Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of 3D-printed foods incorporating riboflavin-loaded whey protein isolate nanostructures: characterization and in vitro digestion†
João F.
Araújo
 a,
Jean-Michel
Fernandes
a,
Jean-Michel
Fernandes
 a,
Daniel
Madalena
a,
Daniel
Madalena
 a,
Raquel F. S.
Gonçalves
a,
Jorge M.
Vieira
ab,
Joana T.
Martins
a,
Raquel F. S.
Gonçalves
a,
Jorge M.
Vieira
ab,
Joana T.
Martins
 ab,
António A.
Vicente
ab,
António A.
Vicente
 ab and
Ana C.
Pinheiro
ab and
Ana C.
Pinheiro
 *ab
*ab
aCEB – Centre of Biological Engineering, University of Minho, Portugal. E-mail: anapinheiro@ceb.uminho.pt
bLABBELS – Associate Laboratory, Braga, Guimarães, Portugal
First published on 20th February 2025
Abstract
3D printing has emerged as a groundbreaking technology, aiming to enhance sensory attributes and improving nutritional/functional aspects. Simultaneously, nano-delivery systems have emerged as an opportunity to protect bioactive compounds against degradation and improve their bioaccessibility. Therefore, a novel concept is underway, involving the 3D printing of perishable healthy foods previously fortified with bioactive compound-loaded nanostructures. As a model concept, whey protein isolate (WPI) nanostructures were associated with riboflavin with an efficiency of 59.2%. Carrot pastes with adequate printability, shape retention and rheological characteristics were formulated. Riboflavin-WPI loaded nanostructures were incorporated into carrot inks and submitted to a static in vitro digestion. There was a notable increase in riboflavin bioaccessibility (+23.1%), suggesting a synergistic interaction between WPI nanostructures and carrot matrix. These results may contribute to validating the use of WPI nanostructures as effective encapsulating systems allied with 3D food printing towards the development of functional foods with personalized structure and nutrition profile.
1. Introduction
The scientific evidence supporting a correlation between dietary elements and prevalent diseases like heart disease, stroke, type 2 diabetes, and cancer has spurred the exploration for functional foods and the use of innovative technologies. Researchers are delving into alternatives to address consumer needs through the creation of functional foods tailored to personalized nutritional profiles. Advancements in food science and technology present solutions, particularly for specific populations with distinct nutritional requirements, such as the elderly (e.g., prone to dysphagia) and children (i.e., requiring increased fruit/vegetable intake). Despite the scientific progress, concerns regarding the sustainability and marketability of functional foods with specific functionalities or personalized nutrition have surfaced due to production costs and consumer acceptance.1,2 In the sense of a healthy and diversified diet, the consumption of fruits and vegetables is of utmost importance for the general population. When considering the diet of the elderly, the organoleptic properties of such foods (e.g., appearance, flavor and mouthfeel) are crucial for their acceptability and regular consumption. Additionally, due to the limited capacity of the elderly to consume foods of any kind, their nutrition can be significantly affected given the low intake of daily required macro- and micro-nutrients (e.g., proteins, fibers, vitamins and minerals).3–6Over recent years, 3D food printing (3DFP) has garnered significant attention, prompting relevant studies aimed at developing 3D-printed foods (3DPF) endowed with distinctive organoleptic and functional features, tailored functional properties for niche markets, and personalized nutritional profiles.7–9 This technology offers a dual benefit in food manufacturing by enhancing the sensorial attributes and nutritional/functional content of functional foods.10,11 3DFP allows users to exert control over various printing parameters, including the quantity and type of printing ingredients, optimizing the food structure to align with consumption preferences. Notably, it facilitates the reduction of food waste by printing only the required amount and utilizing perishable fruits, vegetables, or by-products to craft appealing foods.12–15 This ability to improve sensorial and nutritional aspects of vegetables and fruits is aligned with the criteria of the consumer's choice to purchase food (i.e., appearance, taste, cost, experience, convenience and nutrition) as mentioned by Ringquist et al. (2016).16 Rheological attributes stand as pivotal physicochemical parameters, significantly impacting the printability of a food ink. Among several others, hydrocolloids such as xanthan gum (XG) play a pivotal role in altering the rheological properties and textures of materials.8,17
In this study, carrot was selected as a model perishable food based on two main criteria: (1) due to its valuable nutritional profile (i.e., source of beta-carotene, fibers, vitamin K1, potassium, antioxidants, among others), interesting composition in macronutrients (i.e., carbohydrates); and (2) due to its high water composition (>80%) that makes this healthy vegetable a good candidate for nanostructure dispersion and further formulation for 3DFP.
The innovative approach of fortifying foods with nanostructures encapsulating bioactive compounds aims at developing specifically tailored foods with high protection of the bioactive compounds against environmental factors such as light, pH and temperature, and digestion. Whey protein isolate (WPI) is a promising candidate for devising delivery systems ensuring the controlled release of bioactive compounds.18
In line with this, the convergence of two emerging technologies, 3DFP and nanotechnology, opens opportunities for developing personalized, cartridge-based food formulations with enhanced bioactive compound bioavailability, offering an improved consumption experience and nutrition. However, despite their potential, the utilization of nano-size particles raises concerns about altering their biological fate, potentially posing risks to human health. In compliance with EU regulations, any nano-derived food ingredient must undergo a thorough safety assessment before approval for use.19 A crucial aspect involves comprehending the behavior of nanostructures during digestion/absorption, evaluating their efficiency and safety, facilitating their widespread integration into the realm of food applications. Additionally, considering the influence of the food matrix on the gastrointestinal fate of nanostructures/bioactive compounds, safety assessments should follow the incorporation of nanostructures into food inks.
In this work, WPI nanostructures were prepared through the thermal gelation method, to associate Rb as hydrophilic model compound. WPI nanostructures were then incorporated in carrot inks, prepared with increasing concentrations of XG which were previously characterized in terms of their rheological behavior, aiming at the selection of the ideal carrot ink formulation for further applications. Cell viability studies and an in vitro digestion adapted to the general older adult population were then performed to determine WPI-Rb nanostructures’ safety and Rb bioaccessibility before and after incorporation in the food inks. The use of WPI to produce nanostructures has been extensively studied as reported elsewhere.18,20–23
This research introduces an emerging innovation through the combination of nano delivery systems and 3D food printing, aiming at the development of functional foods with customizable nutritional and rheological profiles. Also, it provides valuable insights into food ink rheological characteristics, offering an understanding of influence of the physical and mechanical aspects of studied inks and finally, the impact on 3DPFs’ shape printability. Furthermore, the positive impact of the encapsulation of WPI nanostructures and their incorporation into the food matrix on Rb bioaccessibility was also validated.
2. Materials and methods
2.1. Chemicals and materials
Rb (≥98%), XG (Xanthomonas campestris), penicillin/streptomycin solution (contains 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units penicillin and 10 mg streptomycin per mL) (PS), trypsin-EDTA, and MTT (3-(4,5–126 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and sodium phosphate tribasic dodecahydrate 98% were purchased from Sigma-Aldrich (St Louis, MO, USA). WPI powder (Lacprodan DI-9212) was kindly supplied by Arla (Arla Foods, Viby, Denmark). In accordance with information provided by the supplier, WPI powder was essentially free of fat (max. 0.2%) and lactose (max. 0.5%). Protein composition, determined by reversed-phase high-performance liquid chromatography (RH-HPLC) as described elsewhere,24 was as follows: α-lactalbumin (α-lac) 22.8%, bovine serum albumin (BSA) 1.7%, β-lgA 44%, β-lgB 30.7%, and immunoglobulins (IG) 1.1%, on a protein basis. Hydrochloric acid and monosodium phosphate were purchased from Panreac (Barcelona, Spain), and sodium phosphate dibasic was obtained from Chem-Lab (Zedelgem, Belgium). Dimethyl sulfoxide (≥99.0%) (DMSO) was purchased from Fisher Scientific (NJ, USA). All chemicals used were of analytical grade. Dulbecco's modified Eagle's medium (DMEM), non-essential amino acids (NEAA) and phosphate-buffered saline (PBS) were obtained from Lonza (Basel, Switzerland). Heat-inactivated fetal bovine serum (FBS) was obtained from Merck (Darmstadt, Germany). Human colon Caco-2 cell line (American Type Culture Collection, ATCC) was kindly provided by the Department of Biology of the University of Minho (Braga, Portugal). Carrots were purchased in a local supermarket (Braga, Portugal), refrigerated at 4 °C for a maximum of two days until used.
000 units penicillin and 10 mg streptomycin per mL) (PS), trypsin-EDTA, and MTT (3-(4,5–126 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and sodium phosphate tribasic dodecahydrate 98% were purchased from Sigma-Aldrich (St Louis, MO, USA). WPI powder (Lacprodan DI-9212) was kindly supplied by Arla (Arla Foods, Viby, Denmark). In accordance with information provided by the supplier, WPI powder was essentially free of fat (max. 0.2%) and lactose (max. 0.5%). Protein composition, determined by reversed-phase high-performance liquid chromatography (RH-HPLC) as described elsewhere,24 was as follows: α-lactalbumin (α-lac) 22.8%, bovine serum albumin (BSA) 1.7%, β-lgA 44%, β-lgB 30.7%, and immunoglobulins (IG) 1.1%, on a protein basis. Hydrochloric acid and monosodium phosphate were purchased from Panreac (Barcelona, Spain), and sodium phosphate dibasic was obtained from Chem-Lab (Zedelgem, Belgium). Dimethyl sulfoxide (≥99.0%) (DMSO) was purchased from Fisher Scientific (NJ, USA). All chemicals used were of analytical grade. Dulbecco's modified Eagle's medium (DMEM), non-essential amino acids (NEAA) and phosphate-buffered saline (PBS) were obtained from Lonza (Basel, Switzerland). Heat-inactivated fetal bovine serum (FBS) was obtained from Merck (Darmstadt, Germany). Human colon Caco-2 cell line (American Type Culture Collection, ATCC) was kindly provided by the Department of Biology of the University of Minho (Braga, Portugal). Carrots were purchased in a local supermarket (Braga, Portugal), refrigerated at 4 °C for a maximum of two days until used.
2.2. Production of WPI-Rb nanostructures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g was conducted for 20 min at 4 °C.26 The spectrophotometric determination of unbound Rb in the filtrate occurred at 437 nm using a calibration curve (y = 26.951x − 7 × 10−5, R2 = 0.99, where y and x represent absorbance and Rb concentration, respectively). These outcomes were employed to calculate the association efficiency (AE) using eqn (1). The concentrate containing Rb-loaded WPI nanostructures, retained in Amicon®, underwent drying at 55 °C for 24 h in a ventilated oven. The loading capacity (LC) was then determined by mass difference, following eqn (2).
000g was conducted for 20 min at 4 °C.26 The spectrophotometric determination of unbound Rb in the filtrate occurred at 437 nm using a calibration curve (y = 26.951x − 7 × 10−5, R2 = 0.99, where y and x represent absorbance and Rb concentration, respectively). These outcomes were employed to calculate the association efficiency (AE) using eqn (1). The concentrate containing Rb-loaded WPI nanostructures, retained in Amicon®, underwent drying at 55 °C for 24 h in a ventilated oven. The loading capacity (LC) was then determined by mass difference, following eqn (2).| AE% = (total Rb − free Rb)/total Rb × 100 | (1) |
| LC% = (total Rb − free Rb)/mass nanoparticles × 100 | (2) |
2.3. Particle characterization
Size, polydispersity index (PdI), and surface charge (ζ-potential) of WPI nanostructures were evaluated through dynamic light scattering (DLS) with a Zetasizer Nano ZS (Malvern Instruments, United Kingdom), equipped with a He–Ne laser with a wavelength of 633 nm. This particle characterization method was applied to assess the effects of the freeze-drying process might have in the Rb-loaded WPI nanostructures in terms of size, PdI, and ζ-potential of WPI. All measurements were conducted at 25 °C in triplicate, with the results presented as the average ± standard deviation of the experimental values.2.4. Cell viability assay
The cell viability assessment was conducted using the MTT conversion assay, following the methodologies outlined by Braz et al. (2017)27 and Tibolla et al. (2019).28 Caco-2 cells (passage number 20–35) in DMEM supplemented with 10% (v/v) FBS, 1% (v/v) PS and 1% (v/v) of NEAA solution were seeded at a density of 4 × 104 cells per well in a 96-well plate and incubated at 37 °C in a 5% CO2 humidified atmosphere for 24 h. Briefly, various concentrations of WPI nanostructures, WPI nanostructures associated with Rb, and free Rb (ranging from 0.015 to 0.1 mg mL−1) were diluted in supplemented DMEM medium and homogenized. Cells treated with DMEM alone served as the control. Subsequently, the DMEM medium was replaced with WPI samples, free Rb, and ethanol, maintaining the cells at 37 °C in a 5% CO2 environment for an additional 24 h. After incubation, the samples were removed, and cells were washed with PBS. Following this, 100 μL of MTT solution (0.5 mg mL−1) was added to each well, and the plate was incubated at 37 °C for 3 h to generate purple formazan crystals. The solubilization of these crystals involved adding 200 μL of DMSO to each well, followed by orbital shaking (100 rpm) for 30 min. The formazan was quantified by measuring the absorbance at 570 nm and 630 nm (used for background subtraction) in a Synergy™ HT Multi-mode Microplate Reader (Biotek Instruments, Winooski, VT, USA). Four replicates of each sample were analyzed. The percentage of cell viability for all samples was determined using eqn (3).| Cell viability% = Abssample/Abscontrol × 100 | (3) |
2.5. 3D food printing of carrot structures
As presented in Table 1, increasing concentrations of XG, ranging from 0 to 1.2%, were added to the carrot pastes and mixed at 1500 rpm with a Hei-TORQUE overhead stirrer equipped with a propeller stirrer 3-blade from Heidolph instruments (Germany), in a water bath at 45 °C, for 20 min, to ensure an initial decline in XG viscosity and proper homogenization within the carrot paste. Prior to XG addition, WPI-Rb nanostructures were added to the carrot paste to ensure proper dispersion, reaching a final concentration of 5.2 mg mL−1, following the previous mixing conditions, during 20 min. This concentration was based on the AE results that can be verified in section 3.1.1, and on Rb’ daily recommended intake (i.e., 1.3 mg day−1 for men and 1.1 mg day−1 for women).29
| Ink 1 | Ink 2 | Ink 3 | Ink 4 | Ink 5 | |
|---|---|---|---|---|---|
| XG (%) | 0 | 0.3 | 0.6 | 1.2 | 0.6 |
| WPI-Rb nanostructures (%) | — | — | — | — | 0.52 |
The obtained carrot inks, free from any particles that could obstruct the nozzle of the printer, were then stored in the refrigerator for future utilization.
The obtained carrot inks were dropped into a 10 mL Luer-lock syringe nozzle coupled with a micro tip (d = 1 mm). Carrot-filled syringes were placed into the print head of a Tissue Scribe 3D bioprinter from 3D Cultures (Philadelphia, USA) and further programmed.
2.6. Carrot inks characterization
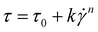 | (4) |
 the shear rate, and n the flow index.
the shear rate, and n the flow index.
Oscillation amplitude sweep tests were performed by applying a stress range from 0.1 to 2000 Pa at a constant frequency of 1 Hz to access the viscoelastic properties of the inks. All experiments were conducted at room temperature, in triplicate.
2.7. In vitro digestion of carrot inks incorporating nanostructures
WPI-Rb nanostructures, free Rb, carrot ink incorporating Rb, and carrot ink incorporating WPI-Rb nanostructures underwent an in vitro digestion process following the INFOGEST static in vitro digestion model adapted to the general older adult population.36 This model simulates the oral, gastric, and intestinal phases of digestion of the elderly, where the rate of gastric emptying is slowed down from 2 to 3 h, the pH of the stomach content is higher (3.7), the amount of gastric and intestinal digestive enzymes and bile salts is lower. In the oral phase, 5 grams of the sample was combined with simulated salivary fluid (SSF) containing KCl, KH2PO4, NaHCO3, MgCl2(H2O)6, (NH4)2CO3, HCl, and CaCl2(H2O)2. α-Amylase (1875 IU mL−1) was added due to the presence of starch in the carrot samples. The mixture underwent a 2 min incubation at 37 °C with orbital agitation at 120 rpm. Moving to the gastric phase, simulated gastric fluid (SGF) containing KCl, KH2PO4, NaHCO3, NaCl, MgCl2(H2O)6, (NH4)2CO3, HCl, CaCl2(H2O)2, and pepsin solution (1200 IU mL−1) was added, adjusting the pH to 3.7. The mixture was incubated for 3 h under similar conditions. Subsequently, the intestinal phase comprised simulated intestinal fluid (SIF) containing KCl, KH2PO4, NaHCO3, NaCl, MgCl2(H2O)6, HCl, CaCl2(H2O)2, bile salts (6.7 mmol L−1), and pancreatin solution (800 IU mL−1). The pH was adjusted to 7.0, and the samples were incubated for an additional 2 h. After the intestinal phase, the reaction was stopped by adding 1 mmol L−1 of enzyme inhibitor pefabloc® SC and samples were cooled in ice for 15 min until further collection.2.8. Riboflavin's bioaccessibility
Rb's bioaccessibility was assessed utilizing a method adapted from Pinheiro et al. (2013).37 10 mL samples of digested carrot inks, nanostructures, and Rb underwent centrifugation (Allegra 64R, Beckman Coulter, USA) at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min at 25 °C. The resulting separation yielded two distinct phases: an opaque sediment phase at the bottom and a supernatant (micelle phase) at the top. The micelle phase, presumed to contain the bioactive compound (i.e., the bioaccessible fraction), was considered available for absorption and metabolic processing.38 Subsequently, 5 mL aliquots of the supernatant were collected to quantify the released bioactive compound post in vitro digestion. Rb concentration was determined through fluorescence spectroscopy using a microplate reader (BioTek™ Cytation™ 3, USA) with excitation at 450 nm and emission at 530 nm. Standard Rb solutions facilitated the creation of a calibration curve (y = 1
000g for 30 min at 25 °C. The resulting separation yielded two distinct phases: an opaque sediment phase at the bottom and a supernatant (micelle phase) at the top. The micelle phase, presumed to contain the bioactive compound (i.e., the bioaccessible fraction), was considered available for absorption and metabolic processing.38 Subsequently, 5 mL aliquots of the supernatant were collected to quantify the released bioactive compound post in vitro digestion. Rb concentration was determined through fluorescence spectroscopy using a microplate reader (BioTek™ Cytation™ 3, USA) with excitation at 450 nm and emission at 530 nm. Standard Rb solutions facilitated the creation of a calibration curve (y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000x + 1006; R2 = 0.97), where y represents fluorescence and x represents Rb concentration. Blank fluorescence subtraction corrected the fluorescence intensity. Rb bioaccessibility (eqn (5)) was determined as reported by Akça et al. (2019)39 using eqn (4):
000x + 1006; R2 = 0.97), where y represents fluorescence and x represents Rb concentration. Blank fluorescence subtraction corrected the fluorescence intensity. Rb bioaccessibility (eqn (5)) was determined as reported by Akça et al. (2019)39 using eqn (4):| Bioaccessibility (%) = CMicelle Phase/CInitial × 100 | (5) |
2.9. Statistical analysis
During this study, statistical analyses were performed to every data from each of the experimental results. Therefore, the mean values and standard deviations of the experimental data were calculated. Statistical analysis of the data was carried out using Analysis of Variance (ANOVA) and Tukey mean comparison test (p < 0.05), resorting to Origin® Pro 2018 software (Massachusetts, USA).3. Results
3.1. Particles characterization
3.2. Cell viability
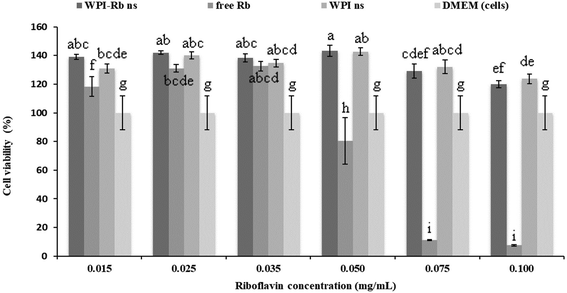
Cell viability is an essential parameter that needs careful assessment, particularly in the case of high bioactive compounds concentration and nanostructures, prior to any application, due to their nano size and large surface area per unit, which may increase the adherence and interaction with cell membranes. These phenomena may result in enhanced cellular uptake, leading to cellular damage and consequently, a decrease in the efficiency of bioactive compound absorption.43 Therefore, the cell viability study was evaluated with WPI-Rb nanostructures, WPI nanostructures, and free Rb using Caco-2 cells with a 24-hour exposure period. In Fig. 1, it can be observed that, starting from a concentration of 0.050 mg mL−1 of free Rb, cellular viability decreases, reaching a low value (<10%) at 0.075 and 0.1 mg mL−1. In contrast, we observed that the number of viable cells present after treatment with increasing concentrations of WPI nanostructures (up to 5 mg mL−1) with and without Rb, after incubation for 24 h, was approximately 100%. This indicated that the nanostructures were non-toxic and reduce significantly free Rb negative effect to the cells at the dilution concentrations and exposure times tested. On one hand, prior investigations have reported the absence of cytotoxic effects on Caco-2 cells exposed to high internal phase Pickering emulsion encapsulating bioactive compounds, previously stabilized with WPI nanoparticles (up to 10 mg mL−1).44 On the other hand, Simões et al. (2020) have shown the adverse effect of β-lactoglobulin nanostructures with or without Rb on Caco-2 cells in a similar range of concentrations, comparing with WPI nanostructures.453.3. 3D printing of carrot inks
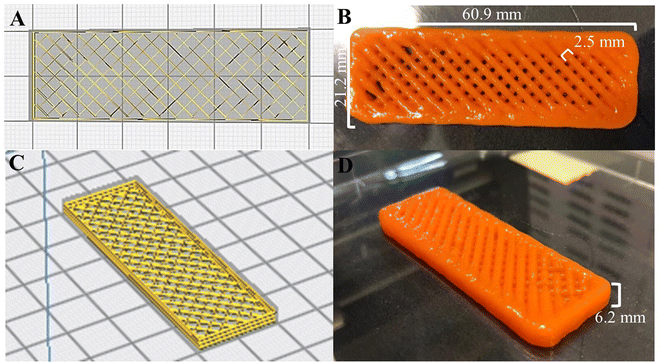 | ||
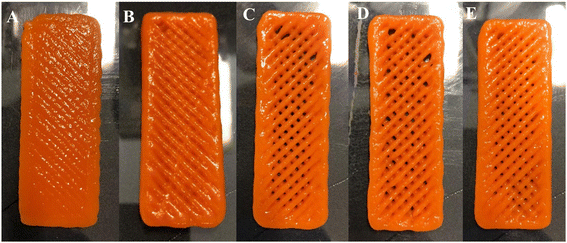
| Fig. 3 3D-food structures in (A) and (C) digital, and (B) and (D) printed formats (ink 3). Bars and letters in the pictures indicate the digital measures of the 3D printed food structures. | ||
Relatively good printability and shape retention were achieved in terms of the printed structure’ area, meaning that the designed area is close to the printed and measured area of the parallelepiped carrot structures (as seen in Fig. 3A–D). One of the most important factors to evaluate the printability and resolution, is the difference between digitally designed filament and the printed ones. Visual differences can be seen by comparing the width of the extruded infill filament (Fig. 4B), which is 2.5 times wider than the designed ones (Fig. 4A). Also, although the height of the printed structures is nearly the same as the designed ones, there is a lack of definition in the 6 projected layers which can also be explained by the abovementioned slumping effect, reported by Heck et al. (2023).46
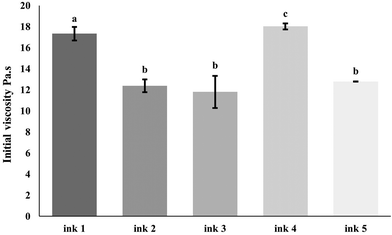
Regardless of the optimization that must be performed in terms of shape fidelity, resolution and printability, the visual inspection and the printability values obtained (Table 3) led to the provisional selection of ink 3 as the best formulation to study and incorporate the nanostructures. As expected, due to the physical properties of the nano structures, as reported in section 3.1.2 (i.e., low size and polydispersity), in terms shape fidelity and resolution and printability, no relevant visual changes can be seen in ink 5, comparing to ink 3 and ink 4, (Fig. 2C, D and E). To confirm these outcomes and the selection of the best formulation, a rheological characterization was performed.
| Ink 1 | Ink 2 | Ink 3 | Ink 4 | Ink 5 | |
|---|---|---|---|---|---|
| Yield stress (Pa) | 79.42 | 39.81 | 56.60 | 125.87 | 71.69 |
| k | 57.77 | 54.83 | 49.98 | 61.32 | 50.18 |
| n | 0.24 | 0.17 | 0.24 | 0.25 | 0.25 |
| R 2 | 0.997 | 0.877 | 0.989 | 0.984 | 0.993 |
| CAD | Ink 1 | Ink 2 | Ink 3 | Ink 4 | Ink 5 | |
|---|---|---|---|---|---|---|
| Width (mm) | 20 | 21.2 | 21.4 | 21.2 | 21.3 | 21.1 |
| Length (mm) | 60 | 61.5 | 61.3 | 60.9 | 61.0 | 59.9 |
| Height (mm) | 6 | 4.9 | 5.2 | 6.2 | 6.1 | 6.1 |
| Printability | 1 | 0.88 | 0.94 | 1.1 | 1.1 | 1.07 |
Following the surpassing of yield stress for flow initiation, the continuous extrusion process is influenced by changes in viscosity with shear rate.51 The shear rate dependency is assessed by fitting shear rate-viscosity profiles using the Herschel–Bulkley model (eqn (4)), enabling the analysis of consistency index (k) and flow index (n). In contrast to shear-thickening fluids (n > 1), shear-thinning fluids (n < 1) are generally preferred for printing applications. Table 2 shows that all inks exhibit non-Newtonian behavior, evident in a conspicuous decrease in viscosity with increasing shear rate (data not shown). The n values for all inks fall within the range of 0.17–0.25, which stands significantly below 1, indicating robust shear-thinning behavior.52 Despite ink 2 theoretically demonstrating the most pronounced shear-thinning behavior (i.e., lowest n value) among increasing XG concentrations, the results from infill resolution and shape assessment (section 3.3.1) revealed that ink 3 (i.e., XG at 0.6%) exhibits superior accuracy in terms of shape fidelity and infill resolution. Ideally, low K and n values are advantageous for easing extrusion through the nozzle by reducing ink viscosity.53 Considering these factors and reports, ink 3, with the lowest k value and a relatively low n value, provides an adequate extrusion. The incorporation of WPI-Rb nanostructures into ink 3 resulted in ink 5. As expected, comparing to ink 3, ink 5 showed no significant differences in terms of its initial viscosity, shear-thinning and viscoelastic behavior, although there was an increase on yield stress value, that can be attributed to the effect of WPI-Rb nanostructures powder on the food ink, that resulted in a slightly rigid matrix, which can be seen as an ideal indicator for better filament formation, as investigated elsewhere.54
In rheology, the loss modulus (G′′) is the ratio of the viscous (out of phase) component to the stress and is related to the material's ability to dissipate stress through heat. The G′ relates to the stored energy arising from the elastic deformation of materials and serves as a crucial metric, not only assessing extrudability but also gauging the shape retention of 3D printing materials during the deposition process.48 The results from angular frequency sweep experiments revealed that G′ for all inks consistently surpassed G′′ values across the entire angular frequency spectrum. As a reflection of this result, the loss tangent or tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (i.e., ratio G′′/G′) was consistently below 1 (between 0.16 and 0.31), indicating that the viscoelastic nature of materials is predominantly solid-like behavior. In 3D printing, shape fidelity and elastic-like behaviors are often attributed to higher G′ and lower tan
δ (i.e., ratio G′′/G′) was consistently below 1 (between 0.16 and 0.31), indicating that the viscoelastic nature of materials is predominantly solid-like behavior. In 3D printing, shape fidelity and elastic-like behaviors are often attributed to higher G′ and lower tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values.55 However, a correlation with yield stress values must be performed to understand the desired balance between extrudability and shape retention.
δ values.55 However, a correlation with yield stress values must be performed to understand the desired balance between extrudability and shape retention.
The assessment of the linear viscoelastic region in a material involves conducting an oscillatory measurement at a constant frequency while gradually increasing stress or strain amplitude. Throughout this process, the measured moduli exhibit linearity until the critical strain threshold is surpassed. The termination of the linear region is marked by a reduction in viscosity or elastic modulus coupled with a rise in the phase shift.55 All studied inks have shown viscoelastic behavior, although, with different G′ and G′′ magnitudes and inflection points along with increasing XG concentration. The G′ and G′′ exhibited relative stability until the inflection point, with G′ surpassing G′′, revealing that all inks presented a viscoelastic solid behavior (G′ > G′′). Jeon et al. (2024) investigated the effect of hydrocolloids incorporation in carrot/squid blends as edible inks for extrusion 3D printing. The authors obtained similar results in oscillatory measurements, and highlighted the inks supplemented with XG as capable of maintaining their elastic microstructure, resulting in the most printable inks.17
The correlation between yield stress and the extrusion performance in 3D printing is significant, as it represents the minimum pressure necessary to initiate fluid flow.56 An increased yield stress imposes a demand for elevated pressure generated by the extrusion unit, facilitating the uninterrupted extrusion of inks throughout the 3D printing process. In this sense, opting for a lower yield stress is typically advantageous for seamless extrusion. In the context of food inks, the yield stress is specifically identified as the point of intersection between G′ and G′′ in a shear stress sweep test. Analyzing Table 2, it is evident that yield stress initially decreased as the XG concentration rises from ink 1 to ink 2. Subsequently, it exhibited an increase with a further elevation in XG from ink 2 to ink 4. Ultimately, these values experienced a reduction upon the introduction of WPI-Rb nanostructures into the selected ink formulation (i.e., ink 5). Also, there are differences in effect on yield stress among hydrocolloids, which can be attributed to distinct physical and chemical interactions between the hydrocolloids and other food components, as well as variations in intermolecular mattings resulting from chain flexibility and conformational changes.46 For example, Xing et al. (2022) demonstrated that the introduction of Arabic gum to a black fungus-based ink resulted in a reduction in yield stress. In contrast, in the present work, the addition of XG increased the yield stress, a phenomenon that can be attributed to distinct water mobilities, and molecular conformations induced by the interaction with these different hydrocolloids.9
3.4. Riboflavin bioaccessibility
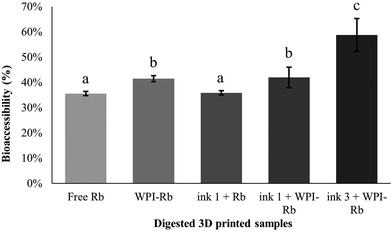
The concept of bioaccessibility relates to the proportion of a bioactive compound liberated from its structure, presenting potential availability for absorption, as elucidated by previous research studies.57,58 The observation of Rb bioaccessibility results (Fig. 5) unveils that 36.4 ± 0.9% of Rb in its free form, reaching the intestinal phase, holds for potential absorption. Fig. 5 highlights a slight increase (+5.8%, p > 0.05) in Rb bioaccessibility when associated with WPI nanostructures (p < 0.05). It is believed that the reduced size of WPI nanostructures enhances solubility and bioavailability, preventing undesired physical and chemical reactions while safeguarding bioactive compounds from degradation. When associated with WPI nanostructures and further incorporated into the carrot ink, Rb bioaccessibility was significantly increased (+23.1%, p > 0.05). This may suggest the occurrence of a synergistic effect between the carrot food matrix and WPI nanostructures, indicating that the absorption of a specific bioactive compound can be maximized through strategic association/encapsulation within a system coupled with an appropriate food matrix. In comparison with relevant scientific publications, the observed synergistic effect between the food matrix and nanostructures aligns with studies emphasizing the crucial role of nano-based delivery systems in enhancing bioavailability. Previous research often reports the incorporation of nanostructures encapsulating bioactive compounds in food matrices such as beverages, dairy, bakery, meat products and plant-based milk analogs with relative success regarding compounds bioactivity, shelf life, and sensory properties.59 For example, Ubeyitogullari et al. (2019) studied the bioaccessibility of novel phytosterols nanoparticles in granola bars and puddings and reported enhanced bioaccessibility values in all digested food matrix.60 This achievement was mainly attributed to the incorporation of phytosterols in nanoporous starch aerogels particles, and no synergistic effect was identified between the food matrices and nanoparticles. In this regard, there is a lack of research reporting the incorporation of nanostructures in 3DPF and moreover, the impact of foods on the bioaccessibility of nanoencapsulated bioactive compounds. Therefore, the present study arises as an opportunity to open this perspective of further research on the interactions between nano-based delivery systems and food matrices.4. Conclusions
This study underscores the promising potential of WPI nanostructures as an effective model of encapsulation of bioactive compounds. These nanostructures, characterized by a particle size of approximately 65 nm and a PDI of 0.392 have proven to be valuable candidates for associating Rb with an AE of approximately 60%. Notably, this association does not compromise the physical characteristics of the nanostructures. The biocompatibility assessment using Caco-2 cells revealed that their viability remained unaffected even at a concentration of 0.1 mg mL−1 of Rb in WPI nanostructures, demonstrating their safety for potential use in food applications. These results position Rb-loaded WPI nanostructures as promising fortifier agents in the food industry, providing a viable solution for enhancing nutritional content. Additionally, a carrot-based food matrix with adequate rheological characteristics for 3D printing has been formulated, in which the introduction of XG emerged as a pivotal factor, positively influencing the printability, structure, and shape of 3D printed carrot inks. This additive enhanced the overall quality of the printed food product. Lastly, the investigation into the 3D printed food matrix revealed a synergistic interaction with WPI nanostructures, particularly in terms of Rb bioaccessibility. This collaborative effect may enhance the overall bioavailability of Rb, highlighting the potential of these combined technologies (i.e., nanotechnology and 3D food printing) for advancing food engineering and nutrition.Author contributions
João Fernandes Araújo: writing – original draft, conceptualization, investigation, formal analysis, writing – review & editing; Jean-Michel Fernandes: writing – review & editing, investigation; Daniel Madalena: writing – review & editing and investigation; Raquel F.S. Gonçalves: writing – review & editing and investigation; Jorge M. Vieira: writing – review & editing, investigation and formal analysis; Joana T. Martins: writing – review & editing, investigation, formal analysis; António A. Vicente: writing – review & editing, supervision and funding acquisition; Ana C. Pinheiro: writing – review & editing, conceptualization, supervision, validation and funding acquisition.Abbreviations
| 3DFP | 3D food printing |
| 3DPF | 3D-printed foods |
| WPI | Whey protein isolate |
| Rb | Riboflavin |
| XG | Xanthan gum. |
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
João Fernandes Araújo, Jean-Michel Fernandes and Raquel F. Gonçalves acknowledge the Foundation for Science and Technology (FCT) for their fellowship (SFRH/BD/09849/2020, SFRH/BD/147286/2019 and SFRH/BD/140182/2018, respectively). Joana T. Martins and Ana C. Pinheiro acknowledge FCT for their Assistant Research contract obtained under the scope of Scientific Stimulus Employment with reference 2022.00788.CEECIND/CP1718/CT0024 and 2023.06513.CEECIND, respectively.This work was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit, and by LABBELS – Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020.
References
- B. Jagadiswaran, V. Alagarasan, P. Palanivelu, R. Theagarajan, J. A. Moses and C. Anandharamakrishnan, Valorization of food industry waste and by-products using 3D printing: A study on the development of value-added functional cookies, Future Foods, 2021, 4, 100036 CrossRef CAS.
- A. Jędrusek-Golińska, D. Górecka, M. Buchowski, K. Wieczorowska-Tobis, A. Gramza-Michałowska and K. Szymandera-Buszka, Recent progress in the use of functional foods for older adults: A narrative review, Compr. Rev. Food Sci. Food Saf., 2020, 19, 835–856 CrossRef PubMed.
- E. S. Yearick, M. S. L. Wang and S. J. Pisias, Nutritional status of the elderly: Dietary and biochemical findings, J. Gerontol., 1980, 35, 663–671 CrossRef CAS PubMed.
- R. K. Chandra, A. Imbach, C. Moore, D. Skelton and D. Woolcott, Nutrition of the elderly, Can. Med. Assoc. J., 1991, 145, 1475–1487 CAS.
- L. Kourkouta, P. Ouzounakis, A. Monios and Ch. Iliadis, Nutritional habits in the elderly, Prog. Health Sci., 2016, 6(2), 155–159 CAS.
- M. L. Wahlqvist, G. S. Savige and W. Lukito, Nutritional disorders in the elderly, Med. J. Aust., 1995, 163, 376–381 CrossRef CAS PubMed.
- A. Derossi, R. Caporizzi, D. Azzollini and C. Severini, Application of 3D printing for customized food. A case on the development of a fruit-based snack for children, J. Food Eng., 2018, 220, 65–75 CrossRef.
- A. Pant, A. Y. Lee, R. Karyappa, C. P. Lee, J. An, M. Hashimoto, U. X. Tan, G. Wong, C. K. Chua and Y. Zhang, 3D food printing of fresh vegetables using food hydrocolloids for dysphagic patients, Food Hydrocolloids, 2021, 114, 106546 CrossRef CAS.
- X. Xing, B. Chitrakar, S. Hati, S. Xie, H. Li, C. Li, Z. Liu and H. Mo, Development of black fungus-based 3D printed foods as dysphagia diet: Effect of gums incorporation, Food Hydrocolloids, 2022, 123, 107173 CrossRef CAS.
- Y. Huang, M. Zhang and P. Pattarapon, Reducing freeze-thaw drip loss of mixed vegetable gel by 3D printing porosity, Innovative Food Sci. Emerging Technol., 2022, 75, 102893 CrossRef CAS.
- Y. O. Kewuyemi, H. Kesa and O. A. Adebo, Trends in functional food development with three-dimensional (3D) food printing technology: prospects for value-added traditionally processed food products, Crit. Rev. Food Sci. Nutr., 2022, 62, 7866–7904 CrossRef CAS PubMed.
- Y. J. Lin, P. Punpongsanon, X. Wen, D. Iwai, K. Sato, M. Obrist and S. Mueller, FoodFab: Creating Food Perception Illusions Using Food 3D Printing, in Conference on Human Factors in Computing Systems - Proceedings, Association for Computing Machinery, 2020.
- R. Wu, J. Jiang, F. An, X. Ma and J. Wu, Research progress of 3D printing technology in functional food, powering the future of food, Trends Food Sci. Technol., 2024, 149, 104545 CrossRef CAS.
- F. Silva, T. Pereira, S. Mendes, L. Gordo and M. M. Gil, Consumer's perceptions and motivations on the consumption of fortified foods and 3D food printing, Future Foods, 2024, 10, 100423 CrossRef CAS.
- I. Tomašević, P. Putnik, F. Valjak, B. Pavlić, B. Šojić, A. Bebek Markovinović and D. Bursać Kovačević, 3D printing as novel tool for fruit-based functional food production, Curr. Opin. Food Sci., 2021, 41, 138–145 CrossRef.
- J. Ringquist, T. Phillips, B. Renner, R. Sides, K. Stuart, M. Baum and J. Flannery, Capitalizing on the shifting consumer food value equation, in Capitalizing on the shifting consumer food value equation, 2016 Search PubMed.
- E. Y. Jeon, Y. G. Chun and B.-K. Kim, Investigation of carrot/squid blends as edible inks for extrusion 3D printing: Effect of hydrocolloids incorporation, J. Food Eng., 2024, 364, 111777 CrossRef CAS.
- O. L. Ramos, R. N. Pereira, L. S. Simões, D. A. Madalena, R. M. Rodrigues, J. A. Teixeira and A. A. Vicente, Nanostructures of whey proteins for encapsulation of food ingredients, in Biopolymer Nanostructures for Food Encapsulation Purposes, 2019, vol. 1, pp. 69–100 Search PubMed.
- V. Amenta, K. Aschberger, M. Arena, H. Bouwmeester, F. Botelho Moniz, P. Brandhoff, S. Gottardo, H. J. P. Marvin, A. Mech, L. Quiros Pesudo, H. Rauscher, R. Schoonjans, M. V. Vettori, S. Weigel and R. J. Peters, Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries, Regul. Toxicol. Pharmacol., 2015, 73, 463–476 CrossRef PubMed.
- A. Abbasi, Z. Emam-Djomeh, M. A. E. Mousavi and D. Davoodi, Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate, Food Chem., 2014, 143, 379–383 CrossRef CAS PubMed.
- O. L. Ramos, R. N. Pereira, R. Rodrigues, J. A. Teixeira, A. A. Vicente and F. Xavier Malcata, Physical effects upon whey protein aggregation for nano-coating production, Food Res. Int., 2014, 66, 344–355 CrossRef CAS.
- O. L. Ramos, R. N. Pereira, A. Martins, R. Rodrigues, C. Fuciños, J. A. Teixeira, L. Pastrana, F. X. Malcata and A. A. Vicente, Design of whey protein nanostructures for incorporation and release of nutraceutical compounds in food, Crit. Rev. Food Sci. Nutr., 2017, 57, 1377–1393 CrossRef CAS PubMed.
- J. Yi, L. Gao, G. Zhong and Y. Fan, Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for β-carotene stabilization and delivery, Food Funct., 2020, 11, 768–778 RSC.
- O. L. Ramos, R. N. Pereira, R. M. Rodrigues, J. A. Teixeira, A. A. Vicente and F. X. Malcata, Whey and Whey Powders: Production and Uses, in Encyclopedia of Food and Health, 1st edn, 2015, vol. 5, pp. 498–505 Search PubMed.
- L. S. Simões, J. F. Araújo, A. A. Vicente and O. L. Ramos, Design of β-lactoglobulin micro- and nanostructures by controlling gelation through physical variables, Food Hydrocolloids, 2020, 100, 105357 CrossRef.
- M. A. Azevedo, A. I. Bourbon, A. A. Vicente and M. A. Cerqueira, Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2, Int. J. Biol. Macromol., 2014, 71, 141–146 CrossRef CAS PubMed.
- L. Braz, A. Grenha, D. Ferreira, A. M. Rosa da Costa, C. Gamazo and B. Sarmento, Chitosan/sulfated locust bean gum nanoparticles: In vitro and in vivo evaluation towards an application in oral immunization, Int. J. Biol. Macromol., 2017, 96, 786–797 CrossRef CAS PubMed.
- H. Tibolla, F. M. Pelissari, J. T. Martins, E. M. Lanzoni, A. A. Vicente, F. C. Menegalli and R. L. Cunha, Banana starch nanocomposite with cellulose nanofibers isolated from banana peel by enzymatic treatment: In vitro cytotoxicity assessment, Carbohydr. Polym., 2019, 207, 169–179 CrossRef CAS PubMed.
- D. Turck, J.-L. Bresson, B. Burlingame, T. Dean, S. Fairweather-, M. Heinonen, K.-I. Hirsch-Ernst, I. Mangelsdorf, H. J. McArdle, A. Naska, G. Nowicka, K. Pentieva, Y. Sanz, A. Siani, M. Stern, D. Tomé, H. Van Loveren, M. Vinceti, P. Willatts, H. Przyrembel, I. Tetens, C. Dumas, L. Fabiani, C. Forss, S. Ioannidou and M. Neuhäuser-Berthold, Dietary Reference Values for riboflavin, EFSA J., 2017, 15(8), 4919 Search PubMed.
- J. M. Habibur, N. Islam, K. Ahmed, A. Khosla, M. Kawakami and H. Furukawa, Heliyon Rheological and mechanical properties of edible gel materials for 3D food printing technology, Heliyon, 2020, 6, e05859 CrossRef PubMed.
- L. Masbernat, S. Berland, C. Leverrier, G. Moulin, C. Michon and G. Almeida, Structuring wheat dough using a thermomechanical process, from liquid food to 3D-printable food material, J. Food Eng., 2021, 310, 110696 CrossRef CAS.
- T. Carranza, P. Guerrero, K. de la Caba and A. Etxabide, Texture-modified soy protein foods: 3D printing design and red cabbage effect, Food Hydrocolloids, 2023, 145, 109141 CrossRef CAS.
- S. B. Kokane, P. R. Anjaly, S. Thangalakshmi and V. K. Arora, Current trends in additive manufacturing based 4D food printing technology: A review, Future Foods, 2024, 10, 100450 CrossRef CAS.
- J. Sun, W. Zhou, L. Yan, D. Huang and L. ya Lin, Extrusion-based food printing for digitalized food design and nutrition control, J. Food Eng., 2018, 220, 1–11 CrossRef.
- S. J. Rowat, R. L. Legge and C. Moresoli, Plant protein in material extrusion 3D printing: Formation, plasticization, prospects, and challenges, J. Food Eng., 2021, 308, 110623 CrossRef CAS.
- O. Menard, U. Lesmes, C. S. Shani-Levi, A. Araiza Calahorra, A. Lavoisier, M. Morzel, A. Rieder, G. Feron, S. Nebbia, L. Mashiah, A. Andres, G. Bornhorst, F. Carrière, L. Egger, S. Gwala, A. Heredia, B. Kirkhus, A. Macierzanka, R. Portman, I. Recio, V. Santé-Lhoutellier, C. Tournier, A. Sarkar, A. Brodkorb, A. Mackie and D. Dupont, Static in vitro digestion model adapted to the general older adult population: an INFOGEST international consensus, Food Funct., 2023, 14, 4569–4582 RSC.
- A. C. Pinheiro, M. Lad, H. D. Silva, M. A. Coimbra, M. Boland and A. A. Vicente, Unravelling the behaviour of curcumin nanoemulsions during in vitro digestion: effect of the surface charge, Soft Matter, 2013, 9, 3147–3154 RSC.
- H. D. Silva, E. Beldíková, J. Poejo, L. Abrunhosa, A. T. Serra, C. M. M. Duarte, T. Brányik, M. A. Cerqueira, A. C. Pinheiro and A. A. Vicente, Evaluating the effect of chitosan layer on bioaccessibility and cellular uptake of curcumin nanoemulsions, J. Food Eng., 2019, 243, 89–100 CrossRef CAS.
- S. N. Akça, H. S. Sargın, Ö. F. Mızrak and M. Yaman, Determination and assessment of the bioaccessibility of vitamins B1, B2, and B3 in commercially available cereal-based baby foods, Microchem. J., 2019, 150, 104192 CrossRef.
- L. S. Simões, L. Abrunhosa, A. A. Vicente and O. L. Ramos, Suitability of β-lactoglobulin micro- and nanostructures for loading and release of bioactive compounds, Food Hydrocolloids, 2020, 101, 105492 CrossRef.
- D. A. Madalena, Ó. L. Ramos, R. N. Pereira, A. I. Bourbon, A. C. Pinheiro, F. X. Malcata, J. A. Teixeira and A. A. Vicente, In vitro digestion and stability assessment of β-lactoglobulin/riboflavin nanostructures, Food Hydrocolloids, 2016, 58, 89–97 CrossRef CAS.
- S. Rikhtehgaran, I. Katouzian, S. M. Jafari, H. Kiani, L. A. Maiorova and H. Takbirgou, Casein-based nanodelivery of olive leaf phenolics: Preparation, characterization and release study, Food Struct., 2021, 30, 100227 CrossRef CAS.
- H.-K. Ha, J. W. Kim, M.-R. Lee, W. Jun and W.-J. Lee, Cellular Uptake and Cytotoxicity of β-Lactoglobulin Nanoparticles: The Effects of Particle Size and Surface Charge, Asian-Australas. J. Anim. Sci., 2015, 28, 420–427 CrossRef CAS PubMed.
- J. Yi, L. Gao, G. Zhong and Y. Fan, Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for β-carotene stabilization and delivery, Food Funct., 2020, 11, 768–778 RSC.
- L. S. Simões, J. T. Martins, A. C. Pinheiro, A. A. Vicente and O. L. Ramos, β-lactoglobulin micro- and nanostructures as bioactive compounds vehicle: In vitro studies, Food Res. Int., 2020, 131, 108979 CrossRef PubMed.
- M. P. Heckl, M. Korber, M. Jekle and T. Becker, Relation between deformation and relaxation of hydrocolloids-starch based bio-inks and 3D printing accuracy, Food Hydrocolloids, 2023, 137, 108326 CrossRef CAS.
- A. R. Fahmy, T. Becker and M. Jekle, 3D printing and additive manufacturing of cereal-based materials: Quality analysis of starch-based systems using a camera-based morphological approach, Innovative Food Sci. Emerging Technol., 2020, 63, 102384 CrossRef CAS.
- S. Zhu, M. A. Stieger, A. J. van der Goot and M. A. I. Schutyser, Extrusion-based 3D printing of food pastes: Correlating rheological properties with printing behaviour, Innovative Food Sci. Emerging Technol., 2019, 58, 102214 CrossRef CAS.
- H. M. Cho and B. Yoo, Rheological Characteristics of Cold Thickened Beverages Containing Xanthan Gum–Based Food Thickeners Used for Dysphagia Diets, J. Acad. Nutr. Diet., 2015, 115, 106–111 CrossRef PubMed.
- W. Jo, J. H. Bak and B. Yoo, Rheological characterizations of concentrated binary gum mixtures with xanthan gum and galactomannans, Int. J. Biol. Macromol., 2018, 114, 263–269 CrossRef CAS PubMed.
- A. Dick, B. Bhandari, X. Dong and S. Prakash, Food Hydrocolloids Feasibility study of hydrocolloid incorporated 3D printed pork as dysphagia food, Food Hydrocolloids, 2020, 107, 105940 CrossRef CAS.
- N. Paxton, W. Smolan, T. Böck, F. Melchels, J. Groll and T. Jungst, Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability, Biofabrication, 2017, 9, 044107 CrossRef PubMed.
- Y. Cheng, Y. Fu, L. Ma, P. L. Yap, D. Losic, H. Wang and Y. Zhang, Rheology of edible food inks from 2D/3D/4D printing, and its role in future 5D/6D printing, Food Hydrocolloids, 2022, 132, 107855 CrossRef CAS.
- A. Schwab, R. Levato, M. D'Este, S. Piluso, D. Eglin and J. Malda, Printability and Shape Fidelity of Bioinks in 3D Bioprinting, J. Am. Chem. Soc., 2020, 120, 11028–11055 CAS.
- J. M. Vieira, F. D. Oliveira, D. B. Salvaro, G. P. Maffezzolli, J. D. B. de Mello, A. A. Vicente and R. L. Cunha, Rheology and soft tribology of thickened dispersions aiming the development of oropharyngeal dysphagia-oriented products, Curr. Res. Food Sci., 2020, 3, 19–29 CrossRef CAS PubMed.
- Y. Liu, X. Liang, A. Saeed, W. Lan and W. Qin, Properties of 3D printed dough and optimization of printing parameters, Innovative Food Sci. Emerging Technol., 2019, 54, 9–18 CrossRef CAS.
- R. F. S. Gonçalves, J. T. Martins, C. M. M. Duarte, A. A. Vicente and A. C. Pinheiro, Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation, Trends Food Sci. Technol., 2018, 78, 270–291 CrossRef.
- B. Demir, M. Gürbüz, J. Çatak, H. Uğur, E. Duman, Y. Beceren and M. Yaman, In vitro bioaccessibility of vitamins B1, B2, and B3 from various vegetables, Food Chem., 2023, 398, 133944 CrossRef CAS PubMed.
- R. F. S. Gonçalves, D. A. Madalena, J. M. Fernandes, M. Marques, A. A. Vicente and A. C. Pinheiro, Application of nanostructured delivery systems in food: From incorporation to detection and characterization, Trends Food Sci. Technol., 2022, 129, 111–125 CrossRef.
- A. Ubeyitogullari and O. N. Ciftci, In vitro bioaccessibility of novel low-crystallinity phytosterol nanoparticles in non-fat and regular-fat foods, Food Res. Int., 2019, 123, 27–35 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo05102e |
| This journal is © The Royal Society of Chemistry 2025 |