Open Access Article
Open Access ArticleFabrication of microcapsules encapsulating L. rhamnosus GG with Eudragit® L100–trehalose and polysaccharides: a study on physicochemical properties and cell adhesion
Yuyan Xuab,
Shuangying Zhu b,
Xinyi Sunb,
Kai Shanb,
Chong Zhangb,
Hongmei Xiaoab,
Xia Fanb and
Chuang Zhang*ab
b,
Xinyi Sunb,
Kai Shanb,
Chong Zhangb,
Hongmei Xiaoab,
Xia Fanb and
Chuang Zhang*ab
aSanya Research Institute, Nanjing Agricultural University, Sanya, Hainan 572025, China. E-mail: zhangchuang@njau.edu.cn
bCollege of Food Science and Technology, Nanjing Agricultural University, Nanjing, Jiangsu 210095, China
First published on 20th May 2025
Abstract
Our previous study revealed the relationship between the droplet-to-particle transition process and the functionality of Lacticaseibacillus rhamnosus GG (LGG) particles encapsulated with Eudragit® L100 (L100)–trehalose (Tre). The main focus was on exploring the effects of convective drying conditions on the targeted delivery of viable bacteria to the intestine, by using a single droplet drying technique to mimic realistic spray drying conditions. In the current study, spray-dried L100–Tre–LGG microcapsules combined with polysaccharides (maltodextrin, inulin, and soluble soy polysaccharides) were fabricated, to investigate the physicochemical properties of powders and the adhesion ability of spray-dried LGG cells. The results showed that L100–Tre powder exhibited better moisture content (4.84%) and hygroscopicity (17.94%) than the other three powders produced with L100–Tre and polysaccharides. Moreover, the LGG in the powders retained a high viability of 9 log CFU g−1 after spray drying and maintained 7 log CFU g−1 after 8 weeks of storage. Notably, all powders exhibited desirable survival rates of 87.4–93% for LGG after in vitro digestion. In addition, spray drying had minimal impact on the cell adhesion ability of LGG, maintaining an adhesion rate of 80% to Caco-2 cells. The L100–Tre–LGG probiotic spray-dried powders exhibit long shelf stability and strong adhesion capacity, providing strong support for the industrial production of probiotic products.
Sustainability spotlightGlobal food systems face challenges of low probiotic viability and high energy consumption. Our L100–trehalose–polysaccharide microencapsulation system for Lacticaseibacillus rhamnosus GG achieves high viability and intestinal adhesion, reducing dosage requirements and manufacturing waste. Innovations include optimized drying for energy efficiency, improved gastrointestinal survival to minimize waste, and the use of biodegradable materials. Aligned with UN Sustainable Development Goals, this system enhances nutrition and health through stable probiotics, reduces carbon emissions, and extends shelf life, advancing sustainable functional food production. |
1. Introduction
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”.1 The concept of the microbiota–brain–gut axis has gained significant recognition, highlighting the importance of successfully delivering probiotics to the intestine to reconstruct the intestinal microenvironment. The gut microbiota communicates with the brain through multiple pathways, including the immune system, tryptophan metabolism, the vagus nerve, and the enteric nervous system.2 For example, short-chain fatty acids (SCFAs), such as butyric acid produced by fermentation of dietary fiber by intestinal microbes, can cross the blood–brain barrier, modulate microglial activity (the brain's resident immune cells), and affect neurodevelopment.3 Oral administration of probiotic supplements has been shown to modulate the composition of the human gut microbiota. Certain probiotic strains, such as Bifidobacterium bifidum, lower intestinal pH by producing SCFAs, thereby creating a favorable environment for the growth of beneficial commensal bacteria, including Faecalibacterium prausnitzii.4 In addition, probiotics help suppress pathogenic bacteria (e.g., Escherichia coli and Salmonella spp.) through several mechanisms: (1) competitive exclusion by occupying mucosal adhesion sites, (2) competition for nutrients, and (3) secretion of antimicrobial compounds, such as bacteriocins, lactic acid, and hydrogen peroxide.5Spray drying, known for its high efficiency and low energy consumption, has emerged as a preferred method for probiotic microencapsulation. Enhancing the quality of probiotic powder preparation is crucial to ensure that probiotics effectively participate in the human body's metabolic and immune processes. One critical factor is maintaining high viability of probiotics upon reaching the intestine. During spray drying, probiotic cells are exposed to heat, dehydration and oxidative stress, all of which can compromise cell integrity and lead to cell death.6,7 Cellular components are particularly vulnerable: membrane damage occurs at about 50 °C, and the 30S ribosomal subunit is impaired at 60 °C, proteins begin to denature at 65 °C, and DNA damage becomes evident around 90 °C.8 The removal of bound water further disrupts the stability of proteins, DNA, lipids, and overall cellular structure.9 For instance, L. helveticus experiences significant loss of viability when the water content drops below around 0.3–0.5 g H2O per g dry weight.10 Beyond the drying process, probiotics are also subject to environmental stresses during storage and digestion.11
The physicochemical properties of the resulting probiotic powder, such as porosity, density and hygroscopicity, are critical in determining its protective capacity.12 Notably, particle size and surface morphology (e.g., hollowness or surface cracks) can significantly affect the effectiveness of microencapsulation.13 Alvarado-Reveles et al.14 reported that encapsulating Lactobacillus rhamnosus GG (LGG) in buttermilk proteins combined with agave fructans yielded particles with an average diameter of 52.4 μm, which improved bacterial survival by shielding against environmental and gastrointestinal stresses while enhancing bioaccessibility. Similarly, Chaikham et al.15 found that increasing maltodextrin content to 20% reduced probiotic particle size and led to wrinkled surfaces, whereas a blend of 10% maltodextrin and 10% inulin produced larger, smoother particles. The mixed formulation also improved bacterial survival, retaining one log CFU g−1 more viable cells after spray drying compared to the maltodextrin-only system.
Selecting an appropriate wall material is crucial for protecting probiotics. Oligosaccharides such as trehalose (Tre) are well known for their protective role during spray drying, where they replace water molecules and help maintain cell membrane integrity.16 However, oligosaccharides alone provide limited protection in the harsh gastric environment, rendering probiotics susceptible to acidic degradation and bile salt inactivation.17 To overcome this limitation, the incorporation of Eudragit® L100 (L100), a pH-responsive polymer, offers a complementary solution.18 Unlike Tre, L100 remains insoluble under acidic gastric conditions but dissolves at intestinal pH, allowing targeted probiotic release in the colon and minimizing inactivation in the stomach. Thus, the combination of Tre and L100 represents a promising strategy for developing an intestinal-targeted probiotic delivery system.
In our previous research, we found that the L100–Tre system could synergistically enhance the viability of probiotics after thermal convective drying. L100, adsorbed on the particle surfaces during evaporation, endowed the powder with intestinal-targeted delivery functions.18 However, there was limited focus on the physiological functions of probiotics and the physicochemical characteristics of the powder. Thus, this study aimed to analyse the physicochemical properties of L100–Tre–LGG particles, optimize the L100–Tre–LGG system, and investigate the physiological functions of microencapsulated probiotics. This study may provide new insights and references regarding the application of spray drying encapsulation in the delivery of probiotics and functional foods.
2. Materials and methods
2.1. Materials
The LGG strain (ATCC 53103) used in this study was obtained from our laboratory collection (College of Food Science and Technology, Nanjing Agricultural University, Nanjing, China). MRS broth powder and MRS agar powder were purchased from Hopebio Ltd (Qingdao, China). Eudragit® L100 (L100) was obtained from Evonik Industries AG (Shanghai, China). Trehalose was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Maltodextrin was kindly provided as a gift by Ingredion Ltd (Shanghai, China). Inulin and soybean soluble polysaccharide were purchased from Henan Gaobao Industry Co. (Henan, China). Pepsin (item no. P7125), pancreatin (item no. P7545), and bile salt (item no. 48305) were all purchased from Sigma-Aldrich (Shanghai, China). Solutions were prepared with ultrapure grade water. Growth media, solutions, and glassware were all sterilized by autoclaving at 115 °C for 20 min.2.2. Bacterial cultivation
The stored LGG cells in a culture tube were transferred onto MRS agar plates, composed of 52 g per L MRS broth powder and 20 g L−1 of agar, and incubated at 37 °C for two days before being stored at 4 °C. The culture was refreshed on new agar media weekly to maintain the viability of the LGG. From these plates, a single colony was selected and introduced into sterile MRS broth and then incubated under static conditions at 37 °C for 12 h. This culture was then inoculated into fresh MRS broth, maintaining an added concentration of 2% (v/v). After 24 h in the same environment, the MRS broth was centrifuged at 8000 g for 10 min to collect LGG cells, which reached a density of 1 × 109 CFU mL−1. To purify, the cells were washed twice using 0.85% sterile saline under the same centrifugal conditions.2.3. Spray dying microencapsulation of probiotics
| Composition | L100 (%) | Trehalose (%) | Maltodextrin (%) | Inulin (%) | Soluble soy polysaccharides (%) | Total mass (%) |
|---|---|---|---|---|---|---|
| a LT: L100–trehalose; LT–MD: L100–maltodextrin; LT–IN: L100–inulin; LT–SP: L100–soluble soy polysaccharides. | ||||||
| LT | 2 | 10 | 12 | |||
| LT–MD | 2 | 5 | 5 | 12 | ||
| LT–IN | 2 | 5 | 5 | 12 | ||
| LT–SP | 2 | 5 | 5 | 12 | ||
2.4. Physicochemical properties of spray-dried microcapsules
2.5. Viable evaluation of LGG after spray drying
The experimental methodology is based on Bhagwat et al.,22 with minor modifications. The cell viability was determined using the viable cell count method. Briefly, the powder samples were dissolved in 0.05 M PBS (pH 6.5), followed by serial dilutions and plate counting. The encapsulation efficiency was calculated using the following formula:| Encapsulation efficiency (%) = E/E0 × 100 | (1) |
2.6. Cell survival in in vitro digestion
The simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were prepared according to Minekus et al.23 with modifications. For SGF, 10 mL was prepared by mixing 0.3 M CaCl2 solution (5 μL), pepsin (50 mg), SGF stock solution (8 mL) and pure water (1.95 mL). The pH of the mixture was adjusted to 2.0. SIF (10 mL) was prepared by combining 0.3 M CaCl2 solution (20 μL), pepsin (125 mg), bile salts (0.08 g), SIF stock solution (8 mL) and pure water (1.95 mL), with the final pH adjusted to 7.0.To conduct the experiment, a mixture of 10 mL of SGF and 0.1 g of spray-dried powder was prepared. Before digestion, the pH of the solution was readjusted to 2.0. The mixture was then subjected to water bath oscillation for 2 h at 37 °C and 50 rpm. Subsequently, 10 mL of SIF was added to the gastric fluid mixture, and the pH of the digestion juice was adjusted to 7.0 before incubating for another 2 h under the same conditions. Samples of 500 μL were collected at 0, 1, 2, 3, and 4 h during continuous digestion. The samples obtained at each time point were diluted and spread onto MRS agar plates for static incubation at 37 °C for 48 h to detect bacterial viability.
2.7. Cell survival during storage
The spray-dried powders were stored in hermetically sealed tubes at 4 ± 1 °C, and the number of viable cells was detected weekly.2.8. Adhesion to Caco-2 monolayer cells
For passage, the cell monolayer was washed with PBS, and 0.5 mL of trypsin–EDTA solution was added for digestion until the cell monolayer was detached into individual cells. Subsequently, 0.5 mL of cell culture medium was added to terminate digestion. The obtained cell suspension was transferred to a sterile centrifuge tube for centrifugation, resuspended in culture medium, and then passaged at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and cultured for 2–3 days for the experiment.
000 cells per well and cultured for 2–3 days for the experiment.The monolayer was used for the evaluation tests of bacterial adhesion ability, following the method described by Xu et al.25 with some modifications. Firstly, cells were collected and resuspended in PBS. After incubation at 37 °C for 2 h, the cells were washed three times with sterile PBS to remove free cells. Next, 0.5 mL trypsin was added to disrupt the monolayer, followed by incubation at 37 °C for 7 min. Finally, 0.5 mL medium was added to deactivate the trypsin. The mixture was then serially diluted for plate colony counting in a centrifuge tube as proposed previously. The viable cells were detected before the Caco-2 adhesion tests. The adhesion rate was expressed as below:
| Adhesion rate (%) = N/N0 | (2) |
2.9. Statistical analysis
All experiments and analyses were conducted in triplicate. The results were expressed as means ± standard deviation (SD), and data analysis was performed using SPSS software (version 26, SPSS Inc., Chicago, USA) with one-way ANOVA (p < 0.05) for statistical significance.3. Results and discussion
3.1. Physicochemical properties of L100–Tre–LGG based microcapsules
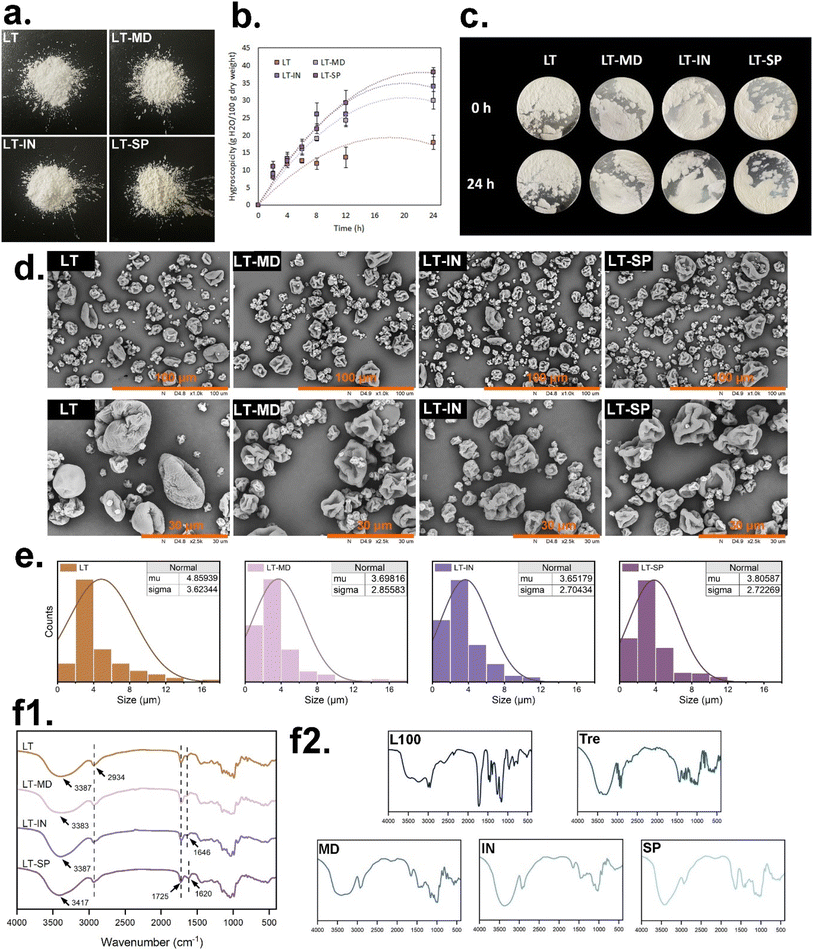
All powders obtained through spray drying were similar in colour, with the LT–SP powder appearing light yellow due to the presence of SSP (Fig. 1a).| Properties | LT | LT–MD | LT–IN | LT–SP |
|---|---|---|---|---|
| a Values followed by the different superscript letters in the same row are significantly different (p < 0.05). | ||||
| Moisture content (%) | 4.84 ± 0.59a | 6.02 ± 0.38 ab | 5.37 ± 0.56 ab | 6.22 ± 0.34b |
| ρt (g mL−1) | 0.29 ± 0.04 ab | 0.27 ± 0.003a | 0.26 ± 0.03a | 0.34 ± 0.003b |
| ρb (g mL−1) | 0.41 ± 0.07a | 0.48 ± 0.04a | 0.51 ± 0.09a | 0.57 ± 0.05a |
The moisture absorption ability of a powder is closely linked to the interaction between its wall material and water molecules. Compared with LT powders, LT–MD, LT–IN, and LT–SP powders exhibited higher water absorption rates. This enhanced absorption can be attributed to the abundance of hydroxyl groups in polysaccharides, which facilitate stronger bonding with water molecules.32 In fact, for powder products, a high moisture absorption rate indicates that the sample is more prone to attracting moisture from the surrounding environment. This, in turn, can negatively impact the storage stability and powder flowability, potentially leading to adhesion and clumping during the shelf life. Moreover, water absorbed from the external environment can accelerate redox reactions within the particles, thereby affecting their biological activity.20 Therefore, controlling the moisture absorption rate is crucial for ensuring the stability, flowability, and bioactivity of powder products.
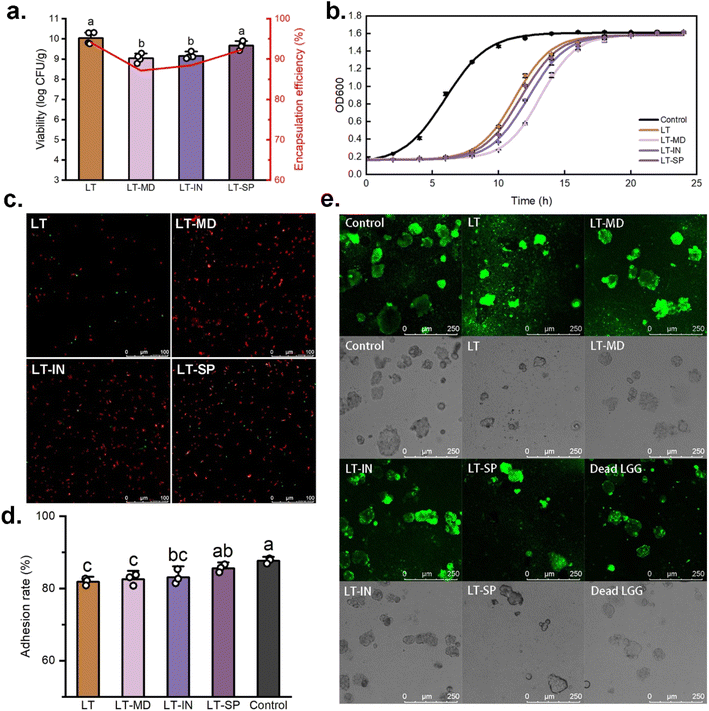
3.2. The viability and adhesion activity of cells in L100–Tre based spray-dried powders
Consistent findings were observed in the adhesion analysis (Fig. 2e). LGG labelled with CFDA-SE adhered to the surface of Caco-2 cells, emitting green fluorescence. Notably, even after high-temperature inactivation, LGG was still able to adhere to Caco-2 cells, though its distribution was less uniform compared to live cells. This may be due to the spontaneous absorption of LGG aggregates by Caco-2 cells. Similar studies have also shown that Lactobacillus acidophilus, following heat treatment at 100–105 °C, can adhere to HeLa 299 cells and inhibit the adhesion of Escherichia coli B41.44 This suggested that even after high-temperature treatment, the surface of LGG cells retained molecular structures that can be recognized by Caco-2 cells.
Golowczyc et al.24 found that spray drying did not significantly alter the adhesion function of Lactobacillus plantarum 83114 and Lactobacillus kefir 8321, but it significantly reduced the adhesion ability of Lactobacillus kefir 8348. This underlines the strain-specific effect of spray drying on bacterial adhesion, as previously reported by Iaconelli et al.45 Therefore, when producing probiotic powders using spray drying, it is crucial to comprehensively evaluate its potential impacts on the specific functionalities of the target strain to ensure optimal performance.
3.3. The stability of cell viability
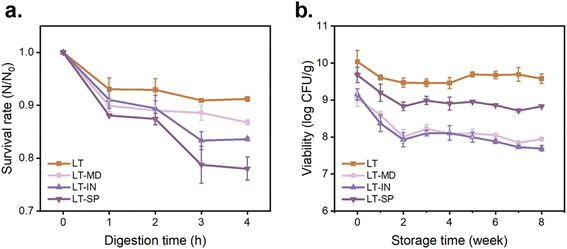 | ||
| Fig. 3 Digestion and storage stability. (a) survival rate of cells in microcapsules after simulated in vitro digestion. (b) Viability of cells in microcapsules stored at 4 °C. | ||
During the simulated intestinal digestion, the bacterial cells encapsulated within the particles were released and had to face the stressful conditions of bile salts and pancreatic enzymes.46 Although no significant difference (p > 0.05) in bacterial viability was observed among the four powders after spray drying, significant variations (p < 0.05) in viability were observed after 4 h of simulated gastrointestinal digestion. The results indicated that probiotics encapsulated with IN or SP were more sensitive to these stresses. Specifically, after 4 h of digestion, the bacterial viability levels in LT–IN and LT–SP powders decreased to 83.6% and 78%, respectively, while LT powder maintained the highest viability retention at 91.2%. This may be attributed to the more severe damage inflicted on the cells during spray drying in LT-–IN and LT–SP powders, making them more vulnerable to the stress of bile salts and trypsin presented in the SIF. Gonzalez-Ferrero et al.47 also emphasized the challenging gut environment for probiotics, discovering that probiotics encapsulated with soy protein concentrate and maltodextrin showed minimal viability loss in SGF but substantial cell death in SIF.
Overall, after 4 h of simulated in vitro digestion, the survival rates of spray-dried LGG in all powders were above 78%, indicating that MD, IN, and SP exhibited certain protective effects during the in vitro digestion of LGG. These findings align with similar studies that have reported the good resistance of probiotics encapsulated in IN and MD against low pH and bile stress.36 These results provide insights into the protective effects of different wall materials for probiotics in simulated digestive environments, which can help optimize the formulation and stability of probiotic products.
It is worth noting that for the four microcapsules, LGG experienced a distinct loss of viability during the initial storage period, and then the viability began to regain, followed by a slower rate of bacterial viability loss (Fig. 3b). This pattern of viability fluctuation has been similarly observed in the study conducted by Reyes et al.50 This phenomenon could be due to the environmental pressure encountered by the bacterial cells upon storage. However, as the storage period progressed, some bacterial cells gradually adapted to these conditions and began to regain viability through their inherent repair mechanisms. In addition, the refrigeration conditions used for storage likely contributed to the improved stability of the cells, potentially by reducing harmful chemical reactions like redox reactions. This finding is consistent with the observations made by Broeckx et al.,51 further confirming the importance of refrigeration in preserving probiotic viability during storage.
4. Conclusion
In this study, we successfully fabricated L100–Tre–LGG microcapsules using a spray drying technique and analysed the physicochemical properties of the resulting powders containing L100–Tre and polysaccharides. The results revealed that the particles exhibited good physicochemical characteristics, and the encapsulated LGG showed desirable viability, re-growth capability, and storage stability. Furthermore, the spray-dried LGG in the L100–Tre-based microcapsules maintained high viability during simulated in vitro digestion, confirming their high digestive stability. In addition, the study investigated the adhesion functionality of the LGG encapsulated in the L100–Tre-based microcapsules and found that the spray drying process had a limited impact on the bacterial adhesion capacity. Overall, these findings demonstrated the high viability retention and adhesion capacities of the L100–Tre–LGG probiotic spray-dried particles, providing strong support for the development and application of spray-dried probiotic powder products.Data availability
The data supporting this paper have been included in the paper.Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.Acknowledgements
The work was supported by funding from the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (Grant No. 2021JJLH0009); Natural Science Foundation of Jiangsu Province (Grant No. BK20210404); Scientific Research Cooperation and High-Level Talent Training Projects with Canada, Australia, New Zealand and Latin America (Grant No. 2022-1007); Jiangsu Provincial Double-Creative Doctorate Plan.References
- C. Hill, F. Guarner, G. Reid, G. R. Gibson, D. J. Merenstein, B. Pot, L. Morelli, R. B. Canani, H. J. Flint, S. Salminen, P. C. Calder and M. E. Sanders, Nat. Rev. Gastroenterol. Hepatol., 2014, 11, 506–514 CrossRef PubMed.
- J. F. Cryan, K. J. O'Riordan, C. S. M. Cowan, K. V. Sandhu, T. F. S. Bastiaanssen, M. Boehme, M. G. Codagnone, S. Cussotto, C. Fulling, A. V. Golubeva, K. E. Guzzetta, M. Jaggar, C. M. Long-Smith, J. M. Lyte, J. A. Martin, A. Molinero-Perez, G. Moloney, E. Morelli, E. Morillas, R. O'Connor, J. S. Cruz-Pereira, V. L. Peterson, K. Rea, N. L. Ritz, E. Sherwin, S. Spichak, E. M. Teichman, M. van de Wouw, A. P. Ventura-Silva, S. E. Wallace-Fitzsimons, N. Hyland, G. Clarke and T. G. Dinan, Physiol. Rev., 2019, 99, 1877–2013 CrossRef CAS PubMed.
- G. Sharon, N. J. Cruz, D. W. Kang, M. J. Gandal, B. Wang, Y. M. Kim, E. M. Zink, C. P. Casey, B. C. Taylor, C. J. Lane, L. M. Bramer, N. G. Isern, D. W. Hoyt, C. Noecker, M. J. Sweredoski, A. Moradian, E. Borenstein, J. K. Jansson, R. Knight, T. O. Metz, C. Lois, D. H. Geschwind, R. Krajmalnik-Brown and S. K. Mazmanian, Cell, 2019, 177, 1600–1618 CrossRef CAS PubMed.
- A. Rivière, M. Selak, D. Lantin, F. Leroy and L. De Vuyst, Front. Microbiol., 2016, 7, 979 Search PubMed.
- A. L. Servin, FEMS Microbiol. Rev., 2004, 28, 405–440 CrossRef CAS PubMed.
- S. Huang, M. L. Vignolles, X. D. Chen, Y. Le Loir, G. Jan, P. Schuck and R. Jeantet, Trends Food Sci. Technol., 2017, 63, 1–17 CrossRef CAS.
- E. Ananta, M. Volkert and D. Knorr, Int. Dairy J., 2005, 15, 399–409 CrossRef CAS.
- P. Teixeira, H. Castro, C. Mohácsi-Farkas and R. Kirby, J. Appl. Microbiol., 1997, 83, 219–226 CrossRef CAS PubMed.
- C. Santivarangkna, M. Wenning, P. Foerst and U. Kulozik, J. Appl. Microbiol., 2007, 102, 748–756 CrossRef CAS PubMed.
- C. Santivarangkna, U. Kulozik and P. Foerst, J. Appl. Microbiol., 2008, 105, 1–13 CrossRef CAS PubMed.
- L. K. Liao, X. Y. Wei, X. Gong, J. H. Li, T. Huang and T. Xiong, LWT--Food Sci. Technol., 2017, 82, 82–89 CrossRef CAS.
- Z. Muhammad, R. Ramzan, R. F. Zhang and M. W. Zhang, Coatings, 2021, 11, 587 CrossRef CAS.
- Y. Xu, M. Dong, H. Xiao, S. Y. Quek, Y. Ogawa, G. Ma and C. Zhang, Crit. Rev. Food Sci. Nutr., 2023, 1–17 Search PubMed.
- O. Alvarado-Reveles, S. Fernandez-Michel, R. Jimenez-Flores, C. Cueto-Wong, L. Vazquez-Moreno and G. R. C. Montfort, Probiotics Antimicrob. Proteins, 2019, 11, 1340–1347 CrossRef CAS PubMed.
- P. Chaikham, V. Kemsawasd and P. Seesuriyachan, LWT, 2017, 78, 31–40 CrossRef CAS.
- Y. Xu, D. Mingsheng, X. Hongmei, Y. Q. Siew, O. Yukiharu, M. Guangyuan and C. and Zhang, Crit. Rev. Food Sci. Nutr., 2024, 64, 11222–11238 CrossRef CAS PubMed.
- S. S. Pinto, S. Verruck, C. R. W. Vieira, E. S. Prudêncio, E. R. Amante and R. D. M. C. Amboni, LWT--Food Sci. Technol., 2015, 64, 1004–1009 CrossRef CAS.
- Y. Xu, S. Zhu, Y. Zeng, C. Zhang, M. Dong and C. Zhang, J. Food Eng., 2024, 369, 111940 CrossRef CAS.
- D. Arepally, R. S. Reddy and T. K. Goswami, Food Funct., 2020, 11, 8694–8706 RSC.
- A. M. Goula and K. G. Adamopoulos, Drying Technol., 2008, 26, 726–737 CrossRef CAS.
- D. Paim, S. D. O. Costa, E. H. M. Walter and R. V. Tonon, LWT--Food Sci. Technol., 2016, 74, 21–25 CrossRef CAS.
- A. Bhagwat, P. Bhushette and U. S. Annapure, Beni-Suef Univ. J. Basic Appl. Sci., 2020, 9, 1–8 CrossRef.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. Wickham, W. Weitschies and A. Brodkorb, Food Funct., 2014, 5, 1113–1124 RSC.
- M. A. Golowczyc, P. T. JoanaSilva, G. L. D. Antoni and A. G. Abraham, Int. J. Food Microbiol., 2011, 144, 556–560 CrossRef PubMed.
- D. Xu, X. Zhao, G. C. Mahsa, K. Ma, C. Zhang, X. Rui, M. Dong and W. Li, Carbohydr. Polym., 2023, 313, 120874 CrossRef CAS PubMed.
- S. Huang, S. Mejean, H. Rabah, A. Dolivet, Y. Le Loir, X. D. Chen, G. Jan, R. Jeantet and P. Schuck, J. Food Eng., 2017, 196, 11–17 CrossRef CAS.
- P. Vaibhav, K. C. Anil and P. S. Surendra, Int. J. Curr. Microbiol. Appl. Sci., 2014, 3, 1224–1237 Search PubMed.
- D. Arepally and T. K. Goswami, LWT--Food Sci. Technol., 2019, 99, 583–593 CrossRef CAS.
- A. Rodklongtan and P. Chitprasert, Food Res. Int., 2017, 100, 276–283 CrossRef CAS PubMed.
- M. F. Zotarelli, V. M. da Silva, A. Durigon, M. D. Hubinger and J. B. Laurindo, Powder Technol., 2017, 305, 447–454 CrossRef CAS.
- S. George, A. Thomas, M. V. P. Kumar, A. S. Kamdod, A. Rajput, T. J. Joshi and S. Abdullah, Eur. Food Res. Technol., 2023, 249, 241–257 CrossRef CAS.
- C. Zhang, S. L. Ada Khoo, X. D. Chen and S. Y. Quek, Powder Technol., 2020, 361, 995–1005 CrossRef CAS.
- P. Barajas-Alvarez, M. Gonzalez-Avila and H. Espinosa-Andrews, LWT--Food Sci. Technol., 2022, 153, 112485 CrossRef CAS.
- R. Rajam and C. Anandharamakrishnan, LWT--Food Sci. Technol., 2015, 60, 773–780 CrossRef CAS.
- S. Thakral, N. K. Thakral and D. K. Majumdar, Expert Opin. Drug Delivery, 2013, 10, 131–149 CrossRef CAS PubMed.
- V. Kumar, J. J. Ahire, R. Amrutha, S. Nain and N. K. Taneja, Probiotics Antimicrob. Proteins, 2023, 10115 Search PubMed.
- R. M. Wang, N. Li, K. Zheng and J. F. Hao, FEMS Microbiol. Lett., 2018, 365, fny217 CAS.
- P. Jayaprakash, C. Gaiani, J.-M. Edorh, F. Borges, E. Beaupeux, A. Maudhuit and S. Desobry, Foods, 2023, 12, 3117 CrossRef CAS PubMed.
- M. Yin, M. Chen, Y. Yuan, F. Liu and F. Zhong, Food Hydrocoll., 2024, 146, 109252 CrossRef CAS.
- Y. Luo, Z. Ma, C. De Souza, S. Wang, F. Qiao, H. Yi, P. Gong, Z. Zhang, T. Liu, L. Zhang and K. Lin, Food Hydrocoll., 2024, 149, 109602 CrossRef CAS.
- M. L. Van Tassell and M. J. Miller, Nutrients, 2011, 3, 613–636 CrossRef CAS PubMed.
- B. Sophatha, S. Piwat and R. Teanpaisan, Arch. Microbiol., 2020, 202, 1349–1357 CrossRef CAS PubMed.
- S. Lebeer, I. Claes, H. L. P. Tytgat, T. L. A. Verhoeven, E. Marien, I. von Ossowski, J. Reunanen, A. Palva, W. M. de Vos, S. C. J. De Keersmaecker and J. Vanderleyden, Appl. Environ. Microbiol., 2012, 78, 185–193 CrossRef CAS PubMed.
- J. Fourniat, C. Colomban, C. Linxe and D. Karam, Ann. Rech. Vet., 1992, 23, 361–370 CAS.
- C. Iaconelli, G. Lemetais, N. Kechaouc, F. Chain, L. G. Bermudez-Humaran, P. Langella, P. Gervais and L. Beney, J. Biotechnol., 2015, 214, 17–26 CrossRef CAS PubMed.
- R. Arevalo-Perez, C. Maderuelo and J. M. Lanao, J. Controlled Release, 2020, 327, 703–724 CrossRef CAS PubMed.
- C. Gonzalez-Ferrero, J. M. Irache and C. J. Gonzalez-Navarro, Food Chem., 2018, 239, 879–888 CrossRef CAS PubMed.
- G. L. Nunes, M. D. Etchepare, A. J. Cichoski, L. Q. Zepka, E. J. Lopes, J. S. Barin, E. M. D. Flores, C. D. da Silva and C. R. de Menezes, LWT--Food Sci. Technol., 2018, 89, 128–133 CrossRef CAS.
- Y. Zhang, J. Lin and Q. X. Zhong, Food Res. Int., 2015, 71, 9–15 CrossRef CAS.
- V. Reyes, A. Chotiko, A. Chouljenko, V. Campbell, C. Liu, C. Theegala and S. Sathivel, Drying Technol., 2018, 36, 1738–1748 CrossRef CAS.
- G. Broeckx, D. Vandenheuvel, T. Henkens, S. Kiekens, M. F. L. van den Broek, S. Lebeer and F. Kiekens, Int. J. Pharm., 2017, 534, 35–41 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |