 Open Access Article
Open Access ArticleProduction and optimization of date fruit and honey wines using Saccharomyces cerevisiae isolated from various palm wine sources
Ositadinma Chinyere Ugbogu *a,
Alloysius Chibuike Ogodob,
Amadike Eziuche Ugboguc,
Kingsley Chukwuemeka Nwachukwua and
Frank Anayo Orjid
*a,
Alloysius Chibuike Ogodob,
Amadike Eziuche Ugboguc,
Kingsley Chukwuemeka Nwachukwua and
Frank Anayo Orjid
aDepartment of Microbiology, Faculty of Biological Sciences, Abia State University, Uturu, Nigeria. E-mail: osita.ugbogu@abiastateuniversity.edu.ng
bDepartment of Microbiology, Faculty of Biosciences, Federal University, Wukari, Nigeria
cDepartment of Biochemistry, Faculty of Biological Sciences, Abia State University, Uturu, Nigeria
dDepartment of Biotechnology, Federal Institute of Industrial Research Oshodi (FIIRO), Nigeria
First published on 19th May 2025
Abstract
Dates and honey are known for their high nutritional values and associated benefits. Wine production with dates and honey will help in value addition to dates and also reduce post-harvest losses. This study aims to produce and evaluate wine produced from dates and honey using yeasts isolated from palm wine. Yeasts were isolated from various samples of palm wine, selected, and identified using molecular techniques. The best-performing yeasts, namely, isolate 065 (Saccharomyces cerevisiae) and isolate 047 (Candida tropicalis), were used to produce date fruit and honey wines combined in the ratios of: date100%, D/H 50%/50%, D/H 40%/60% and D/H 30%/70% for 7 days. The performance results of the yeasts indicated that isolate BFC 065 recorded the highest invertase activity (40.95 μmol min−1), followed by PPE 047 (36.84 μmol min−1), while IPA 151 showed the least invertase activity (4.3 μmol min−1). The alcohol dehydrogenase activity results indicated that PPE 047 had the highest activity (13.67 unit per mL), followed by BFC 065 (11.42 unit per mL), while IPA 151 had the least activity (2.4 unit per mL). The yeast isolate's sugar tolerance properties showed that isolates 065 and 047 had the highest sugar tolerance level at 20% sugar concentration with optical densities (OD) of 1.098 and 0.947, respectively, after 72 h of incubation. In contrast, the isolate BFC 168 showed the least sugar tolerance level (OD = 0.674). The ethanol tolerance potentials of the yeasts showed that the highest ethanol tolerance was observed for BFC 065 (17.5 ± 0.18% v/v), followed by PPE 047 (14.00 ± 0.81% v/v), while IPA 142 showed the least tolerance (4.5 ± 0.47% v/v). The total titratable acidity (TTA) producing potential of the yeasts showed that the highest acid-producing potential was observed for BFC 065 (3.21% ± 0.144%), followed by PPE 047 (2.16% ± 0.35%), while IPA 131 had the least potential (0.27% ± 0.00%). The highest pH tolerance of the yeasts was observed at pH 2. The isolate BFC 168 tolerated the lowest pH (pH 2), while IPA 110 (OD = 0.115) showed the least tolerance. The composite value of the date syrup and honey showed that the energy level of dates was 303.36 kJ, while that of honey was 335.69 kJ; the protein contents were 2.37% and 1.09%, respectively, while the carbohydrate contents were 72.21% and 82.09%, respectively. The essential element composition of dates and honey showed that they contained calcium levels of 468.90 mg and 3.67 mg, respectively, magnesium levels of 117.8 mg and 2.98 mg, respectively, and iron levels of 29.50 mg and 1.34 mg, respectively, while copper and zinc were not detected in honey. There was a gradual decrease in soluble sugar (0Brix), pH, and specific gravity of various wines, while TTA increased with the fermentation time. The alcohol contents of the wines fermented with the isolate 047 were higher than those fermented with the isolate 065. The alcohol content ranged from 7.54% (D/H30/70) and 9.65% (D/H30/70) to 9.98% (date100%) and 12.24% (date100%) for the isolates 065 and 047, respectively. The result of the flavor compounds in the top three developed wines identified using GC-MS indicated that hexadecanoic acid, oleic acid, octadecenoic acid, and methyl ester were present in all the wines. In contrast, cis-vaccenic acid was present only in D/H40/60 fermented with the isolate 065. The sensory evaluation of the wines ranked the commercial wine first, wine 2 second and wine 1 sixth. This shows that acceptable wine could be produced from date fruit and honey blends.
Sustainability spotlightAlthough Nigeria is not a major producer of dates in the world, the crop strives in the northern part of the country particularly in areas that lie above latitude 10° North of the equator. Large quantities of date fruits are disposed of yearly due to the non-availability of, or poor, storage facilities, resulting in loss of the vital nutrients (vitamins) that are associated with them and loss of potential revenue sources. However, if the fruits could be used in wine production, the nutrients that are lost can be harnessed and made available all year round in addition to generating revenue. This aligns with SDG 12, which is to ensure sustainable consumption and production patterns. |
Introduction
Wine is an alcoholic beverage, made of fermented fruit juice, usually grapes. It is the fermented juice of grapes used as a beverage. The natural chemical balance of grapes lets them ferment without the addition of sugars, acids, enzymes, or other nutrients. However, wine can also be defined as an alcoholic drink made by the fermentation of plants or fruits other than grapes.1 In this case, the wine is qualified by the name of the plant or fruit from which it is made, e.g. pineapple wine, mango wine, pawpaw wine, carrot wine, cucumber wine, watermelon wine, banana wine and plantain wine. In this regard, a drink is qualified as wine only if it is derived from the process of fermentation of plant matter. Wine therefore refers to the higher alcoholic content rather than the production process.2From the foregoing and definitions, wine can be divided into two major categories: First, wines made with grapes as the main ingredient, and second, country wines, made from fruits other than grapes, or nuts, grains, herbs, flowers, and vegetables, and seasoned with the creativity and imagination of amateur winemakers through generations.1,3 In general, grapes are the main raw materials that have been used for wine production in the past few decades.4 However, grapes are indigenous to Nigeria and not readily available to the winemaker. Alternative sources, fruits such as banana, cucumber and pineapple which are readily available and in large quantity in Nigeria, are used in wine production.5–9
In recent times, home-made wine production has been practiced with various fruits such as apples, pears, strawberries, cherries, plums, bananas, pineapple, oranges, cucumber, watermelon, and guava using species of Saccharomyces cerevisiae, which converts the sugar in the fruit juices into alcohol and organic acids that later react to form aldehydes, esters and other chemical compounds, which also help to preserve the wine.10,11 This fermentation process could either be spontaneous by the natural flora of the fruits or controlled by introducing industrial strains of yeasts to ferment the juice.12 However, yeasts from other sources such as palm wine could be used.13
Date palm (Phoenix dactylifera L.) production in Nigeria started around the 17th century, but its cultivation and marketing have been at the subsistence level. It was reported that pilgrims brought date palms into Nigeria from North Africa during the trans-Saharan trade.14,15 Though Nigeria is not a major date producer in the world, the crop strives in the northern parts of the country, particularly regions above latitude 100 north of the equator.15 It is propagated by seed, offshoot, and tissue culture. The date palm is a dioecious perennial plant whose females normally begin to bear date fruits after four years depending on the agronomic practices. It is a monocotyledonous plant with no tap root but a fibrous root system. Date production in Nigeria has two fruiting seasons, the dry and wet seasons; however, only the dry season fruit is economically useful. Large quantities of those fruits are disposed of yearly due to the non-availability of, or poor, storage facilities, resulting in the loss of vital nutrients (vitamins) that are associated with them and the loss of potential revenue sources.15 However, if the fruits could be used in wine production, the nutrients that are lost can be harnessed and made available all year round in addition to generating revenue.
Mead is one of the world's oldest alcoholic beverages, containing 8–18% (v/v) of ethanol, which results from the alcoholic fermentation of diluted honey carried out by yeast strains. However, mead is difficult to find in the commercial market. This is because mead producers face several problems such as delayed and arrested fermentation, production of off-flavours by the yeast, and lack of uniformity of the final product.16,17 Honey is a natural product and a highly concentrated solution of a complex mixture of sugars. It also contains small amounts of other constituents such as minerals, proteins, vitamins, organic acids, flavonoids, phenolic acids, enzymes, and other phytochemicals.17 The components in honey responsible for its antioxidative effect are flavonoids, phenolic acids, ascorbic acid, catalase, peroxidase, and carotenoids.18,19 The colour, flavour, aroma, and yeast influence the quality of mead.20 The optimization of process conditions is one of the most critical stages in the development of an efficient and economic bioprocess.21
However, studies have shown that several fruits can be implicated in wine production, including banana, pineapple, plantain, carrot, pawpaw, mango, cucumber, watermelon, and date fruit.1,5,12,15,22–26 However, the production of wine with date fruits and honey is not readily available in the literature. Therefore, the objective of the present study is to produce, optimize, and evaluate the quality of wine produced from the mixture of date fruit and honey using Saccharomyces cerevisiae and Candida tropicalis isolated from palm wine.
Materials and methods
Source of materials
Palm wine samples of Raphia hookeri from palm wine markets in Isuochi (Abia state-Nigeria), Badagri (Lagos-Nigeria), and Papalento (Ogun State Nigeria) were purchased and transported to the laboratory for investigations. The samples of date seeds were purchased from local traders at Mushin Market in Lagos, while samples of the honey were purchased from Mile 2 Market in Lagos State, Nigeria. All analyses using the samples were performed at the World Bank (Step B) Laboratory, Federal Institute of Industrial Research, Oshodi, Lagos State.Determination of the population of palm wine yeasts and isolation of yeasts
The samples of palm wine were diluted serially using a ten-fold dilution technique and 0.1 mL of the diluted sample at various levels of dilution was plated on a yeast extract peptone-dextrose (YEPD) agar medium supplemented with 0.1 mg mL−1 streptomycin sulphate, as previously described by Ugbogu and Okereke.27 The plates were incubated at 25 °C for 24 to 48 hours. Morphologically distinguished colonies were selected using a dissection microscope and then counted. The counts were calculated and expressed as colony-forming units per millilitre (CFU mL−1) of the sample. The distinct yeast cells were isolated and purified by subsequent streaking on the YEPD agar medium. The pure culture of each strain was kept on YEPD agar slants and stored at 4 °C until use.Identification and characterization of yeast cells
Pure yeast isolates were transferred to a yeast extract peptone-dextrose (YEPD) agar medium supplemented with 0.1 mg mL−1 streptomycin sulphate for 24 h. The yeasts were identified based on their cultural characteristics, microscopic examination, and morphology as well as their exhibited pattern of carbohydrate fermentation and assimilation.8Determination of invertase activities of palm wine isolates from various local sources
The invertase activity was determined following the method described by Harkness and Arnason.28 Each test yeast strain was first plated for growth on agar slants. The cells were incubated for 24 hours and then harvested by pouring sterile distilled water into the plates and gently scraping with a wire loop. The cells were washed and centrifuged, and 0.1 g wet weight of each was re-suspended in 50 μL of 50 mM sodium acetate, pH 5.1. Exactly 12.5 μL of 0.5 M sucrose was added to the sample. This was followed by incubation at 37 °C for 10 minutes. The reaction was halted by the addition of 75 μL of 0.2 M K2HPO4, subsequently placed on ice, boiled for 3 minutes and again put on ice for 1 min. Colour reaction was commenced by adding 500 μL of the assay mix, which was made fresh by the addition of 50 μL of 5000 U mL−1 glucose oxidase, 62.5 μL of 1 mg mL−1 peroxidase, and 375 μL 10 mg mL−1 o-dianisidine into 25 mL of 0.1 M potassium phosphate, pH 7.0, as described by Harkness and Arnason.28 The sample was incubated at 37 °C for 10 minutes. This was followed by the addition of 500 μL 6 N HCl for colour development. The cell debris was pelleted and the amount of sucrose converted to glucose was measured using a spectrophotometer at 540 nm. The invertase activity was measured as μM glucose converted per minute per 106 cells.Determination of alcohol dehydrogenase activities of palm wine yeast isolates from various local sources
Alcohol dehydrogenase (ADH) comprises a family of enzymes that catalyze the conversion of alcohol into aldehydes in many organisms. ADH plays an important role in alcohol detoxification and leads to the generation of carcinogenic acetaldehyde, which can be further converted into acetic acid by aldehyde dehydrogenase. Each of the test yeast strains was first plated for growth on agar slants. The cells were then incubated for 24 hours and harvested by pouring sterile distilled water into the plates and gently scraping with a wire loop. The cells were washed and centrifuged, and 0.1 g wet weight of each was re-suspended in a sucrose solution (4% w/v). The ADH assay working reagent based on the number of samples to be measured was prepared. This was done by mixing 60 μL assay buffer, 10 μL developer solution (1×), 5 μL NAD (SCP), 5 μL WST solution, and 10 μL substrate (Sigma-Aldrich). Ninety (90) μL of working reagent was mixed into each well of the 96-well plate containing diluted ADH-positive control, samples, and blank. The set-up was mixed well immediately, and the measurements were recorded at OD440 nm with 3 minute intervals, collecting data every 0.5 min. The absorbance values obtained were compared with a standard ADH calibration curve and then calculated.Determination of sugar tolerance properties of yeasts in increasing sugar concentrations (% w/v)
The procedure reported by Fakruddin et al.29 was employed for the observation of sugar tolerance in this study. A yeast Extract Peptone Dextrose Broth containing 10%, 15%, and 20% sugar (glucose) concentrations was prepared. Each McCartney containing 15 mL of YEPD liquid media with an appropriate concentration of salt and blank media was used as a control. Then each was inoculated with a half-loopful of yeast cells, and the initial optical density was measured at 600 nm, followed by incubation at 30 °C for 48 h. After 48 h of cell solution, the absorbance values were recorded at 600 nm. The increase in optical density in a flask was recorded as evidence of growth and tolerance.Determination of the alcohol tolerance properties of the yeasts
The procedures developed by Fakruddin et al.29 were employed for the observation of sugar tolerance in this study. The yeast Extract Peptone Dextrose Broth autoclaved under standard conditions was used for the determination of ethanol tolerance. The concentrations of absolute ethanol were varied from 5% to 20% (v/v) and then added to different flasks. The initial optical density of each flask was read using a spectrophotometer at 600 nm against the medium as blank. All cultures were incubated at 25 °C for 48 hours. The increase in optical density in a flask was recorded as evidence of growth. The concentration of alcohol at which the growth of yeasts was just inhibited was assessed as the ethanol tolerance of yeasts.Determination of total titratable acidity
The yeast Extract Peptone Dextrose Broth was autoclaved and inoculated with various yeast strains. Incubation in a shaker incubator at 250 °C, 150 rpm was carried out for 72 hours. The total titratable acidity was determined by the alkaline titration method.30 Exactly 5 mL of the hydrolysate was dispensed into a conical flask and 45 mL of distilled water was added to it and mixed well. About 3 drops of phenolphthalein were added as an indicator. This was well mixed and titrated against a dilute alkaline solution (0.1 N NaOH). The appearance of light brick red/purple colour marked the endpoint.Determination of pH tolerance levels of yeasts from local sources
The YEPD broth was prepared at different pH values (2.0 to 6.0). Each McCartney contained 25 mL of YEPD agar media, and was adjusted to different pH values 2.0, 3.0, 4.0, 5.0, and 6.0. Blank media was used as a control. Then each was inoculated with a loopful of yeast cells, and the initial optical density was measured at 600 nm, followed by incubation at 30 °C for 48 h. After 48 h, the cell density was further recorded at 600 nm for growth. The procedures have been previously used by Willaert and Nedovic.31Determination of killer toxin-producing potentials of all the yeast isolates
The Killer Toxin Production Capacity was determined by the method developed by Ribéreau-Gayon et al.32 Yeast Extract Peptone Dextrose Agar was prepared at a pH of 4.2, and 1 mL of sensitive yeast strain solution was aseptically inoculated into a fresh medium at a temperature of 35 °C before it solidifies. This was followed by spot streaking of the test yeast strain, incubation at room temperature for 48 hours, and observation of the zones and pattern of clearance.Yeasts with higher sugar, alcohol, and pH tolerance, and less killer toxin-producing potentials were selected and further identified using molecular techniques. The essence of selecting yeasts that have no or less killer toxin potentials is to avoid death of the yeast cells before completion of the fermentation process. This is very common with yeast cells with a high level of killer toxin expression.
Deoxyribonucleic acid (DNA) extraction
The genomic DNA was extracted using a DNA extraction kit of Zymo. A 24 h culture of the isolates was suspended in 200 μL isotonic buffer (PBS) in a Lysis Tube. Exactly 750 μL Ethylene Diamine Tetra-Acetate (EDTA), a Lysis Solution, was introduced into the tube and then processed at a speed of 3000×g for 5 min. The Lysis Tube was spun using a micro-centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 1 min. Exactly 400 μL of the supernatant was introduced into a Zymo-Spin TM IV Spin Filter in a collection tube, followed by centrifugation at 7000×g for 1 min. The fungal DNA binding buffer (1200 μL) was then added into a collection tube to the filtrate. Exactly 800 μL of the mixture was transferred to a Zymo-Spin TM IIC column and centrifuged at 10
000×g for 1 min. Exactly 400 μL of the supernatant was introduced into a Zymo-Spin TM IV Spin Filter in a collection tube, followed by centrifugation at 7000×g for 1 min. The fungal DNA binding buffer (1200 μL) was then added into a collection tube to the filtrate. Exactly 800 μL of the mixture was transferred to a Zymo-Spin TM IIC column and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 1 min. The centrifugation was repeated after discarding the flow from the collection tube. Exactly 200 μL of the DNA pre-wash buffer was then introduced to the Zymo-Spin TM IIC column in a separate collection tube followed by centrifugation at 10
000×g for 1 min. The centrifugation was repeated after discarding the flow from the collection tube. Exactly 200 μL of the DNA pre-wash buffer was then introduced to the Zymo-Spin TM IIC column in a separate collection tube followed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 1 min. Then 500 μL of Fungal DNA wash buffer was added to the Zymo-Spin TM IIC Column and centrifuged at 10
000×g for 1 min. Then 500 μL of Fungal DNA wash buffer was added to the Zymo-Spin TM IIC Column and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 1 min. This was transferred to a micro-centrifuge tube with the addition of 100 μL DNA elution buffer. This was centrifuged at 10
000×g for 1 min. This was transferred to a micro-centrifuge tube with the addition of 100 μL DNA elution buffer. This was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 30 seconds to elute the DNA.33 The integrity and quality of the extracted DNA was determined using 1.5% Agarose gel by size fractionation, as described by Obidi et al.33
000×g for 30 seconds to elute the DNA.33 The integrity and quality of the extracted DNA was determined using 1.5% Agarose gel by size fractionation, as described by Obidi et al.33
PCR amplification
The amplification by polymerase chain reaction (PCR) of the fungal DNA (18S rDNA) genes was carried out following the method described by Obidi et al.33 The technique of Sodium acetate wash was employed for further amplicon purification before sequencing. ITS 4 (TCCTCCGCTTATTGATATGS) and ITS 5 (GGAAGTAAAAGTCGTAACAAGG) universal primers were used for the reaction.Identification of 18S rDNA sequences
The sequences obtained from the amplified 18 S rDNA were used for the identification of the fungal isolates by comparison with the GenBank database using BLAST (basic local alignment search tool). The representative sequences at the GenBank corresponding to the organisms that have been previously identified in the BLAST were used for the present identification.Development of syrup from date seeds
One kg of date fruits was first de-seeded manually and washed thoroughly with clean water before soaking in 3 liters of clean water for three hours to soften the fleshy fruit. After three hours, the samples were processed in batches with a maximum of about 500 g of the de-seeded date fruit flesh. Then, 50 grams of them were loaded in a big beaker (1000 mL capacity) and 500 mL of sterile water was added. The preparation was transferred to a water bath and the temperature was adjusted to 50 °C (the optimum temperature of the pectinase and cellulase enzymes to be used). Cellulase and pectinase (from the Biotech Department of FIIRO with activities of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units and 26
000 units and 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units, respectively) were added. Then, 10 mL of each of the enzymes were added at 50 °C and then maintained for 2 h 30 min before stopping the reactions. After cooling, the preparation was blended and sieved with a muslin cloth of about 0.027 inches (685.8 micrometres) diameter size, and the residues were filtered off. The syrup was packaged in bottles and kept at room temperature until use.
000 units, respectively) were added. Then, 10 mL of each of the enzymes were added at 50 °C and then maintained for 2 h 30 min before stopping the reactions. After cooling, the preparation was blended and sieved with a muslin cloth of about 0.027 inches (685.8 micrometres) diameter size, and the residues were filtered off. The syrup was packaged in bottles and kept at room temperature until use.
Determination of the chemical composition of the date fruit, honey, and the wines
The chemical composition analysis of the fruits and the wines was performed using the method of the Association of Analytical Chemistry (AOAC).34 to determine moisture, ash, fibre, protein, fat, and carbohydrate contents.Energy value
The total energy (in kcal) was estimated according to the following relation:35| Energy value (kcal/100 g) = (% carbohydrate) × 4 + (% protein) × 4 + (% crude fat) × 9 |
Inoculum propagation
The inoculum development was accomplished at 28 °C in a yeast extract peptone dextrose (YEPD) broth medium (Oxoid). A loopful of each of the six isolates was inoculated with 10 mL of sterile YEPD broth in test tubes, and after 48 h of incubation, the culture broths were transferred into 250 mL Erlenmeyer flasks containing 100 mL of sterile YEPD broth. The YEPD culture broths were incubated in a rotary shaker incubator rotating at 125 rpm. Yeast cells harvested were centrifuged at 5000 g for 4 minutes at 4 °C. The harvested yeast cells were washed twice by suspending them in distilled water and then centrifuged for washing of the cell pellets, and the supernatants were decanted. The sediment of yeast cells was dissolved in distilled water using a vortex mixer and stored at 4 °C.36Supplementation of must/fermentation
Before pitching inoculums into Substrates, the must was supplemented with accessory nutrients of 1.3 g L−1 KH2PO4, 2.0 g L−1 NH4CL, 0.1 g L−1 MgSO4·7H2O, 0.1 g L−1 yeast extract, and 0.7 g L−1 K2HPO4. All the reagents were of analytical grade, and produced and marketed by SCP Science. This fermentation medium was sterilised at a temperature of 121 °C, 15 psi and a holding time of 15 minutes. The stock inoculum was serially diluted in sterilized distilled water and 10–8 test tubes were pitched into 1000 mL of must aseptically. The flasks were caped with cotton wool for the aerobic stage of fermentation, which enhances yeast growth. During the 3 days of aerobic fermentation, the samples were drawn from the flasks for analyses aseptically, after which, the flasks were corked with air-tight caps, which initiated anaerobic fermentation processes. The anaerobic fermentation lasted for 10 days, and the final products were also analysed and allowed to age.37The following experimental conditions were established during the process:
| S. no. | Date![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) honey ratio (v/v) honey ratio (v/v) |
Honey![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) date ratio date ratio |
Yeast isolates used in the fermentation |
|---|---|---|---|
| 1 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
— | Isolate 065 |
| 2 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
— | Isolate 065 |
| 3 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
— | Isolate 065 |
| 4 | 100 | — | Isolate 065 |
| 5 | — | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
Isolate 047 |
| 7 | — | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
Isolate 047 |
| 8 | — | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
Isolate 047 |
| 9 | — | 100 | Isolate 047 |
| Isolate 065: Saccharomyces cerevisiae, yeasts, Isolate 047: Candida tropicalis | |||
Total yeast cell counts during primary fermentation
The fermentation flask was properly shaken and 10 mL of the fermented product must be withdrawn from the flask aseptically. The sample was serially diluted and 0.1 mL of 10−8 was plated on a sterile Petri dish. Semi-solid YEPD agar at a temperature ≤40 °C was aseptically poured into the plate and gently shaken to mix properly and allowed to solidify, followed by incubation at 28 °C for 24 h. From the number of colonies counted, the microbial load can be estimated by calculating the colony forming unit per mL of the sample.38Physicochemical parameters
All the physicochemical analyses were carried out at a temperature of 20 °C, which included pH, total titratable acidity, specific gravity, alcohol content, and sugar content.pH determination
The pH was measured using a pH meter (pH 211 microprocessor, Hanna Instruments). Then, 5–10 mL of sample was dispensed into a beaker and the pH meter was dropped into the beaker. The reading of the pH meter was taken when it was stable.Determination of changes in ethanol content during fermentation
The methods reported earlier by Sayyad and coworkers53 were adopted with slight modification. The dichromate reagent required for study was prepared by dissolving 40 g potassium dichromate in approximately 200 mL of distilled water. Then 270 mL concentrated H2SO4 added cautiously, the resultant solution cooled and volume is adjusted to 500 mL by adding sufficient volume of distilled water.Dichromate oxidation and spectrophotometric analysis
First, 3 mL of wine samples were transferred to a well labelled tube and mixed with 3 mL freshly prepared predichromate reagent by shaking at 150 rpm for 10 minutes.Then, a lower layer was separated and subjected to measurement of absorbance at 595 nm using a spectrophotometer (Shimadzu-1800).
Total titratable acid (TTA) as tartaric acid
A sample of 20 mL was dispensed into a 100 mL conical flask and carbon(IV) oxide was removed by vigorously stirring the sample. Then, 1–2 drops of phenolphthalein indicator were added into the sample, and 50 mL of 0.1 moles of NaOH was poured into the burette and titrated against the sample until the sample turned pink. The volume of the remaining NaOH in the burette – ‘the titer’ was recorded. The TTA was calculated as a percentage of tartaric acid. Then, 1 mL of 0.1 NaOH represents 0.075 g tartaric acid. The total titratable acidity (TTA) is expressed in percentage.39Determination of flavour compounds in the wines using GC-MS
The samples of wines for GC-MS analyses were collected from the experimental cellar and progressed for extraction. An aliquot of 400 μL of wine sample or standard (calibration curve) and 10 μL of C13 (internal standard at 1.28 g L−1 diluted at 50% (v/v) in ethanol) were used for analysis at the World Bank Step B laboratory of the Federal Institute of Industrial Research Oshodi, Lagos-Nigeria. The Method has been previously reported by Gómez-Ariza et al.52Chromatographic conditions
Three microliters of a derivatized sample were injected in split (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) mode into a GC system, SCHIMADZU QP2010 – Ultra, equipped with a Rescekti TM column (60 m × 0.23 mm × 0.25 μm df). Helium was used as the carrier gas at a constant flow of 1.0 mL min−1. The compounds were detected using a mass selective detector. The ionic source and interface temperature was 280 °C, against a threshold of 2200. The MSD data were acquired in the electronic ionization scan mode at 70 eV within the range of 35–650 amu after a solvent delay of 3.0 min and then analyzed using a Chemstation.
10) mode into a GC system, SCHIMADZU QP2010 – Ultra, equipped with a Rescekti TM column (60 m × 0.23 mm × 0.25 μm df). Helium was used as the carrier gas at a constant flow of 1.0 mL min−1. The compounds were detected using a mass selective detector. The ionic source and interface temperature was 280 °C, against a threshold of 2200. The MSD data were acquired in the electronic ionization scan mode at 70 eV within the range of 35–650 amu after a solvent delay of 3.0 min and then analyzed using a Chemstation.
Specific gravity (SG)
The specific gravity was measured using a specific gravity bottle. The empty specific gravity bottle was weighed, filled with 50 mL of distilled water, and reweighed. The bottle was refilled again with 50 mL sample and weighed. The specific gravity of each must was measured using a specific gravity bottle.39Alcohol content
To estimate the final alcoholic content of the final product, write the values of the specific gravity of must and final product omitting the decimal point. Then, subtract the value of the final product specific gravity from the value of must SG; divide the difference by 7.4 (a constant).Sugar content
The method employed to ascertain the residual sugar left after fermentation was adopted by Oti.40 A handheld refractometer was used in measuring the residual sugar in brix (B0). One or two drops of sample were placed on the glass surface of the refractometer and then viewed in the presence of light. When properly adjusted, the calibration of the refractometer indicates the amount present.Determination of the concentrations of elements present in honey and date syrup
To avoid organic impurities and prevent interference during analysis, each 20 mL volume sample was digested using 2.0 mL conc. HNO3 in a 250 mL conical flask placed on a fume cupboard. The samples were covered and heated on a hot plate until the solution was reduced to 10 mL. This was allowed to cool and made up to mark with distilled water before filtering into a 50 mL standard flask, labeled and kept ready for analysis. The blank constituted 5% HNO3. The Atomic Absorption Spectroscopy (AAS) instrument (SCHIMAZU-AA-7000) consisted of a hollow cathode lamp with a slit width of 0.7 nm, and an air acetylene flame was used for this work. The samples were analyzed for sodium, potassium, magnesium, calcium, manganese, copper, and zinc.Sensory data collection
Blind wine tasting data were provided from an experimental part of a sensory evaluation of six different wines produced from various combinations of date syrup and honey, and fermented with different yeast isolates. In this primary research were thirty known wine tasters. The purpose was to identify the wines based on their aromatic characteristics and physical properties. The first six wines were the result of the comparison process of fermentation with yeasts (Saccharomyces cerevisiae and Candida tropicalis), and varied substrates. The 7th wine was commercial. A form was given to the wine tasters about various physical and aromatic properties, after explaining to them how to carry out the study and well guided in the area of filling the forms.Results
Table 1 presents the population of yeast in various palm wine samples from different locations. The result shows that the population of yeast ranged from 9.6 × 108 cfu mL−1 (BADAGRY) to 1.74 × 104 cfu mL−1 (ISUOCHI).| S. no. | Sample | Sample code | Number of colonies | Dilution factor | CFU mL−1 |
|---|---|---|---|---|---|
| 1 | ISUOCHI | IPA | 174 | 102 | 1.74 × 104 |
| 2 | ISUOCHI | IPA | 81 | 105 | 8.1 × 106 |
| 3 | ISUOCHI | IPA | 56 | 107 | 5.6 × 108 |
| 4 | BADAGRY | BFC | 181 | 105 | 1.8 × 107 |
| 5 | BADAGRY | BFC | 96 | 107 | 9.6 × 108 |
| 6 | PAPALANTO | PPE | 195 | 105 | 1.95 × 107 |
| 7 | PAPALANTO | PPE | 79 | 107 | 7.9 × 108 |
The invertase and alcohol dehydrogenase activities of the various yeasts isolated from the different palm wine sources are presented in Table 2. The result shows that the isolate BFC 065 recorded the highest invertase activity (40.95 μmol min−1), followed by isolate PPE 047 (36.84 μmol min−1), while IPA 151 was the least (4.3 μmol min−1). The result of the alcohol dehydrogenase activity shows that PPE 047 had the highest activity (13.67 unit per mL), followed by BFC 065 (11.42 unit per mL), while IPA 151 had the least activity (2.4 unit per mL).
| S. no. | Isolate code | Invertase activities (μmol min−1) | Alcohol dehydrogenase (unit per mL) |
|---|---|---|---|
| 1 | IPA 110 | 12.65 | 7.5 |
| 2 | IPA 111 | 13.11 | 9.0 |
| 3 | IPA 131 | 6.5 | 4.7 |
| 4 | IPA 142 | 7.8 | 4.2 |
| 5 | IPA 149 | 9.2 | 5.0 |
| 6 | IPA 151 | 4.3 | 2.4 |
| 7 | IPA 160 | 5.6 | 3.6 |
| 8 | BFC 065 | 40.95 | 11.42 |
| 9 | BFC 168 | 22.60 | 8.0 |
| 10 | PPE 047 | 36.84 | 13.67 |
| 11 | PPE 049 | 18.64 | 6.5 |
| 12 | PPE 063 | 6.70 | 6.9 |
Table 3 presents the sugar tolerance properties of the yeast isolates. The result shows that isolate 065 and 047 have the highest sugar tolerance level at 20% sugar concentration with optical densities (OD) of 1.098 and 0.947, respectively, after 72 h of incubation. In contrast, the isolate BFC 168 showed the least sugar tolerance level (OD, 0.674).
| S. no. | Isolate code | Optical density (10%) | Optical density (15%) | Optical density (20%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 72 | 0 | 24 | 72 | 0 | 24 | 72 | ||
| 1 | IPA 110 | 0.246 | 0.324 | 0.946 | 0.301 | 0.624 | 0.947 | 0.209 | 0.604 | 0.920 |
| 2 | IPA 111 | 0.212 | 0.336 | 0.847 | 0.147 | 0.426 | 0.861 | 0.216 | 0.512 | 0.809 |
| 3 | IPA 131 | 0.284 | 0.406 | 0.744 | 0.316 | 0.517 | 0.812 | 0.512 | 0.627 | 0.817 |
| 4 | IPA 142 | 0.286 | 0.926 | 1.118 | 0.360 | 0.860 | 1.201 | 0.209 | 0.747 | 0.947 |
| 5 | IPA 149 | 0.333 | 0.947 | 1.096 | 0.114 | 0.474 | 0.920 | 0.216 | 0.336 | 0.880 |
| 6 | IPA 151 | 0.267 | 0.804 | 0.926 | 0.203 | 0.616 | 0.849 | 0.301 | 0.606 | 0.812 |
| 7 | IPA 160 | 0.382 | 0.674 | 0.920 | 0.186 | 0.616 | 0.849 | 0.226 | 0.747 | 0.801 |
| 8 | BFC 065 | 0.202 | 0.696 | 1.980 | 0.313 | 0.575 | 1.474 | 0.247 | 0.847 | 1.098 |
| 9 | BFC 168 | 0.147 | 0.464 | 0.987 | 0.314 | 0.624 | 0.986 | 0.222 | 0.475 | 0.674 |
| 10 | PPE 047 | 0.240 | 0.614 | 1.784 | 0.227 | 0.616 | 1.394 | 0.281 | 0.514 | 0.947 |
| 11 | PPE 049 | 0.116 | 0.374 | 1.860 | 0.260 | 0.485 | 0.677 | 0.287 | 0.620 | 0.801 |
| 12 | PPE 063 | 0.240 | 0.610 | 1.901 | 0.118 | 0.420 | 0.810 | 0.074 | 0.507 | 0.894 |
The ethanol tolerance potentials of the yeast isolated from various palm wine sources are presented in Table 4. The result showed that the highest ethanol tolerance was observed for BFC 065 (17.5% ± 0.18%), followed by PPE 047 (14.00% ± 0.81%), while IPA 142 showed the least (4.5% ± 0.47%).
| S. no. | Isolate code | Highest ethanol tolerance (%) |
|---|---|---|
| 1 | IPA 110 | 10.7 ± 2.50 |
| 2 | IPA 111 | 6.5 ± 2.00 |
| 3 | IPA 131 | 5.0 ± 0.90 |
| 4 | IPA 142 | 4.5 ± 0.47 |
| 5 | IPA 149 | 7.5 ± 0.35 |
| 6 | IPA 151 | 11.0 ± 0.92 |
| 7 | IPA 160 | 12.0 ± 0.40 |
| 8 | BFC 065 | 17.50 ± 0.18 |
| 9 | BFC 168 | 12.50 ± 0.75 |
| 10 | PPE 047 | 14.00 ± 0.81 |
| 11 | PPE 049 | 11.00 ± 0.26 |
| 12 | PPE 063 | 10.50 ± 0.54 |
The total titratable acidity (TTA) producing potential of the yeasts shows that the production of acid ranged from 0.27% ± 0.004% (IPA 137) to 3.21% ± 0.144% (BFC 065). The highest acid-producing potential was observed for BFC 065 (3.21% ± 0.144%), followed by PPE 047 (2.16% ± 0.35%), while IPA 131 had the least (0.27% ± 0.00%) (Table 5).
| S. no. | Isolate code | TTA (mL) |
|---|---|---|
| 1 | IPA 110 | 0.68 ± 0.80 |
| 2 | IPA 111 | 1.22 ± 0.354 |
| 3 | IPA 131 | 0.27 ± 0.00 |
| 4 | IPA 142 | 0.54 ± 0.714 |
| 5 | IPA 149 | 0.97 ± 0.384 |
| 6 | IPA 151 | 0.86 ± 0.141 |
| 7 | IPA 160 | 0.58 ± 0.344 |
| 8 | BFC 065 | 3.21 ± 0.144 |
| 9 | BFC 160 | 0.95 ± 0.453 |
| 10 | BFC 168 | 0.85 ± 0.244 |
| 11 | PPE 047 | 2.16 ± 0.354 |
| 12 | PPE 168 | 1.09 ± 0.816 |
| 13 | PPE 168 | 0.35 ± 0.274 |
| 14 | PPE 063 | 0.49 ± 0.454 |
The highest pH tolerance of the yeasts was observed at pH 2 as shown in Table 6. The isolate BFC 168 tolerated the lowest pH (pH2) with OD 0.404, followed by BFC 065 (OD. 0.347), IPA 131 (OD, 0.314), PPE 049 (OD, 0.311), and then PPE 047 (OD, 0.287), while IPA 110 (OD, 0.115) showed the least tolerance.
| S. no. | Isolate code | O.Da | ||||
|---|---|---|---|---|---|---|
| pH 2.0 | pH 3.0 | pH 4.0 | pH 5.0 | pH 6.0 | ||
| a O.D = Optical densities recorded at a particular pH. | ||||||
| 1 | IPA 110 | 0.115 | 0.286 | 0.747 | 0.926 | 0.847 |
| 2 | IPA 111 | 0.204 | 0.321 | 0.547 | 0.641 | 0.780 |
| 3 | IPA 131 | 0.314 | 0.216 | 0.620 | 0.778 | 0.814 |
| 4 | IPA 142 | 0.151 | 0.261 | 0.320 | 0.450 | 0.634 |
| 5 | IPA 149 | 0.226 | 0.245 | 0.360 | 0.471 | 0.364 |
| 6 | IPA 151 | 0.316 | 0.404 | 0.517 | 0.810 | 0.840 |
| 7 | IPA 160 | 0.320 | 0.450 | 0.620 | 0.711 | 0.926 |
| 8 | BFC 065 | 0.347 | 0.386 | 0.629 | 1.096 | 0.985 |
| 9 | BFC 168 | 0.404 | 0.414 | 0.526 | 0.810 | 0.995 |
| 10 | PPE 047 | 0.287 | 0.426 | 0.715 | 1.371 | 1.086 |
| 11 | PPE 049 | 0.311 | 0.426 | 0.515 | 0.620 | 0.745 |
| 12 | PPE 063 | 0.216 | 0.314 | 0.616 | 0.721 | 0.740 |
Fig. 1 shows the agarose gel electrophoresis, indicating the positive amplification of the fungal samples using the ITS universal primers. The results indicate that the base pair (bp) of the amplified genes of the organisms was above 500 bp.
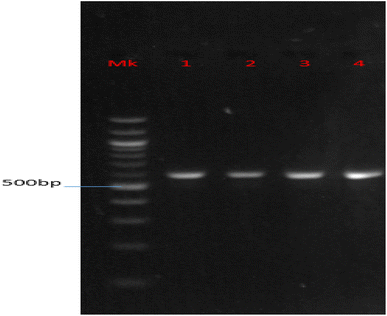 | ||
| Fig. 1 Agarose gel electrophoresis indicating the positive amplification of the fungal samples using ITS universal primers. | ||
The nucleotide sequences of the selected isolates (PPE 047, IPA 110, BFC 065, and IPA 131) are presented in Table 7.
| Isolate code | Sequences |
|---|---|
| Isolate PPE 047 | CCATACTGATTTGCTTAATTGCACCACATGTGTTTTTTATTGAACAAATTTCTTTGGTGGCGGGAGCAATCCTACCGCCAGAGGTTATAACTAAACCAAACTTTTTATTTACAGTCAAACTTGATTTATTATTACAATAGTCAAAACTTTCAACAACGGATCTCTTGGTTCTCGCATCGATGAAGAACGCAGCGAAATGCGATACGTAATATGAATTGCAGATATTCGTGAATCATCGAATCTTTGAACGCACATTGCGCCCTTTGGTATTCCAAAGGGCATGCCTGTTTGAGCGTCATTTCTCCCTCAAACCCCCGGGTTTGGTGTTGAGCAATACGCTAGGTTTGTTTGAAAGAATTTAACGTGGAAACTTATTTTAAGCGACTTAGGTTTATCCAAAAACGCTTATTTTGCTAGTGGCCACCACAATTTATTTCATAACTTTGACCTCAAATCAGGTAGGACTACCCGCTGAACTTAAGCATATCCAT |
| Isolate IPA 110 | CCACATTTTGCATACACACTGATTTGGATTTTAAAACTAACCCAACGTTAAGTTCAACTAAAACAAAAACATAAAACTTTCAACAACGGATCTCTTGGTTCTCGCATCGATGAAGAACGCAGCGAAATGCGATACGTAGTATGACTTGCAGACGTGAATCATCGAATCTTTGAACGCACATTGCGCCTTGGGGTATTCCCCAAGGCATGCCTGTTTGAGCGTGATGTCTTCTCACCAATCTTCGCGGTGGCGTTGCATTCACAAAATTACAGCTTGCACGAAAAAAATCTACGCTTTTTTTTTCGTTTTGTTGTCGCCTCAAATCAGGTAGGACTACCCGCTGAACTTAAGCATATCAATAAGCGGAGGAAAAGAAACCAACAGGGATTGCCTCAGTAACGGCGAGTGAAGCGGCAAGAGCTCAACTTTGGAATCGCTCCGGCGAGTTGTAGTCTGGAGGTGGCCACCACGAGGTGTTCTAGCAGCAGGCAAGTCCTTTGGAACAAGGCGCCAGCGAGGGTGACAGCCCCGTACCTGCTTTTGCTAGTGCTTCCTGTGGCCCACCGACGAGTCGAGTTGTTTGGGAATGCAGCTCTAAGTGGGTGGCCATT |
| Isolate BFC 065 | CACTAATAATTTTGAAAATGGATTTTTTTGTTTTGGCAAGAGCATGAGAGCTTTTACTGGGCAAGAAGACAAGAGATGGAGAGTCCAGCCGGGCCTGCGCTTAAGTGCGCGGTCTTGCTAGGCTTGTAAGTTTCTTTCTTGCTATTCCAAACGGTGAGAGATTTCTGTGCTTTTGTTATAGGACAATTAAAACCGTTTCAATACAACACACTGTGGAGTTTTCATATCTTTGCAACTTTTTCTTTGGGCATTCGAGCAATCGGGGCCCAGAGGTAACAAACACAAACAATTTTATCTATTCATTAAATTTTTGTCAAAAACAAGAATTTTCGTAACTGGAAATTTTAAAAATATTAAAAACTTTCAACAACGGATCTCTTGGTTCTCGCATCGATGAAGAACGCAGCGAAATGCGATACGTAATGTGAATTGCAGAATTCCGTGAATCATCGAATCTTTGAACGCACATTGCCCCCTTGGTATTCCAGGGGGCATGCCTGTTTGAGCGTCATTTCCTTCTCAAACATTCTGTTTGGTAGTGAGTGATACTCTTTGGAGTTAACTTGAAATTGCTGGCCTTTTCATTGGATGTTTTTTTTCCAAAGAGAGGTTTCTCTGCGTGCTTGAGGTATAATGCAAGTACGGTCGTTTTAGGTTTTACCAACTGCGGCTAATCTTTTTTATACTGAGCGTATTGGAACGTTATCGATAAGAAGAGAGCGTCGCATT |
| Isolate IPA 131 | CACTAATAATTTTGAAAATGGATTTTTTTGTTTTGGCAAGAGCATGAGAGCTTTTACTGGGCAAGAAGACAAGAGATGGAGAGTCCAGCCGGGCCTGCGCTTAAGTGCGCGGTCTTGCTAGGCTTGTAAGTTTCTTTCTTGCTATTCCAAACGGTGAGAGATTTCTGTGCTTTTGTTATAGGACAATTAAAACCGTTTCAATACAACACACTGTGGAGTTTTCATATCTTTGCAACTTTTTCTTTGGGCATTCGAGCAATCGGGGCCCAGAGGTAACAAACACAAACAATTTTATCTATTCATTAAATTTTTGTCAAAAACAAGAATTTTCGTAACTGGAAATTTTAAAAATATTAAAAACTTTCAACAACGGATCTCTTGGTTCTCGCATCGATGAAGAACGCAGCGAAATGCGATACGTAATGTGAATTGCAGAATTCCGTGAATCATCGAATCTTTGAACGCACATTGCCCCCTTGGTATTCCAGGGGGCATGCCTGTTTGAGCGTCATTTCCTTCTCAAACATTCTGTTTGGTAGTGAGTGATACTCTTTGGAGTTAACTTGAAATTGCTGGCCTTTTCATTGGATGTTTTTTTTCCAAAGAGAGGTTTCTCTGCGTGCTTGAGGTATAATGCAAGTACGGTCGTTTTAGGTTTTACCAACTGCGGCTAATCTTTTTTATACTGAGCGTATTGGAACGTTATCGATAAGAAGAGAGCGTCGCATT |
Table 8 presents the identification of the organisms using polymerase chain reaction (PCR) amplification products and sequences of the isolates. The organisms were identified as Candida tropicalis Pe 1 (PPE 047), Saccharomyces cerevisiae CBS1171 (BFC 065), Candida auris (IPA110) and Saccharomyces cerevisiae B1B2 (IPA 131).
| S. no. | Isolate code | Percentage similarity | Identity to strain level | Accession number |
|---|---|---|---|---|
| 1 | PPE 047 | 100 | Candida tropicalis Pe 1 | MK752669.1 |
| 2 | BFC 065 | 100 | Saccharomyces cerevisiae strain CBS 1171 | NR111007.1 |
| 3 | IPA110 | 99 | Candida auris | CP043535.1 |
| 4 | IPA 131 | 99.72 | Saccharomyces cerevisiae strain B1B2 |
Table 9 presents the composite values of the date syrup and honey. The result shows that the energy level of dates was 303.36 kJ, while that of honey was 335.69 kJ, the protein contents were 2.37% and 1.09%, respectively, while the carbohydrate contents were 72.21% and 82.09%, respectively.
| Parameters | Date | Honey |
|---|---|---|
| pH | 4.05 | 4.4 |
| Total reducing sugar | 71.90 | 64.0 |
| Crude fiber | 0.06 | 0.03 |
| Ash | 1.8 | 0.78 |
| Fat content | 0.56 | 0.33 |
| Protein content | 2.37 | 1.09 |
| Moisture content | 23.0 | 16.79 |
| Carbohydrate content | 72.21 | 82.09 |
| Energy level | 303.36 | 335.69 |
The essential element compositions of the date and honey are presented in Table 10. The result shows that dates and honey contain calcium levels of 468.90 and 3.67, respectively, magnesium levels of 117.8 and 2.98, respectively, and iron levels of 29.50 and 1.34, respectively, while copper and zinc were not detected in honey.
| Parameters | Dates/mg | Honey/mg |
|---|---|---|
| Ca | 468.90 | 3.67 |
| Mg | 1137.8 | 2.98 |
| K | 1420.0 | 45.50 |
| Na | 747.96 | 4.45 |
| Iron | 29.50 | 1.34 |
| Copper | 0.114 | — |
| Manganese | 0.540 | 1.08 |
| Zinc | 1.980 | — |
Table 11 presents the changes in pH, specific gravity, soluble sugar level, and total titratable acidity (TTA) during wine production using yeast isolates 047 and 065, respectively. The result shows a gradual decrease in soluble sugar (0Brix), pH, and specific gravity of the various wines, while TTA increased with the fermentation time.
| Sample code/days | Soluble sugar, 0Brix | pH value | Specific gravity | TTA (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| 047 | 065 | 047 | 065 | 047 | 065 | 047 | 065 | |
| a D/H = date–honey ratio. | ||||||||
| Day 1 | ||||||||
| Date (100%) | 16 | 16 | 5.38 | 5.35 | 1.065 | 1.065 | 1.83 (5.41%) | 1.84 (5.52%) |
| D/H 30/70 | 16 | 16 | 5.35 | 5.36 | 1.065 | 1.065 | 1.86 (5.58%) | 1.85 (5.54%) |
| D/H 40/60 | 16 | 16 | 5.34 | 5.35 | 1.065 | 1.065 | 1.85 (5.64%) | 1.87 (5.61%) |
| D/H 50/50 | 16 | 16 | 5.36 | 5.36 | 1.065 | 1.065 | 1.87 (5.61%) | 1.86 (5.58%) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Day 2 | ||||||||
| Date (100%) | 10.5 | 11.5 | 4.85 | 4.97 | 1.047 | 1.048 | 2.82 (8.48%) | 2.74 (8.20%) |
| D/H 30/70 | 13.5 | 14 | 5.01 | 5.16 | 1.054 | 1.056 | 2.30 (6.91%) | 2.38 (6.83%) |
| D/H 40/60 | 12.0 | 13.5 | 4.94 | 5.04 | 1.053 | 1.054 | 2.45 (7.36%) | 2.33 (6.98%) |
| D/H 50/50 | 11.5 | 12.5 | 4.91 | 5.0 | 1.048 | 1.053 | 2.67 (8.02%) | 2.36 (7.09%) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Day 3 | ||||||||
| Date (100%) | 8.5 | 10 | 4.78 | 4.88 | 1.032 | 1.045 | 2.95 mL | 2.93 mL |
| D/H 30/70 | 12.5 | 13 | 4.97 | 4.96 | 1.054 | 1.055 | 2.735 mL | 2.70 mL |
| D/H 40/60 | 11.0 | 12 | 4.87 | 4.93 | 1.047 | 1.053 | 2.87 mL | 2.66 mL |
| D/H 50/50 | 10.0 | 11 | 4.86 | 4.90 | 1.045 | 1.047 | 2.88 mL | 2.83 mL |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Day 4 | ||||||||
| Date (100%) | 7.0 | 8.5 | 4.66 | 4.70 | 1.028 | 2.032 | 3.145 (9.49%) | 3.11 (934%) |
| D/H 30/70 | 11.0 | 11.0 | 4.68 | 4.88 | 1.047 | 2.047 | 2.86 (8.59%) | 2.93 (8.80%) |
| D/H 40/60 | 9.5 | 10.5 | 4.72 | 4.86 | 1.037 | 1.046 | 3.02 (9.08%) | 2.95 (8.89%) |
| D/H 50/50 | 9.0 | 10.0 | 4.70 | 4.80 | 1.034 | 1.045 | 3.06 (9.19%) | I2.98 (8.95%) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Day 5 | ||||||||
| Date (100%) | 5.8 | 7.0 | 4.46 | 4.70 | 1.023 | 1.028 | 3.43 (10.30%) | 3.20 (9.62%) |
| D/H 30/70 | 8.0 | 9.5 | 4.67 | 4.76 | 1.034 | 1.045 | 3.20 (9.61%) | 3.16 (9.49%) |
| D/H 40/60 | 7.0 | 9.0 | 4.64 | 4.72 | 1.028 | 1.034 | 3.26 (9.79%) | 3.14 (9.43%) |
| D/H 50/50 | 6.5 | 8.0 | 4.60 | 4.70 | 1.025 | 1.030 | 3.29 (9.86%) | 3.24 (9.73%) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Day 6 | ||||||||
| Date (100%) | 4.4 | 6.5 | 4.06 | 4.43 | 1.018 | 1.035 | 3.71 (11.14%) | 3.46 (10.43%) |
| D/H 30/70 | 7.0 | 9.2 | 4.26 | 5.1 | 1.038 | 1.035 | 3.58 (10.75%) | 3.40 (10.21%) |
| D/H 40/60 | 5.5 | 8.0 | 4.16 | 4.42 | 1.023 | 1.030 | 3.63 (10.90%) | 3.53 (10.60%) |
| D/H 50/50 | 5.0 | 7.5 | 4.12 | 4.36 | 1.021 | 1.036 | 3.66 (10.99%) | 3.57 (10.72%) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Day 7 | ||||||||
| Date (100%) | 3.0 | 5.8 | 4.80 | 4.13 | 1.014 | 1.023 | 3.93 (11.80%) | 3.67 (11.02%) |
| D/H 30/70 | 5.3 | 8.0 | 4.06 | 2.30 | 1.022 | 1.030 | 3.77 (11.18%) | 3.58 (10.75%) |
| D/H 40/60 | 4.8 | 7.8 | 3.98 | 4.22 | 1.021 | 1.030 | 3.84 (11.53%) | 3.62 (10.87%) |
| D/H 50/50 | 4.0 | 7.4 | 3.71 | 4.16 | 1.016 | 1.029 | 3.89 (11.68%) | 3.74 (11.23%) |
Fig. 2 presents the specific gravities (SGs) of the various wines fermented with isolates 047 and 065. The specific gravities of wines fermented with the isolate 065 were greater than those of the isolate 065. It ranged from 0.9833 (date100%) and 0.9861 (date100%) to 0.9866 (D/H30/70) and 0.9903 (D/H40/60) for the isolates 047 and 065, respectively.
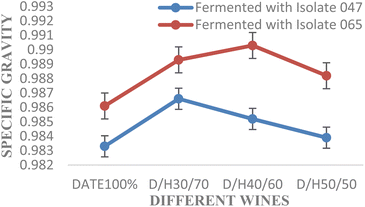 | ||
| Fig. 2 Changes in specific gravity during the timeline in wine production. Error bars represent standard error. D/H = date/honey ratio. | ||
Fig. 3 presents the percentage alcohol contents of the various wines using different isolates. The result shows that the alcohol content of the wines fermented with the isolate 047 was higher than that of the isolate 065. The alcohol content ranged from 7.54% (D/H30/70) and 9.65% (D/H30/70) to 9.98% (date100%) and 12.24% (date100%) for isolates 065 and 047, respectively.
 | ||
| Fig. 3 Changes in alcohol content during the timeline in wine production. Error bars represent percentage errors. D/H = date/honey ratio. | ||
Table 12 presents the changes in reducing sugar during fermentation of the wines. The result showed a gradual decrease in reducing sugar for the various wines using the two different isolates, 047 and 065.
| 0HR | 4th day | 7th day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date (100%) | D/H 30/70 | D/H 40/60 | D/H 50/50 | Date (100%) | D/H 30/70 | D/H 40/60 | D/H 50/50 | Date (100%) | D/H 30/70 | D/H 40/60 | D/H 50/50 | |
| Isolate 047 | 1622.3 | 1621.4 | 1621.8 | 1622.5 | 649.06 | 1083.81 | 894.60 | 876.25 | 209.17 | 368.70 | 333.27 | 289.21 |
| Isolate 065 | 1622.7 | 1621.8 | 1621.6 | 1622.3 | 835.27 | 1091.19 | 986.51 | 957.55 | 576.08 | 795.68 | 781.39 | 483.81 |
Table 13 presents the flavor compounds found in the best three of the developed wines identified using GC-MS. The result showed that hexadecanoic acid, oleic acid, octadecenoic acid, and methyl ester were present in all the wines, while cis-vaccenic acid was present only in D/H40/60 fermented with the isolate 065.
Date![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) honey 70 honey 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 Isolate 065 30 Isolate 065 |
Date![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) honey 70 honey 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 Isolate 047 30 Isolate 047 |
Date![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) honey 40 honey 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 Isolate 065 60 Isolate 065 |
|---|---|---|
| □ Hexadecanoic acid, methyl ester | □ 2,4-Di-tert-butylphenol | □ Dodecanoic acid |
| □ n-Hexadecanoic acid | □ 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | □ Tetradecanoic acid |
| □ 11-Octadecenoic acid, methyl ester | □ Phthalic acid | □ Tetradecanoic acid |
| □ 9,17-Octadecadienal, (Z)- | □ Decyl isobutyl ester | □ p-Heptylbenzonitrile |
| □ 10-Octadecenoic acid, methyl ester | □ Hexadecanoic acid, ethyl ester | □ Oxacycloundecane-2,7-dione |
| □ 9,17-Octadecadienal, (Z)- | □ Methyl 9-cis,11-trans-octadecadienoate | □ 9-Octadecenoic acid (Z)-, methyl ester |
| □ cis-13-Octadecenoic acid | □ 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | □ Oleic acid |
| □ Oleic acid | □ 8,11-Octadecadienoic acid, methyl ester | □ 9-Octadecenoic acid, (E)- |
| □ cis-13-Octadecenoic acid | □ 9-Octadecenoic acid (Z)-, methyl ester | □ cis-Vaccenic acid |
| □ 9,12-Octadecadienoic acid (Z,Z)- | □ cis-13-Octadecenoic acid, methyl ester | □ 9-Octadecenoic acid |
| □ 9,17-Octadecadienal, (Z)- | □ 11-Octadecenoic acid, methyl ester | □ Oleic acid |
| □ Octadecanoic acid | □ Phytol | □ cis-Vaccenic acid |
| □ Methyl hexadecane, 1-iodo-tricosane | □ D-Glucitol, cyclic 1, s(ethylboronate) | |
| □ 2-methyl-bis(2-ethylhexyl) phthalate | □ Cholestan-22(26)-cholestan-22(26)-epoxy | |
| □ Diisooctyl phthalate | □ Aluminum, bis(2-methylpropyl) (2,4- pentanedionato-O,O′)-, (T-4)- | |
| □ Carbamic acid, N-(3-chloro-4-methoxyphenyl)-, glycidyl ester | ||
| □ 4-Piperidinone, 1,3-dimethyl- | ||
| □ 3-Dibenzofuranamine | ||
| □ Lauric anhydride | ||
| □ 1,2-Cyclohexanedicarboxylic acid | ||
| □ Ethyl hexyl ester | ||
| □ Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | ||
| □ Lauric anhydride |
The sensory evaluation of the wines is presented in Table 14. The commercial wine was ranked first, wine 2 was ranked second, while wine 1 was ranked sixth.
| Attributes | Wine 1 | Wine 2 | Wine 3 | Wine 4 | Wine 5 | Wine 6 | Commercial wine |
|---|---|---|---|---|---|---|---|
| Colour/apperance | 8 | 8 | 8 | 8 | 8 | 6 | 8 |
| Taste | 8 | 6 | 8 | 8 | 7 | 4 | 8 |
| After taste | 7 | 6 | 6 | 7 | 8 | 6 | 8 |
| Aroma | 6 | 8 | 7 | 8 | 7 | 6 | 8 |
| Flavour | 6 | 9 | 7 | 9 | 6 | 5 | 9 |
| Full-bodied | 6 | 9 | 7 | 9 | 6 | 5 | 9 |
| Sweetness | 7 | 6 | 8 | 7 | 7 | 3 | 9 |
| Brightness | 8 | 9 | 6 | 8 | 7 | 3 | 8 |
| Sourness | 8 | 8 | 6 | 8 | 7 | 3 | 8 |
| Astringency | 7 | 6 | 8 | 8 | 7 | 6 | 7 |
| Aromatic intensity | 6 | 8 | 8 | 9 | 8 | 7 | 8 |
| Overall acceptibility | 7 | 8 | 8 | 8 | 8 | 6 | 9 |
| Ranking | 6th | 2nd | 4th | 3rd | 5th | 7th | 1st |
Discussion
The process that leads to the conversion of fruits into wine involves a complex interplay of biochemical and ecological processes involving yeasts as fermenting organisms. The yeasts, during fermentation, convert the sugar present in the substrates to alcohol and other aromatic compounds, leading to the final characteristics of the wine.8,11 In the present study, yeasts from different palm wine sources were screened, selected and used to produce different combinations of wines from date fruit and honey blends.The population of yeasts in various palm wine samples from different locations shows that the population of yeast ranged from 9.6 × 108 cfu mL−1 (BADAGRY) to 1.74 × 104 cfu mL−1 (ISUOCHI). The report of Ukwuru and Awah41 indicated high presence of yeasts ranging from 3.7 to 4.8 log10 cfu mL−1, which increases with the increase in storage time of the palm wine. Moreover, Matthew et al.42 reported yeast count ranging from 3.2 × 108 cfu mL−1 to 1.0 × 106 cfu mL−1 in palm wine from different locations in their study ‘Molecular characterization of yeast isolated from palm wine in Alakalis, Rivers State, Nigeria’.
The invertase and alcohol dehydrogenase activities of the various yeasts isolated from different palm wine sources show that the isolate BFC 065 recorded the highest invertase activity (40.95 μmol min−1), followed by the isolate PPE 047 (36.84 μmol min−1), while IPA 151 showed the least (4.3 μmol min−1). The result of the alcohol dehydrogenase activity shows that PPE 047 had the highest activity (13.67 unit per mL), followed by BFC 065 (11.42 unit per mL), while IPA 151 had the least activity (2.4 unit per mL). Saccharomyces cerevisiae has been reported to produce invertase.43 Silveira et al.44 reported invertase production by organisms such as Neurospra crassa, Candida utilis, Fusarium oxysporium, Phytophthora megasperma, Aspergillus niger, Schwanniomyces occidentalis, Schizosaccharomyces pombe and Saccharomyces cerevisiae. Moreover, Saccharomyces cerevisiae has been reported to produce more invertase enzyme than other organisms.33 This corresponds to the present observation.
The sugar tolerance properties of the yeast isolates show that isolates 065 and 047 have the highest sugar tolerance level at 20% sugar concentration with optical densities (OD) of 1.098 and 0.947, respectively, after 72 h of incubation, while the isolate BFC 168 showed the least sugar tolerance level (OD, 0.674). This observation is an indication that the organisms are stable and shall not be inhibited by high sugar levels. It gives the organisms a competitive advantage over contaminating organisms that cannot withstand a higher sugar level.
In the present study, the ethanol tolerance potentials of the yeast isolates from various palm wine sources showed that the highest ethanol tolerance was observed for BFC 065 (17.5% ± 0.18%), followed by PPE 047 (14.00% ± 0.81%), while IPA 142 had the least (4.5% ± 0.47%). A similar observation has been reported by Ukwuru and Awah41 who demonstrated high alcohol tolerance of Saccharomyces cerevisiae from palm wine in the range of 14.7% to 17.2% (v/v). The property of ethanol tolerance in yeasts makes them useful when applied for industrial purposes.
The total titratable acidity (TTA) producing potential of the yeasts shows that the production of acid ranged from 0.27% ± 0.004% (IPA 137) to 3.21% ± 0.144% (BFC 065). The highest acid-producing potential was observed for BFC 065 (3.21% ± 0.144%), followed by PPE 047 (2.16% ± 0.35%), while IPA 131 was the least (0.27% ± 0.00%). The ability of the test yeasts to produce acids is an indication of active viability in the utilization of sugars.41
The highest pH tolerance of the yeasts was observed at pH 2. The isolate BFC 168 tolerated the lowest pH (pH 2), while IPA 110 was the least. The ability of yeasts from palm wine to tolerate low pH gives them an added advantage over contaminants that cannot withstand acidic conditions during wine production.
The pH of the wines fermented with the two yeast isolates was towards acidity. This was irrespective of the percentage mixture of the blends of dates and honey that the pH ranged from 3.78 to 5.38 in wines fermented with the isolate 047 (Candida tropicalis) and 2.30 to 5.36 in wines fermented with the isolate 065 (Saccharomyces cerevisiae). The pH values showed a gradual decrease with the increase in fermentation time. This observation is consistent with the report of Ogodo et al.8 on mixed fruit wines from pawpaw, banana and watermelon. Ogodo et al.9 observed a similar trend of gradual decrease in the pH of mango wine fermented with bakers' yeast (Saccharomyces cerevisiae). The pH has direct effects on the stability of wine, and low pH values during fermentation processes favour fermenting organisms (yeasts) and eliminate contaminants and spoilage bacteria.6,10 The report of Potey et al.45 also showed a gradual decrease in pH towards acidity during the production of banana wine using Saccharomyces cerevisiae. Moreover, low pH values have been reported earlier in some tropical fruit wines such as sapota wine,46 Tendu wine47 and sweet potato wine.48 There is no reported use of isolate 047 (Candida tropicalis) strains in the production of fruit wines and this novel study can further be exploited in wine production.
In the present study, there was a gradual increase in the total titratable acidity (TTA) as the fermentation time progressed. The TTA ranged from 5.42% to 11.68% in Candida tropicalis fermented wines and 5.52% to 11.23% in Saccharomyces cerevisiae fermented wines. The values obtained in this study for TTA are higher than those reported by Ogodo et al.8 and Ogodo et al.,9 which were in the range of 0.41–0.71% and 0.71–0.80% for mixed fruits of pawpaw, banana and watermelon wine and mango wine, respectively. Moreover, the observation in this study on acidity showed a higher value than that of bael wine,49 sweet potato wine48 and sapota wine.46 The progress of fermentation is favoured by high acidity because it gives the fermenting yeast an edge to withstand competition by other undesirable microorganisms.22
The soluble sugar contents of the wines during fermentation by the two isolates, 047 (Candida tropicalis Pe 1) and 065 (Saccharomyces cerevisiae strain CBS), showed a gradual decrease in soluble sugar (0Brix). The soluble sugar contents ranged from 3.00Brix (date100%) to 8.00Brix (D/H 30%/70%). There were significant differences (p < 0.05) when the reducing sugar of the wines fermented with the isolate 047 is compared to wines fermented with the isolate 065. The reduction in soluble sugar content was more in wines fermented with the isolate 047. This is an indication of the capability of isolate 047 in utilizing the fermentable sugars in the substrates. The observation in the present study can be compared to the report by Panda et al.46 and Sahu et al.47 who reported 3.28 g/100 mL and 3.78 g/100 mL for sapota wine and Tendu wine, respectively. However, the present observation is higher than that reported by Ray et al.48 for purple sweet potato wine and Panda et al.47 for bael wine. The result showed that the total sugar contents of the wines in this study are more than 1%, which shows that the wines could be classified as sweet table.
The percentage alcohol contents of the various wines using different isolates. The results of the percentage alcohol content of the various wines produced using different isolates show that the wines fermented with isolate 047 had higher alcohol content than those fermented with isolate 065. The alcohol content ranged from 7.54% (D/H30/70) and 9.65% (D/H30/70) to 9.98% (date100%) and 12.24% (date100%) for isolates 065 and 047, respectively.
Significant amounts of alcohol were produced from the fruit wines during fermentation with the test yeasts in the present study. The result shows that the alcohol content of the wines fermented with the isolate 047 was more than that of the isolate 065. The alcohol content ranged from 7.54% (D/H30/70) and 9.65% (D/H30/70) to 9.98% (date100%) and 12.24% (date100%) for isolates 065 and 047, respectively. This observation agreed with the finding that palm wine yeast isolates may show alcohol tolerance in the range of 10–20%.50 Similarly, a study by Noll (2008)7 showed that yeast strains from palm wine are genetically distinct when compared to the strains that ferment grapes during wine making, and they can survive and continue the fermentation process at an alcohol concentration of 18%. This attribute can be exploited in the production of ethanol for fuel. Moreover, the present study showed that isolate Candida tropicalis Pe 1 (047) produced more alcohol than Saccharomyces cerevisiae strain CBS, and this property can further be exploited in ethanol production. High alcohols have been reported to serve as precursors in pleasant aroma development through the production of esters (Clement-Jimenez et al. 2005).51 Similarly, Reddy and Reddy4 asserted that the concentration of alcohol contributes to the overall characteristic, quality and flavour of wine.
The good flavour obtained from the present wine could be due to the high content of alcohol51 or due to the presence of some aromatic compounds such as hexadecanoic acid, methyl ester, n-hexadecanoic acid, 11-octadecenoic acid, methyl ester, 9,17-octadecadienal, (Z)-10-octadecenoic acid, methyl ester, 9,17-octadecadienal, (Z)-cis-13-octadecenoic acid, and oleic acid, as identified from the various wines. These compounds are capable of imparting good aroma to the finished product.
The sensory attributes of the wines produced in the present study were compared favourably with commercial wines, which was ranked first, wine 2 was ranked second, while wine 1 was ranked sixth. This shows that acceptable wines can be produced from date fruit and honey blends.
Conclusion
The present study which was based on the evaluation of date fruit and honey as substrates for wine production and the efficiency of isolated yeasts from palm wine for mixed fruit wine production has revealed that the test substrates (date fruits and honey) are good for wine production. The biochemical and sensory attributes of the wines were acceptable by the consumers. The study has also given an insight into the efficacy and role of Candida tropicalis Pe 1 and Saccharomyces cerevisiae strain CBS from palm wine in wine production and alcohol production, which can be explored for industrial purposes especially with Candida tropicalis Pe 1. Therefore, nutrients, minerals, vitamins, aroma and taste of dates and honey could be preserved by fermenting them to wines.Data availability
The authors confirm that the data supporting the findings of this study are available within the manuscript.Author contributions
Ositadinma Chinyere Ugbogu and Alloysius Chibuike Ogodo conceived and designed the experiments; Amadike Eziuche Ugbogu, Kingsley Chukwuemeka Nwachukwu and Frank Anayo Orji analyzed the data; Alloysius Chibuike Ogodo wrote the original draft; and Ositadinma Chinyere Ugbogu reviewed and edited the final draft. All authors read and approved the final manuscript.Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
The authors wish to acknowledge the Tertiary Education Trust Fund (TETFUND) for providing funding to the research through the institution-based research (IBR).References
- F. Jacobs, Wine Making from Pineapple. Ihem Davis Press Ltd., Owerri, 2001, pp. 1–79 Search PubMed.
- F. Allen, Barley Wine. Anderson Valley Brewing Company, 2008, http://web.archive.org/web/20080227015623/http://www.avbc.com/news/Bw.html, accessed 24/11/2024 Search PubMed.
- P. J. Akubor, S. O. Obio, K. A. Nwadomere and E. Obiomah, Production and Quality Evaluation of Banana Wine, Plant Foods Hum. Nutr., 2003, 58, 1–6 CAS.
- I. V. Reddy and O. V. S. Reddy, Production, optimization and characterization of wine from mango (Mangifera indica L.), Nat. Product Radiance, 2009, 8(4), 426–435 Search PubMed.
- M. E. Obaedo and M. J. Ikenebomeh, Microbiology and production of banana (Musa sapientum) wine, Niger. J. Microbiol., 2009, 23(1), 1886–1891 Search PubMed.
- C. A. Chilaka, N. Uchechukwu, J. E. Obidiegwu and O. B. Akpor, Evaluation of the efficiency of yeast isolates from palm wine in diverse fruit wine production, Afr. J. Food Sci., 2010, 4(12), 764–774 CAS.
- R. G. Noll, The wines of West Africa: History, technology and tasting notes, J. Wine Econ., 2008, 3(1), 85–94 CrossRef.
- A. C. Ogodo, O. C. Ugbogu, A. E. Ugbogu and C. S. Ezeonu, Production of mixed fruit (pawpaw, banana and watermelon) wine using Sacharomyces cerevisiae isolated from palm wine, SpringerPlus, 2015, 4, 683 CrossRef PubMed.
- A. C. Ogodo, O. C. Ugbogu, D. I. Agwaranze and N. G. Ezeonu, Production and Evaluation of fruit wine from Mangifera indica (cv Peter), Appl. Microbiol., 2018, 4(144), 1–6 Search PubMed.
- G. H. Fleet, Yeast interaction and wine flavour, Int. J. Food Microbiol., 2003, 86, 11–22 CrossRef CAS PubMed.
- W. F. Duarte, D. R. Dias, M. J. Oliveira, J. A. Teixeira, J. D. Silva and R. F. Schwan, Characterization of different fruit wines made from cocoa, Cupuassu, gabiroba, juboticaba and Umbu, Food Sci. Technol., 2010, 30, 1–9 CrossRef.
- C. C. Isitua and I. N. Ibeh, Novel method of wine production from Banana (Musa acuminata) and Pineapple (Ananas cosmosus) waste, Afr. J. Biotechnol., 2010, 9(44), 7521–7524 CrossRef.
- T. E. Ayogu, Evaluation of the performance of yeast isolate from Nigeria palm wine in wine production from pineapple fruits, Bioresour. Technol., 1999, 69, 189–190 CrossRef CAS.
- I. B. Omamor, C. I. Aisagbonhi and D. E. A. Oruade, Present status of Date palm diseases, disorders and pests in Nigeria, in Proceedings of the Date Palm International Symposium, Wind Hoek, Namibia, 2000, p. 237 Search PubMed.
- S. Awe and S. N. Nnadoze, Production and Microbiological Assesment of Date Palm (Phoenix dactylifera L.) Fruit Wine, Br. Microbiol. Res. J., 2015, 8(3), 480–488 CrossRef.
- A. P. Pereira, T. Dias, J. Andrade, E. Ramalhosa and L. M. Estevinho, Mead production: Selection and characterization assays of Saccharomyces cerevisiae strains, Food Chem. Toxicol., 2009, 47, 2057–2063 CrossRef CAS PubMed.
- N. Srimeena, S. Gunasekaran and R. Murugesan, Optimization of fermentation conditions for producing Indian rock bee (Apis dorsata) mead using response surface methodology, J. Appl. Nat. Sci., 2014, 6(2), 366–370 CAS.
- N. Turkmen, F. Sari, E. S. Poyrazoglu and Y. S. Velioglu, Effects of prolonged heating on antioxidant activity and colour of honey, Food Chem., 2006, 95, 653–657 CrossRef CAS.
- J. Bertoncelj, U. Dobersek, M. Jamnik and T. Golob, Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey, Food Chem., 2007, 105, 822–828 CrossRef CAS.
- J. K. Gupta and R. Sharma, Production and quality characteristics of mead and fruit-honey wines: A review, Nat. Product Radiance, 2009, 8(4), 345–355 Search PubMed.
- M. Kuruppaiya, E. Sasikumar, T. Viruthagiri and V. Vijayagopal, Optimization of process parameters using response surface methodology (RSM) for ethanol production from waste cashew apple juice using Zymomonas mobilis, Chem. Eng. Commun., 2009, 196, 1–11 Search PubMed.
- L. V. A. Reddy and O. V. S. Reddy, Production and Characterization of Wine from Mango Fruit (Mangifera indica L), World J. Microbiol., 2005, 21, 1345–1350 CrossRef CAS.
- O. L. Enukainure, O. V. Oke, A. O. Daramola, S. O. Adenekan and E. E. Umanhonlem, Improvement of biochemical properties of watermelon rinds subjected to Saccharomyces cerevisiae solid media fermentation, Pak. J. Nutr., 2010, 9(8), 806–809 CrossRef.
- S. Awe, Production and microbiology of pawpaw (Carica papaya L) wine, Curr. Res. J. Biol. Sci., 2011, 3(5), 443–447 CAS.
- S. G. Kocher and Pooja, Status of wine production from guava (Psidium guajava L.): A traditional fruit of India, Afr. J. Food Sci., 2011, 5(16), 851–860 Search PubMed.
- Z. E. Monsavi, S. H. Mousavi, Z. Emma-Djomel and H. Klani, Fermentation of pomogranate juice by probiotic lactic acid bacteria, World J. Biotechnol., 2011, 27, 123–128 CrossRef.
- O. C. Ugbogu and H. C. Okereke, Identification and characterization of Microorganisms, in Laboratory Guide for Microbiology, ed. A. Onyeagba, Crystal Publishers, Okigwe, 2004, pp. 95–110 Search PubMed.
- A. A. T. Harkness and T. G. Arnason, A Simplified Method for Measuring Secreted Invertase Activity in Saccharomyces cerevisiae, Biochem. Pharmacol.: Open Access, 2014, 3(6), 151, DOI:10.4172/2167-0501.1000151.
- M. D. Fakruddin, I. Ariful, Q. Abdul, M. A. Monzur and C. Nayuum, Characterization of Stress tolerant high potential ethanol producing yeast from agro-industrial waste, Am. J. BioSci., 2013, 1, 24–34 CrossRef CAS.
- G. D. Sadler, and P. A. Murphy, pH and Titratable Acidity in Food Analysis, 3rd edn, 2003, pp. 207–226 Search PubMed.
- R. Willaert and V. A. Nedovic, Primary beer fermentation by immobilised yeast—a review on flavour formation and control strategies, J. Chem. Technol., 2006, 81(8), 1353–1367 CAS.
- P. Ribéreau-Gayon, D. Dubourdieu, B. Donèche and A. Lonvaud, Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd edn, John Wiley & Sons, Ontario, 2006, pp. 22–152 Search PubMed.
- O. F. Obidi, O. O. Awe, A. O. Shogbade and A. F. Akanji, Molecular Characterization of Successive Yeasts Strains and their Optimal Invertase Producing Conditions in Fresh Palm Wine (Raphia hookeri) obtained from Lagos, Nigeria, J. Appl. Sci. Environ. Manage., 2022, 26(12), 2125–2134 Search PubMed.
- AOAC, Official Methods of Analysis, 18th edn, Association of Official Analytical Chemists, Gaithersburg, MD, USA, 2019, pp. 1–34 Search PubMed.
- L. Barros, A. M. Carvelho, J. S. Morais and I. C. F. R. Ferreira, Strawberry tree, blackhorn and rose fruits: Detailed characteristics in nutrients and phytochemicals with antioxidant properties, Food Chem., 2010, 120, 247–254 CrossRef CAS.
- L. M. Souza, K. S. Ferreira, J. B. Chaves and S. L. Teixeira, L-ascorbic acid, β-carotene and lycopene content in papaya fruits (Carica Papaya) with or without physiological skin freckles, Sci. Agric., 2008, 65(3), 246–250 CrossRef.
- M. A. K. Ogunjobi and S. Ogunwolu, Physicochemical and Sensory Properties of Cassava Flour Biscuits Supplemented with Cashew Apple Powder, J. Food Technol., 2010, 8(1), 24–29, DOI:10.3923/jftech.2010.24.29.
- C. Tikka, H. P. Osuru, N. Atluri, P. C. Raghavulu, N. K. Yellapu, I. S. Mannur, U. V. Prasad, S. K. N. V. Aluru and M. Bhaskar, Isolation and characterization of ethanol tolerant yeast strains, Bioinformation, 2013, 9(8), 421–425 CrossRef PubMed.
- F. I. Oyeleke and A. M. Olaniyan, Extraction of juice from some tropical fruits using a small-scale multi-fruit juice extractor, Afr. Crop. Sci. Proc., 2009, 8, 1803–1808 Search PubMed.
- W. J. O. Oti, Using Refractometer to determine the sugar content in soft drinks commonly consumed in Abakaliki, Nigeria, IOSR J. Appl. Chem., 2016, 9(7), 89–91 Search PubMed.
- M. U. Ukwuru and J. I. Awah, Properties of palm wine yeasts and its performance in wine making, Afr. J. Biotechnol., 2013, 12(19), 2670–2677 CAS.
- O. Matthew, C. P. Okoronkwo and E. Effiong, Molecular characterization of yeast isolated from palm wine in Alakahia, Rivers State, Nigeria, World Sci. News, 2019, 130, 297–304 Search PubMed.
- A. S. Ikram-Ul-Haq, Kinetics of invertase production by Saccharomyces cerevisiae in batch culture, Pak. J. Bot., 2007, 39(3), 907–912 Search PubMed.
- M. C. Silveira, E. M. Oliveira, E. Carvajal and E. P. Bon, Nitrogen regulation of Saccharomyces cerevisiae invertase. Role of the URE2 gene, Appl. Biochem. Biotechnol., 2000, 84–86 Search PubMed.
- P. Kapse, A. Chandekar, A. Bawane, P. Patil, L. Potey and S. Waghmare, Formulation and Quality Evaluation of Banana Wine, Pharmaceut. Drug Regul. Affair J., 2023, 6(1), 1–5 Search PubMed.
- S. K. Panda, U. C. Sahu, S. K. Behera and R. C. Ray, Fermentation of sapota (Achras sapota linn.) fruits to functional wine, Nutrafoods, 2014, 13, 179–186 CrossRef CAS.
- U. C. Sahu, S. K. Panda, U. B. Mohapatra and R. C. Ray, Preparation and evaluation of wine from tendu (Diospyros melanoxylon L) fruits with antioxidants, Int. J. Food Ferm. Technol., 2012, 2(2), 167–178 Search PubMed.
- R. C. Ray, S. K. Panda, M. R. Swain and S. P. Sivakumar, Proximate composition and sensory evaluation of anthocyanin-rich purple sweet potato (Ipomoea batatas L.) wine, Int. J. Food Sci. Technol., 2011, 47(3), 452–458 CrossRef.
- S. K. Panda, U. R. Sahu, S. K. Behera and R. C. Ray, Bio-processing of bael [Aegle marmelos L.] fruits into wine with antioxidants, Food Biosci., 2014, 5, 34–41 CrossRef CAS.
- E. E. T. Bechem, C. Omoloko, D. Nwaga and V. P. K. Tilanji, Characterization of palm wine yeasts using osmotic ethanol tolerance and isozyme polymorphism of alcohol dehydrogenase, Afr. J. Biotechnol., 2007, 6(14), 1715–1719 CAS.
- J. M. Clement-Jimenez, L. Mingorance-Cazoria, S. Martinez-Rodriguez, F. J. L. Herasvazquez and F. Rodriguez-Vico, Influence of sequential yeast mixtures in wine fermentation, Int. J. Microbiol., 2005, 98, 301–308 CrossRef PubMed.
- J. L. Gómez-Ariza, T. García-Barrera and F. Lorenzo, Determination of flavour and off-flavour compounds in orange juice by on-line unit to gas chromatography-mass spectrometry, J. Chromatogr. A, 2004, 1047(2), 313–317 CrossRef PubMed.
- S. F. Sayyad, S. R. Chaudhari and B. P. Panda, Quantitative determination of ethanol in arishta by using UV visible spectrophotometer, J. Pharm. Biol. Sci., 2015, 2(5), 204–207 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
