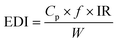Open Access Article
Open Access ArticleMicroplastics in settled dust from university indoor environments: Puerto Colombia, Colombia†
Maria Gabriela
Avilés Valera
 ab,
Victoria Andrea
Arana Rengifo
ab,
Victoria Andrea
Arana Rengifo
 a and
Carlos David
Grande-Tovar
a and
Carlos David
Grande-Tovar
 *b
*b
aGrupo de investigación de Ciencias, Educación y Tecnología – CETIC, Universidad del Atlántico, Carrera 30 Número 8-49, Puerto Colombia, 081001, Colombia
bGrupo de investigación de Fotoquímica y Fotobiología, Universidad del Atlántico, Carrera 30 Número 8-49, Puerto Colombia, 081001, Colombia. E-mail: carlosgrande@mail.uniatlantico.edu.co
First published on 16th January 2025
Abstract
Microplastics (MPs), plastic particles ranging from 1 μm to 5 mm, are contaminants of concern due to their adverse effects on human health. Interest in analyzing their presence in settled dust from indoor environments has increased. However, available data remain limited. This study analyzes the presence of MPs in deposited dust from three indoor university environments: a laboratory, a classroom, and a conference room in Puerto Colombia, Colombia, using a stereomicroscope for quantification and physical analysis and micro-Attenuated Total Reflectance Fourier Transform Infrared spectroscopy (μATR-FT-IR) for chemical characterization. Our findings revealed the highest mean abundance of anthropogenic microparticles and MPs in the laboratory (2070 microparticles per g – 1635 MPs per g), followed by the classroom (1141 microparticles per g – 949 MPs per g) and the conference room (955 microparticles per g – 803 MPs per g). No correlations were found between microparticle abundance and temperature or relative humidity. Fibers were predominant, and most particles fell within the size of 501–1000 μm, with polyethylene terephthalate (PET; 12.2%), polypropylene (PP; 17%), and polyester (32.7%) being the most common polymers across all analyzed samples. μATR-FT-IR analysis also revealed multi-component polymers and weathering on the MPs. Notably, the estimated daily intake (EDI) of MPs was higher among teenagers (mean EDI: 0.47 microparticles per kg – bw per day) than adults, suggesting that dust is a critical exposure pathway. This study calls for increased research on MPs in indoor spaces. It focuses on their transport mechanism and its relationship with climate variables. It also focuses on multi-component and weathered MPs to better understand their dispersion and interaction with the human body and environment.
Environmental significanceMicroplastics (MPs) are ubiquitous contaminants with harmful effects on human health and the environment. However, limited data exist on their prevalence in the atmosphere, particularly in dust, a crucial pathway for airborne MPs in indoor spaces. To the best of our knowledge, this study is the first to analyze MPs in settled dust from indoor university environments in Colombia, providing a valuable baseline of knowledge about MPs in the region. It also addresses important gaps, such as the relationship between abundance of MPs and climatic factors in indoor spaces. This information is essential for developing strategies to mitigate exposure risks of MPs in enclosed environments. |
1 Introduction
Our modern world is surrounded by plastics, one of the most present products in our lives due to their durability and moldability.1 According to Plastics Europe,2 the worldwide production in 2022 (400.3 Mt) exceeded that of 2021 (394 Mt). This increase is concerning since plastics are one of the most recalcitrant planet pollutants, persisting in the environment for up to 1000 years due to their resistance to degradation.3 Plastics whose size ranges from 1 μm to 5 mm are known as microplastics (MPs), which can be synthetic polymers, composites, copolymers, and highly modified natural polymers.4,5 MPs have attracted significant attention due to the adverse impacts they can have on living organisms and the environment.1 Additionally, they are ubiquitous because their small size gives them a high chance of encountering biota and aquatic, terrestrial, and atmospheric environments.6 Unlike macroplastics, which can be easily recognized, pollution by these microparticles cannot become a pervasive challenge.7While pollution by MPs in aquatic ecosystems has received considerable attention, the prevalence of these pollutants in the atmospheric compartment, particularly in dust from indoor urban environments, remains understudied.8,9 This gap is concerning because dust particles, including MPs, can enter the human body through ingestion, inhalation, and dermal absorption.10 Despite the lack of clarity about the impacts of MPs on human health, studies have shown that fibers can cause lung irritation, interstitial lung disease, and an increased risk of cancer due to their persistence in the respiratory system, as some fibers, once in the lungs, cannot be eliminated due to their large surface area.11 Furthermore, MPs may exhibit toxic effects, carcinogenicity, and mutagenicity due to the additives and monomers they may contain.12 MPs can also act as vectors of pathogens and contaminants adsorbed on their surface, such as aromatic hydrocarbons and heavy metals that can cause additional health risks.10
Humans spend more than 70% of their time in indoor spaces, and researchers have found that indoor exposure to MPs exceeds that in outdoor environments.13,14 Universities are characterized by high occupancy and a wide variety of activities, such as laboratory work, constant renovations, and foot traffic, all of which may contribute to the emission and accumulation of MPs; therefore, they could represent focus points for MP exposure.15 The first study to address MPs in settled dust from indoor spaces reported 190–670 fibers per mg in Paris apartments, with 33% being polymers.16 Other studies have reported their prevalence in settled dust from hotels, schools, universities, hospitals, and other indoor spaces in countries like China and Iran.8,17,18 The mechanisms of transportation, deposition, and resuspension of MPs vary according to the indoor environment and its anthropogenic activities, leading to different human exposure patterns.14 Consequently, studying the behavior of MPs in indoor environments across different regions is essential to understand the global situation better. This is particularly important in low-income countries, such as Colombia, which have shown higher MP rates but have not attracted as much attention as high-income countries.19
To address the lack of information on MPs in settled dust and their risk to human health, our study aims to evaluate the prevalence of MPs in settled dust from indoor university spaces in Puerto Colombia, Colombia. We examine the abundance, typology, and chemical composition of the obtained microparticles using a stereo microscope and a μATR-FT-IR and calculate the estimated daily intake (EDI) of MPs in teenagers and adults. To the best of our knowledge, this is the first reported study regarding MPs present in settled dust from a university in Colombia. This research contributes to establishing a baseline of knowledge about MPs in the country. It provides insights into a topic that nowadays still has many gaps in the scientific community, such as MP suspension/transport dynamics and their relation to climate factors in indoor spaces. Effective mitigation strategies cannot be developed if the agent causing the problem is not well understood.
2 Experimental
2.1 Sampling site
Puerto Colombia, a port municipality located in the Atlántico department of Colombia (0° 59′ 52′′ N, 74° 50′ 52′′ W), is part of the metropolitan area of Barranquilla, the fourth-largest city in the country (Fig. 1). The municipality has a total area of 93 km2. Several swamps surround it, including La Ciénega, Manatíes, Aguadulce, Rincón, Salado, and Balboa. In addition, it has several rain-fed streams like Arroyo Grande and beaches on the Caribbean Sea, such as Sabanilla, Salgar, Prado Mar, and others.20 According to the 2018 national census, Puerto Colombia has 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 899 inhabitants, 88% of whom belong to the urban population.21 This study was conducted at the Universidad del Atlántico (11° 1′ 4′′ N, 74° 52′ 27′′ W), a significant focus point and institution within the municipality, with nearly 23
899 inhabitants, 88% of whom belong to the urban population.21 This study was conducted at the Universidad del Atlántico (11° 1′ 4′′ N, 74° 52′ 27′′ W), a significant focus point and institution within the municipality, with nearly 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 people, including students, professors, and administrative staff. The university's high daily traffic of people and its various anthropogenic activities, such as laboratory work and synthetic sports fields, position it as both a potential source of microplastics and a critical site for human exposure.
600 people, including students, professors, and administrative staff. The university's high daily traffic of people and its various anthropogenic activities, such as laboratory work and synthetic sports fields, position it as both a potential source of microplastics and a critical site for human exposure.
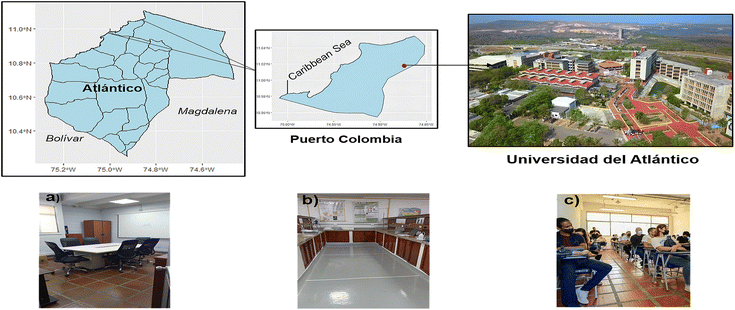 | ||
| Fig. 1 Map of the municipality of Puerto Colombia and location of the sampling places: (a) conference room, (b) laboratory and (c) classroom. The maps were created using RStudio (v. 2023.09.1). | ||
Three locations at the Universidad del Atlántico were chosen for sampling, including a conference room (58.65 m2), a laboratory (104.13 m2), and a classroom (82.35 m2) (Fig. 1). Sampling was carried out twice, covering the entire floor, at each location, with three samples collected per session, giving six samples per place. During the partial and complete rainy season, the sampling period occurred from April 4 to June 16, 2023. Additionally, no sampling area was cleaned 20 to 26 hours before sampling. By sampling during non-working hours, we ensured that the classroom and conference room were not in use during and before the sampling sessions, while the laboratory operated during working hours before and during sample collection. The studied laboratory is a research laboratory, and seminars are regularly held there. The sampling sessions at this location were scheduled on a day when a seminar occurred before and during the sampling. Usually, the laboratory team gathers to prepare before the talk. Detailed information on the sampling conditions at each location is provided in Table 1.
| Sampling session | Daily average occupancy | Type of active AC system | Cleaning (times per day) | Active labor hours | T (°C) | RH% |
|---|---|---|---|---|---|---|
| a Temperature (T); relative humidity (RH%). | ||||||
| Classroom 1 | 111 | Central | 3 | No | 27 | 74 |
| Classroom 2 | 111 | 3 | No | 28 | 81 | |
| Conference room 1 | 2 | Central | 1 | No | 31 | 49 |
| Conference room 2 | 1 | 1 | No | 28 | 58 | |
| Laboratory 1 | 18 | Split | 1 | Yes | 24 | 61 |
| Laboratory 2 | 20 | 1 | Yes | 25 | 48 | |
2.2 Sampling protocol
The sampling methodology employed in this study utilized an active sampling method similar to those described by Abbasi et al.21 and Dehghani et al.1 Additionally, the quadrant technique was applied to ensure systematic distribution and uniform coverage in the sampling areas and guarantee representative sampling collection.22 Once the quadrants were delimited with paper masking tape, the samples were collected using a natural fibers brush and a steel dustpan, then deposited into aluminum bags, and subsequently stored in glass jars to reduce the risk of cross-contamination. Finally, the samples were transported to the laboratory in cardboard boxes. The aluminum bags and glass jars were rinsed thrice with ultrapure Milli-Q (18.2 MΩ cm) water before use. Similarly, the brush and the steel dustpan were also rinsed.2.3 Sample pre-treatment
After sample collection, each sample was carefully weighed with an analytical balance (RADWAG AS 220.R2, Poland) to calculate the total dust collected. Subsequently, the working mass was determined to be 0.25 g ± 0.001 g. Once the mass was established, the samples were sieved through a 5 mm steel sieve into a 250 mL glass beaker to remove non-interest particles, such as coarse debris.2.4 Sample treatment
The sample treatment was carried out based on the proposed methodology by Dehghani et al.1 and Abbasi et al.23 After sieving the samples into glass beakers, 35 mL of H2O2 (30% v/v) was added to remove organic materials. The beakers were then covered with aluminum foil and placed in stirring plates for 72 hours at 60–70 °C and 225 rpm. Following this, the samples were filtered using a vacuum pump (MZ C1, VACUUBRAND®, Germany) and a metal funnel using cellulose fiber filters (Hahnemühle, Germany) of 0.42 μm pore size and 47 mm in diameter to remove the H2O2. The beakers were washed three times with Milli-Q ultrapure water (18.2 MΩ cm) to avoid any loss of microparticles. No effects of H2O2 on the filter integrity or potential fragmentation were observed. Afterward, a density separation procedure was conducted employing ZnCl2 (1.3 kg L−1). Each filter obtained in the previous step was washed with pressure with Milli-Q ultrapure water (18.2 MΩ cm) into a new 250 mL beaker to remove the present microparticles in the filter, and then 100 mL of the ZnCl2 solution was added. The mixture was stirred for 5 min at 400 rpm and left to settle for 24 hours at room temperature. The supernatant was then filtered using a vacuum filtration system and cellulose fiber filters of 0.42 μm pore size and 47 mm diameter. The remaining liquid in the beaker was subjected to the density separation process three more times; four filters were obtained for each treated sample. Finally, the filters were stored in Petri dishes and dried at room temperature to detect and quantify microparticles.2.5 Sample analysis
The physical characterization of microparticles was based on the shape and structure criteria proposed by Hartmann et al.5 and Crawford and Quinn.25
The chemical characterization of microplastics was based on the chemical composition criteria proposed by Hartmann et al.5
2.6 Human exposure to microparticles: estimated daily intake (EDI)
In the present study, human exposure was only estimated for the ingestion pathway, as this is the most likely exposure route for particles ≥50 μm.16,27 The human daily intake was calculated based on that suggested by Zhu et al.18 using the following equation:where Cp is the determined microparticle concentration in indoor dust samples (microparticles per g); f is the time fraction in indoor environment exposure, for example, hours spent in the indoor environment per day; IR (gper day) is the indoor dust ingestion rate for humans; and W represents the body weight. The values for f, IR, and W were obtained from U.S. EPA.28 and Jhonson-Restrepo and Kannan.29
It is important to note that our research focuses on specific age groups due to their relevance to the study. As babies (0.5–1 year), toddlers (1–6 years), and children (6–12 years) are not part of the population that attends university, we have only estimated daily intake values for teenagers (12–19 years) and adults (≥20 years).
2.7 Quality assurance and quality control (QA/QC)
To ensure the integrity of our findings, quality assurance and quality control measures were implemented to minimize cross-contamination. During all sample treatment and analyses, plastic materials were eliminated, and all glassware was washed three times with Milli-Q ultrapure water (18.2 MΩ cm) before use according to the protocols suggested by Soltani et al.30 Additionally, workbenches were cleaned with paper towels and 70% isopropyl alcohol before each procedure.30 Before use, all reagents were filtered using quartz fiber filters (GF/A Whatman) of 1.2 μm pore size and 47 mm diameter. This step ensured that any potential particulate matter or impurities present in the reagents were removed. Blanks were made at each stage of sample treatment, quantification, and characterization of microparticles, as outlined by Dehghani et al.1 To minimize external influences, μATR-FT-IR analysis was conducted in a single-person room, and for optimal performance, the ATR crystal was cleaned with 96% isopropyl alcohol before each reading.2.8 Statistical analysis
The normality of the data set was tested using the Shapiro–Wilk normality test. An ANOVA was employed to compare univariate groups within the data sets, followed by Tukey's test for post hoc analysis. Pearson linear correlations were used to evaluate the relationship between the abundance of microparticles and meteorological factors, such as temperature and humidity. The significance level was set as 0.05. All tests were performed with RStudio (v. 2023.09.1).3 Results and discussion
3.1 Total microparticle abundance and influential factors
After the visual inspection, 6249 microparticles (non-MP and MP particles) were identified in all samples. Our findings revealed that the concentration of microparticles was generally distributed in all interior university environments (p > 0.05). The highest average concentration corresponded to 2070 microparticles per g in the laboratory, where the abundance ranged between 632 and 3424 microparticles per g, followed by 1141 microparticles per g in the classroom, with variations between 516 and 1708 microparticles per g, and finally 955 microparticles per g in the conference room, with an abundance between 200 and 1952 microparticles per g (Fig. 2).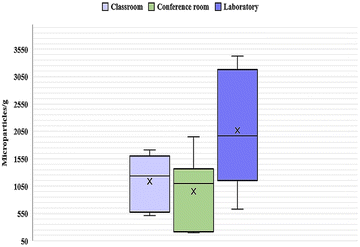 | ||
| Fig. 2 Box and whisker plot (microparticles per g) (mean ± S) of anthropogenic microparticles by the sample location. | ||
After performing the analysis of variance (ANOVA), it was found that the difference between the mean abundances was not statistically significant (p = 0.0554). However, a Tukey test was also conducted since ANOVA does not rule out the possibility of substantial differences among specific pairs of groups. Despite observing a higher mean microparticle abundance in the laboratory compared to the classroom and the conference room, the Tukey test revealed that there were no significant differences when comparing pairs of groups (p > 0.05) (Fig. 2). It is important to note that these findings indicate insufficient evidence to affirm differences between the means. Thus, the ANOVA and Tukey test results do not conclusively demonstrate that the means are identical.
The abundance of microparticles varied among the sampling places, which may be attributed to the varying characteristics of each location. The classroom had the highest average daily occupancy (Table 1). However, the laboratory, which poses a daily average occupation nearly five times lower, exhibited a higher abundance. This observation indicates that other anthropogenic factors beyond the occupancy rate may influence the prevalence of these microparticles. Pratiwi et al.31 and Zhang et al.32 concluded that human activity greatly impacted the abundance of microparticles. This one would be higher in those areas sampled during working hours compared to non-working hours. Our findings coincided with this since the laboratory was the only place where samples were taken during working hours and with people present (Table 1). Cleaning methods and frequency may also influence microparticle concentrations. Nematollahi et al.17 mentioned that conventional cleaning methods like sweeping may contribute to higher microparticle levels in settled dust from indoor environments like classrooms. At the same time, Soltani et al.30 found that frequent vacuum cleaning reduces microparticle numbers in indoor house environments. For this study, no sampling sites were cleaned before sampling. However, the frequency of daily cleaning varies, with the classroom being the site with the most recurring cleaning, which could influence the lower amount of microparticles found at this location than the laboratory despite having a higher daily occupancy (Table 1). Other factors, such as inadequate ventilation and the type of floor covering, also contribute to indoor pollution.16,26
It is worth noting that all sampling locations had active air conditioning (AC). A recent study on filters for indoor split ACs suggests that these devices can act as both sources and sinks of microparticles, especially microfibers since their large surface area increases the possibility of adhering to AC filters.33 However, the AC units' type, age, and duration of use varied among locations, which could influence the accumulative effect of microparticles and also the resuspension mechanism of microparticles.32–34
Additionally, while all the sampling sites were within the same university, variations in sampling days led to different temperature and relative humidity conditions, which influence indoor environments and may contribute to differences in the microparticle resuspension (Table 1).35,36 Pearson correlations were carried out to assess the association between temperature, relative humidity, and microparticle prevalence. The results showed that temperature and relative humidity did not exhibit a significant linear correlation with the microparticle abundance at the sampling sites, presenting p = 0.1393 and p = 0.6956, respectively. This suggests that these factors may not play a primary role in microparticle deposition, implying that other variables, such as the airflow in indoor spaces, may have a more substantial influence.
Our findings align with those of Sharaf Din et al.26 and Su et al.37 They concluded that the environmental and air quality variables, such as wind speed, relative humidity, and temperature, have a weak impact on the abundance of MPs in suspended and settled dust samples from indoor and outdoor spaces, respectively. In contrast, other studies on suspended atmospheric microparticles in outdoor environments have reported a decrease in PET and PP microparticles deposition during nighttime, possibly due to their density and hygroscopic properties, along with higher nocturnal relative humidity conditions.38 Furthermore, it has been found that the deposition of microplastics increases on average with wind speed, stimulated by rising humidity.39 Temperature has been associated with the fragmentation of textiles.40
Most microplastic studies focus on quantifying their abundance, leaving a gap in understanding their transport, dispersion, deposition mechanisms, and association with environmental variables. These variables play an essential role in understanding the spatial dispersion dynamics of microplastics, which affect our exposure risk to MPs.13,32 The present research explored possible associations between temperature, relative humidity, and microparticle abundance, enhancing our understanding of microparticle dynamics in indoor environments. However, further research is warranted to draw more robust conclusions, especially with long-term observation and larger data sets. The microparticle concentrations reported in this study are lower than those found in research conducted at other universities, which reported concentrations of 48.7 items per mg and 139 items per mg.8 Conversely, other studies that also evaluated the presence of MPs in university and school classrooms reported that the average concentration of microplastics in the dust is 209 MPs per g and 195 MPs per g, respectively.17,18 These values are approximately four times lower than our findings for the classroom. These differences in abundance may arise due to variations in environmental conditions, occupancy patterns, and other factors mentioned earlier.
Furthermore, it is essential to highlight that, to date, only two studies have reported the prevalence of MPs and microparticles in fallout and settled dust from indoor spaces in Colombia. However, differences in sampling methods and the lack of consensus on the units used to report microparticle abundance make comparisons challenging. Zhang et al.41 reported concentrations of PET at 1000 μg mg−1 and polycarbonate (PC) at 5.6 μg mg−1 in residential apartments, while Abad-López et al.34 reported 1.1 × 104 to 6.1 × 104 MPs per m2 per day in residential and work areas. Table 2 compares the current study with other global research conducted on settled dust from different indoor environments.
| Sampling method | Country | Location | Microparticle size range | Shapes | Predominant polymers | Mean abundance | Reference | |
|---|---|---|---|---|---|---|---|---|
| a N/A: not available. b Polyethylene terephthalate (PET); polypropylene (PP); polycarbonate (PC); polyethylene (PE); polystyrene (PS); polyamide (PA); polyacrylic (PAC); polyvinyl (PV); polyurethane (PU). c The first values represent the mean abundance of PET and PC in Colombia, while the second reflect the mean abundance across all studied countries. d Japan, USA, Greece, Kuwait, India, China, South Korea, Pakistan, Romania, Vietnam and Saudi Arabia. e Gana, India, Bangladesh, Nepal, Nigeria, Ukraine, Kenya, Indonesia, China, Thailand, Iran, Malaysia, Brazil, Croatia, Australia, Chile, Estonia, Singapore, USA, Germany, Switzerland, Canada, South Korea, Belgium, France, New Zealand, Netherland, Cyprus and Barbados. f High income countries (HI); medium income countries (MI); low-income countries (LI). | ||||||||
| Sweeping and vacuum cleaner | Fiber | 2070 microparticles per g | Current study | |||||
| Fragment | 1635 MPs per g | |||||||
| University laboratory | Film | PETb | 955 microparticles per g | |||||
| Colombia | University conference | 50–5000 μm | Foam | PPb | 803 MPs per g | |||
| University classroom | Conglomerate | Polyester | 1141 microparticles per g | |||||
| Pellet | 949 MPs per g | |||||||
| Micro-Sphere | ||||||||
| 1000 μg g−1c | 41 | |||||||
| PET | 5.6 μg g−1 | |||||||
| Colombia + 11 countriesd | Houses/apartments | N/A | N/A | PCb | 5900 μg g−1c | |||
| 8.8 μg g−1 | ||||||||
| France | Apartments | 50–4850 μm | Fiber | PP | 190–670 | 16 | ||
| Fibers per g | ||||||||
| Apartments and offices | Fiber | PEb | 1174 MPs per g | |||||
| China | Business hotels | ∼10 to ≥1000 μm | Fragment | PP | 896 MPs per g | |||
| University dormitories | Film | Polyester | 843 MPs per g | 18 | ||||
| University classrooms | 775 MPs per g | |||||||
| 209 MPs per g | ||||||||
| Fiber | ||||||||
| Iran | Schools | 50 to ≥1000 μm | Fragment | PET | 195 MPs per g | 17 | ||
| Sheet | PP | |||||||
| Sweeping and Vacuum cleaner | China | Apartments | N/A | Fiber | PET | 190–670 fibers per mg | 13 | |
| Granules | 6–184 particle per mg | |||||||
| 334–351 particles weekday | ||||||||
| 242–252 weekend | ||||||||
| Indonesia | Offices | 3000–5000 μm | Fiber | N/A | 290–321 particles weekday | 42 | ||
| Schools | Fragment | 239–275 weekend | ||||||
| Apartments | Film | 108–133 weekday | ||||||
| 95–127 weekend | ||||||||
| 74 items per mg | ||||||||
| 139 items per mg | ||||||||
| Houses | Fiber | 121.6 items per mg | ||||||
| Iran | Bushehr | Mosque | ∼10 to ≥1000 μm | Fragment | PE | 55.6 items per mg | ||
| Kindergarten | Film | 63.6 items per mg | 8 | |||||
| Shiraz | Hospital | 68.6 items per mg | ||||||
| University | 87.3 items per mg | |||||||
| 134.3 items per mg | ||||||||
| 65 items | ||||||||
| Apartments | 3.2 × 103 to 1.1 × 104 MP per m2 per day | |||||||
| Office | Fiber | 5.2 × 103 MP per m2 per day | ||||||
| Deposition into Petri dishes or stainless steel basins | Colombia | Pastry Shop | 10–5000 μm | Fragment | Polyester | 2.3 × 103 MP per m2 per day | 34 | |
| Gift shop | 7.4 × 103 MP per m2 per day | |||||||
| Paint shop | 6.1 × 103 MP per m2 per day | |||||||
| 29 Countriese | Houses/apartments | 50–5000 μm | Fiber | Polyester | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 fibers per m2 per day (HI)f 507 fibers per m2 per day (HI)f |
19 | ||
| Fragment | 1268 fibers per m2 per day (MI)f | |||||||
| Film | PUb | 3518 fibers per m2 per day (LC)f | ||||||
| Fiber | PET | |||||||
| England | Houses | 5–5000 μm | Fragment | PAb | 1414 MP per m2 per day | 43 | ||
| Spheres | PP | |||||||
| Dormitory | 9.9 × 103 MPs per m2 per day | |||||||
| China | University office | 1.8 × 103 MPs per m2 per day | ||||||
| University corridor | 50–5000 μm | Fiber | Polyester | 1.5 × 103 MPs per m2 per day | 32 | |||
| PE | ||||||||
| PSb | ||||||||
| Fiber | PA | 22–6169 fibers per m2 per day | 30 | |||||
| Australia | Houses | 50–5000 μm | Fragment | PACb | ||||
| Film | PVb | |||||||
| Polyester | ||||||||
3.2 Microparticle shapes and sizes
The shapes identified during visual analysis were fibers, fragments, films, foams, pellets, and microspheres (Fig. S1†). Additionally, we observed fiber conglomerations, which could consist of fibers of the same color or be multicolored, as shown in Fig. 3. Due to the compact nature of many of these conglomerations, accurately counting the individual fibers was challenging. Given this, in the present research, these groups of fibers were classified as ‘conglomerates’ and counted as single units.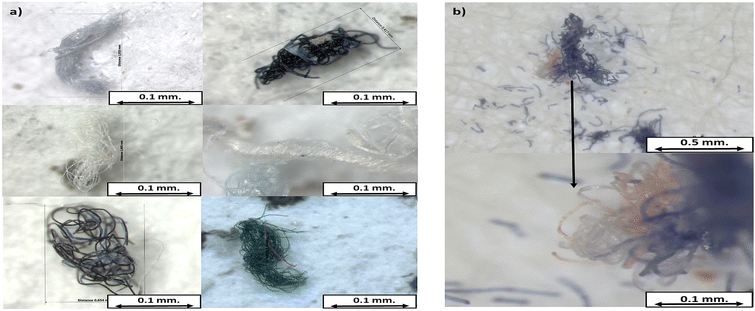 | ||
| Fig. 3 Images of conglomerates obtained with a stereomicroscope: (a) multicolored conglomerates and (b) conglomerate consisting of multicolored fibers. | ||
These clusters have been observed in previous studies, but they were not differentiated from individual fibers.8,16 As seen in Fig. 3, this conglomeration can contain multiple fibers and may release smaller fibers due to weathering. Counting fiber conglomerates as single units could lead to underestimating the total fiber contribution in a sample.
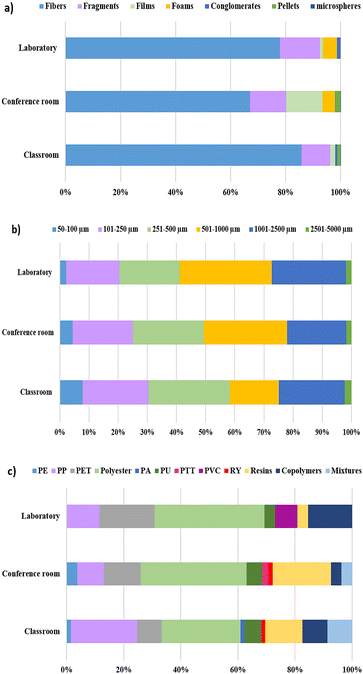
When analyzing the abundance of each shape, we found that fibers were the most prevalent in all sampling sites, contributing the most significant proportion of particles (Fig. 4a). The sampling location with the highest fiber contribution was the laboratory at 85.8%. In comparison, the conference room had the lowest contribution at 67.0%. Following fibers, fragments were the second most predominant shape, contributing between 10.5 and 14.8% in all sampling places. However, the contributions of the fragments and films were very similar in the conference room, with no statistically significant difference (p = 0.99). Although the fragments were the second most abundant shape, ANOVA and the Tukey test indicated that their means abundances differ significantly from fibers (p < 0.05) at all locations. Foams were only observed in the conference room (4.69%) and the laboratory (5.21%). Other shapes, such as pellets and conglomerates, did not contribute more than 1.81% in any studied location. Finally, microspheres were present in all places, but their contribution was minimal (0.13–0.02%).
Our findings regarding shape distribution align with previous studies. Kashfi et al.8 reported that fibers are the predominant typology (85%), followed by fragments (13%) and films (2%) in settled dust samples from various indoor spaces, including a university. A comparable pattern has been observed in dust from schools and other indoor spaces where fibers are consistently the dominant shape, followed by fragments and films (Table 2).16,17,34
The high abundance of fibers in the studied locations may be attributed to their easiness of shedding from different interior items, such as clothes, furniture, and cleaning tools.16 Although none of the sampled spaces had curtains, it is essential to highlight that large curtains, frequently used in such locations, are significant contributors to fiber release.44,45 All indoor garments made of synthetic or semi-synthetic fabrics are potential sources of microfibers.7 Fragments can originate from the breakdown of larger particles from cleaning products, furniture, and plastic containers commonly used in indoor spaces.9 The films contributed with low percentages to the total particle abundance; however, their contribution was similar to fragments in the conference room. The latter may be due to the presence of several plastic-wrapped boxes in the conference room, with films likely obtained from the degradation of this type of packaging material.18 Foams and conglomerates might derive from the abrasion of cleaning tools; however, conglomerates, being groups of fibers, can also originate from textiles. Pellets and microspheres are primary microplastics, usually found in beauty products, such as hand and face cleaners, exfoliants, or plastic resins.46,47 These types of MPs may enter the studied university environments through human use and outdoor sources. The airflow from outdoor into indoor environments can influence the prevalence of indoor MPs.33,48
Regarding the microparticle size, fibers become the predominant shape across all sampling sites as size increases (Fig. S2†). This trend was also observed in Soltani et al.30 and Abad-López et al.34 The fiber size ranged from 50 to 5000 μm in all locations, similar to Dris et al.,16 with the highest abundance in the range of 251–2500 μm. Fragments were the predominant shape in the 50–250 μm range, and their size was found to be between 50 μm and 2500 μm. Films and conglomerates varied between 101 μm and 2500 μm, and foams between 101 μm and 1000 μm. Other shapes, such as microspheres and pellets, were found in the 50–250 μm range.
These results align with the findings on shape abundance. As the size increases, the number of deposited microparticles increases due to larger particles settling faster than smaller particles.15,34 Fibers are predominant at larger sizes. It is to be expected that these were the most abundant shapes overall. Conversely, shapes, such as fragments, are more common at smaller sizes and, therefore, less prevalent in the deposited dust samples.
Size distributions followed a similar trend among all sampling sites (Fig. 4b). The predominant size range was 501–1000 μm across all analyzed samples. These results agree with Dris et al.16 in samples of settled dust. Sharaf Din et al.26 observed the same trend in suspended dust from university environments. In the classroom, however, the prevalent range was 251–500 μm, consistent with Soltani et al.19 The range of 501–1000 μm was the most significant contributor in both the laboratory and conference room. The range with the lowest contribution corresponded to 2501–5000 μm, followed by 50–100 μm in all sampling sites. The slight deviation observed in the classroom may be related to its higher daily occupancy, resulting in increased foot traffic compared to other areas. High foot traffic likely facilitates a constant dust exchange between outdoor and indoor environments via the soles of shoes, potentially affecting the size distribution.17 Additionally, the increased mechanical stress from frequent movement may contribute to the fragmentation of microparticles into smaller sizes.49
These findings suggest a consistent size distribution pattern across settled dust in the different indoor environments. However, these distributions are not identical due to anthropogenic or environmental factors influencing each location. It is important to note that the size ranges reported in the literature for microparticles in dust are highly heterogeneous, which may be attributed to the specific characteristics of each site, as well as the equipment detection limits and sampling methodologies used (Table 2).18,30,50
3.3 Chemical composition of microparticles and MP abundance
μATR-FT-IR analysis revealed that plastics, synthetic, and semi-synthetic polymers constituted most microparticles analyzed, contributing more than 75% of the total microparticles across all sampling locations (Fig. 5). The conference room exhibited the highest contribution at 84.1% (Fig. 5). These findings are consistent with Abad-López et al.34 in Barranquilla, Colombia, who indicated that plastics contributed 41% to 96% to indoor environments. In contrast, other studies reported lower contributions. For example, Dris et al.16 reported that 33% of the fibers analyzed were synthetic polymers, while Soltani et al.30 indicated 39% and Liu et al.13 8%. This suggests that the municipality of Puerto Colombia has a high MP prevalence or that the sample treatment methodology used in this study effectively removed most organic microparticles. As mentioned in earlier studies, it is important to note that none of those performed sample digestion, as was done in this study.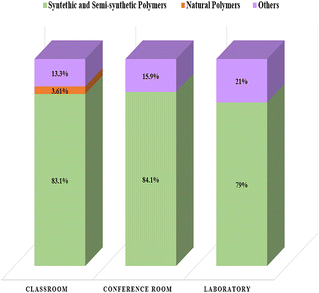 | ||
| Fig. 5 Contribution of MPs to the total number of particles by the sampling location: classroom, conference room, and laboratory. | ||
The abundance of microplastics was also established. The mean MP abundance was 937 MPs per g in the classroom, 803 MPs per g in the conference room, and 1635 MPs per g in the laboratory. Among non-polymer particles, we observed diethanolamines, compounds widely used in the formulation of soaps and surfactants for detergents and cosmetic products,51 and zinc oxides, which may be residues of sample treatment. The classroom was the only location that presented natural polymers, such as cotton, raffia, and ramie, commonly used in the textile industry.
Among the synthetic polymers, eight conventional plastics were identified: polyethylene (PE), polyethylene terephthalate (PET), polypropylene (PP), polyester, polyurethane (PU), polyamide (PA), polytrimethylene terephthalate (PTT) and polyvinyl chloride (PVC). In addition, rayon (RY), high-modified cellulose, polymer resins, polymer mixtures, and copolymers were observed. Polyester, PP, and PET were the most abundant synthetic polymers across all samples, contributing 32.7%, 17%, and 12.2%, respectively. Polyester was the dominant plastic in all sampling locations, contributing 27.5% in the classroom and 38.5% in both the laboratory and conference room (Fig. 4c). It is essential to highlight that polymer resins contributed more than PET in the classroom (13%), while in the conference room, their presence was higher than PP (21.2%). Copolymers also contributed more than PP in the laboratory (15.4%) (Fig. 4c).
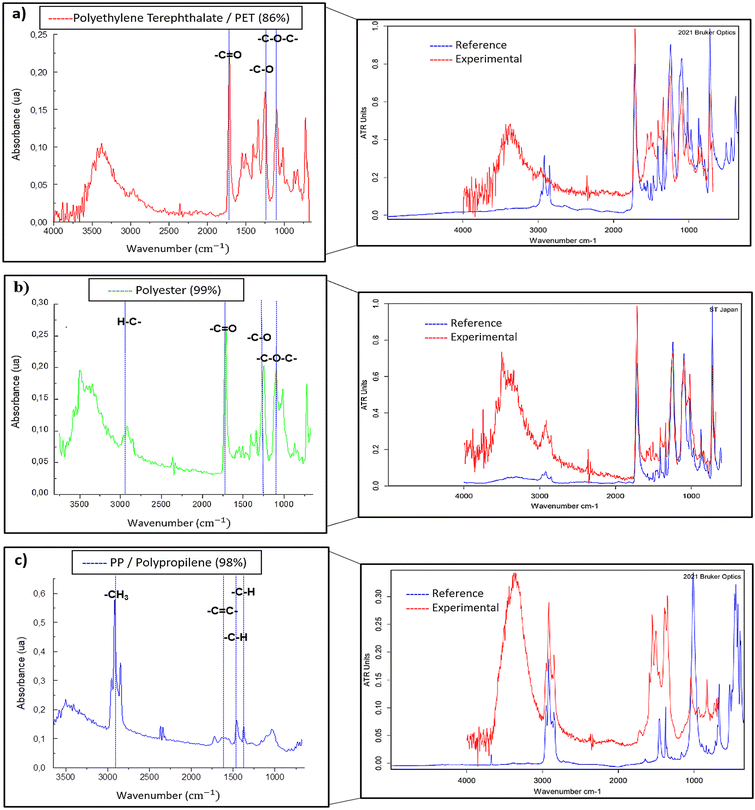
Some representative polymer spectra of PET, polyester, and PP are shown in Fig. 6, with characteristic bands identified for each polymer. For example, the band at 1700–1750 cm−1 related to the stretching of the ester group bond –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, is observed in both PET and polyester. Since polyester is the fibrous form of PET predominantly used in textiles,52 the μATR-FT-IR spectra of these two polymers are expected to be similar. However, physical and chemical modifications during fabrication may introduce spectral variations. Slight differences in the intensity of the C–H stretching bands around 3000–2800 cm−1 are apparent (Fig. 6a and b), which may be associated with the differing molecular orientation of polyester fibers compared to PET.53,54 Additionally, a band at 2950–2970 cm−1, corresponding to –CH3 groups, is observed in PP (Fig. 6c). All spectra exhibited bands around 3250–3500 cm−1. The latter bands are likely related to the presence of OH− from carboxylic acids or alcohol groups formed during weathering through UV exposure.24,55
O, is observed in both PET and polyester. Since polyester is the fibrous form of PET predominantly used in textiles,52 the μATR-FT-IR spectra of these two polymers are expected to be similar. However, physical and chemical modifications during fabrication may introduce spectral variations. Slight differences in the intensity of the C–H stretching bands around 3000–2800 cm−1 are apparent (Fig. 6a and b), which may be associated with the differing molecular orientation of polyester fibers compared to PET.53,54 Additionally, a band at 2950–2970 cm−1, corresponding to –CH3 groups, is observed in PP (Fig. 6c). All spectra exhibited bands around 3250–3500 cm−1. The latter bands are likely related to the presence of OH− from carboxylic acids or alcohol groups formed during weathering through UV exposure.24,55
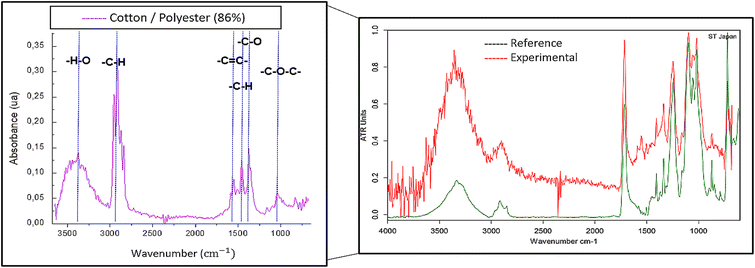
It is essential to mention that we also found mixtures of polymers among the obtained spectra. These mixtures were grouped into different categories since obtaining an accurate quantitative composition from the samples was impossible with μATR-FT-IR due to the inhomogeneity of mixed fibers.55 Therefore, the principal component remained unknown. Among the polymer mixtures, we identified cotton/polyester, polyester/rayon, and cotton/nylon/polyester fibers. In Fig. 7, one example of these multiple-component microparticles is shown. The spectrum exhibited bands around 3375 cm−1 and 2919 cm−1, corresponding to OH− stretching and –CH symmetric stretching, respectively, from cotton. Additionally, there are bands at 1545 cm−1 from –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic ring stretching, and 1350 cm−1 from ester's –C–O stretching related to the polyester.
C aromatic ring stretching, and 1350 cm−1 from ester's –C–O stretching related to the polyester.
The findings in this study are consistent with previous research on microplastics in indoor dust. Zhu et al.18 found that PE, PP, and polyester were the largest contributors to all polymers analyzed, with PE and PP being the most predominant in university classrooms. Other investigations on settled and suspended dust also concluded that PE, polycarbonate (PC), PP, PET, PA, polystyrene (PS), and polyester were the predominant polymers in various indoor locations, reinforcing what was previously stated.8,16 This shows that PET, PP, and polyester consistently appear more significantly across different studies. This aligns with global plastic production, where PP and PET are among the most produced plastics, which explains their high prevalence.26
The notable contributions of polyester, PET, and PP in the dust deposits analyzed may be related to the extensive use of these polymers in textile manufacturing.56 Considering that most observed polymers were fibers, it can be suggested that textiles are a significant source of MPs in settled dust from Puerto Colombia. On the other hand, PP is widely used to manufacture desks, chairs, sofas, and other furniture.57 As seen in Fig. 4, PP contributed more in the classroom than in the other studied sites, possibly due to the more oversized furniture in this space compared to the conference room and the laboratory. PET and PP are used in common-use product production, such as food packaging, water bottles, and wrappers, among others, which appear as potential sources of MPs in indoor environments.26
Polymer resins made an essential contribution in the classroom and the conference room. Among the resins observed, alkyd resins were the most common. These resins are widely used in wall paints, thinners, and metallic and surface coatings.31 Since polymer resins were recurrent among the fragments, it is suggested that fragments of paints from walls or furniture in interior spaces are a potential source of MPs.
Due to the absence of a comprehensive database that chemically characterizes all the furniture and artifacts present at each sampling location and considering the contribution of outdoor environments to indoor dust, identifying the specific sources that contribute to the prevalence of MPs in dust deposits in Puerto Colombia becomes a challenge. Therefore, knowing the composition of MPs provides an overview of the possible sources of these emerging pollutants.
3.4 Human exposure to microparticles and possible risks
In recent years, concerns about human exposure to anthropogenic microparticles, including MPs, have increased.8 This research calculated the Estimated Daily Intake (EDI) for teenagers and adults in various university environments (Table 3). The mean EDI was 0.47 microparticles per kg – bw per day and 0.37 microparticles per kg – bw per day for teenagers and adults, respectively. For teenagers, the EDI ranged between 0.32 and 0.70 microparticles per kg – bw per day, while for adults, it ranged between 0.26 and 0.56 microparticles per kg – bw per day. These EDI values reflect higher ingestion exposure in teenagers than adults, attributable to their lower body weight.| Age groups | Sampling Location | Factors | |||||
|---|---|---|---|---|---|---|---|
| C P (microparticles per g) | W (kg) | IRb (g per day) | f | EDI (microparticles per kg – bw per day) | Mean EDI (microparticles per kg – bw per day) | ||
| a U.S. EPA.28 b Jhonson-Restrepo and Kannan.29 | |||||||
| Teenagers | Classroom | 1141 | 52 | 0.02 | 0.88 | 0.39 | 0.47 ± 0.20 |
| Conference room | 955 | 0.32 | |||||
| Laboratory | 2070 | 0.70 | |||||
| Adults | Classroom | 1141 | 65 | 0.02 | 0.88 | 0.30 | 0.37 ± 0.13 |
| Conference room | 955 | 0.26 | |||||
| Laboratory | 2070 | 0.50 | |||||
Our EDI results exceed those reported in previous research on settled dust from universities and schools.8,17 For example, Zhu et al.18 reported an EDI of 0.22 MPs per kg – bw per day for university students and 0.23 MPs per kg – bw per day for adults in China. Similarly, Kasfhi et al.8 found lower EDIs in Iranian Universities, with values ranging from 0.029 to 0.034 MPs per kg – bw per day for adolescents and 0.02 to 0.029 MPs per kg – bw per day for adults. These findings highlight the regional variability in MP exposure, likely influenced by differences in environmental conditions and anthropogenic activity patterns. The high values of the EDI presented in this study underscore settled dust as an essential route of exposure to MPs and other microparticles in university indoor environments in Puerto Colombia. It should also be noted that ingesting MPs through food, such as vegetables and fruits, and drinking water further contributes to overall exposure.58,59
In the dust samples analyzed, PET, PP, and polyester were the predominant plastics. Ingested PET can undergo biotransformation in the human digestive tract and colon, altering the structure of the microbiota.60,61 PET nanoplastics can alter cell physiology, increasing the toxic effect of lung carcinoma cells (A549 cells) and affecting mitochondrial integrity.10,60,62 Although nanoparticles were not detected in this study due to equipment limitations, the presence of polymers suggests that nanoplastics might be present. On the other hand, PP can damage the liver by causing pyroptosis, oxidative damage, and lipid peroxidation, and it is related to the production of reactive oxygen species.60
When discussing toxicity of MPs, it is essential to account for other agents, such as additives and pollutants that adhere to surfaces of MPs, contributing to the inflammatory response.10,11 For example, bisphenol-A (BPA), a commonly found plasticizer in PET and polyester, is considered estrogenic due to its hormone-disrupting properties in humans, which are associated with obesity, cardiovascular disease, reproductive disorders, and breast cancer.10,63 Additionally, BPA is linked to a higher risk of allergic diseases through prenatal exposure.59,64 Flame retardants frequently added to PP are associated with diabetes, neurobehavioral and developmental disorders, cancer, reproductive health effects, and impaired thyroid function. Furthermore, MPs can carry pathogenic microbial communities on their surface due to their larger surface area.65
Weathering of the MPs is another factor that should be addressed when discussing toxicity of MPs and health risks. Some μATR-FT-IR spectra from this study indicated signs of MP weathering. Although the relationship between the level of weathering of MPs and toxicity remains unclear, a recent study reported that weathered MPs exhibited a more severe inflammatory response in human brain-derived microglial cell lines compared to non-weathered MPs.66,67 Weathering also increases the adsorption capacity of pollutants and microorganisms on the surface of MPs, thereby affecting their toxicity.10,68 Additionally, prolonged aging of MPs can substantially increase their cytotoxicity due to heightened levels of reactive oxygen species.10 These points underscore the relevance of further investigation on aging MPs.
Although MPs were the most abundant microparticles, others, such as cotton, were also observed, and their potential health risks should be addressed. Cotton is bio-persistent because it contains a large amount of cellulose, which has an estimated half-life of up to 3 years in rats and mice. In addition, it has been associated with asthma, bronchitis, and byssinosis.69 This study also calls for addressing the role of toxicity of multi-component MPs in human health since the information on this topic is still scarce.
According to our findings and existing literature, the classroom, the conference room and the laboratory analyzed in this study represent significant environments for ingesting MP and non-MP microparticles, posing potential and concerning health risks. Future research should focus on estimating the health risks associated with weathered and multi-component MPs.
4 Conclusions
This is one of the first research studies in Colombia to address the abundance, shape, and chemical composition of microplastics (MPs) in indoor university environments. Our findings indicate the laboratory had the highest mean abundance of microparticles (2070 microparticles per g) and MPs (1635 MPs per g). The lack of a linear correlation between temperature, relative humidity, and microparticle abundance suggests insufficient evidence to affirm that these factors play a primary role in microparticle deposition. Still, other variables, like airflow, might have a more substantial influence.Fibers were the most common shape across sampling sites, particularly in larger size ranges (500–2500 μm), while fragments were more common in smaller size ranges (50–250 μm). The size range with the highest contribution was between 501 μm and 1000 μm. Additionally, conglomerates were suggested as a new shape category. Moreover, μATR-FT-IR analysis revealed that polymers, such as polyester, PP, and PET, were the most abundant microparticles. Multi-component polymers were also identified but classified separately due to indeterminate predominant components. Weathering signs were also observed on the MPs.
EDI calculations revealed mean exposures of 0.47 microparticles per kg – bw per day for teenagers and 0.37 microparticles per kg – bw per day for adults. These high EDI values highlight settled dust in indoor university spaces from Puerto Colombia as an essential route to anthropogenic microparticles, posing potential health risks. When discussing toxicity of MPs, weathering and multi-component polymers should be considered, as weathering MPs, for example, may aggravate the adverse effects of MPs on health.
Overall, this study provides a valuable baseline for future research and highlights the need to develop targeted mitigation strategies to address MP exposure in indoor environments, public health, and environmental protection.
5 Limitations of the study and recommendations for future research
There are still many gaps related to research on MPs in settled dust and the urban environment. The lack of consensus on units and the standardization of a methodology for sample treatment hinder the comparison of results, making it challenging to understand the general behavior and magnitude of microplastic pollution. Additionally, there is limited knowledge about transport and suspension mechanisms of MPs and their relationship with environmental variables in indoor environments. Regarding health risks, expanding the research on the effects of multi-component and weathered MPs on human health is essential. Therefore, this study encourages future research to take into account the following considerations:• Including larger data sets for statistical analyses: although our study represents a valuable preliminary approach to understanding the dynamics between microparticle concentration and climatic factors, the absence of linear correlations is insufficient evidence to conclude that temperature and relative humidity do not influence microparticle abundance. Therefore, a more significant number of samples to assess these meteorological factors, as well as other variables, like airflow, that were not considered in this study, would expand our knowledge of the influence of these factors on the transport mechanisms of MP and non-MP microparticles.
• Studying microparticles <50 μm: due to limitations on size analysis, only ≥50 μm particles were assessed; therefore, we may be underestimating the contribution of some shapes in the size range <50 μm. Various studies have reported the presence of microparticles <50 μm in indoor environments.8,43 Additionally, nanoparticles have also been observed in settled dust and have been gaining special attention due to their higher health risks.70 Future studies focusing on these smaller microparticles and nanoparticles would contribute to a better understanding of their impact on human health.
• Broadening analysis on multi-component and weathered MPs: this study identified multi-component MPs in settled dust; however, μATR-FT-IR could not provide accurate quantitative composition from the samples. Multi-component MPs raise essential questions: How does an MP with polyester and cotton affect health compared to one with only polyester? Would this impact depend on the proportion of these polymers? Future research might consider using pyrolysis-gas chromatography-mass spectrometry (pyr-GC-MS), a technique known for its accuracy in breaking down and identifying polymers by fragments, potentially offering a better understanding of multi-component MPs.71 Additionally, detailed information is lacking, while weathered MPs are known to increase toxicity. Investigating the chemical composition of polymer mixtures and the chemical and physical changes in weathered MPs may provide valuable insights into their adverse health effects.
This research urges the scientific community to standardize concentration units and sample treatment methodologies for reporting microplastics in dust to improve the comparability of results and support the development of effective public health strategies.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
M. G. A. V.: writing – review & editing, writing – original draft, methodology, investigation, formal analysis, visualization, conceptualization. V. A. A.: writing – review & editing, resources, project administration, investigation, funding acquisition, data curation, conceptualization. C. D. G.-T.: writing – review & editing, supervision, methodology, investigation, formal analysis.Conflicts of interest
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: V. A. A. reports that financial support, equipment, and supplies were provided by Fondo De Ciencia, Tecnología e Innovación del Sistema General de Regalías and Ministerio de Ciencia Tecnología e Innovación (Minciencias). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by Fondo De Ciencia, Tecnología e Innovación del Sistema General de Regalías – Colombia (FCTeI-SGR), grant BPIN 2020000100065, Ministerio de Ciencia Tecnología e Innovación (Minciencias) – Colombia, grant 1116-852-72530 (agreement 80740-485-2020) and the Universidad del Atlántico.References
- S. Dehghani, F. Moore and R. Akhbarizadeh, Microplastic pollution in deposited urban dust, Tehran metropolis, Iran, Environ. Sci. Pollut. Res. Int., 2017, 24(25), 20360–20371 CrossRef CAS PubMed.
- Plastics Europe, https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/, accessed 23 December 2023 Search PubMed.
- F. Wang, Z. Lai, G. Peng, L. Luo, K. Liu and X. Huang, et al., Microplastic abundance and distribution in a Central Asian desert, Sci. Total Environ., 2021, 800, 149529, DOI:10.1016/j.scitotenv.2021.149529.
- J. P. G. L. Frias and R. Nash, Microplastics: finding a consensus on the definition, Mar. Pollut. Bull., 2019, 138, 145–147 CrossRef CAS PubMed.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor and A. E. Daugaard, et al., Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris, Environ. Sci. Technol., 2019, 53(3), 1039–1047 CrossRef CAS PubMed.
- M. B. Alfonso, A. H. Arias, A. C. Ronda and M. C. Piccolo, Continental microplastics: presence, features, and environmental transport pathways, Sci. Total Environ., 2021, 799, 149447, DOI:10.1016/j.scitotenv.2021.149447.
- R. M. Wagterveld, J. C. M. Marijnissen, L. Gradoń and A. Moskal, Synthetic Nano- and Microfibers, Glasstree, 2020 Search PubMed.
- F. S. Kashfi, B. Ramavandi, H. Arfaeinia, A. Mohammadi, R. Saeedi, G. E. De-la-Torre and S. Dobaradaran, Occurrence and exposure assessment of microplastics in indoor dusts of buildings with different applications in Bushehr and Shiraz cities, Iran, Sci. Total Environ., 2022, 829, 8154651, DOI:10.1016/j.scitotenv.2022.154651.
- Q. Wang, C. E. Enyoh, T. Chowdhury and A. H. Chowdhury, Analytical techniques, occurrence and health effects of micro and nano plastics deposited in street dust, Int. J. Environ. Anal. Chem., 2020, 102(18), 6435–6453 CrossRef.
- A. P. Abad López, J. Trilleras, V. A. Arana, L. S. Garcia-Alzate and C. D. Grande-Tovar, Atmospheric microplastics: exposure, toxicity, and detrimental health effects, RSC Adv., 2023, 13(11), 7468–7489 RSC.
- J. C. Prata, Airborne microplastics: consequences to human health?, Environ. Pollut., 2018, 234, 115–126 CrossRef CAS PubMed.
- Y. B. Zhao, P. P. Gao and H. G. Ni, A chemical time bomb: Future risks of microplastics, Water, Air, Soil Pollut., 2019, 230(11) DOI:10.1007/s11270-019-4320-9.
- C. Liu, J. Li, Y. Zhang, L. Wang, J. Deng and Y. Gao, et al., Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure, Environ. Int., 2019, 128, 116–124 CrossRef CAS PubMed.
- Z. Liao, X. Ji, Y. Ma, B. Lv, W. Huang and X. Zhu, et al., Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China, J. Hazard. Mater., 2021, 417, 126007, DOI:10.1016/j.jhazmat.2021.126007.
- M. A. Bhat, Airborne microplastic contamination across diverse university indoor environments: a comprehensive ambient analysis, Air Qual., Atmos. Health, 2024, 17, 1851–1866, DOI:10.1007/s11869-024-01548-9.
- R. Dris, J. Gasperi, C. Mirande, C. Mandin, M. Guerrouache, V. Langlois and B. Tassin, A first overview of textile fibers, including microplastics, in indoor and outdoor environments, Environ. Pollut., 2017, 221, 453–458 CrossRef CAS PubMed.
- M. J. Nematollahi, F. Zarei, B. Keshavarzi, M. Zarei, F. Moore, R. Busquets and F. J. Kelly, Microplastic occurrence in settled indoor dust in schools, Sci. Total Environ., 2022, 807, 150984, DOI:10.1016/j.scitotenv.2021.150984.
- J. Zhu, X. Zhang, K. Liao, P. Wu and H. Jin, Microplastics in dust from different indoor environments, Sci. Total Environ., 2022, 833, 155256, DOI:10.1016/j.scitotenv.2022.155256.
- N. S. Soltani, M. P. Taylor and S. P. Wilson, International quantification of microplastics in indoor dust: prevalence, exposure and risk assessment, Environ. Pollut., 2022, 312, 119957, DOI:10.1016/j.envpol.2022.119957.
- Mi municipio: Información del municipio, https://www.puertocolombiaatlantico.gov.co/MiMunicipio/Paginas/Informacion-delMunicipio.aspx, accessed 18 October 2022 Search PubMed.
- DANE, Censo Nacional de Población y Vivienda 2018, https://www.dane.gov.co/index.php/estadisticas-portema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018, accessed 18 October 2022 Search PubMed.
- U.S. EPA, Guidance on Choosing a Sampling Design for Environmental Data Collection, https://www.epa.gov/sites/default/files/2015-06/documents/g5s-final.pdf, accessed 3 July 2024 Search PubMed.
- S. Abbasi, B. Keshavarzi, F. Moore, A. Turner, F. J. Kelly, A. O. Dominguez and N. Jaafarzadeh, Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran, Environ. Pollut., 2019, 244, 153–164 CrossRef CAS PubMed.
- L. Cai, J. Wang, J. Peng, Z. Tan, Z. Zhan, X. Tan and Q. Chen, Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence, Environ. Sci. Pollut. Res. Int., 2017, 24(32), 24928–24935 CrossRef PubMed.
- C. B. Crawford and B. Quinn, Microplastic Pollutants: Microplastics, Standardisation and Spatial Distribution, Elsevier, 2017 Search PubMed.
- K. Sharaf Din, M. F. Khokhar, S. I. Butt, A. Qadir and F. Younas, Exploration of microplastic concentration in indoor and outdoor air samples: morphological, polymeric, and elemental analysis, Sci. Total Environ., 2024, 908, 168398, DOI:10.1016/j.scitotenv.2023.168398.
- Y. D. Lin, P. H. Huang, Y. W. Chen, C. W. Hsieh, Y. L. Tain and B. H. Lee, et al., Sources, degradation, ingestion and effects of microplastics on humans: a review, Toxics, 2023, 11(9), 747 CrossRef CAS PubMed.
- U.S. EPA, Update for chapter 5 of the exposure factors handbook: soil and dust ingestion, https://www.epa.gov/sites/default/files/2018-01/documents/efh-chapter05_2017.pdf, accessed 6 February 2024 Search PubMed.
- B. Johnson-Restrepo and K. Kannan, An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States, Chemosphere, 2009, 76(4), 542–548 CrossRef CAS PubMed.
- N. S. Soltani, M. P. Taylor and S. P. Wilson, Quantification and exposure assessment of microplastics in Australian indoor house dust, Environ. Pollut., 2021, 283, 117064, DOI:10.1016/j.envpol.2021.117064.
- A. Pratiwi, A. D. Syafei, A. F. Assomadi, R. Boedisantoso and J. Hermana, Microplastic characterization based on the number of occupants, Presented in Part at International Conference on Science and Applied Science (ICSAS2020), 2020 Search PubMed.
- Q. Zhang, Y. Zhao, F. Du, H. Cai, G. Wang and H. Shi, Microplastic fallout in different indoor environments, Environ. Sci. Technol., 2020, 54(11), 6530–6539 CrossRef CAS PubMed.
- Y. Chen, X. Li, X. Zhang, Y. Zhang, W. Gao, R. Wang and D. He, Air conditioner filters become sinks and sources of indoor microplastics fibers, Environ. Pollut., 2022, 292(Pt B), 118465, DOI:10.1016/j.envpol.2021.118465.
- A. P. Abad-López, K. K. Orozco-Pérez, V. A. Arana and C. D. Grande-Tovar, Microplastics suspended in dust from different indoor environments in Barranquilla, Colombia: predominant microparticles?, Environ. Pollut., 2024, 350, 124023, DOI:10.1016/j.envpol.2024.124023.
- American Society of Heating, Refrigerating and Air-Conditioning Engineers, ASHRAE Handbook: Fundamentals, ASHRAE, 2017 Search PubMed.
- A. Serrano-Jiménez, J. Lizana, M. Molina-Huelva and Á. Barrios-Padura, Indoor environmental quality in social housing with elderly occupants in Spain: Measurement results and retrofit opportunities, J. Build. Eng., 2020, 30, 101264, DOI:10.1016/j.jobe.2020.101264.
- L. Su, B. Nan, N. J. Craig and V. Pettigrove, Temporal and spatial variations of microplastics in roadside dust from rural and urban Victoria, Australia: implications for diffuse pollution, Chemosphere, 2020, 252, 126567, DOI:10.1016/j.chemosphere.2020.126567.
- K. Liu, X. Wang, T. Fang, P. Xu, L. Zhu and D. Li, Source and potential risk assessment of suspended atmospheric microplastics in Shanghai, Sci. Total Environ., 2019, 675, 462–471 CrossRef CAS PubMed.
- K. Szewc, B. Graca and A. Dołęga, Atmospheric deposition of microplastics in the coastal zone: characteristics and relationship with meteorological factors, Sci. Total Environ., 2021, 761, 143272, DOI:10.1016/j.scitotenv.2020.143272.
- Y. Huang, T. He, M. Yan, L. Yang, H. Gong and W. Wang, et al., Atmospheric transport and deposition of microplastics in a subtropical urban environment, J. Hazard. Mater., 2021, 416, 126168, DOI:10.1016/j.jhazmat.2021.126168.
- J. Zhang, L. Wang and K. Kannan, Microplastics in house dust from 12 countries and associated human exposure, Environ. Int., 2020, 134, 105314, DOI:10.1016/j.envint.2019.105314.
- I. Bahrina, A. Syafei and R. Satoto, et al., An occupant-based overview of microplastics in indoor environments in the city of surabaya, Indonesia, J. Ecol. Eng., 2020, 21(8), 236–242 CrossRef.
- L. C. Jenner, L. R. Sadofsky, E. Danopoulos and J. M. Rotchell, Household 969 indoor microplastics within the Humber region (United King-970 dom): quantification and chemical characterisation of parti-971 cles present, Atmos. Environ., 2021, 259, 118512, DOI:10.1016/j.atmosenv.2021.118512.
- A. P. Periyasamy and A. Tehrani-Bagha, A review on microplastic emission from textile materials and its reduction techniques, Polym. Degrad. Stab., 2022, 199, 109901, DOI:10.1016/j.polymdegradstab.2022.109901..
- P. K. Noorimotlagh, S. A. Hopke and S. A. Mirzaee, A systematic review of airbone microplastics emissions as emerging contaminants in outdoor and indoor airenvironments, Emerging Contam., 2024, 10(4), 100372, DOI:10.1016/j.emcon.2024.100372.
- V. V. Narmadha, J. Jose, S. Patil, M. O. Farooqui, B. Srimuruganandam, S. Saravanadevi and K. Krishnamurthi, Assessment of microplastics in roadside suspended dust from urban and rural environment of Nagpur, India, Int. J. Environ. Res., 2020, 14(6), 629–640 CrossRef CAS.
- P. Singh, G. Varshney and R. Kaur, Primary microplastics in the ecosystem: ecological effects, risks, and comprehensive perspectives on toxicology and detection methods, J. Environ. Sci. Health, Part C, 2024, 42(4), 314–365 CrossRef CAS PubMed.
- S. O'Brien, C. Rauert and F. Ribeiro, et al., There's something in the air: a review of sources, prevalence and behaviour of microplastics in the atmosphere, Sci. Total Environ., 2023, 874, 162193, DOI:10.1016/j.scitotenv.2023.162193.
- A. L. Andrady, P. W. Barnes and J. F. Bornman, et al., Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation, Sci. Total Environ., 2022, 851(Pt 2), 158022, DOI:10.1016/j.scitotenv.2022.158022.
- L. F. Amato-Lourenço, L. Dos Santos Galvão, H. Wiebeck, R. Carvalho-Oliveira and T. Mauad, Atmospheric microplastic fallout in outdoor and indoor environments in São Paulo megacity, Sci. Total Environ., 2022, 821, 153450, DOI:10.1016/j.scitotenv.2022.153450.
- International Agency for Research on Cancer (IARC), Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-water, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 101, IARC, 2012 Search PubMed.
- P. Piskuła and A. M. Astel, Microplastics in commercial fishes and by-catch from selected FAO major fishing areas of the southern Baltic Sea, Animals, 2023, 13(3), 458 CrossRef PubMed.
- K. C. Cole, J. Guèvremont, A. Ajji and M. M. Dumoulin, Orientation and Conformation in PET: New Information from Specular Reflection FT-IR, Springer, Berlin/Heidelberg, 1997 Search PubMed.
- S. A. Jabarin, Optical properties of thermally crystallized poly(ethylene terephthalate), Polym. Eng. Sci., 1982, 22(13), 815–820, DOI:10.1002/pen.760221305.
- A. L. Andrady, The plastic in microplastics: a review, Mar. Pollut. Bull., 2017, 119(1), 12–22 CrossRef CAS PubMed.
- P. Peets, I. Leito, J. Pelt and S. Vahur, Identification and classification of textile fibres using ATR-FT-IR spectroscopy with chemometric methods, Spectrochim. Acta, Part A, 2017, 173, 175–181 CrossRef CAS PubMed.
- S. O'Brien, E. D. Okoffo, C. Rauert, J. W. O'Brien, F. Ribeiro and S. D. Burrows, et al., Quantification of selected microplastics in Australian urban road dust, J. Hazard. Mater., 2021, 416, 125811, DOI:10.1016/j.jhazmat.2021.125811.
- X. Zhai, H. Zheng, Y. Xu, R. Zhao, W. Wang and H. Guo, Characterization and quantification of microplastics in indoor environments, Heliyon, 2023, 9, e15901, DOI:10.1016/j.heliyon.2023.e15901.
- E. Winiarska, M. Jutel and M. Zemelka-Wiacek, The potential impact of nano- and microplastics on human health: understanding human health risks, Environ. Res., 2024, 251, 118535, DOI:10.1016/j.envres.2024.118535.
- N. Ali, J. Katsouli, E. L. Marczylo, T. W. Gant, S. Wright and J. Bernardino de la Serna, The potential impacts of micro-and-nano plastics on various organ systems in humans, EBioMedicine, 2024, 99, 104901, DOI:10.1016/j.ebiom.2023.104901.
- A. Tamargo, N. Molinero and J. J. Reinosa, et al., PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion, Sci. Rep., 2022, 12, 528, DOI:10.1038/s41598-021-04713-6.
- H. Zhang, S. Zhang, Z. Duan and L. Wang, Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro, Environ. Int., 2022, 162, 107177, DOI:10.1016/j.envint.2022.107177.
- S. Horn, K. M. Mölsä, J. Sorvari, H. Tuovila and P. Heikkilä, Environmental sustainability assessment of a polyester T-shirt - Comparison of circularity strategies, Sci. Total Environ., 2023, 884, 163821, DOI:10.1016/j.scitotenv.2023.163821.
- A. Zhou, H. Chang and W. Huo, et al., Prenatal exposure to bisphenol A and risk of allergic diseases in early life, Pediatr. Res., 2017, 81(6), 851–856 CAS.
- H. K. Ageel, S. Harrad and M. A.-E. Abdallah, Occurrence, human exposure, and risk of microplastics in the indoor environment, Environ. Sci.:Processes Impacts, 2022, 24(1), 17–31 RSC.
- S. Burrows, J. Colwell and S. Costanzo, et al., UV sources and plastic composition influence microplastic surface degradation: Implications for plastic weathering studies, J. Hazard. Mater. Adv., 2024, 14, 100428, DOI:10.1016/j.hazadv.2024.100428.
- H.-Y. Kim, J. Ashim and S. Park, et al., A preliminary study about the potential risks of the UV-weathered microplastic: the proteome-level changes in the brain in response to polystyrene derived weathered microplastics, Environ. Res., 2023, 233, 116411, DOI:10.1016/j.envres.2023.116411.
- J. N. Hanun, F. Hassan and J.-J. Jiang, Occurrence, fate, and sorption behavior of contaminants of emerging concern to microplastics: influence of the weathering/aging process, J. Environ. Chem. Eng., 2021, 9(5), 106290, DOI:10.1016/j.jece.2021.106290.
- S. Wieland, A. Balmes, J. Bender, J. Kitzinger, F. Meyer and A. F. Ramsperger, et al., From properties to toxicity: comparing microplastics to other airborne microparticles, J. Hazard. Mater., 2022, 428, 128151, DOI:10.1016/j.jhazmat.2021.128151.
- L. Tian, E. Skoczynska, D. Siddhanti, R.-J. van Putten, H. A. Leslie and G.-J. M. Gruter, Quantification of polyethylene terephthalate microplastics and nanoplastics in sands, indoor dust and sludge using a simplified in-matrix depolymerization method, Mar. Pollut. Bull., 2022, 175, 113403, DOI:10.1016/j.marpolbul.2022.113403.
- C. Yang, S. Niu, Y. Xia and J. Wu, Microplastics in urban road dust: sampling, analysis, characterization, pollution level, and influencing factors, TrAC, Trends Anal. Chem., 2023, 168, 117348, DOI:10.1016/j.trac.2023.117348.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ea00139g |
| This journal is © The Royal Society of Chemistry 2025 |