Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heavy metal leaching from stormwater control measures – insights into field and lab prestressed media and road-deposited sediments†
Philipp
Stinshoff
,
Yannic
Henn
,
Steffen H.
Rommel
and
Brigitte
Helmreich
 *
*
Chair of Urban Water Systems Engineering, Technical University of Munich, Am Coulombwall 3, 85748 Garching, Germany. E-mail: sww@tum.de; b.helmreich@tum.de; Tel: +49 89 289 13719
First published on 30th October 2024
Abstract
The risk of heavy metal leaching from sorptive filter media in stormwater control measures (SCMs) treating road runoff is mainly assessed through lab-scale studies. In contrast, investigations with filter media prestressed under real conditions are crucial. Therefore, the leaching potential of five traffic-relevant heavy metals (Cr, Cu, Ni, Pb, and Zn) from field-scale and lab-scale prestressed sorptive filter media and road-deposited sediments (RDSs) from a decentralized treatment facility was assessed using quiescent batch leaching tests with and without adding de-icing salts. The hydraulic retention times of a maximum of 7 days should represent prolonged submerged conditions during dry periods. The leaching quantity order was Zn ≫ Cu > Ni, whereas no observed leaching was quantified for Cr and Pb for all tested materials. Considerable loads of Cu only leached from the field-scale prestressed sorptive filter media, which was mainly associated with the presence of dissolved organic matter. Regarding the tested filter media, zeolite and carbonate sand revealed significantly higher leaching of Zn under the influence of de-icing salts. The leaching of Cu and Zn concerning the mobile heavy metal fraction was less than 0.2%. The highest concentrations of heavy metals were observed for the RDSs, where up to 0.3% leached of the potential mobile fraction during one dry cycle.
Water impactStormwater quality improvement devices, which should be a sink for heavy metals from road runoff, can be a source of pollutant emission under unfavorable conditions. The influencing mechanisms regarding the leaching of heavy metals were evaluated with in situ and artificial prestressed filter media. The prestressing conditions were identified as key elements. The results can help predict heavy metal leaching. |
1. Introduction
Traffic emission is a major contributor to the diffuse release of pollutants into the environment. The main pathway of dissolved and particulate contaminants such as polycyclic aromatic hydrocarbons or heavy metals is through the wash-off from the traffic areas during storm events.1–4 Heavy metals can be named as the most relevant pollutants5 and have been the focus of numerous studies for decades due to their prevalence, potential exposure to aquatic and non-aquatic ecosystems, and toxicity.6–10Traffic-related heavy metals are chromium (Cr), copper (Cu), lead (Pb), nickel (Ni), and zinc (Zn), whereof Cu and Zn are found in the highest concentration in the runoff.5,11–13 The partitioning determines the mobility of heavy metals in the environment,14 and also, the toxicity is increased if the metals are dissolved, i.e., bioavailable.15,16
In traffic area runoff, heavy metals are more abundant in particulate form.2,17 Thus, road-deposited sediments (RDSs) are critical parameters to assess the transport of heavy metals from roads.2,18 High RDS or total suspended solids (TSS) concentrations were analyzed regardless of the traffic volumes.19 Winston et al.20 observed in a recent study that the highest and finest loads of RDSs are found on an interstate highway with higher traffic density. In contrast, fewer and coarser particles were analyzed in residential areas. Some could conclude that the speed of vehicles influences the particle size distribution, which this study did not statistically prove. However, the particle size distribution is an essential factor for RDSs as it could be demonstrated that significantly higher metal loads are found in finer fractions.21,22
Generally, the RDS and heavy metal concentrations in traffic area runoff depend on the annual average daily traffic (AADT) and also on other factors like antecedent dry days, rainfall characteristics, and local variables (e.g., wind and land use).3,23,24 Accordingly, the emission has a high spatial and seasonal variation.25–27 In particular, in cold climates, two phenomena can be observed in the winter period: on the one hand, the metals can accumulate in the snow/ice and then be released continually over the melting period.12 On the other hand, peak concentrations are found due to increased tear and wear through studded tires and the application of de-icing salts or gravel.25,26
A recent study found even elevated concentrations of metals in roadside soils near roads with a low traffic density,28 which amplifies the treatment need for stormwater runoff, especially from traffic areas. Decentralized stormwater control measures (SCMs), such as natural systems (e.g., swales) or engineered systems (e.g., permeable pavements or filter channel systems), are efficient ways to manage and treat stormwater from roadways in dense urban areas.29 Technical SCMs are mainly verified based on required pollutant retention30 and commonly consist of a stage to remove particulate matter, e.g., a settling tank and a stage with sorptive filter media mainly for the removal of dissolved substances.29 The diverse removal mechanisms of SCMs are sedimentation, filtration, sorption, ion exchange, and precipitation, whereas the partitioning of heavy metals in the runoff is crucial for the needed retention mechanism.11,15,19,29 The major removal mechanism is determined by the applied filter material.31–34
However, sorption processes are often reversible, although sorption is typically faster than desorption.35,36 Prolonged dry periods in SCMs operating under permanent submerged conditions could lead to unfavorable conditions. The high hydraulic retention time results in low redox conditions through oxygen consumption, increasing heavy metal remobilization risks. In winter, deicing salts also influences the mobility of metals. Hereby, desorption mechanisms contributing to the mobilization are cation exchange, chloride complex formation, and colloid dispersion, and further, a critical parameter is time.37–41 Thus, the NaCl-induced heavy metal mobilization can harm groundwater quality.42
An appropriate lab method to gain insight into the mobility of heavy metals is to determine the strength of the association through a sequential extraction procedure (SEP).43,44 Utilizing SEPs, it is possible to determine potential mobile fractions and assess the potential risk of heavy metal leaching.45,46 Although SEPs have limitations,47–49 SEPs contributed to the scientific knowledge regarding the mobility of heavy metals and have been applied to traffic-related media like RDSs, roadside soils, and soakaway sediment.22,38,45,50,51
Moreover, a SEP revealed a higher mobile fraction of lab prestressed sorptive filter media than that of field prestressed media. Prestressed means, in this context, that the filter media already received polluted road runoff over a certain period. The authors concluded that heavy metal mobilization is possibly overestimated through lab prestressed media.50 To the best of our knowledge, no study examined the possible risk of heavy metal remobilization from in situ and lab prestressed sorptive filter media with known mobile fractions. Nevertheless, gaining deeper knowledge and obtaining more accurate estimates of the leaching potential of heavy metals from filter media is essential. We conducted leaching experiments with in situ prestressed filter media compared to lab prestressed filter media under different influencing factors to investigate this issue. In summary, the main objectives of this paper are (i) to identify the heavy metal leaching and the main influencing factors of road-deposited sediments and in situ prestressed sorptive filter media in comparison to lab prestressed filter media in a long-term (7 days) quiescent batch leaching test, (ii) to ascertain the relation of the mobile fraction of heavy metals detected by sequential extraction and the analyzed leached heavy metal fraction regarding a possible overestimation through lab scale studies, and (iii) to compare the quiescent column leaching test to a shaken batch extraction test (DIN 19529). Therefore, we formulate the following hypothesis, which we aim to prove in this article: heavy metals bound in the potential mobile fraction (the sum of acid-extractable, reducible and oxidizable fractions) on sorptive filter media evaluated by SEPs tend to remobilize during dry periods in SCMs (= high hydraulic retention time) operated under permanent impoundment (>5% within 7 days).
2. Materials and methods
2.1. Study site and stormwater control measures
The field-scale prestressed filter media were withdrawn from three real-scale SCMs after 2.75 years of operation on a highly trafficked road in Munich (AADT: 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 vehicles per day).52 The first SCM was a filter substratum channel, Drainfix Clean 300 (SCM-D, Hauraton, Germany), filled with carbonate sand and a catchment of 165 m2. The filter media of SCM-D falls completely dry after a storm event in the real application.
000 vehicles per day).52 The first SCM was a filter substratum channel, Drainfix Clean 300 (SCM-D, Hauraton, Germany), filled with carbonate sand and a catchment of 165 m2. The filter media of SCM-D falls completely dry after a storm event in the real application.
The second and third SCMs, SediSubstrator XL 600/12 (SCM-S, Fränkische Rohrwerke, Germany) and ViaPlus 500 (SCM-V, Mall, Germany), were underground structures (manhole devices) with a two-stage setup consisting of a sedimentation stage and a downstream filter stage. SCM-S used a permanently submerged iron-based medium with added granular activated lignite and had a catchment of 1660 m2. In contrast, clinoptilolite, a zeolite from SCM-V, was not permanently submerged and had a catchment area of 473 m2. The order of the catchment area-to-filter surface area ratios was: SCM-S > SCM-V ≫ SCM-D.
2.2. Sampling procedure
After 2.75 years of operation, we sampled the filter media of each SCM in 5 cm increments in the flow direction with a plastic spatula or stainless-steel soilcore-sampler, homogenized, stored them in a cool and dark place, and dried them at 38 ± 2 °C for 24 h prior to the leaching experiment. Only the first 5 cm of media, in the flow direction, was used to evaluate the leaching behavior since the highest heavy metal load can be found in the first centimeters.53,54 Additionally, we sampled the filter cake of SCM-D with a plastic spatula, which had a thickness of ca. 4 cm and was accumulated on top of the 15 cm thick carbonate sand layer (cf. Fig. S1†). The filter cake was sieved to 2 mm to remove unrepresentative debris like pebbles and cigarette butts. The filter cake (further referred to as RDS) serves as an estimation of the leaching potential of the RDS, because of the high particulate retention capacity of SCM-D observed in the field and its comparable heavy metal content to other studies.22,55 Real sediments trapped in the SCM-V and SCM-S sedimentation step were no longer available for investigation.The same sampling and pretreatment procedure was applied to the artificial prestressed filter samples (section 2.3) in lab-scale. Then, the materials are labeled RDS, D_field, S_field, V_field, as well as D_lab, S_lab, and V_lab, according to the prestressing method and respective SCM.
2.3. Prestressing of filter media in lab-scale
The lab-scale prestressed filter media were produced using the blank filter media of the SCMs in a column test setup using synthetic road runoff. The lab-scale prestressing of the filter media was done according to Huber et al.,56 a technique to evaluate the service life of technical SCMs.Zn and Cu were used for the artificial prestressing as the most relevant heavy metals in traffic area runoff. In summary, annual dissolved metal loads of 15.5 mg m−2 a−1 Cu and 135 mg m−2 a−1 Zn were applied depending on the connected catchment area and years of operation (here 2.75 years) under consideration of the model scale factor (field filter-surface/lab filter surface, cf. Table S1†). Also, the feed solution volume was derived by the catchment area from a chosen rain intensity of 10 L s−1 ha−1. The column setup was adapted to the conditions of the full-scale SCM regarding the flow direction and filter height and to produce a sufficient amount of sorptive filter media mass. The synthetic road runoff was applied with spray nozzles from the top onto the SCM-D and -V columns, unlike the SCM-S column, fed from the bottom to the top, to mimic the permanent impoundment present in the real scale SCM.
The synthetic road runoff was made by spiking copper(II) sulfate pentahydrate (CuSO4·5H2O, Merck, Darmstadt Germany) and zinc sulfate heptahydrate (ZnSO4·7H2O, Merck, Darmstadt Germany) to reach the target concentrations of 3.67 mg L−1 Cu and 32.0 mg L−1 Zn in deionized water. The pH was adjusted to 5.0 ± 0.1 in the feed. Cu and Zn effluent concentrations were measured using photometric cuvette tests (LCK360 and LCK329, Hach Lange, Düsseldorf, Germany). The prestressing was successful if more than 90% of the metals were retained. Otherwise, the pH was readjusted, and the procedure was repeated. A more detailed description can be found elsewhere.50 An overview of the prestressing conditions for the SCM filter media and further parameters are in Table S1.†
2.4. Leaching experiment of filter media and RDS
The comprehensive quiescent column leaching test descriptions were reported before.57 Briefly, 40 g of the substrate and 80 mL of synthetic road runoff (SRR) (liquid to solid ratio of 2 L kg−1 dry mass) are used in a chromatography column (Ø 20 mm) with 10 mm of glass beads (Ø 2 mm) beneath and on top of the substrate. The substrate mass was reduced to 40 g instead of 50 g due to the limited availability of the field substrates. The composition of the SRR was mainly adapted from Barrett et al. (see Table S2†).11 The concentrations of Zn and Cu were 100 and 50 μg L−1, respectively.Two SRRs representing summer and winter conditions without and with the addition of a de-icing salt (NaCl), respectively, were used and further referred to as SRR and SRR + NaCl. The leachate was withdrawn at 4, 48, and 168 h and performed as duplicates.
Additionally, blank samples without filter media were obtained with every batch to exclude cross-contamination. The examinations of the blank samples did not show any conspicuity. During the experiments, a constant room temperature of 19 ± 2 °C was ensured and the columns were wrapped with aluminum foil to avoid any photodegradation of DOM.
2.5. Chemical analysis
A shaken batch extraction test (DIN 19529) was conducted for the two substrates D_field and D_lab to identify the difference between shaken and quiescent batch leaching experiments with the same SRRs, sorptive filter media mass and liquid-to-solid ratio. Only the filter media of SCM-D were used, due to the limited availability of the other materials and as this is primarily a comparison of the two methods used.The pH and electric conductivity (EC) (WTW MultiLine® Multi 3620 IDS, SenTix® 940 pH electrode, TetraCon® 925 EC probe, Xylem Analytics) were determined in all liquid samples. Dissolved organic carbon (DOC) was measured after filtration (0.45 μm, PES syringe filter) and acidification with 1% of 32% HCl, and the samples for the dissolved heavy metals were acidified to pH < 2 with HNO3. All presented metal concentrations are in dissolved form. The concentration of Pb was analyzed using an inductively coupled plasma mass spectrometer (ICP-MS, NexION 300D, Perkin Elmer, Waltham, USA) and the concentrations of Cr, Cu, Ni, and Zn with an inductively coupled plasma optical emission spectrometer (ICP-OES, Ultima II, Horiba Jobin Yvon, Kyoto, Japan). The limits of quantification (LOQs) for Cr, Cu, Ni, Pb, and Zn are 10, 10, 10, 1, and 2 μg L−1, respectively. The DOC was measured in the liquid phase after filtration using a varioTOC Cube analyzer (Elementar, Langenselbold, Germany) with an LOQ of 0.25 mg L−1 (DIN EN 1484). The heavy metal Cd was not measured, because already the concentrations in the sorptive filter media were under the LOQ in preliminary experiments.
The loss on ignition (LOI) was analyzed according to DIN EN 15935 and determined in double determination in the 168 h sample of each tested media.
2.6. Data analysis
If the measured concentration was below the LOQ, we set the value to half of the corresponding LOQ.58 Out of 96 samples, 14 samples for Cu and 1 for Zn were below the LOQ without considering the blank and quality assurance measurements. For Ni, we evaluated 18 out of 48 measurements under the LOQ. The results from one of the duplicates of the sample V_field with SRR + NaCl at a timestep of 4 h were neglected because the measured concentrations for Cu, Zn, and Ni differed considerably. To analyze and evaluate the leached heavy metal loads from the sorptive filter media, raw data for the same media from the study of Rommel et al.50 were used to compare with the potential mobile fraction evaluated by a SEP. The potential mobile fraction was defined as the sum of the heavy metals analyzed in the SEP's acid-extractable, reducible, and oxidizable extraction steps.49 The method can be found in the ESI.†The data were assessed for normal distribution before conducting the statistical analysis. The evaluation was carried out using the Shapiro–Wilk normality test. Due to the non-normal distribution of specific parameters, the correlation analysis was performed using non-parametric Spearman rank-order correlation coefficients. Additionally, a permutation test (n = 1000) was conducted for the correlation analysis to account for the small sample size.
Further, the non-parametric Kruskal–Wallis test was used to identify significant differences among groups. In case of a significant difference, if more than two groups were evaluated, a following Conover post hoc test with Bonferroni-p-adjustment was performed to determine which group differed. The statistical analysis was done in Python with the libraries Scikit-Posthocs (0.7.0)59 and Scipy (1.10.1)60 unless stated otherwise. A significance level of α = 0.05 was applied for all hypothesis tests. A principal component analysis (PCA) was conducted with the Scikit-Learn (1.3.0) library in Python.61 Prior to the PCA, the data were standardized, and the number of principal components was set to 2. The PCA considered the following parameters: leached Cu and Zn [mg kg−1], LOI, pH, EC, and DOC. Other parameters like the timesteps, Ni, Cr, and Pb were not used due to insufficient data points or low influence on the total variance.
3. Results and discussion
3.1. Comparison of leaching in filter media prestressed in field and lab-scales and the RDS
The eluent of the SRR had an EC of 853 μS cm−1, SRR + NaCl had an EC of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 μS cm−1 and both have a pH value of 8.1. The pH and EC in the leachates changed for some of the investigated materials but remained constant over the three investigated timesteps: 4, 48, and 168 hours (Fig. S2†).
406 μS cm−1 and both have a pH value of 8.1. The pH and EC in the leachates changed for some of the investigated materials but remained constant over the three investigated timesteps: 4, 48, and 168 hours (Fig. S2†).
The highest EC was measured in the SRR leachate of RDS with 2510 μS cm−1, mostly over twice as high as from the sorptive filter media (Table 2). The EC in the field-tested media was significantly higher (p < 0.002) compared to the lab prestressed media. This observation can mainly be explained by the fact that the field filter media also contains fine RDS retained by mainly physical filtration. An increased EC was only observed in the leachate of the lab filter media from SCM-S which can be attributed to the dissolution of the mineral phases (iron-based medium).
For the eluate SRR + NaCl, the EC was comparable throughout all samples and mainly influenced by the NaCl concentrations, with still the highest value for the RDS with 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 605 μS cm−1. Formerly, a study found considerable amounts of cations (Ca, Mg, Na, and Fe) leaching from comparable RDS of a settling tank.57
605 μS cm−1. Formerly, a study found considerable amounts of cations (Ca, Mg, Na, and Fe) leaching from comparable RDS of a settling tank.57
The pH values differed significantly (p < 0.001) between the field and lab media and were generally higher for the lab media. Overall, the pH value for the SRR varied between 7.8 and 8.0 for the field, 8.0 and 8.3 for the lab filter media and slightly change from the initial pH.
Generally, lower pH values were found under the influence of the de-icing salt, most likely due to the release of H3O+ ions caused by the high Na+ concentrations through cation exchange38 with a minor negative correlation of −0.4 (Fig. S3†). Therefore, the most substantial effect on remobilization was expected for the zeolite, as the primary retention mechanism is cation exchange.
For the LOI, it can be concluded that over the 2.75 years of field operation, the sorptive filter media received enrichment in organic matter through RDS. This fact is supported by the exceptionally higher and significantly different DOC leaching of the field media. In contrast, the media of SCM-S contain granular activated lignite and have a high initial LOI (cf.Table 1), and the enrichment with RDS apparently leads to a decrease in LOI. However, the DOC concentration of S_field increased by many folds compared to S_lab.
| Media | RDS | D_lab | D_field | S_lab | S_field | V_lab | V_field | |
|---|---|---|---|---|---|---|---|---|
| Road-deposited sediments | Carbonate sand | Iron-based medium with granular activated lignite | Zeolite | |||||
| a Measured concentration was below the LOQ, we set the value to half of the corresponding LOQ. b Important note: the percentage potential mobile fraction (pMF) may not be directly linked to the mass concentrations from the aqua regia digestion. Please refer to ref. 50. | ||||||||
| Leaching test | a | a, b | a, b | a | a | a | a | |
| Cr | mg kg−1 | 90.2 | — | 22.4 | — | 6.2b | — | 16.6 |
| pMFa | 9.3 | — | 2.9 | — | 65.7 | — | 68.1 | |
| Cu | mg kg−1 | 214 | 52.9 | 41.1 | 1282 | 32.9 | 444 | 64.6 |
| pMF%a | 56.7 | 55.1 | 20.9 | 81.5 | 83.2 | 99.1 | 96.2 | |
| Ni | mg kg−1 | 23.2 | — | 11.7 | — | 42.0 | — | 8.0 |
| pMF%a | 11.6 | — | 3.5 | — | 74.8 | — | 75.5 | |
| Pb | mg kg−1 | 24.7 | — | 5.0b | — | 5.0b | — | 42.0 |
| pMF%a | 65.1 | — | 20.9 | — | 57.9 | — | 92.0 | |
| Zn | mg kg−1 | 1521 | 444 | 333 | 8204 | 274 | 3370 | 260 |
| pMF%a | 88.3 | 85.5 | 31.5 | 99.3 | 78.7 | 98.6 | 86.9 | |
| LOI | % | 10.2 | 0.5 | 2.0 | 26 | 23.6 | 5.2 | 6.6 |
| SCM | EC [μS cm−1] | pH [−] | DOC [mg L−1] | |||
|---|---|---|---|---|---|---|
| SRR | SRR + NaCl | SRR | SRR + NaCl | SRR | SRR + NaCl | |
| Eluent | 853 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 406 |
8.1 | 8.1 | 1.0 | 1.0 |
| D | ||||||
|---|---|---|---|---|---|---|
| RDS | 2510 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 605 605 |
7.8 | 7.6 | 87.1 | 104 |
| Lab | 873 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 448 448 |
8.0 | 8.2 | 0.9 | 1.1 |
| Field | 1395 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
7.8 | 7.8 | 21.2 | 22.6 |
| S | ||||||
|---|---|---|---|---|---|---|
| Lab | 1079 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476 |
8.2 | 8 | 2.7 | 3.5 |
| Field | 1302 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 413 413 |
8.0 | 7.9 | 26.7 | 22.2 |
| V | ||||||
|---|---|---|---|---|---|---|
| Lab | 846 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 425 |
8.3 | 6.6 | 2.2 | 0.9 |
| Field | 1430 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516 516 |
8.0 | 7.5 | 24.6 | 37 |
A considerably high organic content with an LOI of 10.2% was analyzed in the RDS, with a severe leaching of DOC > 85 mg L−1. It is known that RDS can carry a wide variety of organic constituents, acting as a source of pollutants,17,62,63 but they can also affect the physical structure of RDS through aggregation and the leaching of particles.64
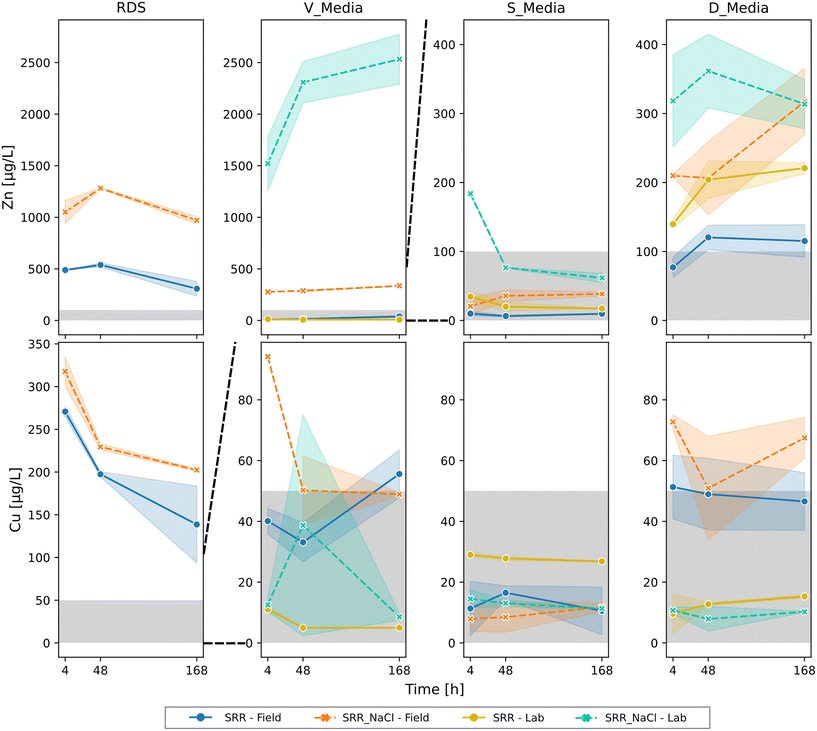
The following general observations can be stated for the leaching of heavy metals in the quiescent column leaching test.
The heavy metals Cu, Ni, and Zn had a slight positive correlation among themselves and were negatively correlated to pH (Fig. S3†), either due to higher dissolution at lower pH values or precipitation effects. In particular, under the influence of NaCl, the concentrations for Zn in the leachates of the tested filter media, beside SCM-S, exceeded the initial concentrations of 100 μg L−1, indicating leaching. Further, significant positive correlation (0.6; p < 0.0001) and leaching in the presence of NaCl were evaluated.
In contrast, no or only minor leaching of Cu was identified for all filter media. Most concentrations lay below the initial concentration of 50 μg L−1, represented by the gray area in Fig. 1. A significant influence of DOC on Cu can explain the comparable higher dissolved concentrations of copper in the field filter media compared to the lab prestressed one. Cu is known to have a high adsorption affinity to organic matter,35,65 and former studies identified complexation as an essential factor in the retention of metals from road runoff.11,63,66–68 Only a minor effect of NaCl on the Cu leaching was found, in contrast to Adhikari et al.,66 who described a negative correlation between Na and Cu in a green stormwater infrastructure. The authors attributed this to the influence of de-icing salts.
Neither Zn, Ni, nor Cu showed a time-dependent leaching, indicating a fast adjustment of equilibrium conditions or reaction. However, the RDS had declining concentrations of Zn and Cu, corresponding to an increasing pH value over time. In summary, the most important parameters controlling the heavy metal leaching from sorptive filter media and RDS were DOC, NaCl, and pH. Other studies also reported similar findings.45,63,69,70
A deeper look into the tested media reveals that the leaching of both heavy metals, Cu and Zn, was observed only for the RDS (Fig. 1). The RDS also had the highest leaching of Zn and Cu beside Zn for V_lab with SRR_NaCl. Higher concentrations in the leachate were found with NaCl, but more pronounced for Zn with approximately 100% higher values. Due to its finer particle size distribution than the sorptive filter media, it can be assumed that a larger inter-phase surface area contributes to the increased desorption. For RDS, a similar leaching order Zn > Cu > Ni > Cr was also found by Zafra et al.4 A previous study reported higher concentrations of Zn, Cu, and Ni leaching from the sediment of a settling tank (SCM-V) in a similar experimental setup.57 However, the total metal contents for Cr, Ni, and Zn were comparable, whereby Pb and Cu were about half in the RDS of this study. Therefore, a higher risk of RDS trapped in settling tanks, which are permanently submerged and subject to anaerobic processes during longer dry periods, can be assumed. Further, a higher mobile fraction was identified for Cu, Cr, Ni, and Pb in the sediments, which was also attributed to the different redox conditions in the settling tanks. Another notable difference between the sediments was the LOI, which was twice as high (∼20%) compared to the RDS in this study.50
A more diverse image can be seen for the lab and field filter media. For D_media, Zn leaching with and without de-icing salt was observed, which increased with NaCl. A possible reason might be the pH decrease with additional high amounts of Cl−. Thus, chloride complexes are formed, which increase with lower pH and compete with hydroxides and carbonate complexes.71,72 Zn-ions bound in chloride complexes are unavailable for sorption processes and precipitation and can therefore leach from the carbonate sand. The fluctuation of the Zn concentrations in Fig. 1 matches well with the development of the pH over the experiment (Fig. S2†). Overall, D_lab leached more Zn but less Cu compared to D_field. As already elaborated, this can be attributed to the higher DOC concentrations in the D_field leachate. Nevertheless, it must be pointed out that SCM-D and SCM-V are not operated with permanent submerged filter media, demonstrating that leaching during long hydraulic retention times does not pose a real risk in these systems.
As expected, a significant influence of the de-icing salts on the Zn leaching in V_media was observed, with a concentration of up to 2704 μg L−1 in the V_lab leachate. This strong Zn remobilization can be attributed to the main retention mechanism of zeolites – ion exchange. Due to the high flushing of Na+-ions, the Zn2+-ions were washed off from the exchange sites. It is known that NaCl can be used for the regeneration and activation of zeolites.73–76 In contrast, there was no leaching of Zn with SRR and only partial leaching of Cu. Similar to D_media, the lab prestressed media had higher Zn but lower Cu concentrations in the leachates.
Interestingly, the S_media showed no leaching, even though the SCM had the highest catchment area-to-filter surface area ratio. Thus, the highest total concentrations of Cu and Zn were achieved in the substrate S_lab with the highest discrepancy between the lab and field. As S_media consists of a mixture of granular ferric hydroxide with granular activated lignite, leaching for S_lab was expected as former studies showed heavy metal leaching under the influence of de-icing salts from prestressed ferric hydroxide.31
In addition to Cu and Zn, the prestressed filter media were analyzed for Cr, Pb, and Ni. All samples for Cr and Pb were below the LOQ. Relevant leaching is, therefore, unlikely. In contrast, elevated concentrations of Ni were determined for all media in the leachate with SRR + NaCl. Only the RDS had Ni concentrations over the LOQ with SRR. The highest concentrations were found in the RDS and V_field with 50.8 μg L−1 and 65.4 μg L−1, respectively, and more than twice as high as S_field and D_field (Fig. S4†). A significant positive correlation was calculated between the EC and Ni, indicating a strong influence of NaCl on the Ni leaching. The effect of de-icing salts on the mobility of Ni was already reported by others.31,77–79
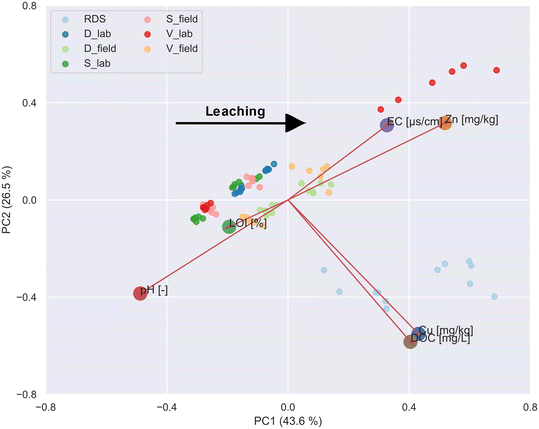
The PCA results with the identified six main parameters explaining the leaching behavior are presented in Fig. 2. In summary, 70% of the variability is explained by the principal components PC1 and PC2, with 43.6% and 26.5%, respectively. PC1 can be interpreted as a leaching vector; it increases with increasing leached Zn and Cu. Therefore, the clustering of the EC and Zn loadings is well associated with the positive influence of the de-icing salt on the leaching of Zn. The most pronounced effect of EC and Zn leaching can be determined for the V_lab substrate. This is in good agreement with the correlation analysis and is explained by the already elaborated reasons. The same applies to the cluster of DOC and Cu concentrations. Higher leaching of Cu is accompanied by increased DOC concentrations, which supports the former findings that the mobility of Cu is mainly influenced by dissolved organic matter and less through de-icing salts. Adverse effects on the leaching can be attributed to the parameters such as the pH and the LOI. A low pH leads to a dissolution of the investigated metals and can, therefore, leach from the sorptive filter media. The effect of the LOI regarding the decreased leaching of Zn and Cu can be linked to sorption processes onto the organic matter and their reactive functional groups.33 However, the LOI loading is way below the other five parameters and is therefore less relevant.
It is essential to highlight that the resulting total heavy metal content for the lab and field prestressed media differed significantly (p = <0.001). The large discrepancy can be explained by the assumptions made for the prestressing method. Either there is an overestimation regarding the applied dissolved loads of Zn and Cu, or the removal efficiency of the SCMs under in situ conditions does not meet the requirement of 90% removal chosen for the lab scale prestressing. Interestingly, the loads of D_lab and D_field are at a comparable level. This finding can be attributed to higher removal efficiency compared to SCM-S and SCM-V or a higher enrichment of particulate-bound metals through the translocation of fine sediments from the filter cake. Predominantly, the heavy metal accumulation in the different sorptive filter media can be mainly attributed to the physical filtration of particulate matter, also demonstrated by other studies for bioretention facilities.54
Generally speaking, lab studies try to mimic similar heavy metal loads, but applied in a short time, resulting in significantly increased influent concentrations, which consequently results in different loads and strengths of association.50
It is important to note that SCM-D and SCM-V are not operated under permanent impoundment and the chosen high retention times (>4 h) do not reflect the real conditions in these systems. Nevertheless, it is relevant to examine different filter media, especially as their temporal and spatial prestressing conditions were equivalent and an impoundment for roughly 4 h is conceivable during heavy, prolonged storm events. Furthermore, comparing SCM-D with a directly connected filter substratum channel without an upstream sedimentation stage against SCM-V and SCM-S with a two-stage setup is of high interest.
The following recommendations can be concluded for sorptive filter media: firstly, ion exchangers, e.g., zeolite, are unsuitable for treating traffic area runoff if de-icing salts are applied. Further, the leaching of Zn could be identified after short contact times, whereby it is also expected to happen if SCMs are not operated under permanent impoundment. Secondly, a pronounced influence of the de-icing salt on the Zn leaching for the carbonate sand also suggests an application on roads without salts in the influent.
3.2. Relationship between the leached heavy metal loads and the identified potential mobile heavy metal fraction
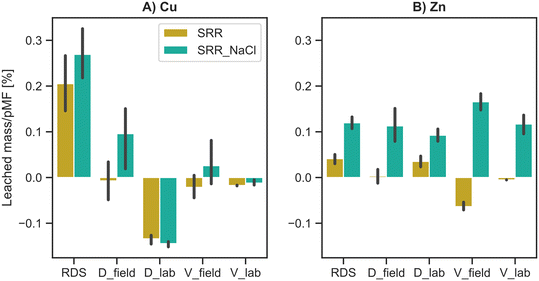
To further elaborate on the results obtained with the leaching experiments, the analyzed leached heavy metal loads were brought in relation to the total heavy metal load in the mobile fraction (Fig. 3). The total leached mass for RDS, D_field and V_field was 0.11–0.56 mg Cu per kg, 0.0–0.05 mg Cu per kg and 0.0–0.09 mg Cu per kg, respectively.For the field sorptive media, Cu-leaching occurred with only a share of less than 0.1% of the pMF. The lab media showed no leaching of Cu. Interestingly, the RDS not only had the highest released concentrations of Cu, but also shows the highest tendency to leach, concerning its potential mobile fraction.
As described in the previous section, the general leaching of Zn was only found under the influence of a de-icing salt. On average, we evaluated notable leaching loads of 2.00 mg Zn per kg for RDS, 0.29 mg Zn per kg for D_field, 0.46 mg Zn per kg for D_lab, 0.41 mg Zn per kg for V_field and 4.04 mg Zn per kg for V_lab. Despite significant differences in the leached mass and total pMF, a uniform leaching ratio of Zn was observed across all sorptive filter media with around 0.1%.
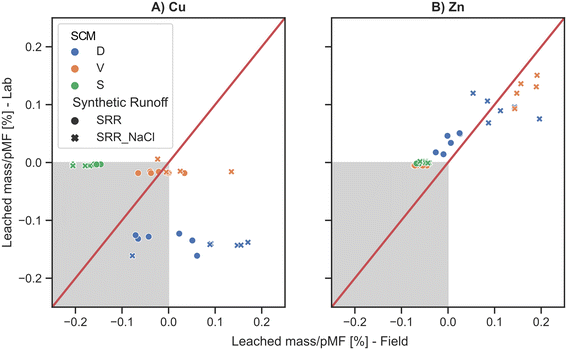
Therefore, the hypothesis of Rommel et al.50 that experiments with prestressed filter media in lab-scale likely overestimated the leaching of heavy metals cannot be confirmed with the performed quiescent column experiments on the assumption that the pMF is known. This circumstance becomes even more apparent if the leaching behaviors of Cu and Zn from the lab and in situ prestressed sorptive filter media are directly compared and standardized to their actual mobile fraction (Fig. 4).
For Cu, it can be clearly seen that when leaching occurred, the lab filter media tended to underestimate the percentage of leaching of the pMF. In this study, this is again due to the fact that DOM contamination was not taken into account in the lab-scale prestressing procedure. Yet, it is essential to mention that DOM's properties can influence the interaction with heavy metals,80,81 which must be considered when designing suitable lab experiments. The leaching prediction of the Zn loads was overall in good agreement, with an R2 of 0.89.
In summary, the mobile fraction identified through the sequential extraction tends not to leach in a high proportion in quiescent column experiments. The leaching of Cu and Zn in relation to the total mobile fraction in the sorptive filter media is less than 0.2%. Nevertheless, it needs to be considered that the experiment carried out here only represents one dry cycle. Based on these results, further research is needed to determine the development of heavy metal leaching during the life cycle of an SCM and what proportion of the beforehand retained heavy metal load is leached in the consecutive dry period. Moreover, due to the evaluated fast desorption of <4 h, the findings are also valid for less intense storm events where challenging conditions and a sufficient retention time are present in the SCM. Still, through the analyzed leaching dependence of Cu and Zn on DOM and de-icing salts, it cannot be assumed that there is permanent leaching throughout the year but rather seasonal peaks.
3.3. Comparison of the quiescent column leaching test and DIN 19529
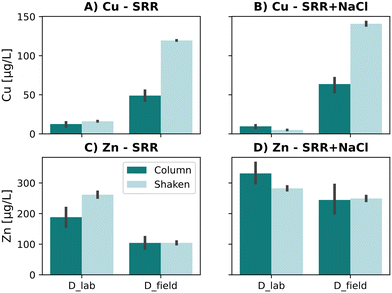
The results obtained from the quiescent column leaching experiments were compared to shaken batch leaching experiments according to German Standard DIN 19529 to understand better which mechanisms influence the Zn and Cu leaching behavior. The analyzed concentrations of Zn and Cu for the in situ or lab prestressed sorptive filter material D are presented in Fig. 5. Interestingly, no distinct difference in leached Zn was observed, particularly for D_field. Higher Zn concentrations were only analyzed in D_lab with SRR but not with SRR + NaCl. Therefore, the shaken batch test revealed no generally increased Zn concentrations. Previous studies indicated a possible overestimation compared to quiescent experiments through decreasing dispersion limitations (high solid/liquid movement) and possible physical altering of the media.57 In summary, we concluded that the contact time, surface interaction, and mixing are not limiting factors for the Zn leaching in sorptive filter media, i.e. carbonate sand.The same conclusion can be drawn for Cu leaching in D_lab. No influence of the shaken batch test on the leaching neither for the SRR nor SRR + NaCl was observed. In contrast, a remarkable increase of the Cu concentration is determined for D_field with 144% and 121% for SRR and SRR + NaCl, respectively. The pH could be excluded as a driver of the mobilization as all experiments showed similar pH values. A possible explanation could be the higher concentrations of DOC found in the eluate of the shaken batch being 38.2 mg L−1 compared to 21.9 mg L−1 in the quiescent column leaching experiments. The high DOC concentrations are directly linked to Cu leaching due to the aforementioned tendency of Cu to form complexes with organic matter. Therefore, in reality, Cu leaching in sorptive filter media is expected due to the presence of DOM which is not reflected by the lab-scale prestressed filter media.
4. Conclusion
The main findings of our study are:• The highest leaching was determined for Zn in the RDSs and filter media with a leaching order: Zn ≫ Cu > Ni. No leaching was observed for Cr or Pb. We evaluated no general increased leaching of the lab prestressed media compared to the field filter media, especially not for Cu. Leaching depended on factors like filter media properties, NaCl concentration, DOM in the liquid phase or prestressing conditions, not hydraulic retention time. The PCA revealed a strong relationship between Cu–DOM and Zn–NaCl, which also depended on the properties of the raw material. This also indicates strong seasonal differences in leaching.
• RDSs leached significantly higher heavy metal loads than the filter media and had the highest shares considering the possible mobile fraction. The higher heavy metal contents and potential mobile fraction in the lab-scale prestressed sorptive filter media did not necessarily lead to higher metal leaching or leaching at all. A leaching assessment via the determination of the potentially mobile fraction seems insufficient.
• No or only minor difference of the Zn leaching by the shaken batch compared to quiescent column leaching experiments suggests only minor limitations due to the contact time and surface interaction. In contrast, higher Cu leaching in the in situ prestressed filter media is linked to higher mobilization of DOM.
• The aforementioned formulated hypothesis has to be rejected, i.e. that over 5% of the heavy metals bound in the potential mobile fraction on sorptive filter media evaluated by a SEP tend to remobilize during dry periods in SCMs operated under permanent impoundment.
• Ion exchangers and also carbonate sand showed increased leaching under the influence of NaCl and can, therefore, not be recommended on roads with a high application of de-icing salts.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Philipp Stinshoff: conceptualization, investigation, methodology, validation, data curation, and writing – original draft. Yannic Henn: investigation, methodology, validation, data curation, and writing – review & editing. Steffen H. Rommel: conceptualization, methodology, and writing – review & editing. Brigitte Helmreich: resources, writing – review & editing, supervision, project administration, and funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We would like to thank Andreas Gmell and Harald Hilbig of the Centre for Building Materials, Technical University of Munich, for providing the analysis. We would also like to thank the SCM manufacturers, Fränkische Rohrwerke, Hauraton, and Mall, for allowing us to publish the data. Our thanks go to Marion Schiffer for the graphic realization of the abstract.References
- J. Gasperi, J. Le Roux, S. Deshayes, S. Ayrault, L. Bordier, L. Boudahmane, H. Budzinski, E. Caupos, N. Caubrière, K. Flanagan, M. Guillon, N. Huynh, P. Labadie, L. Meffray, P. Neveu, C. Partibane, J. Paupardin, M. Saad, L. Varnede and M.-C. Gromaire, Micropollutants in Urban Runoff from Traffic Areas: Target and Non-Target Screening on Four Contrasted Sites, Water, 2022, 14, 394 CrossRef CAS.
- M. Kayhanian, B. D. Fruchtman, J. S. Gulliver, C. Montanaro, E. Ranieri and S. Wuertz, Review of highway runoff characteristics, Water Res., 2012, 46, 6609–6624 CrossRef CAS PubMed.
- A. Müller, H. Österlund, J. Marsalek and M. Viklander, The pollution conveyed by urban runoff: A review of sources, Sci. Total Environ., 2020, 709, 136125 CrossRef.
- C. Zafra, J. Temprano and J. Suárez, A simplified method for determining potential heavy metal loads washed-off by stormwater runoff from road-deposited sediments, Sci. Total Environ., 2017, 601–602, 260–270 CrossRef CAS PubMed.
- M. Huber, A. Welker and B. Helmreich, Critical review of heavy metal pollution of traffic area runoff, Sci. Total Environ., 2016, 541, 895–919 CrossRef CAS PubMed.
- J. N. Brown and B. M. Peake, Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff, Sci. Total Environ., 2006, 359, 145–155 CrossRef CAS PubMed.
- İ. S. Kravkaz Kuşçu, M. Kılıç Bayraktar and B. Tunçer, Determination of Heavy Metal (Cr, Co, and Ni) Accumulation in Selected Vegetables Depending on Traffic Density, Water, Air, Soil Pollut., 2022, 233, 224 CrossRef.
- U. Kummer, J. Pacyna, E. Pacyna and R. Friedrich, Assessment of heavy metal releases from the use phase of road transport in Europe, Atmos. Environ., 2009, 43, 640–647 CrossRef CAS.
- D. S. Manta, M. Angelone, A. Bellanca, R. Neri and M. Sprovieri, Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy, Sci. Total Environ., 2002, 300, 229–243 CrossRef CAS PubMed.
- S. Meland, T. Gomes, K. Petersen, J. Håll, E. Lund, A. Kringstad and M. Grung, Road related pollutants induced DNA damage in dragonfly nymphs (Odonata, Anisoptera) living in highway sedimentation ponds, Sci. Rep., 2019, 9, 16002 CrossRef PubMed.
- M. Barrett, L. Katz, S. Taylor, J. Sansalone and M. Stevenson, Measuring and Removing Dissolved Metals from Stormwater in Highly Urbanized Areas, Transportation Research Board, Washington, D.C., 2014 Search PubMed.
- S. Gavrić, G. Leonhardt, H. Österlund, J. Marsalek and M. Viklander, Metal enrichment of soils in three urban drainage grass swales used for seasonal snow storage, Sci. Total Environ., 2021, 760, 144136 CrossRef PubMed.
- J. Zakharova, H. Pouran and A. Wheatley, Metal distribution in first flush in highway runoff of one of the busiest motorway junctions in the UK, Environ. Sci.: Water Res. Technol., 2023, 9, 3290–3301 RSC.
- Chemical Speciation in the Environment, ed. A. M. Ure and C. M. Davidson, Blackwell Science Ltd, Oxford, UK, 2002 Search PubMed.
- G. H. LeFevre, K. H. Paus, P. Natarajan, J. S. Gulliver, P. J. Novak and R. M. Hozalski, Review of Dissolved Pollutants in Urban Storm Water and Their Removal and Fate in Bioretention Cells, J. Environ. Eng., 2015, 141, 04014050 CrossRef CAS.
- K. Turpin-Nagel and T. M. Vadas, Controls on metal exposure to aquatic organisms in urban streams, Environ. Sci.: Processes Impacts, 2016, 18, 956–967 RSC.
- M. Borris, H. Österlund, J. Marsalek and M. Viklander, Contribution of coarse particles from road surfaces to dissolved and particle-bound heavy metal loads in runoff: A laboratory leaching study with synthetic stormwater, Sci. Total Environ., 2016, 573, 212–221 CrossRef CAS PubMed.
- P. Loganathan, S. Vigneswaran and J. Kandasamy, Road-Deposited Sediment Pollutants: A Critical Review of their Characteristics, Source Apportionment, and Management, Crit. Rev. Environ. Sci. Technol., 2013, 43, 1315–1348 CrossRef CAS.
- F. J. Charters, T. A. Cochrane and A. D. O'Sullivan, The influence of urban surface type and characteristics on runoff water quality, Sci. Total Environ., 2021, 755, 142470 CrossRef CAS PubMed.
- R. J. Winston, J. D. Witter and R. A. Tirpak, Measuring sediment loads and particle size distribution in road runoff: Implications for sediment removal by stormwater control measures, Sci. Total Environ., 2023, 902, 166071 CrossRef CAS.
- P. Baum, B. Kuch and U. Dittmer, Adsorption of Metals to Particles in Urban Stormwater Runoff—Does Size Really Matter?, Water, 2021, 13, 309 CrossRef CAS.
- R. A. Sutherland, F. M. G. Tack and A. D. Ziegler, Road-deposited sediments in an urban environment: A first look at sequentially extracted element loads in grain size fractions, J. Hazard. Mater., 2012, 225–226, 54–62 CrossRef CAS PubMed.
- A. R. Bakr, G. Y. Fu and D. Hedeen, Water quality impacts of bridge stormwater runoff from scupper drains on receiving waters: A review, Sci. Total Environ., 2020, 726, 138068 CrossRef CAS.
- T. Opher, A. Ostfeld and E. Friedler, Modeling highway runoff pollutant levels using a data driven model, Water Sci. Technol., 2009, 60, 19–28 CrossRef CAS.
- M. Hallberg, G. Renman and T. Lundbom, Seasonal Variations of Ten Metals in Highway Runoff and their Partition between Dissolved and Particulate Matter, Water, Air, Soil Pollut., 2007, 181, 183–191 CrossRef CAS.
- B. Helmreich, R. Hilliges, A. Schriewer and H. Horn, Runoff pollutants of a highly trafficked urban road--correlation analysis and seasonal influences, Chemosphere, 2010, 80, 991–997 CrossRef CAS PubMed.
- J. M. Wang, C.-H. Jeong, N. Hilker, R. M. Healy, U. Sofowote, J. Debosz, Y. Su, A. Munoz and G. J. Evans, Quantifying metal emissions from vehicular traffic using real world emission factors, Environ. Pollut., 2021, 268, 115805 CrossRef CAS PubMed.
- E. S. Rødland, L. S. Heier, O. C. Lind and S. Meland, High levels of tire wear particles in soils along low traffic roads, Sci. Total Environ., 2023, 903, 166470 CrossRef.
- C. Dierkes, T. Lucke and B. Helmreich, General Technical Approvals for Decentralised Sustainable Urban Drainage Systems (SUDS)—The Current Situation in Germany, Sustainability, 2015, 7, 3031–3051 CrossRef.
- T. Lucke, P. Nichols, E. Shaver, J. Lenhart, A. Welker and M. Huber, Pathways for the Evaluation of Stormwater Quality Improvement Devices − the Experience of Six Countries, Clean: Soil, Air, Water, 2017, 45, 1600596 Search PubMed.
- M. Huber, H. Hilbig, S. C. Badenberg, J. Fassnacht, J. E. Drewes and B. Helmreich, Heavy metal removal mechanisms of sorptive filter materials for road runoff treatment and remobilization under de-icing salt applications, Water Res., 2016, 102, 453–463 CrossRef CAS PubMed.
- D. Liu, J. J. Sansalone and F. K. Cartledge, Comparison of Sorptive Filter Media for Treatment of Metals in Runoff, J. Environ. Eng., 2005, 131, 1178–1186 CrossRef CAS.
- F. E. K. Okaikue-Woodi, K. Cherukumilli and J. R. Ray, A critical review of contaminant removal by conventional and emerging media for urban stormwater treatment in the United States, Water Res., 2020, 187, 116434 CrossRef CAS.
- G. Prabhukumar, G. S. Bhupal and K. R. Pagilla, Laboratory Evaluation of Sorptive Filtration Media Mixtures for Targeted Pollutant Removals from Simulated Stormwater, Water Environ. Res., 2015, 87, 789–795 CrossRef CAS PubMed.
- H.-P. Blume, G. W. Brümmer, H. Fleige, R. Horn, E. Kandeler, I. Kögel-Knabner, R. Kretzschmar, K. Stahr and B.-M. Wilke, Scheffer/Schachtschabel: Soil Science, Springer Berlin Heidelberg, Berlin, Heidelberg, s.l., 1st edn, 2016, p. 2016 Search PubMed.
- D. Tedoldi, G. Chebbo, D. Pierlot, Y. Kovacs and M.-C. Gromaire, Impact of runoff infiltration on contaminant accumulation and transport in the soil/filter media of Sustainable Urban Drainage Systems, Sci. Total Environ., 2016, 569–570, 904–926 CrossRef CAS.
- J. A. Acosta, B. Jansen, K. Kalbitz, A. Faz and S. Martínez-Martínez, Salinity increases mobility of heavy metals in soils, Chemosphere, 2011, 85, 1318–1324 CrossRef CAS PubMed.
- M. Bäckström, S. Karlsson and B. Allard, Metal Leachability and Anthropogenic Signal in Roadside Soils Estimated from Sequential Extraction and Stable Lead Isotopes, Environ. Monit. Assess., 2004, 90, 135–160 CrossRef PubMed.
- M. Huber, S. Badenberg, M. Wulff, J. Drewes and B. Helmreich, Evaluation of Factors Influencing Lab-Scale Studies to Determine Heavy Metal Removal by Six Sorbents for Stormwater Treatment, Water, 2016, 8, 62 CrossRef.
- S. Nelson, D. Yonge and M. Barber, Effects of Road Salts on Heavy Metal Mobility in Two Eastern Washington Soils, J. Environ. Eng., 2009, 135, 505–510 CrossRef CAS.
- A. C. Norrström, Metal mobility by de-icing salt from an infiltration trench for highway runoff, Appl. Geochem., 2005, 20, 1907–1919 CrossRef.
- A. Lazur, T. VanDerwerker and K. Koepenick, Review of Implications of Road Salt Use on Groundwater Quality—Corrosivity and Mobilization of Heavy Metals and Radionuclides, Water, Air, Soil Pollut., 2020, 231, 474 CrossRef CAS.
- J. M. Martin, P. Nirel and A. J. Thomas, Sequential extraction techniques: Promises and problems, Mar. Chem., 1987, 22, 313–341 CrossRef CAS.
- M. Pueyo, J. Mateu, A. Rigol, M. Vidal, J. F. López-Sánchez and G. Rauret, Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils, Environ. Pollut., 2008, 152, 330–341 CrossRef CAS PubMed.
- M. Kumar, H. Furumai, F. Kurisu and I. Kasuga, Potential mobility of heavy metals through coupled application of sequential extraction and isotopic exchange: Comparison of leaching tests applied to soil and soakaway sediment, Chemosphere, 2013, 90, 796–804 CrossRef CAS PubMed.
- R. A. Sutherland, BCR®-701: A review of 10-years of sequential extraction analyses, Anal. Chim. Acta, 2010, 680, 10–20 CrossRef CAS PubMed.
- M. A. Caraballo, A. Serna, F. Macías, R. Pérez-López, C. Ruiz-Cánovas, P. Richter and M. Becerra-Herrera, Uncertainty in the measurement of toxic metals mobility in mining/mineral wastes by standardized BCR®SEP, J. Hazard. Mater., 2018, 360, 587–593 CrossRef CAS PubMed.
- J. J. D'Amore, S. R. Al-Abed, K. G. Scheckel and J. A. Ryan, Methods for speciation of metals in soils, J. Environ. Qual., 2005, 34, 1707–1745 CrossRef PubMed.
- J. R. Bacon and C. M. Davidson, Is there a future for sequential chemical extraction?, Analyst, 2008, 133, 25–46 RSC.
- S. H. Rommel, P. Stinshoff and B. Helmreich, Sequential extraction of heavy metals from sorptive filter media and sediments trapped in stormwater quality improvement devices for road runoff, Sci. Total Environ., 2021, 12 Search PubMed.
- R. A. Sutherland, Comparison between non-residual Al, Co, Cu, Fe, Mn, Ni, Pb and Zn released by a three-step sequential extraction procedure and a dilute hydrochloric acid leach for soil and road deposited sediment, Appl. Geochem., 2002, 17, 353–365 CrossRef CAS.
- S. Rommel and B. Helmreich, Influence of Temperature and De-Icing Salt on the Sedimentation of Particulate Matter in Traffic Area Runoff, Water, 2018, 10, 1738 CrossRef CAS.
- M. Al-Ameri, B. Hatt, S. Le Coustumer, T. Fletcher, E. Payne and A. Deletic, Accumulation of heavy metals in stormwater bioretention media: A field study of temporal and spatial variation, J. Hydrol., 2018, 567, 721–731 CrossRef CAS.
- R. Furén, H. Österlund, R. J. Winston, R. A. Tirpak, J. D. Dorsey, J. Smith, M. Viklander and G.-T. Blecken, Concentration, distribution, and fractionation of metals in the filter material of 29 bioretention facilities: a field study, Environ. Sci.: Water Res. Technol., 2023, 9, 3158–3173 RSC.
- H. Li, X. Qian, W. Hu, Y. Wang and H. Gao, Chemical speciation and human health risk of trace metals in urban street dusts from a metropolitan city, Nanjing, SE China, Sci. Total Environ., 2013, 456–457, 212–221 CrossRef CAS.
- M. Huber, A. Welker, M. Dierschke, J. E. Drewes and B. Helmreich, A novel test method to determine the filter material service life of decentralized systems treating runoff from traffic areas, J. Environ. Manage., 2016, 179, 66–75 CrossRef.
- S. H. Rommel, L. Noceti, P. Stinshoff and B. Helmreich, Leaching potential of heavy metals from road-deposited sediment and sorptive media during dry periods in storm water quality improvement devices, Environ. Sci.: Water Res. Technol., 2020, 6, 1890–1901 RSC.
- R. W. Hornung and L. D. Reed, Estimation of Average Concentration in the Presence of Nondetectable Values, Appl. Occup. Environ. Hyg., 1990, 5, 46–51 Search PubMed.
- M. Terpilowski, scikit-posthocs: Pairwise multiple comparison tests in Python, J. Open Source Softw., 2019, 4, 1169 Search PubMed.
- P. Virtanen, R. Gommers, T. E. Oliphant, M. Haberland, T. Reddy, D. Cournapeau, E. Burovski, P. Peterson, W. Weckesser, J. Bright, S. J. Van Der Walt, M. Brett, J. Wilson, K. J. Millman, N. Mayorov, A. R. J. Nelson, E. Jones, R. Kern, E. Larson, C. J. Carey, İ. Polat, Y. Feng, E. W. Moore, J. VanderPlas, D. Laxalde, J. Perktold, R. Cimrman, I. Henriksen, E. A. Quintero, C. R. Harris, A. M. Archibald, A. H. Ribeiro, F. Pedregosa, P. Van Mulbregt, SciPy 1.0 Contributors, A. Vijaykumar, A. P. Bardelli, A. Rothberg, A. Hilboll, A. Kloeckner, A. Scopatz, A. Lee, A. Rokem, C. N. Woods, C. Fulton, C. Masson, C. Häggström, C. Fitzgerald, D. A. Nicholson, D. R. Hagen, D. V. Pasechnik, E. Olivetti, E. Martin, E. Wieser, F. Silva, F. Lenders, F. Wilhelm, G. Young, G. A. Price, G.-L. Ingold, G. E. Allen, G. R. Lee, H. Audren, I. Probst, J. P. Dietrich, J. Silterra, J. T. Webber, J. Slavič, J. Nothman, J. Buchner, J. Kulick, J. L. Schönberger, J. V. De Miranda Cardoso, J. Reimer, J. Harrington, J. L. C. Rodríguez, J. Nunez-Iglesias, J. Kuczynski, K. Tritz, M. Thoma, M. Newville, M. Kümmerer, M. Bolingbroke, M. Tartre, M. Pak, N. J. Smith, N. Nowaczyk, N. Shebanov, O. Pavlyk, P. A. Brodtkorb, P. Lee, R. T. McGibbon, R. Feldbauer, S. Lewis, S. Tygier, S. Sievert, S. Vigna, S. Peterson, S. More, T. Pudlik, T. Oshima, T. J. Pingel, T. P. Robitaille, T. Spura, T. R. Jones, T. Cera, T. Leslie, T. Zito, T. Krauss, U. Upadhyay, Y. O. Halchenko and Y. Vázquez-Baeza, SciPy 1.0: fundamental algorithms for scientific computing in Python, Nat. Methods, 2020, 17, 261–272 CrossRef CAS PubMed.
- F. Pedregosa, G. Varoquaux, A. Gramfort, V. Michel, B. Thirion, O. Grisel, M. Blondel, P. Prettenhofer, R. Weiss, V. Dubourg, J. Vanderplas, A. Passos, D. Cournapeau, M. Brucher, M. Perrot and E. Duchesnay, Scikit-learn: Machine Learning in Python, J. Mach. Learn. Res., 2011, 12, 2825–2830 Search PubMed.
- H.-M. Hwang, M. J. Fiala, T. L. Wade and D. Park, Review of pollutants in urban road dust: Part II. Organic contaminants from vehicles and road management, Int. J. Urban Sci., 2019, 23, 445–463 CrossRef.
- J. Zhang, P. Hua and P. Krebs, The influences of dissolved organic matter and surfactant on the desorption of Cu and Zn from road-deposited sediment, Chemosphere, 2016, 150, 63–70 CrossRef CAS PubMed.
- A.-L. Badin, P. Faure, J.-P. Bedell and C. Delolme, Distribution of organic pollutants and natural organic matter in urban storm water sediments as a function of grain size, Sci. Total Environ., 2008, 403, 178–187 CrossRef CAS PubMed.
- P. Zhu, O. Knoop and B. Helmreich, Interaction of heavy metals and biocide/herbicide from stormwater runoff of buildings with dissolved organic matter, Sci. Total Environ., 2022, 814, 152599 CrossRef CAS PubMed.
- B. Adhikari, R. Perlman, A. Rigden, M. T. Walter, S. Clark and L. McPhillips, Field assessment of metal and base cation accumulation in green stormwater infrastructure soils, Sci. Total Environ., 2023, 875, 162500 CrossRef CAS PubMed.
- N. Esfandiar, R. Suri and E. R. McKenzie, Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH, J. Hazard. Mater., 2022, 423, 126938 CrossRef CAS.
- H. Genç-Fuhrman, P. S. Mikkelsen and A. Ledin, Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater, Water Res., 2007, 41, 591–602 CrossRef PubMed.
- F. S. Degaffe and A. Turner, Leaching of zinc from tire wear particles under simulated estuarine conditions, Chemosphere, 2011, 85, 738–743 CrossRef CAS PubMed.
- M. J. A. Rijkenberg and C. V. Depree, Heavy metal stabilization in contaminated road-derived sediments, Sci. Total Environ., 2010, 408, 1212–1220 CrossRef CAS PubMed.
- M. M. Benjamin, Water chemistry, Waveland Press, Inc., Long Grove, Illinois, 2nd edn, 2015 Search PubMed.
- W. Stumm and J. J. Morgan, Aquatic chemistry: chemical equilibria and rates in natural waters, Wiley, New York, 3rd edn, 1996 Search PubMed.
- A. Alshameri, C. Yan, Y. Al-Ani, A. S. Dawood, A. Ibrahim, C. Zhou and H. Wang, An investigation into the adsorption removal of ammonium by salt activated Chinese (Hulaodu) natural zeolite: Kinetics, isotherms, and thermodynamics, J. Taiwan Inst. Chem. Eng., 2014, 45, 554–564 CrossRef CAS.
- K. Athanasiadis and B. Helmreich, Influence of chemical conditioning on the ion exchange capacity and on kinetic of zinc uptake by clinoptilolite, Water Res., 2005, 39, 1527–1532 CrossRef CAS PubMed.
- V. J. Inglezakis, M. M. Loizidou and H. P. Grigoropoulou, Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility, J. Colloid Interface Sci., 2004, 275, 570–576 CrossRef CAS PubMed.
- S. Kesraoui-Ouki, C. R. Cheeseman and R. Perry, Natural zeolite utilisation in pollution control: A review of applications to metals' effluents, J. Chem. Technol. Biotechnol., 1994, 59, 121–126 CrossRef CAS.
- M. Linde, I. Öborn and J. P. Gustafsson, Effects of Changed Soil Conditions on the Mobility of Trace Metals in Moderately Contaminated Urban Soils, Water, Air, Soil Pollut., 2007, 183, 69–83 CrossRef CAS.
- K. Tromp, A. T. Lima, A. Barendregt and J. T. A. Verhoeven, Retention of heavy metals and poly-aromatic hydrocarbons from road water in a constructed wetland and the effect of de-icing, J. Hazard. Mater., 2012, 203–204, 290–298 CrossRef CAS PubMed.
- L. Weng, E. J. M. Temminghoff, S. Lofts, E. Tipping and W. H. Van Riemsdijk, Complexation with Dissolved Organic Matter and Solubility Control of Heavy Metals in a Sandy Soil, Environ. Sci. Technol., 2002, 36, 4804–4810 CrossRef CAS PubMed.
- Y. Ding, M. Liu, S. Peng, J. Li, Y. Liang and Z. Shi, Binding characteristics of heavy metals to humic acid before and after fractionation by ferrihydrite, Chemosphere, 2019, 226, 140–148 CrossRef CAS PubMed.
- X. Guo, X. Xie, Y. Liu, C. Wang, M. Yang and Y. Huang, Effects of digestate DOM on chemical behavior of soil heavy metals in an abandoned copper mining areas, J. Hazard. Mater., 2020, 393, 122436 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ew00388h |
| This journal is © The Royal Society of Chemistry 2025 |