Open Access Article
Open Access ArticleComparing conventional and novel extraction methods for chia seed mucilage as a sustainable vegan thickening agent†
Qiu Yi
Tan
a,
Divyang
Solanki
a,
Regis
Badin
ab and
Sangeeta
Prakash
 *a
*a
aSchool of Agriculture and Food Sustainability, The University of Queensland, St. Lucia, QLD 4072, Australia. E-mail: s.prakash@uq.edu.au
bUniversité de Lorraine, LIBio, Nancy, F-54000, France
First published on 27th December 2024
Abstract
Chia seed mucilage (CSM) is a remarkable food hydrocolloid with exceptional functional properties. However, variations in extraction conditions across studies impede reliable comparisons of CSM quality. This study investigates the influence of extraction conditions on the yield, physical properties, and functional properties of non-purified CSM. Three extraction methods – regular soaking (R), hot water soaking (H), and microwave-assisted (M) – were evaluated, with R serving as the control. The H method produced the highest yield (8.45 ± 0.22%), followed by M (5.76 ± 0.42%) and R (5.23 ± 0.21%). The CSM extracted via H yielded a darker colour and stronger tint than R and M's milky-white appearance. Regarding moisture content, R had the highest content (10.02 ± 0.82%), followed by M (8.4 ± 0.82%) and H (6.33 ± 0.42%). All samples displayed shear-thinning flow behaviour and viscoelastic properties, with M and H showing similar viscosity, while R demonstrated higher viscosity than both. The water holding capacity of H (117.03 ± 2.31 g g−1) and M (108.28 ± 1.37 g g−1) was significantly lower than that of R (152.88 ± 5.48 g g−1). The oil holding capacity varied significantly among R (29.32 ± 1.11 g g−1), H (18.15 ± 0.09 g g−1), and M (25.61 ± 0.8 g g−1). The emulsion capacity of R (91.74 ± 2.42%) was significantly higher than those of H (85.4 ± 2.54%) and M (92.97 ± 1.72%). Microwave-based CSM has shown the highest emulsion stability (96.71 ± 0.58%), followed by R (93.25 ± 0.46%) and H (92.97 ± 1.72%). The solubility of CSM did not differ significantly among the methods (78.1–82.48%). In conclusion, our findings emphasize the significant impact of extraction methods on the overall quality of CSM.
Sustainability spotlightThis is the first study which compared the extraction of non-purified CSM and its functional properties for microwave and other conventional methods in detail. Aiming at sustainable food ingredient development, use of non-thermal technologies like microwaves provides promising results as compared to conventional hot-water extraction-based methods. Crude or non-purified CSM can be a promising hydrocolloid with varied functional properties in food and non-food sectors. This crude CSM can replace many animal-based purified hydrocolloids envisaging promising revolution in plant-based food ingredients. This will provide an understanding for industrialists and researchers to select a suitable technique to extract a novel food hydrocolloid which is having a beneficial impact on food properties. This study focused on the utilization of sustainable technologies without the use of thermal treatments to extract CSM with minimal loss of nutritional qualities. This will open possibilities for developing new food ingredients, as well as processing, encapsulation and packaging applications for chia seeds. |
1. Introduction
Chia (Salvia hispanica L.), a plant species belonging to the Lamiaceae family, is known for its exceptionally high nutritional value. Generally, chia seeds contain significant amounts of carbohydrate (37–45%), fat (31–34%),1 dietary fibre (23–35%), and protein (16–26%).2 Upon hydration, chia seeds produce a fibre-rich transparent gel known as chia seed mucilage (CSM),3 which has demonstrated diverse functional properties, including acting as a fat replacer, stabiliser, emulsifier, and texture modifier.4–6There has been an increase in health concerns and demand for fat reduction in food products in recent years. The multifunctional nature of CSM positions it as a potential candidate for enhancing the textural attributes of various future food products. For example, maintaining the desirable texture while reducing the fat content in ice cream, which typically contains 10–16% fat, often results in undesirable textural characteristics, such as iciness, coarseness, and brittleness.7 However, CSM has shown promise in addressing these textural challenges, serving as an emulsifier and a stabiliser in ice cream,8 a fat replacer in cookies and biscuits9,10 and an additive in yogurt.8
CSM extraction involves three basic steps: hydration, separation, and recovery.4 However, the conditions for these steps vary widely among studies, including differences in the hydration time, temperature, pH of water, separation technique, and drying technique, making meaningful comparisons challenging. Recent reviews from Solanki et al.,9 Chiang et al.,4 and Mensah et al.10 have shown the clear impact of these parameters, along with the impact of non-thermal technologies on the CSM yield, composition, and functional properties. Overall, the higher extraction temperature and pH (alkaline) support the extraction yield. Previously, it has been confirmed that after 2 h of hydration, the total weight of the seeds remained constant following water absorption. The seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio also proved to affect the viscosity of the CSM.11 For example, Muñoz et al.12 used 2 hours of stirring at 20 °C with a 1
water ratio also proved to affect the viscosity of the CSM.11 For example, Muñoz et al.12 used 2 hours of stirring at 20 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 seed-to-deionized water ratio, whereas Punia & Dhull13 used 3 hours of stirring at 85 °C with a 1
40 seed-to-deionized water ratio, whereas Punia & Dhull13 used 3 hours of stirring at 85 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 seed-to-pH 6 adjusted water ratio. Additionally, Muñoz et al.12 dried CSM at 50 °C for 10 hours and sieved it through a 40-mesh screen, while Punia & Dhull13 filtered the fresh suspension through a 200-mesh cheesecloth, precipitated it with ethanol, and dried it at 45 °C for 12 hours before grinding it into a fine powder. Such variations hinder meaningful comparisons between the properties of CSM obtained through different methods. The functional properties of non-purified CSM varied based on many factors such as drying, seed
10 seed-to-pH 6 adjusted water ratio. Additionally, Muñoz et al.12 dried CSM at 50 °C for 10 hours and sieved it through a 40-mesh screen, while Punia & Dhull13 filtered the fresh suspension through a 200-mesh cheesecloth, precipitated it with ethanol, and dried it at 45 °C for 12 hours before grinding it into a fine powder. Such variations hinder meaningful comparisons between the properties of CSM obtained through different methods. The functional properties of non-purified CSM varied based on many factors such as drying, seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio, separation technique, temperature, pH, and genetic variety of seeds.9 Additionally, there is a lack of information on microwave-assisted extraction as there was only one study conducted using this method, which was by Sameera and Subba.14 Their study lacks insights into the proximate composition, functional properties of crude CSM, CLSM profiling, microstructure, and rheological properties. This is the first study that bridges the research gaps for the future use of non-purified CSM by comparing the extraction conditions with the same seed
water ratio, separation technique, temperature, pH, and genetic variety of seeds.9 Additionally, there is a lack of information on microwave-assisted extraction as there was only one study conducted using this method, which was by Sameera and Subba.14 Their study lacks insights into the proximate composition, functional properties of crude CSM, CLSM profiling, microstructure, and rheological properties. This is the first study that bridges the research gaps for the future use of non-purified CSM by comparing the extraction conditions with the same seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio. Recently, Solanki et al.9 mentioned the prospects of extracting crude CSM through various novel non-thermal technologies, which fosters this research in the direction of sustainable food ingredient production.
water ratio. Recently, Solanki et al.9 mentioned the prospects of extracting crude CSM through various novel non-thermal technologies, which fosters this research in the direction of sustainable food ingredient production.
This is the first study that bridges the research gaps for the future use of non-purified CSM by comparing the extraction conditions with the same seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio. Recent applications of non-purified CSM include complex coacervation of bioactive compounds in various food products to facilitate the efficiency of fortification as a carrier system.15 The complex coacervation process can be induced through electrostatic, intramolecular, or intermolecular interactions and combinations. Protein and polysaccharides create complex coacervates to encapsulate essential oils and bioactive compounds such as quercetin fortified in set yogurt.15 CSM is also used as a stabilizer in ice cream, as a fat replacer for bakery products, as an emulsifier, and for developing packaging films.10
water ratio. Recent applications of non-purified CSM include complex coacervation of bioactive compounds in various food products to facilitate the efficiency of fortification as a carrier system.15 The complex coacervation process can be induced through electrostatic, intramolecular, or intermolecular interactions and combinations. Protein and polysaccharides create complex coacervates to encapsulate essential oils and bioactive compounds such as quercetin fortified in set yogurt.15 CSM is also used as a stabilizer in ice cream, as a fat replacer for bakery products, as an emulsifier, and for developing packaging films.10
This research investigates the influence of various extraction methods on the yield, physical properties, and functional properties of non-purified CSM. This study is the first to systematically compare three extraction methods (microwave-assisted extraction, hot-water-based extraction, and regular soaking) with the same hydration ratios while considering their temperature differences. This study is the pioneer for observing CSM under confocal laser scanning microscopy and analysing non-purified CSM using Fourier transform infrared spectroscopy for crude CSM extracted through microwave treatment. By conducting a comprehensive analysis, we aim to elucidate the effects of temperature on CSM extraction and provide valuable insights into optimizing the extraction process to produce high-quality CSM for diverse food applications. In particular, the use of microwaves for CSM extraction gives future aspects for the commercialization of non-purified CSM. Our findings will address the gap in comparative studies on CSM extraction methods, enhancing the understanding of how different extraction conditions affect CSM functional properties.
2. Materials and methods
2.1 Materials
Chia seeds (Salvia hispanica L.) and sunflower oil were purchased from the Macro Wholefoods Market and MOI International (Australia), respectively. Analytical grade sodium hydroxide (NaOH), calcofluor white, rhodamine B, Nile red, and safranin O were obtained from Sigma-Aldrich (Australia). Deionised water was used for all the experiments.2.2 Methods
2.2.1.1 Regular soaking (control). The methodology for hydration, separation, drying, and storage of CSM was adapted from Tavares et al.16 and Feizi et al.17 To initiate hydration, whole chia seeds were soaked in water with a seed-to-water ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and a pH of 8 at a speed of 1000 rpm (IKA RW 20 Digital Model, Germany) for 1 hour and 40 minutes, followed by an additional 20 min at a higher speed of 1300 rpm to remove CSM from the surface of the hydrated seeds. Sodium hydroxide (NaOH) was added to adjust the pH, and a water bath (Thermoline Digital Water Bath, Australia) was utilised to control the temperature at 25 °C.
20 and a pH of 8 at a speed of 1000 rpm (IKA RW 20 Digital Model, Germany) for 1 hour and 40 minutes, followed by an additional 20 min at a higher speed of 1300 rpm to remove CSM from the surface of the hydrated seeds. Sodium hydroxide (NaOH) was added to adjust the pH, and a water bath (Thermoline Digital Water Bath, Australia) was utilised to control the temperature at 25 °C.
Subsequently, the suspension was subjected to ultracentrifugation (Beckman Coulter Centrifuge Avanti JXN-30, Australia) at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 20 minutes at room temperature (25 °C). The resulting supernatant, containing the mucilage layer, was collected. To facilitate preservation, the mucilage layer was frozen overnight at −20 °C and then subjected to freeze drying (BenchTop Pro with Omnitronics, SP Scientific, Gardiner, New York, USA) for 48 hours at −63.5 °C temperature and 55 μB vacuum. The dried mucilage was then sieved into powder using a 500 μm sieve and stored in a sealed container at −20 °C until further use. Sodium hydroxide (NaOH) was added to adjust the pH, and a water bath (Thermoline Digital Water Bath Model) was utilised to control the temperature at 25 °C.
000×g for 20 minutes at room temperature (25 °C). The resulting supernatant, containing the mucilage layer, was collected. To facilitate preservation, the mucilage layer was frozen overnight at −20 °C and then subjected to freeze drying (BenchTop Pro with Omnitronics, SP Scientific, Gardiner, New York, USA) for 48 hours at −63.5 °C temperature and 55 μB vacuum. The dried mucilage was then sieved into powder using a 500 μm sieve and stored in a sealed container at −20 °C until further use. Sodium hydroxide (NaOH) was added to adjust the pH, and a water bath (Thermoline Digital Water Bath Model) was utilised to control the temperature at 25 °C.
2.2.1.2 Hot water soaking. Whole chia seeds were soaked in water with a pH of 8, using a seed-to-water ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The suspension was subject to mechanical stirring for 2 hours at a high temperature of 80 °C, utilising a stirrer. NaOH was added to the water to adjust the pH, and the temperature was maintained using a water bath. The stirring speed was consistent with that of the control samples. Following the hydration process, the subsequent steps of ultracentrifugation, freezing, freeze-drying, and storage were performed in the same manner as described for the control sample (Section 2.2.1.1).
20. The suspension was subject to mechanical stirring for 2 hours at a high temperature of 80 °C, utilising a stirrer. NaOH was added to the water to adjust the pH, and the temperature was maintained using a water bath. The stirring speed was consistent with that of the control samples. Following the hydration process, the subsequent steps of ultracentrifugation, freezing, freeze-drying, and storage were performed in the same manner as described for the control sample (Section 2.2.1.1).
2.2.1.3 Microwave-assisted extraction. Whole chia seeds were immersed in water with a pH of 8, maintaining a seed-to-water ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. NaOH was added to adjust the pH of the water. The microwave-assisted extraction method was adapted from Sameera and Subba.14 The suspension was subjected to microwave treatment at level 10 power (1000 W) using a microwave (Samsung ME6104W1 Model, Malaysia) until the temperature reached 80 ± 2 °C. To ensure even heating, the suspension was gently stirred at 1 minute intervals, and the temperature was measured using a probe thermometer after each stir. Once the desired temperature was achieved, the suspension was mechanically stirred for 2 hours at room temperature (25 °C), with temperature control achieved using a water bath. The stirring speed was consistent with that of the control sample. Following that, the suspension underwent ultracentrifugation, freezing, freeze-drying and storage procedures, following the same methodology as the control sample (Section 2.2.1.1).
20. NaOH was added to adjust the pH of the water. The microwave-assisted extraction method was adapted from Sameera and Subba.14 The suspension was subjected to microwave treatment at level 10 power (1000 W) using a microwave (Samsung ME6104W1 Model, Malaysia) until the temperature reached 80 ± 2 °C. To ensure even heating, the suspension was gently stirred at 1 minute intervals, and the temperature was measured using a probe thermometer after each stir. Once the desired temperature was achieved, the suspension was mechanically stirred for 2 hours at room temperature (25 °C), with temperature control achieved using a water bath. The stirring speed was consistent with that of the control sample. Following that, the suspension underwent ultracentrifugation, freezing, freeze-drying and storage procedures, following the same methodology as the control sample (Section 2.2.1.1).
2.2.3.1 Yield. The freshly freeze-dried and sieved CSM powder was weighed. The yield of CSM was determined using the equation provided by Sameera and Subba.14
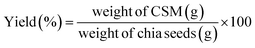 | (1) |
2.2.4.1 Colour. The colour analysis methods were adapted from Tiwari et al. with modification.19 The CSM powder was placed in a sample holder and measured using a colorimeter (Konica Minolta, CR-400, Japan). The parameters L*, a*, and b* were recorded in the CIELAB colour scale. The colour difference (ΔE), chroma (C), and hue angle (h°) were calculated using the following equations:
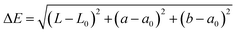 | (2) |
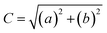 | (3) |
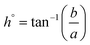 | (4) |
The colour difference value was assessed based on the values provided in Table 1 obtained from Campos et al.20
| Imperceptible | ΔE < 1 |
| Minimal | 1 ≤ ΔE ≤ 2 |
| Just perceptible | 2 ≤ ΔE ≤ 3 |
| Perceptible | 3 ≤ ΔE ≤ 5 |
| Strong difference | 5 ≤ ΔE ≤ 12 |
| Different colour | ΔE ≥ 12 |
2.2.4.2 Microstructure analysis.
2.2.4.2.1 Light microscopy. The CSM was rehydrated to a concentration of 0.3% (w/v) using a 0.02% safranin solution following the method described by Brütsch et al.18
The structure of CSM strands was observed using a light microscope (Olympus CX41 model, Japan) at 20× magnification. Images of observation were captured using a digital camera (Olympus U-CMAD3 Model, Tokyo, Japan).
2.2.4.2.2 Confocal laser scanning microscopy (CLSM). Due to the absence of images showing rehydrated CSM using CLSM, we employed methods adapted from Phan et al.21 to observe the structure of polysaccharides, fats, and proteins using CLSM. Undiluted calcofluor white, 0.05% Rhodamine B, and 0.2% Nile red were used to visualise starch, fats, and proteins, respectively. The CSM was rehydrated to a concentration of 1% (w/v) and observed at a magnification of 25×. To eliminate unwanted solid particles, the dispersion was subjected to centrifugation at 300×g for 30 minutes, and only the upper half of the centrifuged dispersion was collected for sampling. To stain the CSM, 100 μL of CSM was mixed with 5 μL of calcofluor white and 10 μL of Nile red and allowed to stand for 10 minutes. Subsequently, 10 μL of rhodamine B was added.
2.2.4.2.3 Field emission scanning electron microscopy (FE-SEM). FE-SEM was used to observe the morphology of the freeze-dried CSM powder.22 A small pinch of CSM powder was gently dropped on a double-sided carbon tape set on an SEM mount. The excess particles were then removed by gently tapping on the SEM mount. After that, samples were oven dried under vacuum at 30 °C overnight to outgas them. Samples were then coated using a Quorumtech 150TS platinum sputter coater (Quorum Technologies, Lewes, United Kingdom) under argon gas to achieve a platinum coating thickness of 15 nm. Finally, the samples were imaged at magnifications of 50× and 150× using a JEOL 7100 FE-SEM (JEOL Ltd, Akishima, Tokyo, Japan) with the voltage set to 5 kV and a probe current of 4 pA.
2.2.4.3 Moisture, protein, starch, and soluble sugar (soluble carbohydrate) contents. The moisture content of the CSM powder was determined following the procedure outlined in AOAC Official Method 925.09.23 The weight of the samples before and after drying at 100 °C was considered for the moisture content determination (eqn (5)).
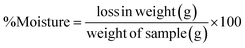 | (5) |
The protein content of Chia seed mucilage was determined using the Dumas method, in which the nitrogen content was analysed using a Leco CNS 928 and further multiplied to 6.25 to get the protein content.22,24
The determination of soluble carbohydrates and starches was done by following the principles of McCleary and Codd25 and Karkalas,26 which were hydrolysing soluble carbohydrates to reducing sugars and reacting with ferricyanide, breaking down starch enzymatically to glucose and reacting with the GOD/POD (glucose oxidase/peroxidase) reagent.
2.2.4.4 Rheological properties. Based on the method described by Silva et al.,6 the rheological evaluation involved rehydrating the CSM to a concentration of 1% (w/v). The rheological properties of the CSM were assessed using a probe rheometer (AR-G2, TA Instruments, New Castle-DE, USA) equipped with a parallel plate measuring 40 mm in diameter and a 100 μm gap. The measurements were conducted at a temperature of 25 °C.
2.2.4.4.1 Flow behaviour. The method for assessment of the flow behaviour of rehydrated CSM was adapted from Brütsch et al.18 The flow measurements were performed over a shear rate range spanning from 0.1 to 100 s−1. The obtained data were then fitted to the power law model to generate curves for comparison:
| σ = Kγn | (6) |
2.2.4.4.2 Viscoelasticity. The linear viscoelastic region (LVR) of the CSM samples was assessed using the procedure outlined by Brütsch et al.18 Amplitude sweeps ranging from 0.1 to 100% strain were applied at a frequency of 1 rad s−1. The method described by Silva et al.6 was followed for the frequency sweep test. The frequency range was set between 0.1 and 10 Hz, selecting values within the determined LVR, with a fixed strain of 1% strain.
2.2.5.1 Fourier transform infrared (FTIR) spectroscopy. Structural properties of the mucilage can be studied using FTIR spectroscopy. For this study, the structural-functional groups were characterized by FTIR spectroscopy using an Agilent Cary 630 FTIR spectrometer (USA) at absorbance wavelengths between 400 and 4000 cm−1 and a resolution of 4 cm−1 with 32 sample scans for each spectrum.6 Air was taken as a background to derive the spectra, and the functional groups were evaluated.
2.2.5.2 Water holding capacity (WHC) and oil holding capacity (OHC). The WHC and OHC were determined following the methods adapted from Wang et al.27 Initially, a Falcon tube and its lid were weighed. Then, 0.1 g of the sample was weighed into the tube, followed by the addition of 20 g of either deionised water or sunflower oil for WHC and OHC, respectively. The suspension was gently shaken and allowed to stand for 10 minutes to ensure the dissolution of CSM powder in the liquid. Subsequently, the suspension was centrifuged (Eppendorf 5702 R, Germany) at 3000×g for 30 minutes, and the weight of the supernatant was recorded. WHC and OHC were calculated using the following equations:
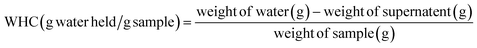 | (7) |
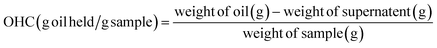 | (8) |
2.2.5.3 Emulsifying properties. The emulsifying properties were determined by following the methods from Sciarini et al.28 For this analysis, the CSM was rehydrated to a concentration of 0.25% (w/v) using deionised water, which was adjusted to pH 7 by adding NaOH.
2.2.5.3.1 Emulsion capacity (EC). 60 mL of rehydrated CSM was mixed with 6 mL of sunflower oil using a homogeniser (IKA Ultra Turrax T25 Digital, Germany) at 6600 rpm for 3 minutes. The resulting suspension was centrifuged for 10 minutes at 800×g, and the EC was calculated using the following formula:
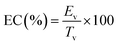 | (9) |
2.2.5.3.2 Emulsion stability (ES). To determine the stability of the emulsion against high temperatures, the emulsion formed was then subjected to heating by placing it in a water bath set at 80 °C for 30 minutes. After 30 minutes, the emulsion was centrifuged for 10 minutes at 800×g, and ES was calculated using the following formula:
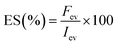 | (10) |
2.2.5.4 Solubility. In this study, the method described by Timilsena et al.29 was adapted to determine the solubility of CSM in water. A total of 0.3 g of CSM powder was rehydrated in 100 mL of deionised water, which had been adjusted to a pH of 7 using NaOH. Subsequently, the suspension was centrifuged at 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 15 minutes, and the resulting supernatant was collected in a separate beaker. 30 mL of the supernatant was weighed in an aluminium dish, which had been previously dried in an oven for 5 hours. The sample in the dish was then dried in an oven at 120 °C until a constant weight was obtained. The solubility of CSM was calculated as follows:
000×g for 15 minutes, and the resulting supernatant was collected in a separate beaker. 30 mL of the supernatant was weighed in an aluminium dish, which had been previously dried in an oven for 5 hours. The sample in the dish was then dried in an oven at 120 °C until a constant weight was obtained. The solubility of CSM was calculated as follows: | (11) |
3. Results and discussion
CSM is a plant-based hydrocolloid with a proven role in food and non-food industries. Consumers' need for plant-based food has aroused research interest in plant-seed mucilage, which can replace commercial hydrocolloids. This study emphasizes the impact of three different extraction methods on the physical and functional properties of crude CSM. In this study, hot water soaking extraction (H) and microwave-assisted extraction (M) were compared with regular soaking extraction (R).3.1 Yield
In this study, hot water extraction gave the significantly high (p < 0.05) CSM yield (8.45%), followed by M (5.76%) and R (5.23%), as presented in Table 2. The results of this study align with the findings of Timilsena et al.29,30 for the R method, although the studies do not specify the hydration temperature of CSM. Goh et al.,31 who employed a hydration temperature of 25 °C, reported a lower yield despite using similar hydration conditions as R, potentially due to additional precipitation or purification processes, as highlighted in a report of Timilsena et al.29 A significant reduction in yield due to ethanol precipitation in taro mucilage was also observed by Tosif et al.32 For the H method, the yield was comparable to that reported by da Silveira Ramos et al.33(7.9 ± 1.5%) and Sameera & Subba14 (8%), and both of them employed a hydration temperature of 80 °C. Sameera and Subba14 conducted CSM extraction using a microwave and reported a higher yield than the M method. As Sameera and Subba14 had mentioned, with the lack of study on the optimization of power levels for CSM extraction, the possible cause for the lower yield might be the power difference the seeds suspension was subjected to, which was 1.3 W g−1. In contrast, in this study, the power was fixed at 1000 W and not varied by watt per gram.| Parameter | R | H | M |
|---|---|---|---|
| a For ΔE value, the R method was set as a control for calculation. Superscript letters indicate significant differences (p < 0.05) in mean values between extraction methods, where the same letters in the same row indicate no significant difference (p > 0.05). | |||
| Yield (%) | 5.23 ± 0.21a | 8.45 ± 0.22b | 5.76 ± 0.42a |
| L* | 77.81 ± 0.59a | 71.74 ± 1.02b | 77.99 ± 1.53a |
| a* | 1.28 ± 0.16a | 2.37 ± 0.26b | 1.13 ± 0.22a |
| b* | 11.63 ± 0.58a | 13.45 ± 0.61b | 11.77 ± 0.75a |
| Chroma, C | 11.7 ± 0.59a | 13.66 ± 0.63b | 11.83 ± 0.76a |
| Hue angle (°) | 83.72 ± 0.23a | 79.99 ± 0.56b | 84.55 ± 0.41a |
| ΔE | — | 6.49 ± 1.11 | 1.03 ± 0.6 |
| Moisture (%) | 10.02 ± 0.82a | 6.33 ± 0.42b | 8.4 ± 0.82a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Composition | |||
| Soluble carbohydrates (% glucose equivalent) | 2.93 | 17.41 | 7.52 |
| Starch (%) | 0.36 | 0.74 | 0.41 |
| Total C (%) | 30.9 | 35.1 | 33.8 |
| Total N (%) | 1.01 | 2.07 | 1.19 |
| Crude protein (%) | 6.31 | 12.92 | 7.44 |
| Consistency index, K (Pa sn) | 1.66 ± 0.13 | 1.06 ± 0.51 | 1.18 ± 0.16 |
| Flow behaviour index, n | 0.36 ± 0.01 | 0.35 ± 0.03 | 0.38 ± 0.01 |
| WHC (g water/g CSM) | 152.88 ± 5.48a | 117.03 ± 2.31b | 108.28 ± 1.37b |
| OHC (g oil/g CSM) | 29.32 ± 1.11a | 18.15 ± 0.09b | 25.61 ± 0.8c |
| Emulsion capacity, EC (%) | 91.74 ± 2.42a | 85.40 ± 2.54b | 87.72 ± 0.49b |
| Emulsion stability, ES (%) | 93.25 ± 0.46a | 92.97 ± 1.72a | 96.71 ± 0.58b |
| Solubility (%) | 82.23 ± 4.93a | 82.48 ± 0.83a | 78.18 ± 2.60a |
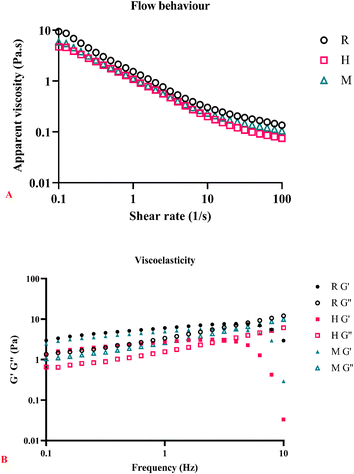
Our findings indicate that the H method yield is significantly (p < 0.05) more than that of R and M methods. This can be attributed to the lower viscosity of CSM at higher temperatures, facilitating easier separation from the seed coat during centrifugation, as noted by Silva et al.6 and Wang et al.27 Higher temperatures enhance the solubilisation of CSM polysaccharides in water, aligning with da Silveira Ramos et al.33 observations. Furthermore, Goh et al.31 noted that separation at room temperature with shear could only partially separate the gel from the seed. Fig. 1 shows the separation of CSM after centrifugation for each extraction method.
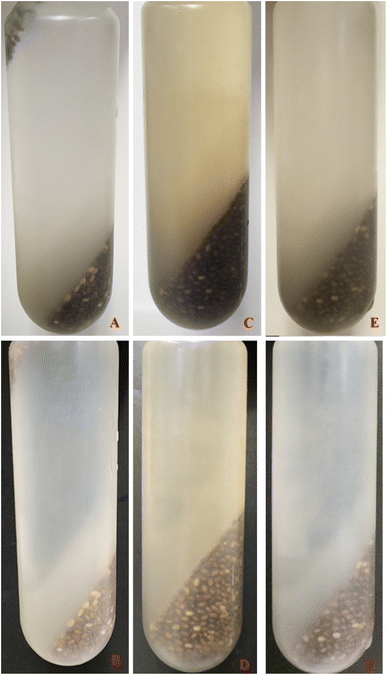 | ||
| Fig. 1 CSM separation after the centrifugation process for R, H, and M against a white and black background. R: A and B; H: C and D; M: E and F. | ||
As Goh et al.31 have suggested, the CSM from the R method did not completely separate from the seed (Fig. 1) at room temperature, with residual mucilage still attached, resulting in seeds being suspended in the upper layer of the supernatant. The R method exhibited the most visible residual mucilage around the seeds, followed by M and H. The presence of residual mucilage on the seed likely affected the yield of CSM, as its weight is not included in the final CSM mass.
The yields for the R and M methods were similar to those reported by Fernandes and Salas-Mellado,34 who obtained 5.81 g of CSM using a freeze-drying method. Contrary to our findings, Tosif et al.32 reported lower yields for taro mucilage at higher temperatures than room temperature extractions, suggesting that growth conditions affecting the taro composition could contribute to the difference in the mucilage yield. Their previous research indicated that acidic and alkaline conditions produced higher yields than water extraction due to variations in monosaccharide structures.35 These findings align with previous research, indicating that a higher pH and lower temperature produce higher yields.20,36
Our findings demonstrate that temperature has significant impacts on the yield of CSM when using the same hydration duration, seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio, pH, extraction method and drying conditions. Specifically, higher temperatures result in higher yields, and microwave-assisted extraction does not significantly affect the yield. Santos et al.37 reported that higher drying temperatures (50 °C, 60 °C and 70 °C) produced more desirable mucilage regarding physicochemical properties, aligning with our results.
water ratio, pH, extraction method and drying conditions. Specifically, higher temperatures result in higher yields, and microwave-assisted extraction does not significantly affect the yield. Santos et al.37 reported that higher drying temperatures (50 °C, 60 °C and 70 °C) produced more desirable mucilage regarding physicochemical properties, aligning with our results.
Our results highlight the importance of temperature in CSM extraction, with higher temperatures resulting in greater yields. These findings provide critical insights for optimising CSM extraction processes to achieve high-quality CSM for various food applications.
3.2 Colour
As presented in Table 2, there is no significant difference in the colour of extracted CSM between the R and M methods, while the H method differs significantly from both. In particular, R and M exhibit higher L* values but lower a* and b* values than H. Our findings indicate that extraction at higher temperatures, as observed with H, leads to a decrease in the L* value and an increase in the a* and b* values. Additionally, our results demonstrate that higher temperatures increase the chroma (C) value and decrease the hue angle. Specifically, the H method exhibits significantly higher chroma, indicating a more intense colour but a lower hue angle than R and M. This suggests that H has a lower level of greyness than R and M. The colour difference (ΔE) between H and R is substantial, with a value of 6.49, whereas the difference between M and R, with an ΔE of 1.03 (Table 1). Fig. 2 displays images of CSM powder extracted using the three different methods. | ||
| Fig. 2 CSM powder. R, H, and M are represented from left to right. A, B, and C show samples in containers used for colorimetry measurements, while D, E, and F show samples in metal dishes. | ||
The R samples have a lower L* value but higher a* and b* values compared to the findings of Timilsena et al.,29 who reported the colour profile for purified chia seed polysaccharides. The difference could be attributed to high-speed stirring during hydration in our samples, which may have caused the breakdown of micro-sized seed coats into the suspension. Similarly, Farahnaky et al.38 suggested that microscopic shearing forces generated by sonication could damage the chia seed coat, allowing more natural pigment to diffuse into the aqueous solution and resulting in a darker CSM powder. The colour of mucilage can be attributed to Maillard browning39 or alkaline conditions.40 When comparing our findings to the 80 °C heat extraction described by Wang et al.27 the H samples exhibit lower lightness. Koocheki et al.41 proposed that the colour of mucilage can be influenced by the presence of impurities (such as pigments or tannic substances) from the seed coat. They claimed that longer extraction times and higher temperatures caused more colour to diffuse into the water, resulting in a darker CSM powder. According to the report of Campos et al.,20 the extraction temperature has a pronounced effect on the colour of CSM, along with the seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio and a longer extraction time.
water ratio and a longer extraction time.
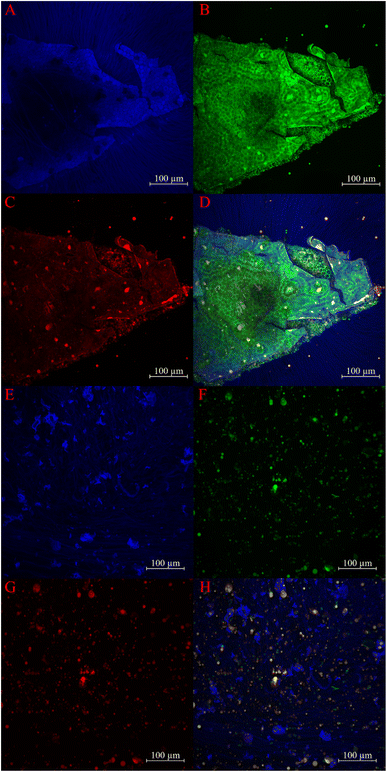
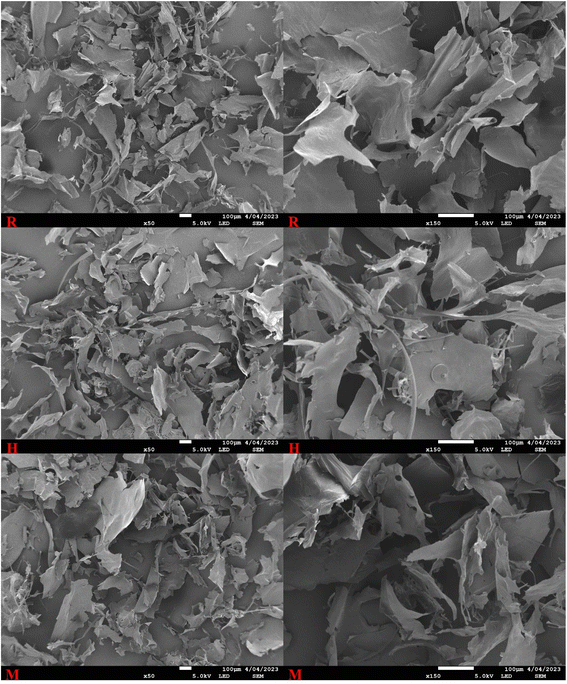
3.3 Light microscopy, CLSM and SEM
The microstructure of rehydrated CSM and hydrated chia seeds was examined using light microscopy, CLSM and SEM. The following analysis (Fig. 3–5) provides detailed observations of these structures, comparing the findings with previous studies and evaluating the effects of various extraction methods.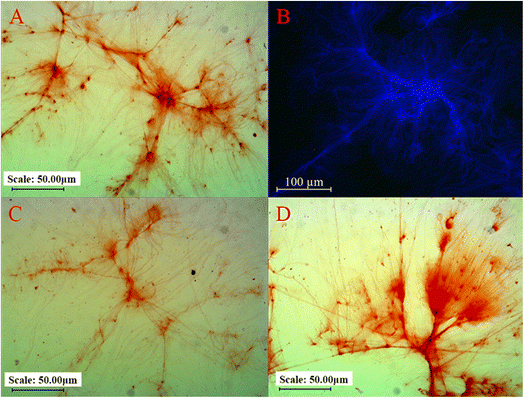 | ||
| Fig. 3 Light microscopy images of rehydrated CSM at 20× magnification: (A) R, (C) H, and (D) M. (B) CLSM image of rehydrated R at 25× magnification. | ||
A suspension of ground chia seeds hydrated with water was also examined under a CLSM to validate these observations. The suspension displayed traces of starch, protein and fat, represented by blue, green, and red colours, respectively (Fig. 4E–H). This indicates that the extracted CSM mainly comprises starch, with calcofluor white used as a starch-specific stain. Further examination of a hydrated fragment of crushed chia seeds (Fig. 4A–D) showed that the polysaccharide strands extending from the seed fragment consisted solely of starch, aligning with the absence of proteins and fats in the extracted CSM. The hexagonal structures observed in the seed fragments align with SEM observations by Muñoz et al.36 and Salgado-Cruz et al.42
The findings suggest that different extraction methods have minimal impact on the overall network structure of CSM. Microwaves lead to the loss of the curly, thinly cracked laminar structure of CSM, which is observed in the present study as in the study on flaxseed gum.44 This can be attributed to the partial changes in the structure of the microwave-based CSM as compared to regular soaking.44 The detailed analysis confirms the predominance of starch in CSM, with no significant presentation of fats or proteins, highlighting the consistency of these observations across different microscopy techniques.
3.4 Moisture, protein, and soluble sugar (soluble carbohydrate) contents
Moisture content refers to the presence of water within a sample. The moisture content of R and M is 10.02% and 8.4%, respectively, showing no significant difference between them (Table 2). However, sample H exhibits a significantly lower moisture content at 6.33% compared to both R and M. Our results align with the literature that calculates moisture on a wet basis. For instance, Fernandes and Salas-Mellado34 reported a similar moisture content of 10.74 ± 0.29% for freeze-dried CSM. Our results are closer to those reported by Solanki et al.22 who extracted and separated crude CSM through a novel technique.Timilsena et al.29 reported much lower moisture (3.9% on a dry basis) for CSM using the same seed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio, hydration duration and drying method as employed in this study, although with a neutral pH and additional purification process. The lower moisture content in their study could be attributed to the extended drying time and removal of fat, protein, and insoluble cell walls (i.e., cellulose, hemicellulose, and lignin) during purification.29 The variation in the moisture content between our CSM and theirs suggests that different components of CSM influence moisture levels. Factors such as seed origin, extraction conditions, and methods can significantly affect the CSM composition.45,46 For H, which underwent high-temperature treatment, the lower moisture content might result from the disruption of polysaccharide, protein, and fat structures during the hydration process, reducing their water-retention capacity.
water ratio, hydration duration and drying method as employed in this study, although with a neutral pH and additional purification process. The lower moisture content in their study could be attributed to the extended drying time and removal of fat, protein, and insoluble cell walls (i.e., cellulose, hemicellulose, and lignin) during purification.29 The variation in the moisture content between our CSM and theirs suggests that different components of CSM influence moisture levels. Factors such as seed origin, extraction conditions, and methods can significantly affect the CSM composition.45,46 For H, which underwent high-temperature treatment, the lower moisture content might result from the disruption of polysaccharide, protein, and fat structures during the hydration process, reducing their water-retention capacity.
Contrary to our findings, Wang et al.27 reported that temperature and extraction time did not significantly affect the moisture content of their CSM samples. Similarly, Hussain et al.47 found no significant influence of temperature and agitation speed on the moisture content of Ocimum basilicuum L. mucilage during hydration. The discrepancy between our results and these studies might be due to the relatively modest temperature difference (20–30 °C) and short hydration duration (≤1 hour) in their experiments, which may not have been sufficient to produce a significant difference in the moisture content. The minimal difference in moisture content between R and M further supports this, as M was heated to 80 °C for a very short duration, while the significant temperature difference (55 °C) and extended heating time (2 hours) between R and H likely contributed to the lower moisture content in H.
Sample H had the highest soluble carbohydrate, starch, and crude protein content, followed by M and then R (Table 2). The crude protein % of H was comparable to white-spotted CSM but significantly higher than black-spotted CSM reported by Muñoz et al.,36 which were 12.84 ± 0.13% and 9.54 ± 1.48%, respectively. The glucose equivalent percentage in H was similar to that of purified CSM, which was reported by Timilsena et al.29 as 19.6 ± 3.2%. Comparatively, Silva et al.6 determined that the total protein and carbohydrate contents on a dry basis ranged between 16.15–25.2% and 66.83–78.9%, respectively. Their study indicated that a longer stirring time or extended exposure to ultrasound increased the protein content but decreased the carbohydrate content. This finding is further supported by comparing the glucose equivalent percentage of H with that of da Silveira Ramos et al.,33 who reported a higher glucose content of 26.6% with a shorter hydration time.
Both R and M had significantly (p < 0.05) lower protein and soluble carbohydrate contents compared to H, although M showed slightly higher values than R. This suggests that both heat and microwave treatments can extract more protein and soluble carbohydrates from the seeds into suspension. Although H had the most nutritious composition, its functionality might be compromised due to the denaturation of structures, which will be discussed in the following sections.
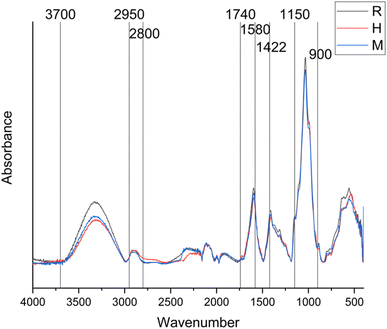
3.5 FTIR spectroscopy
FTIR spectroscopy was employed to investigate the molecular composition and structural differences, if any, among differently extracted CSM. Fig. 6 shows the FTIR spectra, where the absorbance of R, H and M samples was plotted against wavenumbers ranging from 4400 to 400 cm−1. Table 3 presents the specific absorption bands corresponding to molecular vibrations at certain wavenumbers, as referenced in Ellerbrock et al.48 and Muñoz et al.12| Wavenumber (cm−1) | Bands |
|---|---|
| 3700–2700 | O–H stretch |
| 2950–2800 | C–H stretch symmetric |
| 2800–2700 | C–H asymmetric |
| 1740–1698 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch O stretch |
| 1680–1580 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch C stretch |
| 1422 | C–H, C–O–H (carbohydrates) |
| 1150–900 | C–O–C |
Analysis of Fig. 6 indicates that the FTIR spectra for all three samples (R, H, and M) are relatively similar, with a major variation in the peaks within the wavenumber range of 3700 to 2950 cm−1, corresponding to the O–H stretch. This observation suggests that thermal exposure and microwave treatment might influence the quantity of hydroxyl groups extracted into the CSM or could cause partial degradation of these hydroxyl groups under harsh conditions. The variations in the hydroxyl group content might affect the functional properties of CSM depending on the extraction method employed. Furthermore, differences in the moisture content among the CSM samples could impact the O–H stretch region, as residual moisture can interfere with the FTIR spectrum.49
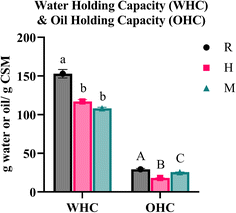
3.6 WHC and OHC
The water holding capacity (WHC) refers to the maximum quantity of water absorbed by a rehydrated sample under the influence of an external force, while the oil holding capacity (OHC) represents the amount of oil absorbed through nonpolar sites on the sample.50,51 According to Table 2, the WHC values for samples R, H and M are 152.88 g g−1, 117.03 g g−1 and 108.28 g g−1, respectively, while the OHC values are 29.32 g g−1, 18.15 g g−1, and 25.61 g g−1, respectively. These results are presented in Fig. 7.Interestingly, the results of WHC and OHC of this study align with the observations of Segura-Campos et al.52 for WHC (103.2 g g−1) and OHC (25.79 g g−1). Additionally, our WHC values slightly exceed those reported by Punia and Dhull,13 who attributed the water-holding capabilities of CSM to the presence of free hydroxyl groups capable of forming bonds with water molecules. Compared with the study of Wang et al.,27 our WHC was tremendously higher as their highest reported WHC was only 18.52 to 39.39 g g−1; however, our OHC findings were similar to their reported range of 26.23 to 34.31 g g−1.
In contrast, Coorey et al.50 reported approximately twice the WHC and OHC values compared to our findings. The differences can be attributed to differences in the extraction process. Coorey et al.50 only collected the middle layer after centrifugation. In contrast, we collected the supernatant's layers (top layer and middle layer), based on previous trials, indicating a significant amount of CSM in the top layer. The lower WHC observed in our findings could be due to the lower concentration of gel fibres in the top layer, which appeared less viscous during the extraction (Fig. 3). Ragab et al.53 explained that fibres and protein are crucial for water binding; thus, higher fibre and protein contents typically increases the WHC. Our lower WHC might result from a reduced concentration of gel fibres.
The significant differences in WHC and OHC values across the three extraction methods suggest that heat and microwave treatments can alter the interaction between CSM fibres and water. Factors such as ingredient–water interactions, hydration positions, and protein configurations can affect the WHC.54 Exposure to heat generally decreases the WHC, whereas a higher concentration of polysaccharides/soluble fibres and proteins increases it.27,29 A study on the basil seed gel by Hussain et al.47 suggested that impurities such as fat, protein, fibre, and natural pigments could impact water absorption, thereby altering the WHC. Regarding OHC, factors such as fat content, particle size, protein composition and hemicellulose influence CSM's ability to entrap oils, thereby affecting the OHC.27,55 The compositional differences, resulting from the degree of seed coat shedding, influenced by the extraction method, could account for the observed variations in WHC and OHC between the different extraction methods. ESI Fig. 1† displays a series of images illustrating the before and after stages of WHC and OHC analyses. The separation line between CSM and oil is clearly defined in all OHC analyses. However, in the WHC analysis, a distinct separation is only noticeable in R, less prominent in H, and indiscernible in M. This pattern is consistent across all replicates. The lack of clear separation in sample M could affect the reliability of the results, necessitating further investigation to gain a better understanding of these observations and to refine the extraction methods to optimise the WHC and OHC of CSM. This will help understand how different CSM components interact with water and oil, contributing to its functional properties in various applications.
3.7 Rheological properties
Rheology is employed to study and understand the flow and deformation characteristics of substances. Rheological properties are determined by observing the deformation over time in relation to the applied stress.56Furthermore, Timilsena et al.30 observed that the purified CSM at the concentration of 0.05% (w/v) and below exhibit Newtonian behaviour at low shear rates, but a significant decrease in viscosity is observed when approaching high shear rates. However, this Newtonian plateau is absent for concentrations above 0.1% w/v. In line with their results, our samples do not display any plateau. Similar behaviour was reported for taro mucilage, where Newtonian flow behaviour was observed at a concentration below 10%.32
Contrary to the studies conducted by Tavares et al.16 and Wang et al.,27 our findings indicate that exposure to heat (H and M) still exhibits shear-thinning and pseudoplastic behaviour. The high n value observed for heat extraction can be attributed to the degradation of proteins and polysaccharide chains during extraction.16 However, our samples exhibit a low n value, suggesting that the polysaccharide chains may only be partially degraded and have retained enough structure to maintain non-Newtonian flow behaviour. A more comprehensive investigation into the degradation rate and interaction of polysaccharide chains is required to fully understand these findings. Fig. 8A shows that R has a higher apparent viscosity than H and M. Unlike CSM, basil seed mucilage studied by Hussain et al.47 demonstrated that the extraction temperature and processing did not affect the viscosity. In fact, a higher extraction temperature resulted in a more viscous basil seed gel, although the difference was insignificant.
At lower frequencies, G′ > G′′ indicates that the structure of CSM consists of a weak network of polysaccharides. Goh et al.31 proposed that a weak transient gel network is formed during the dispersion of swollen microgel particles within a polysaccharide solution. A significant G′ decrease is observed at higher frequencies, particularly in sample H. This could be due to the formation of a fragile network in the dispersion.58 Brütsch et al.18 suggested that the stability of the gel decreases when the freeze-drying method is employed, as the formation of water crystals during freezing can impact the network structure.
Sample H exhibits the most pronounced drop in G′ after crossing over with G′′. Brütsch et al.18 mentioned that protein denaturation from drying at 50 °C could affect its structure and functionality. Similarly, Solanki et al.22 also observed the impact of high temperature of drying on viscoelastic properties of crude CSM. Hence, the more significant drop in G′ observed in sample H could be due to structural damage caused by heat. The crossover point first occurs in sample H, followed by M, and then R. Capitani et al.57 suggested that the crossover point is related to the disruption of the CSM structure. Mezger59 stated that crossover points occurring at higher frequencies could be contributed by low molar mass, which causes changes in the molecular arrangement. However, our findings show the opposite trend, where sample R (assuming its structure is most intact, resulting in the highest average molar mass) has a crossover point at a higher frequency than H and M, which were subjected to heating.
The results regarding the viscoelasticity of CSM have yet to be consistent across different literature sources, indicating the need for more comprehensive research to better understand the factors influencing its rheological properties.
3.8 Emulsion properties
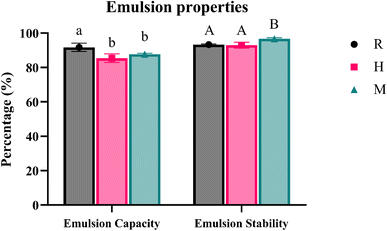 | ||
| Fig. 9 EC and ES in % for different extraction methods, where R is regular soaking, H is hot-water soaking, and M is microwave-assisted extraction. | ||
Our results indicate that sample R has significantly higher EC than H and M, while sample M has significantly higher ES than samples R and H. The differences in EC and ES can be attributed to compositional variations between the samples. Sciarini et al.28 suggested that a higher protein content could enhance the emulsion activity. They found that different dispersion concentrations affected EC and ES results for Gleditsia triacanthos gum, indicating that different extraction methods perform better at different concentrations. This could explain the differences observed among our samples.
It was expected that CSM extracted at higher temperatures would have lower stability due to a reduced quantity of the polysaccharide content.20,27 However, our findings did not align with these expectations, as samples R and H did not show a significant difference in ES. Wang et al.27 reported that their CSM samples contained 5.1–5.7% protein content, which might contribute to the surface activity and stability of emulsions. Coorey et al.50 suggested that temperature-dependent changes in configuration and orientation within the water–oil interphase could influence CSM's ability to form a viscous continuous phase, thereby increasing the stability of the water-based emulsion. These findings suggest that differences in ES may be influenced by the protein content (presence of seed coat) and the quantity and type of polysaccharide molecules present in CSM. Further studies are required to understand these interactions and optimise extraction methods to enhance emulsion properties.
3.9 Solubility
In our study, the solubility values for samples R, H and M were 82.23%, 82.48% and 78.18%, respectively. Statistical analysis indicated no significant difference (p > 0.05) among the different CSM samples (Table 2). These findings differ from those reported by Timilsena et al.,29 who observed a solubility of approximately 94% for CSM at pH 7 and hydrated at 20 °C. The higher solubility observed by Timilsena et al.29 can likely be attributed to their use of purified gum, whereas our study utilised less processed CSM. Solubility in this study was found to be better than that in the previous report from Solanki et al.22 Furthermore, Solanki et al.9,22 also mentioned the influence of purification on the solubility of CSM.Additionally, our results contrast with those of Muñoz et al.,12 who reported a solubility of about 68% for CSM at temperatures ranging from 30 to 60 °C, with a peak solubility of 80.65% at 70 °C, decreasing to 66.78% at 80 °C. Their findings were lower than ours, possibly due to differences in temperature conditions and experimental protocols. Sciarini et al.28 also observed significant differences in solubility for Gleditsia triacanthos gum at low temperatures (30 °C), with solubility values ranging from 20% for overnight swollen-extracted CSM to approximately 40% for NaOH-treated extracted gum, and approximately 55% for hot water-extracted gum. These results highlight that CSM exhibits superior solubility compared to Gleditsia triacanthos gum.
Furthermore, Wang et al.27 found that the extraction temperature and duration significantly affect the solubility. They observed a solubility of approximately 70% for CSM extracted at 80 °C for 60 minutes at pH 7. This significantly differed from other extraction conditions (50 °C for 30 minutes, 50 °C for 60 minutes, and 80 °C for 30 minutes), which yielded 76% to 80% solubility. The variation in results may be due to differences in chia seed origin and the use of proteinase during extraction and the purification processes. Higher purity CSM might result in larger differences in solubility depending on the extraction method employed. Further investigation is required into the correlation between extraction methods and full solubility in CSM.
4. Conclusion
In conclusion, different extraction methods significantly influence the quality of CSM, except for solubility. Our findings indicate that prolonged exposure to heat at 80 °C during hydration of CSM (hot water-soaked extraction) increases the yield of CSM but potentially leads to polysaccharide degradation, which alters its physical and functional properties. While a higher yield is desirable, the associated changes, such as reduced viscosity and water-holding capacity, are undesirable for CSM's use as a functional ingredient. Conversely, short-term exposure to heat at 80 °C, as seen with microwave-assisted extraction, does not yield the same effects. Additionally, microwaves have been shown to enhance certain properties, such as emulsion stability, although they still reduce water and oil holding capacities. Therefore, using heat to facilitate the separation of CSM from seed coat requires further assessment regarding its effectiveness and suitability for producing high-quality CSM. Non-thermal technologies like microwaves can open a bright future for preparing and processing chia seed-based ingredients. However, further study is necessary to determine the impact of varying power, temperature, and seed ratio on microwave-assisted extracted CSM and its effect on gelling and other functional properties. Our study concludes that the regular extraction method (R) generally yields more favourable functional properties than both hot-water (H) and microwave-assisted (M) methods, except in terms of emulsion stability and solubility.Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts to declare.References
- V.-O. Alfredo, R.-R. Gabriel, C.-G. Luis and B.-A. David, LWT–Food Sci. Technol., 2009, 42, 168–173 CrossRef.
- K. Marcinek and Z. Krejpcio, Rocz. Panstw. Zakl. Hig., 2017, 68, 123–129 Search PubMed.
- S. Ribes, N. Peña, A. Fuentes, P. Talens and J. M. Barat, J. Dairy Sci., 2021, 104, 2822–2833 CrossRef PubMed.
- J. H. Chiang, D. S. M. Ong, F. S. K. Ng, X. Y. Hua, W. L. W. Tay and C. J. Henry, Trends Food Sci. Technol., 2021, 115, 105–116 CrossRef.
- L. L. Garcia e Silva, R. Alves Bastos, G. V. Souza Lima, L. de Souza Soares, J. Selia dos Reis Coimbra, M. Arêdes Martins and R. de Castro Santana, Food Biophys., 2022, 17, 568–574 CrossRef.
- L. A. Silva, P. Sinnecker, A. A. Cavalari, A. C. K. Sato and F. A. Perrechil, Food Chem. Adv., 2022, 1, 100024 CrossRef.
- M. Akbari, M. H. Eskandari and Z. Davoudi, Trends Food Sci. Technol., 2019, 86, 34–40 CrossRef CAS.
- D. S. Atik, T. Demirci, H. İ. Öztürk, S. Demirci, D. Sert and N. Akın, Braz. Arch. Biol. Technol., 2020, 63, e20190702 CrossRef CAS.
- D. Solanki, I. Oey, S. Prakash, B. Bhandari and J. K. Sahu, Sustainable Food Technol., 2024, 2, 993–1010 RSC.
- E. O. Mensah, E. O. Oludipe, Y. H. Gebremeskal, L. A. Nadtochii and D. Baranenko, Food Hydrocolloids, 2024, 153, 110051 CrossRef.
- K. Saporittis, J. Marasco, R. Morales, M. J. Martinez and A. M. Pilosof, J. Sci. Food Agric., 2023, 103, 3860–3870 CrossRef PubMed.
- L. A. Muñoz, N. Vera C, M. C. Zúñiga-López, M. Moncada and C. M. Haros, J. Food Compos. Anal., 2021, 104, 104138 CrossRef.
- S. Punia and S. B. Dhull, Int. J. Biol. Macromol., 2019, 140, 1084–1090 CrossRef PubMed.
- N. Sameera and D. Subba Rao, Int. J. Eng. Res. Ind. Appl., 2020, V9 Search PubMed.
- M. R. I. Shishir, H. Suo, F. S. Taip and K.-W. Cheng, Food Chem., 2024, 456, 139818 CrossRef PubMed.
- L. S. Tavares, L. A. Junqueira, Í. C. De Oliveira Guimarães and J. V. De Resende, J. Food Sci. Technol., 2018, 55, 457–466 CrossRef PubMed.
- R. Feizi, K. K. T. Goh and A. N. Mutukumira, Int. Dairy J., 2021, 120, 105087 CrossRef.
- L. Brütsch, F. J. Stringer, S. Kuster, E. J. Windhab and P. Fischer, Food Funct., 2019, 10, 4854–4860 RSC.
- A. Tiwari, G. Singh, V. Sharma, R. K. Srivastava and S. Sharma, LWT, 2021, 150, 111913 CrossRef.
- B. E. Campos, T. Dias Ruivo, M. R. da Silva Scapim, G. S. Madrona and R. C. Bergamasco, LWT–Food Sci. Technol., 2016, 65, 874–883 CrossRef CAS.
- J. L. Phan and R. A. Burton, in Annual Plant Reviews online, 2018, pp. 63–104, DOI:10.1002/9781119312994.apr0606.
- D. Solanki, P. Dhungana, Q. Y. Tan, R. Badin, B. Bhandari, J. K. Sahu and S. Prakash, Food Hydrocolloids, 2024, 110342, DOI:10.1016/j.foodhyd.2024.110342.
- W. Horwitz, Official methods of analysis of AOAC International. Volume I, agricultural chemicals, contaminants, drugs, ed. W. Horwitz, AOAC International, Gaithersburg, Maryland, 2010 Search PubMed.
- T. Kaewmanee, L. Bagnasco, S. Benjakul, S. Lanteri, C. F. Morelli, G. Speranza and M. E. Cosulich, Food Chem., 2014, 148, 60–69 CrossRef CAS.
- B. V. McCleary and R. Codd, J. Sci. Food Agric., 1991, 55, 303–312 CrossRef CAS.
- J. Karkalas, J. Sci. Food Agric., 1985, 36, 1019–1027 CrossRef CAS.
- W.-H. Wang, C.-P. Lu and M.-I. Kuo, Processes, 2022, 10, 519 CrossRef CAS.
- L. S. Sciarini, F. Maldonado, P. D. Ribotta, G. T. Pérez and A. E. León, Food Hydrocolloids, 2009, 23, 306–313 CrossRef.
- Y. P. Timilsena, R. Adhikari, S. Kasapis and B. Adhikari, Carbohydr. Polym., 2016, 136, 128–136 CrossRef PubMed.
- Y. P. Timilsena, R. Adhikari, S. Kasapis and B. Adhikari, Int. J. Biol. Macromol., 2015, 81, 991–999 CrossRef.
- K. K. T. Goh, L. Matia-Merino, J. H. Chiang, R. Quek, S. J. B. Soh and R. G. Lentle, Carbohydr. Polym., 2016, 149, 297–307 CrossRef.
- M. M. Tosif, A. Najda, J. Klepacka, A. Bains, P. Chawla, A. Kumar, M. Sharma, K. Sridhar, S. P. Gautam and R. Kaushik, Polymers, 2022, 14, 1163 CrossRef.
- I. F. da Silveira Ramos, L. M. Magalhães, C. do O Pessoa, P. M. Pinheiro Ferreira, M. dos Santos Rizzo, J. A. Osajima, E. C. Silva-Filho, C. Nunes, F. Raposo, M. A. Coimbra, A. B. Ribeiro and M. P. Costa, Ind. Crops Prod., 2021, 171, 113981 CrossRef.
- S. S. Fernandes and M. d. l. M. Salas-Mellado, Food Chem., 2017, 227, 237–244 CrossRef PubMed.
- M. M. Tosif, A. Najda, A. Bains, T. C. Krishna, P. Chawla, M. Dyduch-Siemińska, J. Klepacka and R. Kaushik, Int. J. Mol. Sci., 2021, 22, 13548 CrossRef PubMed.
- L. A. Muñoz, A. Cobos, O. Diaz and J. M. Aguilera, J. Food Eng., 2012, 108, 216–224 CrossRef.
- F. S. d. Santos, R. M. F. d. Figueirêdo, A. J. d. M. Queiroz, Y. F. Paiva, H. V. Moura, E. T. d. V. Silva, J. P. d. L. Ferreira, B. A. d. Melo, A. J. d. B. A. Carvalho and M. d. S. Lima, Foods, 2023, 12, 569 CrossRef PubMed.
- A. Farahnaky, S. Bakhshizadeh-Shirazi, G. Mesbahi, M. Majzoobi, E. Rezvani and G. Schleining, Innovative Food Sci. Emerging Technol., 2013, 20, 182–190 CrossRef.
- K. Y. Qian, S. W. Cui, Y. Wu and H. D. Goff, Food hydrocolloids, 2012, 28, 275–283 CrossRef.
- B. T. Amid and H. Mirhosseini, Food Chem., 2012, 132, 1258–1268 CrossRef PubMed.
- A. Koocheki, A. R. Taherian, S. M. Razavi and A. Bostan, Food Hydrocolloids, 2009, 23, 2369–2379 CrossRef.
- M. d. l. P. Salgado-Cruz, G. Calderón-Domínguez, J. Chanona-Pérez, R. R. Farrera-Rebollo, J. V. Méndez-Méndez and M. Díaz-Ramírez, Ind. Crops Prod., 2013, 51, 453–462 CrossRef.
- A. M. G. Darwish, R. E. Khalifa and S. A. El Sohaimy, Alexandria Sci. Exch. J., 2018, 39, 450–459 CrossRef.
- X. Yu, S. Huang, F. Yang, X. Qin, C. Nie, Q. Deng, F. Huang, Q. Xiang, Y. Zhu and F. Geng, Food Hydrocolloids, 2022, 125, 107447 CrossRef.
- M. I. Capitani, S. M. Nolasco and M. C. Tomás, Food Hydrocolloids, 2016, 61, 537–546 CrossRef.
- C. de Campo, P. P. dos Santos, T. M. H. Costa, K. Paese, S. S. Guterres, A. d. O. Rios and S. H. Flôres, Food Chem., 2017, 234, 1–9 CrossRef.
- N. Hussain, I. Ishak, M. F. Abdullah, A. Abd Rauh and N. Azhar, Malays. Appl. Biol., 2019, 48, 97–101 Search PubMed.
- R. H. Ellerbrock, M. A. Ahmed and H. H. Gerke, J. Plant Nutr. Soil Sci., 2019, 182, 888–895 CrossRef.
- X. Zhang, A. He, R. Guo, Y. Zhao, L. Yang, S. Morita, Y. Xu, I. Noda and Y. Ozaki, Spectrochim. Acta, Part A, 2022, 265, 120373 CrossRef.
- R. Coorey, A. Tjoe and V. Jayasena, J. Food Sci., 2014, 79, E859–E866 CrossRef PubMed.
- B. L. Olivos-Lugo, M. Á. Valdivia-López and A. Tecante, Food Sci. Technol. Int., 2010, 16, 89–96 CrossRef PubMed.
- M. R. Segura-Campos, N. Ciau-Solís, G. Rosado-Rubio, L. Chel-Guerrero and D. Betancur-Ancona, Agric. Sci., 2014, 2014 Search PubMed.
- D. M. Ragab, E. E. Babiker and A. H. Eltinay, Food Chem., 2004, 84, 207–212 CrossRef.
- N. R. Galla and G. R. Dubasi, Food Hydrocolloids, 2010, 24, 479–485 CrossRef.
- M. R. Segura-Campos, N. Ciau-Solís, G. Rosado-Rubio, L. Chel-Guerrero and D. Betancur-Ancona, Int. J. Food Sci., 2014, 2014, 241053 Search PubMed.
- S. Kasapis and A. Bannikova, in Advances in Food Rheology and Its Applications, Elsevier, 2017, pp. 7–46 Search PubMed.
- M. I. Capitani, L. J. Corzo-Rios, L. A. Chel-Guerrero, D. A. Betancur-Ancona, S. M. Nolasco and M. C. Tomás, J. Food Eng., 2015, 149, 70–77 CrossRef.
- L. Feng, Y. Zhou, T. J. Ashaolu, F. Ye and G. Zhao, Int. J. Biol. Macromol., 2019, 128, 629–637 CrossRef.
- T. G. Mezger, Applied Rheology: with Joe Flow on Rheology Road, Anton Paar, 2015 Search PubMed.
- M. I. Capitani, S. M. Nolasco and M. C. Tomás, Food Ind., 2013, 19, 421–437 Search PubMed.
- S. Boostani, S. M. H. Hosseini, M.-T. Golmakani, A. Marefati, N. B. A. Hadi and M. Rayner, Food Hydrocolloids, 2020, 101, 105520 CrossRef.
- S. S. Teh and S. H. Mah, J. Oleo Sci., 2018, 67, 1381–1387 CrossRef.
- Y. Maphosa and V. A. Jideani, Science and Technology behind Nanoemulsions, 2018, p. 65 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00309h |
| This journal is © The Royal Society of Chemistry 2025 |