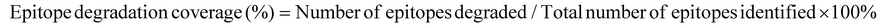Insight into the effect of lactic acid fermentation on soy protein immunoreactivity: emphasizing the difference in epitope destruction of varied fermentation terminal pH levels during gastrointestinal digestion
Yaqiong
Wang
,
Qiyuan
Lu
,
Qiuqin
Zhang
,
Runqiang
Yang
,
Wei
Li
 and
Xin
Rui
and
Xin
Rui
 *
*
College of Food Science and Technology, Nanjing Agricultural University, Nanjing, Jiangsu Province, P R China. E-mail: ruix@njau.edu.cn; Fax: +86 25 84399090; Tel: +86 15651661026
First published on 26th March 2025
Abstract
This study delves into the immunoreactivity of lactic acid bacterium (LAB)-fermented soy protein isolates (FSPIs), with various structural features, during gastrointestinal digestion and absorption. Additionally, the structural breakdown and epitope degradation of major allergens were investigated for their gastrointestinal behavior. Allergenicity and IgE-binding capacity assays revealed that at the initial and middle stages of digestion, FSPI-5.0/4.0 exhibited greater ability to reduce immunoreactivity than FSPI-7.0/6.0 and reduced immunoreactivity by 1.9%–36.6%. Caco-2 model transport studies further suggested that FSPI-5.0/4.0 T-5 and T-30 samples decreased immunoreactivity by inducing Th1-dominant differentiation. Peptidomics and bioinformatics analyses corroborated these findings, revealing that FSPI-5.0/4.0 promoted the epitope destruction of basic 7S globulin, β-conglycinin, P34, and glycinin and was dominated by secondary structures consisting of α-helices and β-sheets. Therefore, structural control through LAB fermentation represents a promising strategy for regulating the degradation of allergen epitopes.
1. Introduction
Allergy to soy is a common health problem and has a prevalence of ∼0.5% in the general population.1 Soybean, along with egg and cow's milk, is one of the three major allergens impacting children, affecting 0.4% of the child population.2 Allergic reactions triggered by soybean are mainly caused by proteins and depend on epitopes. Forty-four proteins have been identified as soybean allergens.3 They include major allergens, namely, glycinin, β-conglycinin, P34, lectin, and trypsin inhibitor. They are highly resistant to digestive enzymes and are thus considered highly correlated with severe reactions in patients with soybean allergies.4Food processing may regulate the structural features of soy proteins, leading to allergenicity reduction. Fermentation by lactic acid bacteria (LAB) is an innovative processing approach that could cause acidic gelation and regulate the structure of soy proteins to reduce soy allergenicity.5 A previous study showed that LAB fermentation might cause changes to the primary structure (degradation), further triggering the breakdown of allergen epitopes.6 Additional research suggested that acid gelation by LAB remarkably diminished the solubility of soy protein isolate (SPI), indicating that alterations in higher molecular structures might contribute to allergenicity reduction.7 Our previous study further suggested that LAB can regulate the secondary and tertiary structures of soy protein, corresponding to an overall increase in β-strands and surface hydrophobicity, thus contributing to allergenicity reduction.3 However, whether the reduction of immunoreactivity is associated with protein acidic gelation induced by LAB and its potential role in epitope disruption remains unclear.
The growth of LAB gradually releases protons from soy proteins, leading to a reduction in pH and triggering protein gelation.8 This process results in the formation of a gel network that comprises thick strands and large pores and is different from the gel network formed by acid coagulants.9 The conformational structure of soy proteins is greatly influenced by pH changes during gelation. Therefore, the terminal pH in LAB fermentation is vital for the structural modulation and immunoreactivity of soy protein.
The gastrointestinal fate of soybean allergens is crucial for determining their immunoreactivity because only allergen epitopes that persist in their intact state following digestion possess the capability to traverse the epithelial layer, eventually binding to immunoglobulin E (IgE) and triggering allergic reactions. Interestingly, structural changes induced by gelation greatly regulate the digestive behavior of soybean allergens that are notoriously impervious to enzymatic breakdown in their native configuration.10 However, studies exploring the effects and underlying mechanisms of structural changes induced by the protein gelation process in the degradation of soy allergen epitopes during gastrointestinal digestion are scant. A previous study suggested that the terminal pH of fermentation greatly affects the structural features of fermented soy protein isolates (FSPIs); these features range from a loosely arranged layer to a densely packed gel network.11 The structural rearrangement induced by LAB has been hypothesized to favor the gastrointestinal exposure and potential disruption of major allergen epitopes, contributing to a remarkable decline in soy allergenicity. Therefore, in the present study, FSPIs were prepared at different terminal pH levels of fermentation (pH 7.0, 6.0, 5.0, and 4.0) to represent varied structural features. A dynamic gastrointestinal model and a Caco-2 cell model were further applied to evaluate the epitope degradation and absorption of intestinal digestates using digestomics and bioinformatics tools. This study offers pivotal insights into the underlying mechanisms of the structural changes involved in promoting epitope degradation and thus reducing soy immunoreactivity.
2. Materials and methods
2.1. Materials
Soybeans were purchased from a local supermarket in Nanjing, China, and kept refrigerated at 4 °C during storage. All chemical reagents used in the experiments were of analytical grade and sourced from Sigma-Aldrich Co. (St Louis, MO, USA).The Lactiplantibacillus plantarum B1–6 strain was isolated from Xingjiang Kirgiz boza, a traditional cereal-based beverage from the Xinjiang Region of China.
2.2 Preparation of FSPIs
2.3 Solubility and turbidity
The Bradford method was employed to determine solubility. Initially, 100 μL of the sample was suspended in distilled water to a final concentration of 1% and then centrifuged at 4500g for 15 min. The absorbance of the supernatant was then measured at 595 nm and characterized by protein concentration (mg mL−1).Subsequently, the samples were dissolved in distilled water to a final concentration of 6%. The turbidity of the samples was subsequently characterized by measuring their absorbance at a wavelength of 660 nm.
2.4 Dynamic gastrointestinal digestion
Dynamic gastrointestinal digestion was conducted by using a mechanized soft rat model. The enzyme cocktails, simulated gastric fluid (SGF), and simulated intestinal fluid (SIF) used in this study were identical to those applied in previous research.12 To the FSPIs, 0.6 mL of SGF was added and the mixture transferred to an artificial rat stomach before digestion. The temperature was set at 37 °C. Gastrointestinal digestion was conducted for 3 h, and artificial gastric juice and intestinal fluid were added using a syringe pump. The FSPI digestates collected at 5, 30, and 180 min during intestinal digestion were designated as I-5, I-30, and I-180, respectively, and subsequently boiled for 5 min. The entire gastrointestinal digestion was performed in duplicate.2.5 Immunoassays
Immunoassays were carried out using an ELISA test kit according to a previous protocol.13 A total of 100 μL of the diluted solutions were transferred into primary antibody-coated microtiter plate wells. The plate was then incubated at 25 °C for 10 min and washed three times with a PBS solution containing 0.05% Tween 20 (v/v) (PBST). Then, 100 μL of secondary antibody was added to the plate and its was incubated at 25 °C for 10 min. Subsequently, the plate was washed again, and the calorimetric reaction was initiated by adding 100 μL of the substrate. The plate was then incubated in the dark at 25 °C for 10 min, and the reaction was terminated by adding 100 μL of the stop solution. The absorbance of the samples was measured at 450 nm. Three replicates were performed.2.6 IgE enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was conducted according to a previous method.14 Human sera with five levels of allergic reactions to soybeans were purchased from Chongqing Wolcavi Biological Technology Co., Ltd. The human serum samples were abbreviated as HS1, HS2, HS3, HS4, and HS5. Their specific IgE levels were 5.11, 6.07, 6.83, 7.62, and 12.09 KU L−1. The samples were dissolved in sodium carbonate buffer and then added to ELISA plates for incubation at 4 °C for 22 h. Nonspecific sites were rinsed three times with PBST and blocked using 250 μL of bovine serum albumin (2.5%, w/v, PBS) per well at 37 °C for 60 min. The ELISA plates were washed three times with PBST and incubated with 100 μL of human sera per well at 37 °C for 60 min. They were washed and incubated with 100 μL of antihuman IgE-ε-chain-specific peroxidase. The plate was incubated at 37 °C for 60 min, washed again, 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate solution added, and incubated at 37 °C for 20 min. Finally, the plate was loaded with sulfuric acid to terminate the reaction. Absorbance was measured at 450 nm, and three replicates were performed.2.7 Transepithelial transport study
The human colon cancer cell line Caco-2 was obtained from the American Type Culture Collection (ATCC). Caco-2 cells were propagated in Dulbecco's modified Eagle's medium and cultured in a humidified environment under 5% CO2 at 37 °C. Subsequently, the cells were seeded into 12-well plates at a density of 4 × 105 cells per cm2. Only monolayers with transepithelial electrical resistance (TEER) values exceeding 300 Ω cm2, indicating a well-formed epithelial barrier, were selected for the subsequent transport experiments.15 Next, the Caco-2 monolayers were rinsed three times with Hank's balanced salt solution (HBSS) and preincubated at 37 °C for 30 min. After preincubation, FSPI digestates were resuspended in a 5-fold volume of HBSS to create a suspension. The suspension was added to the apical (AP) side, and transport assays were then conducted at 37 °C for 120 min.2.8 RBL-2H3 cell assay
RBL-2H3 cells sourced from Proccell, Wuhan, China, were cultivated in a humidified 5% CO2 environment at 37 °C. They were grown in minimum essential medium and harvested through trypsinization. Subsequently, 5 × 105 cells were seeded into 96-well plates. Human antisera, which were obtained by pooling sera from five patients with soy allergies in equal proportions, were added to the plates and incubated for 1 d at 37 °C. The cells were then stimulated with 100 μL per well test samples and further incubated for 4 h at 37 °C. Interleukin (IL)-4, IL-13, histamine, and interferon (IFN)-γ levels were measured using a previously described method.16 Additionally, β-hexosaminidase levels were investigated in accordance with a previous method with minor modifications.17 Following the treatment of RBL-2H3 cells with a test sample solution, the resultant supernatant was aliquoted into a 96-well plate and incubated with 1 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide in citrate buffer (0.1 mol L−1) at 37 °C for 1 h. The enzymatic reaction was terminated by adding 200 μL of Na2CO3/NaHCO3 solution (0.1 mol L−1) and adjusted to pH 10.0. The absorbance was measured at 405 nm.2.9 Bioinformatics analysis of FSPI digestates
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min. The peptide solution was desalted using a desalination column and then acidified with 0.1% formic acid. Subsequently, the acidified solution was loaded onto a nano trap column using 2% acetonitrile and eluted from the trap column onto an analytical column using a linear gradient elution of 3%–45% buffer solution (acetonitrile with 0.1% formic acid) at a flow rate of 0.3 μL min−1. Mass spectrometry (MS) scans were conducted within the range of 350 m/z to 1800 m/z, whereas MS/MS scans were performed from 150 m/z to 1800 m/z. The identified peptide sequences of the allergens were retrieved from the UniProt database.
000g for 15 min. The peptide solution was desalted using a desalination column and then acidified with 0.1% formic acid. Subsequently, the acidified solution was loaded onto a nano trap column using 2% acetonitrile and eluted from the trap column onto an analytical column using a linear gradient elution of 3%–45% buffer solution (acetonitrile with 0.1% formic acid) at a flow rate of 0.3 μL min−1. Mass spectrometry (MS) scans were conducted within the range of 350 m/z to 1800 m/z, whereas MS/MS scans were performed from 150 m/z to 1800 m/z. The identified peptide sequences of the allergens were retrieved from the UniProt database.
Three-dimensional models of glycinin G5 (PDB ID: AF-P04347-F1), the β-conglycinin α subunit (PDB ID: AF-P0DO16-F1), P34 (PDB ID: AF-O64458-F1), and basic 7S globulin (Bg7S, PDB ID: AF-Q8RVH5-F1) were used to predict the spatial structures of major allergens.
2.10 Statistical analysis
The one-way analysis of variance and Duncan's multiple comparison test were used to evaluate significant differences among the means (P < 0.05).3. Results and discussion
3.1 Antigenicity and IgE-binding capacity of fermented soy proteins
During fermentation, the growth of LAB in the SPI food matrix gradually decreases pH and leads to protein gelation.8 LAB-induced gelation, which is specified as acid gelation, is triggered by the acids produced by LAB, exerting a considerable influence on the physicochemical and structural properties of proteins. The increase in L. plantarum B1–6 FSPI gradually reduced the pH and decreased the protein solubility at pH 6.0 (30.4% ± 0.01%), 5.0 (9.4% ± 0.92%), and 4.0 (11.0% ± 0.15%) (Fig. 1A). The turbidity of FSPI peaked at pH 5.0 and 4.0 and was 2-fold higher than that at pH 7.0. This result indicates that gel structure formation at pH 5.0 and 4.0 changed soy protein from soluble to insoluble, resulting in a dramatic increase in turbidity and a sharp decline in solubility (Fig. 1A). No significant difference (P > 0.05) was observed between the turbidity of FSPI gels with fermentation terminal pH levels of 5.0 and 4.0. The findings of our previous study on microstructure are consistent with the current results, demonstrating that FSPI-7.0 exhibits a protein network characterized by large hollows (18 μm), whereas FSPI-6.0 displays a similar structure with smaller hollows (7 μm), and FSPI-5.0/4.0 shows a dense 3D gel network with 1.8 μm pores.11 Previous studies have shown that LAB-induced SPI has a gelation pH of 5.5 and is accompanied by a decrease in electrostatic repulsion between soy proteins.10,19 Therefore, FSPIs were collected at the terminal pH levels of 7.0, 6.0, 5.0, and 4.0 and utilized for subsequent analyses. These FSPIs are abbreviated as FSPI-7.0, FSPI-6.0, FSPI-5.0, and FSPI-4.0. | ||
| Fig. 1 Solubility and turbidity (A), antigenicity (B) and IgE-binding activity (C) of FSPIs fermented at varied terminal pH values. | ||
Antigenicity (Fig. 1B) and IgE-binding capacity (Fig. 1C) gradually declined during the gelation of soy protein. The antigenicity values of FSPI-6.0, FSPI-5.0, and FSPI-4.0 were 37.9%, 57.2%, and 60.8% lower than that of FSPI-7.0, respectively. This finding suggests that LAB fermentation promoted the decline in protein allergenicity, particularly for gel structures that formed at low terminal pH levels. The decline in IgE-binding capacity was similar, albeit less potent when compared with that of antigenicity, which is likely due to individual differences. The IgE-binding capacities of FSPI-6.0, FSPI-5.0, and FSPI-4.0 were 23.4%, 31.7%, and 42.3% lower than that of FSPI-7.0, respectively. Similarly, LAB fermentation has been shown to reduce the immunoreactivity (67.7%–77.3%) of peanut and cow's milk protein effectively.20,21
3.2 Changes in the antigenicity and IgE-binding capacity of FSPIs during gastrointestinal digestion and absorption
Upon gastroduodenal digestion, all assayed samples demonstrated a dramatic decline in antigenicity. At D-5 and D-30, FSPI-7.0 digestates, followed by FSPI-6.0 (13.2%–5.0%), presented the highest antigenicity (16.2%–5.9%), whereas FSPI-5.0 and FSPI-4.0 had lower antigenicity (2.8%–4.1%) among digestates. The observed increase in antigenicity for FSPI-5.0 and FSPI-4.0 from D-5 to D-30 may be attributed to the exposure of allergenic epitopes during digestion. Comparable findings have been previously described for α-lactalbumin.22 At the late stage of intestinal digestion (D-180), the antigenicities of FSPI-6.0, FSPI-5.0, and FSPI-4.0 digestates were comparable (0.6% ± 0.1%) but were significantly lower than that of FSPI-7.0 (1.3% ± 0.2%). This finding indicates that gel structure formation promoted a sudden decrease in protein immunoreactivity at the early stage of gastrointestinal digestion (0–30 min).
The decline in IgE-binding capacity was not as dramatic as that in antigenicity, and the differences between FSPI samples showed dynamic changes with digestion time. At D-5, the FSPI-7.0 digestate showed the highest IgE-binding capacity (47.5% ± 2.9%), which was 1.1-, 1.3-, and 1.4-fold higher than the IgE-binding capacities of FSPI-6.0, FSPI-5.0 and FSPI-4.0 digestates, respectively. Subsequently, at D-30, the differences between FSPI digestates were significantly reduced. FSPI-7.0 and FSPI-6.0 showed comparable IgE-binding capacities (40.9%–41.6%), which were 1.1–1.2-fold higher than those shown by FSPI-5.0 and FSPI-4.0 (35.7%–36.6%). This finding is similar to the result for antigenicity and further confirmed that FSPI gels promoted IgE-binding capacity. The formation of a gel structure might unfold higher structures and expose catalytic sites by regulating the gastrointestinal pH environment, thus promoting the hydrolysis of soy proteins and reducing the IgE-binding capacity.11 FSPI-5.0 and FSPI-4.0 exhibit similar gel networks, which may lead to their similar digestive pattern and immunoreactivity. At D-180, the IgE-binding capacity of all investigated samples further decreased to <12% and lacked significant differences (P > 0.05) likely due to the increase in protein hydrolysis.
FSPI-7.0 and FSPI-6.0 T-5 samples showed comparable antigenicities (3.2%–3.4%), which were 1.6–1.8-fold higher than those exhibited by FSPI-5.0 and FSPI-4.0 samples (1.9%–2.0%). Subsequently, the difference between FSPI samples significantly increased at T-30. Among the samples, the FSPI-7.0 sample showed the highest antigenicity (4.3% ± 0.4%), which was 1.3-, 2.5-, and 2.8-fold higher than those of FSPI-6.0, FSPI-5.0, and FSPI-4.0, respectively. FSPIs collected at low terminal pH levels of 5.0 and 4.0 demonstrated a sudden decline in antigenicity during 0–30 min of gastrointestinal digestion and transport. This result reflects the vital role of gel structure formation in the control of soy antigenicity during LAB fermentation. After the transport of the late digestates (T-180), the difference between FSPI samples significantly decreased. The antigenicities of the FSPI-6.0/5.0/4.0 samples were comparable but significantly lower (P > 0.05) than that of FSPI-7.0.
The IgE-binding capacity of FSPI samples demonstrated a similar trend to that of antigenicity. The IgE-binding capacities (10.3%–11.1%) of FSPI-7.0 and FSPI-6.0 samples at T-5 were comparable and 1.1–1.2-fold higher than those of FSPI-5.0 and FSPI-4.0 samples (9.4%). Subsequently, the IgE-binding capacity (10.4% ± 0.9%) of the FSPI-7.0 sample was the highest and 1.1-, 1.3-, and 1.3-fold higher than those of FSPI-6.0, FSPI-5.0, and FSPI-4.0, respectively. This finding suggests that gel structure formation promoted the decline in immunoreactivity after the transport of early and medium digestates. Gel structure formation might break the steric hindrance of allergen epitopes during digestion, thus facilitating entry into the Caco-2 cell monolayer and leading to a considerable decline in IgE-binding capacity. After the transport of the late intestinal digestates (T-180), the IgE-binding capacities of all assayed FSPI samples after transport further decreased to <10% and became comparable.
3.3 IgE-mediated allergic responses in RBL-2H3 cells
Peptide fragments collected from intestinal digestates on the BL side were treated with RBL-2H3 cells to further delve into IgE-mediated allergic responses. Fig. 3 shows the levels of histamine (Fig. 3A), β-hexosaminidase (Fig. 3B), IL-4 (Fig. 3C), IL-13 (Fig. 3D), IFN-γ (Fig. 3E), and IFN-γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL-4 (Fig. 3F) released from RBL-2H3 cells.
IL-4 (Fig. 3F) released from RBL-2H3 cells.
At T-5 and T-30, FSPI-7.0 and FSPI-6.0 stimulated the release of comparable histamine and β-hexosaminidase levels (2.3–2.7 ng mL−1) without significant differences (P > 0.05), whereas FSPI-5.0 and FSPI-4.0 resulted in the release of low histamine and β-hexosaminidase levels (1.0–1.6 ng mL−1). Subsequently, all FSPI T-180 samples presented comparable histamine and β-hexosaminidase levels without significant differences (P > 0.05). Histamine and β-hexosaminidase are the key substances observed during allergic inflammation and are produced by the degranulation of mast cells.24,25 The above result indicates that FSPI gels have a potential role in the prevention of mast cell degranulation by decreasing the levels of histamine and β-hexosaminidase.
At T-5, all assayed FSPI samples elicited comparable IL-4 and IL-13 levels that ranged from 3.1 pg mL−1 to 3.7 pg mL−1 without significant differences (P > 0.05). However, IFN-γ levels were notably higher in cells sensitized with FSPI-6.0/5.0/4.0 T-5 samples, exhibiting 1.7-, 2.5-, and 2.5-fold increments compared with those sensitized with FSPI-7.0 (92.0 ± 9.3 pg mL−1). The trend of IFN-γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL-4 ratios followed a similar pattern as that of IFN-γ levels. Subsequently, at T-30, FSPI-7.0 and FSPI-6.0 had comparable IL-4 and IL-13 levels (3.6 pg mL−1) without significant differences (P > 0.05), whereas FSPI-5.0 and FSPI-4.0 showed significantly reduced levels of IL-4 and IL-13 (3.1–3.3 pg mL−1). Notably, IFN-γ levels in cells sensitized with FSPI-6.0/5.0/4.0 T-30 samples were 2.9-, 4.4-, and 4.3-fold higher than those in cells sensitized with FSPI-7.0. Moreover, the IFN-γ
IL-4 ratios followed a similar pattern as that of IFN-γ levels. Subsequently, at T-30, FSPI-7.0 and FSPI-6.0 had comparable IL-4 and IL-13 levels (3.6 pg mL−1) without significant differences (P > 0.05), whereas FSPI-5.0 and FSPI-4.0 showed significantly reduced levels of IL-4 and IL-13 (3.1–3.3 pg mL−1). Notably, IFN-γ levels in cells sensitized with FSPI-6.0/5.0/4.0 T-30 samples were 2.9-, 4.4-, and 4.3-fold higher than those in cells sensitized with FSPI-7.0. Moreover, the IFN-γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL-4 ratios in these cells exhibited considerable increases and were 3.3-, 8.2-, and 7.5-fold higher than those in cells sensitized by FSPI-7.0. At T-180, all assayed FSPI samples exhibited comparable levels of IL-4, IL-13, IFN-γ, and IFN-γ
IL-4 ratios in these cells exhibited considerable increases and were 3.3-, 8.2-, and 7.5-fold higher than those in cells sensitized by FSPI-7.0. At T-180, all assayed FSPI samples exhibited comparable levels of IL-4, IL-13, IFN-γ, and IFN-γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL-4 without significant differences (P > 0.05). This finding is consistent with the results for histamine and β-hexosaminidase.
IL-4 without significant differences (P > 0.05). This finding is consistent with the results for histamine and β-hexosaminidase.
IgE-mediated allergic reactions are related to Th1 and Th2 imbalance. IL-4 and IL-13 are proinflammatory factors produced by Th2 cells, basophils, and activated mast cells in allergic reactions.26,27 IFN-γ acts as an anti-inflammatory factor that regulates the differentiation of Th1 cells.28 The differentiation status of Th1 and Th2 cells is predominantly dependent on IL-4, IL-13, and IFN-γ levels. Our result illustrates that FSPI-6.0 up-regulated IFN-γ levels but had a minor effect on IL-4 levels, whereas FSPI gels (FSPI-5.0 and FSPI-4.0) simultaneously induced IFN-γ production and down-regulated IL-4 levels compared to FSPI-7.0. These phenomena might further favor the dominant differentiation of Th1:Th2, thus reducing immunoreactivity.
3.4 Bioinformatics analysis of allergen epitope degradation
The reduction in the immunoreactivity of a protein is intricately linked to its epitope degradation pattern. Therefore, we evaluated epitope degradation by using a bioinformatics approach that statistically analyzes the digestive peptidome on the basis of protein levels.29The difference in the epitope degradation coverage of FSPIs is dependent on the time of gastrointestinal digestion. At I-5, digestates derived from FSPI-5.0 and FSPI-4.0 showed significantly higher epitope degradation coverage (47.8%–100%) than FSPI-7.0 and FSPI-6.0 (40.6%–100%). FSPI-6.0 showed a higher epitope degradation coverage for glycinin G1 (27/34), glycinin G4 (18/23), the β-conglycinin α subunit (24/30), and the β subunit (71/75) than FSPI-7.0, whereas FSPI-5.0 and FSPI-4.0 elicited a potent elevation in the epitope degradation coverage of the β-conglycinin α/β subunit, glycinin G1/G3/G4/G5, and P34. The epitope degradation coverages of FSPI gel digestates were 26/30 (α subunit), 70–71/75 (β subunit), 27/34 (G1), 13/22 (G3), 18/23 (G4), 17–18/32 (G5), and 14/21 (P34) and were 1.2–1.4-fold higher than those of FSPI-7.0 (20/30, 52/75, 24/34, 11/22, 15/23, 15/32, and 13/21).
At I-30, the epitope degradation coverage of FSPI-6.0 for glycinin G5 (12/32) was high and 1.2-fold higher than that of FSPI-7.0 (10/32). FSPI-5.0 and 4.0 demonstrated the increased degradation of the allergens glycinin G2/G5, P34, and Bg7S. The epitope degradation coverages of FSPI-5.0 and 4.0 were 44/53, 16–17/32, 14/21, and 22–24/38, which were 1.1–1.7-fold higher than that of FSPI-7.0 (43/53, 10/32, 13/21, and 19/38).
At I-180, FSPI-6.0 demonstrated epitope degradation coverage similar to that of FSPI-7.0. However, the degradation rate of FSPI-6.0 for Bg7S (12/38) was 1.2-fold higher than that of FSPI-7.0 (10/38). FSPI gels (pH 5.0/4.0) showed higher epitope degradation coverage for the β-conglycinin α′ subunit, glycinin G5, and Bg7S. The epitope degradation coverages of the FSPI gel digestates were 23–25/46, 12–15/32, and 19–21/38, which were 1.1–3.5-fold higher than that of FSPI-7.0 (21/46, 10/32, and 6/38).
In summary, FSPI-5.0 and FSPI-4.0 promoted the rapid degradation of epitopes from β-conglycinin α/β subunits and glycinin G1/G3/G4/G5 at the early digestion stage and elicited the high epitope degradation coverage of Bg7S/P34 and the β-conglycinin α′ subunit mainly at the middle and late stages of digestion, respectively. The recognition rate for glycinin G5 in children allergic to soy proteins is 91% (20 out of 22), which is considerably higher than that for glycinin G1–G4.30 The comparison of α′ or β subunits revealed that the α subunit of β-conglycinin is the strongest allergenic protein subunit, affecting 25% of patients with soy allergies.31,32 P34 is an immunodominant allergen, causing allergic reactions in more than 65% of patients.33 Bg7S, a novel globulin, has been noted for its extreme recalcitrance to enzymatic digestion.34 Its swift degradation could potentially play a pivotal role in minimizing soy immunoreactivity in FSPI-5.0 and FSPI-4.0 samples, which were selected for the further prediction of 3D structures.
3.4.2.1 Glycinin G5. Fig. 5A depicts the 3D structure of glycinin G5. A total of 32 epitopes were identified in glycinin G5. They were dispersed across two distinct regions: the acidic (I25–N344) and basic (G345–P516) subunits. The variation in the cleavage frequency of these epitopes among FSPIs was most notable in 11 epitopes, which clustered in six regions as determined by their 3D structural adjacent relationships. Regions 1 (epitope D194–T200), 2 (epitope W275–D282), 3 (epitopes E311–P320, E313–P324, Q317–G322, and P320–Q325), and 4 (epitope Q332–G337) were distributed in the acidic subunit region, and regions 5 (epitopes L490–N495 and N492–G497) and 6 (epitopes N509–N515 and G511–N515) were distributed in the basic subunit region. The other 20 epitopes exhibited little significant variations among FSPIs. Most of them predominantly resided in the acidic subunit region, and a few were scattered in the basic subunit region.
At I-5, in contrast to FSPI-7.0, the FSPI-6.0 digestate promoted the degradation of epitopes in region 2, whereas FSPI-5.0 and FSPI-4.0 resulted in the substantial degradation of epitopes in regions 1–4 of glycinin G5 (highlighted in red in Fig. 5A1). These epitopes were positioned on the exterior random coil and α-helix structures of the acidic subunit. As intestinal digestion progressed, additional degradation in region 6 was observed at I-30 (highlighted in yellow in Fig. 5A2) beyond the variations observed in regions 2–4. The FSPI-6.0 digestate enhanced the degradation of epitopes located in region 6, whereas FSPI-5.0 and FSPI-4.0 showed a high cleavage frequency of epitopes in regions 2–4 and 6. Epitopes in region 6 (N509–N515 and G511–N515) were degraded completely in FSPI-6.0/5.0/4.0 but not in FSPI-7.0. These epitopes were situated on the exterior of the basic subunit with a random coil structure. In addition, the degradation of epitopes located on the exterior random coil structure of the acidic subunit, namely, regions 3 and 4, was promoted by FSPI-5.0/4.0 but was barely promoted by FSPI-6.0/7.0. At I-180, discrepancies between variations in different samples were limited to region 5 (highlighted with green lines in Fig. 5A3). The promoted degradation of epitopes in this region was observed in FSPI-5.0 and FSPI-4.0, which were related to the exterior α-helix structure of the basic subunit. These results indicate that the formation of gel structure might elicit increased epitope degradation in the acidic subunit of glycinin at the early digestion stage but promote the epitope degradation of basic subunits in the middle and late digestion stages.
3.4.2.2 The β-conglycinin α subunit. Fig. 5B illustrates the 3D structure of the β-conglycinin α subunit. In total, 24 epitopes were situated along its chain from residues V63 to Y605. The notable differences in the cleavage frequency of these epitopes among all FSPI samples were primarily observed in eight epitopes, which were grouped into five distinct regions: regions 1 (epitope E67–E95), 2 (epitopes R102–R127 and H117–E142), 3 (epitope D172–L197), 4 (epitope F322–E343), and 5 (epitopes E382–N407, S401–N430, and D412–D437). The other 16 epitopes exhibited few notable differences among FSPI samples and were predominantly located in α-helix and β-sheet structures.
At I-5, in contrast to FSPI-7.0, the FSPI-6.0 digestate enhanced the degradation of epitopes situated in regions 2 and 3, whereas FSPI-5.0 and FSPI-4.0 resulted in the robust degradation of seven epitopes spanning regions 1 to 3 of the β-conglycinin α subunit (highlighted with red lines in Fig. 5B1). These epitopes were positioned on the exterior of the spatial structure and characterized by a secondary structure comprising α-helices and random coils. This finding was consistent with the result for glycinin G5. The epitope E67–E95 located in region 1 at the exterior of the spatial structure exhibited a random coil structure and was degraded in FSPI gels, but not in FSPI-7.0, throughout intestinal digestion.
As intestinal digestion progressed, further variations in region 4 were observed at I-30 (highlighted with yellow lines in Fig. 5B2). The FSPI-6.0 digestate promoted the degradation of epitopes located in regions 2–4, whereas FSPI-5.0 and FSPI-4.0 digestates showed a high cleavage frequency of epitopes in regions 1–5. The cleavage frequencies of epitopes R102–R127 and H117–E142 in region 2 of the FSPI-5.0 and FSPI-4.0 digestates were 2.1–3.1- and 2.6–5.1-fold higher than those in FSPI-7.0, respectively. Epitope H117–E142 in region 2 is a potent epitope and was recognized in all investigated patients in a previous study.35 At I-180, additional variations in region 5 became apparent among the FSPIs (highlighted with green lines in Fig. 5B3). The promoting effect on FSPI-5.0 and FSPI-4.0 significantly reduced, and these changes were most prominent in region 5, followed by those in regions 3 and 2 in descending order.
3.4.2.3 P34. Fig. 5C depicts the 3D structure of P34. A total of 21 epitopes, spanning residues S18 to L379, were identified along its main chain. Of these epitopes, four showed marked differences in cleavage frequency among FSPI samples. These four epitopes corresponded to two distinct regions, namely, regions 1 (epitopes D242–S253, D251–F262, and T254–A265) and 2 (epitope A366–L379). The other 17 epitopes exhibited few significant variations among FSPIs and were predominantly located in random coil and α-helix regions.
At I-5 and I-30, the FSPI-6.0 digestate facilitated the degradation of three epitopes situated in region 1. FSPI-5.0 and FSPI-4.0 exhibited high cleavage frequencies in epitopes at regions 1 and 2. They were positioned on the exterior of P34 and exhibited a secondary structure comprising α-helices, β-sheets, and random coils. At I-180, the promoting effect on epitope degradation in FSPI-5.0 and FSPI-4.0 significantly reduced, with the most notable changes observed in region 1. A previous study indicated that the α-helix is the primary structure of cross-reactive allergen epitopes, with a considerable number of such epitopes predominantly located within the Nt domain.32 The development of the gel network might cause the rearrangement and exposure of the secondary structure of α-helices and thus elicit rapid and extensive epitope degradation.
3.4.2.4 Bg7S. Fig. 5D illustrates the 3D structure of Bg7S. Thirty-eight epitopes were identified. The variance in the degradation among all FSPI samples was most apparent in six epitopes, which corresponded to six distinct regions: regions 1 (epitope Q66–D72), 2 (epitope H77–E83), 3 (epitope T156–L162), 4 (epitope G332–N338), 5 (epitope V367–G372), and 6 (epitope G392–E397). The other 32 epitopes exhibited few significant differences among FSPIs. They were primarily positioned within the interior of Bg7S and characterized by a β-sheet secondary structure.
At I-5 and I-30, the epitope T156–L162 in region 3 was degraded in the FSPIs obtained at low fermentation pH levels (6.0/5.0/4.0). However, FSPI-7.0 showed no degradation. The epitope G392–E397 in region 6 was degraded only in FSPI-5.0 and FSPI-4.0. Compared with FSPI-7.0 and FSPI-6.0, FSPI-5.0 and FSPI-4.0 showed greater similarities and promoted the degradation of epitopes buried in the interior of Bg7S with a secondary structure consisting of β-sheets and random coils. The epitope G332–N338 in region 4 was degraded only in FSPI-5.0, whereas epitope V367–G372 in region 5 was degraded only in FSPI-4.0. These two epitopes were positioned on the exterior β-sheet structure of Bg7S (highlighted in red and yellow in Fig. 5D1 and D2), indicating that several exterior discrepancies existed between FSPI gels. At I-180, FSPI-6.0 markedly enhanced the degradation of an epitope situated in region 3, whereas the promotion of epitope degradation by FSPI-5.0 and FSPI-4.0 significantly decreased and was mainly reflected at regions 1–3 (highlighted in green in Fig. 5D3).
3.4.2.5 Difference in the gastrointestinal degradation of major allergens between FSPIs. Compared with FSPI-7.0, FSPI-6.0 allowed a more intensive degradation of epitopes with random coil and α-helix structures, whereas FSPI-5.0 and FSPI-4.0 samples promoted the extensive degradation of epitopes with α-helix, random coil, and β-sheet structures. This result indicates that epitopes with random coil and α-helix structures can be potentially modified flexibly by LAB fermentation, whereas β-sheets can be altered during the formation of FSPI gels. The development of a gel network potentially disrupts the conformational constraints of β-sheet structures in major allergens, facilitating the exposure of cleavage sites within allergen epitopes during gastrointestinal digestion. This process enables the degradation of allergens into small peptides, which can then traverse the Caco-2 monolayer. Consequently, these phenomena contribute to a reduction of the immunoreactivity in the gastrointestinal tract. This finding is consistent with our previous results,10 and further proves that the gel structure fermented at terminal pH values of 5.0–4.0 plays a key role in promoting the epitope degradation of interior β-sheet structures, while FSPI fermented at terminal pH 6.0 does not show this promoting effect.
The variations between FSPI-5.0 and FSPI-4.0 were minimal and dependent on specific allergens. FSPI-5.0/4.0 showed a discrepancy in the epitope degradation of Bg7S but displayed comparable degradation patterns for β-conglycinin, glycinin, and P34, thereby highlighting a remarkable similarity in the degradation pattern of major allergens within the gel structure.
4. Conclusion
This study delved into the effect of protein structural features on the epitope degradation of various soybean allergens during digestion and transport. The terminal pH of fermentation regulated protein structural shifts. During gastrointestinal digestion and transport, soybean allergens in gel matrices prepared with terminal pH levels of 5.0–4.0 showed extensive epitope destruction that contributed to the remarkable reduction in immunoreactivity. In addition, FSPI-5.0 and FSPI-4.0 triggered the considerable degradation of exterior epitopes at I-5 and promoted the extensive degradation of several epitopes embedded within the interior of major allergens at I-30. The similar effects observed at pH 5.0 and 4.0 can be attributed to the formation of gel networks when the pH is at or lower than 5.5. Specifically, FSPI-5.0 and FSPI-4.0 develop comparable, densely structured three-dimensional gel networks with analogous pore sizes. This structural similarity leads to consistent epitope degradation patterns during gastrointestinal digestion. The gel structure formed during LAB fermentation potentially broke conformational hindrances and modulated the gastrointestinal degradation pattern of soy proteins, resulting in the rapid degradation of linear epitopes and reduction in immunoreactivity. This research provides crucial insights into how fermentation terminal pH levels affect the digestion and transport of soy allergen epitopes, offering valuable information for improving processing methods to develop hypoallergenic products.Author contributions
Yaqiong Wang: investigation, writing – original draft, and data curation. Qiyuan Lu: methodology, validation. Qiuqin Zhang: writing – review & editing. Runqiang Yang: formal analysis. Wei Li: supervision. Xin Rui: conceptualization, resources, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.Ethical statement
All experiments involving human antisera were conducted in strict accordance with the “Ethical Guidelines for Biomedical Research Involving Human Subjects, National Health Commission of China”. The experiments were approved by the ethics committee at Nanjing Agricultural University. Informed consent was obtained from human participants in this study.Data availability
The data supporting this article have been included as part of the ESI.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was co-financed by the National Natural Science Foundation of China (No. 32072337 and 32372440), the Fundamental Research Funds for the Central Universities (CXCYL2023005), the Jiangsu Key R&D Program Project (BE2023318), and the Yangzhou Key R&D Plan Project (YZ2023044). The authors would also like to acknowledge a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- X. Pi, Y. Sun, G. Fu, Z. Wu and J. Cheng, Effect of processing on soybean allergens and their allergenicity, Trends Food Sci. Technol., 2021, 118, 316–327 CrossRef CAS
.
- J. Riascos, S. Weissinger, A. Weissinger, M. Kulis, A. Burks and L. Pons, The Seed Biotinylated Protein of Soybean (Glycine max): A Boiling-Resistant New Allergen (Gly m 7) with the Capacity to Induce IgE-Mediated Allergic Responses, J. Agric. Food Chem., 2016, 64, 3890–3900 CrossRef CAS
.
- X. Rui, J. Huang, G. Xing, Q. Zhang, W. Li and M. Dong, Changes in soy protein immunoglobulin E reactivity, protein degradation, and conformation through fermentation with Lactobacillus plantarum strains, LWT–Food Sci. Technol., 2019, 99, 156–165 CrossRef CAS
.
- M. Berneder, M. Bublin, K. Hoffmann-Sommergruber, T. Hawranek and R. Lang, Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe foodallergic reactions to soy, Int. Arch. Allergy Appl. Immunol., 2013, 161, 229–233 CrossRef CAS PubMed
.
- J. Xia, Q. Zu, A. Yang, Z. Wu, X. Li, P. Tong and H. Chen, Allergenicity reduction and rheology property of lactobacillus -fermented soymilk, J. Sci. Food Agric., 2019, 99, 6841–6849 CrossRef CAS PubMed
.
- V. Biscola, A. R. de Olmos, Y. Choiset, H. Rabesona, M. S. Garro, F. Mozzi and B. Franco, Soymilk fermentation by Enterococcus faecalis VB43 leads to reduction in the immunoreactivity of allergenic proteins betaconglycinin (7S) and glycinin (11S), Benefic. Microbes, 2017, 8, 635–643 CAS
.
- P. Meinlschmidt, E. Ueberham, J. Lehmann, U. Schweiggert-Weisz and P. Eisner, Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate, Food Chem., 2016, 205, 229–238 CrossRef CAS PubMed
.
- A. Grygorczyk, L. Duizer and I. Lesschaeve, Gelation of recombined soymilk and cow's milk gels: Effect of homogenization order and mode of gelation on microstructure and texture of the final matrix, Food Hydrocolloids, 2014, 35, 69–77 CrossRef CAS
.
- X. Yang, Y. Ren, H. Liu, C. Huo and L. Li, Differences in the physicochemical, digestion and microstructural characteristics of soy protein gel acidified with lactic acid bacteria, glucono-δ-lactone and organic acid, Int. J. Biol. Macromol., 2021, 185, 462–470 CrossRef CAS
.
- Y. Wang, Y. Fu, W. Li, K. Benjiamin and X. Rui, Modulation of soy protein immunoreactivity by different matrix structures of lactic acid bacterium-induced soy protein gels: Epitope destruction during in vitro, gastroduodenal digestion and absorption, Food Res. Int., 2023, 173, 113281 CrossRef CAS PubMed
.
- Y. Wang, Y. Fu, E. Azarpazhooh, W. Li, Q. Liu and X. Rui, Assessment of In Vitro Digestive Behavior of Lactic-AciI-Bacteria Fermented Soy Proteins: A Study Comparing Colloidal Solutions and Curds, Molecules, 2022, 27, 7652 CrossRef CAS PubMed
.
- Y. Wang, W. Sun, Y. Zhang, W. Li, Q. Zhang and X. Rui, Assessment of dynamic digestion fate of soy protein gel induced by lactic acid bacteria: A protein digestomics research, Food Hydrocolloids, 2023, 136, 108309 CrossRef CAS
.
- Y. Li and S. Damodaran,
In vitro digestibility and IgE reactivity of enzymatically cross-linked heterologous protein polymers, Food Chem., 2017, 221, 1151–1157 CrossRef CAS PubMed
.
- Y. Song, Z. Li and H. Lin, Effect of malondialdehyde treatment on the IgE binding capacity and conformational structure of shrimp tropomyosin, Food Chem., 2015, 175, 374–380 CrossRef CAS PubMed
.
- D. Regazzo, D. Mollé, G. Gabai, D. Tomé, D. Dupont, J. Leonil and R. Boutrou, The (193–209) 17-residues peptide of bovine β-casein is transported through Caco-2 monolayer, Mol. Nutr. Food Res., 2010, 54, 1428–1435 CrossRef CAS PubMed
.
- J. Ma, P. Tong, Y. Chen, Y. Wang, H. Ren, Z. Gao and F. Long, The inhibition of pectin oligosaccharides on degranulation of RBL-2H3 cells from apple pectin with high hydrostatic pressure assisted enzyme treatment, Food Chem., 2022, 371, 131097 Search PubMed
.
- W. Wang, Q. Zhou, L. Liu and K. Zou, Anti-allergic activity of emodin on IgE-mediated activation in RBL-2H3 cells, Pharmacol. Rep., 2012, 64, 1216–1222 CrossRef CAS PubMed
.
- J. Izquierdo-González, F. Amil-Ruiz, S. Zazzu, R. Sánchez-Lucas, C. Fuentes-Almagro and M. Rodríguez-Ortega, Proteomic analysis of goat milk kefir: Profiling the fermentation-time dependent protein digestion and identification of potential peptides with biological activity, Food Chem., 2019, 295, 456–465 CrossRef PubMed
.
- Z. Pang, R. Xu, Y. Zhu, H. Li, N. Bansal and X. Liu, Comparison of rheological, tribological, and microstructural properties of soymilk gels acidified with glucono-δ-lactone or culture, Food Res. Int., 2018, 121, 798–805 CrossRef PubMed
.
- X. Pi, G. Fu, B. Dong, Y. Yang, Y. Wan and M. Xie, Effects of fermentation with Bacillus natto on the allergenicity of peanut, LWT–Food Sci. Technol., 2021, 141, 110862 CrossRef CAS
.
- Y. Xu, F. Zhang, R. Ma, Z. Zhang, L. Chi, Y. Li and X. Zhu, Effects of Lacticaseibacillus paracasei XJ-003 on the allergenicity and antigenicity of milk proteins during fermentation, Food Biosci., 2024, 59, 103967 CrossRef CAS
.
- X. Wang, Z. Tu, G. Liu, H. Wang, Y. Hu and T. Huang, Investigation on the anaphylaxis and anti-digestive stable peptides identification of ultrasound-treated α-lactalbumin during in vitro gastroduodenal digestion, Foods, 2021, 10, 2760 CrossRef CAS PubMed
.
- G. Picariello, G. Iacomino, G. Mamone, P. Ferranti, O. Fierro, C. Gianfrani and F. Addeo, Transport across Caco-2 monolayers of peptides arising from in vitro digestion of bovine milk proteins, Food Chem., 2013, 139, 203–212 CAS
.
- J. Chen, R. Liang, W. Liu, T. Li, C. Liu, S. Wu and Z. Wang, Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties, Carbohydr. Polym., 2012, 91, 175–182 CrossRef PubMed
.
- M. Jutel, M. Akdis and C. Akdis, Histamine, histamine receptors and their role in immune pathology, Clin. Exp. Allergy, 2009, 39, 1786–1800 CAS
.
- S. Romagnani, Immunologic influences on allergy and the TH1/TH2 balance, J. Allergy Clin. Immunol., 2004, 113, P395–P400 Search PubMed
.
- G. Arthur and P. Bradding, New Developments in Mast Cell Biology, Chest, 2016, 150, P680–P693 Search PubMed
.
- E. Pohjavuori, M. Viljanen, R. Korpela, M. Kuitunen, M. Tiittanen, O. Vaarala and E. Savilahti, Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy, J. Allergy Clin. Immunol., 2004, 114, 131–136 CrossRef CAS PubMed
.
- K. Kiyotani, Y. Toyoshima, K. Nemoto and Y. Nakamura, Bioinformatic prediction of potential T cell epitopes for SARS-Cov-2, J. Hum. Genet., 2020, 65, 569–575 CAS
.
- S. Sjolander, F. Bernhardsson, P. Brostedt, M. Borres, A. Tanaka, K. Ito and M. Poorafshar, High IgE Reactivity to Subunit G5 from the Soybean Legumin Allergen Gly m 6 in Sera from Soy Allergic Japanese Children, J. Allergy Clin. Immunol., 2010, 125, AB220 Search PubMed
.
- B. Liu, D. Teng, Y. Yang, X. Wang and J. Wang, Development of a competitive ELISA for the detection of soybean α subunit of β-conglycinin, Process Biochem., 2012, 47, 280–287 CAS
.
- H. B. Krishnan, W. S. Kim, S. Jang and M. S. Kerley, All Three Subunits of Soybean β-Conglycinin Are Potential Food Allergens, J. Agric. Food Chem., 2009, 57, 938–943 CAS
.
- A. M. Candreva, P. L. Smaldini, A. Cauerhff, S. Petruccelli and G. H. Docena, A novel approach to ameliorate experimental milk allergy based on the oral administration of a short soy cross-reactive peptide, Food Chem., 2020, 346, 128926 Search PubMed
.
- C. Magni, F. Sessa, J. Capraro, M. Duranti, E. Maffioli and A. Scarafoni, Structural and functional insights into the basic globulin 7S of soybean seeds by using trypsin as a molecular probe, Biochem. Biophys. Res. Commun., 2018, 496, 89–94 CAS
.
- X. Sun, X. Shan, Z. Yan, Y. Zhang and L. Guan, Prediction and characterization of the linear IgE epitopes for the major soybean allergen beta-conglycinin using immunoinformatics tools, Food Chem. Toxicol., 2013, 56, 254–260 CAS
.
| This journal is © The Royal Society of Chemistry 2025 |