Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review on the extraction of polyphenols from pomegranate peel for punicalagin purification: techniques, applications, and future prospects
Zhirong Huang,
Su Chern Foo and
Wee Sim Choo *
*
School of Science, Monash University Malaysia, Jalan Lagoon Selatan, Bandar Sunway, 47500, Selangor, Malaysia. E-mail: choo.wee.sim@monash.edu
First published on 4th February 2025
Abstract
Pomegranate peel is rich in polyphenols with punicalagin as the dominant compound but is always regarded as agricultural waste. This review focuses on the extraction of polyphenols as well as the purification, food industry applications and health effects of punicalagin. Considering polyphenol extraction, solvent extraction is the most commonly used method because it offers the highest total phenolic content; however, it is time consuming and energy intensive. Therefore, advanced methods are used to enhance its extraction efficiency, such as enzyme-assisted solvent extraction, resulting in the highest extraction yield. As for the purification of punicalagin from polyphenols, liquid chromatography is the most widely used method, and the highest purity is achieved with semi-high-pressure liquid chromatography (HPLC). Medium-pressure liquid chromatography (MPLC) and high-speed countercurrent chromatography (HSCCC) have similar effects, but relatively fewer studies have adopted these two methods. Besides, punicalagin has outstanding antioxidant properties and can thus be added to functional foods to extend their shelf life. Moreover, it shows great antibacterial effects on drug-resistant pathogens. Its anti-inflammatory potential is governed by its ability to treat infection and hyperimmune-related disorders. This work provides a comprehensive review of methods for extracting and purifying valuable compounds from pomegranate peel, particularly punicalagin, and highlights its potential applications in functional foods and health therapies.
Sustainability spotlightPomegranate peel, a major byproduct of the food industry, is generated at an estimated 1.5 million tons annually according to the Food Agricultural Organization (FAO). This review explores sustainable methods to repurpose this waste by extracting polyphenols and purifying punicalagin from pomegranate peel. Potential applications of punicalagin include its use as a natural food preservative, antioxidant in nutraceuticals, and agent in energy storage. This work aligns with the UN's sustainable development goals 2 (zero hunger), 3 (good health and well-being), 9 (industry and innovation), and 12 (responsible consumption and production). The re-utilization of food waste not only contributes to reducing environmental waste but promotes the development of preservatives and nutraceuticals, supporting a circular economy in the food and pharmaceutical industries. |
1. Introduction
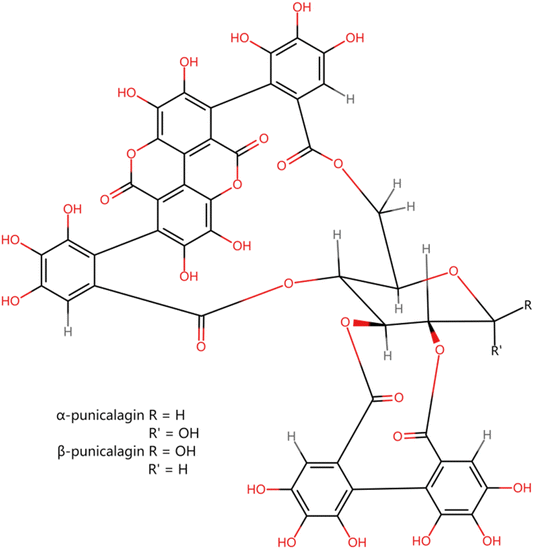
Pomegranate (Punica granatum L.), a deciduous shrub or small tree belonging to the Punicaceae family, is commonly grown and consumed in the Middle East, Europe, and Southeast Asia.1 The pomegranate fruit possesses significant commercial value and finds extensive utilization in the fruit processing and beverage industries, particularly for the manufacturing of juice and soft drinks. However, a significant amount of nonedible waste is generated from this fruit during its processing, which is usually disposed of as agro-waste.2,3 According to the Food Agricultural Organization (FAO), industrial pomegranate-processing centers generate an estimated 1.5 million tons of waste annually.4 The fruit consists of three parts, i.e., leathery exocarp (peel), fleshy mesocarp, and seeds, including arils.5 After juicing, a combination of peel, fleshy mesocarp, seeds, and aril residues remain. The combination can be manually fractionated into the following: peel (hard and tough) and seed residuals (a mixture of fleshy mesocarp, seeds, and aril residues). The peel accounts for about 40–50% of the total weight of the fruit.6 According to Bar-Ya'akov et al.,7 pomegranate peel is a source of several important bioactive compounds, such as phenolic compounds including ellagitannins, flavonoids, and anthocyanins, which are known to have potent antioxidant activities, thus suggesting its good potential for health-related applications. Thus, pomegranate waste can be valorized for use in the nutraceutical and pharmaceutical industries.8Polyphenols belong to an organic chemical class with multiple phenolic structural units. Different parts of the pomegranate fruit, including seeds and peel, consist of up to 48 phenolic compounds, including flavonoids, anthocyanins, gallotannins, hydroxycinnamic acid, hydroxybenzoic acid, and hydrolyzed tannins such as ellagitannins and gallagyl esters.9 Various biological activities of phenolic compounds in pomegranate peel extracts have been reported. These compounds regulate gene expression, reduce inflammation, and function as antioxidants, helping support immune functions in humans.10,11 Pomegranate peel extract has antimicrobial, antioxidant, anti-inflammatory, hepatoprotective, and antigenotoxic activities.12–14 The important health properties of pomegranate products are mainly due to the strong biological activity of water-soluble hydrolyzable ellagitannins, whereby punicalagin accounts for up to 70% of the polyphenols found in commercially available pomegranate juice.15 A major polyphenol of pomegranate is punicalagin, which exists in the α and β forms (Fig. 1). This is a unique high-molecular-weight water-soluble compound, serving as the predominant ellagitannin in the pomegranate fruit.16–18 Punicalagin shows multiple bioactivities, including antibacterial, anti-inflammatory, antioxidant, and anti-atherosclerosis activities.19–22 These properties suggest that punicalagin has significant potential for use in functional foods and nutritional supplements.23
In the food industry, there is an increasing interest in mild, non-thermal approaches that effectively reduce microbial activity, while preserving the physical, chemical, nutritional, and sensory characteristics of raw materials.24 In most current studies, researchers are focused on the extraction of polyphenols from pomegranate peel, while targeted extraction methods for specific compounds such as punicalagin during the crude extraction stage remain relatively limited. Punicalagin makes up the majority of polyphenols in pomegranate peel, and therefore extraction methods targeting polyphenols as a group can also serve as an indirect reflection of punicalagin content. In this respect, initially this review introduces the methods for the crude extraction of polyphenols from pomegranate peel, followed by approaches for the purification of punicalagin. Although the traditional methods of maceration and solvent extraction are commonly used, currently active research in the area of extraction includes new and environmentally friendly methods such as supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), pressurized liquid extraction (PLE), and enzyme-assisted extraction (EAE). The techniques employed to purify punicalagin include liquid chromatography (LC), high-speed countercurrent chromatography (HSCCC), and medium-pressure liquid chromatography (MPLC). Overall, this review aims to explore the various extraction methods for obtaining polyphenols from pomegranate peel, with punicalagin being the predominant compound, together with the purification techniques targeting punicalagin and its applications in the food industry and pharmaceutical use. The overall framework and structure of this review are summarized in Fig. 2.
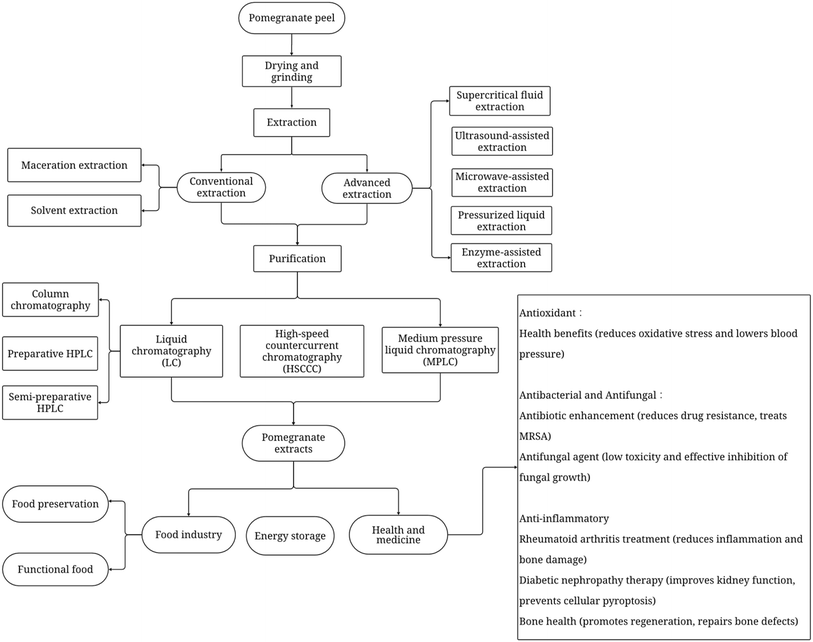 | ||
| Fig. 2 Overview of techniques used in pomegranate peel processing for extraction of polyphenols and purification of punicalagin. | ||
2. Extraction techniques
2.1 Conventional extraction methods
El-Beltagi et al.26 obtained pomegranate peel powder using ethanol, methanol, and water. Briefly, 20 g powdered pomegranate peel was macerated in 100 mL of the above-mentioned solvents separately, shaking at 200 rpm at 37 °C for 24 h three times. The results indicated that the best extraction of phenolic compounds was in distilled water, with the highest content of total phenols of 513.8 ± 4.0 mg gallic acid equivalent (GAE)/100 g dry weight, flavonoids of 45.3 ± 0.5 mg quercetin equivalent (QE) per g dry weight, and extraction yield of 0.55 g/10 g peel. During the maceration process described by Ranjha et al.,27 mixed solvents were used, involving different concentrations of methanol, ethanol, and acetone. The sample-to-solvent ratio was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 at a temperature of 40 °C in a shaking water bath for a period of 20 h. Their findings indicated that 50% methanol gave the highest extraction yield at 30.87%, while 75% methanolic extract exhibited the highest total phenolic content of 51.04 mg GAE per g and the total flavonoids reached as high as 27.61 mg QE per g. Thus, the type of solvent used is very important during the process of maceration. For example, water is a good solvent in extracting phenolic compounds from pomegranate peel, as reported by El-Beltagi et al.,26 while 50% methanol gives superior extraction yields in comparison with 75% methanol according to Ranjha et al.27 Alternatively, Ranjha et al.27 compared the conventional maceration technique with sonication, where the yields obtained using 50% methanol in sonication were the highest at about 31.45%. More importantly, both the phenolic and flavonoid contents increased by about 40% in the sonication method compared to that from maceration, indicating a significant improvement over traditional maceration methods.
15 at a temperature of 40 °C in a shaking water bath for a period of 20 h. Their findings indicated that 50% methanol gave the highest extraction yield at 30.87%, while 75% methanolic extract exhibited the highest total phenolic content of 51.04 mg GAE per g and the total flavonoids reached as high as 27.61 mg QE per g. Thus, the type of solvent used is very important during the process of maceration. For example, water is a good solvent in extracting phenolic compounds from pomegranate peel, as reported by El-Beltagi et al.,26 while 50% methanol gives superior extraction yields in comparison with 75% methanol according to Ranjha et al.27 Alternatively, Ranjha et al.27 compared the conventional maceration technique with sonication, where the yields obtained using 50% methanol in sonication were the highest at about 31.45%. More importantly, both the phenolic and flavonoid contents increased by about 40% in the sonication method compared to that from maceration, indicating a significant improvement over traditional maceration methods.
In summary, the advantage of maceration is its inherent simplicity and low cost. Dynamic processes such as shaking or magnetic stirring may improve the interaction of the solvent with the plant material. Thus, the integration of dynamic processes, the choice of solvent, and advanced technologies such as ultrasonication can enhance its efficiency to a large degree. Therefore, to obtain the highest yield and quality of extracted bioactive chemicals using maceration, further procedures should be employed.
Negi and Jayaprakasha30 extracted pomegranate peels using ethyl acetate, acetone, methanol, and water. Among these solvents, methanol extraction showed the most promising result, returning an extract yield of 9.4% compared with the minimal yield of 1.04% using ethyl acetate. The phenolic content in water, ethyl acetate, acetone and methanol extracts was found to be 140, 170, 400, and 460 mg per g catechin equivalent, respectively.
Wang et al.31 reported that methanol was the most effective solvent for extracting phenolics from pomegranate peels, giving the highest total extract yield of 8.26% compared to that of water of 5.90%, ethanol of 1.55%, acetone of 0.37%, and ethyl acetate of 0.18% at a low temperature of 40 °C. However, although ethyl acetate resulted in the lowest extract yield, it produced the highest content of total phenolics in the extracts, reaching 20.24%, which suggests that ethyl acetate can have a better recovery and preservation of phenolics despite its lower yield. Meanwhile, considering toxicity and cost, water has a comparable value with methanol or ethyl acetate as a better extraction solvent. Under similar conditions but at higher temperatures of >95 °C, water could also achieve a high extractive yield of 11.15%. There is a delicate balance between the characteristics of the solvent and the operating conditions, both of which significantly influence the extraction outcome. Generally, methanol and water appeared to be good solvents for recovering phenolic contents from pomegranate peels, and this also refers to their mixture. Kennas and Amellal-Chibane32 also depicted that 50% aqueous methanol resulted in the highest extraction yield of 37.33% ± 5.3% and total phenolics of 625.525 ± 6.83 mg GAE per g compared to ethanol, methanol, acetone, and water (Table 1).
| Extraction method | Sample preparation | Optimal conditions | Extraction yield | Ref. |
|---|---|---|---|---|
| Maceration extraction | Purchase location: supermarket, Assiut, Egypt | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v) 5 (w/v) |
Extraction yield: 0.55 g/10 g peel | 26 |
| Treatment: peels dried at 50 °C in an oven | Solvent: distilled water | Total phenolic content: 513.8 ± 4.0 mg gallic acid equivalent (GAE)/100 g dry weight | ||
| Ripeness: fully ripe, no injury or infection | Shaking speed: 200 rpm at 37 °C for 24 h | Total flavonoid content: 45.3 ± 0.5 mg quercetin equivalent (QE) per g dry weight | ||
| Form of sample: peel powder, stored at room temperature in dark plastic bags | ||||
| Maceration extraction | Purchase location: local fruit market, Islamabad, Pakistan | Solvent: 50% methanol | 50% methanol: extraction yield: 30.87% | 27 |
| Treatment: peels dried at 50 °C for 48 h to less than 10% moisture | Solvent: 75% methanol | 75% methanol: total phenolic content: 51.04 mg GAE per g | ||
| Ripeness: fully matured and ripened | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
75% methanol: total flavonoid content: 27.61 mg QE per g | ||
| Form of sample: peel powder, stored in airtight polyethylene zip bags at 4–6 °C | Temperature: 40 °C | |||
| Shaking water bath: 20 h | ||||
| Solvent extraction | Purchase location: local markets, India | Solvent: methanol | Highest extraction yield: 9.4% | 30 |
| Treatment: Peels dried under natural conditions for 5 days | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/v) 4 (w/v) |
Phenolic content in methanol extract: 460 mg g−1 in catechin equivalents | ||
| Form of sample: peel powder (60-mesh), extracted with multiple solvents | Temperature: 30 °C | |||
| Stirring speed: 200 rpm on a magnetic stirrer | ||||
| Duration: 1 h | ||||
| Solvent extraction | Purchase location: stiebs Pomegranate Products (Madera, CA, USA) | Solvent and temperature: methanol, 40 °C | Methanol, 40 °C, 240 min: highest extract yield of total phenolics 8.26% | 31 |
| Treatment: peels dried at 40 °C to 8% moisture | Solvent and temperature: water, 95 °C | Water, 95 °C, 2 min: highest extract yield 11.15% | ||
| Form of sample: ground peel, stored at −18 °C, separated by mesh size | Solvent and temperature: ethyl acetate, 40 °C | Ethyl acetate, 40 °C, 240 min: highest content of the total phenolics in extract 20.24% | ||
| Solvent extraction | Purchase location: Bouira, Algeria | Optimized solvent: 50% aqueous methanol | Highest extraction yield: 37.33% ± 5.3% | 32 |
| Treatment: peels air-dried for 2 weeks (moisture content: 8.64%) | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) 20 (w/v) |
Total phenolics: 625.525 ± 6.83 mg GAE per g | ||
| Ripeness: collected between October and November | Temperature: room | Flavonoids: 52.68 ± 1.97 μg QE per mg of dry extract | ||
| Form of sample: peel powder, stored in dark conditions at 4–6 °C | Shaking speed: 500 rpm | |||
| Time: overnight | ||||
| Supercritical CO2 extraction | Purchase location: local producer, Vicuña, Valle de Elqui, Chile | Solvent: 20% ethanol | 300 bar, 50 °C: extract yield: 8.5% | 33 |
| Treatment: peels freeze-dried for 72 h | Pressure: 300 bar, 400 bar | 400 bar, 40 °C: punicalagin: 9.5% | ||
| Ripeness: not specified | Temperature: 40 °C, 50 °C | 400 bar, 40 °C: total phenolic content: 8.94 mg GAE per g | ||
| Form of sample: ground peels (30–40 mesh), stored at −20 °C | ||||
| Supercritical fluid extraction | Purchase location: Meybod, Yazd, Iran | Methanol volume: 150 μL | Oleic acid, palmitic acid and (−)-borneol was major compound in extracts | 34 |
| Treatment: peels air-dried for 2 weeks | Pressure: 350 atm | The optimum extraction yield was 1.18% (w/w) for SFE | ||
| Ripeness: obtained in October 2012 | Temperature: 55 °C | |||
| Form of sample: ground peels (0.5 mm), stored at 4 °C | Extraction time: 30 min | |||
| Supercritical fluid extraction | Purchase location: Alfred Galke GmbH, Bad Grund, Germany | Fluid: SC CO2 | 20 MPa: polyphenolic components: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 561.84 μg g−1 561.84 μg g−1 |
35 |
| Treatment: lyophilized peels | Co-solvent: ethanol | Predominant compound: ellagic acid content of 7492.53 μg g−1 | ||
| Form of sample: ground lyophilized peels, used for extractions with various solvents | Pressure: 20 MPa, 10 MPa | 10 MPa: the highest SFE yield (12.34%) | ||
| Temperature: 40 °C | ||||
| Ultrasound extraction | Purchase location: local markets | Power: 140 w | Solvent: 70% ethanol–water mixture, temperature: 60 °C, extraction time: 30 min | 36 |
| Treatment: peels dried at 40 °C with air circulation | Solvent: 70% ethanol–water mixture | Extraction yield: 45.38% | ||
| Form of sample: ground peels, stored at −20 °C, fractioned by sieves | Temperature: 60 °C | Ferric reducing antioxidant power: 63.37 mmol Fe2+/100 g | ||
| Extraction time: 30 min | Solvent: 50% ethanol–water mixture, temperature: 45 °C, extraction time: 30 min | |||
| Power: 140 W | Total phenolics: 8923.24 mg GA/100 g dry weight | |||
| Solvent: 50% ethanol–water mixture | ||||
| Temperature: 45 °C | ||||
| Extraction time: 30 min | ||||
| Ultrasonic extraction | Purchase location: Sichuan Huili Guoguo Fruit Food Co., Ltd., Chengdu, China | Ethanol concentration: 53% | Yield of punicalagin: 505.89 ± 1.73 mg per g dry weight | 37 |
| Treatment: lyophilized peels, passed through a 100-mesh sieve | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (w/v) 25 (w/v) |
|||
| Ripeness: full-ripe stage | Ultrasonic power: 757 W | |||
| Form of sample: ground lyophilized peel powder, stored at 4 °C | Extraction time: 25 min | |||
| Pulsed ultrasound extraction | Purchase location: Sahakari Bhandar, a local fruit market, Mumbai, India | Solvent type: 50% ethanol (v/v) | Yield: 0.48 g g−1 | 38 |
| Treatment: peels dried at 45 °C in a vacuum oven for 36 h | Sample and solvent ratio: 2.17 g/100 mL, sonication power: 116 W | Total phenolics: 177.54 mg GAE per g | ||
| Form of sample: peel powder, passed through a 500 μm mesh sieve and stored in a water and airtight high-density polyethylene (HDPE) pouch at −18 °C | Duty cycle: 80% | Total flavonoids: 35.71 mg QE per g | ||
| Extraction time: 6 min | Antioxidant capacity: 160.54 mg GAE per g | |||
| Anthocyanin content: 21.65 mg cyn-3-glc/100 g with browning index: 54.92 | ||||
| Ultrasonication with dynamic maceration extraction | Purchase location: pomegranates (Rabab-e-Neiriz cultivar) were obtained from local shop in Tabriz, Iran | Solvent: ethanol–water 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v) 40 (v/v) |
Temperature: 61.8 °C, stirring speed: 1000 rpm, yield of extract (%) 38.14% | 39 |
| Treatment: peels dried at 25 °C for 5 days | Sample and solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 g mL−1 50 g mL−1 |
Temperature: 56.2 °C, stirring speed, 515 rpm: DPPH scavenging activity 90.35% | ||
| Form of sample: peel powder, passed through a 325-mesh screen and stored in a plastic zip-lock bag at −18 °C | Sonication time: 70 min | Temperature: 62.2 °C, stirring speed, 1000 rpm: TPC 283.18 mg GAE/100 g dry weight pomegranate peel extract | ||
| Power: 400 W | ||||
| Temperature: 61.8 °C, 56.2 °C, 62.2 °C | ||||
| Dynamic maceration conditions: 24 h at 25 °C, stirring speed: 1000 rpm, 515 rpm | ||||
| Vacuum microwave-assisted aqueous extraction | Purchase location: pomegranate peels were collected after juicing process conducted at “Rodones S.A.” (Greece) using the “wonderful” pomegranate fruit variety | Solvent: water | Extraction temperature: 61.48 °C, extraction time: 10 min, microwave power: 3797.24 W, water to raw material ratio: 39.92 L kg−1 | 40 |
| Treatment: peels frozen after juice processing, milled into 2 kg samples | Temperature: 61.48 °C | High total polyphenol content (5.542 mg GAE per g fresh pomegranate peel/min) | ||
| Form of sample: peel powder, stored at −20 °C until extraction | Time: 10 min | |||
| Microwave power: 3797.24 W | ||||
| Water to raw material ratio: 39.92 L kg−1 | ||||
| Microwave-assisted extraction | Purchase location: local producer, Rodi Hellas, Greece | Solvent type: 50% aqueous ethanol | Yield: 199.4 mg GAE per g dry peel | 41 |
| Treatment: peels dried at 40 °C for 48 h | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (w/v) 60 (w/v) |
Antioxidant activity: 94.91% radical scavenging activity | ||
| Form of sample: ground peels, stored at −30 °C, with mean particle size of about 0.1 mm | Power: 600 W | Punicalagin content: 143.64 mg per g dry weight | ||
| Process time: 4 min | ||||
| Microwave-assisted extraction | Purchase location: a natural locality in village do, Bosnia and Herzegovina | Irradiation power: 470 W | Irradiation power: 470 W | 42 |
| Treatment: peels air-dried at room temperature for 4–6 days | Irradiation power: 800 W | Extraction yield (EY): 41.31% | ||
| Ripeness: collected in November | Solvent: 50% ethanol | Total phenolics (TP): 20.61 g GAE/100 g | ||
| Form of sample: ground peels, with particle size between 0.75–2 mm | Total flavonoids (TF): 3.29 g CE/100 g, gallic acid: 53.54 mg/100 g | |||
| Punicalin: 747.89 mg/100 g | ||||
| Punicalagin: 218.32 mg/100 g | ||||
| Irradiation power: 800 W: ellagic acid: 96.69 mg/100 g | ||||
| Ultrasound and pressurized liquid extraction | Purchase location: pomegranate fruits (wonderful variety) cultivated in California (USA), purchased at an establishment in Limeira-SP (Brazil) | Solvent type: 70% aqueous ethanol | Water, 70 °C, 480 W and 3 cycles: highest yield of 61.72 ± 7.70 mg g−1 | 43 |
| Treatment: peels dried at 70 °C for 48 h | Solvent type: water, ethanol 70% | Water, 70 °C, 400 W: α-punicalagin: 11.48 ± 0.21 mg per g dry weight | ||
| Ripeness: not specified | Temperature: 70 °C, 60 °C | Water, 70 °C, 400 W: β-punicalagin: 24.55 ± 0.61 mg per g dry weight | ||
| Form of sample: ground peels, with particle sizes of 0.177–1.410 mm, stored at −20 °C | Power: 480 W, 400 W | Ethanol 70%, 60 °C, 400 W: ellagic acid: 1.28 ± 0.09 mg per g dry weight | ||
| Pressure: 10 MPa | Water, 70 °C, 400 W: sum of phenolic compounds: 43.27 ± 3.03 mg per g dry weight | |||
| Pressurized liquid extraction | Purchase location: commercial farm, Vallenar, Atacama Region, Chile | Process temperature: 200 °C | Total phenolic content (TPC) of 164.3 ± 10.7 mg GAE per g dry weight | 44 |
| Treatment: peels dried at 60 °C for 16 h in an air-drying tunnel | Solvent: 77% ethanol | Punicalagin content of 17 ± 3.6 mg per g dry weight | ||
| Ripeness: ripening stage, collected in April 2017 | At a pressure of 1500 psi for 20 min | |||
| Form of sample: ground peel powder, passed through a 20 mesh sieve, stored at room temperature | ||||
| Pressurized liquid extraction | Purchase location: Ite district, Jorge Basadre province, Tacna, Peru | Solvent: ethanol | Pressure: 40 bar: α-punicalagin: 48 ± 2 mg/100 g | 45 |
| Treatment: peels dried at 40 °C for 72 h | Temperature: 60 °C | β-Punicalagin: 146 ± 11 mg/100 g | ||
| Ripeness: not specified | Pressure: 40 bar, 80 bar | Ellagic acid: 25.6 ± 0.3 mg/100 g | ||
| Form of sample: ground peels, with average particle size of 0.50 mm, stored at −20 °C | Pressure: 80 bar: global extraction yield: 45% on dry basis | |||
| Pressurized liquids assisted by ultrasound combined with an expansion gas | Purchase location: pomegranates grown in California (USA) of the variety wonderful were purchased from a market located in Limeira-SP | Solvent: water | 100 bar, 5 bar N2-Pi, 400 W: α-punicalagin: 14.87 ± 0.36 mg g−1 | 46 |
| Treatment: peels dried at 40 °C for 48 h | Temperature: 40 °C | 100 bar, 5 bar N2-Pi, 400 W: β-punicalagin: 37.13 ± 1.44 mg g−1 | ||
| Form of sample: ground peels, with particle sizes of 0.5–1.0 mm and >1.0 mm, stored at −20 °C | Pressure: 100 bar, 200 bar | 200 bar, 5 bar N2-Pi, 400 W: ellagic acid: 0.96 ± 0.05 mg g−1 | ||
| N2-Pi: 5 bar | 100 bar, 5 bar N2-Pi, 400 W: SPC (sum of phenolic compounds): 53.11 ± 1.79 mg g−1 | |||
| US-Pwr: 400 W | ||||
| Enzymatic extraction combined with high pressure | Purchase location: Serpa, Portugal | Solvent: water | Total extraction yield: 41% ± 1.9% (w/w) per gram of dried pomegranate peel | 47 |
| Treatment: peels dried at 40 °C until reaching 6% moisture | High pressure: 300 MPa, 15 min | Antioxidant activity: 334 ± 2.8 mg trolox equivalents per g dry weight | ||
| Ripeness: ripe | Enzymatic: 4% (v/v) pectinase (novozyme) and 4% (v/v) cellulase (novozyme) | Total phenolic compounds: 207 ± 2.8 mg GAE per g dry weight | ||
| Form of sample: ground peels, stored at −20 °C | ||||
| Enzyme-assisted extraction combined with microwave extraction | Treatment: dried PP powder treated with viscozyme for 1 h, followed by microwave-assisted extraction | Solvent: 30% ethanol | Predicted value | 48 |
| Ripeness: not specified | Enzymatic treatment: 0.6% (w/v) viscozyme for 1 h | TPC: 305.5 mg GAE per g | ||
| Form of sample: ethanol extract | Response surface methodology (RSM) optimal condition: power 443.5 W, time: 131.0 min, and sample to solvent ratio: 23.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Antioxidant activity using ferric reducing antioxidant power (FRAP): 1788 μmol TE per mg | ||
| Antioxidant activity using cupric reducing antioxidant activity (CUPRAC): 2641 μmol TE per mg | ||||
| Actual experimental value: TPC: 326.7 ± 21.3 mg GAE per g | ||||
| Antioxidant activity using FRAP: 1751 ± 64 μmol TE per mg | ||||
| Antioxidant activity using CUPRAC: 2589 ± 87 μmol TE per mg | ||||
| Enzyme-assisted extraction combined with supercritical fluid extraction or solvent extraction | Purchase location: local agro-processing units, Faisalabad, Pakistan | Enzyme pre-treatment: enzyme: cocktail enzyme (a mixture of cellulase, pectinase, and protease; 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) at 3.8% concentration 25) at 3.8% concentration |
Enzyme- assisted solvent extraction: extract yield: 65.89% ± 2.64% | 49 |
| Treatment: peels dried under ambient conditions | Temperature: 49 °C | Total phenolics: 218.74 ± 7.15 mg GAE per g | ||
| Form of sample: peel powder, passed through an 80-mesh sieve | pH: 6.7 | |||
| Time: 85 min | ||||
| Supercritical fluid extraction: CO2 flow rate: 2 g min−1 | Enzyme-assisted supercritical fluid extraction: extract yield: 32.19% ± 1.26% | |||
| Ethanol injection rate: 0.2 g min−1 | Total phenolics: 301.53 ± 7.86 mg GAE per g | |||
| Temperature: 55 °C | ||||
| Pressure: 300 bar | ||||
| Solvent extraction as comparison: solvent: 80% ethanol | ||||
| Enzyme-assisted extraction combined with ultrasound extraction | Purchase location: local market, Tezpur, Assam, India | Solvent: water | TPC (mg GAE per g dry solid): 19.77 | 50 |
| Treatment: peels dried at 45 °C for 48 h | Sample to solvent ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
TFC (mg QE per g dry solid): 17.97 | ||
| Ripeness: maturity | Ultrasound treatment: 41.45 min | Radical scavenging activity: 74.213% | ||
| Form of sample: peel powder, stored at 4 °C | Enzyme: viscozyme | |||
| Enzyme concentration: 1.32 mL/100 mL | ||||
| Incubation temperature: 44.85 °C | ||||
| Incubation time: 1.82 h |
These studies highlight the relevance of the proper selection of solvent and extraction conditions according to the target compounds. Specifically, under the optimal conditions of any extraction technology, the extraction efficiency of different solvents would be different due to the interaction between the extraction technology and chemical properties. In general, selecting a suitable solvent and optimizing the extraction conditions are two main factors that can maximize the yields of valuable compounds separated from pomegranate peel.
2.2 Advanced extraction methods
Panja51 also explained that carbon dioxide is the most attractive fluid for SFE due to its non-toxic, non-flammable and chemically inert properties, which is also available in high quality and large quantities. CO2 has a low critical temperature of 31 °C, indicating that little heating will be needed to achieve its critical point, making it economically feasible both for laboratory and pilot plant operations. Notably, the critical pressure of CO2 is very high, i.e., 73 atm. Despite this high pressure, the benefits of using CO2 as a solvent, particularly in terms of safety and environmental impact, often outweigh the challenges associated with high-pressure conditions.
The research by Bustamante et al.33 showed that small changes in pressure and temperature, together with the addition of cosolvents such as ethanol, can effectively change the solvency power of supercritical fluids, thereby influencing the yield and quality of extracts. These researchers demonstrated that operating within the range of 300–400 bar and 40–50 °C with 20% ethanol optimized the extraction of punicalagin from pomegranate residues. Modifying these parameters affects the density and solvent capacity of supercritical CO2, impacting the dissolution rates of specific bioactive compounds.
Ara and Raofie34 analyzed the volatile chemicals and essential oils extracted from pomegranate peels and found that pressure had a significant impact on the yield of the extraction process. It was noticed that supercritical CO2 extraction required less time compared to the traditional hydro-distillation method. As a result, it is more efficient given that it requires less energy to operate, making SFE more sustainable. The response surface approach shows that there is room for refinement in the extraction parameters during the optimization of the SFE settings. This means that each extraction parameter will need to be systematically modified to achieve the highest yields and purity. This approach illustrates the great applicability and efficacy of SFE in extracting various phytochemicals such as polyphenols and essential oils. Kupnik et al.35 emphasized pressure as the main parameter increasing the solubility of bioactive compounds in a relevant mixture of supercritical CO2 and ethanol. Under the optimal conditions of 20 MPa, the highest levels of ellagic acid were obtained from lyophilized pomegranate peel.
SFE technology is characterized by the use of nontoxic and non-flammable CO2, combined with co-solvents, mostly ethanol. Using the optimum pressure and temperature conditions, the extraction efficiency and bioactive compound yields can be greatly enhanced. Thus, SFE technology has wide applicability and effectiveness in the extraction of various phytochemicals and essential oils.
Tabaraki et al.36 investigated the UAE conditions for extracting phenolic-rich by-products from pomegranate peel using response surface methodology with central composite design. They achieved an extraction yield of 45.38% and ferric reducing antioxidant power of 63.37 mmol Fe2+/100 g under the optimum extraction conditions of power of 140 W, 70% ethanol–water mixture, temperature of 60 °C, and extraction time of 30 min. Employing a 50% ethanol–water mixture at 45 °C for 30 min, they obtained the highest total phenolic content of 8923.24 mg GAE/100 g dry weight. The extraction yield increased by 6.38% when the concentration of ethanol was increased from 30% to 70%. Thus, an appropriate ethanol content was found to significantly improve the extraction efficiency.
Similarly, Liu et al.37 investigated the UAE conditions for punicalagin, which is the most abundant polyphenol in pomegranate peel, using response surface methodology coupled with a Box-Behnken experimental design. The optimized conditions were an ethanol concentration of 53%, a sample-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 w/v, an ultrasonic power of 757 W, and an extraction duration of 25 min. These extraction parameters resulted in the extraction of a high punicalagin content of 505.89 ± 1.73 mg per g dry weight. Combined with statistical optimization techniques such as response surface methodology (RSM), UAE becomes a highly adaptable method for the optimization of the extraction parameters to achieve elevated yields and purity. This method is effective both for the broad-spectrum extraction of phenolics and the isolation of particular polyphenols, such as punicalagin.
25 w/v, an ultrasonic power of 757 W, and an extraction duration of 25 min. These extraction parameters resulted in the extraction of a high punicalagin content of 505.89 ± 1.73 mg per g dry weight. Combined with statistical optimization techniques such as response surface methodology (RSM), UAE becomes a highly adaptable method for the optimization of the extraction parameters to achieve elevated yields and purity. This method is effective both for the broad-spectrum extraction of phenolics and the isolation of particular polyphenols, such as punicalagin.
More and Arya38 introduced pulsed UAE, which enhanced the traditional UAE by reducing the energy consumption, while maintaining a high extraction efficacy. Using a solvent of 50% ethanol (v/v) to extract bio-actives from pomegranate peel, but with a lower sonication power of 116 W and an 80% duty cycle for a brief 6 min duration, a high extraction yield of 48% was achieved, with a total phenolic content of 177.54 mg GAE per g.
Another novel approach in this regard is the research on ultrasonication in conjunction with other modes of extraction. As depicted by Andishmand et al.,39 ultrasonication combined with dynamic maceration was employed to recover phenolic compounds from pomegranate peel, with the optimal conditions identified using response surface methodology. A sonication time of 70 min at 400 W, coupled with dynamic maceration for 24 h at 25 °C and a stirring speed of 1000 rpm yielded the highest phenolic extract of 38.14% and a total phenolic content of 283.18 mg GAE per 100 g dry weight at approximately 60 °C. This synergistic approach demonstrated the potential of integrating ultrasonic energy with mechanical agitation to enhance the extraction efficiency. The combined method outperformed its individual techniques when used independently, offering a more effective strategy for the recovery of bioactive compounds in industrial applications.
The cumulative results show the huge potential of ultrasound technologies in terms of increasing the yield, shortening the processing time, and greater sustainability in industry. This technology is considered to be innovative for the extraction of natural compounds for the production of functional foods and health products because of the adaptability of UAE and its derivatives, including pulsed UAE, and the way it combines dynamic maceration with other approaches.
Basically, there are two mechanisms for microwave extraction, i.e., dielectric heating and ionic conduction. Dielectric heating is based on the principle that the rotation of dipole moments in molecules rapidly aligns and realigns with the changing electromagnetic field to generate heat via molecular friction. Alternatively, when ions in solution align with an electromagnetic field, their movement through the solvent generates heat. These mechanisms enhance effective heat generation inside the solvent, hence improving the extraction process. This indicates that microwave-assisted extraction promotes effective heat generation inside the extracting media, hence improving the extraction process.53–55
Kaderides et al.41 reported that the optimized microwave-assisted extraction conditions for phenolics from pomegranate peels were 50% aqueous ethanol, a ratio of 60/1 mL g−1 for solvent/solid and 600 W for the microwave power. This setup significantly shortened the process time to a mere 4 min with an extraction yield of 199.4 mg GAE per g dry peel and punicalagin content of 143.63 mg per g dry matter. Therefore, the short processing time and excellent efficiency of microwave-assisted extraction make it more attractive compared to approaches such as ultrasound-assisted extraction, requiring more time to produce similar results.
Skenderidis et al.40 reported the vacuum microwave-assisted extraction (MAE) of pomegranate peels, presenting a valuable tool for the pomegranate juice industry to execute effective and economically viable green extraction technology on an industrial scale. This was aimed at increasing the phenolic content and antioxidant activity using merely water, avoiding organic solvents. It was found that the optimum conditions for extraction temperature, duration, microwave power, and water-to-raw material ratio are 61.48 °C, 10 min, 3797.24 W, and 39.92 L kg−1, respectively, amounting to a high total polyphenol content of 5.542 mg GAE per g fresh pomegranate peel per min. By reducing the process time, energy, and solvent costs, while maximizing the quality (indicated by total phenolic content and antioxidant capacity) and avoiding harmful solvents, this method effectively converts a fruit by-product into a functional ingredient with high antioxidant activity.
Additionally, Vladić et al.42 compared the impact of subcritical water and microwave-assisted extraction on the phenolic compounds in pomegranate peel. This study proved that microwave-assisted extraction using a lower microwave power and 50% ethanol was more efficient than subcritical water extraction. High-quality polyphenol-rich extracts with no presence of 5-hydroxymethylfurfural (HMF), which is associated with high temperature processes, were produced. In this work, microwave-assisted extraction was described as a low-cost green methodology that supports the circular economy due to the optimization of natural resource utilization and reducing food waste.
Overall, microwave-assisted extraction has been demonstrated to be a successful method for extracting bioactive chemicals from a considerable number of natural products, including pomegranate peel. In this process, the solvents are rapidly heated, which drastically reduces the extraction time and maintains the integrity of thermally sensitive compounds. Some other benefits include the fact that it is a relatively green methodology, uses less solvent, and high cleanliness, all aligning with the principles of a circular economy.
PLE and its advanced versions, such as ultrasound-assisted PLE (UAPLE), represent very promising approaches for the recovery of bioactive compounds from fruit by-products, where the economic evaluation of UAPLE indicates its viability for industrial-scale application.56 According to Toledo-Merma et al.,45 high extraction yields of α-punicalagin (48 ± 2 mg/100 g), β-punicalagin (146 ± 11 mg/100 g), and ellagic acid (25.6 ± 0.3 mg/100 g) could be achieved using PLE by modifying the temperature to about 60 °C and pressure to 40 bar. At higher pressures, such as 80 bar, it could recover even greater quantities. This simply proves that PLE can be tailored to suit the diverse requirements of compounds, making this process more economically viable.
Alternatively, Sumere et al.43 reported an improvement in extraction efficiency from pomegranate peel with the introduction of ultrasound into PLE. Using 70% aqueous ethanol, temperature of 70 °C, ultrasound power of 480 W and pressure of 10 MPa, the highest extraction yield of 61.72 ± 7.70 mg g−1 was achieved. Santos et al.46 further improved UAPLE by adding an expansion gas and maintained the heat-sensitive chemicals. With a lower temperature of 40 °C, Santos et al.46 obtained an α-punicalagin content of 14.87 ± 0.36 mg g−1 and β-punicalagin content of 37.13 ± 1.44 mg g−1 from pomegranate peel with a much lower temperature than 200 °C employed by García et al.44 and greater extraction yield than that obtained by Toledo-Merma et al.45
Overall, UAPLE with expansion gas seems to offer good extraction yields and effectiveness with the least amount of damage to the environment. Further research on the improvement of these methods should still be done, mainly by adding ultrasound and searching for the best concentrations of solvents and temperatures to make them even more useful.
EAE is an advanced approach based on the ability of enzymes to catalyze reactions with high specificity and regioselectivity in aqueous media and mild conditions, which would not damage the integrity of the bioactive compounds, as reported by Gardossi et al.58 Physical parameters such as temperature and pressure are crucial for maximizing the extraction efficiency. For instance, Alexandre et al.47 employed a combination of pectinase and cellulase under high-pressure conditions at 300 MPa for 15 min, achieving the highest total extraction yield of 41% ± 1.9% (w/w) per gram of dried pomegranate peel and a total phenolic content of 207 ± 2.8 mg GAE per g dry weight.
Similarly, physical extraction methods can also increase the efficiency when combined with EAE. The combination of cellulolytic enzymes with microwave-assisted extraction was reported to highly increase the phenolic yield and antioxidant activities of pomegranate peel extracts.48 According to Mushtaq et al.,49 the combination of enzymes and supercritical fluid extraction resulted in a high level of total phenolics of 301.53 ± 7.86 mg GAE per g and the combination of enzymes and solvent extraction resulted in a high yield of 65.89% ± 2.64%, which is also the highest yield among the methods in Table 1. Again, Nag and Sit50 combined ultrasound with the enzymatic method to recover the total phenolic content (TPC) of 19.77 mg GAE per g dry solid and total flavonoid content of (TFC) 17.97 mg quercetin equivalent per g dry solid from pomegranate peels. This study demonstrated that ultrasound-assisted enzymatic extraction is a promising method for obtaining polyphenols from pomegranate peels as well as other agricultural and food waste, offering an effective alternative to chemical solvent extraction. The selection of enzymes is crucial, given that the aforementioned studies identified that pectinase, cellulase, and Viscozyme are effective enzymes for extracting bioactive compounds from pomegranate peel.47–49
Therefore, EAE is an easy and time-effective way for extracting bioactive compounds from pomegranate peel. The ability of EAE to act in synergy with other extraction technologies, together with its environmental and economic advantages, make it one of the best options among the advanced methods. Enzyme cost is one of the major hurdles in the commercial application of enzyme technology.59 In this respect, the combination of enzymes with other modern technologies such as ultrasound can balance the cost to a great extent, while using ultrasound and enzymes can be even more cost-effective given that the extraction yield of polyphenols is higher, with the energy consumed by ultrasonic equipment being much lower.50 Thus, future research should focus on optimizing enzyme combinations and exploring new physical methods that will further enhance the yield and reduce the cost of EAE for its wide application in the food and pharmaceutical industries.
3. Purification
Purification is an essential step following the extraction procedure for the commercial use of bioactive chemicals.60 Chromatography effectively separates, detects, identifies, and measures the components in complex mixtures61 with its performance influenced by factors such as adsorption and molecular weight. Liquid chromatography (LC) is a common method that has been applied to substances such as pomegranate peel extract because of its ability to separate and identify biomolecules and other active components with the use of a liquid mobile phase and a stationary phase.62 Within LC, various techniques have been greatly utilized, such as high-performance liquid chromatography (HPLC) and supercritical fluid chromatography.63 Recent studies emphasized the use of liquid chromatography (LC), high-speed countercurrent chromatography (HSCCC), and medium-pressure liquid chromatography (MPLC) to obtain high-purity products from pomegranate peel extracts.3.1 Liquid chromatography (LC)
Liquid chromatography (LC) includes preparative high-performance liquid chromatography and semi-preparative HPLC. LC is the most feasible compound isolation method. Nowadays, a set of equipment is combined into a single purification system for increasing the yield of purified compounds. Macroporous resins are high loading, permeable and multifunctional, and hence they are combined with LC as a pretreatment.64 For example, HPD-300 resin possesses an excellent capacity to enrich total polyphenols, where the maximum loading concentration improved the extraction ratio of crude pomegranate from 18.02% to 68.45%.65 Subsequently, the most effective macroporous resin for punicalagin was assessed. Liu et al.37 examined six standard macroporous resins (201-7, D101, AB-8, HPD-100, HPD-300, and HPD-826), and the results of the adsorption studies showed that D101 is the optimum. The purity of punicalagin reached 71.85% by UAE combined with D101.The integration of other purification procedures, such as preparative HPLC (Table 2), may result in improvements in both the purity and yield of the target compounds. Preparative high-performance liquid chromatography (prep-HPLC) serves as an important approach for the large-scale separation of natural products. Big columns, high flow rates, and small particle sizes are combined to obtain high-resolution and efficient separation. The term “preparative” normally implies that the process is done on a large scale, and its improved capabilities, together with the reduction in cost make it affordable by most research organizations, allowing the exact purification of complicated mixtures.71,72 Prep-HPLC can be used with macroporous resins to purify polyphenols from plant extracts. Liu et al.37 achieved punicalagin with a purity of 71.85% by purification using D101 macroporous resin. The purity of the extract increased to 92.15%, while the yield reached 58.90 ± 1.10 mg when a pre-HPLC on C18 column was used for purification of the initial extract. According to Fischer et al.,66 the purification of punicalagin involved lyophilizing and extracting pomegranate peels with aqueous methanol, followed by preparative HPLC using a C18 column with gradient elution, and detection at 280 nm, achieving a purity of 93%. Lu et al.67 achieved even greater purity with a one-step purification using prep-HPLC. The optimum choice for purifying punicalagin was the combination of methanol and trifluoroacetic acid (TFA) in water as the mobile phase, from which 81.7 mg of punicalagin with a purity of 98.05% was obtained from 300 mg of crude pomegranate husk extract. Considering this high purity, the parameters of the mobile phase, stationary phase and the flow rate can be used as a reference for the purification of punicalagin. Similarly, Oudane et al.68 achieved an extraordinarily high purity with a comparable mobile phase. They extracted 20 g of ground Punica granatum with methanol, and then processed the crude extract with a series of steps, and finally obtained 23 mg of >99% pure punicalagin using semi-preparative HPLC with a reversed-phase C18 column and a linear gradient of methanol and TFA. The combination of column chromatography with prep-HPLC has been proven to be very efficient for the isolation of high-purity molecules such as punicalagin.
| Method | Material conditions | Operation process | Purity | Ref. |
|---|---|---|---|---|
| D101 resin-based column chromatography and prep-HPLC | D101 resin column with 10–80% methanol, C18 column with 5–30% methanol | Using a D101 resin column, sequentially eluted with water and 10–80% methanol (gradient) at a flow rate of 30 mL min−1, then purified using a prep-HPLC system with a C18 column and a gradient of 5–30% methanol (gradient) over 60 min at a flow rate of 20 mL min−1 | D101 resin-based column chromatography: 71.85% prep-HPLC: 92.15% | 37 |
| Preparative HPLC | Phenomenex Aqua C18 column, 2% acetic acid in water, 0.5% acetic acid in water and methanol (10/90, v/v) | The purification of punicalagin and pedunculagin I involved lyophilizing and extracting pomegranate peels with aqueous methanol, followed by preparative HPLC using a C18 column with a gradient elution, and detection at 280 nm | 93% of punicalagin | 66 |
| Preparative HPLC | Reversed phase C18 column (19 × 300 mm, 7 μm, symmetry Prep™, methanol (eluent A) and 0.1% TFA in water (eluent B) | Extracting ground pomegranate husk with 50% acetone, concentrating the extract, and using preparative liquid chromatography with a C18 column | 81.7 mg punicalagin at 98.05% purity was obtained from 300 mg crude extract containing 30% punicalagin | 67 |
| The optimum purification condition for punicalagin purification was 14% methanol in 0.1% trifluoroacetic acid solution as the mobile phase. The flow rate was 12 mL min−1 and isolated punicalagin was detected at 378 nm | ||||
| Semi-preparative HPLC purification | Reversed-phase C18 column (250 mm 10 mm, 5 mm, Grace-Vydac) | The purification process involved extracting 20 g of ground Punica granatum with methanol, evaporating, dissolving in water, centrifuging, filtering, lyophilizing, and finally purifying 100 mg of the crude extract using semi-preparative HPLC with a linear gradient of methanol and TFA, resulting in 23 mg of pure punicalagin | >99% based on HPLC analysis at 254 nm | 68 |
| The mobile phase consisted of a linear gradient of eluent A (0.1% TFA aqueous) and eluent B (0.09% TFA in 70% aqueous acetonitrile) | ||||
| High-speed countercurrent chromatography (HSc) | Multiplayer coiled column) | Filling the column with the lower phase, pumping the upper phase at 2.0 mL min−1 while running at 800 rpm, injecting the sample after hydrodynamic equilibrium, and monitoring the effluent with a UV detector at 254 nm to collect peak fractions | A 350 mg amount of the crude extract was separated, yielding 105 mg of punicalagin at a high-purity of over 92% | 69 |
Solvent system: mixing butyl alcohol–TFA–water (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v) 100, v/v) |
||||
| Medium pressure liquid chromatography (MPLC) | C18 column. The mobile phase was composed of 3% aqueous acetic acid (solvent A) and methanol (solvent B) | Ellagitannins were purified by injecting pomegranate polyphenols into a C18 column using an MPLC system, with isocratic elution (5% methanol), followed by column washing and reconditioning, collecting fractions, drying, suspending in water, freezing, freeze-drying, and characterizing by HPLC-ESI-MS | Isolation of punicalagin with a purity exceeding 97.9% | 70 |
3.2 High-speed countercurrent chromatography (HSCCC)
High-speed countercurrent chromatography (HSCCC) has been developed since the mid-1980s with its objective to increase the efficiency and stability of countercurrent chromatography and shorten the separation time by using liquid–liquid partitioning for efficient preparative separation.73,74 Its low solvent usage, good reproducibility, and large loading capacity for solutes make this technique popular for the separation of phenolics in natural compound extraction.37,75 HSCCC has been extensively used to separate the ingredients from natural products and pharmaceuticals, especially polyphenols in various plant materials.76 For example, Sun et al.77 achieved over 95% purity for many acids and flavonoids from Sorbus pohuashanensis fruits. Besides, HSCCC was used to separate phenolic acids from jackfruit peels,78 apple pomace79 and grape skins.80It is worth noting that thus far, only a few reports describing the applicability of the HSCCC technique in the purification of punicalagin from pomegranates has been found. Lu et al.69 extracted punicalagin from pomegranate husk with HSCCC using a butyl alcohol-TFA-water (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:100, v/v) solvent solution. They purified 105 mg of punicalagin (92% purity) and 80 mg of gallic acid (75% purity) from a 350 mg crude sample.
1:100, v/v) solvent solution. They purified 105 mg of punicalagin (92% purity) and 80 mg of gallic acid (75% purity) from a 350 mg crude sample.
Other improvements have been made to the system including better detection devices, column design, proper solvent selection, and modeling of the process, all imparting an improved efficiency and cost-effectiveness to HSCCC. Innovations such as improved online monitoring and non-aqueous solvent solutions for the highly hydrophobic polyphenols are expected to promote this to an even higher standard. Future improvements in model-based process design and user-friendly chromatography modeling software are expected to enhance the industrial adoption of HSCCC technologies, making them simpler and more convenient for large-scale use.76
3.3 Medium-pressure liquid chromatography (MPLC)
Medium-pressure liquid chromatography (MPLC) is a preparative technique developed in the 1970s that enables the separation of organic compounds efficiently. It operates under a certain pressure, allowing the use of smaller particle size and a wider range of stationary phases, overcoming the limiting sample loading of low-pressure liquid chromatography (LPLC) and giving separations that are faster and more accurate.81Aguilar-Zárate et al.70 isolated punicalagin from pomegranate husk using MPLC, obtaining 97.9% purity and displaying significant antioxidant capacity with IC50 values of 109.53 and 151.50 μg mL−1 for 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals, respectively. Similarly, Wang et al.82 reported similar results with a 5% methanol and 0.1% TFA in water mobile phase to extract 339 mg of punicalin at 95.9% purity in 40 min, as well as 59.7 mg of gallic acid with 78% purity. To achieve the extraction of compounds possessing substantial antioxidant properties, MPLC can be applied in the food, pharmaceutical, and healthcare industries to a great extent. Although there are few reports on purifying punicalagin using MPLC, existing research still shows its efficacy. Future research should be aimed at optimization of the MPLC conditions and the application of the process on an industrial scale to get closer towards the possible purification of punicalagin and other major compounds of chemical interest from natural sources.
4. Applications
Punicalagin is a polyphenol compound found in Punica granatum, Lafoensia pacari, and the genus Terminalia.83 Punicalagin was isolated from Lafoensia pacari leaves for the first time by Carneiro et al.84 It was obtained at a concentration of 197 mg g−1 of dried leaf, which is higher than the average amount found in pomegranate husk, reported to be 82.4 mg g−1.85 In Terminalia ferdinandiana, punicalagin was found to be 74 mg/100 g dry weight in fruits and 49 mg/100 g dry weight in leaves.86 It appears that Punica granatum and Lafoensia pacari have higher levels of punicalagin, emerging as the main sources of punicalagin. Additionally, pomegranate peel is a common material for obtaining punicalagin due to its low cost, mass production, and widespread availability.Punicalagin has attracted great interest due to its antibacterial capabilities and potential for application in multiple areas. An overall summary of its applications is presented in Fig. 3. Gosset-Erard et al.87 found that punicalagin is a key antibacterial agent in pomegranate peel extracts, with minimum inhibitory concentration (MIC) values ranging from 0.3 to 1.2 μg mL−1, effectively targeting 10 out of 13 Gram-positive bacteria, 2 out of 3 Gram-negative bacteria, and one yeast strain. Hayrapetyan et al.88 discovered that a 7.5% v/v liquid pomegranate extract (24.7 mg dry pomegranate extract per mL) demonstrated significant antimicrobial activity. In meat pâté refrigerated at 4 °C, pomegranate extract at its minimal bactericidal concentration (MBC) reduced the growth of L. monocytogenes by 4.1 log CFU g−1 over 46 days, compared to that of the control of log 9.2 CFU g−1 by day 18. Demir89 also found that adding 1% punicalagin to meatballs had considerable antibacterial activity, with an MIC of 1.87 mg mL−1 against L. monocytogenes and Salmonella typhimurium. In addition, punicalagin considerably reduced the free fatty acid (FFA), peroxide value (POV), and thiobarbituric acid reactive substance (TBARS) levels during storage. Cooper et al.90 explored the mechanistic effects of punicalagin on bacterial cells. Using high-throughput mass spectrometry and quantitative isobaric labeling, the researchers discovered that punicalagin impairs iron homeostasis in Staphylococcus aureus, causing major changes in the bacterial proteome. Punicalagin therapy inhibited the accumulation of proteins and enzymes required for iron uptake, while inducing an SOS response to damaged deoxyribonucleic acid (DNA), which indicates the usage of punicalagin in limiting bacterial colonization by interfering with critical metabolic pathways. Moreover, punicalagin has antibacterial properties by enhancing the efficacy of antibiotics. For instance, punicalagin inhibited Escherichia coli ATP synthase, which was helpful to avoid antimicrobial resistance.91 Compared with traditional prescriptions, patients took lower dosage of punicalagin for recovering from bacterial infections. Furthermore, punicalagin enhanced the sensitivity towards methicillin-resistant Staphylococcus aureus (MRSA) by muting related genes.92 Punicalagin together with cefotaxime eliminated the symptoms of MRSA-induced pneumonia in mice.93 These therapeutic effects prove the feasibility of applying punicalagin in treating drug-resistant bacterial infections. Also, punicalagin can be a strong antifungal drug with low side effects and is appropriate for application in pharmaceutical practice. Punicalagin exhibited significant antifungal activity with MIC values in the range of 0.5 to 4.0 μg mL−1 against C. neoformans complex. Additionally, it showed low cytotoxicity in human cell lines and no haemolytic potential in animal cells, further confirming its good potential as a safe antifungal agent.94 The results of inhibition of different bacteria demonstrate the potential of punicalagin as a highly effective natural bio-preservative, which possesses the ability to improve the shelf life and safety of meat products. In summary, punicalagin exhibits strong antibacterial and antifungal effects.
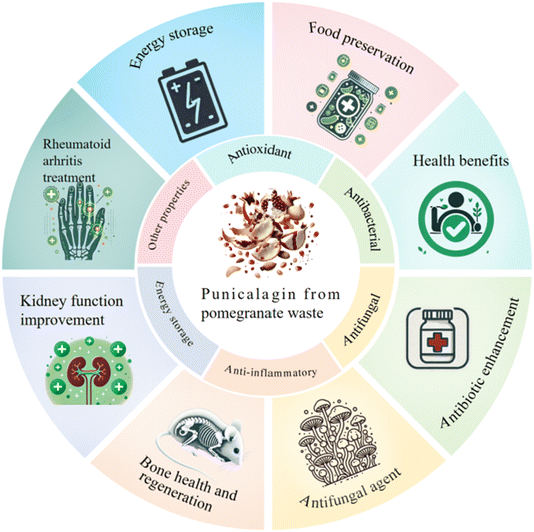 | ||
| Fig. 3 Overview of the applications of punicalagin in foods, health products, and energy storage.86–100 | ||
Punicalagin has attracted significant attention as a plant-derived anti-inflammatory agent due to its strong anti-inflammatory effects and potential applications in medical treatments and disease management.95 Studies have shown that punicalagin effectively reduces inflammatory markers, including NO, TNF-α, and IL-6, in a concentration-dependent manner, thereby inhibiting inflammation and protecting cartilage cells.96 In rheumatoid arthritis treatment, punicalagin alleviated the disease progression in mouse models, mitigating the arthritis severity and minimizing bone destruction.101 Punicalagin exhibited significant renoprotective and anti-inflammatory effects in diabetic nephropathy. In diabetic mice induced by a high-fat diet (HFD) and streptozotocin (STZ), 8 weeks of punicalagin administration led to a significant reduction in blood urea nitrogen (BUN), serum creatinine (CREA), and urinary albumin/creatinine ratio (UACR). It also alleviated glomerular hypertrophy and interstitial hyperplasia, improving kidney function. Furthermore, punicalagin inhibited inflammatory proteins such as IL-1β and caspase-1, effectively suppressing inflammation and preventing pyroptosis.97,102 Additionally, punicalagin supports bone health and shows promise in treating periodontal diseases. It promotes bone regeneration and repairs defects caused by inflammation, making it a safe and promising candidate for bone repair therapy. Specifically, punicalagin stimulated osteogenic activity in MC3T3-E1 cells under periodontal inflammatory conditions and inhibited osteoclast formation in bone marrow-derived macrophages (BMMs) through the RANKL/OPG pathway.103 In conclusion, punicalagin exhibits strong anti-inflammatory and therapeutic effects, offering potential applications in treating rheumatoid arthritis, diabetic nephropathy, and bone-related diseases through its ability to reduce inflammation, protect tissues, and promote regeneration.
Meanwhile, punicalagin also possesses a considerable antioxidant effect. According to Gil et al.,98 HPLC-DAD and HPLC-MS analyses revealed that commercial juices made from pomegranate arils containing 1500–1900 mg L−1 of punicalagin possessed three times higher antioxidant activity than red wine and green tea. Also, da Silva Veloso et al.99 explored the use of pomegranate epicarp extracts as a natural functionalizing ingredient in Brazilian pastry products. Fourteen phenolic compounds were identified through HPLC-DAD-ESI/MS. Besides, the antioxidant properties of punicalagin also contribute to its potential health benefits. According to Wang et al.,100 punicalagin demonstrated effects on angiogenesis and oxidative stress in pregnancy-induced hypertensive rats. It was shown that punicalagin significantly reduced the diastolic, systolic, and mean arterial blood pressure in the affected rats.
Alternatively, Talekar et al.104 demonstrated the use of punicalagin-rich phenolics in energy storage applications. The yield of punicalagin-rich phenolics amounted to 71.2% of the total phenolic extract. They were further processed to develop hard carbon electrodes, which performed well in sodium batteries and showed a good electrochemical performance, while displaying the sustainability and efficiency of using the wastes of pomegranate peels in high-performance applications. Overall, punicalagin is extracted from the peels of pomegranate and used for a wide range of applications, including natural antibacterial, anti-fungal, anti-inflammatory, antioxidant agents, and as a compound in energy storage systems.
5. Conclusion and future perspectives
This study emphasizes the potential of pomegranate peel, a by-product of pomegranate processing, as a great source of bioactive compounds with commercial value. Repurposing these materials can convert waste into viable resources for functional foods and health goods. Punicalagin, the predominant and crucial polyphenol in pomegranate peel, was the focus of this review. This review discussed the extraction of polyphenols (punicalagin being the most abundant component), purification, and prospective applications of punicalagin, considering its many bioactive properties.Regarding extraction procedures, this review addressed both conventional and advanced methods. Among the methods, solvent extraction using 50% aqueous methanol attained an astonishingly high total phenolic content of 625.525 ± 6.83 mg GAE per g. However, conventional extraction methods are usually time and energy consuming, which reduces their cost-effectiveness. Alternatively, a combination of traditional and advanced methods, enzyme-assisted solvent extraction, exhibited higher effectiveness and attained the highest extraction yield of 65.89% ± 2.64% compared to others.
Chromatographic technologies are the most common techniques for purification and often with promising results. Prep-HPLC can achieve a quite high purity of 92.15–98.05%, and semi-HPLC can even reach an extremely high purity of 99%. Other methods, including medium-pressure liquid chromatography (MPLC) and high-speed countercurrent chromatography (HSCCC), also result in purities between 92% and 97.9%. Overall, prep-HPLC and semi-HPLC are recommended due to their higher purification efficiency.
Punicalagin has promising potential in both the food industry and health potential given that various studies support its antioxidant, antibacterial, antifungal, and anti-inflammatory properties. Punicalagin possesses the potential to be a natural preservative, improving the shelf life, functional qualities, and nutritional value of food products. Moreover, its antibacterial and anti-inflammatory properties indicate its possible therapeutic uses, including the mitigation of inflammation-related disorders, reduction in antibiotic use, and treatment of drug-resistant bacterial infections.
However, although punicalagin shows great potential in the food and medical field, its massive production is still immature. Thus, future research should focus on developing cost-effective extraction and purification techniques to enable its mass production and commercialization, thereby optimizing the use of pomegranate peels and delivering their health benefits to consumers. Meanwhile, given that most therapeutic trials have been conducted on mice or in vitro cell models, further studies are needed to confirm its safety and effective dosage. More research is also required to ensure the safe and efficient use of punicalagin in enhancing medical therapies and promoting human health.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by Monash University Malaysia.References
- A. E. El-Hadary and M. Taha, Food Sci. Nutr., 2020, 8, 1798–1811 CrossRef CAS PubMed.
- N. H. Alsubhi, D. A. Al-Quwaie, G. I. Alrefaei, M. Alharbi, N. Binothman, M. Aljadani, S. H. Qahl, F. A. Jaber, M. Huwaikem, H. M. Sheikh, J. Alrahimi, A. N. Abd Elhafez and A. Saad, Bioengineering, 2022, 9, 735 CrossRef CAS PubMed.
- A. K. Das, P. K. Nanda, N. R. Chowdhury, P. Dandapat, M. Gagaoua, P. Chauhan, M. Pateiro and J. M. Lorenzo, Molecules, 2021, 26, 467 CrossRef CAS PubMed.
- A. Gholipour, A. Sadegheih, A. Mostafaeipour and M. B. Fakhrzad, Environ. Dev. Sustainability, 2024, 26, 3993–4027 CrossRef PubMed.
- A. Paul and M. Radhakrishnan, Trends Food Sci. Technol., 2020, 105, 273–283 CrossRef CAS.
- P. Gullón, G. Astray, B. Gullón, I. Tomasevic and J. M. Lorenzo, Molecules, 2020, 25, 2859 CrossRef PubMed.
- I. Bar-Ya’akov, L. Tian, R. Amir and D. Holland, Front. Plant Sci., 2019, 10, 620 CrossRef PubMed.
- H. A. Fahmy and M. A. Farag, J. Food Biochem., 2022, 46, e14024 CrossRef CAS PubMed.
- S. Akhtar, T. Ismail, D. Fraternale and P. Sestili, Food Chem., 2015, 174, 417–425 CrossRef CAS PubMed.
- S. Tota, H. Awasthi, P. K. Kamat, C. Nath and K. Hanif, Behav. Brain Res., 2010, 209, 73–79 CrossRef CAS PubMed.
- N. J. Kang, S. H. Shin, H. J. Lee and K. W. Lee, Pharmacol. Ther., 2011, 130, 310–324 CrossRef CAS PubMed.
- F. Aqil, R. Munagala, M. V. Vadhanam, H. Kausar, J. Jeyabalan, D. J. Schultz and R. C. Gupta, Food Res. Int., 2012, 49, 345–353 CrossRef CAS PubMed.
- S. K. Devatkal, P. Jaiswal, S. N. Jha, R. Bharadwaj and K. N. Viswas, J. Food Sci. Technol., 2013, 50, 555–560 CrossRef PubMed.
- P. Jing, T. Ye, H. Shi, Y. Sheng, M. Slavin, B. Gao, L. Liu and L. L. Yu, Food Chem., 2012, 132, 1457–1464 CrossRef CAS PubMed.
- D. Bialonska, S. G. Kasimsetty, K. K. Schrader and D. Ferreira, J. Agric. Food Chem., 2009, 57, 8344–8349 CrossRef CAS PubMed.
- L. Feng, Y. Yin, X. Yang, H. Tang and Q. Jiao, Hortic. J., 2019, 88, 444–454 CrossRef CAS.
- D. Heber, R. N. Schulman and N. P. Seeram, Pomegranates: Ancient Roots to Modern Medicine, CRC Press, 2006 Search PubMed.
- X.-R. Zhang, J. S. Kaunda, H.-T. Zhu, D. Wang, C.-R. Yang and Y.-J. Zhang, Nat. Prod. Bioprospect., 2019, 9, 357–392 CrossRef CAS PubMed.
- X. Cheng, X. Yao, S. Xu, J. Pan, H. Yu, J. Bao, H. Guan, R. Lu and L. Zhang, Biomed. Pharmacother., 2018, 103, 490–498 CrossRef CAS PubMed.
- A. K. Farha, Q.-Q. Yang, G. Kim, H.-B. Li, F. Zhu, H.-Y. Liu, R.-Y. Gan and H. Corke, Food Biosci., 2020, 38, 100751 CrossRef CAS.
- G.-Y. Sun, C. Wang, Y.-Q. Luo, Y.-X. Zhao, J. Yang, Z.-S. Liu and H. A. Aisa, J. Sep. Sci., 2016, 39, 1963–1970 CrossRef CAS PubMed.
- S.-g. Wang, M.-h. Huang, J.-h. Li, F.-i. Lai, H.-m. Lee and Y.-n. Hsu, Acta Pharmacol. Sin., 2013, 34, 1411–1419 CrossRef CAS PubMed.
- M. Kazemi, R. Karim, H. Mirhosseini and A. Abdul Hamid, Food Chem., 2016, 206, 156–166 CrossRef CAS PubMed.
- H. B. Jadhav, U. S. Annapure and R. R. Deshmukh, Front. Nutr., 2021, 8, 657090 CrossRef PubMed.
- R. Wardany, Patmawati and D. Nirmala, IOP Conf. Ser.: Earth Environ. Sci., 2023, 1273, 012011 CrossRef.
- H. S. El-Beltagi, N. S. Eshak, H. I. Mohamed, E. S. A. Bendary and A. W. Danial, Plants, 2022, 11, 1740 CrossRef CAS PubMed.
- M. M. A. N. Ranjha, S. Amjad, S. Ashraf, L. Khawar, M. N. Safdar, S. Jabbar, M. Nadeem, S. Mahmood and M. A. Murtaza, Int. J. Fruit Sci., 2020, 20, S1201–S1221 CrossRef.
- E. F. Aransiola, E. O. Ehinmitola, A. I. Adebimpe, T. D. Shittu and B. O. Solomon, in Advances in Eco-Fuels for a Sustainable Environment, ed. K. Azad, Woodhead Publishing, 2019, pp. 53–87 Search PubMed.
- S. Moldoveanu and V. David, in Modern Sample Preparation for Chromatography, Elsevier, 2015, pp. 131–189 Search PubMed.
- P. S. Negi and G. K. Jayaprakasha, J. Food Sci., 2003, 68, 1473–1477 CrossRef CAS.
- Z. Wang, Z. Pan, H. Ma and G. G. Atungulu, Open Food Sci. J., 2011, 5, 17–25 CrossRef CAS.
- A. Kennas and H. Amellal-Chibane, North Afr. J. Food Nutr. Res., 2019, 3, 140–147 CAS.
- A. Bustamante, A. Hinojosa, P. Robert and V. Escalona, Int. J. Food Sci. Technol., 2017, 52, 1452–1462 CrossRef CAS.
- K. M. Ara and F. Raofie, J. Food Sci. Technol., 2016, 53, 3113–3121 CrossRef CAS PubMed.
- K. Kupnik, M. Leitgeb, M. Primožič, V. Postružnik, P. Kotnik, N. Kučuk, Ž. Knez and M. K. Marevci, Plants, 2022, 11, 928 CrossRef CAS PubMed.
- R. Tabaraki, E. Heidarizadi and A. Benvidi, Sep. Purif. Technol., 2012, 98, 16–23 CrossRef CAS.
- Y. Liu, K. W. Kong, D.-T. Wu, H.-Y. Liu, H.-B. Li, J.-R. Zhang and R.-Y. Gan, Food Chem., 2022, 374, 131635 CrossRef CAS PubMed.
- P. R. More and S. S. Arya, Ultrason. Sonochem., 2021, 72, 105423 CrossRef CAS PubMed.
- H. Andishmand, B. Masoumi, M. Torbati, A. Homayouni-Rad, S. Azadmard-Damirchi and H. Hamishehkar, Food Sci. Nutr., 2023, 11, 7160–7171 CrossRef CAS PubMed.
- P. Skenderidis, S. Leontopoulos, K. Petrotos and I. Giavasis, Foods, 2020, 9, 1655 CrossRef CAS PubMed.
- K. Kaderides, L. Papaoikonomou, M. Serafim and A. M. Goula, Chem. Eng. Process. Process Intensif., 2019, 137, 1–11 CrossRef CAS.
- J. Vladić, T. Janković, J. Živković, M. Tomić, G. Zdunić, K. Šavikin and S. Vidović, Plant Foods Hum. Nutr., 2020, 75, 553–560 CrossRef PubMed.
- B. R. Sumere, M. C. De Souza, M. P. Dos Santos, R. M. N. Bezerra, D. T. Da Cunha, J. Martinez and M. A. Rostagno, Ultrason. Sonochem., 2018, 48, 151–162 Search PubMed.
- P. García, C. Fredes, I. Cea, J. Lozano-Sánchez, F. J. Leyva-Jiménez, P. Robert, C. Vergara and P. Jiménez, Foods, 2021, 10 Search PubMed.
- P. R. Toledo-Merma, M. H. Cornejo-Figueroa, A. d. R. Crisosto-Fuster, M. M. Strieder, L. O. Chañi-Paucar, G. Náthia-Neves, H. Rodríguez-Papuico, M. A. Rostagno, M. A. A. Meireles and S. C. Alcázar-Alay, Foods, 2022, 11, 1070 Search PubMed.
- M. P. Santos, M. C. Souza, B. R. Sumere, L. C. Da Silva, D. T. Cunha, R. M. N. Bezerra and M. A. Rostagno, Ultrason. Sonochem., 2019, 54, 11–17 CrossRef CAS PubMed.
- E. M. C. Alexandre, S. Silva, S. A. O. Santos, A. J. D. Silvestre, M. F. Duarte, J. A. Saraiva and M. Pintado, Food Res. Int., 2019, 115, 167–176 CrossRef CAS PubMed.
- M. Kumar, M. Tomar, S. Punia, R. Amarowicz and C. Kaur, Plant Foods Hum. Nutr., 2020, 75, 614–620 CrossRef CAS PubMed.
- M. Mushtaq, B. Sultana, F. Anwar, A. Adnan and S. S. H. Rizvi, J. Supercrit. Fluids, 2015, 104, 122–131 CrossRef CAS.
- S. Nag and N. Sit, J. Food Meas. Charact., 2018, 12, 1734–1743 CrossRef.
- P. Panja, Curr. Opin. Food Sci., 2018, 23, 173–182 CrossRef.
- F. Chemat, Z. e-Huma and M. K. Khan, Ultrason. Sonochem., 2011, 18, 813–835 CrossRef CAS PubMed.
- L. Jasie, R. Revesz, T. Kierstead and E. Hasty, Microwave-assisted solvent extraction, in Microwave-Enhanced Chemistry, Fundamentals, Sample Preparation and Applications, ed. H. M. S. Kingston and S. J. Haswell, American Chemical Society, Washington, DC, 1997, pp. 569–609 Search PubMed.
- H. M. Kingston and L. B. Jassie, Introduction to Microwave Sample Preparation: Theory and Practice, 1988 Search PubMed.
- J. Thuéry, in Microwaves: Industrial, Scientific, and Medical Applications, ed. E. H. Grant, Artech House, 1992 Search PubMed.
- D. T. V. Pereira, G. L. Zabot, F. G. R. Reyes, A. H. Iglesias and J. Martínez, Innovative Food Sci. Emerging Technol., 2021, 67, 102549 CrossRef CAS.
- M. Puri, D. Sharma and C. J. Barrow, Trends Biotechnol., 2012, 30, 37–44 CrossRef CAS PubMed.
- L. Gardossi, P. B. Poulsen, A. Ballesteros, K. Hult, V. K. Svedas, D. Vasić-Racki, G. Carrea, A. Magnusson, A. Schmid, R. Wohlgemuth, P. J. Halling and European Federation of Biotechnology Section on Applied Biocatalysis, Trends Biotechnol., 2010, 28, 171–180 CrossRef CAS PubMed.
- S. S. Nadar, P. Rao and V. K. Rathod, Food Res. Int., 2018, 108, 309–330 CrossRef CAS PubMed.
- G. A. D. Vichakshana, D. J. Young and W. S. Choo, Qual. Assur. Saf. Crops Foods, 2022, 14, 67–83 CrossRef CAS.
- M. P. B. Mourão, A. H. J. Kolk and H.-G. Janssen, in Hyphenations of Capillary Chromatography with Mass Spectrometry, ed. P. Q. Tranchida and L. Mondello, Elsevier, 2020, pp. 3–74 Search PubMed.
- J. W. Dolan and L. R. Snyder, in Liquid Chromatography, ed. S. Fanali, P. R. Haddad, C. F. Poole, P. Schoenmakers and D. Lloyd, Elsevier, Amsterdam, 2013, pp. 251–267 Search PubMed.
- V. B. C. Kumari, S. M. Patil, R. Ramu, P. S. Shirahatti, N. Kumar, B. P. Sowmya, C. Egbuna, C. Z. Uche and K. C. Patrick-Iwuanyanwu, in Analytical Techniques in Biosciences, Elsevier, 2022, pp. 73–101 Search PubMed.
- L. Lin, H. Zhao, Y. Dong, B. Yang and M. Zhao, Food Chem., 2012, 130, 417–424 CrossRef CAS.
- R. Abdulla, S. Mansur, H. Lai, A. Ubul, G. Sun, G. Huang and H. A. Aisa, Phytochem. Anal., 2017, 28, 465–473 CrossRef CAS PubMed.
- U. A. Fischer, R. Carle and D. R. Kammerer, Food Chem., 2011, 127, 807–821 CrossRef CAS PubMed.
- J. Lu, K. Ding and Q. Yuan, Sep. Sci. Technol., 2010, 46, 147–154 CrossRef.
- B. Oudane, D. Boudemagh, M. Bounekhel, W. Sobhi, M. Vidal and S. Broussy, J. Mol. Struct., 2018, 1156, 390–396 CrossRef CAS.
- J. Lu, Y. Wei and Q. Yuan, J. Chromatogr. B, 2007, 857, 175–179 CrossRef CAS PubMed.
- P. Aguilar-Zárate, J. E. Wong-Paz, M. Michel, J. Buenrostro-Figueroa, H. R. Díaz, J. A. Ascacio, J. C. Contreras-Esquivel, G. Gutiérrez-Sánchez and C. N. Aguilar, Phytochem. Anal., 2017, 28, 433–438 CrossRef PubMed.
- Z. Latif and S. D. Sarker, in Natural Products Isolation, ed. S. D. Sarker and L. Nahar, Humana Press, Totowa, NJ, 2012, pp. 255–274 Search PubMed.
- Q.-W. Zhang, L.-G. Lin and W.-C. Ye, Chin. Med., 2018, 13, 20 CrossRef PubMed.
- Y. Ito, Adv. Chromatogr., 1984, 24, 181–226 CAS.
- Y. Ito, J. Sandlin and W. G. Bowers, J. Chromatogr. A, 1982, 244, 247–258 CrossRef CAS.
- Y. Ito and R. L. Bowman, Science, 1971, 173, 420–422 CrossRef CAS PubMed.
- L. Li, J. Zhao, T. Yang and B. Sun, Food Res. Int., 2022, 153, 110956 CrossRef CAS PubMed.
- S. Sun, X. Xin, L. Zhu, L. Chen, Z. Xu and Y. Liu, J. Chromatogr. B, 2021, 1172, 122620 Search PubMed.
- Z. Jiang and Y. Wang, Anal. Methods, 2020, 12, 4674–4681 RSC.
- X. Cao, C. Wang, H. Pei and B. Sun, J. Chromatogr. A, 2009, 1216, 4268–4274 CrossRef CAS PubMed.
- L. Luo, Y. Cui, S. Zhang, L. Li, Y. Li, P. Zhou and B. Sun, Food Chem., 2016, 212, 712–721 CrossRef CAS PubMed.
- K. Hostettmann and C. Terreaux, in Encyclopedia of Separation Science, Elsevier, 2000, pp. 3296–3303 Search PubMed.
- Y. Wang, H. Zhang, H. Liang and Q. Yuan, Food Chem., 2013, 138, 437–443 CrossRef CAS PubMed.
- N. Rozadi, S. Oktavia and F. Fauziah, J. Drug Deliv. Ther., 2022, 12, 148–155 CrossRef CAS.
- C. C. Carneiro, S. da Costa Santos, R. de Souza Lino, M. T. F. Bara, B. A. Chaibub, P. R. de Melo Reis, D. A. Chaves, A. J. R. da Silva, L. S. Silva, D. de Melo e Silva and L. Chen-Chen, Toxicol. Appl. Pharmacol., 2016, 310, 1–8 CrossRef CAS PubMed.
- J. Lu, K. Ding and Q. Yuan, Chromatographia, 2008, 68, 303–306 CrossRef CAS.
- S. Akter, H. Hong, M. Netzel, U. Tinggi, M. Fletcher, S. Osborne and Y. Sultanbawa, Food Anal. Methods, 2021, 14, 2534–2544 CrossRef.
- C. Gosset-Erard, M. Zhao, S. Lordel-Madeleine and S. Ennahar, Food Chem., 2021, 352, 129396 CrossRef CAS PubMed.
- H. Hayrapetyan, W. C. Hazeleger and R. R. Beumer, Food Control, 2012, 23, 66–72 Search PubMed.
- T. Demir, Molecules, 2021, 26, 4052 CrossRef CAS PubMed.
- B. Cooper, N. Islam, Y. Xu, H. S. Beard, W. M. Garrett, G. Gu and X. Nou, Proteomics, 2018, 18, 1700461 CrossRef PubMed.
- M. Lakhani, S. Azim, S. Akhtar and Z. Ahmad, Int. J. Biol. Macromol., 2022, 213, 195–209 Search PubMed.
- S.-H. Mun, O.-H. Kang, R. Kong, T. Zhou, S.-A. Kim, D.-W. Shin and D.-Y. Kwon, J. Pharmacol. Sci., 2018, 137, 317–323 CrossRef CAS PubMed.
- W. Song, L. Wang, M. Jin, X. Guo, X. Wang, J. Guan and Y. Zhao, Antimicrob. Agents Chemother., 2022, 66, e00222–e00224 Search PubMed.
- T. C. Silva, R. I. de Ávila, A. L. S. A. Zara, A. S. Santos, F. Ataídes, V. A. Q. Freitas, C. R. Costa, M. C. Valadares and M. D. R. Silva, Rev. Inst. Med. Trop. São Paulo, 2018, 60, e60 Search PubMed.
- S. Aerken and G. Awuti, Int. J. Front. Med., 2023, 5, 78–82 Search PubMed.
- Y. Cao, J. Chen, G. Ren, Y. Zhang, X. Tan and L. Yang, Nutrients, 2019, 11, 2794 CrossRef CAS PubMed.
- D. Jin, B. Zhang, Q. Li, J. Tu and B. Zhou, Food Funct., 2020, 11, 10617–10634 Search PubMed.
- M. I. Gil, F. A. Tomás-Barberán, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, J. Agric. Food Chem., 2000, 48, 4581–4589 CrossRef CAS PubMed.
- F. da Silva Veloso, C. Caleja, R. C. Calhelha, T. C. S. Pires, M. J. Alves, L. Barros, A. K. Genena, J. C. M. Barreira and I. C. F. R. Ferreira, Molecules, 2020, 25, 1481 CrossRef PubMed.
- Y. Wang, M. Huang, X. Yang, Z. Yang, L. Li and J. Mei, Korean J. Physiol. Pharmacol., 2018, 22, 409–417 Search PubMed.
- M. Huang, K. Wu, S. Zeng, W. Liu, T. Cui, Z. Chen, L. Lin, D. Chen and H. Ouyang, J. Inflamm. Res., 2021, 14, 1901–1913 Search PubMed.
- X. An, Y. Zhang, Y. Cao, J. Chen, H. Qin and L. Yang, Nutrients, 2020, 12, 1516 CrossRef CAS PubMed.
- Q. Wang, G. Ge, X. Liang, J. Bai, W. Wang, W. Zhang, K. Zheng, S. Yang, M. Wei, H. Yang, Y. Xu, B. Liu and D. Geng, Biomater. Sci., 2020, 8, 5157–5171 RSC.
- S. Talekar, A. F. Patti, R. Vijayraghavan and A. Arora, ACS Sustainable Chem. Eng., 2018, 6, 16363–16374 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |