Beyond campus borders: wastewater surveillance sheds light on university COVID-19 interventions and their community impact†
David
Lisboa
 a,
Devrim
Kaya
ab,
Michael
Harry
a,
Casey
Kanalos
a,
Gabriel
Davis
a,
Oumaima
Hachimi
a,
Shana
Jaaf
a,
David
Mickle
a,
Dana
Alegre
c,
Katherine
Carter
c,
Steven
Carrell
c,
Mark
Dasenko
c,
Nathan
Davidson
c,
Justin
Elser
c,
Matthew
Geniza
c,
Anne-Marie
Girard
c,
Brent
Kronmiller
c,
Matthew
Peterson
a,
Devrim
Kaya
ab,
Michael
Harry
a,
Casey
Kanalos
a,
Gabriel
Davis
a,
Oumaima
Hachimi
a,
Shana
Jaaf
a,
David
Mickle
a,
Dana
Alegre
c,
Katherine
Carter
c,
Steven
Carrell
c,
Mark
Dasenko
c,
Nathan
Davidson
c,
Justin
Elser
c,
Matthew
Geniza
c,
Anne-Marie
Girard
c,
Brent
Kronmiller
c,
Matthew
Peterson
 c,
Elizabeth
Zepeda
c,
Christine
Kelly
a and
Tyler S.
Radniecki
c,
Elizabeth
Zepeda
c,
Christine
Kelly
a and
Tyler S.
Radniecki
 *a
*a
aSchool of Chemical, Biological and Environmental Engineering, Oregon State University, Corvallis, OR, USA. E-mail: tyler.radniecki@oregonstate.edu
bSchool of Public Health and SDSU-IV, San Diego State University, San Diego, CA, USA
cCenter for Quantitative Life Sciences, Oregon State University, Corvallis, OR, USA
First published on 5th July 2024
Abstract
The evaluation of COVID-19 policy effectiveness on university campuses, particularly in mitigating spread to neighboring cities (i.e., “campus spill-over”), is challenging due to asymptomatic transmission, biases in case reporting, and spatial case reporting limitations. Wastewater surveillance offers a less biased and more spatially precise alternative to conventional clinical surveillance, thus providing reliable data for university COVID-19 policy evaluation. Wastewater surveillance data spanning the academic terms from Fall 2020 through Spring 2022 was used to evaluate the impact of university COVID-19 policies. During the campus closure to external visitors (09/21/2020–9/15/2021), campus viral concentrations and variant compositions were dissimilar from those of the host and neighboring cities (MAPE = 0.25 ± 0.14; Bray–Curtis = 0.68 ± 0.1, respectively), indicating relative isolation of the campus from its surroundings. Upon the campus reopening to visitors (9/15/2021–2/27/2022), the viral concentrations and variant compositions matched more closely with the host and neighboring cities (MAPE = 0.21 ± 0.1; Bray–Curtis = 0.14 ± 0.08, respectively). Furthermore, post-lifting of campus and state mask mandates (2/27/2022–6/12/2022), the campus, host and neighboring city viral concentrations and variant compositions became indistinguishable (MAPE = 0.06 ± 0.02; Bray–Curtis = 0.07 ± 0.05, respectively). This data suggests that university COVID-19 policies effectively prevented campus-spill over, with no significant contribution to COVID-19 spread into the surrounding communities. Conversely, it was the surrounding communities that led to the spread of COVID-19 onto the campus. Therefore, wastewater surveillance proves instrumental in monitoring COVID-19 trends in surrounding areas, aiding in predicting the impact of easing campus restrictions on campus health.
Water impactThis study utilized wastewater surveillance to assess the efficacy of university COVID-19 policies to protect campus and community health. The findings demonstrated that university COVID-19 policies were effective at preventing campus spill-over to neighboring communities. This research highlights wastewater surveillance's potential as a vital tool in evaluating the effectiveness of university public health policies. |
1. Introduction
The implementation of university COVID-19 restrictions and policies has been heavily influenced by clinical testing as well as recommendations from local, city, and federal health officials. These policies, designed to mitigate the suspected and feared spread of COVID-19 into its surrounding communities (i.e. campus spill-over), included shifting to online-only instruction, enforcing social distancing, and mandating the use of masks on campus.1 While the effects of these COVID-19 policies in universities have been proven to reduce transmission,2–4 there has been limited effort in understanding how effective these policies were at preventing campus spill-over nor at how easing these restrictions affects the campus and surrounding communities.Traditional clinical surveillance methods, such as PCR testing, contact tracing, and isolating, have been essential in both identifying and isolating cases to prevent COVID-19 spread.5 However, there are significant limitations within this. These include asymptomatic transmission, underreporting, test availability, and time delays due to infection-to-symptom onset.6 Additionally, early COVID-19 testing were shown to disproportionately represent both Black and Hispanic populations due to geographic disparities of testing sites.7–9 Although clinical surveillance is useful in monitoring county and state-wide data, it often fails to capture the whole picture of COVID-19 viral transmission, especially within dynamic populations such as university campuses.
In contrast, wastewater surveillance offers a less biased and more spatially precise alternative.10–12 This method uses PCR-based approaches to quantify pathogens, like the SARS-CoV-2 virus, that are shed in an infected individual's stool and enters the waste stream after flushing. Additionally, pathogen variant composition can be identified via amplicon sequencing.13 This tool has been used to provide an accurate representation of the pathogenic activity within a neighborhood or community, providing an additional source of data to assist public health officials in making informed decisions on how to respond to the pandemic dynamics.14–18 Wastewater surveillance as a whole is cost-effective, non-invasive, and can be performed in areas where clinical testing is constrained.19,20
Despite its advantages, wastewater surveillance is also linked to potential biases and constraints such as variations in water use, rainwater infiltration and inundation (I and I), and sampling frequency. This study addresses these challenges through regular maintenance of autosamplers and excluding data from significant rainfall events. Additionally, SARS-CoV-2 quantification underwent rigorous quality control measures to mitigate the risk of inaccurate data.
Wastewater surveillance has been utilized to identify COVID-19 hotspots at the community scale12 and to provide initial screening efforts at the building scale.21 At the university level, wastewater surveillance has been successful in monitoring the viral burden of a campus community.20,22 Furthermore, wastewater surveillance has been effective as a tool to identify locations of positive individuals and provide intervention to contain outbreaks.21,23–25 However, to date, wastewater surveillance has not been used to evaluate the effectiveness of university COVID-19 policies.
A common concern of many living in university host cities was the increased risk of COVID-19 spreading through the community and neighboring communities due to the influx of out-of-area students.26–29 Universities responded to this concern, in addition to concern for the well-being of their students, faculty and staff, with a variety of COVID-19 policies. These policies included campus closures, isolation policies in residential halls for infected students, and mask mandates. However, quantifying the impact of college students entering into a community, as well as the effectiveness of university COVID-19 policies to reduce SARS-CoV-2 transmission, can be challenging using traditional clinical surveillance metrics due to the small-geographic scale of a university campus,26,30 as well as the clinical testing biases mentioned above.
This study utilized wastewater surveillance to investigate the effectiveness of university COVID-19 policies at Oregon State University (OSU) in reducing the spread of COVID-19 on campus. Additionally, it evaluated the effects of rainwater infiltration and inundation (I and I) as well as autosampler maintenance times to make recommendations towards more accurate data collection. Furthermore, wastewater surveillance data of the university host city (Corvallis, OR) and that of a neighboring city (Albany, OR) were compared to the campus community to help determine the effectiveness of university COVID-19 policies in preventing the spread of COVID-19 from the campus into its surrounding communities, (i.e. campus spill-over).
2. Materials and methods
2.1 Wastewater sampling sites and collection
Untreated influent wastewater was collected from the Corvallis and Albany wastewater reclamation plants (WWRPs) located in Benton and Linn County, Oregon, respectively. Samples consisted of 24 h time-weighted composites following Layton, et al.16 and were collected 4 times and 2 times per week from the Corvallis and Albany WWRP, respectively. Additionally, 24 hour time-weighted composite samples, with a 15 minute sampling interval, were collected from manhole locations at 15 on-campus sites (Fig. S1†) twice per week (Table 1). All samples were collected between September 2020 and June 2022.| Group | Location | Number of samples | Sampling period |
|---|---|---|---|
| Main line | East Campus | 127 | 09/21/20–06/04/22 |
| International Living-Learning Center (ILLC) | 133 | 09/27/20–06/18/22 | |
| Arnold Dining | 142 | 10/04/20–06/11/22 | |
| Building clusters | Poling/NW Weatherford | 114 | 09/27/20–06/11/22 |
| Goss Stadium | 139 | 10/04/20–06/04/22 | |
| Halsell | 109 | 09/27/20–06/11/22 | |
| Beth Ray Center | 135 | 10/07/20–06/04/22 | |
| Isolated buildings | Sackett | 115 | 09/21/20–06/11/22 |
| West | 112 | 09/30/20–06/08/22 | |
| Finley | 149 | 09/21/20–06/18/22 | |
| Callahan | 123 | 09/21/20–06/08/22 | |
| Gem | 118 | 09/27/20–06/11/22 | |
| Hawley/Buxton | 118 | 09/21/20–06/08/22 | |
| Wilson/McNary/Tebeau | 118 | 09/21/20–06/11/22 | |
| SE Weatherford | 112 | 09/30/20–06/11/22 | |
| WW treatment plants | Corvallis | 374 | 04/21/20–06/05/22 |
| Albany | 151 | 09/23/20–08/12/22 |
Campus sampling sites were divided into three categories: main line, building clusters, and isolated buildings. Main line locations were those in which most of the wastewater effluents from buildings across campus intersected and flowed. Building clusters contained wastewater effluent from at least ten campus buildings which included a mix of residential halls and academic buildings. Finally, isolated buildings were those in which wastewater effluent contributing to the sample was only from residential halls. Isolated buildings were primarily a single residential hall but could also include residential hall groups (e.g. Hawley/Buxton and Wilson/McNary/Tebeau). The International Living-Learning Center (ILLC) and East Campus locations were used to represent the entire campus' wastewater data as the majority of the university's effluent flowed through these points before exiting to the city. The East Campus location was used as the primary source for representing the entire campus while the ILLC location was used when sampling failures occurred at the East Campus location.
2.2 Wastewater sample collection and SARS-CoV-2 quantification
All wastewater samples were processed as described in Layton et al.16 In brief, during collection, all composite wastewater samples were kept on ice. Within 8 hours of collection, 30 mL of each wastewater composite was vacuum filtered through a 0.45 μm electronegative HA membrane filter. The filter was placed into 2 mL tubes containing 0.7 mm garnet beads and 1 mL of DNA/RNA Shield (Zymo Research, Irvine, CA) and were bead beaten for 2 min. The samples were extracted from 200–400 μL of lysate using the MagMAX Viral/Pathogen kit on a KingFisher Flex automated instrument per manufacturer's instructions (catalog #A48310 ThermoFisher Scientific).SARS-CoV-2 targets (N1 and N2) and Human RNaseP (internal control, RP) were quantified via RT-ddPCR using BioRad's 2019-nCoV CDC ddPCR Triplex Probe Assay and the One-Step RT-ddPCR Advanced Kit for Probes (Bio-Rad Laboratories, Hercules, CA) on a QX-200 ddPCR system with an automated droplet generator and droplet reader, per manufacturer's instructions (Bio-Rad Laboratories). All assay conditions were performed as specified in the Bio-Rad assay protocol with a template concentration of 5.5 μL of RNA per reaction.31 One-step thermal cycling conditions were as follows: reverse transcription at 50 °C for 60 min, enzyme activation at 95 °C for 10 min; 40 cycles of denaturation at 94 °C for 30s and annealing/extension at 55 °C for 60 s; enzyme inactivation at 98 °C for 10 min; droplet stabilization at 4 °C for 30 min to a maximum of overnight.
A minimum of three positive droplets was required for a sample to be identified as positive for SARS-CoV-2. Samples negative for SARS-CoV-2 detection were assigned a concentration of ½ the limit of detection. All samples were run in duplicate and the N1 and N2 concentrations were averaged together for a final concentration per sample. As determined by Layton et al., the limit of blank for N1 and N2 were 2.0 and 4.2 copies per reaction and the estimated limit of detection for N1 and N2 were 4 and 12 copies per reaction, respectively.16 Additionally, the process recovery efficiency (using bovine coronavirus as a surrogate) was determined to be 57%.
Each ddPCR plate contains the following controls in duplicate; field blanks (FBs), extraction blanks (EBs), negative control reactions (containing human genomic DNA), positive control reactions (containing synthetic RNA of N1 and N2 and synthetic DNA of RP), and no template controls (NTCs). For a ddPCR run to be considered valid, a minimum of 6000 droplets per reaction must have been produced, all negative controls (FBs, EBs, NTCs and negative control reactions) must have less than 3 positive droplets, and all positive controls (synthetic RNA of N1 and N2 and synthetic DNA of RP) must have passed the three-positive droplet threshold. Per manufacturer's instructions, for an individual ddPCR reaction to be considered valid, at least one of the targets (N1, N2 or RP) must have passed the three-positive droplet threshold. Reactions that did not pass this threshold were excluded from further analyses.
2.3 Sequencing of wastewater SARS-CoV-2
All wastewater samples containing SARS-CoV-2 at a concentration of 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 gc L−1 or greater were sequenced to identify their SARS-CoV-2 variant composition, as previously described.32 A minimum sequencing template concentration of 4.0
log10 gc L−1 or greater were sequenced to identify their SARS-CoV-2 variant composition, as previously described.32 A minimum sequencing template concentration of 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 gc L−1 was required to achieve sufficient sequencing depth. In brief, an amplicon-based sequencing approach was taken to amplify the entire SARS-CoV-2 genome using the Swift Amplicon SARS-CoV-2 Panel and Amplicon Combinatorial Dual indexed adapters (Swift Biosciences, Ann Arbor, MI), per manufacturer's instructions. The average amplicon length was 150 bp which were sequenced on either a HiSeq 3000 or NextSeq 2000 sequencer (Illumina, San Diego, CA) to an average depth of 4–5 million sequence reads per sample.
log10 gc L−1 was required to achieve sufficient sequencing depth. In brief, an amplicon-based sequencing approach was taken to amplify the entire SARS-CoV-2 genome using the Swift Amplicon SARS-CoV-2 Panel and Amplicon Combinatorial Dual indexed adapters (Swift Biosciences, Ann Arbor, MI), per manufacturer's instructions. The average amplicon length was 150 bp which were sequenced on either a HiSeq 3000 or NextSeq 2000 sequencer (Illumina, San Diego, CA) to an average depth of 4–5 million sequence reads per sample.
Sequence reads were demultiplexed, trimmed to the reference sequence (Wuhan-Hu1, GenBank accession no. NC_045512.2), and coordinate-sorted with SAMtools version 1.10 (Genome Research Limited, http://www.sanger.ac.uk). The primer sequences were trimmed and GATK version 4.2.0.0 (Broad Institute, https://www.broadinstitute.org) was used to identify mutations compared to the reference genome sequence.
A multilocus sequence typing approach was then used to identify SARS-CoV-2 variants by matching amplicon mutations to known SARS-CoV-2 variant mutation sequences. For a positive identification of a variant, a lower limit of 5% of sequence reads (with a minimum of six total reads) was required to span the mutation site. Additionally, a minimum of two different mutations unique to each variant was required.
2.4 Rainfall and wastewater conductivity data
Rainfall totals were obtained for Corvallis from the Hyslop Weather Station. Wastewater collection occurred over 24 hours that spanned over two days. Therefore, 48 h rainfall totals, that included the days the wastewater collection started and ended for each sample, were used for correlation analyses (section 2.6.2). Wastewater conductivity of the 24 h composite samples was measured using a VWR conductivity electrode (catalog #89231-618) and symphony B40PCID benchtop meter (VWR SympHony B40PCID) within 4 hours of sample collection.To determine the effects of rainwater inundation and infiltration (I and I), spearman correlations were conducted between 48 h rainfall totals and wastewater conductivity. Additionally, regression analyses were performed comparing East Campus wastewater SARS-CoV-2 concentrations to reported on-campus COVID-19 cases under two conditions: 1) using all samples regardless of rainfall and 2) removing samples if the 48 h rainfall total was greater than 15 mm. This cutoff was chosen using the IQR method of outlier detection.
2.5 Campus policies
During Spring Term of the 2020–2021 academic year, OSU closed its campus to outside visitors, held remote classes and only allowed essential staff and researchers onto campus. Additionally, the residential halls were open to students at 50% capacity, housing ∼2200 students in 13 residential halls. For students living in the OSU residential halls at this time, the following policies were enforced: residents and visitors were required to wear face coverings at all times in shared spaces, except for their room as long as no visitors were present; residents were required to maintain a distance of 6 feet at all times; residents could only host visitors in their room if the guest is a resident of the same building; and residents could not attend social gatherings of more than 10 people at any location within Oregon. Additionally, residents who showed COVID-19 symptoms, had a positive COVID-19 diagnosis, or had been exposed to someone who tested positive for COVID-19 were required to isolate until ten days had passed since the onset of symptoms.33During the 2021–2022 academic year, the OSU Corvallis Campus was opened to outside visitors, non-essential employees, and non-resident hall students, and policies followed guidelines provided by the CDC. These requirements included: receiving the first set of vaccinations before arriving on campus, weekly testing for those exempt from vaccination, wearing face coverings at all times in shared spaces, and maintaining a distance of 6 feet at all times.34 During Spring 2022, the mask mandate was lifted, and all other policies remained in place. During the 2021–2022 academic year, the OSU Corvallis Campus was opened to outside visitors, non-essential employees, and non-resident hall students. Classes were held in person and the resident hall population increased to ∼4700 students across the 13 residential halls. A state-wide mask mandate remained in place until March 12, 2022, requiring masks to be worn inside.
2.6 Transmission and vaccination rates
Community transmission levels for Linn and Benton County were received from historical data provided by the CDC.35 Transmission was given a category of Low, Moderate, Substantial, and High based on two criteria; total new cases per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons in the last 7 days, and percentage of NAATs (nucleic acid amplification tests) that are positive during the past 7 days.36 Community vaccination rates for Linn and Benton county were received from historical data provided by the CDC.37 An individual was considered vaccinated if they had received at least their first dose of their vaccination series.
000 persons in the last 7 days, and percentage of NAATs (nucleic acid amplification tests) that are positive during the past 7 days.36 Community vaccination rates for Linn and Benton county were received from historical data provided by the CDC.37 An individual was considered vaccinated if they had received at least their first dose of their vaccination series.
2.7 Statistical analysis
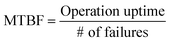 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons vs. wastewater SARS-CoV-2 concentrations; and viral concentrations across three communities, Corvallis, Albany, and OSU.39 The following categories were assigned to indicate the strength of the correlation: rs values greater than 0.7 were considered “strong”, rs values from 0.5–0.7 were “moderate”, rs values from 0.3 to 0.5 were “weak” and rs <0.3 were “none” or “very weak”.
000 persons vs. wastewater SARS-CoV-2 concentrations; and viral concentrations across three communities, Corvallis, Albany, and OSU.39 The following categories were assigned to indicate the strength of the correlation: rs values greater than 0.7 were considered “strong”, rs values from 0.5–0.7 were “moderate”, rs values from 0.3 to 0.5 were “weak” and rs <0.3 were “none” or “very weak”.
In the comparison of rainfall and wastewater conductivity, the interquartile range (IQR) method of outlier detection was used to identify and filter out outliers that would skew the data. This method defines an outlier as a data point in which its value is 1.5 times greater than or 1.5 times less than the IQR.40
Additionally, the Fisher's Z-transformation was utilized when comparing between different correlations. This test transforms the samples to become normally distributed and then returns a “z-score” that allows for the testing of the significance of the difference between two correlation coefficients.41 The Fisher's Z-transformation was used to assess whether rainfall events resulted in significant variations in the wastewater data.
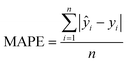 | (2) |
y i = log-transformed SARS-CoV-2 concentration of another community
n = number of samples collected
The Bray–Curtis dissimilarity was used to quantify the differences between communities based on the variant composition of the wastewater samples (eqn (3)). Bray–Curtis returns a value in the range of 0–1 where 1 indicates the populations have complete dissimilarity from each other.
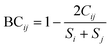 | (3) |
S i = sum of total variant percentage counted in the community i
S j = sum of total variance percentage counted in community j
C ij = the sum of only the lesser counts for each variant in both sites
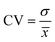 | (4) |
σ = standard deviation
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) = sample mean
= sample mean
3. Results and discussion
3.1 Wastewater surveillance reliability metrics
These failure events impacted the data collected by creating gaps in the data, potentially leading to an underrepresentation of the viral load during those times. To mitigate this impact, data from the days with failure events (7 out of 144 samples) were excluded from this study to ensure that the analysis accounted for these missing points. Thus, autosamplers used in campus wastewater surveillance efforts should undergo preventative maintenance approximately every 4–5 weeks to minimize risk of these disruptions and biases.
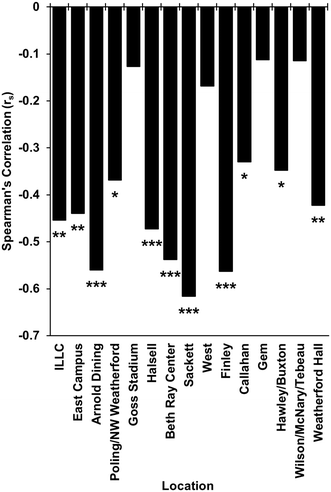 | ||
| Fig. 1 Correlation between conductivity and rainfall at different sampling points across campus. * = p < 0.05, ** = p < 0.01, *** = p < 0.001. | ||
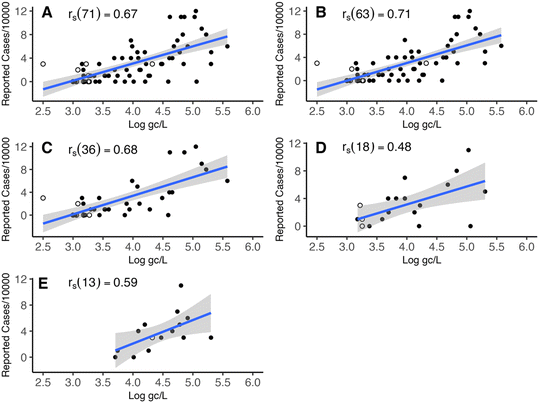
The removal of samples with 48 h rainfall totals greater than 15 mm increased the correlation from rs(71) = 0.67 to rs(63) = 0.71 (Fig. 2A and B). However, a Fisher's r to z transformation identified that these values were not significantly different from each other (p > 0.05). Additionally, 71% of sampling events occurred during periods of little-to-no precipitation, and more than 90% of the rainfalls were less than 15 mm over 48 hours. Thus, it was concluded that rainfall events were not of significant consequence to this study, and no further transformation of the data was necessary.
It should also be noted that while the influence of I and I on wastewater surveillance results is often ignored, it is also highly site specific. For instance, in new housing developments with new separated sewer systems, the influence of I and I on wastewater surveillance would be negligible. However, when conducting wastewater surveillance in established communities with combined sewer systems or older conveyance lines, the influence of I and I can be substantial. Especially if a sizable area drains to that location. Thus, we recommend that the influence of I and I be investigated at all sampling locations through identifying potential sources of I and I as well as through measuring wastewater parameters, such as conductivity, that may indicate if I and I is occurring during the sampling period.
3.2 Exploring the campus spill-over hypothesis
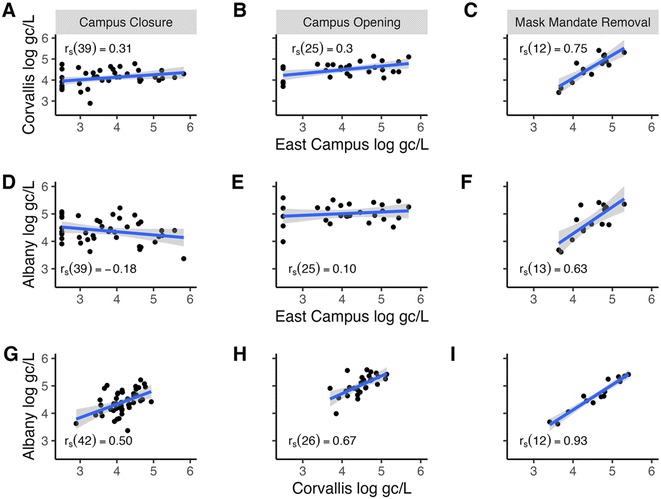
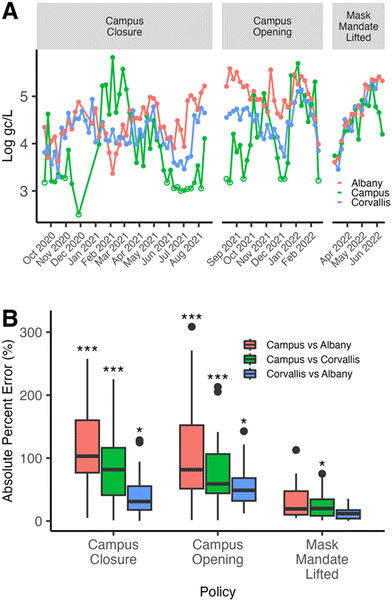
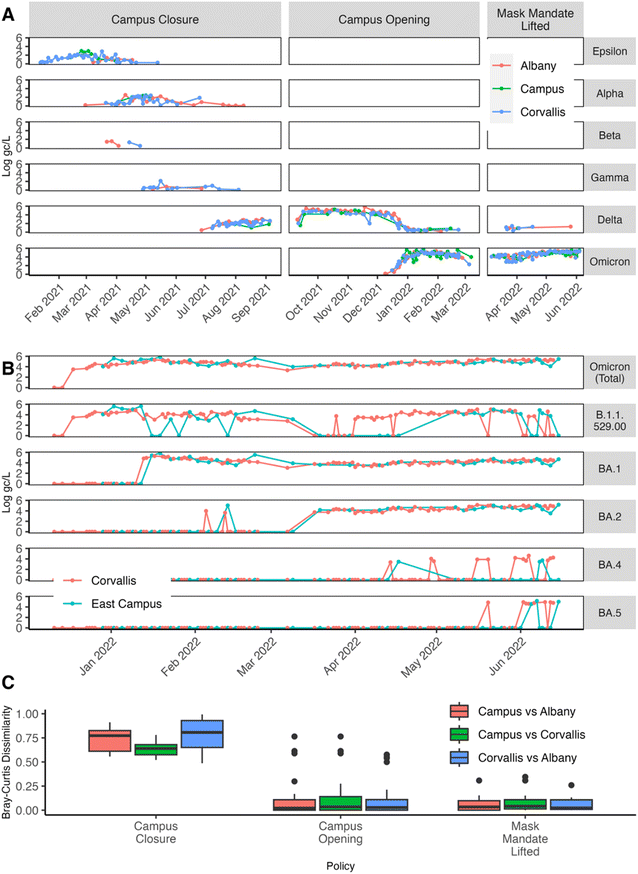
Additionally, during the campus closure, the viral wastewater concentrations at the East Campus location were weakly correlated with those in Corvallis and Albany, showing r values of 0.31 and −0.18 (Fig. 3A and D) and MAPE values of 0.87 and 1.16, respectively (Fig. 4B). Notably, except for the period from January 17, 2021, to March 14, 2021, the East Campus wastewater viral concentrations were generally lower than those observed in Corvallis or Albany. Meanwhile, during the campus closure, the correlation of wastewater viral concentrations between Corvallis and Albany remained moderate, with a rs value of 0.50 and a MAPE of 0.39 (Fig. 3G and 4B), indicating some interaction between these communities. Finally, during the campus closure, the wastewater SARS-CoV-2 variant composition remained distinct for the East Campus location compared with the Corvallis and Albany wastewater SARS-CoV-2 variant compositions with a Bray Curtis dissimilarity index of 0.63 and 0.73, respectively (Fig. 5C).
The high correlation between East Campus SARS-CoV-2 wastewater concentrations and the reported campus COVID-19 cases suggests that the wastewater accurately captured the campus disease burden. Additionally, the moderate correlation between the campus and Corvallis wastewater SARS-CoV-2 concentrations in combination with the low correlation between the wastewater SARS-CoV-2 concentrations and variant composition with the cities of Corvallis and Albany suggests that the campus was insular from the outside communities and did not strongly influence the adjacent communities. This is unlike Corvallis and Albany themselves, which have moderate correlations between wastewater SARS-CoV-2 concentrations and variant compositions suggesting that there was an influence of these two communities on one another.
These results are consistent with other non-wastewater studies that estimated the effects of opening the campus to the surrounding community. Arnold et al.,26 conducted a clinical study that quantified the presence of anti-SARS-CoV-2 antibodies of Pennsylvania State University residents and those residing in the surrounding neighborhoods during the time students were allowed to return to campus. There was little evidence of a significant increase in COVID-19 cases in the surrounding community following the return of students to campus. Similarly, Valesano et al.,28 conducted a genomic analysis of SARS-CoV-2 in positive individuals both on-campus and within the community. This study found that most of the variants present in the outside community were not linked to variants within the campus population. Thus, these findings collectively demonstrate minimal evidence of a campus spillover into the surrounding communities.
The weakened correlation between reported campus COVID-19 cases and East Campus wastewater SARS-CoV-2 concentrations was indicative of the campus community becoming less insular from the surrounding communities. In particular, the shallower slope between wastewater SARS-CoV-2 concentrations and reported campus COVID-19 cases (i.e. fewer reported COVID-19 cases per wastewater SARS-CoV-2 concentration) indicated that infected individuals arrived on campus, contributed to SARS-CoV-2 signals in the wastewater but were not reported as campus-based COVID-19 infections, since their residences were located off-campus.
Similarly, the increased correlation between East Campus and Corvallis wastewater SARS-CoV-2 concentrations suggests a greater interaction between these two communities while the improved, but still weak, correlation between East Campus and Albany SARS-CoV-2 wastewater concentrations suggests that these two communities are still largely insular from one another. Additionally, the increased similarity of the wastewater SARS-CoV-2 variant composition also indicates increased interactions between the three communities (Fig. 5). However, the rapid rise of the Delta variant being dominant across the state at this time weakens the usefulness of this comparison.16 Nonetheless, various Omicron variants were detected in the Corvallis wastewater before the East Campus wastewater, indicating that these variants did not enter the city communities through the campus community members (Fig. 5B).
Taken as a whole, the increased correlation between East Campus and Corvallis indicates that either East Campus is influencing Corvallis or vice versa. However, the increased correlation between Corvallis and Albany combined with the weakened correlation between East Campus and Albany suggests that there was not a campus spill-over effect into either Corvallis or Albany. Instead, this data indicates the exact opposite with Corvallis spilling over onto campus. These findings coincide with a study performed by Platt et al.,4 where in-class instruction with a mask mandate did not increase viral transmission on campus, but students who had become infected had potential exposure points from interactions with the outer community.
This theory of the surrounding communities increasing the COVID-19 burden on campus is also supported by the transmission rates and vaccination coverage reported in the Corvallis (Benton County) and Albany (Linn County) communities during this time. The non-detections of SARS-CoV-2 in the East Campus wastewater at the start of the campus opening period indicated a low level of COVID-19 transmission on campus (Fig. 4A). This was likely due to the near 100% vaccination level of university students, faculty, and staff at that time, as required by university policy. In contrast, at this time the COVID-19 transmission rates in Corvallis (Benton County) and Albany (Linn County) were classified as “high” to “substantial” and the single dose vaccination rates in Corvallis and Albany, were much lower at rates of 68% and 49%, respectively (Fig. S3†).35,37 Thus, the introduction of unvaccinated individuals from communities with relatively high transmission rates onto campus may have likely contributed to the onto-campus spill-over effect.
The further increase in correlation between East Campus wastewater SARS-CoV-2 concentrations with those from both Corvallis and Albany suggests an even greater interaction between the three communities after the mask mandate was lifted (Fig. 4). At this time, transmission rates in both communities remained classified as “high” to “substantial” and the single dose vaccination rates in Corvallis and Albany were still lower than the university campus at 80% and 60%, respectively (Fig. S3†). Similar to before the mask mandate was lifted, the stronger correlation between Corvallis and Albany compared to East Campus indicates that campus spillover was not occurring but rather the surrounding communities were spilling over into the campus. Finally, the wastewater SARS-CoV-2 variant composition of East Campus, Corvallis, and Albany also increased in similarity after the mask mandate was lifted (Fig. 5). However, just as with Delta, a single variant, Omicron, was dominant across the state at this time weakening the usefulness of this comparison.16
4. Conclusion
This study used wastewater surveillance to evaluate how university COVID-19 policies influenced SARS-CoV-2 viral burdens on campus in the surrounding communities. The findings suggest that regular autosampler maintenance every 4–6 weeks can minimize the risk of missed sampling due to equipment failure. Additionally, data normalization due to rainwater infiltration and inundation (I and I) effects, was not necessary for this study as there were no significant differences in data when samples collected during large rainfall events were removed.This study also demonstrated that the enactment of campus closures and mask mandates were highly effective in reducing SARS-CoV-2 viral burdens and dampened the influence of campus residents on the surrounding communities and vice versa. Additionally, the findings of this study indicate that the campus spill-over hypothesis can be dispelled, showing that the increase in the SARS-CoV-2 viral load on the campus primarily originated from the surrounding community rather than from the campus spreading the virus to the community.
As the campus COVID-19 restrictions on campus were adjusted, the viral dynamics on the campus began to mirror those of the host city. This suggests that university policymakers can use the viral activity patterns of the host city as a benchmark to estimate the consequences of lifting COVID-19 restrictions on campus. Additionally, through wastewater surveillance's ability to track viral trends down to the neighborhood and building scale, this method could be utilized to create targeted interventions that alleviate transmission potential from building to building. On a university-scale, this could include enforcing testing and isolation of that area, without having to spend resources to test the entire campus. Overall, wastewater surveillance in conjunction with traditional clinical surveillance methods, can serve as a valuable tool towards refining policy decisions based on accurate, real-time data.
Data availability
Numerical data for Campus Reported Cases, Rainfall, Wastewater Conductivity, and the SARS-CoV-2 log10 gc L−1 for East Campus, Corvallis and Albany is provided in the ESI† section (Numerical_Data_Archive.xlsx). All wastewater sequences were deposited in the National Center for Biotechnology Information's short read archive, under BioProject ID # PRJNA1121307.Author contributions
David Lisboa – formal analyses, data curation, writing – original draft, review and editing, visualization; Devrim Kaya – methodology, validation, formal analyses, investigation, data curation, writing – review and editing; Michael Harry – methodology, validation, investigation; Casey Kanalos – investigation; Gabriel Davis – methodology, validation, investigation; Oumaima Hachimi – methodology, validation, investigation, writing – review and editing; Shana Jaaf – methodology, validation, investigation; David Mickle – methodology, validation, investigation, writing – review and editing; Dana Alegre –methodology, validation, investigation; Katherine Carter – methodology, validation, investigation, writing – review and editing; Steven Carrell – investigation, writing – review and editing; Mark Dasenko – methodology, validation, investigation, writing – review and editing; Nathan Davidson – investigation; Justin Elser – investigation, writing – review and editing; Matthew Geniza – methodology, validation, investigation; Anne-Marie Girard – methodology, validation, investigation, writing – review and editing; Brent Kronmiller – methodology, validation, investigation, writing – review and editing; Matthew Peterson – methodology, validation, investigation; Elizabeth Zepeda – methodology, validation, investigation, writing – review and editing; Christine Kelly – conceptualization, investigation, supervision, project administration, funding acquisition, writing – review and editing; Tyler S. Radniecki – conceptualization, investigation, supervision, project administration, funding acquisition, writing – original draft, writing – review and editing.Conflicts of interest
None to report.Acknowledgements
This work was supported by Oregon State University. We are grateful to the Corvallis Wastewater Treatment Facility, especially Gloria Zeller, John Hoppner, and Max Hildebrand for their participation in our wastewater surveillance program. We would also like to thank David Gilby and the rest of the team at the Albany Wastewater Treatment Facility for their participation. Additionally, we would like to thank our OSU wastewater collection undergraduate team, especially Jim Gallagher, and the TRACE OSU COVID-19 team, especially Jeff Bethel, Ben Dalziel, Roy Haggerty, Kathy Higley, Jane Lubchenco, Katie McLaughlin, Allison Meyers, Javier Nieto and Justin Sanders, for their support and advice for field collection, sample preparation and data analysis activities, and Leslie Dietz for proofreading.References
- A. Hatibie, S. Majumdar, M. Pyarali, R. Koch, A. Wood and T. Hale, Variation in US states' responses to COVID-19 Search PubMed.
- J. M. Brauner, S. Mindermann, M. Sharma, D. Johnston, J. Salvatier, T. Gavenčiak, A. B. Stephenson, G. Leech, G. Altman, V. Mikulik, A. J. Norman, J. T. Monrad, T. Besiroglu, H. Ge, M. A. Hartwick, Y. W. Teh, L. Chindelevitch, Y. Gal and J. Kulveit, Inferring the effectiveness of government interventions against COVID-19, Science, 2021, 371, eabd9338 CrossRef CAS
.
- A. Mendez-Brito, C. El Bcheraoui and F. Pozo-Martin, Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19, J. Infect., 2021, 83, 281–293 CrossRef CAS
.
- K. Kuhfeldt, J. Turcinovic, M. Sullivan, L. Landaverde, L. Doucette-Stamm, D. H. Hamer, J. T. Platt, C. Klapperich, H. E. Landsberg and J. H. Connor, Examination of SARS-CoV-2 In-Class Transmission at a Large Urban University With Public Health Mandates Using Epidemiological and Genomic Methodology, JAMA Netw. Open, 2022, 5, e2225430 CrossRef
.
- H. Littlecott, C. Herd, J. O'Rourke, L. T. Chaparro, M. Keeling, G. James Rubin and E. Fearon, Effectiveness of testing, contact tracing and isolation interventions among the general population on reducing transmission of SARS-CoV-2: a systematic review, Philos. Trans. R. Soc., A, 2023, 381, 20230131 CrossRef
.
- K. Sherratt, S. Abbott, S. R. Meakin, J. Hellewell, J. D. Munday, N. Bosse, M. Jit and S. Funk, Exploring surveillance data biases when estimating the reproduction number: with insights into subpopulation transmission of COVID-19 in England, Philos. Trans. R. Soc., B, 2021, 376, 20200283 CrossRef CAS PubMed
.
- D. S. Grigsby-Toussaint, J. C. Shin and A. Jones, Disparities in the distribution of COVID-19 testing sites in black and Latino areas in new York City, Prev. Med., 2021, 147, 106463 CrossRef
.
- E. N. Pond, L. Rutkow, B. Blauer, A. Aliseda Alonso, S. Bertran de Lis and J. B. Nuzzo, Disparities in SARS-CoV-2 Testing for Hispanic/Latino Populations: An Analysis of State-Published Demographic Data, J. Public Health Manag. Pract., 2022, 28, 330 CrossRef PubMed
.
- N. P. Dalva-Baird, W. M. Alobuia, E. Bendavid and J. Bhattacharya, Racial and ethnic inequities in the early distribution of U.S. COVID-19 testing sites and mortality, Eur. J. Clin. Invest., 2021, 51, e13669 CrossRef CAS PubMed
.
- D. A. Larsen and K. R. Wigginton, Tracking COVID-19 with wastewater, Nat. Biotechnol., 2020, 38, 1151–1153 CrossRef CAS PubMed
.
- C. Hoar, F. Chauvin, A. Clare, H. McGibbon, E. Castro, S. Patinella, D. Katehis, J. J. Dennehy, M. Trujillo, D. S. Smyth and A. I. Silverman, Monitoring SARS-CoV-2 in wastewater during New York City's second wave of COVID-19: sewershed-level trends and relationships to publicly available clinical testing data, Environ. Sci.: Water Res. Technol., 2022, 8, 1021–1035 RSC
.
- B. J. Paterson and D. N. Durrheim, Wastewater surveillance: an effective and adaptable surveillance tool in settings with a low prevalence of COVID-19, Lancet Planet. Health, 2022, 6, e87–e88 CrossRef PubMed
.
- D. Kaya, R. Falender, T. Radniecki, M. Geniza, P. Cieslak, C. Kelly, N. Lininger and M. Sutton, Correlation between Clinical and Wastewater SARS-CoV-2 Genomic Surveillance, Oregon, USA - Volume 28, Number 9—September 2022, Emerg. Infect. Dis., 2022, 28(9), 1906–1908 CrossRef CAS
.
- CDC, National Wastewater Surveillance System, https://www.cdc.gov/nwss/wastewater-surveillance/index.html, (accessed 28 February 2023).
- M. B. Diamond, A. Keshaviah, A. I. Bento, O. Conroy-Ben, E. M. Driver, K. B. Ensor, R. U. Halden, L. P. Hopkins, K. G. Kuhn, C. L. Moe, E. C. Rouchka, T. Smith, B. S. Stevenson, Z. Susswein, J. R. Vogel, M. K. Wolfe, L. B. Stadler and S. V. Scarpino, Wastewater surveillance of pathogens can inform public health responses, Nat. Med., 2022, 28, 1992–1995 CrossRef CAS PubMed
.
- B. A. Layton, D. Kaya, C. Kelly, K. J. Williamson, D. Alegre, S. M. Bachhuber, P. G. Banwarth, J. W. Bethel, K. Carter, B. D. Dalziel, M. Dasenko, M. Geniza, A. George, A.-M. Girard, R. Haggerty, K. A. Higley, D. M. Hynes, J. Lubchenco, K. R. McLaughlin, F. J. Nieto, A. Noakes, M. Peterson, A. D. Piemonti, J. L. Sanders, B. M. Tyler and T. S. Radniecki, Evaluation of a Wastewater-Based Epidemiological Approach to Estimate the Prevalence of SARS-CoV-2 Infections and the Detection of Viral Variants in Disparate Oregon Communities at City and Neighborhood Scales, Environ. Health Perspect., 2022, 130(6), 067010 CrossRef CAS
.
- A. E. Kirby, M. S. Walters, W. C. Jennings, R. Fugitt, N. LaCross, M. Mattioli, Z. A. Marsh, V. A. Roberts, J. W. Mercante, J. Yoder and V. R. Hill, Using Wastewater Surveillance Data to Support the COVID-19 Response — United States, 2020–2021, MMWR Morb. Mortal. Wkly. Rep., 2021, 70, 1242–1244 CrossRef CAS PubMed
.
- T. Prado, T. M. Fumian, C. F. Mannarino, P. C. Resende, F. C. Motta, A. L. F. Eppinghaus, V. H. Chagas do Vale, R. M. S. Braz, J. da S. R. de Andrade, A. G. Maranhão and M. P. Miagostovich, Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil, Water Res., 2021, 191, 116810 CrossRef CAS PubMed
.
- S. Shrestha, E. Yoshinaga, S. K. Chapagain, G. Mohan, A. Gasparatos and K. Fukushi, Wastewater-Based Epidemiology for Cost-Effective Mass Surveillance of COVID-19 in Low- and Middle-Income Countries: Challenges and Opportunities, Water, 2021, 13, 2897 CrossRef CAS
.
- P. Liu, M. Ibaraki, J. VanTassell, K. Geith, M. Cavallo, R. Kann, L. Guo and C. L. Moe, A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level, Sci. Total Environ., 2022, 807, 151047 CrossRef CAS PubMed
.
- A. L. Rainey, K. Buschang, A. O'Connor, D. Love, A. M. Wormington, R. L. Messcher, J. C. Loeb, S. E. Robinson, H. Ponder, S. Waldo, R. Williams, J. Shapiro, E. B. McAlister, M. Lauzardo, J. A. Lednicky, A. T. Maurelli, T. Sabo-Attwood and J. H. Bisesi, Retrospective Analysis of Wastewater-Based Epidemiology of SARS-CoV-2 in Residences on a Large College Campus: Relationships between Wastewater Outcomes and COVID-19 Cases across Two Semesters with Different COVID-19 Mitigation Policies, ACS EST Water, 2023, 3(1), 16–29 CrossRef CAS PubMed
.
- S. Harris-Lovett, K. L. Nelson, P. Beamer, H. N. Bischel, A. Bivins, A. Bruder, C. Butler, T. D. Camenisch, S. K. De Long, S. Karthikeyan, D. A. Larsen, K. Meierdiercks, P. J. Mouser, S. Pagsuyoin, S. M. Prasek, T. S. Radniecki, J. L. Ram, D. K. Roper, H. Safford, S. P. Sherchan, W. Shuster, T. Stalder, R. T. Wheeler and K. S. Korfmacher, Wastewater Surveillance for SARS-CoV-2 on College Campuses: Initial Efforts, Lessons Learned, and Research Needs, Int. J. Environ. Res. Public Health, 2021, 18, 4455 CrossRef CAS PubMed
.
- C. M. Welling, D. R. Singleton, S. B. Haase, C. H. Browning, B. R. Stoner, C. K. Gunsch and S. Grego, Predictive values of time-dense SARS-CoV-2 wastewater analysis in university campus buildings, Sci. Total Environ., 2022, 835, 155401 CrossRef CAS PubMed
.
- W. Q. Betancourt, B. W. Schmitz, G. K. Innes, S. M. Prasek, K. M. Pogreba Brown, E. R. Stark, A. R. Foster, R. S. Sprissler, D. T. Harris, S. P. Sherchan, C. P. Gerba and I. L. Pepper, COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention, Sci. Total Environ., 2021, 779, 146408 CrossRef CAS
.
- L. C. Scott, A. Aubee, L. Babahaji, K. Vigil, S. Tims and T. G. Aw, Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation, Environ. Res., 2021, 200, 111374 CrossRef CAS PubMed
.
- C. R. K. Arnold, S. Srinivasan, S. Rodriguez, N. Rydzak, C. M. Herzog, A. Gontu, N. Bharti, M. Small, C. J. Rogers, M. M. Schade, S. V. Kuchipudi, V. Kapur, A. F. Read and M. J. Ferrari, A longitudinal study of the impact of university student return to campus on the SARS-CoV-2 seroprevalence among the community members, Sci. Rep., 2022, 12, 8586 CrossRef CAS PubMed
.
- L. E. Cipriano, W. M. R. Haddara, G. S. Zaric and E. A. Enns, Impact of university re-opening on total community COVID-19 burden, PLoS One, 2021, 16, e0255782 CrossRef CAS PubMed
.
- A. L. Valesano, W. J. Fitzsimmons, C. N. Blair, R. J. Woods, J. Gilbert, D. Rudnik, L. Mortenson, T. C. Friedrich, D. H. O'Connor, D. R. MacCannell, J. G. Petrie, E. T. Martin and A. S. Lauring, SARS-CoV-2 Genomic Surveillance Reveals Little Spread From a Large University Campus to the Surrounding Community, Open Forum Infect. Dis., 2021, 8, ofab518 CrossRef PubMed
.
- N. Bharti, B. Lambert, C. Exten, C. Faust, M. Ferrari and A. Robinson, Large university with high COVID-19 incidence is not associated with excess cases in non-student population, Sci. Rep., 2022, 12, 3313 CrossRef CAS PubMed
.
- C. J. Courtemanche, A. H. Le, A. Yelowitz and R. Zimmer, 2021.
- Bio-Rad, Instructions for Use: SARS-CoV-2 ddPCR Kit - Part Number 12013743, https://www.bio-rad.com/webroot/web/pdf/lsr/literature/10000130776.pdf, (accessed 1 May 2020).
- M. Sutton, T. S. Radniecki, D. Kaya, D. Alegre, M. Geniza, A.-M. Girard, K. Carter, M. Dasenko, J. L. Sanders, P. R. Cieslak, C. Kelly and B. M. Tyler, Detection of SARS-CoV-2 B.1.351 (Beta) Variant through Wastewater Surveillance before Case Detection in a Community, Oregon, USA, Emerg. Infect. Dis., 2022, 28, 1101–1109 CrossRef CAS PubMed
.
- UHDS, The Student Policy and Information Guide, https://uhds.oregonstate.edu/sites/uhds.oregonstate.edu/files/uhds_policy_guide_sept_2020.pdf, (accessed 15 January 2023).
- COVID-19 Information, https://studenthealth.oregonstate.edu/covid-19-info, (accessed 23 May 2024).
- United States COVID-19 County Level of Community Transmission Historical Changes - ARCHIVED|Data|Centers for Disease Control and Prevention, https://data.cdc.gov/Public-Health-Surveillance/United-States-COVID-19-County-Level-of-Community-T/nra9-vzzn, (accessed 28 May 2024).
- A. Christie, Guidance for Implementing COVID-19 Prevention Strategies in the Context of Varying Community Transmission Levels and Vaccination Coverage, MMWR Morb. Mortal. Wkly. Rep., 2021, 70(30), 1044–1047 CrossRef CAS PubMed
.
- COVID-19 Vaccinations in the United States, County|Data|Centers for Disease Control and Prevention, https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-County/8xkx-amqh/explore/, (accessed 28 May 2024).
- What is Mean Time Between Failure MTBF?, https://www.upkeep.com/learning/mean-time-between-failure/, (accessed 22 May 2023).
-
D. Freedman, R. Pisani and R. Purves, Statistics, W.W. Norton & Co, New York, 4th edn, 2007 Search PubMed
.
- Zach, How to Find Outliers Using the Interquartile Range, https://www.statology.org/find-outliers-with-iqr/, (accessed 18 July 2023).
- Fisher Z-Transformation - Statistics How To, https://www.statisticshowto.com/fisher-z/, (accessed 22 May 2023).
- A. D. George, D. Kaya, B. A. Layton, K. Bailey, S. Mansell, C. Kelly, K. J. Williamson and T. S. Radniecki, Impact of Sampling Type, Frequency, and Scale of the Collection System on SARS-CoV-2 Quantification Fidelity, Environ. Sci. Technol. Lett., 2022, 9, 160–165 CrossRef CAS PubMed
.
- Stephanie, Bray Curtis Dissimilarity, https://www.statisticshowto.com/bray-curtis-dissimilarity/, (accessed 22 May 2023).
- How to Find a Coefficient of Variation, https://www.statisticshowto.com/probability-and-statistics/how-to-find-a-coefficient-of-variation/, (accessed 5 June 2023).
- B. Nguyen Quoc, P. Saingam, R. RedCorn, J. A. Carter, T. Jain, P. Candry, M. Gattuso, M.-L. W. Huang, A. L. Greninger, J. S. Meschke, A. Bryan and M. K. H. Winkler, Case Study: Impact of Diurnal Variations and Stormwater Dilution on SARS-CoV-2 RNA Signal Intensity at Neighborhood Scale Wastewater Pumping Stations, ACS EST Water, 2022, 2, 1964–1975 CrossRef CAS
.
- Neighbourhood-scale wastewater-based epidemiology for covid-19: Opportunities and challenges|Journal of Hydrology (New Zealand), https://search.informit.org/doi/abs/10.3316/informit.548064549025013, (accessed 28 February 2023).
- A. Lastra, J. Botello, A. Pinilla, J. I. Urrutia, J. Canora, J. Sánchez, P. Fernández, F. J. Candel, A. Zapatero, M. Ortega and J. Flores, SARS-CoV-2 detection in wastewater as an early warning indicator for COVID-19 pandemic. Madrid region case study, Environ. Res., 2022, 203, 111852 CrossRef CAS
.
- S. Arora, A. Nag, A. Kalra, V. Sinha, E. Meena, S. Saxena, D. Sutaria, M. Kaur, T. Pamnani, K. Sharma, S. Saxena, S. K. Shrivastava, A. B. Gupta, X. Li and G. Jiang, Successful application of wastewater-based epidemiology in prediction and monitoring of the second wave of COVID-19 with fragmented sewerage systems–a case study of Jaipur (India), Environ. Monit. Assess., 2022, 194, 342 CrossRef CAS PubMed
.
- B. Layton, D. Kaya, C. Kelly, K. Williamson, S. Bachhuber, P. Banwarth, J. Bethel, K. Carter, B. Dalziel, M. Dasenko, M. Geniza, D. Gibbon, A.-M. Girard, R. Haggerty, K. Higley, D. Hynes, J. Lubchenco, K. McLaughlin, F. J. Nieto, A. Noakes, M. Peterson, A. Piemonti, J. Sanders, B. Tyler and T. Radniecki, Wastewater-based epidemiology predicts COVID-19 community prevalence, In Review, 2021 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ew00168k |
| This journal is © The Royal Society of Chemistry 2025 |