Open Access Article
Open Access ArticleRed wine consumption activates the erythropoietin–erythroferrone–hepcidin erythropoietic pathway in both apparently healthy individuals and patients with type 2 diabetes†
Jurica
Nazlić
a,
Diana
Gujinović
b,
Ivana
Mudnić
b,
Zvonimir
Boban
c,
Ana Marija
Dželalija
b,
Leida
Tandara
d,
Katarina
Gugo
d,
Maja
Radman
e,
Vedran
Kovačić
a and
Mladen
Boban
 *b
*b
aDepartment of Intensive Medicine and Clinical Pharmacology, University Hospital of Split, Šoltanska 1, Split 21000, Croatia
bDepartment of Pharmacology, University of Split School of Medicine, Šoltanska 2a, Split 21000, Croatia. E-mail: mladen.boban@mefst.hr
cDepartment of Medical Physics and Biophysics, University of Split School of Medicine, Šoltanska 2a, Split 21000, Croatia
dDepartment of Medical Laboratory Diagnostics, University Hospital of Split, Spinčićeva 1 and University of Split School of Medicine, Šoltanska 2a, Split 21000, Croatia
eDepartment of Endocrinology, Diabetes and Metabolic Diseases, University Hospital of Split, Šoltanska 1, Split 21000, Croatia
First published on 28th January 2025
Abstract
Alcohol consumption is associated with reduced expression of hepcidin, a key iron-regulatory hormone, which may lead to accumulation of iron in the body. Although polyphenols from wine may have effects on hepcidin expression and iron absorption contrary to that of alcohol, we recently showed that consumption of 300 ml of red wine for 3 weeks, after an alcohol-free lead-in period of 2 weeks, resulted in decreased serum hepcidin in apparently healthy individuals (n = 13) and subjects with type 2 diabetes (T2D) (n = 18). To determine the mechanism of decrease in hepcidin after wine intervention, additional biochemical analyses of spare serum samples from the same subjects were performed. The decrease in hepcidin was accompanied by increased erythropoietin levels in both groups, while the increase in erythroferrone reached statistical significance only in the T2D group. These results suggest activation of the erythropoietin–erythroferrone–hepcidin pathway by red wine consumption. As an indicator of the activation of the erythropoietin–erythroferrone–hepcidin pathway we observed an increase in the red cell distribution width in both groups and in the reticulocyte count in the T2D group, while serum ferritin decreased. Our study reveals a novel biological effect of wine that may be important in conditions influencing iron homeostasis and functions of hepcidin in general.
Introduction
It is well known that alcohol consumption facilitates the accumulation of iron in the body.1,2 Depending on the circumstances, this can be beneficial or harmful to human health. For example, the risk of iron deficiency anemia is 40% lower in people who consume alcohol.3 On the other hand, alcohol in combination with the accumulation of iron in the liver promotes inflammation, fibrinogenesis and carcinogenesis.4Alcohol-induced gastric acid secretion was proposed to be the mechanism of alcohol-induced facilitation of iron absorption from food.1 Newer findings suggest that alcohol increases iron absorption indirectly, by reduction of hepcidin expression in the liver.5,6
That mechanism was confirmed in animal models,7,8 and in people with alcoholic liver disease it was observed that they have lower levels of hepcidin.9
Hepcidin is a peptide hormone predominantly secreted by hepatocytes and plays a crucial role in iron homeostasis.10 Hepcidin reduces iron bioavailability by triggering the internalization and degradation of ferroportin, the sole known cellular iron exporter.11 Since erythropoiesis is by far the largest consumer of iron in the body, various conditions influencing erythropoiesis can affect the kinetics of hepcidin as well.12,13
Different types of alcoholic beverages may have different effects on iron absorption and indicators of iron homeostasis.2,14,15 In this context, the effects of red wine consumption may be of particular interest. Namely, polyphenols that are abundantly present in red wine may have effects on the expression of hepcidin and on the absorption of iron contrary to that of alcohol.5,16,17
In our recent publication, we showed that moderate consumption of red wine for 3 weeks was accompanied by a decrease in serum hepcidin levels in both apparently healthy individuals and type 2 diabetic patients.18 However, the mechanism of decrease of hepcidin levels associated with red wine consumption remained unclear. We hypothesized that the reduction in hepcidin could be the result of activation of the erythropoietin–erythroferrone–hepcidin erythropoietic pathway. Therefore, we performed post hoc biochemical analyses of spare serum samples from the same subjects, primarily focusing on the levels of erythropoietin (EPO) and erythroferrone (ERFE) before and after wine consumption. This paper presents and discusses integrated measurement results from both studies.
Materials and methods
Study design
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Split School of Medicine, Croatia (No. 2181-198-03-04-13-0042). Participants were divided into 2 groups: apparently healthy subjects and patients with type 2 diabetes. Inclusion criteria for both groups were: (1) males, (2) aged between 40 and 65 years, (3) non-severely obese (BMI < 35 kg m−2), (4) non-smokers, and (5) willing to give consent and carry out all study-related procedures.Participants from both groups were matched for age and BMI. Subjects from the healthy group had fasting plasma glucose ≤6.9 mmol L−1, while diabetic subjects were eligible if they had controlled glycemia (HbA1c value ≤7.5% (58 mmol mol−1)) under treatment with metformin alone or in combination with other oral glucose-lowering medications.
Exclusion criteria were: (1) medical history of atherosclerotic cardiovascular disease or venous thromboembolism, (2) current evidence of acute or chronic inflammatory or infective disease, (3) liver disease, (4) malignancy, (5) dysregulation of iron homeostasis (anemia or hereditary hemochromatosis), (6) previous alcohol or substance abuse, and (7) introduction of new pharmacological agents during the study period.
After a lead-in period of 2 weeks, in which consumption of any alcoholic beverage was prohibited, subjects in both groups started to drink 300 ml of red wine daily for 3 weeks. The daily consumption amount had to be split between lunch and dinner and taken with meals. Red wine used in this study was produced from the Croatian autochthonous red cultivar Plavac Mali (Vitis vinifera L.), Volarevic winery, vintage 2016. It contained 14.6 vol% of ethanol, and total phenolic content was 2186 mg gallic acid equivalent (GAE) per L. Detailed information on physicochemical properties of the wine and its phenolic composition is available from our previous publication.18
A total of 31 participants completed the study protocol: 18 subjects with type 2 diabetes and 13 apparently healthy subjects.
Anthropometric assessments and blood sampling
At the end of the lead-in and intervention periods, participants’ weight, height, and body circumference were measured. The body mass index (BMI) was calculated as the ratio of weight and the square of height (kg m−2). Fasting blood samples were taken early in the morning and, depending on the type of laboratory parameter, were analyzed the same day or stored at −80 °C for later analysis. Three types of vacutainers were used: (1) one with K3EDTA, to determine the complete blood count and HbA1c; (2) a container with fluoride/EDTA, to estimate fasting plasma glucose concentration; and (3) one with silica (clot activator)/gel, to separate serum required for the analysis of liver and kidney function, inflammation, and serum-based indicators of iron status (UIBC, TIBC, iron, EPO, ERFE, hepcidin, ferritin, soluble transferrin receptors) and glycemic control (fructosamine). Levels of soluble transferrin receptors (sTfR) were measured using a nephelometric method on a BN ProSpec analyzer (Siemens, ProSpec, Erlangen, Germany). Serum hepcidin was quantified by using a commercially available competitive ELISA kit (Hepcidin 25 (bioactive) HS, DRG Instruments GmbH, Germany). EPO and ERFE concentrations were measured using commercially available ELISA tests: Quantikine® IVD® Human Erythropoietin ELISA (R&D Systems Inc) and Intrinsic Erythroferrone IETM ELISA (Intrinsic LifeSciences). Analyses were performed according to the manufacturers’ instructions.Statistical analysis
Depending on whether or not the distribution of values was normal, the data were presented as a mean ± standard deviation (SD) or a median with 95% confidence interval (CI). The normality of distributions was checked by inspecting the Q–Q plots and using the Shapiro–Wilk test. The within-group comparisons before and after the intervention were performed using the paired-samples t-test or its nonparametric equivalent – the Wilcoxon signed rank test. Between-group comparisons were made using the two-sample t-test for independent samples or its nonparametric equivalent – the Mann–Whitney test. The comparison of absolute parameter values between groups was performed only for anthropometric measurements before the intervention. All other between-group tests compared only the magnitude of response induced by the treatment. P-values less than 0.05 were considered statistically significant. The R programming language for statistical computing version 4.2.1 was used for statistical analyses and data visualization.Results
Anthropometric data of all participants at the baseline before the intervention are shown in Table 1. Subjects from the healthy group were comparable with the subjects with type 2 diabetes with regard to age, weight, height, BMI value, waist, and hip circumference. Table 2 shows basic biochemical data of participants in both groups in relation to parameters of hepatic and kidney function, the grade of inflammation and glucose levels at the baseline and after the wine intervention. All parameters in both groups (except fasting glucose levels in participants from the T2D group) were within the reference range at the baseline and remained normal and basically unchanged after the intervention.| Parameter | Healthy (n = 13) | T2D (n = 18) | P-value |
|---|---|---|---|
| Normally distributed variables are presented as mean ± SD, whilst non-normally distributed variables are presented as median with 95% CIs. The P-values show the significance of between-group tests. P-values less than 0.05 were considered statistically significant. Abbreviations: BMI, body mass index; N/A, not applicable; T2D, type 2 diabetes. | |||
| Age [years] | 50.5 ± 5.9 | 54.6 ± 6.2 | 0.075 |
| Age at T2D onset [years] | N/A | 50.6 ± 6.7 | N/A |
| Weight [kg] | 100.3 (84.7, 105.5) | 98.5 (87.2, 107.0) | 0.617 |
| Height [cm] | 186.8 ± 5.6 | 184.5 ± 10.1 | 0.427 |
| Waist circumference [cm] | 106.0 (95.8, 109.5) | 107.0 (98.8, 112.4) | 0.458 |
| Hip circumference [cm] | 108.0 ± 5.6 | 105.0 ± 7.5 | 0.237 |
| Upper arm circumference [cm] | 35.2 ± 2.9 | 32.2 ± 3.4 | 0.015 |
| Neck circumference [cm] | 42.0 (40.3, 44.0) | 38.5 (37.0, 41.2) | 0.051 |
| BMI [kg m−2] | 27.2 ± 2.7 | 29.8 ± 4.1 | 0.062 |
| Laboratory parameter | Healthy (n = 13) | T2D (n = 18) | ||||
|---|---|---|---|---|---|---|
| Baseline | After red wine | P-value | Baseline | After red wine | P-value | |
| Normally distributed variables are presented as mean ± SD, whilst non-normally distributed variables are presented as median with 95% CIs. The P-values show the significance of within-group tests. P-values less than 0.05 were considered statistically significant. Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; hsCRP, high-sensitivity C-reactive protein; T2D, type 2 diabetes. One participant from the healthy group was excluded from the albumin analysis and two were excluded from the total bilirubin analysis due to lacking data for the 2nd measurement. | ||||||
| AST [IU L−1] | 27.1 ± 7.1 | 24.8 ± 5.6 | 0.324 | 22.1 ± 4.9 | 22.4 ± 5.2 | 0.740 |
| ALT [IU L−1] | 25.0 (19.5, 39.2) | 24 (20.0, 38.0) | 0.321 | 25.5 (16.4, 29.6) | 22.5 (20.5, 35.5) | 0.868 |
| GGT [IU L−1] | 28.0 (23.5, 43.7) | 31 (26, 50) | 0.074 | 25.5 (20.0, 32.2) | 27.5 (24.0, 34.0) | 0.486 |
| Albumin [g L−1] | 45.0 (45.0, 46.0) | 45 (42.5, 45.0) | 0.012 | 43.0 (41.4, 44.0) | 42.0 (41.0, 43.5) | 0.164 |
| Total bilirubin [μmol L−1] | 13.8 ± 4.0 | 14.6 ± 6.7 | 0.441 | 12.4 ± 4.6 | 12.0 ± 4.6 | 0.559 |
| Urates [μmol L−1] | 328.9 ± 43.8 | 346 ± 38 | 0.078 | 359.6 ± 81.2 | 352 ± 56 | 0.543 |
| hsCRP [mg L−1] | 1.3 (0.7, 1.9) | 1.2 (0.7, 2.8) | 0.129 | 1.5 (0.8, 2.9) | 1.3 (0.9, 2.5) | 0.918 |
| Urea [mmol L−1] | 5.8 ± 1.1 | 5.7 ± 1.4 | 0.250 | 6.2 ± 1.6 | 6.1 ± 1.3 | 0.84 |
| Creatinine [μmol L−1] | 78.5 ± 8.1 | 83 ± 13 | 0.051 | 83.5 ± 9.2 | 80 ± 12 | 0.72 |
| Fasting glucose [mmol L−1] | 5.3 ± 0.4 | 5.7 ± 0.4 | 0.115 | 7.5 ± 1.4 | 7.3 ± 1.4 | 0.294 |
Hematological and biochemical markers of the iron status in the diabetic and healthy groups, at the baseline and post-intervention, are presented in Table 3.
| Laboratory parameter | Healthy (n = 13) | T2D (n = 18) | ||||
|---|---|---|---|---|---|---|
| Baseline | After red wine | P-value | Baseline | After red wine | P-value | |
| Normally distributed variables are presented as mean ± SD, whilst non-normally distributed variables are presented as median with 95% CIs. The P-values show the significance of within-group tests. P-values less than 0.05 were considered statistically significant. Abbreviations: MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RDW, red cell distribution width; RBC, red blood cell; sTfR, soluble transferrin receptor; TIBC, total iron-binding capacity; T2D, type 2 diabetes; UIBC, unsaturated iron-binding capacity. In the healthy group one participant had undetectable levels of sTfR, and was thus excluded from the analysis, and for another values of erythropoietin and erythroferrone at the baseline were missing due to a deficient sample. | ||||||
| RBC [× 1012 L−1] | 5.2 ± 0.5 | 5.1 ± 0.4 | 0.077 | 5.0 ± 0.3 | 4.9 ± 0.3 | 0.888 |
| Hematocrit [L L−1] | 0.45 ± 0.039 | 0.45 ± 0.034 | 0.388 | 0.44 ± 0.019 | 0.44 ± 0.021 | 0.749 |
| Hemoglobin [g L−1] | 154.2 ± 12.6 | 153.0 ± 10.6 | 0.348 | 151.4 ± 6.2 | 151.5 ± 6.5 | 0.969 |
| Reticulocytes [× 109 L−1] | — | 60 ± 16 | 70 ± 15 | 0.005 | ||
| RDW [%] | 13.0 ± 0.5 | 13.2 ± 0.5 | 0.013 | 13.5 ± 0.4 | 13.6 ± 0.5 | 0.033 |
| MCH [pg] | 29.6 ± 1.0 | 29.9 ± 1.0 | 0.047 | 30.7 ± 1.3 | 30.7 ± 1.4 | 0.709 |
| MCHC [g L−1] | 337.5 ± 7.3 | 339.5 ± 8.8 | 0.261 | 341.4 ± 5.5 | 342.7 ± 6.8 | 0.349 |
| MCV [fL] | 87.1 (84.9–90.1) | 87.2 (85.2–91.5) | 0.675 | 89.8 ± 3.6 | 89.6 ± 3.5 | 0.495 |
| Serum iron [μmol L−1] | 21.8 ± 7.1 | 19.6 ± 6.9 | 0.328 | 15.0 (13.8–18.7) | 16.4 (14.6–20.9) | 0.177 |
| TIBC [μmol L−1] | 54.2 ± 9.3 | 53.8 ± 12.1 | 0.767 | 57.8 ± 7.2 | 58.2 ± 7.6 | 0.434 |
| UIBC [μmol L−1] | 31.6 ± 11.6 | 34.2 ± 11.4 | 0.109 | 39.4 (37.5–45.5) | 41.6 (36.8–46.5) | 0.453 |
| Transferrin saturation [%] | 40.6 ± 12.9 | 37.0 ± 11.3 | 0.307 | 26.7 (22.6–29.9) | 29.3 (24.5–32.7) | 0.265 |
| sTfR [mg L−1] | 1.20 ± 0.22 | 1.24 ± 0.25 | 0.734 | 1.05 ± 0.17 | 1.04 ± 0.20 | 0.830 |
| Ferritin [ng mL−1] | 173.0 (126.4–259.8) | 118.0 (90.5–232.6) | 0.017 | 209.5 ± 141.5 | 198.8 ± 139.4 | 0.215 |
| Erythropoietin [mlU ml−1] | 10.6 (7.7, 12.1) | 12.5 (10.2, 14.1) | 0.001 | 7.6 (6.4, 9.4) | 8.9 (8.4, 11.1) | 0.024 |
| Erythroferone [ng ml−1] | 0.11 ± 0.14 | 0.17 ± 0.19 | 0.270 | 0.044 ± 0.075 | 0.12 ± 0.12 | 0.028 |
| Hepcidin [ng mL−1] | 30.0 ± 17.3 | 21.0 ± 12.1 | 0.045 | 17.9 (11.9–25.2) | 13.2 (8.2–18.3) | 0.001 |
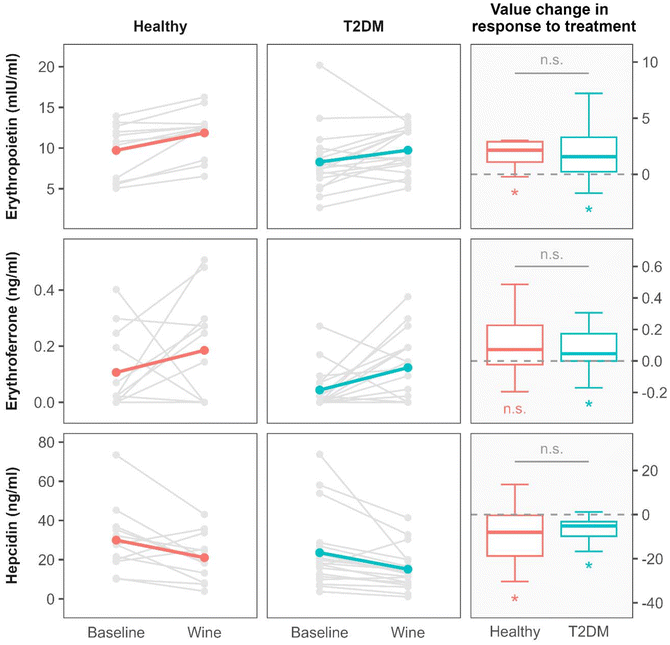
After 3 weeks of red wine consumption, a significant decrease in hepcidin levels was observed within both groups. It was accompanied by a significant increase in EPO levels in both groups while the increase in ERFE levels reached statistical significance only in the T2D group (Fig. 1 and Table 3). The magnitude of change in EPO, ERFE and hepcidin levels as a response to the intervention with wine did not differ between the groups (Fig. 1).
Changes in EPO, ERFE and hepcidin levels were not accompanied by changes in serum iron and other standard Fe-related parameters, which remained largely unaffected after the intervention with wine consumption (Table 3).
However, after red wine consumption, two significant increases were observed: of the red cell distribution width (RDW) in both study groups; and of the reticulocyte count in the T2D group (Table 3). The reticulocyte count in the healthy group was not measured.
Discussion
Although hepcidin is implicated in multiple biological functions and its kinetics can be influenced by different (patho)physiological conditions, our results imply that the decrease in serum levels of hepcidin following consumption of red wine for 3 weeks might be the result of an activation of the erythropoietin–erythroferrone–hepcidin erythropoietic pathway in both apparently healthy individuals and in patients with type 2 diabetes. To our knowledge, this paper is the first to describe the effect of red wine consumption on the erythropoietin–erythroferrone–hepcidin pathway in humans.Despite the fact that full understanding of all mechanisms involved in regulation of this erythropoietic pathway is lacking, it is generally recognized that the main iron-regulatory hormone hepcidin is suppressed by ERFE, a hormone synthesized and secreted by erythroid precursors in the bone marrow in response to EPO. The result of hepcidin inhibition is enhanced iron mobilization and sustained erythropoiesis.10,19 Our results are in accordance with the described mechanisms. After the intervention with wine consumption, EPO and ERFE increased and hepcidin decreased in both groups (Fig. 1 and Table 3). In the healthy group, however, the increase in the ERFE level did not reach statistical significance as the small size of the group could not compensate for the wider distribution of ERFE values observed in the healthy participants.
As an indicator of erythropoietic response to the activation of the EPO–ERFE–hepcidin pathway following wine-drinking intervention, we observed a significant increase in the reticulocyte count in the T2D group (Table 3). The baseline value of the absolute reticulocyte count in the T2D group was within the reference interval that typically ranges from 50 to 100 × 109 L−1 (ref. 20). An interesting finding in that regard is also an increase in the red cell distribution width (RDW) that occurred in both study groups after the intervention (Table 3). The RDW is a calculated erythrocyte parameter reflecting the degree of heterogeneity of red blood cell volumes in the blood sample. As such it can be considered a marker of altered erythrocyte biology associated with various (para)physiological and pathological challenges.21 Among these and in the context of our results, it is worth noting that an increase in RDW was associated with erythropoietic activity induced by exogenous EPO in healthy volunteers,22 while in some pathological conditions, such as coronary artery disease, RDW was significantly related to endogenous EPO levels.23
Changes in kinetics of EPO, ERFE and hepcidin indicative of erythropoiesis activation were not reflected in changes of the serum iron. Moreover, other standard laboratory indicators of the iron status also remained mostly unchanged after the intervention (Table 3). While the decrease in serum hepcidin after the wine intervention did not result in changes of the serum iron levels, we found that the mean levels of serum ferritin decreased after red wine consumption in both groups, with statistical significance reached only in the healthy group. A possible explanation for this observation is that the decrease in hepcidin levels leads to increased cellular iron export. This in turn may be associated with decreased ferritin production and lower circulating ferritin. Indeed, a linear relationship between serum hepcidin and ferritin is well established.24 The mobilized iron could be used at higher rates, likely because of increased erythropoiesis. It might be that the described processes take longer than 3 weeks to significantly influence the iron status indicators. Nevertheless, the effects of red wine consumption on the body iron indices are influenced by more complex regulatory mechanisms that extend beyond the sole effects of ethanol. Namely, red wine is a rich source of polyphenolic compounds that may inhibit absorption of dietary iron through formation of chelates with iron in the gastrointestinal tract, making the iron less available for absorption.5,25 In addition to these direct inhibitory effects on iron absorption, some phenolic compounds found in wine were shown to induce hepcidin expression, thereby reducing systemic iron levels16 and preventing ethanol-induced iron overload.17
Apart from being the main regulator of iron homeostasis, hepcidin is a type II acute-phase protein and its synthesis is stimulated in inflammatory disorders.10,26 The potential influence of this mechanism on the changes in serum hepcidin levels in our study is not likely. Namely, the levels of high-sensitivity C-reactive protein remained low in both participant groups indicating the absence of acute inflammatory processes during the experimental period (Table 2).
Another pathogenic mechanism that may influence serum hepcidin levels is chronic kidney disease, which may impair renal clearance of hepcidin.27 This mechanism can also be excluded as an interfering factor with respect to the observed results. Namely, urea and creatinine levels were in the reference range and did not change over the course of the intervention period indicating stable kidney function of the participants.
Complex relationships between wine phenolics, ethanol and iron homeostasis are of particular importance for patients with diseases in which the pathophysiology of iron plays a role, such as T2D and genetic hemochromatosis.
Iron overload is a well-established risk factor for T2D, indicating a potential role of hepcidin in its pathogenesis.28 Indeed, conditions that are characterized by iron overload, such as genetic hemochromatosis, are associated with increased risk of T2D.29 In hemochromatosis, absorption of dietary iron is enhanced due to a decreased expression of hepcidin. So, red wine rich in polyphenols that may inhibit the effects of alcohol on iron uptake may be a better choice in hemochromatosis than alcoholic beverages with no or low polyphenolic content.30
In contrast to hemochromatosis that is a low-hepcidin condition, T2D may also be associated with pathologies characterized by elevated hepcidin levels. It is hypothesized that high hepcidin downregulates the cellular iron exporter ferroportin, causing iron overload in cells and tissues with ferroprotein expression.28,31 Excess free iron is associated with an increased level of oxidative stress, which is considered an important contributor to the loss of insulin secretory capacity in diabetes. Moreover, the risk of diabetes related to iron is evident in most or all tissues that determine diabetes phenotypes.28
In the described setting, it is possible that moderate consumption of red wine may positively influence the maintenance of iron homeostasis. This in turn could contribute to the cardiometabolic benefits of the moderate consumption of red wine in diabetic patients as has been described in numerous epidemiological and interventional human studies.32
Indeed, moderate consumption of red wine is an important component of the Mediterranean diet,33 which is considered one of the healthiest dietary patterns34 that has been proven beneficial in preventing and controlling diet-related non-communicable diseases including cardiovascular diseases and type 2 diabetes.35
Taken together, the present study provides evidence of an important biological effect of moderate red wine consumption. It may enhance our understanding of the role of wine in conditions that are associated with iron homeostasis and with the biological effects of hepcidin in general. Due to objective limitations, our study cannot offer far-reaching and definitive answers. Limitations of our study include the small size of the experimental groups and relatively short duration of intervention and follow-up periods. Also, our study cannot offer information on possible mechanisms of how constituents of red wine might directly or indirectly influence synthesis and release of each component of the erythropoietin–erythroferrone–hepcidin pathway. Therefore, studies with a larger number of subjects and a longer duration that will test a greater number of outcomes related to iron homeostasis and the biology of hepcidin are warranted.
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CI | Confidence interval |
| ELISA | Enzyme-linked immunosorbent assay |
| EPO | Erythropoietin |
| ERFE | Erythroferrone |
| GAE | Gallic acid equivalent |
| GGT | Gamma-glutamyl transferase |
| HbA1c | Hemoglobin A1c |
| hsCRP | High-sensitivity C-reactive protein |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| N/A | Not applicable |
| n.s. | Not significant |
| RBC | Red blood cell |
| RDW | Red cell distribution width |
| SD | Standard deviation |
| sTfR | Soluble transferrin receptor |
| TIBC | Total iron binding capacity |
| T2D | Type 2 diabetes |
| UIBC | Unsaturated iron binding capacity |
Author contributions
Conceptualization, J. N., I. M., L. T. and M. B.; methodology, J. N., D. G., A. M. DŽ., V. K., M. R. and L. T.; software, Z. B.; validation J. N., K. G., L. T., Z. B. and M. B.; formal analysis, Z. B. and V. K.; investigation, J. N., M. R., V. K., I. M., A. M. DŽ., D. G., K. G., L. T.; resources, M. B. and L. T.; data curation, D. G., A. M. DŽ., Z. B., V. K., L. T. and K. G.; writing—original draft preparation, J. N. and M. B.; writing—review and editing, J. N., I. M., D. G., A. M. DŽ., L. T., M. R., Z. B., M. B.; visualization, Z. B.; supervision, M. B. and L. T.; project administration, J. N., V. K., M. R., D. G., I. M. and A. M. DŽ.; funding acquisition, M. B.; all authors have read and agreed to the published version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the Croatian Science Foundation (“Biological effects of wine: the influence of vinification technology, dealcoholization and aging of wine”) under project no. IP-2013-11-8652, with M.B. as the project leader. The funder had no role in the design of this study, its execution, the analyses, the interpretation of the data, or the decision to submit the results.References
- R. W. Charlton, P. Jacobs, H. Seftel and T. H. Bothwell, Effect of Alcohol on Iron Absorption, Br. Med. J., 1964, 2, 1427–1429 CrossRef CAS PubMed.
- J. B. Whitfield, G. Zhu, A. C. Heath, L. W. Powell and N. G. Martin, Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers, Alcohol.: Clin. Exp. Res., 2001, 25, 1037–1045 CrossRef CAS PubMed.
- G. N. Ioannou, J. A. Dominitz, N. S. Weiss, P. J. Heagerty and K. V. Kowdley, The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia, Gastroenterology, 2004, 126, 1293–1301 CrossRef CAS.
- V. Scotet, M.-C. Mérour, A.-Y. Mercier, B. Chanu, T. Le Faou, O. Raguénes, G. Le Gac, C. Mura, J.-B. Nousbaum and C. Férec, Hereditary hemochromatosis: effect of excessive alcohol consumption on disease expression in patients homozygous for the C282Y mutation., Am. J. Epidemiol., 2003, 158, 129–134 CrossRef PubMed.
- N. T. Milman, A Review of Nutrients and Compounds, Which Promote or Inhibit Intestinal Iron Absorption: Making a Platform for Dietary Measures That Can Reduce Iron Uptake in Patients with Genetic Haemochromatosis, J. Nutr. Metab., 2020, 2020, 1–15 Search PubMed.
- D. D. Harrison-Findik, Role of alcohol in the regulation of iron metabolism, World J. Gastroenterol., 2007, 13, 4925 CrossRef CAS PubMed.
- D. D. Harrison-Findik, E. Klein, C. Crist, J. Evans, N. Timchenko and J. Gollan, Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol, Hepatology, 2007, 46, 1979–1985 CrossRef CAS PubMed.
- K. R. Bridle, T. Cheung, T. L. Murphy, M. M. Walters, G. J. Anderson, D. H. G. Crawford and L. M. Fletcher, Hepcidin Is Down–regulated in Alcoholic Liver Injury: Implications for the Pathogenesis of Alcoholic Liver Disease, Alcohol.: Clin. Exp. Res., 2006, 30, 106–112 CrossRef CAS PubMed.
- D.-D. Harrison-Findik, Is the iron regulatory hormone hepcidin a risk factor for alcoholic liver disease?, World J. Gastroenterol., 2009, 15, 1186 CrossRef CAS.
- E. Nemeth and T. Ganz, Hepcidin and Iron in Health and Disease, Annu. Rev. Med., 2023, 74, 261–277 CrossRef CAS PubMed.
- E. Nemeth, M. S. Tuttle, J. Powelson, M. B. Vaughn, A. Donovan, D. M. Ward, T. Ganz and J. Kaplan, Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization, Science, 2004, 306, 2090–2093 CrossRef CAS.
- L. Kautz and E. Nemeth, Molecular liaisons between erythropoiesis and iron metabolism, Blood, 2014, 124, 479–482 CrossRef CAS.
- D. N. Srole and T. Ganz, Erythroferrone structure, function, and physiology: Iron homeostasis and beyond, J. Cell Physiol., 2021, 236, 4888–4901 CrossRef CAS PubMed.
- W. R. Bezwoda, J. D. Torrance, T. H. Bothwell, A. P. Macphail, B. Graham and W. Mills, Iron absorption from red and white wines, Scand. J. Haematol., 1985, 34, 121–127 CrossRef CAS PubMed.
- J. Cook, M. Reddy and R. Hurrell, The effect of red and white wines on nonheme-iron absorption in humans, Am. J. Clin. Nutr., 1995, 61, 800–804 CrossRef CAS.
- H. K. Bayele, S. Balesaria and S. K. S. Srai, Phytoestrogens modulate hepcidin expression by Nrf2: Implications for dietary control of iron absorption, Free Radicals Biol. Med., 2015, 89, 1192–1202 CrossRef CAS.
- Y. Tang, Y. Li, H. Yu, C. Gao, L. Liu, S. Chen, M. Xing, L. Liu and P. Yao, Quercetin prevents ethanol-induced iron overload by regulating hepcidin through the BMP6/SMAD4 signaling pathway, J. Nutr. Biochem., 2014, 25, 675–682 CrossRef CAS PubMed.
- J. Nazlić, D. Jurić, I. Mudnić, Z. Boban, A. M. Dželalija, L. Tandara, D. Šupe-Domić, K. Gugo and M. Boban, Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus, Foods, 2022, 11, 1881 CrossRef.
- T. Ganz, Erythropoietic regulators of iron metabolism, Free Radicals Biol. Med., 2019, 133, 69–74 CrossRef CAS PubMed.
- M. Buttarello, P. Bulian, G. Farina, V. Temporin, L. Toffolo, E. Trabuio and P. Rizzotti, Flow Cytometric Reticulocyte Counting, Am. J. Clin. Pathol., 2001, 115, 100–111 CrossRef CAS PubMed.
- G. Lippi, C. Mattiuzzi and G. Cervellin, Learning more and spending less with neglected laboratory parameters: the paradigmatic case of red blood cell distribution width, Acta Biomed., 2016, 87, 323–328 CAS.
- G. D. Miller, J. Husk, A. K. Crouch and D. Eichner, EPO and the athlete biological passport: Hematological results from a placebo–controlled, boosting and microdose EPO administration in male recreational athletes, Drug Test. Anal., 2022, 14, 1962–1973 CrossRef CAS PubMed.
- Y. Li, M. Li, Y. Teng, C. Zhang, Q. Liu and H. Hou, The association between red cell distribution width, erythropoietin levels, and coronary artery disease, Coron. Artery Dis., 2018, 29, 74–80 CrossRef PubMed.
- T. E. Galesloot, S. H. Vermeulen, A. J. Geurts-Moespot, S. M. Klaver, J. J. Kroot, D. van Tienoven, J. F. M. Wetzels, L. A. L. M. Kiemeney, F. C. Sweep, M. den Heijer and D. W. Swinkels, Serum hepcidin: reference ranges and biochemical correlates in the general population, Blood, 2011, 117, e218–e225 CrossRef CAS.
- X. Wang, Y. Li, L. Han, J. Li, C. Liu and C. Sun, Role of Flavonoids in the Treatment of Iron Overload, Front. Cell Dev. Biol., 2021, 9, 685364 CrossRef PubMed.
- E. Nemeth, E. V. Valore, M. Territo, G. Schiller, A. Lichtenstein and T. Ganz, Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein, Blood, 2003, 101, 2461–2463 CrossRef CAS.
- H. P. Peters, C. M. Laarakkers, P. Pickkers, R. Masereeuw, O. C. Boerman, A. Eek, E. A. Cornelissen, D. W. Swinkels and J. F. Wetzels, Tubular reabsorption and local production of urine hepcidin-25, BMC Nephrol., 2013, 14, 70 CrossRef CAS.
- A. V. Harrison, F. R. Lorenzo and D. A. McClain, Iron and the Pathophysiology of Diabetes, Annu. Rev. Physiol., 2023, 85, 339–362 CrossRef CAS.
- K. M. Utzschneider and K. V. Kowdley, Hereditary hemochromatosis and diabetes mellitus: implications for clinical practice, Nat. Rev. Endocrinol., 2010, 6, 26–33 CrossRef CAS PubMed.
- N. T. Milman, Managing Genetic Hemochromatosis: An Overview of Dietary Measures, Which May Reduce Intestinal Iron Absorption in Persons With Iron Overload, Gastroenterol. Res., 2021, 14, 66–80 CrossRef CAS PubMed.
- M. Andrews, N. Soto and M. Arredondo-Olguín, Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity, Nutrition, 2015, 31, 51–57 CrossRef CAS PubMed.
- M. A. Martin, L. Goya and S. Ramos, Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies, Food Chem. Toxicol., 2017, 109, 302–314 CrossRef CAS.
- D. Eleftheriou, V. Benetou, A. Trichopoulou, C. La Vecchia and C. Bamia, Mediterranean diet and its components in relation to all-cause mortality: meta-analysis, Br. J. Nutr., 2018, 120, 1081–1097 CrossRef CAS.
- U.S. News & World Report., Best diets overall 2025 [Internet]. U.S. News & World Report [accessed on Jan 3, 2025]. Available at: https://health.usnews.com/best-diet/best-diets-overall.
- WHO Regional Office for Europe, What National and Subnational Interventions and Policies Based on Mediterranean and Nordic Diets are Recommended or Implemented in the WHO European Region, and is there Evidence of Effectiveness in Reducing Noncommunicable Diseases? [Internet]. WHO Regional Office for Europe, Copenhagen; 2018 [accessed on Jan 3, 2025]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK519076/ Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04555f |
| This journal is © The Royal Society of Chemistry 2025 |