. Schumers, G. De Bo and I. E. Markó, Highly β-(E)-Selective Hydrosilylation of Terminal and Internal Alkynes Catalyzed by a (IPr)Pt(Diene) Complex, J. Org. Chem., 2008, 73(11), 4190–4197, DOI:10.1021/jo800411e.
- S. Dierick, E. Vercruysse, G. Berthon-Gelloz and I. E. Markó, User-Friendly Platinum Catalysts for the Highly Stereoselective Hydrosilylation of Alkynes and Alkenes, Chem. – Eur. J., 2015, 21(47), 17073–17078, DOI:10.1002/chem.201502643.
- U. Kanbur, R. J. Witzke, J. Xu, M. S. Ferrandon, T. A. Goetjen, A. J. Kropf, F. A. Perras, C. Liu, T. D. Tilley, D. M. Kaphan and M. Delferro, Supported Electrophilic Organoruthenium Catalyst for the Hydrosilylation of Olefins, ACS Catal., 2023, 13(20), 13383–13394, DOI:10.1021/acscatal.3c03399.
- C. B. Reddy, A. K. Shil, N. R. Guha, D. Sharma and P. Das, Solid Supported Palladium(0) Nanoparticles: An Efficient Heterogeneous Catalyst for Regioselective Hydrosilylation of Alkynes and Suzuki Coupling of β-Arylvinyl Iodides, Catal. Lett., 2014, 144(9), 1530–1536, DOI:10.1007/s10562-014-1311-8.
- J. Fotie, M. E. Agbo, F. Qu and T. Tolar, Dichloro(Ethylenediamine)Platinum(II), a Water-Soluble Analog of the Antitumor Cisplatin, as a Heterogeneous Catalyst for a Stereoselective Hydrosilylation of Alkynes under Neat Conditions, Tetrahedron Lett., 2020, 61(36), 152300, DOI:10.1016/j.tetlet.2020.152300.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00680e |
| This journal is © The Royal Society of Chemistry 2025 |