 Open Access Article
Open Access ArticleFormulation and characterization of hybrid milk containing bovine and Spirulina proteins
Jayani Samarathunga *a,
Thi Phuong Linh Lea,
Max Gabardb,
Katrina Strazdinsb,
Jeroen Rensb and
Benu Adhikari
*a,
Thi Phuong Linh Lea,
Max Gabardb,
Katrina Strazdinsb,
Jeroen Rensb and
Benu Adhikari *a
*a
aSchool of Science, RMIT University, Melbourne, VIC 3083, Australia. E-mail: S3971507@student.rmit.edu.au; benu.adhikari@rmit.edu.au
bBega Group, Melbourne, VIC 3008, Australia
First published on 15th May 2025
Abstract
The incorporation of microalgal protein into dairy products presents a sustainable and innovative approach to address rising global protein demands. This study developed model hybrid milk formulations (HMFs) by combining Spirulina protein concentrate (SPC) and milk protein concentrate (MPC), in which SPC replaced 25%, 50%, 75%, and 100% of the total protein content (w/w). A formulation with 100% MPC was used as the control. The fat and mineral contents of the HMFs were standardized using ghee and simulated milk ultrafiltrate (SMUF) respectively. Confocal laser scanning microscopy revealed a uniform distribution of fat globules in all formulations, characterized by small initial globule sizes; however, their size increased significantly after 15 days of storage. Increasing SPC levels led to a significant shift toward a greenish-brown hue. HMFs containing 25–75% SPC exhibited significantly higher creaming than those with either 100% MPC or 100% SPC. Increasing SPC levels led to higher viscosity and reduced thermal stability, with gelation occurring at lower temperatures. This reduction in thermal stability was supported by lower protein denaturation temperatures observed for SPC compared to MPC. Corresponding structural analysis showed a progressive loss of ordered conformation, with decreased β-sheet and increased random coil content, which contributed to the altered gelation behaviour. Among the formulations, the 25% SPC (75% MPC) blend most closely resembled the 100% MPC in terms of color, creaming, viscosity, thermal stability, and structural integrity, making it the optimal HMF developed using SPC and MPC.
Sustainability spotlightThis study supports the transition toward a more sustainable food system by formulating hybrid milk using bovine and Spirulina proteins. The hybrid formulations offer greatly increased nutritional value. Thus, the content of this paper aligns with United Nations Sustainable Development Goals (SDGs) particularly, zero hunger (SDG 2) and good health and well-being (SDG 3). By partially substituting dairy protein with Spirulina, the research supports more responsible consumption and production patterns (SDG 12) and contributes to climate action by reducing the environmental footprint of dairy products (SDG 13). Furthermore, the work promotes innovation in food systems (SDG 9), demonstrating the potential of alternative proteins to drive a more resilient and sustainable future for the dairy-food industry. |
1 Introduction
Bovine milk is a widely consumed, nutritionally balanced staple in diets across the globe. Yet, rising consumer demand and evolving preferences have led to the growing popularity of mixed milk formulations containing blends of milk proteins with other protein sources, as well as dairy alternatives.1 Among alternative protein sources, Spirulina platensis is of great interest due to its high protein content (60–67% dry weight) and the sustainable ways it can be cultivated.2 Spirulina protein has been shown to interact compatibly with dairy proteins, suggesting potential applications in model milk and gelled products. To incorporate Spirulina protein and dairy proteins into a mixed food system, the protein from Spirulina must first be extracted from the biomass. Additionally, its techno-functional properties such as solubility, water and oil absorption capacities, and foaming and emulsifying properties require improvement.3 The techno-functional properties of Spirulina protein can be influenced by interactions with other proteins, solvents, and solutes. These properties are also influenced by processing parameters; for example, hydrophobic, hydrophilic, and electrostatic interactions in milk and plant protein systems are impacted by ionic strength, pH, and temperature.4 Chen et al.5 showed that proteins extracted from Chlorella pyrenoidosa and S. platensis using a phosphate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v, pH 7) and ultrasonication (300 W, 30 min) exhibited minimal foam capacity, stability, solubility, and emulsifying activity at pH 5. Understanding these interactions is important for developing innovative products with greater consumer acceptability.
10 w/v, pH 7) and ultrasonication (300 W, 30 min) exhibited minimal foam capacity, stability, solubility, and emulsifying activity at pH 5. Understanding these interactions is important for developing innovative products with greater consumer acceptability.
To date, the whole biomass of S. platensis has been used in the food industry as a nutraceutical,6 a coloring agent (phycocyanin for blue and phycoerythrin for red),7 and as a food supplement.6,8,9 Benelhadj et al.9 determined three major protein fractions in Arthrospira platensis (the most commonly studied species of Spirulina)—albumins (51.5%), globulins (2.4%), and prolamins (46.1%)—using the Osborne sequential extraction method. These proteins, which are derived from what is commonly known as Spirulina, are characterized by their amino acid composition. This gives Spirulina protein its mildly acidic nature and a net negative charge at neutral pH, due to higher acidic-to-basic amino acid ratios.10 These protein fractions significantly influence the physicochemical and techno-functional properties in food applications. Since albumins are soluble in water, glutelins in alkaline solutions, globulins in saline, and prolamins in water/alcohol mixtures,9,11 the Spirulina protein concentrate is expected to contain water-soluble, alkaline-soluble, and salt-soluble fractions.
The native conformation of Spirulina protein is altered by denaturation during food processing. In this study, we evaluated how the physicochemical and techno-functional properties are influenced by partial unfolding of Spirulina protein during microfluidization and its cross-linking with other components in hybrid milk formulations. Lozober et al.12 demonstrated that high-pressure homogenization of Spirulina protein concentrate (5% w/v) at 50 MPa (pH 6.5, 25 °C) enhanced protein solubility, which in turn promoted gel formation at a low gelation point, resulting in a stiff gel. In industrial milk production (pasteurized, UHT, and sterilized milk), thermal treatments are applied to extend shelf life. Similarly, in Spirulina protein-containing hybrid milk production, understanding the thermal stability and heat-induced gelation of Spirulina protein is essential. Additionally, high pressure homogenization/microfluidization, a key process in producing Spirulina protein-containing model milk, may have an impact on gelation, potentially posing challenges. This study, therefore, focused on the thermal stability of Spirulina protein in hybrid milk formulations.
Partially replacing bovine milk with alternative milk, or milk proteins with alternative proteins in milk systems, remains challenging, as protein–protein interactions are weak due to their non-covalent nature.1 This can lead to phase separation through co-aggregation, driven by incompatibilities in internal factors such as structure, molecular weight, and free thiol groups, as well as external factors like pH, temperature, and ionic strength. Studies have been conducted to evaluate the application of cereal-based proteins (pea, soy, quinoa, barley, and rice proteins) as milk protein alternatives.13,14 However, research on partially or fully replacing bovine protein with microalgal protein in milk systems remains scarce. Published studies have so far utilized whole algal biomass powder to produce dairy-mimetic products. For example, cheese analogues have been made from Chlorella vulgaris biomass powder,15 yogurts fortified with Spirulina powder,16 cheese enriched with Spirulina powder,17 and fermented milk incorporated with Spirulina biomass.18
So far, there is no study on the behavior of Spirulina protein in milk systems. Given its potential as a sustainably sourced protein, it is essential to investigate how Spirulina protein interacts with milk fat, lactose, and minerals to mimic the properties of bovine milk proteins. This work aims to optimize hybrid milk formulations designed to mimic the composition of natural milk for research purposes containing Spirulina protein and compare them to those made with bovine protein with respect to their physicochemical properties, techno-functional characteristics, and microstructure. The findings of this study will provide food producers with a scientific basis to utilize Spirulina protein in food formulations as a sustainable alternative to milk proteins.
2 Materials and methods
2.1. Materials
Spirulina biomass powder (BIOGLAN organic, New South Wales, Australia) was purchased from a local chemist warehouse outlet (Melbourne, Australia). Milk protein concentrate (MPC) was donated by Tatura milk industries Ltd, Victoria, Australia. According to the manufacturer, it contained 85.5% protein on dry basis of which 67.5% was casein and 16.2% whey protein. Ghee (clarified butter), labelled as containing 99.9% fat by the manufacturer, was purchased from the local supermarket (Maharajah's Choice, Melbourne, Australia). Food grade D-lactose monohydrate with purity ≥ 98% was purchased from Sigma-Aldrich Pty Ltd (New South Wales, Australia) and was used as the source of sugar for all Spirulina protein-based milk formulations. All other chemicals used were of analytical grade and were purchased from Sigma-Aldrich Pty Ltd Milli-Q water was used to prepare all of the milk mimetic formulations.2.2. Protein extraction from Spirulina biomass
Preparation of Spirulina protein concentrate (SPC) was carried out according to Zhang et al.19 with minor modification and the procedure is shown in the Fig. 1.2.3. Determining proximate composition of freeze dried SPC powder
The crude protein content (Kjeldahl method; AOAC Method 991.20),20 moisture (AOAC method 925.10),21 fat (AOAC method 920.85)22 and ash content (AOAC method 923.03)23 of freeze dried SPC powder were determined. The mineral content (calcium-Ca, sodium-Na, magnesium-Mg, and potassium-K, iron-Fe, zinc-Zn and copper-Cu) of the freeze dried SPC powder, and MPC were analyzed using ICP-MS method (7700®, Agilent Technologies, Santa Clara, USA). This instrument was able to detect upper limit of 10 mg L−1 (10 ppm) for Na, K, Mg, Ca and Fe and upper limit of 100 μg L−1 (100 ppb) for Cu and Zn. In brief, a 2.0 g sample of powder was subjected to dry ashing in a muffle furnace (Thermo Scientific Thermolyne Muffle Furnace, USA) at 550 °C for 16 hours. The resulting ash was dissolved in 2% HNO3 to prepare the analyte solution, which was then filtered through a 0.45 μm membrane filter.2.4. Formulation of milk
Milk was formulated to mimic the composition of cow's milk (fat, protein, sugar and minerals). Typically, cow's milk contains 3.5% fat, 3.3% protein, and 5% lactose.24 The pure ghee was used as the source milk fat after melting it at 40 °C. The mineral composition of milk was replicated using simulated milk ultrafiltrate (SMUF), prepared according to the protocol of Jenness25 with minor modifications as described in de Groot et al.26 Formulation of the Spirulina-based milk was carried out by replacing milk proteins (casein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whey; 80
whey; 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) using SPC at 0%, 25%, 50%, 75% and 100% (w/w). All the ingredients (Table 1) were homogenized under 62 MPa in 3 passes using a microfluidizer (Microfluidics®-M110L-UHPH, Germany). Experiments were carried out at 25 °C and stored at 4 ± 1 °C for further analysis.
20) using SPC at 0%, 25%, 50%, 75% and 100% (w/w). All the ingredients (Table 1) were homogenized under 62 MPa in 3 passes using a microfluidizer (Microfluidics®-M110L-UHPH, Germany). Experiments were carried out at 25 °C and stored at 4 ± 1 °C for further analysis.
| Sample | Description | Protein | Fat (melted ghee) | Lactose | SMUF (minerals) |
|---|---|---|---|---|---|
| a 100% MPC – formulation with 100% MPC; 25% SPC – formulation having 25% SPC; 50% SPC – formulation having 50% SPC; 75% SPC – formulation having 75% SPC and 100% SPC – formulation having 100% SPC. | |||||
| 100% MPC | 100% Milk protein (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; casein 20; casein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whey) whey) |
3.3% (w/v) | 3.5% (v/v) | 5% (w/v) | 500 mL |
| 25% SPC | 25% Spirulina protein + 75% dairy protein | 3.3% (w/v) | 3.5% (v/v) | 5% (w/v) | 500 mL |
| 50% SPC | 50% Spirulina protein + 50% dairy protein | 3.3% (w/v) | 3.5% (v/v) | 5% (w/v) | 500 mL |
| 75% SPC | 75% Spirulina protein + 25% dairy protein | 3.3% (w/v) | 3.5% (v/v) | 5% (w/v) | 500 mL |
| 100% SPC | 100% Spirulina protein | 3.3% (w/v) | 3.5% (v/v) | 5% (w/v) | 500 mL |
The SPC and MPC contents (33.3 g protein/1000 mL) required for each milk formulation system was calculated based on the protein content in them. Prior to homogenization, the protein component was dissolved in 500 mL SMUF and mixed by agitating at 600 rpm for 2 h at 35 ± 2 °C. The pH of the protein solutions was adjusted to 6.70 using 1 M NaOH/1 M HCl to match the pH of bovine milk. These protein solutions were stored at 4 °C overnight for complete hydration. Then, course emulsions were prepared by mixing these protein solutions (30 °C) with melted ghee using Ultra Turrax high shear homogenizer (IKA T25 digital® Ultra-Turrax, Malaysia) at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 s. Finally, model milk samples were produced by passing the course emulsions through microfluidizer three times. Temperature during the microfluidization process was maintained within 20 ± 5 °C using an ice bath. The milk samples produced in this way were stored at 4 ± 1 °C for further analysis.
000 rpm for 30 s. Finally, model milk samples were produced by passing the course emulsions through microfluidizer three times. Temperature during the microfluidization process was maintained within 20 ± 5 °C using an ice bath. The milk samples produced in this way were stored at 4 ± 1 °C for further analysis.
2.5. Physicochemical properties of milk formulations
 | (1) |
2.6. Microstructure of the formulated milk
To understand the fat and protein distribution within the coarse emulsion and the model milk emulsions, their microstructure was observed using a light microscope under 40× magnification to assess droplet size and phase separation, and a confocal laser scanning microscope (CLSM) (A1 HD25, Nikon Instruments Inc., Japan) under 100× magnification with oil immersion to examine fat and protein distribution. These measurements provided valuable information on the droplet size, concentration, aggregation nature, and nature of the oil-in-water emulsion of the model milk samples. For CLSM, protein in the model milk was stained with fast green and was excited at 635 nm. The fat component was stained with nile red excited at 488 nm. A 200 μL sample was stained with 4 μL of nile red (1 mg/1 mL) and fast green (1 mg/1 mL) each and mixed well. Samples were placed on a glass slide and observed using CLSM while maintaining a dark environment to protect samples from photobleaching.2.7. Particle size distribution of milk formulations
Particle size distribution of the fat globules was used to evaluate the stability of model milk for 15 days.29 Particle size of the fat globules of the formulations was measured by laser diffraction method using Mastersizer 3000 attached to a HydroMV sample handling unit (Malvern Mastersizer liquid cell-3000-ATA scientific instruments, Australia). The refractive indices of milk fat and the dispersant (SMUF) were considered as 1.460 and 1.334, respectively, at 25 °C.2.8. Techno-functional properties
2.9. Determination of changes of protein conformation
Fourier transform infrared spectrometric spectra (FTIR) were recorded in 4000–400 cm−1 range and at a resolution of 2 cm−1 of all milk formulations using a FTIR spectrometer (PerkinElmer, CT, USA). The spectra in the amide I region (1700–1600 cm−1) were analyzed to determine the changes in the protein's conformation. Milli-Q water was used to acquire reference (background) spectra. Sixty-four scans were taken and averaged for each sample.2.10. Statistical analysis
All measurements were made in triplicate in terms of sample preparation and tests unless otherwise specified above. Results are presented as mean ± standard deviation (SD). The analysis was performed using MINITAB 17 software. One way ANOVA test (Tukey test) was performed to determine the significant difference. The significant difference between any two mean values were determined at 95% confidence level (p < 0.05).3 Results and discussion
3.1. Proximate composition
An amount of 24.37 g of SPC was extracted from 100 g of Spirulina biomass powder. The extracted SPC contained 81.53% crude protein (dry basis), 7.80% moisture, 1.90% lipid, and 7.81% total ash. The mineral content of SPC and MPC was compared to assess the impact of mineral salts in milk-based systems. Notably, SPC showed significantly higher sodium levels (p < 0.05) than MPC (Table 2), likely due to the use of sodium hydroxide during the extraction process. Elevated sodium levels in SPC can impact the flavor profile of HMFs, potentially leading to undesirable levels of saltiness and reduced consumer acceptability. For reference, bovine milk typically contains 39.1–64.4 mg/100 g of sodium and 121.2–168.1 mg/100 g of potassium, which together contribute to its naturally balanced salty taste.32 Beyond sensory effects, high sodium intake is linked to adverse health outcomes, including hypertension and kidney strain.33 Sodium content in SPC can be reduced through ultrafiltration, diafiltration, or dialysis. Additionally, increasing both the number of washing steps and the volume of washing solution used during centrifugation has been shown to effectively lower residual sodium levels. MPC had higher levels of magnesium, calcium, and potassium (Table 2), with these differences being statistically significant (p < 0.05). These variations highlight the influence of composition and processing methods on the mineral composition of protein concentrates. Mineral composition of SPC is rarely found in literature despite its importance and this information can broaden its application, for example, to enrich iron (Table 2). SMUF was used in this study to replicate the ionic environment of bovine milk, as the mineral profile plays a crucial role in the buffering capacity of milk systems.34 This is because minerals like ionic calcium and phosphate release hydrogen ions during heat treatment, causing a decrease in pH.| Mineral | SPC freeze dried powder | MPC powder |
|---|---|---|
| a Results are represented as an average of triplicates ± SD. Different letters (superscript) indicate significant differences within a row (p < 0.05). | ||
| Macro (mg/100 g) | ||
| Na | 15.22 ± 0.10a | 0.64 ± 0.03b |
| Ca | 0.26 ± 0.15a | 10.98 ± 0.41b |
| Mg | 0.14 ± 0.07a | 0.62 ± 0.11b |
| K | 0.62 ± 1.30a | 1.91 ± 0.05b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Micro (mg/100 g) | ||
| Fe | 2.50 ± 0.05a | <0.001b |
| Zn | <0.001 | <0.001 |
| Cu | <0.001 | <0.001 |
3.2. Physicochemical properties of HMFs
| Sample | L* | a* | b* | Whiteness index | Zeta potential (mV) |
|---|---|---|---|---|---|
| a Results are represented as an average of triplicates ± SD. Different letters (superscript) indicate significant differences within a column (p < 0.05). | |||||
| 100% MPC | 87.11 ± 0.06a | −3.00 ± 0.15a | 4.66 ± 0.07a | 85.97 ± 0.27a | −35.2 ± 0.3a |
| 25% SPC | 55.41 ± 0.10b | −2.07 ± 0.04b | 24.64 ± 0.45b | 49.01 ± 0.55b | −35.8 ± 0.1b |
| 50% SPC | 44.84 ± 0.23c | −1.63 ± 0.01c | 21.94 ± 0.03c | 40.61 ± 0.83c | −37.4 ± 0.7c |
| 75% SPC | 38.26 ± 0.03d | −1.17 ± 0.20d | 19.67 ± 0.11d | 35.19 ± 0.08d | −37.5 ± 0.5d |
| 100% SPC | 32.52 ± 0.01e | −1.18 ± 0.05e | 15.96 ± 0.34e | 30.65 ± 0.72e | −40.2 ± 0.3e |
3.3. Microstructure
Freshly prepared five milk formulations were observed under light microscope (40×) and CLSM (100× oil immersion). The microscopic images of preliminary emulsions in all HMFs were similar. Hence, image of preliminary emulsion of 100% SPC is presented in Fig. 3A as representative of preliminary emulsion of all milk formulations. High pressure (62 MPa) prevailing in the microfluidization promoted formation of a stable emulsion. SPC and MPC are soluble in aqueous medium at pH 6.70 at 25 °C.37,43 Thus, protein component (stained with fast green) was visible in green color and was found to disperse uniformly. In both model milks produced using MPC and SPC, fat droplets (stained with nile red) were covered by protein film or layer. The coverage by protein layer improved the stability of these O/W emulsions. The larger protein aggregates formed in the preliminary emulsion were further broken-down during homogenization as indicated by the breakdown of larger bright green areas in the pre-emulsion (Fig. 3A) to small areas in HMFs.3.4. Particle size
The bimodal particle size distribution of preliminary emulsions was changed to monomodal distribution in milk formulations after third pass of microfluidization. As observed in Fig. 4A, bimodal particle size distribution is observed in the preliminary-emulsion and also in all milk formulations after first and second pass of microfluidization. Hence, microfluidization at 62 MPa and three passes were found to be optimum homogenisation conditions to produce milk formulations. Microfluidization helps to form stable emulsions by minimizing the oil droplet size and facilitating their coverage with proteins or emulsifiers.40 The average particle size (volume-weighted mean diameter, D[4, 3]) of fat globules in the pre-emulsions was fairly consistent in these formulations, ranging from 27.5 μm (in 100% MPC) to 29.5 μm (in 100% SPC). After microfluidization (62 MPa, 3 passes), the globule size was significantly reduced to the nanoscale, with mean diameters remaining between 359 nm (in 100% MPC) and 411 nm (in 100% SPC) as the diameter increased with higher level of SPC. The D[4, 3] values of all HMFs increased over the storage period. By the 15th day, the 100% MPC (circled area in Fig. 4B) exhibited a bimodal particle distribution, indicating the presence of aggregates. In comparison, the fat particle size distribution of HMFs containing SPC had a much wider span than that of 100% MPC. The nature of protein adsorption influences the particle size distribution of fat in oil/water emulsions.44 The particle size results suggested that the interaction between milk protein–fat and Spirulina protein–fat was different and indicated that this protein–fat interaction is an important factor in determining the particle size of the emulsions.45 Spirulina protein showed a lower ability to adsorb to fat droplet surfaces compared to milk protein, likely due to its lower solubility at pH 6.0–7.0 and greater susceptibility to unfolding under pressure. Consequently, the particle size distribution span of 100% SPC was broader than that of 100% MPC. Despite the differences in particle size distribution, the emulsion stability of 100% SPC was comparable to that of 100% MPC throughout the storage period, as indicated by the creaming index results (Fig. 4A). Both 100% MPC and 100% SPC exhibited low creaming compared to hybrid milk (HMFs). In HMFs, the interfacial protein layer appeared more heterogeneous and discontinuous, with regions of incomplete coverage exposing the fat core. This disruption was particularly evident at intermediate SPC levels (25–75%), where the reduced continuity of the interfacial layer likely contributed to increased creaming due to insufficient droplet stabilization. However, as the SPC proportion increased—reaching 100% in the SPC-only formulation—the interfacial layer became more uniform and continuous, indicating improved coverage of fat droplets. This enhanced interfacial stability in the 100% SPC formulation corresponded with its lower creaming index compared to the HMFs (Fig. 5A). In 100% MPC, casein micelles form a stable network to envelop fat droplets, facilitated by calcium and phosphate ions in SMUF and contribute to the emulsion's stability.46 This would also be one major reason due to which the size of fat droplets in 100% MPC was smaller compared to that in 100% SPC. In contrast, the interaction between Spirulina protein and fat droplets is less defined because it lacks this robust enveloping capability or is influenced by other factors, such as mineral salts.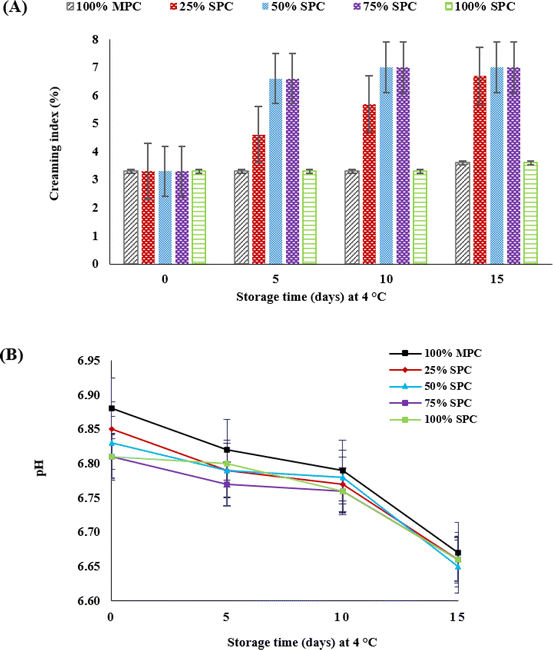 | ||
| Fig. 5 (A) and (B) represent the creaming index and pH changes of milk formulations during storage (mean ± SD; n = 3). | ||
3.5. Creaming index and pH of HMFs during storage
The pH of HMFs was measured and adjusted at different stages of their preparation. The pH of SPC solutions was higher than that of MPC solutions and was adjusted to 6.67 (milk pH) before mixing with melted ghee. The slightly lower pH in MPC can be attributed to its higher calcium ion content, which lowers the pH. However, the pH of all HMFs increased slightly after microfluidization (Fig. 5B; day 0), which is likely due to the unfolding of the protein structures. During this process, the milk and Spirulina proteins in the HMFs (25% SPC, 50% SPC, and 75% SPC) were expected to interact, forming an envelope around the fat droplets to stabilize the emulsion. The strength of these protein–protein interactions depends on factors such as temperature, pH, ionic strength, and the availability of free thiol groups.1 Notably, the solubility of milk and Spirulina proteins differed at pH values between 6.0 and 7.0, which is close to the natural pH of milk. This reduced solubility of Spirulina protein likely contributed to the partial settling observed in the hybrid formulations after being left overnight for hydration. In hybrid protein formulations, Spirulina protein showed lower solubility in SMUF compared to milk proteins, leading to partial settling. The protein content in SPC was 81.53%, with the remaining portion consisting of non-protein components, which could influence the overall solubility. During storage, the pH of all milk formulations gradually decreased, although the pH of the 100% MPC formulation consistently maintained a slightly higher pH than the SPC-based HMFs.The creaming index measures the gravitational separation of dense and light phases in an oil-in-water emulsion.47,48 The creaming index of 25% SPC, 50% SPC, and 75% SPC increased during storage (Fig. 5A). For the 100% SPC and 100% MPC formulations, the creaming index remained stable until the 10th day, while a noticeable increase was observed by the 15th day (Fig. 5A). Microfluidization enhances emulsion stability by reducing oil droplet size and helping to increase the coverage of fat globules by proteins, both of which help prevent phase separation. However, cooling the emulsions can lead to partial crystallization of the fat phase, promoting phase separation. The presence of these two different protein types in the formulations appeared to increase the tendency for phase separation due to the aggregation of unabsorbed proteins.
3.6. Techno-functional properties
The protein denaturation temperature serves as an indicator of thermal stability/instability; thus, the denaturation temperatures of SPC and MPC were assessed to evaluate the thermal behavior of milk formulations (Fig. 6). The thermal analysis of SPC at pH 7 (Fig. 6A) revealed two distinct endothermic events within the 20–200 °C range, indicating multistage decomposition due to the presence of multiple protein fractions with different thermal stabilities. The first broad peak occurred between 58.3 °C (onset) and 97.5 °C (end-set), with a peak temperature (Tpeak) of 89.9 °C and an enthalpy change (ΔH) of 6.1 J g−1. A second, sharper peak was observed from 154.3 °C (onset) to 185.9 °C (end-set), with a Tpeak of 177.2 °C and ΔH of 114.7 J g−1. This dual-stage behavior was found in the studies of Ramírez-Rodrigues et al.52 and Chronakis,10 who reported dual peaks at 89 °C and 173 °C, and 67 °C and 109 °C, respectively, in SPC, indicating the presence of proteins with varying molecular weights and structural properties that contribute to different levels of thermal resistance. The thermal stability of proteins is influenced by the nature of bonding, purity, extraction method, and pH.10,52 The purity of the Spirulina protein extracted in the current study showed higher purity (81.5% dw) than the previously reported (76.1% (dw) and 78.6% (dw)).10,52 Ramírez-Rodrigues et al.52 reported that SPC produced by alkaline solubilization, and isoelectric precipitation had molecular weights ranging from 12 to 100 kDa. Additionally, phycocyanin, a phycobiliprotein in Spirulina, was found to have molecular weights of 17 kDa and 21 kDa. These variations in molecular weight help explain the multiple decomposition (denaturation) peaks observed in the thermograms of the current study (Fig. 6). In the thermograph of MPC (Fig. 6B), containing casein and whey proteins at a ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, two endothermic peaks were observed within the 20–200 °C range. The first peak occurred between 69 °C (onset) and 125 °C (end-set), with a Tpeak of 113.4 °C and an enthalpy change (ΔH) of 26.4 J g−1. This peak could be attributed with the unfolding of protein, protein aggregation and moisture lost from the sample.53 A previous study reported that MPC with the same protein content (85%) began to undergo protein denaturation at a lower temperature (63 °C) to that observed in the current study.53 The second peak appeared from 145 °C (onset) to 157 °C (end-set), with a Tpeak of 148 °C and ΔH of 146.4 J g−1 that may occur with the denaturation of protein and sample decomposition.53,54 Ptiček Siročić et al.54 determined the thermal behavior of casein by scanning between 25 and 600 °C at 10 °C min−1 and observed transitions around 40–150 °C for the first stage and 150–250 °C for the second stage. This revealed that different casein fractions exhibit different thermal stabilities. MPC has a heterogeneous structure due to the presence of caseins and non-casein proteins. The casein component is composed of α1, α2, β, and κ-casein, which extend the thermal stability up to 150–250 °C.54 In contrast, whey proteins, including β-lactoglobulin (Td: 88.5 °C), α-lactalbumin (Td: 56.7 °C), and immunoglobulins (Td: 73.3 °C), have lower thermal stability.50,54 Therefore, the thermal stability of MPC depends on the casein-to-whey ratio.53 In the current study, the ΔH and Tpeak values of the first peak indicate that SPC (Fig. 6A) has comparatively lower thermal stability than MPC (Fig. 6B).
20, two endothermic peaks were observed within the 20–200 °C range. The first peak occurred between 69 °C (onset) and 125 °C (end-set), with a Tpeak of 113.4 °C and an enthalpy change (ΔH) of 26.4 J g−1. This peak could be attributed with the unfolding of protein, protein aggregation and moisture lost from the sample.53 A previous study reported that MPC with the same protein content (85%) began to undergo protein denaturation at a lower temperature (63 °C) to that observed in the current study.53 The second peak appeared from 145 °C (onset) to 157 °C (end-set), with a Tpeak of 148 °C and ΔH of 146.4 J g−1 that may occur with the denaturation of protein and sample decomposition.53,54 Ptiček Siročić et al.54 determined the thermal behavior of casein by scanning between 25 and 600 °C at 10 °C min−1 and observed transitions around 40–150 °C for the first stage and 150–250 °C for the second stage. This revealed that different casein fractions exhibit different thermal stabilities. MPC has a heterogeneous structure due to the presence of caseins and non-casein proteins. The casein component is composed of α1, α2, β, and κ-casein, which extend the thermal stability up to 150–250 °C.54 In contrast, whey proteins, including β-lactoglobulin (Td: 88.5 °C), α-lactalbumin (Td: 56.7 °C), and immunoglobulins (Td: 73.3 °C), have lower thermal stability.50,54 Therefore, the thermal stability of MPC depends on the casein-to-whey ratio.53 In the current study, the ΔH and Tpeak values of the first peak indicate that SPC (Fig. 6A) has comparatively lower thermal stability than MPC (Fig. 6B).
 | ||
| Fig. 6 Thermograms of (A) SPC and (B) MPC obtained from differential scanning calorimetry by heating in the range 20–200 °C at 5 °C min−1. | ||
DSC analyses of milk formulations were performed to examine thermal transitions resulting from the physicochemical interactions of the milk components during heating. A pronounced endothermic peak was observed in all milk formulations between 125 and 161 °C along with distinct thermal transitions occurring at different temperatures across the five formulations (Table 4). The endothermic peak could be attributed due to dehydration55–57 and interaction between lactose and amino group of proteins.57 Maillard browning is known to occur during heating of protein–lactose mixtures.58,59 It is initiated by a reaction between reducing sugar (lactose) and amino acid (lysine) leading to the formation of various compounds that influence the color, flavor, and texture of the food. The DSC analysis of the Maillard reaction has not been extensively studied in recent years. A study revealed the reaction between glucose and lysine is characterized by an endothermic peak between 100 and 150 °C, followed by a second endothermic peak at 200–260 °C upon further heating.60 Maillard reaction is influenced by factors like nature of the heat treatment, pH, salts (metal ions), water activity, sugar ratio, amino acid type and emulsion structure.61 The HMFs in this study form complex systems with various milk components, making it challenging to fully understand their thermal behavior. The thermal transitions of lactose are known to occur at different temperatures according to its isomeric properties. For example, DSC analysis of 96% w/w α-lactose monohydrate powder revealed two endothermic peaks at 146.9 °C and 219.8 °C, corresponding to water loss from α-lactose and the melting point, respectively.55 The reduction in water content lowers the water activity in milk formulations, creating ideal conditions for the Maillard reaction.62 Physical treatments such as high pressure applied to milk can influence the protein–protein and protein–lactose interactions.40 In particular, the application of high pressure (62 MPa) during the formulation of HMFs may have affected the structural changes in proteins, which, in turn, impacted lactose solubility. In SPC based milk formulations, higher energy was required for the dehydration due to the resistance of water molecules bound to the milk system with high water-binding capacity of SPC compared to MPC. Additionally, SPC increased the viscosity in HMFs, thereby restricting water mobility. This effect explains the observed increase in enthalpy at higher SPC levels (Table 4). The lower thermal stability of SPC compared to MPC (Fig. 6) is a key factor contributing to structural changes in SPC-based milk systems at lower temperatures. This effect was evident in the thermal transitions observed in the DSC analysis of milk formulations, where the onset occurred at lower temperatures as the SPC-to-MPC ratio increased (Table 4).
| Sample | Ton-set (°C) | Toff-set (°C) | Tpeak (°C) | ΔH (J g−1) |
|---|---|---|---|---|
| a Results are represented as an average of triplicates ± SD. Different letters (superscript) indicate significant differences within a column (p < 0.05). | ||||
| 100%MPC | 148.0 ± 0.3a | 161.0 ± 1.4a | 151.0 ± 0.6a | 2425 ± 0.7a |
| 25%SPC | 145.6 ± 0.1b | 160.5 ± 0.1b | 149.6 ± 0.5b | 2921 ± 4.5b |
| 50%SPC | 128.4 ± 7.2c | 140.0 ± 8.6c | 137.1 ± 0.1c | 3011 ± 0.1c |
| 75%SPC | 129.7 ± 2.4d | 136.2 ± 4.1d | 133.5 ± 0.8d | 3127 ± 0.3d |
| 100%SPC | 125.0 ± 0.1e | 140.1 ± 0.8e | 130.1 ± 0.2e | 3678 ± 0.2e |
In the dairy industry, milk undergoes various heat treatments, such as pasteurization, sterilization, and drying, which can alter its physicochemical properties due to changes in molecular structure and interactions among the components, especially at high temperatures (>100 °C).59 These conditions can cause protein denaturation and increased interactions with fat, lactose, and calcium phosphate. Therefore, understanding heat-induced effects, such as denaturation, gelation, and crystallization of SPC based HMFs, is important for optimizing the process parameters of HMF production.
3.7. Heat-induced gelation
Understanding the rheology and gel formation of formulated HMFs during heating and cooling is essential for controlling the fluidity and texture of products that may be produced from it (e.g., yogurt, cheese, ice cream). The heat-induced gelation curves (Fig. 7) show that replacing bovine proteins with Spirulina protein affects the rheology of the HMFs, as indicated by changes in the storage modulus (G′) of the gel network formed during heat-induced gelation.Heating was followed by cooling to assess the stability of the gel. The lower thermal stability of SPC HMFs, observed in the DSC thermogram, was also reflected in the heat-induced gelation curves, where SPC HMFs showed a rapid increase in G′ at lower temperatures compared to 100% MPC. During heating, both bovine and Spirulina proteins in the HMFs unfolded from their native state, forming an intermolecular network with water, sugar, and fat. Gelation occurred earlier in HMFs with higher SPC content (50–100% SPC) compared to those with lower SPC content (25% SPC) and 100% MPC. The gelling points of milk formulations decreased as MPC was increasingly replaced with SPC, with 50% and 100% replacement showing gelling points of 62.5 °C and 56.0 °C, respectively, while 100% MPC had a gelling point of 90.1 °C.
These results show that SPC and MPC have different gelling characteristics, likely due to the differing influence of secondary protein structure, pH (release of hydrogen ions) and mineral salts (release of calcium ions).34,63,64 This explains why HMFs with higher SPC concentrations (50–100%) gelled at lower temperatures compared to 100% MPC. The findings suggest that SPC could be useful in developing low-temperature gelled products. At the same time, formulations with lower SPC content (up to 25%) retained thermal stability comparable to the 100% MPC control, indicating that functional integrity can be maintained at modest substitution levels. To avoid premature gelation, low temperatures (≤63 °C) should be used to pasteurize HMFs containing high levels of SPC. This implies that utilization of Spirulina protein as a dairy mimetic protein requires low temperature to attribute similar textures with bovine milk in developing yogurt and cheese products. This aligns with industry trends focusing on energy efficiency, reduced processing inputs, and minimal additives. The gelation properties of SPC-based HMFs make them suitable for producing gelled dairy products, such as cheese and yogurt, with lower energy input and without the need for added calcium ions compared to 100% MPC. Thermal processing of dairy products like drying and pasteurization accounts for physicochemical changes like Maillard reaction (glycation between lysine and lactose), protein denaturation and aggregation, conversion of soluble calcium and phosphate into colloids, structural changes in fat globules and decrease in pH.32 Thus, in the dairy industry, heat treatments are carefully designed with these considerations in mind. Similarly, HMFs developed incorporating Spirulina protein require thorough evaluation under various thermal processing conditions to optimize their properties. Low-temperature or moderate drying methods, such as freeze drying or vacuum drying, can be preferred to preserve the structural and functional integrity of microalgal proteins. Understanding the thermal behavior of Spirulina protein will provide valuable insights for designing optimal processing protocols tailored to the needs of the dairy industry. Most plant protein-based milk products incorporate stabilizers and food hydrocolloids for creating desired texture to address challenges associated with their low thermal stability.65 Spirulina protein-based milk products, as a newer innovation, require further research on the incorporation of hydrocolloids, similar to those in plant protein-based products, to achieve optimal quality and stability.
3.8. Spectral properties, molecular interaction, and secondary structure of proteins in HMFs
Zhao et al.67 identified FTIR spectroscopic peaks in bovine milk at 1159 cm−1 and 1076 cm−1, corresponding to the C–O–C stretching of the 1 → 4 glycosidic bond and the C–OH bending of lactose, respectively. Peaks in the 1200–1000 cm−1 range are linked to C–N bonds formed due to Maillard reactions between lysine and lactose.19,67 Similar spectral features were observed in our milk formulations, with peaks at 1079 cm−1 and 1078 cm−1 attributed to MPC and SPC, indicating that these proteins are phosphate-containing. The peak at 1075 cm−1 has been ascribed to phosphate groups in casein, consistent with previous findings.68,69
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations) was used to assess changes in the secondary structure of proteins in HMFs (Fig. 8). Major secondary structures (α-helix, β-sheet, β-turns, and random coils) were determined through second derivative and deconvolution analysis of the spectra. Microfluidization is known to induce partial protein unfolding, promoting intermolecular aggregation and rearrangement, which affects viscoelastic properties.70–72 These aggregates may form via non-covalent hydrophobic interactions and covalent disulfide bonds.51 A decrease in α-helices and an increase in random coils are associated with higher surface hydrophobicity.9 Among the five milk formulations, the α-helix structure was detected only in 100% SPC at 1657 cm−1. The varying amounts of random coils help explain the differences in solubility among the HMFs. As MPC was gradually replaced with SPC, the total β-sheet content decreased while the random coil content increased (Fig. 8). The higher proportion of random coils observed in 100% SPC HMFs could be due to a greater degree of partial unfolding of SPC compared to MPC during microfluidization. These random coils likely contributed to the aggregation of SPC, leading to increased viscosity in SPC-containing HMFs compared to 100% MPC. The β-sheet peaks around 1691 cm−1 or 1689 cm−1 were observed only in HMFs containing SPC.
O stretching vibrations) was used to assess changes in the secondary structure of proteins in HMFs (Fig. 8). Major secondary structures (α-helix, β-sheet, β-turns, and random coils) were determined through second derivative and deconvolution analysis of the spectra. Microfluidization is known to induce partial protein unfolding, promoting intermolecular aggregation and rearrangement, which affects viscoelastic properties.70–72 These aggregates may form via non-covalent hydrophobic interactions and covalent disulfide bonds.51 A decrease in α-helices and an increase in random coils are associated with higher surface hydrophobicity.9 Among the five milk formulations, the α-helix structure was detected only in 100% SPC at 1657 cm−1. The varying amounts of random coils help explain the differences in solubility among the HMFs. As MPC was gradually replaced with SPC, the total β-sheet content decreased while the random coil content increased (Fig. 8). The higher proportion of random coils observed in 100% SPC HMFs could be due to a greater degree of partial unfolding of SPC compared to MPC during microfluidization. These random coils likely contributed to the aggregation of SPC, leading to increased viscosity in SPC-containing HMFs compared to 100% MPC. The β-sheet peaks around 1691 cm−1 or 1689 cm−1 were observed only in HMFs containing SPC.4 Conclusion
This study explored the potential of formulating a hybrid milk using Spirulina protein concentrate (SPC) and milk protein concentrate (MPC). The SPC content in the formulation was progressively increased to identify an optimal balance. The results indicated that SPC significantly influenced the physicochemical and functional properties of the hybrid milk, with its impact intensifying at higher concentrations. The purity of SPC was identified as a critical factor, as residual non-protein components, including native polysaccharides and pigments, affected milk functionality. Specifically, polysaccharides contributed to viscosity and water-binding capacity, while pigments influenced color under varying pH conditions. The hybrid milk formulation containing 25% SPC and 75% MPC showed promising texture and flavor. Additionally, SPC exhibited performance comparable to MPC in terms of solubility at neutral pH (6.0–7.0) and emulsifying properties. To enhance the purity of SPC, further refinement through filtration or sequential extraction is recommended, as these methods are feasible for industrial applications. For large-scale processing of SPC-containing milk, thermal treatments should not exceed 63 °C for 30 minutes (or equivalent) to prevent heat-induced gelation.Ethics statement
The authors confirm that this manuscript does not include any studies involving human or animal subjects.Data availability
The manuscript includes all essential data supporting its findings, statements, and conclusions. Spreadsheet data can be provided upon request. Any relevant data sourced from the literature have been properly cited and acknowledged where applicable.Author contributions
Jayani Samarathunga: conceptualization, writing—original draft. Thi Phuong Linh Le: writing, reviewing and editing. Max Gabard: supervision. Katrina Strazdins: supervision and reviewing. Jeroen Rens: conceptualization and supervision. Benu Adhikari: conceptualization, supervision, reviewing and editing.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
The authors acknowledge the financial support of BDD Australia Pty Ltd (part of the Bega Group of Companies) towards scholarship of Samarathunga and Linh Le.References
- C. Alves, J. Silva, S. Pinteus, H. Gaspar, M. C. Alpoim, L. M. Botana and R. Pedrosa, Front. Pharmacol., 2018, 9, 777 CrossRef PubMed.
- S. Grosshagauer, K. Kraemer and V. Somoza, J. Agric. Food Chem., 2020, 68(14), 4109–4115 CrossRef CAS PubMed.
- A. L. L. Menegotto, L. E. S. de Souza, L. M. Colla, J. A. V. Costa, E. Sehn, P. R. S. Bittencourt, É. L. de Moraes Flores, C. Canan and E. Colla, LWT–Food Sci. Technol., 2019, 114, 108267 CrossRef.
- L. G. Lima Nascimento, D. Odelli, A. Fernandes de Carvalho, E. Martins, G. Delaplace, P. Peres de sá Peixoto Júnior, N. F. Nogueira Silva and F. Casanova, Foods, 2023, 12(12), 2385 CrossRef CAS PubMed.
- Y. Chen, J. Chen, C. Chang, J. Chen, F. Cao, J. Zhao, Y. Zheng and J. Zhu, Food Hydrocolloids, 2019, 96, 510–517 CrossRef CAS.
- T. Lafarga, J. M. Fernández-Sevilla, C. González-López and F. G. Acién-Fernández, Food Res. Int., 2020, 137, 109356 CrossRef CAS PubMed.
- A. B. García, E. Longo and R. Bermejo, Appl. Psychol., 2021, 33(5), 3059–3070 Search PubMed.
- S.-E. Jin, S. J. Lee and C.-Y. Park, Food Chem., 2020, 325, 126751 CrossRef CAS PubMed.
- S. Benelhadj, S. Douiri, A. Ghouilli, R. B. Hassen, S. M. Keshk, A. El-Kott, H. Attia and D. Ghorbel, J. Food Compos. Anal., 2023, 115, 104984 CrossRef CAS.
- I. S. Chronakis, J. Agric. Food Chem., 2001, 49(2), 888–898 CrossRef CAS PubMed.
- T. B. Osborne, Nature, 1924, 114, 822 Search PubMed.
- H. S. Lozober, Z. Okun and A. Shpigelman, Innovative Food Sci. Emerging Technol., 2021, 74, 102857 CrossRef.
- S. Jeske, E. Zannini, M. F. Cronin and E. K. Arendt, Food Funct., 2018, 9(6), 3500–3508 RSC.
- M. Vogelsang-O'Dwyer, E. Zannini and E. K. Arendt, Trends Food Sci. Technol., 2021, 110, 364–374 CrossRef.
- A. Mohamed, B. Abo-El-Khair and S. M. Shalaby, World Appl. Sci. J., 2013, 23(7), 914–925 CAS.
- R. Arslan and S. Aksay, J. Food Process. Preserv., 2022, 46(6), e15941 CAS.
- M.-T. Golmakani, S. Soleimanian-Zad, N. Alavi, E. Nazari and M. H. Eskandari, J. Appl. Phycol., 2019, 31, 1085–1094 CrossRef CAS.
- L. Varga, J. Szigeti, R. Kovács, T. Földes and S. Buti, J. Dairy Sci., 2002, 85(5), 1031–1038 CrossRef CAS PubMed.
- Z. Zhang, G. Holden, B. Wang and B. Adhikari, Food Chem., 2023, 406, 134931 CrossRef CAS PubMed.
- AOAC, Official Methods: Sec. 33.2.1, Method No. 991.20, Gaithersburg MD, 16th edn, 1995 Search PubMed.
- AOAC, Official Methods: Method No. 925.10, Rockville, 20th edn, 2016 Search PubMed.
- AOAC, Official Methods: 780 Method No. 920.85, Arlington, VA, USA, 15th edn, 1990 Search PubMed.
- AOAC, Official Methods: Method No. 923.03, Rockville, 20th edn, 2016 Search PubMed.
- A. Foroutan, A. C. Guo, R. Vazquez-Fresno, M. Lipfert, L. Zhang, J. Zheng, H. Badran, Z. Budinski, R. Mandal and B. N. Ametaj, J. Agric. Food Chem., 2019, 67(17), 4897–4914 CrossRef CAS PubMed.
- R. Jenness, Neth. Milk Dairy J., 1962, 16, 153–164 CAS.
- A. de Groot, E. Bijl and L. Sagis, Food Hydrocolloids, 2024, 154, 110085 CrossRef CAS.
- J. Glusac, S. Moguiliansky, A. Fishman and M. Davidovich-Pinhas, Food Struct., 2024, 41, 100378 CrossRef CAS.
- C. Cano-Sarmiento, D. Téllez-Medina, R. Viveros-Contreras, M. Cornejo-Mazón, C. Figueroa-Hernández, E. García-Armenta, L. Alamilla-Beltrán, H. García and G. Gutiérrez-López, Food Eng. Rev., 2018, 10, 113–138 CrossRef.
- K. Briviba, V. Gräf, E. Walz, B. Guamis and P. Butz, Food Chem., 2016, 192, 82–89 CrossRef CAS PubMed.
- N. Bernat, M. Chafer, J. Rodríguez-García, A. Chiralt and C. González-Martínez, LWT–Food Sci. Technol., 2015, 62(1), 488–496 CrossRef CAS.
- J. V. Silva, R. Cochereau, C. Schmitt, C. Chassenieux and T. Nicolai, Food Res. Int., 2019, 116, 1135–1143 CrossRef CAS PubMed.
- K. Pegu, S. S. Arya and J. Agric, Food Res., 2023, 14, 100730 CAS.
- V. Perez and E. T. Chang, Adv. Nutr., 2014, 5(6), 712–741 CrossRef CAS PubMed.
- T. Aydogdu, J. A. O'Mahony and N. A. McCarthy, Int. Dairy J., 2022, 131, 105383 CrossRef CAS.
- S. Alotaiby, X. Zhao, C. Boesch and N. N. Sergeeva, Food Chem., 2024, 436, 137653 CrossRef CAS PubMed.
- E. Nouri, H. Abbasi and E. Rahimi, Qual. Assur. Saf. Crop Foods, 2018, 10(4), 335–349 CrossRef CAS.
- S. C. Silva, T. Almeida, G. Colucci, A. Santamaria-Echart, Y. A. Manrique, M. M. Dias, L. Barros, Â. Fernandes, E. Colla and M. F. Barreiro, Colloids Surf., A, 2022, 648, 129264 CrossRef CAS.
- M. T. Sejersen, T. Salomonsen, R. Ipsen, R. Clark, C. Rolin and S. B. Engelsen, Int. Dairy J., 2007, 17(4), 302–307 CrossRef.
- N. Li, P. Wang, S. Wang, C. Wang, H. Zhou, S. Kapur, J. Zhang and Y. Song, J. Environ. Chem. Eng., 2022, 10(3), 107516 CrossRef CAS.
- O. K. Ozturk and H. Turasan, Trends Food Sci. Technol., 2021, 116, 609–625 CrossRef CAS.
- S. Benelhadj, A. Gharsallaoui, P. Degraeve, H. Attia and D. Ghorbel, Food Chem., 2016, 194, 1056–1063 CrossRef CAS PubMed.
- M. Du, J. Xie, B. Gong, X. Xu, W. Tang, X. Li, C. Li and M. Xie, Food Hydrocolloids, 2018, 76, 131–140 CrossRef CAS.
- V. Sikand, P. Tong, S. Roy, L. Rodriguez-Saona and B. Murray, J. Dairy Sci., 2011, 94(12), 6194–6202 CrossRef CAS PubMed.
- L. Buchmann, P. Bertsch, L. Böcker, U. Krähenmann and P. Fischer, Food Hydrocolloids, 2019, 97, 105182 CrossRef CAS.
- P. Bertsch, L. Böcker, A. Mathys and P. Fischer, Trends Food Sci. Technol., 2021, 108, 326–342 CrossRef CAS.
- D. S. Horne, In Milk Proteins, ed. M. Boland, and H. Singh, Academic Press, Cambridge, MA, USA, 3rd edn, 2020, 213–250 Search PubMed.
- D. J. McClements, Adv. Colloid Interface Sci., 2015, 219, 27–53 CrossRef CAS PubMed.
- D. J. McClements, Curr. Opin. Colloid Interface Sci., 2004, 9(5), 305–313 CrossRef CAS.
- A. J. Vasbinder, A. C. Alting and K. G. de Kruif, Colloids Surf., B, 2003, 31(1–4), 115–123 CrossRef CAS.
- C. C. Jennings, M. Freidenberger, S. A. Christensen, J. Conlin, O. Freidenberger and J. D. Kenealey, Food Chem., 2024, 441, 138347 CrossRef CAS PubMed.
- Z. Yüksel and Y. K. Erdem, J. Food Eng., 2005, 67(3), 301–308 CrossRef.
- M. M. Ramírez-Rodrigues, C. Estrada-Beristain, J. Metri-Ojeda, A. Pérez-Alva and D. K. Baigts-Allende, Sustainability, 2021, 13(12), 6849 CrossRef.
- M. Khalesi and R. J. FitzGerald, Food Res. Int., 2021, 147, 110576 CrossRef CAS PubMed.
- A. Ptiček Siročić, L. Kratofil Krehula, Z. Katančić and Z. Hrnjak-Murgić, Chem. Biochem. Eng. Q., 2016, 30(4), 501–509 CrossRef.
- M. J. Altamimi, K. Wolff, A. Nokhodchi, G. P. Martin and P. G. Royall, Int. J. Pharm., 2019, 555, 237–249 CrossRef CAS PubMed.
- S. Garnier, S. Petit, F. Mallet, M.-N. Petit, D. Lemarchand, S. Coste, J. Lefebvre and G. Coquerel, Int. J. Pharm., 2008, 361(1–2), 131–140 CrossRef CAS PubMed.
- J.-B. J. Manuel, R.-M. Jesús, H.-L. Erasmo, A.-C. Andrés, M.-V. V. Manuel and H.-S. Betsabé, Dairy Foods, 2022, 233–260 Search PubMed.
- S. Arena, G. Renzone, C. D'Ambrosio, A. M. Salzano and A. Scaloni, Food Chem., 2017, 219, 477–489 CrossRef CAS PubMed.
- P. Fox, T. Uniacke-Lowe, P. McSweeney, J. O'Mahony, P. Fox, T. Uniacke-Lowe, P. McSweeney and J. O'Mahony, Dairy Chem. Biochem., 2015, 345–375 Search PubMed.
- L. Manzocco, M. C. Nicoli and E. Maltini, J. Food Process. Preserv., 1999, 23(4), 317–328 CrossRef CAS.
- S. Stojanovska, N. Gruevska, J. Tomovska and J. Tasevska, Chem. Res. J., 2017, 2(6), 139–145 CAS.
- M. N. Lund and C. A. Ray, J. Agric. Food Chem., 2017, 65(23), 4537–4552 CrossRef CAS PubMed.
- X. D. Sun and S. D. Arntfield, Food Res. Int., 2010, 43(2), 509–515 CrossRef CAS.
- M. Wang, Z. Yin, W. Sun, Q. Zhong, Y. Zhang and M. Zeng, Food Hydrocolloids, 2023, 136, 108244 CrossRef CAS.
- T. Mehany, S. A. Siddiqui, B. Olawoye, O. Olabisi Popoola, A. Hassoun, M. F. Manzoor and S. Punia Bangar, Crit. Rev. Food Sci. Nutr., 2024, 64(20), 7237–7267 CrossRef PubMed.
- A. Barth, Biochim. Biophys. Acta, Bioenerg., 2007, 1767(9), 1073–1101 CrossRef CAS PubMed.
- Y. Zhao, J. Saxena, V. Cherian, M. Silva, T. Truong and J. Chandrapala, Int. J. Food Sci. Technol., 2024, 59(2), 1037–1050 CrossRef CAS.
- P. Jaiswal, S. N. Jha, A. Borah, A. Gautam, M. K. Grewal and G. Jindal, Food Chem., 2015, 168, 41–47 CrossRef CAS PubMed.
- T. Markoska, T. Huppertz, M. K. Grewal and T. Vasiljevic, LWT–Food Sci. Technol., 2019, 102, 64–70 CrossRef CAS.
- N. Zhang, Z. Xiong, W. Xue, R. He, X. Ju and Z. Wang, Innovative Food Sci. Emerging Technol., 2022, 80, 103091 CrossRef CAS.
- I. Lange, S. Mleko, M. Tomczynska-Mleko, G. Polishchuk, P. Janas and L. Ozimek, Ukr. Food J., 2020, 9(1), 7–35 CrossRef CAS.
- P. Paquin, Int. Dairy J., 1999, 9(3–6), 329–335 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |






