Integrating wastewater analysis and targeted clinical testing for early disease outbreak detection and an enhanced public health response
Vicente
Contreras
*ac,
Vander
Georgeff
ac,
Gabriela
Iglesias-Mendoza
ac,
Tara
Nicklay
ac,
Matthew
Rutherford
d,
Nancy
Lorenzon
b,
Keith
Miller
e,
Sarah
Watamura
f,
Corinne
Lengsfeld
a and
Phillip
Danielson
 *b
*b
aResearch and Sponsored Programs, University of Denver, Denver, CO, USA. E-mail: Vicente.Contreras@du.edu
bDepartment of Biological Sciences, University of Denver, Denver, CO, USA. E-mail: Phil.Danielson@du.edu
cColorado National Wastewater Surveillance Center of Excellence, Denver, CO, USA
dDepartment of Computer Science, University of Denver, Denver, CO, USA
eDepartment of Chemistry and Biochemistry, University of Denver, Denver, CO, USA
fDepartment of Psychology, University of Denver, Denver, CO, USA
First published on 5th December 2024
Abstract
The COVID-19 pandemic provided an unprecedented opportunity to assess the value of wastewater based epidemiology (WBE) as a tool to complement clinical testing in efforts to monitor and mitigate disease outbreaks. This study presents a retrospective assessment of a WBE approach that integrated WBE from congregate living facilities with high-frequency, rapid-turnaround clinical testing within a university setting. By focusing on communal living spaces, such as dormitories, this approach made it possible to rapidly identify and counter the spread of SARS-CoV-2 as well as to monitor the efficacy of campus-focused public health measures throughout the pandemic. Beginning in 2020, the University of Denver (DU) implemented a campus-wide, dual-prong COVID-19 response that combined WBE with frequent high-sensitivity testing (FHST) of individuals by RT-qPCR. Wastewater monitoring at the building level was employed in an effort to facilitate the early detection of SARS-CoV-2 spread and thereby make it possible to more confidently and precisely allocate limited clinical testing resources to identify and isolate infected individuals. This data-driven approach to WBE-informed targeting of FHST resources contributed to markedly and consistently lower SARS-CoV-2 positivity rates on campus compared to the surrounding metropolitan area. Analyses of data from multiple dormitories, and spanning several early-stage disease outbreaks, have highlighted the potential of WBE to optimize limited clinical resources for detecting, containing, and resolving the spread of communicable diseases. The information gained from DU's COVID-19 response can help to guide the development of future public health strategies in other communities confronting similar challenges.
Water impactThis study examines the synergistic benefits of integrating wastewater-based epidemiology with high-frequency clinical testing for enhanced public health interventions. The successful management of COVID-19 in congregate living facilities serves as a useful case study, illustrating the potential for swift and sustainable development of effective public health responses to some types of communicable disease transmission. |
Introduction
Integrating wastewater based epidemiology (WBE) derived data with targeted clinical testing offers a powerful approach for early disease outbreak detection and more effective public health responses. Its use during the COVID-19 pandemic contributed to an evolution in wastewater pathogen monitoring that spans decades.1 The implementation of WBE strategies during the global pandemic has demonstrated its utility as a means of efficiently tracking community SARS-CoV-2 trends, often predicting outbreaks and waves of emerging variants days before infected individuals sought medical assistance and received confirmed diagnoses at hospitals and other clinical facilities.2 This has accelerated advancements in wastewater sampling and pathogen concentration and detection technologies, propelling WBE derived data to the forefront of the public health response.3With roots tracing back to poliovirus detection in the 1940s, the use of wastewater testing has expanded over the decades to encompass a broader diversity of potential public health markers. While the testing of wastewater for pollutants, pharmaceuticals, and illicit drugs remains more abundant in the professional literature due to its broader historical scope, regulatory drivers, and ecological focus, the testing of wastewater for disease organisms (e.g., enteroviruses, rotavirus, and SARS-CoV-2) and antibiotic resistance genes has gained prominence, especially with the increased interest during the COVID-19 pandemic.1,4 Demonstrating its potential to specifically impact public health policy, aside from SARS-CoV-2, WBE has also facilitated the confirmation of vaccine-derived poliovirus transmission.5 Still, questions and concerns regarding the value of wastewater disease monitoring remain unresolved. These include the extent to which wastewater testing can be adapted for other pathogens, how such data can be effectively translated into actionable insights for public health and healthcare decision-makers, and whether the public health interests of populations not served by centralized sewer systems can nonetheless benefit from WBE-derived data.4
The urgency of the COVID-19 pandemic accelerated advancements in molecular diagnostics but also strained the infrastructure for clinical testing worldwide. In this context, wastewater-based epidemiology (WBE) offered the promise of providing a more reliable snapshot of community health, unaffected by factors such as the appearance of clinically apparent infections and healthcare access.4 Data collected from large-scale implementations of wastewater testing often reflected viral trends days earlier than trends based on confirmed diagnoses of symptomatic individuals in clinical settings. Although WBE's capacity to “forecast” the ebb and flow of disease prevalence may have assisted healthcare providers in preparing for increasing patient numbers, it made less of a contribution in terms of enabling targeted public health interventions aimed at directly and immediately curbing the spread of disease.6
Maximizing the potential utility of WBE as a public health tool requires more than its application as a forecasting mechanism; it necessitates effective integration with clinical testing activities and rapid data turnaround to enable public health decision-makers to implement timely countermeasures against disease transmission.7 By integrating WBE with traditional epidemiological tools, there is an opportunity to gain a more comprehensive understanding of community disease spread and to better monitor the effectiveness of public health interventions.8–10 While WBE has the potential to serve as a more valuable component of modern public health strategies, translating wastewater data into actionable policies remains a complex challenge. It is one that calls for integration with complementary clinical testing activities, sophisticated approaches to interpreting trends compensating for the heterogeneity of wastewater as a matrix, and the resolution of a host of other technical barriers. Simultaneously, there is growing recognition of the need for a more sustainable and less labor-intensive early warning system capable of enhancing community capacity to detect and mitigate the spread of communicable diseases.3
This paper examines the dual-pronged strategy employed at the University of Denver (DU) during the COVID-19 pandemic, one that combined WBE with frequent high-sensitivity testing (FHST). This integrated approach enabled real-time monitoring of viral loads at the building level. This allowed for the identification of early-stage disease outbreaks and the more targeted allocation of clinical testing resources on top of the university's general screening of the campus population. DU's strategy, with its emphasis on rapid-turnaround testing and robust contact tracing, highlights the potential of WBE to serve not only as an early warning system but also as a tool to assist decision makers in refining and validating the efficacy of public health interventions.11
While several other studies have shown that WBE-derived, building-level data from university campuses can be combined with clinical data the majority of these studies were focused on evaluating trends and identifying correlations between SARS-CoV-2 quantitation in the wastewater and the incidence of clinically diagnosed infections in individual buildings.12–15 The University of Denver employed WBE as a decision-making component for the targeted allocation of clinical diagnostic resources above and beyond the baseline level of campus-wide clinical testing. Focused on congregate living settings, such as university dormitories where close contact among residents facilitates the transmission of infectious agents, DU's COVID-19 response strategy sought to swiftly detect and mitigate disease outbreaks. By integrating building-level wastewater analysis with individual-level clinical data, DU demonstrated the synergy between these methods, creating a responsive and adaptive public health surveillance framework. This retrospective analysis evaluates DU's strategy in managing COVID-19 and explores the lessons learned with an eye to the potential for broader applicability of such integrated approaches to other pathogens such as influenza and respiratory syncytial virus.16,17 Through this case study, we highlight the potential of WBE to evolve from a sentinel surveillance tool into a dynamic instrument for guiding public health policy, optimizing resource allocation, and enhancing disease outbreak mitigation strategies in high-risk settings.2
Methods and materials
A two-pronged COVID-19 response strategy was implemented at DU in 2020. This combined wastewater surveillance with clinical testing to monitor and control the spread of SARS-CoV-2 on campus. As part of this response strategy, informed consent was obtained from over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 students, faculty, and staff to allow use of their test data for research purposes. This research was conducted in compliance with U.S. Federal Policy for the Protection of Human Subjects (56 FR 28003). Informed consent was obtained from all participants for testing and data collection. The privacy and confidentiality of individuals were maintained throughout the study. Ethical approval was granted by the DU Institutional Review Board for Research Involving Human Subjects (IRB 1675365-4).
000 students, faculty, and staff to allow use of their test data for research purposes. This research was conducted in compliance with U.S. Federal Policy for the Protection of Human Subjects (56 FR 28003). Informed consent was obtained from all participants for testing and data collection. The privacy and confidentiality of individuals were maintained throughout the study. Ethical approval was granted by the DU Institutional Review Board for Research Involving Human Subjects (IRB 1675365-4).
Site selection and wastewater collection
Six dormitories on the DU campus with an average population of 287 were selected for building-level wastewater testing based on sewer line accessibility and specificity of the effluent flow to the selected buildings. Dorms 1–3 (shared bathroom) consisted of rooms where a single bathroom was shared by two or three rooms. Dorms 4–6 (communal bathroom) featured communal bathroom facilities shared by twenty or more rooms. The wastewater sampling sites for dorm 3 and dorm 4 also received some effluent from additional non-residential campus buildings and an adjacent residential city block, respectively.Wastewater samples were collected twice weekly (typically Tuesday and Thursday mornings) with data from September 2020 to April 2022 being the focus of the current study. Full-size ISCO 6712 portable samplers (Teledyne ISCO, Lincoln NE) were placed in maintenance holes and programmed to collect 80 mL samples at 15-minute intervals (3 PM to 9 AM), aligning with high-volume flow rates and overnight occupancy. Grab samples were also collected from the campus COVID-19 isolation dormitory (when occupied) which housed students who had tested positive for SARS-CoV-2 on a clinical test during their requisite isolation period.
Viral RNA extraction and quantitation
Wastewater samples (45 mL) were stored on ice and transported to an accredited testing laboratory for viral RNA extraction and SARS-CoV-2 quantitation. Outsourced samples were tested by GT Molecular (Fort Collins, CO). Samples tested in house were processed by the DU Molecular Diagnostics Laboratory using the GeneCount® SARS-CoV-2 Wastewater RT-qPCR Assay Kit on a GeneCount® Q-8 qPCR (LuminUltra, New Brunswick, Canada) per the manufacturer's instructions.Clinical testing
The clinical molecular diagnostic testing component involved a 3-tier screening strategy to identify and manage COVID-19 cases including the detection of asymptomatic and pre-symptomatic cases that might otherwise go undetected. On the first tier, all students and campus employees were tested individually regardless of symptoms with the frequency of testing being based on their roles and living arrangements. Students residing in campus dormitories or participating in Greek life were tested twice a week. High-contact individuals, such as those in athletics, collection site operators, custodial and dormitory maintenance workers, and students living off campus were tested once a week. All other individuals were tested once every three weeks. On the second tier, individuals exhibiting symptoms consistent with COVID-19, such as fever, coughing, or other respiratory symptoms, were promptly tested, typically within 24 hours of symptom onset to ensure the early detection and isolation of individuals with symptomatic infections. On the third tier, contact tracing assessments were used to identify individuals at elevated risk of SARS-CoV-2 transmission due primarily to close contacts with confirmed cases. These individuals were also prioritized and tested, typically within 48 hours of contact. Individuals who had tested positive for SARS-CoV-2 within the past 90 days, were excluded from testing to avoid the potential designating residual viral RNA as a new infection.Nasopharyngeal swabs were primarily used at the onset of the program in 2020. Due to the resource-intensive nature of collecting nasopharyngeal swabs, testing of raw saliva samples was introduced in January 2021 to complement, and then replace, nasopharyngeal swabs. Saliva testing offered a less invasive and more sustainable alternative which more than quadrupled testing capacity, shortened sample-to-result turnaround times and enabled the earlier and more consistent detection of SARS-CoV-2 infections.18,19
Testing of nasopharyngeal swabs was carried out by the accredited and CLIA-certified Advanced Diagnostic Laboratories at National Jewish Health (NJH) (Denver, CO). Samples, collected by trained medical staff, were assigned barcodes linked to unique test codes and delivered to NJH for processing. RNA extraction and RT-qPCR analysis employed the Thermo Fisher TaqPath™ COVID-19 Combo Kit (EUA2010/A002) per the manufacturer's instructions. Test results were securely transferred electronically from NJH to DU patient medical records within 48 hours of sample collection. Tests that returned positive or inconclusive indications of SARS-CoV-2 infection were reported to contact tracing for processing.
Testing of saliva samples was conducted by the accredited and CLIA-certified Molecular Diagnostics Laboratory (MDL) at DU. Individuals were advised to avoid eating, drinking, or using nicotine products for 30 minutes prior to collection. A minimum of 2 mL of saliva was then self-collected into a 50 mL conical tube for analysis. Samples were assigned barcodes linked to unique test codes and transported in coolers with cold packs to the MDL for processing using a validated lab-developed test. Briefly, saliva samples were thermally inactivated at 95 °C for 15 minutes. RNA was then extracted from 200 μL aliquots using the RNAdvance Viral Kit on a BioMek i5 Automated Workstation (Beckman Coulter, Brea, CA) according to the manufacturer's instructions. Detection and quantitation of SARS-CoV-2 by RT-qPCR employed the thermal profile, master mix, and primer/probe sets from the TaqPath™ COVID-19 Combo Kit (EUA200010/A002) on a QuantStudio™ 5 Real-Time PCR detection platform (Thermo Fisher Scientific, Waltham, MA). Viral counts were quantified using a standard curve spanning a range of 5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 genome copy equivalents (GCE) of SARS-CoV-2 per reaction. Test results were securely transferred electronically from the MDL to DU patient medical records within 24 hours of sample collection. Positive and inconclusive results were reported to contact tracing teams for immediate follow up.
000 genome copy equivalents (GCE) of SARS-CoV-2 per reaction. Test results were securely transferred electronically from the MDL to DU patient medical records within 24 hours of sample collection. Positive and inconclusive results were reported to contact tracing teams for immediate follow up.
Contact tracing
The detection of SARS-CoV-2 in wastewater samples collected from any of the six dormitories being monitored served as a building-level indicator of a potential SARS-CoV-2 outbreak. Contact tracing was then employed to refine the utility of this information by identifying high-probability transmission groups and allocating resources accordingly. This leveraged data on social connections and on-campus presence as key inputs for identifying high-risk individuals and potential transmission pathways. The DU COVID-19 response and contact tracing teams analyzed new and recent cases, combining information from interviews, living locations, course registrations, extracurricular networks, and clinical data to identify likely exposures.Identified members of potential outbreak groups were contacted for clinical testing within 48 hours, with the samples collected being prioritized for rapid lab processing. An outbreak was defined, in accordance with the Denver Department of Public Health and Environment (DDPHE), as three or more related COVID-19 cases within a 14 day period occurring in a specific “group.” At DU, a “group” referred to any set of individuals who regularly interacted, such as roommates, students in the same course, or employees working in the same office. The process was iterative, with continuous data collection and analysis to adapt to new cases and emerging patterns of transmission. Wastewater data, clinical testing results, and contact tracing information were regularly reviewed to refine the focus of surveillance and intervention efforts.
Statistical analyses
Statistical analyses, including linear regression and calculation of Pearson's correlation coefficient, were performed using Microsoft Excel (version 16.0) with the Data Analysis ToolPak and JMP (version 18.0.1). A Pearson's r value of less than 0.10 was considered indicative of a negligible correlation. Graphical representations of the data were also generated using Microsoft Excel.Results and discussion
SARS-CoV-2 infections in dormitories and viral concentration in wastewater
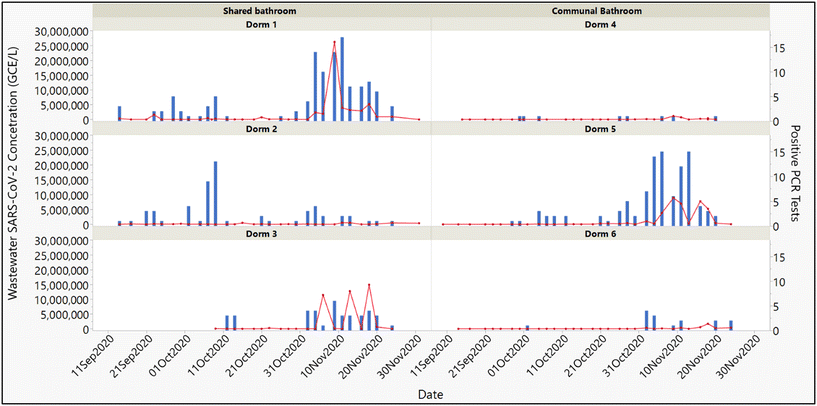
The temporal relationship between SARS-CoV-2 concentrations in building-level composite wastewater samples and the number of infected individuals residing in a given dormitory is illustrated in Fig. 1. As a part of the DU COVID-19 response plan, approximately 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 individual molecular diagnostic nucleic acid amplification tests (NAATs) for SARS-CoV-2 were performed during the period represented by this figure (September through November 2020). While only a subset of these tests were performed in response to the detection of SARS-CoV-2 in wastewater samples, this larger clinical dataset facilities a more probing retrospective analysis of the relationship between SARS-CoV-2 transmission during the initial stages of disease outbreaks and the appearance of detectable levels of virus in building-level wastewater samples. Characterizing the temporal characteristics of this relationship is paramount to effectively utilizing wastewater surveillance to assess disease transmission in high-density residential environments. This is because knowledge of how closely the detection of shed virus in wastewater tracks to the clinical detectability of an infected individual can help to inform the timing and deployment of targeted public health interventions. Understanding this relationship enables decision-makers to optimize the allocation of clinical testing resources, improve the accuracy of outbreak predictions, and implement timely containment measures. By correlating wastewater viral loads with clinical testing data, public health strategies can be refined to address the dynamics of high-density residential environments, where rapid transmission of communicable diseases is of concern.
000 individual molecular diagnostic nucleic acid amplification tests (NAATs) for SARS-CoV-2 were performed during the period represented by this figure (September through November 2020). While only a subset of these tests were performed in response to the detection of SARS-CoV-2 in wastewater samples, this larger clinical dataset facilities a more probing retrospective analysis of the relationship between SARS-CoV-2 transmission during the initial stages of disease outbreaks and the appearance of detectable levels of virus in building-level wastewater samples. Characterizing the temporal characteristics of this relationship is paramount to effectively utilizing wastewater surveillance to assess disease transmission in high-density residential environments. This is because knowledge of how closely the detection of shed virus in wastewater tracks to the clinical detectability of an infected individual can help to inform the timing and deployment of targeted public health interventions. Understanding this relationship enables decision-makers to optimize the allocation of clinical testing resources, improve the accuracy of outbreak predictions, and implement timely containment measures. By correlating wastewater viral loads with clinical testing data, public health strategies can be refined to address the dynamics of high-density residential environments, where rapid transmission of communicable diseases is of concern.
In dormitories, and similar congregate living facilities, close person-to-person proximity, centralized infrastructure and shared facilities are well suited to the transmission of communicable pathogens. The data presented in Fig. 1 shows a trend between the number of infected residents during the initial stages of an outbreak and the subsequent increases in wastewater viral concentrations. This is particularly evident when the number of new infections exceeds approximately ten per day for multiple consecutive days. This can be seen in dorm 5 (communal bathroom), dorm 1 (shared bathroom) and to a lesser extent in dorm 3 (shared bathroom). These results suggest that disease transmission within congregate living facilities is not a function of the type of bathroom facility nor the number of persons sharing it. This is perhaps not surprising given that bathrooms are often disinfected more thoroughly and frequently than other living areas due to their higher risk of pathogen transmission. More importantly, the results support the reliability of wastewater surveillance as a near real-time indicator of early-stage disease transmission. At the same time, outbreaks involving smaller numbers of infected individuals resulted in less pronounced changes in wastewater viral load. This can be seen in dorms 4 and 6 (communal bathrooms). This likely reflects the challenge of detecting very low levels of virus being shed into the wastewater in such situations.
The results from dorm 2 (shared bathroom) illustrate some of the complexities associated with wastewater surveillance. Despite a persistent number of infections over several days, viral concentrations in wastewater did not rise significantly. One possible explanation for this is the discharge of large quantities of “graywater” into the building's wastewater effluent by an on-site commercial kitchen. It is hypothesized that this may dilute the viral concentration to levels below the limit of detection of the SARS-CoV-2 assay. In addition, industrial detergents, and other chemicals potentially present in graywater, may inhibit PCR-based quantitation and/or detection methods.20,21 This underscores the need to consider the potential impact of any building-specific operational characteristics when selecting sampling sites and evaluating the wastewater data collected.
Aside from this isolated case, however, the association between the initial occurrence and spread of SARS-CoV-2 infections and a subsequent increase in SARS-CoV-2 concentrations in wastewater samples shows only a slight lag in time. This initial 2–3 day “lag period” may stem from a combination of factors including delays and variation in viral shedding, variation the rate of epidemiological spread and technical limits of detection.22,23 This supports the notion that monitoring trends in wastewater viral loads over time is essential to reliably detect disease spread.
The temporal relationship between wastewater viral concentrations and COVID-19 prevalence has been a topic of debate, with studies reporting wastewater as both a leading and a lagging indicator of disease trends. For example, one study in North Carolina found that wastewater viral trends often preceded reported clinical cases by a median of six days.24 In contrast, the current retrospective study observed wastewater viral detection as a lagging indicator of disease spread. This apparent “discrepancy”, though, may largely stem from differences in study design. The North Carolina study correlated trends in wastewater data with symptomatic clinical cases reported to the state's Department of Health and Human Services. From that perspective, wastewater data serve as leading indicators of clinically apparent disease. In our study, (using sample collection dates) data from FHST screening, was compared to wastewater data to assess the temporal relationship between the clinical detecting of SARS-CoV-2 and its appearance in wastewater from their dormitory. From this viewpoint, wastewater serves as a lagging indicator of presymptomatic disease transmission.
Despite the differences in study design, these findings support the proposition that wastewater-based epidemiology can serve as an effective marker of disease transmission. Moreover, compared to large scale clinical screening by NAATs, the comparatively low cost of monitoring a community for the presence of infected individuals by wastewater testing may be particularly well suited to environments where technical and/or financial resources are more limited.25–27 Given the extended turnaround times for individual clinical testing that were often encountered during the COVID-19 pandemic, the use of wastewater-derived data has the potential to provide valuable insights for public health officials, enabling more timely interventions even before cases are confirmed through clinical testing. This is based on the expectation that faster turnaround times for wastewater samples are likely to be more readily achieved due to the need to process far fewer samples compared to individualized clinical testing. The retrospective analysis of SARS-CoV-2 concentrations in building-level wastewater samples on the DU campus and the number of infected individuals residing in a given dormitory further reveal that once the infected individuals have been identified and are isolated, the viral load present in the wastewater similarly decreases. This finding suggests that wastewater testing may be a reliable indicator of the effectiveness of public health interventions in slowing or halting transmission in other congregate living facilities that share characteristics with dormitories such as nursing homes, apartment complexes, and the like.
Wastewater viral loads in a COVID-19 isolation dormitory
During the COVID-19 pandemic, FHST was used to identify and isolate SARS-CoV-2 infected individuals from otherwise healthy populations. These individuals were given the option of isolating either off-campus or in a designated isolation dormitory on campus. This isolation dormitory was also included in the university's wastewater surveillance program. In contrast to the observed association between early disease outbreaks and subsequent increases in SARS-CoV-2 concentrations in wastewater in regular dormitories, a negligible correlation was found between the number of infected individuals and viral load in the wastewater effluent from the university's isolation dormitory (Fig. 2). | ||
| Fig. 2 Linear regression analysis of the relationship between the concentration of SARS-CoV-2 in wastewater and the number of infected individuals in an isolation dormitory at different time points after having contracted the virus. The x-axis shows the concentration of target virus (GCE L−1) in the building's wastewater effluent and the y-axis shows the number of SARS-CoV-2 positive residents. The data, collected from the isolation dormitory, show a negligible strength of correlation between these variables (R2 = 0.0055; Pearson's correlation coefficient = 0.074). This result is consistent with the observation that the rate of viral shedding changes substantially between individuals and throughout the course of an infection.28,29 | ||
This lack of a strong correlation in data collected from the isolation dormitory was not unexpected. It is hypothesized that this likely reflects significant differences in the temporal dynamics of viral shedding between residents in the regular (i.e., non-isolation) dormitories versus those residing in the isolation dormitory. Specifically, in the regular dormitories the integrated wastewater and FHST screening protocols typically identified newly infected individuals shedding in the early stages of the infection when viral shedding is lower than at the peak of the infection. In contrast to this, residents in the isolation dormitory comprised a population of individuals at all stages of disease progression from pre- to post-symptomatic. Since the rate of SARS-CoV-2 shedding can vary widely not only as a function of one's stage of infection but also due to age, immune status and viral variant there is little if any basis for expecting viral concentrations in wastewater to be reflective of the number of individuals shedding the virus in an isolation dormitory setting.22,23,28
This finding has important epidemiological implications, suggesting that, at the building level, changes in wastewater concentrations of a target viruses may be more quantitatively useful at the beginning of an outbreak when infected individuals are more likely to be mostly in the earliest stages of disease spread and thus somewhat more consistent with each other in terms of viral shedding. This is in comparison to a building-level population experiencing multiple temporally separate outbreaks and a more sustained state of disease transmission spanning a greater duration of time (i.e., a state similar to that in the isolation dormitory). This is not to suggest that wastewater-derived data is less informative outside of the beginning of an initial outbreak. Rather, it may simply be that its informational value shifts somewhat from being more “quantitative” to being more “qualitative” if viral transmission progresses without effective mitigation.
Optimizing resource allocation through wastewater surveillance
During the COVID-19 pandemic, DU employed a dual strategy of clinical diagnostic testing for students, faculty, and staff alongside wastewater testing of dormitories. Early in the pandemic (autumn 2020), clinical testing resources were limited. In this context, wastewater testing proved invaluable by providing collective information for larger portions of the campus population than individual clinical testing could cover. With a turnaround time of 1.5 to 2 days, equal to or faster than clinical testing, wastewater data guided the allocation and prioritization of clinical testing resources. Dormitories with SARS-CoV-2 detected in wastewater were prioritized for individual clinical testing using nasopharyngeal or saliva samples, while random screening in buildings testing negative for SARS-CoV-2 was temporarily reduced until outbreaks in other areas were mitigated.Although wastewater surveillance effectively detected early stage COVID-19 outbreaks, its granularity was limited to identifying specific dormitories. With an average of 287 residents per dormitory, further refinement was necessary to avoid overly broad testing mandates. A data-driven approach that then integrated information such as employment categories, course schedules, social contact networks, and attendance at high-contact events facilitated the further refinement of targeted testing. By prioritizing clinical testing for high-risk individuals, in buildings with SARS-CoV-2 positive wastewater, the university achieved rapid identification of cases, swift isolation, and timely contact tracing. Expedited processing of RT-qPCR tests (approximately 11 hours on average) by the DU Molecular Diagnostics Laboratory of clinical samples from targeted groups minimized the window of opportunity for viral spread. This underscored the importance of efficient laboratory workflows and rapid testing turnaround times for effective public health responses.
Despite these advantages, the university recognized the limitations of relying primarily on wastewater testing. Factors such as the relatively early stage of wastewater SARS-CoV-2 testing development and the substantial potential costs of a large-scale outbreak necessitated a cautious approach. Given the large number of students, faculty, and staff who did not live on campus full time, the university opted to maintain a more proven FHST strategy alongside the wastewater testing program to ensure comprehensive coverage.
Consistent with the findings of other researchers a retrospective view of DU's COVID-19 response also supports the reliability of building-level wastewater surveillance as an early indicator of SARS-CoV-2 transmission in congregate living facilities.14,30 Wastewater data from multiple dormitories appeared to track shortly behind the occurrence of new outbreaks and quickly returned to baseline (i.e., no virus detected) shortly following the isolation of individuals identified by focused clinical testing were isolated away from the dormitory. Based on these observations, and the fact that the University of Denver campus comprises over 40 buildings, an expansion in the number of building-level wastewater collection sites combined with rapid sample-to-result turnaround times wastewater-derived data could have been employed more broadly to quickly detect and mitigate COVID-19 outbreaks. A greater reliance on wastewater monitoring could have substantially reduced the overall volume of individual clinical tests required to identify infected individuals; thereby conserving personnel, testing kits, and laboratory resources. This is consistent with mathematical models used to simulate the use of wastewater monitoring to reduce clinical testing intensity while maintaining reliable measurements of diseases incidence.30 This has also indicated the potential for significant cost savings through wastewater data driven optimization of public health resource allocation.
Effectiveness of wastewater surveillance integrated with FHST on COVID-19 positivity
The overall effectiveness of integrating building-level wastewater testing data with FHST to rapidly identify and isolate SARS-CoV-2 positive individuals is illustrated in Fig. 3. Between August of 2020 and April of 2022, the DU COVID-19 response program conducted twice weekly wastewater testing of six non-isolation dormitories and approximately 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 clinical diagnostic tests across campus. Throughout this time period the percent SARS-CoV-2 positivity of the campus population was tracked and compared the positivity rate for the surrounding city and county of Denver. The data demonstrate that the integrated approach to mitigating the impact of SARS-CoV-2 allowed for the early identification and isolation of infected individuals, which was crucial in preventing significant outbreaks on campus. This resulted in a level of SARS-CoV-2 positivity that was one quarter to one tenth that of the surrounding general population where efforts to limit the transmission of the virus centered largely on guidance from a series of Public Health Orders issued by the Colorado Department of Health and Environment. These included social distancing, masking, and size limitations on indoor gatherings.
000 clinical diagnostic tests across campus. Throughout this time period the percent SARS-CoV-2 positivity of the campus population was tracked and compared the positivity rate for the surrounding city and county of Denver. The data demonstrate that the integrated approach to mitigating the impact of SARS-CoV-2 allowed for the early identification and isolation of infected individuals, which was crucial in preventing significant outbreaks on campus. This resulted in a level of SARS-CoV-2 positivity that was one quarter to one tenth that of the surrounding general population where efforts to limit the transmission of the virus centered largely on guidance from a series of Public Health Orders issued by the Colorado Department of Health and Environment. These included social distancing, masking, and size limitations on indoor gatherings.
The integration of building-level wastewater surveillance and FHST during DU's COVID-19 response provided critical insights into resource optimization for public health interventions, both during the pandemic and through retrospective analysis. Other studies further validate the complementary role of wastewater surveillance in integrated public health strategies. For example, a study at the University of Massachusetts Amherst demonstrated that wastewater surveillance provided valuable real-time data, confirming the success of public health interventions and emphasizing the importance of integrating wastewater surveillance with systematic clinical testing.31 Similarly, the Tokyo 2020 Olympic and Paralympic Games effectively leveraged this dual approach to prevent significant outbreaks and reduce viral transmission through the combination of high-frequency clinical testing and wastewater monitoring.32
The lessons learned from DU's integrated approach highlight the value of wastewater surveillance in augmenting targeted clinical testing to enhance the effectiveness of disease outbreak detection and mitigation. This strategy minimized disruption to the campus community while maintaining a COVID-19 response and mitigation strategy that was highly effective relative to the rates of disease transmission in the surrounding community. The lessons learned from this approach may help to inform future efforts to optimize the use and allocation of resources for disease outbreak detection and mitigation strategies.
Conclusions
This study assessed the effectiveness of wastewater surveillance combined with FHST in preventing widespread outbreaks of SARS-CoV-2 on the DU campus during the COVID-19 pandemic. This two-pronged approach not only allowed for rapid identification and isolation of infected individuals but also maintained significantly lower positivity rates on campus compared to the surrounding metropolitan Denver community. These findings highlight the benefits of integrating wastewater-based epidemiology with targeted clinical testing as a key strategy for infectious disease management in communal living facilities like university dormitories.Key findings from this study show an association between new outbreaks of SARS-CoV-2 and detectable increases in building-level wastewater viral concentrations, particularly when infections exceeded ten individuals per day over consecutive days. Although smaller outbreaks resulted in less pronounced changes in viral load, these findings still demonstrate the reliability of wastewater surveillance as a near real-time indicator of disease transmission. It is important to note that building-specific factors such as graywater discharge may influence the ability to detect a target disease organism. This underscores some of the nuances with regard to the effective use of wastewater data and the importance of considering building-specific characteristics when interpreting data.
The observed lag of 2–3 days between initial outbreaks and detectable increases in wastewater viral concentrations underscores the importance of wastewater monitoring at regular intervals over time. Effective use of wastewater data also requires rapid data analysis and reporting so as to facilitate optimal resource allocation, and timely public health responses. Following the successful implementation of DU's two-pronged COVID-19 response strategy, deviation from a wastewater baseline status of “SARS-CoV-2 not detected” in the dormitories where wastewater was being monitored contributed to the precise and accurate tracking of the relatively few subsequent outbreaks of SARS-CoV-2 infections in these congregate living facilities. In toto, the integrated use of wastewater monitoring and targeted clinical testing coupled with swift analytical workflows, has the potential to more effectively detect and interrupt outbreaks thereby preventing broader disease transmission in congregate living facilities.
Lessons learned and recommendations
The successful containment of outbreaks and prevention of widespread disease transmission on campus demonstrated the effectiveness and efficiency of DU's approach to integrating wastewater surveillance data into the overall COVID-19 pandemic response plan. The lessons learned highlighted the importance of swift analytical workflows, refined group targeting and smart resource allocation. Future research and development efforts should focus on:Enhanced data integration: leveraging multiple data sources to refine target groups more precisely and improve resource allocation.
Rapid response protocols: developing and implementing rapid testing and isolation protocols informed by wastewater data.
Continual monitoring and adaptation: developing sentinel systems for continuous wastewater monitoring to better inform public health stakeholders.
Data availability
The data that support the findings of this study which are stored in a secure data repository at the University of Denver's are available from the corresponding author, Dr. Phil Danielson, upon reasonable request. Due to ethical and legal restrictions, access to some data (e.g., personal identifiers and location-specific details) is restricted to protect participant confidentiality. Requests for access to these data should be made in writing and will be subject to review by the University of Denver's Institutional Review Board. Data will be made available to qualified researchers who agree to sign a data use agreement outlining the responsibilities and conditions for data use, including restrictions on the use of data for non-commercial research purposes only.Author contributions
Vicente Contreras: conceptualization, investigation, formal analysis writing – original draft, writing – reviewing and editing Vander Georgeff: investigation, data curation, visualization, Gabriela Iglesias-Mendoza: investigation, data curation Tara Nicklay: investigation, data curation Matthew Rutherford: software Nancy Lorenzon: project administration, resources Keith Miller: conceptualization, methodology, resources Sarah Watamura: project administration, funding acquisition Corinne Lengsfeld: project administration, funding acquisition Phillip Danielson: writing – reviewing and editing, methodology, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This article was funded in part by the Colorado National Wastewater Surveillance System Center of Excellence, which is supported by the Epidemiology and Laboratory Capacity for Infectious Disease Cooperative Agreement through the Centers for Disease Control and Prevention (Grant No. NU50CK000552). A portion of the funding for this work also came from the University of Denver COVID Response Fund; a University of Denver and National Jewish Health seed funding grant; and a PROF grant from the University of Denver. The authors would like to acknowledge Sarah Johnson and Dr. David Odell from the University of Denver Health and Counselling Center and Roo Hiremath in the University of Denver Housing and Residential Education department for their technical expertise and willingness to gather data or store our HIPPA compliant data set for this study. Without their help with our dataset, this paper would not have been possible. This work had approval through the University of Denver IBC and IRB committees specifically: DU IRB protocol number 1680792 and DU IBC protocol number. 1675365-1.Notes and references
- H. R. Safford, K. Shapiro and H. N. Bischel, Wastewater analysis can be a powerful public health tool—if it's done sensibly, Proc. Natl. Acad. Sci. U. S. A., 2022, 119(6), e2119600119 CrossRef CAS PubMed.
- K. Bibby, A. Bivins, Z. Wu and D. North, Making waves: Plausible lead time for wastewater based epidemiology as an early warning system for COVID-19, Water Res., 2021, 202, 117438 CrossRef CAS PubMed.
- C. G. Daughton, Wastewater surveillance for population-wide Covid-19: The present and future, Sci. Total Environ., 2020, 736, 139631 CrossRef CAS PubMed.
- I. Xagoraraki and E. O'Brien, Wastewater-Based Epidemiology for Early Detection of Viral Outbreaks, in Women in Water Quality: Investigations by Prominent Female Engineers [Internet], ed. D. J. O'Bannon, Springer International Publishing, Cham, 2020, pp. 75–97, DOI:10.1007/978-3-030-17819-2_5.
- H. Asghar, O. M. Diop, G. Weldegebriel, F. Malik, S. Shetty and L. El Bassioni, et al., Environmental Surveillance for Polioviruses in the Global Polio Eradication Initiative, J. Infect. Dis., 2014, 210(suppl_1), S294–S303 CrossRef PubMed.
- A. E. Kirby, Using Wastewater Surveillance Data to Support the COVID-19 Response — United States, 2020–2021, Morb. Mortal. Wkly. Rep., 2021, 70(36), 1242–1244 CrossRef CAS PubMed , available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7036a2.htm.
- J. Faraway, J. Boxall-Clasby, E. J. Feil, M. J. Gibbon, O. Hatfield and B. Kasprzyk-Hordern, et al., Challenges in realising the potential of wastewater-based epidemiology to quantitatively monitor and predict the spread of disease, J. Water Health, 2022, 20(7), 1038–1050 CrossRef PubMed.
- W. Ahmed, B. Tscharke, P. M. Bertsch, K. Bibby, A. Bivins and P. Choi, et al., SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: A temporal case study, Sci. Total Environ., 2021, 761, 144216 CrossRef CAS PubMed.
- M. V. A. Corpuz, A. Buonerba, G. Vigliotta, T. Zarra, F. Ballesteros and P. Campiglia, et al., Viruses in wastewater: occurrence, abundance and detection methods, Sci. Total Environ., 2020, 745, 140910 CrossRef CAS PubMed.
- National Academies of Sciences, Engineering, and Medicine, Wastewater-based Disease Surveillance for Public Health Action [Internet], National Academies Press, Washington, D.C., 2023, available from: https://www.nap.edu/catalog/26767 Search PubMed.
- L. C. Scott, A. Aubee, L. Babahaji, K. Vigil, S. Tims and T. G. Aw, Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation, Environ. Res., 2021, 200, 111374 CrossRef CAS PubMed.
- A. Cohen, A. Maile-Moskowitz, C. Grubb, R. A. Gonzalez, A. Ceci and A. Darling, et al., Subsewershed SARS-CoV-2 Wastewater Surveillance and COVID-19 Epidemiology Using Building-Specific Occupancy and Case Data, ACS ES&T Water, 2022, 2(11), 2047–2059 Search PubMed.
- S. C. Sellers, E. Gosnell, D. Bryant, S. Belmonte, S. Self and M. S. J. McCarter, et al., Building-level wastewater surveillance of SARS-CoV-2 is associated with transmission and variant trends in a university setting, Environ. Res., 2022, 215, 114277 CrossRef CAS PubMed.
- W. Q. Betancourt, B. W. Schmitz, G. K. Innes, S. M. Prasek, K. M. Pogreba Brown and E. R. Stark, et al., COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention, Sci. Total Environ., 2021, 779, 146408 CrossRef CAS PubMed.
- H. M. Solo-Gabriele, S. Kumar, S. Abelson, J. Penso, J. Contreras and K. M. Babler, et al., Predicting COVID-19 cases using SARS-CoV-2 RNA in air, surface swab and wastewater samples, Sci. Total Environ., 2023, 857, 159188 CrossRef CAS PubMed.
- M. K. Wolfe, D. Duong, K. M. Bakker, M. Ammerman, L. Mortenson and B. Hughes, et al., Wastewater-Based Detection of Two Influenza Outbreaks, Environ. Sci. Technol. Lett., 2022, 9(8), 687–692 CrossRef CAS.
- P. M. DeJonge, C. Adams, I. Pray, M. K. Schussman, R. B. Fahney and M. Shafer, et al., Wastewater Surveillance Data as a Complement to Emergency Department Visit Data for Tracking Incidence of Influenza A and Respiratory Syncytial Virus — Wisconsin, August 2022–March 2023, Morb. Mortal. Wkly. Rep., 2023, 72(37), 1005–1009 CrossRef PubMed.
- M. Migueres, J. M. Mansuy, S. Vasseur, N. Claverie, C. Lougarre and F. Soulier, et al., Omicron Wave SARS-CoV-2 Diagnosis: Evaluation of Saliva, Anterior Nasal, and Nasopharyngeal Swab Samples, Microbiol. Spectrum, 2022, 10(6), e0252122 CrossRef PubMed.
- D. Sakanashi, N. Asai, A. Nakamura, N. Miyazaki, Y. Kawamoto and T. Ohno, et al., Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19, J. Infect. Chemother., 2021, 27(1), 126–129 CrossRef CAS PubMed.
- C. Rock, A. Alum and M. Abbaszadegan, PCR Inhibitor Levels in Concentrates of Biosolid Samples Predicted by a New Method Based on Excitation-Emission Matrix Spectroscopy, Appl. Environ. Microbiol., 2010, 76(24), 8102–8109 CrossRef CAS PubMed.
- C. Schrader, A. Schielke, L. Ellerbroek and R. Johne, PCR inhibitors – occurrence, properties and removal, J. Appl. Microbiol., 2012, 113(5), 1014–1026 CrossRef CAS PubMed.
- P. J. Arts, J. D. Kelly, C. M. Midgley, K. Anglin, S. Lu and G. R. Abedi, et al., Longitudinal and quantitative fecal shedding dynamics of SARS-CoV-2, pepper mild mottle virus, and crAssphage, mSphere, 2023, 8(4), e0013223 CrossRef PubMed.
- T. Braeye, K. Proesmans, D. Van Cauteren, R. Brondeel, N. Hens and E. Vermeiren, et al., Personal characteristics and transmission dynamics associated with SARS-CoV-2 semi-quantitative PCR test results: an observational study from Belgium, 2021–2022, Front Public Health, 2024, 12, 1429021 CrossRef PubMed.
- K. Hoffman, D. Holcomb, S. Reckling, T. Clerkin, D. Blackwood and R. Beattie, et al., Using detrending to assess SARS-CoV-2 wastewater loads as a leading indicator of fluctuations in COVID-19 cases at fine temporal scales: Correlations across twenty sewersheds in North Carolina, PLOS Water, 2023, 2(10), e0000140 CrossRef.
- P. Liu, M. Ibaraki, J. VanTassell, K. Geith, M. Cavallo and R. Kann, et al., A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level, Sci. Total Environ., 2022, 807, 151047 CrossRef CAS PubMed.
- S. Ali, E. K. Gudina, A. Gize, A. Aliy, B. T. Adankie and W. Tsegaye, et al., Community Wastewater-Based Surveillance Can Be a Cost-Effective Approach to Track COVID-19 Outbreak in Low-Resource Settings: Feasibility Assessment for Ethiopia Context, Int. J. Environ. Res. Public Health, 2022, 19(14), 8515 CrossRef CAS PubMed.
- P. Kilaru, D. Hill, K. Anderson, M. B. Collins, H. Green and B. L. Kmush, et al., Wastewater Surveillance for Infectious Disease: A Systematic Review, Am. J. Epidemiol., 2023, 192(2), 305–322 CrossRef PubMed.
- K. Owens, S. Esmaeili and J. T. Schiffer, Heterogeneous SARS-CoV-2 kinetics due to variable timing and intensity of immune responses, JCI Insight, 2024, 9(9), e176286 CrossRef PubMed.
- O. Puhach, B. Meyer and I. Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat. Rev. Microbiol., 2022, 21, 147–161 Search PubMed , available from: https://www.nature.com/articles/s41579-022-00822-w.
- A. Amirali, K. M. Babler, M. E. Sharkey, C. C. Beaver, M. M. Boone and S. Comerford, et al., Wastewater based surveillance can be used to reduce clinical testing intensity on a university campus, Sci. Total Environ., 2024, 918, 170452 CrossRef CAS PubMed.
- P. T. Acer, L. M. Kelly, A. A. Lover and C. S. Butler, Quantifying the Relationship between SARS-CoV-2 Wastewater Concentrations and Building-Level COVID-19 Prevalence at an Isolation Residence: A Passive Sampling Approach, Int. J. Environ. Res. Public Health, 2022, 19(18), 11245 CrossRef CAS PubMed.
- M. Kitajima, M. Murakami, S. S. Kadoya, H. Ando, T. Kuroita and H. Katayama, et al., Association of SARS-CoV-2 Load in Wastewater With Reported COVID-19 Cases in the Tokyo 2020 Olympic and Paralympic Village From July to September 2021, JAMA Netw. Open, 2022, 5(8), e2226822 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |