Assembling 4f and 3d–4f clusters as single-molecule magnets by automatic fixation of atmospheric CO2
Cai-Ming
Liu
 ab
ab
aBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory for Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: cmliu@iccas.ac.cn
bSchool of Chemical Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 26th May 2025
Abstract
Emerging methods for cluster assembly through fixation of CO2 in air provide an innovative approach for the development of novel single-molecule magnets (SMMs). Both 4f cluster SMMs and 3d–4f cluster SMMs may be assembled using this green pathway. Even after the introduction of chirality and/or intermolecular hydrogen bonds, such SMMs can be further made into multifunctional molecular materials at the nanoscale. In this paper, 4f cluster SMMs and 3d–4f cluster SMMs assembled by the fixation of CO2 in air are briefly reviewed, and an outlook of the promising future prospects in this field is provided.
1. Introduction
Global warming due to the massive emission of CO2 has attracted widespread attention. By innovating energy technologies and reducing tailpipe emissions, we can directly reduce the greenhouse effect. On the other hand, some progress has been made in fixing and converting atmospheric CO2 into organic molecules1 and CO,2 which can “turn waste into treasure” and realize the carbon cycle to reduce the greenhouse effect too. However, further costs need to be decreased. In the field of coordination chemistry, chemists may imitate the permanent carbon fixation method of “mineral carbonization”,3 so that the CO2 in air directly reacts in situ with solvents or ligands to form bridging ligands, such as carbonate,4–7 monomethyl anion carbonate,8 carbamate,9,10etc., which are then self-assembled into complexes; since the structures of such complexes are even different from those of complexes formed when reactants such as carbonates are directly used,11 a unique green pathway is provided for the construction of new functional complexes, especially for single-molecule magnets (SMMs).SMMs are molecule-based magnets with magnetic bistability at the nanoscale,12 and have shown potential applications in the fields of high-density information storage, molecular spintronics and quantum computing. SMMs require both large ground-state spin values and obvious magnetic anisotropies. Lanthanide(III) ions, such as the Dy(III) ion, naturally meet these two necessary conditions, and are generally used to construct SMMs, including 4f SMMs13 and 3d–4f SMMs.14 It is important to note that for the cluster complexes containing multiple Ln(III) ions it is often hard to exhibit good SMM performance due to the difficulty in maintaining consistent magnetic axis orientation across all Ln(III) ions.15,16 Therefore, it is particularly important to select an appropriate bridging ligand to link cations such as the Ln(III) ions. The carbonate anion, the most common product of CO2 fixation, is exactly a suitable ligand that may bridge three or more Ln(III) ions.17 It can also transfer ferromagnetic interactions, which is beneficial to obtain zero-field SMMs.18 In addition, it is an excellent functional structural unit of nonlinear optical double-frequency effects,19 and is thus suitable for the construction of multifunctional molecular materials. What's more, under suitable conditions, the in situ reaction process of immobilizing CO2 to form the carbonate or other bridging ligands can be perfectly matched to the self-assembly process of 4f and 3d–4f SMMs, whose single crystals can be directly grown and easily separated. Therefore, the fixation of atmospheric CO2 provides a unique green approach for the development of novel SMMs. Herein related zero-field SMMs and multifunctional SMMs are focused.
2. Fixation of atmospheric CO2 for the assembly of 4f cluster SMMs
Since CO2 is a weakly acidic gas, an alkaline solution helps in its automatic capture and fixation.20 The commonly used alkaline reagents include NaOH, NaOMe, LiOH, KOH, KOBut, Et3N, Me4NOH·5H2O, pyridine and so on. In some cases, organic amines with hydroxyl groups such as triethanolamine21 are even used as alkaline reagents. There is no doubt that suitable organic ligands play a critical role in assembling 4f cluster SMMs by the fixation of atmospheric CO2. Schiff bases, especially those formed by the condensation of hydrazide and salicylaldehyde (or salicylaldehyde with substituents) (Scheme 1), show unique advantages in this area. H2L1 in Scheme 1 was reacted with DyCl3·6H2O and Et3N in MeOH–CH2Cl2, which resulted in the formation of a Dy6 SMM based on vertex- and edge-sharing Dy3 triangles, [Dy6(μ3-OH)3(μ3-CO3)(μ-OMe)(HL1)6(MeOH)4(H2O)2]·3MeOH·2H2O (1), which contains an unusual η2:η2-μ3-CO32− carbonate bridging ligand sourced from the atmospheric CO2 and shows two clear relaxation regimes, with U/k values of 5.6 K and 37.9 K at 0 Oe;22 this ligand could also be reacted with Dy(OAc)3·6H2O and Et3N in MeOH–CH2Cl2, yielding another Dy6 SMM, [Dy6(μ4-CO3)3(μ3-H2O)(L1)6(MeOH)6(H2O)3]·4MeOH·3H2O (2), in which three CO32− groups derived from the fixation of CO2 in air are located on the sides of the triangular prism of Dy6;23 complex 2 also displays double magnetic relaxation, with U/k values of 5.4 K and 186.8 K at 0 Oe.23 In H2L2 (Scheme 1), there is an additional methoxy group compared to H2L1. When H2L2 was reacted with Dy(OAc)3·6H2O and Et3N in MeOH–EtOH–CH2Cl2, a trigonal prism Dy6 cluster, [Dy6(OAc)3(μ3-CO3)2(L2)5(HL2)(MeOH)2]·4H2O·5MeOH·EtOH (3), could be produced by the fixation of CO2 in air,24 in which two CO32− anions are located on the two bases of the triangular prism and three AcO− anions are involved in coordination; complex 3 is a zero-field SMM, with an U/k value of 56 K.24 Interestingly, when Dy(OAc)3·6H2O was replaced with DyCl3·6H2O and MeOH–EtOH–CH2Cl2 with MeOH–CH2Cl2, a quadruple-CO32− bridged Dy8 cluster, [Dy8(μ4-CO3)4(L2)8(H2O)8]·10MeOH·2H2O (4), could be yielded by fixating atmospheric CO2 too, where four CO32− anions are located on the four lateral faces of the square prismoid Dy8;25 complex 4 shows intramolecular ferromagnetic interactions and is a zero-field SMM with an U/k value of 74.2 K; in addition, it has an obvious hysteresis loop at 1.9 K.25 Furthermore, when H2L3 (Scheme 1), a Schiff base ligand similar to H2L2 but with a pyridine ring instead of a pyrazine ring, was reacted with DyCl3·6H2O and Et3N in MeOH–MeCN, another double-CO32− bridged trigonal prism Dy6 cluster, [Dy6(L3)4(HL3)2Cl4(H2O)2(CO3)2]·CH3OH·H2O·MeCN (5), might be obtained,11 in which four Cl− anions are involved in coordination; complex 5 is also a zero-field SMM with an U/k value of 76 K.11 Thus, the anions can alter the structures and compositions of 4f cluster SMMs assembled by the fixation of atmospheric CO2. | ||
| Scheme 1 Some Schiff base ligands for the assembly of 4f cluster SMMs by the automatic fixation of atmospheric CO2. | ||
The choice of anions also determines whether the reaction of Dy(III) cluster SMMs assembled by the immobilization of atmospheric CO2 can be carried out. H2L4 (Scheme 1) was reacted with different dysprosium(III) salts in an alkaline solution to assemble Dy(III) clusters with different nuclei, depending on whether the anion used is NO3− or Cl−. The double-CO32− bridged trigonal prism Dy6 cluster [Dy6(CO3)2(L4)6(H2O)3(MeOH)Cl2]·5MeOH (6) was formed by fixing CO2 in air, in which two Cl− anions participate in coordination, and complex 6 is a zero-field SMM, with an U/k value of 150.9 K;26 however, when the reaction was carried out with Dy(NO3)3·5H2O, a Dy4 cluster SMM was obtained, which does not involve the fixation of CO2 in air.26 A similar trend was observed when H2L5 (Scheme 1) was used to construct Dy(III) SMMs: when DyCl3·6H2O was used, a propeller-shaped Dy6 cluster, [Dy6(H2L5)3(μ3-OH)(μ3-CO3)3(CH3OH)4(H2O)8]·5Cl·3H2O (7), was obtained, in which each CO32− group derived from the CO2 fixation is linked to two Dy3+ ions from both the small triangular Dy3 and the large triangular Dy3, and complex 7 is a SMM at 0 Oe, showing double relaxation of magnetization, with U/k values of 2 K and 62.4 K;27 however, when Dy(NO3)3·5H2O was used, a Dy2 SMM without the CO2 fixation was yielded.27
The reaction solvent also has an effect on the construction of Dy(III) SMMs assembled by the fixation of atmospheric CO2. When H2L6 (Scheme 1) was used to construct Dy(III) cluster SMMs containing the CO2 immobilized bridging ligands, it was surprising that the small differences between the MeOH and EtOH solvents led to a dramatic change in the structures of the Dy(III) cluster complexes (Fig. 1).28 When MeOH participated in the reaction, a trapezoidal pyramidal Dy5 pentanuclear cluster, [Dy5(L6)5(OH)2(CO3)(O2COMe)(MeOH)3(H2O)]·3MeOH·3.5H2O (8), was obtained,28 in which both the carbonate anion and the monomethyl carbonate anion are formed by the atmospheric CO2 fixation, and complex 8 is a zero-field SMM, with an U/k value of 93.2 K;28 however, when EtOH participated in the reaction, a triangular prism Dy6 cluster, [Dy6(L6)6(CO3)2(EtOH)2(H2O)2Cl2]·6EtOH (9), was obtained,28 in which only the carbonate anion exists, and complex 9 is also a zero-field SMM, with an U/k value of 133.5 K.28 Notably, 8 and 9 can form hysteresis loops at 1.9 K and 2.0 K, respectively.28 Moreover, the coordination solvents also have an effect on the magnetic properties of 4f cluster SMMs involved in the CO2 immobilization. In different mixed solvents, two parallelogram Dy4 SMMs could be obtained by fixing CO2 in air using H2L7 (Scheme 1), [Dy4(CO3)(L7)4(acac)2(H2O)4]·2CH3CN (10) and [Dy4(CO3)(L7)4(acac)2(CH3OH)2(H2O)2]·CH3OH·H2O (11),29 where two MeOH molecules in 11 are coordinated instead of two H2O molecules in 10, and consequently, the U/k value increases obviously from 2.7 K at 0 Oe in 10 to 23.8 K at 0 Oe in 11.29
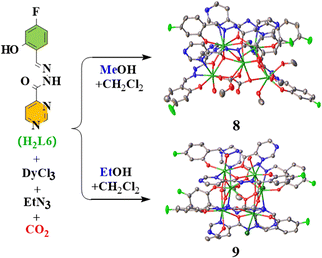 | ||
| Fig. 1 MeOH and EtOH solvents have a dramatic effect on the construction of Dy(III) cluster SMMs (8 and 9) assembled by the fixation of atmospheric CO2. | ||
The Schiff bases derived from organic amines with hydroxyl groups can also be used to assemble Ln(III) cluster SMMs with the fixation of atmospheric CO2. For example, H2L8 (Scheme 1) was treated with Dy(ClO4)3·6H2O and Me4NOH·5H2O in MeOH to produce a metal-centred trigonal prismatic Dy7 cluster, [Dy7(OH)6(CO3)3(L8)3(HL8)3(MeOH)6] (12),30 in which three CO32− anions derived from the CO2 fixation are located on the sides of the triangular prism; complex 12 displays weak SMM properties, with a small U/k value of ∼1.7 K.30
Interestingly, homochiral Ln(III) cluster SMMs formed by the fixation of atmospheric CO2 can also be constructed with the Schiff base ligand. For example, H2L9 (Scheme 1) and L/D-proline were used to construct a pair of homochiral triangular Dy6 cluster complexes, [Dy6(CO3)(L/D-Pro)6(L9)4(HL9)2]·5DMA·2H2O (L-13 and D-13),31 which contain a centre CO32− bridging ligand that originated from the fixation of atmospheric CO2. Although only small U/k values of ∼6.5–8.3 K are observed for L-13/D-13, they have clear magneto-optical Faraday effects and show a large SHG response (1.0× KDP).31 Therefore, the immobilization of atmospheric CO2 can be used to assemble homochiral multifunctional 4f cluster complexes.
3. Fixation of atmospheric CO2 for the assembly of 3d–4f cluster SMMs
Schiff bases and their hydrogenated derivatives or analogues also play a leading role in the assembly of 3d–4f SMMs by the immobilization of atmospheric CO2 in alkaline media.32–41 H2L10 (Scheme 2) and tetramethylheptanedione (Hthd) were used to assemble a Cu–Tb heterometalilic SMM, Cu(L10)(O2COMe)Tb(thd)2 (14),33 in which the monomethyl carbonate ligand was formed by the fixation of atmospheric CO2 in MeOH in the presence of LiOH·H2O, and complex 14 is a zero-field SMM, with an U/k value of 13.8 K.33 | ||
| Scheme 2 Some ligands for the assembly of 3d–4f cluster SMMs by the automatic fixation of atmospheric CO2. | ||
The coordination solvents also have an effect on the magnetic properties of 3f–4f SMMs produced by atmospheric CO2 fixation.34,35 In MeOH–Me2CO, H2L11 (Scheme 2) was used to construct Ni2Ln2 complexes [(μ4-CO3)2{Ni(L11)(MeOH)Tb(NO3)}2] (15) and [(μ4-CO3)2{Ni(L11)(MeOH)Dy(NO3)}2] (16);35 however, in MeCN–H2O, other two Ni2Ln2 complexes [(μ4-CO3)2{Ni(L11)(H2O)Tb(NO3)}2] (17) and [(μ4-CO3)2{Ni(L11)(H2O)Dy(NO3)}2] (18) were formed,35 in which the coordinated H2O molecules take the place of the coordinated MeOH molecules in 15 and 16. Two Ni(II)–Ln(III) units in 15–18 are bridged by two carbonate ligands from the atmospheric CO2 fixation. The U/k value of 15 (12.2 K at 1000 Oe) is larger than that of 17 (6.1 K at 1000 Oe),35 similarly, the U/k value of 16 (18.1 K at 1000 Oe) is larger than that of 18 (14.5 K at 1000 Oe), and 16 even can show SMM behaviour at 0 Oe, with an U/k value of 6.6 K.35 These results indicate that the coordinated MeOH molecule is better for this type of SMM performance than the coordinated H2O molecule.
The salen ligand H2L11 (Scheme 2) was also used to assemble Zn2Ln2 SMMs by the immobilization of CO2 in air. Two Zn2Ln2 cluster complexes Zn2Dy2(μ3-CO3)2(L11)2(NO3)2(MeOH)2 (19) and Zn2Tb2(μ3-CO3)2(L11)2(NO3)2(MeOH)2 (20) were synthesized using this ligand;36 similarly, another salen ligand H2L12 (Scheme 2) was used to construct two other Zn2Ln2 cluster complexes, [Zn2Dy2(μ3-CO3)2(L12)2(NO3)2]·2MeOH (21) and [Zn2Tb2(μ3-CO3)2(L12)2(NO3)2]·2MeOH (22);3619 shows double magnetic relaxation at 1500 Oe, with U/k values of 18.8 K and 41.0 K, while 20 shows double magnetic relaxation at 1200 Oe, with U/k values of 12.4 K and 31.4 K; 21 exhibits SMM behaviour at 2000 Oe, with an U/k value of 54.0 K, while 22 shows SMM behaviour at 1200 Oe, with an U/k value of 26.9 K; interestingly, 21 and 22 display characteristic fluorescence of the Tb(III) ions, and the lifetime (τ) of 21 (20.6 μs) is longer than that of 22 (4.6 μs).36 These results indicate that the structures, magnetic and luminescence properties of these Zn–Ln cluster SMMs may be adjusted by the bisimine chain of the Schiff base ligands.
Another salen ligand, H2L13 (Scheme 2), was used to synthesize a similar Zn2Dy2 SMM containing the CO32− bridging ligand from CO2, [Dy2Zn2(L13)2(OAc)2(CO3)2]·10CH3OH (23); it is a zero-field SMM, with an U/k value of 34 K.37 Surprisingly, when H2L14 (Scheme 2) was adopted to prepare 3f–4f SMMs by the fixation of atmospheric CO2, a carbamate ligand (Lcarbamate) was formed automatically through an in situ ligand reaction of H2L14, and both [Zn4Dy2(L14)2(Lcarbamate)2(N3)2]Cl2·2H2O (24) and [Zn4Tb2(L14)2(Lcarbamate)2(Cl)2][ZnN3Cl3]·2H2O (25) show SMM behaviours under a dc field, with U/k values of 30.67 K at 1000 Oe for 24 and 8.9 K at 2000 Oe for 25.10
Asymmetric Schiff bases have also been used in the synthesis of 3d–4f cluster SMMs involving CO2 fixation.38–40 The Ni2+ complex precursor derived from H2L15 (Scheme 2), NiL15, was pre-synthesized; it was then reacted with DyCl3·6H2O in MeOH–MeCN to obtain a Ni4Dy2 cluster, [Ni4Dy2(CO3)2Cl2(L15)2(L′)2(MeCN)2]·4MeCN·2H2O (H2L′ = N,N′-bis(salicylidene)-1,3propanediamine) (26),38 which contains the carbonate bridging ligand from the CO2 immobilization and shows SMM behaviour at 2000 Oe, with an U/k value of ∼40 K.38 When H2L16 (Scheme 2) was treated with Dy(NO3)3·5H2O, Ni(NO3)3·6H2O and Et3N in MeOH, another Ni2Dy2 cluster, [Ni2Dy2(L16)2(o-vanillin)2(CO3)2(NO3)2(MeOH)2] (27), was yielded, which exhibits possibility of SMM behaviour.39 Notably, the co-ligand may play an important role in the assembly of such 3d–4f cluster SMMs; for example, when di-2-pyridyl ketone (dpk) was treated with H2L16 (Scheme 2), Ni(NO3)2·6H2O, Dy(NO3)3·5H2O and Et3N, a Ni4Dy4 cluster, [Ni4Dy4(L17)6(L′)2{(py)2C(OCH3)O}2(μ3-CO3)2(CH3OH)2]·10CH3OH·13H2O (28), was obtained,40 in which the new ligand (py)2C(OCH3)O was generated by an in situ ligand reaction of dpk, and the latter also provides an alkaline reaction environment for the immobilization of CO2; complex 28 is a zero-field SMM, with an U/k value of 14.9 K.40
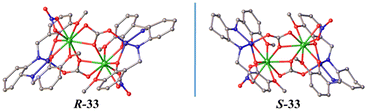
Schiff base analogues have also been successfully used to assemble several Zn2Ln2 SMMs involving atmospheric CO2 fixation.41–43 Two such luminescent Zn2Ln2 SMMs, {(μ3-CO3)2[Zn(μ-L18)Dy(NO3)]2}·4CH3OH (29)41 and {(μ3-CO3)2[Zn(μ-L18)Yb(H2O)]2}(NO3)2·4CH3OH (30),42 were obtained using H2L18 (Scheme 2): both 29 and 30 are field-induced SMMs,41,42 and the U/k value of 29 (24 K at 1000 Oe) is larger than that of 30 (19.4 K at 1000 Oe); however, 29 shows yellow luminescence of the Dy3+ ion,41 while 30 displays near-infrared Dy3+-based luminescence.42 A Zn3Dy3 triangular cluster containing a central μ6-CO32− bridging ligand from the fixation of CO2 in air, [Zn3Dy3(μ6-CO3)(μ3-OH)3(L19)3(H2O)3]·3ClO4·NO3 (31), was obtained using H2L19 (Scheme 2), whose SMM behaviors were studied at 0 Oe and 1000 Oe, with an U/k value of 48 K at 1000 Oe.43 Moreover, H2L20 (Scheme 2) was chosen to prepare another Zn2Dy2 SMM with the CO32− anion derived from the CO2 fixation, {Zn2Dy2(μ3-CO3)2(L20)(acacF6)2}·CH3OH (32),44 which contains hexafluoroacetylacetone terminal ligands; complex 32 shows magnetic relaxation at 1500 Oe, with an U/k value of 83 K.44 Importantly, homochiral Schiff base analogues R-H2L21 and S-H2L21 (Scheme 2) were utilized to construct a pair of homochiral Zn2Ln2 multifunctional SMMs, [Zn2Ln2(R-L21)2(CO3)2(NO3)2]·2CH3OH (R-33) and [Zn2Ln2(S-L21)2(CO3)2(NO3)2]·2CH3OH (S-33),18 which exhibit typical zero-field SMM properties with an U/k value of 19.61 K, display the characteristic fluorescence of the Dy(III) ion, and show a weak SHG response (0.051× KDP) (Fig. 2).18
4. Conclusion and outlook
In this review, 4f cluster SMMs and 3d–4f cluster SMMs assembled by the automatic fixation of atmospheric CO2 were focused. The high thermodynamic stability of CO2 and its low concentration in air continue to pose challenges for the synthesis of such SMMs. Many factors, such as basic reagents, anion types, solvents, substituents on ligands, etc., can not only directly affect the occurrence of atmospheric CO2 fixation, but also affect the structures and properties of 4f cluster SMMs and 3d–4f cluster SMMs after fixing atmospheric CO2. Notably, in addition to the direct bridging of metal ions as described earlier, the specific structural units of new ligands, which are obtained from the in situ reaction of CO2 in air (such as hydrazine carboxylate45), can also be coordinated with metal ions in a non-bridging manner when constructing SMMs, but multi-nuclear (≥3) molecular systems are yet to be developed. Furthermore, the introduction of chirality into SMMs can further add new physical properties such as second-order nonlinear optics, ferroelectricity, circularly polarized luminescence and magnetochiral dichroism, and the formation of intermolecular hydrogen bonds has the potential to lead to proton conductivity.46 Looking to the future, these molecular engineering and crystal engineering strategies may bring new prospects for the development of nanoscale multifunctional SMMs involving atmospheric CO2 fixation.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was made possible as a result of a generous grant from the National Natural Science Foundation of China (Grant Numbers 22271289 and 21871274).References
- J. Ye, N. Dimitratos, L. M. Rossi, N. Thonemann, A. M. Beale and R. Wojcieszak, Science, 2025, 387, eadn9388 CrossRef CAS PubMed.
- W.-P. Chen, P.-Q. Liao, P.-B. Jin, L. Zhang, B.-K. Ling, S.-C. Wang, Y.-T. Chan, X.-M. Chen and Y.-Z. Zheng, J. Am. Chem. Soc., 2020, 142, 4663–4670 CrossRef CAS PubMed.
- Y. Chen and M. W. Kanan, Nature, 2025, 638, 972–979 CrossRef CAS PubMed.
- X.-J. Kong, Y.-P. Ren, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, J. Am. Chem. Soc., 2007, 129, 7016–7017 CrossRef CAS PubMed.
- J. Vallejo, J. Cano, I. Castro, M. Julve, F. Lloret, O. Fabelo, L. Cañadillas-Delgadozcd and E. Pardo, Chem. Commun., 2012, 48, 7726–7728 RSC.
- K. Ehama, Y. Ohmichi, S. Sakamoto, T. Fujinami, N. Matsumoto, N. Mochida, T. Ishida, Y. Sunatsuki, M. Tsuchimoto and N. Re, Inorg. Chem., 2013, 52, 12828–12841 CrossRef CAS PubMed.
- M. Hołyńska, R. Clérac and M. Rouzières, Chem. – Eur. J., 2015, 21, 13321–13329 CrossRef PubMed.
- C.-M. Liu, X. Hao, Z.-B. Hu and H.-R. Wen, Dalton Trans., 2025, 54, 96–107 RSC.
- E. M. Pineda, Y. Lan, O. Fuhr and W. Wernsdorfer, Chem. Sci., 2017, 8, 1178–1185 RSC.
- C.-L. Yin, Z.-B. Hu, Q.-Q. Long, H.-S. Wang, J. Li, Y. Song, Z.-C. Zhang, Y.-Q. Zhang and Z.-Q. Pan, Dalton Trans., 2019, 48, 512–522 RSC.
- Y.-N. Guo, X.-H. Chen, S. Xue and J. Tang, Inorg. Chem., 2012, 51, 4035–4042 CrossRef CAS PubMed.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268–297 CrossRef CAS PubMed.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed.
- K. Liu, W. Shi and P. Cheng, Coord. Chem. Rev., 2015, 289, 74–122 CrossRef.
- Y.-S. Meng, S.-D. Jiang, B.-W. Wang and S. Gao, Acc. Chem. Res., 2016, 49, 2381–2389 CrossRef CAS PubMed.
- C.-M. Liu, D.-Q. Zhang, X. Hao and D.-B. Zhu, Inorg. Chem., 2018, 57, 6803–6806 CrossRef CAS PubMed.
- G. Lu, Y. Liu, W. Deng, G.-Z. Huang, Y.-C. Chen, J.-L. Liu, Z.-P. Ni, M. Giansiracusa, N. F. Chilton and M.-L. Tong, Inorg. Chem. Front., 2020, 7, 2941–2948 RSC.
- H.-R. Wen, J.-J. Hu, K. Yang, J.-L. Zhang, S.-J. Liu, J.-S. Liao and C.-M. Liu, Inorg. Chem., 2020, 59, 2811–2824 CrossRef CAS PubMed.
- X. Liu, L. Kang, P. Gong and Z. Lin, Angew. Chem., Int. Ed., 2021, 60, 13574–13578 CrossRef CAS PubMed.
- H.-L. Wang, T. Liu, Z.-H. Zhu, J.-M. Peng, H.-H. Zou and F.-P. Liang, Inorg. Chem. Front., 2021, 8, 3134–3140 RSC.
- S. K. Langley, I. A. Gass, B. Moubaraki and K. S. Murray, Inorg. Chem., 2012, 51, 3947–3949 CrossRef CAS PubMed.
- H. Tian, Y.-N. Guo, L. Zhao, J. Tang and Z. Liu, Inorg. Chem., 2011, 50, 8688–8690 CrossRef CAS PubMed.
- H. Tian, L. Zhao and J. Tang, Cryst. Growth Des., 2018, 18, 1173–1181 CrossRef CAS.
- H. Tian, L. Zhao, Y.-N. Guo, Y. Guo, J. Tang and Z. Liu, Chem. Commun., 2012, 48, 708–710 RSC.
- H. Tian, M. Wang, L. Zhao, Y.-N. Guo, Y. Guo, J. Tang and Z. Liu, Chem. – Eur. J., 2012, 18, 442–445 CrossRef CAS PubMed.
- C.-M. Liu and X. Hao, New J. Chem., 2023, 47, 18849–18855 RSC.
- P. Kumar, A. Swain, J. Acharya, Y. Li, V. Kumar, G. Rajaraman, E. Colacio and V. Chandrasekhar, Inorg. Chem., 2022, 61, 11600–11621 CrossRef CAS PubMed.
- C.-M. Liu, X. Hao, Z.-B. Hu and H.-R. Wen, Dalton Trans., 2025, 54, 96–107 RSC.
- W.-M. Wang, Z.-L. Wu, Y.-X. Zhang, H.-Y. Wei, H.-L. Gao and J.-Z. Cui, Inorg. Chem. Front., 2018, 5, 2346–2354 RSC.
- E. C. Mazarakioti, K. M. Poole, L. Cunha-Silva, G. Christou and T. C. Stamatatos, Dalton Trans., 2014, 43, 11456–11460 RSC.
- C.-M. Liu, X. Hao and X.-L. Li, Molecules, 2024, 29, 3402 CrossRef CAS PubMed.
- L. Jiang, Y. Liu, X. Liu, J. Tian and S. Yan, Dalton Trans., 2017, 46, 12558–12573 RSC.
- J.-P. Costes, F. Dahan and W. Wernsdorfer, Inorg. Chem., 2006, 45, 5–7 CrossRef CAS PubMed.
- K. Ke, S. Zhang, W. Zhu, G. Xie and S. Chen, J. Coord. Chem., 2015, 68, 808–822 CrossRef.
- S. Sakamoto, T. Fujinami, K. Nishi, N. Matsumoto, N. Mochida, T. Ishida, Y. Sunatsuki and N. Re, Inorg. Chem., 2013, 52, 7218–7229 CrossRef CAS PubMed.
- C.-M. Liu, X. Hao and D.-Q. Zhang, Appl. Organomet. Chem., 2020, 34, e5893 CrossRef CAS.
- P. Zhang, L. Zhang, S.-Y. Lin and J. Tang, Inorg. Chem., 2013, 52, 6595–6602 CrossRef CAS PubMed.
- S. Maity, T. K. Ghosh, S. Ito, P. Bhunia, T. Ishida and A. Ghosh, Cryst. Growth Des., 2022, 22, 4332–4342 CrossRef CAS.
- A. Upadhyay, C. Das, S. K. Langley, K. S. Murray, A. K. Srivastava and M. Shanmugam, Dalton Trans., 2016, 45, 3616–3626 RSC.
- Y.-J. Zhu, H.-S. Wang, Y. Chen, P. Zhou, Y. Wu and Y.-Q. Zhang, New J. Chem., 2024, 48, 3438–3446 RSC.
- S. Titos-Padilla, J. Ruiz, J. M. Herrera, E. K. Brechin, W. Wersndorfer, F. Lloret and E. Colacio, Inorg. Chem., 2013, 52, 9620–9626 CrossRef CAS PubMed.
- J. Ruiz, G. Lorusso, M. Evangelisti, E. K. Brechin, S. J. A. Pope and E. Colacio, Inorg. Chem., 2014, 53, 3586–3594 CrossRef CAS PubMed.
- J. Goura, E. Colacio, J. M. Herrera, E. A. Suturina, I. Kuprov, Y. Lan, W. Wernsdorfer and V. Chandrasekhar, Chem. – Eur. J., 2017, 23, 16621–16636 CrossRef CAS PubMed.
- C.-M. Liu, D.-Q. Zhang, X. Hao and D.-B. Zhu, Dalton Trans., 2020, 49, 2121–2128 RSC.
- K. Zhang, F.-S. Guo and Y.-Y. Wang, Dalton Trans., 2017, 46, 1753–1756 RSC.
- C.-M. Liu, H.-H. Zou, Y.-Q. Zhang, X. Hao and X.-M. Ren, Chin. J. Chem., 2025, 43, 1051–1058 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |