Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biodegradable plastics in soils: sources, degradation, and effects
Piumi Amasha Withana†
 ab,
Xiangzhou Yuan†
ab,
Xiangzhou Yuan† c,
Darvin Im
c,
Darvin Im d,
Yujin Choid,
Michael S. Bank
d,
Yujin Choid,
Michael S. Bank ef,
Carol Sze Ki Lin
ef,
Carol Sze Ki Lin g,
Sung Yeon Hwang*d and
Yong Sik Ok
g,
Sung Yeon Hwang*d and
Yong Sik Ok *ab
*ab
aKorea Biochar Research Center, Division of Environmental Science and Ecological Engineering, Korea University, Seoul, 02841, Republic of Korea. E-mail: yongsikok@korea.ac.kr
bInternational ESG Association (IESGA), Seoul, 06621, Korea
cMinistry of Education of Key Laboratory of Energy Thermal Conversion and Control, School of Energy and Environment, Southeast University, Nanjing 210096, China
dDepartment of Plant & Environmental New Resources, Graduate School of Biotechnology, Kyung Hee University, Gyeonggi-do 17104, Republic of Korea. E-mail: crew75@khu.ac.kr
eInstitute of Marine Research, Bergen, Norway
fUniversity of Massachusetts Amherst, Amherst, MA, USA
gSchool of Energy and Environment, City University of Hong Kong, Tat Chee Avenue, Kowloon Tong, Kowloon, Hong Kong
First published on 29th May 2025
Abstract
Biodegradable plastics (BPs) are increasingly marketed as sustainable alternatives to conventional plastics, yet their environmental impacts on soil ecosystems remain uncertain. Attention to plastic-related policies, global treaties, and initiatives assessing industrial sustainability are growing, and thus there is an urgent need for scientific data on the life cycle of BPs in soils to determine their viability as a truly sustainable alternative. BPs enter soil through agricultural applications, waste disposal, and landfills, undergoing complex degradation processes influenced by soil properties, environmental conditions, and polymer characteristics. However, the release of degradation by-products, including potential toxins and microplastics, raises concerns about soil health and plant growth. Furthermore, discrepancies in biodegradability claims and the lack of standardized assessment methods hinder the reliable evaluation of BP sustainability. To ensure the environmental viability of BPs, rigorous long-term studies and standardized testing protocols are necessary to validate their degradation, in situ, under environmentally relevant soil conditions. Without robust scientific evidence demonstrating the safe and effective degradation of BPs in soils, the expansion of their production and investment in these materials may be limited. This review highlights the urgent necessity for integrated approaches to support effective BP assessment, to bridge scientific research, industrial deployment, and policy frameworks, which are beneficial for mitigating potential unintended environmental consequences and achieving the relevant UN Sustainable Development Goals (SDGs).
Environmental significanceBiodegradable plastics (BPs) are emerging as a sustainable alternative to petroleum-based plastics, which are non-biodegradable, persist in the environment, and contribute to microplastic pollution. Agriculture, as a major user of BPs, has turned soils into long-term sinks for these materials and their residues. Although marketed as 100% degradable, evidence indicates incomplete degradation and the release of microplastics. In soils, BPs undergo chemical, physical, and biological degradation, producing CO2, H2O, and biomass, while intermediate by-products may affect soil fertility and plant growth. This review identifies BP sources in soils, explores their degradation mechanisms, and assesses their impacts on soil properties. We also highlight a methodology for evaluating BP degradation in soils and propose future research directions to address existing knowledge gaps. |
1. Introduction
Plastic pollution has significant environmental impacts, potentially jeopardizing human health, and damaging ecosystems essential for species survival. Over 430 million tons of plastic are produced annually, with two-thirds discarded after a single use. If this trend continues, plastic waste is likely to triple by 2060,1 negatively affecting ecosystems and human health. Even with prompt and coordinated efforts, approximately 710 million metric tons of plastic waste are projected to enter both aquatic and terrestrial environments.2 Recent studies have highlighted agricultural soils as significant reservoirs of microplastics (MPs).3 MPs enter soils mainly through compost, sewage sludge, agricultural mulching, littering, and atmospheric deposition.4 Environmental issues related to plastic pollution, particularly MP pollution, have attracted significant attention from scientists, civil society, and policymakers. MPs are plastic particles ranging from 100 nm to 5 mm in size. These can originate either as primary MPs produced in microscopic sizes or as secondary MPs resulting from the breakdown of larger plastic fragments.5 In March 2022, over 170 United Nations member states agreed to forge a new global plastic treaty on plastic pollution and to date negotiations are still ongoing. The UN treaty emphasizes the necessity for global scientific and policy communities to collaborate and adopt a life-cycle approach to address the issues from production to disposal.1 Plastic recycling policies have been adopted by many countries to protect their plastic industries and to prevent, gradually reduce, and eliminate plastic pollution throughout the life cycle by 2040. This demonstrates that conventional plastics have insufficient cyclability, which is essential for establishing a carbon-neutral and eco-friendly society based on a truly circular economy.Bioplastics constitute an important advancement in sustainable material development. Bioplastics display properties similar to those of conventional plastics while providing additional advantages such as a lower carbon footprint and more diverse waste management prospects such as composting. The use of bioplastics can contribute to reducing the reliance on fossil fuels, lowering greenhouse gas (GHG) emissions, promoting a sustainable and circular economy, and attaining a “Cradle to Cradle” design.6 However, there has been considerable uncertainty and distrust regarding claims of biodegradability associated with plastic products. This is mainly because of instances where materials marketed as biodegradable failed to degrade as anticipated. Biodegradable plastics (BPs) are a subset of a broader category known as bioplastics. However, the ambiguity regarding the term “bioplastics” is widespread. A prevalent misconception is that materials derived from biomass would biodegrade. However, the utilization of biological feedstock does not ensure complete biodegradability of the final product. For example, bio-based plastics such as polyethylene (PE) are not biodegradable, and BPs such as polycaprolactone (PCL) are not derived from biomass (Havstad, 2020).7 Plastic materials can originate from bio-based (renewable), fossil (petroleum), or mixed resources. Their end-of-life degradation determines whether these are classified as biodegradable or nonbiodegradable plastics (Fig. 1).
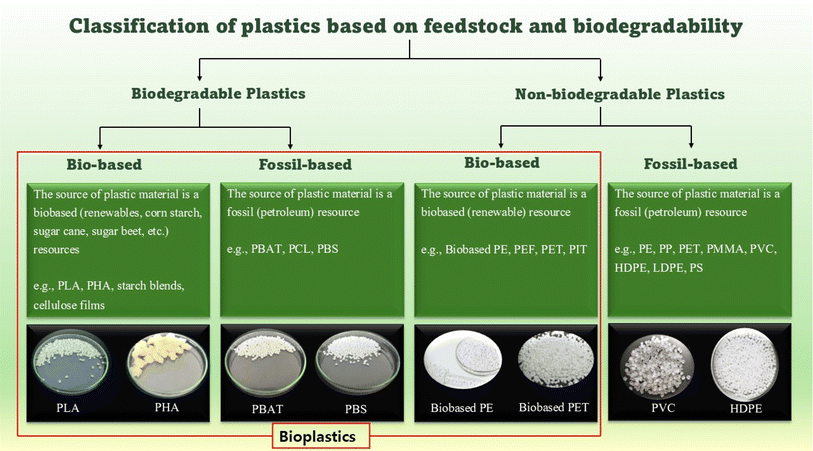 | ||
| Fig. 1 Plastic types based on their raw material origin and biodegradability.8,9 (PLA = poly(lactic) acid, PHA = polyhydroxyalkanoates, PBAT = polybutylene adipate terephthalate, PCL = polycaprolactone, PBS = poly(butylene succinate), PE = polyethylene, PEF = polyethylene furanoate, PET = polyethylene terephthalate, PIT = poly(isosorbide terephthalate), PMMA = polymethyl methacrylate, PVC = polyvinyl chloride, HDPE = high-density polyethylene, LDPE = low-density polyethylene, PS = polystyrene). | ||
Although the BP manufacturing processes vary, these do not affect the overall biodegradability of the material. The common procedures include producing plastics from a mechanically or chemically processed bio-based polymer (e.g., destructurized starch-based plastics) and chemically synthesizing a polymer from a monomer obtained through the biotechnological conversion of a renewable resource (e.g., using lactic acid from sugar fermentation for polylactic acid (PLA) production). Additionally, biotechnological procedures can be used to produce polymers from renewable resources, such as sugar fermentation by microorganisms to synthesize aliphatic polyesters (e.g., polyhydroxybutyrate (PHB)). Another common method is to chemically synthesize polymers from components obtained via petrochemical processes from non-renewable resource (e.g., polyvinyl alcohol (PVA)) polymerization.10–13
Currently, the market is dominated by commercially available bio-based BPs such as PLA, PBS (poly(butylene succinate)), its derivative PBSA, polyhydroxyalkanoate (PHA), and polybutylene adipate terephthalate (PBAT),14 where BPs constitute 0.5% of the total annual plastic production and is >400 million tons. Following a period of stagnation due to the COVID-19 pandemic, global plastic production began to rebound in 2023, driven by rising demand and the development of more advanced applications and products.15 This growth is also evident in the bioplastics sector, where production capacity is expected to increase significantly, from approximately 2.18 million tonnes in 2023 to around 7.43 million tonnes by 2028. More recent projections indicate an increase from 2.47 million tonnes in 2024 to approximately 5.73 million tonnes by 2029. These trends underscore the continuous expansion of both conventional plastics and bioplastics, fueled by technological advancements and evolving market demands.9 (Fig. 2).
 | ||
| Fig. 2 Global production capacities of biodegradable plastics (biobased) in 2024 and 2029 (%).15 (PLA = poly(lactic) acid, SCPC = starch-containing polymer compounds, PBAT = polybutylene adipate terephthalate, PHA = polyhydroxyalkanoates, CR = regenerated cellulose films, PBS = poly(butylene succinate), and CP = casein polymers). | ||
The agriculture and horticulture sectors have emerged as major drivers of the BP market, leading to higher BP production. These account for 98.6 t (in 1000 tons) of market production in 2023.9 This increase indicates that large quantities of BPs would ultimately reach soils. Although labeled as “biodegradable,” existing research emphasizes the complexities and challenges in achieving complete degradation within desired timeframes (typically between six months to one year) and generates concerns regarding the generation of BP-based MPs (BMPs), which pose additional ecological risks. However, annual sustainability reports of global industries engaged in the development of BPs and related products reveal significant challenges. The current evaluations focus predominantly on marine ecosystems or composting facilities. These do not have comprehensive data on the full biodegradability of these plastics under realistic soil conditions. This presents a severe problem, and industries may inaccurately assert their products' recyclability, compostability, and biodegradability. This can potentially lead to false sustainability claims and the “greenwashing” of these products.
It is our understanding that, with plastic-related regulations and standards still evolving, along with the UN's introduction of new plastic regulations and the recognition of BPs by the United Nations Conference on Trade and Development,16 there is an urgent need for scientifically validated, research-based information on BPs. This includes their sources, degradation in soils, associated byproducts, and overall impact on soil fertility to ensure the long-term sustainability of BPs in soils. Furthermore, as the Environmental, Social, and Governance (ESG) framework gains traction in assessing industry sustainability, global industries should prioritize the production of more environmentally sustainable products. Considering these global initiatives, stronger collaboration between the scientific and policy communities is essential. Therefore, this review provides a timely and comprehensive analysis of BPs in soils, offering important scientific insights into their lifecycle in soils for various stakeholders, including those involved in product development, policy initiatives and more. Specifically, this review discusses BP sources, degradation mechanisms, effects on soil, and associated challenges. The specific objectives of this study were to: (1) provide critical insights into the sources, fate, transport, and impacts of BPs on soil ecosystems, including degradation mechanisms and resultant byproducts; (2) discuss social and political considerations regarding the use and application of BPs to support sustainable development practices, and (3) propose novel research areas that can inform public policies while suggesting new methodologies for analyzing BP degradation in soils.
2. Biodegradable plastics in soils
BPs are frequently used in agriculture activities, including mulch films,13,17 shielded greenhouse tunnels, soil stabilizers, seed coatings, seedling pot pesticide bottles, and fertilizer bags.18 BPs, particularly PBAT, PLA, and PBS, are preferred for agricultural mulches because of their high non-recyclability. Notwithstanding its good mechanical and processing properties as well as biodegradability, PBAT has limitations in toughness and photoaging resistance for large scale applications.19 In contrast, PLA (known for its good mechanical properties, processability, and biodegradability) has become a successful rigid BP widely used in agriculture for mulch, drip irrigation pipes, and shielded greenhouse tunnels.20,21 With the low thermal stability and processing properties of PHA, recent studies have evaluated its potential in agriculture, primarily as a biocarrier for mulch and slow-release fertilizers.22 Additionally, polypropylene carbonate has been applied in the field of slow-release fertilizer materials. It exhibits effective slow-release properties and a capacity for water retention.23PLA and PBAT are also extensively utilized in the production of textile fibers. Additionally, BPs play a significant role in the medical field, where they are used for manufacturing medical equipment, personal protective equipment (PPE) including gloves, and blood storage containers. Their biodegradable nature makes them particularly suitable for biomedical applications, avoiding extra treatment for surgical removal. Additionally, these materials are incorporated into cardiovascular devices, burn and wound dressings, drug delivery systems, and dental implants.24 If not managed properly, waste from the biomedical industry poses a significant risk of accumulating in the environment. This issue became more prominent during the COVID-19 pandemic, which led to a substantial rise in biomedical plastic waste, mainly due to the extensive use of PPE (i.e., masks, gloves, face shields, and sanitizer bottles). The surge in plastic waste has placed immense pressure on existing waste management systems. Improper disposal, particularly in open environments like roadsides, has further contributed to the accumulation of biomedical plastic waste on land, exacerbating environmental concerns.25
BPs also enter soils through landfill waste, if they are not disposed of or composed effectively, and then they degrade and release their byproducts into soil ecosystems. Composting is a common practice for managing plastic waste, and when performed effectively, BPs can break down and contribute nutrients to the soil. However, improper composting may result in incomplete degradation, leading to plastic contamination (mainly through the introduction of BMPs) in soils. Littering is another source of BP contamination, as these plastics can accumulate in soils. Once littered, BPs degrade over time, releasing their byproducts into soil ecosystems.13 When BPs degrade in soils, evidence suggests that BMPs can eventually enter water bodies. Riverine inputs and surface runoff serve as pathways for the transportation of BMPs from terrestrial environments into aquatic systems. Sediments, which act as receptors for various pollutants including metals, organic contaminants, and MPs, carry BMPs along waterways. As a result, these plastics accumulate in water bodies, contributing to their contamination.26
3. Biodegradable plastic degradation in soils
The fate of BPs in soils depends on several factors, including their physicochemical properties, soil and environmental conditions, soil management practices, and the duration for which the plastic has been in the soil. In general, BPs can undergo several processes in soils, including fragmentation and mineralization.27 Degradation is the process by which microorganisms break down plastics into simpler organic compounds. In soils, microorganisms (such as bacteria and fungi) can degrade plastics by secreting enzymes that break down polymer chains into smaller molecules (monomers). These molecules can be broken down further into CO2, water, and other compounds.27 The rate and extent of degradation depend on various environmental factors such as the temperature, moisture, and availability of O2 and nutrients. Fragmentation is another process that occurs when BPs are exposed to soils. This process involves the physical breakdown of plastics into smaller pieces. Compared with petroleum-based plastics, BPs in soils are significantly more reactive. Additionally, their effects on the chemical, physical, and biological properties of soils also differ significantly.17,27,28 Understanding the degradation processes and the fate of byproducts is essential for assessing the environmental impacts and effectiveness of BPs as sustainable and viable alternatives to fossil-based plastics.3.1. Factors affecting the biodegradable plastic degradation in soils
The degradation of BPs in soil is primarily affected by BP and soil properties, and environmental factors. Consequently, the ecological safety of plastic materials cannot be assessed by considering individual influencing factors. Rather, these should be evaluated simultaneously.29Furthermore, it was observed that soil texture influenced enzymatic activities, with loamy soil showing higher values due to stronger enzyme adsorption onto clay particles.31 Also, soil texture can influence the degradation of BPs, due to their impacts on microbial activity and soil moisture retention. Loamy soils, which have a balanced mixture of sand, silt, and clay, typically exhibit higher microbial activity and better moisture retention compared to sandy soils. This enhanced microbial presence and sustained moisture create favorable conditions for the breakdown of BPs. In contrast, sandy soils, with larger particle sizes and lower water-holding capacity, may support reduced microbial activity, potentially leading to slower degradation rates of these materials.32
Chen et al.33 demonstrated that incorporating clay minerals like kaolinite and montmorillonite into artificial soil increased CO2–C emissions from MPs, including PE, polypropylene, polystyrene, and polyvinyl chloride. This enhancement is due to clay minerals serving as microbial habitats and providing a larger surface area for microbial colonization. Ding et al.34 further supported this mechanism, reporting that kaolinite, in particular, accelerated MP oxidation and led to the production of reactive oxygen species (e.g., ˙OH and O2˙−). The negatively charged surfaces of clay minerals may also play a role in stabilizing MP radical cations, preventing their recombination with hydrated electrons, and promoting ˙OH radical formation in oxygen-rich environments.34
The soil moisture content is essential for determining the rate and extent of plastic degradation. Adequate moisture promotes conditions favorable for microbial activity, which is essential for BP degradation.27 Optimal moisture levels promote microbial growth, enzymatic activity, and nutrient availability, that could accelerate plastic degradation. PLA-based films demonstrated increased weight loss when exposed to wet conditions, showing significant degradation after a year. Under wet conditions, the percentages of weight loss were 86.4 ± 2.8 for clay soil and 89.4 ± 1.8 for loam soil. Conversely, under dry conditions, the weight loss percentages were 69.3 ± 10.6 for clay soil and 84.9 ± 2.3 for loam soil.32 Excessively dry or waterlogged soils can hinder microbial activity. Insufficient moisture limits the microbial breakdown of plastics, whereas waterlogged conditions impede aerobic degradation owing to restricted O2 availability. Generally, anaerobic metabolic pathways are less efficient than aerobic respiration, and yield lower degradation rates.35 Identifying preferable soil moisture conditions is essential for optimizing plastic degradation via providing adequate moisture for microbial activity without saturation. Moisture can enhance BP degradation and promote sustainable plastic waste management in soils.32
Temperature is another critical factor governing the degradation of BPs in soils, primarily because it regulates microbial activity, enzyme secretion, and abiotic hydrolysis processes. Studies have shown that under industrial composting conditions, BP degradation rates are significantly high due to elevated temperatures (>58 °C) and the extensive growth of thermophilic microorganisms capable of breaking down plastic polymers. However, such high-temperature and moisture conditions are not feasible in natural environmental settings, leading to much slower degradation rates in soils, particularly in cooler climates.36 Generally, the degradation rate of BPs increases with increasing temperatures due to enhanced microbial metabolism. However, the extent of this effect depends on the type of BP and the temperature tolerance of the microbial communities involved.37
Laboratory degradation tests in soils are typically conducted at stable temperatures between 25 and 28 °C, but the average temperatures in many temperate regions are lower. This suggests that real-world degradation rates may be significantly slower than those observed in laboratory studies. A study by Huo et al.38 highlights the strong influence of temperature variations within the mesophilic range (20–40 °C) on the degradation of PBAT and starch-based BPs. The study found that mesophilic microorganisms, which thrive at moderate temperatures (30–40 °C), exhibit optimal enzyme secretion, particularly hydrolytic enzymes such as lipases and cutinases. These enzymes accelerate the breakdown of ester bonds in PBAT, generating hydrolysis products such as terephthalic acid, 1,4-butanediol, and adipic acid. Similarly, Sintim et al.39 observed that mulch films derived from PLA/PHA degraded more rapidly during summer than winter, emphasizing the role of temperature in enhancing microbial degradation. Fig. 3 schematically illustrates the variability of BP degradation in soils depending on temperature (Dissanayake et al., 2024).40
 | ||
| Fig. 3 Cumulative CO2 efflux during incubation experiments. PBAT and PLA = poly(butylene adipate-co-terephthalate) = poly(lactic acid). RT and HT = room temperature (25 °C) and high temperature (58 °C), respectively. Figure adapted and reprinted from ref. 40. | ||
Despite these observations, laboratory experiments often do not fully capture real-world temperature fluctuations. In controlled environments, stable temperatures create optimal conditions for microbial activity and enzymatic hydrolysis, leading to consistent and predictable degradation rates. Laboratory tests typically maintain uniform temperatures (20–40 °C), providing valuable insights into biodegradation mechanisms. However, natural field conditions are far more variable daily and seasonal temperature fluctuations significantly influence microbial metabolism, often slowing degradation compared to laboratory predictions. Even in warmer climates, laboratory tests tend to underestimate actual degradation timelines, highlighting temperature as a key factor contributing to discrepancies between controlled studies and real-world observations.36 Understanding these temperature-dependent differences is important for improving the accuracy of biodegradation assessments in environmental settings.
The degradation of BPs in soils is largely governed by the biological activity of soil macroorganisms, such as earthworms and insects, and a diverse range of microorganisms, including bacteria and fungi. These organisms play a major role in breaking down BPs into simpler compounds, eventually converting them into CO2, water, and biomass under favorable environmental conditions. Typically, environments rich in microorganisms, particularly those with high temperatures and humidity levels, exhibit accelerated degradation rates.41
Microorganisms, particularly plastic-degrading bacteria and fungi, colonize BP surfaces and form biofilms that secrete specialized enzymes. These enzymes facilitate the breakdown of plastic polymers into smaller fragments, such as oligomers, dimers, and monomers, through a series of distinct stages including biodeterioration, depolymerization, assimilation, and mineralization. The microbial biomass present in the soil plays a critical role in the degradation of various BPs, such as PBS, PBS–starch blends, and PLA. Over extended periods, ranging from 28 days to two years, higher microbial biomass levels have been directly linked to increased degradation rates, highlighting the strong relationship between soil microbial activity and BP breakdown.41
Certain microbial groups exhibit unique contributions to BP degradation. For instance, nitrogen-fixing bacteria have been shown to facilitate the microbial degradation of polybutylene succinate-co-adipate (PBSA). Their activity promotes fungal proliferation, which enhances the production of enzymes involved in plastic degradation and influences fungal community interactions essential for breaking down plastics.28 The presence of these microorganisms accelerates enzymatic activity, leading to more efficient BP degradation.
Beyond microorganisms, soil macroorganisms such as earthworms also play a significant role in BP degradation. Earthworms interact with biodegradable mulch films buried in the soil for six to twelve months, ingesting these films and promoting degradation through processes like bioturbation and vermicomposting. These activities enhance BP fragmentation and increase microbial exposure to the degraded material, further accelerating decomposition.42
O2 availability also plays an important role in the degradation of BPs in soils. Aerobic degradation occurs in the presence of O2. It is typically the preferred pathway for degradation because it is more efficient and yields higher degradation rates. Under aerobic conditions, microorganisms utilize O2 to break down BPs into CO2, water, and biomass through enzymatic reactions. Microorganisms in anaerobic environments may adopt alternative metabolic pathways such as fermentation or methanogenesis. These result in the production of CH4 and other organic compounds. These pathways are generally less effective in breaking down BPs than aerobic degradation, potentially resulting in prolonged persistence of plastic materials in soils. For example, considering the O2 content, BP degradation decelerates under anaerobic conditions within the soil profile compared with the soil surfaces,43 suggesting that the O2 availability directly influences the extent of BP degradation in soils. Factors such as soil porosity, moisture content, and microbial activity can affect the O2 diffusion and availability within soils and thereby, the degradation kinetics of BPs. In environments where O2 is abundant, aerobic degradation processes are dominant.
Additionally, UV light exposure can induce the photodegradation of BPs. This reduces their molecular weight, potentially influencing microbial activity on BPs. These plastics are particularly vulnerable to degradation initiated by natural light, particularly in the near-UV band ranging from 290 to 400 nm. Photons in this range carry energy that can induce unstable states in polymer macromolecules and even cleave carbon–carbon bonds.44 The photodegradation of BPs generally involves Norrish reactions, which include the stages of photoionization and chain scission, as well as crosslinking reactions or oxidative processes. Furthermore, photodegradation can cause a decrease in the molecular weight and mechanical properties of BPs.44
Temporal factors also influence the dynamics of soil BP degradation. Degradation typically occurs gradually over extended periods. The duration of exposure to soil conditions significantly influences the degradation rate and extent. Over time, BPs undergo physical, chemical, and biological transformations in soil ecosystems. Microorganisms colonize plastic surfaces and produce extracellular enzymes that break down polymer chains into smaller molecules. These molecules are assimilated by microorganisms as carbon and energy sources.
BP blends are being produced to enhance mechanical properties while maintaining degradability and striving for improved performance and environmental sustainability. Thus, the degradation degrees are as follows: pure BPs > BP blends > claimed “BP” ≈ non-BP (PE).44 The degradation performance of both the PLA blend (with PE) and PBAT blend (with PLA) was inferior to that of their respective pure BPs. These differences can be attributed to the enhanced mechanical properties resulting from the blending. These improved properties hinder the polymers from fragmenting or cracking into smaller pieces. Previous studies have indicated that the plastic surface hydrophobicity of BP blends (i.e., a PLA blend with PE) can undergo alterations complicating the degree of degradation.44 MD40, marketed as a “BP” exhibited minimal indications of degradation compared with nonbiodegradable PE. This is because MD40 is composed of 40% mineral-doped PE plastic, which implies that it may pose a higher challenge to degradation than regular PE.44
The size and shape of plastic can influence its surface area and thereby, the rate of microbial colonization and enzyme activity. As reported, larger contact surface area of plastic blends results in a higher degradation rate.38,46 A comparison of the sizes of biodegradable mulch films at 1% and 2% (w/w) content levels revealed that macro-sized pieces exhibited higher porosity than micro-sized particles in soils.47 Over a period of 140 days at 28 °C under dark conditions with a moisture level of 14.6%, rapid degradation was observed in smaller-sized BP pellets (50–75 μm) compared with larger-sized pellets. This phenomenon can be attributed to the larger surface areas of materials with smaller particle sizes loaded with more microorganisms.48 During a 10–12 month burial at a depth of 15 cm, the PHA BPs including PHB and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) (both in pellet and film forms) underwent degradation where films exhibited faster degradation than pellets. Notably, microorganisms attached more readily to the surfaces of PHA films. This indicates a potential correlation between surface properties and microbial activity during degradation.
The concentrations of BPs in soils play a significant role in the degradation process, influencing microbial activity and soil respiration. It has been observed that the highest dose of BPs (1%) caused a noticeable increase in CO2 emissions from the soil, particularly in sandy soil, highlighting the effect of plastic concentration on microbial respiration.31 The increased CO2 release can be attributed to both the microbial degradation of the BPs and a potential priming effect. When high doses of carbon-rich materials like BPs are added to soil, they can stimulate microbial activity, often leading to an acceleration of microbial biomass turnover or changes in the mineralization of soil organic matter (SOM), a phenomenon known as the priming effect.31 This priming effect is particularly pronounced when the added BP is nitrogen-poor, as microorganisms may rely on native SOM to meet their nitrogen needs, further accelerating CO2 emissions. Mazzon et al.31 presented that the highest BP dose supplied a large amount of carbon but lacked nitrogen, leading to increased soil respiration due to both the degradation of BPs and enhanced microbial activity from the priming effect. In contrast, lower doses of BPs did not significantly affect CO2 emissions, as they did not induce sufficient changes in microbial dynamics or nutrient balance to trigger the priming effect. Overall, the concentration of BPs directly influences their degradation rate in soils, with higher concentrations stimulating greater microbial activity and carbon cycling. The stoichiometric imbalance caused by adding a nitrogen-poor plastic material to soil ecosystems determines how effectively the microbial community degrades plastic. Thus, plastic concentration is a crucial factor in BP degradation, influencing microbial dynamics, nutrient cycling, and overall carbon turnover in soils.31
Another factor influencing BP degradation in soil is the presence of various additives, including plasticizers, fillers, oxidants, colorants, and stabilizers. These additives serve specific functions, shaping the mechanical properties, longevity, and environmental impacts of BPs.49,50 Plasticizers, used to enhance flexibility, may inadvertently hinder microbial degradation by forming protective barriers around polymer chains or leaching into soil, potentially affecting soil health.51 Fillers, incorporated to improve mechanical strength, can either promote or obstruct degradation based on their composition. Organic fillers such as starch enhance microbial activity, whereas inorganic fillers like clay may limit microbial access to the polymer matrix. Oxidants facilitate degradation by breaking polymer chains into small fragments, accelerating the decomposition process. Colorants, commonly added for aesthetics or UV protection, may contain toxic compounds that disrupt soil microbial communities or alter the thermal properties of plastics, affecting degradation kinetics. Other additives, such as stabilizers, antioxidants, pro-oxidants, and surfactants, further influence the breakdown process by prolonging plastic lifespan, preventing oxidation, or promoting surface interactions.28,51,52
Surface charge can also play an important role in determining the behavior and fate of BPs, MPs, NPs, and BMPs in soil environments. Plastics are generally positively charged in soils; however, environmental weathering introduces carbonyl functional groups, leading to an overall negative surface charge on the particles.53 These changes in surface charge significantly influence interactions between plastics and environmental contaminants. For instance, positively charged NPs have been shown to exert a stronger impact on plant roots than negatively charged ones. Sun et al.54 found that while positively charged NPs accumulated at lower levels in root tips, they induced higher levels of reactive oxygen species, ultimately inhibiting plant growth and seedling development more severely than negatively charged, sulfonic-acid-modified NPs. In contrast, negatively charged NPs were predominantly transported through the apoplast and xylem, suggesting differences in uptake pathways and potential ecological effects. Both positively and negatively charged MPs exhibit strong attachment to soil particles, primarily due to electrostatic interactions and physical trapping. The extent of this attachment is closely related to the soil's zeta potential and is influenced by several factors including pH and ionic strength. These interactions ultimately affect the degradation, mobility, bioavailability, and potential risks associated with MPs, NPs, and BMPs in terrestrial ecosystems.
3.2. Pathways of biodegradable plastic degradation in soils
Enzymatic hydrolysis is the first step in this process. Microorganisms in soils produce enzymes such as lipases, esterases, and proteases. These can hydrolyze esters or other chemical bonds in the polymer chains of plastic and thus, break these down into smaller oligomers. As an example, for PLA, hydrolysis occurs in the presence of moisture at temperatures above 30 °C releasing smaller oligomers and monomers. This process begins in the amorphous regions of the polymer and causes an increase in the number of carboxylic acid chain ends.27 In the second step, oligomer degradation occurs. Here, oligomers are broken down further by microorganisms through enzymatic reactions such as dehydrogenation and decarboxylation. These cleave the carbon–carbon and carbon–oxygen bonds in oligomers. In the monomer formation step, oligomers are broken down into monomers such as lactic acid, glycolic acid, or 3-hydroxybutyrate. These can be utilized by microorganisms as a source of energy and carbon. The monomers are degraded further through a process called mineralization and involves the complete oxidation of carbon to CO2 and the release of water and other organic compounds. This process is performed by microorganisms that use O2 as an electron acceptor.27
Enzymatic processes play a critical role in breaking down specific types of bioplastics. For instance, polysaccharide-based bioplastics, such as starch and cellulose, undergo enzymatic hydrolysis facilitated by amylases and cellulases. These enzymes cleave ester bonds and release carboxylic acids and alcohols as byproducts.60 The degradation is driven by a catalytic triad mechanism involving serine, histidine, and aspartate amino acids, where serine and histidine interact to form nucleophilic alcohol groups that facilitate ester bond cleavage. For other types of BPs, such as PBS and PCL, the enzymatic breakdown is mediated by lipases and cutinases. Lipases attack ester bonds in PBS through hydrolysis, forming water-soluble intermediates like 4-hydroxy butylene succinates, which are then mineralized.61,62
It is important to acknowledge that the degradation of BPs in soils involves multiple pathways including biological, physical, and chemical processes that are not mutually exclusive. Therefore, achieving the complete degradation of BPs in soils generally entails a combination of these pathways working collaboratively. Fig. 4 illustrates the degradation pathways of BPs in soils. It outlines the various processes involved, including the biological, physical, and chemical pathways (Fig. 4).
4. By-products and waste generated by degradation of biodegradable plastics
Studies indicate that the degradation of BPs does not pose significant harmful effects.14 However, there remains limited knowledge regarding the chemical safety of BPs. Specifically, the composition of chemical compounds used in these materials and the potential toxicity of their leachates in both ecosystems and human health are poorly understood. Research suggests that BPs often contain similar additives to conventional plastics and may exhibit comparable levels of toxicity.51 During the degradation of BPs in soils, various by-products and waste can be generated depending on the specific plastic and degradation processes involved. CO2 and water are common by-products of degradation because microorganisms oxidize the carbon in plastics to CO2. Organic acids such as acetic acid, propionic acid, and butyric acid, and volatile organic compounds such as ethanol, methanol, and acetone can be generated during the degradation of BPs.63 CH4 can be produced during anaerobic degradation of certain BPs under O2 deficit conditions.64Plastic manufacturing intentionally incorporates various compounds including additives such as plasticizers, antioxidants, and stabilizers (which enhance their functionality), as well as solvents and catalysts that facilitate their production.50 So, there is a possibility that these compounds may be released in soil environments upon the degradation of BPs. Because most of these compounds are not covalently bonded to polymers, these can potentially migrate to surrounding air, solids (e.g., packaged goods or soil), or liquids (e.g., beverages) via chemical migration.
Organic additives commonly found in plastics include bisphenols, phthalates, brominated flame retardants, organotin compounds, alkylphenols, formaldehyde, antimicrobials, and azo colorants.65 Many of these substances are recognized or suspected endocrine-disrupting chemicals (EDCs), suggesting that they interfere with hormonal regulation and potentially lead to various adverse health effects.66 In addition to organic additives, trace metals are frequently used as inorganic additives in plastics.67 Trace metals such as lead, mercury, and cadmium accumulate in soils at varying rates, depending on plastic types and environmental conditions and can also be toxic to soil organisms and inhibit plant growth, homeostasis, and reproduction.29 Certain oligomers and monomers generated during BP degradation are toxic. For example, terephthalic acid (a monomer contained in PBAT) functions as an EDC that can damage the endocrine system of organisms. The migration and potential health risks associated with non-intentionally added substances (NIAS) including oligomers formed during polymerization and monomers that are not covalently bonded to the polymer, have long been acknowledged.17 Additionally, other substances are present, both intentionally (e.g., unreacted monomers) and unintentionally (NIAS, side or breakdown products).
Regarding BP degradation, most scientific attention has been focused on the formation of BMPs. If BPs are not degraded completely in soil, there is a possibility of residual plastic fragments or MPs being left behind. These can persist in the environment and cause long-term environmental problems.29 The application of film coatings containing fungicides and/or insecticides on agricultural seeds has gained approval as a method for improving seed germination and overall seedling health. Consequently, fragments of the coating can detach and enter the soil in varying amounts. Previous studies have indicated that these detached seed-coat fragments typically have dimensions less than 5 mm. This makes these comparable to MPs in size and appearance.18 The environmental impacts of the MPs and NPs generated from BPs are likely to be similar to those of petroleum-based plastic derived MPs and NPs.
MPs also act as vectors of various toxic substances. They have a strong affinity for pollutants such as polycyclic aromatic hydrocarbons (PAHs), pesticides, heavy metals, and antibiotics, which are commonly found in agricultural soils.68 When MPs undergo oxidation in soil, their ability to bind pesticides increases, potentially altering the chemical behavior and bioavailability of these toxic compounds in unpredictable ways. Consequently, MPs not only serve as pollutants themselves but also enhance the mobility and persistence of hazardous contaminants, further compromising crop quality and posing risks to food security and human health.68
Evidence has shown the presence of MPs in the human body, which can enter through multiple pathways, including contaminated food, inhalation, drinking water, and even skin absorption.69 Given these risks, it is crucial to expand research efforts beyond MPs alone and investigate other byproducts and waste generated during the degradation of BPs. Understanding their potential migration from soils to the human body is of utmost importance, especially considering the unknown risks of bioaccumulation and biomagnification within the food chain. Without comprehensive studies, the long-term implications of these contaminants for human health remain uncertain, emphasizing the urgency for further research in this field.
5. Impact of degradation of biodegradable plastics
5.1. Impacts on soil properties
The degradation of BPs can release essential nutrients such as carbon, nitrogen, and phosphorus, making them available for uptake by soil microorganisms and plants. However, studies have shown that microplastics, including BMPs, can negatively impact soil nitrogen dynamics by disrupting both soil carbon and nitrogen cycles.72,73 PBAT–MPs may also have a stronger inhibitory effect on the nitrification of ammonium nitrogen (NH4+–N) compared to PE–MPs. Both types of MPs contribute to reductions in total nitrogen and available potassium in soils while increasing the available phosphorus.74 Additionally, the presence of PLA–MPs has been observed to promote the accumulation of NH4+–N. Specifically, NH4+–N concentrations increased by 185.8% and 156.8% in soils containing 0.5% and 1.5% (w/w) PLA MPs, respectively.75
BP degradation in soils contributes to the formation of SOM as microbial activity breaks down plastics and incorporates them into SOM. This process can improve the soil structure, water-holding capacity, and nutrient retention. The addition of PLA–MPs significantly increased soil total organic carbon and dissolved organic carbon (DOC) (p < 0.05).75 Additionally, BPs can serve as carbon sources for soil microorganisms, potentially influencing microbial composition, activity, and function in the long term. Chen et al.37 observed a higher rate of NH4+ transformation in PLA MP-treated soils compared to pure soils, suggesting that PLA–MPs function as a carbon source in soil ecosystems. A study by Zhou et al.76 examined the biochemical transformations induced by BP amendments in a plant–soil system. They found significant increases in microbial biomass carbon and DOC with PHBV, a common BP. These increases are likely the result of microbial assimilation of BPs. The study also demonstrated nitrogen immobilization, as evidenced by decreased dissolved organic nitrogen (DON) and increased microbial biomass nitrogen. Sanz-Lázaro et al.77 also observed substantial increases in the carbon![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen ratio and changes in nitrogen cycling induced by PLA compared to conventional plastics.
nitrogen ratio and changes in nitrogen cycling induced by PLA compared to conventional plastics.
As discussed, certain BPs may contain heavy metals that can be released during degradation, making them bioavailable for plant uptake and potentially toxic to soil microorganisms. The mechanisms involved in the sorption of MPs, including BMPs, are primarily attributed to hydrophobic interactions, electrostatic forces, π–π interactions, hydrogen bonding, and other effects.78 However, few field investigations and experimental studies have verified the sorption behaviors and mechanisms of MPs and BMPs. BMPs exhibit higher affinities for chemical substances than MPs. For example, PBAT–MPs show the highest affinity for phenanthrene in aqueous solutions of PBAT, PE, and PS MPs due to the low crystallinity of PBAT.79 Similar observations have been made regarding the adsorption of oxytetracycline (OTC) onto PLA–BMPs under different environmental conditions.80 It was shown that biofilm-formed PLA–BMPs had a significantly stronger affinity for OTC due to their increased surface area, the generation of oxygen-containing functional groups, and enhanced hydrogen bonding, among other interactions with biofilms. Moreover, OTC desorption behavior was more pronounced for biofilm-formed PLA–BMPs.80
BP degradation can also positively impact soil water-holding capacity by increasing soil organic matter and improving the soil structure. Qi et al.83 observed that the presence of macro-sized BP fragments at 1% and 2% levels increased soil field capacity. Additionally, alterations in porosity, saturated hydraulic conductivity, and soil water repellency were observed in the presence of these fragments. The random dispersion of MPs in soils also created water-resistant barriers, hindering soil pore function and altering the water flow direction. Jiang et al.84 observed that residual MP fragments altered various soil properties such as soil water content, bulk density, hydraulic conductivity, and porosity, which affected soil water distribution near plant roots.
BP degradation can reduce soil bulk density as the formation of soil aggregates increases soil porosity. Furthermore, BPs can alter soil aeration by reducing soil compaction and improving the soil structure. This, in turn, facilitates better gas exchange between the soil and the atmosphere. Qi et al.83 compared the impact of macro-sized BP fragments at 1% and 2% levels on porosity, observing that macro-sized fragments resulted in higher porosity compared to micro-sized particles.
The degradation of BPs can thus enhance soil biodiversity as microbial populations increase.32 According to Zhou et al.76 the introduction of bioavailable carbon (via the degradation products of PHBV) resulted in a two-fold increase in enzyme activities in hotspots compared to bulk soils. This increased microbial activity was anticipated, considering that poly-3-hydroxybutyrate is a common compound produced by diverse groups of microorganisms, particularly in response to nitrogen deficiency and cold stress. The addition of PHBV affected the activities of carbon- and nitrogen-degrading enzymes, with activity differences between hotspots and bulk soil being 2–10 times greater when PHBV was introduced. This indicated its direct influence on carbon and nitrogen cycling.76 According to the authors, the higher maximum velocity (Vmax) of β-glucosidase in the soil plastisphere, compared to the rhizosphere, can be attributed to the faster growth of biomass following PHBV additions. This correlation is supported by the positive relationship observed between active microbial biomass and the Vmax of β-glucosidase (R2 = 0.7). Additionally, the increase in β-glucosidase activity suggests that PHBV stimulates the breakdown of other common soil polymers, such as cellulose. Furthermore, PHBV can be broken down by depolymerases, releasing hydroxybutyric acid monomers that serve as fuel for the production of energetically expensive exoenzymes, such as leucine aminopeptidase.76 These exoenzymes are capable of degrading soil organic matter to acquire nitrogen for microbial growth, indicating a positive priming effect.
Although the alteration of soil properties by BP degradation has been discussed separately, soil should be considered as a complex ecosystem. While plastic degradation may directly alter one soil property, it can also indirectly affect others. Therefore, we emphasize that when conducting soil research, ecosystem complexity should be considered. Investigators should consider the overall variations in soil properties following degradation and their potential impacts on crop growth. Table 1 summarizes the mechanisms by which BPs and their degradation affect soil properties.
| Soil property | Mechanisms of impact of biodegradable plastics on soil properties | References |
|---|---|---|
| Total organic carbon (TOC) | The degradation of BPs in soil can alter TOC concentration in soil. That is, the polymeric units of BPs, BMPs, and MPs are approximately 80% carbon and thus their introduction into soil can increase its storage of organic carbon | 86 |
| Rapid production of BPs may lead to the accumulation of these materials in soil ecosystems. This accumulation may disrupt soil biogeochemical cycles, particularly the carbon and nitrogen cycles acting as an exogenous carbon input | 87 | |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio N ratio |
BPs affect soil carbon cycling due to their high carbon content, resulting in increased ranges of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratios, which may adversely affect the soil nitrogen and carbon cycles N ratios, which may adversely affect the soil nitrogen and carbon cycles |
87 |
| Dissolved organic carbon (DOC) | BPs are more readily degraded by soil microbes, leading to the release of BP-derived DOC into soil. Additionally, soil water molecules may attack the ester bonds of BPs (e.g., PLA), facilitating the leaching of soluble low-molecular-weight oligomers into soil. Both these forms of BP degradation can contribute to the alteration of DOC concentrations in soil | 76, 88 and 89 |
| Microorganisms colonize the surfaces of BPs and produce enzymes that initiate the breakdown of polymers into smaller molecules, including DOC and other organic residues which serve as nutrients thus assimilated by microorganisms. | 76 | |
| The concentrations of DOC in soil are altered by direct impacts on the carbon and nitrogen cycles resulting from the degradation of BPs in soil | ||
| Biomass C | When BPs degrade in soils, microorganisms metabolize the resulting degradation products and thus, incorporate some of the products' C into their biomass. Therefore, the biomass C concentration in microorganisms may increase due to their assimilation | 76 |
| The microorganism activity stimulated by the presence of BPs can lead to an increase in the decomposition of organic matter in soils, thereby releasing additional carbon into soil and further contributing to changes in biomass carbon concentrations | ||
| Dissolved organic nitrogen (DON) | Microorganisms that colonize and degrade BPs may also utilize nitrogen-containing compounds released during the degradation process as a source of nitrogen for their growth and metabolism. Thus, some of the DON in soil may be assimilated by these microorganisms, leading to a decrease in DON concentrations | 76 |
| The degradation of BPs can alter soil properties, such as pH, water content, and O2 availability. These alterations can influence the chemical and biological processes that control nitrogen cycling in soils, potentially leading to alterations in the forms and concentrations of DON | ||
| The presence of BPs in soil increases the availability of carbon and other nutrients, which can increase microbial activity. This may lead to increased nitrogen mineralization, whereby organic nitrogen compounds are converted into inorganic nitrogen compounds (such as NH4+ ions), that are subsequently taken up by plants or immobilized by microbes, potentially reducing DON concentrations in soil | ||
| NO3−–N and NH4+–N content | BPs, especially MPs derived from the breakdown of PBAT may influence soil nitrogen dynamics, potentially affecting the nitrification process | 90 |
| N losses | BPs may alter the soil structure and porosity, leading to increases in the abundances of denitrifying bacteria and thus increases in nitrogen loss | 91 |
| pH | When BPs are broken down into macro-sized particles and micro-sized particles (i.e., MPs) that infiltrate soil, these particles' large surface area enable them to effect cation exchange processes, leading to changes in soil pH | 66 |
| The presence of MPs may indirectly influence soil pH by modifying the structure and function of soil microbial communities. Specifically, changes in microbial dynamics can have cascading effects on soil processes that may lead to shifts in soil pH | ||
| Trace elements mobility in soils | Compared with petroleum-based plastic (i.e., PE) MPs, BMPs (i.e., PBAT) are smaller and have rougher surfaces and different cracking patterns and functional groups, which can lead to their higher sorption capacities for heavy metals, such as copper. This can result in alterations in the mobility and availability of heavy metals in soil containing BMPs | 90 |
| Microbial community richness and diversity | The disintegration and formation of biofilms were found to be more pronounced on BMPs than on petroleum-based MPs, which could result in greater alterations in microbial community structures in soils | 66 and 83 |
| The distinct chemical compositions and surface characteristics of BPs can favor the proliferation of different microorganism species in soil, resulting in variations in their abundance in soils | ||
| Alterations in soil physicochemical properties resulting from the degradation of BPs may indirectly impact their microbial activities and community structures | 92 | |
| Increases in the concentrations of available carbon due to the release of BP degradation by-products into soils result in the growth of soil microorganisms. Moreover, as BPs are exogenous carbon sources, they provide selective niches for soil microorganisms | 76 and 93 | |
| Degradation of BPs in soil could affect airflow in soils and contribute to the high abundance of ammonia-oxidizing archaea-like organisms, which are involved in nitrification | 94 | |
| BPs can stimulate nutrient turnover, thereby increasing bacterial growth over time | 95 | |
| The presence of BP mulch films can create favorable microclimatic conditions and optimized soil water storage, thereby increasing bacterial diversity | 96 | |
| Soil enzymes | Changes in enzyme activities in soil are largely induced by changes in the physicochemical properties of soil that occur because of BP degradation | 94 and 97 |
| Impacts on animals and plants | Degradation of BPs in soil can cause changes in the physiochemical and biological properties of soil that negatively affect plant health | 98 |
| Degradation of BPs in soil can increase the intracellular concentrations of reactive oxygen species and impair membrane integrity or functioning of organisms | 99 and 100 | |
| Particulate-induced toxicity of PLA–MPs in organisms such as zebrafish (Danio rerio) and a species of water flea (Daphnia magna) may be due to the release of additives, monomers, and toxic intermediates during the relatively rapid degradation of BPs | 51 and 101 | |
| Changes in surface properties (e.g., increases in hydrophilicity) or the introduction of oxygen-containing surface groups during the degradation of PLA-like MPs may lead to oxidative damage in organisms such as Danio rerio. Thus, the ecotoxicity of MPs can increase during their degradation | 100 | |
| Products and secondary metabolites formed during the degradation of BPs may inhibit seed germination and plant growth, and adversely affect root health | 102 and 103 | |
| Reductions in shoot and root growth, and fruit biomass of common bean-like crops are attributable to BP-induced alterations in rhizosphere bacterial communities | 104 | |
| Compared with normal degradation of BPs, accelerated degradation of BPs can lead to greater alterations in the soil structure and stability and greater biological effects, all of which can affect soil flora and fauna | 105 |
5.2. Effects of BP degradation on crop growth
The impact of BP degradation on crop growth is a multifaceted issue that requires a comprehensive examination within the context of the broader soil ecosystem. As BPs degrade in soils, they release diverse byproducts and breakdown components, which influence soil health and, consequently, plant growth. These effects manifest through changes in the soil structure, nutrient availability, water retention capacity, microbial activity, and various soil processes. Depending on the specific BP type and environmental conditions, these alterations can either promote or inhibit crop growth.One of the major concerns regarding BP degradation is its influence on soil physical properties, which directly affect plant growth. The physical presence of degraded plastic residues in soil can alter root penetration, soil aeration, and moisture distribution, critical factors in determining crop productivity.66,106–108 The formation of BMPs from BP breakdown may lead to soil compaction, reduced porosity, and restricted root development, ultimately impeding nutrient absorption and water uptake. Furthermore, weathered BP fragments can create barriers in soil pores, affecting water infiltration and retention, which may further impact plant growth and yield.
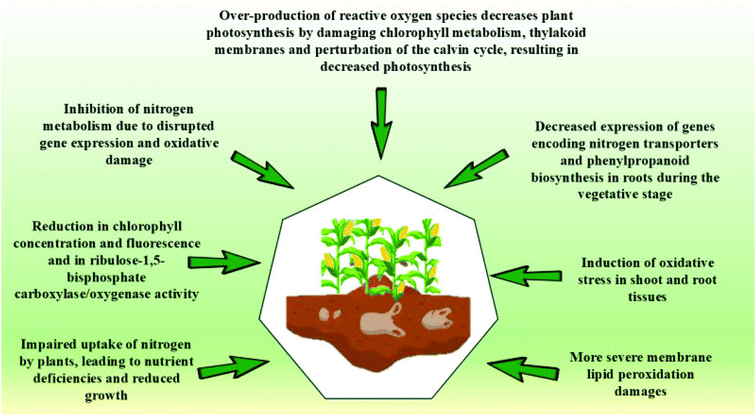
Beyond physical modifications, BP degradation also releases chemical compounds that may interact with plant physiological processes. While some degradation byproducts may act as growth stimulants, others could inhibit seed germination, root elongation, or nutrient uptake. Yang and Gao108 found that MPs derived from PBAT-based biodegradable mulch films altered nitrogen metabolism and photosynthesis in rice plants. Exposure to these MPs downregulated genes responsible for nitrogen transportation and phenylpropanoid biosynthesis in rice roots, leading to oxidative stress in both roots and shoots. Similarly, Liu et al.106 demonstrated that 2% PBAT-derived MPs had a more pronounced inhibitory effect on Arabidopsis thaliana's growth and photosynthetic system compared to PE–MPs after 14 and 28 days.
Further evidence suggests that biodegradable mulch film residues can significantly hinder plant growth. Serrano-Ruiz et al.109 reported that fragments of polyhydroxybutyrate-based (PHB) biodegradable mulch films reduced tomato and lettuce growth by 90% and 95%, respectively. Additionally, both pristine and field-weathered BP fragments delayed lettuce development, with weathered fragments exhibiting more severe effects than unweathered ones. These findings suggest that the impact of BP degradation on plants is influenced not only by their physical presence but also by their chemical composition and environmental changes.
Another pressing concern is the potential uptake of MPs by plants, which raises food security and safety issues. While most research has focused on MP contamination in soil and its effects on microbial communities, recent studies indicate that MPs and plastic nanoparticles (<100 nm) can be absorbed by plant roots and translocated to aboveground tissues.68 This has been corroborated by the detection of MPs in commonly consumed fruits and vegetables such as apples, pears, broccoli, lettuce, and carrots from local markets.110 MPs follow similar absorption and translocation pathways as carbon nanomaterials, infiltrating plant tissues through root uptake, seed penetration, and capillary action. The accumulation of MPs in edible crops increases the risk of human exposure through dietary consumption, emphasizing the need for further studies on the potential health implications of BP degradation products entering the food chain.
Given these complexities, it is essential to consider the degradation of BPs not as an isolated event but as part of a dynamic soil–plant system (Fig. 5). While BP mulch films and other BPs offer potential benefits for sustainable agriculture, their long-term effects on soil health and crop productivity remain an area of active research and interest. Investigations should aim to understand how BP degradation products influence plant–soil interactions over time and whether mitigation strategies can minimize negative impacts while maximizing the benefits of biodegradable materials in agricultural applications.
6. Analysis of biodegradable plastic degradation in soils
The assessment of BPs in terms of their degradation is critical for ensuring their sustainability and credibility. Accredited agencies play an important role in evaluating these materials, adhering to international standards to ensure reproducibility and reliability of results. However, the assessment methods may vary across different nations and regulatory bodies, reflecting regional differences in climate, soil productivity, and acidity. Globally, several standardization frameworks guide the evaluation of BP degradation. In Europe, EN standards regulate biodegradability testing, while the ASTM standards apply in the United States, AS standards in Australia, NFT standards in France, and ISO standards internationally. Compliance with these standards is verified by certification marks such as Germany's DIN CERTCO, Belgium's OK Compost, and the European Union's Seedling logo, which indicate adherence to specific biodegradability requirements.BPs undergo degradation in both terrestrial and marine environments, each requiring distinct evaluation methods. Terrestrial biodegradability is assessed using standards such as ISO 14855, EN 13432, ASTM D6400, EN 14995, and ISO 17088, which outline protocols for measuring degradation under controlled composting conditions. Among these, ISO 14855 is widely emphasized for its ability to replicate anaerobic composting environments, measuring CO2 evolution to assess the breakdown of organic compounds.
For an analysis under ISO 14855 to be considered valid, the following four criteria must be met:
(1) Stable CO2 emissions throughout the testing period.
(2) Biodegradability exceeding 70% within 45 days.
(3) Minimal variability (<20%) in biodegradability among different test materials.
(4) CO2 emissions between 50 and 150 mg g−1 of volatile solids over a 10 day period.
In contrast, the evaluation of marine biodegradability remains less standardized and universally accepted. Standards such as ASTM D6691, ASTM D7473, and ISO 16221 guide the assessment of BP degradation in marine ecosystems. However, due to the variability in oceanic conditions, such as temperature fluctuations, microbial diversity, and water movement, marine degradation results are often inconsistent and more challenging to validate compared to terrestrial environments.
To quantify the extent of BP degradation in soils, researchers employ a variety of analytical methods. The mass-balance method, which calculates the percentage of mass reduction before and after degradation, is a widely used approach. This method involves weight loss measurements using an analytical balance, a simple yet effective technique, though it poses challenges in extrapolation and upscaling. To complement mass-balance analysis, scientists employ advanced analytical techniques to gain deeper insights into BP degradation mechanisms. Respirometric methods are widely used to indirectly measure microbial respiration rates by monitoring CO2 emissions, providing valuable data on biodegradation kinetics.111 Fourier Transform Infrared Spectroscopy (FTIR) helps identify changes in chemical bonds, offering insights into the breakdown of polymer structures, while Nuclear Magnetic Resonance (NMR) spectroscopy provides molecular-level information on the chemical transformations occurring during degradation.27 Additionally, mechanical property assessments evaluate variations in tensile strength, elasticity, flexibility, and surface morphology, revealing structural changes in BPs over time. Among these techniques, respirometry plays a particularly important role by precisely measuring CO2 evolution as an indicator of microbial activity. This method typically employs sensors and computerized control programs to ensure accurate data collection and analysis, enhancing the reliability of biodegradability assessments.111
By integrating multiple analytical approaches, researchers can achieve a comprehensive understanding of BP degradation in soils, underlying mechanisms, and effects on its mechanical and chemical properties. This knowledge is essential for refining biodegradability assessment protocols and guiding the development of sustainable BP materials that minimize environmental impacts.
7. Laboratory-based certification protocols for biodegradable plastics
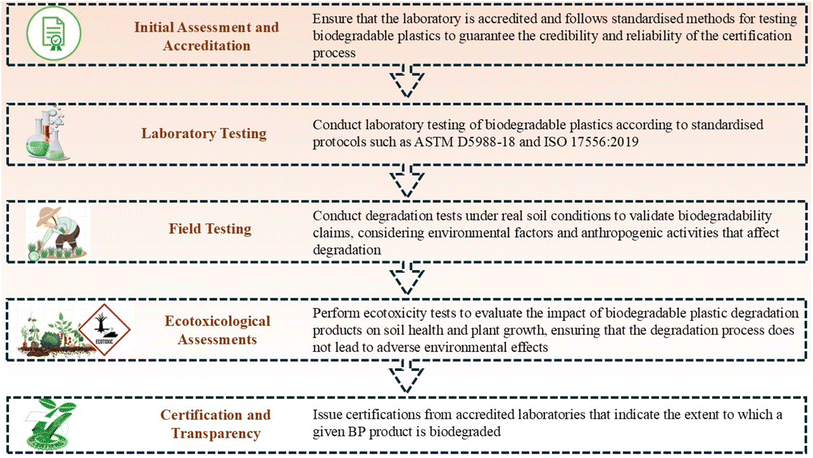
Global industries generally label their BP-based products as “100% biodegradable”, “marine biodegradable”, or “soil biodegradable” without providing valid scientific data to support these assertions. It is important to analyze BP degradation in real soil environments rather than under controlled industrial conditions with high temperatures and overly abundant microbial communities. Real soil conditions in natural environments differ significantly from controlled settings and soils are the primary reservoir of waste including BPs.Therefore, we proposed a laboratory-based certification protocol for BPs to ensure their degradation and sustainability in soil environments (Fig. 6). This certification should be conducted in accredited laboratories by universally acknowledged methods to ensure consistency across tests. Products claiming 100% biodegradability should be assessed under actual soil conditions to validate their claims. The current standard methodologies for assessing plastic degradation in soil, such as ASTM D5988-18 (ref. 112) and ISO 17556:2019,113 involve quantifying CO2 production and monitoring O2 consumption over time. These methods include burying the BP samples in closed vessels with prepared soil to create conditions conducive to microbial growth. However, variation in testing procedures has yielded a wide range of reported degradation values for similar biomaterials. This challenges the establishment of unique degradation values for each material. Furthermore, these standards may not include all variables influencing plastic degradation.111
To address these challenges, a synergistic approach utilizing various methodologies is necessary, and establishing an international standard that complements the existing ISO and ASTM standards is imperative. This would ensure a more accurate characterization of the biodegradability of each BP entering the market. This, in turn, can support informed decisions regarding disposal techniques to ensure complete and safe degradation. To further verify the test results, degradation tests should be conducted under field conditions. In real soil environments, the numerous environmental and soil management factors are not controlled as in laboratory settings. Factors such as soil erosion, water flow, drought, and human activities including machinery use, plowing, and soil tillage can directly impact the disintegration and degradation of BPs in soils. Unlike laboratory conditions, where optimal parameters such as moisture (e.g., 60% of water holding capacity) and temperature (e.g., 25 °C) are maintained consistently throughout the testing period, real environmental conditions are in constant flux, highly variable, and unpredictable. Soil ecosystems are subject to extreme weather events such as droughts and floods which may further complicate degradation processes. Moreover, soil properties vary significantly across sites. This adds to the complexity of understanding soil characteristics that are mostly conducive to BP degradation. Given these challenges, conducting degradation experiments under both laboratory and field conditions is recommended to obtain a comprehensive understanding of the degradation process and its environmental implications.
For BPs to serve as viable alternatives to petroleum-based plastics, it is important to ensure their complete degradability without causing hazardous ecotoxicological effects. As BPs break down, they release various elements into soils that can hinder or promote plant growth. This highlights the importance of robust ecotoxicological assessments. Seed germination and plant growth bioassays are commonly used only to assess plant ecotoxicity. In these tests, biopolymers are buried in soil at high concentrations and left to degrade naturally. Soil samples are collected periodically and tested for ecotoxicity using bioassays. Control samples, both exposed and unexposed to biopolymers are included. A substance generally recognized as safe (GRAS), such as cellulose, is used as a benchmark. The overall objective is to identify the significant differences between the test and control samples to ensure that degradation did not result in adverse ecotoxic effects. This rigorous assessment helped determine the safety and environmental impact of BP degradation and associated products.114
The degradation test under real field conditions should focus on the following:
(1) Variation in soil physical, chemical, and biological properties.
(2) Variation in mechanical properties of BPs/products.
(3) Toxicity test results associated with secondary products released during degradation.
These test results provide a comprehensive understanding of the effects of BP degradation in soils. This ensures that degradation and associated products do not adversely affect soil properties, soil health, crop growth, and overall food security. Based on research data (laboratory- and field-based) obtained from accredited laboratories, a certification can be issued for the respective BP or product to indicate the degree of degradation achieved (e.g., “60% degradation” or “75% degradation”). Thus, this is essential to prevent stating misleading information such as “100% degradation” unless it can be fully verified and substantiated. The certification report should also include quality assurance details including the analysis conditions such as soil properties, experimental conditions, and test duration. This information should be made available to all parties interested in utilizing these products. The test results should also be accessible to these parties. This ensures complete transparency and informs consumers regarding product sustainability. Importantly, these test results can be included in the annual sustainability reports released by industries.
8. Knowledge gaps related to biodegradable plastics in soils and future research directions
While BPs are gaining increasing attention as a sustainable alternative to conventional plastics, significant knowledge gaps remain regarding their degradation in soils. One critical area of uncertainty is how soil properties are affected by BP degradation. Current research primarily focuses on BP degradation in controlled environments, such as compost and marine settings, but the environmental impacts of BP degradation in soils, where conditions are more variable, remain understudied. Soil ecosystems are complex, and factors such as the microbial community structure, soil texture, and moisture levels have not been adequately studied in relation to BP degradation. This highlights the urgent need for more comprehensive research that extends beyond laboratory models to include in situ field experiments. Field-based studies should be prioritized to validate laboratory results and provide a better understanding of how BPs degrade under environmentally relevant soil conditions.Another significant knowledge gap is the lack of understanding regarding which soil properties are most conducive to the complete degradation of BPs. For example, it remains uncertain whether sandy soils or soils with high organic content are more favorable for BP degradation. Similarly, the optimal soil pH for BP degradation is still not well understood. Future studies should focus on how soil characteristics, such as soil texture and moisture content influence BP degradation.
Additionally, research should explore how the surface properties of BP fragments, including micro- and nanoscale particles, affect their behavior in soils. These particles may act as contaminant carriers, influencing the environmental behavior of BP degradation products. It is essential to utilize environmentally relevant conditions to assess the real-world impacts of BP fragments on soil ecosystems.54 Field studies should be conducted at various sites with different soil properties (e.g., pH and organic content) to validate laboratory findings on BP degradation. BP samples can be buried at different soil depths and monitored over time for the degradation rate, microbial community shifts, and environmental factors like moisture and temperature. Then the soils can be collected periodically to assess changes in chemical properties and BP degradation.
Another area that requires attention is the environmental impact of BP degradation. Large-scale BP-producing industries are important in global production, yet their expansion raises concerns. Despite claims of 100% biodegradability, many BP products are composites, combining BPs with petrochemical-based plastics. This raises questions about the transparency of industry claims, and the potential for greenwashing, where products are marketed as environmentally friendly without meeting stringent environmental standards. Thus, future research should focus on scrutinizing the transparency and accuracy of industry claims, establishing clear guidelines for BP labeling, and investigating the true biodegradability of these products under various environmental conditions. The development of more stringent certification protocols is necessary to ensure that BP products meet regulatory guidelines.
Moreover, a significant gap in the current body of research is the absence of standardized methods for identifying and quantifying BP degradation in soils. Most current methods were designed for controlled laboratory conditions and cannot be directly applied to the diverse array of environments found in soils. It is essential to develop and validate methods that can accurately assess BP degradation in natural soil environments. This includes developing protocols to measure not just polymer breakdown but also the resulting biomass, an often-neglected aspect of biodegradation. Incorporating these protocols will help in understanding the fate of carbon during the degradation process, allowing for accurate carbon balance assessments. Recent studies, such as that by Trapp et al.115 have highlighted the importance of accounting for microbial biomass incorporation in degradation tests to prevent underestimating the persistence of chemicals in the environment. They evaluated the microbial turnover to biomass (MTB) approach for estimating biogenic non-extractable residues (bioNER) in degradation tests.
Furthermore, there is a pressing need for research into the impacts of climate change on BP degradation in soils. Climate change alters soil properties and microbial activity, potentially affecting the degradation rates and environmental impacts of BPs. For instance, increased temperatures or changing moisture levels could accelerate or hinder BP degradation, influencing GHG emissions and carbon footprints. Future studies should include climate change scenarios to assess the long-term degradation of BPs and their potential to contribute to or mitigate climate change.
For instance, Posen et al.116 developed a Life-Cycle Assessment (LCA) model featuring five pathways for plastic production in the United States, including both petroleum and renewable energy sources for biobased plastic production. Their findings indicate that corn-based bioplastics, manufactured using conventional energy, reduce GHG emission by 16 Mt CO2e per year. This reduction could potentially reach 38 Mt CO2e per year with the utilization of low-carbon energy during production. Based on MRIO-based assessment by Jin et al.117 an 18.4 Mt CO2e emission reduction from the use of biobased plastics was suggested, aligning closely with the reported estimate of 16 Mt CO2e. Another study employing the LCA methodology indicates that substituting a significant portion of petroleum-based plastics with biobased alternatives could lead to emission reductions, ranging between 241 and 316 Mt CO2e per year.118 Transitioning to a 20% bioplastic adoption rate could result in a reduction of 77 Mt of GHG emissions. Notably, while completely replacing petroleum-based plastics with bioplastics could yield a reduction of 369 Mt in GHG emissions, it may also lead to increased land use and job creation. However, expanding the cultivation areas for bioplastics may inadvertently lead to additional carbon emissions.117 Overall, the transition from traditional plastics to bioplastics appears to offer environmental benefits in terms of reduced GHG emission, albeit with associated increases in land use and job opportunities. However, careful consideration of the potential impacts on land use and carbon emissions is necessary, particularly with regards to the expansion of cultivation areas for bioplastic production.
In terms of waste management, the presence of BP products that are not purely biodegradable but are composed of a mixture of petrochemical plastics presents significant challenges. Research must focus on developing strategies to manage these mixed BP products, as they complicate recycling and waste disposal efforts. Laboratory trials and field-scale studies are essential to better understand the fate, occurrence, distribution, and release pathways of these mixed BP products.13 A comprehensive certification protocol for BPs, as proposed in this study (Fig. 6), could be valuable in ensuring that BP products undergo proper testing before entering the market. Additionally, to promote responsible and informed decision-making, there is a need for a standardized method to analyze BP degradation in soils, which industries could adopt before releasing BP-based products. Further research aimed at identifying effective strategies for handling and disposing of these mixed BP products is strongly encouraged.
In addition, ecotoxicological studies on BP and BMPs in soil ecosystems are currently limited. Much of the existing research has focused on common materials like PLA and PBAT, with limited attention given to a broader range of BP types and their ecological impacts. Future research should explore the potential biotoxicity of BMPs, investigating how they interact with soil microorganisms and their potential to release harmful chemicals during degradation. This research should extend beyond single-particle toxicity studies to encompass the impacts of weathering processes and co-exposure of other environmental pollutants. Such studies will help determine whether BMPs pose stronger ecological threats than traditional petrochemical-based MPs. For example, to evaluate the ecological risks of BP degradation, experiments can be designed to expose soil organisms (i.e., earthworms and nematodes) to soils undergoing BP degradation. Toxicity will be assessed by measuring the survival, growth, and reproduction rates of the organisms, along with changes in microbial diversity. Additionally, the potential leaching of harmful substances from BP materials and their bioaccumulation in soil organisms can be studied.
Furthermore, expanding the range of polymer types, sizes, and species in these studies is essential. Not all BPs behave similarly, and it is important to assess whether specific BP types are viable substitutes for conventional plastics. Studies should consider the full life cycle of BPs, examining not only their degradation but also their potential long-term environmental effects and the socio-economic implications of their widespread adoption. The surface charge of BP particles can be modified through chemical treatments to assess its impact on their mobility and interactions with contaminants. Column leaching experiments are proposed to track the movement of BP particles through soil columns, while sorption tests will measure how these particles interact with various contaminants. These studies are essential for understanding the behavior of BP particles in soil environments and their potential role as vectors for contaminant transport.
Addressing these knowledge gaps will be critical for ensuring the sustainable development of biodegradable plastics in soil and agricultural environments. Standardizing testing methods, understanding long-term environmental impacts, and conducting LCA are key to guiding future research and policymaking in this field. Additionally, public education efforts should be ramped up to correct misconceptions about the environmental friendliness of BPs and prevent improper waste disposal. By addressing these challenges and ensuring transparency in BP production and labeling, we can ensure the responsible development and use of BPs.
Data availability
All data used during the study appear in the submitted article.Author contributions
Piumi Amasha Withana: writing – original draft, writing – review & editing, validation, methodology, investigation. Xiangzhou Yuan: writing – review & editing. Darvin Im: writing – review & editing, writing – original draft. Yujin Choi: writing – review & editing, writing – original draft. Michael S. Bank: writing – review & editing, writing – original draft. Carol Sze Ki Lin: writing – review & editing. Sung Yeon Hwang: writing – review & editing, writing – original draft, methodology, supervision. Yong Sik Ok: writing – review & editing, writing – original draft, validation, supervision, methodology, investigation.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2025-00555967). This work was supported by the Technology Innovation Program (Project No. 00432915, Development of biodegradable polymer and their applications using high-performance enzyme activation technologies for acceleration of biodegradability) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2021-NR060142).References
- United Nations, End Plastic Pollution: Towards an International Legally Binding Instrument, 2022, cited 2024. 10.12, available from: https://www.unep.org/inc-plastic-pollution Search PubMed.
- W. W. Lau, Y. Shiran, R. M. Bailey, E. Cook, M. R. Stuchtey, J. Koskella, C. A. Velis, L. Godfrey, J. Boucher, M. B. Murphy and R. C. Thompson, Evaluating scenarios toward zero plastic pollution, Science, 2020, 369(6510), 1455–1461 CrossRef CAS PubMed.
- C. Preininger, E. Hackl and V. Stagl, NETmicroplastic in agricultural soil and its impact on soil properties, Eur. J. Soil Sci., 2024, 75(3), e13496 CrossRef.
- I. Evangelou, D. Tatsii, S. Bucci and A. Stohl, Atmospheric resuspension of microplastics from bare soil regions, Environ. Sci. Technol., 2024, 58(22), 9741–9749 CrossRef CAS PubMed.
- Y. Yu, A. K. Battu, T. Varga, A. C. Denny, T. M. Zahid, I. Chowdhury and M. Flury, Minimal impacts of microplastics on soil physical properties under environmentally relevant concentrations, Environ. Sci. Technol., 2023, 57(13), 5296–5304 Search PubMed.
- N. Simon, K. Raubenheimer, N. Urho, S. Unger, D. Azoulay, T. Farrelly, J. Sousa, H. van Asselt, G. Carlini, C. Sekomo and M. L. Schulte, A binding global agreement to address the life cycle of plastics, Science, 2021, 373(6550), 43–47 Search PubMed.
- M. R. Havstad, Biodegradable Plastics, InPlastic Waste and Recycling, Academic Press, 2020, pp. 97–129 Search PubMed.
- L. G. Chem, HDPE (High Density Polyethylene), 2024, cited 2024. 10.12, available from: https://www.lgchem.com/product/PD00000006 Search PubMed.
- European Bioplastics, Bioplastics Market Development Update 2023, European Bioplastics, Berlin, 2024 Search PubMed.
- K. R. Hwang, W. Jeon, S. Y. Lee, M. S. Kim and Y. K. Park, Sustainable bioplastics: recent progress in the production of bio-building blocks for the bio-based next-generation polymer PEF, Chem. Eng. J., 2020, 390, 124636 Search PubMed.
- L. T. Korley, T. H. Epps III, B. A. Helms and A. J. Ryan, Toward polymer upcycling—adding value and tackling circularity, Science, 2021, 373(6550), 66–69 Search PubMed.
- Q. Xia, C. Chen, Y. Yao, J. Li, S. He, Y. Zhou, T. Li, X. Pan, Y. Yao and L. Hu, A strong, biodegradable and recyclable lignocellulosic bioplastic, Nat Sustainability, 2021, 4(7), 627–635 Search PubMed.
- N. M. Ainali, D. Kalaronis, E. Evgenidou, G. Z. Kyzas, D. C. Bobori, M. Kaloyianni, X. Yang, D. N. Bikiaris and D. A. Lambropoulou, Do poly (lactic acid) microplastics instigate a threat? A perception for their dynamic towards environmental pollution and toxicity, Sci. Total Environ., 2022, 832, 155014 CrossRef CAS PubMed.
- T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Plastics of the future? The impact of biodegradable polymers on the environment and on society, Angew. Chem., Int. Ed., 2019, 58(1), 50–62 Search PubMed.
- European Bioplastics, Bioplastics Market Development Update 2024, European Bioplastics, Berlin, 2025 Search PubMed.
- United Nations Trade and Development, UN Agencies at COP27 Urge Action to Tackle Impact of Plastic on Climate, UNCTAD, Geneva, 2022, cited 2024.10.12, available from: https://unctad.org/news/un-agencies-cop27-urge-action-tackle-impact-plastic-climate Search PubMed.
- L. Hu, Y. Zhou, Z. Chen, D. Zhang and X. Pan, Oligomers and monomers from biodegradable plastics: an important but neglected threat to ecosystems, Environ. Sci. Technol., 2023, 57(27), 9895–9897 CrossRef CAS PubMed.
- C. Accinelli, H. K. Abbas, W. T. Shier, A. Vicari, N. S. Little, M. R. Aloise and S. Giacomini, Degradation of microplastic seed film-coating fragments in soil, Chemosphere, 2019, 226, 645–650 Search PubMed.
- A. Mo, Y. Zhang, W. Gao, J. Jiang and D. He, Environmental fate and impacts of biodegradable plastics in agricultural soil ecosystems, Appl. Soil Ecol., 2023, 181, 104667 Search PubMed.
- K. J. Jem and B. Tan, The development and challenges of poly (lactic acid) and poly (glycolic acid), Adv. Ind. Eng. Polym. Res., 2020, 3(2), 60–70 Search PubMed.
- S. Mangaraj, A. Yadav, L. M. Bal, S. K. Dash and N. K. Mahanti, Application of biodegradable polymers in food packaging industry: a comprehensive review, J. Packag. Technol. Res., 2019, 3, 77–96 Search PubMed.
- V. U. Reddy, S. V. Ramanaiah, M. V. Reddy and Y. C. Chang, Review of the developments of bacterial medium-chain-length polyhydroxyalkanoates (mcl-PHAs), Bioengineering, 2022, 9(5), 225 Search PubMed.
- H. Li, L. Sui and Y. Niu, Preparation and properties of a double-coated slow-release urea fertilizer with poly (propylene carbonate), a sodium polyacrylate hydroscopicity resin and sodium alginate, ChemistrySelect, 2018, 3(26), 7643–7647 Search PubMed.
- E. Moshkbid, D. E. Cree, L. Bradford and W. Zhang, Biodegradable alternatives to plastic in medical equipment: current state, challenges, and the future, J. Compos. Sci., 2024, 8(9), 342 CrossRef CAS.
- S. Sahoo, W. Rathod, H. Vardikar, M. Biswal, S. Mohanty and S. K. Nayak, Biomedical waste plastic: bacteria, disinfection and recycling technologies—a comprehensive review, Int. J. Environ. Sci. Technol., 2024, 21(1), 1141–1158 CrossRef CAS PubMed.
- I. Damikouka and O. Georgiadou, Plastics, Bioplastics and Water Pollution, Environ. Sci. Proc., 2023, 26(1), 144 Search PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, Degradation rates of plastics in the environment, ACS Sustain. Chem. Eng., 2020, 8(9), 3494–3511 Search PubMed.
- B. Tanunchai, S. Kalkhof, V. Guliyev, S. F. Wahdan, D. Krstic, M. Schädler, A. Geissler, B. Glaser, F. Buscot, E. Blagodatskaya and M. Noll, Nitrogen fixing bacteria facilitate microbial biodegradation of a bio-based and biodegradable plastic in soils under ambient and future climatic conditions, Environ. Sci.: Processes Impacts, 2022, 24(2), 233–241 Search PubMed.
- M. Qin, C. Chen, B. Song, M. Shen, W. Cao, H. Yang, G. Zeng and J. Gong, A review of biodegradable plastics to biodegradable microplastics: another ecological threat to soil environments?, J. Clean. Prod., 2021, 312, 127816 CrossRef CAS.
- M. E. César, P. D. Mariani, L. H. Innocentini-Mei and E. J. Cardoso, Particle size and concentration of poly (ε-caprolactone) and adipate modified starch blend on mineralization in soils with differing textures, Polym. Test., 2009, 28(7), 680–687 Search PubMed.
- M. Mazzon, P. Gioacchini, D. Montecchio, S. Rapisarda, C. Ciavatta and C. Marzadori, Biodegradable plastics: effects on functionality and fertility of two different soils, Appl. Soil Ecol., 2022, 169, 104216 Search PubMed.
- A. Beltrán-Sanahuja, A. Benito-Kaesbach, N. Sánchez-García and C. Sanz-Lázaro, Degradation of conventional and biobased plastics in soil under contrasting environmental conditions, Sci. Total Environ., 2021, 787, 147678 Search PubMed.
- Y. Chen, B. Gao, Y. Yang, Z. Pan, J. Liu, K. Sun and B. Xing, Tracking microplastics biodegradation through CO2 emission: role of photoaging and mineral addition, J. Hazard. Mater., 2022, 439, 129615 CrossRef CAS PubMed.
- L. Ding, X. Yu, X. Guo, Y. Zhang, Z. Ouyang, P. Liu, C. Zhang, T. Wang, H. Jia and L. Zhu, The photodegradation processes and mechanisms of polyvinyl chloride and polyethylene terephthalate microplastic in aquatic environments: important role of clay minerals, Water Res., 2022, 208, 117879 Search PubMed.
- A. O. El, Anaerobic biodegradation: the anaerobic digestion process, in Handbook of Biodegradable Materials, Springer International Publishing, Cham, 2022, pp. 1–26 Search PubMed.
- D. Griffin-LaHue, S. Ghimire, Y. Yu, E. J. Scheenstra, C. A. Miles and M. Flury, In-field degradation of soil-biodegradable plastic mulch films in a Mediterranean climate, Sci. Total Environ., 2022, 806, 150238 Search PubMed.
- H. Chen, Y. Wang, X. Sun, Y. Peng and L. Xiao, Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function, Chemosphere, 2020, 243, 125271 Search PubMed.
- Y. Huo, F. A. Dijkstra, M. Possell and B. Singh, Mineralisation and priming effects of a biodegradable plastic mulch film in soils: influence of soil type, temperature and plastic particle size, Soil Biol. Biochem., 2024, 189, 109257 CrossRef CAS.
- H. Y. Sintim, A. I. Bary, D. G. Hayes, L. C. Wadsworth, M. B. Anunciado, M. E. English, S. Bandopadhyay, S. M. Schaeffer, J. M. DeBruyn, C. A. Miles and J. P. Reganold, In situ degradation of biodegradable plastic mulch films in compost and agricultural soils, Sci. Total Environ., 2020, 727, 138668 CrossRef CAS PubMed.
- P. D. Dissanayake, P. A. Withana, M. K. Sang, Y. Cho, J. Park, D. X. Oh, S. X. Chang, C. S. Lin, M. S. Bank, S. Y. Hwang and Y. S. Ok, Effects of biodegradable poly (butylene adipate-co-terephthalate) and poly (lactic acid) plastic degradation on soil ecosystems, Soil Use Manage., 2024, 40(2), e13055 Search PubMed.
- D. Adhikari, M. Mukai, K. Kubota, T. Kai, N. Kaneko, K. S. Araki and M. Kubo, Degradation of bioplastics in soil and their degradation effects on environmental microorganisms, J. Agric. Chem. Environ., 2016, 5(1), 23–34 Search PubMed.
- J. C. Sanchez-Hernandez, Y. Capowiez and K. S. Ro, Potential use of earthworms to enhance decaying of biodegradable plastics, ACS Sustain. Chem. Eng., 2020, 8(11), 4292–4316 Search PubMed.
- I. E. Napper and R. C. Thompson, Environmental deterioration of biodegradable, oxo-biodegradable, compostable, and conventional plastic carrier bags in the sea, soil, and open-air over a 3-year period, Environ. Sci. Technol., 2019, 53(9), 4775–4783 Search PubMed.
- J. Liao and Q. Chen, Biodegradable plastics in the air and soil environment: low degradation rate and high microplastics formation, J. Hazard Mater., 2021, 418, 126329 Search PubMed.
- R. Kaur and I. Chauhan, Biodegradable plastics: mechanisms of degradation and generated bio microplastic impact on soil health, Biodegradation, 2024, 35(6), 863–892 Search PubMed.
- P. A. Withana, J. Li, S. S. Senadheera, C. Fan, Y. Wang and Y. S. Ok, Machine learning prediction and interpretation of the impact of microplastics on soil properties, Environ. Pollut., 2024, 341, 122833 Search PubMed.
- Y. Qi, A. Ossowicki, X. Yang, E. H. Lwanga, F. Dini-Andreote, V. Geissen and P. Garbeva, Effects of plastic mulch film residues on wheat rhizosphere and soil properties, J. Hazard. Mater., 2020, 387, 121711 CrossRef CAS PubMed.
- S. Chinaglia, M. Tosin and F. Degli-Innocenti, Biodegradation rate of biodegradable plastics at molecular level, Polym. Degrad. Stabil., 2018, 147, 237–244 CrossRef CAS.
- K. Gunaalan, E. Fabbri and M. Capolupo, The hidden threat of plastic leachates: a critical review on their impacts on aquatic organisms, Water Res., 2020, 184, 116170 CrossRef CAS PubMed.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard Mater., 2018, 344, 179–199 Search PubMed.
- L. Zimmermann, S. Göttlich, J. Oehlmann, M. Wagner and C. Völker, What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna, Environ. Pollut., 2020, 267, 115392 Search PubMed.
- M. Rujnić-Sokele and A. Pilipović, Challenges and opportunities of biodegradable plastics: a mini review, Waste Manag. Res., 2017, 35(2), 132–140 CrossRef PubMed.
- Y. Wang, F. Wang, L. Xiang, Y. Bian, Z. Wang, P. Srivastava, X. Jiang and B. Xing, Attachment of positively and negatively charged submicron polystyrene plastics on nine typical soils, J. Hazard. Mater., 2022, 431, 128566 CrossRef CAS PubMed.
- X. D. Sun, X. Z. Yuan, Y. Jia, L. J. Feng, F. P. Zhu, S. S. Dong, J. Liu, X. Kong, H. Tian, J. L. Duan and Z. Ding, Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana, Nat. Nanotechnol., 2020, 15(9), 755–760 CrossRef CAS PubMed.
- E. A. MacAfee and A. J. Löhr, Multi-scalar interactions between mismanaged plastic waste and urban flooding in an era of climate change and rapid urbanization, Wiley Interdiscip. Rev.:Water, 2024, 11(2), e1708 CrossRef.
- M. Tosin, A. Pischedda and F. Degli-Innocenti, Biodegradation kinetics in soil of a multi-constituent biodegradable plastic, Polym. Degrad. Stab., 2019, 166, 213–218 CrossRef CAS.
- M. T. Zumstein, A. Schintlmeister, T. F. Nelson, R. Baumgartner, D. Woebken, M. Wagner, H. P. Kohler, K. McNeill and M. Sander, Biodegradation of synthetic polymers in soils: tracking carbon into CO2 and microbial biomass, Sci. Adv., 2018, 4(7), eaas9024 CrossRef CAS PubMed.
- M. Sander, Biodegradation of polymeric mulch films in agricultural soils: concepts, knowledge gaps, and future research directions, Environ. Sci. Technol., 2019, 53(5), 2304–2315 CrossRef CAS PubMed.
- F. Muroi, Y. Tachibana, Y. Kobayashi, T. Sakurai and K. I. Kasuya, Influences of poly (butylene adipate-co-terephthalate) on soil microbiota and plant growth, Polym. Degrad. Stab., 2016, 129, 338–346 Search PubMed.
- M. M. Abe, M. C. Branciforti and M. Brienzo, Biodegradation of hemicellulose-cellulose-starch-based bioplastics and microbial polyesters, Recycling, 2021, 6(1), 22 Search PubMed.
- S. Bi, B. Tan, J. L. Soule and M. J. Sobkowicz, Enzymatic degradation of poly (butylene succinate-co-hexamethylene succinate), Polym. Degrad. Stab., 2018, 155, 9–14 CrossRef CAS.
- M. Liu, T. Zhang, L. Long, R. Zhang and S. Ding, Efficient enzymatic degradation of poly (ε-caprolactone) by an engineered bifunctional lipase-cutinase, Polym. Degrad. Stab., 2019, 160, 120–125 CrossRef CAS.
- N. A. Mahmoud, A. M. Yasien, D. H. Swilam, M. M. Gamil and S. T. Ahmed, Impacts of biodegradable plastic on the environment, in Handbook of Biodegradable Materials, Springer International Publishing, Cham, 2023, pp. 811–837 Search PubMed.
- Y. Jin, F. Cai, C. Song, G. Liu and C. Chen, Degradation of biodegradable plastics by anaerobic digestion: morphological, micro-structural changes and microbial community dynamics, Sci. Total Environ., 2022, 834, 155167 Search PubMed.
- Y. Luo, Z. Zhang, R. Naidu, X. Zhang and C. Fang, Raman imaging of microplastics and nanoplastics released from the printed toner powders burned by a mimicked bushfire, Sci. Total Environ., 2022, 849, 157686 Search PubMed.
- F. Wang, X. Zhang, S. Zhang, S. Zhang and Y. Sun, Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil, Chemosphere, 2020, 254, 126791 CrossRef CAS PubMed.
- M. Capolupo, L. Sørensen, K. D. Jayasena, A. M. Booth and E. Fabbri, Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms, Water Res., 2020, 169, 115270 Search PubMed.
- B. Jadhav and A. Medyńska-Juraszek, Microplastic and nanoplastic in crops: possible adverse effects to crop production and contaminant transfer in the food chain, Plants, 2024, 13(17), 2526 Search PubMed.
- Y. Li, L. Chen, N. Zhou, Y. Chen, Z. Ling and P. Xiang, Microplastics in the human body: a comprehensive review of exposure, distribution, migration mechanisms, and toxicity, Sci. Total Environ., 2024, 22, 174215 CrossRef PubMed.
- H. Jia, M. Zhang, Y. Weng, Y. Zhao, C. Li and A. Kanwal, Degradation of poly (butylene adipate-co-terephthalate) by Stenotrophomonas sp. YCJ1 isolated from farmland soil, J. Environ. Sci., 2021, 103, 50–58 CrossRef CAS PubMed.
- K. Janczak, K. Hrynkiewicz, Z. Znajewska and G. Dąbrowska, Use of rhizosphere microorganisms in the biodegradation of PLA and PET polymers in compost soil, Int. Biodeterior. Biodegrad., 2018, 130, 65–75 CrossRef CAS.
- S. Guo, L. Mu, S. Sun, X. Hou, M. Yao and X. Hu, Concurrence of microplastics and heat waves reduces rice yields and disturbs the agroecosystem nitrogen cycle, J. Hazard. Mater., 2023, 452, 131340 Search PubMed.
- S. Huang, T. Guo, Z. Feng, B. Li, Y. Cai, D. Ouyang, W. Gustave, C. Ying and H. Zhang, Polyethylene and polyvinyl chloride microplastics promote soil nitrification and alter the composition of key nitrogen functional bacterial groups, J. Hazard. Mater., 2023, 453, 131391 CrossRef CAS PubMed.
- W. Wang, Y. Xie, H. Li, H. Dong, B. Li, Y. Guo, Y. Wang, X. Guo, T. Yin, X. Liu and W. Zhou, Responses of lettuce (Lactuca sativa L.) growth and soil properties to conventional non-biodegradable and new biodegradable microplastics, Environ. Pollut., 2024, 341, 122897 CrossRef CAS PubMed.
- Z. Zhang, W. Wang, J. Liu and H. Wu, Discrepant responses of bacterial community and enzyme activities to conventional and biodegradable microplastics in paddy soil, Sci. Total Environ., 2024, 909, 168513 Search PubMed.
- J. Zhou, H. Gui, C. C. Banfield, Y. Wen, H. Zang, M. A. Dippold, A. Charlton and D. L. Jones, The microplastisphere: biodegradable microplastics addition alters soil microbial community structure and function, Soil Biol. Biochem., 2021, 156, 108211 Search PubMed.
- C. Sanz-Lázaro, N. Casado-Coy and A. Beltrán-Sanahuja, Biodegradable plastics can alter carbon and nitrogen cycles to a greater extent than conventional plastics in marine sediment, Sci. Total Environ., 2021, 756, 143978 Search PubMed.
- M. Shen, B. Song, G. Zeng, Y. Zhang, F. Teng and C. Zhou, Surfactant changes lead adsorption behaviors and mechanisms on microplastics, Chem. Eng. J., 2021, 405, 126989 CrossRef CAS.
- L. Z. Zuo, H. X. Li, L. Lin, Y. X. Sun, Z. H. Diao, S. Liu, Z. Y. Zhang and X. R. Xu, Sorption and desorption of phenanthrene on biodegradable poly (butylene adipate co-terephtalate) microplastics, Chemosphere, 2019, 215, 25–32 Search PubMed.
- Y. Sun, X. Wang, S. Xia and J. Zhao, New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation, Chem. Eng. J., 2021, 416, 129085 Search PubMed.
- Y. Wan, C. Wu, Q. Xue and X. Hui, Effects of plastic contamination on water evaporation and desiccation cracking in soil, Sci. Total Environ., 2019, 654, 576–582 Search PubMed.
- H. Y. Sintim, S. Bandopadhyay, M. E. English, A. Bary, J. E. y González, J. M. DeBruyn, S. M. Schaeffer, C. A. Miles and M. Flury, Four years of continuous use of soil-biodegradable plastic mulch: impact on soil and groundwater quality, Geoderma, 2021, 381, 114665 Search PubMed.
- Y. Qi, N. Beriot, G. Gort, E. H. Lwanga, H. Gooren, X. Yang and V. Geissen, Impact of plastic mulch film debris on soil physicochemical and hydrological properties, Environ. Pollut., 2020, 266, 115097 Search PubMed.
- X. J. Jiang, W. Liu, E. Wang, T. Zhou and P. Xin, Residual plastic mulch fragments effects on soil physical properties and water flow behavior in the Minqin Oasis, northwestern China, Soil Tillage Res., 2017, 166, 100–107 Search PubMed.
- M. C. Rillig, S. W. Kim and Y. G. Zhu, The soil plastisphere, Nat. Rev. Microbiol., 2024, 22(2), 64–74 Search PubMed.
- M. C. Rillig, Microplastic disguising as soil carbon storage, Science, 2018, 361(6400), 6079–6080 Search PubMed.
- B. Boots, C. W. Russell and D. S. Green, Effects of microplastics in soil ecosystems: above and below ground, Environ. Sci. Technol., 2019, 53(19), 11496–11506 CrossRef CAS PubMed.
- K. Gopalakrishnan and D. R. Kashian, Extracellular polymeric substances in green alga facilitate microplastic deposition, Chemosphere, 2022, 286, 131814 CrossRef CAS PubMed.
- Y. Sun, C. Duan, N. Cao, C. Ding, Y. Huang and J. Wang, Biodegradable and conventional microplastics exhibit distinct microbiome, functionality, and metabolome changes in soil, J. Hazard. Mater., 2022, 424, 127282 Search PubMed.
- R. Li, Y. Liu, Y. Sheng, Q. Xiang, Y. Zhou and J. V. Cizdziel, Effect of prothioconazole on the degradation of microplastics derived from mulching plastic film: apparent change and interaction with heavy metals in soil, Environ. Pollut., 2020, 260, 113988 Search PubMed.
- R. Ingraffia, G. Amato, M. Iovino, M. C. Rillig, D. Giambalvo and A. S. Frenda, Polyester microplastic fibers in soil increase nitrogen loss via leaching and decrease plant biomass production and N uptake, Environ. Res. Lett., 2022, 17(5), 054012 Search PubMed.
- B. Xu, F. Liu, Z. Cryder, D. Huang, Z. Lu, Y. He, H. Wang, Z. Lu, P. C. Brookes, C. Tang and J. Gan, Microplastics in the soil environment: occurrence, risks, interactions and fate–a review, Crit. Rev. Environ. Sci. Technol., 2020, 50(21), 2175–2222 CrossRef CAS.
- S. Bandopadhyay, L. Martin-Closas, A. M. Pelacho and J. M. DeBruyn, Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions, Front. Microbiol., 2018, 9, 819 Search PubMed.
- A. A. de Souza Machado, C. W. Lau, J. Till, W. Kloas, A. Lehmann, R. Becker and M. C. Rillig, Impacts of microplastics on the soil biophysical environment, Environ. Sci. Technol., 2018, 52(17), 9656–9665 Search PubMed.
- Y. Sun, C. Duan, N. Cao, X. Li, X. Li, Y. Chen, Y. Huang and J. Wang, Effects of microplastics on soil microbiome: the impacts of polymer type, shape, and concentration, Sci. Total Environ., 2022, 806, 150516 Search PubMed.
- S. Li, F. Ding, M. Flury, Z. Wang, L. Xu, S. Li, D. L. Jones and J. Wang, Macro-and microplastic accumulation in soil after 32 years of plastic film mulching, Environ. Pollut., 2022, 300, 118945 Search PubMed.
- Y. Fei, S. Huang, H. Zhang, Y. Tong, D. Wen, X. Xia, H. Wang, Y. Luo and D. Barceló, Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil, Sci. Total Environ., 2020, 707, 135634 CrossRef CAS PubMed.
- X. Jiang, H. Chen, Y. Liao, Z. Ye, M. Li and G. Klobučar, Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba, Environ. Pollut., 2019, 250, 831–838 CrossRef CAS PubMed.
- N. González-Soto, J. Hatfield, A. Katsumiti, N. Duroudier, J. M. Lacave, E. Bilbao, A. Orbea, E. Navarro and M. P. Cajaraville, Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis, Sci. Total Environ., 2019, 684, 548–566 Search PubMed.
- X. Zhang, M. Xia, X. Su, P. Yuan, X. Li, C. Zhou, Z. Wan and W. Zou, Photolytic degradation elevated the toxicity of polylactic acid microplastics to developing zebrafish by triggering mitochondrial dysfunction and apoptosis, J. Hazard. Mater., 2021, 413, 125321 Search PubMed.
- H. Serrano-Ruiz, L. Martin-Closas and A. M. Pelacho, Biodegradable plastic mulches: impact on the agricultural biotic environment, Sci. Total Environ., 2021, 750, 141228 Search PubMed.
- H. Serrano-Ruiz, L. Martin-Closas and A. M. Pelacho, Application of an in vitro plant ecotoxicity test to unused biodegradable mulches, Polym. Degrad. Stab., 2018, 158, 102–110 Search PubMed.
- W. Yang, P. Cheng, C. A. Adams, S. Zhang, Y. Sun, H. Yu and F. Wang, Effects of microplastics on plant growth and arbuscular mycorrhizal fungal communities in a soil spiked with ZnO nanoparticles, Soil Biol. Biochem., 2021, 155, 108179 Search PubMed.
- F. Meng, X. Yang, M. Riksen, M. Xu and V. Geissen, Response of common bean (Phaseolus vulgaris L.) growth to soil contaminated with microplastics, Sci. Total Environ., 2021, 755, 142516 CrossRef CAS PubMed.
- Y. Qi, X. Yang, A. M. Pelaez, E. H. Lwanga, N. Beriot, H. Gertsen, P. Garbeva and V. Geissen, Macro-and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth, Sci. Total Environ., 2018, 645, 1048–1056 Search PubMed.
- J. Liu, P. Wang, Y. Wang, Y. Zhang, T. Xu, Y. Zhang, J. Xi, L. Hou, L. Li, Z. Zhang and Y. Lin, Negative effects of poly (butylene adipate-co-terephthalate) microplastics on Arabidopsis and its root-associated microbiome, J. Hazard Mater., 2022, 437, 129294 Search PubMed.
- H. Y. Sintim and M. Flury, Is biodegradable plastic mulch the solution to agriculture's plastic problem?, Environ. Sci. Technol., 2017, 51(19), 1068–1069 Search PubMed.
- C. Yang and X. Gao, Impact of microplastics from polyethylene and biodegradable mulch films on rice (Oryza sativa L.), Sci. Total Environ., 2022, 828, 154579 Search PubMed.
- H. Serrano-Ruiz, L. Martin-Closas and A. M. Pelacho, Impact of buried debris from agricultural biodegradable plastic mulches on two horticultural crop plants: tomato and lettuce, Sci. Total Environ., 2023, 856, 159167 Search PubMed.
- G. O. Conti, M. Ferrante, M. Banni, C. Favara, I. Nicolosi, A. Cristaldi, M. Fiore and P. Zuccarello, Micro-and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population, Environmental Res., 2020, 187, 109677 CrossRef PubMed.
- S. Baidurah, Methods of analyses for biodegradable polymers: a review, Polymers, 2022, 14(22), 4928 Search PubMed.
- ASTM International, Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil, ASTM International, West Conshohocken, PA, 2018, pp. 1–6 Search PubMed.
- ISO, Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved, International Organization for Standardization, Geneva, 2019, ISO 17556:2019 Search PubMed.
- J. R. Pires, V. G. Souza, P. Fuciños, L. Pastrana and A. L. Fernando, Methodologies to assess the biodegradability of bio-based polymers—current knowledge and existing gaps, Polymers, 2022, 14(7), 1359 Search PubMed.
- S. Trapp, A. L. Brock, M. Kästner, A. Schäffer and D. Hennecke, Critical evaluation of the microbial turnover to biomass approach for the estimation of biogenic non-extractable residues (NER), Environ. Sci. Eur., 2022, 34(1), 15 Search PubMed.
- I. D. Posen, P. Jaramillo, A. E. Landis and W. M. Griffin, Greenhouse gas mitigation for US plastics production: energy first, feedstocks later, Environ. Res. Lett., 2017, 12(3), 034024 Search PubMed.
- Y. Jin, M. Lenzen, A. Montoya, B. Laycock, Z. Yuan, P. Lant, M. Li, R. Wood and A. Malik, Greenhouse gas emissions, land use and employment in a future global bioplastics economy, Resour. Conserv. Recycl., 2023, 193, 106950 Search PubMed.
- S. Spierling, E. Knüpffer, H. Behnsen, M. Mudersbach, H. Krieg, S. Springer, S. Albrecht, C. Herrmann and H. J. Endres, Bio-based plastics-a review of environmental, social and economic impact assessments, J. Clean. Prod., 2018, 185, 476–491 Search PubMed.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |