Open Access Article
Open Access ArticleExtraction of metal ions from water using a novel liquid membrane containing ZIF-8 nanoparticles, an ionic liquid, and benzo-18-crown-6
Arash Adhami *a,
Jafar Towfighi
*a,
Jafar Towfighi b,
Hamid Kazemzadehb and
Vahid Kazemzadehc
b,
Hamid Kazemzadehb and
Vahid Kazemzadehc
aDepartment of Chemical and Biological Engineering, Drexel University, Philadelphia, PA 19104, USA. E-mail: arash.adhamijamali@drexel.edu; Tel: +989356820028
bDepartment of Chemical Engineering, Tarbiat Modares University, P.O.Box 14115-143, Tehran, Iran
cDepartment of Chemical Engineering, Amirkabir University of Technology, Tehran, Iran
First published on 3rd January 2025
Abstract
As a promising type of separation membrane, liquid membranes have been broadly studied. Developing and designing a liquid membrane that shows high performance and stability for ion removal is a challenge that needs to be addressed. Here, to demonstrate the effectiveness and generalization of our method, a novel polymer inclusion membrane (PIM) was developed as a liquid membrane to separate Ca2+. The PIM contains three major components: green polyol (GPO) as the base polymer, 1-butyl-3-methylimidazolium chloride (BMIMCl) as the plasticizer, and benzo-18-crown-6 as the carrier. Due to interesting characteristics of metal–organic frameworks (MOFs), such as their highly porous structure, they have attracted attention for separating metal ions. In this work, zeolitic imidazolate framework (ZIF) nanoparticles having a highly porous structure were used to improve the flux of Ca2+ through the PIM. The impact of each constituting component of the membrane was studied. The best membrane, which resulted in optimal flux, consisted of 1 g GPO, 1.5 g ionic liquid, 0.75 g crown ether, and 0.15 g ZIF-8 (a subclass of ZIF). Additionally, the employed membrane shows a higher flux compared with other similar studies. Efficaciously separating Ca2+ can imply that any ions can be separated using our approach while tailored compositions are provided.
Water impactRemoving metal ions from water is crucial. Employing this novel hydrophobic polymer inclusion membrane, any metal ions can be removed from water providing that well-suited composing ingredients of the membrane are used. The ZIF-8 nanoparticles in the PIM structure would increase the extraction of cations from water. This developed selective membrane system is potentiated to be scaled-up for industrial water treatment. |
1. Introduction
As a solution including various components, seawater normally consists of various cations such as potassium (K+), sodium (Na+), magnesium (Mg2+), and calcium (Ca2+). A byproduct of desalination operation is brine having different dissolved salts.1 The chemical composition of brine, post-desalination, resembles that of seawater, prior to desalination, albeit with varying concentrations of dissolved salts. As an extractable component found in both brine and seawater, calcium finds extensive applications across a wide range of scientific and industrial domains. It is an essential element with significant benefits for human life.2 Further, one of the remarkable applications of calcium is in the development of Ca-ion batteries, which are far more promising compared to Li-ion batteries.3 Calcium is also available in various rocks and minerals such as stalagmites, stalactites, fluorite, limestone, gypsum, and dolomite.4 For the separation and removal of metal ions, liquid membranes have gained attention. They can be employed to effectively separate calcium ions.5 Compared with conventional liquid–liquid extraction (SX), these membranes offer more desirable characteristics, such as reducing intermediate steps and eliminating volatile solvents in conventional separation processes.6 Generally, three categories of liquid membranes are suggested: emulsion liquid membranes (ELMs), supported liquid membranes (SLMs), and bulk liquid membranes (BLMs). The essential drawback of ELMs is emulsion instability, whereas a small surface area and slow mass transfer rates are the drawbacks of BLMs.6–10 Polymer inclusion membranes (PIMs) are a developed type of SLM for tackling the problem of instability of SLMs. A PIM is generally composed of three main components: a carrier, a polymer support material, and a plasticizer.11,12 Compared with conventional types of liquid membranes, PIMs have several more favorable traits, rendering researchers interested in using PIMs for removing metal ions. The chief motives could be limited loss of carriers, high stability of PIMs, high selectivity, and transport efficiency.11,13 Several researchers have studied the reusability and stability of PIMs by conducting repeated experiments using the same membrane. As a result, PIMs showed desired stability and flux. During these experiments, the permeability values of the PIMs changed slightly in the first several cycles, while no membrane structural weakness was observed.13–15Numerous studies have explored the use of PIMs for ion separation, typically utilizing base polymers such as polyvinyl chloride (PVC), polyvinylidene fluoride (PVDF), and cellulose derivatives like cellulose triacetate (CTA). Nevertheless, many polymers possess desirable properties, such as providing a flexible thin film, improving membrane stability, mechanically supporting membranes, and minimally hindering the transport of components through the membranes.16–19 As a result, many researchers have made efforts to design and prepare PIMs to surmount the challenge of poor mechanical strength by manipulating the membrane composition.20 Cellulosic derivatives have desirable stability and show favorable flux due to insolubility in water and high melting temperature, therefore, they are useful for PIMs.21,22 However, cellulosic derivatives suffer from disadvantages including their mild hydrophilic character and vulnerability to acid hydrolysis.15,23,24 To mitigate these disadvantages, the structure of cellulosic materials such as cellulose acetate (CA) can be modified so that they will be less susceptible to hydrolysis.23,25,26 Chemical modifications to the polymer structure can be employed to retain all desirable properties while enhancing its mechanical strength.19,27 Furthermore, membrane characteristics can be further modified by employing an ionic liquid rather than traditional plasticizers.28,29 Hence, it is of paramount importance to find a well-suited composition for PIMs to perform separation desirably. Improved mechanical and chemical stability and higher surface roughness could be the results of a logical combination of a tailored polymer and plasticizer. These results can enhance the facilitated transport of components that are targeted.30–32 Lastly, as effective plasticizers for PIMs, ionic liquids (ILs) have been suggested to generate far more stable and softer membranes.33,34 The most frequently used ILs are pyridinium- and imidazolium-based ionic liquids ought to their compatibility with base polymers.5,33,35,36 Plasticizers are commonly employed to enhance the segmental mobility of polymers, therefore, ion flux across membranes increases. Additionally, they are used to impart flexibility to the polymeric material. As a core component of PIMs, carriers can be classified as neutral such as phosphoric acid esters,37 macrocyclic and macromolecular such as imidazole azothia-crown ethers,38 basic such as quaternary amines,39 acidic and chelating such as sulfonic acids.39,40 To transport alkali metals such as K+, macromolecular and macrocyclic crown ethers are normally employed as carriers.15,17,41 For their particular host–guest complexation behavior, the target ion transport across the PIM is allowed. To further improve the performance of membranes, metal–organic frameworks (MOFs) have been widely investigated. However, the integration of these materials has typically been limited to PIMs that do not prioritize sustainability. Furthermore, few studies have systematically explored the effects of membrane surface properties, such as roughness and hydrophobicity, on the transport of specific ions like Ca2+. A subcategory of metal–organic frameworks is zeolitic imidazolate framework (ZIF).42–44 This crystalline porous material exhibit desirable characteristics such as chemical and thermal stability, high surface area, and microporosity, which surpass those of other types of MOFs. ZIF nanocrystals are synthesized by linking zinc or cobalt ions with nitrogen atoms from various imidazole-based groups to form neutral frameworks. The nanosized pores, which are tunable, are made of four-, six-, eight-, or twelve-membered ring CoN4 and ZnN4 tetrahedra.45,46 For their desirable characteristics, ZIFs have an array of applications in metal removal,47 water treatment,48 sensing,49 gas separation,46,50 and gas storage.51 Zeolitic imidazolate framework-8 (ZIF-8), a member of the ZIF family, has garnered attention for its advantageous properties such as thermal and chemical stability, ease of synthesis, regular micro-channels, rapid synthesis time, and high synthesis yield.52–54 ZIF-8 has a broad spectrum of applications, including as a luminescent material for detecting Co2+, Cd2+, Ni2+, and Fe3+,42,55,56 as a catalyst for synthesizing styrene carbonate,57 as a medium for the effective separation of propylene/ propane58 and CO2/CH4,59 and for seawater desalination.60 Additional applications of ZIF-8 can be found in the literature.46,61
The current research addresses these gaps by utilizing a novel green polyol (GPO) derived from renewable resources as the base polymer. The composition of the PIM is optimized by integrating 1-butyl-3-methylimidazolium chloride (BMIMCl) as the plasticizer, benzo-18-crown-6 as the carrier, and ZIF-8 nanoparticles to achieve higher flux and selectivity. This study also investigates the role of surface roughness and hydrophobicity in enhancing the facilitated transport of Ca2+ while minimizing the effects of competitive ions.
The primary objective of this study is to develop an advanced PIM that can selectively separate Ca2+ from aqueous solutions with higher efficiency than existing membranes. This is achieved by leveraging the unique properties of the newly synthesized green polyol, ionic liquids, and MOFs. The novelty of the study lies in several aspects: the use of a green polyol (GPO), synthesized from cellulose acetate and epoxidized castor oil, provides a sustainable and biodegradable alternative. Compared with other base polymers used in PIM studies, GPO is intrinsically more hydrophobic. Consequently, it is expected that a lower quantity of organic liquid will be required in the novel membrane. In this work, the PIM was composed of environmentally friendly components, including a base polymer (GPO), a carrier (benzo-18-crown-6 (B18C6)), a plasticizer (1-butyl-3-methylimidazolium chloride (BMIMCl)), and ZIF-8. The integration of these compatible components offers a synergistic effect that improves the membrane's flux, selectivity, and stability. This shift towards environmentally friendly membrane materials is significant, offering enhanced membrane composition. Additionally, the optimized PIM enables the separation of Ca2+ from other ions in a single step, unlike traditional liquid–liquid extraction techniques that require multiple stages. This contributes to lower energy consumption and operational costs.
The study systematically examines how changes in membrane composition affect surface roughness, hydrophobicity, and their influence on ion transport mechanisms, providing new insights into designing PIMs with tailored properties for specific ion separation. Previous studies have focused on various PIM compositions; however, the incorporation of all these environmentally friendly components is relatively rare. Notably, the resulting PIM exhibits improved flux (63.01 μmol cm−2 h−1) and selectivity for Ca2+ compared to traditional membranes made from PVDF, CA, and PVC. These improvements are attributed to the enhanced surface roughness and hydrophobicity of the membrane, facilitating better ion transport mechanisms. This work demonstrates a significant advancement in developing sustainable and efficient ion separation technologies, addressing environmental concerns often associated with conventional methods.
To study the effectiveness of the developed membrane, Ca2+ was extracted from an aqueous phase. Moreover, it is anticipated that the use of this novel PIM will significantly reduce the likelihood of ion transport via simple diffusion. As a result, facilitated transport is anticipated to become the predominant transport mechanism across the membrane. The crown ether employed in this work was one of the carriers able to specifically extract calcium ions. As a result, the effective separation of Ca2+ demonstrates that our proposed method is potentially applicable for separating various cations from aqueous environments, provided that an appropriate PIM composition and carrier are used. The ultimate aim of our study is to leverage these findings to enrich specific isotopes of calcium.
The rest of this paper is organized as follows: in section 2, the required materials, experimental steps, characterization methods, and synthesis processes are described. In section 3, the results and findings of this work are explained. Finally, in section 4 the conclusions are drawn.
2. Experimental and methods
2.1. Materials
Cellulose acetate (CA, a molecular weight of ∼78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g per mole and a degree of acetylation of 2.87), sodium chloride (NaCl), calcium chloride (CaCl2), potassium chloride (KCl), and magnesium chloride (MgCl2) were purchased from Sigma-Aldrich. In addition, the ionic liquid, i.e., 1-butyl-3-methylimidazolium chloride (BMIMCl), used in this work was purchased from Sigma-Aldrich. Also, benzo-18-crown-6 (B18C6) and isophorone diisocyanate were purchased from Sigma-Aldrich. Methanol, 2-methylimidazole, zinc nitrate hexahydrate, hydrochloric acid, and N,N-dimethylacetamide (DMAc) were purchased from Merck Company. Castor oil (the viscosity of 950–1050 mPa at 20 °C, iodine value ≈ 90) was purchased from M/s SD Fine-Chem Limited, India.
000 g per mole and a degree of acetylation of 2.87), sodium chloride (NaCl), calcium chloride (CaCl2), potassium chloride (KCl), and magnesium chloride (MgCl2) were purchased from Sigma-Aldrich. In addition, the ionic liquid, i.e., 1-butyl-3-methylimidazolium chloride (BMIMCl), used in this work was purchased from Sigma-Aldrich. Also, benzo-18-crown-6 (B18C6) and isophorone diisocyanate were purchased from Sigma-Aldrich. Methanol, 2-methylimidazole, zinc nitrate hexahydrate, hydrochloric acid, and N,N-dimethylacetamide (DMAc) were purchased from Merck Company. Castor oil (the viscosity of 950–1050 mPa at 20 °C, iodine value ≈ 90) was purchased from M/s SD Fine-Chem Limited, India.
2.2. ZIF-8 preparation
In recent years, many researchers have widely studied zeolitic imidazolate frameworks (ZIFs). In addition to the frameworks' variety and their high chemical, hydrothermal, and thermal stability, as a subclass of MOFs, ZIFs have the benefits of MOFs, i.e., well-suited linkers, transition metal centers, and high porosity.62 Possessing regular microchannels and micropores, ZIF-8 is a form of ZIFs. As a benefit of having a regular microporous structure, ZIF-8 nanoparticles are capable of selectively separating molecules based on size and shape. Following the mentioned fact, researchers have shown increased interest in the applications of ZIF-8 nanoparticles in membrane technology.63,64 The details of the synthesis process of ZIF-8 can be found somewhere else.65 In summary, the experimental procedure involved the preparation of two distinct solutions. The first solution comprised 10 mmol (3 g) of zinc nitrate hexahydrate [Zn(NO3)2·6H2O, 98%, Sigma-Aldrich] dissolved in 100 mL of methanol. The second solution consisted of 80 mmol (6.6 g) of 2-methylimidazole in 100 mL of methanol. These solutions were subsequently combined and subjected to vigorous stirring at 50 °C for a duration of 2 hours to facilitate the reaction. Following the stirring process, a centrifugation step was conducted at 7000 rpm for 20 minutes to effectively isolate the resulting ZIF-8 nanocrystals from the reaction mixture. Prior to the second centrifugation, the obtained crystals were thoroughly washed with pure methanol to remove any unreacted precursors. The solid material was then allowed to dry completely at ambient temperature overnight. Finally, any residual solvent was evaporated using a vacuum oven maintained at 70 °C for a period of 24 hours, ensuring the complete removal of volatile components.2.3. Preparation steps of the PIM
2.3.1.1. Epoxidizing castor oil. Comprehensive information regarding the epoxidation of castor oil is available in the existing literature.66 To summarize, the procedure commenced with the combination of ethyl acetate, a catalyst (γ-alumina), castor oil, and an oxidizing agent (hydrogen peroxide) in a 500 mL vessel fitted with a condenser. Subsequently, the mixture was subjected to vigorous stirring while being heated. The catalytic reaction was then performed under a nitrogen (N2) atmosphere, where the mixture was maintained in a glycerin bath at 80 °C for a duration of 6 hours. The epoxidized castor oil was ultimately purified through a filtration process that utilized a Dean–Stark apparatus.
2.3.1.2. Synthesis step of green polyol (GPO). A GPO (glycidyl polyol) is synthesized through the reaction of primary hydroxyl groups in cellulose acetate with epoxy groups present in epoxidized castor oil. The process begins by dissolving cellulose acetate, fluoroboric acid, and isopropanol in DMAc at 60 °C to create a homogeneous solution in a flask placed on a stirrer. Following this, the previously epoxidized castor oil is gradually added to the solution in a dropwise manner while maintaining vigorous stirring. Notably, there are 2 moles of oxirane groups in the castor oil for every mole of hydroxyl group present in cellulose acetate.67 Upon completion of the reaction after 2 hours, the resulting polyol is obtained through the use of a rotary evaporator.
2.3.1.3. Membrane preparation. Initially, a solution containing 1 g of GPO was prepared in 10 mL of a volatile organic solvent, specifically DMAc. Subsequently, the synthesized ZIF-8, benzo-18-crown-6 (B18C6), and BMIMCl were incorporated into the solution in predetermined amounts. The resulting mixture was stirred at room temperature for 24 hours. Concurrently, while maintaining stirring, cross-linking agents, namely dibutyltin dilaurate and isophorone diisocyanate, were added to the solution. Following 20 minutes of stirring, the mixture was poured onto a smooth glass surface to cast a flat-sheet membrane using an aluminum casting knife equipped with a steel blade. After allowing the solvent to evaporate under ambient conditions overnight, a flexible polymer inclusion membrane (PIM) was obtained, with an approximate thickness of 15 μm. The membrane was then immediately submerged in a cold pure water bath to facilitate its removal from the glass. After drying, the membrane was cut into sections for use in a flat-sheet module to evaluate its separation performance.
2.4. Characterization techniques
To study the resulting membranes' surface morphology, atomic force microscopy (AFM) and field emission scanning electron microscopy (FE-SEM) were conducted. Moreover, by exploiting FE-SEM analysis, the membrane's thickness was measured. Fourier transform infrared spectroscopy (FTIR) spectra were recorded, to show the functional groups of samples. BET analysis has been conducted to discover the traits of MOF particles. To study the wettability (i.e., hydrophobicity) of the membranes, contact angle measurements were done using deionized water at room temperature. A KRÜSS DSA 10-MK2 shape analysis system was used to record the contact angle measurements which were the average of five times measuring the contact angles of five locations of each PIM sample. A Philips X-ray diffractometer (XRD) instrument (model X-pert) was used to analyze the crystallinity of each structure by recording the XRD patterns of each sample. To obtain the thermogravimetric analysis (TGA) data of each sample, a thermal analysis system (SETARAM TG 96) was exploited. These data were used to evaluate the thermal stability of each sample. At room temperature, the measurements of dumbbell shape membrane samples having 10 mm width and 82 mm length were accomplished.2.5. Design and experimental optimization of the PIM
As shown in Table 1, PIMs with different compositions of constituting components were investigated to find the optimal liquid membrane that selectively separates Ca2+ from an equimolar K+, Na+, and Mg2+ solution as the feed phase.| PIM name | Polymer BMIMCl (g) | Carrier (g) | MOF (g) | Flux (μmol cm−2 h−1) |
|---|---|---|---|---|
| CAP | 1 g CA 0.0 | 0.0 | 0.0 | 0.25 |
| GN | 1 g GPO (non-cured) 0.0 | 0.0 | 0.0 | 0.05 |
| GC1 | 1 g GPO (cured) 0.0 | 0.0 | 0.0 | 0.00 |
| GC2 | 1 g GPO (cured) 0.5 | 0.0 | 0.0 | 0.84 |
| GC3 | 1 g GPO (cured) 1.0 | 0.0 | 0.0 | 1.95 |
| GC4 | 1 g GPO (cured) 1.5 | 0.0 | 0.0 | 3.11 |
| GC5 | 1 g GPO (cured) 2.0 | 0.0 | 0.0 | — |
| PIMCr6 | 1 g GPO (cured) 1.5 | 0.25 | 0.0 | 18.79 |
| PIMCr7 | 1 g GPO (cured) 1.5 | 0.5 | 0.0 | 24.83 |
| PIMCr8 | 1 g GPO (cured) 1.5 | 0.75 | 0.0 | 45.72 |
| PIMCr9 | 1 g GPO (cured) 1.5 | 1 | 0.0 | 6.56 |
| ZPIM10 | 1 g GPO (cured) 1.5 | 0.75 | 0.05 | 24.81 |
| ZPIM11 | 1 g GPO (cured) 1.5 | 0.75 | 0.10 | 45.13 |
| ZPIM12 | 1 g GPO (cured) 1.5 | 0.75 | 0.15 | 63.01 |
| ZPIM13 | 1 g GPO (cured) 1.5 | 0.75 | 0.20 | 39.21 |
| PIMCA | 1 g CA 1.5 | 0.75 | 0.0 | 23.52 |
| PIMPVDF | 1 g PVDF 1.5 | 0.75 | 0.0 | 47.2 |
| PIMPVC | 1 g PVC 1.5 | 0.75 | 0.0 | 44.3 |
As illustrated in Fig. 1, the transport experiments were conducted at room temperature using a two-compartment glass cell, with an average volume of approximately 250 mL for each compartment. Circular liquid membranes, each with a diameter of about 7 cm, were precisely cut from the prepared membranes and positioned between the two cells. To create the feed phase, a solution of alkali metal chloride salts was prepared, maintaining a consistent molar concentration of 0.15 M. In the opposing cell, distilled deionized water served as the stripping phase. To ensure that the feed phase remained alkaline, a 0.05 M solution of tris(hydroxymethyl)aminomethane was added, resulting in a pH of approximately 8.01. A magnetic stirring bar was utilized to maintain homogeneity in both the feed and stripping phases continuously. Every six hours, 2 mL samples were collected from each phase—feed and stripping. The concentrations of cations in each sample were subsequently analyzed using the ICP/MS system (PerkinElmer Elan 9000). Each experiment was repeated three times, and the average values were reported.
Ion flux (Ji, μmol cm−2 h−1)68–70 and selectivity (αij)71,72 can be calculated by governing equations given as:
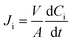 | (1) |
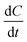 is the cation concentration change over time.
is the cation concentration change over time.
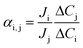 | (2) |
3. Results and discussion
3.1. Characterization results
Fig. 2(a) displays the X-ray diffraction patterns of the synthesized ZIF nanoparticles. Miller indices proportional to the crystallographic patterns of (002), (112), (022), (013), (222), (123), (114), (233), (224), (134), (125), (044), and (011), ascribed to the nanoparticles, are pertained to 2θ angles of 10.3, 12.7, 14.7, 16.4, 18, 19.1, 22.5, 24.6, 26, 26.7, 28.45, 29.7, and 7.3, respectively. All patterns of the nanoparticles match those reported in the literature.64,73,74 After using FESEM, it was revealed that the size of the ZIF-8 nanoparticle was roughly 200 nanometers. As shown in Fig. 2(b), the typical shape of a ZIF-8 nanoparticle is six-sided. Fig. 2(c) demonstrates the upshots of the TGA analysis of ZIF-8. As is clear, the first weight loss takes place at 120 °C as a result of solvent evaporation. Next, unreacted species start to decompose. Afterward, ZIF-8 is decomposed at 380 °C and thoroughly decomposed at 506 °C. Additionally, the purity of the nanoparticles was confirmed via the TGA analysis, as the results aligned perfectly with those of the TGA analysis of pure ZIF-8 reported in the literature.75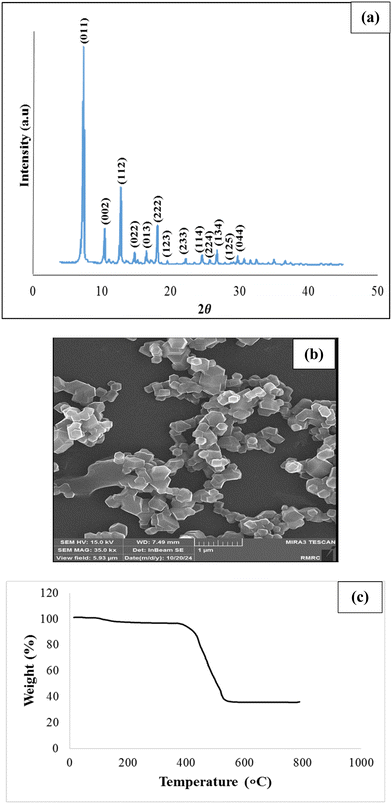 | ||
| Fig. 2 (a) The results of the XRD pattern for ZIF-8 nanoparticles, (b) FESEM of ZIF-8, and (c) TGA upshots for ZIF-8. | ||
The structural properties of ZIF-8 nanoparticles were studied using BET analysis at P/P0 = 0.99. The results show that the average pore size is 13.2 Å. Additionally, Langmuir and BET surface areas are 1871 and 1283 m2 g−1, respectively.
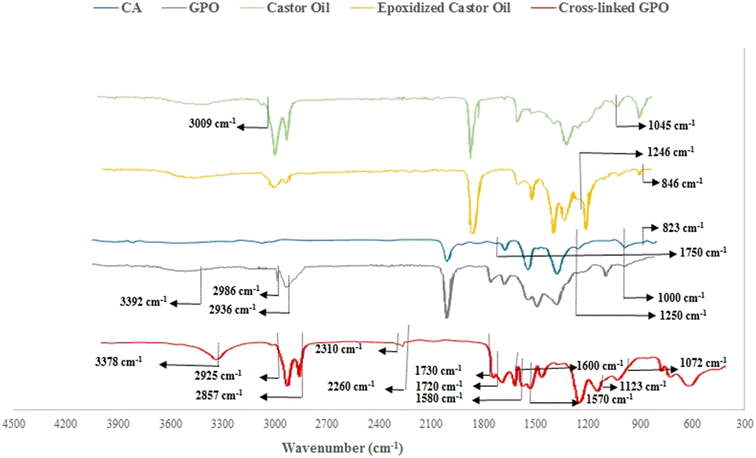
Following the epoxidation of castor oil, FTIR analysis confirmed the formation of epoxy groups. Fig. 3 presents the FTIR absorption spectrum, which reveals a new peak at 846 cm−1 attributed to the epoxy group, while the peak associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond at 3009 cm−1 significantly diminished. The peak at 1045 cm−1 results from symmetrical axial bending, and the oxirane group is responsible for the peak observed around 1246 cm−1. The figure also illustrates the FTIR results for the synthesized polyol, which is produced when the ring of epoxidized castor oil opens and reacts with cellulose acetate (CA). In the FTIR spectrum of the glycidyl polyol (GPO), the vibrational stretching of C–H bonds in CH3 and CH2 is indicated by peaks at 2936 cm−1 and 2986 cm−1, respectively. Additionally, the peak at 1750 cm−1 corresponds to the stretching vibration of C
C bond at 3009 cm−1 significantly diminished. The peak at 1045 cm−1 results from symmetrical axial bending, and the oxirane group is responsible for the peak observed around 1246 cm−1. The figure also illustrates the FTIR results for the synthesized polyol, which is produced when the ring of epoxidized castor oil opens and reacts with cellulose acetate (CA). In the FTIR spectrum of the glycidyl polyol (GPO), the vibrational stretching of C–H bonds in CH3 and CH2 is indicated by peaks at 2936 cm−1 and 2986 cm−1, respectively. Additionally, the peak at 1750 cm−1 corresponds to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in CA. A comparison of the two FTIR spectra indicates that the peak associated with the oxirane group at 823 cm−1 is considerably diminished, while a new peak emerges at 3392 cm−1, signifying the substitution of the hydroxyl group for the previously opened epoxy ring. Notable changes in the region from 1250 to 1000 cm−1 suggest an increase in the intensity of the C–O stretching vibrations (ether bonds) in the GPO, reflecting the transformation of hydroxyl groups into ether groups. Upon cross-linking the GPO, the associated FTIR absorption spectrum is depicted in Fig. 3. The FTIR spectrum reveals bands related to the cyanate group within the range of 2310 to 2260 cm−1, where the absence of peaks indicates that no free NCO groups are present in the sample. This observation confirms that the hydroxyl groups have fully reacted with isophorone diisocyanate.
O in CA. A comparison of the two FTIR spectra indicates that the peak associated with the oxirane group at 823 cm−1 is considerably diminished, while a new peak emerges at 3392 cm−1, signifying the substitution of the hydroxyl group for the previously opened epoxy ring. Notable changes in the region from 1250 to 1000 cm−1 suggest an increase in the intensity of the C–O stretching vibrations (ether bonds) in the GPO, reflecting the transformation of hydroxyl groups into ether groups. Upon cross-linking the GPO, the associated FTIR absorption spectrum is depicted in Fig. 3. The FTIR spectrum reveals bands related to the cyanate group within the range of 2310 to 2260 cm−1, where the absence of peaks indicates that no free NCO groups are present in the sample. This observation confirms that the hydroxyl groups have fully reacted with isophorone diisocyanate.
The symmetric and anti-symmetric stretching vibrations of CH2, N–H bending, and C–N stretching cause peaks in the range of 2857–2925 cm−1 and 1557–1580 cm−1. Likewise, peaks at 1720–1730 cm−1 are ascribed to the stretching vibrations of C–O related to urethane groups. Majorly, these peaks reveal that after isophorone diisocyanate cross-linked the GPO, a urethane linkage (NH–COO) was formed. The stretching of N–H and O–H in the urethane group, stretching vibration of C–C, stretching vibrations of C–O, and stretching vibrations of C–N cause peaks at 3378 cm−1, 1600 cm−1, 1123 cm−1, and 1072 cm−1, respectively.
Fig. 4 shows the SEM images of the surface and cross-section of membrane samples. The dense cross-sections and surface morphologies can be seen in these images. As shown in Fig. 4(c), the GPO-based membrane has spotted areas that are desirable rooms to load more ionic liquid content. As a result, the flux will grow higher. After adding the synthesized nanoparticles to the composition of the GPO-based liquid membrane containing BMIMCl (the ionic liquid), they can be seen on the surface of the membrane. It follows that the surface roughness of the membrane considerably grows higher. Because the nanoparticles are intrinsically porous possessing regular micropores, the ionic liquid storage capacity of the PIM is expected to increase.
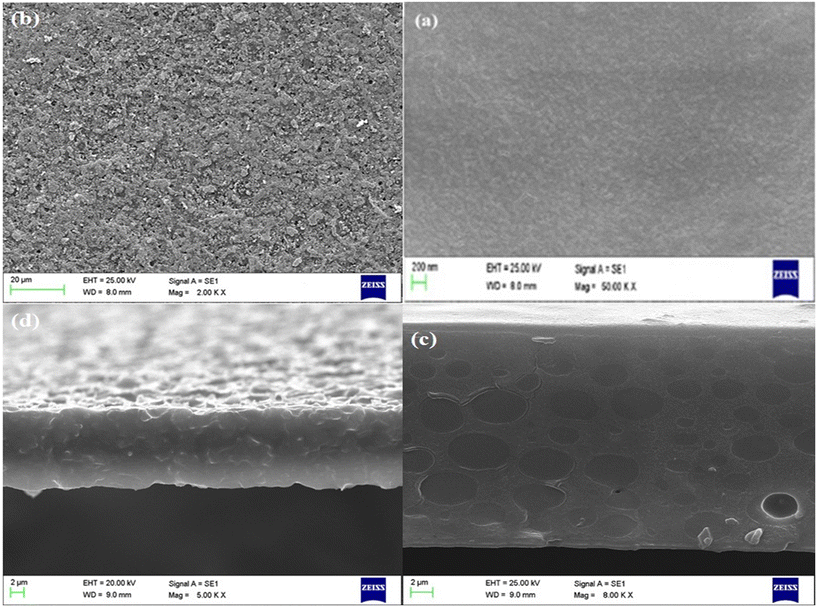 | ||
| Fig. 4 The results of scanning electron microscopy (SEM) images. (a) Surface of PIMCr8, (b) surface of ZPIM12, (c) cross-section of PIMCr8, and (d) cross-section of ZPIM12. | ||
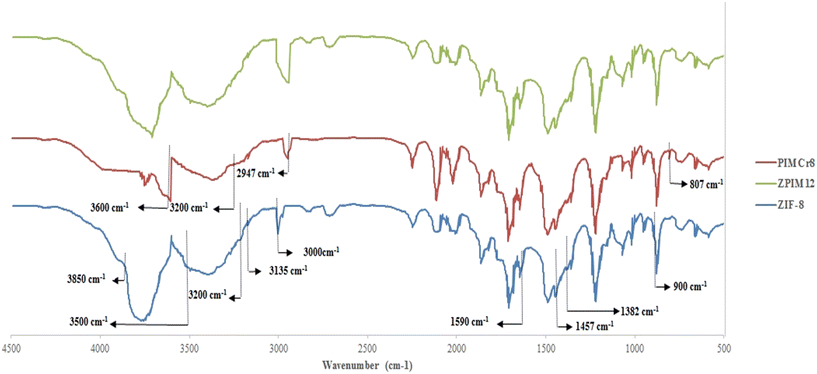
The FTIR upshots of pure nanoparticles can be found in Fig. 5. According to this figure, the N–H bond stretching causes a peak between 3500–3200 cm−1. The peaks in the range of 3000–3850 cm−1 and the peak at 3135 cm−1 are assigned to the methyl group of the linker and C–H stretching vibrational modes of the imidazole ring, respectively. The peak at 1590 cm−1 is because of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch modes. The peaks at 1457 and 1382 cm−1 are the results of the entire ring stretching. The peaks in the range of 1350 to 900 cm−1 are credited to the in-plane bending of the ring. The peaks at 760 and 690 cm−1 are assigned to aromatic sp2 C–H bending. The peak close to 420 cm−1 is the consequence of the Zn–N stretching. The FTIR analysis of PIMCr8 is also shown in Fig. 5. As is shown, the aliphatic asymmetric vibrational stretching of the C–H bond in methyl groups brings about the peak at 2947 cm−1. Likewise, the peak at 807 cm−1 is attributed to the aliphatic asymmetric stretching vibration of the C–N bond in the ionic liquid. The formation of chlorine in BMIMCl with quaternary amine salt causes a peak between 3200 and 3600 cm−1.
N stretch modes. The peaks at 1457 and 1382 cm−1 are the results of the entire ring stretching. The peaks in the range of 1350 to 900 cm−1 are credited to the in-plane bending of the ring. The peaks at 760 and 690 cm−1 are assigned to aromatic sp2 C–H bending. The peak close to 420 cm−1 is the consequence of the Zn–N stretching. The FTIR analysis of PIMCr8 is also shown in Fig. 5. As is shown, the aliphatic asymmetric vibrational stretching of the C–H bond in methyl groups brings about the peak at 2947 cm−1. Likewise, the peak at 807 cm−1 is attributed to the aliphatic asymmetric stretching vibration of the C–N bond in the ionic liquid. The formation of chlorine in BMIMCl with quaternary amine salt causes a peak between 3200 and 3600 cm−1.
This figure also demonstrates the FTIR spectrum of ZPIM12. Some differences can be distinguished between 2500 and 3550 cm−1 which are ascribed to C–H, O–H, and N–H groups in the structure of ZIF-8.
3.2. Results of design and experimental optimization of the PIM
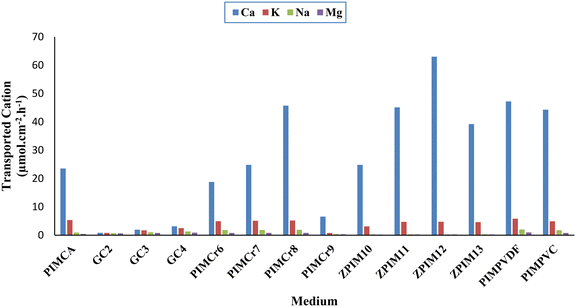
To determine the optimal polymer inclusion membrane (PIM) for achieving the highest ion flux, various membranes with different compositions of the constituent ingredients (GPO, B18C6, BMIMCl, and ZIF-8) were prepared, as shown in Table 1. Each ingredient was considered a factor that could potentially influence the flux of the target cation. To design the experimental optimization framework, at each step, one factor was varied while keeping the other factors constant. Each resulting membrane was then used in transport experiments to measure the flux of Ca2+ ions through them. Based on the data presented in Table 1, the membrane identified as ZPIM12 demonstrated the highest flux, achieving a value of 63.01 μmol cm−2 h−1.3.2.1.1. Impact of cross-linking GPO on membrane performance. To study the effect of cross-linking the GPO on the calcium flux, a membrane composed of a not cross-linked GPO and another one composed of a cross-linked GPO as the base polymers were prepared while other constituting components were not added, i.e. carrier, ionic liquid, and MOF. Generally, curing (cross-linking) can affect polymer chains and intermolecular spaces which may affect ion transport across membranes.76 As a rule of thumb, it can be mentioned that as long as the pores of a membrane matrix are larger than the ions' sizes, ions can move through the membrane. CA is almost hydrophobic having both crystalline and amorphous regions.21,77 Hydrated cations can be adsorbed onto the amorphous regions of CA by making hydrogen bonds. As can be seen in Fig. 6, the pure cellulose acetate-based membrane does not completely prohibit the passage of cations, whereas GC1 completely prohibits such passage. Structure modification of cellulose acetate and curing the GPO triggers an increase in the membrane hydrophobicity. Consequently, the targeted cation cannot pass (through simple diffusion) across the liquid membrane composed of the cross-linked GPO. Hence, the dominant transport mechanism is facilitated transport. It should be noted that this characteristic improves the membrane's selectivity.
3.2.1.2. Ionic liquid impact on membrane performance. According to Fig. 6, after introducing the ionic liquid into the membrane composition, the cation flux increased where the PIM's flexibility increased. The gaps between the polymer's chains were enlarged once the ionic liquid content increased, therefore, the Ca2+ flux increased. As explained before, once the ionic liquid content increased the cation flux across the membrane increased. It is important to note that ionic liquid contents exceeding 1.5 g resulted in an unstable base polymer. This instability may be attributed to the excessive flexibility imparted to the polymer, which could increase the distance between polymer chains and thereby hinder the formation of a stable polymer structure. Therefore, no membrane film could be formed. In this regard, the best weight for the ionic liquid was 1.5 g.
3.2.1.3. Carrier impact on membrane performance. To investigate the effect of the carrier (B18C6) on the membrane's performance in terms of flux and selectivity, miscellaneous membranes were prepared in which the ionic liquid content was kept constant (the optimal content determined in the previous step), incorporating various proportions of crown ether into the membrane structure. Then, the membranes' performances were evaluated. According to Fig. 6, these investigations revealed that the targeted cation (Ca2+) flux increased drastically after introducing B18C6. After incorporating the optimal content of the carrier into the membrane matrix, the cation flux increased by approximately 16-fold compared to PIMs including only the ionic liquid. After adding the carrier, the flux of the targeted cation (Ca2+) increased because the carrier formed a stable complex with the cation. To form such a complex, the hydrated radius of the ion and the cavity of the crown ether should be almost equal. Otherwise stated, the ion has to perfectly fit into the carrier's cavity to generate an efficacious complex. In general, crown ethers interact with cations in different ways. The most significant factors influencing the type and stoichiometry of cation/crown ether complex are the size of the crown ether cavity and the size of the cation. Typically, an effective complex, referred to as a nesting complex in this work, is formed when the size ratio of the crown ether cavity to the cation is approximately 1. The cavity can be slightly larger than the cation to facilitate this formation. Alternatively, a perching complex is formed when the crown ether cavity is moderately smaller than the targeted cation. Furthermore, to form a sandwich complex, the targeted cation must be significantly larger than the crown ether cavity, with a ratio of approximately 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
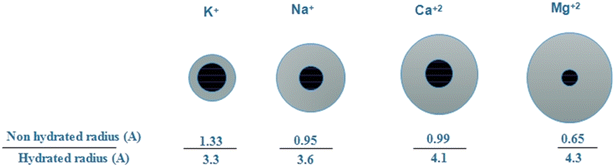
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Similarly, if the crown ether's cavity is much larger than the cation, the composition of the crown ether will change, and the cation will not interact with all the available oxygens in the crown ether structure.78 As shown in Fig. 7, the ionic radius and hydrated radius of different cations considered in this work were compared. Based on the explanations provided above, a cation with a size matching that of the carrier cavity contributed to achieving higher fluxes through the liquid membrane used in this work. According to hydrated radii reported in the literature79 for the cations considered in this work, Ca2+ registers higher flux in comparison with the other competitive cations studied in this work. According to Table 1, the maximum flux of calcium through the membrane was peculiar to the PIM containing 0.75 g of B18C6. Yet, further quantities of the carrier have reverse impact on the flux of the cation. It could be for the fact that the crown ether could not dissolve in the membrane phase, therefore, with amounts above 0.75 g it was deposited in the membrane phase and formed crystals.
1. Similarly, if the crown ether's cavity is much larger than the cation, the composition of the crown ether will change, and the cation will not interact with all the available oxygens in the crown ether structure.78 As shown in Fig. 7, the ionic radius and hydrated radius of different cations considered in this work were compared. Based on the explanations provided above, a cation with a size matching that of the carrier cavity contributed to achieving higher fluxes through the liquid membrane used in this work. According to hydrated radii reported in the literature79 for the cations considered in this work, Ca2+ registers higher flux in comparison with the other competitive cations studied in this work. According to Table 1, the maximum flux of calcium through the membrane was peculiar to the PIM containing 0.75 g of B18C6. Yet, further quantities of the carrier have reverse impact on the flux of the cation. It could be for the fact that the crown ether could not dissolve in the membrane phase, therefore, with amounts above 0.75 g it was deposited in the membrane phase and formed crystals.
3.2.1.4. Effect of ZIF-8 on membrane performance. Following the incorporation of MOF nanocrystals, the flux of Ca2+ ions exhibited notable changes, as depicted in Fig. 6. Initially, in membranes devoid of MOF particles, the calcium–crown ether complex was primarily transported through the polymer chains. However, upon the introduction of MOF nanoparticles, the transport pathways transitioned to the microchannels of the MOFs, rather than relying solely on the interstitial spaces between the polymer chains.
When 0.50 g of nanoparticles was added to the membrane composition, a decrease in calcium flux was observed compared to the membranes without nanoparticles. This reduction can be attributed to the presence of more confined passage pathways created by the 0.50 g of nanoparticles, which limited ion transport compared to the membranes lacking nanoparticles. Interestingly, membranes containing 0.10 g of nanoparticles exhibited higher flux than those with 0.50 g, although both still showed lower flux compared to membranes without nanoparticles. This higher flux in the 0.10 g membranes was due to a greater number of transport pathways formed by the nanoparticles' microchannels, despite these pathways being considerably more constrained than the inter-polymer chain spaces in the nanoparticle-free membranes.
Upon the addition of 0.15 g of nanoparticles, an increase in flux was recorded, achieving the highest value among all tested concentrations. This indicates that the transport pathways formed by the microchannels of the 0.15 g nanoparticles were more numerous than in previous cases. Conversely, further increasing the nanoparticle mass to 0.20 g led to a decline in flux, likely due to the aggregation of MOF nanoparticles within the membrane matrix, which may have resulted in the blockage of certain microchannels. Consequently, the incorporation of more than 0.15 g of nanoparticles limited the passage pathways provided by the MOF microchannels. Thus, membranes containing over 0.15 g of nanoparticles exhibited lower flux compared to those without MOF particles, indicating that the pathways became significantly more restricted with the addition of 0.20 g of nanoparticles compared to membranes lacking MOF components.
3.2.1.5. Effect of competing cations' concentration on membrane performance. To study the impacts of cations existing in the feed phase, initially, a solution containing calcium in the absence of other competitive cations was prepared. Using this solution, it was seen that the system reached an equilibrium state. Otherwise stated, the concentrations of calcium ions in both the feed and receiving phases were the same. A sample was taken after 24 h. It was found that the calcium ion concentration was slightly higher than its concentration in the feed phase. To explain this phenomenon, it can be stated that the concentration change across the membrane caused more calcium ions to be transported from the feed phase to the receiving phase. After the equilibrium was established between three available phases (the feed, receiving, and membrane phases), the calcium concentration in the feed phase was equal to the receiving phase. Otherwise stated, the calcium\crown ether complexes passed a reverse direction across the membrane. After adding other competitive cations to the feed phase, the calcium flux reduced from roughly 63.01 to 58 (μmol cm−2 h−1). It may be justified by the fact that other competitive cations slightly interact with the crown ether, therefore, they can partially form a complex, which interrupts the formation of a complex between the crown ether and calcium ions. Hence, the calcium flux reduces slightly.
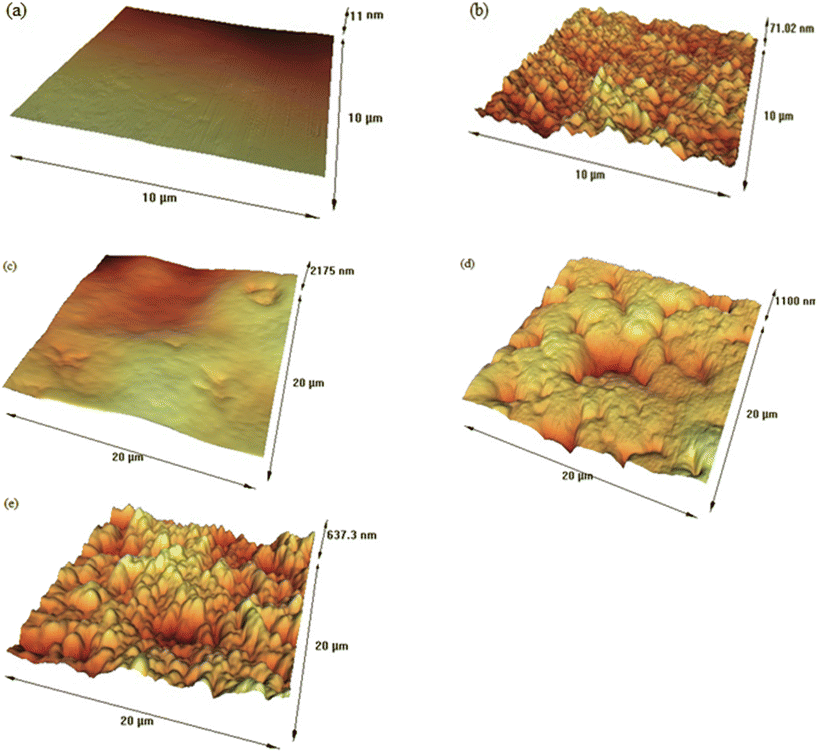
3.2.1.6. Impact of hydrophobicity and surface roughness on membrane performance. Atomic force microscopy (AFM) analysis was employed to study the surface roughness of the various prepared membranes, with the results presented in Fig. 8. In atomic force microscopy (AFM) studies, several roughness parameters are commonly reported to describe the surface texture and properties of a sample. Some of the most frequently reported AFM roughness parameters are:80 (1) average roughness (Ra): the arithmetic mean of the absolute values of the surface height deviations measured from the mean plane, (2) root mean square roughness (Rq or RMS): the square root of the average of the squares of the surface height deviations from the mean plane, and (3) maximum peak-to-valley height (Rz): the vertical distance between the highest peak and the lowest valley in the scanned area.
The roughness parameters, including average roughness (Ra), root mean square roughness (Rq), and maximum peak-to-valley height (Rz), reveal significant variations in surface texture across the samples. The Ra values ranged from 1.60 nm for the CAP membrane to 31.20 nm for the ZPIM12 membrane, indicating a substantial increase in surface roughness when additional components, such as the plasticizer, carrier, and ZIF-8 nanoparticles, were incorporated. The corresponding Rq values ranged from 2.00 nm to 39.00 nm, while Rz values spanned from 8.00 nm to 156.00 nm, with the highest values observed in the ZPIM12 membrane. The increase in roughness parameters (Ra, Rq, and Rz) with the addition of components such as BMIMCl, benzo-18-crown-6, and ZIF-8 nanoparticles can be attributed to the more heterogeneous and porous membrane surface morphology. This enhanced roughness improves the membrane's hydrophobicity and creates additional microchannels, facilitating ion transport across the membrane. For example, the ZPIM12 membrane, with the highest Ra and Rz values, exhibited the highest flux of Ca2+ ions (63.01 μmol cm−2 h−1), suggesting a strong correlation between surface roughness and ion transport efficiency. In contrast, the smoother surfaces of membranes like CAP (Ra = 1.60 nm) and GC1 (Ra = 11.20 nm), which lacked the ionic liquid, carrier, or ZIF-8 components, exhibited significantly lower fluxes (0.25 μmol cm−2 h−1 for CAP and 0.00 μmol cm−2 h−1 for GC1). These results indicate that the lack of surface features limited the pathways for ion transport, leading to lower membrane performance.
The increased roughness is beneficial in this context because it enhances the facilitated transport mechanism for Ca2+ ions. Rougher surfaces provide more interaction sites for the carrier molecules (benzo-18-crown-6), promoting complex formation with the target ions and improving their transport across the membrane. Additionally, the increased surface roughness caused by the ZIF-8 nanoparticles boosts the membrane's selectivity (as shown in Table 2) by making it more difficult for competing ions (e.g., Na+, Mg2+, K+) to permeate, as they are more likely to rely on simple diffusion rather than facilitated transport. The significant increase in roughness parameters observed with the incorporation of the ionic liquid, carrier, and nanoparticles in the ZPIM12 membrane demonstrates how these modifications can overcome the limitations of conventional membranes and contribute to the development of high-performance polymer inclusion membranes.
| Membrane | αCa/K | αCa/Na | αCa/Mg |
|---|---|---|---|
| PIMCr8 | 88.80 | 247.50 | 856.10 |
| ZPIM12 | 138.67 | 392.52 | 1256.00 |
| PIMCA | 17.12 | 33.41 | 79.56 |
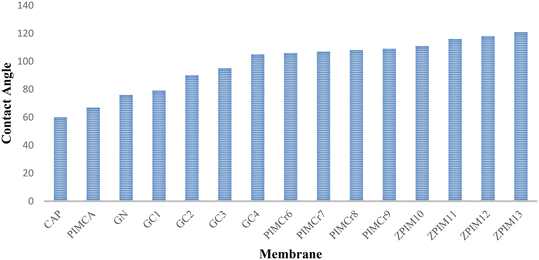
To study the membrane surface hydrophobicity, contact angle analysis was exploited whose results are demonstrated in Fig. 9. As is seen, by comparing the contact angle measurements of pure CA (CAP) with CA containing the ionic liquid and carriers (PIMCA), the hydrophobicity of the membrane surface increased. The contact angle of the CAP measured around 60°, whereas this measurement for PIMCA, the membrane containing only GPO (GC1), and the membrane containing GPO and IL (GC4) was 67°, nearly 79°, and 105°, respectively. Also, the contact angle of the PIM including MOF particles (ZPIM12) measured over 115°. Notably, GC1 showed a higher contact angle measurement than PIMCA due to the hydrophobic nature of the GPO. Additionally, the hydrophobic nature of the IL changes the roughness of the membrane surface, i.e., the membrane turns more hydrophobic than the membrane without any IL. In the SEM images (Fig. 4), this change can be patently seen. Additionally, the ZIF-8 nanocrystals change the membrane surface morphology and also amplify the membrane surface roughness, therefore, a significant increase in the membrane's surface hydrophobicity results.
This section indicates that increased membrane hydrophobicity enhances selectivity, leading to a higher flux of the target cation (Ca2+). Membranes devoid of the ingredients (carrier, ionic liquid, and ZIF-8) rely exclusively on simple diffusion for the transport of all cations, including Ca2+. Due to their hydrophobic nature, these membranes exhibit minimal flux for all cations and are thus non-selective. Conversely, the addition of these ingredients improves the conditions for facilitated transport of Ca2+, significantly increasing its flux. Competing cations show negligible changes in flux since they continue to pass through the membrane via simple diffusion, as no specific carriers are designated for them. Consequently, membranes with greater hydrophobicity are more selective for Ca2+.
3.2.1.7. Membrane selectivity. Selectivity is determined by comparing the flux of transported calcium ions to the flux of other competing cations.
Based on the calculated fluxes of the prepared PIMs, the selectivity results for various membranes are presented in Table 2. Incorporating the nanoparticles into the membrane structure increases the surface roughness, leading to a more hydrophobic membrane. This increased hydrophobicity significantly enhances the membrane's selectivity after adding ZIF-8 nanocrystals to the PIM matrix. The increased hydrophobicity of the membrane impedes the passage of cations other than calcium ions, as these competing cations have a reduced likelihood of permeating the membrane. This phenomenon can be attributed to the altered hydrophilic nature of calcium ions when they form a complex with the carrier (the calcium–crown ether complex being hydrophobic). Consequently, the passage of calcium ions across the membrane is substantially enhanced compared to other competing cations. This suggests that facilitated transport is the dominant mechanism for the targeted cation. Additionally, more hydrophobic PIMs impede the transport of hydrated cations via simple diffusion, thus favoring transport through carriers. This behavior is illustrated by comparing the fluxes of the competing cations through PIMCA with PIMCr8 and ZPIM12, as shown in Fig. 6.
3.3. Membrane stability and reusability
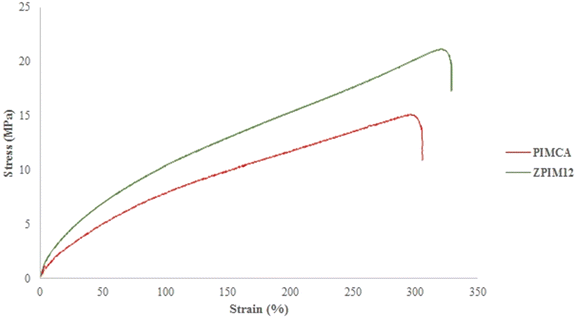
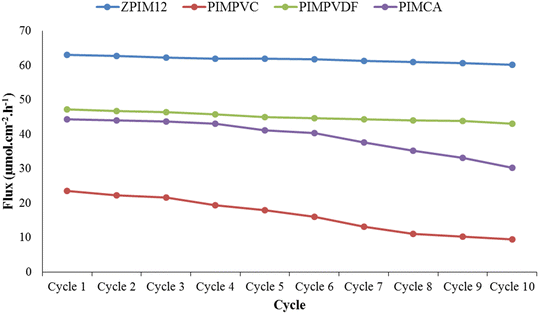
Fig. 10 demonstrates the relationship between stress and strain related to ZPIM12 and PIMCA. As is seen, the moduli of elasticity for PIMCA and ZPIM12 were around 59 and 96 MPa, respectively. As can be found from the figure, for intermolecular crosslinking in GPO, the PIM containing GPO as the base polymer (ZPIM12) is generally of better mechanical traits, i.e., more desirable modulus, greater elongation at break, and bigger ultimate strength.The chemical stability of the PIMs was studied by the membrane reusability test. To do so, transport experiments were repeated over a specified period whereas the PIM was not altered. During repeating the experiments, the feed and receiving phases were renewed over time. The results of this test are shown in Fig. 11. As can be deduced, the GPO-based membrane shows significantly higher stability compared with other base polymers, i.e. CA, PVDF, and PVC. According to Fig. 11, after repeating the transport experiments for 10 cycles, the flux of Ca decreased by only less than 5% by employing the PIM containing GPO, whereas the flux decreased by about 60%, 9%, and 32% by employing PIMs based on CA, PVDF, and PVC, respectively. It follows that using the membrane containing GPO, the flux stays almost unchanged in more cycles and far more stable performance is obtained compared with other membranes including PVC, PVDF, and CA as base polymers.
3.4. Comparison of membranes' performances in terms of flux and recovery factor
According to the studies reported thus far, the most frequently used base polymers in PIMs were PVDF, PVC, and CTA.15,41,81 Thus, the fluxes of different target cations transported across PIMs based on PVDF, PVC, and cellulosic derivatives (like CA and CTA) were compared with the flux of the target ion transported across the PIM prepared in the current work in Table 3.| Base polymer | Solvent | Plasticizer | Carrier | Target ion | Flux (μmol cm−2 h−1) |
|---|---|---|---|---|---|
| PVC82 | THF | 2NPOE | Aliquat 336 | Cr | 1.120 |
| CTA83 | Dichloromethane | O-NPPE | EDAB-acac | Zn | 3.950 |
| PVDF84 | DMF | 2NPOE | Aliquat 336 | Cr | 4.430 |
| CTA/PBAT85 | Chloroform | Aliquat 336 | Aliquat 336 | Cr | 2.840 |
| CTA86 | Dichloromethane | TBP | Aliquat 336 | Co | 2.600 |
| CTA87 | Dichloromethane | o-NPPE | 1-Decyl-imidazole | Zn | 0.954 |
| PVC88 | THF | ADO | Acac | Zn | 0.122 |
| PVC88 | THF | ADO | 3-Propyl-acac | Zn | 0.138 |
| PVC88 | THF | ADO | 3-Benzyl-acac | Zn | 0.106 |
| PVDF89 | DMF | [C8mim][PF6] | Cyphos IL 104 | Cr | 1.404 |
| PVDF89 | DMF | [C8mim][BF4] | Cyphos IL 104 | Cr | 1.008 |
| CTA90 | Dichloromethane | — | TTA and TOPO | Li | 1.040 |
| CTA91 | Dichloromethane | 2NPOE | HBTA | Sc | 0.030 |
| CTA91 | Dichloromethane | 2NPOE | HBTA | La | 0.030 |
| CTA92 | Dichloromethane | DOP | PC-88A & Versatic 10 | Sc | 0.010 |
| CTA92 | Dichloromethane | DOP | PC-88A & Versatic 10 | La | 0.020 |
| CTA93 | Dichloromethane | DOP | D2EHAG | Sc | 0.137 |
| CTA93 | Dichloromethane | DOP | D2EHAG | La | 0.090 |
| CTA93 | Dichloromethane | 2NPOE | D2EHAF | Sc | 0.650 |
| CTA93 | Dichloromethane | 2NPOE | D2EHAF | La | 0.007 |
| GPO (in the current work) | DMAc | BMIMCl | B18C6 | Ca | 63.01 |
As illustrated, the flux of the target ion, Ca2+, through the GPO-based PIM was significantly higher compared to the flux of other cations through PIMs based on different polymers such as PVDF, PVC, and CTA. Consequently, to study the impact of different base polymers on the performance of the membrane developed in this work, various membrane compositions were prepared using the optimum composition achieved for the GPO-based PIM. In this regard, CA-, PVC-, and PVDF-based PIMs were prepared whose compositions were the same as PIMCr8, and transport experiments were accomplished employing the mentioned PIMs. By comparing the results shown in Table 4, it is evident that the PVC- and PVDF-based membranes demonstrate the best performance for calcium ion separation. However, GPO-based PIMs are more desirable due to their superior durability (both chemically and mechanically) and greater hydrophobicity compared to other base polymers. For instance, the flux of Ca2+ through the GPO-based membrane with the optimal composition is approximately twice as high as that through a CA-based membrane with the same composition.
| Membrane | Calcium flux (μmol cm−2 h−1) |
|---|---|
| PIMCr8 | 45.72 |
| ZPIM12 | 63.01 |
| PIMCA | 23.52 |
| PIMPVDF | 47.2 |
| PIMPVC | 44.3 |
Moreover, to show how efficiently the target ion was removed from the aqueous feed solution, the recovery factor (RF) can be calculated as follows:
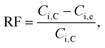 | (3) |
| Ions in the feed solution | Membrane | Recovery factor |
|---|---|---|
| Ca | PIMCr8 | 0.95 |
| Ca | ZPIM12 | 0.99 |
| Ca, Mg | PIMCr8 | 0.93 |
| Ca, Mg | ZPIM12 | 0.98 |
| Ca, Mg, Na | PIMCr8 | 0.91 |
| Ca, Mg, Na | ZPIM12 | 0.95 |
| Ca, Mg, Na, K | PIMCr8 | 0.9 |
| Ca, Mg, Na, K | ZPIM12 | 0.94 |
4. Conclusion
FTIR analysis revealed that cellulose acetate reacted with epoxidized castor oil to produce a novel green polyol (GPO) as the base polymer in the polymer inclusion membrane (PIM) used in this study. This analysis also confirmed the compatibility of all PIM components. XRD patterns showed that the novel polyol is fully amorphous, and TGA analysis indicated that its decomposition temperatures are higher than those of cellulose acetate. It was found that increasing the masses of the constituting components of the PIM up to their optimal amounts (1.50 g ionic liquid, 0.75 g carrier, and 0.15 g ZIF-8) enhanced the flux of Ca2+ over the other competitive cations (Na+, Mg2+, and K+). ZIF-8 nanoparticles with regular porosity enhanced the performance of the PIM in terms of flux and selectivity. The BET analysis revealed that the average pore size of the nanoparticles was significantly larger than the calcium ion size, considerably improving the ion transport through the PIM. AFM analysis demonstrated that increased membrane roughness enhanced performance. The best performance in terms of flux and selectivity was shown by the PIM containing 0.75 g B18C6, 1.00 g cross-linked GPO, 1.50 g BMIMCl, and 0.15 g ZIF-8. By using the recovery factor of Ca2+ calculated in the presence of other competing cations, it was further demonstrated that the competitive cations had adverse effects on the membrane's performance. Based on the results from the recovery factor and selectivity studies, it was concluded that facilitated transport was the predominant mechanism through the membrane. This indicates that the transport of competing cations was significantly hindered, as they primarily pass through the membrane via simple diffusion. This method can be adopted to effectively remove other cations while utilizing a tailored composition.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
There are no conflicts to declare.References
- M. Figueira, D. Rodríguez-Jiménez, J. López, M. Reig, J. L. Cortina and C. Valderrama, Evaluation of the nanofiltration of brines from seawater desalination plants as pre-treatment in a multimineral brine extraction process, Sep. Purif. Technol., 2023, 124232, DOI:10.1016/j.seppur.2023.124232.
- S. Aditya, J. Stephen and M. Radhakrishnan, Utilization of eggshell waste in calcium-fortified foods and other industrial applications: A review, Trends Food Sci. Technol., 2021, 115, 422–432 CrossRef CAS.
- A. Taghavi-Kahagh, H. Roghani-Mamaqani and M. Salami-Kalajahi, Powering the future: A comprehensive review on calcium-ion batteries, J. Energy Chem., 2023, 90, 77–97 CrossRef.
- N. B. Singh and B. Middendorf, Geopolymers as an alternative to Portland cement: An overview, Constr. Build. Mater., 2020, 237, 117455 CrossRef CAS.
- S. Chen, Y. Dong, J. Sun, P. Gu, J. Wang and S. Zhang, Ionic Liquids Membranes for Liquid Separation: Status and Challenge, Green Chem., 2023, 25(15), 5813–5835 RSC.
- S. Imdad and R. K. Dohare, A critical review on heavy metals removal using ionic liquid membranes from the industrial wastewater, Chem. Eng. Process.: Process Intensif., 2022, 173, 108812 CrossRef CAS.
- H. C. Ting, H. W. Khan, A. V. B. Reddy, M. Goto and M. Moniruzzaman, Extraction of salicylic acid from wastewater using ionic liquid-based green emulsion liquid membrane: COSMO-RS prediction and experimental verification, J. Mol. Liq., 2022, 347, 118280 CrossRef.
- J. D. Way, R. D. Noble, T. M. Flynn and E. D. Sloan, Liquid membrane transport: a survey, J. Membr. Sci., 1982, 12, 239–259 CrossRef CAS.
- H. K. Admawi and A. A. Mohammed, A comprehensive review of emulsion liquid membrane for toxic contaminants removal: An overview on emulsion stability and extraction efficiency, J. Environ. Chem. Eng., 2023, 109936 CrossRef CAS.
- N. S. W. Zulkefeli, S. K. Weng and N. S. Abdul Halim, Removal of heavy metals by polymer inclusion membranes, Curr. Pollut. Rep., 2018, 4, 84–92 CrossRef CAS.
- Z. Habibi, C. Youssef, M. Riri, S. Majid, K. Touaj and M. Hlaibi, A Novel Polymer Inclusion Membrane Containing an Organometallic Complex Carrier for The Extraction and Recovery of Chromium and Nickel from Wastewater, J. Membr. Sci. Res., 2023, 9(1), 546447–546453 CAS.
- C. Zhang, Y. Mu, S. Zhao, W. Zhang and Y. Wang, Lithium extraction from synthetic brine with high Mg2+/Li+ ratio using the polymer inclusion membrane, Desalination, 2020, 496, 114710 CrossRef CAS.
- N. Wu, M. I. G. Almeida, S. Simeonova, T. G. Spassov, A. Rangelov, R. W. Cattrall, M. Datcheva and S. D. Kolev, Preparation and characterization of very thin polymer inclusion membranes (PIMs) and their application to the transport of thiocyanate, J. Membr. Sci., 2023, 668, 121249 CrossRef CAS.
- S. Bensaadi, N. Drai, O. Arous, Y. Berbar, Z. E. Hammache, M. Amara and B. Van Der Bruggen, A study of chromium (VI) ions fixation and transport using polymer inclusion membrane containing D2EHPA as complexing agent, J. Membr. Sci. Res., 2022, 8, 531653–531659 CAS.
- B. Keskin, A. Yuksekdag, B. Zeytuncu and I. Koyuncu, Development of polymer inclusion membranes for palladium recovery: Effect of base polymer, carriers, and plasticizers on structure and performance, J. Water Process Eng., 2023, 52, 103576 CrossRef.
- K. Maiphetlho, K. Netshiongolwe, H. Tutu, L. Chimuka and H. Richards, Optimisation of semi-upscaled polymer inclusion membrane (PIMs) based device for passive remediation of metal ions in acid mine drainage (AMD), J. Water Process Eng., 2022, 49, 103061 CrossRef.
- B. Keskin, B. Zeytuncu-Gökoğlu and I. Koyuncu, Polymer inclusion membrane applications for transport of metal ions: A critical review, Chemosphere, 2021, 279, 130604 CrossRef CAS PubMed.
- A. Olasupo and F. B. M. Suah, Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes, J. Hazard. Mater., 2021, 406, 124317 CrossRef CAS PubMed.
- F. Nitti, O. T. E. Selan, B. Hoque, D. Tambaru and M. C. Djunaidi, Improving the performance of polymer inclusion membranes in separation process using alternative base polymers: A review, Indones. J. Chem., 2022, 22, 284–302 CrossRef CAS.
- G. Zante, M. Boltoeva, A. Masmoudi, R. Barillon and D. Trébouet, Supported ionic liquid and polymer inclusion membranes for metal separation, Sep. Purif. Rev., 2022, 51, 100–116 CrossRef CAS.
- V. Vatanpour, M. E. Pasaoglu, H. Barzegar, O. O. Teber, R. Kaya, M. Bastug, A. Khataee and I. Koyuncu, Cellulose acetate in fabrication of polymeric membranes: A review, Chemosphere, 2022, 295, 133914 CrossRef CAS PubMed.
- A. K. Rana, V. K. Gupta, A. K. Saini, S. I. Voicu, M. H. Abdellattifaand and V. K. Thakur, Water desalination using nanocelluloses/cellulose derivatives based membranes for sustainable future, Desalination, 2021, 520, 115359 CrossRef CAS.
- T. Ohshima, S. Kagaya, M. Gemmei-Ide, R. W. Cattrall and S. D. Kolev, The use of a polymer inclusion membrane as a sorbent for online preconcentration in the flow injection determination of thiocyanate impurity in ammonium sulfate fertilizer, Talanta, 2014, 129, 560–564 CrossRef CAS PubMed.
- N. Ghaderi, L. Dolatyari, D. Kazemi, H. R. Sharafi, H. Shayani-Jam and M. R. Yaftian, Application of a polymer inclusion membrane made of cellulose triacetate base polymer and trioctylamine for the selective extraction of bismuth (III) from chloride solutions, J. Appl. Polym. Sci., 2022, 139, 51480 CrossRef CAS.
- B. M. Jayawardane, R. W. Cattrall and S. D. Kolev, The use of a polymer inclusion membrane in a paper-based sensor for the selective determination of Cu (II), Anal. Chim. Acta, 2013, 803, 106–112 CrossRef CAS PubMed.
- A. Makowka and B. Pospiech, Synthesis of polymer inclusion membranes based on cellulose triacetate for recovery of lanthanum (III) from aqueous solutions, Autex Res. J., 2019, 19, 288–292 CrossRef CAS.
- B. Hoque, M. I. G. Almeida, R. W. Cattrall, T. G. Gopakumar and S. D. Kolev, Improving the extraction performance of polymer inclusion membranes by cross-linking their polymeric backbone, React. Funct. Polym., 2021, 160, 104813 CrossRef CAS.
- L. Xu, X. Zeng, Q. He, T. Deng, C. Zhang and W. Zhang, Stable ionic liquid-based polymer inclusion membranes for lithium and magnesium separation, Sep. Purif. Technol., 2022, 288, 120626 CrossRef CAS.
- R. Vera, E. Anticó, J. I. Eguiazábal, N. Aranburu and C. Fontàs, First report on a solvent-free preparation of polymer inclusion membranes with an ionic liquid, Molecules, 2019, 24, 1845 CrossRef CAS PubMed.
- G. Arena, A. Contino, A. Magri, D. Sciotto and J. Lamb, Selective transport of cesium and strontium ions through polymer inclusion membranes containing calixarenes as carriers, Supramol. Chem., 1998, 10, 5–15 CrossRef CAS.
- C. Onac, H. K. Alpoguz, E. Akceylan and M. Yilmaz, Facilitated transport of Cr (VI) through polymer inclusion membrane system containing calix [4] arene derivative as carrier agent, J. Macromol. Sci., Part A:Pure Appl. Chem., 2013, 50, 1013–1021 CrossRef CAS.
- Y. Su, S. Cong, M. Shan and Y. Zhang, Enhanced propylene/propane separation in facilitated transport membranes containing multisilver complex, AIChE J., 2022, 68, e17410 CrossRef CAS.
- F. Tomás-Alonso, A. M. Rubio, R. Álvarez and J. A. Ortuño, Dynamic potential response and SEM-EDX studies of polymeric inclusion membranes based on ionic liquids, Int. J. Electrochem. Sci., 2013, 8, 4955–4969 CrossRef.
- B. Pospiech, Highly efficient facilitated membrane transport of palladium (II) ions from hydrochloric acid solutions through plasticizer membranes with Cyanex 471X, Physicochem. Probl. Miner. Process., 2015, 51, 281–291 Search PubMed.
- A. Kaya, C. Onac, H. K. Alpoguz, A. Yilmaz and N. Atar, Removal of Cr (VI) through calixarene based polymer inclusion membrane from chrome plating bath water, Chem. Eng. J., 2016, 283, 141–149 CrossRef CAS.
- V. Eyupoglu, A. Unal, E. Polat, B. Eren and R. A. Kumbasar, An efficient cobalt separation using PVDF-co-HFP based ultrafiltration polymer inclusion membrane by room temperature ionic liquids, Sep. Purif. Technol., 2022, 303, 122201 CrossRef CAS.
- R. Jha, G. Mishra, M. Agrawal, M. Dhanunjaya Rao, A. Meshram and K. K. Singh, Opportunities for an en-route to polymer inclusion membrane approach from conventional hydrometallurgical recycling of WPCBs: a mini-review, Can. Metall. Q., 2023, 62, 810–824 CrossRef CAS.
- M. A. Bourouai, A. Bouchoucha and O. Arous, Sodium and Lead (II) Extraction and Transport in Polymer Inclusion Membrane Using 2-Hydroxy-5-dodecylbenzaldehyde and 15-Crown-ether-5 as Mobile Carriers, Macromol. Symp., 2023, 408, 2200061 CrossRef CAS.
- H. Dahdah, F. Sellami, S. Dekkouche, M. Benamor and O. Senhadji-Kebiche, Stability study of polymer inclusion membranes (PIMs) based on acidic (D2EHPA), basic (Aliquat 336) and neutral (TOPO) carriers: effect of membrane composition and aqueous solution, Polym. Bull., 2023, 80, 6495–6525 CrossRef CAS.
- D. Kazemi and M. R. Yaftian, Selective transport-recovery of bismuth (III) by a polymer inclusion membrane containing polyvinyl chloride base polymer and bis (2-ethylhexyl) phosphoric acid, Sep. Purif. Technol., 2022, 285, 120375 CrossRef CAS.
- M. A. Kaczorowska, The use of polymer inclusion membranes for the removal of metal ions from aqueous solutions—The latest achievements and potential industrial applications: A Review, Membranes, 2022, 12, 1135 CrossRef CAS PubMed.
- Z. Mo, D. Tai, H. Zhang and A. Shahab, A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water, Chem. Eng. J., 2022, 443, 136320 CrossRef CAS.
- T. Shi, S. Hussain, C. Ge, G. Liu, M. Wang and G. Qiao, ZIF-X (8, 67) based nanostructures for gas-sensing applications, Rev. Chem. Eng., 2023, 39, 911–939 CrossRef CAS.
- H. S. Jadhav, H. A. Bandal, S. Ramakrishna and H. Kim, Critical review, recent updates on zeolitic imidazolate framework-67 (ZIF-67) and its derivatives for electrochemical water splitting, Adv. Mater., 2022, 34, 2107072 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- Y. Sun, N. Zhang, Y. Yue, J. Xiao, X. Huang and A. Ishag, Recent advances in the application of zeolitic imidazolate frameworks (ZIFs) in environmental remediation: a review, Environ. Sci.: Nano, 2022, 9, 4069–4092 RSC.
- K. Li, N. Miwornunyuie, L. Chen, H. Jingyu, P. S. Amaniampong, D. Ato Koomson, D. Ewusi-Mensah, W. Xue, G. Li and H. Lu, Sustainable Application of ZIF-8 for Heavy-Metal Removal in Aqueous Solutions, Sustainability, 2021, 13, 984 CrossRef CAS.
- Z. Abbasi, E. Shamsaei, S. K. Leong, B. Ladewig, X. Zhang and H. Wang, Effect of carbonization temperature on adsorption property of ZIF-8 derived nanoporous carbon for water treatment, Microporous Mesoporous Mater., 2016, 236, 28–37 CrossRef CAS.
- A. Paul, I. K. Banga, S. Muthukumar and S. Prasad, Engineering the ZIF-8 pore for electrochemical sensor applications—a mini review, ACS Omega, 2022, 7, 26993–27003 CrossRef CAS PubMed.
- A. Imtiaz, M. H. D. Othman, A. Jilani, I. U. Khan, R. Kamaludin and O. Samuel, ZIF-filler incorporated mixed matrix membranes (MMMs) for efficient gas separation: a review, J. Environ. Chem. Eng., 2022, 108541 CrossRef CAS.
- Y. Ding, H. Wang, M. Yu, W. Zheng, X. Ruan, X. Li, Y. Xi, Y. Dai, H. Liu and G. He, Amine group graft ZIF-93 to create gas storage space to improve the gas separation performance of Pebax-1657 MMMs, Sep. Purif. Technol., 2023, 309, 122949 CrossRef CAS.
- Z. Lai, Development of ZIF-8 membranes: opportunities and challenges for commercial applications, Curr. Opin. Chem. Eng., 2018, 20, 78–85 CrossRef.
- Y.-R. Lee, M.-S. Jang, H.-Y. Cho, H.-J. Kwon, S. Kim and W.-S. Ahn, ZIF-8: A comparison of synthesis methods, Chem. Eng. J., 2015, 271, 276–280 CrossRef CAS.
- Q. Wang, Y. Sun, S. Li, P. Zhang and Q. Yao, Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy, RSC Adv., 2020, 10, 37600–37620 RSC.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- B. Niu, Z. Zhai, J. Wang and C. Li, Preparation of ZIF-8/PAN composite nanofiber membrane and its application in acetone gas monitoring, Nanotechnology, 2023, 34, 245710 CrossRef CAS PubMed.
- S. Liu, Z. Xiang, Z. Hu, X. Zheng and D. Cao, Zeolitic imidazolate framework-8 as a luminescent material for the sensing of metal ions and small molecules, J. Mater. Chem., 2011, 21, 6649–6653 RSC.
- F. Banihashemi and J. Y. S. Lin, Synthesis of ZIF-8 Membranes on γ-Alumina Supports for Separation of Propylene/Propane Gas Mixture, Ind. Eng. Chem. Res., 2022, 61, 4125–4133 CrossRef CAS.
- A. Jonnalagedda and B. V. R. Kuncharam, Investigation of ZIF-8, amine-modified ZIF-8 and polysulfone based mixed matrix membranes for CO2/CH4 separation, J. Appl. Polym. Sci., 2023, 140, e54650 CrossRef CAS.
- T. Li, Y. Wang, X. Wang, C. Cheng, K. Zhang, J. Yang, G. Han, Z. Wang, X. Wang and L. Wang, Desalination characteristics of cellulose acetate FO membrane incorporated with ZIF-8 nanoparticles, Membranes, 2022, 12, 122 CrossRef CAS PubMed.
- M. El Ouardi, H. A. Ahsaine, M. Zbair, A. BaQais and M. Saadi, ZIF-8 metal organic framework composites as hydrogen evolution reaction photocatalyst: A review of the current state, Chemosphere, 2022, 136483 CrossRef CAS PubMed.
- Y. Li, T. Fan, W. Cui, X. Wang, S. Ramakrishna and Y. Long, Harsh environment-tolerant and robust PTFE@ ZIF-8 fibrous membrane for efficient photocatalytic organic pollutants degradation and oil/water separation, Sep. Purif. Technol., 2023, 306, 122586 CrossRef CAS.
- V. Vatanpour, A. Yuksekdag, M. Ağtaş, M. Mehrabi, E. Salehi, R. Castro-Muñoz and I. Koyuncu, Zeolitic imidazolate framework (ZIF-8) modified cellulose acetate NF membranes for potential water treatment application, Carbohydr. Polym., 2023, 299, 120230 CrossRef CAS PubMed.
- A. V. Sonawane and Z. Murthy, Synthesis and characterization of ZIF-8-based PVDF mixed matrix membranes and application to treat pulp and paper industry wastewater using a membrane bioreactor, Environ. Sci.: Water Res. Technol., 2022, 8, 881–896 RSC.
- S. Z. N. Ahmad, W. N. W. Salleh, N. Yusof, M. Z. M. Yusop, R. Hamdan and A. F. Ismail, Synthesis of zeolitic imidazolate framework-8 (ZIF-8) using different solvents for lead and cadmium adsorption, Appl. Nanosci., 2023, 13, 4005–4019 CrossRef CAS.
- N. L. Parada Hernandez, A. J. Bonon, J. O. Bahú, M. I. R. Barbosa, M. R. Wolf Maciel and R. M. Filho, Epoxy monomers obtained from castor oil using a toxicity-free catalytic system, J. Mol. Catal. A: Chem., 2017, 426, 550–556 CrossRef CAS.
- C. J. Malm, L. J. Tanghe, B. C. Laird and G. D. Smith, Determination of Total and Primary Hydroxyl in Cellulose Esters by Ultraviolet Absorption Methods, Anal. Chem., 1954, 26, 188–190 CrossRef CAS.
- F. Gholami, S. Zinadini, A. A. Zinatizadeh and A. R. Abbasi, TMU-5 metal-organic frameworks (MOFs) as a novel nanofiller for flux increment and fouling mitigation in PES ultrafiltration membrane, Sep. Purif. Technol., 2018, 194, 272–280 CrossRef CAS.
- S. Leaper, A. Abdel-Karim, B. Faki, J. M. Luque-Alled, M. Alberto, A. Vijayaraghavan, S. M. Holmes, G. Szekely, M. I. Badawy, N. Shokri and P. Gorgojo, Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation, J. Membr. Sci., 2018, 554, 309–323 CrossRef CAS.
- Y. Jin, Y. Zhao, H. Liu, A. Sotto, C. Gao and J. Shen, A durable and antifouling monovalent selective anion exchange membrane modified by polydopamine and sulfonated reduced graphene oxide, Sep. Purif. Technol., 2018, 207, 116–123 CrossRef CAS.
- D. Sun, M. Meng, Y. Qiao, Y. Zhao, Y. Yan and C. Li, Synthesis of ion imprinted nanocomposite membranes for selective adsorption of lithium, Sep. Purif. Technol., 2018, 194, 64–72 CrossRef CAS.
- M. Mokhtar, S. E. Dickson, Y. Kim and W. Mekky, Preparation and characterization of ion selective membrane and its application for Cu2+ removal, J. Ind. Eng. Chem., 2018, 60, 475–484 CrossRef CAS.
- R. M. Abdelhameed, M. Hasanin, H. Abdel-Gawad and B. Hegazi, Engineering ZIF-8 Hybridization by Extracted Lignin with Antibacterial Property for Uptake of Methomyl Residues from Wastewater, Sep. Sci. Technol., 2022, 57, 3023–3034 CrossRef.
- X. Jin, H. Li, X. Zhu, N. Li, G. Owens and Z. Chen, Enhanced removal of oxytetracycline from wastewater using bimetallic Fe/Ni nanoparticles combined with ZIF-8 nanocomposites, J. Environ. Manage., 2022, 318, 115526 CrossRef CAS PubMed.
- A. Deacon, L. Briquet, M. Malankowska, F. Massingberd-Mundy, S. Rudić, T. l. Hyde, H. Cavaye, J. Coronas, S. Poulston and T. Johnson, Understanding the ZIF-L to ZIF-8 transformation from fundamentals to fully costed kilogram-scale production, Commun. Chem., 2022, 5, 18 CrossRef CAS PubMed.
- S. Li, G. Cai, S. Wu, A. Raut, W. Borges, P. R. Sharma, S. K. Sharma, B. S. Hsiao and M. Rafailovich, Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells, Int. J. Mol. Sci., 2022, 23, 15245 CrossRef CAS PubMed.
- A. Rehman, Z. Jahan, F. Sher, T. Noor, M. B. K. Niazi, M. A. Akram and E. K. Sher, Cellulose acetate based sustainable nanostructured membranes for environmental remediation, Chemosphere, 2022, 307, 135736 CrossRef CAS PubMed.
- Y. Yahmin, H. D. Pranowo and R. Armunanto, AB initio investigation of 12-crown-4 and benzo-12-crown-4 complexes with Li+, Na+, K+, Zn 2+, Cd 2+, and Hg 2+, Indones. J. Chem., 2010, 10, 106–109 CrossRef.
- J. W. Steed, First-and second-sphere coordination chemistry of alkali metal crown ether complexes, Coord. Chem. Rev., 2001, 215, 171–221 CrossRef CAS.
- R. De Oliveira, D. Albuquerque, T. Cruz, F. Yamaji and F. Leite, Measurement of the nanoscale roughness by atomic force microscopy: basic principles and applications, Atomic force microscopy-imaging, measuring and manipulating surfaces at the atomic scale, 2012, vol. 3 Search PubMed.
- E. A. Nagul, C. Fontàs, I. D. McKelvie, R. W. Cattrall and S. D. Kolev, The use of a polymer inclusion membrane for separation and preconcentration of orthophosphate in flow analysis, Anal. Chim. Acta, 2013, 803, 82–90 CrossRef CAS PubMed.
- P. Kunene, O. Akinbami, N. Motsoane, H. Tutu, L. Chimuka and H. Richards, Feasibility of Polysulfone as Base Polymer in a Polymer Inclusion Membrane: Synthesis and Characterisation, J. Membr. Sci. Res., 2020, 6, 203–210 CAS.
- I. Pyszka and E. Radzyminska-Lenarcik, New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions, Membranes, 2020, 10, 385 CrossRef CAS PubMed.
- F. Sellami, O. Kebiche-Senhadji, S. Marais, L. Colasse and K. Fatyeyeva, Enhanced removal of Cr(VI) by polymer inclusion membrane based on poly(vinylidene fluoride) and Aliquat 336, Sep. Purif. Technol., 2020, 248, 117038 CrossRef CAS.
- F. Sellami, O. Kebiche-Senhadji, S. Marais, N. Couvrat and K. Fatyeyeva, Polymer inclusion membranes based on CTA/PBAT blend containing Aliquat 336 as extractant for removal of Cr (VI): Efficiency, stability and selectivity, React. Funct. Polym., 2019, 139, 120–132 CrossRef CAS.
- Y. Yıldız, A. Manzak and O. Tutkun, Selective extraction of cobalt ions through polymer inclusion membrane containing Aliquat 336 as a carrier, Desalin. Water Treat., 2016, 57, 4616–4623 CrossRef.
- E. Radzyminska-Lenarcik and M. Ulewicz, The application of polymer inclusion membranes based on CTA with 1-alkylimidazole for the separation of zinc (II) and manganese (II) ions from aqueous solutions, Polymer, 2019, 11, 242 Search PubMed.
- K. Witt, E. Radzyminska-Lenarcik and W. Urbaniak, Selective transport of zinc ions through novel polymer inclusion membranes (PIMS) containing β-diketone derivatives as carrier reagents, Sep. Sci. Technol., 2016, 51, 2620–2627 CrossRef CAS.
- L. Guo, Y. Liu, C. Zhang and J. Chen, Preparation of PVDF-based polymer inclusion membrane using ionic liquid plasticizer and Cyphos IL 104 carrier for Cr (VI) transport, J. Membr. Sci., 2011, 372, 314–321 CrossRef CAS.
- C. Cai, F. Yang, Z. Zhao, Q. Liao, R. Bai, W. Guo, P. Chen, Y. Zhang and H. Zhang, Promising transport and high-selective separation of Li (I) from Na (I) and K (I) by a functional polymer inclusion membrane (PIM) system, J. Membr. Sci., 2019, 579, 1–10 CrossRef CAS.
- M. Sugiura, M. Kikkawa and S. Urita, Carrier-mediated transport of rare earth ions through cellulose triacetate membranes, J. Membr. Sci., 1989, 42, 47–55 CrossRef CAS.
- M. Sharaf, W. Yoshida, F. Kubota, S. D. Kolev and M. Goto, A polymer inclusion membrane composed of the binary carrier PC-88A and Versatic 10 for the selective separation and recovery of Sc, RSC Adv., 2018, 8, 8631–8637 RSC.
- W. Yoshida, Y. Baba, F. Kubota, S. D. Kolev and M. Goto, Selective transport of scandium (III) across polymer inclusion membranes with improved stability which contain an amic acid carrier, J. Membr. Sci., 2019, 572, 291–299 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |