Open Access Article
Open Access ArticleRelationships of sarcopenia symptoms and dietary patterns with lung cancer risk: a prospective cohort study†
Huijun
Zhou‡
a,
Fubin
Liu‡
a,
Jingyi
Xu‡
b,
Xixuan
Wang
a,
Yu
Peng
a,
Peng
Wang
a,
Changyu
Si
a,
Jianxiao
Gong
a,
Jiale
Gu
a,
Ailing
Qin
a and
Fangfang
Song
 *a
*a
aDepartment of Epidemiology and Biostatistics, Key Laboratory of Molecular Cancer Epidemiology, Key Laboratory of Prevention and Control of Major Diseases in the Population, Ministry of Education, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin, 300060, China. E-mail: songfangfang@tmu.edu.cn; Fax: +86 (0)2223372231; Tel: +86 (0)2223372231
bDepartment of Biochemistry and Molecular Biology, Tianjin Key Laboratory of Medical Epigenetics, School of Basic Medical Sciences, Tianjin Medical University, Tianjin, 300070, China
First published on 17th February 2025
Abstract
Background: Few studies focused on the effects of sarcopenia on lung cancer in the general population and optimizing nutritional intake may be a feasible way to manage sarcopenia. We sought to systematically investigate the associations of sarcopenia symptoms with lung cancer incidence and mortality in the general population, and whether dietary patterns could modify these risks. Methods: A total of 361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 participants (mean age: 56.2 years; and men: 46.7%) were included in this prospective UK Biobank study. Sarcopenia symptoms (low handgrip strength, low muscle mass and slow walking pace) were determined according to European Working Group of Sarcopenia in Older People 2 (EWGSOP2) criteria. Individuals without any of the three sarcopenia symptoms were classified as the normal control group. Based on the baseline data from food frequency questionnaires, primary dietary patterns were identified through principal component analysis. Cox proportional hazards models were used to examine associations between sarcopenia symptoms as well as dietary patterns and lung cancer. Then we explored the joint effects of sarcopenia symptoms and dietary patterns on lung cancer risk. Results: A total of 3532 incident lung cancer cases and 2073 deaths were documented during a median follow-up of 12 years. All the sarcopenia symptoms were associated with a higher risk of lung cancer incidence than the normal control group, especially in people aged <60 (Pinteraction < 0.05). Particularly, a stronger association was observed for slow walking pace with incidence (hazard ratio [HR]: 1.49, 95% confidence interval [CI]: 1.34–1.65) and mortality (HR: 1.54, 95% CI: 1.35–1.75) of lung cancer. Higher adherence to the wholegrain pattern was associated with a greater reduction in the risk of lung cancer incidence (HRQ4vs.Q1: 0.71, 95% CI: 0.65–0.79) and mortality (HRQ4vs.Q1: 0.68, 95% CI: 0.60–0.77). The joint analysis demonstrated that the risk of lung cancer-related outcomes associated with low handgrip strength gradually reduced as the quartile of wholegrain pattern scores increased (Ptrend < 0.05). Conclusions: Our study indicated that individuals with sarcopenia symptoms suffered a higher risk of lung cancer incidence and mortality even in younger age. A diet abundant in whole grains may help to improve sarcopenia symptoms and reduce adverse lung cancer-related outcomes associated with low handgrip strength.
763 participants (mean age: 56.2 years; and men: 46.7%) were included in this prospective UK Biobank study. Sarcopenia symptoms (low handgrip strength, low muscle mass and slow walking pace) were determined according to European Working Group of Sarcopenia in Older People 2 (EWGSOP2) criteria. Individuals without any of the three sarcopenia symptoms were classified as the normal control group. Based on the baseline data from food frequency questionnaires, primary dietary patterns were identified through principal component analysis. Cox proportional hazards models were used to examine associations between sarcopenia symptoms as well as dietary patterns and lung cancer. Then we explored the joint effects of sarcopenia symptoms and dietary patterns on lung cancer risk. Results: A total of 3532 incident lung cancer cases and 2073 deaths were documented during a median follow-up of 12 years. All the sarcopenia symptoms were associated with a higher risk of lung cancer incidence than the normal control group, especially in people aged <60 (Pinteraction < 0.05). Particularly, a stronger association was observed for slow walking pace with incidence (hazard ratio [HR]: 1.49, 95% confidence interval [CI]: 1.34–1.65) and mortality (HR: 1.54, 95% CI: 1.35–1.75) of lung cancer. Higher adherence to the wholegrain pattern was associated with a greater reduction in the risk of lung cancer incidence (HRQ4vs.Q1: 0.71, 95% CI: 0.65–0.79) and mortality (HRQ4vs.Q1: 0.68, 95% CI: 0.60–0.77). The joint analysis demonstrated that the risk of lung cancer-related outcomes associated with low handgrip strength gradually reduced as the quartile of wholegrain pattern scores increased (Ptrend < 0.05). Conclusions: Our study indicated that individuals with sarcopenia symptoms suffered a higher risk of lung cancer incidence and mortality even in younger age. A diet abundant in whole grains may help to improve sarcopenia symptoms and reduce adverse lung cancer-related outcomes associated with low handgrip strength.
Introduction
Sarcopenia is a progressive and systemic disorder of skeletal muscle characterized by decreased skeletal muscle mass, strength, and function, which commonly occurs with advancing age, but can also occur earlier in life.1 Longitudinal data show that muscle mass declines at a rate of 8% per decade from the age of 40, reaching 15% per decade after the age of 70. Muscle strength weakens at a greater rate than muscle mass, with a 10% to 15% decline per decade starting at age 40 and a faster rate of 25% to 40% per decade after age 70.2In the general population, few studies have reported the prevalence of sarcopenia in people under 60 years old; however, it was estimated that approximately 10% to 16% of participants aged 60 years and older worldwide suffer from sarcopenia, which is increasing with the quick rate of population aging.3 Sarcopenia has been linked to an increased likelihood of various adverse consequences, including mortality of multiple site-specific cancers, such as digestive system, lung, breast, etc.3–5The intensifying aging trend of the global population also imposes an ever-increasing incidence of cancer. With almost 2.5 million new cases and over 1.8 million deaths worldwide, lung cancer is the leading cause of cancer morbidity and mortality in 2022.6 Research on lung cancer has revealed that patients with sarcopenia prior to treatment exhibit an elevated susceptibility to toxicity from chemotherapy, a poorer prognosis, and post-operative complications.7,8 Specifically, a meta-analysis has found that lung cancer patients have the highest risk of poor overall survival concerning sarcopenia-related mortality and survival rate and other consequences.3 Epidemiological evidence has implicated chronic inflammation, higher insulin levels, and insulin resistance in lung cancer etiology,9,10 which are also related to sarcopenia.2 Although this suggests that lung cancer and sarcopenia may share similar pathophysiological mechanisms, studies on the correlations of sarcopenia with the risk of lung cancer in the general population are still lacking.
There are no licensed medications for sarcopenia currently. Optimizing nutritional intake may be an effective intervention in sarcopenic patients. The current evidence from nutritional epidemiology has indicated a beneficial link between a healthier dietary pattern, e.g., the Mediterranean diet (MD), and improved muscle function.11 Notably, previous studies have demonstrated that higher adherence to the MD was linked to a lower risk of lung cancer.12 The relationship between data-driven dietary patterns and the risk of sarcopenia is a topic of current debate. One meta-analysis13 has revealed that the maximal adherence to the “western/meat” dietary pattern was associated with a substantial increase in lung cancer risk, whereas the highest adherence to the “healthy/prudent” pattern demonstrated the opposite effect. To the best of our knowledge, there is a scarcity of research to investigate the combined influence of dietary patterns and sarcopenia on lung cancer.
In this study, we utilized a large-scale sample size and a longitudinal perspective afforded by the UK Biobank to examine the associations between sarcopenia symptoms and the risk of lung cancer incidence and mortality. Additionally, we investigated the joint association of dietary patterns and sarcopenia symptoms with lung cancer-related outcomes.
Materials and methods
Study design and participants
Over 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 participants (5.5% response rate), aged 37 to 73 years, were recruited from the general population between 2006 and 2010 to participate in the UK Biobank. Detailed information on study design, implementation, and data acquisition can be found at https://www.ukbiobank.ac.uk.14 The dataset encompassed details of sociodemographic characteristics, lifestyle factors, physical examinations, and medical histories, acquired through a touchscreen questionnaire and a brief verbal interview. Written informed consent was obtained from all participants, and ethical approval for the UK Biobank study was granted by the North West–Haydock Research Ethics Committee (Approval No: 16/NW/0274). The research adhered to the ethical principles delineated in the Declaration of Helsinki.
000 participants (5.5% response rate), aged 37 to 73 years, were recruited from the general population between 2006 and 2010 to participate in the UK Biobank. Detailed information on study design, implementation, and data acquisition can be found at https://www.ukbiobank.ac.uk.14 The dataset encompassed details of sociodemographic characteristics, lifestyle factors, physical examinations, and medical histories, acquired through a touchscreen questionnaire and a brief verbal interview. Written informed consent was obtained from all participants, and ethical approval for the UK Biobank study was granted by the North West–Haydock Research Ethics Committee (Approval No: 16/NW/0274). The research adhered to the ethical principles delineated in the Declaration of Helsinki.
In the present analysis, we excluded pregnant women (n = 371), and participants with diagnosed cancer (except for non-melanoma skin cancer) from the baseline (n = 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995). Accounting for ethnic variations in diagnostic criteria values for sarcopenia, participants in this study were limited to those of White European descent by excluding 29
995). Accounting for ethnic variations in diagnostic criteria values for sarcopenia, participants in this study were limited to those of White European descent by excluding 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781 non-White participants. Among 428
781 non-White participants. Among 428![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 cancer-free participants, we excluded those without data of sarcopenia-defining parameters (n = 4395) and complete dietary information (n = 39
224 cancer-free participants, we excluded those without data of sarcopenia-defining parameters (n = 4395) and complete dietary information (n = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883), leaving 361
883), leaving 361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 adults in the final analyses (Fig. S1†).
763 adults in the final analyses (Fig. S1†).
Outcome ascertainment
The focal outcomes of this study were the incidence and mortality of lung cancer. Data pertaining to lung cancer registration and death were procured through record linkage with the National Health Service (NHS) digital and NHS Central Register. Additionally, data on hospital admissions were linked to the Health Episode Statistics and Scottish Morbidity Records.Individuals with self-reported or diagnosed cancer either prior to or on the day of UK Biobank biological sampling were treated as having baseline cancer. Incident cancer cases refer to those diagnosed subsequent to this initial assessment. Participants were prospectively monitored from the date of attendance at the recruitment assessment center until the occurrence of lung cancer diagnosis, date of death, or the last follow-up date (30 September 2021 for England, 31 July 2021 for Scotland, and 28 February 2018 for Wales), whichever came first. The type of lung cancer was defined as the International Classification of Diseases, ninth revision (ICD-9) 162, and tenth revision (ICD-10) C34, as well as self-reported data fields 1001, 1027, and 1028.
Assessment of sarcopenia
Three sarcopenic thresholds, defined by the European Working Group of Sarcopenia in Older People 2 (EWGSOP2) standard,1 and now the most extensively utilized globally, were as follows: (1) probable sarcopenia (low handgrip strength), (2) sarcopenia (low handgrip strength and muscle mass), and (3) severe sarcopenia (low handgrip strength, low muscle mass and slow gait speed) (Table S1†).Handgrip strength was measured utilizing a Jamar J00105 hydraulic hand dynamometer by a trained research nurse. One measurement was conducted for each arm, with participants seated in an upright position, maintaining a 90° elbow flexion, and situating the forearm on armrests in a mid-prone position. The average of the right and left values was derived and expressed in absolute units (kg). The cut-off points applied to define low hand grip were <27 kg in men and <16 kg in women.1
Muscle mass was assessed by appendicular lean mass (ALM) estimated from bioelectrical impedance analysis (BIA) – derived predicted mass (Tanita BC418MA, Tokyo, Japan). ALM was expressed relative to height2 (m2) using standing height measured with a SECA 2020 height measuring stadiometer. The cut-off points applied to define low muscle mass were <7 kg m−2 in men and <5.5 kg m−2 in women.1
The UK Biobank lacks an objective measure of gait speed as per the specifications outlined in the EWGSOP2. Consequently, self-reported walking pace was employed as a surrogate for gait speed, categorized into three levels: slow, average, or brisk. A previous investigation demonstrated that self-reported walking pace is a reliable indicator of walking speed.15 As a surrogate marker of gait speed, the walking pace was then dichotomized as slow or normal (average or brisk pace).
Due to the small numbers of individuals with sarcopenia (n = 632) and severe sarcopenia (n = 129), the main exposure variables in our study were the aforementioned three sarcopenia symptoms: low handgrip strength, low muscle mass and slow walking pace.
Assessment of dietary intake and dietary patterns
Dietary data on fresh fruits, dried fruits, raw vegetables, cooked vegetables, processed meats, oily fish, non-oily fish, lamb, pork, poultry, beef, cheese, whole grain bread, other bread, grain cereal, other cereal, tea, coffee and water were collected using the Food Frequency Questionnaire (FFQ) in the UK Biobank (category 100052). Details about the coding of intake for each food are shown in Table S2.†Dietary patterns were discerned by standardizing the consumption frequencies of aforementioned 19 food items, which were subsequently incorporated into a principal component analysis (PCA) with varimax rotation. The scree plot, eigenvalue (>1.5) and interpretability were used to determine the retention factor16 (Fig. S2†). Rotated factor loadings with an absolute value ≥0.30 were considered to exert a substantial influence on the identified dietary pattern. A greater factor loading symbolized a more robust association between specific food items and the discerned dietary patterns.17 Ultimately, based on the characteristics of food items exhibiting high factor loadings, major dietary patterns were identified.
Covariates
The covariates in our analysis were selected based on both theoretical and empirical considerations, by referring to relevant literature and previous studies, as well as by using a Directed Acyclic Graph (DAG) (Fig. S3†), to ensure that the model adjusted for potential confounding factors that could impact the associations being tested. Age, sex, family history of cancer and lifestyle factors (alcohol consumption frequency, smoking status, physical activity, and sleep duration) were self-reported at the baseline assessment. The family history of cancer refers to the participant's father, mother or siblings who suffered from lung cancer, bowel cancer, breast cancer and prostate cancer. The Townsend deprivation index functioned as a metric for assessing socioeconomic status, where high scores denoted great levels of socioeconomic deprivation.18 Alcohol consumption frequency was categorized into six groups (daily or almost daily, three or four times a week, once or twice a week, one to three times a month, special occasions only, and never). Smoking status was classified as current smoker, former smoker, and non-smoker. Physical activity was divided into three groups (low, moderate, vigorous) according to the guidelines of 75 min of vigorous activity per week or 150 min of moderate activity. Total sleep duration was recorded as the number of reported hours of sleep within a 24 hour period, including naps. Body mass index (BMI) was calculated from weight and height (kilogram per square meter) and classified as <18.5, 18.5–24.9, 25–29.9, and ≥30 kg m−2. The long-term conditions were originally formulated for an extensive cross-sectional study conducted in Scotland and were subsequently modified for application in the UK Biobank.19 Detailed definitions can be found in Table S3.† The number of long-term conditions was divided into six groups (0, 1, 2, 3, 4, ≥5).Statistical analyses
Descriptive characteristics are expressed as median (inter quartile range) for quantitative variables, and as counts (percentage) for categorical variables. Nonparametric tests were used for continuous variables and chi-square tests were used for categorical variables.We tested the proportional hazards assumption based on Schoenfeld residuals and no statistically significant deviations were observed. Cox proportional hazards models were used to examine overall, age-stratified and sex-stratified hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of three sarcopenia symptoms with lung cancer incidence and mortality. The normal control group (individuals with the three sarcopenia-defining parameters in the normal range defined by the EWGSOP2 criteria) was treated as the reference group.
The continuous dietary pattern scores were subsequently categorized into quartiles. We used the Cox proportional hazards model to assess associations of dietary patterns with risks of lung cancer incidence and mortality. Then chi-square tests and multivariate logistic regression models were employed to investigate the correlation between dietary patterns and sarcopenia symptoms. Eventually, the joint effects of sarcopenia symptoms and dietary patterns on lung cancer incidence and mortality were also taken into consideration. Throughout, our analyses, the minimally-adjusted model was adjusted for age, sex and Townsend deprivation index; the fully-adjusted model was adjusted for covariates included in the minimally adjusted model plus BMI, alcohol frequency, smoking status, family history of cancer, no. of long-term conditions, physical activity and sleep duration.
Secondary and sensitivity analyses were conducted. Firstly, we respectively explored the relationships of sarcopenia symptoms and dietary patterns with the incidence of lung cancer subtypes, including lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LSCC), and small cell lung cancer (SCLC). Secondly, the associations of the above exposure variables with lung cancer risks were analyzed independently stratified by subgroups. The interaction test was used to examine the statistical significance of the difference between subgroups. Finally, in order to mitigate the risk of potential reverse causation, the primary analyses were reperformed after excluding participants who either developed lung cancer or died within a 2-year period from the baseline (n = 361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422).
422).
SAS version 9.4 (SAS Institute, USA) and R software (The R Foundation, https://www.r-project.org, version 4.0.2) were used for all analyses. For two-sided P values, a threshold of less than 0.05 was deemed statistically significant.
Results
Characteristics of the study population
After excluding participants who did not meet the inclusion criteria, we included a total of 361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 participants in the final analysis, among which 0.2% (n = 632) suffered from sarcopenia at the baseline. The prevalence of low handgrip strength, low muscle mass and slow walking pace was 7.4% (n = 26
763 participants in the final analysis, among which 0.2% (n = 632) suffered from sarcopenia at the baseline. The prevalence of low handgrip strength, low muscle mass and slow walking pace was 7.4% (n = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 811), 1.3% (n = 4734) and 6.3% (n = 22
811), 1.3% (n = 4734) and 6.3% (n = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932), respectively, in the general population, and increased with age irrespective of gender (Fig. S4†). In detail, low handgrip strength, low muscle mass and slow walking pace accounted for 4.9%, 0.9% and 4.9% of people aged <60 while 10.9%, 1.9% and 8.4% of people aged ≥60 respectively.
932), respectively, in the general population, and increased with age irrespective of gender (Fig. S4†). In detail, low handgrip strength, low muscle mass and slow walking pace accounted for 4.9%, 0.9% and 4.9% of people aged <60 while 10.9%, 1.9% and 8.4% of people aged ≥60 respectively.
The baseline characteristics of participants by sarcopenia symptoms are shown in Table 1. Briefly, compared with the normal control group, those with sarcopenia symptoms were more likely to be older, more deprived, less educated, current smokers, physically inactive, have a lower income, more long-term conditions and family history of cancer.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 participants in UK Biobank by sarcopenia symptomsa
763 participants in UK Biobank by sarcopenia symptomsa
| Sarcopenia symptoms | ||||
|---|---|---|---|---|
| Normal control group | Low handgrip strength | Low muscle mass | Slow walking pace | |
| Abbreviation: BMI, body mass index; IQR, inter quartile range.a Data were expressed as median (IQR) or number of participants (proportion). Nonparametric tests were used for continuous variables and chi-square tests were used for categorical variables, all P value <0.05. | ||||
| Number of participants, n (%) | 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462 (86.6) 462 (86.6) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 811 (7.4) 811 (7.4) |
4734 (1.3) | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 (6.3) 932 (6.3) |
| Age, median (IQR), years | 57.0 (49.0, 62.0) | 61.0 (56.0, 65.0) | 61.0 (56.0, 66.0) | 60.0 (54.0, 65.0) |
| Men, n (%) | 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227 (47.3) 227 (47.3) |
9511 (35.5) | 3423 (72.3) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 257 (44.7) 257 (44.7) |
| Townsend deprivation index, median (IQR) | −2.4 (−3.8, −0.1) | −1.9 (−3.5, 1.0) | −2.0 (−3.7, 0.9) | −1.3 (−3.2, 2.0) |
| Education, n (%) | ||||
| College or university | 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 (35.8) 291 (35.8) |
6610 (24.7) | 1769 (37.4) | 4621 (20.2) |
| Vocational | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 674 (11.4) 674 (11.4) |
3151 (11.8) | 581 (12.3) | 2893 (12.6) |
| Upper secondary | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 (12.2) 261 (12.2) |
2701 (10.1) | 518 (10.9) | 2125 (9.3) |
| Lower secondary | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 748 (27.4) 748 (27.4) |
7273 (27.1) | 986 (20.8) | 6054 (26.4) |
| Others | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 813 (12.7) 813 (12.7) |
6837 (25.5) | 852 (18.0) | 7025 (30.6) |
| Unknown | 1675 (0.5) | 239 (0.9) | 28 (0.6) | 214 (1.0) |
| Household income, n (%), £ | ||||
<18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 837 (15.3) 837 (15.3) |
7959 (29.7) | 1223 (25.8) | 8476 (37.0) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30 000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999 999 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 (21.8) 341 (21.8) |
6305 (23.5) | 1223 (25.8) | 5073 (22.1) |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–51 000–51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999 999 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 (25.1) 569 (25.1) |
4691 (17.5) | 944 (19.9) | 3599 (15.7) |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100 000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 (21.2) 332 (21.2) |
2752 (10.3) | 620 (13.1) | 1849 (8.1) |
>100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068 (5.8) 068 (5.8) |
609 (2.3) | 150 (3.2) | 362 (1.6) |
| Unknown | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 (11.0) 315 (11.0) |
4495 (16.8) | 574 (12.1) | 3573 (15.6) |
| BMI, median (IQR), kg m−2 | 26.5 (24.1, 29.4) | 27.1 (24.3, 30.7) | 20.3 (19.1, 21.6) | 30.7 (26.9, 35.0) |
| Alcohol frequency, n (%) | ||||
| Daily or almost daily | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828 (22.3) 828 (22.3) |
4801 (17.8) | 1456 (30.7) | 3722 (16.2) |
| 3 or 4 times a week | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 (25.9) 054 (25.9) |
5191 (19.2) | 1068 (22.5) | 3611 (15.8) |
| 1 or 2 times a week | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 745 (26.7) 745 (26.7) |
6817 (25.3) | 964 (20.3) | 5378 (23.5) |
| 1 to 3 times a month | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 (11.0) 422 (11.0) |
3138 (11.6) | 409 (8.6) | 2894 (12.6) |
| Special occasions only | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 (9.0) 286 (9.0) |
4111 (15.2) | 490 (10.3) | 4239 (18.5) |
| Never | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037 (5.1) 037 (5.1) |
2899 (10.8) | 358 (7.5) | 3067 (13.4) |
| Current smokers, n (%) | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 311 (9.4) 311 (9.4) |
2706 (10.1) | 932 (19.7) | 3901 (17.0) |
| Physical activity, n (%) | ||||
| Vigorous | 178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 (57.1) 917 (57.1) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705 (47.4) 705 (47.4) |
2478 (52.3) | 7155 (31.2) |
| Moderate | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235 (12.2) 235 (12.2) |
3096 (11.6) | 582 (12.3) | 2436 (10.6) |
| Low | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 (30.7) 310 (30.7) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 (41.1) 010 (41.1) |
1674 (35.4) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 (58.2) 341 (58.2) |
| Family history of cancer, n (%) | 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362 (36.5) 362 (36.5) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (38.8) 400 (38.8) |
1809 (38.2) | 8984 (39.2) |
| No. of long-term conditions, n (%) | ||||
| None | 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 (41.4) 620 (41.4) |
6262 (23.4) | 1899 (40.1) | 2808 (12.2) |
| One | 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267 (33.6) 267 (33.6) |
8106 (30.2) | 1570 (33.2) | 5664 (24.7) |
| Two or more | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575(25.1) 575(25.1) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 (46.4) 443 (46.4) |
1265 (26.7) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 (63.1) 460 (63.1) |
Overall age-stratified and sex-stratified lung cancer risk by sarcopenia symptoms
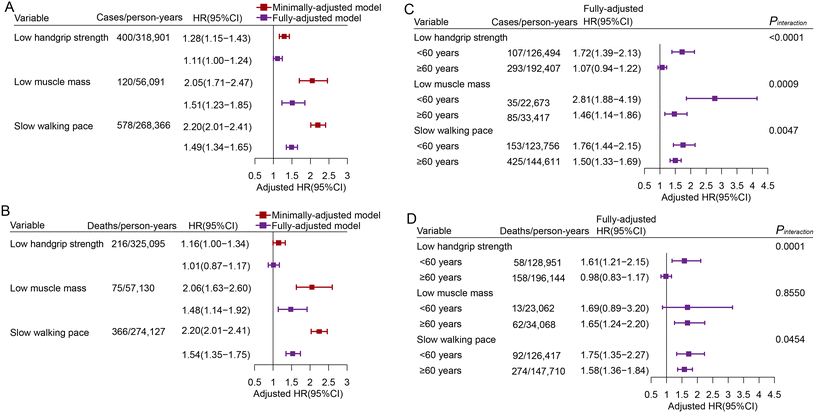
After a median follow-up of 12 years, 3532 incident cases and 2073 deaths from lung cancer occurred. We assessed the associations between sarcopenia symptoms and lung cancer-related outcomes using three separate models, with individuals lacking all three sarcopenia symptoms serving as the reference group (Fig. 1A, B and Table S4†). In the minimally-adjusted model, low handgrip strength, low muscle mass and slow walking pace were independently associated with 1.28-, 2.05- and 2.20-fold risk of lung cancer incidence, as well as 1.16-, 2.06- and 2.20-fold risk of lung cancer mortality, respectively, in contrast to the normal control group. In the fully-adjusted model, the associations of low handgrip strength (HR: 1.11, 95% CI: 1.00–1.24), low muscle mass (HR: 1.51, 95%CI: 1.23–1.85) and slow walking pace (HR: 1.49, 95%CI: 1.34–1.65) with lung cancer incidence, although attenuated, remained significant. Similarly, a higher risk of lung cancer mortality was observed in low muscle mass (HR: 1.48, 95% CI: 1.14–1.92) and slow walking pace individuals (HR: 1.54, 95% CI: 1.35–1.75).Age-stratified and sex-stratified associations of sarcopenia symptoms with the risk of lung cancer-related outcomes are shown in Fig. 1C, D and Tables S5–S8,† respectively. We found significant interactions between sarcopenia symptoms and age groups on lung cancer risk (Pinteraction < 0.05), but not the sex group. Sarcopenia symptoms contributed more to an augmented risk of lung cancer incidence in participants under 60 years old than in those aged 60 and over. Furthermore, low handgrip strength (HR: 1.61, 95% CI: 1.21–2.15) was observed to be significantly associated with elevated risks of lung cancer mortality only in younger individuals.
PCA-derived dietary patterns and lung cancer risk
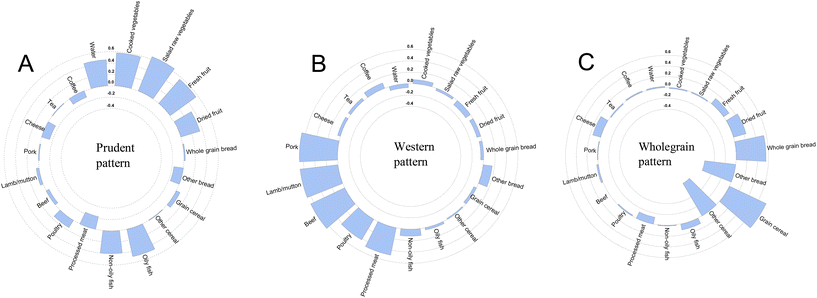
Three primary dietary patterns were discerned through PCA and were designated as the prudent pattern, western pattern, and wholegrain pattern (Fig. 2 and Table S9†). These three dietary patterns accounted for 29.4% of the total variance in the frequency of consumption of 19 food groups. The prudent pattern was positively loaded by the consumption of cooked or salad raw vegetables, fresh or dried fruits, oily or non-oily fish, and water. The western pattern exhibited a diet high in processed meat, poultry, beef, lamb/mutton, and pork. The wholegrain pattern was characterized by a high consumption of whole grain bread and grain cereal, coupled with a low intake of other bread and cereal.Subsequently, we assessed the links between dietary patterns derived using PCA and the hazards of lung cancer incidence and mortality (Table 2). Dose-dependent relationships of the prudent pattern and wholegrain pattern scores with decreased risk of lung cancer incidence and mortality were observed, while the western pattern showed the opposite effect (all Ptrend < 0.05). In the fully-adjusted model, the highest quartile (Q4) of both the prudent pattern (HR: 0.85, 95% CI: 0.77–0.93) and wholegrain pattern (HR: 0.71, 95% CI: 0.65–0.79) demonstrated a lower risk of lung cancer incidence, respectively, when compared to the lowest quartile (Q1). In contrast, the risk of lung cancer incidence was significantly elevated in participants with the Q4 level of the western pattern score compared to those with the Q1 level (HR: 1.27, 95% CI: 1.15–1.40). Similarly, the greatest adherence to either the prudent (HR: 0.84, 95% CI: 0.74–0.95) or wholegrain (HR: 0.68, 95% CI: 0.60–0.77) pattern was autonomously associated with a decreased risk of lung cancer mortality. Whereas, the highest quartile of the western pattern (HR: 1.37, 95% CI: 1.20–1.55) was associated with a higher mortality risk of lung cancer than the lowest quartile. It was noteworthy that the wholegrain consumption pattern led to a greater reduction in the risks of lung cancer. Associations between carbohydrate types, which exhibited high factor loadings on the wholegrain pattern, and the risks of lung cancer were also calculated (Table S10†). The results showed that whole grains (whole-grain bread and grain cereal) were linked to lower risks of lung cancer incidence and mortality.
| Dietary pattern | Lung cancer cases/person-years | Minimally-adjusted model | Fully-adjusted model | Lung cancer deaths/person-years | Minimally-adjusted model | Fully-adjusted model | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P trend | HR (95% CI) | P trend | HR (95% CI) | P trend | HR (95% CI) | P trend | |||
| The cox proportional hazards regression was performed to examine the associations between dietary patterns and the risk of lung cancer incidence and mortality. Minimally-adjusted model was adjusted for age (continuous), sex (men, women) and Townsend deprivation index (in quintiles). Fully-adjusted model was adjusted for covariates included in minimally-adjusted model plus body mass index (<18.5, 18.5–24.9, 25–29.9, or ≥30 kg m−2), alcohol frequency (daily or almost daily, 3 or 4 times a week, 1 or 2 times a week, 1 to 3 times a month, special occasions only, never, or unknown), smoking status (never, former, current, or unknown), family history of cancer (yes, no, or unknown), no. of long-term conditions (0, 1, 2, 3, 4, ≥5), physical activity (vigorous, moderate, low). Abbreviations: CI, confidence interval; HR, hazard ratio; Q, quartile; Ref, reference; SD, standard deviation. | ||||||||||
| Prudent pattern | ||||||||||
| Q1 | 1069/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238 238 |
Ref. | <0.0001 | Ref. | 0.0001 | 656/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 392 392 |
Ref. | <0.0001 | Ref. | 0.0001 |
| Q2 | 861/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
0.78(0.71–0.86) | 0.93(0.85–1.02) | 511/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 711 711 |
0.77(0.68–0.86) | 0.94(0.83–1.05) | ||||
| Q3 | 798/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 339 339 |
0.69(0.63–0.76) | 0.86(0.78–0.95) | 445/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 129 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 984 984 |
0.64(0.57–0.72) | 0.82(0.73–0.93) | ||||
| Q4 | 804/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
0.67(0.61–0.73) | 0.85(0.77–0.93) | 461 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 129 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071 071 |
0.63(0.56–0.72) | 0.84(0.74–0.95) | ||||
| Per 1 SD increase | 0.85(0.82–0.88) | 0.94(0.91–0.97) | 0.84(0.80–0.88) | 0.94(0.90–0.98) | ||||||
| Western pattern | ||||||||||
| Q1 | 726/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 979 979 |
Ref. | <0.0001 | Ref. | <0.0001 | 399/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132 132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 412 412 |
Ref. | <0.0001 | Ref. | <0.0001 |
| Q2 | 802/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 573 573 |
1.08(0.98–1.20) | 1.06(0.96–1.17) | 460/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 129 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 109 |
1.13(0.99–1.29) | 1.09(0.96–1.25) | ||||
| Q3 | 870/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
1.17(1.06–1.29) | 1.10(1.00–1.22) | 517/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473 |
1.25(1.10–1.43) | 1.17(1.02–1.33) | ||||
| Q4 | 1134 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
1.45(1.32–1.60) | 1.27(1.15–1.40) | 697/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 164 164 |
1.60(1.41–1.81) | 1.37(1.20–1.55) | ||||
| Per 1 SD increase | 1.16(1.12–1.19) | 1.10(1.06–1.13) | 1.18(1.14–1.23) | 1.11(1.07–1.16) | ||||||
| Wholegrain pattern | ||||||||||
| Q1 | 1145/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808 808 |
Ref. | <0.0001 | Ref. | <0.0001 | 712/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 816 |
Ref. | <0.0001 | Ref. | <0.0001 |
| Q2 | 1002/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 847 847 |
0.92(0.84–1.00) | 0.94(0.86–1.02) | 589/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
0.88(0.79–0.98) | 0.90(0.81–1.00) | ||||
| Q3 | 750/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 977 977 |
0.68(0.62–0.74) | 0.80(0.73–0.88) | 415/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443 443 |
0.61(0.54–0.69) | 0.74(0.66–0.84) | ||||
| Q4 | 635/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
0.53(0.48–0.58) | 0.71(0.65–0.79) | 357/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
0.48(0.43–0.55) | 0.68(0.60–0.77) | ||||
| Per 1 SD increase | 0.80(0.78–0.83) | 0.89(0.86–0.92) | 0.77(0.74–0.81) | 0.87(0.83–0.90) | ||||||
The combined effect of PCA-derived dietary patterns and sarcopenia symptoms on lung cancer incidence and mortality
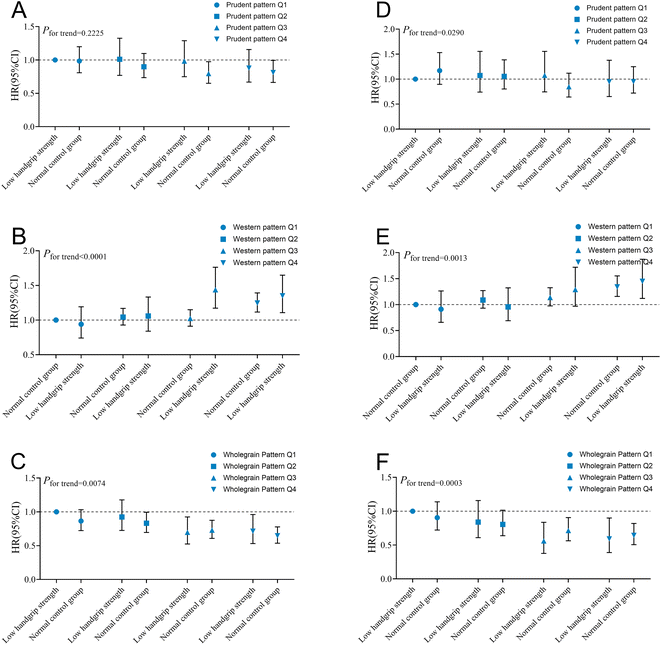
The relationships between sarcopenia symptoms and dietary patterns were calculated (Fig. S5 and Table S11†). Briefly, our study indicated that the prudent and wholegrain patterns exhibited negative associations with sarcopenia symptoms, while the western pattern displayed a positive association. We calculated additional correlations between carbohydrate types and the risk of sarcopenia symptoms. As shown in Table S12,† the results further suggested that consumption of whole grains was negatively correlated with sarcopenia symptoms, while consumption of refined grains showed a positive correlation.Besides, we explored the joint effect of PCA-derived dietary patterns and low handgrip strength on lung cancer incidence (Fig. 3A–C) and mortality (Fig. 3D–F). Of note, the risk of lung cancer incidence and mortality gradually decreased as the quartile of wholegrain pattern scores increased, while it progressively increased as the quartile of the western pattern scores increased (all Ptrend < 0.05). Compared to low handgrip strength with wholegrain pattern Q1 level, low handgrip strength with the Q4 level exhibited a 28.6% (95% CI: 0.53–0.96) reduction in incidence risk and a 40.9% (95% CI: 0.39–0.90) reduction in mortality risk. However, compared to the normal control group with the western pattern Q1 level, there was an increased risk of 1.35(95% CI: 1.11–1.65) and 1.45 times (95% CI: 1.12–1.87), respectively, for lung cancer incidence and mortality in low handgrip strength ones with the Q4 level. The combined effects of low muscle mass and slow walking pace with dietary patterns were similar to low handgrip strength (Fig. S6 and S7†). Specifically, we discovered that low muscle mass combined with the Q4 level of the wholegrain pattern was associated with a 72% and 62% reduction in the risk of lung cancer incidence and mortality, respectively, compared to low muscle mass with the Q1 level.
Secondary and sensitivity analyses
In secondary analyses, sarcopenia symptoms were found to be risk factors for non-small cell lung cancer (NSCLC), specifically LSCC (Table S13†). However, the results regarding the relationships between dietary patterns and incident lung cancer subtypes were similar (Table S14†).In stratified analyses, the associations of sarcopenia symptoms with lung cancer incidence and mortality risk remained persistent in most subgroups (Tables S15 and S16†). Taking the normal control group as the reference, low handgrip strength was linked to an enhanced risk of cancer incidence among current smokers (Pinteraction = 0.01). Notably, slow walking pace was related to risk elevation especially in higher Townsend deprivation index groups and participants with lower BMI (Pinteraction < 0.05). The effects of dietary patterns on lung cancer risk were generally similar across subgroups (Tables S17 and S18†). Finally, sensitivity analyses were performed by excluding participants who developed lung cancer or died within two years of follow-up and produced consistent results (n = 361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422) (Table S19†).
422) (Table S19†).
Discussion
In this large-scale population-based prospective cohort study, approximately 7.4%, 1.3% and 6.3% of participants suffered from low handgrip strength, low muscle mass and slow walking pace, respectively. Our findings indicated that individuals with these sarcopenia symptoms carried a significantly higher risk of lung cancer incidence and mortality with an age-specific pattern. In particular, slow walking pace had a greater impact on lung cancer incidence and mortality. We further observed that both the prudent and wholegrain patterns were negatively linked to the risk of lung cancer-related outcomes in a dose-dependent manner, in contrast to the western pattern. Furthermore, our study discovered that the wholegrain pattern improved lung cancer-related outcomes associated with low handgrip strength.The prevalence of sarcopenia varies widely in different studies because a single diagnostic criterion has not yet been established. Several attempts to standardize the operational diagnostic criterion and cut-off points for sarcopenia have been proposed, most of which have used combinations of measuring muscle mass, muscle strength, and walking pace speed. However, within these definitions, the cut-off values applied differ due to study populations or applied measurements (i.e., BIA or dual-energy X-ray absorptiometry [DXA]), which lead to a wide range of prevalence of sarcopenia in the majority of meta-analyses.20 The current EWGSOP2 recommendations are based on European populations.1 Additionally, in Stuck et al.'s review, the authors tested the predictive validity of 10 sarcopenia definitions, including those defined by the Sarcopenia Definition and Outcome Consortium (SDOC), EWGSOP2, and others, for 13 different clinical outcomes.21 Although the review did not identify which definition had the strongest predictive validity, the EWGSOP2 definition was the most widely applied, with predictive validity tested in 11 studies covering all 13 outcomes. In contrast, the SDOC definition was only investigated in one study on fractures in men. Therefore, we herein adopted the EWGSOP2 criteria for the definition of sarcopenia. What's more, one study compared the prevalence of sarcopenia using twelve different definitions in a large, multinational European population of community-dwelling older adults. It reported that the prevalence of sarcopenia using the EWGSOP2 criteria was 0.7%,22 which closely aligns with our findings.
Inflammatory pathways and insulin resistance played a key role in angiogenesis, cell proliferation, and apoptosis suppression during the development of lung cancer.23 The role of sarcopenia in lung carcinogenesis has not been fully elucidated, but studies suggested they may share similar pathophysiological mechanisms. Increased visceral fat is one of the body composition changes associated with sarcopenia.24 Abnormal accumulation of visceral fat activates inflammatory pathways that produce pro-inflammatory cytokines, which play a key role in angiogenesis, promotion of cell proliferation, and inhibition of apoptosis during lung cancer development.9 Chronic low-grade inflammation also contributes to the diminishment of muscle mass, strength and functionality, which is the key attributes of sarcopenia.25 On the other hand, reduction in skeletal muscle mass can lower insulin-mediated glucose uptake in myocytes, leading to insulin resistance,26 which can elevate insulin-like growth factor and then promote the development of lung cancer.10 Consequently, sarcopenia symptoms could constitute independent risk factors for lung cancer.
As yet, a vast majority of studies aim to systematically review the prognostic value of sarcopenia and its symptoms in lung cancer patients, especially in older patients. Only a limited number of studies have assessed the link between sarcopenia and the risk of lung cancer incidence and mortality in the general population. A long-term cohort study using physical examination data from the National Health Insurance Research Database (NHIRD) showed that patients with sarcopenia had a significantly higher risk of lung cancer compared with the non-sarcopenia group.27 This study applied the propensity score matched (PSM) method, which eliminated bias between the two groups and led to more reliable results. Correspondingly, our findings suggested that sarcopenia symptoms were associated with higher risk of lung cancer incidence (mainly LSCC). In particular, slow walking pace exhibited a more risky effect on lung cancer-related outcomes. Various research showed that walking pace may be a more effective indicator of sarcopenia severity and its risk to health,28–30 which were consistent with our findings. Hence, considering the convenient and quick detection method of walking pace, definitions and diagnoses for sarcopenia relying on reduced walking pace may be more meaningful in clinical practice and research.
Furthermore, age differences were also observed in the links between sarcopenia symptoms and lung cancer incidence and mortality, with a more evident association amongst individuals aged <60 than their older counterparts. Although sarcopenia symptoms are common in the elderly, its screening and improvement in the younger population is also critical to prevent lung cancer-related outcomes.
Dietary pattern analysis takes into account the interaction between nutrients and food, which are closer to real-world data, providing the most direct evidence for disease prevention and the revision of dietary guidelines. In particular, posterior dietary patterns, derived from all available dietary data in the study population using statistical techniques, may better reflect the usual diet of the population. Of these techniques, PCA has been most often used in the nutritional epidemiology literature.31 In accordance with previous studies,32–37 our findings supported that the Prudent dietary pattern was negatively associated with lung cancer risk, whereas the western dietary pattern demonstrated the opposite effect. Interestingly, our findings also demonstrated a stronger correlation of wholegrain dietary pattern with the reduced incidence and mortality risks of lung cancer. To date, there is a dearth of data connecting whole grains to the risk of lung cancer. The National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study indicated an inverse relationship between whole grains and incidence of lung cancer,38 which is consistent with our findings. One case-control study among Polish adults found that whole grains help to reduce insulin-like growth factors and insulin resistance, which are both also related to lung cancer risk.37 Additionally, in Roager et al.'s randomized cross-over trial, the whole grain diet, compared with the refined grain diet, decreased serum inflammatory markers, interleukin-6 and C-reactive protein. This reduction in systemic low-grade inflammation may help decrease the risk of lung cancer.39 Furthermore, given whole grains are a good source of fiber, animal studies have shown that the composition of the lung microbiota can be altered by dietary fermentable fiber to reshape the immune environment, which may be helpful for lung cancer prevention.40 Even so, the exact mechanism needs to be further explored in detail.
The present cross-sectional analysis revealed that dietary patterns were correlated with sarcopenia symptoms, with particularly strong negative associations between the wholegrain pattern and sarcopenia symptoms. Furthermore, we found that the lung cancer risk was decreased by about 30% by the combined impacts of the wholegrain pattern and low handgrip strength. Whole grains refer to the whole, powdered, fragmented or pressed pieces of cereal glume whose proportion of endosperm, germ and bran is substantially the same as in the intact glume. In contrast to refined grains, whole grains contain unique bioactive components like dietary fiber, β-glucan, phenolics, carotenoids, tocotrienol and γ-oryzanol.41 Importantly, growing evidence demonstrates that bioactive substances derived from whole grains could enhance myogenesis, muscle mass and metabolic function.41 To be specific, gut microbiota can produce short-chain fatty acids (SCFAs) through fiber fermentation, which may have a protective effect against lung cancer42 and sarcopenia symptoms.43 An observational study involving older adult men found higher levels of SCFAs in individuals with greater muscle mass, better physical function, and higher dietary fiber density (grams of fiber consumed per 100 calories).44 Additionally, γ-oryzanol supplementation has been evidenced to improve grip strength in aged mice, possibly by promoting the formation of slow-twitch muscle fibers.41 Moreover, epidemiological assessments using the National Health and Nutrition Examination Survey database indicated that whole grain intake was associated with greater walking pace.45 It may be because whole grains consumption favorably enhances protein turnover and promotes net protein balance in adults.45 Thus, increasing whole grains may help the efforts to improve sarcopenia symptoms to better avert adverse outcomes. More prospective data are warranted to confirm these findings in the future.
This study has some strengths. The prospective study design and the substantial sample size constitute the principal strengths of this study. Furthermore, to the best of our knowledge, this represented the first longitudinal study to comprehensively examine the correlations between sarcopenia symptoms, PCA-derived dietary patterns and lung cancer-related outcomes within the general population. Nonetheless, several limitations also need to be considered. Firstly, the primary exposure variables in our analysis were sarcopenia symptoms, due to a few cases of sarcopenia and severe sarcopenia. Secondly, walking pace and dietary data were self-reported at the baseline and may be prone to recall bias. Additionally, handgrip strength and ALM were measured only once at baseline, which may result in exposure misclassification and measurement errors. Thirdly, we used only the baseline data for sarcopenia symptoms and dietary intake, and did not track dynamic changes during the follow-up period, which may limit the interpretation of our results. Fourthly, we cannot estimate total energy intake because the FFQ only covered some commonly consumed foods, which do not represent the full range of dietary intake, and therefore, total energy intake was not adjusted. Fifthly, caution should be exercised in generalizing our findings to other racial or ethnic groups, as the participants were all White. Furthermore, despite extensive adjustments for potential confounders considered in our study, the possibility of residual confounding by either unmeasured or unknown confounders cannot be completely excluded.
Conclusions
Our findings indicated that all three sarcopenia symptoms, especially slow walking pace, might serve as significant risk factors for lung cancer in the general population even those under 60 years. Individuals with high adherence to a Prudent or wholegrain pattern were more likely to be free of sarcopenia and have a lower risk of lung cancer-related outcomes. Additionally, the lung cancer risk associated with low handgrip strength gradually decreased with the increase in the quartile of wholegrain pattern scores. Thus, assessment of sarcopenia symptoms may be important for lung cancer prevention in the general population, particularly younger individuals. Furthermore, adopting a dietary pattern rich in whole grains could be a novel approach to improve low handgrip strength and adverse lung cancer-related outcomes.Abbreviations
| ALM | Appendicular lean mass |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| CI | Confidence interval |
| EWGSOP | European Working Group of Sarcopenia in Older People |
| FFQ | Food frequency questionnaire |
| HR | Hazard ratio |
| ICD-9 | International Classification of Diseases, ninth revision |
| ICD-10 | International classification of diseases, tenth revision |
| IQR | Inter quartile range |
| LSCC | Lung squamous cell carcinoma |
| LUAD | Lung adenocarcinoma |
| MD | Mediterranean diet |
| NSCLC | Non-small cell lung cancer |
| PCA | Principal component analysis |
| Q | Quartile |
| Ref | Reference |
| SCLC | Small cell lung cancer |
| SD | Standard deviation |
Author contributions
Conceptualization: FS; data curation: HZ and FL; formal analysis: HZ, YP and PW; funding acquisition: FS; project administration: FS; software: HZ, JX, CS and XW; supervision: FS; validation: JXG, CS and XW; visualization: HZ, JX, JLG and AQ; writing – original draft: HZ; writing – review and editing: FL, JX, YP, PW, CS, XW, JXG, JLG, AQ, and FS. All authors read and approved the final manuscript.Ethics approval and consent to participate
The study protocol was approved by the North West Multicenter Research Ethics Committee (16/NW/0274) in the United Kingdom. All participants provided written consent to their participation in the UK biobank.Data availability
Data from the UK Biobank could be requested by researchers for approved projects through https://www.ukbiobank.ac.uk/.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFC2500401), the National Natural Science Foundation of China (81974488), the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A), and Tianjin Science and Technology Committee Foundation (24JCZDJC00590). The funding agencies had no role in study design, data collection, analysis, decision to publish, or manuscript preparation. We are grateful to all participants and project teams in the UK Biobank study. This research was conducted using the UK Biobank resource under approved project 76092.References
- A. J. Cruz-Jentoft, G. Bahat, J. Bauer, Y. Boirie, O. Bruyère, T. Cederholm, C. Cooper, F. Landi, Y. Rolland, A. A. Sayer, S. M. Schneider, C. C. Sieber, E. Topinkova, M. Vandewoude, M. Visser, M. Zamboni, I. Bautmans, J.-P. Baeyens, M. Cesari, A. Cherubini, J. Kanis, M. Maggio, F. Martin, J.-P. Michel, K. Pitkala, J.-Y. Reginster, R. Rizzoli, D. Sánchez-Rodríguez and J. Schols, Sarcopenia: revised European consensus on definition and diagnosis, Age Ageing, 2019, 48, 16–31 CrossRef PubMed.
- T. N. Kim and K. M. Choi, Sarcopenia: definition, epidemiology, and pathophysiology, J. Bone Metab., 2013, 20, 1–10 CrossRef PubMed.
- S. Yuan and S. C. Larsson, Epidemiology of sarcopenia: Prevalence, risk factors, and consequences, Metabolism, 2023, 144, 155533 CrossRef CAS PubMed.
- M. Y. Sun, C. L. Chang, C. Y. Lu, S. Y. Wu and J. Q. Zhang, Sarcopenia as an Independent Risk Factor for Specific Cancers: A Propensity Score-Matched Asian Population-Based Cohort Study, Nutrients, 2022, 14, 1910 CrossRef PubMed.
- L. Xia, R. Zhao, Q. Wan, Y. Wu, Y. Zhou, Y. Wang, Y. Cui, X. Shen and X. Wu, Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies, Cancer Med., 2020, 9, 7964–7978 CrossRef PubMed.
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA-Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed.
- U. Jogiat, Z. Jimoh, S. R. Turner, V. Baracos, D. Eurich and E. L. R. Bedard, Sarcopenia in Lung Cancer: A Narrative Review, Nutr. Cancer, 2023, 75, 1485–1498 CrossRef CAS PubMed.
- M. Yang, Y. Shen, L. Tan and W. Li, Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis, Chest, 2019, 156, 101–111 CrossRef PubMed.
- M. S. Shiels, H. A. Katki, A. Hildesheim, R. M. Pfeiffer, E. A. Engels, M. Williams, T. J. Kemp, N. E. Caporaso, L. A. Pinto and A. K. Chaturvedi, Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification, J. Natl. Cancer Inst., 2015, 107, djv199 CrossRef PubMed.
- I. Argirion, S. J. Weinstein, S. Mannisto, D. Albanes and A. M. Mondul, Serum Insulin, Glucose, Indices of Insulin Resistance, and Risk of Lung Cancer, Cancer Epidemiol. Biomarkers Prev., 2017, 26, 1519–1524 CrossRef CAS PubMed.
- A. Granic, A. A. Sayer and S. M. Robinson, Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults, Nutrients, 2019, 11, 745 CrossRef PubMed.
- A. Bahrami, S. Khalesi, E. Makiabadi, S. Alibeyk, M. Hajigholam-Saryazdi and E. Hejazi, Adherence to the Mediterranean diet and the risk of lung cancer: a systematic review and dose-response meta-analysis of observational studies, Nutr. Rev., 2022, 80, 1118–1128 CrossRef PubMed.
- R. Fabiani, G. La Porta, L. Li Cavoli, P. Rosignoli and M. Chiavarini, Adherence to Data-Driven Dietary Patterns and Lung Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis, Nutrients, 2023, 15, 4406 CrossRef PubMed.
- C. Sudlow, J. Gallacher, N. Allen, V. Beral, P. Burton, J. Danesh, P. Downey, P. Elliott, J. Green, M. Landray, B. Liu, P. Matthews, G. Ong, J. Pell, A. Silman, A. Young, T. Sprosen, T. Peakman and R. Collins, UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age, PLoS Med., 2015, 12, e1001779 CrossRef PubMed.
- H. E. Syddall, L. D. Westbury, C. Cooper and A. A. Sayer, Self-Reported Walking Speed: A Useful Marker of Physical Performance Among Community-Dwelling Older People?, J. Am. Med. Dir. Assoc., 2015, 16, 323–328 CrossRef PubMed.
- C. Yu, Z. Shi, J. Lv, H. Du, L. Qi, Y. Guo, Z. Bian, L. Chang, X. Tang, Q. Jiang, H. Mu, D. Pan, J. Chen, Z. Chen and L. Li, Major Dietary Patterns in Relation to General and Central Obesity among Chinese Adults, Nutrients, 2015, 7, 5834–5849 CrossRef PubMed.
- A. N. Previdelli, S. C. de Andrade, R. M. Fisberg and D. M. Marchioni, Using Two Different Approaches to Assess Dietary Patterns: Hypothesis-Driven and Data-Driven Analysis, Nutrients, 2016, 8, 593 CrossRef PubMed.
- H. M. E. Foster, C. A. Celis-Morales, B. I. Nicholl, F. Petermann-Rocha, J. P. Pell, J. M. R. Gill, C. A. O'Donnell and F. S. Mair, The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort, Lancet Public Health, 2018, 3, e576–e585 CrossRef PubMed.
- K. Barnett, S. W. Mercer, M. Norbury, G. Watt, S. Wyke and B. Guthrie, Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study, Lancet, 2012, 380, 37–43 CrossRef PubMed.
- F. Petermann-Rocha, V. Balntzi, S. R. Gray, J. Lara, F. K. Ho, J. P. Pell and C. Celis-Morales, Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis, J. Cachexia Sarcopenia Muscle, 2022, 13, 86–99 CrossRef PubMed.
- A. K. Stuck, G. Basile, G. Freystaetter, C. de Godoi Rezende Costa Molino, W. Lang and H. A. Bischoff-Ferrari, Predictive validity of current sarcopenia definitions (EWGSOP2, SDOC, and AWGS2) for clinical outcomes: A scoping review, J. Cachexia Sarcopenia Muscle, 2023, 14, 71–83 CrossRef PubMed.
- A. K. Stuck, L. T. Tsai, G. Freystaetter, B. Vellas, J. A. Kanis, R. Rizzoli, K. S. Kressig, G. Armbrecht, J. A. P. Da Silva, B. Dawson-Hughes, A. Egli and H. A. Bischoff-Ferrari, Comparing Prevalence of Sarcopenia Using Twelve Sarcopenia Definitions in a Large Multinational European Population of Community-Dwelling Older Adults, J. Nutr. Health Aging, 2023, 27, 205–212 CrossRef CAS PubMed.
- B. C. Bade and C. S. Dela Cruz, Lung Cancer 2020: Epidemiology, Etiology, and Prevention, Clin. Chest Med., 2020, 41, 1–24 CrossRef PubMed.
- M. J. Gomes, P. F. Martinez, L. U. Pagan, R. L. Damatto, M. D. M. Cezar, A. R. R. Lima, K. Okoshi and M. P. Okoshi, Skeletal muscle aging: influence of oxidative stress and physical exercise, Oncotarget, 2017, 8, 20428–20440 CrossRef PubMed.
- S. Dalle, L. Rossmeislova and K. Koppo, The Role of Inflammation in Age-Related Sarcopenia, Front. Physiol., 2017, 8, 1045 CrossRef PubMed.
- S. W. Lee, Y. Youm, W. J. Lee, W. Choi, S. H. Chu, Y. R. Park and H. C. Kim, Appendicular skeletal muscle mass and insulin resistance in an elderly korean population: the korean social life, health and aging project-health examination cohort, Diabetes Metab. J., 2015, 39, 37–45 CrossRef PubMed.
- M. Y. Sun, C. L. Chang, C. Y. Lu, S. Y. Wu and J. Q. Zhang, Sarcopenia as an Independent Risk Factor for Specific Cancers: A Propensity Score-Matched Asian Population-Based Cohort Study, Nutrients, 2022, 14, 1910 CrossRef PubMed.
- F. Petermann-Rocha, F. K. Ho, P. Welsh, D. Mackay, R. Brown, J. M. R. Gill, N. Sattar, S. R. Gray, J. P. Pell and C. A. Celis-Morales, Physical capability markers used to define sarcopenia and their association with cardiovascular and respiratory outcomes and all-cause mortality: A prospective study from UK Biobank, Maturitas, 2020, 138, 69–75 CrossRef PubMed.
- N. P. Bachettini, R. M. Bielemann, T. G. Barbosa-Silva, A. M. B. Menezes, E. Tomasi and M. C. Gonzalez, Sarcopenia as a mortality predictor in community-dwelling older adults: a comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People, Eur. J. Clin. Nutr., 2020, 74, 573–580 CrossRef CAS PubMed.
- A. Ganna and E. Ingelsson, 5 years mortality predictors in 498,103 UK Biobank participants: a prospective population-based study, Lancet, 2015, 386, 533–540 CrossRef PubMed.
- S. E. Steck and E. A. Murphy, Dietary patterns and cancer risk, Nat. Rev. Cancer, 2020, 20, 125–138 CrossRef CAS PubMed.
- Y. Sun, Z. Li, J. Li, Z. Li and J. Han, A Healthy Dietary Pattern Reduces Lung Cancer Risk: A Systematic Review and Meta-Analysis, Nutrients, 2016, 8, 134 CrossRef PubMed.
- H. Tu, J. V. Heymach, C. P. Wen, Y. Ye, J. A. Pierzynski, J. A. Roth and X. Wu, Different dietary patterns and reduction of lung cancer risk: A large case-control study in the U.S, Sci. Rep., 2016, 6, 26760 CrossRef CAS PubMed.
- F. He, R. D. Xiao, T. Lin, W. M. Xiong, Q. P. Xu, X. Li, Z. Q. Liu, B. C. He, Z. J. Hu and L. Cai, Dietary patterns, BCMO1 polymorphisms, and primary lung cancer risk in a Han Chinese population: a case-control study in Southeast China, BMC Cancer, 2018, 18, 445 CrossRef PubMed.
- E. De Stefani, A. L. Ronco, H. Deneo-Pellegrini, P. Correa, P. Boffetta, G. Acosta and M. Mendilaharsu, Dietary patterns and risk of adenocarcinoma of the lung in males: a factor analysis in Uruguay, Nutr. Cancer, 2011, 63, 699–706 CrossRef PubMed.
- X. Wei, C. Zhu, M. Ji, J. Fan, J. Xie, Y. Huang, X. Jiang, J. Xu, R. Yin, L. Du, Y. Wang, J. Dai, G. Jin, L. Xu, Z. Hu, H. Shen, M. Zhu and H. Ma, Diet and Risk of Incident Lung Cancer: A Large Prospective Cohort Study in UK Biobank, Am. J. Clin. Nutr., 2021, 114, 2043–2051 CrossRef CAS PubMed.
- B. Krusinska, I. Hawrysz, L. Wadolowska, M. A. Slowinska, M. Biernacki, A. Czerwinska and J. J. Golota, Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study, Nutrients, 2018, 10, 470 CrossRef PubMed.
- G. M. Anic, Y. Park, A. F. Subar, T. E. Schap and J. Reedy, Index-based dietary patterns and risk of lung cancer in the NIH-AARP diet and health study, Eur. J. Clin. Nutr., 2016, 70, 123–129 CrossRef CAS PubMed.
- H. M. Roager, J. K. Vogt, M. Kristensen, L. B. S. Hansen, S. Ibrugger, R. B. Maerkedahl, M. I. Bahl, M. V. Lind, R. L. Nielsen, H. Frokiaer, R. J. Gobel, R. Landberg, A. B. Ross, S. Brix, J. Holck, A. S. Meyer, M. H. Sparholt, A. F. Christensen, V. Carvalho, B. Hartmann, J. J. Holst, J. J. Rumessen, A. Linneberg, T. Sicheritz-Ponten, M. D. Dalgaard, A. Blennow, H. L. Frandsen, S. Villas-Boas, K. Kristiansen, H. Vestergaard, T. Hansen, C. T. Ekstrom, C. Ritz, H. B. Nielsen, O. B. Pedersen, R. Gupta, L. Lauritzen and T. R. Licht, Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial, Gut, 2019, 68, 83–93 CrossRef CAS PubMed.
- A. Trompette, E. S. Gollwitzer, K. Yadava, A. K. Sichelstiel, N. Sprenger, C. Ngom-Bru, C. Blanchard, T. Junt, L. P. Nicod, N. L. Harris and B. J. Marsland, Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis, Nat. Med., 2014, 20, 159–166 CrossRef CAS PubMed.
- Q. Li, H. Yang, S. Song, J. Liu, Z. Wang and J. Wang, Bioactive Components in Whole Grains for the Regulation of Skeletal Muscle Function, Foods, 2022, 11, 2752 CrossRef CAS PubMed.
- A. Koh, F. De Vadder, P. Kovatcheva-Datchary and F. Backhed, From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites, Cell, 2016, 165, 1332–1345 CrossRef CAS PubMed.
- C. Liu, W. H. Cheung, J. Li, S. K. Chow, J. Yu, S. H. Wong, M. Ip, J. J. Y. Sung and R. M. Y. Wong, Understanding the gut microbiota and sarcopenia: a systematic review, J. Cachexia Sarcopenia Muscle, 2021, 12, 1393–1407 CrossRef PubMed.
- K. Barger, L. Langsetmo, E. S. Orwoll and M. S. Lustgarten, Investigation of the Diet-Gut-Muscle Axis in the Osteoporotic Fractures in Men Study, J. Nutr., Health Aging, 2020, 24, 445–452 CrossRef CAS PubMed.
- J. T. Mey, J. P. Godin, A. R. Scelsi, E. L. Kullman, S. K. Malin, S. Yang, Z. E. Floyd, A. Poulev, R. A. Fielding, A. B. Ross and J. P. Kirwan, A Whole-Grain Diet Increases Whole-Body Protein Balance Compared with a Macronutrient-Matched Refined-Grain Diet, Curr. Dev. Nutr., 2021, 5, nzab121 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo03332a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |