Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of Al and Si substituted polyoxometalates†
Jan-Christian
Raabe
 ab,
Saurabh S.
Chitnis
ab,
Saurabh S.
Chitnis
 b and
Maximilian J.
Poller
b and
Maximilian J.
Poller
 *a
*a
aInstitute for Technical and Macromolecular Chemistry, Universität Hamburg, Bundesstraße 45, 20146 Hamburg, Germany. E-mail: JanChristian.Raabe@uni-hamburg.de; Maximilian.Poller@uni-hamburg.de
bDepartment of Chemistry, Dalhousie University, Coburg Road 6274, 15000 Halifax, Nova Scotia, Canada. E-mail: Jan-Christian.Raabe@dal.ca; Saurabh.Chitnis@dal.ca
First published on 27th May 2025
Abstract
Polyoxometalates with different transition-metals substituting the main framework element have recently been applied as promising catalysts. In comparison, polyoxometalates, featuring main-group elements in their framework are extremely rare. In this work, we present two main-group element substituted Keggin-type phosphotungstates containing Al or Si, and their characterization using spectroscopic and crystallographic methods. Al substituted POMs are rare examples, which means that the investigation of such systems deserves special attention. Guided by electronic-structure calculations, the reactivity of the Al substituted cluster with the electrophile benzyl bromide was investigated. This reactivity study together with the results of computational simulations illustrate new reaction behaviour for main-group element substituted polyoxometalates, setting the stage for future applications.
Polyoxometalates (POMs) are inorganic polyanionic cluster compounds, formed by transition-elements of group five (V, Nb, Ta) and six (Mo, W) of the periodic table, mostly in their highest oxidation states.1 Formally, POMs can be understood as oxometal complexes, in which the oxo ligand can coordinate the metal M in a terminal M
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or two metals in a bridging bond motif M–O–M (Fig. S1, ESI†).2–4 In general, the M–O units form defined octahedra MO6, that are connected via common edges or corners during POM formation. POM formation corresponds to the formation of M–O–M bonds, so-called bridging of metals by oxo ligands, and is achieved in an acidic medium. A cleavage of these bonds is possible in a basic reaction medium. A POM structure can be decomposed here to so-called monometallates MO42−. However, a controlled decomposition of the cluster is also possible in basic reaction media, where individual MO42− anions are selectively released from the cluster. So-called defects or vacancies remain in the POM structure. Such structure-types are called Lacunary-type structures and are known for Keggin– and Wells–Dawson-type POM structures. Thus, intact POM structures can be distinguished from those of the defect structures (Lacunary-type). Lacunary-type structures therefore do not represent a separate structure-type but can be derived from any POM-type structure.
O or two metals in a bridging bond motif M–O–M (Fig. S1, ESI†).2–4 In general, the M–O units form defined octahedra MO6, that are connected via common edges or corners during POM formation. POM formation corresponds to the formation of M–O–M bonds, so-called bridging of metals by oxo ligands, and is achieved in an acidic medium. A cleavage of these bonds is possible in a basic reaction medium. A POM structure can be decomposed here to so-called monometallates MO42−. However, a controlled decomposition of the cluster is also possible in basic reaction media, where individual MO42− anions are selectively released from the cluster. So-called defects or vacancies remain in the POM structure. Such structure-types are called Lacunary-type structures and are known for Keggin– and Wells–Dawson-type POM structures. Thus, intact POM structures can be distinguished from those of the defect structures (Lacunary-type). Lacunary-type structures therefore do not represent a separate structure-type but can be derived from any POM-type structure.
A large variety of structure-types for POMs are known (Fig. S2, ESI†).1,5 The most important one is the Keggin-type ([XM12O40]n−), with a heteroelement X at the centre of its structure.6 The framework metals M are usually Mo and W, but can be partially substituted.1,7–15 Fundamentally, there are four distinct atom positions in a Keggin-type POM molecule: the heteroelement position (usually main-group elements like P, Si, S, Sb, or Te), the framework-element position (mostly Mo, W including foreign-elements), the oxo ligands, and the cations compensating the charge (Fig. S3, ESI†).6,16–18 POMs with hard cation such as protons or light alkali cations are known for their high water solubility.19 In contrast, soft cations such as Cs+, form POM salts that are insoluble practically all solvents.20,21 To achieve solubility in organic solvents, POMs can also be combined with organic cations, such as tetrabutylammonium (TBA). These organic cation salts are soluble in polar organic solvents such as acetonitrile (Fig. S4, ESI†). With this strategy, the POM chemistry can be successfully transferred from aqueous to the organic reaction media.22–25
Foreign-element substitution in POM chemistry is an emerging and active research field that significantly diversifies the composition and structural possibilities. Selective substitution of one or more framework-elements by different transition-elements like vanadium, niobium, or cobalt, give the POM cluster new electronic properties, making POMs interesting candidates for applications in the fields of biomedicine or catalysis.26–33 Most examples of foreign-element substituted POMs contain different transition-metals in the framework-element position. In contrast, only few examples are known with main-group elements incorporated in the POM structure in positions other than the central heteroelement.18 The majority of these are POMs with main-group cations,34,35 which sometimes form coordination polymers, in which the individual POM clusters are linked via coordination to main-group element cations.36–40 Incorporation of main-group elements as substitutional elements into the framework-element position is extremely rare.41
Given the increasing importance of transition-metal substituted POMs, we believe that expanding this concept to main-group substituted POMs is a promising step towards a more versatile application of main-group elements. Therefore, we investigated the incorporation of two elements (Al and Si) into the Keggin-type phosphotungstate structure, utilizing organo-ammonium salts to enable the use of moisture sensitive main-group precursors in organic solvents.
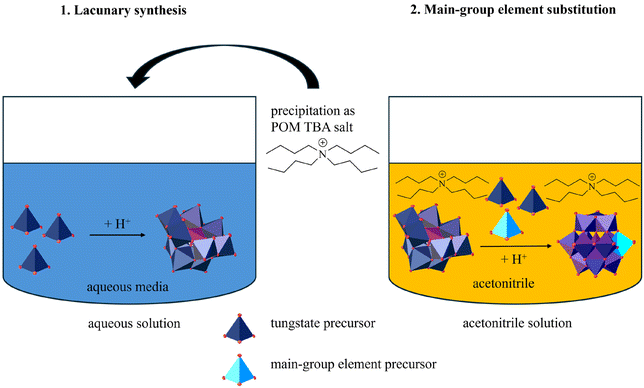
We started from a Lacunary–Keggin-type POM structure with a defined number of vacancies, as a precursor. The anion [PW9O34]9− was initially synthesized from phosphate and tungstate.42 Tungsten was chosen as the framework-element, as W-based POM structures are generally more stable than Mo-based structures. This becomes clear from the Mo-based Lacunary–Keggin-type POM [PMo9O34]9−, which tends to dimerize.28,43 However, since most of the main-group element precursors required (aluminum trichloride and tetraethyl orthosilicate, TEOS) are sensitive to hydrolysis, the Lacunary-POM precursor was precipitated from aqueous solution with TBA cations and transferred to acetonitrile. Within the anhydrous organic medium, a precursor for the main-group element (1 equivalent) and WO42− precursor for the framework-element (2 equivalents) were added stoichiometrically to the Lacunary-type structure [PW9O34]9−, generating the fully intact Keggin-type structure. This strategy for obtaining main-group element-substituted POMs is illustrated in Fig. 1.
The resulting products were analysed with ICP-OES and C, H, N analysis to determine their elemental composition (Table 1 and Table S1†).
| POM | Expected stoichiometry | Cations found | P | Al | Si | W |
|---|---|---|---|---|---|---|
| nBu4NPW | 3 (C16H36N)+ [PW12O40]3− | 3.00 (C16H36N)+ | 1.04 | — | — | 12.0 |
| nBu4NPAlW | 6 (C16H36N)+ [PAlW11O40]6− | 4.00 (C16H36N)+ | 1.09 | 0.916 | — | 11.0 |
| nBu4NPSiW | 5 (C16H36N)+ P/Si/W [PSiW11O40]5− | 3.37 (C16H36N)+ | 0.927 | — | 0.818 | 11.0 |
For both, PAlW and PSiW, we found P, Al, Si, and W in the expected ratios, indicating successful integration of Al and Si respectively. If the main-group elements are incorporated into the framework-element position, we would expect anionic charges of −6 for PAlW and −5 for PSiW. The reduced values for C/H/N could indicate that the negative charge of the anions is not compensated by the TBA cations alone, but additional protons might be present, resulting in acidic compounds of the type TBAxHn−x[PMW11O40]. Stoichiometrically, four TBA cations and two protons are present for POM PAlW and 3.37 TBA cations and 1.63 protons for POM PSiW, which compensate for the anionic charges of −6 (PAlW) and −5 (PSiW). This results in stoichiometric formulas of (TBA)4H2[PAlW11O40] and (TBA)3.37H1.63[PSiW11O40]. The remaining protons originate from the acidic pH medium during the synthetic procedure and protonate the terminal oxo ligands.44 Interestingly, 4.623 TBA cations were found for the precursor, the anion [PW9O34]9−, with the rest of the ninefold anionic charge having to be balanced by 4377 protons. This results in a stoichiometric molecular formula of (TBA)4.623H4.377[PW9O34]. The raw data of the elemental analysis (CHNS and ICP-OES) can be found in the ESI† under the respective experimental procedures, chapter 2.2.
The integrity of the structure-types was verified by KBr-IR and Raman spectroscopy. All spectra are shown in Fig. 2 and S5–S7 in the ESI.† IR/Raman data in Fig. 2 were compared to unsubstituted POM acid H3[PW12O40] (abbreviated as HPW), which contains significant amounts of hydration water (peaks in IR at 1613 and 3134 to 3650 cm−1).45 Both main-group element substituted POMs show splitting of the P–O vibrational bands, indicating reduced symmetry.
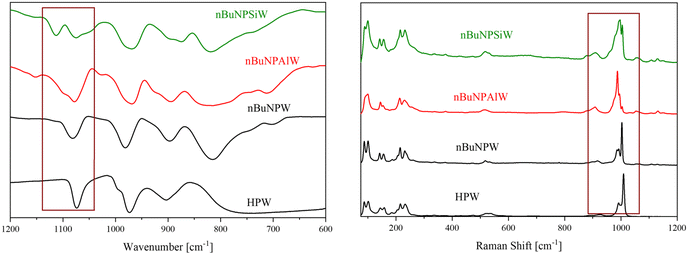 | ||
| Fig. 2 IR (left) and Raman data (right) of all investigated POMs, compared to the POM acid HPW without TBA cation modification. | ||
If the IR spectrum of POM PSiW is compared with that of the commercially available H4[SiW12O40] (SiW), it can be concluded that the Si(IV) atom has not substituted the heteroelement position, since no split Si–O band would be expected for [SiW12O40]4− (see Fig. S8, ESI†).
Using single-crystal X-ray diffraction (sc-XRD) the solid-state structure of compound PAlW was investigated, as shown in Fig. 3. In agreement with the vibrational data the solid-state structure was identified as a disordered (inversion at P1) Keggin-type anion. A short discussion for the refinement details is provided in the ESI† together with a justification of the atom assignement (see Fig. S9†). Table S2† shows observed bond lengths in comparison to the sum of covalent radii.46 A comparison of the bond lengths with those of the anion [PW12O40]3− shows (see ESI†) that in particular the O1–M1 and M1–O2 bond lengths in PAlW are shortened by 0.039 and 0.019 Å, which indicates that the Al atom is incorporated into the framework-element position.
 | ||
Fig. 3 Refinement of the crystallographic data of anion PAlW in Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. Color code: red – oxygen (ligand position), purple – phosphorous (heteroelement position) and turquoise – metals (W, Al; framework-element position). R1: 5.71%, wR2: 10.46%, GooF: 1.052 and Rint: 9.58%. Full .cif file is available through the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service (deposition number: 2441126†). m. Color code: red – oxygen (ligand position), purple – phosphorous (heteroelement position) and turquoise – metals (W, Al; framework-element position). R1: 5.71%, wR2: 10.46%, GooF: 1.052 and Rint: 9.58%. Full .cif file is available through the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service (deposition number: 2441126†). | ||
For further confirmation of the successful incorporation of Al, the product was investigated using 31P and 27Al NMR spectroscopy and electrochemistry, as shown in Fig. S11–S20, S29 and S30, ESI.† In the 27Al NMR data (Fig. S29†), a peak at 103.8 ppm was identified, indicating that the Al atom is located in a tetrahedral environment.47 This is contrary to the other analytical findings. However, we believe it can be explained by dissociation of the Al in solution, a behaviour which is commonly observed in transition-metal substituted POMs.28–30
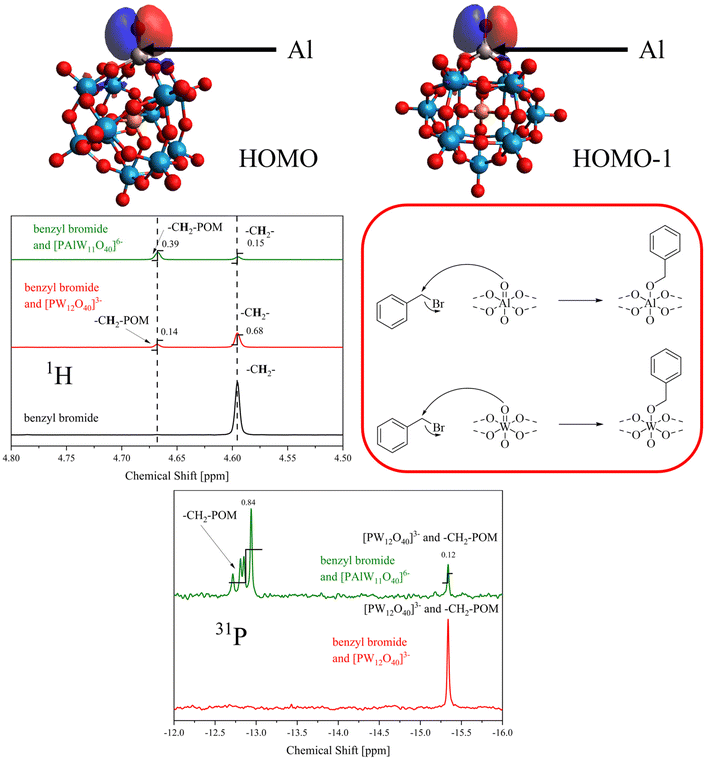
From our DFT calculations we were able to see, in comparison to POM PW, that the HOMO and HOMO−1 are strongly localized on the terminal oxygen atom of the main-group foreign-element (Fig. 4, top), if we assume that one of the tungsten framework-elements has been successfully replaced with Al. This observation implies that this oxygen atom acts as a nucleophile and could react with the LUMO of a suitable electrophile. For POM PW an AlW, both the HOMO and the HOMO−1 appear to be delocalized over the entire cluster (Fig. S31 and S32†). Therefore, benzyl bromide was added to a solution of nBu4NPAlW and nBu4NPW in acetonitrile. The solution was then analyzed with 1H, 13C and 31P NMR, as shown in Fig. 4 (bottom).
The 1H NMR in Fig. 4 (middle left) clearly shows a shift of the –CH2– protons of the benzyl bromide to higher values. While the –CH2– protons of the benzyl bromide appear at 4.60 ppm, the –CH2– protons of the benzyl bromide-modified POMs PW and PAlW each have a peak at 4.67 ppm with different intensities. These results suggest that the unsubstituted POM PW is also reactive, but to a lesser extent than PAlW, which is consistent with the DFT prediction that the molecular orbitals HOMO−1 and HOMO of the former are both delocalized over the entire cluster (see experimental procedure ESI, 2.2.6†). For POM PAlW, the peak at 4.67 ppm is significantly more intense, which agrees with the DFT prediction for a higher degree of reactivity at the terminal oxo ligand of the Al atom. For both POMs it was also shown that unreacted benzyl bromide is still present, which means that the conversion is not complete. From the integrals, the ratio of modified to unmodified POM is 0.206 for PW and 2.6 for PAlW, which is consistent with the predicted higher reactivity at the oxo ligand from Al. Similar observations were also made in the 31P NMR data (bottom right). After the addition of benzyl bromide to the terminal oxo ligand of Al, the nucleophilicity of the POM anion could increase, resulting in multiple addition of benzyl bromide to other terminal oxo ligands of W. Therefore, several signals can be seen in the 31P NMR in Fig. 4 (bottom). The complete NMR spectra can be found in the ESI, Fig. S21–S28.† It can be concluded from the discussion of the elemenal analysis that further protons are present in the solid-state structure of the POM anions PAlW and PSiW, which protonate the terminal oxo ligands. From the discussion presented here, it can be concluded that the protons preferentially occupy the terminal oxo ligands that contain the foreign-elements, as their nucleophilicity is significantly increased.44
From the collected data, it can be concluded that the main-group elements Al and Si can be successfully incorporated into the Keggin-type POM structure. The vibrational spectra clearly indicate a successful incorporation of those elements in the position of the framework element. 27Al NMR measurements show a tetrahedrally coordinated Al, which indicates dissociation of the Al substituted POM in solution. DFT calculations predicted high nucleophilic reactivity at the Al atom for an [PAlW11O40]6− anion, as the HOMOs are much more delocalized for [PW12O40]3−. This reactivity has been experimentally verified by a reaction with benzyl bromide. Thereby it is confirmed that adding an electropositive main-group metal as the foreign-element is that the HOMO becomes localized at the substitution site, allowing for well-defined reactions and enhanced nucleophilicity relative to unsubstituted derivatives. Thus, main-group substitution presents a highly promising strategy to introduce special reactivity to Keggin-type POMs, revealing a new frontier in the chemistry of the main group elements. Collectively, these synthetic, structural, and reactivity studies provide a foundation for more application focussed studies into main-group and transition-metal hybrid HPAs in the future.
Author contributions
Jan-Christian Raabe: writing – original draft, review & editing, investigation, conceptualization, methodology, visualization. Saurabh Chitnis: conceptualization, methodology, resources, supervision, writing – original draft, review & editing, funding acquisition. Maximilian J. Poller: conceptualization, methodology, supervision, project administration, writing – original draft, review & editing.Data availability
The full crystallographic information is available as a .cif file through the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service (deposition number: 2441126†).Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors gratefully thank MITACS (the Mathematics of Information Technology and Complex Systems) for financing Jan-Christian Raabe with a Mitacs Globalink Research Award to Canada. We thank all analytical departments that were involved in our project.References
- R. Dehghani, S. Aber and F. Mahdizadeh, Clean: Soil, Air, Water, 2018, 46, 1800413 Search PubMed.
- M. T. Pope and A. Müller, Angew. Chem., 1991, 103, 56–70 CrossRef CAS.
- J. J. Borrás-Almenar, E. Coronado, A. Müller and M. Pope, Polyoxometalate Molecular Science, Springer Netherlands, Dordrecht, 2003 Search PubMed.
- H. J. Lunk and H. Hartl, ChemTexts, 2021, 7, 1–30 CrossRef.
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- J.-C. Raabe, F. Jameel, M. Stein, J. Albert and M. J. Poller, Dalton Trans., 2024, 53, 454–466 RSC.
- J. F. Keggin, Nature, 1933, 131, 908–909 Search PubMed.
- M. X. Xu, S. Lin, L.-M. Xu and S.-L. Zhen, Transition Met. Chem., 2004, 29, 332–335 Search PubMed.
- L. M. Sanchez, Á. G. Sathicq, G. T. Baronetti, H. J. Thomas and G. P. Romanelli, Catal. Lett., 2014, 144, 172–180 Search PubMed.
- I.-M. Mbomekalle, Y. W. Lu, B. Keita and L. Nadjo, Inorg. Chem. Commun., 2004, 7, 86–90 CrossRef CAS.
- A. F. Wells, Structural Inorganic Chemistry, Oxford, 1984 Search PubMed.
- B. Dawson, Acta Crystallogr., 1953, 6, 113–126 CrossRef CAS.
- J. S. Anderson, Nature, 1937, 140, 1937 Search PubMed.
- H. T. Evans, J. Am. Chem. Soc., 1948, 70, 1291–1292 CrossRef CAS.
- H. T. Evans, Inorg. Chem., 1966, 5, 967–977 CrossRef CAS.
- A. Patel, N. Narkhede, S. Singh and S. Pathan, Catal. Rev. - Sci. Eng., 2016, 58, 337–370 CrossRef CAS.
- J. Breibeck, N. I. Gumerova and A. Rompel, ACS Org. Inorg. Au, 2022, 2, 477–495 CrossRef CAS PubMed.
- K. Y. Monakhov, C. Gourlaouen, R. Pattacini and P. Braunstein, Inorg. Chem., 2012, 51, 1562–1568 CrossRef CAS PubMed.
- A. Misra, K. Kozma, C. Streb and M. Nyman, Angew. Chem., 2020, 132, 606–623 Search PubMed.
- D. J. Sures, S. K. Sahu, P. I. Molina, A. Navrotsky and M. Nyman, ChemistrySelect, 2016, 1, 1858–1862 CrossRef CAS.
- D. J. Sures, P. I. Molina, P. Miró, L. N. Zakharov and M. Nyman, New J. Chem., 2016, 40, 928–936 RSC.
- C. Li, N. Mizuno, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2019, 141, 7687–7692 CrossRef CAS PubMed.
- C. Li, K. Yamaguchi and K. Suzuki, Chem. Commun., 2021, 57, 7882–7885 RSC.
- Y. Koizumi, K. Yonesato, K. Yamaguchi and K. Suzuki, Inorg. Chem., 2022, 61, 9841–9848 CrossRef CAS PubMed.
- D. Gabb, C. P. Pradeep, T. Boyd, S. G. Mitchell, H. N. Miras, D.-L. Long and L. Cronin, Polyhedron, 2013, 52, 159–164 Search PubMed.
- J. C. Raabe, J. Albert and M. J. Poller, Chem. – Eur. J., 2022, 28, e202201084 CrossRef CAS PubMed.
- S. D. Mürtz, J.-C. Raabe, M. J. Poller, R. Palkovits, J. Albert and N. Kurig, ChemCatChem, 2024, 16, e202301632 CrossRef.
- J.-C. Raabe, T. Esser, F. Jameel, M. Stein, J. Albert and M. J. Poller, Inorg. Chem. Front., 2023, 10, 4854–4868 RSC.
- J.-C. Raabe, J. Aceituno Cruz, J. Albert and M. J. Poller, Inorganics, 2023, 11, 138 CrossRef CAS.
- J.-C. Raabe, M. J. Poller, D. Voß and J. Albert, ChemSusChem, 2023, 16, 2013–2015 CrossRef PubMed.
- J.-C. Raabe, L. Hombach, M. J. Poller, A. Collauto, M. M. Roessler, A. Vorholt, A. K. Beine and J. Albert, ChemCatChem, 2024, 16, e202400395 CrossRef CAS.
- M. J. Poller, S. Bönisch, B. Bertleff, J.-C. Raabe, A. Görling and J. Albert, Chem. Eng. Sci., 2022, 264, 118143 CrossRef CAS.
- T. Esser, A. Wassenberg, J. Raabe, D. Voß and J. Albert, ACS Sustainable Chem. Eng., 2024, 12, 543–560 CrossRef CAS.
- H. Aliyan, R. Fazaeli and N. Habibollahi, J. Korean Chem. Soc., 2012, 56, 591–596 CrossRef CAS.
- I. C. B. Martins, D. Al-Sabbagh, U. Bentrup, J. Marquardt, T. Schmid, E. Scoppola, W. Kraus, T. M. Stawski, A. Guilherme Buzanich, K. V. Yusenko, S. Weidner and F. Emmerling, Chem. – Eur. J., 2022, 28, e202200079 CrossRef CAS PubMed.
- N. Ncube, S. Bhattacharya, D. Thiam, J. Goura, A. S. Mougharbel and U. Kortz, Eur. J. Inorg. Chem., 2019, 2019, 363–366 CrossRef CAS.
- R. Khoshnavazi, L. Bahrami and M. Rezaei, RSC Adv., 2017, 7, 45495–45503 Search PubMed.
- T. Hanaya, K. Suzuki, R. Sato, K. Yamaguchi and N. Mizuno, Dalton Trans., 2017, 46, 7384–7387 RSC.
- L. Bahrami, R. Khoshnavazi and A. Rostami, J. Coord. Chem., 2015, 68, 4143–4158 CrossRef CAS.
- S. Matsunaga, T. Otaki, Y. Inoue, K. Mihara and K. Nomiya, Inorganics, 2016, 4, 16 CrossRef.
- Z. Han, H. Zhang, J. Yan and X. Zhai, Dalton Trans., 2016, 45, 14044–14048 RSC.
- R. G. Finke, M. W. Droege and P. J. Domaille, Inorg. Chem., 1987, 26, 3886–3896 CrossRef CAS.
- C. Marchal-Roch, E. Ayrault, L. Lisnard, J. Marrot, F.-X. Liu and F. Sécheresse, J. Cluster Sci., 2006, 17, 283–290 CrossRef CAS.
- D.-L. Long, C. Streb, Y.-F. Song, S. Mitchell and L. Cronin, J. Am. Chem. Soc., 2008, 130, 1830–1832 CrossRef CAS PubMed.
- V. Sasca, M. Stefanescu and A. Popa, J. Therm. Anal. Calorim., 2003, 72, 311–322 CrossRef CAS.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 186–197 CrossRef PubMed.
- M. Haouas, F. Taulelle and C. Martineau, Prog. Nucl. Magn. Reson. Spectrosc., 2016, 94–95, 11–36 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2441126. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00894h |
| This journal is © The Royal Society of Chemistry 2025 |