A sustainable infrared dry peeling method for shallots (Allium cepa L. aggregatum): comparison of nutritional, enzymatic and sensory characteristics of infrared and conventional peeling methods
Received
9th December 2024
, Accepted 7th February 2025
First published on 10th February 2025
Abstract
Traditional peeling methods involve the usage of a high amount of water which leads to effluent generation, high biological oxygen demand and salinity and need for waste water treatment plants. However, infrared peeling can be an alternative to overcome the problems as it does not involve water. Thus, phytochemical, antioxidant, functional and sensory parameters were compared between infrared and conventional peeling methods (steam, lye, hot-water, flame and untreated hand peeling). Phytochemical analysis revealed that infrared peeled shallots exhibited similar quality attributes to hand peeled shallots. Peroxidase activity was higher in hand-peeled and the lowest in flame peeled shallots, and the activity in infrared peeled shallots was similar to that in other traditional treatments. During Fourier transform infrared analysis, it was observed that both infrared-peeled and hand peeled samples shared similar functional group compositions that are responsible for the aromatic nature of the shallots. X-Ray diffraction analysis showed the typical agricultural characteristics (amorphous and crystalline) that are inherent in shallots. The acceptance was higher for hand peeled and infrared peeled shallots determined by sensory evaluation and analysed by the fuzzy logic method. This research highlights the potential of infrared dry peeling as a sustainable alternative, offering promising outcomes in quality preservation.
Sustainability spotlight
This study introduces infrared peeling as a water-free, eco-friendly alternative to conventional shallot peeling methods, which are resource-intensive and generate wastewater with high biological oxygen demand and salinity. Infrared peeling aligns with the United Nations Sustainable Development Goals (SDGs) by promoting responsible consumption and production (SDG 12) and ensuring the sustainable management of water resources (SDG 6). By eliminating water use and reducing environmental impact, the process addresses industrial challenges while preserving product quality, as demonstrated through phytochemical, functional, and sensory analyses. This advancement emphasizes the potential for integrating sustainable technologies in food processing, fostering a transition toward greener practices and reducing the environmental footprint of agricultural processing industries.
|
1 Introduction
The genus Allium is recognised for its typically high contents of organo-sulfides, like polysulfides and thiosulfinates, that are usually generated when the alliums are crushed or damaged by the reaction of the precursor present in the cytoplasm, non-volatile alk(en)yl-cysteine-sulfoxide and alliinase, the vacuole enzyme.1 Shallots, in particular, could be a potential source of organic polysulfides. They are typically grown in southeast Asian countries like Indonesia, Thailand, Vietnam, Malaysia, Philippines, and India, where it has been suggested that they are superior to regular onions (Allium cepa L. var. cepa) due to their peculiar taste and resilience to pests and disease.2,3 Shallots are highly valued for their characteristic pungency and nutritional values and are consumed either pickled, dried, or fried, or used as a flavour.1
The Aggregatum group produces clusters by vegetative propagation and is smaller than the size of an ordinary onion.4 Gallic acid, quercetin, isoquercetin, apigenin, rutin, eriodictyol, catechin, tannic acid, and kaempferol constitute some phenolic compounds found in shallots. Quercetin is mainly found in shallots as glycosides. When shallots are processed, the presence of quercetin in the shallot changes, which has grabbed attention in recent years.5Allium plants include a range of carbohydrates, amino acids, vitamins, minerals, flavonoids, sulphurous compounds, enzymes, and saponins.6 Thiosulfinates are sensitive to heat depending on the time and intensity of the processing conditions. Researchers have reported that total phenol and anti-oxidant contents are positively related in shallots and onions.7 Allicin is usually unavailable in whole shallots, while it is swiftly produced when the cells of the shallots are disrupted. After disruption, the enzyme alliinase comes in contact with alliin and converts alliin to allicin and pyruvic acid. Likewise, allicin is unstable in the presence of heat or organic solvents, though it typically degrades on crushing. It rapidly breaks down into various degradation products, including vinyldithins, allylsulfides, and ajoenes.8 Analysis of the shallots' properties is of prime importance as it reflects the effect of processing on the product. Structural and functional properties are highly influenced by the peeling conditions, which directly affects the quality of the finished product, impacting their commercial applications.9 The structural alterations are related to the nutritional and functional attributes of the product.10
Peeling is a crucial step in food processing, which involves the removal of the inedible peel.11 Additionally, peeling has several advantages such as enhancing palatability, reducing residues of pesticides, and preparing the food product for the next processing step. Since peeling is a critical operation in many industries, it can also impact product processing costs and waste management expenses.12 Peeling not only causes mechanical damage to inner edible layers but also influences the physical integrity of the product, which elevates microbial contamination and oxidative and enzymatic deterioration, leading to the risk of flesh degradation.13 Furthermore, using improper peeling methods will lead to low process efficiency, high peeling losses, high water and energy consumption, high nutritional property damage, and low consumer acceptability.14 Thus, selecting suitable peeling methods based on the product is vital for commercial processing of high-quality products with reduced production cost and environmental impact.15
Even though these methods are easy to adapt and can be used on a large scale, they tend to cause a negative environmental footprint due to intensive water consumption of most peeling methods.16 Additionally, they face many problems like low yield, quality damage, loss of product integrity, lower acceptance, and reduced nutritional composition due to heat damage.17,18 The infrared dry-peeling method can overcome these challenges as it directly heats the food product without heating the atmosphere.16,19 Moreover, sustainable dry infrared peeling has recently been adapted for effective peeling with less peeling loss and better quality retention.20,21 Infrared peeling has already been adapted for food products like tomatoes,19,22 pears,23 kiwifruits,24 shallots,16 hazelnuts,20 jujubes,25 ginger,26 potatoes27 and clingstone peaches.28 However, there are very few published papers on the effect of infrared and traditional peeling on quality of the agricultural products.
Sensory evaluation provides vital and practical information to the food industry concerning the sensory and quality characteristics of the product. Sensory evaluation is a scientific method used to invoke, assess, examine, and decipher the responses to products that are perceived by senses of smell, sight, taste, hearing, and touch.29 Sensory analyses are generally performed by a set of trained panellists. It is utilised at various stages of product development and for comparison of similar types of products. The scoring or ranking of the products is collected in crisp form and analysed statistically.30 Human perception can be fuzzy, and the evaluator's natural judgement may be in linguistic form. A product cannot be accepted or rejected directly as it cannot provide the strengths and weaknesses of the quality parameter. Thus, it is more realistic to use linguistic labels than numeric scores for quality attributes (colour, aroma, taste, mouthfeel, etc.). The linguistic parameters of sensory evaluation without proper training tend to be uncertain, ambiguous, imprecise, and vague.31 To circumvent the problems that arise during sensory evaluation analysis, fuzzy logic can be applied to evaluate the sensory scores. Fuzzy model systems can be used where judgement, human perception, decision, and reasoning are involved.32 Fuzzy logic enables the enumeration of linguistic terms of the judge's opinion. They are used to bridge the individual and dependent variables using linguistic variables. Fuzzy logic quantifies an individual's semantic expression, thereby enabling the quantification of primary and inexplicit data acquired from sensory tests.33 This rational analysis method can be utilised to use codes for all the linguistic parameters. After the progress of numerical control, every item is provided with a solitary score. Based on the choices of the panellists, the scores are additionally de-fuzzified to get the semantic term.32 There is a lack of research addressing the impact of traditional and IR peeling on quality characteristics of shallots. A previously published paper16 demonstrated that infrared peeling has better peelability, peeling efficiency and yield, but there is no detailed information on the effect of hot water, steam, flame, lye, knife and the novel non-water infrared peeling on quality attributes of the peeled shallots. Therefore, this study demonstrates that the advantages of infrared peeling extend beyond operational efficiency to include better preservation of product quality, thereby evaluating its suitability as an alternative to traditional peeling methods.
2 Materials and methods
2.1. Sample preparation
Shallots have been purchased from the local markets of Villupuram, Tamilnadu, India. Furthermore, the shallots were subjected to five different peeling treatments: steam peeling, hot water peeling, lye peeling, IR peeling and flame peeling. The moisture content and diameter of the shallots used for experimentation were 85.1 ± 1.1 (% wb) and 20 to 23 (mm), respectively.
Traditional peeling conditions were selected based on preliminary trials and the literature. Knife or hand peeling, flame peeling, lye peeling, and steam and hot water peeling were the selected peeling methods for shallots due to their widespread use as traditional methods.23 Knife peeling is the most traditional and manual method, involving no use of heat and chemicals but it is labour intensive.34 Flame peeling is often used for root vegetables, which involves direct exposure to high heat to char the outer tunics and is suitable for rapid processing.35 On the other hand, lye peeling involves the use of an alkaline solution to break down peels chemically and is suitable for tough skinned products.36 Steam peeling applies steam to loosen peels with minimal or no chemical use. Hot water peeling is a straight forward and cost-effective method which uses heat to soften peels, making it appropriate for applications where scalability is the priority.34
In hot-water peeling, shallots were submerged in 60 °C water for 5 min and for steam peeling, shallots were exposed to steam for 5 min, and the peel was removed manually. For lye peeling, shallots were dipped in a 2% NaOH solution at 60 °C for 5 min, rinsed in distilled water, and blotted dry to avoid cooking. In flame peeling, shallots were exposed to flame for 30 seconds, burning off the outer peel, which was further manually removed. Shallots peeled manually with a knife served as the control. IR peeling was conducted at a 60 mm distance between the infrared source and product, with a 60% IR power level and 15 min treatment time, using a ceramic lamp with a peak wavelength of 8.16 μm. Detailed procedures for all the treatments and optimisation of IR treatment are mentioned in ref. 16.
2.2. Allicin content
The allicin content in shallot bulbs was measured by the method given by Feng et al. (2019).37 The shallot samples were homogenised with 10 mL of 50 mM Hepes buffer at pH 7.5, and were further incubated at 25 ± 1 °C for 15 min and later centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. Afterwards, the solution was filtered, and the supernatant was used for allicin determination. For analysis, 1 mL of the sample extract (Hepes buffer for blank) was mixed with 5 mL of 10 mM cysteine solution and was left to rest for 15 min at 25 ± 1 °C. Furthermore, 1 mL of the reaction mixture was made up to 100 mL with the Hepes buffer solution. Later, 4.5 mL of the diluted solution was made to react with 0.5 mL of 1.5 mM DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) and left to stand for 15 min at 25 ± 1 °C. Subsequently, the absorbance was measured at 412 nm using a spectrophotometer, as A for samples and A0 for blank. The quantification of allicin was calculated as follows:
000 rpm for 5 min. Afterwards, the solution was filtered, and the supernatant was used for allicin determination. For analysis, 1 mL of the sample extract (Hepes buffer for blank) was mixed with 5 mL of 10 mM cysteine solution and was left to rest for 15 min at 25 ± 1 °C. Furthermore, 1 mL of the reaction mixture was made up to 100 mL with the Hepes buffer solution. Later, 4.5 mL of the diluted solution was made to react with 0.5 mL of 1.5 mM DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) and left to stand for 15 min at 25 ± 1 °C. Subsequently, the absorbance was measured at 412 nm using a spectrophotometer, as A for samples and A0 for blank. The quantification of allicin was calculated as follows:| | 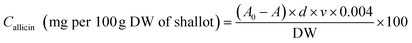 | (1) |
where d is the dilution factor, v is the extracted volume, and DW is the dry weight of the sample taken.
2.3. Pyruvate content
The shallot sample was ground with distilled water, and from that, 5 g of the shallot puree was blended with 5 mL of trichloroacetic acid (10%). The solution was vigorously shaken and left to stand for 1 h, after which it was further centrifuged and filtered. The aliquot was collected and made up to 100 mL using distilled water. The pyruvate analysis was performed as given by Aslam et al. (2021).38 Briefly, 1 mL of the extract was reacted with 0.0125% DNPH in 2 N HCl. The mixture was incubated at 37 °C for 10 min. Furthermore, 5 mL of NaOH was added to the solution, and the absorbance was measured at 420 nm using a UV/visible spectrophotometer. The pyruvate content was quantified using the standard curves for sodium pyruvate, expressed as μmol pyruvate per g DW of shallot.
2.4. Extraction for phytochemical analysis
The phytochemical components of the shallots were extracted using methanol (80%, v/v) as a solvent. The sample was well dispersed in the solvent in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v). Furthermore, it was extracted by ultrasound treatment (40 kHz) for 30 min at 30 ± 2 °C. Additionally, the solution was centrifuged at 5000 × g for 15 min and filtered. The extract is the collected supernatant, and it was stored at 4 °C until analysis.39
20 (w/v). Furthermore, it was extracted by ultrasound treatment (40 kHz) for 30 min at 30 ± 2 °C. Additionally, the solution was centrifuged at 5000 × g for 15 min and filtered. The extract is the collected supernatant, and it was stored at 4 °C until analysis.39
2.5. Total flavonoid content
The total flavonoid (quercetin) content of shallot bulbs was measured using the method reported by Sharma et al. (2018).40 Methanolic extract of the samples (0.5 mL) was mixed with 1.5 mL of methanol, 100 μL of 10% aluminium chloride, 100 μL of 1 M potassium acetate and 2.8 mL of distilled water (to make up the volume to 5 mL). The reaction mixture was incubated for 30 min at room temperature. Blank solution was prepared by replacing methanolic extract with distilled water. The absorbance was measured at 415 nm using a UV/visible spectrophotometer (Shimadzu – UV, Japan). The calibration curve was determined by measuring the absorbance of quercetin solutions at different concentrations. The results were represented as mg of quercetin equivalent per gram dry weight of shallot. TFC values were obtained from the calibration curve (y = 0.0034x − 0.1506 with R2 = 0.80 where x is the absorbance and y is the concentration of quercetin).
2.6. Total phenol content
The total phenol content in peeled shallot bulb extract was analysed by Folin–Ciocalteu assay. Briefly, 1 mL of the extract was mixed with 1 mL Folin–Ciocalteu reagent and shaken well, and then allowed to rest for 5 min. Furthermore, 10 mL of 7% Na2CO3 was added to the reaction mixture, and the volume was made up to 25 mL using distilled water immediately. Later, the solution was incubated at 23 °C for 90 min in a dark room. Furthermore, the absorbance was measured at 750 nm using a UV/visible spectrophotometer. The standard curve was generated by determining the absorbance of gallic acid at different concentrations (50, 100, 150 and 250 mg L−1). The total phenol content present in peeled shallot bulbs was expressed in terms of gallic acid equivalent (GAE) based on the standard curve attained from different concentrations of gallic acid.41 TPC values were obtained from the calibration curve (y = 0.0038x + 0.024 with R2 = 0.99 where x is the absorbance and y is the concentration of gallic acid (μg mL−1)) and it is expressed as mg of GAE per g DW.
2.7. Antioxidant activity (DPPH)
The antioxidant activity of peeled shallots was determined using a DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay.42 Briefly, 1 mL of methanolic extract was added to 2 mL of 0.002% DPPH methanolic solution, and the mixture was further shaken vigorously. The mixture was incubated at ambient temperature (30 ± 2 °C) for 30 min, and the absorbance was measured using a UV/visible spectrophotometer at 517 nm. The quantification of antioxidant activity in the peeled shallots was carried out using the following eqn (2).| | 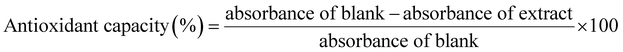 | (2) |
The absorbance value of blank solution was 0.147.
2.8. Ascorbic acid content
The ascorbic acid content in peeled shallot samples was determined by the indophenol dye titrimetric method based on AOAC method 967.21.43 In this method, the ascorbic acid present in the product reduces 2,6-dichlorophenol indophenol dye to a colourless leuco-base due to the oxidation of ascorbic acid to dehydroascorbic acid. The excess unreduced dye turns into a pink solution in an acid medium at the endpoint. Briefly, the shallot bulb (around 2 g) was homogenised with meta-phosphoric acid–acetic acid (MPAA) solution and the volume was made up to 50 mL with MPAA. The extracted solution was titrated against 2,6-dichlorophenol indophenol dye until the solution turned mild pink. Titration was performed in the presence of MPAA solution to maintain pH and avoid auto-oxidation of ascorbic acid at high pH.
MPAA solution was prepared by mixing 200 mL of distilled water, 40 mL acetic acid and 14 g of meta-phosphoric acid, and further making up the volume to 500 mL. The solution was filtered and stored under refrigerated conditions until use. Likewise, 2,6-dichlorophenol indophenol dye was prepared by diluting 42 mg of sodium bicarbonate and 50 mg 2,6-dichlorophenol indophenol salt such that the final volume was 200 mL.
The dye was standardised by titrating 5 mL MPAA and 2 mL of standard ascorbic acid solution (0.1% ascorbic acid in MPAA) against the prepared dye until light but distinct mild pink color that persists for more than 5 s was obtained. Blank titration was performed by titrating 7 mL of MPAA against dye. The titre value was calculated by using the data obtained from the standardisation of dye and the following formula:
| | 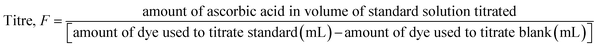 | (3) |
The numerator was calculated by using (mg of ascorbic acid/50 mL) × 2 mL. The ascorbic acid content in peeled shallots was determined by using the following eqn (4).
| | | Ascorbic acid content (mg ascorbic acid per g DW) = (S − B) × (F/W) × (V/Y) | (4) |
where
S is the amount of dye used to titrate the sample (mL),
B is the amount of dye used to titrate the blank (mL),
F is the titre of the dye,
W is the dry weight of the sample (g),
V is the volume of the initial assay solution (7 mL), and
Y is the volume of the sample aliquot titrated (7 mL).
2.9. Peroxidase content
The sample was blended with phosphate buffer (pH 6.0) in a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v). It was homogenised under chilled conditions for 1 min, and the homogenate was centrifuged in polypropylene tubes at 6000 × g at 4 °C for 20 min. The centrifuged solution was filtered, and the supernatant was collected. The supernatants were kept under refrigerated conditions until further analysis.
5 (w/v). It was homogenised under chilled conditions for 1 min, and the homogenate was centrifuged in polypropylene tubes at 6000 × g at 4 °C for 20 min. The centrifuged solution was filtered, and the supernatant was collected. The supernatants were kept under refrigerated conditions until further analysis.
The substrate for peroxidase analysis was prepared by mixing 100 μL of guaiacol and 100 μL of hydrogen peroxide (30%) and the volume was made up to 100 mL using phosphate buffer (0.1 M, pH 6.5). The assays for enzyme analysis were prepared by pipetting 120 μL of enzyme extract and 3.48 mL of the substrate solution in a quartz cuvette. The absorbance of the reaction solution was immediately measured at 470 nm using a UV/visible spectrophotometer for 10 min.17 One unit of POD was described as the amount of enzyme that induces a 0.01 increment in absorbance per min.44
2.10. XRD
XRD patterns of the shallot bulbs were characterised using an X-ray diffractometer (D8 Advance A25, Bruker, USA) operated at 40 kV and 40 mA. The diffractometer consists of components such as an X-ray source, optics, sample stages and specimen holders, a non-ambient chamber, and a detector. The sample was dispersed onto a stub and placed within the chamber of an analytical X-ray diffractometer. Measurements were performed at room temperature with a scanning rate of 10° min−1 and step size of 0.02° in the diffraction angle range of 3° to 80°. The angle of incidence of the X-ray beam on the sample (theta) was maintained in the 2θ range. The properties can be determined by considering the angstrom range of the XRD wavelengths and the extreme energy used for penetration at an atomic level. The data were analysed using the software OriginPro 2020b. The crystallinity index was calculated as follows:45| | 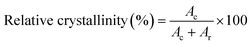 | (5) |
where Ac and Ar are the area of the crystalline peak and area of the amorphous peak, respectively.
2.11. FTIR
FTIR spectra were measured for the peeled shallot samples using a Fourier transform infrared spectrometer (Bruker, Alpha E FTIR, Germany) at room temperature. The spectral profile was recorded from wavenumber 4000 to 500 cm−1 with a resolution of 4 cm−1. Before conducting each sample scan, a preliminary background scan was executed to minimise the impact of environmental factors on the spectral data, thereby reducing noise. The data were analysed using the software OriginPro 2020b.
2.12. Sensory evaluation by fuzzy logic
After preliminary screening, 10 trained healthy non-smoking assessors (6 females and 4 males, aged 22 to 31) were selected as panellists from the Department of Food Process Engineering, NIT Rourkela. During the screening process, individuals with excessive fatigue, tension, excitement, or disease were excluded. Likewise, other researchers have used the 10 final participants to measure acceptability of food samples,29,30,46–48 All participants were given proper instructions on scoring procedures and they voluntarily participated in the study after providing informed consent. All the samples were presented in similar plates and under similar conditions. Panellists were requested to tick the appropriate box of the sensory scale for each quality attribute and sample. Furthermore, they were asked to select the choice of quality attributes in general. The evaluation of peeled shallots was conducted for four main categories, including colour, odour, texture, and appearance.
Linguistic data obtained from the sensory analysis were used for the fuzzy analysis. The triangular fuzzy membership distribution function was used to rank the peeled shallot bulbs. Sensory scores collected with fuzzy scorecards were translated into triplets, which were later used to determine the similarity values. Visual representation of the sensory evaluation procedure using fuzzy logic is illustrated in Fig. 1.
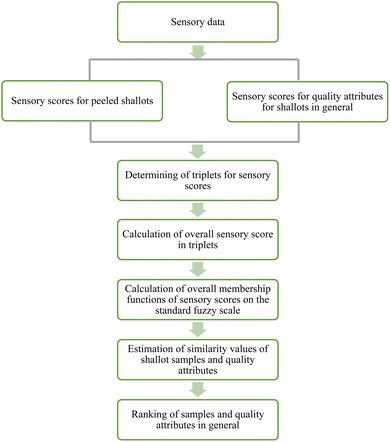 |
| | Fig. 1 Representation of the sensory evaluation performed by fuzzy logic. | |
2.12.1. Triangular fuzzy number and fuzzy arithmetic operations.
The “triplet” gives the collection of three integers used to represent the distribution pattern of a triangular membership function across sensory scales and the distribution pattern of 5-point sensory scales that consists of “excellent/extremely important”, “good/highly important”, “medium/important”, “fair/somewhat important”, and “not satisfactory/not at all important”. Fig. 2 depicts the values of the triangular membership function distribution pattern and the triplets linked to the fuzzy logic five-point sensory scale. To assess the similarity values, the triplets were used, and ranking of the samples was performed. The following steps were followed for fuzzy analysis of the sensory scores: (i) triplet determination based on sensory scores, (ii) triplet determination with respect to samples and all attributes, (iii) triplet estimation associated with the relative weightage of the traits, (iv) overall sensory score based triplet estimation, (v) overall membership function estimation for sensory scores, (vi) similarity value estimation for various sample and quality attributes and (vii) final ranking of the samples and their associated attributes.
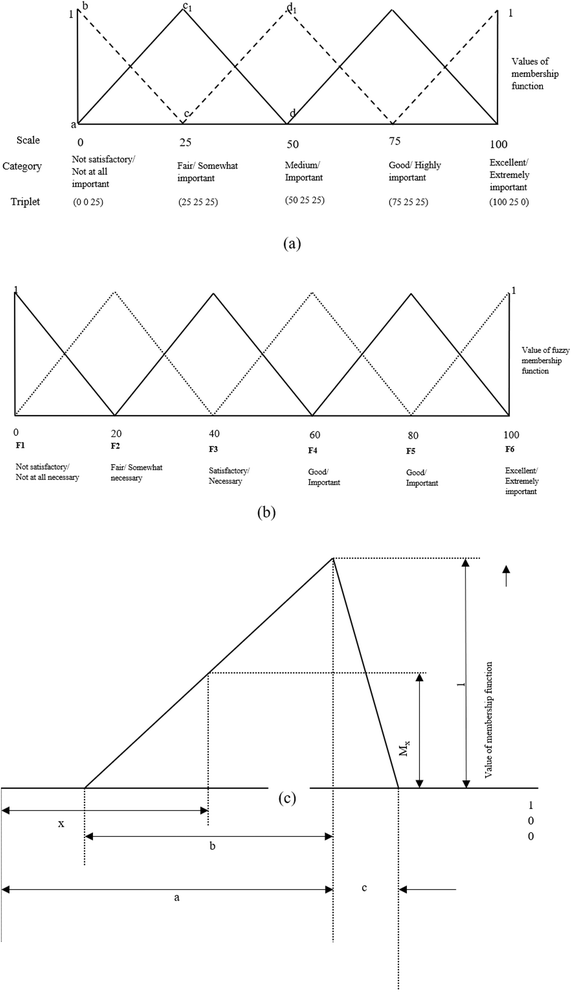 |
| | Fig. 2 Sensory evaluation using fuzzy logic (a) values of triplets associated with the triangular membership distribution for five-point sensory scales, (b) standard fuzzy scale and (c) triplet (a–c) and its membership function. | |
The distribution pattern for the triangular membership function of the sensory scale is also depicted in Fig. 2(b). Triangular fuzzy number is the triplet associated with the sensory scale. At this point, ‘a’ (first number of triplets) is called the mean value of the fuzzy number, and it denotes the co-ordinate of the abscissa at which the membership function value is 1. Likewise, ‘b’ and ‘c’ are the left and right spread, respectively, for which the membership functions are 0. The distance from the first integer to the place on the left side of the first number where the membership function is 0 is represented by the second number. The third number in the triplet represents the distance to the right of the first number when the membership function is 0.
2.12.2. Calculation of triplets.
The three-number set (triplet) values for the sensory scores of a particular quality attribute of each sample were acquired from the sum of sensory scores, triplets associated with sensory score and number of judges.
For example, in the case of the colour attribute of a sample, when the total number of judges is j1 + j2 + j3 + j4 + j5, and j1 judges give a ‘not satisfactory’ score, j2 judges give a ‘fair’ score, j3 judges give a ‘medium’ score, j4 judges give a ‘good’ score and j5 judges give and ‘excellent’ score, the calculation is performed as follows.
| | 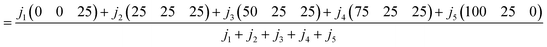 | (6) |
To determine the triplets for the overall sensory score of the peeled shallots, it is necessary to resolve the relative weights of quality attributes. To calculate Qsum, it is essential to sum the first value from each triplet.
The triplet for relative weightage of the colour attribute will be
Similarly, the relative weightage for other quality attributes was calculated. Overall sensory scores for sample 1 were determined by using the following eqn (8)
| | | POi = PiC × QCrel + PiO × QOrel + PiT × QTrel + PiA × QArel | (8) |
where
C is colour,
O is odour,
T is texture,
A is appearance, and
i is the sample number.
2.12.3. Assessment of the membership function for the standard fuzzy scale.
The membership function has a set of membership values for each triangular distribution pattern, which are defined by a set of ten numbers,| |  | (9) |
2.12.4. Estimation of the overall membership function of sensory scores on the standard fuzzy logic scale.
The overall membership function for different peeled shallot samples was calculated using the following equations:| | 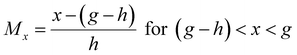 | (10) |
| | 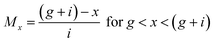 | (11) |
| Mx = 0 for all other values of x |
where Mx is the membership function of sensory scores and g, h, and i are the triplets. Mx for each sample was calculated at x = 0 to 100.
2.12.5. Estimation of similarity values.
The membership function (M1, M2, M3, M4, M5 and M6) was compared with the standard fuzzy scale (F1, F2, F3, F4, F5 and F6). Fx and Mx are single-row matrices. Similarity values were obtained by using eqn (12)| | 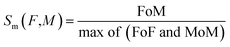 | (12) |
where FoM is the multiplication of matrix F with the transpose of matrix M. Similarly, FoF is the multiplication of matrix F with the transpose of F, and MoM is the multiplication of matrix M with the transpose of M. Similar procedures were followed for the general quality attributes of the samples.
2.13. Browning index
The colour of the peeled surface of the shallots was measured using a colorimeter (Hunter Associate Laboratories Inc., Virginia, USA). The L*-values denote the range from light to dark, a*-values represent the spectrum from red to green, and b*-values signify the spectrum from yellow to blue. The browning index was determined using the following eqn (13) and (14)| | | BI = [100(Y − 0.31)]/0.172 | (13) |
| | | where Y = (a* + 1.75L*)/(5.645L* + a* − 3.012b*) | (14) |
2.14. Statistical analysis
The data were analysed using one-way ANOVA in IBM SPSS Statistics (version 28) to evaluate differences among groups, with triplicates used for each treatment. Post hoc comparisons were conducted using the Tukey test to identify significant group differences. The statistical analysis was performed at a significance level of 0.05.
3 Results and discussion
3.1. Allicin content
Allicin is one of the most distinctive organosulfur compounds in shallots, which signifies the presence of flavour and pungency. The allicin content was estimated in peeled products to evaluate the pungency of the shallots after peeling by various methods. The allicin content after different peeling methods is presented in Fig. 3(a). As expected, allicin content was higher in the untreated hand peeled sample, having a value of 6.43 ± 0.04 mg g−1 of dry weight. It was significantly (p < 0.05) higher than all the treated peeled samples. Following hand peeled shallots, IR-treated shallots had the highest allicin content among the treated samples, with a value of 6.08 ± 0.18 mg g−1 of DW. Steam peeling, hot water peeling, and lye peeling showed insignificant differences in allicin content, with values of 5.25 ± 0.15, 5.36 ± 0.11, and 5.1 ± 0.12 mg per g DW, respectively. Flame peeling had the lowest value, 4.57 ± 0.23 mg per g DW. The lowest values were observed since the organic compound allicin decomposes rapidly at high temperatures. Since the disulfide bond found in allicin is 40% less strong than the C–C and C–H bonds, it is the most vulnerable element in the allicin structure. This makes it particularly prone to breaking when exposed to elevated temperatures.49 Also, the results were in accordance with Mansor et al. (2016),49 who found that allicin is more stable at 30 °C and decomposes rapidly at around 70 to 80 °C. Likewise, Mathialagan et al. (2017) (ref. 50) reported that after increasing the extraction temperature above 35 °C, the deterioration of allicin was significant. Also, flame-catalytic infrared peeling of tomatoes led to the most minor degradation of cellulose, hemicellulose, pectin, and firmness compared to lye and hot water peeling.51
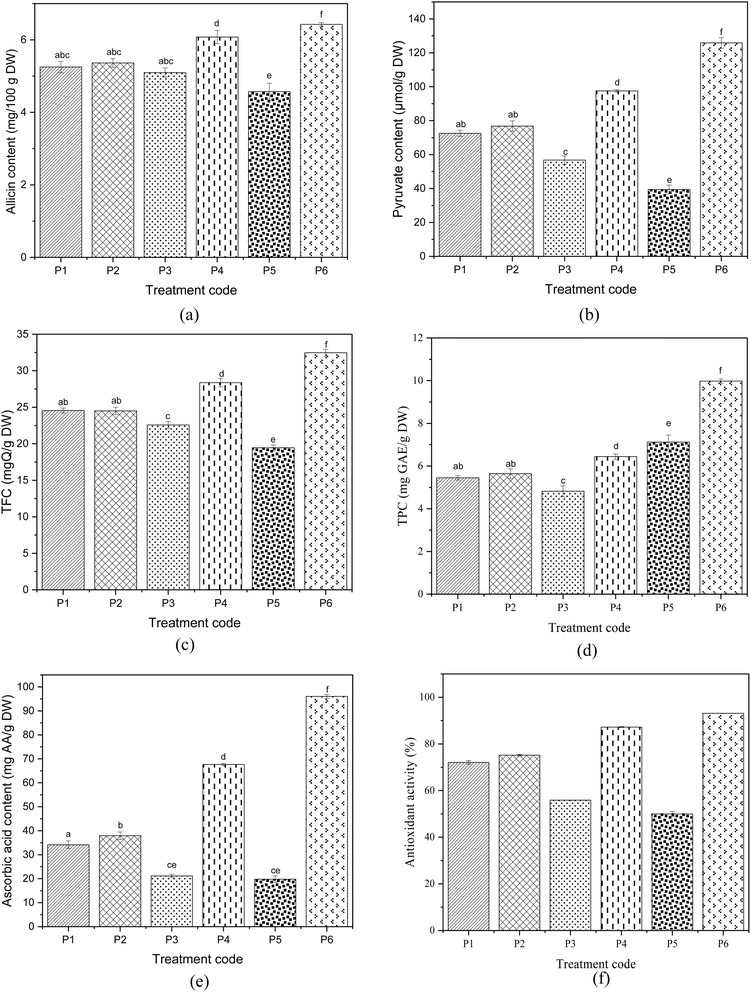 |
| | Fig. 3 Effect of different peeling treatments on quality parameters: (a) allicin content, (b) pyruvate content, (c) total flavonoid content, (d) total phenol content, (e) ascorbic acid content and (f) antioxidant activity. *Different letters indicate significant differences (p < 0.05) according to the Tukey test. | |
3.2. Pyruvate content
Pyruvate content was estimated to understand the effect of peeling treatments on the thiosulfate components of the shallots. Reduction in pyruvate content is related to the alliinase produced by alliin. Alliinase gets damaged by disrupting the cells during cutting and at higher temperatures. It can be observed from the results that the pyruvate content degradation pattern was very similar to that of allicin content. The pyruvate content after different peeling methods is presented in Fig. 3(b). Pyruvate content was highly affected by high temperatures during the treatment. The highest pyruvate content value was determined in untreated hand peeled shallots, with 125.86 ± 3.13 μmol per g DW of shallot. Following hand peeled shallots, IR peeling had higher values of 97.57 ± 0.8 μmol per g DW. Lye peeling had lower values (56.74 ± 2.5 μmol per g DW) than hot water and steam peeling. Pyruvate content in hot water and steam peeling were statistically insignificant. Similarly, it has been reported that kiwifruit had softer tissues and higher moisture loss for lye peeling compared to hot water peeling.52 Flame peeling had the lowest value (39.42 ± 2.56 μmol per g DW) because of the relatively higher temperature.
Shallots' health benefits mainly depend on flavonoids and alk(en)yl cysteine sulfoxides (ACSOs). The latter are non-proteinogenic sulfur amino acids particular to the tissues of Allium species and are responsible for the typical characteristic flavour and odour. Isoalliin is the most abundant ACSO in shallots, and it is the precursor of the lachromatory factor. ACSOs are intact in tissues while they undergo rapid hydrolysis by alliinase when subjected to disruption. They form sulfenic acids and α-iminopropionic acids, where the former condenses to thiosulfanates and the latter hydrolyses to ammonia and pyruvate. Since pyruvate is the final product of maceration, it is used as a measure to indicate the pungency of the shallots. Kim et al. (1992) (ref. 53) found that the pyruvate content decreased during the drying of garlic with prolonged drying time and higher temperatures.
3.3. Total flavonoid content
Flavonoids are water-soluble components common in plants, and quercetin is a natural flavonoid with potential health benefits for humans. Shallots are one of the natural sources of quercetin. The results have shown that a hand peeled fresh shallot bulb contains 32.47 ± 0.47 mgQ per g DW. The flavonoid content has reduced during the peeling treatments, and the results are presented in Fig. 3(c). Compared to the treatments, IR peeling is the least affected (28.37 ± 0.57 mgQ per g DW), and flame peeling is highly affected (19.45 ± 0.4 mgQ per g DW). The difference in quercetin content for steam and hot water peeling is insignificant. In contrast, there is a significant difference between other treatments and untreated hand peeling. Lye peeling and flame peeling have highly affected the quercetin content in shallots. During lye peeling, the hot alkaline solution penetrates through the outer skin and into the hypodermal cells; the α-1,4 bonds in the galacturonic units of pectin may be cleaved, which weakens the cellulosic and hemicellulosic network supported by a pectin polysaccharide unit in the cell wall. A similar mechanism occurs in steam and hot water peeling due to high temperature, leading to skin separation and rupture.54
3.4. Total phenolic content
Phenolic compounds, widely distributed among plant species, hold significant importance as antioxidants. They are considered one of the most crucial groups of antioxidants due to their potent ability to scavenge free radicals. These compounds play a vital role in plants, protecting against environmental stresses like UV radiation, pathogens, and oxidative damage.
The total phenolic content after different peeling methods is presented in Fig. 3(d). Untreated hand peeled samples (9.97 ± 0.1 mg GAE per g DW) have the highest total phenol content compared to the peeling methods. Unlike other parameters, flame peeling has higher TPC (7.12 ± 0.32 mg GAE per g DW) than other peeling methods including IR peeling. Since the absorbance value of the samples directly converts to the total phenol content, a difference could have occurred. The colour value of the flame-peeled sample is higher, and the absorbance has been measured at 715 nm, which detects colour compared to the other absorbance wavelength. Besides flame peeling, IR peeling has higher TPC, which may be due to the comparatively lower product temperature, followed by hot water peeling (5.64 ± 0.22 mg GAE per g DW) and steam peeling (5.45 ± 0.1 mg GAE per g DW). The difference in TPC in hot water and steam peeling is statistically insignificant (p > 0.05). Hot water peeling can cause a considerable amount of soluble nutrient substances like water-soluble vitamins, minerals and carbohydrates.55 Lye peeling has the lowest TPC value (4.82 ± 0.26 mg GAE per g DW). The decrease in phenol content may result from the heat or thermal treatment. Chlopika et al. (2012) (ref. 56) also reported degradation of TPC as a result of heat treatment.
3.5. Ascorbic acid content
Ascorbic acid is one of the main components contributing to the antioxidant activity of the food product. Like other phytochemical components, ascorbic acid content is also affected by high temperatures. The same has been observed in the results. The ascorbic acid content after different peeling methods is presented in Fig. 3(e). The highest ascorbic acid content has been observed in hand peeled samples, 96.05 ± 0.84 mg per g DW. The retention of ascorbic acid is dependent on the high texture retention during the treatment.57 Among different peeling methods, IR peeling leads to a higher ascorbic acid value of 67.64 ± 0.48 mg per g DW. Steam and hot water peeling led to relatively low ascorbic acid values of 34.16 ± 1.56 mg per g DW and 37.91 ± 1.57 mg per g DW, respectively. This may be due to the higher temperatures employed during the treatment. Likewise, Garcia and Barrett, 2006 (ref. 52) have found that even under optimised conditions, the textural damage of steam peeling was higher compared to hand peeling. The mushy texture after steam and hot peeling may be due to under and over-scalding, respectively.15 The lowest values were for flame peeling (19.79 ± 1.33 mg per g DW) and lye peeling (21.16 mg per g DW), and their difference was insignificant.
3.6. Antioxidant activity (DPPH)
Total antioxidant capacity provides a more adequate representation of the collective impact of phenolics, flavonoids and other reducing compounds in plant extracts. Shallots contain components with high antioxidant activity. Antioxidant activity after different peeling treatments was determined and is depicted in Fig. 3(f). All the treatments have significantly affected the antioxidant activity. As expected, the hand peeled sample has higher antioxidant activity (93.1%), followed by the IR peeled sample (87.24%). After that, hot water peeling led to an antioxidant activity of 75.17%, and steam peeling led to 72.05%. Steam peeling led to softening of tissue and quality losses of the product due to over-scalding caused by a low rate of steam heat.55 Lye peeling has also affected the antioxidant activity of shallots (55.86%). Even though similar temperature conditions were maintained for hot water peeling and lye peeling, the usage of NaOH has decreased the antioxidant properties. For fruits and vegetables with epicuticular waxes like shallots, the alkaline solution dissolves the wax layer and destroys the microstructure of the epidermis. If the wax layers are intact, NaOH cannot penetrate inside.
The flame peeling method has highly affected the antioxidant activity due to the high-temperature treatment. Since the peel is almost burnt, it directly heats the outer fleshy layer of the shallot, thereby causing heat damage. Dissolution of the wax layer can also result from the liquefaction of the wax layer at high temperatures.
3.7. Peroxidase content
Peroxidase content in fresh shallot causes undesirable flavor, texture, color and nutrition changes. A significant reduction in the peroxide content has been observed in all the peeling treatments. In general, POD is a heat-stable enzyme, and it has been used as an indicator to measure the efficacy of thermal blanching. The antioxidant activity of the bulbs decreases as POD catalyses the direct oxidation of flavonoids and phenolics. The residual activity of the peroxidase enzyme in shallot bulbs peeled by different methods has been determined. The results indicate that the inactivation of enzymes depended on the temperature and time of the treatment. All the treatments significantly affected the selected enzyme's residual activity.
The data were normalised in relation to the specific activity observed in the fresh product (A/A0). The POD activity for untreated hand peeled shallots was 12.45 U mL−1 min−1, the highest compared to the treated samples. The highest enzyme activity inactivation was determined in the flame treated sample (0.57 U mL−1 min−1), where the reduction was 95.41% with respect to the hand peeled sample. Likewise, there was significant enzyme inactivation for all treated samples. The inactivation of peroxidase in infrared peeled shallot was comparably similar to the traditionally peeled samples. Infrared, hot water and lye peeled shallots had an enzyme activity of 1.71, 1.65 and 1.42 U mL−1 min−1, respectively. Steam peeling led to a significantly (p < 0.005) higher enzyme activity value of 2.05 U mL−1 min−1 than that of all the thermal treatments. The change in enzyme activity of treated samples compared to the untreated samples is presented in Fig. 4. Additionally, the correlation between the response variables is presented in Fig. 5. It depicts that all the variables are interrelated with each other, which means that they are directly proportional. This indicates that changes in one variable are likely to influence the other variable.
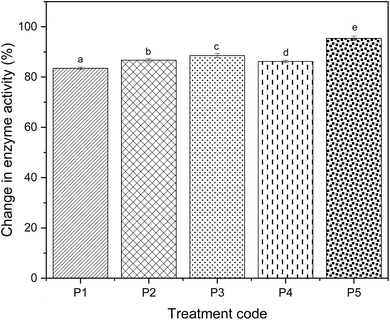 |
| | Fig. 4 Effect of different peeling treatments on changes in peroxidase activity. *Different letters indicate significant differences (p < 0.05) according to the Tukey test. | |
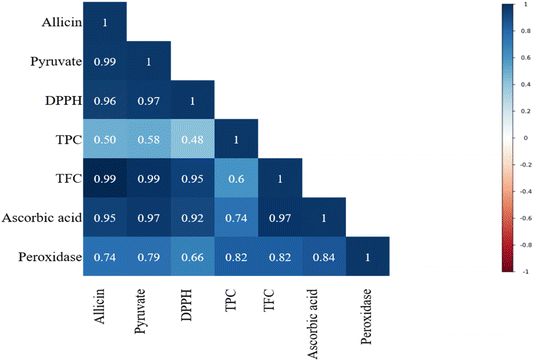 |
| | Fig. 5 Correlation matrix between the dependent variables. | |
3.8. XRD
The X-ray diffraction images of peeled shallot bulbs are depicted in Fig. 6. They show both crystalline and amorphous behaviour. Changes in the crystalline index mainly depend on variables like process conditions, compositional deformation and structural modification in peeled shallot samples. All the samples were more amorphous than crystalline, as the peaks were more curved, whereas purely crystalline substances have sharp peaks. The crystallinity was higher for lye peeling, which was 64.15%. Lye peeling leads to a higher crystallinity value, but the peak is more curved, demonstrating its amorphous nature. Similarly, the peak at 32.09° differed from other samples, likely due to the presence of sodium ions.58 Likewise, researchers have observed the effect of heat treatment on the changes in the structure of corn,59 potato60 and rice starch61 through XRD analysis.
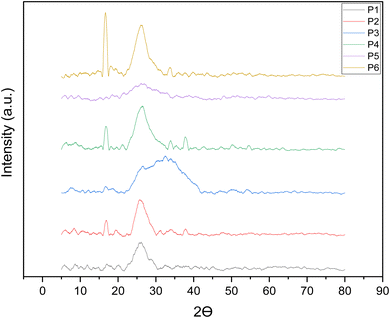 |
| | Fig. 6 XRD characterisation of shallots peeled by different treatments. | |
Moreover, even though the shallots fall in between amorphous and crystalline structures, fresh knife peeled shallots are more crystalline than the other peeling treated samples. Meanwhile, for other peeling methods, the peak was at around 16.6° and 25.9°, which was prominent in the fresh untreated sample, followed by IR peeled samples. The crystallinity after hot water peeling was 42.7%, while those after steam peeling and flame peeling were similar, 32.5% and 32.6%, respectively. From the data, we can observe that the shallots lose their crystalline nature due to higher temperatures. During heat treatment, the thermal energy causes the breaking of hydrogen bonds and other intermolecular interactions that stabilize the crystalline regions. This results in the transition from a highly ordered crystalline state to an amorphous or disordered structure.62
3.9. FTIR
The spectral features of the treated and untreated peeled shallots are given in Fig. 7. The bands between the fingerprint region (1800–750 cm−1) represent the primary biochemical and macronutrients, especially the moieties of carbohydrates, primary secondary structures (α-helix, β-sheet and random coil), lipids and polyphenols in plants.
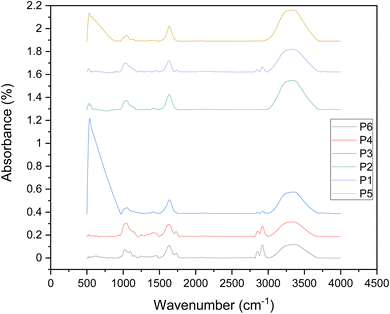 |
| | Fig. 7 FTIR absorbance spectrum of shallots peeled by different treatments. | |
The functional groups detected using the FTIR spectral band and their corresponding wavelengths are given in Table 1. This tends to denote the presence of higher levels of polyphenolic compounds predominant in Allium plants. It has been noted that many peaks were detected in the hand peeled sample as it was devoid of heat treatment. Similarly, infrared-treated samples have higher peaks which has comparatively lower product temperature. It can be observed from Table 1 that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C aromatic ring stretch and aromatic combination bands are present only in infrared-treated and hand peeled samples. Likewise, aldehydes and esters are present in IR-treated and hand peeled samples, which constitute the major volatile compounds in shallots (and other Allium species).63
C–C aromatic ring stretch and aromatic combination bands are present only in infrared-treated and hand peeled samples. Likewise, aldehydes and esters are present in IR-treated and hand peeled samples, which constitute the major volatile compounds in shallots (and other Allium species).63
Table 1 Functional group analysis of peeled shallots treated by different peeling methods based on the FTIR spectrum
| Sample |
Functional group/assignment |
| P6 |
Aromatic C–H in-plane bend; skeletal C–C vibrations |
| P6 |
Alkyl-substituted ether, C–O stretch |
| P6 |
Tertiary amine, CN stretch; cyanate (–OCN and C–OCN stretch) |
| P4, P6 |
Cyclic ethers, large rings, C–O stretch |
| P4, P6 |
Secondary amine, CN stretch; dialkyl/aryl sulfones; sulfonates |
| P4, P6 |
Organic sulfates; carbonate ion |
| P4, P6 |
Ammonium ion |
| P4, P6 |
Methyl C–H asym./sym. bend; methylene C–H bend; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C aromatic ring stretch C–C aromatic ring stretch |
| P4, P6 |
Aldehyde; ester; aromatic combination bands |
| P1, P4, P6 |
Methylene C–H asym./sym. stretch |
| P1, P4, P6 |
Aliphatic fluoro compounds, C–F stretch |
| P1, P2, P6 |
Phosphate |
| P1, P2, P6 |
Amide |
| P2, P3, P5 |
Aliphatic iodo compounds, C–I stretch |
| P1, P4, P5, P6 |
Quinone or conjugated ketone |
| P1, P2, P4, P6 |
Hydroxyl group, H– bonded OH stretch |
| P1, P2, P4, P6 |
Silicate ion; organic siloxane or silicone (Si–O–Si); aliphatic phosphates (P–O–C stretch); primary amine, CN stretch; cyclohexane ring vibrations |
| P1, P2, P4, P6 |
Secondary amine, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–H bend; open-chain imino (–C N–H bend; open-chain imino (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) N–) |
| P1, P2, P4, P5, P6 |
Organic nitrates |
| P1, P2, P4, P5, P6 |
Primary amine, NH bend; alkenyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch C stretch |
| P1, P2, P3, P4, P6 |
Polymeric OH stretch |
| P1, P2, P4, P5, P6 |
Aliphatic primary amine, NH stretch |
| P1, P2, P3, P4, P5 |
Aliphatic secondary amine, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–H stretch; imino compounds, N–H stretch; imino compounds, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–H stretch N–H stretch |
3.10. Sensory evaluation by fuzzy logic
The sensory preferences of the peeled shallots were evaluated using different methods (P1, P2, P3, P4, P5, and P6), and the quality characteristics were evaluated using the fuzzy sensory scale. The triplets associated with the quality characteristics and sensory scores were computed. The triplet values were further converted to a regular fuzzy scale and applied to the overall membership functions. The similarity values for the quality characteristics and samples were computed and employed for ranking purposes. Sensory evaluation plays a major role in the consumer's acceptance. To overcome the influence of artificial tendency in sensory evaluation, the fuzzy logic technique was put in use to calculate the sensory scores. By the fuzzy technique, the idealised mathematical relationship between the sensory attributes was constructed, and specific numerical values can quantify the contribution weights of different sensory quality attributes. Thus, the highly and least important quality attributes of peeled shallots were obtained. The sum of the panellist's choices and the relative importance of the quality of peeled shallots in general are given in Table 2.
Table 2 Sum of sensory scores and triplets associated with sensory scales for quality attributes of peeled shallots
| Sensory quality attribute of different peeling methods |
Not satisfactory |
Fair |
Medium |
Good |
Excellent |
Triplets of sensory score |
|
Colour
|
| P1C |
6 |
3 |
1 |
0 |
0 |
(12.5 10 25) |
| P2C |
0 |
1 |
3 |
6 |
0 |
(62.5 25 25) |
| P3C |
9 |
1 |
0 |
0 |
0 |
(2.5 2.5 25) |
| P4C |
0 |
0 |
0 |
4 |
6 |
(90 25 10) |
| P5C |
9 |
1 |
0 |
0 |
0 |
(2.5 2.5 25) |
| P6C |
0 |
0 |
0 |
2 |
8 |
(95 25 5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Odor
|
| P1O |
3 |
4 |
2 |
1 |
0 |
(27.5 17.5 25) |
| P2O |
0 |
4 |
2 |
4 |
0 |
(50 25 25) |
| P3O |
8 |
2 |
0 |
0 |
0 |
(5 5 25) |
| P4O |
0 |
0 |
1 |
4 |
5 |
(85 25 12.5) |
| P5O |
6 |
4 |
0 |
0 |
0 |
(10 10 25) |
| P6O |
0 |
0 |
0 |
5 |
5 |
(87.5 25 12.5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Texture
|
| P1T |
2 |
7 |
1 |
0 |
0 |
(22.5 20 25) |
| P2T |
1 |
3 |
4 |
2 |
0 |
(42.5 22.5 25) |
| P3T |
7 |
3 |
0 |
0 |
0 |
(7.5 7.5 25) |
| P4T |
0 |
0 |
2 |
2 |
6 |
(85 25 10) |
| P5T |
8 |
1 |
1 |
0 |
0 |
(7.5 5 25) |
| P6T |
0 |
0 |
0 |
4 |
6 |
(90 25 10) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Appearance
|
| P1A |
3 |
4 |
3 |
0 |
0 |
(25 17.5 25) |
| P2A |
1 |
2 |
3 |
4 |
0 |
(50 22.5 25) |
| P3A |
8 |
2 |
0 |
0 |
0 |
(5 5 25) |
| P4A |
0 |
0 |
1 |
4 |
5 |
(85 25 12.5) |
| P5A |
9 |
1 |
0 |
0 |
0 |
(2.5 2.5 25) |
| P6A |
0 |
0 |
0 |
3 |
7 |
(92.5 25 7.5) |
3.10.1. Overall sensory scores of peeled shallots.
Evaluation of the sensory score was performed for various peeling treatments. Responses from 10 evaluators were taken to analyse the sensory scores of the quality attributes of peeled shallots and the quality attributes of peeled shallots in general. Sample P6 was hand-peeled shallot without any treatment; samples P1, P2, P3, P4 and P5 are steam, hot water, lye, infrared, and flame-peeled shallot bulbs. The sensory scores and ratings of the evaluators for shallots peeled with various methods ranged widely. Rankings were given for quality characteristic parameters, such as color, odor, texture, and appearance of the peeled shallot bulbs in general. The overall sensory score for each peeled shallot bulb was calculated using eqn (8) with the help of triplets for sensory scores of samples and relative weightage of the quality characteristic parameters. The overall sensory score of each peeled bulb P1, P2, P3, P4, P5 and P6 is represented as PO1, PO2, PO3, PO4, PO5 and PO6, respectively.
The sensory scores given by the panellists for each sample were converted to the triplets according to eqn (6) and were obtained as follows:
| | | PO1 = (19.98 22.74 30.76) | (15) |
| | | PO2 = (52.52 41.19 36.57) | (16) |
| | | PO3 = (4.76 6.48 26.33) | (17) |
| | | PO4 = (86.81 54.62 31.26) | (18) |
| | | PO5 = (4.33 5.43 26.69) | (19) |
| | | PO6 = (92.4 56.43 29.00) | (20) |
Accordingly, the triplets for their scores on all the quality attributes of the peeled shallots were obtained. The individual quality attributes of peeled shallots were calculated by using eqn (6), and the relative weightages were obtained from eqn (7)
The sum of sensory scores and triplets associated with sensory scales of peeled shallots in general is given in Table 3. The 6-point scale membership function values F1, F2, F3, F4, F5 and F6 expressed on a standard fuzzy scale are presented in eqn (9). The value of the membership function can be indicated for a particular peeled shallot sample ‘x’ on the abscissa. The value of the overall membership function for peeled shallot sample triplets at x = 0 to 100 with an even interval of ten was assessed with the aid of membership function values on a standard scale. For the peeled samples, the overall membership is denoted as Mn, where n represents the sample number.
Table 3 Sum of sensory scores and triplets associated with sensory scales of peeled shallots in general
| Quality attributes |
Not important |
Somewhat important |
Important |
Highly important |
Extremely important |
Triplet score |
| Color |
0 |
0 |
0 |
2 |
8 |
(95 25 5) |
| Odor |
4 |
5 |
1 |
0 |
0 |
(17.5 15 25) |
| Texture |
0 |
0 |
4 |
4 |
2 |
(70 25 20) |
| Appearance |
0 |
1 |
1 |
3 |
5 |
(80 25 12.5) |
Likewise, the overall membership values of sensory scores on standard fuzzy scales were obtained, and the value of Mx at x = 0, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 for individual samples was obtained as:
| | | M1 = (0.56 1 0.99 0.67 0.35 0.02 0 0 0 0) | (25) |
| | | M2 = (0 0.21 0.45 0.69 0.94 1 0.79 0.52 0.25 0) | (26) |
| | | M3 = (1 0.80 0.42 0.04 0 0 0 0 0 0) | (27) |
| | | M4 = (0 0 0 0.14 0.33 0.51 0.69 0.87 1 0.89) | (28) |
| | | M5 = (1 0.78 0.41 0.04 0 0 0 0 0 0) | (29) |
| | | M6 = (0 0 0 0.07 0.25 0.42 0.6 0.78 0.96 1) | (30) |
Likewise, overall membership values for quality attributes in general on a standard fuzzy scale were obtained as follows:
| | | MC = (0 0 0 0 0 0 0.067 0.205 0.343 0.482) | (31) |
| | | MO = (1 0.6 0 0 0 0 0 0 0 0) | (32) |
| | | MT = (0 0 0.163 0.35 0.538 0.725 0.913 1 0.641 0.406) | (33) |
| | | MA = (0 0 0 0.056 0.220 0.384 0.548 0.713 0.877 1) | (34) |
3.10.2. Similarity values of the peeled samples and their ranking.
Similarity (Sm) values were calculated using the membership function of the standard fuzzy scale and overall membership values of sensory scores. As shown in Table 4, similarity values Sm (F, M) for sample M were calculated using eqn (12). Table 4 shows the similarity value of peeled shallots. The ranking order was obtained as follows:
| P6 > P4 > P2 > P1 > P3 > P5 |
| 0.80 (very good) > 0.77 (very good) > 0.70 (good) > 0.90 (fair) > 0.76 (not satisfactory) > 0.77 (not satisfactory) |
| Hand peeling > infrared heating > hot water peeling > steam peeling > lye peeling > flame peeling |
Table 4 Similarity values of peeled shallot samples
|
|
P1 |
P2 |
P3 |
P4 |
P5 |
P6 |
| Not satisfactory, F1 |
0.3672 |
0.0294 |
0.7691
|
0 |
0.7776
|
0 |
| Fair, F2 |
0.9054
|
0.2824 |
0.6973 |
0.0208 |
0.6880 |
0.0114 |
| Satisfactory, F3 |
0.5310 |
0.6591 |
0.1009 |
0.2105 |
0.0979 |
0.1698 |
| Good, F4 |
0.0687 |
0.7051
|
0 |
0.5242 |
0 |
0.4919 |
| Very good, F5 |
0 |
0.3262 |
0 |
0.7768
|
0 |
0.8094
|
| Excellent, F6 |
0 |
0.0347 |
0 |
0.4067 |
0 |
0.4714 |
As expected, the overall acceptance of untreated hand-peeled shallots is very good, as no heat treatment was applied. Among the treated bulbs, infrared peeling has higher acceptability. Flame peeling was not accepted by the panellists as it formed a dark layer by burning the epithelial tissues. Likewise, lye peeling turned a few layers of the bulb tissues to yellow. The color change has naturally influenced the panellists to rank low.
Moreover, the high temperature and extended treatment time in all conventional peeling methods have caused the disruption of tissues, leading to the melting of the cells. The change in quality and functional values has been reciprocated through sensory scores. From the scores given by the panellists, the colour values are better for untreated hand and infrared peeled shallots.
The quality attributes preferred for peeled shallots were colour, texture, appearance, and odour. The ranking of the quality attributes of peeled shallots in general, was accomplished by calculating similarity values for all the selected quality attributes using membership function values on the standard fuzzy scale and overall membership function values of quality attributes. Qsum = 262.5
| | | QCrel = (0.361 0.095 0.019) | (35) |
| | | QOrel = (0.066 0.057 0.095) | (36) |
| | | QTrel = (0.266 0.095 0.076) | (37) |
| | | QArel = (0.304 0.095 0.047) | (38) |
The similarity values for the selected quality attributes, in general, are shown in Table 5.
| Colour > appearance > texture > odour |
| 0.52 > 0.85 > 0.71 > 0.95 |
| Extremely important > highly important > important > not at all important |
Table 5 Similarity values of quality attributes of peeled shallot samples in general
|
|
Color |
Odor |
Texture |
Appearance |
| Not at all necessary, F1 |
0 |
0.9559
|
0 |
0 |
| Somewhat necessary, F2 |
0 |
0.4400 |
0.1001 |
0.0101 |
| Necessary, F3 |
0 |
0 |
0.3949 |
0.1688 |
| Important, F4 |
0.0679 |
0 |
0.7137
|
0.5040 |
| Highly important, F5 |
0.3292 |
0 |
0.6822 |
0.8513
|
| Extremely important, F6 |
0.5226
|
0 |
0.2155 |
0.5181 |
The colour is the major quality attribute and is considered the most significant quality parameter for peeled shallot bulbs. Odour was the least important (not at all important) quality attribute for peeled shallots.
3.11. Colour values and the browning index of peeled shallot bulbs
The colour values (L*, a* and b*) of the peeled shallot bulbs are represented in Fig. 8. The a* value of P3 falls in the negative category since the bulb has turned yellowish after lye treatment. The browning index was calculated based on eqn (13) and (14). It was found that lye peeling had a higher browning index, which had a value of 89.58. Similarly, flame peeling showed a higher value of 57.51. The lowest value was for hand peeling (14.98), as expected. The second lowest value was for IR peeled shallot, which had a browning index of 19.29. Steam peeling and hot water peeling had browning index values of 38.75 and 36.95, respectively. For better understanding, the visual representation of shallot bulbs peeled by different methods is illustrated in Fig. 9. Non-enzymatic browning is one of the primary reasons for the difference in the browning effect of peeled shallots. Similarly, non-enzymatic browning has been reported during onion slice drying.64
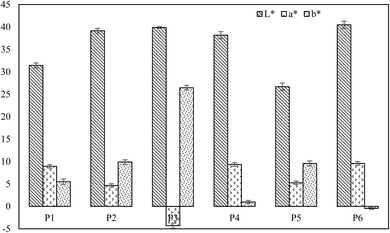 |
| | Fig. 8 Color values of shallots peeled by different peeling treatments. | |
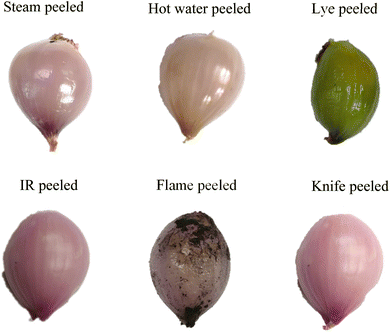 |
| | Fig. 9 Shallot bulbs peeled using different peeling methods (steam, hot water, lye, IR, flame and knife peeling). | |
4 Summary and conclusions
The shallots were peeled by six methods, namely steam peeling, hot water peeling, lye peeling, flame peeling, hand or knife peeling and infrared peeling. Chemical analyses were conducted to determine allicin content, pyruvate content, total flavonoid content, antioxidant activity, total phenolic content, ascorbic acid content and peroxidase activity in the peeled shallots. The results proved that the hand peeled shallots had higher nutritional value. Among the thermal-treated peeling methods, infrared peeling had higher values, whereas flame peeling and lye peeling had lower values. Similar results were observed in the XRD and FTIR profile analyses.
From fuzzy logic analysis, the shallot bulbs peeled by hand (untreated) have higher sensory ranking as no heat treatment was applied. Following this, infrared peeling has higher acceptance compared to other conventionally peeled samples. Furthermore, flame peeling, which is commonly used to remove the tunics from the bulb in onions and shallots, had the least acceptance. Likewise, while analysing the importance of the quality attributes of the peeled shallots, it was found that the colour parameter was extremely important. Odour was not at all important. The sensory scores directly reciprocate the quality damage and the consumer acceptance.
This study provides insights into the impact of various peeling methods on nutritional content, sensory attributes, and quality of shallots, aiding the food processing industry in optimizing processes and meeting consumer preferences. Infrared peeling can be an environmentally friendly peeling method as it avoids usage of water, thereby reducing the problems associated with waste water generation. Future research will focus on investigating long-term effects, scaling up optimized methods for industrial applications, and application of the technology on other food products.
Ethical statement
Participants gave informed consent via the statement “I am aware that my responses are confidential, and I agree to participate in this survey” where an affirmative reply was required to enter the survey. They were able to withdraw from the survey at any time without giving a reason. The products tested were safe for consumption.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
D. S.: conceptualisation, formal analysis, investigation, methodology, writing the original draft. M. W.: investigation, writing – review and editing. P. P. S.: investigation, fund acquisition, resources, writing – review and editing, supervision.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to the Science and Engineering Research Board, India, (grant/award number: IMP/2018/002120), the Central Research Facility, NIT Rourkela, India, and the Ministry of Education, India.
References
- R. Tocmo, Y. Lin and D. Huang, J. Agric. Food Chem., 2014, 62, 5296–5304 CrossRef CAS PubMed.
- R. Pangestuti, E. Sulistyaningsih, B. Kurniasih, R. H. Murti, S. Harper and S. Subandiyah, Ann. Appl. Biol., 2023, 182, 257–266 CrossRef CAS.
-
M. Jay, Onions and Garlic: A Global History, Reaktion Books, 2016 Search PubMed.
- F. L. G. L. Saos, A. Hourmant, F. Esnault and J.-E. Chauvin, Ann. Bot., 2002, 89, 419–425 CrossRef PubMed.
- K. Nemeth and M. Piskula, Crit. Rev. Food Sci. Nutr., 2007, 47, 397–409 CrossRef CAS PubMed.
- J. Mlcek, M. Valsikova, H. Druzbikova, P. Ryant, T. Jurikova, J. Sochor and M. Borkovcova, Turk. J. Agric. For., 2015, 39, 999–1004 CrossRef CAS.
- X. Lu, J. Wang, H. M. Al-Qadiri, C. F. Ross, J. R. Powers, J. Tang and B. A. Rasco, Food Chem., 2011, 129, 637–644 CrossRef CAS PubMed.
- R. Thuwapanichayanan, S. Prachayawarakorn and S. Soponronnarit, J. Food Eng., 2014, 136, 34–41 CrossRef CAS.
- S. Khushbu, C. K. Sunil, D. V. Chidanand and R. Jaganmohan, J. Food Process Eng., 2020, 43, e13237 CrossRef.
- S. Khushbu and C. Sunil, J. Food Sci. Technol., 2020, 57, 3601–3610 CrossRef CAS PubMed.
-
Z. Pan, X. Li, C. Venkitasamy and Y. Shen, Reference Module in Food Science, 2015, pp. 1–9 Search PubMed.
- M. Gavahian and S. K. Sastry, Trends Food Sci. Technol., 2020, 106, 345–354 CrossRef CAS.
- A. F. Vinha, R. C. Alves, S. V. Barreira, A. Castro, A. S. Costa and M. B. P. Oliveira, Food Sci. Technol., 2014, 55, 197–202 CAS.
- P. Prudhvi, S. Deepika and P. Sutar, J. Food Eng., 2022, 318, 110891 CrossRef CAS.
- Y.-H. Zhou, S. K. Vidyarthi, X.-H. Yang, X. Duan, Z.-L. Liu, A. S. Mujumdar and H.-W. Xiao, Innovative Food Sci. Emerging Technol., 2022, 102961 CrossRef.
- S. Deepika and P. P. Sutar, J. Food Sci., 2023, 88, 1506–1522 CrossRef CAS PubMed.
- S. Deepika and P. Sutar, Drying Technol., 2018, 36, 1719–1737 CrossRef CAS.
-
S. Deepika and P. Sutar, Drying Technologies for Foods: Fundamentals and Applications (Part I), 2015, pp. 121–154 Search PubMed.
- S. K. Vidyarthi, H. M. El Mashad, R. Khir, S. K. Upadhyaya, S. K. Singh, R. Zhang, R. Tiwari and Z. Pan, Biosyst. Eng., 2019, 186, 106–117 CrossRef.
- J. Eskandari, A. M. Kermani, S. Kouravand and P. Zarafshan, Lwt, 2018, 90, 570–576 CrossRef CAS.
- S. Deepika and P. P. Sutar, Crit. Rev. Food Sci. Nutr., 2024, 64, 10749–10771 CrossRef CAS PubMed.
- X. Li, Z. Pan, G. G. Atungulu, D. Wood and T. McHugh, J. Food Eng., 2014, 128, 79–87 CrossRef.
- Y. Shen, R. Khir, D. Wood, T. H. McHugh and Z. Pan, Innovative Food Sci. Emerging Technol., 2020, 65, 102474 CrossRef CAS.
- Z. Mohammadi, M. Kashaninejad, A. M. Ziaiifar and M. Ghorbani, Lwt, 2019, 99, 128–137 CrossRef CAS.
- B. Wang, C. Venkitasamy, F. Zhang, L. Zhao, R. Khir and Z. Pan, Food Sci. Technol., 2016, 69, 458–467 CrossRef CAS.
- A. Kate and P. Sutar, Innovative Food Sci. Emerging Technol., 2018, 48, 111–121 CrossRef CAS.
- S. Zhao, S. Wang, H. Ding, Z. Guo, M. B. Simelane, Q. Liu, T. Tao, L. Guo, L. Chang and C. Ding, J. Food Eng., 2023, 357, 111631 CrossRef CAS.
- X. Li, A. Zhang, G. G. Atungulu, M. Delwiche, R. Milczarek, D. Wood, T. Williams, T. McHugh and Z. Pan, Food Sci. Technol., 2014, 55, 34–42 CAS.
-
M. K. Sharif, M. S. Butt, H. R. Sharif and M. Nasir, Handbook of Food Science and Technology, 2017, pp. 361–386 Search PubMed.
-
H. Heymann and H. T. Lawless, Sensory Evaluation of Food: Principles and Practices, Springer Science & Business Media, 2013 Search PubMed.
- P. Yu, M. Y. Low and W. Zhou, Trends Food Sci. Technol., 2018, 71, 202–215 CrossRef CAS.
- K. Vivek, K. Subbarao, W. Routray, N. Kamini and K. K. Dash, Food Bioprocess Technol., 2020, 13, 1–29 CrossRef.
- Z. S. Zolfaghari, M. Mohebbi and M. Najariyan, Appl. Soft Comput., 2014, 22, 417–423 CrossRef.
- D. Kohli, P. S. Champawat, V. D. Mudgal, S. K. Jain and B. K. Tiwari, J. Food Process Eng., 2021, 44, e13826 CrossRef CAS.
-
V. Lebot, Tropical Root and Tuber Crops, Cabi, 2019 Search PubMed.
- Y.-H. Zhou, S. K. Vidyarthi, X.-H. Yang, X. Duan, Z.-L. Liu, A. S. Mujumdar and H.-W. Xiao, Innovative Food Sci. Emerging Technol., 2022, 77, 102961 CrossRef.
- Y. Feng, C. Zhou, A. E. A. Yagoub, Y. Sun, P. Owusu-Ansah, X. Yu, X. Wang, X. Xu, J. Zhang and Z. Ren, Lwt, 2019, 116, 108577 CrossRef CAS.
- R. Aslam, M. S. Alam, S. Singh and S. Kumar, LWT, 2021, 151, 112183 CrossRef CAS.
- C. Leishangthem and P. Sutar, Drying Technol., 2024, 42, 238–252 CrossRef CAS.
- K. Sharma, N. Mahato and Y. R. Lee, J. Food Drug Anal., 2018, 26, 518–528 CrossRef CAS PubMed.
- K. M. Yoo, K. W. Lee, J. B. Park, H. J. Lee and I. K. Hwang, J. Agric. Food Chem., 2004, 52, 5907–5913 CrossRef CAS PubMed.
- S. K. Lee, Z. Mbwambo, H. Chung, L. Luyengi, E. Gamez, R. Mehta, A. Kinghorn and J. Pezzuto, Comb. Chem. High Throughput Screening, 1998, 1, 35–46 CrossRef CAS PubMed.
-
W. Horwitz, Official Methods of Analysis of AOAC International, edn 17, 2000 Search PubMed.
- K. Subrahmanyam, K. Gul, R. Sehrawat and F. M. Allai, Food Biosci., 2023, 52, 102425 CrossRef CAS.
- S. Murakonda and M. Dwivedi, Biomass Convers. Biorefin., 2022, 1–19 Search PubMed.
- L. J. Yin, C. L. Pan and S. T. Jiang, J. Food Sci., 2002, 67, 786–792 CrossRef CAS.
- M. L. Corollaro, E. Aprea, I. Endrizzi, E. Betta, M. L. Demattè, M. Charles, M. Bergamaschi, F. Costa, F. Biasioli and L. C. Grappadelli, Postharvest Biol. Technol., 2014, 96, 135–144 CrossRef.
-
M. K. Sharif, M. S. Butt, H. R. Sharif and M. Nasir, Handbook of Food Science and Technology, 2017, vol. 10, pp. 362–386 Search PubMed.
- N. Mansor, H. J. Herng, S. J. Samsudin, S. Sufian and Y. Uemura, J. Med. Bioeng., 2016, 5, 24–27 CAS.
- R. Mathialagan, N. Mansor, M. R. Shamsuddin, Y. Uemura and Z. Majeed, Chem. Eng. Trans., 2017, 56, 1747–1752 Search PubMed.
- W. Qu, Y. Liu, Y. Feng and H. Ma, LWT, 2022, 163, 113542 CrossRef CAS.
- E. Garcia and D. M. Barrett, J. Food Process. Preserv., 2006, 30, 3–14 CrossRef.
- H.-K. Kim, K.-S. Jo, D.-Y. Kwon and M.-H. Park, Appl. Biol. Chem., 1992, 35, 6–9 CAS.
- C. Rock, W. Yang, R. Goodrich-Schneider and H. Feng, Food Eng. Rev., 2012, 4, 1–15 CrossRef.
- L.-Z. Deng, A. S. Mujumdar, Q. Zhang, X.-H. Yang, J. Wang, Z.-A. Zheng, Z.-J. Gao and H.-W. Xiao, Crit. Rev. Food Sci. Nutr., 2019, 59, 1408–1432 CrossRef CAS PubMed.
- J. Chlopicka, P. Pasko, S. Gorinstein, A. Jedryas and P. Zagrodzki, Food Sci. Technol., 2012, 46, 548–555 CAS.
- L. Zhang, L. Chen, C. Zhou, A. T. Mustapha and H. Wahia, Food Rev. Int., 2023, 1–18 Search PubMed.
- F.-h. Li, H.-j. Hu, R.-s. Yao, H. Wang and M.-m. Li, Ind. Eng. Chem. Res., 2012, 51, 6270–6274 CrossRef CAS.
- Z. Liu, C. Wang, X. Liao and Q. Shen, Food Hydrocolloids, 2020, 108, 106081 CrossRef CAS.
- E. Bidzińska, M. Michalec and D. Pawcenis, Magn. Reson. Chem., 2015, 53, 1051–1056 CrossRef PubMed.
- M. Witek, W. Węglarz, L. De Jong, G. Van Dalen, J. Blonk, P. Heussen, E. Van Velzen, H. Van As and J. Van Duynhoven, Food Chem., 2010, 120, 1031–1040 CrossRef CAS.
- A. Newman and G. Zografi, Mol. Pharmaceutics, 2020, 17, 1761–1777 CrossRef CAS PubMed.
- G. Liu, Y. Wang, L. Hu and H. He, Foods, 2022, 11, 3829 CrossRef CAS PubMed.
- J. Mitra, S. L. Shrivastava and P. S. Rao, Int. Agrophys., 2015, 29, 91–100 CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Min
Wu
b and
P. P.
Sutar
*a
a,
Min
Wu
b and
P. P.
Sutar
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. Afterwards, the solution was filtered, and the supernatant was used for allicin determination. For analysis, 1 mL of the sample extract (Hepes buffer for blank) was mixed with 5 mL of 10 mM cysteine solution and was left to rest for 15 min at 25 ± 1 °C. Furthermore, 1 mL of the reaction mixture was made up to 100 mL with the Hepes buffer solution. Later, 4.5 mL of the diluted solution was made to react with 0.5 mL of 1.5 mM DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) and left to stand for 15 min at 25 ± 1 °C. Subsequently, the absorbance was measured at 412 nm using a spectrophotometer, as A for samples and A0 for blank. The quantification of allicin was calculated as follows:
000 rpm for 5 min. Afterwards, the solution was filtered, and the supernatant was used for allicin determination. For analysis, 1 mL of the sample extract (Hepes buffer for blank) was mixed with 5 mL of 10 mM cysteine solution and was left to rest for 15 min at 25 ± 1 °C. Furthermore, 1 mL of the reaction mixture was made up to 100 mL with the Hepes buffer solution. Later, 4.5 mL of the diluted solution was made to react with 0.5 mL of 1.5 mM DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) and left to stand for 15 min at 25 ± 1 °C. Subsequently, the absorbance was measured at 412 nm using a spectrophotometer, as A for samples and A0 for blank. The quantification of allicin was calculated as follows: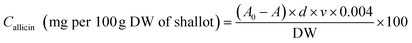
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v). Furthermore, it was extracted by ultrasound treatment (40 kHz) for 30 min at 30 ± 2 °C. Additionally, the solution was centrifuged at 5000 × g for 15 min and filtered. The extract is the collected supernatant, and it was stored at 4 °C until analysis.39
20 (w/v). Furthermore, it was extracted by ultrasound treatment (40 kHz) for 30 min at 30 ± 2 °C. Additionally, the solution was centrifuged at 5000 × g for 15 min and filtered. The extract is the collected supernatant, and it was stored at 4 °C until analysis.39
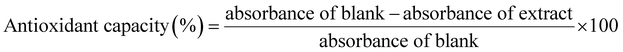
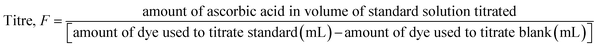
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v). It was homogenised under chilled conditions for 1 min, and the homogenate was centrifuged in polypropylene tubes at 6000 × g at 4 °C for 20 min. The centrifuged solution was filtered, and the supernatant was collected. The supernatants were kept under refrigerated conditions until further analysis.
5 (w/v). It was homogenised under chilled conditions for 1 min, and the homogenate was centrifuged in polypropylene tubes at 6000 × g at 4 °C for 20 min. The centrifuged solution was filtered, and the supernatant was collected. The supernatants were kept under refrigerated conditions until further analysis.
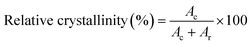
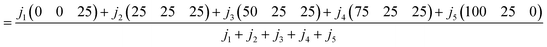

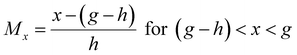
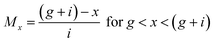
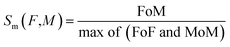

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C aromatic ring stretch and aromatic combination bands are present only in infrared-treated and hand peeled samples. Likewise, aldehydes and esters are present in IR-treated and hand peeled samples, which constitute the major volatile compounds in shallots (and other Allium species).63
C–C aromatic ring stretch and aromatic combination bands are present only in infrared-treated and hand peeled samples. Likewise, aldehydes and esters are present in IR-treated and hand peeled samples, which constitute the major volatile compounds in shallots (and other Allium species).63![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C aromatic ring stretch
C–C aromatic ring stretch![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–H bend; open-chain imino (–C
N–H bend; open-chain imino (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–)
N–)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch
C stretch![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–H stretch; imino compounds,
N–H stretch; imino compounds, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–H stretch
N–H stretch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)








