Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Natural polymer-based food packaging: paving the way to a greener future – a review
Chun Bong Yuen
 ,
Hio Lam Chong
,
Man-Hin Kwok
* and
To Ngai
,
Hio Lam Chong
,
Man-Hin Kwok
* and
To Ngai
 *
*
Department of Chemistry, The Chinese University of Hong Kong, Shatin, Hong Kong, China. E-mail: manhinkwok@cuhk.edu.hk; tongai@cuhk.edu.hk
First published on 5th May 2025
Abstract
Food packaging materials play a major role in the current plastics industry. To pursue environmental protection and sustainability, the search for alternatives to replace conventional plastics has emerged as an industry-wide imperative. This article offers a comprehensive comparison of different food packaging materials, analysing both synthetic and natural polymers across key performance criteria. The analysis reveals that natural polymers demonstrate superior biodegradability, tensile strength, and oxygen barrier properties. Conversely, synthetic packaging exhibits significant advantages in elongation performance, mass production, and costs due to mature manufacturing technology. However, this review also highlights the need to overcome key challenges related to their production scalability, mechanical and barrier properties, and waste treatment. Realizing their full potential will require close collaboration among researchers, industry stakeholders, and policymakers to promote sustainable solutions.
Sustainability spotlightThe widespread use of synthetic polymer-based food packaging materials has been a long-standing practice. However, growing environmental concerns have prompted increased scrutiny in recent times. As the global community recognizes the need for sustainable practices, research into eco-friendly alternatives has gained momentum. One area of focus is natural polymer-based food packaging materials, which have shown promise as a viable replacement for synthetic polymers. Nevertheless, significant challenges remain to be overcome in terms of post-processing. This article provides a comprehensive analysis of the advantages and disadvantages of current natural polymer-based food packaging materials, with a particular emphasis on their production, usage, and treatment. Through comparing various materials, we assess the feasibility of replacing synthetic polymer-based materials with natural polymers, providing a valuable resource for decision-makers and stakeholders. |
1. Background
The food packaging industry plays a crucial role in ensuring the quality and safety of food products. Packaging acts as a critical shield for preserving food. It protects against moisture, oxygen, and carbon dioxide, thereby helping to maintain its flavours, aromas, nutrients, and colors.1Historically, natural materials like gourds, shells, and leaves were used to contain food in ancient civilizations.2 However, with the advent of paper packaging in the 17th century, a lightweight and versatile alternative emerged. This innovation offered printability, portability, and disposability, but it struggled to ensure product quality preservation due to limitations in protection and durability. The 20th century marked a significant shift with the emergence of plastic packaging – a revolutionary development that reshaped the market. Plastics, mainly being synthetic polymer, began to replace conventional packaging materials due to their lightweight, flexible, and water-resistant properties, as well as their exceptional polymeric elongation and tensile strength. Furthermore, the stable chemical structure and non-biodegradable nature of plastics led to a significant extension of shelf life.
The environmental costs associated with plastic packaging are multifaceted and far-reaching, stemming from its durability and persistence in the environment.3 Rapid development and population growth have significantly increased the demand for single-use plastics, driven by our preference for convenience. However, the lack of effective global waste management systems has created a stark imbalance between supply and demand, leading to improper disposal and exacerbating plastic pollution.4
Plastic manufacturing relies heavily on finite petroleum resources, generating toxic byproducts such as phthalates, bisphenols, alkylphenols, and biocides.5 To address these complex challenges, a holistic approach is necessary, balancing convenience with sustainable practices and effective waste management solutions.
The widespread use of plastic food packaging has led to a significant annual increase in plastic waste discard, with single-use packaging plastic waste constituting approximately 40% of the market by weight.6 Removing this persistent waste from the environment is incredibly time-consuming and energy-intensive. To accelerate decomposition, common approaches include physical, thermal/chemical, and biological treatments.7–9 Natural degradation can be extremely slow, with plastics decomposing as little as 10 micrometers per year. In contrast, according to industry standard EN 17033, biodegradable plastic mulches must decompose in soil by at least 90% within two years. However, few plastic alternatives have successfully achieved a balance between performance and cost.10,11
Physical treatment methods for plastic waste include polymer size reduction and UV/photo-oxidative treatment,12 with some processes utilized in material recycling to create new products from used plastics. However, recycling efforts have struggled to achieve widespread success due to policy enforcement challenges.13 Thermal treatment approaches like incineration, pyrolysis, and gasification demand advanced processing systems and meticulous waste sorting for optimal efficiency. This exportation increases the risk of improper waste management practices, a problem often exacerbated by the widespread reliance on plastic packaging.14
Given these challenges, the development of natural polymer-based materials (NPMs) is emphasizing biodegradability, composability, and a reduced carbon footprint. As consumer demand for sustainable solutions grows, the market for biomaterial-based food packaging is expanding. This growth is driven by a focus on replacing traditional single-use plastic packaging with recyclable and biodegradable alternatives, reflecting a broader shift towards a more sustainable future.15
Despite its potential, the market growth of NPMs has fallen short of expectations.16 A major hindrance is the need for industrial-scale investment and practical differentiation from synthetic polymer-based materials (SPMs) in terms of environmental impact.17 Some commercially available NPMs promote biodegradability but require specific waste treatment processes. This necessitates waste sorting and collection to meet the conditions necessary for degradation. Conversely, soil degradation may be a more viable option depending on the availability of suitable NPMs and landfill space.18 However, comprehensive studies on the soil and aquatic decomposition of these materials are still lacking,19–22 which has limited their adoption as a common solution. Therefore, it is crucial to analyse the complete life cycle of bio-based food packaging materials when considering the potential of bioplastics.
Fig. 1 illustrates the typical lifecycle of food packaging materials derived from natural and petroleum sources. Although both types undergo processes like extrusion, moulding, or lamination, SPMs cannot be repurposed as nutrients for new natural resources after disposal. Conversely, NPMs can reintegrate into the lifecycle due to their characteristics, highlighting their significant potential as sustainable materials. This capability has garnered considerable attention in recent years.
Biodegradable films formed by natural polymers have emerged as a promising alternative to traditional plastic packaging, offering a viable solution to address the growing issue of plastic pollution. Despite their potential benefits, including reduced environmental impact and increased sustainability, biodegradable films also present several challenges, including resource utilization, production processes, material properties, and biodegradability. This review aims to critically evaluate the current state of biodegradable films, highlighting their strengths and limitations, and exploring future directions for their development and implementation in the food packaging industry.
2. Introduction of common food packaging materials (FPMs)
FPMs can be classified into two main types: synthetic polymer and natural polymers, sourced from either petroleum-based or bio-based sources that adjusted chemical and physical properties. Synthetic polymers are man-made polymers created through chemical reactions, typically involving the combination of monomers. They are produced through industrial processes and have unique properties such as strength, flexibility, and resistance to heat. Natural polymers are a type of macromolecule that occurs naturally in the environment, or can be produced through biological processes. These polymers are often found in living organisms such as plants and animals, or can be derived from renewable biomass sources. They are biodegradable and renewable, and often exhibit unique structures and properties derived from their natural sources. Fig. 2a illustrates the production process of FPMs from these two sources. Also, Fig. 2b presents the outlook of these natural polymer based materials.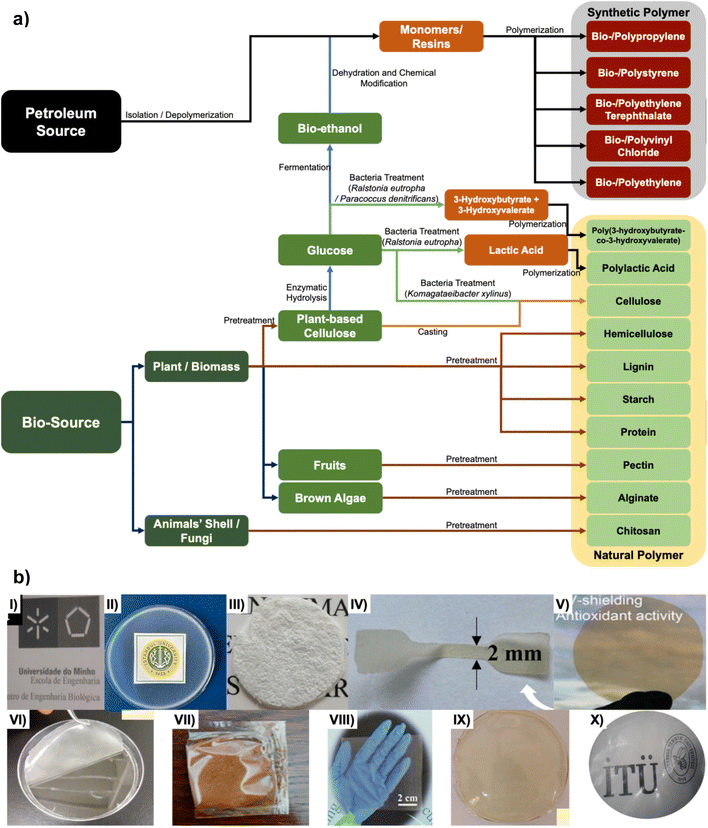 | ||
| Fig. 2 (a) The production routes of different food packaging materials, and (b) appearance of a (I) poly(3-hydroxybutyrate-co-3-hydroxyvalerate) film23 (Reprinted with permission from Elsevier), (II) polylactic acid film24 (Reprinted with permission from Elsevier), (III) cellulose-based film25 (Reprinted with permission from SNCSC), (IV) hemicellulose-based film26 (Reprinted with permission from Elsevier), (V) lignin-based film27 (Reprinted from with permission from SNCSC), (VI) starch-based film28 (Reprinted with permission from SNCSC), (VII) protein-based film29 (Reprinted with permission from SNCSC), (VIII) pectin-based film30 (Reprinted with permission from Elsevier), (IX) alginate-based film31 (Reprinted with permission from MDPI), and (X) chitosan-based film32 (Reprinted with permission from Elsevier). | ||
2.1. Synthetic polymer-based materials (SPMs)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 829 million pounds of PP in 2022, ranking second after polyethylene.34 Traditional PP is derived from petroleum-based sources, but researchers have developed alternative methods to produce propene monomers from plant or biomass sources.35 This approach creates bio-polypropylene (Bio-PP), which reduces environmental impact.36 However, both PP and Bio-PP exhibit non-biodegradable characteristics under natural conditions, making further research necessary to address technical and economic challenges associated with sustainable production of PP.
829 million pounds of PP in 2022, ranking second after polyethylene.34 Traditional PP is derived from petroleum-based sources, but researchers have developed alternative methods to produce propene monomers from plant or biomass sources.35 This approach creates bio-polypropylene (Bio-PP), which reduces environmental impact.36 However, both PP and Bio-PP exhibit non-biodegradable characteristics under natural conditions, making further research necessary to address technical and economic challenges associated with sustainable production of PP.2.2. Natural polymer-based materials (NPMs)
PLA exhibits good transparency and mechanical strength, which are comparable to those of traditional plastics like PE and PP.53 However, PLA has a relatively low melting point, limiting its use in high-temperature applications. One of the key advantages of PLA is its biodegradability.53 Under suitable composting conditions, PLA can break down into naturally occurring substances such as carbon dioxide and water.52 This makes PLA an environmentally friendly alternative to conventional SPMs, such as PET, which is commonly used for bottles, cups, trays and films of disposable cutleries.54
2.2.3.1. Cellulose nanofibers (CNFs). Nanocellulose can be produced through chemical, biological and mechanical approaches.65 Regardless of the method employed, the resulting CNFs typically undergo high-pressure homogenization during pretreatment and post-treatment to achieve desirable functionalities and fiber sizes.66
Traditionally, CNFs have been extracted from wood cellulose through kraft pulping treatment. This process requires pretreatment to remove lignin and hemicellulose components, which are then discharged into the black liquor waste stream.67 TEMPO-oxidation is an energy-efficient approach to chemically obtain CNFs from the plant sources, which oxidises cellulose hydroxyl groups, enabling easier subsequent treatment under basic and neutral conditions.68 Other common downsizing methods include soft acid hydrolysis, enzymatic treatment, and mechanical beating. Studies have shown that TEMPO-oxidized CNFs offer the highest yield in nanofiber content compared to the other types of CNFs.69 In recent years, there has been growing interest in using non-wood sources for CNF production, as they can offer rapid generation and a lower starting value. Aside from their high aspect ratio and nano-scale dimensions, the residual elements in CNFs have demonstrated potential to enhance fibrillation efficiency. As a result, maintaining an optimal level of residual elements is an ongoing area of research.
On the other hand, CNFs possess several desirable properties, including a high specific surface area, high compressive strength, low density, a small expansion coefficient, and an easily modifiable polyhydroxy surface structure.70 These characteristics make CNFs attractive for various applications. To obtain specific desired properties, such as antibacterial, emulsifying, and stabilizer properties, CNFs are often grafted with new functional groups.71
2.2.3.2. Cellulose nanocrystals (CNCs). CNCs are rod-like or needle-shaped particles with dimensions less than 100 nm and high crystallinity. Similar to other cellulose derivatives, CNCs possess desirable properties such as high strength, stiffness, mouldability, and transparency, making them suitable for applications in the transparent packaging market.72–74 The preparation of CNCs typically begins with acid hydrolysis, but other methods like enzymatic hydrolysis, mechanical refining, ionic liquid treatment, subcritical water hydrolysis, and oxidation methods are also employed.75 The goal of these processes is to cleave the glycosidic bonds between cellulose fibers, resulting in CNCs with a high surface-to-volume ratio, which facilitates surface functionalization.76 Through composition and chemical modifications, CNCs can acquire drug delivery capabilities,77 aerogel and foam production,78,79 and maintain food quality with PHBV and PLA composites.80
2.2.3.3. Bacterial cellulose (BC). BC is a newly developed bio-based material synthesized by using specific bacteria, notably Komagataeibacter xylinus. Although BC exhibits the same chemical structure as plant cellulose, it exhibits higher crystallinity, which imparts superior physical and mechanical properties. Like typical plant cellulose, BC comprises a linear chain of β-1,4-linked D-glucose units, represented by the molecular formula (C6H10O5)n. It forms a three-dimensional network of ultrafine fibers characterized by high yield, purity, biocompatibility, and biodegradability. These attributes position BC as a promising material for diverse applications, particularly in food and biomedical engineering.81
However, the abundance of hydroxyl groups in BC results in high water sensitivity, low barrier properties, a complex structure, low solubility, and an unpleasant odor that depends on the medium.82 These drawbacks reduce the performance of BC films under humid conditions and limit their application for oxygen-sensitive foods, such as strawberries and meat.83 To address these challenges, various strategies have been developed to modify the surface or structure of BC films, including blending, cross-linking, coating, or grafting with different agents or polymers, in order to introduce new properties, such as antimicrobial, antioxidant, or bioactive effects.84–86
The production of BC films from a carbon source involves three main steps: hydrolysis, fermentation, and oxidation. This process can be classified as a typical bottom-up production method. The quality and quantity of BC films depend on various factors, such as the type and concentration of tea, the duration and temperature of fermentation, and the strain and inoculum size of bacteria.87 Recently, several studies have investigated the potential applications of BC films for food and biomedical purposes,82,83,88,89 reassuring their potential in use as FPMs.
Thermoplastic starches (TPSs) have been investigated with blends of PVA and LDPE.105–107 By replacing water with glycerol, sugars, and glycols, the gelatinization temperature will vary according to viscosity, diffusion, and hydrogen bonding.104 Although TPSs have many benefits and applications, without modification, they are water-soluble and have poor mechanical properties. Therefore, reinforcement with different biopolymers to enhance strength and reduce water absorption has become a common approach for TPS blends.108
Pectin is typically a white to yellowish powder that dissolves in pure water and at high concentration showcasing pseudo-plastic characteristics.110 It offers good compatibility with different polymers, and pectin films are mainly prepared with glycerol as the plasticizer and formed through solution casting.111,112 Additionally, pectin-based nanocomposites films can be used in packaging to provide barriers against water, gases and contaminants.113 These films are biodegradable, renewable, and customizable with additives or other biopolymers to enhance specific properties, aligning with sustainability goals. However, stability and mechanical properties still have to be addressed.114
One of the notable applications of SPI is in food packaging, as it is also considered safe for food contact. It can be utilized to produce edible films and coatings that act as a barrier between the food and its surrounding environment, thereby extending the shelf life of perishable food items. Studies have shown that SPI possesses polymerization properties, and blending with collagen can enhance hydrophobicity.121 Combined with plasticizers and antimicrobial nanoparticles, the composite films made from SPI offer protection against moisture, oxygen, and microbial contamination, ensuring the quality and safety of packaged food.122
In addition to SPI, another natural protein with unique properties and various applications is zein, which is derived from corn (maize). It is obtained through a process called wet milling, which involves separating the corn protein from other components such as starch and fiber. Zein has exceptional hydrophobicity due to its non-polar amino acids.123 Through electrospinning, zein polymer solutions are prepared as fibers that possess antimicrobial properties.124 Moreover, blending with other biopolymers can achieve thin, flexible films that serve as coatings or packaging materials.125
As discussed in the previous sections, various NPMs and SPMs have been explored for their potential use in food packaging. These polymers exhibit distinct properties and characteristics that can impact the performance of food packaging materials. To better understand the extent to which these polymers can replace SPMs, a comparison of their properties has been provided in the section below.
3. Comparison of different FPMs
3.1. Production and cost
The global production of thermoplastics has experienced significant growth, with a compound annual growth rate (CAGR) of 8.4% from 2 million tons in 1950 to 159 million tons in 2023.132 The production of NPMs is driven by increasing demand and investment, which currently account for a global production capacity of 4 Mt and has a CAGR of 17.04%.133,134 This highlights the great potential of biodegradable packaging in the market, driven by increasing attention to severe plastic pollution and restrictions on plastic use.135While both legislative and non-legislative actions have been found to be effective in reducing single-use plastic consumption, finding suitable alternatives to single-use plastics remains a challenge. The production of such alternatives is often considered complex and small-scale, with high costs, but their performance still does not compare to that of conventional synthetic polymers.
Table 1 illustrates that SPMs have mature manufacturing techniques, resulting in a larger production scale and lower costs. However, their CO2 emissions remain significantly higher, and despite efforts to recycle and reduce energy consumption, the recycling rate remains low.13 The production scale refers to the total annual production volume, while energy consumption is influenced by factors such as viscosity, dissolution, and drying, which impact polymer processing costs.159 In a cradle-to-gate analysis of plastics with an annual consumption exceeding 1 million metric tons, energy consumption was assessed.
| Type of food packaging material | Cost (USD per kg) | Annual energy consumption (PJ per year) | CO2 emission (kg kg−1) | Scale | Reference |
|---|---|---|---|---|---|
| PP | 0.32–1.12 | 560 | 0.63 | Industrial, kiloton | 136 and 137 |
| PS | 0.32–1.33 | 270 | 0.98 | Industrial, kiloton | 138 |
| PET | 0.13–1.17 | 340 | 5 | Industrial, kiloton | 139 and 140 |
| PVC | 0.27–0.91 | 340 | 2.82 | Industrial, kiloton | 141 and 142 |
| HDPE | 0.01–1.21 | 830 | 2.5 | Industrial, kiloton | 142 and 143 |
| LDPE | 0.21–0.74 | 3 | Industrial, kiloton | 142 and 144 | |
| PHBV-based | 2.05 | 10 | 2 | Industrial, kiloton | 145–147 |
| PLA-based | 12–15 | 587 | 1.8 | Industrial, kiloton | 148–150 |
| Cellulose-based | 1300 | 0.22 | 1.2–3.7 | Industrial, kiloton | 69 and 151–153 |
| Hemicellulose-based | — | — | — | Lab scale | |
| Lignin-based | — | — | — | Lab scale | |
| Starch-based | 4081 | 6.2 × 10−7 | 1.14 | Industrial, kiloton | 154 and 155 |
| Protein-based | — | — | — | Lab scale | |
| Pectin-based | — | — | — | Lab scale | |
| Alginate | 2358–5623 | — | 2.6 | Lab scale, kg | 156 |
| Chitosan | 20 | 0.03–0.16 | 0.7 | Lab scale, kg | 126, 153, 157 and 158 |
Although NPMs have achieved adequate reduced CO2 emissions compared with some SPMs, challenges persist in scaling up and cost reduction of the production process. NPM sources struggle to secure a stable resource chain, affecting material quality and limiting their application fields, particularly in terms of moldability and elasticity. Despite the success of PLA, its flexibility remains relatively low compared to plastics without additives.160 Therefore, scientists are focusing on other naturally occurring elastic materials, such as starch and chitosan, which are projected to reach a global production of 225.82 and 365 tonnes in the coming decade, respectively, with a compound annual growth rate (CAGR) of 7.10%, and 11.93% respectively.161–163 New generations of these materials have been developed, but their inherent fragility and instability have led to the introduction of composites such as cellulose, fibers, waxes, lignin and metal oxides.164–168 Among them, bacterial cellulose (BC) has gained popularity due to its non-wood source and rapid growth, which proves to be economically feasible, with a predicted CAGR of 12.6%.169,170
Currently, the production cost of NPMs is primarily limited by small-scale production and immature manufacturing technology. This results in NPMs lacking a clear cost advantage compared to traditional SPMs, which have undergone decades of development.
A similar situation exists regarding CO2 emissions. Consequently, at this stage, NPMs' primary advantage lies in energy consumption, particularly for bio-based NPMs like cellulose and starch, which benefit from established production methods. However, due to cost and production constraints, NPMs have yet to fully replace traditional SPMs.
To achieve significant reductions in production and treatment costs associated with SPMs, it is crucial to allocate more resources towards improving development procedures. Prioritizing the development of natural resources is essential to advance NPMs and realize cost-reduction goals. Furthermore, addressing the challenges related to transparency and water resistance in NPMs has the potential to attract further investment and drive progress in this field. The production and recycling of bio-based plastics pose significant challenges due to their complex production processes and limited recyclability.
3.2. Mechanical properties
The mechanical properties, specifically tensile strength and maximum elongation, are critical in determining the effectiveness of food packaging materials in separating and protecting food from the surrounding environment. Specifically, the tensile strength and elongation at break are vital parameters.171 A higher elongation results in a higher possibility of achieving close contact between the food and packaging, thereby enhancing protection and enabling denser packaging. Fig. 3 illustrates a comparison of the tensile strength and elongation at break for various FPMs, including materials with additives or modifications.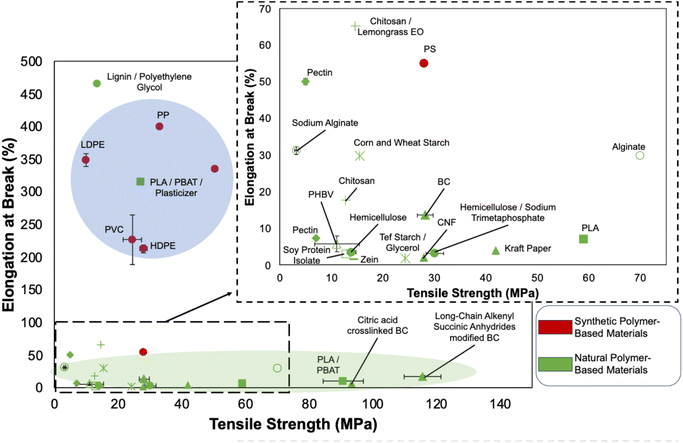 | ||
| Fig. 3 The comparison of tensile strength and elongation at break of different film-based materials.83,98,172–195 | ||
Observations reveal that typical SPMs are distributed in a region characterized by lower tensile strength and higher elongation at break (blue region). NPMs primarily occupy the low-elongation region, with some displaying higher tensile strength than typical SPMs (green region). This clear distinction highlights the distinct functional characteristics of these material types.
It is essential to note that, at the current stage, NPMs cannot fully replace SPMs. In contrast, historical applications of cellulose have primarily involved composites, relying on other elastic polymers to expand its utility.65 Government policies promoting sustainability have led to a review of paper-plastic composites and coatings like PE, PP, and PVC, which enhance water, oil, and grease resistance, and durability in paper packaging.
The development of BC presents a possible solution to break the limitation, as demonstrated by Jiang et al.'s study on BC films modified with long-chain alkenyl succinic anhydrides.83 This material exhibited impressive tensile strength (115.6 MPa), which is twice that of a PET film and nearly three times higher than that of a neat BC film. However, its stretchability falls short compared to conventional SPMs, limiting the applicability of BC in certain applications such as plastic wraps that demand high elasticity.
Apart from the ultra-performance in terms of tensile strength for modified BC, the lignin-poly polyethylene glycol polyurethane bio-plastic developed by Chen et al.98 has shown an extremely high elongation compared to other NPMs. Its elongation property closely resembles that of synthetic polymers. The biodegradable semicrystalline polyester, polyethylene glycol, acts as a soft segment, contributing to the material's high elongation performance. This method enhances the elongation of certain NPMs, although it may not be suitable for those that can form films independently.
It is noteworthy that the elongation at break of the PLA/PBAT/Plasticizer film (315.4%), developed by Slezak et al.179 exhibits a staggering 44 times higher value compared to that of a pure PLA film (7%). The addition of a plasticizer has significantly improved its mechanical properties, aligning them closely with those of typical SPMs and providing an exceptionally high elongation compared to other NPMs. This factor has contributed to making PLA a common material for food packaging in recent years. It is important to point out that this method improves the elongation performance of the material through the additive, which differs from lignin-poly polyethylene glycol polyurethane discussed above, where the material can form a film without an additive. Hence, the method of additive addition will be suitable for most film formations of different natural polymers. Apart from this, PHBV is found to be another promoting bioplastic other than PLA in recent years; however, it showed a weak tensile strength and elongation at break compared with other NPMs. This limited its application, and additives will be necessary in future development to enhance the tensile strength and elongation at break, similar to PLA.
This underscores the possibility that, through continuous development, the extensibility of natural source materials can be further enhanced, ultimately leading to the replacement of outdated SPMs. This aligns more closely with the characteristics and practical requirements of existing NPMs, marking a promising evolution in the field.
3.3. Oxygen and water vapor permeability
Food spoilage poses a significant threat to food safety, characterized by undesirable changes in food quality due to chemical processes. Chemical oxidation, arising from the interaction between food and oxygen, emerges as a primary pathway for food spoilage.196 Additionally, mold, yeast, and other harmful fungi thrive in oxygen-rich and high-humidity environments,197 underscoring the importance of isolating food from oxygen and water vapor in the atmosphere to preserve both food quality and safety.198,199 Fig. 4 illustrates some examples of biobased materials used as food packaging, designed to prevent food spoilage. | ||
| Fig. 4 (a) The use of food packaging materials to prevent quality changes in food, and the example of using (b) chitosan-based200 (Reprinted with permission from MDPI), (c) PLA201 (Reprinted with permission from Springer Nature), and (d) bacterial cellulose-based food packaging materials83 (Reprinted with permission from American Chemical Society). | ||
Table 2 presents the oxygen and water vapor permeability of various packaging materials, with lower permeability values indicating superior barrier properties. For NPMs, raw forms without modification are selected, while for SPMs, additives or cross-linkers are included.
| Materials | Oxygen permeability (mL per mm2 per day per Pa) | Condition | Water vapor permeability (g per mm2 per day per Pa) | Condition | Reference |
|---|---|---|---|---|---|
| a RH = relative humidity. | |||||
| PP/Bio-PP | 4.94 × 10−7 | 50% RH, 23 °C | 8.24 × 10−11 | 85% RH, 23 °C | 22 and 202 |
| PS/Bio-PS | 9.87 × 10−7 | 50% RH, 23 °C | 4.12 × 10−10 | 85% RH, 23 °C | 22 and 202 |
| PET/Bio-PET | 9.80 × 10−5 | 50% RH, 23 °C | 6.14 × 10−10 | 50% RH 23 °C | 22, 175 and 203 |
| PVC/Bio-PVC | 1.97 × 10−8 | 20 °C | 4.12 × 10−10 | 90% RH, 38 °C | 22 and 202 |
| HDPE/Bio-HDPE | 7.13 × 10−7 | 25 °C | 1.46 × 10−10 | 90% RH, 38 °C | 22 and 204 |
| LDPE/Bio-LDPE | 4.48 × 10−8 | 25 °C | 2.25 × 10−5 | 100% RH, 38 °C | 22 and 175 |
| PHBV-based | 7.88 × 10−3 | 0% RH, 23 °C | 9.78 × 10−4 | 100% RH, 37.8 °C | 205 |
| PLA-based | 8.64 × 10−9 | 0% RH, 23 °C | 1.56 × 10−6 | 50% RH, 25 °C | 206 and 207 |
| Cellulose-based | 1.12 × 10−9 | 50% RH, 25 °C | 1.72 × 10−3 | 50% RH, 23 °C | 208 and 209 |
| BC-based | 5.87 × 10−8 | 54% RH, 25 °C | 8.79 × 10−7 | — | 82, 83, 89, 210 and 211 |
| Paper | — | — | 6.96 × 10−9 | 38 °C | 212 |
| Hemicellulose-based | 3.72 × 10−9 | 50% RH, 23 °C | 2.46 × 10−5 | 100% RH, 20 °C | 191 |
| Lignin-based | 5.28 × 10−7 | — | 3.14 × 10−7 | — | 213 |
| Starch-based | 1 × 10−3 | 75% RH | 7.41 × 10−3 | 75% RH | 214 and 215 |
| Protein-based | 5.37 × 10−8 | — | 3.14 × 10−2 | 20% RH, 24 °C | 216 and 217 |
| Pectin-based | 3.39 × 10−7 | 53% RH, 25 °C | 1.33 × 10−6 | 25 °C | 181 and 218 |
| Alginate-based | 1.11 × 10−10 | 0% RH, 25 °C | 1.09 × 10−5 | — | 219 |
| Chitosan-based | 6.21 × 10−9 | 55% RH, 25 °C | 8.50 × 10−5 | 55% RH, 23 °C | 220 |
The permeability of materials to water vapor and oxygen is influenced by several factors, including their chemical composition, structure, crystallinity, and cross-linking level at the raw material level, as well as the content of additives and fillers during the manufacturing process. The literature suggests a direct correlation between crystallinity and permeability: materials with higher crystallinity demonstrate lower permeability.221,222 Conversely, materials with lower crystallinity feature greater internal spaces, which facilitate the passage of oxygen and moisture, leading to a high permeability.223
Apart from crystallinity, the hydroxyl group may form hydrogen bonding with the water molecules passing through the materials,224 the interaction provided the ability of water molecule catching and further, a very low water vapor passing rate for the materials. Thus, the permeability characteristics of different raw materials are complex and multifactorial.
In Table 2, we can observe that synthetic materials generally have relatively low permeability, especially in terms of water vapor. The performance of NPMs in terms of water vapor permeability is not as good as that of most synthetic materials, such as PP and HDPE. Crystallinity is not the only factor affecting water vapor permeability in these film materials; additives and fillers in synthetic materials also play an important role.
The hydrophobic nature and chemical structure of the polymer are found to be other key factors in determining the water vapor barrier properties,225 with most synthetic polymers not containing hydroxyl groups in their chemical structure. The more hydrophobic the polymer, the lower its water vapor permeability.226 Unlike “catching” by hydroxyl groups, the impermeability of these materials prevents water from passing inside the material, resulting in low water permeability. Therefore, apart from LDPE, which has a high water vapor permeability due to its low density, other synthetic polymers exhibit a lower water vapor permeability than NPM.
Apart from the synthetic materials, we can also observe that paper, lignin-based materials, and BC have outstanding performance in preventing water vapor penetration (6.96 × 10−9 g per mm2 per day per Pa, 3.14 × 10−7 g per mm2 per day per Pa, and 8.79 × 10−7 g per mm2 per day per Pa respectively). For Kraft paper and BC, the crystallinity was found to be 69.81% and 74%, respectively;227,228 the high crystallinity of cellulose creates a highly ordered, three-dimensional framework that traps water molecules. This limits the ability of water to penetrate the material. Besides this, the free hydroxyl group inside the film, provided water molecule capture ability, ultimately leading to a lower water permeability.229 Apart from this, the abundance of aromatic rings and various non-polar functional groups led to a high hydrophobicity of lignin, resulting in a high water vapor barrier ability of the materials.230 Therefore, the water vapor permeability of these materials can become lower than that of other NPMs.
Apart from the interaction between a hydroxyl group and water molecules, lignin-based materials have a different mechanism to lead to a low water vapor permeability. Lignin has a unique three-dimensional long chain network structure, consisting of aromatic rings, benzene groups, and ether bonds; this led to a highly branched and heterogeneous structure of the lignin-based material, which creates a complex network of molecules. This complexity limits the ability of water molecules to penetrate the material and disrupt the hydrogen bonding network. This decreases the interaction between lignin and water, resulting in hydrophobic properties and low water vapor permeability.231,232
Interestingly, we can observe very different situations in terms of oxygen permeability. NPMs such as PLA (8.64 × 10−9 mL per mm2 per day per Pa), alginate (1.11 × 10−10 mL per mm2 per day per Pa) and chitosan (6.21 × 10−9 mL per mm2 per day per Pa) show better oxygen barrier properties than traditional SPMs in the table. This is probably due to the molecular linear structure, which has no branches and allows each layer of molecules to be arranged neatly giving these materials a certain degree of hydrophilicity; the tighter crystal structure reduces the chance of oxygen passing through the film, so they can have a better performance for the oxygen barrier. Starch-based materials have the opportunity to contain a branched chain structure of amylose and amylopectin,233 which leads to large numbers of alternating layers of crystalline and amorphous areas in the hydrogen bonding network, forming holes that allow oxygen to pass through the film. A similar situation occurs due to its water permeability. Additionally, protein-based materials have relatively high oxygen and water vapor permeability due to their unique three-dimensional structure. The unique structure prevents the proteins from arranging themselves neatly, leading to the formation of voids during film generation. Generally, similar to starch-based materials, this may also weaken the interaction between the polymer backbone and increase permeability.234 Hence, oxygen and water vapor can pass easily though those voids, resulting in a high oxygen permeability and water vapor permeability.
Therefore, understanding a packaging material's permeability properties is crucial for designing effective food packaging solutions that preserve food quality and safety. While synthetic materials generally exhibit lower permeability, exceptions exist among natural materials, highlighting the importance of considering material-specific characteristics.
The properties of packaging materials play a significant role in determining their effectiveness in preserving food quality and safety. NPMs, such as lignin-based materials, Kraft paper, and chitosan, exhibit high water vapor barrier properties and potential low oxygen permeability. In contrast, SPMs, such as PP and HDPE, generally possess lower permeability to water vapor and oxygen. The hydrophobic nature of synthetic materials contributes to their low water vapor permeability, while additives and fillers can impact the material's overall performance. Material-specific characteristics are crucial for designing effective food packaging solutions, and further investigation can lead to innovative developments. Further investigation into these factors will enable researchers to contribute to the development of innovative packaging materials that tackle the challenges of food spoilage and improve food preservation techniques.
3.4. Waste treatment
The waste treatment of FPMs after use plays a crucial role in their life cycle. Currently, there are three major waste treatment methods: recycling, landfilling, and incineration, which accounted for treatment of 9%, 79%, and 12% of FPM waste dominated by plastics in 2015, respectively.9 It is worth noting that natural polymer-based materials exhibit distinct differences in comparison to synthetic polymers and polymers with natural building blocks. This led to the different results between SPMs and NPMs after they have undergone these treatments, specifically landfilling. This underscores the importance of considering material-specific characteristics when designing effective food packaging solutions.Mechanical recycling involves washing, shredding, melting, and remolding waste into a new product. However, only materials that exhibit thermoplastic-like properties, such as polyethylene (PE) and polyethylene terephthalate (PET), can undergo mechanical recycling.235
Natural polymers, like starch-based films, also demonstrate thermoplastic-like characteristics, making them suitable for mechanical recycling.236 For example, PLA can be mechanically recycled, but the process may lead to depolymerization, altering its mechanical properties.237,238
Chemical recycling offers an alternative method that converts waste into valuable products, often involving depolymerization. Pyrolysis, pyrolysis-reforming, and gasification are examples of chemical recycling methods that selectively produce gases, fuels, or waxes through catalysed thermo-processing. Chemolysis, also known as chemolytic depolymerization, is a specific method suitable for PET, where the waste yields monomers of the polymer, allowing them to be reused in polymerization.239,240
Both synthetic polymer and natural polymer-based FPMs can undergo recycling through these two methods. However, NPMs follow a distinct path. Xia's group reported a lignocellulosic bioplastic produced through the casting method, using deep eutectic solvent (DES) as a dispersing medium for cellulose–lignin-based materials.241 This bioplastic can be recycled and reformed using DES as the solvent without changing its chemical structure. On the other hand, Cibinel presented alginate composites using ethylenediaminetetraacetic acid as a solvent, demonstrating the ability to produce and recycle new composite materials.242
Ren et al. introduced a pectin-based film that follows a similar method of production and recycling.30 It is noteworthy that for NPMs, the use of solvents is a critical aspect of both casting and recycling, distinguishing them from synthetic polymers. However, it's essential to highlight that recycling NPMs requires careful selection methods. Each material must be matched with a suitable recycling method based on its manufacturing process.
This is in contrast to traditional synthetic polymers, which commonly use heat as a generic recycling method following the separation of different types of synthetic polymers. As a result, the recycling of NPMs presents increased complexity, leading to a low recycling rate in today's society.
Typically, ideal biodegradable materials require specific environmental conditions such as temperature, light, moisture, and oxygen along with the addition of enzymes or bacteria to enhance biodegradation rates. However, incorporating functionalized materials into FPMs to achieve desired properties such as water resistance, flexibility, and anti-barrier characteristics often hinders the degradation process.244,245 This can affect the formation of final fragments and raise concerns about the overall reliability of biodegradable FPMs.246,247 But generally bio-based modified FPMs have higher degradability than conventional plastics under natural terrestrial composting.248,249
Recent studies have shed light on the biodegradation rates of various natural polymer-based materials. Fig. 5a illustrates the time required for biodegradation and the resultant degradation products in different environments with various additives. The results show that, excluding SPMs, most other biobased materials tend to decompose into substances with a lower environmental impact within a year, provided that ideal and suitable conditions are met.
 | ||
| Fig. 5 (a) Biodegradation time of different FPMs,10,26,82,111,178,179,250–263 (b) degradation of bacterial cellulose264 (Reprinted with permission from MDPI), and (c) degradation of lignin-based films255 (Reprinted with permission from Elsevier). | ||
Additionally, Fig. 5b and c demonstrate the degradation of bacterial cellulose and lignin-based materials in soil, where biofragmentation can be observed. The materials break down to become smaller in size and undergo assimilation, making them unobservable in the soil. Chitosan-based, HPBV-based, and hemicellulose-based materials have been shown to be highly biodegradable, taking as little as 0.8–2 weeks to degrade in the soil.
These findings highlight the potential of natural polymer-based materials as alternatives to traditional synthetic plastics. Paper, a common food packaging material, has significant advantages in terms of biodegradability but is limited by its inherent characteristics. Despite these limitations, natural polymer-based materials demonstrate remarkable biodegradability and environmental benefits.
Table 3 provides details on the biodegradation conditions required for various FPMs, including environmental conditions and whether enzymatic additives can enhance the degradation rate. Upon examining the table, it becomes clear that PLA requires relatively stringent conditions to achieve complete biodegradation within six weeks. Specifically, a temperature of 58 °C and a relative humidity of 55% are necessary for swift degradation. However, temperatures between 20 °C and 25 °C fail to meet these environmental conditions, restricting the process to controlled factory or laboratory environments.
| Material | Condition | Additive to enhance the biodegradation rate | Reference |
|---|---|---|---|
| a RH = relative humidity. | |||
| PP/Bio-PP | Nonbiodegradable | Nonbiodegradable | 265 |
| PS/Bio-PS | Nonbiodegradable | Nonbiodegradable | 265 |
| PET/Bio-PET | Nonbiodegradable | Nonbiodegradable | 265 |
| PVC/Bio-PVC | Nonbiodegradable | Nonbiodegradable | 265 |
| HDPE/Bio-HDPE | Nonbiodegradable | Nonbiodegradable | 265 |
| LDPE/Bio-LDPE | Nonbiodegradable | Nonbiodegradable | 265 |
| PHBV-based | Soil, 25 °C, 80% moisture content | Esterases, lipases, cutinases, peroxidases, and laccases | 266 and 267 |
| PLA-based | Soil, 58 °C, and 55% RHa | Proteinase K | 247 and 268 |
| Cellulose-based | Soil, 25–45 °C, 12% moisture content | Cellulase | 258 |
| BC-based | Soil, 35.1 °C, 10–20% moisture content | Cellulase | 264 |
| Paper | Soil, 40 °C | Cellulase, mannanase, xylanase, and β-glucosidase enzyme | 269 |
| Hemicellulose-based | Soil | — | 26 |
| Lignin-based | Soil | White-rot fungi | 255 |
| Starch-based | Soil, 27 °C, 70% moisture content | Amylase | 270 and 271 |
| Protein-based | Aerobic soil with volatile, pH 6–6.5, 25 °C, and 40% RHa | Proteases | 272 and 273 |
| Pectin-based | Soil, 28 °C, 50% RHa/sea water, 25 °C | Polygalacturonase, pectinesterase, and pectin lyase | 111, 253 and 274 |
| Alginate-based | Soil, 25 °C, and 75% RH/beach sand, 25 °C, and 75% RHa | Alginate lyase | 178, 250 and 275 |
| Chitosan-based | Vineyard soil | Lysozyme | 254 |
In contrast, typical cellulose, BC, pectin, SPI, and alginate-based materials exhibit more favourable biodegradation conditions, at temperatures closer to room temperature (20–25 °C). It is important to point out that for some types of NPMs, the addition of enzymatic additives can enhance the degradation rate, providing a more outstanding biodegradability. Among these materials, cellulose and BC stand out due to their lower humidity requirements. When supplemented with enzymes, these materials can biodegrade under normal environmental conditions, highlighting their significant biodegradability.
This finding underscores the importance of considering environmental factors and additives when designing sustainable packaging solutions for the future. The challenging biodegradation conditions and rates for various FPMs emphasize the need for solutions that meet current environmental needs.
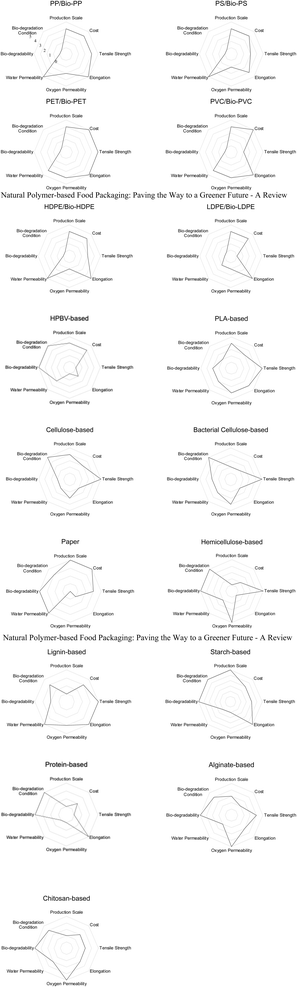
4. Discussion, challenges and future developments
Understanding the lifecycle of FPMs requires considering their production methods, usage characteristics, and post-use treatment strategies. Each material possesses unique features, which we have thoroughly evaluated and scored, resulting in the summarized findings presented in Fig. 6.The primary feature of NPMs is their biodegradability. When degraded, these products typically form monomer materials that can be utilized as nutrients for plant growth, fostering the production of new natural polymers within the plant. This allows them to quickly re-enter the lifecycle. For instance, starch-based materials can degrade into glucose and maltose within 5.4 weeks, serving as nutrients for soil bacteria and enhancing bacterial activity, thereby perpetuating a sustainable lifecycle for natural polymers.276,277 In contrast, synthetic polymers are difficult to biodegrade and often break down into microplastics due to UV radiation, water, and earthworms.
In contrast, synthetic polymers are difficult to biodegrade and often break down into microplastics due to UV radiation, heat, and water.278 These microplastics can have a detrimental impact on the surrounding environment, inducing functional changes in soil and affecting the growth of soil microorganisms and bacteria.
Despite the readily biodegradable nature of NPMs under composting conditions, factors such as form, size, and the type of additives used can influence the degree of biodegradability. This variability may lead to incomplete degradation.249,279 However, studies have shown that composites containing additives are gradually compostable in natural terrestrial environments, landfills, and water bodies. Notably, temperature plays a crucial role in this process, as optimal temperatures allow for complete cellulose degradation, enhancing the suitability of NPMs for various applications.249
Conversely, introducing additives has proven complex due to their overall impact on biodegradability. For example, studies have shown that antimicrobial thermoplastic starch composites exhibit high antimicrobial efficacy. However, there is limited evidence regarding their biodegradation impact.280–282 Furthermore, Carmen et al. highlighted that while some enzymatic activities may be disrupted by phenolic additives, the overall enzymatic biodegradation of these composites under laboratory conditions generally remains unaffected.283 Therefore, it is crucial to assess the biodegradation rates of polymers modified with biodegradable agents before making any claims about their environmental sustainability.
Furthermore, NPMs exhibit notable tensile strength, with nearly half exceeding that of conventional synthetic materials. While products like plastic wrap and food boxes have relatively modest strength requirements, synthetic polymers often struggle in this domain. However, achieving optimal elongation performance in NPMs remains a challenge that must be addressed before they can fully replace unsustainable SPMs as food packaging in the future.
NPMs also exhibit exceptional oxygen barrier characteristics, which are crucial for food preservation. Maintaining a balance between oxygen and water vapor barrier properties is essential when selecting materials to effectively replace SPMs.
Upon reviewing the material properties, we conclude that lignin-based materials possess mechanical attributes comparable to current SPMs, notably exhibiting the highest elongation among all NPMs discussed. However, their extended biodegradation duration remains a notable limitation, warranting further research focus in this area.
Conversely, BC presents another promising avenue. Cellulose-based materials can exhibit excellent tensile strength after modification and possess good oxygen and water vapor barrier properties. Their relatively short biodegradation time and environmentally friendly biodegrade conditions, with only the addition of enzymes needed, make them a potential candidate for future development.
HPBV, one of the NPMs gaining attention in recent years, exhibits significant biodegradability. However, its mechanical properties, water vapor barrier properties, and oxygen barrier properties lag behind those of existing SPMs and other NPMs. These limitations hinder its widespread practical application and development. Future research will likely focus on expanding the functional capabilities of HPBV to overcome these challenges and propel it into mainstream use.
While we have identified three potential alternatives to SPMs, scaling up material manufacturing presents a significant obstacle to their complete replacement. A primary hurdle in NPM production is establishing large-scale manufacturing processes. Currently, limited production capacity hinders this development.
The production capacity of green materials is significantly lower than that of conventional plastics. This limitation stems from factors such as the availability of raw materials, specialized equipment, and unique processing requirements.
Producing green materials often incurs higher costs due to limited economies of scale. For example, extracting chitosan from food waste can be both challenging and inefficient, requiring effective extraction methods.126 However, as the demand for sustainable alternatives grows, advancements in production techniques and the achievement of larger economies of scale are expected to reduce costs and facilitate wider adoption.
Similar to cellulose, the production of green materials typically involves a series of extraction processes, including pulping, dissolution, film formation, purification, drying, coating, and sheeting. This results in NPMs being weaker than SPMs in terms of production costs and production quantities. Furthermore, after pre-treatments, these materials are often limited to bottom-up film production methods, which can compromise their strength and barrier properties, such as in solution casting and solvent dissolution of starch, pectin, alginate, and cellulose.284–286 Additionally, modifications are required to improve processability but this may lead to the reduction of their biodegradability as observed in cellulose acetate at high degrees of modification,287 which changed the properties of biodegradability, making complete biodegradation and disposal challenging.
Despite the diverse chemical properties of NPMs, there is a strong demand for solutions that minimize plasticizer content and ensure compatibility with existing machinery. Improving transparency, controllable smoothness, thickness, color, and high-speed operation will make NPMs more competitive with conventional plastics. Adjusting the fiber size and molar mass of bio-based materials used in NPM production is necessary. These adjustments are closely related to pretreatment methods, morphology control, functionalization strategies, and the feasibility of pelletization for applications in the packaging field.288,289
In the future, producing multilayer structures may be a promising method for minimizing the need for extensive modification.290 This can be achieved by utilizing plant-based materials and tailoring structure–function relationships through pretreatment and fibrillation to achieve desired properties. Conversely, enhancing NPM processability with existing machinery and reducing toxicant usage are crucial parameters.291 Exploring the impact of surface energy on moulds and drying surfaces can help address issues such as film shrinkage, adhesion during the drying process, and challenges posed by solid content in sample preparation.292
In conclusion, while NPMs offer several advantages compared to SPMs, their production, usage characteristics, and end-of-life treatment require careful consideration. Addressing practical considerations like regulatory barriers, industry resistance, and consumer acceptance will necessitate collaboration among stakeholders.
5. Conclusion
The food packaging industry is currently dominated by SPMs. These materials are widely adopted due to their well-established manufacturing processes, high production volumes, and relatively low costs. However, the environmental impact of SPMs, including their significant contribution to greenhouse gas emissions and limited recycling rates, underscores the urgent need for more sustainable alternatives.In contrast, NPMs, such as starch-based and cellulose-based materials, offer promising properties with the potential to meet industry demands. NPMs, like BC films, exhibit promising biodegradability and tensile strength compared to SPMs. However, challenges remain in scaling up production, securing resources, and expanding application fields.
Recent advances have been made in thermoplastic starch, chitosan, and lignin-based composites; however, issues such as fragility and instability persist, necessitating further research and development. The mechanical properties, as well as oxygen and water vapor permeability, of materials play crucial roles in food packaging effectiveness. SPMs excel in these areas, but NPMs, such as BC and lignin-based materials, show potential for enhancing mechanical strength and barrier properties. Achieving a balance between strength, elasticity, and barrier properties remains a challenge.
SPMs and NPMs differ in their waste treatment methods, including recycling and biodegradation. SPMs can be recycled mechanically or chemically. NPMs, however, may require specific solvents, such as deep eutectic solvents (DESs), to dissolve them and be reformed. While the biodegradability of NPMs presents a key advantage, challenges remain in achieving rapid degradation under diverse conditions.
Several regulatory barriers and industry resistance must be addressed to enable the widespread adoption of NPMs. For example, infrastructure changes are needed to support recycling programs for NPMs, potentially requiring specialized equipment and facilities. Industry stakeholders must be willing to invest in developing new production lines and manufacturing processes that can efficiently scale up NPM production. It's crucial to note that most NPMs are still under investigation; further research on mass production, energy consumption, and cost reduction is necessary for their future promotion.
Consumer acceptance is crucial for the commercial success of NPMs. Studies indicate that consumers often favour traditional SPMs due to familiarity and perceived performance. To overcome this resistance, industry stakeholders should invest in education and awareness programs highlighting the benefits of NPMs, such as reduced waste and environmental sustainability.
The authors hope that this review will provide an overview of different FPMs by analysing various material performances and manufacturing conditions. This analysis aims to provide momentum and direction for future research on NPMs, ultimately enabling NPMs to replace traditional SPMs and contribute to a more sustainable and environmentally friendly future.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
We declare that we have no financial or personal relationship with other people or organizations that could inappropriately influence our work. Furthermore, we have no professional or personal interests of any nature in any product, service or company that could be perceived as influencing the position presented in, or the review of, the manuscript entitled "Natural Polymer-based Food Packaging: Paving the Way to a Greener Future - A Review".Acknowledgements
The financial support of the Research Matching Grant Scheme at CUHK (8601309) is acknowledged.References
- A. Agarwal, B. Shaida, M. Rastogi and N. B. Singh, Chem. Afr., 2023, 6, 117–144 CrossRef CAS.
- J. K. Boateng, E. J. K. Attiogbe, A. Stahl, W. Apoh, C. Boadi and W. O. Frimpong, Cogent Soc. Sci., 2022, 8, 2137962 Search PubMed.
- A. A. Abdillah and A. L. Charles, Int. J. Biol. Macromol., 2021, 191, 618–626 CrossRef CAS PubMed.
- S. Obebe and A. Adamu, Int. J. Appl. Sci. Eng. Technol., 2020, 4, 85–95 Search PubMed.
- N. M. A. Roland Weber, N. Aurisano, Z. Wang, M. Outters, K. De Miguel, M. Schlummer, M. Blepp, H. Wiesinger, H. Andrade, M. Scheringer and P. Fantke, Chemicals in Plastic – A Technical Report, 2023 Search PubMed.
- Y. Chen, A. K. Awasthi, F. Wei, Q. Tan and J. Li, Sci. Total Environ., 2021, 752, 141772 CrossRef CAS PubMed.
- D. Yao, H. Li, Y. Dai and C.-H. Wang, Chem. Eng. J., 2021, 408, 127268 CrossRef CAS.
- S. Dey, G. Veerendra, P. A. Babu, A. P. Manoj and K. Nagarjuna, Biomed. Mater. & Devices, 2024, 2, 70–112 Search PubMed.
- I. Wojnowska-Baryła, K. Bernat and M. Zaborowska, Int. J. Environ. Res. Public Health, 2022, 19, 13223 CrossRef PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustain. Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- D. Griffin-LaHue, S. Ghimire, Y. Yu, E. J. Scheenstra, C. A. Miles and M. Flury, Sci. Total Environ., 2022, 806, 150238 CrossRef CAS PubMed.
- K. V. S. Rajmohan, C. Ramya, M. Raja Viswanathan and S. Varjani, Curr. Opin. Environ. Sci. Health, 2019, 12, 72–84 CrossRef.
- R. Hossain, M. T. Islam, A. Ghose and V. Sahajwalla, J. Cleaner Prod., 2022, 368, 133127 CrossRef CAS.
- C. A. Velis and E. Cook, Environ. Sci. Technol., 2021, 55, 7186–7207 CrossRef CAS PubMed.
- M. Verma, S. Shakya, P. Kumar, J. Madhavi, J. Murugaiyan and M. Rao, J. Environ. Sci. Technol., 2021, 1–14 Search PubMed.
- M. Shen, B. Song, G. Zeng, Y. Zhang, W. Huang, X. Wen and W. Tang, Environ. Pollut., 2020, 263, 114469 CrossRef CAS PubMed.
- T. D. Moshood, G. Nawanir, F. Mahmud, F. Mohamad, M. H. Ahmad and A. Abdul Ghani, Sustainability, 2021, 13, 6170 CrossRef CAS.
- H. Y. Sintim, A. I. Bary, D. G. Hayes, L. C. Wadsworth, M. B. Anunciado, M. E. English, S. Bandopadhyay, S. M. Schaeffer, J. M. DeBruyn, C. A. Miles, J. P. Reganold and M. Flury, Sci. Total Environ., 2020, 727, 138668 CrossRef CAS PubMed.
- P. Rizzarelli, M. Rapisarda, L. Ascione, F. D. Innocenti and F. P. La Mantia, Polym. Degrad. Stab., 2021, 188, 109578 CrossRef CAS.
- N. Mahmoudi, G. F. Slater and A. L. Juhasz, Water, Air, Soil Pollut., 2013, 224, 1411 CrossRef.
- L. Ribba, M. Lopretti, G. M. de Oca-Vásquez, D. Batista, S. Goyanes and J. R. Vega-Baudrit, Environ. Res. Lett., 2022, 17, 033003 CrossRef CAS.
- L. Bastarrachea, S. Dhawan and S. S. Sablani, Food Eng. Rev., 2011, 3, 79–93 CrossRef.
- M. J. Costa, L. M. Pastrana, J. A. Teixeira, S. M. Sillankorva and M. A. Cerqueira, Innovative Food Sci. Emerging Technol., 2021, 69, 102646 CrossRef CAS.
- Z. Hasanoglu, N. Sivri, M. B. Alanalp and A. Durmus, Int. J. Biol. Macromol., 2024, 257, 128631 CrossRef CAS PubMed.
- C. Chen, S. Wen, Z. Wang, D. Zhang, J. Zhang, C. Yan and W. Cong, J. Polym. Res., 2022, 29, 36 CrossRef CAS.
- J. Li, W. Wang, H. Wu, F. Peng, H. Gao and Y. Guan, Int. J. Biol. Macromol., 2024, 254, 127795 CrossRef CAS PubMed.
- D. Tian, J. Hu, J. Bao, R. P. Chandra, J. N. Saddler and C. Lu, Biotechnol. Biofuels, 2017, 10, 1–11 CrossRef PubMed.
- P. S. Hornung, K. Masisi, L. N. Malunga, T. Beta and R. H. Ribani, Polym. Bull., 2018, 75, 4735–4752 CrossRef CAS.
- D. Nogueira and V. G. Martins, J. Polym. Environ., 2019, 27, 2027–2039 CrossRef CAS.
- W. Ren, T. Qiang and L. Chen, Food Hydrocolloids, 2022, 129, 107643 CrossRef CAS.
- R. Gheorghita, G. Gutt and S. Amariei, Coatings, 2020, 10, 166 CrossRef CAS.
- Z. Kalaycıoğlu, E. Torlak, G. Akın-Evingür, İ. Özen and F. B. Erim, Int. J. Biol. Macromol., 2017, 101, 882–888 CrossRef PubMed.
- Z. Liu, J. Chen, Y. Hua, L. Qian, J. Sun, H. Li, X. Gu and S. Zhang, Polym. Degrad. Stab., 2022, 206, 110195 CrossRef CAS.
- US Resin Production & Sales 2022 vs. 2021, Plastics industry producers, statistics group, ACC, 2023 Search PubMed.
- E. Aparicio, R. M. Rodríguez-Jasso, C. D. Pinales-Márquez, A. Loredo-Treviño, A. Robledo-Olivo, C. N. Aguilar, E. T. Kostas and H. A. Ruiz, Bioresour. Technol., 2021, 329, 124935 CrossRef CAS PubMed.
- T. K. Phung, T. L. M. Pham, K. B. Vu and G. Busca, J. Environ. Chem. Eng., 2021, 9, 105673 CrossRef CAS.
- J. R. Wünsch, Polystyrene: Synthesis, production and applications, 2000 Search PubMed.
- G. Koerner, Y. Hsuan and R. Koerner, Geosynthetics in Civil Engineering, 2007, pp. 36–65 Search PubMed.
- C. M. Mendieta, M. E. Vallejos, F. E. Felissia, G. Chinga-Carrasco and M. C. Area, J. Polym. Environ., 2020, 28, 1–16 CrossRef CAS.
- L. Qiao, X. Yan, H. Tan, S. Dong, G. Ju, H. Shen and Z. Ren, Polymers, 2022, 14, 2892 CrossRef CAS PubMed.
- S. B. Jaime, R. M. Alves and P. F. Bócoli, J. Drug Delivery Sci. Technol., 2022, 71, 103330 CAS.
- E. E. Kwon and J. Lee, J. Cleaner Prod., 2024, 143210 CrossRef CAS.
- P. T. Benavides, J. B. Dunn, J. Han, M. Biddy and J. Markham, ACS Sustain. Chem. Eng., 2018, 6, 9725–9733 CrossRef CAS.
- S. M. Khan, N. Gull, R. U. Khan and M. T. Z. Butt, Polyvinylchloride-based Blends: Preparation, Characterization and Applications, 2022, pp. 19–47 Search PubMed.
- G. Zichittella, A. Ceruti, G. Guillén-Gosálbez and J. Pérez-Ramírez, Chem, 2022, 8, 883–885 CAS.
- M. Shahsavar, N. Ghadami, M. Akrami, R. Aghlmand and M. Gheibi, Ann. Environ. Sci. Toxicol., 2021, 5, 099–0102 Search PubMed.
- S. Kobayashi and K. Müllen, Encyclopedia of Polymeric Nanomaterials, Springer Berlin Heidelberg Berlin Heidelberg, 2015 Search PubMed.
- O. H. Gunawardene, C. Gunathilake, S. M. Amaraweera, N. M. Fernando, D. B. Wanninayaka, A. Manamperi, A. K. Kulatunga, S. M. Rajapaksha, R. S. Dassanayake and C. A. Fernando, J. Compos. Sci., 2021, 5, 300 CrossRef CAS.
- N. A. Asri, N. A. A. Sezali, H. L. Ong, M. H. Mohd Pisal, Y. H. Lim and J. Fang, Macromol. Rapid Commun., 2024, 45, 2400475 CrossRef CAS PubMed.
- C. Bonnenfant, N. Gontard and C. Aouf, Polymer, 2023, 270, 125784 CrossRef CAS.
- M. I. Ibrahim, D. Alsafadi, K. A. Alamry and M. A. Hussein, J. Polym. Environ., 2021, 29, 1010–1030 CrossRef CAS.
- E. Rezvani Ghomi, F. Khosravi, A. Saedi Ardahaei, Y. Dai, R. E. Neisiany, F. Foroughi, M. Wu, O. Das and S. Ramakrishna, Polymers, 2021, 13, 1854 CrossRef PubMed.
- N.-A. A. B. Taib, M. R. Rahman, D. Huda, K. K. Kuok, S. Hamdan, M. K. B. Bakri, M. R. M. B. Julaihi and A. Khan, Polym. Bull., 2023, 80, 1179–1213 CrossRef CAS.
- T. A. Swetha, A. Bora, K. Mohanrasu, P. Balaji, R. Raja, K. Ponnuchamy, G. Muthusamy and A. Arun, Int. J. Biol. Macromol., 2023, 234, 123715 CrossRef CAS PubMed.
- Y. Liu, S. Ahmed, D. E. Sameen, Y. Wang, R. Lu, J. Dai, S. Li and W. Qin, Trends Food Sci. Technol., 2021, 112, 532–546 CrossRef CAS.
- A. Alemdar and M. Sain, Bioresour. Technol., 2008, 99, 1664–1671 CrossRef CAS PubMed.
- E. de Morais Teixeira, A. C. Corrêa, A. Manzoli, F. de Lima Leite, C. R. de Oliveira and L. H. C. Mattoso, Cellulose, 2010, 17, 595–606 CrossRef.
- S. LakshmiBalasubramaniam, M. Tajvidi and D. Skonberg, Food Chem., 2024, 458, 140220 CrossRef CAS PubMed.
- B. Li, C. Xu, L. Liu, J. Yu and Y. Fan, Green Chem., 2021, 23, 479–489 RSC.
- J. Zhang, Z. Guo, S. Chen, H. Dong, X. Zhang, Y. Qin, C. Yao and F. Xu, Cellulose, 2021, 28, 4371–4384 CrossRef CAS.
- R. Puscaselu, G. Gutt and S. Amariei, Molecules, 2019, 24, 3136 CrossRef CAS PubMed.
- T. Stanton, P. Kay, M. Johnson, F. K. S. Chan, R. L. Gomes, J. Hughes, W. Meredith, H. G. Orr, C. E. Snape, M. Taylor, J. Weeks, H. Wood and Y. Xu, WIREs Water, 2021, 8, e1490 CrossRef.
- B. Khan, M. Bilal Khan Niazi, G. Samin and Z. Jahan, J. Food Process Eng., 2017, 40, e12447 CrossRef.
- K. Radotić and M. Mićić, in Sample Preparation Techniques for Soil, Plant, and Animal Samples, ed. M. Micic, Springer New York, New York, NY, 2016, pp. 365–376, DOI:10.1007/978-1-4939-3185-9_26.
- Y. Teramoto, Molecules, 2015, 20, 5487–5527 CrossRef CAS PubMed.
- K. Copenhaver, K. Li, L. Wang, M. Lamm, X. Zhao, M. Korey, D. Neivandt, B. Dixon, S. Sultana and P. Kelly, Cellulose, 2022, 29, 4835–4876 CrossRef CAS.
- J. Pennells, I. D. Godwin, N. Amiralian and D. J. Martin, Cellulose, 2020, 27, 575–593 CrossRef CAS.
- S. Liu, Z. X. Low, Z. Xie and H. Wang, Adv. Mater. Technol., 2021, 6, 2001180 CrossRef CAS.
- M. Delgado Aguilar, I. González Tovar, J. A. Tarrés Farrés, M. Alcalà Vilavella, M. À. Pèlach Serra and P. Mutjé Pujol, BioResources, 2015, 10, 5345–5355 CAS.
- K. Li, C. M. Clarkson, L. Wang, Y. Liu, M. Lamm, Z. Pang, Y. Zhou, J. Qian, M. Tajvidi, D. J. Gardner, H. Tekinalp, L. Hu, T. Li, A. J. Ragauskas, J. P. Youngblood and S. Ozcan, ACS Nano, 2021, 15, 3646–3673 CrossRef CAS PubMed.
- Y. Chu, Y. Sun, W. Wu and H. Xiao, Carbohydr. Polym., 2020, 250, 116892 CrossRef CAS PubMed.
- M. S. Andrade, O. H. Ishikawa, R. S. Costa, M. V. Seixas, R. C. Rodrigues and E. A. Moura, Food Packag. Shelf Life, 2022, 31, 100807 CrossRef CAS.
- K. Bahsaine, B. El Allaoui, H. Benzeid, M. El Achaby, N. Zari, A. el Kacem Qaiss and R. Bouhfid, RSC Adv., 2023, 13, 33294–33304 RSC.
- C. Chen, W. Sun, L. Wang, M. Tajvidi, J. Wang and D. J. Gardner, ACS Sustain. Chem. Eng., 2022, 10, 9419–9430 CrossRef CAS.
- M. Raza and B. Abu-Jdayil, Cellulose, 2022, 29, 685–722 CrossRef CAS.
- T. Ghosh, S. Roy, A. Khan, K. Mondal, P. Ezati and J.-W. Rhim, Food Hydrocolloids, 2024, 110141 CrossRef CAS.
- M. Yusefi, M. L.-K. Soon, S.-Y. Teow, E. I. Monchouguy, B. N. H. M. Neerooa, Z. Izadiyan, H. Jahangirian, R. Rafiee-Moghaddam, T. J. Webster and K. Shameli, Int. J. Biol. Macromol., 2022, 199, 372–385 CrossRef CAS PubMed.
- M. Cheng, J. Hu, J. Xia, Q. Liu, T. Wei, Y. Ling, W. Li and B. Liu, Chem. Eng. J., 2022, 431, 133935 CrossRef CAS.
- S. Zhang, K. Sun, H. Liu, X. Chen, Y. Zheng, X. Shi, D. Zhang, L. Mi, C. Liu and C. Shen, Chem. Eng. J., 2020, 387, 124045 CrossRef.
- M. Pracella, C. Mura and G. Galli, ACS Appl. Nano Mater., 2021, 4, 260–270 CrossRef CAS.
- S. M. Choi, K. M. Rao, S. M. Zo, E. J. Shin and S. S. Han, Polymers, 2022, 14, 1080 CrossRef CAS PubMed.
- K. M. Cheung, Z. Jiang and T. Ngai, J. Sci. Food Agric., 2023, 103, 6625–6639 CrossRef CAS PubMed.
- Z. Jiang, Z. Shi, K. M. Cheung and T. Ngai, ACS Sustain. Chem. Eng., 2023, 11, 2486–2498 CrossRef CAS.
- X. Zhou, X. Liu, Q. Wang, G. Lin, H. Yang, D. Yu, S. W. Cui and W. Xia, Food Packag. Shelf Life, 2022, 33, 100866 CrossRef CAS.
- O. M. Atta, S. Manan, M. Ul-Islam, A. A. Q. Ahmed, M. W. Ullah and G. Yang, ES Food Agrofor., 2021, 6, 12–26 CAS.
- A. Salama, A. K. Saleh, I. Cruz-Maya and V. Guarino, J. Funct. Biomater., 2023, 14, 60 CrossRef CAS PubMed.
- R. R. Singhania, A. K. Patel, M.-L. Tsai, C.-W. Chen and C. Di Dong, Bioengineered, 2021, 12, 6793–6807 CrossRef CAS PubMed.
- K. M. Cheung, H. L. Chong, Z. Jiang and T. Ngai, Soft Matter, 2023, 19, 7696–7707 RSC.
- H. L. Chong, K. M. Cheung, Z. Jiang and T. Ngai, Adv. Energy Sustainability Res., 2023, 2300117 Search PubMed.
- S. A. Hussain, M. P. Yadav, B. K. Sharma, P. X. Qi and T. Z. Jin, Polymers, 2024, 16, 3171 CrossRef CAS PubMed.
- V. Puchart, K. Šuchová and P. Biely, Polysaccharide-Degrading Biocatalysts, 2023, pp. 139–176 Search PubMed.
- M. M. Abe, M. C. Branciforti and M. Brienzo, Recycling, 2021, 6, 22 CrossRef.
- P. Nechita, R. Mirela and F. Ciolacu, Sustainability, 2021, 13, 13504 CrossRef CAS.
- Y. Zhao, H. Sun, B. Yang and Y. Weng, Polymers, 2020, 12, 1775 CrossRef CAS PubMed.
- Y. Zhang and M. Naebe, ACS Sustain. Chem. Eng., 2021, 9, 1427–1442 CrossRef CAS.
- R.-C. Sun, ChemSusChem, 2020, 13, 4385–4393 CrossRef CAS PubMed.
- R. D. Patria, S. Rehman, C.-B. Yuen, D.-J. Lee, A. K. Vuppaladadiyam and S.-Y. Leu, Appl. Energy, 2024, 357, 122543 CrossRef CAS.
- X. Chen, Z. Li, L. Zhang, H. Wang, C. Qiu, X. Fan and S. Sun, Ind. Crops Prod., 2021, 164, 113396 CrossRef CAS.
- M. H. Lahtinen, E. Kynkäänniemi, C. Jian, A. Salonen, A. M. Pajari and K. S. Mikkonen, Mol. Nutr. Food Res., 2023, 67, 2300201 CrossRef CAS PubMed.
- Anushikha and K. K. Gaikwad, Biomass Convers. Biorefin., 2024, 14, 16755–16767 CrossRef CAS.
- A. Avella, M. Ruda, C. Gioia, V. Sessini, T. Roulin, C. Carrick, J. Verendel and G. L. Re, Chem. Eng. J., 2023, 463, 142245 CrossRef CAS.
- H.-M. Wang, T.-Q. Yuan, G.-Y. Song and R.-C. Sun, Green Chem., 2021, 23, 3790–3817 RSC.
- H. Makroo, S. Naqash, J. Saxena, S. Sharma, D. Majid and B. Dar, Appl. Food Res., 2021, 1, 100001 CrossRef CAS.
- D. Donmez, L. Pinho, B. Patel, P. Desam and O. H. Campanella, Curr. Opin. Food Sci., 2021, 39, 103–109 CrossRef CAS.
- Y. Zhang, C. Rempel and Q. Liu, Crit. Rev. Food Sci. Nutr., 2014, 54, 1353–1370 CrossRef CAS PubMed.
- H. Zaman and M. D. H. Beg, Prog. Appl. Sci. Tech., 2021, 11, 53–65 Search PubMed.
- C. Ge, B. Lansing and C. L. Lewis, Food Packag. Shelf Life, 2021, 27, 100610 CrossRef CAS.
- V. O. Bulatović, V. Mandić, D. Kučić Grgić and A. Ivančić, J. Polym. Environ., 2021, 29, 492–508 CrossRef.
- D. Pasarin, A.-I. Ghizdareanu, F. Teodorescu, C. Rovinaru and A. Banu, Fermentation, 2023, 9, 312 CrossRef CAS.
- C. Mellinas, M. Ramos, A. Jiménez and M. C. Garrigós, Materials, 2020, 13, 673 CrossRef CAS PubMed.
- T. Qiang, W. Ren and L. Chen, Food Hydrocolloids, 2024, 149, 109539 CrossRef CAS.
- T. I. A. Gouveia, K. Biernacki, M. C. R. Castro, M. P. Gonçalves and H. K. S. Souza, Food Hydrocolloids, 2019, 97, 105175 CrossRef CAS.
- V. G. L. Souza, I. P. Mello, O. Khalid, J. R. A. Pires, C. Rodrigues, M. M. Alves, C. Santos, A. L. Fernando and I. Coelhoso, Coatings, 2022, 12, 108 CrossRef CAS.
- C. M. P. Freitas, J. S. R. Coimbra, V. G. L. Souza and R. C. S. Sousa, Coatings, 2021, 11, 922 CrossRef CAS.
- S. Kaidi, F. Bentiss, C. Jama, K. Khaya, Z. Belattmania, A. Reani and B. Sabour, Colloids Interfaces, 2022, 6, 51 CrossRef CAS.
- S. H. Rashedy, M. S. Abd El Hafez, M. A. Dar, J. Cotas and L. Pereira, Appl. Sci., 2021, 11, 6290 CrossRef CAS.
- Y. Qin, G. Zhang and H. Chen, J. Nutr. Food Sci., 2020, 3, 100013 Search PubMed.
- H. Li, Z. Wang, F. Zhu and G. Li, Int. J. Biol. Macromol., 2024, 135441 CrossRef PubMed.
- R. Gheorghita Puscaselu, A. Lobiuc, M. Dimian and M. Covasa, Polymers, 2020, 12, 2417 CrossRef PubMed.
- M. Hadidi, S. Jafarzadeh, M. Forough, F. Garavand, S. Alizadeh, A. Salehabadi, A. M. Khaneghah and S. M. Jafari, Trends Food Sci. Technol., 2022, 120, 154–173 CrossRef CAS.
- M. Ahmad, N. P. Nirmal, M. Danish, J. Chuprom and S. Jafarzedeh, RSC Adv., 2016, 6, 82191–82204 RSC.
- R. Bhaskar, S. M. Zo, K. B. Narayanan, S. D. Purohit, M. K. Gupta and S. S. Han, Polym. Test., 2023, 124, 108097 CrossRef CAS.
- J. Glusac and A. Fishman, Trends Food Sci. Technol., 2021, 112, 507–517 CrossRef CAS.
- Z. Aytac, R. Huang, N. Vaze, T. Xu, B. D. Eitzer, W. Krol, L. A. MacQueen, H. Chang, D. W. Bousfield, M. B. Chan-Park, K. W. Ng, K. K. Parker, J. C. White and P. Demokritou, ACS Sustain. Chem. Eng., 2020, 8, 15354–15365 CrossRef CAS.
- C. Cui, L. Gao, L. Dai, N. Ji, Y. Qin, R. Shi, Y. Qiao, L. Xiong and Q. Sun, Food Eng. Rev., 2023, 15, 360–379 CrossRef CAS.
- S. G. Kou, L. M. Peters and M. R. Mucalo, Int. J. Biol. Macromol., 2021, 169, 85–94 CrossRef CAS PubMed.
- D. E. Azofeifa, H. J. Arguedas and W. E. Vargas, Opt. Mater., 2012, 35, 175–183 CrossRef CAS.
- O. J. Pemble, M. Bardosova, I. M. Povey and M. E. Pemble, Polymers, 2021, 13, 1566 CrossRef CAS PubMed.
- M. Barik, G. BhagyaRaj, K. K. Dash and R. Shams, J. Agric. Food Res., 2024, 101164 CAS.
- C. Thambiliyagodage, M. Jayanetti, A. Mendis, G. Ekanayake, H. Liyanaarachchi and S. Vigneswaran, Materials, 2023, 16, 2073 CrossRef CAS PubMed.
- N. Oladzadabbasabadi, A. M. Nafchi, F. Ariffin, M. J. O. Wijekoon, A. Al-Hassan, M. A. Dheyab and M. Ghasemlou, Carbohydr. Polym., 2022, 277, 118876 CrossRef CAS PubMed.
- K. Hiraga, I. Taniguchi, S. Yoshida, Y. Kimura and K. Oda, EMBO Rep., 2019, 20, e49365 CrossRef CAS PubMed.
- H. Raclavská, J. Růžičková, B. Švédová, M. Kucbel, M. Šafář, K. Raclavský and E. L. D. S. Abel, Biodegradable Waste Management in the Circular Economy: Challenges and Opportunities, 2022, pp. 69–153 Search PubMed.
- S. Shaikh, M. Yaqoob and P. Aggarwal, Curr. Res. Food Sci., 2021, 4, 503–520 CrossRef CAS PubMed.
- D. Knoblauch and L. Mederake, Curr. Opin. Toxicol., 2021, 28, 87–96 CrossRef CAS.
- H. A. Maddah, Am. J. Polym. Sci., 2016, 6, 1–11 CAS.
- C. Moretti, M. Junginger and L. Shen, Resour., Conserv. Recycl., 2020, 157, 104750 CrossRef.
- S. Feng, L. Yao, M. Feng, H. Cai, X. He, M. He, X. Bu, Y. Zhou and T. Zhang, J. Cleaner Prod., 2024, 434, 140361 CrossRef CAS.
- M. P. Hekkert, L. A. Joosten, E. Worrell and W. C. Turkenburg, Resour., Conserv. Recycl., 2000, 29, 33–64 CrossRef.
- M. Tamoor, N. A. Samak, M. Yang and J. Xing, Molecules, 2022, 27, 1599 CrossRef CAS PubMed.
- Y. Saeki and T. Emura, Prog. Polym. Sci., 2002, 27, 2055–2131 CrossRef CAS.
- J. An, F. Wu, D. Wang and J. You, Resour., Conserv. Recycl., 2022, 180, 106161 CrossRef CAS.
- S. Chiarakorn, C. K. Permpoonwiwat and P. Nanthachatchavankul, Econ. Publ. Pol. J., 2012, 3, 56–85 Search PubMed.
- C. Santagata, G. Iaquaniello, A. Salladini, E. Agostini, M. Capocelli and M. De Falco, J. Cleaner Prod., 2020, 253, 119837 CrossRef CAS.
- T. T. Nhu, L. Boone, V. Guillard, L. Chatellard, M. Reis, M. Matos and J. Dewulf, J. Environ. Manage., 2024, 356, 120522 CrossRef CAS PubMed.
- L. Atarés, A. Chiralt, C. González-Martínez and M. Vargas, Foods, 2024, 13, 950 CrossRef PubMed.
- C. Amabile, T. Abate, S. Chianese, R. Munoz and D. Musmarra, Chem. Eng. Trans., 2024, 110, 259–264 Search PubMed.
- C. Wellenreuther, A. Wolf and N. Zander, Clean Eng. Technol., 2022, 6, 100411 CrossRef.
- E. T. Vink, D. A. Glassner, J. J. Kolstad, R. J. Wooley and R. P. O'Connor, Ind. Biotechnol., 2007, 3, 58–81 CrossRef CAS.
- U. Suwanmanee, T. Leejarkpai, Y. Rudeekit and T. Mungcharoen, Agric. Nat. Resour., 2010, 44, 703–716 CAS.
- T. T. V. T. T., Scale-Up Nanoparticles in Modern Papermaking, https://cordis.europa.eu/project/id/228802, (accessed 21 Feb, 2024).
- D. Moon, T. Yagishita, T. Minowa and X.-Z. Sun, Trans. ASABE, 2013, 56, 1061–1067 Search PubMed.
- S. R. Djafari Petroudy, B. Chabot, E. Loranger, M. Naebe, J. Shojaeiarani, S. Gharehkhani, B. Ahvazi, J. Hu and S. Thomas, Energies, 2021, 14, 6792 CrossRef CAS.
- N. d. C. L. Beluci, J. d. Santos, F. A. de Carvalho and F. Yamashita, Carbohydr. Polym. Technol. Appl., 2023, 5, 100274 CAS.
- C. Schulze, M. Juraschek, C. Herrmann and S. Thiede, Proced. CIRP, 2017, 61, 600–605 CrossRef.
- T. Lopez-Arenas, M. González-Contreras, O. Anaya-Reza and M. Sales-Cruz, Comput. Chem. Eng., 2017, 107, 140–150 CrossRef CAS.
- L. A. Cira, S. Huerta, G. M. Hall and K. Shirai, Process Biochem., 2002, 37, 1359–1366 CrossRef CAS.
- A. Riofrio, T. Alcivar and H. Baykara, ACS Omega, 2021, 6, 23038–23051 CrossRef CAS PubMed.
- Z. O. G. Schyns and M. P. Shaver, Macromol. Rapid Commun., 2021, 42, 2000415 CrossRef CAS PubMed.
- T. T. Thiyagu, J. V. Sai-Prasanna-Kumar, P. Gurusamy, V. Sathiyamoorthy, T. Maridurai and V. R. Arun-Prakash, Biomass Convers. Biorefin., 2023, 13, 11841–11851 CrossRef CAS.
- D. Gómez-Ríos, R. Barrera-Zapata and R. Ríos-Estepa, Food Bioprod. Process., 2017, 103, 49–57 CrossRef.
- F. A. Nasirov, V. M. Abbasov, E. M. Suleymanova, A. M. Aslanbeyli and S. Z. Ahmedova, Processes Petrochem. Oil Refin., 2023, 24(4), 778–824 CAS.
- A. Surendren, A. K. Mohanty, Q. Liu and M. Misra, Green Chem., 2022, 8606–8636 RSC.
- A. d. S. de Freitas, J. S. Rodrigues, C. C. Maciel, A. A. Pires, A. P. Lemes, M. Ferreira and V. R. Botaro, Int. J. Biol. Macromol., 2021, 184, 863–873 CrossRef PubMed.
- T. G. Ambaye, M. Vaccari, S. Prasad, E. D. van Hullebusch and S. Rtimi, J. Environ. Manage., 2022, 301, 113850 CrossRef CAS PubMed.
- C. Xue and L. D. Wilson, J. Compos. Sci., 2021, 5, 160 CrossRef CAS.
- Z. Diyana, R. Jumaidin, M. Z. Selamat, I. Ghazali, N. Julmohammad, N. Huda and R. Ilyas, Polymers, 2021, 13, 1396 CrossRef CAS PubMed.
- R. Jiang, H.-Y. Zhu, X. Zang, Y.-Q. Fu, S.-T. Jiang, J.-B. Li and Q. Wang, Int. J. Biol. Macromol., 2023, 127887 Search PubMed.
- B. Behera, D. Laavanya and P. Balasubramanian, Bioresour. Technol., 2022, 346, 126659 CrossRef CAS PubMed.
- V. Potočnik, S. Gorgieva and J. Trček, Polymers, 2023, 15, 3466 CrossRef PubMed.
- Y. A. Shah, S. Bhatia, A. Al-Harrasi, M. Afzaal, F. Saeed, M. K. Anwer, M. R. Khan, M. Jawad, N. Akram and Z. Faisal, Polymers, 2023, 15, 1724 CrossRef CAS PubMed.
- K. F. Hasan, P. G. Horváth, G. Markó and T. Alpár, Green Mater., 2021, 10, 1–10 Search PubMed.
- P. Hongdilokkul, K. Keeratipinit, S. Chawthai, B. Hararak, M. Seadan and S. Suttiruengwong, IOP Conf. Ser.: Mater. Sci. Eng., 2015, 87, 012112 Search PubMed.
- S. Mooninta, S. Poompradub and P. Prasassarakich, J. Polym. Environ., 2020, 28, 3116–3128 CrossRef CAS.
- A. Sangroniz, J.-B. Zhu, X. Tang, A. Etxeberria, E. Y.-X. Chen and H. Sardon, Nat. Commun., 2019, 10, 3559 CrossRef PubMed.
- N. Rahman, N. C. Dafader and P. Banu, J. Polym. Sci. Technol., 2017, 2, 20–35 Search PubMed.
- J.-W. Rhim, Food Sci. Biotechnol., 2010, 19, 243–247 CrossRef CAS.
- L. G. Santos, G. F. Alves-Silva and V. G. Martins, Int. J. Biol. Macromol., 2022, 220, 866–877 CrossRef CAS PubMed.
- R. Slezak, L. Krzystek, M. Puchalski, I. Krucińska and A. Sitarski, Sci. Total Environ., 2023, 866, 161401 CrossRef CAS PubMed.
- V. Morillon, F. Debeaufort, G. Blond, M. Capelle and A. Voilley, Crit. Rev. Food Sci. Nutr., 2002, 42, 67–89 CrossRef CAS PubMed.
- T. M. P. Ngo, T. H. Nguyen, T. M. Q. Dang, T. X. Tran and P. Rachtanapun, Int. J. Mol. Sci., 2020, 21, 2224 CrossRef CAS PubMed.
- E. Shlush and M. Davidovich-Pinhas, Trends Food Sci. Technol., 2022, 125, 66–80 CrossRef CAS.
- K. D. Tafa, N. Satheesh and W. Abera, Heliyon, 2023, 9, e13160 CrossRef CAS PubMed.
- F. Han Lyn and Z. Nur Hanani, J. Packag. Technol. Res., 2020, 4, 33–44 CrossRef.
- S. Gupta, M. Dixit, K. Sharma and N. Saxena, Surf. Coat. Technol., 2009, 204, 661–666 CrossRef CAS.
- A. Shebani, A. Klash, R. Elhabishi, S. Abdsalam, H. Elbreki and W. Elhrari, Res. Dev. Mater. Sci., 2018, 7, 791–797 Search PubMed.
- Q. Yu, L. Yang, S. Wang, L. Zhang and D. Sun, Cellulose, 2023, 30, 10273–10284 CrossRef CAS.
- Z. Jiang, K. M. Cheung and T. Ngai, RSC Sustainability, 2024, 2, 139–152 RSC.
- S. S. Patil and H. M. Jena, Polym. Test., 2021, 101, 107271 CrossRef CAS.
- S.-Y. Liao, X.-Y. Wang, X.-M. Li, Y.-J. Wan, T. Zhao, Y.-G. Hu, P.-L. Zhu, R. Sun and C.-P. Wong, Chem. Eng. J., 2021, 422, 129962 CrossRef CAS.
- Y. Zhao, H. Sun, B. Yang, B. Fan, H. Zhang and Y. Weng, Polymers, 2021, 13, 927 CrossRef CAS PubMed.
- P. Rachtanapun, W. Klunklin, P. Jantrawut, K. Jantanasakulwong, Y. Phimolsiripol, P. Seesuriyachan, N. Leksawasdi, T. Chaiyaso, W. Ruksiriwanich and S. Phongthai, Polymers, 2021, 13, 963 CrossRef CAS PubMed.
- J. R. Nastasi, M. A. Fitzgerald and V. Kontogiorgos, Int. J. Biol. Macromol., 2023, 253, 127536 CrossRef CAS PubMed.
- Y. Wei, Z. e. Huang, Z. Yu, C. Han and C. Yang, Materials, 2021, 14, 5436 CrossRef CAS PubMed.
- T. Erceg, S. Rackov, P. Terek and B. Pilić, Polymers, 2023, 15, 4694 CrossRef CAS PubMed.
- R. V. Saini, P. Vaid, N. K. Saini, S. S. Siwal, V. K. Gupta, V. K. Thakur and A. K. Saini, J. Funct. Biomater., 2021, 12, 67 CrossRef CAS PubMed.
- Y. Han, X. Huang, Y. Wang, J. Du, K. Ma, Y. Chen, N. Li, Z. Zhang and J. Pan, Front. Microbiol., 2021, 11, 609475 CrossRef PubMed.
- M. Zabihzadeh Khajavi, A. Ebrahimi, M. Yousefi, S. Ahmadi, M. Farhoodi, A. Mirza Alizadeh and M. Taslikh, Food Eng. Rev., 2020, 12, 346–363 CrossRef CAS.
- J. Long, W. Zhang, M. Zhao and C.-Q. Ruan, Carbohydr. Polym., 2023, 121267 CrossRef CAS PubMed.
- Z. Zhao, Y. Li and Z. Du, Sustainability, 2022, 14, 16579 CrossRef CAS.
- A. G. de Souza, R. F. d. S. Barbosa, Y. M. Quispe and D. d. S. Rosa, J. Polym. Environ., 2022, 30, 3307–3315 CrossRef CAS.
- J. Lange and Y. Wyser, Packag. Technol. Sci., 2003, 16, 149–158 CrossRef CAS.
- A. Apicella, P. Scarfato, L. Di Maio and L. Incarnato, Front. Mater., 2019, 6, 243 CrossRef.
- P. E. Keller and R. T. Kouzes, Water Vapor Permeation in Plastics, Pacific Northwest National Lab (PNNL), Richland, WA (United States), 2017 Search PubMed.
- F. Wu, D. Feng, Y.-h. Xie, D. Xie and Y. Mei, Ind. Eng. Chem. Res., 2022, 61, 5464–5474 CrossRef CAS.
- M. Guivier, G. Almeida, S. Domenek and C. Chevigny, Carbohydr. Polym., 2023, 312, 120761 CrossRef CAS PubMed.
- J.-W. Rhim, S.-I. Hong and C.-S. Ha, LWT-Food Sci. Technol., 2009, 42, 612–617 CrossRef CAS.
- I. Hasan, J. Wang and M. Tajvidi, Cellulose, 2021, 28, 11345–11366 CrossRef CAS.
- S. Saedi, M. Shokri, J. T. Kim and G. H. Shin, J. Food Eng., 2021, 307, 110665 CrossRef CAS.
- C. Campano, V. Rivero-Buceta, M. J. Fabra and M. A. Prieto, Int. J. Biol. Macromol., 2022, 223, 1495–1505 CrossRef CAS PubMed.
- P. Cazón, G. Velázquez and M. Vázquez, Food Packag. Shelf Life, 2020, 25, 100526 CrossRef.
- F. Larotonda, K. N. Matsui, P. J. d. A. Sobral and J. Laurindo, J. Food Eng., 2005, 71, 394–402 CrossRef.
- Y. Xue, Q. Xu, G. Jiang and D. Teng, Arab. J. Chem., 2024, 17, 105471 CrossRef CAS.
- M. Dong, G. Mastroianni, E. Bilotti, H. Zhang and D. G. Papageorgiou, ACS Sustain. Chem. Eng., 2024, 12, 11056–11066 CrossRef CAS.
- T. Li, R. Li, H. Luo, L. Peng, J. Wang, S. Li, M. Zhou, X. Yuan, Z. Zhang and H. Wu, Food Hydrocolloids, 2024, 150, 109632 CrossRef CAS.
- B. Gökkaya Erdem, S. Dıblan and S. Kaya, Food Bioprocess Technol., 2021, 14, 2161–2179 CrossRef.
- R. Tao, J. Sedman and A. Ismail, Food Hydrocolloids, 2022, 122, 107091 CrossRef CAS.
- L. Rivera-Hernández, N. Chavarría-Hernández, M. d. R. López Cuellar, V. M. Martínez-Juárez and A.-I. Rodríguez-Hernández, J. Food Sci. Technol., 2021, 58, 2973–2981 CrossRef PubMed.
- A. Hadi, A. Nawab, F. Alam and S. Naqvi, Sustainable Food Technol., 2023, 1, 863–873 RSC.
- G. Singh, S. Singh, B. Kumar and K. K. Gaikwad, J. Food Meas. Char., 2021, 15, 585–593 CrossRef.
- H. Nguyen, M.-Y. Hsiao, K. Nagai and H. Lin, Polymer, 2020, 205, 122790 CrossRef CAS.
- Y. Su, H. Lv, W. Zhou and C. Zhang, World Elec. Veh. J., 2021, 12, 130 CrossRef.
- X. Cheng, S. Shen, G. Wei, C. Wang, L. Luo and J. Zhang, Adv. Mater. Technol., 2022, 7, 2200228 CrossRef CAS.
- J. Zhou, S. Lin, H. Zeng, J. Liu, B. Li, Y. Xu, X. Zhao and G. Chen, Mater. Horiz., 2020, 7, 2936–2943 RSC.
- E. Pasquier, B. D. Mattos, H. Koivula, A. Khakalo, M. N. Belgacem, O. J. Rojas and J. Bras, ACS Appl. Mater. Interfaces, 2022, 14, 30236–30245 CrossRef CAS PubMed.
- Y. Chang and F. Liu, Materials, 2023, 16, 5339 CrossRef CAS PubMed.
- R. Setnescu, L. Badicu, L. Dumitran, P. Notingher and T. Setnescu, Cellulose, 2014, 21, 823–833 CrossRef CAS.
- D. Atila, A. Karataş, D. Keskin and A. Tezcaner, Int. J. Biol. Macromol., 2022, 218, 760–774 CrossRef CAS PubMed.
- S. V. Kononova, G. K. Lebedeva, G. N. Gubanova, E. V. Kruchinina, E. N. Vlasova, N. V. Afanas’ eva, E. N. Popova, A. Y. Volkov, E. N. Bykova and N. V. Zakharova, Membranes, 2023, 13, 716 CrossRef CAS PubMed.
- X. Liu, H. J. Zhang, S. Xi, Y. Zhang, P. Rao, X. You and S. Qu, Adv. Funct. Mater., 2025, 35, 2413464 CrossRef CAS.
- N. Alwadani, N. Ghavidel and P. Fatehi, Colloids Surf., A, 2021, 609, 125656 CrossRef CAS.
- X. Liu, Q. Liu, S. Wang, Z. Liu, G. Yang, H. Wang, W. Xiong, P. Li, F. Xu and Y. Xi, Int. J. Biol. Macromol., 2022, 215, 132–140 CrossRef CAS PubMed.
- A. Apriyanto, J. Compart and J. Fettke, Plant Sci., 2022, 318, 111223 CrossRef CAS PubMed.
- M. Yu, N. Ji, Y. Wang, L. Dai, L. Xiong and Q. Sun, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1075–1100 CrossRef CAS PubMed.
- A. J. Zervoudakis, C. S. Sample, X. Peng, D. Lake, M. A. Hillmyer and C. J. Ellison, ACS Macro Lett., 2022, 11, 1396–1402 CrossRef CAS PubMed.
- D. Rahardiyan, E. M. Moko, T. J. Shun and L. C. Keong, Enzyme Microb. Technol., 2023, 110260 CrossRef CAS PubMed.
- F. Beltrán, M. De La Orden and J. Martínez Urreaga, J. Polym. Environ., 2018, 26, 4046–4055 CrossRef.
- M. F. Cosate de Andrade, P. M. Souza, O. Cavalett and A. R. Morales, J. Polym. Environ., 2016, 24, 372–384 CrossRef CAS.
- Y.-J. Luo, J.-Y. Sun and Z. Li, Green Chem. Eng., 2023, 5, 257–265 CrossRef.
- E. Barnard, J. J. R. Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- Q. Xia, C. Chen, Y. Yao, J. Li, S. He, Y. Zhou, T. Li, X. Pan, Y. Yao and L. Hu, Nat Sustainability, 2021, 4, 627–635 CrossRef.
- M. Cibinel, G. Pugliese, D. Porrelli, L. Marsich and V. Lughi, Carbohydr. Polym., 2021, 251, 116995 CrossRef CAS PubMed.
- N. G. El Menofy and A. M. Khattab, in Handbook of Biodegradable Materials, Springer, 2023, pp. 571–600 Search PubMed.
- R. R. Pagar, S. R. Musale, G. Pawar, D. Kulkarni and P. S. Giram, ACS Biomater. Sci. Eng., 2022, 8, 2161–2195 CrossRef CAS PubMed.
- S. Kalidhasan, M.-S. Kang, J. Choi and H.-Y. Lee, J. Cleaner Prod., 2024, 469, 143150 CrossRef CAS.
- S. Cesur, J. Polym. Environ., 2018, 26, 1425–1444 CrossRef CAS.
- A. Bher, Y. Cho and R. Auras, Macromol. Rapid Commun., 2023, 44, 2200769 CrossRef CAS PubMed.
- P. Gururani, P. Bhatnagar, P. Dogra, H. C. Joshi, P. Chauhan, M. S. Vlaskin, N. C. Joshi, A. Kurbatova, A. Irina and V. Kumar, Curr. Res. Green Sustainable Chem., 2023, 7, 100384 CrossRef CAS.
- A. Beltrán-Sanahuja, A. Benito-Kaesbach, N. Sánchez-García and C. Sanz-Lázaro, Sci. Total Environ., 2021, 787, 147678 CrossRef.
- Q. Li, L. Zheng, Z. Guo, T. Tang and B. Zhu, Crit. Rev. Biotechnol., 2021, 41, 953–968 CrossRef CAS PubMed.
- X. Gao, C. Fu, M. Li, X. Qi and X. Jia, Int. J. Environ. Res. Public Health, 2022, 19, 8631 CrossRef CAS PubMed.
- J. P. Maran, V. Sivakumar, K. Thirugnanasambandham and R. Sridhar, Carbohydr. Polym., 2014, 101, 20–28 CrossRef CAS PubMed.
- D. G. Pereira, J. M. Vieira, A. A. Vicente and R. M. Cruz, Polymers, 2021, 13, 2632 CrossRef CAS PubMed.
- A. Oberlintner, M. Bajić, G. Kalčíková, B. Likozar and U. Novak, Environ. Technol. Innovation, 2021, 21, 101318 CrossRef CAS.
- Y. Zhang, J. Wang, X. Fang, J. Liao, X. Zhou, S. Zhou, F. Bai and S. Peng, Polymer, 2019, 178, 121572 CrossRef CAS.
- G. Liu, K. Shi and H. Sun, Polymers, 2023, 15, 979 CrossRef CAS PubMed.
- J. Puls, S. A. Wilson and D. Hölter, J. Polym. Environ., 2011, 19, 152–165 CrossRef CAS.
- S. S. Rumi, S. Liyanage and N. Abidi, Sci. Rep., 2024, 14, 6921 CrossRef CAS PubMed.
- K. Chaisuwan, D. Anurakumphan, S. Hemmanee, J. Ruamcharoen and M. Leelakriangsak, J. Sustainability Sci. Manage., 2023, 18, 110–124 CrossRef CAS.
- Y. Sun, W. Yang, H. Shi, S. K. Tanveer and J. Hai, Front. Bioeng. Biotechnol., 2022, 10, 1006388 CrossRef PubMed.
- D. S. Kocabaş, M. E. Akçelik, E. Bahçegül and H. N. Özbek, Ind. Crops Prod., 2021, 171, 113847 CrossRef.
- J. Kim, N. S. Gupta, L. B. Bezek, J. Linn, K. K. Bejagam, S. Banerjee, J. H. Dumont, S. Y. Nam, H. W. Kang and C. H. Park, Int. J. Mol. Sci., 2023, 24, 7638 CrossRef CAS PubMed.
- M. K. Reay, M. Graf, L. M. Greenfield, R. Bargiela, C. Onyije, C. E. Lloyd, I. D. Bull, R. P. Evershed, P. N. Golyshin and D. R. Chadwick, Environ. Sci.: Adv., 2025, 4, 133–146 CAS.
- K. Potivara and M. Phisalaphong, Materials, 2019, 12, 2323 CrossRef CAS PubMed.
- M. H. Rahman and P. R. Bhoi, J. Cleaner Prod., 2021, 294, 126218 CrossRef CAS.
- M. van der Zee, M. Zijlstra, L. J. Kuijpers, M. Hilhorst, K. Molenveld and W. Post, Polym. Test., 2024, 140, 108601 CrossRef CAS.
- N. S. Payanthoth, N. N. N. Mut, P. Samanta, G. Li and J. Jung, Appl. Biol. Chem., 2024, 67, 110 CrossRef CAS.
- P. C. Mayekar and R. Auras, Macromol. Rapid Commun., 2024, 45, 2300641 CrossRef CAS PubMed.
- A. Korpela, A. Tanaka and J. Asikainen, BioResources, 2023, 18, 6336–6347 CAS.
- E. Malekzadeh, A. Tatari and M. D. Firouzabadi, Carbohydr. Polym., 2023, 309, 120699 CrossRef CAS PubMed.
- J. Yang, S. Xu, X. Ran, Y. C. Ching, X. Sui and Y. Wei, Polym. Adv. Technol., 2024, 35, e6325 CrossRef CAS.
- J.-F. Su, X.-Y. Yuan, Z. Huang and W.-L. Xia, Polym. Degrad. Stab., 2010, 95, 1226–1237 CrossRef CAS.
- N. Raj, S. Selvakumar, B. Soundara and P. Kulanthaivel, Environ. Sci. Pollut. Res., 2023, 30, 118117–118132 CrossRef CAS PubMed.
- H. Rehman, A. H. Baloch and M. A. Nawaz, Trends Pept. Protein Sci., 2021, 6, 1–16 Search PubMed.
- B. Zhu and H. Yin, Bioengineered, 2015, 6, 125–131 CrossRef CAS PubMed.
- W. Zhou, X. Qin, D. Lyu and S. Qin, Hortic. Plant J., 2021, 7, 307–317 CrossRef CAS.
- R. Hayat, S. Ali, U. Amara, R. Khalid and I. Ahmed, Ann. Microbiol., 2010, 60, 579–598 CrossRef.
- K. Zhang, A. H. Hamidian, A. Tubić, Y. Zhang, J. K. Fang, C. Wu and P. K. Lam, Environ. Pollut., 2021, 274, 116554 CrossRef CAS PubMed.
- G. Singh, C. Chandoha-Lee, W. Zhang, S. Renneckar, P. J. Vikesland and A. Pruden, Water Res., 2016, 104, 137–146 CrossRef CAS PubMed.
- H. Wang, D. Wei, A. Zheng and H. Xiao, Polym. Degrad. Stab., 2015, 116, 14–22 CrossRef CAS.
- Y. Tian, Q. Lei, F. Yang, J. Xie and C. Chen, Int. J. Biol. Macromol., 2024, 263, 130048 CrossRef CAS PubMed.
- S. Ganjizadeh Zavareh, M. Javanmard Dakheli and B. Tajeddin, Food Sci. Nutr., 2021, 9, 6762–6775 CrossRef CAS PubMed.
- S. Carmen, J. Hazard. Mater., 2021, 402, 123534 CrossRef CAS PubMed.
- E. Pasquier, PhD Thesis, Université Grenoble Alpes, 2022.
- J. Wang, D. J. Gardner, N. M. Stark, D. W. Bousfield, M. Tajvidi and Z. Cai, ACS Sustain. Chem. Eng., 2018, 6, 49–70 CrossRef CAS.
- K. E. Helanto, L. Matikainen, R. Talja and O. J. Rojas, BioResources, 2019, 14, 4902–4951 Search PubMed.
- N. Yadav and M. Hakkarainen, Chemosphere, 2021, 265, 128731 CrossRef CAS PubMed.
- A. Balea, E. Fuente, Q. Tarrés, M. À. Pèlach, P. Mutjé, M. Delgado-Aguilar, A. Blanco and C. Negro, Cellulose, 2021, 28, 9187–9206 CrossRef CAS.
- T. Min, L. Zhou, X. Sun, H. Du, Z. Zhu and Y. Wen, Food Chem., 2022, 391, 133239 CrossRef CAS PubMed.
- A. Alias, M. K. Wan and N. Sarbon, Food Control, 2022, 136, 108875 CrossRef CAS.
- M. Alamri, A. A. Qasem, A. A. Mohamed, S. Hussain, M. A. Ibraheem, G. Shamlan, H. A. Alqah and A. S. Qasha, Saudi J. Biol. Sci., 2021, 28, 4490–4499 CrossRef CAS PubMed.
- T. Mäkelä, M. Kainlauri, P. Willberg-Keyriläinen, T. Tammelin and U. Forsström, Microelectron. Eng., 2016, 163, 1–6 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |