Open Access Article
Open Access ArticleAdvanced research on electrolytes for regulating the SEI in high-performance LMBs
Huaping
Wang
and
Hailong
Wang
 *
*
Advanced Energy Storage Materials and Devices Lab, School of Materials and New Energy, Ningxia University, Yinchuan, 750021 China. E-mail: wanghailong@nxu.edu.cn
First published on 12th February 2025
Abstract
Rechargeable lithium metal batteries (LMBs) with a lithium anode have attracted wide attention as next-generation energy storage systems due to their high specific energy density and wide operating voltage range. Electrolytes not only facilitate the transport of lithium ions but also play an important role in the formation of the solid electrolyte interphase (SEI) on the lithium anode surface, which affects the coulombic efficiency of LMBs. To date, extensive research on electrolyte modification has been devoted to adjusting the structure and composition of the SEI to inhibit dendrite growth on the rechargeable lithium anode. In order to guide further research on electrolytes, this paper summarizes the latest advancements in electrolytes for lithium metal anodes, with an emphasis on constructing a stable SEI on the lithium anode surface. Finally, a perspective on the future development of LMBs is proposed, which will help guide researchers in designing advanced LMBs.
Broader contextThe solid electrolyte interphase (SEI) is formed in various energy storage batteries, including lithium (Li), sodium (Na), potassium (K), and magnesium (Mg) ion batteries. It is a primary factor contributing to the low coulombic efficiency of the anode and is the key to controlling ion diffusion on the anode surface. Enhancing the electronic insulation and ionic conductivity of the SEI can effectively address these issues and improve the electrochemical performance of metallic anode energy storage batteries. This review provides a comprehensive summary of advanced electrolytes employed to modulate the structure and phase composition of the SEI. Additionally, it addresses a key question: is an inorganic-rich interface or an organic-rich interface more favorable for lithium metal anodes? This offers a promising pathway for designing SEIs that can effectively mitigate lithium dendrite formation in future applications. |
Introduction
Lithium-ion batteries (LIBs) with the highest energy density currently cannot meet the growing demands for power density and capacity density in power batteries, due to the relatively low energy density of graphite anodes (372 mA h g−1).1 On the contrary, rechargeable lithium metal batteries (LMBs) with a metallic lithium (Li) anode have been regarded as the “holy grail” for energy storage.2 Li metal is considered the most ideal anode material due to its high theoretical specific capacity (3860 mA h g−1) and the lowest redox potential (−3.04 V vs. SHE).3 However, the Li metal anode suffers from substantial challenges in cycle life, safety and sustainability, which hinder its practical applications.4 The main reason for the low lifespan of the Li metal anode (LMA) is uncontrolled dendrite growth during repeated deposition/stripping processes, leading to complex side reactions and the risk of diaphragm puncture.5 Therefore, in order to achieve practical LMBs, it is crucial to suppress the growth of Li dendrites.Various methods have been proposed to suppress Li dendrites, such as current collector modification, alloy arrays, solid-state electrolytes, electrolyte engineering and electrochemical artificial SEI.6 After extensive research and discussion, it is widely recognized that electrolyte engineering plays an important role in stabilizing the LMA.7 This is because the physicochemical properties of the electrolyte not only control the migration behaviour of Li ions but also affect the properties of the solid electrolyte interphase (SEI) formed by the reaction between the electrolyte and Li metal on the anode surface.8 The SEI, as a protective layer, can block further parasitic reactions between the electrolyte and Li metal and prevent the growth of Li dendrites.9 Constructing a suitable SEI is necessary for the LMA to achieve long cycle life and safety.10 An ideal SEI should satisfy the following requirements: (1) it must be ion-conducting and electron-insulating; (2) it should have high surface energy and mechanical strength; (3) it must tolerate the huge volume changes of the LMA; and (4) it should have a homogeneous morphology and structure.11 Regulating the structure and composition of the SEI through electrolyte engineering to meet the above requirements is the simplest and most efficient method.
In this review, we summarize recent advanced research on electrolyte engineering. We briefly introduce the formation mechanism of the SEI on the surface of the LMA and the application history of liquid electrolytes in Li battery systems. Then, we summarize the electrolyte chemistry on the surface of the LMA, as well as the mechanisms for regulating the structure and composition of the SEI to suppress Li dendrites. Finally, we discuss and forecast the future development directions of liquid electrolytes. We hope that this review will promote the investigation of advanced electrolytes for high electrochemical performance LMBs.
Formation mechanism of the SEI on the LMA surface
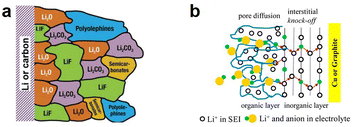
The electrolyte is an important component of LMBs, which is not only used to connect the anode and cathode but also serves as a medium for dissolving Li ions. However, it faces serious challenges on the surface of LMA. Due to its high reducibility, a spontaneous reduction reaction occurs when the LMA contacts the liquid electrolytes, forming a passive layer on the LMA surface, which is known as the SEI.12 The composition, structure, and physicochemical properties of the SEI are closely related to the electroplating morphology of Li ions on the LMA surface. Goodenough et al. proposed a formation mechanism for the SEI on the LMA. As shown in Fig. 1, assuming the electrochemical potentials of the anode and cathode are μa and μc, respectively, when the lowest unoccupied molecular orbital (LUMO) of the electrolyte molecules is lower than the anode electrochemical potential μa, electrons tend to transfer to the unoccupied orbitals of the electrolyte, causing a reduction reaction on the surface of the LMA. This reaction will only terminate when the obtained SEI can completely passivate the LMA.13 In fact, due to the lowest reduction potential of the LMA, the side reaction between the LMA and electrolyte is always unavoidable, so an SEI layer will spontaneously form on the surface of the LMA. In contrast, the SEI on the graphite anode can only form after charging due to the high potential, which causes different structures and component distributions in the formed SEI.14 To obtain long-life LMBs, it is necessary to understand the structure, composition, and physicochemical properties of the SEI and how they influence the growth of Li dendrites.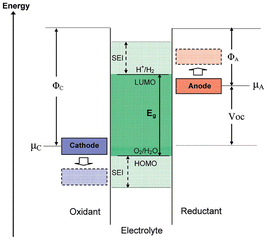 | ||
| Fig. 1 Formation mechanism of the SEI on the Li metal anode surface.13 | ||
Electrolyte derived SEI for suppressing Li dendrite growth on the LMA
Mechanism of the SEI suppressing Li dendrites
The SEI plays an important role in the repeated charging and discharging processes of LMBs. As shown in Fig. 2, the SEI generated from electrolyte decomposition is usually fragile and easily punctured by the initial nucleation of Li, leading to new metal reduction sites, thus promoting the growth of filamentous, mossy, and dendritic Li metal. Moreover, the inherent physical and chemical properties of the SEI can also easily cause dendrite growth.15 During the charging and discharging processes of the LMA, Li ions need to pass through the SEI. However, the non-uniform Li-ion channels in the SEI result in non-uniform initial Li nucleation. Subsequently, this could cause puncturing of the SEI, with further growth along the axial direction through the tip effect, eventually leading to the formation of a dendritic morphology.16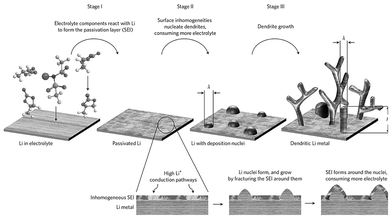 | ||
| Fig. 2 Schematic of the SEI controlling Li dendrite growth.16 | ||
With the continuous growth of Li dendrites, the decomposition reaction between the Li metal and electrolyte will also continue to occur, causing a rapid increase in overpotential. In addition, for each charging and discharging cycle, the growth of Li dendrites consumes a large amount of active Li metal, which inevitably leads to a decrease in capacity and low coulombic efficiency (CE).17 Therefore, altering the SEI structure and composition to regulate the physical and chemical properties of the SEI is a key method for suppressing Li dendrites.18
In principle, an ideal SEI should have the following properties: it should have high mechanical strength, which can prevent further side reactions caused by initial Li nucleation and puncture; it should be conducive to Li-ion conduction, thereby promoting uniform Li-ion flow and achieving uniform initial Li nucleation; the SEI should be as uniform as possible to reduce heterogeneous nucleation during Li metal deposition; and the SEI should have high electronic insulation to reduce excessive electrolyte decomposition and the consumption of Li metal. In order to achieve the above properties, regulating the structure and composition of the SEI, such as by introducing LiF to improve the mechanical strength of the SEI, has been proven to be effective.19 With the increasing mechanical strength of the SEI, it can effectively prevent the SEI from being punctured by Li metal nucleation, thus reducing the growth sites of Li dendrites (Fig. 3a). On the other hand, introducing components with good Li-ion conductivity into the SEI can improve the Li nucleation homogeneity. In this case, as more Li ions can simultaneously reach the LMA surface during the deposition process, the uniformity of the initial Li nucleation is improved, and subsequent dendrite growth is suppressed (Fig. 3b).20 Therefore, by regulating the SEI components to improve mechanical stability, Li-ion conductivity, uniformity, and other physical and chemical properties, the growth of Li dendrites can be effectively suppressed, thereby improving the electrochemical CE of the LMA.21
Inorganic-rich SEI to supress dendrite growth
The electrolyte is the main component involved in the formation of an SEI on the LMA surface, and the main method to change the structure and composition of the SEI is by regulating the components of the electrolyte solvents, Li salts, and additives.22 Generally, an inorganic-rich SEI, generated by the decomposition of anions, is widely regarded as having high stability and surface energy, which can effectively inhibit the growth of Li dendrites.23 In this section, we summarize recent research on engineering liquid electrolytes to regulate the inorganic-rich SEI in LMBs.Carbonate electrolytes have been widely used in Li-ion batteries over the past few decades due to their excellent physical and chemical properties, such as a high dielectric constant, high Li salt solubility, and high oxidation resistance. Therefore, they can provide high ionic conductivity and low resistance. However, their stability on the LMA surface is poor, and they cannot effectively inhibit the growth of Li dendrites. In contrast, ether electrolytes exhibit higher compatibility with the LMA but cannot tolerate high voltages. In order to suppress Li dendrites and improve the electrochemical performance of LMBs, many studies have been conducted by researchers, including strategies, such as electrolyte additives, high-concentration electrolytes (HCEs), and localized-high-concentration electrolytes (LHCEs), among others.24
Usually, the Li salt concentration in electrolytes is 0.8–1.2 M, which exhibits high ionic conductivity, lower viscosity, and low cost, making it widely used in LIBs. However, it exhibits poor compatibility and high safety risks in LMBs. Due to the formation of more Li dendrites, the CE of the LMA in dilute carbonate electrolytes is usually lower than 90.0%, resulting in poor cycling performance. This is because the SEI formed in carbonate electrolytes mainly contains heterogeneous organic alkyl carbonate lithium (ROCO2Li), inorganic lithium carbonate (Li2CO3), lithium oxide (Li2O), lithium fluoride (LiF), and others.25 Generally, these components are unevenly distributed in the SEI, as depicted by the mosaic model (Fig. 4).26 There are two distinct migration pathways for Li+ in the SEI: through the inorganic species crystals or along the grain boundaries of different components, which ultimately results in the deposition of Li+ on the anode surface (Fig. 5).27 Generally, the more complex the migration path, the more uneven the Li deposition morphology. Therefore, many studies have been devoted to improving the uniformity of the species distribution in the SEI to enhance the uniformity of Li metal deposition. In addition, it is also possible to improve the mechanical stability, chemical stability, and Li-ion conductivity of the SEI by increasing the content of inorganic species in the SEI to suppress the growth of Li dendrites.
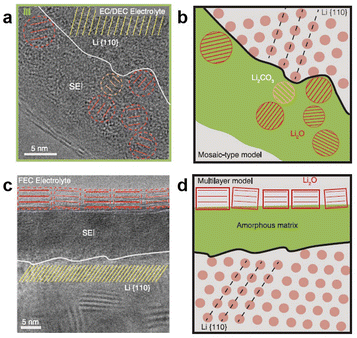 | ||
| Fig. 4 SEI (a and b) mosaic and (c and d) multilayer structure models.26 | ||
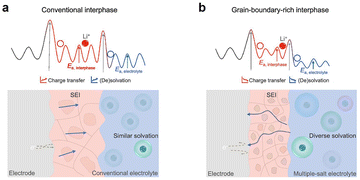 | ||
| Fig. 5 Migration pathways of Li ions in the SEI in the (a) intracrystalline migration model and (b) grain boundary migration model.27 | ||
An effective method to increase the content of inorganic components in the SEI is through the strategy of including additives in the electrolyte.28 Although additives are usually present in very low amounts in electrolytes, they can effectively regulate the components of the SEI. Among all the additives, lithium nitrate (LiNO3) has exhibited excellent inhibitory effects on Li dendrites, even at lower concentrations.29 LiNO3 can decompose into lithium oxide (Li2O) and lithium nitride (Li3N) on the LMA surface, which are components with ultra-high ion conductivity, accelerating the transport of Li ions in the SEI.30 For example, Zhang and his colleagues increased the solubility of LiNO3 in traditional carbonate electrolytes by using LiNO3 as an additive and CuF2 as a co-solvent, promoting LiNO3 participation in the formation of a Li3N-rich SEI. Compared to the blank electrolyte, the LMA with this SEI exhibited a high CE and cycling stability (Fig. 6a).31 Besides increasing the solubility of LiNO3 in the electrolyte, integrating LiNO3 into the separator or polymer molecular array can result in a sustained slow release of LiNO3, thus supporting its continuous participation in the formation of the SEI, ultimately improving the CE and cycle life of the LMA (Fig. 6b and c).32 Besides LiNO3, other nitrates have also been used to form a Li3N-rich SEI and inhibit the growth of Li dendrites (Fig. 6d).33 These results indicate that the method of using additives to form an inorganic-rich SEI to improve its Li-ion conductivity can effectively inhibit the growth of Li dendrites.
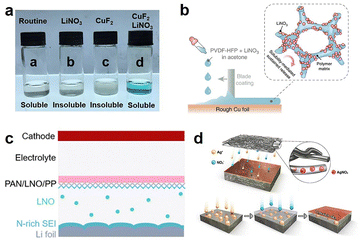 | ||
| Fig. 6 Various methods to produce a Li3N-rich SEI in carbonate electrolytes: (a) by increasing the solubility of LiNO3 in the electrolyte,31 (b and c) through solubility-mediated sustained release, and ref. 32 (d) by the addition of high solubility nitrates.33a | ||
Molecular additives are also important components in forming a stable SEI.34 For example, new carbonate solvents, such as fluorinated ethylene carbonate (FEC), can be decomposed to produce lithium fluoride (LiF). LiF is a recognized substance that can improve the mechanical stability of the SEI, which has a positive effect on the cycling performance of the LMA and LMBs.35 LiF has various positive effects on the SEI, including its high bandgap (13.6 eV), which can improve the electronic insulation of the SEI and reduce the thickness of SEI formation; high mechanical strength, which can improve the mechanical and electrochemical stability of the SEI; and its high interfacial energy, which is beneficial to Li metal deposition along the interface between the SEI and LMA, thereby reducing dendrite growth. In addition, the formation of heterojunctions between LiF and other inorganic components can enhance the Li-ion conductivity of the SEI.36 The ability of the SEI to suppress Li dendrites based on a high content of LiF can be further improved by designing a high fluorine content electrolyte. For example, Wang's team designed a perfluorinated electrolyte (FEC/FEM/HFE (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) with the aim to construct a LiF-rich SEI, which induced the growth morphology of large Li particles and low porosity. This highly dense arrangement of large Li particles greatly reduced the specific surface area of the LMA and reduced side reactions between the electrolyte and Li metal,37 enabling a high CE of 99.2% to be achieved in this system.
2)) with the aim to construct a LiF-rich SEI, which induced the growth morphology of large Li particles and low porosity. This highly dense arrangement of large Li particles greatly reduced the specific surface area of the LMA and reduced side reactions between the electrolyte and Li metal,37 enabling a high CE of 99.2% to be achieved in this system.
In addition to the above-mentioned additives, many additives that can produce special components, such as Li2S, LiBxOy, and LiSxOy, during SEI formation have been widely studied in carbonate electrolytes.38 For example, Zheng's team designed a new additive, vinyltrimethylsilane (VTMS), that led to the construction of an excellent SEI, which could greatly improve the electrochemical performance of LMBs.39 In another study, methyl methanesulfonate (MMDS) and 1,3-propanesultone were applied to generate Li2S components with high Li-ion conductivity in the SEI, thereby improving the uniformity of Li metal deposition and reducing dendrite growth.40 The introduction of these additives improved the stability of the SEI and Li-ion conductivity, which effectively supressed the growth of Li dendrites. In addition, additives containing unique elements, such as silicon, boron, and magnesium, can form special SEI alloys on the LMA and also exhibit an inhibitory effect on the growth of Li dendrites.
A high-concentration electrolyte (HCE), relative to dilute electrolytes, is a system with a salt concentration exceeding 3.0 M. Due to the high Li salt concentration, it exhibits high thermodynamic stability and promotes Li salt participation in the formation of the SEI.41 The composition of Li-ion solvation sheaths is completely different from that in dilute electrolytes in HCEs. In dilute electrolytes, Li ions are completely surrounded by solvents, but in HCEs, the solvation structure of Li ions contains more anions (Fig. 7a).42 As the number of anions in the Li-ion solvation sheath increases, it tends to form a highly stable anion-driven SEI, which reduces electrolyte decomposition and suppresses the growth of Li dendrites. For example, in a 7.0 M LiFSI-FEC solution, the LMA exhibited a high CE of 99.6% (Fig. 7b).43 In the 10.0 M LiFSI-EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolyte, more FSI anions were involved in the formation of the SEI, with more LiF and Li2S components, resulting in a high CE of 99.2% for the LMA (Fig. 7c).44 It is worth noting that not all high concentrations of Li salts can improve the dendrite suppression effect of the LMA, and more work is needed to clarify the differences in SEI formation among different Li salts in HCEs.
1) electrolyte, more FSI anions were involved in the formation of the SEI, with more LiF and Li2S components, resulting in a high CE of 99.2% for the LMA (Fig. 7c).44 It is worth noting that not all high concentrations of Li salts can improve the dendrite suppression effect of the LMA, and more work is needed to clarify the differences in SEI formation among different Li salts in HCEs.
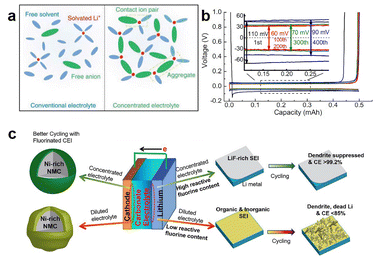 | ||
| Fig. 7 Structure and influences of HCEs: (a) Structure schematic of HCEs 42; (b) and (c) HCE LiFSI-FEC and HCE LiFSI-EC/DMC improve Coulombic Efficiency for LMA, respectively 43,44. | ||
Although HCEs can contribute to the formation of a highly inorganic SEI and effectively supress Li dendrite growth, these electrolytes often show low Li-ion conductivity, high viscosity, poor electrode wettability, and incur a high cost due to their high Li salt concentration. To address these issues, localized-high-concentration electrolytes (LHCEs) have been proposed as alternative electrolyte systems.45 LHCEs are typically composed of electrolytes and diluents that do not dissolve Li salts. Since the diluents do not participate in the Li-ion solvation sheath, they can add more anions into the Li-ion solvation, achieving an anion-driven inorganic-rich SEI. This high-concentration effect system, which does not require high salt concentration but is achieved through diluents, is called an LHCE. The most commonly used diluents in such systems are fluorinated ethers, such as 1,1,2,2-tetrafluoroethyl-2,2,3-tetrafluoropropyl ether (TTE) and bis (2,2,2-trifluoroethyl) ether (BTFE).46
Fluorinated ethers have ultra-low viscosity, which not only reduces electrolyte viscosity and improves Li-ion conductivity but also increases the participation of anions in Li-ion solvation groups, thus improving the content of inorganic species in the SEI.47 By applying the LHCE strategy, the LMA can achieve a high CE of over 99.0%. A high Coulombic efficiency (CE) indicates that lithium metal is deposited in the form of large particles rather than dendrites. For example, the 1.2 M LiFSI-DMC-BTFE (1/2) LHCEs designed by Zhang's team exhibited a high CE of 99.5% for the LMA, indicating that the growth of Li dendrites was significantly inhibited. The deposition layer formed in the designed LHCEs is shown in Fig. 8a–c, indicating that a dense Li deposition layer with a thickness of only 10 μm was achieved in LHCEs, while the deposition layer on the LMA reached 30 μm in dilute electrolyte. This demonstrates that the LHCE strategy effectively inhibits the growth of Li dendrites and improves the cycling stability of the LMA.48 The high CE in these LHCEs is mainly attributed to the formation of an anion-driven inorganic-rich SEI. As shown in Fig. 8d, an SEI with higher Li3N and LiF content was formed on the surface of the LMA in LHCEs, which enhanced the Li-ion conductivity and reduced the growth of Li dendrites, thus exhibiting a high CE of 98.2%.49 It should be noted that while the inorganic-rich SEI can inhibit the growth of Li dendrites, the current system is still difficult to implement in practical LMBs, and further research is needed. Additionally, it is worth noting that the cathode material significantly influences the SEI of the Li metal anode. The properties of cathode materials, particularly the degradation products of transition metal ions, can significantly impact the formation and stability of the SEI. For example, certain transition metal ions, such as Mn2+, form a thick, soft, and unstable SEI, which ultimately results in the degradation of battery performance. Furthermore, nickel-rich cathode materials may also cause instability of the SEI due to their increased reactivity with the electrolyte, thereby affecting the cycling life and stability of the batteries.
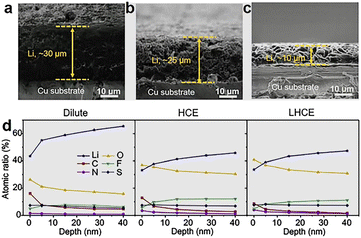 | ||
Fig. 8 Thickness of Li deposition in (a) 1.0 M LiPF6/EC-EMC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), (b) 1.2 M LiFSI/DMC, and (c) 1.2 M LiFSI/DMC-BTFE (1 6), (b) 1.2 M LiFSI/DMC, and (c) 1.2 M LiFSI/DMC-BTFE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) electrolytes.48 (d) Element distribution of SEIs formed in different electrolytes.49 2) electrolytes.48 (d) Element distribution of SEIs formed in different electrolytes.49 | ||
Inorganic-polymerized organic hybrid SEI to suppress dendrite growth
Recent research has shown that a highly inorganic SEI may not necessarily be beneficial, as excessive inorganic components could lead to poor mechanical stability due to inadequate interconnection between the inorganic particles. Polymerized organic components are usually more flexible and can accommodate significant volume changes during the Li metal deposition process.50 High-molecular-weight polymerized organic materials also exhibit high viscosity, which can strongly support adhering the fine inorganic nanoparticles to the Li anode surface.51 Consequently, the inorganic-polymerized organic hybrid structure of the SEI demonstrates high stability due to the high mechanical strength of the inorganic components and the high toughness of the organic components. This combination allows the SEI to withstand Li metal volume expansion and inhibit the growth of Li dendrites.52 Furthermore, the inorganic-polymerized organic hybrid SEI structure can easily form a double-layer structure, which offers greater uniformity than the “mosaic” structure and maximizes the avoidance of functional interference between the different layers (Table 1). Therefore, the hybridization of inorganic and polymerized organic components is essential for achieving the ideal uniformity, flexibility, and mechanical stability of the SEI.53Since 2020, numerous studies have demonstrated the significance of electrolyte polymerization in the SEI, with “film-forming” serving as a fundamental mechanism to inhibit Li dendrite growth in non-aqueous electrolytes. For example, Guo et al. and He et al. utilized an anion-induced ring-opening polymerization of 1,3-dioxolane (DOL) to form a pDOL polymer, which exhibited high Li metal tolerance, thus mitigating the adverse reactions between the Li metal and electrolyte, thereby enhancing the utilization rate of the active Li metal (Fig. 9a).54 However, polymer molecules typically exhibit low Li-ion conductivity and poor Li affinity. To address these limitations, various additives, such as LiNO3 and AlF3, have been introduced into pDOL to build an inorganic-polymerized organic hybrid SEI to improve its Li-ion conductivity and Li affinity, facilitating uniform Li metal deposition.54b
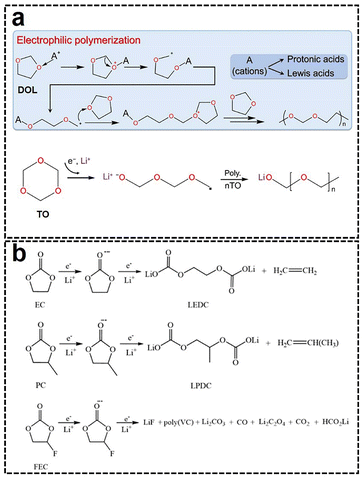 | ||
| Fig. 9 (a) In situ polymerization pathway of ether solvents on the LMA surface.54 (b) In situ polymerization pathways of carbonate solvents on the LMA surface.55 | ||
For liquid electrolyte systems in LMBs that can achieve a high CE, it is evident that a common component of the electrolyte is a film-forming agent, which should readily undergo polymerization. For example, cyclic carbonates in traditional carbonate electrolytes are the main components in SEI formation, producing a large number of glycol-based Li decarbonates and poly-lithium carbonates by ring-opening polymerization (Fig. 9b).55 In this type of SEI, the CE of the LMA can reach 95%. Chen's group developed a 1.0 M LiTFSI-VC electrolyte, which formed a stable SEI due to the polymerization of VC on the surface of the LMA (Fig. 10).56 This SEI exhibited high Li-ion conductivity (1.39 × 10−6 S cm−1) and high mechanical strength (34 GPa), resulting in a high CE (97.0%). It could also maintain low polarization and high cycling stability even at high current densities of 5 mA cm−2 and 10 mA cm−2. Zhao et al. utilized lithium difluorooxalate borate (LiDFOB) as an additive to alter the decomposition pathway of difluoroethylene carbonate (DFEC) molecules, ensuring the formation of inorganic LiF by inducing a direct defluorination of DFEC. The defluorinated DFEC was prone to polymerization into polyvinyl carbonate, which enhanced the elasticity of the SEI. This polymer-interleaved LiF-dominated hybrid SEI showed high ionic conductivity and mechanical stability, effectively accelerating the kinetics of the electrode reactions and facilitating more efficient reversible deposition and stripping of the LMA.
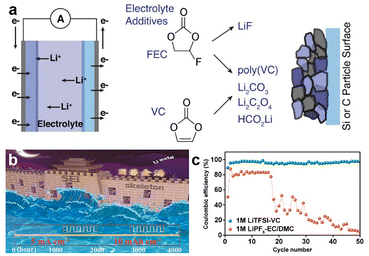 | ||
| Fig. 10 (a) Polymerization pathways of VC and FEC to form an SEI. (b and c) Polymerization of VC effectively supresses the growth of Li dendrites and improves the CE of the LMA.56 | ||
The in situ polymerization of ether solvents can significantly inhibit the growth of Li metal dendrites.57 Wang et al. proposed the use of dipropylene glycol dimethyl ether, a non-toxic and non-flammable ether solvent, in a Li–S@pPAN battery.58 This solvent undergoes in situ electrochemical polymerization during the cycling process, effectively addressing the low-polarization interphase and alleviating interfacial side reactions. The electrochemical polymerization of this non-flammable electrolyte exhibited excellent stability for over 3000 hours of operation with the LMA. Huang et al. also introduced in situ polymerization of a trioxane additive on the surface of an LMA, which was easier to polymerize than DOL (Fig. 11), enabling a customized dual-layer SEI structure to be obtained.59 The inner layer was dominated by LiF to enhance mechanical stability, while the outer layer contained lithium polyformalin to improve uniformity. Together, these layers synergistically facilitated reversible Li deposition/stripping with an ultra-high CE of 99.4%. The above discussion indicates that the in situ polymerization of the electrolyte on the LMA surface is also important for suppressing Li dendrites.60
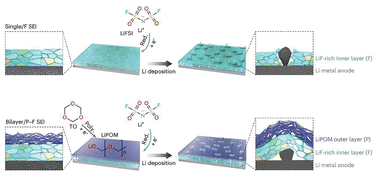 | ||
| Fig. 11 Schematic of the bilayer SEI structure formed by the in situ polymerization of trioxane.60 | ||
The latest research suggests that the structure and composition of the SEI are usually controlled by the potential of the anode. High potential conditions tend to preferentially form an organic-dominated SEI, while low potential conditions typically generate an inorganic-dominated SEI.61 Therefore, understanding the underlying mechanisms of SEI formation can enable the rational design of inorganic-polymerized organic hybrid SEIs, which can more effectively suppress the growth of dendrites on lithium metal anodes. This may offer a viable approach for achieving lithium anodes with coulombic efficiencies exceeding 99.5%.
Conclusions and perspectives
Since the birth of LIBs in 1990, the accessible capacity of graphite anodes has reached its theoretical limit. Pursing higher energy density forces us to explore the possibility of LMAs. However, the highly reactive nature of LMAs and the associated electrolytes poses enormous challenges for practical applications. A spontaneously formed SEI on the surface of the LMA significantly influences the growth morphology of the deposited Li metal, ultimately leading to the growth of Li dendrites. Therefore, the main method for suppressing Li dendrite formation entails the establishment of a stable and homogeneous SEI, which helps mitigate the non-uniform nucleation of Li metal and the subsequent rupture of the SEI. Although the fundamental mechanism by which the SEI suppresses Li dendrites remains elusive, several related connections have been established:(1) A high ionic conductivity of the SEI can accelerate the migration rate of Li ions, allowing more Li ions to reach the anode surface simultaneously, thereby reducing the non-uniform nucleation caused by concentration polarization. Uniform initial Li nucleation promotes subsequent uniform deposition, thus reducing the growth of Li dendrites.
(2) The thermodynamic and mechanical properties of the SEI can mitigate the disordered growth of the LMA. On the one hand, the significant expansion in volume and surface area that occurs during the growth of Li dendrites results in a high surface energy. The trend of dendrite growth can be alleviated by the enhanced thermodynamic surface energy of the SEI. On the other hand, the high mechanical strength of the SEI can suppress the intense stress generated by Li dendrites, thereby suppressing their growth.
Based on the aforementioned considerations, the most pressing tasks in electrolyte design are to enhance the thermodynamic stability, mechanical strength, and ionic conductivity of the SEI. In summary, an inorganic-polymer hybrid SEI with a dual-layer structure is likely to meet all these requirements simultaneously, thus effectively suppressing Li dendrites. This approach offers a promising pathway for the development of practical LMBs in the future.
Author contributions
Dr. Huaping Wang drafted the initial manuscript. Prof. Hailong Wang directed the research project and revised the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial supports from the Natural Science Foundation of Ningxia Province (No. 2023AAC02009), National Natural Science Foundation of China (No. 22379076), and the Ningxia Key Research and Development Project (No. 2024BEE02001).References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed; H. Dai, K. Xi, X. Liu, C. Lai and S. Zhang, J. Am. Chem. Soc., 2018, 140, 17515–17521 Search PubMed; J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef PubMed; N. Xu, X. Ma, M. Wang, T. Qian, J. Liang, W. Yang, Y. Wang, J. Hu and C. Yan, Electrochim. Acta, 2016, 203, 171–177 CrossRef.
- J. Offermann, A. Paolella, R. Adelung and M. Abdollahifar, Chem. Eng. J., 2024, 502, 157920 CrossRef CAS; J. Liu, Z. Bao, Y. Cui, E. J. Dufek, J. B. Goodenough, P. Khalifah, Q. Li, B. Y. Liaw, P. Liu, A. Manthiram, Y. S. Meng, V. R. Subramanian, M. F. Toney, V. V. Viswanathan, M. S. Whittingham, J. Xiao, W. Xu, J. Yang, X.-Q. Yang and J.-G. Zhang, Nat. Energy, 2019, 4, 180–186 CrossRef.
- Y. Ji, L. Dong, J. Liu, H. Xie, S. Zhong, C. Yang, J. Han and W. He, Energy Environ. Sci., 2024, 17, 4078–4089 RSC; L. Xiao, Z. Zeng, X. Liu, Y. Fang, X. Jiang, Y. Shao, L. Zhuang, X. Ai, H. Yang, Y. Cao and J. Liu, ACS Energy Lett., 2019, 4, 483–488 CrossRef CAS.
- S. K. Heiskanen, J. Kim and B. L. Lucht, Joule, 2019, 3, 2322–2333 CrossRef CAS; H. Yang, C. Guo, J. Chen, A. Naveed, J. Yang, Y. Nuli and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 791–795 CrossRef PubMed.
- Y. Zhang, T.-T. Zuo, J. Popovic, K. Lim, Y.-X. Yin, J. Maier and Y.-G. Guo, Mater. Today, 2020, 33, 56–74 CrossRef CAS.
- H. Ye, Z.-J. Zheng, H.-R. Yao, S.-C. Liu, T.-T. Zuo, X.-W. Wu, Y.-X. Yin, N.-W. Li, J.-J. Gu, F.-F. Cao and Y.-G. Guo, Angew. Chem., Int. Ed., 2019, 58, 1094–1099 CrossRef CAS PubMed; C. Wu, H. Huang, W. Lu, Z. Wei, X. Ni, F. Sun, P. Qing, Z. Liu, J. Ma, W. Wei, L. Chen, C. Yan and L. Mai, Adv. Sci., 2020, 1902643 CrossRef PubMed; J. Duan, L. Huang, T. Wang, Y. Huang, H. Fu, W. Wu, W. Luo and Y. Huang, Adv. Funct. Mater., 2020, 30, 1908701 CrossRef; M. K. Majeed, A. Hussain, G. Hussain, M. U. Majeed, M. Z. Ashfaq, R. Iqbal and A. Saleem, Small, 2024, 20, 2406357 CrossRef PubMed; M. R. Shaik, Y. Park, Y.-K. Jung and W. B. Im, J. Energy Chem., 2024, 97, 120–127 CrossRef; Y. J. Gong, S. Pyo, H. Kim, J. Cho, H. Yun, H. Kim, S. Ryu, J. Yoo and Y. S. Kim, Energy Environ. Sci., 2021, 14, 940–954 RSC.
- C.-X. Bi, N. Yao, X.-Y. Li, Q.-K. Zhang, X. Chen, X.-Q. Zhang, B.-Q. Li and J.-Q. Huang, Adv. Mater., 2024, 36, 2411197 CrossRef CAS PubMed; H. Su, P. Liu, Y. Liu, S. Liu, Y. Zhong, X. Xia, X. Wang and J. Tu, Nano Energy, 2023, 115, 108722 CrossRef; F. Mushtaq, H. Tu, L. Zhao, L. Wang, B. Tang, Z. He, Y. Cao, Z. Hou, J. Ran, J. Wang, M. Zahid, Y. Zhang and M. Liu, Energy Storage Mater., 2024, 73, 103854 CrossRef.
- B. Han, Z. Zhang, Y. Zou, K. Xu, G. Xu, H. Wang, H. Meng, Y. Deng, J. Li and M. Gu, Adv. Mater., 2021, 33, 2100404 CrossRef CAS PubMed; H. Su, H. Zhang, Z. Chen, M. Li, J. Zhao, H. Xun, J. Sun and Y. Xu, Chin. Chem. Lett., 2023, 34, 108640 CrossRef.
- P. Liu, S. Shen, Z. Qiu, T. Yang, Y. Liu, H. Su, Y. Zhang, J. Li, F. Cao, Y. Zhong, X. Liang, M. Chen, X. He, Y. Xia, C. Wang, W. Wan, J. Tu, W. Zhang and X. Xia, Adv. Mater., 2024, 36, 2312812 CrossRef CAS PubMed.
- Y. Wu, C. Wang, C. Wang, Y. Zhang, J. Liu, Y. Jin, H. Wang and Q. Zhang, Mater. Horiz., 2024, 11, 388–407 RSC; Q. Wang, J. Yang, X. Huang, Z. Zhai, J. Tang, J. You, C. Shi, W. Li, P. Dai, W. Zheng, L. Huang and S. Sun, Adv. Energy Mater., 2022, 12, 2103972 CrossRef CAS; D. Wang, C. Luan, W. Zhang, X. Liu, L. Sun, Q. Liang, T. Qin, Z. Zhao, Y. Zhou, P. Wang and W. Zheng, Adv. Energy Mater., 2018, 8, 1800650 CrossRef.
- X. Yin, R. Zhu, X. Hu, H. Zhao, X. Li, L. Liu, S. Niu, J. Wang, Y. Meng, Y. Su, S. Ding and W. Yu, Adv. Funct. Mater., 2024, 34, 2310358 CrossRef CAS; O. B. Chae and B. L. Lucht, Adv. Energy Mater., 2023, 13, 2203791 CrossRef; Y. Gao and B. Zhang, Adv. Mater., 2023, 35, 2205421 CrossRef PubMed; C. Chen, Q. Liang, G. Wang, D. Liu and X. Xiong, Adv. Funct. Mater., 2022, 32, 2107249 CrossRef; N.-W. Li, Y. Shi, Y.-X. Yin, X.-X. Zeng, J.-Y. Li, C.-J. Li, L.-J. Wan, R. Wen and Y.-G. Guo, Angew. Chem., Int. Ed., 2018, 57, 1505–1509 CrossRef PubMed.
- H. J. Chang, A. J. Ilott, N. M. Trease, M. Mohammadi, A. Jerschow and C. P. Grey, J. Am. Chem. Soc., 2015, 137, 15209–15216 CrossRef CAS PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- S.-Y. Sun, N. Yao, C.-B. Jin, J. Xie, X.-Y. Li, M.-Y. Zhou, X. Chen, B.-Q. Li, X.-Q. Zhang and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208743 CrossRef CAS PubMed.
- X. Shen, R. Zhang, X. Chen, X.-B. Cheng, X. Li and Q. Zhang, Adv. Energy Mater., 2020, 10, 1903645 CrossRef CAS.
- M. D. Tikekar, S. Choudhury, Z. Tu and L. A. Archer, Nat. Energy, 2016, 1, 16114 CrossRef CAS.
- E. Peled, J. Electrochem. Soc., 1979, 126, 2047 CrossRef CAS; D. Aurbach, M. L. Daroux, P. W. Faguy and E. Yeager, J. Electrochem. Soc., 1987, 134, 1611 CrossRef; B. D. Adams, J. Zheng, X. Ren, W. Xu and J.-G. Zhang, Adv. Energy Mater., 2018, 8, 1702097 CrossRef.
- A. Cipolla, C. Barchasz, B. Mathieu, B. Chavillon and S. Martinet, J. Power Sources, 2022, 545, 231898 CrossRef CAS; J. Pokharel, A. Cresce, B. Pant, M. Y. Yang, A. Gurung, W. He, A. Baniya, B. S. Lamsal, Z. Yang, S. Gent, X. Xian, Y. Cao, W. A. Goddard, K. Xu and Y. Zhou, Nat. Commun., 2024, 15, 3085 CrossRef PubMed; D. Luo, L. Zheng, Z. Zhang, M. Li, Z. Chen, R. Cui, Y. Shen, G. Li, R. Feng, S. Zhang, G. Jiang, L. Chen, A. Yu and X. Wang, Nat. Commun., 2021, 12, 186 CrossRef PubMed.
- H. Zhuang, H. Xiao, T. Zhang, F. Zhang, P. Han, M. Xu, W. Dai, J. Jiao, L. Jiang and Q. Gao, Angew. Chem., Int. Ed., 2024, 63, e202407315 CrossRef CAS PubMed.
- X.-B. Cheng, R. Zhang, C.-Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- C. Zhu, C. Sun, R. Li, S. Weng, L. Fan, X. Wang, L. Chen, M. Noked and X. Fan, ACS Energy Lett., 2022, 7, 1338–1347 CrossRef CAS.
- Y. S. Cohen, Y. Cohen and D. Aurbach, J. Phys. Chem. B, 2000, 104, 12282–12291 Search PubMed; S. Basu and G. S. Hwang, ACS Appl. Mater. Interfaces, 2023, 15, 59494–59501 CrossRef CAS.
- W.-H. Hou, P. Zhou, H. Gu, Y. Ou, Y. Xia, X. Song, Y. Lu, S. Yan, Q. Cao, H. Liu, F. Liu and K. Liu, ACS Nano, 2023, 17, 17527–17535 Search PubMed; M. Li, C. Chen, H. Luo, Q. Xu, K. Yan, Y. Qiu and G. Zhou, J. Mater. Chem. A, 2024, 12, 10072–10080 RSC.
- H. Li, Z. Chen, L. Zheng, J. Wang, H. Adenusi, S. Passerini and H. Zhang, Small Methods, 2024, 8, 2300554 CrossRef CAS; H. Wan, J. Xu and C. Wang, Nat. Rev. Chem., 2024, 8, 30–44 CrossRef PubMed.
- J. Wu, M. Ihsan-Ul-Haq, Y. Chen and J.-K. Kim, Nano Energy, 2021, 89, 106489 Search PubMed; M. Nie, D. Chalasani, D. P. Abraham, Y. Chen, A. Bose and B. L. Lucht, J. Phys. Chem. C, 2013, 117, 1257–1267 Search PubMed; G. V. Zhuang, K. Xu, H. Yang, T. R. Jow and P. N. Ross, J. Phys. Chem. B, 2005, 109, 17567–17573 CrossRef CAS PubMed; D. Aurbach, Y. Ein-Eli, B. Markovsky, A. Zaban, S. Luski, Y. Carmeli and H. Yamin, J. Electrochem. Soc., 1995, 142, 2882 CrossRef; H. Ota, Y. Sakata, X. Wang, J. Sasahara and E. Yasukawa, J. Electrochem. Soc., 2004, 151, A437 Search PubMed.
- E. Peled, D. Golodnitsky and G. Ardel, J. Electrochem. Soc., 1997, 144, L208 CrossRef CAS; Y. Li, Y. Li, A. Pei, K. Yan, Y. Sun, C.-L. Wu, L.-M. Joubert, R. Chin, A. L. Koh, Y. Yu, J. Perrino, B. Butz, S. Chu and Y. Cui, Science, 2017, 358, 506–510 CrossRef PubMed.
- Q. Wang, C. Zhao, X. Hu, J. Wang, S. Ganapathy, S. Eustace, X. Bai, B. Li, H. Li, D. Aurbach and M. Wagemaker, J. Am. Chem. Soc., 2024, 146, 31778–31787 CrossRef CAS PubMed.
- (a) T. Cai, Q. Sun, Z. Cao, Z. Ma, W. Wahyudi, L. Cavallo, Q. Li and J. Ming, J. Phys. Chem. C, 2022, 126, 20302–20313 Search PubMed; (b) Y. Zhang, Y. Wu, H. Li, J. Chen, D. Lei and C. Wang, Nat. Commun., 2022, 13, 1297 Search PubMed; (c) R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267–278 Search PubMed.
- (a) J. Fu, X. Ji, J. Chen, L. Chen, X. Fan, D. Mu and C. Wang, Angew. Chem., Int. Ed., 2020, 59, 22194–22201 CrossRef CAS PubMed; (b) E. Winter, M. Briccola, T. J. Schmidt and S. Trabesinger, Appl. Res., 2024, 3, e202200096 Search PubMed; (c) S. Liu, X. Ji, N. Piao, J. Chen, N. Eidson, J. Xu, P. Wang, L. Chen, J. Zhang, T. Deng, S. Hou, T. Jin, H. Wan, J. Li, J. Tu and C. Wang, Angew. Chem., Int. Ed., 2021, 60, 3661–3671 CrossRef CAS PubMed; (d) K. Peng, X. Wang and X. Yan, Chin. Chem. Lett., 2024, 35, 109274 Search PubMed.
- M. S. Kim, Z. Zhang, J. Wang, S. T. Oyakhire, S. C. Kim, Z. Yu, Y. Chen, D. T. Boyle, Y. Ye, Z. Huang, W. Zhang, R. Xu, P. Sayavong, S. F. Bent, J. Qin, Z. Bao and Y. Cui, ACS Nano, 2023, 17, 3168–3180 Search PubMed.
- C. Yan, Y. X. Yao, X. Chen, X. B. Cheng, X. Q. Zhang, J. Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2018, 57, 14055–14059 Search PubMed.
- Y. Liu, D. Lin, Y. Li, G. Chen, A. Pei, O. Nix, Y. Li and Y. Cui, Nat. Commun., 2018, 9, 3656 Search PubMed; S. Li, Y. Liu, K. Wang, X. Hu, W. Guan, Z. Du, H. Du and W. Ai, Chem. Commun., 2024, 60, 2649–2652 Search PubMed.
- (a) G. Huang, G. Chen, X. Jin, K. Ge, M. Guan and Y. Li, J. Power Sources, 2023, 556, 232497 Search PubMed; (b) C. Liao, L. Han, X. Mu, Y. Zhu, N. Wu, J. Lu, Y. Zhao, X. Li, Y. Hu, Y. Kan and L. Song, ACS Appl. Mater. Interfaces, 2021, 13, 46783–46793 CrossRef CAS PubMed; (c) C. Chen, Q. Zhou, X. Li, B. Zhao, Y. Chen and X. Xiong, Small Methods, 2024, 8, 2300839 CrossRef CAS PubMed; (d) H. Kang, T.-H. Kim, G. Hwang, G. H. Shin, J. Lee, G. Kim and E. Cho, Chem. Eng. J., 2024, 484, 149510 CrossRef CAS.
- X. Ren, Y. Zhang, M. H. Engelhard, Q. Li, J.-G. Zhang and W. Xu, ACS Energy Lett., 2018, 3, 14–19 Search PubMed.
- T. Hou, G. Yang, N. N. Rajput, J. Self, S.-W. Park, J. Nanda and K. A. Persson, Nano Energy, 2019, 64, 103881 CrossRef CAS; L. Liu, S. Wang, Z. Zhang, J. Fan, W. Qi and S. Chen, Ionics, 2019, 25, 1035–1043 Search PubMed.
- Y.-X. Lin, Z. Liu, K. Leung, L.-Q. Chen, P. Lu and Y. Qi, J. Power Sources, 2016, 309, 221–230 CrossRef CAS; Y. C. Chen, C. Y. Ouyang, L. J. Song and Z. L. Sun, J. Phys. Chem. C, 2011, 115, 7044–7049 CrossRef; S. Zhou, Y. Zhu, H. Hu, C. Li, J. Jiang, J. Huang and B. Zhang, J. Mater. Chem. A, 2023, 11, 5636–5644 RSC.
- X. Fan, L. Chen, O. Borodin, X. Ji, J. Chen, S. Hou, T. Deng, J. Zheng, C. Yang, S.-C. Liou, K. Amine, K. Xu and C. Wang, Nat. Nanotechnol., 2018, 13, 715–722 Search PubMed.
- F. Liu, L. Wang, Z. Zhang, P. Shi, Y. Feng, Y. Yao, S. Ye, H. Wang, X. Wu and Y. Yu, Adv. Funct. Mater., 2020, 30, 2001607 Search PubMed; Z. Wu, S. Li, Y. Zheng, Z. Zhang, E. Umesh, B. Zheng, X. Zheng and Y. Yang, J. Electrochem. Soc., 2018, 165, A2792 Search PubMed; B. Tong, Z. Song, H. Wan, W. Feng, M. Armand, J. Liu, H. Zhang and Z. Zhou, InfoMat, 2021, 3, 1364–1392 Search PubMed; J. Li, L. Zhang, L. Yu, W. Fan, Z. Wang, X. Yang, Y. Lin, L. Xing, M. Xu and W. Li, J. Phys. Chem. C, 2016, 120, 26899–26907 Search PubMed; X. Yang, J. Li, L. Xing, Y. Liao, M. Xu, Q. Huang and W. Li, Electrochim. Acta, 2017, 227, 24–32 Search PubMed; F. An, H. Zhao, W. Zhou, Y. Ma and P. Li, Sci. Rep., 2019, 9, 14108 CrossRef PubMed.
- Y. Li, Q. Qu, L. Lv, J. Shao and H. Zheng, Adv. Funct. Mater., 2024, 34, 2314100 CrossRef CAS.
- Z. Huang, T. Huang, X. Ye, X. Feng, X. Yang, J. Liang, S. Ye, Y. Li, X. Ren, W. Xiong, X. Ouyang, Q. Zhang and J. Liu, Appl. Surf. Sci., 2022, 605, 154586 Search PubMed; E. G. Leggesse and J.-C. Jiang, RSC Adv., 2012, 2, 5439–5446 RSC.
- L.-L. Jiang, C. Yan, Y.-X. Yao, W. Cai, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 3402–3406 Search PubMed; Z. Jiang, Z. Zeng, W. Hu, Z. Han, S. Cheng and J. Xie, Energy Storage Mater., 2021, 36, 333–340 CrossRef CAS; Z. Peng, X. Cao, P. Gao, H. Jia, X. Ren, S. Roy, Z. Li, Y. Zhu, W. Xie, D. Liu, Q. Li, D. Wang, W. Xu and J.-G. Zhang, Adv. Funct. Mater., 2020, 30, 2001285 CrossRef.
- Y. Yamada, J. Wang, S. Ko, E. Watanabe and A. Yamada, Nat. Energy, 2019, 4, 427–427 CrossRef.
- L. Suo, W. Xue, M. Gobet, S. G. Greenbaum, C. Wang, Y. Chen, W. Yang, Y. Li and J. Li, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 1156–1161 CrossRef CAS PubMed.
- X. Fan, L. Chen, X. Ji, T. Deng, S. Hou, J. Chen, J. Zheng, F. Wang, J. Jiang, K. Xu and C. Wang, Chem, 2018, 4, 174–185 CAS.
- X. Cao, P. Gao, X. Ren, L. Zou, M. H. Engelhard, B. E. Matthews, J. Hu, C. Niu, D. Liu, B. W. Arey, C. Wang, J. Xiao, J. Liu, W. Xu and J.-G. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020357118 CrossRef CAS PubMed; W. van Ekeren, A. Hall, K. Lahtinen and R. Younesi, ChemElectroChem, 2024, 11, e202400050 CrossRef.
- X. Kong, Y. Kong, Y. Zheng, L. He, D. Wang and Y. Zhao, Small, 2022, 18, 2205017 CrossRef CAS PubMed; C.-C. Su, J. Shi, R. Amine, M. He, S.-B. Son, J. Guo, M. Jiang and K. Amine, Nano Energy, 2023, 110, 108335 CrossRef.
- Q. Liu, Y. Liu, Z. Chen, Q. Ma, Y. Hong, J. Wang, Y. Xu, W. Zhao, Z. Hu, X. Hong, J. Wang, X. Fan and H. B. Wu, Adv. Funct. Mater., 2023, 33, 2209725 CrossRef CAS.
- S. Chen, J. Zheng, D. Mei, K. S. Han, M. H. Engelhard, W. Zhao, W. Xu, J. Liu and J.-G. Zhang, Adv. Mater., 2018, 30, 1706102 CrossRef PubMed.
- X. Ren, S. Chen, H. Lee, D. Mei, M. H. Engelhard, S. D. Burton, W. Zhao, J. Zheng, Q. Li, M. S. Ding, M. Schroeder, J. Alvarado, K. Xu, Y. S. Meng, J. Liu, J.-G. Zhang and W. Xu, Chem, 2018, 4, 1877–1892 CAS.
- Y. Chen, Y. Cui, S. Wang, Y. Xiao, J. Niu, J. Huang, F. Wang and S. Chen, Adv. Mater., 2023, 35, 2300982 CrossRef CAS PubMed; D. Kang, S. Sardar, R. Zhang, H. Noam, J. Chen, L. Ma, W. Liang, C. Shi and J. P. Lemmon, Energy Storage Mater., 2020, 27, 69–77 CrossRef; Y. Zhu, Y. Zheng, J. Liu, L. Zhang, X. Ni, J. Zhou, M. Wang, T. Qian and C. Yan, J. Phys. Chem. Lett., 2024, 15, 733–743 CrossRef PubMed.
- Y. Zhao, G. Li, Y. Gao, D. Wang, Q. Huang and D. Wang, ACS Energy Lett., 2019, 4, 1271–1278 CrossRef CAS.
- W. Cao, J. Lu, K. Zhou, G. Sun, J. Zheng, Z. Geng and H. Li, Nano Energy, 2022, 95, 106983 CrossRef CAS.
- D. Han, Z. Wang, S. Chen, J. Zhou, S. Chen, M. Wang, D. Wu, X. Meng, C. W. Bielawski and J. Geng, Small, 2024, 20, 2405453 CrossRef CAS PubMed.
- (a) F.-Q. Liu, W.-P. Wang, Y.-X. Yin, S.-F. Zhang, J.-L. Shi, L. Wang, X.-D. Zhang, Y. Zheng, J.-J. Zhou, L. Li and Y.-G. Guo, Sci. Adv., 2018, 4, eaat5383 CrossRef CAS PubMed; (b) H. Yang, M. Jing, L. Wang, H. Xu, X. Yan and X. He, Nano-Micro Lett., 2024, 16, 127 CrossRef PubMed.
- M. Yeddala, L. Rynearson and B. L. Lucht, ACS Energy Lett., 2023, 8, 4782–4793 CrossRef CAS.
- (a) A. L. Michan, B. S. Parimalam, M. Leskes, R. N. Kerber, T. Yoon, C. P. Grey and B. L. Lucht, Chem. Mater., 2016, 28, 8149–8159 CrossRef CAS; (b) Z. Hu, S. Zhang, S. Dong, Q. Li, G. Cui and L. Chen, Chem. Mater., 2018, 30, 4039–4047 CrossRef CAS.
- X. Peng, T. Wang, B. Liu, Y. Li and T. Zhao, Energy Environ. Sci., 2022, 15, 5350–5361 RSC.
- J. Chen, H. Lu, X. Zhang, Y. Zhang, J. Yang, Y. Nuli, Y. Huang and J. Wang, Energy Storage Mater., 2022, 50, 387–394 CrossRef.
- Q.-K. Zhang, X.-Q. Zhang, J. Wan, N. Yao, T.-L. Song, J. Xie, L.-P. Hou, M.-Y. Zhou, X. Chen, B.-Q. Li, R. Wen, H.-J. Peng, Q. Zhang and J.-Q. Huang, Nat. Energy, 2023, 8, 725–735 Search PubMed.
- X. Hu, Y. Ma, J. Qian, W. Qu, Y. Li, R. Luo, H. Wang, A. Zhou, Y. Chen, K. Shi, L. Li, F. Wu and R. Chen, Adv. Mater., 2024, 36, 2303710 CrossRef CAS PubMed.
- Y. Lu, Q. Cao, W. Zhang, T. Zeng, Y. Ou, S. Yan, H. Liu, X. Song, H. Zhou, W. Hou, P. Zhou, N. Hu, Q. Feng, Y. Li and K. Liu, Nat. Energy, 2024 DOI:10.1038/s41560-024-01679-4.
| This journal is © The Royal Society of Chemistry 2025 |