Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent strategies for controlling the white mould fungal pathogen (Sclerotinia sclerotiorum) using gene silencing, botanical fungicides and nanomaterials
Timothy O.
Ajiboye
 *,
H. G.
Visser
,
E.
Erasmus
*,
H. G.
Visser
,
E.
Erasmus
 and
M.
Schutte-Smith
and
M.
Schutte-Smith

Department of Chemistry, University of the Free State, Bloemfontein, 9300, South Africa. E-mail: timaji2003@gmail.com
First published on 17th February 2025
Abstract
Sclerotinia sclerotiorum is a fungal pathogen that causes white mould diseases in several plants of economic importance. In the form of sclerotia, the pathogen is soilborne and can survive for a long period of time. White mould is considered yield burdening to soybean and poses a threat to over 600 host plant species; hence, there is an increasing effort to manage it by using various strategies. One of the common methods of controlling the pathogen is the use of chemical fungicides; however, concerns remain regarding environmental and human safety. Additionally, the pathogen has demonstrated potential to develop resistance to chemical fungicides and some of these chemical fungicides are being removed from the market due to threat to the environment, and the use of environmentally friendly biofungicides and gene silencing are examined. Another recent strategy that has been adopted for controlling the pathogen is the use of nanomaterials. Hence, the use of metallic nanoparticles, metal oxide nanoparticles, carbon-based materials, and their composites for combating the Sclerotinia sclerotiorum fungal pathogen are comprehensively reviewed. Finally, future perspectives on the control of the fungal pathogen are suggested.
Sustainability spotlightWhite mould is considered yield burdening to soybean and poses a threat to over 600 host plant species; hence, there is an increasing effort to manage it by using various strategies. One of the common methods of controlling the pathogen is the use of chemical fungicides; however, concerns remain regarding environmental and human safety. Additionally, the pathogen has demonstrated potential to develop resistance to chemical fungicides and some of these chemical fungicides are being removed from the market due to threat to the environment. Without controlling this pathogen, the sustainable development goal of the United Nations (Goal 2) would not be achieved. Therefore, the current research is important for scientists to know the way forward on the control of this destructive pathogen. |
1 Introduction
Sclerotinia sclerotiorum (Lib.) de Bary is a fungal pathogen responsible for white mould that affects plants such as canola, brassica oilseed1,2 soybean,3 sunflower,4 mustard,5 tomato 6 and other plants (over 600 plant species in total).2 It is a devastating disease of plants, and its pathogen is not only ubiquitous but also has potential to feed as a hemi-biotroph on these plants.2,7 In lettuce, it impacts the anthocyanins, flavonoids and phenolic contents leading to reduction in the yield of the plant.8 Similarly, a significant decrease in the biochemical activities, yield of oil, growth, dry weight and fresh weight of Mentha arvensis infected by this fungal pathogen has been reported.9 The reduction in these parameters was found to increase when the initial inoculum levels of S. sclerotiorum were increased. This showed that the pathogen is a threat to food security and must be controlled.Apart from white mould, different names are associated with diseases caused by this pathogen, i.e., blossom blight, crown rot, watery soft rot, cottony rot and Sclerotinia stem rot.10 The management of Sclerotinia sclerotiorum is challenging due to the fact that it affects such a wide range of host plants coupled with long-term survivability and genetic variability.11 Additionally, the pathogen exhibits two infection mechanisms. It can damage the basal stem through soil-borne mycelia via a myceliogenic germination mechanism. It can also infect the upper part of the plant, i.e., stems, leaves and reproductive organs through ascospore dispersal, resulting from carpogenic germination (Fig. 1).2 Taxonomically, it belongs to the kingdom, phylum, class, order, family and genus of Fungi, Ascomycota, Discomycetes, Helotiales, Sclerotiniaceae, and Sclerotinia respectively.13 Its presence in the plant cultivars is often detected via the use of techniques such as polymerase chain reaction (PCR)-based techniques,14 immunochemical detection methods15 and leaf-reflectance spectroscopy.16
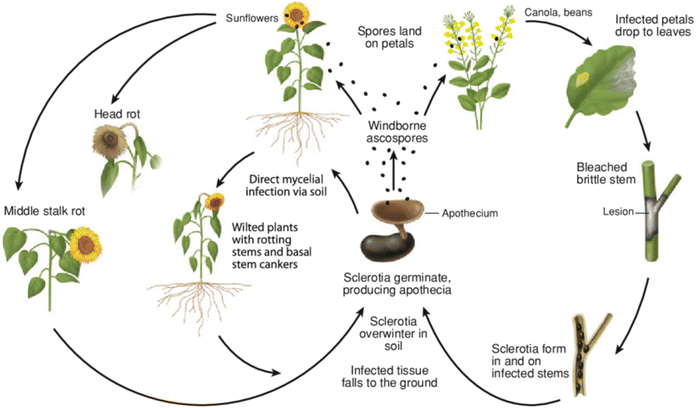 | ||
| Fig. 1 The disease life cycle of the white mould pathogen (Sclerotinia sclerotiorum) on canola and sunflower. Reproduced with permission from ref. 12. Copyright (2014), Springer Nature, License number: 5964070527457. | ||
The use of fungicides to manage Sclerotinia not only aims to protect current yields by mitigating disease establishment and severity but also seeks to safeguard future yields by reducing the accumulation of sclerotia in the soil, thereby preventing the initiation of new infection cycles. However, heavy reliance on expensive commercial fungicides, along with repeated, poorly timed, and sometimes indiscriminate use, has resulted in the pathogen developing an insensitivity against the active ingredient/s, leading to a decreased efficacy of the fungicide in controlling the pathogen. The risk of Sclerotinia sclerotiorum continuing to develop resistance to common fungicides, for example benomyl, carbendazim,,17–19 procymidone, fluazinam, and pyraclostrobin, has prompted researchers to explore alternative management strategies against white mould. Besides, most of these fungicides are being removed from the market as they have been found to be highly carcinogenic by inhibiting thyroid peroxidase in animals and some of them release the ethylene thiourea metabolite.20,21 Another strategy that has been used is the use of cultural practices such as crop rotation, tillage, canopy management, controlled seedling rate, widening row spacing during planting, weed control, changing planting dates, reducing nitrogen-rich fertilizers, planting small grains along with cover crops, avoiding excessive irrigation and the use of resistance varieties. However, all these have been found to reduce the overall yield of the plants.22 The limitations of some of the other strategies that have been adopted are shown in Table 1.
| Control method | Descriptions | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Biofumigation | Adding fresh plant biomass to the soil that releases chemicals which destroy soil-borne pathogens | The properties of the soil are improved; the activities of beneficial microbes are enhanced | It is costly; the efficacy is time dependent since there is ascopore dispersal when applied to flowering plants at a particular stage of development; unreliable | 1, 23 and 24 |
| Organic amendment | The use of manure, municipal solid waste compost and biosolids to replace or supplement mineral fertilizers | Enhancement of soil fertility and yields; reduces the cost of the fertilizers | Safety concerns such as accumulation of metals in the soil and groundwater contamination with organic compounds; health impacts from viruses and other pathogens | 21 and 25 |
| Crop monitoring via unmanned aerial systems | The use of remote sensing technology such as unmanned aerial vehicles, manned aircraft and satellites to study diseases and pests | More efficient than ground-based surveys; high mobility with near real-time application | Low endurance time (less than 1 h); air safety concern; involves the use of professional software for complex data analysis | 26 and 27 |
| Biological fungicides | Spraying bio-fungicides on the growing plants, addition to the soil or treatment of plant materials | Minimal toxicity; ecologically pure | Reapplication is required to obtain high efficiency; they are not tolerant to temperature regimes; their response to phytopathogenic organisms is not rapid | 28 |
| Use of ultraviolet/gamma radiation | UV-B, gamma and UV-C are used to kill pathogens in plants | Uniformity of the dose allowing products with different shapes and sizes to be treated; high penetrability | Cannot treat radiation microbial population; mutation of the microbes to a more/less pathogenicity from parent organisms | 29 |
There are over 2567 research articles on the general investigations involving Sclerotinia sclerotiorum between the years 2013–2023. An upward trend in the research was observed from 2017 to 2022 with 325 papers published in the year 2022 alone, indicating increasing interest in the research on Sclerotinia sclerotiorum. Most of the control strategies within these periods are the use of antagonistic fungi (such as Clonostachys rosea,30Aspergillus flavus strain NJC04,31Aspergillus pseudoelegans, Aspergillus niger, and Aspergillus japonicus32), the use of Trichoderma spp. (such as T. afroharzianum and T. asperelloides33), the use of mycoparasites such as Coniothyrium minitans,34 and the use of antagonistic bacteria (such as P. fluorescens, P. commune and T. asperellum35). Other strategies that have been adopted are the use of fungicides, gene silencing and nanomaterials. While the use of antagonistic organisms for controlling this pathogen has been reviewed,36–38 the use of fungicides, gene silencing and nanomaterials has not been reviewed. To understand the work that has been done in this regard and how these strategies disrupt the lifecycle of the pathogen, a comprehensive report is required. Hence, the present review examines the recent developments in the alternative control of S. sclerotiorum to create a greater array of tools for producers to utilise for integrated pest management.
1.1 Life cycle, mechanism of action and morphology of S. sclerotiorum
S. sclerotiorum spends most of its lifetime in the soil where it exists in a sclerotium form (Fig. 1). Although sclerotia are the main structures indicating the end of the disease cycle, they are also the nutritional and structural bases for myceliogenic and apothecial germination for the start of a new disease cycle.12 The size of sclerotia can range from a few millimetres to several centimetres, depending on the host plant, where larger sclerotia have been associated with greater numbers of apothecia.39–41 Ascospores are forcibly ejected into the air from apothecia and are the primary inoculum which initiates disease. The secretion of oxalic acid by S. sclerotiorum at infection sites, i.e., canola petals or heads of sunflower, initiates the breakdown of plant tissue, reducing the pH and triggering the production of polygalacturonase, an enzyme responsible for the depolymerization of pectates in plant cell walls.42,43 This biochemical reaction leads to the disease symptoms of water-soaked lesions and ultimately the formation of mycelia and sclerotia. Without the secretion of oxalic acid, programmed cell death by oxalate oxidase cannot occur, preventing the formation of sclerotia.44,45 The entire disease life cycle of the white mould fungus on canola and sunflower is shown in Fig. 1. There is a positive correlation between flowering and susceptibility to the pathogen, as petals are infection courts for ascospores, and fungicide applications need to target this tissue prior to ascospore deposition.Infection of the stems results in greater risk of yield loss as lesions impair the plant's ability to transport water and nutrients through the xylem and phloem, and in cases of severe infection stems may break and result in no to little yield.46 The sign of the disease is the formation of white fluffy mycelia which melanise and form sclerotia as the disease progresses. The sclerotium is an irregular to round-shaped structure (Fig. 2a) that becomes encased in hyphae within a week. Subsequently, the apex of the inner hyphae swells and extends towards the surface to form a melanised rind47 (Fig. 2b–f). Other fungi such as basidiomycetous Athelia rolfsii have spherical sclerotia. However, the most common shape for the sclerotia of white mold is an irregular shape.
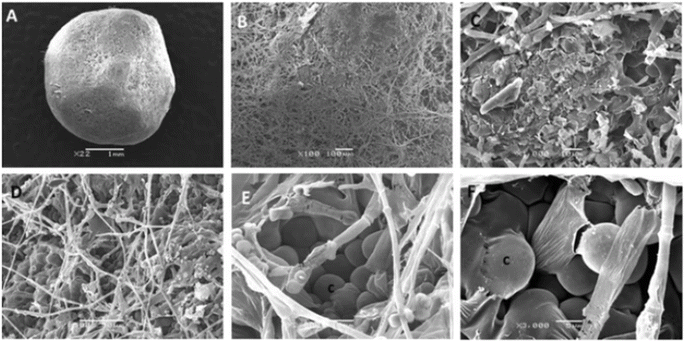 | ||
| Fig. 2 Scanning electron images of the sclerotium after 8 days in the maturation stage. (a) The round structure of the mycelium; (b) sclerotium covered by the mycelium; (c) layer of membrane developing on the surface of the sclerotium. (d) Hyphae projecting into the surface (e and f) swelling cells on the tips (c) of hyphae. Reproduced with permission from ref. 47. Copyright (2014), Springer Nature-License number: 5964071117957. | ||
In the form of sclerotia S. sclerotiorum can remain dormant in the soil for 10 years until conducive weather events permit either carpogenic or myceliogenic germination.48 The ability of sclerotia to withstand adverse environmental conditions in the soil and in the absence of a host plant is associated with the melanized rind (outermost layer of the cortex) unlike the inner region of the cortex which is hyalinized.49 The melanin produced by S. sclerotiorum has been confirmed to be dihydroxynaphthalene which is an arene stabilized by resonance.49 The presence of melanin in sclerotia enhances their survival in soil by providing protection against UV radiation and microbial degradation. This resilience allows sclerotia to successfully germinate and produce ascospores, making timely fungicide applications essential for effective disease management as these ascospores are released from the apothecia.
2 Alternative strategies to control Sclerotinia sclerotiorum
Current methods of managing S. sclerotiorum focus on limiting infections through (1) reducing sclerotial viability and germination ability, (2) inhibiting ascospore germination and (3) enhancing the plant’s ability to withstand penetration mitigating or restricting disease establishment. However, the need to combat S. sclerotiorum requires novel approaches which disrupt the disease cycle unconventionally, i.e., targeting sclerotial formation.2.1 Use of a gene silencing target to control Sclerotinia sclerotiorum
Host-induced gene silencing has been adopted for controlling the fungal pathogen. This approach depends on the potential of eukaryotes to silence the transcription of genes through a transcriptional gene silencing (TGS) or post-transcriptional gene silencing (PTGS) process.50 TGS involves promoter methylation leading to a decrease in the synthesis of RNA while PTGS involves degradation of RNA for a specific sequence.51 Irrespective of the process involved, the response of the plants to the external stimuli and their nature are altered by knocking down the gene expression. To use this strategy in controlling pathogens, it requires identifying and silencing the genes in the pathogens that are responsible for pathogenicity, development and growth.52 By following this, Andrade et al.53 silenced the gene that was responsible for the synthesis of chitin in the white mould pathogen. The specific selection of the gene was carried out in such a way that specific phytopathogens were efficiently targeted without any impact on other organisms in multiple cultures. Generally, some of the effectors that have been identified in S. sclerotiorum for silencing are Ss-caF1 which is a putative Ca+ binding protein; SsiTL which is an integrin; SspG1d which is an endopolygalacturonase that targets a protein involved in calcium signalling known as iPG-1 and SsSSVP1, that targets QCR8 in the cytochrome of the mitochondria.50In an investigation, a forward genetic approach was used to identify a protein that plays a critical role in the formation of sclerotia. The protein is SsGAP1 and it is responsible for activating RAS-GTPase. Considering the role played by the RAS signalling component in the formation of sclerotia, it has been used for host-induced gene silencing for reducing the virulence of white mould diseases in Arabidopsis thaliana and Nicotiana benthamiana.54,55 Knocking down SsOah1 (SS1G_08218) and SsCyp51 (SS1G_04805) genes of S. sclerotiorum using spray-induced gene silencing led to slowing down of the disease progression within two hosts (B. juncea and N. benthamiana). Lesion development reduction and disease initiation delay were also observed. Depending on the host, the growth and morphology of the host have been altered when this gene was deleted.56 Gene deletion can also be used to identify the exact gene that contributes to the parasitic nature of a particular strain against the white mold pathogen. For instance, Lv et al. identified S8 serine protease-encoding gene CrKP43 which has been important for the parasitic nature of Clonostachys chloroleuca against Sclerotinia sclerotiorum. With the deletion of this gene, inhibition of conidiation and deformation of the fungal morphology and cell wall were observed. Their findings further revealed that deletion of CrKP43 weakens the antagonistic mycoparasite ability of Fusarium oxysporum against Sclerotinia sclerotiorum. Other genes that have been deleted and found to be suitable for the control of Sclerotinia sclerotiorum through host-induced gene silencing are shown in Table 2. Despite the prospect of this technique, Nunes and Deans62 reported some limitations for its feasibility and real-world application. One of the limitations is the challenge of identifying fungal targets. Apart from the points raised by these scientists, the technique is expensive and requires a deep understanding of sequencing data and protein–protein interactions.
| Gene silenced | Role of the gene | Method of investigation | Effect of the gene silenced | References |
|---|---|---|---|---|
| SsCak1(a putative protein kinase) | Involved in the growth and pathogenicity of S. sclerotiorum | Next-generation sequencing and forward genetic screen | Complete loss of virulence; defective sclerotia and mycelium development; penetration of the host; formation of appressoria | 57 |
| CrGlu6 (glucose-6-phosphate 1-epimerase) in Clonostachys chloroleuca | Generates energy for the growth of Clonostachys chloroleuca (mycoparasite of S. sclerotiorum) | Protein–protein interaction | Significant conidiation reduction; abnormal hyphal morphology; decreased control efficiency, mycoparasitic activity and antifungal activity against white mould disease | 58 |
| SsTOR | Target of rapamycin (TOR) regulates stress responses of eukaryotes and controls intracellular metabolism and cell growth | Use of pathogenicity assays; study of cell wall integrity; phosphorylation analysis | Retardation of the hyphal growth; disruption of compound appressoria and sclerotium formation | 59 |
| SsPDE2 | Regulation of the accumulation of oxalic acid; virulence and infection cushion functionality; sclerotium formation | Next-generation sequencing; forward genetics approach | Sclerotium formation impacted | 60 |
| MAPK (mitogen-activated protein kinase cascade) | Controls hyphal fusion, compound appressoria formation, sclerotium development and mycelial growth | Forward genetic screen | Deformed appressoria; impaired apothecia formation; attenuated host penetration | 61 |
2.2 The use of botanical fungicides to control Sclerotinia sclerotiorum
Safety and environmental friendliness are the two factors that triggered the investigation of natural products for controlling Sclerotinia sclerotiorum.63 Different botanical fungicides (antifungal agents sourced from the extracts of plants) have been tested against Sclerotinia sclerotiorum. Some of them are vapours from peppermint oil and sweet basil oil. The two essential oils inhibited the growth of the fungus in a dose-dependent manner. This led to further investigations into the antifungal potential of the major constituents of these essential oils (eugenol and linalool in sweet basil oil; menthone and menthol in peppermint oil). It was however noted that while linalool alone displayed a moderate antifungal activity, eugenol alone did not show any antifungal activity at all. Surprisingly, the mixture of the two major constituents at a ratio equal to their natural existence in sweet basil oil showed a synergistic antifungal property. Similarly, there was synergistic antifungal activity when a menthone and menthol mixture was used whereas menthone did not show any antifungal activity and menthol displayed dose-dependent antifungal activity.64An antimycotic natural ingredient that is commonly found in soil, known as natamycin, has also been investigated for controlling Sclerotinia sclerotiorum. Natamycin showed an inhibitory effect on the growth of the mycelia of the pathogen with the half-maximal effective concentration (EC50) ranging between 0.53 and 4.04 μg mL−1. The mechanism of action of natamycin was found to involve an increase in malondialdehyde content and reactive oxygen species (ROS). Also, there was a reduction in the ergosterol and oxalic acid content. In the structural alteration, the integrity of the cell membrane, formation of sclerotia and formation of hyphae were significantly impacted.65
Hinokitiol is a natural monoterpenoid that is present in the wood of the Cupressaceae family and has also been used as an antifungal agent against Sclerotinia sclerotiorum. Through its antifungal properties, the shelf-life of carrot was increased. This is due to enhancement of antioxidant activities, prevention of the formation of malondialdehyde, and reduction of total phenolic, carotenoid and ascorbic acid contents. The formation of sclerotia was also prevented by interfering with the cell membrane formation and altering the gene expression of the enzymes responsible for sclerotium formation. The synthesis of exopolysaccharides and oxalic acid was also suppressed leading to a reduction of its pathogenicity.63
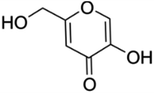
5-Hydroxy-2-hydroxymethyl-γ-pyrone also known as kojic acid is a metabolite derived from several strains of fungi. This natural bioactive compound has been used to protect the skin from UV radiation and make the skin lighter in the cosmetic industry. It has also been used as a food additive due to its non-toxic nature and environmental friendliness.66 It has earlier been established that kojic acid inhibits the biosynthesis of melanin in the cells of humans.67 This prompted its investigation for controlling S. sclerotiorum. In an investigation,68 kojic acid displayed good antifungal activities against S. sclerotiorum by inhibiting the synthesis of melanin and chitin. Its antifungal activities were not affected by the change in pH but the presence of metal ions reduced its antifungal activities. This may be due to the formation of chelates with the metal ions. In soybean for instance, application of 7.1 mg mL−1 of kojic acid completely inhibited S. sclerotiorum. Apart from using kojic acid alone, its addition to commercial fungicides such as carbendazim and prochloraz boosted the antifungal activities of these fungicides.
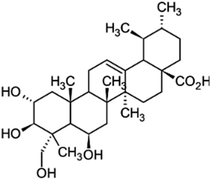
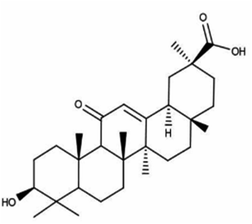
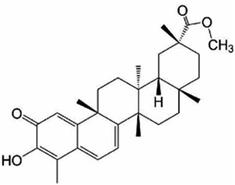
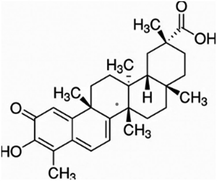
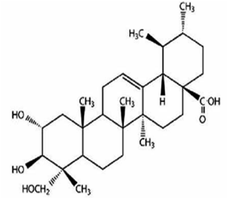
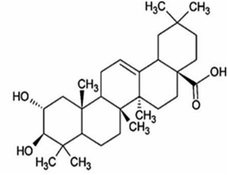
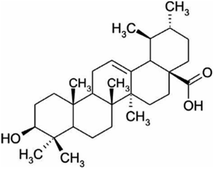
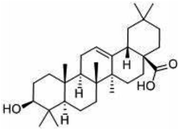
Other natural compounds that have effectively controlled the white mould fungus are the hexane, butylalcohol, heptane and methanol extracts of different parts of Dactyloctenium aegyptium;69 a natural bioactive compound known as kojic acid;70 a series of new quinoline derivatives (cryptolepine alkaloid);71 two alkaloids (rutaecarpine and evodiamine) of the isolates of Evodia rutaecarpa which is a traditional Chinese herbal medicine72 and ten pentacyclic triterpenoids including corosolic acid, madecassic acid, glycyrrhetinic acid, quillaic acid, oleanic acid, ursolic acid, maslinic acid, asiatic acid, pristimerin and celastrol.73 The structure, molecular weight and sources of these natural acids are shown in Table 3. The use of these natural antifungal agents caused disruption in the enzyme activities, initiated the accumulation of reactive oxygen species leading to mycelial abnormalities in the pathogen, distortion of its mitochondria and/or inhibition of either germination or formation of sclerotia. In short, they alter the functions of the mitochondria, cell walls, cell membrane, DNA and RNA. The mechanisms of action of some of these natural antifungal agents are shown in Fig. 3.
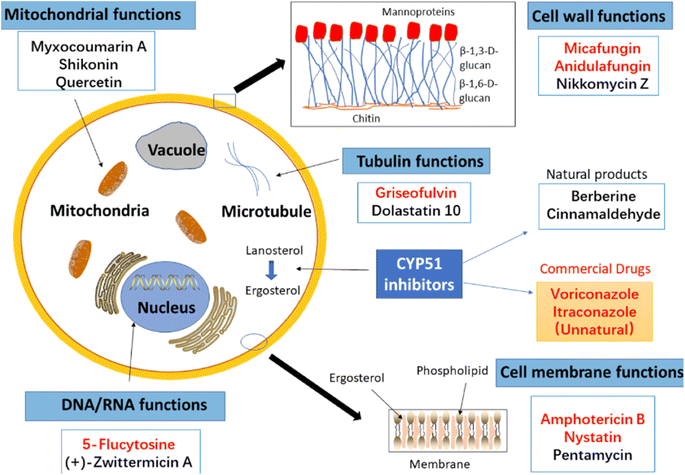 | ||
| Fig. 3 Some antifungal agents from natural sources with some fungal cells that they target. Reproduced from ref. 74. Elsevier (Open Access under Creative Commons Agreement). | ||
One of several advantages botanical fungicides have over synthetic fungicides is environmental friendliness. Unlike the synthetic fungicide that could persist in the environment leading to environmental pollution, botanical fungicides are biodegradable since they are products from nature.75 In addition, they are selective and effective and can impact multiple mechanisms of action since plant extracts contain multiple bioactive components that can act simultaneously. They are not expensive unlike synthetic fungicides, and they are readily available. The fact that they contain an array of chemical families diversifies the molecular and biochemical targets towards fungi. Therefore, there is a delay in the resistance phenomenon.75,76
2.3 Use of nanomaterials to control Sclerotinia sclerotiorum
Nanotechnology is a field that involves the synthesis of nanoparticles and utilising nanoparticles to create new materials. Materials are manipulated at the molecular and atomic levels and extremely small sized nanoparticles are employed in applications such as environmental remediation, catalysis, engineering, medicine, and agriculture.77 Nanoparticles have also been found to be effective in fighting the pathogens of crops by releasing metal ions, photocatalysis, interference with RNA synthesis, inhibition of DNA replication, inhibition of enzyme activities, disruption of energy transduction, generation of reactive oxygen species and/or disruption of the cell wall/cell membrane.78,79 Their action also depends on their size, shape and concentration when applied.78 As also pointed out by Ray et al.80 the mechanism of action of nanoparticles against pathogens can be broadly classified into five. The first is protein oxidation, membrane oxidation and energy production leading to enhanced and distinctive antibiotic activity. The second is the release of toxic ions by the nanoparticles which leads to genotoxicity and cell death in the pathogen. The third is the induction of oxidative and cellular stress on cell pathogens through the reactive oxygen species released by the nanoparticles (Fig. 4). The fourth is the damage caused by the nanoparticles on the nutrient absorption and membrane transporter system. The last is the alteration of the activity and permeability of the membrane proteins initiated by the toxic ions released from the nanoparticles.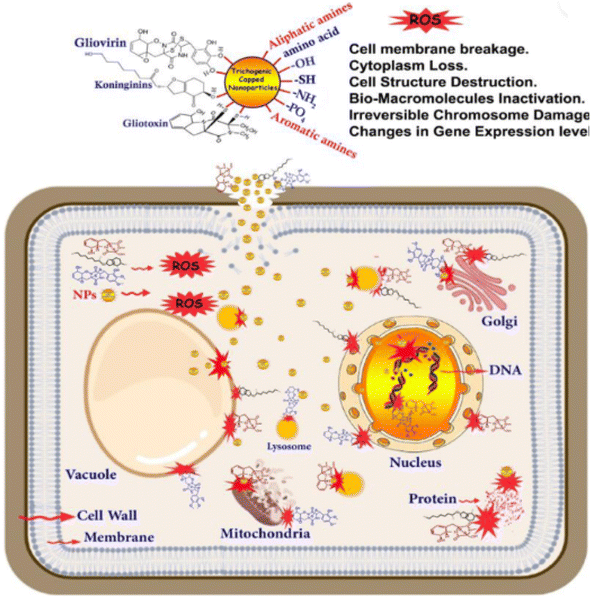 | ||
| Fig. 4 Schematic illustration of the mechanism of antifungal activity of nanoparticles. Reproduced from ref. 81. MDPI Open Access. | ||
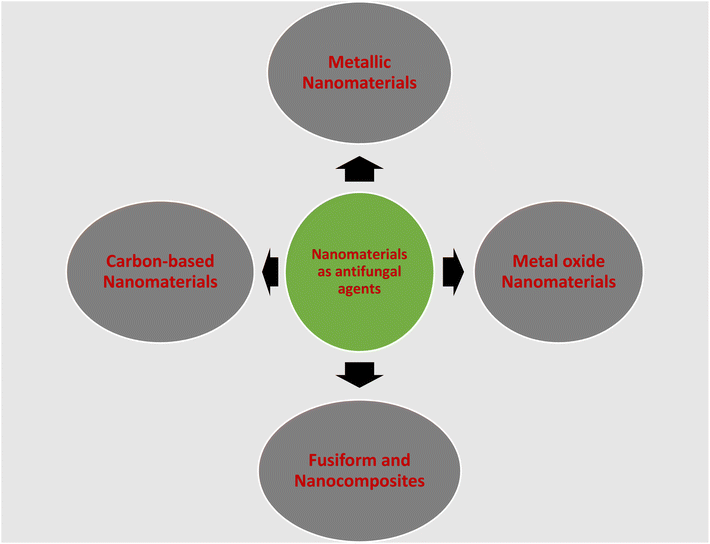
In this section, the nanomaterials that have been used as antifungal agents against Sclerotinia sclerotiorum and discussed are shown in Fig. 5.
Another study examined the effect of capping on the Trichoderma harzianum-derived silver nanoparticles against Sclerotinia sclerotiorum. It was observed that the capped silver nanoparticles contained chitinase and acid protease, the hydrolytic enzymes NAGase, β-1,3-glucanase, protein bands and functional groups of biomolecules from Trichoderma harzianum. The uncapped nanoparticles were not effective against the pathogen, but the capped silver nanoparticles were effective against Sclerotinia sclerotiorum which showed that capping played a significant role in the activities of silver nanoparticles, though there was no significant difference in the cytotoxicity activity of the capped and uncapped silver nanoparticles.84
Fusarium culmorum strain JTW1 has also been used as a source of stable, highly crystalline, spherical, and approximately 16 nm silver nanoparticles. Apart from the fact that the size of the silver nanoparticle produced via this method is smaller than the size obtained when Trichoderma harzianum filtrate was used, with 32 μg mL−1, there were zones of growth inhibition of 3.41 mm.85 Gupta and Saxena similarly used enzyme amylase that is present in Aspergillus oryzae MTCC No. 3107 for reducing silver salt to silver nanoparticles. There was complete inhibition of Sclerotinia sclerotiorum when a 100 μg mL−1 concentration of the silver nanoparticles was used.86
Spherical silver nanoparticles that were produced from the filtrate of gliotoxin-producing T. virens HZA14 demonstrated 100, 93.8 and 100% activities against myceliogenic germination of sclerotia, sclerotial formation and hyphal growth. These performances were observed when the concentration of the nanoparticle assay was 200 μg mL−1. As revealed by the EDS and SEM analysis, there was direct interaction between the cells of the fungi and the nanoparticles leading to fissure and micropore formation on the cell wall of the fungi and fragmentation of its lamella.87 Mitta et al. utilized Tinospora cordifolia leaf extract to reduce silver nitrate to spherical silver nanoparticles with a particle size of 50 nm. The bioactive compounds in the extract were also used as capping agents for the nanoparticles. The capped silver nanoparticles were effective against Sclerotinia sclerotiorum (MTCC 8785).88
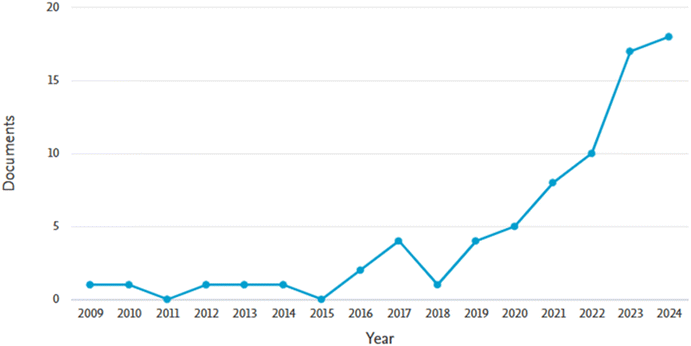
A warm water extract of Chlorella vulgaris and algae Chroococcus dispersus was also used to reduce silver nitrate to silver nanoparticles having particle sizes lower than 55 nm. The antifungal activities of the assay of the produced silver nanoparticles were investigated using the disc diffusion method against Sclerotinia sclerotiorum and (Alternaria alternata, Helminthosporium sp., Rhizoctonia solani, Fusarium oxysporum and Fusarium solani). The results obtained were compared to the performance of generic antibiotics (streptomycin, ampicillin, and gentamycin). It was observed that the antifungal activities of the silver nanoparticles were nine fold better than those of the antibiotics.89 Generally, the inhibitory performance of silver nanoparticles is active against three common sclerotium-forming species (S. minor, Sclerotinia sclerotiorum and Rhizoctonia solani). However, the inhibitory effect against hyphal growth of Sclerotinia sclerotiorum is more than that of S. minor while the hyphal growth inhibition is the greatest in Rhizoctonia solani. Conversely, sclerotial germination growth inhibition against Sclerotinia sclerotiorum is the most pronounced out of the three sclerotium-forming species.90 Specifically, the first article on the use of nanoparticles for controlling Sclerotinia sclerotiorum was written in the year 2009 and there is a significant increase from 2018 onwards as shown in Fig. 6. Overall, more than 51 research articles have been published on the control of the pathogen using different nanomaterials.
Apart from titanium nanoparticles, copper nanoparticles have also been investigated as antifungal agents against Sclerotinia sclerotiorum. Sadek et al.92 compared the antifungal performance of copper sulphate solution and sulphur nanoparticles to that of copper nanoparticles. To achieve this, in vivo and in vitro applications of these materials in cucumber at varied temperatures were carried out by these researchers. The findings showed that copper sulphate displayed the lowest antifungal activities against Sclerotinia sclerotiorum. Even when the concentration of the copper sulphate nanoparticles assay was raised to 4000 μg mL−1, the antifungal activities were still comparatively lower than when 100 μg mL−1 assay of the copper nanoparticles was used. Notedly, the sulphur nanoparticles showed better antifungal performance than copper nanoparticles. For the two nanoparticles (sulphur and copper), as the concentration of the nanoparticles increased, the antifungal activities also increased. Also, the temperature impacted the antifungal activities of these nanoparticles. As the temperature was decreased, there was a significant drop in the fungal growth. Sulphur nanoparticles also caused lower cytotoxic effects compared to copper nanoparticles. Even though there are sulphur and copper in copper sulphate solution, the antifungal activity of the solution is far less than that of sulphur and copper nanoparticles.
Selenium is a beneficial element to plants which has also been employed as a good antifungal agent against Sclerotinia sclerotiorum. It has been experimentally proven that when between 0.1 and 0.5 mg kg−1 of selenium nanoparticles are applied to soil, the diseases caused by Sclerotinia sclerotiorum are reduced in oilseed rape. This is because the presence of selenium nanoparticles increases the transpiration rate, stomatal conductance, the antioxidant system of leaves and the net photosynthetic rate. As revealed by the metabolome analysis, the presence of selenium boosts the amino acids and energy of the oilseed rape leaves infected by Sclerotinia sclerotiorum.93 In another investigation, there were soluble protein leakage and severe damage to the hyphae of Sclerotinia sclerotiorum when the soil used to plant rape straw was treated with selenium. This led to an increase in the oxalic acid secretion and a decrease in cell-wall degrading enzymes in the pathogen with an overall increase in the Sclerotinia sclerotiorum fungal inhibition.94
In a different study,95 nano-silicon was found to inhibit the growth of the mycelium of the pathogen depending on the dose of the nanoparticle applied. The disease index was significantly reduced by a maximum value of approximately 52% when the nano-silicon was used for the field trial on Brassica napus. This was linked to the promotion of minerals such as calcium, potassium and silicon when silicon nanoparticles were applied while at the same time suppressing the absorption of sodium. Besides, the lignin and soluble sugar levels were boosted when silicon nanoparticles were applied unlike the level of cellulose that was reduced in the stems and the leaves of Brassica napus. This is clearly shown in the diameter of lesions with and without silicon nanoparticles.
The effect of zinc oxide nanoparticles in inhibiting Sclerotinia sclerotiorum has also been studied and compared with the inhibiting ability of Benomyl (a known antifungal). As shown by the diameter of the colony of Sclerotinia sclerotiorum grown on the potato dextrose agar and broth, after applying the nanoparticles and the fungicide, there was a 9 cm diameter after 0.3 mL within 5 days of inoculation which was better than that of the fungicide. Besides, it caused the sclerotia with sizes between 25 micrometers and 2 millimeters to disappear.98 When zinc oxide was coated with ethylene glycol and used for inhibiting Sclerotinia sclerotiorum in lettuce, an increased quantum yield rate and net photosynthetic rate were observed. There was no phytotoxic effect on lettuce after the application of this nanoparticle. In short, the impact of the pathogen was reduced due to the application of the nanoparticles.99 In seeds of chickpea treated with zinc oxide made by using the leaf extract of Carica papaya, compared to other fungal pathogens (R. necatrix and Fusarium species), Sclerotinia sclerotiorum was significantly inhibited by 59.7%. This further buttressed the fact that zinc oxide nanoparticles are effective antifungal agents against Sclerotinia sclerotiorum.100 A similar trend was observed when zinc oxide was used as an antifungal agent in potato. There was 60.5% inhibition against Sclerotinia sclerotiorum compared to 55.2% and 37.5% that were reported for inhibition against Fusarium spp and Rosellinia necatrix respectively.101
Apart from zinc oxide nanoparticles, other metal oxide nanoparticles have been reported for combating Sclerotinia sclerotiorum. One of these is titanium oxide synthesized by using shell extracts of pawpaw. Varied concentrations of the nanoparticle were used to preserve the germs of pea from Sclerotinia sclerotiorum. There was a 60.5% inhibition ratio of the pathogen and increases in catalase activities and superoxide dismutase. Besides the mechanisms of inhibition, there was increased plant and root growth, enhanced seed germination, and improved antioxidant enzyme activities and root interval.102
Iron oxide nanoparticles have also been used to control the pathogen. They are particularly used for the plants' investigations. This is because iron is an important micronutrient in plants that helps with boosting the chlorophyll, protein carbohydrates and plant growth.103 Bilesky-José et al. used Trichoderma harzianum as a stabilizing agent for synthesizing iron oxide nanoparticles. The as-synthesized nanoparticles displayed an inhibitory effect on Sclerotinia sclerotiorum without hindering the growth of the plants. In addition, the mitotic index and cell viability of the plants were not altered by the application of iron oxide nanoparticles compared to the controls.104Trichoderma harzianum has also been used as both a stabilizing agent and a reducing agent for the synthesis of copper oxide that was used for controlling Sclerotinia sclerotiorum. Copper oxide had no effect on the size of the mycelia of the pathogen but there was alteration in the size and distribution of the sclerotia due to the application of copper oxide nanoparticles.96
In another research study, copper oxide nanoparticles applied to green bean pods induced an inhibition against Sclerotinia sclerotiorum. In response to the application of copper nanoparticles, high amounts of phaseollin, 6-α-hydroxyphaseollin, phaseollidin, coumestrol, kievitone, and phytoalexins were detected. There was also an increase in the transcriptomic levels of pvgluc, PvGIP, PR1, DOX and pk20 with non-genotoxic impact on the DNA of the green bean. As a result of the inhibition of the pathogen by copper oxide nanoparticles, the shelf life of the green bean pods was extended to 21 days when the storage temperature was maintained at 7 ± 1 °C.105 Silicon oxide capped with a network containing phenol and copper has also proven to be effective in controlling Sclerotinia sclerotiorum in oilseed rape plants. The nanoparticles were found to be environmentally safe and efficient as revealed by the toxicity test carried out on zebrafish.106
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for hydroxyapatite, graphitic carbon nitride and chitosan respectively, there was an encapsulation efficiency of 82%. This combination had an in vitro minimum inhibitory concentration of 187.5 μg mL−1 against Sclerotinia sclerotiorum that is present in kiwifruit. On artificially inoculated fruit, higher doses of 375 μg mL−1 were required for the effective control of the same pathogen.108
5 for hydroxyapatite, graphitic carbon nitride and chitosan respectively, there was an encapsulation efficiency of 82%. This combination had an in vitro minimum inhibitory concentration of 187.5 μg mL−1 against Sclerotinia sclerotiorum that is present in kiwifruit. On artificially inoculated fruit, higher doses of 375 μg mL−1 were required for the effective control of the same pathogen.108
The need to reduce the toxicity of mancozeb which is a commercial antifungal agent led to the fabrication of chitosan-gum acacia polymers loaded with mancozeb. When the loading of mancozeb was 1.0 mg mL−1, there was 100% inhibition of Sclerotinia sclerotiorum through the mycelium inhibition method. The disease-control efficiency against stem rot diseases in pathogen-treated plants was 60.2 ± 1.4%. However, commercial mancozeb also showed 100% inhibition against Sclerotinia sclerotiorum, but the toxicity is comparatively higher than the one loaded on the chitosan–gum acacia polymers as shown when applied on Vero cell lines.109 This same group of researchers loaded mancozeb on chitosan–carrageenan combinations. Mancozeb was entrapped and loaded on the composite carbon-based material. With 1.5 ppm loading of nanomancozeb, there was 100% inhibition of Sclerotinia sclerotiorum. The nano formulation also showed lower toxicity compared to commercial mancozeb only.110
Another formulation is the poly(lactic acid) and cellulose nanocrystal made into spherical beads and combined with methoxylated sucrose soyate polyols and used along with a commercial antifungal against Sclerotinia sclerotiorum (Lib.). The strategy involves loading the commercial antifungal on the nanoformulated material prior to application on the plant. To demonstrate the functionality of this strategy, a model fungicide (azoxystrobin) was loaded on the prepared nanoformulation with canola being the model plant. The design is unique because there is controlled porosity in the cellulose nanocrystals allowing diffusion of the fungicides to the environment via the beads. The material is also cheaply available and biocompatible and has good water dispersibility, the presence of several hydroxyl functional groups that can interact via hydrogen bonds, large surface area and a high aspect ratio. This nanoformulation loaded with fungicides was preferred to ordinary commercial fungicides as commercial fungicides do not persist for a long period of time on the plant before being washed away due to exposure to air flow, rain, and other environmental conditions.17 Without a delivery system like this nanoformulation, less than 0.1% of the applied fungicides get to their biological targets while the remaining are lost through runoff, leaching, degradation, photolysis, and volatilization.111 This is not only economically wasteful for farmers, but it also affects non-target beneficial organisms.17
Graphene oxide is another carbon-based material that has been used to suppress the growth of Sclerotinia sclerotiorum. Treatment of rapeseed seedlings with 15 mg L−1 graphene oxide or treatment of the seeds with the same concentration significantly inhibited the growth of Sclerotinia sclerotiorum compared to the control samples. At this concentration, the growth of rapeseed was not affected.112 Hydrophobic interactions between polyhexamethylene biguanide and fenhexamid fungicides mixed in a ratio of 7 to 10 at 60 °C resulted in stable, spherical nanoparticles. There was a synergistic inhibitory effect on Sclerotinia sclerotiorum compared to when only a polyhexamethylene biguanide or fenhexamid fungicide was used under the same conditions. The introduction of polyhexamethylene biguanide into the fenhexamid fungicide did not increase the cytotoxicity of fenhexamid as revealed by Vicia faba genotoxicity evaluations.113
The use of covalent organic frameworks for controlling the pathogen has also been reported. In particular, zeolite imidazole framework-8 has been investigated due to its easy decomposition at acidic pH, its adjustable nano-scaled size, its large surface area and its good stability. In an investigation, a model pesticide, pyraclostrobin, was loaded on the zeolite imidazole framework made from dimethylimidazole zinc. The release rate of the combination was found to be pH dependent. In a pot experiment, addition of 0.1 μg mL−1 of the nanoformulated material led to the inhibition of Sclerotinia sclerotiorum by 9.4%. However, when the experiment was repeated in vitro, there was 16.4% inhibition of Sclerotinia sclerotiorum. This shows that both the pot experiment and vitro investigations showed inhibition of the pathogen by the nanoformulation and there was slow release of the highly efficient nanomaterial.114
Apart from the use of nanocomposites as delivery systems, they have also been used as antifungal agents against Sclerotinia sclerotiorum. In a study, polyethylene glycol and diethylene glycol were separately composited with copper-doped zinc oxide. The two nanocomposites were tested against Sclerotinia sclerotiorum via foliar spray on lettuce and it was found that they showed antifungal activities of 260 and 278 μg mL−1 against S. sclerotiorum, respectively. Their application also led to an increase in phenolic compound contents and antioxidant activities of the lettuce plants. Overall, the nanocomposite was effective against the fungal pathogens.116
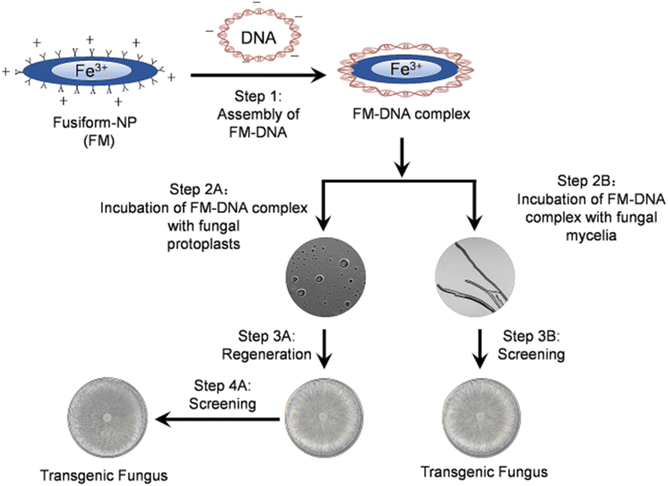 | ||
| Fig. 7 Schemes of the transformation route in S. sclerotiorum using Fusiform nanoparticles.118 Springer Nature-Open Access. | ||
3 Revisit of biological methods of controlling Sclerotinia sclerotiorum
3.1 Use of antagonistic micro-organisms to control Sclerotinia sclerotiorum
The sclerotia of S. sclerotiorum have been used as the source of some biological strains to serve as mycoparasites for controlling the same fungus (S. sclerotiorum). Examples of fungal strains isolated from S. sclerotiorum and used for controlling S. sclerotiorum are T. asperellum TRI 15, Cl. rosea TNAU-CR 02 and Co. minitans LU112 plants.119–121 The isolation of viruses from S. sclerotiorum for biocontrol of S. sclerotiorum is not very common. However, the use of mycoviruses (viruses that infect fungi) such as mycovirus SsNSRV-1, DNA mycovirus SsHADV-1, and deltaflexivirus SsDFV2 has been reported.122–124 In the case of bacterial biocontrol agents, the common sources are soil and plants.119 In this section, the fungi and bacteria that have been used for controlling S. sclerotiorum will be discussed.Aspergillus flavus strain NJC04 isolated from kiwifruit was also used for controlling Sclerotinia sclerotiorum in soybean. This fungal strain competed for nutrients and space with the pathogen leading to the inhibition of sclerotial development and inhibition of the growth of mycelia. Another cause of inhibition is the formation of antifungal kojic acid. In the culture medium, 87 ± 4 mg L−1 was detected. However, the reduction of the symptom of white rot depends on the number of spores per millilitres of the flavus strain that was applied to the soybean plant. For instance, there was 100% and 83.2% white rot symptom suppression when 1 × 108 and 1 × 107 NJC04 spores per mL were used respectively.31 Atallah et al. extended the investigation beyond Aspergillus flavus to check the efficiency of other species of Aspergillus against Sclerotinia sclerotiorum. The species investigated are Aspergillus pseudoelegans, Aspergillus flavus, Aspergillus niger, and Aspergillus japonicus. Under greenhouse conditions, their potential to suppress white mold disease was examined. It was observed that beans treated with Aspergillus niger and Aspergillus japonicus displayed the best rate of survival when compared with the bean plants that were not treated. Another observation was that the sporulation of the Aspergillus isolates is a function of the temperature. This is because Aspergillus became inhibited as the temperature lowered which made its colour change to white. In short, in carrying out investigations involving all the tested Aspergillus spp., the temperature factor should be considered. Interestingly, none of the Aspergillus species tested was aflatoxigenic and none was infectious to the legume that was used for the investigation.32
3.1.1.1 The use of Trichoderma spp. for controlling Sclerotinia sclerotiorum. Trichoderma isolate has been used for the treatment of soil that was used for planting in a bid to control Sclerotinia sclerotiorum. In the study carried out by Montalvão et al.,126 thirty-four Trichoderma antagonist fungal strains were used to treat soil. The T. afroharzianum species obtained from the soil cultivated with cotton performed better than other strains of Trichoderma. Though, all the strains tested led to hyphal penetration, deformation and folding against Sclerotinia sclerotiorum as revealed by the microscopy studies, notedly, the isolate of Trichoderma (CEN281 and CEN126) from tomato seedlings had the best antagonistic potential against the disease. Relatedly, two indigenous strains of Trichoderma which originated from Brazil (T. lentiforme CMAA 1585 and T. asperelloides CMAA 1584) were investigated for controlling Sclerotinia sclerotiorum. The two strains were able to release volatile organic carbons by solubilizing phosphorus minerals. The released volatile organic compounds were potent enough to impair the growth of the mycelia of the pathogen in the cotton plant. They equally reduced the number of sclerotia formed and the growth rate of Sclerotinia sclerotiorum on the plant. Treatment of sclerotia with the conidial suspensions of the two strains led to significant inhibition in myceliogenic germination. It was however observed that the efficiency of T. asperelloides CMAA 1584 was clearly better than that of T. lentiforme CMAA 1585 in controlling Sclerotinia sclerotiorum. In contrast, T. lentiforme CMAA 1585 was more efficient as a biostimulant than T. asperelloides CMAA 1584 as it contributes more to the growth of the cotton. Therefore, application of these two strains of Trichoderma will not only control the white rot diseases, but also contribute to the growth of cotton plants. This will reduce the amount of fungicides and synthetic fertilizers being applied to the cotton plants.33 Other investigations involving control of Sclerotinia sclerotiorum are shown in Table 4.
| Trichoderma investigated | Plant used | Methodology adopted | Key findings | Ref. |
|---|---|---|---|---|
| Trichoderma asperellum, Trichoderma longibrachiatum and Trichoderma harzianum | Egyptian clover (Trifolium alexandrinum L.) | Field experiments and in vitro investigations | The performance of Trichoderma harzianum stood out as it displayed an inhibition of 80.61% compared to the control; 1/10 (1.24 cm) was the best concentration of Trichoderma harzianum that gave the optimal response; 66% sclerotial inhibition; reduction of disease severity from 73.5% (untreated/control) to 51.7% (treated with Trichoderma harzianum); an increase in dry fodder weight and green weight compared to the control | 127 |
| Trichoderma harzianum, Trichoderma viride and other 69 Trichoderma spp. | Clover | Laboratory investigations involving the use of volatile and non-volatile metabolites, followed by greenhouse experiments | Trichoderma harzianum was more active than other isolates showing 20% sclerotiorum sclerotia hypha reduction; 20.37% inhibition of the growth of S. sclerotiorum mycelia was observed with 5% non-volatile metabolite isolate of T. viride; 15% and 25% concentrations of T. harzianum displayed an inhibition of 62.22 and 73.7% inhibition with non-volatile metabolite isolate; enhancement of seeds per pod, height of plants, fresh weight of root, shoot dry weight, branch number, pod diameter and total grain weight | 128 |
| Trichoderma virens, Trichoderma atroviride, Trichoderma koningii and Trichoderma harzianum | Canola | Seeds were coated with a Trichoderma conidia cell suspension and Trichoderma mixed with soil was also used; the use of nanocides with Trichoderma was also investigated in the green house | Trichoderma harzianum showed the best inhibition against the pathogen; no statistical difference between the use of Trichoderma alone and Trichoderma with nanocides; Trichoderma alone boosted the growth parameters compared with Trichoderma with nanocides | 129 |
| Trichoderma harzianum | Tomato | Control of Sclerotium rolfsii, Sclerotium cepivorum and Sclerotinia sclerotiorum using Trichoderma harzianum by laboratory investigations. Mycelial growth analysis was carried out by using the Petri dish pairing method | Reduction in the phytopathogen growth rate; no effect on the total area covered by the pathogen; the three strains showed similar conidium production | 130 |
3.1.1.2 The use of Coniothyrium minitans for controlling Sclerotinia sclerotiorum. The mycoparasitic attack of Sclerotinia by C. minitans has been reported.131 The mechanism of action of this mycoparasite is the generation of enzymes such as glucanases and chitinases which have the ability to degrade the cell walls of Sclerotinia sclerotiorum. Molecular studies have also revealed an increase in the β-1,3-glucanase gene cmg1 in C. minitans when it attacks Sclerotinia sclerotiorum132. Different plants have been used to investigate the efficiency of the mycoparasite in controlling Sclerotinia sclerotiorum. For instance, aerial application of this mycoparasite both in vivo and in vitro to rapeseed plants revealed that there was restriction in the growth of the mycelia of Sclerotinia sclerotiorum34. The efficiency of this mycoparasite has also been confirmed by using it to control Sclerotinia sclerotiorum in peanuts,133 tomato134 and cabbage.135 Yang et al.136 reported that application of this mycoparasite along with N–P–K fertilizers in rapeseed resulted in dosage-dependent mycelial growth inhibition and a maximum of 10% conidial germination inhibition in Sclerotinia sclerotiorum. This combined strategy is advantageous because it reduces the cost of labour and boosts the yield of the plant. Another combined strategy involves the combination of Trichoderma species and Coniothyrium minitans for controlling Sclerotinia sclerotiorum. In an investigation, there was 30–50% reduction in white mould disease (compared with the untreated control) when C. minitans LU112 was combined with T. virens LU555 and T. hamatum LU595. This was clearly better than the disease reduction observed when carbendazim fungicide treatment was used under similar conditions.137
| Bacteria used for controlling the pathogen | Plant used | Instrumentation and technique used for the study | Key findings | Ref. |
|---|---|---|---|---|
| Bacillus cereus CF4-51 | Multiple crops | Gas chromatography–mass spectrometry (GC-MS), a scanning electron microscope (SEM), quantitative reverse transcription polymerase chain reaction (qRT-PCR), and whole-genome sequencing | Volatile organic compounds (VOCs) released by CF4-51 (including 2-pentadecanone, 6,10,14-trimethyl-,1,2-heptadecane, cyclododecane, dibutyl phthalate and bis(2-methylpropyl) ester) inhibited the formation of sclerotia and damaged the hyphae of S. sclerotiorum | 139 |
| Pseudomonas spp (18 isolates) | Potato and rice | Broth-based in vitro dual culturing; analysis of plant growth promoting rhizobacteria (PGPR) | Mycelial growth inhibition ranging from 68.9 to 42.6%; higher phosphorus solubilizing efficiency; inhibition of black scurf severity up to 67.59% compared to the control | 140 |
| Bacillus species A5F | Soybean | ‘One-variable-at-a-time’ (OVAT) approach; high resolution liquid chromatography–mass spectrometry (HR LC-MS); a transmission electron microscope (TEM) and qRT-PCR | Significant effects on seed weight, number of pods, shoot biomass and chlorophyll content; reduction in the disease incidence of S. sclerotiorum infected soybean plants; ITS gene copy number reduction of 16% compared to the control | 141 |
| Streptomyces albulus CK-15 (using its secondary metabolite (wuyiencin) | Soybean | Seed-soaking method | Spraying wuyiencin (200 μg mL−1) three times was the best with an inhibitory percentage of 64.0% but inferior to the 77.6% obtained when 200 μg mL−1 dimethachlon was used | 142 |
| Streptomyces albulus CK-15 (using its secondary metabolite wuyiencin) | Soybean | In vitro | 78.3% inhibition of mycelial growth; inhibition of the formation of sclerotia; an increase in cell membrane permeability; a decrease in the content of pectin methyl-galacturonic enzymes, oxalic acid and activities of polygalacturonase | 143 |
| Five Bacillus strains | Potato | HPLC; the radial growth method for antifungal evaluation; a Zeiss light microscope | Ergosterol (in the cell wall) was blocked; inhibition of sclerotial formation and 100% growth inhibition; polyene compound production by the bacterial strains | 144 |
| Three Streptomyces species (S. sampsonii, S. rochei and S. griseus) | Green beans | Nucleotide sequencing; PCR amplification; in vitro and in vivo investigations; light microscopy and SEM | S. rochei showed the best inhibition among the three bacteria followed by S. griseus; S. sampsonii was more efficient as a bioagent in reducing mycelial growth pathogen by 84.50% | 145 |
| Bacillus sp. FSQ1 | Bean (Phaseolus vulgaris) | Sequencing | Inhibition of the growth of the phytopathogen by 2.4 ± 0.2 cm through in vitro antagonism | 146 |
| Streptomyces | — | RT-qPCR and RNA-seq | ε-Poly-L-lysine is produced; inhibition of the mycelial growth with EC50 values of 283 μg mL−1 | 147 |
4 Conclusion and future perspectives
To achieve sustainable global food security, efforts must be geared towards the fight against plant diseases. Studies on the use of gene editing strategy (usually modifying susceptible genes or effector targeted genes), transgenic strategy (such as genes from microorganisms), and spraying small RNA induced gene silence (SIGS) should be focussed on reducing the menace of white mold caused by Sclerotinia sclerotiorum. The limitations to the commercial utilization of gene silencing are a lack of general approaches that can be used for all plant species and off-target silencing. More research is needed to overcome these challenges. Using UV radiation for controlling the pathogen without harming beneficial microbes through mutation should be investigated. Also, the use of plants that are resistant to white mould along with the pretreatment of the seed prior to planting should be considered.Although different natural compounds have been used for the control of white mould, milsana, cinnamaldehyde and jojoba oil have not been used for controlling Sclerotinia sclerotiorum. Therefore, the use of these natural compounds for controlling white mould diseases should be investigated. Apart from using ordinary nanoparticles for the control of the pathogen, combinations of different nanoparticles with other methods such as gene silencing and the use of antagonistic micro-organisms should be studied. Before this can be achieved, further research needs to be conducted to understand the best conditions that will work for the hybrid control strategies. Also, increasing the storage life of the fungicides derived from the botanical source to compete with the chemical fungicide is still a challenge which requires serious research attention.
Despite the advantages of botanical fungicides over synthetic fungicides, large scale commercialization has not been achieved. This could be due to variation of the active component of the extract with climatic conditions, speedy degradation of botanical pesticides, and low awareness among the farmers.76 These challenges could be solved by focussing research on these limitations. Research studies that are urgently required in this aspect are quicker market introduction with price control, improving the awareness of their usage to farmers, establishment of regulatory guidelines, establishment of possible pathways to obtain maximum extraction of bioactive compounds from plants, and carrying out in vivo experiments in open fields and greenhouses to understand phytotoxicity and toxicity to other living organisms. Future research will also require understanding the shelf-life of grains stored by using these botanical fungicides. Some of these would require collaborative research between sciences, humanities and social sciences.
As discussed in this review, new strategies are emerging, but the use of nanomaterials is one of the major strategies that could significantly reduce fungal diseases in plants (particularly white mold fungal diseases caused by Sclerotinia sclerotiorum). However, the impacts of nanoparticles on plants must be fully understood before they would be used for antifungal applications. This is necessary to prevent the applied nanoparticles from having detrimental effects on the anatomy and physiological attributes of plants. Even though some metallic and metal oxide nanoparticles have been investigated, ternary oxides and quaternary oxide nanoparticles have not been investigated as antifungal agents against fungal pathogens. In addition, the potency of several sulphide nanoparticles has not been studied. Besides, the use of carbon-based materials such as activated carbon, carbon nanotubes, cyclodextrins, fullerenes, gels (xerogels, aerogels, cryogels, and hydrogels), polymers, alginates, reduced graphene oxide, biochar and their composites has not been studied. Chemists and materials scientists will have significant roles to play in ensuring that these materials and nanocomposites are efficiently produced. Simultaneous control and detection of the pathogens can be done by using different nanoparticles/nanocomposites. Finally, most studies on the control of Sclerotinia sclerotiorum were conducted via in vitro methods. It is necessary to carry out these investigations using in vivo methods because laboratory environments are not the same as what is obtainable in the field. When this has been done, commercial applications of nanoparticles in the field for controlling fungal pathogens will be feasible.
Data availability
All data used for analysis in this manuscript are available from the corresponding author or first author upon reasonable request.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Timothy O. Ajiboye gratefully acknowledges the postdoctoral funding support from the University of the Free State, South Africa. Lisa Ann Rothmann (Plant Sciences: Plant Pathology, University of the Free State, Bloemfontein, South Africa) is acknowledged for reading and correcting the manuscript.References
- M. A. Khan, W. A. Cowling, S. S. Banga, M. J. Barbetti, A. Y. Cantila, J. C. Amas, W. J. Thomas, M. P. You, V. Tyagi and B. Bharti, Genetic and molecular analysis of stem rot (Sclerotinia sclerotiorum) resistance in Brassica napus (canola type), Heliyon, 2023, 9, 19237 CrossRef PubMed.
- M. Singh, R. Avtar, N. Kumar, R. Punia, N. Lakra, N. Kumari, M. Bishnoi, R. Rohit, R. R. Choudhary, R. S. Khedwal, R. K. Meena, A. Dhillon and V. K. Singh, Assessment of Sclerotinia Stem and Leaf Rot Resistance and its Association with Physical Strength Attributes in Brassicaceae with Special Emphasis on Brassica Juncea, J. Plant Growth Regul., 2023, 42, 6021–6037 CrossRef CAS.
- R. W. Webster, B. D. Mueller, S. P. Conley and D. L. Smith, Integration of Soybean (Glycine max) Resistance Levels to Sclerotinia Stem Rot into Predictive Sclerotinia sclerotiorum Apothecial Models, Plant Dis., 2023, 107, 2763–2768 CrossRef CAS PubMed.
- R. Darvishzadeh, Association of SSR markers with partial resistance to Sclerotinia sclerotiorum isolates in sunflower ('Helianthus annuus' L.), Aust. J. Crop Sci., 2012, 6, 276–282 CAS.
- S. Trivedi, P. P. Jambhulkar, S. Kumar and P. Niranjan, A modified and effective stem inoculation technique for artificial screening against Sclerotinia sclerotiorum in mustard, J. Phytopathol., 2023, 171, 258–264 CrossRef CAS.
- M. T. Abdullah, N. Y. Ali and P. Suleman, Biological control of Sclerotinia sclerotiorum (Lib.) de Bary with Trichoderma harzianum and Bacillus amyloliquefaciens, Crop Prot., 2008, 27, 1354–1359 CrossRef.
- M. Kabbage, O. Yarden and M. B. Dickman, Pathogenic attributes of Sclerotinia sclerotiorum: Switching from a biotrophic to necrotrophic lifestyle, Plant Sci., 2015, 233, 53–60 CrossRef CAS PubMed.
- V. K. Macioszek, P. Marciniak and A. K. Kononowicz, Impact of Sclerotinia sclerotiorum Infection on Lettuce (Lactuca sativa L.) Survival and Phenolics Content—A Case Study in a Horticulture Farm in Poland, Pathogens, 2023, 12, 1416 CrossRef CAS PubMed.
- K. Perveen, A. Haseeb and P. K. Shukla, Effect of Sclerotinia sclerotiorum on the disease development, growth, oil yield and biochemical changes in plants of Mentha arvensis, Saudi J. Biol. Sci., 2010, 17, 291–294 CrossRef CAS PubMed.
- M. D. Bolton, B. P. Thomma and B. D. Nelson, Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen, Mol. Plant Pathol., 2006, 7, 1–16 CrossRef CAS PubMed.
- P. Mazumdar, Sclerotinia stem rot in tomato: a review on biology, pathogenicity, disease management and future research priorities, J. Plant Dis. Prot., 2021, 128, 1403–1431 CrossRef.
- J. Rollins, C. Cuomo, M. Dickman and L. Kohn, Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens, 2014, pp. 1–17 Search PubMed.
- J. Willbur, M. McCaghey, M. Kabbage and D. L. Smith, An overview of the Sclerotinia sclerotiorum pathosystem in soybean: impact, fungal biology, and current management strategies, Trop. Plant Pathol., 2019, 44, 3–11 CrossRef.
- M. R. Ojaghian, J.-Z. Zhang, F. Zhang, W. Qiu, X.-L. Li, G.-L. Xie and S.-J. Zhu, Early detection of white mold caused by Sclerotinia sclerotiorum in potato fields using real-time PCR, Mycological Progress, 2016, 15, 959–965 CrossRef.
- S. Li, R. Marquardt and D. Abramson, Immunochemical detection of molds: a review, J. Food Prot., 2000, 63, 281–291 CrossRef CAS PubMed.
- M. L. Machado, F. A. Pinto, T. J. d. Paula, D. M. d. Queiroz and O. A. Cerqueira, White mold detection in common beans through leaf reflectance spectroscopy, Eng. Agric., 2015, 35, 1117–1126 Search PubMed.
- R. S. Hazra, J. Roy, L. Jiang, D. C. Webster, M. M. Rahman and M. Quadir, Biobased, Macro-, and Nanoscale Fungicide Delivery Approaches for Plant Fungi Control, ACS Appl. Bio Mater., 2023, 6, 2698–2711 CrossRef CAS PubMed.
- H.-X. Ma, Y. Chen, J.-X. Wang, W.-Y. Yu, Z.-H. Tang, C.-J. Chen and M.-G. Zhou, Activity of carbendazim, dimethachlon, iprodione, procymidone and boscalid against Sclerotinia stem rot in Jiangsu Province of China, Phytoparasitica, 2009, 37, 421–429 CrossRef CAS.
- S. Zhiqi, Z. Mingguo and Y. Zhongyin, Resistance of Sclerotinia sclerotiorum to carbendazim and dimethachlon, Chin. J. Oil Crop Sci., 2000, 22, 54–57 Search PubMed.
- S. K. Goswami, V. Singh, H. Chakdar and P. Choudhary, Harmful effects of fungicides-Current status, Int. J. Agri. Environ. Biotechnol., 2018, 11, 1011–1019 Search PubMed.
- P. Cocco, Time for Re-Evaluating the Human Carcinogenicity of Ethylenedithiocarbamate Fungicides? A Systematic Review, Int. J. Environ. Res. Public Health, 2022, 19, 2632 CrossRef CAS PubMed.
- A. J. Peltier, C. A. Bradley, M. I. Chilvers, D. K. Malvick, D. S. Mueller, K. A. Wise and P. D. Esker, Biology, Yield loss and Control of Sclerotinia Stem Rot of Soybean, J. Integrat. Pest Manage., 2012, 3, B1–B7 CrossRef.
- M. A. Khan, W. Cowling, S. S. Banga, M. P. You, V. Tyagi, B. Bharti and M. J. Barbetti, Inheritance of leaf resistance to Sclerotinia sclerotiorum in Brassica napus and its genetic correlation with cotyledon resistance, Euphytica, 2020, 216, 188 CrossRef CAS.
- J. N. Matthiessen and J. A. Kirkegaard, Biofumigation and enhanced biodegradation: opportunity and challenge in soilborne pest and disease management, Crit. Rev. Plant Sci., 2006, 25(3), 235–265 CrossRef CAS.
- L. H. Moss, E. Epstein and T. Logan, Comparing the characteristics, risks and benefits of soil amendments and fertilizers used in agriculture, Residuals and Biosolids Conference 2002, Water Environment Federation, 2002, pp. 602–623 Search PubMed.
- H. Zhang, L. Wang, T. Tian and J. Yin, A Review of Unmanned Aerial Vehicle Low-Altitude Remote Sensing (UAV-LARS) Use in Agricultural Monitoring in China, Remote Sens., 2021, 13, 1221 CrossRef.
- K. Neupane and F. Baysal-Gurel, Automatic Identification and Monitoring of Plant Diseases Using Unmanned Aerial Vehicles: A Review, Remote Sens., 2021, 13, 3841 CrossRef.
- A. A. Khakimov, A. U. Omonlikov and S. B. U. Utaganov, Current status and prospects of the use of biofungicides against plant diseases, GSC Biol. Pharm. Sci., 2020, 13, 119–126 CrossRef.
- C. Temur and O. Tiryaki, Irradiation alone or combined with other alternative treatments to control postharvest diseases, Afr. J. Agric. Res., 2013, 8, 421–434 Search PubMed.
- G. M. Mascarin, A. V. R. da Silva, T. P. da Silva, N. N. Kobori, M. A. B. Morandi and W. Bettiol, Clonostachys rosea: Production by Submerged Culture and Bioactivity Against Sclerotinia sclerotiorum and Bemisia tabaci, Front. Microbiol., 2022, 13, 851000 CrossRef PubMed.
- G. Y. Zhu, X. C. Shi, D. D. Herrera-Balandrano, S. Y. Wang and P. Laborda, Non-aflatoxigenic kojic acid-producing Aspergillus flavus NJC04 reduces the symptoms of Sclerotinia sclerotiorum in soybean, Biol. Control, 2022, 176, 105064 CrossRef CAS.
- O. O. Atallah, Y. S. A. Mazrou, M. M. Atia, Y. Nehela, A. S. Abdelrhim and M. M. Nader, Polyphasic Characterization of Four Aspergillus Species as Potential Biocontrol Agents for White Mold Disease of Bean, J. Fungi, 2022, 8, 626 CrossRef CAS PubMed.
- L. G. Silva, R. C. Camargo, G. M. Mascarin, P. S. D. O. Nunes, C. Dunlap and W. Bettiol, Dual functionality of Trichoderma: Biocontrol of Sclerotinia sclerotiorum and biostimulant of cotton plants, Front. Plant Sci., 2022, 13, 983127 CrossRef PubMed.
- G. Q. Li, H. C. Huang, H. J. Miao, R. S. Erickson, D. H. Jiang and Y. N. Xiao, Biological Control of Sclerotinia Diseases of Rapeseed by Aerial Applications of the Mycoparasite Coniothyrium minitans, Eur. J. Plant Pathol., 2006, 114, 345–355 CrossRef.
- K. Z. K. Al-Karaawi and D. S. Al-Waily, Integrated Control of White Rot in Eggplant by using Pseudomonas flourescens, Penicillium Commune and Trichoderma asperellum in Iraq, IOP Conf. Ser.: Earth Environ. Sci., 2023, 1158, 072010 CrossRef.
- V.-C. Han, P. J. Michael, B. Swift and S. J. Bennett, Biological control of Sclerotinia sclerotiorum: Modes of action of biocontrol agents, soil organic amendments, and soil microbiome manipulation, Biol. Control, 2023, 186, 105346 CrossRef CAS.
- U. Smolińska and B. Kowalska, Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum––a review, J. Plant Pathol., 2018, 100, 1–12 CrossRef.
- P. Sharma, P. Meena, P. Verma, G. Saharan, N. Mehta, D. Singh and A. Kumar, Sclerotinia sclerotiorum (Lib) de Bary causing Sclerotinia rot in oilseed Brassicas: a review, J. Oilseed Brassica, 2016, 1, 1–44 Search PubMed.
- U. Smolińska and B. Kowalska, Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum –– a review, J. Plant Pathol., 2018, 100, 1–12 CrossRef.
- M. D. Bolton, B. P. H. J. Thomma and B. D. Nelson, Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen, Mol. Plant Pathol., 2006, 7, 1–16 CrossRef CAS PubMed.
- L. Liu, X. Lyu, Z. Pan, Q. Wang, W. Mu, U. Benny, J. A. Rollins and H. Pan, The C2H2 transcription factor SsZFH1 regulates the size, number, and development of apothecia in Sclerotinia sclerotiorum, Phytopathology, 2022, 112, 1476–1485 CrossRef CAS PubMed.
- P. Cotton, Z. Kasza, C. Bruel, C. Rascle and M. Fèvre, Ambient pH controls the expression of endopolygalacturonase genes in the necrotrophic fungus Sclerotinia sclerotiorum, FEMS Microbiol. Lett., 2003, 227, 163–169 CrossRef CAS PubMed.
- S. G. Cessna, V. E. Sears, M. B. Dickman and P. S. Low, Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant, Plant Cell, 2000, 12, 2191–2199 CrossRef CAS PubMed.
- B. Williams, M. Kabbage, H.-J. Kim, R. Britt and M. B. Dickman, Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment, PLoS Pathogens, 2011, 7, e1002107 CrossRef CAS PubMed.
- Y. Wang, Y.-B. Duan and M.-G. Zhou, Molecular and biochemical characterization of boscalid resistance in laboratory mutants of Sclerotinia sclerotiorum, Plant Pathol., 2015, 64, 101–108 CrossRef CAS.
- Y. Cao, X. Yan, S. Ran, J. Ralph, R. A. Smith, X. Chen, C. Qu, J. Li and L. Liu, Knockout of the lignin pathway gene BnF5H decreases the S/G lignin compositional ratio and improves Sclerotinia sclerotiorum resistance in Brassica napus, Plant, Cell Environ., 2022, 45, 248–261 CrossRef CAS PubMed.
- C. Ordóñez-Valencia, R. Ferrera-Cerrato, R. E. Quintanar-Zúñiga, C. M. Flores-Ortiz, G. J. M. Guzmán, A. Alarcón, J. Larsen and O. García-Barradas, Morphological development of sclerotia by Sclerotinia sclerotiorum: a view from light and scanning electron microscopy, Ann. Microbiol., 2015, 65, 765–770 CrossRef.
- Y. Ben-Yephet, A. Genizi and E. Siti, Sclerotial survival and apothecial production by Sclerotinia sclerotiorum following outbreaks of lettuce drop, Phytopathology, 1993, 83, 509–513 CrossRef.
- M. J. Butler, R. B. Gardiner and A. W. Day, Melanin synthesis by Sclerotinia sclerotiorum, Mycologia, 2009, 101, 296–304 CrossRef CAS PubMed.
- M. R. Maximiano, L. S. Santos, C. Santos, F. J. L. Aragão, S. C. Dias, O. L. Franco and A. Mehta, Host induced gene silencing of Sclerotinia sclerotiorum effector genes for the control of white mold, Biocatal. Agric. Biotechnol., 2022, 40, 102302 CrossRef CAS.
- T. Sijen, I. Vijn, A. Rebocho, R. van Blokland, D. Roelofs, J. N. Mol and J. M. Kooter, Transcriptional and posttranscriptional gene silencing are mechanistically related, Curr. Biol., 2001, 11, 436–440 CrossRef CAS PubMed.
- S. B. Ghag, Host induced gene silencing, an emerging science to engineer crop resistance against harmful plant pathogens, Physiological and, Mol. Plant Pathol., 2017, 100, 242–254 CrossRef.
- C. Andrade, M. L. Tinoco, A. Rieth, F. Maia and F. Aragão, Host-induced gene silencing in the necrotrophic fungal pathogen Sclerotinia sclerotiorum, Plant Pathol., 2016, 65, 626–632 CrossRef CAS.
- Y. Xu, J. Tan, J. Lu, Y. Zhang and X. Li, RAS signalling genes can be used as host-induced gene silencing targets to control fungal diseases caused by Sclerotinia sclerotiorum and Botrytis cinerea, Plant Biotechnol. J., 2024, 22, 262–277 CrossRef CAS PubMed.
- B. Lv, X. Zhao, Y. Guo, S. Li and M. Sun, Serine protease CrKP43 interacts with MAPK and regulates fungal development and mycoparasitism in Clonostachys chloroleuca, Microbiol. Spectrum, 2023, 11, 2448–2523 Search PubMed.
- P. Pant and J. Kaur, Spray-Induced Gene Silencing of SsOah1 and SsCyp51 confers protection to Nicotiana benthamiana and Brassica juncea against Sclerotinia sclerotiorum, Physiol. Mol. Plant Pathol., 2023, 127, 102109 CrossRef CAS.
- L. Qin, J. Nong, K. Cui, X. Tang, X. Gong, Y. Xia, Y. Xu, Y. Qiu, X. Li and S. Xia, SsCak1 Regulates Growth and Pathogenicity in Sclerotinia sclerotiorum, Int. J. Mol. Sci., 2023, 24, 12610 CrossRef CAS PubMed.
- B. Lv, Y. Guo, X. Zhao, S. Li and M. Sun, Glucose-6-phosphate 1-Epimerase CrGlu6 Contributes to Development and Biocontrol Efficiency in Clonostachys chloroleuca, J. Fungi, 2023, 9, 764 CrossRef CAS PubMed.
- W. Jiao, W. Ding, J. A. Rollins, J. Liu, Y. Zhang, X. Zhang and H. Pan, Cross-Talk and Multiple Control of Target of Rapamycin (TOR) in Sclerotinia sclerotiorum, Microbiol. Spectrum, 2023, 11, 00013–00023 Search PubMed.
- Y. Xu, Y. Qiu, Y. Zhang and X. Li, A cAMP phosphodiesterase is essential for sclerotia formation and virulence in Sclerotinia sclerotiorum, Front. Plant Sci., 2023, 14, 1175552 CrossRef PubMed.
- L. Tian, J. Li, Y. Xu, Y. Qiu, Y. Zhang and X. Li, A MAP kinase cascade broadly regulates the lifestyle of Sclerotinia sclerotiorum and can be targeted by HIGS for disease control, Plant J., 2023, 324–344 Search PubMed.
- C. C. NUNES and R. A. DEAN, Host-induced gene silencing: a tool for understanding fungal host interaction and for developing novel disease control strategies, Mol. Plant Pathol., 2012, 13, 519–529 CrossRef CAS PubMed.
- Y. Qiao, M. Zhang, Y. Cao, Q. Mi, S. Liang, J. Feng and Y. Wang, Postharvest sclerotinia rot control in carrot by the natural product hinokitiol and the potential mechanisms involved, Int. J. Food Microbiol., 2022, 383, 109939 CrossRef CAS PubMed.
- A. E. Edris and E. S. Farrag, Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase, Food/Nahrung, 2003, 47, 117–121 CrossRef CAS PubMed.
- Y. Cao, X. Zhang, X. Song, W. Li, Z. Ren, J. Feng, Z. Ma, X. Liu and Y. Wang, Efficacy and toxic action of the natural product natamycin against Sclerotinia sclerotiorum, Pest Manage. Sci., 2023, 1981–1990 CAS.
- P. Devi, R. Parvatkar, R. Rajamanikam, S. Wahidullah and N. Thakur, Kojic Acid from Aspergillus wentii: A Journey from Isolation to Application, Fungi Bioactive Metabolites, Integration of Pharmaceutical Applications, Springer, 2024, pp. 709–722 Search PubMed.
- M. Saeedi, M. Eslamifar and K. Khezri, Kojic acid applications in cosmetic and pharmaceutical preparations, Biomed. Pharmacother., 2019, 110, 582–593 CrossRef CAS PubMed.
- G.-Y. Zhu, X.-C. Shi, S.-Y. Wang, B. Wang and P. Laborda, Antifungal Mechanism and Efficacy of Kojic Acid for the Control of Sclerotinia sclerotiorum in Soybean, Front. Plant Sci., 2022, 13, 845698 CrossRef PubMed.
- A. Sahrawat, J. Sharma and S. K. Jawla, Impact of Dactyloctenium aegyptium Weed Extracts on Soil Borne Fungal Phytopathogens and Legume Crop-Vigna, Indian J. Agric. Res., 2022, 56, 751–754 Search PubMed.
- G.-Y. Zhu, X.-C. Shi, S.-Y. Wang, B. Wang and P. Laborda, Antifungal mechanism and efficacy of kojic acid for the control of Sclerotinia sclerotiorum in soybean, Front. Plant Sci., 2022, 13, 845698 CrossRef PubMed.
- Q. R. Chu, Y. H. He, C. Tang, Z. J. Zhang, X. F. Luo, B. Q. Zhang, Y. Zhou, T. L. Wu, S. S. Du, C. J. Yang and Y. Q. Liu, Design, Synthesis, and Antimicrobial Activity of Quindoline Derivatives Inspired by the Cryptolepine Alkaloid, J. Agric. Food Chem., 2022, 70, 2851–2863 CrossRef CAS PubMed.
- C.-J. Yang, H.-x. Li, J.-R. Wang, Z.-J. Zhang, T.-L. Wu, Y.-Q. Liu, C. Tang, Q.-R. Chu, S.-S. Du and Y.-H. He, Design, synthesis and biological evaluation of novel evodiamine and rutaecarpine derivatives against phytopathogenic fungi, Eur. J. Med. Chem., 2022, 227, 113937 CrossRef CAS PubMed.
- W.-B. Zhao, Z.-M. Zhao, Y. Ma, A.-P. Li, Z.-J. Zhang, Y.-M. Hu, Y. Zhou, R. Wang, X.-F. Luo, B.-Q. Zhang, Y.-L. Wang, G.-F. Hu and Y.-Q. Liu, Antifungal activity and preliminary mechanism of pristimerin against Sclerotinia sclerotiorum, Ind. Crops Prod., 2022, 185, 115124 CrossRef CAS.
- C.-W. Zhang, X.-J. Zhong, Y.-S. Zhao, M. S. R. Rajoka, M. H. Hashmi, P. Zhai and X. Song, Antifungal natural products and their derivatives: A review of their activity and mechanism of actions, Pharmacol. Res., 2023, 7, 100262 Search PubMed.
- C. Regnault-Roger and B. J. Philogène, Past and current prospects for the use of botanicals and plant allelochemicals in integrated pest management, Pharm. Biol., 2008, 46, 41–52 CrossRef CAS.
- E. M. Deresa and T. F. Diriba, Phytochemicals as alternative fungicides for controlling plant diseases: A comprehensive review of their efficacy, commercial representatives, advantages, challenges for adoption, and possible solutions, Heliyon, 2023, 9, 13810 CrossRef PubMed.
- A. S. Kashyap, N. Manzar, S. K. Vishwakarma, C. Mahajan and U. Dey, Tiny but mighty: metal nanoparticles as effective antimicrobial agents for plant pathogen control, World J. Microbiol. Biotechnol., 2024, 40, 104 CrossRef PubMed.
- M. Gul, R. S. Khan, Z. U. Islam, S. Khan, A. Shumaila, S. Umar, S. Khan, Brekhna, M. Zahoor and A. Ditta, Nanoparticles in plant resistance against bacterial pathogens: current status and future prospects, Mol. Biol. Rep., 2024, 51, 92 CrossRef CAS PubMed.
- T. O. Ajiboye, S. O. Babalola and D. C. Onwudiwe, Photocatalytic Inactivation as a Method of Elimination of E. coli from Drinking Water, Appl. Sci., 2021, 11, 1313 CrossRef CAS.
- M. K. Ray, A. K. Mishra, Y. K. Mohanta, S. Mahanta, I. Chakrabartty, N. A. Kungwani, S. K. Avula, J. Panda and R. N. Pudake, Nanotechnology as a Promising Tool against Phytopathogens: A Futuristic Approach to Agriculture, Agriculture, 2023, 13, 1856 CrossRef CAS.
- A. A. Tomah, Z. Zhang, I. S. A. Alamer, A. A. Khattak, T. Ahmed, M. Hu, D. Wang, L. Xu, B. Li and Y. Wang, The Potential of Trichoderma-Mediated Nanotechnology Application in Sustainable Development Scopes, Nanomaterials, 2023, 13, 2475 CrossRef CAS PubMed.
- R. M. S. El-Ashmony, N. S. S. Zaghloul, M. Milošević, M. Mohany, S. S. Al-Rejaie, Y. Abdallah and A. A. Galal, The Biogenically Efficient Synthesis of Silver Nanoparticles Using the Fungus Trichoderma harzianum and Their Antifungal Efficacy against Sclerotinia sclerotiorum and Sclerotium rolfsii, J. Fungi, 2022, 8, 597 CrossRef CAS PubMed.
- M. Guilger, T. Pasquoto-Stigliani, N. Bilesky-Jose, R. Grillo, P. C. Abhilash, L. F. Fraceto and R. De Lima, Biogenic silver nanoparticles based on trichoderma harzianum: Synthesis, characterization, toxicity evaluation and biological activity, Sci. Rep., 2017, 7, 44421 CrossRef CAS PubMed.
- M. Guilger-Casagrande, T. Germano-Costa, N. Bilesky-José, T. Pasquoto-Stigliani, L. Carvalho, L. F. Fraceto and R. de Lima, Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards Sclerotinia sclerotiorum, J. Nanobiotechnol., 2021, 19, 1–18 CrossRef PubMed.
- J. Trzcińska-Wencel, M. Wypij, M. Rai and P. Golińska, Biogenic nanosilver bearing antimicrobial and antibiofilm activities and its potential for application in agriculture and industry, Front. Microbiol., 2023, 14, 1125685 CrossRef PubMed.
- T. Gupta and J. Saxena, Biogenic synthesis of silver nanoparticles from aspergillus oryzae MTCC 3107 against plant pathogenic fungi sclerotinia sclerotiorum MTCC 8785, J. Microbiol., Biotechnol. Food Sci., 2023, 12, 9387 Search PubMed.
- A. A. Tomah, I. S. A. Alamer, B. Li and J. Z. Zhang, Mycosynthesis of silver nanoparticles using screened trichoderma isolates and their antifungal activity against sclerotinia sclerotiorum, Nanomaterials, 2020, 10, 1–15 CrossRef PubMed.
- J. Mittal, A. Singh, A. Batra and M. M. Sharma, Synthesis and characterization of silver nanoparticles and their antimicrobial efficacy, Part. Sci. Technol., 2017, 35, 338–345 CrossRef CAS.
- E. E. Hafez and S. S. Kabeil, Antimicrobial activity of nano-silver particles produced by micro algae, J. Pure Appl. Microbiol., 2013, 7, 35–42 CAS.
- J. S. Min, K. S. Kim, S. W. Kim, J. H. Jung, K. Lamsal, S. B. Kim, M. Jung and Y. S. Lee, Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi, Plant Pathol. J., 2009, 25, 376–380 CrossRef CAS.
- T. Pasquoto-Stigliani, M. Guilger-Casagrande, E. V. R. Campos, T. Germano-Costa, N. Bilesky-José, B. B. Migliorini, L. O. Feitosa, B. T. Sousa, H. C. de Oliveira, L. F. Fraceto and R. Lima, Titanium biogenic nanoparticles to help the growth of Trichoderma harzianum to be used in biological control, J. Nanobiotechnol., 2023, 21, 166 CrossRef CAS PubMed.
- M. E. Sadek, Y. M. Shabana, K. Sayed-Ahmed and A. H. Abou Tabl, Antifungal Activities of Sulfur and Copper Nanoparticles against Cucumber Postharvest Diseases Caused by Botrytis cinerea and Sclerotinia sclerotiorum, J. Fungi, 2022, 8, 412 CrossRef CAS PubMed.
- J. Xu, W. Jia, C. Hu, M. Nie, J. Ming, Q. Cheng, M. Cai, X. Sun, X. Li, X. Zheng, J. Wang and X. Zhao, Selenium as a potential fungicide could protect oilseed rape leaves from Sclerotinia sclerotiorum infection, Environ. Pollut., 2020, 257, 113495 CrossRef CAS PubMed.
- W. Jia, C. Hu, J. Xu, J. Ming, Y. Zhao, M. Cai, X. Sun, X. Liu and X. Zhao, Dissolved organic matter derived from rape straw pretreated with selenium in soil improves the inhibition of Sclerotinia sclerotiorum growth, J. Hazard. Mater., 2019, 369, 601–610 CrossRef CAS PubMed.
- Q. Zhang, J. Wang, J. Wang, M. Liu, X. Ma, Y. Bai, Q. Chen, S. Sheng and F. Wang, Nano-Silicon Triggers Rapid Transcriptomic Reprogramming and Biochemical Defenses in Brassica napus Challenged with Sclerotinia sclerotiorum, J. Fungi, 2023, 9, 1108 CrossRef CAS PubMed.
- V. F. Consolo, A. Torres-Nicolini, V. A. Alvarez and M. Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens, Sci. Rep., 2020, 10, 20499 CrossRef CAS PubMed.
- Z. A. M. Al-Tememe, R. G. Abdalmoohsin, M. T. Mohammadali, M. M. Al-Mosawy and Z. M. Al-Masoudi, Molecular diagnosis of the fungus Sclerotinia sclerotiorum: A causal agent of white rot disease in Solanum melongena L. and its control using zinc oxide nanoparticles, Biopestic. Int., 2019, 15, 51–56 Search PubMed.
- H. A. Mehdi, B. T. Mohammed and A. M. Bashy, Effect of nano-silvers, nano-zinc oxide and Benomyl pesticide on some physiological properties of Sclerotinia sclerotiorum, Biochem. Cell. Arch., 2018, 18, 2181–2196 Search PubMed.
- P. Tryfon, N. N. Kamou, S. Mourdikoudis, K. Karamanoli, U. Menkissoglu-Spiroudi and C. Dendrinou-Samara, CuZn and ZnO Nanoflowers as Nano-Fungicides against Botrytis cinerea and Sclerotinia sclerotiorum: Phytoprotection, Translocation, and Impact after Foliar Application, Materials, 2021, 14, 7600 CrossRef CAS PubMed.
- K. Dulta, G. Koşarsoy Ağçeli, P. Chauhan, R. Jasrotia and P. K. Chauhan, Ecofriendly Synthesis of Zinc Oxide Nanoparticles by Carica papaya Leaf Extract and Their Applications, J. Cluster Sci., 2022, 33, 603–617 CrossRef CAS.
- K. Dulta, J. Aman, M. Kamran, A. Trehan, K. Sharma, S. Kulwanshi, S. Kumari and P. Chauhan, ZnO Nanoparticles Synthesis using Potato Peel Waste and their Antifungal Activity, Malays. J. Biochem. Mol. Biol., 2023, 25, 176–183 Search PubMed.
- A. Saka, Y. Shifera, L. T. Jule, B. Badassa, N. Nagaprasad, R. Shanmugam, L. Priyanka Dwarampudi, V. Seenivasan and K. Ramaswamy, Biosynthesis of TiO2 nanoparticles by Caricaceae (Papaya) shell extracts for antifungal application, Sci. Rep., 2022, 12, 15960 CrossRef CAS PubMed.
- H. R. Roosta, A. Estaji and F. Niknam, Effect of iron, zinc and manganese shortage-induced change on photosynthetic pigments, some osmoregulators and chlorophyll fluorescence parameters in lettuce, Photosynthetica, 2018, 56, 606–615 CrossRef CAS.
- N. Bilesky-José, C. Maruyama, T. Germano-Costa, E. Campos, L. Carvalho, R. Grillo, L. F. Fraceto and R. De Lima, Biogenic α-Fe2O3Nanoparticles Enhance the Biological Activity of Trichoderma against the Plant Pathogen Sclerotinia sclerotiorum, ACS Sustainable Chem. Eng., 2021, 9, 1669–1683 CrossRef.
- H. A. S. El-Garhy, A. A. Elsisi, S. A. Mohamed, O. M. Morsy, G. Osman and F. A. Abdel-Rahman, Transcriptomic changes in green bean pods against grey mould and white rot diseases via field application of chemical elicitor nanoparticles, IET Nanobiotechnol., 2020, 14, 574–583 CrossRef PubMed.
- L. Shi, Q. Liang, Q. Zang, Z. Lv, X. Meng and J. Feng, Construction of Prochloraz-Loaded Hollow Mesoporous Silica Nanoparticles Coated with Metal–Phenolic Networks for Precise Release and Improved Biosafety of Pesticides, Nanomaterials, 2022, 12, 2885 CrossRef CAS PubMed.
- R. El-Mohamedya, M. Abd El-Aziz and S. Kamel, Antifungal activity of chitosan nanoparticles against some plant pathogenic fungi in vitro, Agric. Eng. Int. CIGR J., 2019, 21, 201–209 Search PubMed.
- A. Santiago-Aliste, E. Sánchez-Hernández, L. Buzón-Durán, J. L. Marcos-Robles, J. Martín-Gil and P. Martín-Ramos, Uncaria tomentosa-Loaded Chitosan Oligomers–Hydroxyapatite–Carbon Nitride Nanocarriers for Postharvest Fruit Protection, Agronomy, 2023, 13, 2189 CrossRef CAS.
- R. Kumar, J. S. Duhan, A. Manuja, P. Kaur, B. Kumar and P. K. Sadh, Toxicity Assessment and Control of Early Blight and Stem Rot of Solanum tuberosum L. by Mancozeb-Loaded Chitosan–Gum Acacia Nanocomposites, J. Xenobiot., 2022, 12, 74–90 CrossRef CAS PubMed.
- R. Kumar, A. Najda, J. S. Duhan, B. Kumar, P. Chawla, J. Klepacka, S. Malawski, P. K. Sadh and A. K. Poonia, Assessment of antifungal efficacy and release behavior of fungicide-loaded chitosan-carrageenan nanoparticles against phytopathogenic fungi, Polymers, 2022, 14, 41 CrossRef CAS PubMed.
- R. Kaur, D. Singh, A. Kumari, G. Sharma, S. Rajput, S. Arora and R. Kaur, Pesticide residues degradation strategies in soil and water: a review, Int. J. Environ. Sci. Technol., 2023, 20, 3537–3560 CrossRef CAS.
- Y. Liu, C. Yuan, Y. Cheng, G. Yao, L. Xie and B. Xu, Graphene oxide affects growth and resistance to Sclerotinia sclerotiorum in Brassica napus L, J. Nanosci. Nanotechnol., 2018, 18, 8345–8351 CrossRef CAS PubMed.
- G. Tang, Y. Tian, J. Niu, J. Tang, J. Yang, Y. Gao, X. Chen, X. Li, H. Wang and Y. Cao, Development of carrier-free self-assembled nanoparticles based on fenhexamid and polyhexamethylene biguanide for sustainable plant disease management, Green Chem., 2021, 23, 2531–2540 RSC.
- C. Jingli, X. Douxin, L. Wenlong, F. Xun, Z. Jiadong and Z. Jinhao, Synthesis and fungicidal activity of pH-responsive pyraclostrobin/zeolite imidazole ester framework material nanoparticles, Chin. J. Pestic. Sci., 2022, 24, 105–113 Search PubMed.
- Y. Huang, Y. Yang, B. Liang, S. Lu, X. Yuan, Z. Jia, J. Liu and Y. Liu, Green Nanopesticide: pH-Responsive Eco-Friendly Pillar[5]arene-Modified Selenium Nanoparticles for Smart Delivery of Carbendazim to Suppress Sclerotinia Diseases, ACS Appl. Mater. Interfaces, 2023, 15, 16448–16459 CrossRef CAS PubMed.
- P. Tryfon, N. N. Kamou, N. Ntalli, S. Mourdikoudis, K. Karamanoli, D. Karfaridis, U. Menkissoglu-Spiroudi and C. Dendrinou-Samara, Coated Cu-doped ZnO and Cu nanoparticles as control agents against plant pathogenic fungi and nematodes, NanoImpact, 2022, 28, 100430 CrossRef CAS PubMed.
- X. Zhang, S. Hou, M. Liang, J. Xu, M. Ye, Y. Wang, F. Wen, Z. Xu and S. Liu, Engineering nanofusiform Iron-doped polydiaminopyridine boost intratumoral penetration for immunogenic cell Death-mediated synergistic Photothermal/Chemo therapy, Chem. Eng. J., 2023, 462, 142159 CrossRef CAS.
- Y. Ding, N. Yang, Y. Lu, J. Xu, K. Rana, Y. Chen, Z. Xu, W. Qian and H. Wan, Fusiform nanoparticle boosts efficient genetic transformation in Sclerotinia sclerotiorum, J. Nanobiotechnol., 2024, 22, 494 CrossRef CAS PubMed.
- S.-Y. Wang, Y.-H. Jiang, X. Chen, D. D. Herrera-Balandrano, M. F. Simoes, X.-C. Shi and P. Laborda, Biocontrol strategies for the management of Sclerotinia sclerotiorum in Brassica species: A review, Physiol. Mol. Plant Pathol., 2024, 130, 102239 CrossRef CAS.
- R. M. Venkatesan, K. Muthusamy, J. Iruthayasamy, B. Prithiviraj, P. V. Kumaresan, P. Lakshmanan and I. V. Perianadar, First Report of Clonostachys rosea as a Mycoparasite on Sclerotinia sclerotiorum Causing Head Rot of Cabbage in India, Plants, 2023, 12, 199 CrossRef CAS PubMed.
- E. E. Jones, N. Rabeendran and A. Stewart, Biocontrol of Sclerotinia sclerotiorum infection of cabbage by Coniothyrium minitans and Trichoderma spp, Biocontrol Sci. Technol., 2014, 24, 1363–1382 CrossRef.
- M. R. Hamid, J. Xie, S. Wu, S. K. Maria, D. Zheng, A. Assane Hamidou, Q. Wang, J. Cheng, Y. Fu and D. Jiang, A Novel Deltaflexivirus that Infects the Plant Fungal Pathogen, Sclerotinia sclerotiorum, Can Be Transmitted Among Host Vegetative Incompatible Strains, Viruses, 2018, 10, 295 CrossRef PubMed.
- X. Yu, B. Li, Y. Fu, J. Xie, J. Cheng, S. A. Ghabrial, G. Li, X. Yi and D. Jiang, Extracellular transmission of a DNA mycovirus and its use as a natural fungicide, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 1452–1457 CrossRef CAS PubMed.
- Z. Gao, J. Wu, D. Jiang, J. Xie, J. Cheng and Y. Lin, ORF I of Mycovirus SsNSRV-1 is Associated with Debilitating Symptoms of Sclerotinia sclerotiorum, Viruses, 2020, 12, 456 CrossRef CAS PubMed.
- R. M. Venkatesan, K. Muthusamy, J. Iruthayasamy, B. Prithiviraj, P. V. Kumaresan, P. Lakshmanan and I. V. Perianadar, First Report of Clonostachys rosea as a Mycoparasite on Sclerotinia sclerotiorum Causing Head Rot of Cabbage in India, Plants, 2023, 12, 199 CrossRef CAS PubMed.
- S. C. L. Montalvão, E. Marques, I. Martins, J. P. da Silva and S. C. M. de Mello, Suppression of the phytopathogens Sclerotinia sclerotiorum and Sclerotium rolfsii by Trichoderma spp, Biologia, 2023, 78, 2941–2952 CrossRef.
- A. Faraz, I. U. Haq, S. Ijaz, S. T. Sahi and I. Khan, Antimycotic potential assessment of trichoderma species and fungicides for sustainable management of sclerotinia trifoliorum causing stem and crown rot of Trifolium Alexandrinum L, Int. J. Phytopathol., 2022, 11, 195–205 Search PubMed.
- M. R. Safari Motlagh and M. Abolghasemi, The effect of Trichoderma spp. isolates on some morphological traits of canola inoculated with Sclerotinia sclerotiorum and evaluation of their efficacy in biological control of pathogen, J. Saudi Soc. Agric. Sci., 2022, 21, 217–231 Search PubMed.
- J.-W. OH, S. C. Chun and M. Chandrasekaran, Preparation and In Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato, Agronomy, 2019, 9, 21 CrossRef CAS.
- P. H. S. Medrado, A. L. Graf Junior, G. C. Dos Santos, J. B. Tolentino Júnior and A. T. Itako, Efficiency of different strains of Trichoderma on the control of Sclerotinia sclerotiorum, Sclerotium rolfsii and Sclerotium cepivorum, Acta Sci., Biol. Sci., 2022, 44, 60072 CrossRef.
- J. M. Whipps, S. Sreenivasaprasad, S. Muthumeenakshi, C. W. Rogers and M. P. Challen, Use of Coniothyrium minitans as a biocontrol agent and some molecular aspects of sclerotial mycoparasitism, Eur. J. Plant Pathol., 2008, 121, 323–330 CrossRef.
- G. Giczey, Z. Kerényi, L. S. Fülöp and L. S. Hornok, Expression of cmg1, an exo-β-1, 3-glucanase gene from Coniothyrium minitans, increases during sclerotial parasitism, Appl. Environ. Microbiol., 2001, 67, 865–871 CrossRef CAS PubMed.
- D. E. Partridge, T. B. Sutton, D. L. Jordan, V. L. Curtis and J. E. Bailey, Management of Sclerotinia Blight of Peanut with the Biological Control Agent Coniothyrium minitans, Plant Dis., 2006, 90, 957–963 CrossRef CAS PubMed.
- M. Gerlagh, J. Whipps, S. Budge and H. Goossen-Van de Geijn, Efficiency of isolates of Coniothyrium minitans as mycoparasites of Sclerotinia sclerotiorum, Sclerotium cepivorum and Botrytis cinerea on tomato stem pieces, Eur. J. Plant Pathol., 1996, 102, 787–793 CrossRef.
- K. Sivagnanapazham, M. Karthikeyan, T. Raguchander, R. Swarna Priya and A. Kamalakannan, Biological control of cabbage head rot (Sclerotinia sclerotiorum) by Coniothyrium minitans, Egypt. J. Biol. Pest Control, 2022, 32, 132 CrossRef.
- L. Yang, G. Li, J. Zhang, D. Jiang and W. Chen, Compatibility of Coniothyrium minitans with compound fertilizer in suppression of Sclerotinia sclerotiorum, Biol. Control, 2011, 59, 221–227 CrossRef.
- N. Rabeendran, E. E. Jones, D. J. Moot and A. Stewart, Biocontrol of Sclerotinia lettuce drop by Coniothyrium minitans and Trichoderma hamatum, Biol. Control, 2006, 39, 352–362 CrossRef.
- A. P. Mattos, B. B. Rissato, A. T. Itako, J. B. Tolentino Júnior and K. R. F. S. Estrada, Bacillus amyloliquefaciens PKM16 acts as an antagonist of white mold and an inducer of defense enzymes in tomato plants, Acta Sci., Agron., 2023, 45, e59586 CrossRef.
- J. Hu, B. Dong, D. Wang, H. Meng, X. Li and H. Zhou, Genomic and metabolic features of Bacillus cereus, inhibiting the growth of Sclerotinia sclerotiorum by synthesizing secondary metabolites, Arch. Microbiol., 2023, 205, 8 CrossRef CAS PubMed.
- M. Lal, A. Kumar, S. Chaudhary, R. K. Singh, S. Sharma and M. Kumar, Antagonistic and growth enhancement activities of native Pseudomonas spp. against soil and tuber-borne diseases of potato (Solanum tuberosum L.), Egypt. J. Biol. Pest Control, 2022, 32, 22 CrossRef.
- A. Bajpai, R. Agnihotri, A. Prakash and B. N. Johri, Biosurfactant from Bacillus sp. A5F Reduces Disease Incidence of Sclerotinia sclerotiorum in Soybean Crop, Curr. Microbiol., 2022, 79, 206 CrossRef CAS PubMed.
- M. Yang, X. Han, J. Xie, S. Zhang, Z. Lv, B. Li, L. Shi, K. Zhang and B. Ge, Field application of wuyiencin against Sclerotinia stem rot in soybean, Front. Sustain. Food Syst., 2022, 6, 930079 CrossRef.
- M. Yang, X. Han, J. Xie, S. Zhang, Z. Lv, B. Li, L. Shi, K. Zhang and B. Ge, Field Application of Wuyiencin Against Sclerotinia Stem Rot in Soybean, Front. Sustain. Food Syst., 2022, 6, 930079 CrossRef.
- V. V. Yaderets, N. V. Karpova, E. V. Glagoleva, A. I. Ovchinnikov, K. S. Petrova and V. V. Dzhavakhiya, Inhibition of the Growth and Development of Sclerotinia sclerotiorum (Lib.) De Bary by Combining Azoxystrobin, Penicillium chrysogenum VKM F-4876d, and Bacillus Strains, Agronomy, 2021, 11, 2520 CrossRef CAS.
- D. A. S. Gebily, G. A. M. Ghanem, M. M. Ragab, A. M. Ali, N. E. D. K. Soliman and T. H. Abd El-Moity, Characterization and potential antifungal activities of three Streptomyces spp. as biocontrol agents against Sclerotinia sclerotiorum (Lib.) de Bary infecting green bean, Egypt. J. Biol. Pest Control, 2021, 31, 1–15 CrossRef.
- F. I. Parra-Cota, L. A. Cira-Chávez, M. I. Estrada-Alvarado and S. de los Santos-Villalobos, Bacillus sp. FSQ1: a Promising Biological Control Agent Against Sclerotinia sclerotiorum, the Causal Agent of white Mold in Common Bean (Phaseolus vulgaris L.), Biol. Bull., 2021, 48, 729–739 CrossRef.
- T. Zhou, H. Liu, Y. Huang, Z. Wang, Y. Shan, Y. Yue, Z. Xia, Y. Liang, M. An and Y. Wu, ε-poly-L-lysine Affects the Vegetative Growth, Pathogenicity and Expression Regulation of Necrotrophic Pathogen Sclerotinia sclerotiorum and Botrytis cinerea, J. Fungi, 2021, 7, 821 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |