Efficiency and mechanisms of combined persulfate and nanofiltration for the removal of typical perfluorinated compounds
Lihua
Sun
 *a,
Yan
Zhang
b,
Zixuan
Xi
c,
Ruiying
Li
b and
Kaiquan
Zhang
b
*a,
Yan
Zhang
b,
Zixuan
Xi
c,
Ruiying
Li
b and
Kaiquan
Zhang
b
aKey Laboratory of Urban Stormwater System and Water Environment, Ministry of Education, Beijing University of Civil Engineering and Architecture, Beijing 100044, China. E-mail: sunlihuashd@163.com
bSchool of Environmental and Energy Engineering, Beijing University of Civil Engineering and Architecture, Beijing 100044, China
cNational Academy for Mayors of China, Beijing 100029, China
First published on 18th December 2024
Abstract
Perfluorinated compounds (PFCs) are a class of emerging pollutants that are commonly detected in surface water and pose significant risks to both the environment and public health. This study investigates a combined treatment method for removing perfluorooctanoic acid (PFOA), a prevalent PFC found in micro-polluted surface water. The method integrates nanoscale zero-valent iron (nFe)-activated persulfate (PS) pre-oxidation with conventional water treatment processes—coagulation, sedimentation, and sand filtration—combined with nanofiltration (NF). This study primarily aims to evaluate the efficiency of this combined process for PFOA removal and to elucidate the mechanisms underlying PS oxidation and NF separation. The treatment sequence, comprising nFe/PS pre-oxidation, conventional treatment, and NF, was strategically designed considering the specific roles of each process in PFOA removal. In the initial stage, nFe-activated PS generates sulfate radicals (SO4−·) and hydroxyl radicals (OH·), which oxidize and degrade PFOA. The subsequent conventional treatment removes the majority of degradation byproducts and suspended solids. Finally, NF retains both PFOA and its oxidation products, thereby ensuring high removal efficiency. Experimental results indicate that an optimal PS dosage of 0.2 mM and an nFe-to-PS molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 achieved the maximum efficiency for PFOA removal. Among the tested sequences, “nFe/PS pre-oxidation + conventional treatment + NF” achieved the highest removal rate, exceeding 99%. Furthermore, this sequence resulted in the lowest surface potential of the NF membrane, which enhanced electrostatic interactions between the membrane and PFOA. This reduction in surface potential, combined with the formation of C–O bonds between PFOA and the NF membrane, further enhanced PFOA adsorption onto the membrane surface. The combined process of nFe/PS pre-oxidation, conventional treatment, and nanofiltration effectively removes PFOA from micro-polluted surface water, thereby contributing to improved drinking water safety.
1 achieved the maximum efficiency for PFOA removal. Among the tested sequences, “nFe/PS pre-oxidation + conventional treatment + NF” achieved the highest removal rate, exceeding 99%. Furthermore, this sequence resulted in the lowest surface potential of the NF membrane, which enhanced electrostatic interactions between the membrane and PFOA. This reduction in surface potential, combined with the formation of C–O bonds between PFOA and the NF membrane, further enhanced PFOA adsorption onto the membrane surface. The combined process of nFe/PS pre-oxidation, conventional treatment, and nanofiltration effectively removes PFOA from micro-polluted surface water, thereby contributing to improved drinking water safety.
Water impactIn this study, persulfate (PS) is activated using nanoscale zero-valent iron (nFe), and perfluorooctanoic acid (PFOA) in micro-polluted surface water was treated using a combination of nanofiltration (NF), pre-oxidation technology, and conventional treatment (coagulation, sedimentation, and sand filtration) in water treatment plants, ensuring the safety of drinking water. |
1. Introduction
Perfluorinated or polyfluorinated compounds (PFCs) are the organic compounds in which all hydrogen atoms bonded to carbon atoms in the molecular structure are replaced by fluorine atoms. The carbon–fluorine bond is the strongest known chemical bond in nature, making these compounds exceptionally resistant to degradation in both the human body and the natural environment, including water, soil, and the atmosphere.1 Due to their unique properties—such as hydrophobicity, oleophobicity, chemical resistance, and durability—PFCs have become pervasive in both everyday life and the environment.2,3 PFCs are persistent environmental pollutants known for their high bioaccumulation potential and systemic toxicity,4–9 with long biological half-lives in humans (3.8 to 5.4 years).10 Even at low concentrations, PFCs pose both acute and chronic toxicity risks to aquatic life and can adversely affect human health.11 Studies have linked PFC exposure to increased risks of asthma and respiratory issues, particularly when present in drinking water.12 Perfluorooctanoic acid (PFOA), the most prevalent PFC in the environment, was officially included in the Stockholm Convention list in 2019. In 2023, a drinking water safety limit of 80 ng L−1 for PFOA was introduced.13 Effective treatment methods are essential to control PFOA levels and ensure drinking water safety. Recent studies indicate that sulfate radicals can degrade PFOA. Approaches such as B/N-co-doped diamond electrodes have shown promising results in achieving complete mineralization through gradual oxidation.14 In a separate study, Lee et al.15 used iron-modified activated carbon as a catalyst to activate persulfate, achieving a 61.7% removal rate of PFOA and a 41.9% defluorination rate. These findings suggest that iron-modified activated carbon, in conjunction with persulfate (PS), can reduce the activation energy required for PFOA removal and defluorination, allowing these processes to occur at lower temperatures and within shorter reaction times. Meanwhile, researchers have further investigated the mechanisms of PFC oxidation by persulfate. Xu et al.16 found that persulfate oxidation generates sulfate and hydroxyl radicals, which, in the process of removing PFCs, not only cleave carbon–carbon chains but also break carbon-fluorine bonds and detach head groups. Song17 explained the oxidation process of PFCs from the perspective of oxidative potential. His study revealed that fluorine, as the most electronegative atom with a reduction potential of 3.6 V, strongly resists oxidation reactions. The oxidation of PFOA and Perfluorooctane sulfonate (PFOS) is particularly resistant due to the complete substitution of hydrogen atoms with fluorine atoms, making these compounds highly stable. Cleavage of the carbon skeleton during oxidation often results in shorter-chain perfluorinated compounds.Nanofiltration (NF) is one of the primary membrane technologies for reducing PFC concentrations in water and has been extensively studied. Current methods for PFC removal using NF include standalone NF filtration, integrated NF processes, and advanced NF membranes incorporating novel materials. For example, Zhao et al.18 developed a polyamide/metal–organic framework (MOF) composite NF membrane through capillary-assisted interfacial polymerization (CAIP). This membrane demonstrated enhanced selectivity between nutrient ions and PFCs, enabling its use in fertilizer production and controlled-environment agriculture. Similarly, Liu19 reported that NF270 membranes achieved a retention rate exceeding 97% for 42 different types of PFCs. Other studies have compared the effectiveness of various NF membranes and investigated their materials and adsorption capacities, offering valuable insights into the interaction between PFCs and membrane surfaces. Additionally, NF has been combined with other technologies to overcome the limitations of standalone NF separation.20 For instance, Rattanaoudom et al.21 reported that standalone NF removed 60–85% of PFOA, but the addition of magnesium aluminum carbonate increased the removal rate to 95%. Soriano et al.22 effectively removed PFOA from industrial wastewater by combining NF with electrocatalytic oxidation. These integrated approaches have further advanced the practical applications of NF for PFC removal.
Although NF membranes are highly effective in removing PFOA from water, their practical application is limited by several challenges. Despite achieving high removal rates, NF membranes have small apertures and are highly susceptible to fouling by pollutants, which leads to decreased membrane flux, increased operating costs, and reduced operational lifespan. As a result, membrane fouling remains a major factor limiting the advancement of NF technology.23 To address these limitations, pretreatment methods are considered a potential solution. NF pretreatment methods primarily include conventional treatment, preoxidation, and adsorption. Conventional treatment typically involves coagulation, sedimentation, and filtration. Fujioka et al.24 found that thorough coagulation significantly reduced membrane fouling, with the lowest level of fouling achieved under the optimal dosage of polyaluminum chloride, thereby effectively mitigating membrane fouling.
Building on the identified challenges of NF membrane fouling, this study investigates the treatment of PFOA in micro-polluted surface water using a persulfate-nanofiltration (PS-NF) combined process. The research focuses on evaluating the performance and impact of three treatment processes: conventional treatment (coagulation—sedimentation—filtration) + NF, PS + conventional treatment + NF, and nFe/PS + conventional treatment + NF. Additionally, the study explores the removal mechanisms to provide deeper insights into the role of nFe/PS in enhancing PFOA removal efficiency compared to other methods.
2. Test materials and methods
2.1. Principal reagent
The primary chemical reagents used in this experiment included PFOA powder, methanol, sodium persulfate, nFe, and polyaluminum chloride. nFe and PFOA powders were purchased from Shanghai Macklin Biochemical Co., Ltd. The nFe had a purity greater than 99.9% with a particle size of 50 nm, while the PFOA powder had a purity of 96%. Sodium persulfate, sourced from Fuchen (Tianjin) Chemical Reagent Co., Ltd., was of analytical grade purity, ensuring both experimental reliability and accuracy. Its chemical formula is Na2S2O8, and its molecular weight is 238.104 g mol−1. To ensure safe handling, sodium persulfate was stored in a cool, ventilated environment away from heat and flames, to prevent accidents and maintain its stability and effectiveness.The PFOA stock solution was prepared as follows: First, 0.0100 g of PFOA powder was accurately weighed using a high-precision electronic balance and dissolved in 10 mL of methanol. The solution was then vortexed for at least 60 seconds to ensure complete dissolution. Finally, the solution was diluted to 100 mL with ultrapure water in a volumetric flask, yielding a PFOA solution with a concentration of approximately 100 μg L−1, which was used as the experimental stock solution. To maintain stability, the stock solution was stored in a dark environment at 4 °C. All containers used in the experiment were made of polypropylene (PP) to ensure the accuracy and reliability of the results.
2.2. Test material
In this experiment, real micro-polluted surface water was selected as the research subject. Water samples were collected from Ming Lake on the Daxing Campus of Beijing University of Civil Engineering and Architecture. To preserve the stability of the raw water and prevent its degradation, the samples were stored at 4 °C in a refrigerator. Prior to use, the samples were thoroughly shaken to ensure uniform distribution of trace substances, thereby improving the accuracy and reliability of the tests. The quality parameters of the water samples are shown in Table 1.| Name of indicator | Dissolved organic carbon(DOC) | UV absorbance (cm−1) | pH | Turbidity (NTU) |
|---|---|---|---|---|
| Numerical value (average) | 12.09 | 0.095 | 7.3 | 5.72 |
2.3. Nanofiltration membrane
An NF270 low-pressure NF, with a diameter of 80 mm and a molecular weight cutoff of 300 Da, was used in this experiment. To ensure the membrane's purity and performance, it was soaked in deionized water for 24 hours to remove any residual impurities. The pretreated membrane was then installed in the membrane cell and subjected to a 1-hour pressurization with pure water to ensure operational stability during the experiment. This process allowed the membrane to adapt to experimental conditions, ensuring reliable and accurate results.2.4. Test devices and methods
A simulation setup was used for the laboratory testing, and Fig. 1 illustrates the schematic diagram of the combined process setup.The ZR4-6 coagulation test mixer was employed for the coagulation and precipitation of suspended solids, while a 65 cm-tall filter column was used for the filtration operation. This setup replicates the traditional treatment process and efficiently removes impurities and suspended solids from the water, providing a clean water quality foundation for subsequent treatment. The TYLG-18 low-pressure plate NF membrane test apparatus was utilized in the NF process section. The filtered liquid was collected using an electronic balance and a beaker, and the filtered water quality data were displayed in real time.
2.5. Detection instruments and analytical methods
3. Results and discussions
3.1. Efficacy of combined processes for removal of perfluorooctanoic acid (PFOA)
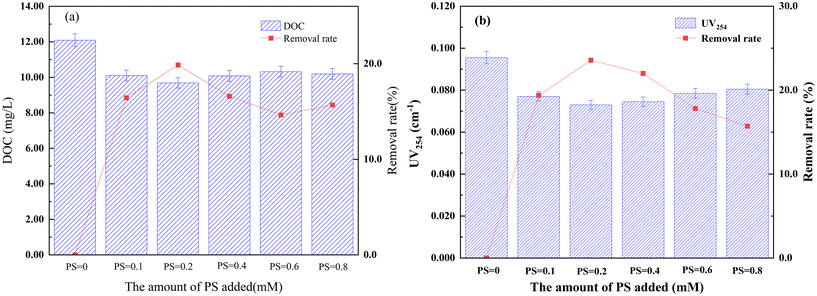
The DOC content in the micro-polluted surface water was 12.09 mg L−1, as shown in Fig. 2(a). After oxidation with varying PS concentrations, the DOC content decreased, following a trend of initially decreasing and then increasing. At a 0.2 mM PS dosage, the DOC concentration decreased to 9.69 mg L−1, corresponding to a removal rate of 19.9%. The UV254 content in the raw water was 0.095 cm−1, as shown in Fig. 2(b). After PS oxidation, the UV254 content decreased, but the overall trend mirrored that of the DOC: first decreasing and then increasing. At the 0.2 mM PS dosage, the UV254 content was reduced to 0.073 cm−1, corresponding to a removal rate of 23.6%. Based on these variations in DOC and UV254, the optimal dosage of PS was determined to be 0.2 mM.
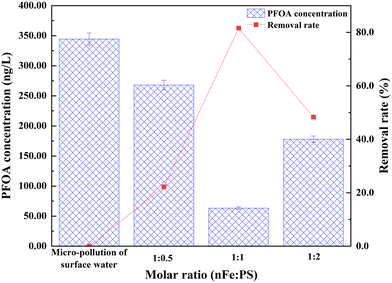
The test results demonstrate that PS had a notable effect on removing PFOA from the micro-polluted surface water at different activation levels (Fig. 3). The initial concentration of PFOA in the micro-polluted surface water was 344.35 ng L−1. After activation with three different molar ratios of nFe to PS—1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2—the PFOA concentration decreased to 267.98 ng L−1, 63.34 ng L−1, and 177.96 ng L−1, respectively. It is evident from the experimental results that the best removal rate of 81.6% was achieved at a molar ratio of 1
2—the PFOA concentration decreased to 267.98 ng L−1, 63.34 ng L−1, and 177.96 ng L−1, respectively. It is evident from the experimental results that the best removal rate of 81.6% was achieved at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between nFe and PS. This can be attributed to the fact that, after the addition of nFe, Fe0 continuously and efficiently releases Fe2+ ions into the solution.26 When Fe2+ interacts with PS, two active substances, Fe3+ and SO4−·, are generated, as described by eqn (1)–(3). These reactions form the basis for understanding how Fe0 facilitates the continuous activation of PS and the subsequent generation of effective oxidizing agents. Furthermore, Fe3+ can react with unreacted Fe0 to regenerate Fe2+, establishing a self-sustaining reaction cycle that further enhances the activation of PS. This indicates that PS retains its oxidizing capacity throughout the reaction and is continually activated, thereby facilitating the oxidative removal of the target compound. This mechanism for maintaining continuous activity helps to increase the reaction's durability and efficiency, resulting in more thorough and stable target removal of PFOA and, ultimately, better water treatment performance.
1 between nFe and PS. This can be attributed to the fact that, after the addition of nFe, Fe0 continuously and efficiently releases Fe2+ ions into the solution.26 When Fe2+ interacts with PS, two active substances, Fe3+ and SO4−·, are generated, as described by eqn (1)–(3). These reactions form the basis for understanding how Fe0 facilitates the continuous activation of PS and the subsequent generation of effective oxidizing agents. Furthermore, Fe3+ can react with unreacted Fe0 to regenerate Fe2+, establishing a self-sustaining reaction cycle that further enhances the activation of PS. This indicates that PS retains its oxidizing capacity throughout the reaction and is continually activated, thereby facilitating the oxidative removal of the target compound. This mechanism for maintaining continuous activity helps to increase the reaction's durability and efficiency, resulting in more thorough and stable target removal of PFOA and, ultimately, better water treatment performance.
| Fe0 + S2O82− → Fe2+ + 2SO42− | (1) |
| Fe2+ + S2O82− → Fe3+ + SO42− + SO4−· | (2) |
| Fe0 + 2Fe3+ → 3Fe2+ | (3) |
The removal of PFOA was 22.2% and 48.3% at molar ratios of nFe to PS of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively, which represented a decrease of approximately 59.4 and 33.3 percentage points compared to the removal efficiency observed at a molar ratio of 1
2, respectively, which represented a decrease of approximately 59.4 and 33.3 percentage points compared to the removal efficiency observed at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This reduction can be attributed to the excessive dosage of PS, which may cause SO4−· to react with the residual S2O82− in the PS, forming SO42− as shown in eqn (4). In the case of excess PS, the PS that is not activated by Fe3+ can react with itself, producing SO42− and thereby depleting the available SO4−·. This phenomenon is consistent with the findings reported by Zhang et al.26 In oxidation systems, when the activator dosage is fixed, only a proportion of PS can be effectively activated. Therefore, an excessive PS dosage does not enhance the degradation efficiency.27 Previous studies have shown that an excess of PS can lead to the quenching of free radicals in the reaction system eqn (4), negatively affecting the degradation performance.28 Additionally, when the nFe dosage is too high, a large amount of Fe2+ is released, which consumes some of the SO4−· radicals, The results in the generation of Fe3+ and SO42−eqn (5), thereby inhibiting the oxidizing effect.29,30 As a consequence, the removal efficiency of PFOA may decrease. This is because SO4−· is a crucial active oxidizing agent, and its reduction may slow down or limit the reaction, diminishing the effective removal of the target compound. Thus, based on the observed PFOA removal efficiency, the optimal molar ratio of nFe to PS was determined to be 1
1. This reduction can be attributed to the excessive dosage of PS, which may cause SO4−· to react with the residual S2O82− in the PS, forming SO42− as shown in eqn (4). In the case of excess PS, the PS that is not activated by Fe3+ can react with itself, producing SO42− and thereby depleting the available SO4−·. This phenomenon is consistent with the findings reported by Zhang et al.26 In oxidation systems, when the activator dosage is fixed, only a proportion of PS can be effectively activated. Therefore, an excessive PS dosage does not enhance the degradation efficiency.27 Previous studies have shown that an excess of PS can lead to the quenching of free radicals in the reaction system eqn (4), negatively affecting the degradation performance.28 Additionally, when the nFe dosage is too high, a large amount of Fe2+ is released, which consumes some of the SO4−· radicals, The results in the generation of Fe3+ and SO42−eqn (5), thereby inhibiting the oxidizing effect.29,30 As a consequence, the removal efficiency of PFOA may decrease. This is because SO4−· is a crucial active oxidizing agent, and its reduction may slow down or limit the reaction, diminishing the effective removal of the target compound. Thus, based on the observed PFOA removal efficiency, the optimal molar ratio of nFe to PS was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| SO4−· + S2O82− → S2O8−· + SO42− | (4) |
| SO4−· + Fe2+ → Fe3+ + SO42− | (5) |
| Removal amount (ng L−1) | Removal rate (%) | ||
|---|---|---|---|
| Conventional treatment + NF | Membrane influent | 23.76 | 6.9% |
| Membrane effluent | 344.08 | 97.0% | |
| PS + conventional treatment + NF | Membrane influent | 26.09 | 7.6% |
| Membrane effluent | 388.66 | 98.4% | |
| nFe/PS + conventional treatment + NF | Membrane influent | 284.07 | 82.5% |
| Membrane effluent | 341.38 | 99.1% |
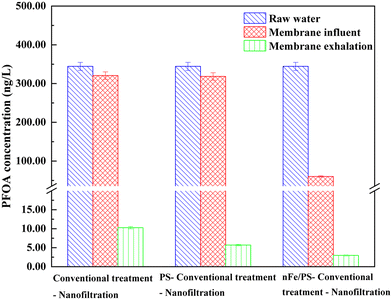
As shown in Fig. 4 and Table 2, the removal rate of PFOA in the micro-polluted surface water exceeded 97% after the three combined processes, demonstrating exceptional treatment performance. Initially, the micro-polluted surface water contained a PFOA concentration of 344.35 ng L−1. After conventional treatment and PS + conventional treatments, the PFOA concentrations decreased to 320.56 ng L−1 and 318.26 ng L−1, respectively, corresponding to removal rates of 6.9% and 7.6%. Previous research has shown that traditional methods for removing PFCs are often inadequate, leading to low removal rates.31 This is consistent with our findings, as the C–F bond in PFOA is difficult to break using conventional treatment methods. Furthermore, the degradation reaction of PS alone follows quasi-first-order reaction kinetics,32 with a reaction rate constant of 0.0026 min−1 in the absence of an activator. This low reaction rate results in insufficient generation of free radicals, causing slow degradation and poor defluorination efficiency, which may require several hours or even up to hundreds of hours.25 However, after applying the nFe/PS + conventional treatment, the PFOA concentration decreased to 60.28 ng L−1, achieving a significant removal rate of 82.5%. This improvement can be attributed to the role of nFe. As an electron donor, nFe is easily oxidized to Fe2+ or Fe3+, forming a unique core-shell with surface complexation and electrostatic attraction on the surface of nFe. The oxidized film structure adsorbs PFOA. Moreover, since Fe0 is the primary active site for persulfate activation, it facilitates the dissolution of Fe2+. This activation of persulfate generates additional active sites, which in turn produce more SO4−·,33 effectively oxidizing PFOA.34
After conventional, PS + conventional, and nFe/PS + conventional treatment, the micro-polluted surface water was filtered using an NF membrane. The removal rate of PFOA was significantly enhanced compared to when NF was not used, with increases of 90.1%, 90.8%, and 16.6%, respectively. This improvement can be attributed to the small pore size of the NF membrane, which effectively blocks the passage of relatively small organic molecules, thereby enhancing the efficient removal of pollutants.20 Additionally, PFOA, with a molecular weight of 414.07 Da, is effectively retained by the NF membrane, contributing to the significant increase in removal efficiency.
3.2. Study of the oxidation mechanism of perfluorooctanoic acid by persulfates
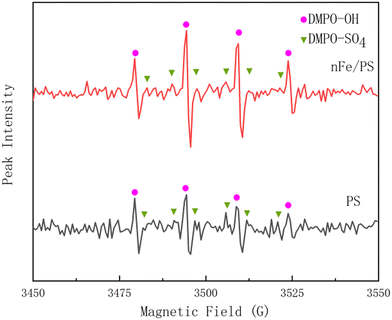
To investigate this, the experiment measured the free radical species produced during the oxidation of PS and nFe/PS separately. The results are shown in Fig. 5, which shows the EPR spectra for the different oxidation processes.
Distinct peaks for SO4−· and OH· were observed in the EPR spectra, as shown in Fig. 5,37,38 confirming the presence of both radicals during the oxidation of PS and nFe/PS. This indicates that SO4−· introduced into the water may react with anions and water molecules, leading to the formation of OH·, as illustrated by the reactions in eqn (6) and (7).
| SO4−· + H2O → OH· + SO42− + H+ | (6) |
| SO4−· + OH− → SO42− + OH· | (7) |
Unactivated PS has a limited oxidation capacity, with the characteristic peaks for SO4−· and OH· are relatively small. However, after the nFe/PS activation, these distinctive peaks became more prominent, indicating that both radicals significantly contributed to the PFOA removal. The activation of PS by nFe resulted in a substantial increase in the concentrations of SO4−· and OH· radicals, thereby amplifying the PFOA removal efficiency. This highlights the effectiveness of the nFe/PS oxidation process in improving pollutant removal. This also explains why conventional nFe/PS + treatment achieves a higher PFOA removal rate compared to PS + treatment alone.
| Radical bursting agent (chemistry) | K m/SO4−· (M−1 s−1) | K m/OH· (M−1 s−1) |
|---|---|---|
| Tertiary butyl alcohol | 4 × 105–9.1 × 105 | 1.9 × 109 |
| Methanol | 1.23 × 107 | 0.97 × 109 |
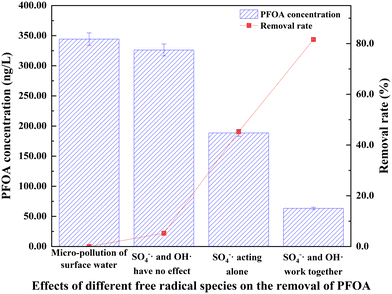
The results, shown in Fig. 6, revealed that when both SO4−· and OH· were inhibited, the PFOA concentration was 326.26 ng L−1, corresponding to a removal rate of 5.3%. When only SO4−· was active, the concentration of PFOA dropped to 188.69 ng L−1, with a removal rate of 45.2%. When both radicals were present, the PFOA concentration further reduced to 63.34 ng L−1 yielding an impressive removal rate of 81.6%. These findings indicate a significant difference of 36.4% in removal efficiency between SO4−· acting alone and the contribution of both radicals. This suggests that both radicals contribute to PFOA removal, with SO4−· playing a more dominant role. This dominance is likely because PS is activated to generate a high concentration of SO4−, which specifically targets the head groups of PFCs, making it more effective in the oxidation process.42
The oxidation process led to the formation of several compounds, including perfluoropropionic acid, perfluoroheptanoic acid, difluoropropionaldehyde, heptafluoropropane, and perfluorovaleric acid. This outcome is likely due to the attack of SO4−· on PFOA, causing decarboxylation and forming perfluoroalkyl radicals (C7F15·).43 These radicals can then interact with OH· in water to form perfluoroalcohols (C6F13CF2OH), or under the influence of SO4−·, form perfluoropentane sulfonate (C6F13CF2OSO3−·) which is subsequently hydrolyzed, to release SO42− and generating C6F13CF2OH. The first stage of the defluorination and decarboxylation occurs as C6F13CF2OH forms perfluorooctanoic acid (PFHpA) through elimination and hydrolysis reactions.44,45 PFHpA is then attacked by SO4−·, leading to the decarboxylation and further breakdown of the compound.46 This process repeats multiple times, with each cycle removing one CF2 unit. By analogy, it is postulated that under conditions of sufficient active free radicals, similar reactions can continue until complete defluorination occurs, ultimately yielding the final products F− and CO2.47–49
3.3. Mechanistic study of the action of nanofiltration on PFOA
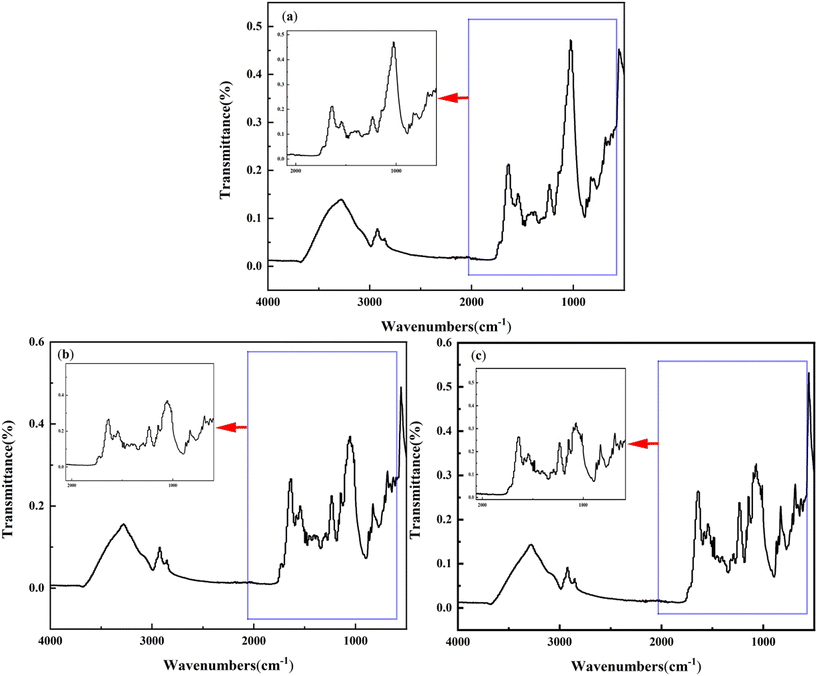 | ||
| Fig. 8 Bonding of NF membrane surfaces with PFOA in different combination processes (a) conventional treatment + NF; (b) PS + conventional treatment + NF; (c) nFe/PS + conventional treatment + NF. | ||
Based on the peak data analysis in Fig. 8, distinct combinations of treatment processes showed prominent peaks around 3300 cm−1 and 2915 cm−1, which are attributed to the C–H bond of alkynes and the C–H stretching vibration of alkanes, respectively. A highly significant peak also appeared around 1032 cm−1, corresponding to the stretching vibration of the C–O bond found in polysaccharides and similar substances,50 suggesting that additional removal of organic matter occurs during the nanofiltration process. Furthermore, peaks between 1275 cm−1 and 1020 cm−1 were observed, representing the stretching vibrations of ethers and aliphatic ethers. These peaks are indicative of carbon–oxygen–carbon (C–O–C) bonds in ethers, which are characteristic of certain organic substances forming C–O–C bonds with the functional groups of PFOA. Additionally, in situ chemical reactions may occur via carboxyl and phenol carboxyl groups, causing PFOA to form carboxylic acid and ester derivatives.51 These reactions increase the molecular size of PFOA, making it more easily retained by the NF membrane.
After the PS + conventional treatment + NF process (Fig. 8b), several new peaks appeared between 1350 cm−1 and 1100 cm−1, a region associated with the C–F bond. This indicates that SO4−· breaks the C–F bonds which are effectively retained by the NF membrane. Additionally, nFe/PS + conventional treatment + NF process (Fig. 8c), a new peak near 1010 cm−1 was observed following nFe/PS, conventional treatment, and NF treatment. This peak corresponds to the C–O bonds in sterols, suggesting that the oxidation of PFOA by nFe/PS results in the formation of new chemical bonds on the NF membrane. These new chemical bonds increases the membrane's adsorption capacity for PFOA, enabling more efficient sequestration of larger PFOA molecules.52 Consequently, the NF membrane not only retains these larger molecules but also adsorbs the exposed chemical bonds of PFOA that are not bound to organic matter, thus enhancing the overall PFOA removal rate.
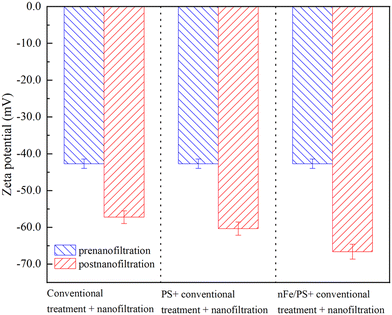
As illustrated in Fig. 9, the NF membrane surface initially exhibited a zeta potential of −42.7 mV, indicating its negatively charged state. After the traditional treatment of micro-polluted surface water followed by the NF process, the zeta potential decreased further to −57.2 mV. This change can be attributed to the negatively charged nature of PFOA at neutral pH (pH = 7), which creates electrostatic repulsion with negatively charged surface of the NF membrane.53 Additionally, the polyamide NF membranes used in this experiment are rich in carboxylate groups, which further contribute to the negative charge on the membrane surface.54 As a result, the negative charges on both the membrane surface and PFOA intensifies the electrostatic repulsion, increasing increase in the retention rate of PFOA and hindering its passage through the NF membrane.
After PS + conventional treatment + NF treatment, the zeta potential of the membrane surface of the micro-polluted surface water decreased to −60.3 mV, which is lower than that observed in the absence of the oxidizer. This reduction could be attributed to the oxidation of organic matter in the water, leading to the formation of small organic molecules enriched with oxygenated functional groups. These oxygenated groups increase the negative charge density of the organic matter,55 which, in turn, decreases the membrane's surface zeta potential during the NF process. As a result, the membrane surface becomes more negatively charged, enhancing the electrostatic repulsion between the membrane and the organic matter, particularly PFOA. This modification improves the membrane's ability to retain organic matter during the NF process.56
Further analysis reveals that after nFe/PS + conventional treatment + NF, the micro-polluted surface water exhibited the lowest zeta potential on the membrane surface, at −66.6 mV. This can be explained from two perspectives: first, Fe3+, produced during nFe activation of PS, is removed during the conventional treatment, and second, the increases in Fe3+ concentration generates a significant amount of Fe(OH)3, leading to a decrease in pH. This results in a sharp reduction in Fe3+ concentration and weakens the electric neutralization effect on the membrane surface.57 Additionally, the bridging of cations increases the size of the PFOA molecules, enhancing their removal rate.58 Conversely, the activation of PS also produces a significant amount of SO42−, and as its concentration rises, the membrane surface becomes more negatively charged. This increased negative charge facilitates the retention and removal of negatively charged PFOA molecules.
4. Conclusion
(1) Optimal conditions for PFOA removal: the study identified the optimal conditions for PFOA removal, with the highest performance achieved at a persulfate dosage of 0.2 mM and an nFe/PS molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All three combined processes demonstrated high PFOA removal efficiencies, exceeding 97%. Among them, the nFe/PS + conventional treatment + NF process achieved the highest removal rate.
1. All three combined processes demonstrated high PFOA removal efficiencies, exceeding 97%. Among them, the nFe/PS + conventional treatment + NF process achieved the highest removal rate.
(2) Role of free radicals: two types of free radicals, SO4−· and OH·, were involved in the oxidation reactions of both the PS and nFe/PS systems. Following the activation of PS with nFe, the characteristic peaks of both SO4−· and ·OH became more prominent. However, SO4−· played the dominant role in the oxidative degradation of PFOA, driving the majority of its removal.
(3) Membrane surface zeta potential: the combined processes reduced the zeta potential of the membrane surface, enhancing its ability to retain PFOA and its oxidation products. The nFe/PS + conventional treatment + NF process achieved the lowest membrane surface zeta potential, decreasing from −42.7 mV to −66.6 mV. This increase in the negative charge on the membrane surface enhanced the electrostatic repulsion between the membrane and PFOA, significantly improving the retention efficiency. Furthermore, the interaction between PFOA and the membrane surface led to the formation of C–O bonds in primary alcohols, promoting the adsorption of PFOA and further increasing the removal efficiency.
In conclusion, the results demonstrate the effectiveness of the nFe/PS—conventional treatment—NF process in removing PFOA from micro-polluted surface water. The study analyzed the mechanisms behind PFOA removal in both the oxidative and NF stages of the combined process. It provides a detailed understanding of the removal mechanisms, highlighting the potential of integrating persulfate oxidation with NF for effective PFOA removal and ensuring safer drinking water.
Data availability
The data used to support the findings of this study are included within the article.Author contributions
Lihua Sun: writing – methodology, writing – review & editing, funding acquisition, resources, supervision, project administration. Yan Zhang: writing – original draft, validation, formal analysis, visualization. Zixuan Xi: data curation. Ruiying Li: investigation. Kaiquan Zhang: investigation.Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Thanks to the National Natural Science Foundation of China (52370004), the National Natural Science Foundation of China (52070011) and the Beijing University of Civil Engineering and Architecture Post Graduate Innovation Project (PG2024085).References
- H. Wang, S. Masters, Y. Hong, J. Stallings, J. O. Falkinham, M. A. Edwards and A. Pruden, Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, Mycobacteria, Pseudomonas aeruginosa, and two amoebas, Environ. Sci. Technol., 2012, 46, 11566–11574 Search PubMed.
- K. Gao, Y. Chen, Q. Xue, J. Fu, K. Fu, J. Fu, A. Zhang, Z. Cai and G. Jiang, Trends and perspectives in per- and polyfluorinated alkyl substances (PFASs) determination: Faster and broader, TrAC, Trends Anal. Chem., 2020, 133, 116114 Search PubMed.
- G. M. Sinclair, S. M. Long and O. A. H. Jones, What are the effects of PFAS exposure at environmentally relevant concentrations?, Chemosphere, 2020, 258, 127340 Search PubMed.
- A. Ritscher, Z. Wang, M. Scheringer, J. M. Boucher, L. Ahrens, U. Berger, S. Bintein, S. K. Bopp, D. Borg, A. M. Buser, I. Cousins, J. DeWitt, T. Fletcher, C. Green, D. Herzke, C. Higgins, J. Huang, H. Hung, T. Knepper, C. S. Lau, E. Leinala, A. B. Lindstrom, J. Liu, M. Miller, K. Ohno, N. Perkola, Y. Shi, L. Småstuen Haug, X. Trier, S. Valsecchi, K. Van Der Jagt and L. Vierke, Zürich statement on future actions on per- and polyfluoroalkyl substances (PFASs), Environ. Health Perspect., 2018, 126, 84502 Search PubMed.
- J. L. Domingo and M. Nadal, Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature, Environ. Res., 2019, 177, 108648 Search PubMed.
- Y. Cao, Y. H. Zhang, C. W. Lei and Z. T. Liu, Environmental pollution and ecological toxicity of perfluorinated chemicals: A review, J. Environ. Health, 2012, 29, 561–567 Search PubMed.
- K. Sznajder-Katarzyńska, M. Surma and I. Cieślik, A review of perfluoroalkyl acids (PFAAs) in terms of sources, applications, human exposure, dietary intake, toxicity, legal regulation, and methods of determination, J. Chem., 2019, 2019, 2717528 Search PubMed.
- C. A. McDonough, S. Choyke, K. E. Barton, S. Mass, A. P. Starling, J. L. Adgate and C. P. Higgins, Unsaturated PFOS and other PFASs in human serum and drinking water from an AFFF-impacted community, Environ. Sci. Technol., 2021, 55, 8139–8148 Search PubMed.
- M. P. Krafft and J. G. Riess, Per- and polyfluorinated substances (PFASs): Environmental challenges, Curr. Opin. Colloid Interface Sci., 2015, 20, 192–212 Search PubMed.
- X. M. Ren, L. Y. Zhang and L. H. Guo, Molecular mechanism study on the toxicological effects of polybrominated diphenyl ethers and perfluoroalkyl acids, Environ. Chem., 2014, 1662–1671 Search PubMed.
- J. W. Yang, X. D. Qin, Y. L. Li and G. H. Dong, Comparative study of the effect of perfluoroalkyl substances exposure on children's lung function, Zhongguo Xuexiao Weisheng, 2017, 38, 1035–1038 Search PubMed.
- P. Anderson-Mahoney, J. Kotlerman, H. Takhar, D. Gray and J. Dahlgren, Self-reported health effects among community residents exposed to perfluorooctanoate, New Solut., 2008, 18, 129–143 Search PubMed.
- Market Regulation of the People's Republic of China and Standardization Administration of the People's Republic of China, 2022.
- Y. Liu, X. Fan, X. Quan, Y. Fan, S. Chen and X. Zhao, Enhanced perfluorooctanoic acid degradation by electrochemical activation of sulfate solution on B/N codoped diamond, Environ. Sci. Technol., 2019, 53, 5195–5201 Search PubMed.
- Y.-C. Lee, Y. Li, M.-J. Chen, Y.-C. Chen, J. Kuo and S.-L. Lo, Efficient decomposition of perfluorooctanic acid by persulfate with iron-modified activated carbon, Water Res., 2020, 174, 115618 Search PubMed.
- B. Xu, J. L. Zhou, A. Altaee, M. B. Ahmed, M. A. H. Johir, J. Ren and X. Li, Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate, Chemosphere, 2020, 239, 124722 Search PubMed.
- Z. Song, H. Tang, N. Wang and L. Zhu, Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system, J. Hazard. Mater., 2013, 262, 332–338 Search PubMed.
- Y. Zhao, X. Tong, J. Kim, T. Tong, C.-H. Huang and Y. Chen, Capillary-assisted fabrication of thin-film nanocomposite membranes for improved solute–solute separation, Environ. Sci. Technol., 2022, 56, 5849–5859 Search PubMed.
- C. J. Liu, T. J. Strathmann and C. Bellona, Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes, Water Res., 2021, 188, 116546 Search PubMed.
- X. J. Zhuang and G. Feng, Progress of membrane separation technology dealing with the removal of per- and polyfluoroalkyl substances in water, Zhongguo Jishui Paishui, 2023, 49(920–925), 935 Search PubMed.
- R. Rattanaoudom and C. Visvanathan, Removal of PFOA by hybrid membrane filtration using PAC and hydrotalcite, Desalin. Water Treat., 2011, 32, 262–270 Search PubMed.
- Á. Soriano, D. Gorri and A. Urtiaga, Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate, Water Res., 2017, 112, 147–156 Search PubMed.
- Y. J. Huai, X. L. Yue, T. Wu, H. J. Fan, Y. F. Qiao, B. L. Zhu and M. Y. Zhang, Preparation and properties of Ag3PO4/g-C3N4 polyamide composite nanofiltration membrane, Huagong Xinxing Cailiao, 2024, 52, 116–121 Search PubMed.
- T. Fujioka, C. T. L. Ung, T. Okuda and S. Boivin, Controlling membrane fouling of nanofiltration using poly-aluminum chloride and Moringa oleifera coagulants, Sep. Purif. Technol., 2024, 334, 126016 Search PubMed.
- Y. H. Li, Master thesis, Zhejiang University, 2020 Search PubMed.
- F. N. Zhang, G. L. Jing, Z. N. Sun, H. J. Li and B. Yan, Research progress on degradation of pollutants in water by iron activated persulfate, Xiandai Huagong, 2023, 43, 74–78 Search PubMed.
- S. F. Lai, J. Z. Liang, K. B. Xiao, F. H. Li, X. D. Jiang, W. C. Xu, H. L. Wang, X. Chen, Y. Hu and X. M. Liang, Visible light assisted peroxymonosulfate activation on Ag modified graphite phase carbon nitride (g-C3N4) for Rhodamine B degradation, Acta Sci. Circumstantiae, 2021, 41, 1847–1858 Search PubMed.
- Q. Jiang, S. Jiang, H. Li, R. Zhang, Z. Jiang and Y. Zhang, A stable biochar supported S-nZVI to activate persulfate for effective dichlorination of atrazine, Chem. Eng. J., 2022, 431, 133937 Search PubMed.
- X. Li, Y. Y. Ren and L. J. Ding, Degradation of diclofenac sodium by premagnetized zero-valent iron-catalyzed persulfate, Huanjing Gongcheng Xuebao, 2019, 13, 2808–2815 Search PubMed.
- H. B. Long, H. F. Gao, C. Li, H. Yan, Z. L. Diao and W. Jiang, Review on remediation of petroleum hydrocarbon contaminated soil with iron activated persulfate, Huagong Huanbao, 2019, 39, 241–246 Search PubMed.
- T. T. Zhong, T. Lin and W. Liu, Distribution, transformation, and fate of per- and polyfluoroalkyl substances in drinking water treatment, Huanjing Kexue, 2023, 44, 2613–2621 Search PubMed.
- Y. Yu, Master thesis, Changzhou University, 2021 Search PubMed.
- R. H. Zhang, Doctoral dissertation, Guilin University of Technology, 2023 Search PubMed.
- T. Tran, L. Abrell, M. L. Brusseau and J. Chorover, Iron-activated persulfate oxidation degrades aqueous perfluorooctanoic acid (PFOA) at ambient temperature, Chemosphere, 2021, 281, 130824 Search PubMed.
- B. Liu, W. Guo, H. Wang, Q. Si, Q. Zhao, H. Luo and N. Ren, Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment, J. Hazard. Mater., 2020, 398, 122768 Search PubMed.
- B. S. Peng, Master thesis, Hunan Agricultural University, 2021 Search PubMed.
- D. Xia, R. Yin, J. Sun, T. An, G. Li, W. Wang, H. Zhao and P. K. Wong, Natural magnetic pyrrhotite as a high-efficient persulfate activator for micropollutants degradation: Radicals identification and toxicity evaluation, J. Hazard. Mater., 2017, 340, 435–444 Search PubMed.
- Y. Wu, X. Chen, Y. Han, D. Yue, X. Cao, Y. Zhao and X. Qian, Highly efficient utilization of nano-Fe0 embedded in mesoporous carbon for activation of peroxydisulfate, Environ. Sci. Technol., 2019, 53, 9081–9090 Search PubMed.
- X. X. Cheng, Doctoral dissertation, Harbin Institute of Technology, 2017 Search PubMed.
- Z. K. Guo, Master thesis, Harbin Institute of Technology, 2014 Search PubMed.
- J. Fu, Master thesis, Xi'an Shiyou University, 2022 Search PubMed.
- J. Chen and P. Zhang, Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate, Water Sci. Technol., 2006, 54, 317–325 Search PubMed.
- H. Tao, D. Yu, Y. Gu and T. Lin, Degradation mechanism of PFOA by Fe2+/PMS/UF process, Environ. Sci. Technol., 2023, 36, 24–30 Search PubMed.
- T. A. Bruton and D. L. Sedlak, Treatment of perfluoroalkyl acids by heat-activated persulfate under conditions representative of in situ chemical oxidation, Chemosphere, 2018, 206, 457–464 Search PubMed.
- T.-H. Kim, S.-H. Lee, H. Y. Kim, K. Doudrick, S. Yu and S. D. Kim, Decomposition of perfluorooctane sulfonate (PFOS) using a hybrid process with electron beam and chemical oxidants, Chem. Eng. J., 2019, 361, 1363–1370 Search PubMed.
- X. Zhang, H. X. Li, T. Y. Zhang, Z. F. Li, W. J. Sun and X. W. Ao, Degradation of per- and polyfluoroalkyl substances in water by UV-based advanced oxidation or advanced reduction processes, Huagong Jinzhan, 2024, 43, 4587–4600 Search PubMed.
- P. Yin, Z. Hu, X. Song, J. Liu and N. Lin, Activated persulfate oxidation of perfluorooctanoic acid (PFOA) in groundwater under acidic conditions, Int. J. Environ. Res. Public Health, 2016, 13, 602 Search PubMed.
- Y. H. Zhan, Y. X. Qin, B. L. Chen, Z. Zhou, H. M. Cao and Y. Liang, Photodegradation technology and mechanism of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS): A critical review, Environ. Chem., 2022, 41, 46–56 Search PubMed.
- H. V. Lutze, J. Brekenfeld, S. Naumov, C. Von Sonntag and T. C. Schmidt, Degradation of perfluorinated compounds by sulfate radicals – New mechanistic aspects and economical considerations, Water Res., 2018, 129, 509–519 Search PubMed.
- D. P. Xu, W. Yao, Y. Xue and X. J. Feng, Adsorption of perfluorooctanoic acid on humic acid from black soil, Anquan Yu Huanjing Xuebao, 2024, 24, 2390–2398 Search PubMed.
- X. Guo, B. Tu, J. Ge, C. Yang, X. Song and Z. Dang, Sorption of tylosin and sulfamethazine on solid humic acid, J. Environ. Sci., 2016, 43, 208–215 Search PubMed.
- M. Li, Master thesis, Harbin Institute of Technology, 2019 Search PubMed.
- M. Jiang, G. Zeng, Z. Chen, Q. Yu, J. Yu and C. Zhang, Photochemical degradation of PFOA using UV irradiation: A critical review, Zhongguo Kexue:Huaxue, 2011, 41, 964–975 Search PubMed.
- J. Wang, L. Wang, R. Miao, Y. Lv, X. Wang, X. Meng, R. Yang and X. Zhang, Enhanced gypsum scaling by organic fouling layer on nanofiltration membrane: Characteristics and mechanisms, Water Res., 2016, 91, 203–213 Search PubMed.
- M. W. Liu, Master thesis, Xi'an University of Architecture and Technology, 2023 Search PubMed.
- X. L. Yue, Master thesis, Xi'an University of Architecture and Technology, 2019 Search PubMed.
- P. R. Fan, Master thesis, Xi'an University of Architecture and Technology, 2020 Search PubMed.
- C. Zhao, G. Hu, D. Hou, L. Yu, Y. Zhao, J. Wang, A. Cao and Y. Zhai, Study on the effects of cations and anions on the removal of perfluorooctane sulphonate by nanofiltration membrane, Sep. Purif. Technol., 2018, 202, 385–396 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |