Open Access Article
Open Access ArticleA review of the capacity of xylooligosaccharides to modulate gut microbiota and promote health
Natalia-María Nogueira-Prieto†
 abc,
Carmen Ansín-Vallejo†
abc,
Carmen Ansín-Vallejo† abc,
Manuel Becerra-Fernández
abc,
Manuel Becerra-Fernández abc and
María-Isabel González-Siso
abc and
María-Isabel González-Siso *abc
*abc
aEXPRELA Research Group, CICA - Centro Interdisciplinar de Química e Bioloxía, Universidade da Coruña, Campus de Elviña, As Carballeiras, s/n, 15071 A Coruña, Spain. E-mail: isabel.gsiso@udc.es
bEXPRELA Research Group, Departamento de Bioloxía, Facultade de Ciencias, Universidade da Coruña, Campus da Zapateira, Rúa da Fraga, 10, 15071 A Coruña, Spain
cEXPRELA Research Group, Instituto de Investigación Biomédica de A Coruña (INIBIC), As Xubias de Arriba 84, 15006 A Coruña, Spain
First published on 21st May 2025
Abstract
Xylooligosaccharides (XOS), derived from lignocellulosic biomass and algae, have emerged as promising prebiotics due to their ability to selectively modulate gut microbiota and confer various health benefits. XOS are composed of β-D-xylopyranose units linked by β-glycosidic bonds and are resistant to mammalian digestion but fermentable by beneficial gut bacteria. Research results indicate that XOS enhance the growth of probiotics, having a bifidogenic effect, which stimulates the production of short-chain fatty acids (SCFAs), and suppress the proliferation of pathogens. In vitro and in vivo studies demonstrate their potential to alleviate metabolic disorders, improve lipid profiles, reduce inflammation, and restore gut homeostasis. Several studies in humans or animal models reveal positive outcomes on gut microbiota diversity, immune function, and metabolic parameters in both healthy and diseased individuals, including improvements in bowel health, obesity, and type 2 diabetes markers. Additionally, XOS exhibit promising anti-inflammatory and anticancer properties, with evidence of their role in reducing tumour cell proliferation and enhancing oxidative stress resistance. Despite these promising findings, challenges remain in cost-effective production and large-scale application. Advances in biotechnological methods and regulatory approvals are expected to drive the expansion of the XOS market, projected to grow significantly over the next decade. This review highlights the potential of XOS as a functional dietary component with applications in gut health and disease prevention, warranting further clinical studies to confirm their therapeutic efficacy in humans.
Introduction
Gut microbiota is a community of microorganisms that inhabit the gastrointestinal tract, covering a wide range of fungi, virus, archaea and, especially, bacteria. This community is notably condensed in the large intestine, where up to 1010 to 1012 microorganisms per gram can be found.1,2Gut microbiota is a vital component of the gastrointestinal environment due to its contribution to digestion and fermentation of diet fibre, as well as its role in stimulating the development of microvilli. This community is also implicated in the stimulation and regulation of the immune system and in the maintenance of metabolic homeostasis, as it contributes to cholesterol and bile acid metabolism and the production of short chain fatty acids (SCFAs).1,3 The correct functioning of gut microbiota can also protect its host against growth and colonization of pathogens.1
The establishment of this complex ecosystem starts right after birth, the two first years of life being the most important. The conformation of the infant microbiota will depend on the type of delivery, health of the mother, drug use and diet around these first two years.3 In the case of adult gut microbiota, the composition is primarily affected by diet, but it can also be affected by other factors (exercise, sleep patterns, stress, etc.).1,2
An imbalance in microbiota composition, also known as dysbiosis, has been linked to a wide spectrum of diseases (type 2 diabetes, obesity, inflammatory bowel disease (IBD), non-alcoholic fatty liver disease (NAFLD) and colorectal cancer).1,2
As commented before, the gut microbiota composition can be influenced by diet; this is the reason why many dietary approaches have been formulated. Amongst these, prebiotics are a very desirable candidate due to their proven effect and safety.2
The term prebiotic was introduced in 1995 by Gibson and Roberfroid, and it has been subjected to many changes through the years.1–9 In 2017, the International Scientific Association of Probiotics and Prebiotics (ISAPP) described the term prebiotic as ‘a substrate that is selectively utilized by host microbiota conferring a health benefit to the host’.1,6,7,10
In this regard, the concept of prebiotic can be used to describe non-digestible carbohydrates of low degrees of polymerization, from 2 to 20 anhydrosugar units (glycans, oligosaccharides, polysaccharides, and polyols), as well as some polyphenols and polyunsaturated fatty acids.2,4–7,9,10 The anhydrosugar moieties that conform these carbohydrates are linked by β-glycosidic bonds, which usually cannot be hydrolysed by mammalian hydrolases but can be fermented by beneficial microbiota.4,5
The fermentation of these substrates can foster the growth and maintenance of gut microbiota, promoting metabolic and digestive activity and improving the absorption of nutrients, the activity of the immune system and the intestinal integrity, just as it inhibits the growth of pathogens.2,7,9,10 These beneficial effects stem from the production of different metabolites after fermentation, especially the production of SCFAs (mainly acetic, propionic and butyric acids).2,4–7,9
Most used and studied prebiotics are galactooligosaccharides (GOS), fructooligosaccharides (FOS), oligofructose and inulin, which carry a long history of safe use.1–10 Apart from the well-established prebiotics, there are many other candidates with promising results, namely mannan-oligosaccharides, soybean oligosaccharides, lactulose, pectin-oligosaccharides, resistant starch, polydextrose and, particularly, xylooligosaccharides (XOS).1–10
XOS are non-digestible oligosaccharides composed of β-D-xylopyranose (β-D-xylose) residues linked by β-(1 → 4) and β-(1 → 3) glycosidic bonds and sometimes branched with different side groups. These carbohydrates have a molecular weight that ranges between 300 and 2000 kDa and a degree of polymerization (DP) ≤20 residues.1,7,11–18
XOS can be obtained from agro-industrial lignocellulosic residues, which are abundant, inexpensive and renewable. Another renewable source of XOS is algae residues, which contain β-(1 → 3) glycosidic bonds, whose use can decrease the production cost due to the lower recalcitrance of the material in comparison with lignocellulosic biomass11–15,17–22 (Fig. 1).
These prebiotics have been used in the treatment of various diseases such as IBD, constipation, colorectal cancer and obesity.2,3,6,8–10 Due to this fact, their global market is expected to grow at a compound annual growth rate (CAGR) of 7% from 2023 to 2033.23
In this review, we focus on summarising the current knowledge about the beneficial effects of prebiotic XOS in the improvement and prevention of some diseases. While prior reviews tend to focus on the production, characterization and prebiotic potential of XOS from different sources, this review offers an integrated analysis across multiple health conditions (obesity, type 2 diabetes mellitus, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) and colorectal cancer), connecting gut microbiota modulation to systemic outcomes like metabolic and immune changes. Additionally, this review addresses market trends and regulatory developments, offering a broader perspective on XOS commercialization.
Prebiotic action of XOS in human microbiota
The structure of XOS, their degree of polymerization (DP) and the present linkages vary depending on the source and method used for their production.24 These structural characteristics have a direct relationship with the prebiotic effect of XOS.25,26Generally, XOS with DP 2–3 are metabolised faster by probiotic bacteria than those with DP ≥4. Also, the substituents that are present among the xylose monomers will determine which probiotics are able to break XOS β(1 → 4) bonds, according to the catalytic activity of the enzymes they possess. For example, Bifidobacterium adolescentis can use both non-substituted XOS and arabinose-substituted XOS (AXOS) as a source of carbon, while Lactobacillus brevis cannot ferment ramified AXOS.25
Furthermore, depending on the complexity of some substituents, unsubstituted and arabinose-substituted XOS are metabolized much faster than XOS substituted with acetyl or methylglucuronic groups due to the complex structure that the latter can form.26
In vitro studies for prebiotic potential of XOS
Most in vitro studies for analysing the prebiotic potential of XOS have focused on measuring the growth, SCFA production and XOS consumption of Lactobacillus (Firmicutes) and Bifidobacterium (Actinobacteria) strains, as many of them are well-established probiotics. It has been seen that some Bifidobacterium species that are present in the adult intestine like Bifidobacterium longum subsp. longum, Bifidobacterium adolescentis or Bifidobacterium animalis are able to assimilate arabinoxylooligosaccharides, while Lactobacillus spp. and Weissella confusa/cibaria are not.27–30 For the utilization of AXOS, these bacteria should have any type of xylosidases in order to break the main chain of XOS. Besides these enzymes, it has been seen that Bifidobacterium species that can metabolise AXOS produce enzymes called arabinofuranosidases, which can remove arabinosyl substituents and are not present in Lactobacillus and Weissella probiotics. These metabolic differences lead to cross-feeding, an important mechanism that occurs in the gut ecosystem which allows microbiota to consume complex substrates as AXOS: in this context, Bifidobacteria strains will produce arabinofuranosidases, enabling them to use arabinose as a carbon source, whilst other probiotics only capable of producing xylosidases can consume the unsubstituted XOS released.31On the other hand, there are also some in vitro studies that evaluate the selectivity of XOS to favour probiotics growth and prevent the proliferation of pathogens.27,29,32–34
In vitro tests have generally been performed in two ways:
The first one is growing individual bacterial species with XOS as the only carbon source, and measuring the absorbance at 600–650 nm and the pH usually for 24–48 hours, depending on the strain. After fermentation, XOS consumption and production of SCFAs are measured by high-performance liquid chromatography with refractive index detection (HPLC-RID) or high-performance anion-exchange chromatography/pulsed amperometric detection (HPAEC-PAD).35–37
The second approach consists of performing fermentations with human faeces inoculum in the presence of the prebiotic and afterwards studying changes in the microbiota composition and SCFA production and comparing those results to a blank fermentation. The changes in faecal microbiota are then analysed using different sequencing techniques and HPLC in order to measure the changes in the SCFA profile.38–40
In this section, a compilation of the most outstanding results achieved with both types of assays28,41–44 over the last ten years has been made.
Mathew et al.27 obtained XOS from wheat bran and observed that B. adolescentis and L. brevis could use them as a source of carbon, as an increase in OD 600 and a decrease in pH were observed. In contrast, the pathogenic bacteria E. coli did not show any of these changes, indicating that it is not able to grow in the presence of these prebiotics, thus showing how selective XOS are.27 This selectivity has also been observed for birch wood, rye wood, sugarcane bagasse and corn crop XOS, which do not allow the growth of E. coli.29,32,33 Other pathogens such as Enterobacter aerogenes, Salmonella typhimurium and Staphylococcus aureus also showed a reduction in their growth when XOS were the only source of carbon.33,34,48
Furthermore, to assess the bifidogenic effect of XOS, other species of Bifidobacterium have been tested using commercial corn cob XOS. In this case, it was concluded that B. animalis subsp. lactis, B. bifidum and B. breve can grow using these XOS.48 When compared with well-established prebiotics, corn cob XOS were metabolised by B. longum subsp. infantis with the same efficacy as inulin,32 whilst sugarcane bagasse XOS promoted medium growth 1.5-fold greater than that of inulin for both B. longum subsp. infantis and B. longum subsp. longum.33 It has also been found that B. breve grows better in the presence of commercial XOS than in the presence of fructooligosaccharides (FOS), reaching absorbances as high as those obtained with glucose.34 It should be noted that not all tested Bifidobacteria species grow as efficiently as with XOS. This is the case for B. longum strain BL 05 Danisco, which grows better using FOS than with commercial XOS.34
Besides, XOS can enhance the growth of other probiotic genera such as Bacteroides,32 Lactobacillus26–28,32,33,48–50 and Weissella.27,31,45 In the case of Bacteroides, corn cob XOS promote their growth with the same efficiency as inulin.32 On the other hand, some Lactobacillus are also able to use XOS from different sources, achieving strong, moderate or low absorbances depending on the strain and origin of XOS.26,48,49 For example, L. acidophilus has been shown to achieve higher growth levels with sugarcane XOS than those reached with inulin.33
Although XOS are most studied as prebiotics for gut bacteria, they have also been shown to contribute to the growth of probiotic bacteria in the oral microbiota. This is the case of Streptococcus salivarius, a bacterium that reduces halitosis and the risk of pharyngitis, which has already been administered as a symbiotic together with inulin in a clinical trial.51 This strain has been shown to have a much greater ability to grow in the presence of XOS than with inulin, so XOS could be a better alternative to administer probiotics of the oral microbiota.32
Results from all these studies are summarised in Table 1.
| Study | XOS source | Bacteria species | Growth response using XOS as only source of carbon |
|---|---|---|---|
| Mathew et al.27 | Wheat bran | Bifidobacterium adolescentis | Utilize XOS (DP 2 and 3 almost completely consumed, while from DP 4 to 6 are consumed to a lower extent) and AXOS |
| Mathew et al.27 | Wheat bran | Lactobacillus brevis | Utilize XOS (DP 2 and 3 almost completely consumed, while from DP 4 to 6 are consumed to a lower extent) but no AXOS |
| Mathew et al.27 | Wheat bran | Escherichia coli (pathogen) | Unable to utilize XOS and AXOS |
| Salas-Veizaga et al.46 | Quinoa | Bifidobacterium adolescentis | Consumption of glucuronosylated XOS (GXOS), AXOS and unsubstituted XOS with DP 2–6. Production of acetate, lactate, propionate, formate and butyrate. |
| Salas-Veizaga et al.46 | Quinoa | Weissella cibaria | Consumption of linear XOS with DP 2–4 |
| Falck et al.29 | Birch wood and rye wood | Bifidobacterium adolescentis | Utilization of the XOS fraction (DP 2–5) and AXOS from the rye arabinoxylan hydrolysate |
| Falck et al.29 | Birch wood and rye wood | Lactobacillus brevis | Utilization of only the XOS fraction (DP 2–5) |
| Falck et al.29 | Birch wood and rye wood | Escherichia coli (pathogen) | No growth with XOS or AXOS (did grow with its respective monomers) |
| Immerzeel et al.28 | Wheat bran | Lactobacillus brevis | Significant increase in cell density and acetic and lactic acid production. Consumes X2 completely, but no AXOS |
| Immerzeel et al.28 | Wheat bran | Bifidobacterium adolescentis | Significant increase in cell density and acetic and lactic acid production. Completely consumes X2, X3 XOS and AXOS to some extent |
| Immerzeel et al.28 | Wheat bran | Weissella cibaria/confusa | Significant increase in cell density and acetic and lactic acid production. X2 and X3 are completely metabolised, |
| Driss et al.47 | Corn cobs | Bifidobacterium adolescentis | Able to grow better than L. acidophilus |
| Driss et al.47 | Corn cobs | Lactobacillus acidoplilus | Able to grow but less than B. adolescentis in terms of OD and cell density. |
| Geetha and Gunasekaran52 | Wheat bran | Lactobacillus brevis | Able to grow. Maximum growth rate of all tested strains (only growth rate was measured) |
| Geetha and Gunasekaran52 | Wheat bran | Lactobacillus lactis | Low growth rate but able to use wheat bran XOS (only growth rate was measured) |
| Geetha and Gunasekaran52 | Wheat bran | Lactobacillus acidoplilus | Able to grow (only growth rate was measured) |
| Geetha and Gunasekaran52 | Wheat bran | Lactobacillus casei | Able to grow (only growth rate was measured) |
| Geetha and Gunasekaran52 | Wheat bran | Lactobacillus collinoides | Able to grow (only growth rate was measured) |
| Geetha and Gunasekaran52 | Wheat bran | Bacillus clausii | Able to grow (only growth rate was measured) |
| Schropp et al.32 | Corn crop | Bifidobacterium longum subsp. infantis | Increase in rmax (maximal growth rate), ODmax, and AUC (area under growth curve) |
| Schropp et al.32 | Corn crop | Bacteroides fragilis | Increase in rmax, ODmax, and AUC |
| Schropp et al.32 | Corn crop | Bacteroides uniformis | Increase in rmax, ODmax, and AUC |
| Schropp et al.32 | Corn crop | Lactobacillus plantarum | Non-significant growth |
| Schropp et al.32 | Corn crop | Weissella confusa | Only the ODmax increases |
| Schropp et al.32 | Corn crop | Escherichia coli (pathogen) | Significantly slower growth in the presence of XOS than with inulin |
| Schropp et al.32 | Corn crop | S. salivarius (oral microbiota) | Greater increase in ODmax and AUC with XOS than with inulin. Could be used as a symbiotic with XOS. |
| Schropp et al.32 | Corn crop | S. parasanguinis (oral microbiota) | Reduced growth rate with XOS |
| Ghosh et al.33 | Sugarcane bagasse | Bifidobacterium longum subsp. infantis | Higher growth rate with XOS than with inulin |
| Ghosh et al.33 | Sugarcane bagasse | Bifidobacterium longum NCC 2705 | Higher growth rate with XOS than with inulin |
| Ghosh et al.33 | Sugarcane bagasse | Lactobacillus acidophilus | Higher growth rate with XOS than with inulin |
| Ghosh et al.33 | Sugarcane bagasse | Escherichia coli (pathogen) | No significant growth with either XOS or inulin |
| Ghosh et al.33 | Sugarcane bagasse | Enterobacter aerogenes (pathogen) | No significant growth with either XOS or inulin |
| De Figueiredo et al.34 | Commercial lineal XOS | Bifidobacterium breve | Better growth with XOS than with FOS. Use XOS as efficiently as glucose |
| De Figueiredo et al.34 | Commercial lineal XOS | Bifidobacterium animalis subsp. lactis | Very low growth with both XOS and FOS |
| De Figueiredo et al.34 | Commercial lineal XOS | Bifidobacterium longum | Less growth stimulation with XOS compared to FOS |
| De Figueiredo et al.34 | Commercial lineal XOS | Lactobacillus brevis | XOS metabolised better than FOS |
| De Figueiredo et al.34 | Commercial lineal XOS | Lactobacillus acidophilus | FOS metabolised better than XOS |
| De Figueiredo et al.34 | Commercial lineal XOS | Salmonella typhimurium (pathogen) | No growth in the media with XOS or FOS |
| Sun et al.48 | Commercial lineal XOS | Bifidobacterium strains | Growth promotion |
| Sun et al.48 | Commercial lineal XOS | Staphylococcus aureus | Growth inhibition |
| Kumari et al.49 | Wheat bran | L. brevis | Absorbance increases with 1% XOS as the carbon source. |
| Kumari et al.49 | Wheat bran | L. rhamnosus | Highest absorbance increase of the three tested strains with 1% XOS as the carbon source. |
| Kumari et al.49 | Wheat bran | L. plantarum | Limited growth with 1% XOS as the only carbon source |
| Nascimento et al.53 | Sugarcane bagasse | L. rhamnosus | High absorbance with XOS |
| Nascimento et al.53 | Sugarcane bagasse | L. casei | High absorbance with XOS |
| Nascimento et al.53 | Sugarcane bagasse | L. fermentum | High absorbance with XOS |
| Nascimento et al.53 | Sugarcane bagasse | L. bulgaricus | High absorbance with XOS |
| Nascimento et al.53 | Sugarcane bagasse | L. casei | High absorbance with XOS |
| Jaichakan et al.50 | Rice husk/straw | L. brevis | Greater growth with XOS than glucose |
| Jaichakan et al.50 | Rice husk/straw | L. sakei | Greater growth with XOS than glucose |
| Jaichakan et al.50 | Rice husk/straw | L. reuteri | Moderate growth with XOS |
| Jaichakan et al.50 | Rice husk/straw | L. bulgaricus | Moderate growth with XOS |
Like other well-known prebiotics (FOS, inulin, GOS and 2′-fucosyllactose), commercial XOS have shown to produce a significant increase in the Gut Microbiome Wellness Index (GMWI) after faecal fermentation for 24 hours, indicating a beneficial effect on gut microbiota wellness. The GMWI is a quantitative tool to measure the impact of a substance on the gut microbiota, taking into account the relative abundances of 50 microbial species found to be associated with human health. The health-prevalent species B. adolescentis, B. catenulatum and Sutterella wadsworthensis increased their relative abundances after 24 h of fermentation of XOS (as well as FOS, inulin and 2′-fucosyllactose), while most of the 28 health-scarce species decreased or showed low abundances after prebiotic fermentations.40
Álvarez et al.54 carried out in vitro human faecal fermentations in the presence of barley straw XOS using FOS as a positive control. To determine SCFA production, HPLC was the selected technique. After 24 h of fermentation, the concentration of total SCFAs in the media was significantly higher than those achieved with FOS. Acetate accounted for almost 70% of total SCFAs, while butyrate was the second most abundant.54 Also, by performing faecal fermentations, Ho et al.,55 Chen et al.38 and Gautério et al.39 found that acetate was the most abundant SCFA followed, in all cases, by propionate and butyrate when using oil palm, corn cob, beechwood and rice husk XOS as the carbon source. In the study of Victoria et al.,39 the XOS-induced production of lactate and SCFAs was similar to that obtained with lactulose, a confirmed prebiotic,39 and to that of XOS from barley straw tested by Álvarez et al.54
Each SCFA has different properties that lead to benefits for host's health: acetate contributes to normal epithelial cell division; butyrate is considered one of the most essential colon metabolites, as it induces differentiation and apoptosis in host cells, constitutes an energy source for colonocytes, and has anti-inflammatory properties;54 and propionate has been related to the reduction of lipogenesis and serum cholesterol level.56
It is important to highlight that the profile of SCFAs produced after fermentation varies depending on the origin of XOS and the donor of the faecal inoculum for performing the fermentation, since each person's microbiota is different, like a fingerprint.
Besides SCFA production, total bacteria and Bifidobacteria counts are usually assessed as well in this kind of study. The bifidogenic effect was confirmed via the FISH technique by Álvarez et al.54 for barley straw XOS, which increased Bifidobacteria population in a similar level to FOS. This effect was also confirmed with FISH by Ho et al.,55 using XOS obtained from oil palm, and Moniz et al., using corn straw XOS.57 On the other hand, Pham et al.58 observed this increase using 16S rRNA sequencing and qPCR after fermentation with commercial corn cob XOS.
Broader changes in intestinal microbiota caused by XOS were analysed by 16S rRNA sequencing by different authors. For corn cob XOS, it was concluded that Actinobacteria and Bacteroidetes phyla were enriched, while most Firmicutes phylum members declined (except for Lactobacillus, Megasphaera and Megamonas).37,39 Moreover, a decrease in the pathogenic Streptococcus species was observed after the faecal fermentation of these XOS.38 In the case of oil palm XOS, it was observed that hydrolysates reduced the abundance of Proteobacteria, associated with gut diseases, methanogenic archaea of the Methanobacteriaceae family and ammonia producing bacteria such as Clostridium species.55 On the other hand, probiotic Megamonas funiformis, an important producer of SCFAs, increased.39
As a conclusion, the prebiotic potential of XOS from many different sources has been widely confirmed both in in vitro cultures with individual strains and in fermentations of faeces.
In vivo human studies for prebiotic potential of XOS
There are not many studies in humans about the effects of XOS consumption on gut microbiota, but the ones available have obtained promising results. Human clinical trials assessing the prebiotic potential of XOS collectively suggest positive effects on gut microbiota composition and certain systemic parameters, yet a synthesized and mechanistic interpretation of these findings is still emerging.Across two double-blind, randomized, placebo-controlled studies on healthy persons who consumed commercial corn cob XOS,59 the bifidogenic effect was consistently demonstrated. Faecal samples were collected before the consumption and several times after starting the consumption of XOS. Both studies differ in the size of the cohort (32 versus 41), the duration of XOS supplementation (8 weeks versus 3 weeks) and the daily dose of XOS tested (1.4 or 2.8 g day−1 versus 8 g day−1) but achieve the same conclusions with respect to the selectivity of XOS for allowing Bifidobacteria spp. growth.58 The study with the smaller cohort size measured stool pH variations and performed 16S sequencing of members of Enterobacteriaceae, Bacteroides fragilis groups, Clostridium, Bifidobacterium, and Lactobacillus and analysis of the SCFA profile by gas liquid chromatography.59
The Bifidobacterium counts for individuals with a high dose were significantly higher than the values at the baseline at 4, 8 and 10 weeks, being 21% above the baseline in the high dose group at 4 weeks and 17% at 8 weeks.
Also, the total anaerobic flora counts of the subjects after high dose intervention were higher than the baseline at 4 and 8 weeks. Bacteroides fragilis also increased significantly at lower and higher doses, while no significant difference was found for Lactobacillus (member of the Firmicutes group) and Clostridium. Furthermore, Enterobacteriaceae was significantly lower at week 10 for the placebo group. These data show the selectivity of XOS.59
As commented previously, it has been observed in individuals with obesity that their associated gut microbiota has increased numbers of Lactobacillus and, consequently, an increased Firmicutes/Bacteroidetes ratio. Because of that, and in view of the results of this study, XOS supplementation would be positive for persons with obesity.59
The study with the bigger cohort size60 also studied microbiota changes, but using fluorescence in situ hybridisation. Also, variations in plasma lipid concentrations and determination of immune cell types and phenotypes were studied in fresh blood samples and bowel habits and mood were reported by the participants.60
Besides the increase in Bifidobacteria, an increment in bowel movements per day was also observed. Also, self-reported vitality and happiness increased, and HDL concentrations were higher, lowering total cholesterol. Regarding the immune system, XOS cause lower expression of CD16/56 on natural killer T cells related to the activation of the immune system. Furthermore, a lower use of analgesics during the XOS supplementation period was observed.60
Another study by Yang et al.61 tested the effects of commercial XOS in individuals with prediabetes and healthy ones, as it has been suggested that gut microbiota is altered in type 2 diabetes mellitus (T2DM) patients. The study population was composed of 16 healthy subjects and 14 with prediabetes. During an 8-week period, both healthy subjects and subjects with prediabetes were randomly assigned to take 2 g of commercial XOS or placebo. A total of two faecal samples were collected from each individual: at the baseline and at week 8 of XOS consumption. 16S rRNA sequencing was conducted to compare the gut microbial composition of healthy individuals to the ones with prediabetes.60
Before XOS consumption, several differences were observed: the abundance of the Synergistota phylum, related to T2DM, was higher in subjects with prediabetes. Besides, the abundances of Allisonella, Cloacibacillus, Enterorhabdus, Howardella, Megamonas, and Slackia were significantly higher, while Adlercreutzia, Anaerococcus, Ethanoligenens, Gordonibacter, Lactococcus, Parasutterella, and Tissierella were greatly reduced in Pre-DM compared with healthy subjects.
The phylum Firmicutes showed a 20% increase in abundance over 8 weeks in placebo groups of healthy subjects, while XOS intervention significantly reversed this increase. Also, XOS intervention inhibited the growth of infection-related Streptococcus and Subdoligranulum. At the genus level, the high abundance of Blautia, Anaerotruncus, Dialister, and Oscillospira decreased after XOS intervention. XOS supplementation significantly decreased or reversed the abundance of Howardella, Enterorhabdus, and Slackia observed in both healthy subjects and subjects with prediabetes. All these four genera tend to be more abundant in individuals with prediabetes. Also, XOS intervention increased the abundance of Blautia hydrogenotrophica, which was lower in subjects with prediabetes.
On the other hand, insulin levels were studied and compared in subjects with prediabetes after the consumption of XOS and placebo and, as there was a tendency to reduce oral glucose 2-hour insulin tolerance, it was not statistically significant.
Overall, the study demonstrated that XOS supplementation could modulate the gut microbiota composition in both healthy individuals and individuals with prediabetes and may have potential benefits in reversing changes in gut microbiota associated with the development of diabetes.61
A different in vivo experiment with humans62 studied the effects of rice porridge enriched with 1.2 g of XOS. The cohort was divided into a placebo group of ten individuals and a test group of the same size, which had to consume porridge every day for 6 weeks. Changes in microbiota were analysed using faecal samples from each subject at weeks 0, 1, 3, 4, 6, and 7, seeding Bifidobacteria, Lactobacillus, Clostridium perfringens and coliforms with selective media on the plate.
Key findings of this study included significant increases in faecal counts of Lactobacillus spp. and Bifidobacterium spp. compared to the placebo group. Also, the XOS group exhibited decreased levels of Clostridium perfringens, a harmful pathogen. This decrease could be due to the production of SCFAs that decrease gut pH, creating an unfavourable environment for such bacteria.60
On the other hand, total anaerobic bacterial counts remained unchanged, unlike in the case of the Finegold et al. study,59 indicating that enriched porridge selectively promoted beneficial bacteria without disrupting overall microbial balance. This difference can be associated with the porridge composition as porridge is a prebiotic food by itself because of its fibre content.
Finally, both groups showed variable coliform counts, likely influenced by dietary factors outside the study's control. Overall, the study supports the incorporation of XOS into functional foods to improve gut health and calls for further research on its broader applications.62
Although these studies collectively demonstrate consistent microbial benefits, they are limited by small cohort sizes, short durations, and variability in XOS sources and dosages. Furthermore, the outcomes beyond microbiota composition—such as improvements in metabolic, inflammatory, or gastrointestinal symptoms—often can be influenced by many factors other than prebiotics or are subjective measures and lack mechanistic clarity. On the other hand, no other in vivo studies of XOS efficacy in clinical populations have been found beyond the one conducted in individuals with prediabetes.
In conclusion, in vivo human studies offer a promising but still incomplete picture of XOS as a functional prebiotic. To advance in this field, future trials should employ larger, stratified populations, integrate multi-omic analyses, and target well-defined clinical endpoints, particularly in metabolically compromised or gastrointestinally vulnerable groups.
XOS and obesity
Obesity is a disease characterized by an excessive accumulation of fat that has its origin in a positive energy imbalance, and which has been associated with many comorbidities, from cardiovascular diseases to type 2 diabetes and non-alcoholic fatty liver disease (NAFLD).63–65Obesity has reached epidemic proportions in the last decade, triplicating the number of patients from 1975 until the present. The World Health Organization (WHO) estimated in 2017 that obesity was linked to approximately 4 million deaths per year.63–65
The aetiology of obesity is complex, as it entails several factors (diet, physical activity, genetics, socioeconomic factors, etc.) with varying levels of involvement, the most prominent contributors being poor dietary patterns and physical inactivity.64,66
In this context, many authors have investigated the link between diet and a sedentary lifestyle in relation to the homeostasis of the intestinal microenvironment. It has been found that patients with obesity suffer from low degree inflammation and poor glycolipid metabolism, which have been linked to gut microbiota dysbiosis.65,67,68
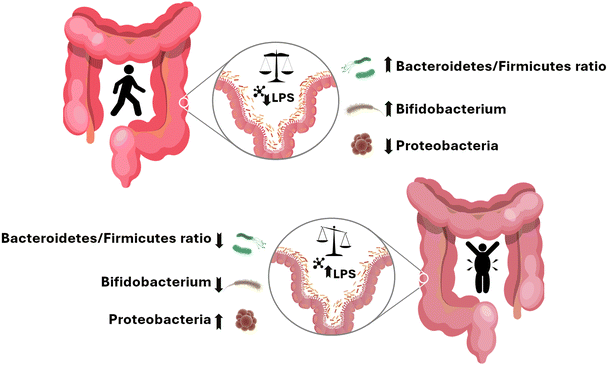
In healthy individuals, the gut is mainly colonized by a higher proportion of Bacteroidetes than Firmicutes, as well as a balanced proportion of Actinobacteria and Proteobacteria. In contrast, in patients with obesity, there is a shift in the main phyla, Firmicutes being the most prominent with respect to the decreased counts of Bacteroidetes. Moreover, there is a spike in Proteobacteria relative abundance compared to the decrease of Actinobacteria counts3 (Fig. 2).
It has been suggested that this disequilibrium affects gut barrier permeability, leading to the development of a leaky gut.69,70 Furthermore, the higher Proteobacteria counts lead to an increased release of lipopolysaccharides (LPS) which, in the context of a leaky gut, can enter systemic circulation. This fact could be linked to the low-grade inflammation mentioned before69,70 (Fig. 2).
In addition, this shift in the composition of gut microbiota has been related to changes in its own functionality, which could explain the impairment of glycolipid metabolism in individuals with obesity.71,72 Some researchers have proposed the regulation of patient's gut microbiota through a diet-based strategy using dietary supplements such as prebiotics.66,68,72,73
Taking that into account, it is imperative to search for prebiotics that promote only the growth of those phyla whose counts are lower in patients with obesity (Actinobacteria and Bacteroidetes). In this regard, XOS have been shown to possess the potential to increase the counts of important Actinobacteria genera, such as Bifidobacterium, without influencing Firmicutes counts.67,68,73,74
Effect of XOS on obesity
Research has primarily focused on the effect of XOS on different physiological characteristics associated with obesity. In this regard, a series of experiments based on the induction of obesity via a high-fat diet (HFD)67,68,73–82 or others72,83 with the same effect have been performed using different rodent models (male C57BL/6J mice, male Sprague Dawley rats and male Wistar rats). Additionally, although to a lesser extent, some studies have also been carried out in humans and in vitro.84,85Different in vivo studies on rodent models have found that XOS supplementation is associated with body weight reduction when compared to obesity-induced controls.67,73–78,82,83 This tendency is sometimes coupled with a decrease in total, epididymal, perirenal or mesenteric fat.67,72–78,81–83 Combinations of XOS with other prebiotics, such as FOS, have been shown to promote both effects, whilst the combination of XOS with probiotics only induced weight reduction.68,81
It should be noted that experiment duration is a variable that can affect the impact of the obtained results. This can be observed when comparing results from studies such as Li Y. et al. (3 week duration)68 and Fei et al.75 (15 week duration). Both studies were carried out by inducing obesity via a HFD in male Sprague Dawley rats and studying the XOS supplementation effect. Li Y. et al.68,75 found that, even though there was a significant reduction in body weight, epididymal and perirenal fat percentages did not change significantly, while in the Fei et al.75 study, a consistent reduction of body weight, perirenal fat and epididymal fat could be observed. These results could indicate the need to centre efforts on experiments of longer duration to determine the extent of the effects of these prebiotics with time.
On human individuals with a BMI > 30, McFarlin et al.85 studied the combined effect of XOS, Bacillus subtilis HU58 and Bacillus coagulans SC-208 supplementation. In this experiment, researchers found that supplementation with the symbiotic induced a significant 35% reduction in the visceral adipose tissue.
Besides weight gain, glucose metabolism markers tend to be altered in patients with obesity. The studies on animal models with diet induced obesity have shown that XOS supplementation, alone or in combination with other prebiotics or probiotics, is able to significantly reduce insulin blood levels. Moreover, they also tend to improve glucose metabolism as shown by the significant reduction in the results of the Oral Glucose Tolerance Test (OGTT) and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR).67,73,76–78,81–83 What is more, there are some studies that have found a significant reduction in glucose blood levels when XOS are included in animal models fed with a HFD.67,83
Besides blood biomarkers, some researchers have focused their studies on the effect of XOS supplementation on gene expression and phosphorylation of proteins related to glucose metabolism. In this regard, Li T. et al.81 found that supplementation with hawthorn leathers combined with 75% FOS and 25% XOS in male C57BL/6J mice fed with a HFD improved the expression of PI3Kr1, Akt1 and mTOR. Khat-udomkiri et al.83 also found that HFD-fed male Wistar rats supplemented with XOS showed an improvement in protein expression of GLUT4 and phosphorylation of pAktser473 and pAmpkthr172 in soleus muscle. The aforementioned proteins and genes have been demonstrated to take part in metabolic routes associated with insulin signalling and glucose metabolism. These results are important as these pathways are downregulated in obesity and type 2 diabetes.81,83
Lipid levels in blood samples are also important biomarkers for the study of obesity, along with the levels of some hormones implicated in lipid metabolism. In obese animal models, supplementation with XOS seems to favour the decrease of cholesterol levels as well as low-density lipoprotein cholesterol (LDL-C) levels in blood samples when compared to obesity-induced animals without supplementation.68,74,76–78,80,81,83
In contrast, triglyceride levels in blood appear to be a point of contention: some studies show no significant effect,72,76–78 while others exhibit a significant reduction.68,74,80,81,83 An analogous situation happens with high-density lipoprotein cholesterol (HDL-C), as only the assays performed by Li T. et al.81 and Khat-udomkiri et al.83 seemed to find a significant increase in HDL-C blood levels.
Long et al.72 and Khat-udomkiri et al.83 also found that XOS supplementation induces a reduction in leptin levels, a mediator involved in metabolic homeostasis regulation. Its levels are proportional to fat mass, so a decrease is indicative of fat loss.72
Several researchers also found that XOS supplementation affected the expression of lipolytic and lipogenic genes. In this sense, XOS seem to improve the expression of Lipe, PPARα, AMPK and CPT-1 genes. Lipe, PPARα and CPT-1 are genes involved in the process of lipolysis, while the AMPK gene has been known to inhibit lipogenesis.74,81
On the other hand, XOS have also been shown to reduce mRNA expression of PPARγ, FASN and C/EBPα genes. These genes are known to be involved in adipogenesis and fatty acid synthesis, which in combination with the increased levels of lipolytic genes show the promising capability of XOS to improve lipid metabolism in obesity models.72,74,81,84
Taken together, the different outcomes of XOS supplementation in relation to glucose and lipid metabolism, as well as their effects on weight reduction, reflect the potential of XOS as a tool to tackle obesity consequences.
In addition to blood and metabolic biomarkers, gut microbiota dysbiosis appears to play a key role in the development and deterioration of obesity as well as other metabolic disorders.
Researchers have found that XOS supplementation increased the counts of different families and genera from the Bacteroidia class (Prevotellaceae family and Bacteroides and Parabacteroides genera), Lachnospiraceae family (Roseburia, Blautia and Anaerostipes genera) and specially Bifidobacterium and Lactobacillus genera.67,68,72,75,82–84 These bacteria are known to produce SCFAs needed for the normal function of the gut and to produce different metabolites that are essential for the correct functioning of host metabolism.72,75,82–84
Furthermore, XOS supplementation appears to reduce the counts of different deleterious and pathogenic taxa such as the Oscillospiracea family (Ruminococcus, Oscillospira and Oscillobacter genera), Clostridiaceae family and Desulfovibrionaceae family (Bilophila genus).68,72,75,79
Some of the reported effects of gut dysbiosis associated with obesity are the loss of gut barrier integrity and the development of local and systemic low-grade inflammation. In this regard, Fei et al.75 studied the effect of XOS supplementation on male Sprague Dawley rats fed with a HFD and observed a decrease in colonic crypt damage when compared to the HFD control. The same tendency, although not statistically significant, was observed in the Lensu et al.80 and Khat-udomkiri et al.83 studies with different animal models. Moreover, Li F. et al.74 found that XOS supplementation to HFD-fed male C57BL/6J mice induced an increase in the expression of different tight junction genes (occludin, claudin-1 and ZO-1). Taken together, these results seem to suggest that XOS supplementation could improve gut barrier integrity, reducing the damage produced by a leaky gut.
Additionally, animal model studies have also found that XOS are able to significantly reduce blood LPS levels68,75–78,83 as well as the expression of some inflammatory cytokines locally (Tumour Necrosis Factor α, TNFα; and Interleukin-6, IL-6) and systemically (Monocyte Chemoattractant Protein 1, MCP1; TNFα; IL-1β; IL-6; interferon γ, IFN-γ; Nitric Oxide Synthase 2, NOS2; etc.).72,74,75,77,81 McFarlin et al.85 have also observed that XOS are involved in the downregulation of inflammatory biomarker expression (IL-18; Chemokine Ligands 4, CCL4; Cluster of Differentiation 80, CD80, etc.) in human individuals suffering from obesity.86 Taken together, these results seem to suggest that XOS supplementation could improve gut barrier integrity, reducing the damage produced by a leaky gut and inflammation induced by LPS filtration.
In conclusion, XOS supplementation demonstrates promising effects in alleviating various obesity-related physiological alterations. Rodent studies have consistently shown reductions in body weight and fat mass, and improvements in glucose metabolism, lipid profiles, and inflammatory markers. Additionally, XOS appear to positively influence gut microbiota composition, increasing beneficial bacteria numbers and improving gut barrier integrity. While some variability exists in the results, particularly concerning the duration of supplementation, the collective evidence suggests that XOS may offer a beneficial strategy for managing obesity and its associated metabolic disturbances. However, further studies, particularly in humans, are needed to optimise its therapeutic potential.
XOS and bowel diseases
IBD covers two major diseases, Crohn's disease (CD) and ulcerative colitis (UC), which severely affect the gastrointestinal tract, resulting in chronic symptoms and flares.86 CD produces intense inflammation located most commonly in the colon and ileum, while UC is characterized by colonic inflammation and mucosal damage. Some symptoms are similar in both conditions: abdominal pain and diarrhoea, as well as hematochezia, fever and weight loss, but there are also several differences: strictures (fibrotic and stenotic), fissure, fistulas and perianal disease are common in CD but absent in UC, while pseudopolyps and shortened colon only appear in patients suffering from UC.87Systematic analysis of the Global Burden of Diseases, Injuries and Risk Factors Study (GBD) dataset revealed that there were 4.9 million cases of IBD globally in 2019, implying high costs and burden for health systems because of the high prevalence of these diseases.7
Current treatments for these pathologies include antibiotics, corticosteroids, amino salicylates and immunomodulatory drugs, which have serious long-term side effects such as infections, malignant tumours, thrombosis,88 hypertension, headache and nausea.89 Because of these side effects, there is a necessity for safer strategies for treating IBD.
One of the new approaches for treatment is through dietary interventions, e.g. using prebiotics and probiotics as prophylactics to prevent and ameliorate symptoms.89 UC and CD patients exhibit pathological changes in the gut, including changes in the gut microbiota. These changes imply a reduction in the proportion of beneficial bacteria and an increase in harmful bacteria, which lead to a decrease in the production of SCFAs. Besides, a decrease in mucus secretion and loss of integrity of the epithelial barrier takes place in UC patients, with decreased expression of tight junction proteins. This fact increases intestinal mucosal permeability, making it more likely for bacteria in the gut to invade the mucus layer and contact intestinal mucosal cells, stimulating the recruitment of immune cells and release of proinflammatory cytokines.90
Although there are not many studies dealing with the effect of XOS on inflammatory bowel syndrome (IBS), some of them have obtained very interesting results that should be considered for the use of XOS as a supplement to improve the symptoms of these diseases.
Pham VT et al.91 carried out in vitro faecal fermentations using samples from three healthy donors to study the effect of a hydrolysate from corn cob xylan composed of a 70% XOS. After incubating a co-culture of Caco-2 and HT29-MTX-E12 epithelial cells with the supernatant from 24 h fermentation, an increase in the tightness of the gut barrier was observed, as transepithelial electrical resistance (TEER) increased compared to the control. Furthermore, mucus-secreting goblet cells (HT29-MTX-E12) in monoculture were incubated with the 24 h fermentation supernatant from XOS diluted 40 times and stained with Alcian Blue to evaluate the influence of XOS on mucus production. In this case, mucus production was significantly increased after treating cells with the supernatant from 24 h XOS fermentation for two of the three donors.91
Zongwei Li, Zhengpeng Li et al.92 also evaluated the prebiotic effect of commercial XOS with faecal fermentations. In this case, the donors were five patients with UC in clinical remission diagnosed by colonoscopy and five healthy volunteers. Faeces were fermented for 48 h and diluted in YCFA medium in the presence and absence of XOS. Afterwards, 16S rRNA sequencing was performed to evaluate gut microbiota composition. Differences in the gut microbiota between healthy volunteers and UC patients in clinical remission were detected, as expected. Moreover, in UC patients, 16S rRNA sequencing results showed that XOS fermentation promoted the growth of some probiotic bacterial groups such as Roseburia, Bifidobacterium and Lactobacillus, which is beneficial for the recovery of intestinal diseases. These results suggest that XOS can relieve dysbiosis in the faecal microbiota of UC patients in clinical remission and thus represent a potential prebiotic material for maintaining remission. Despite the very positive results of this study, the sample size is small, and the results might need to be confirmed in vivo, by performing clinical tests. It is also important to highlight that during relapses, it has been seen that some prebiotics might not have any beneficial effect on UC patients.92
In the light of this background and moving to an in vivo approach, Chen MMed, Z. et al.88 studied the influence of XOS on mucus bacteria penetration early in the occurrence of colitis in interleukin-10 gene-deficient mice (Il10−/−), which is a classical model for IBD. The study was performed using male wild-type (WT) mice and Il10−/− mice. The latter ones were divided into two groups of 6–8 individuals; a group that received corncob XOS of 95% purity (1.0 g kg−1 day−1) for 4 weeks and a control which did not (IL-10-KO).
Histologically, it was proven that XOS ameliorate Il10−/− mice inflammation. Il10−/− developed obvious bowel inflammation with some accumulation of immune cells in the mucosa and damaged intestinal structure, while Il10−/− mice treated with XOS had significant mild colonic inflammation with a small amount of inflammatory cell infiltration and glandular lesions in the intestine.
Also, in order to measure the neutrophil leukocyte infiltration and accumulation in the colon of mice, myeloperoxidase (MPO) was used as a marker and its concentration was evaluated using an ELISA assay kit. As expected, Il10−/− mice had a higher MPO concentration than WT mice, and it was significantly reduced after XOS treatment. Other markers of inflammation analysed by qPCR gave results in line with those of MPO, as the levels of TNF-α, IL-1β, IFN-γ, and IL-17A were lower in mice of the XOS group when compared with those in the IL-10-KO group. On the other hand, the IL-6 blood level, erythrocyte sedimentation rate and C-reactive protein were increased in Il10−/− mice four weeks after the start of the study, while the increase was not obvious in the XOS-fed group.
The research also found that the XOS intervention reduced the penetration of bacteria into the mucus layer. In IL10-KO mice, there is direct contact between epithelial cells and bacteria but in the XOS group, bacterial penetration was significantly reduced after XOS treatment, and the healthy structure was partially restored. Furthermore, IL-10-KO mice showed reduced mucus-filled theca area of goblet cells, which is significantly increased in Il10−/− mice treated with XOS.
Finally, Chen MMed, Z. et al.88 studied the levels of the autophagy markers LC3, as a high ratio of LC3-I and LC3-II is an indicator of autophagy induction, and p62, whose expression increase indicates autophagy blocking. LC3-II/I was decreased and p62 was increased in IL-10-KO mice and the levels were restored to the near WT level with XOS, which might be associated with the ameliorated goblet mucus secretion seen before.88
In conclusion, XOS intervention ameliorated spontaneous colitis in Il10−/− mice at an early stage of colitis development, making them an interesting potential complement for IBD patients, but research in human IBD patients is needed to confirm those beneficial effects.
Taken together, in vitro assays suggest that XOS supplementation promotes the rebalance of UC associated gut microbiota dysbiosis, as well as the tendency of these prebiotics to ameliorate gut barrier and mucus production in cell line models. On the other hand, in vivo assays on spontaneous colitis animal models seem to corroborate these findings, as XOS had the capacity to improve gut barrier by reducing tissue lesions and augmenting mucus production. In this regard, the betterment of gut barrier function helps in decreasing immune cell infiltration and therefore, it can reduce inflammation.
Overall, these results point to XOS supplementation as a potential tool to alleviate and promote the improvement of IBD associated symptomatology. However, it should be noted that there are few studies regarding this topic which, given its complexity, requires further study to validate these results.
As it has been established, no studies have been found in which XOS have been tested in vivo in humans suffering from inflammatory bowel disease, but in healthy individuals it has been seen that XOS supplementation increased faecal bifidobacterial content, while there were no significant changes in the count of other genus investigated. In Fig. 3, all benefits from XOS consumption by inflammatory bowel disease patients are summarised.
 | ||
| Fig. 3 Summary of symptoms caused by inflammatory bowel diseases and positive effects of dietary intervention with XOS observed in vitro and in vivo. | ||
In addition to the effect of XOS on improving IBD, it is important to also mention the use of XOS in other intestinal conditions such as irritable bowel and gut dysbiosis.
Our gut microbiota changes throughout life, and as we get older, it is common to see a significant reduction in Bifidobacteria and an increase in harmful bacteria such as Enterobacteriaceae and Clostridium perfringens. This imbalance leads to symptoms such as constipation, which is very common in the elderly.
For this reason, the effects of consuming 4 g of XOS per day for 3 weeks in subjects over 65 years of age were studied. From the analysis of the patients’ faeces after supplementation with XOS and comparisons with control patients, it was concluded that XOS increases the amount of Bifidobacteria in the intestine and also the amount of water in the faeces, which may improve the symptoms that occur in old age.93
At present, prebiotics containing XOS are already commercialized in Europe, such as Gelsectan®, which has been shown to have benefits for patients with irritable bowel prone to diarrhoea, normalizing stool consistency in 87% of patients after 28 days of XOS supplementation. The subjects who participated in the study also self-assessed the changes in the level of abdominal pain and swelling, which were also reduced, but these data should not be considered as they are totally subjective. It is also important to note that the beneficial effects of these prebiotics disappear quickly once supplementation is stopped (reduction from 87% to 23% in 28 days), which may indicate that other excipients of the supplement (pea protein and xyloglucan) and not XOS could be those that produce the improvement in the consistency of the stools by the protective layer that forms in the intestine.94 It would be advisable to conduct clinical studies in patients with irritable bowel supplementing them with XOS only.
XOS and cancer
Cancer is a global health problem that has affected over 20 million people worldwide, as per a study conducted in 2022 by the World Health Organization (WHO).95 Incidence of cancer increases significantly after 60 years of age. This group of population has 75% of the total cancer patients.96Some prebiotics have been shown to reduce or prevent the growth of some tumours due to their ability to maintain a healthy microbiome. They have also been shown to increase the effectiveness of some treatments or reduce their side effects.96
Gut microbiota affects immune response by the production of certain metabolites. Depending on its microbial composition, innate and adaptive immunity can be regulated at various levels. Therefore, considering that prebiotics improve the composition of gut microbiota, they can also improve the host's immune system that responds against emerging tumours. In order to block cancer development, both innate and adaptive immunity are essential to prevent tumour cell metabolism and to enhance T-cells, which are the main target in cancer treatment. In particular, CD8+ T-cells are very important in reducing cancer growth.96
In conclusion, balancing the gut microbiota with the help of prebiotics can be useful as a treatment enhancer.96
Focusing on the anti-tumour properties of XOS, several in vitro and in vivo (in rats with induced colon tumours) approaches have been conducted, and their conclusions are promising. In vitro, XOS have shown to reduce the growth of several tumor cell lines, and, in vivo, XOS supplementation in rats reduced the level of lipid peroxidation and increased the activities of glutathione-S-transferase and catalase in the colonic mucosa and liver. These facts may have contributed to the inhibition of colon carcinogenesis.13 Despite these results, further research is necessary to understand the anti-tumour effects of XOS and to apply them as nutraceuticals for cancer patients.97,98
In the study by Ghosh et al.,33 XOS produced from sugarcane bagasse that included xylobiose, xylotriose and xylotetraose were incubated with two types of colon cancer cell lines to test their antiproliferative activity. A colorimetric assay with 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) was chosen to carry the test. The results showed that XOS at a concentration of 300 μg mL−1 inhibited the proliferation of colon cancer cell lines HT-29 and Caco-2 by 90% and 75%, respectively, after 48 h of incubation. These results suggest that XOS have high anti-proliferation activity against colon cancer cells.33
Batsalova et al.97 also evaluated the cytotoxic effect of XOS against different human tumour cell lines in vitro. In this case, commercially available corn XOS were used. The main oligosaccharide in these XOS was xylohexaose. This paper establishes more complete conclusions about the cytotoxic capacity of XOS than the one mentioned before, as the researchers compared the cytotoxic effect in several tumour cell lines with that achieved in a normal human fibroblast cell line.
The assayed human cell lines were MRC-5 lung fibroblasts derived from normal tissue, A549 lung adenocarcinoma, HT-29 colon adenocarcinoma, and U-937 histiocytic lymphoma. Again, the MTT assay was selected to study the cell viability in the presence of XOS, but also the neutral red (NR) uptake test was used. The MTT assay allows the study of the metabolic activity and the NR assay evaluates the lysosomal functionality.
The results obtained indicate the antitumor potential of the commercial corn XOS as cytotoxic effects in tumour cell lines were stronger than those in MRC-5 fibroblasts. XOS cytotoxic effects are time- and concentration-dependent. Moreover, tumour cell inhibition was higher in NR assays than in the MTT assay, suggesting a lysosome-specific mechanism of cytotoxicity. Half-maximal inhibitory concentrations (IC50) for NR assays were between 52 and 150 μg mL−1 in tumour cell lines, while the value was 367.3 μg mL−1 for MRC-5 cells. These values confirmed the selective tumour cytotoxic effect of the tested XOS.
Furthermore, this report revealed a possible mode of antitumour action of XOS by altering the cellular redox state, affecting glutathione homeostasis:
The reduced form of the tripeptide glutathione (GSH) is an important antioxidant in all cell types and, during oxidative stress, GSH is converted to an oxidized form called GSSG. As a result, the glutathione levels and the GSH/GSSG ratio are indicators of cell health.
In this study, the influence of commercially available XOS on the redox state of the cell was tested by measuring the GSH levels and GSH/GSSG ratio in A549, HT-29, MRC-5 and U-937 cell lines cultured with 200 μg mL−1 XOS for 24 hours.
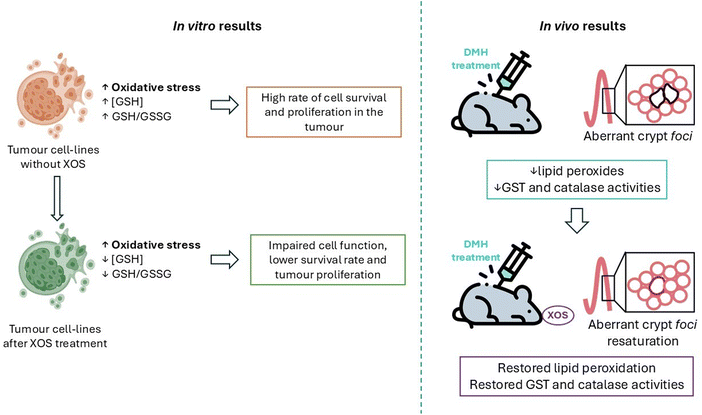
Tumour cells treated with XOS had reduced levels of GSH compared with those cultured without the prebiotics, whilst MRC-5 cells showed no difference in the GSH concentration. Moreover, all tumour cell lines had a reduced GSH/GSSG ratio, but MRC-5 had a higher value. Those results indicate that, when treated with XOS, tumour cells have a low capacity to convert oxidized glutathione to its reduced form (Fig. 4). Elevated oxidative stress is a property of tumour cells, so a low capacity for glutathione reduction could produce an imbalance in the redox homeostasis and lead to bad cell function, affecting the survival and development of tumour cells, which have an elevated level of oxidative stress due to their high metabolic rate.97
Aachary et al.98 studied the effect of corncob XOS in vivo, using male Wistar rats with procarcinogenic lesions induced by the compound 1,2-dimethylhydrazine (DMH). XOS were introduced in their diet for 45 days at 5% and 10% (w/w) in order to observe the possible beneficial effects produced by them.
DMH produced aberrant crypt foci in the colon and XOS supplementation reduced their incidence and multiplicity. A strong correlation between aberrant crypt foci formation and colon carcinogenesis has previously been confirmed, so the results of this study suggest that diet supplementation with XOS may affect the initial state of colon carcinogenesis. Dietary XOS supplementation restored normalcy in the colonic epithelial cells, which suggests that XOS mediated the reparation of preneoplastic lesions. It has been proven before that the administration of Bifidobacteria and Lactobacillus acidophilus decreases aberrant crypt foci formation so the most probable mechanism of action of XOS to produce the same effects as those of probiotics is acting as prebiotics. In fact, the dietary XOS supplementation at 5% and 10% (w/w) increased the population of Bifidobacteria in the rats’ gut, while DMH treatments markedly reduced it and increased the population of C. perfringens and E. coli pathogens.
On the other hand, XOS restored the level of lipid peroxides, which were decreased in the colon after DMH treatment. Glutathione S-transferase and catalase activities were also decreased in the colon due to DMH administration. Both results indicate an increase in oxidative stress after DMH induced lesions. With XOS supplementation, the enzymatic activities mentioned before were increased in the colonic mucosa of rats. It is important to highlight that cancer cells usually proliferate faster when the lipid peroxidation level is low, so it is a very significant result that XOS improve lipid peroxidation levels near normal values in DMH treated rats (Fig. 4).
In conclusion, in this work a protective effect against the formation of aberrant crypt foci and metabolic abnormalities has been observed when the diet of the rats is supplemented with XOS, suggesting an anticarcinogenic effect of the prebiotics.98
Prospects for the XOS market
Awareness about prebiotics has gained popularity in recent years, stimulating scientific as well as industrial interest. Economic forecasts predict that the size of the prebiotic market is expected to grow from US$ 6676 million in 2023 at a CAGR (compound annual growth rate) of 6.3% to reach a value of US$ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 million in 2033.23 Among the emerging prebiotics, XOS are starting to stand out because of their good physicochemical properties and the low dose needed to exhibit health benefits. Because of this, a report from Future Market Insights Inc. has projected that the XOS global market will expand with a CAGR of 7% and their valuation will increase from US$ 74 million in 2023 to US$ 144.5 million by 2033.23
902 million in 2033.23 Among the emerging prebiotics, XOS are starting to stand out because of their good physicochemical properties and the low dose needed to exhibit health benefits. Because of this, a report from Future Market Insights Inc. has projected that the XOS global market will expand with a CAGR of 7% and their valuation will increase from US$ 74 million in 2023 to US$ 144.5 million by 2033.23
At a global level, the most common use of XOS is in the feed industry (49.6%), followed by health and medical products (25.4%), food and beverage (23.2%), and other applications (1.8%). In 2023, China was the leading country in the production of xylooligosaccharides. The principal companies are Shandong Longlive Bio-Technology Co., Ltd, Kangwei Biologic Co., Ltd, Yibin Yatai Biotechnology Co., Ltd, and Henan Yuanlong Biological Technology, all located in China, occupying more than 60% of global production.99
Although FOS, GOS and inulin are currently the most well-established prebiotics, with the most significant global participation in the market,100 the XOS market is poised to increase significantly in the next few years because of their good characteristics. For example, XOS exhibit pH (from 2.5 to 8) and temperature stability (up to 100 °C) in a higher range compared to today's most popular prebiotics. Moreover, XOS are more resistant to digestion and have good organoleptic properties. Besides these excellent properties, the reported beneficial effects on health may also be involved in their future success in the prebiotics market: prevention of diabetes, reduction of inflammation, antioxidant capacity, prevention of colon cancer101 and strengthening the immune system are several of the bioactivities found in XOS.102
Despite all the advantages of XOS to be used as prebiotics, there are also some challenges that need to be overcome in order to gain market share. One of the biggest handicaps is the high cost of their production101 as enzymatic methods are commonly utilized to obtain XOS and the required chemical pretreatments and enzymes are expensive. The choice of these methods, despite their elevated cost, lies in their high yields and specificity in generating the prebiotics, as well as the use of milder conditions when compared with methods like autohydrolysis. The latter methodology has been discarded at the industrial level due to the production of undesired by-products, with the associated necessity of subsequent expensive purification steps to make the product suitable for consumption.103,104
To minimise the costs of production, the strategy in recent years has been to use lignocellulosic residues from industry as a raw material to produce XOS, since lignocellulosic biomass (LCB) is the most abundant agricultural residue available in the world and is sold at low prices.18 Many of these wastes would otherwise be discarded, so besides lowering the cost of prebiotics production, this is also a good way to revalorise them. In the near future, the prebiotic industry will be ready to decrease the price of XOS even more, turning the obtention processes more efficient with the elimination of the pretreatment phase by the use of integrated strategies like direct fermentation101 in which a microorganism has the capacity to extract xylan and hydrolyse it.
The beneficial effects of XOS have been observed at lower doses (1–4 grams per day) compared to other prebiotics such as FOS, GOS and inulin and this fact could be decisive in order to offset production costs. Furthermore, the lower dose required for clinical efficacy gives advantages in terms of product formulation and format.105
Given the current state of XOS in the market, one factor that will be key to achieving the expected growth of XOS in the coming years is the approval of newly obtained XOS by regional regulatory authorities. For this to happen, investment in R&D is essential to prove that XOS are safe as food ingredients and also have beneficial health effects in humans and/or animals. In this way, there will be a greater demand for XOS, and their commercial value will increase.101
In Europe, the latest development that has been made at the level of approval of XOS for food consumption by EFSA occurred in 2018 with Regulation (EU) 2015/2283.106 By this regulation, corn cob-derived and suitably purified XOS obtained by Longlive Europe Food Division Ltd were designated as safe for food consumption at levels set according to the food to which they are added (Regulation 2020/916).107 Thus, they are permitted for use in food but are in no case guaranteed to have beneficial effects on health (unlike some types of inulins that have already obtained EFSA approval for prebiotic health claims).101
Conclusion
XOS represent a promising prebiotic, demonstrating the capacity to selectively modulate gut microbiota, support metabolic and digestive health, and even contribute to disease prevention. Their ability to selectively modulate gut microbiota exerting a bifidogenic effect has been observed in vitro and in vivo, enhancing the production of short-chain fatty acids, and improving gut barrier integrity. Besides the prebiotic effect, some studies have demonstrated that XOS can have some other capacities.In the context of obesity, XOS supplementation in animal models has demonstrated reductions in body weight and fat mass, along with improvements in glucose and lipid metabolism, and inflammatory markers. Despite these promising results, only one study in humans with obesity has been carried out, where XOS were supplied along with the probiotic Bacillus coagulans.
For colon cancer, XOS exhibit antiproliferative effects against tumour cell lines such as HT-29 and Caco-2 in vitro. These effects are mediated by mechanisms like redox homeostasis disruption, as XOS lower glutathione levels in cancer cells, inducing oxidative stress. In animal models, XOS reduce aberrant crypt foci, an early marker of colon carcinogenesis, and restore antioxidant enzyme activity, further supporting their role in cancer prevention.
For both anti-obesity and anti-carcinogenic effects, there is a need to perform more studies in humans to confirm what has been observed in vitro and in animal models. To do that, it would be necessary to approve the use of XOS from origins other than corn cob, which are the only ones allowed for human consumption at the moment.
For inflammatory bowel disease (IBD), XOS have shown significant benefits in both in vitro and in vivo studies. They improve the gut barrier function by increasing tight junction protein expression and mucus production, while reducing bacterial penetration into the epithelial layer. In animal models of colitis, XOS supplementation attenuated inflammation, reducing pro-inflammatory cytokine levels. These findings suggest XOS may be effective in managing IBD symptoms and maintaining remission, though further clinical trials are needed.
Looking forward, the potential applications of XOS are broad and continually expanding, driven by innovations in biotechnology, the growing demand for functional foods, and the pursuit of nutraceutical interventions for chronic conditions.
One promising direction for the use of XOS is personalized nutrition. With the growing understanding of the human microbiome and interindividual variability in gut microbial composition, XOS could be tailored to target specific microbiota profiles and improve conditions such as metabolic syndrome, inflammatory diseases, or age-related dysbiosis more effectively.61,62,93
Food and beverage innovation is another important field. Due to their stability across pH and temperature ranges and their sweet, low-calorie nature, XOS are increasingly explored as functional ingredients in health-oriented products. Their efficacy at low doses (1–4 g day−1) compared to other prebiotics like inulin or FOS makes them attractive for widespread incorporation into consumer products.101
The combination of XOS with probiotics (symbiotics) also represents an underexploited opportunity. Given their ability to selectively enhance the growth of beneficial strains like Bifidobacterium, Lactobacillus and Weissella spp., XOS could be paired with targeted probiotics to improve and prevent gut dysbiosis.27
The animal feed industry constitutes another area of development as XOS can improve the gut's commensal bacteria balance. A balanced microbiota is a crucial part of an animal's digestive system and is involved in metabolic processes such as cholesterol absorption, blood pressure management, and glucose metabolism. The increasing demand for natural ingredients in animal feed, along with the expansion of the livestock sector, opens this part of the market to prebiotics in general and XOS in particular.23
Additionally, XOS are gaining attention in the cosmeceutical sector for their potential to support the skin microbiome, antioxidant capacity and the capacity to retain water. Although research in this area is still scarce, the prebiotic concept is being extended beyond the gut, and XOS could become valuable in skin health products.23
Given their broad functionality and low effective dosage, XOS are well positioned to gain greater market share. Their future success will depend on ongoing research, further clinical validation, and harmonization of regulatory approvals for novel sources and formulations, especially within regions like Europe and North America.23,107
Author contributions
Conceptualization: Manuel Becerra Fernández, Natalia Nogueira Prieto, Carmen Ansín Vallejo and María Isabel González Siso; literature research and data analysis: Natalia Nogueira Prieto and Carmen Ansín Vallejo; funding acquisition: Manuel Becerra Fernández and María Isabel González Siso; project administration: Manuel Becerra Fernández and María Isabel González Siso; supervision: Manuel Becerra Fernández and María Isabel González Siso; writing—original draft: Natalia Nogueira Prieto and Carmen Ansín Vallejo; and writing—review and editing: Manuel Becerra Fernández, Natalia Nogueira Prieto, Carmen Ansín Vallejo and María Isabel González Siso. All authors have read and agreed to the published version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Ministerio de Ciencia e Innovación, Gobierno de España (grant no. PID2021-126194OB-C22) and by the Xunta de Galicia (Ayudas para la consolidación y estructuración de unidades de investigación competitivas y otras acciones de fomento en las universidades del SUG – GRC, grant no. ED431C 2024/01). Natalia Nogueira Prieto acknowledges the Ministry of Science, Innovation, and Universities of Spain for funding through an FPU fellowship (FPU21/05026).References
- C. Bedu-Ferrari, P. Biscarrat, P. Langella and C. Cherbuy, Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health, Nutrients, 2022, 14(10), 2096 CrossRef CAS PubMed.
- D. Davani-Davari, M. Negahdaripour, I. Karimzadeh, M. Seifan, M. Mohkam and S. Masoumi, et al., Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications, Foods, 2019, 8(3), 92 CrossRef CAS PubMed.
- R. Barczyńska, K. Bandurska, K. Śliżewska, M. Litwin, M. Szalecki and Z. Libudzisz, et al., Intestinal Microbiota, Obesity and Prebiotics, Pol. J. Microbiol., 2015, 64(2), 93–100 Search PubMed.
- H. L. Lauzon, A. Dimitroglou, D. L. Merrifield, E. Ringø and S. J. Davies, Probiotics and prebiotics: Concepts, definitions and history, in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, Wiley, 2014, pp. 169–184 Search PubMed.
- S. Chowdhury, S. Das, D. Bhattacharjee, P. K. Saha, S. Mukherjee and B. K. Bhattacharyya, Prebiotics-Clinical relevance, Indian J. Nat. Prod. Resour., 2015, 6(2), 91–97 Search PubMed.
- R. Erhardt, J. E. Harnett, E. Steels and K. J. Steadman, Functional constipation and the effect of prebiotics on the gut microbiota: a review, Br. J. Nutr., 2023, 130(6), 1015–1023 CrossRef CAS PubMed.
- K. Dharni, A. Singh, S. Sharma, V. Midha, K. Kaur and R. Mahajan, et al., Trends of inflammatory bowel disease from the Global Burden of Disease Study (1990–2019), Indian J. Gastroenterol., 2024, 43(1), 188–198 CrossRef PubMed.
- K. R. Pandey, S. R. Naik and B. V. Vakil, Probiotics, prebiotics and synbiotics- a review, J. Food Sci. Technol., 2015, 52(12), 7577–7587 CrossRef CAS PubMed.
- L. G. Ooi and M. T. Liong, Cholesterol-lowering effects of probiotics and prebiotics: A review of in Vivo and in Vitro Findings, Int. J. Mol. Sci., 2010, 11(6), 2499–2522 CrossRef CAS PubMed.
- S. Lockyer and S. Stanner, Prebiotics – an added benefit of some fibre types, Nutr. Bull., 2019, 44(1), 74–91 CrossRef.
- A. F. A. Carvalho, P. d. O. Neto, D. F. da Silva and G. M. Pastore, Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis, Food Res. Int., 2013, 51(1), 75–85 CrossRef CAS.
- B. Yan, C. Huang, C. Lai, Z. Ling and Q. Yong, Production of prebiotic xylooligosaccharides from industrial-derived xylan residue by organic acid treatment, Carbohydr. Polym., 2022, 292, 119641 CrossRef CAS PubMed.
- Y. Chen, Y. Xie, K. M. Ajuwon, R. Zhong, T. Li and L. Chen, et al., Xylo-Oligosaccharides, Preparation and Application to Human and Animal Health: A Review, Front. Nutr., 2021, 8, 1–10 CAS.
- M. Martins, W. G. Sganzerla, T. Forster-Carneiro and R. Goldbeck, Recent advances in xylo-oligosaccharides production and applications: A comprehensive review and bibliometric analysis, Biocatal. Agric. Biotechnol., 2023, 47, 102608 CrossRef CAS.
- Y. Yamamoto, H. Kishimura, Y. Kinoshita, W. Saburi, Y. Kumagai and H. Yasui, et al., Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan, Process Biochem., 2019, 82, 117–122 CrossRef CAS.
- Q. P. Yuan, H. Zhang, Z. M. Qian and X. J. Yang, Pilot-plant production of xylo-oligosaccharides from corncob by steaming, enzymatic hydrolysis and nanofiltration, J. Chem. Technol. Biotechnol., 2004, 79(10), 1073–1079 CrossRef CAS.
- G. Precup, J. Venus, M. Heiermann, R. Schneider, I. D. Pop and D. C. Vodnar, Chemical and Enzymatic Synthesis of Biobased Xylo-Oligosaccharides and Fermentable Sugars from Wheat Straw for Food Applications, Polymers, 2022, 14(7), 1336 CrossRef CAS PubMed.
- L. Santibáñez, C. Henríquez, R. Corro-Tejeda, S. Bernal, B. Armijo and O. Salazar, Xylooligosaccharides from lignocellulosic biomass: A comprehensive review, Carbohydr. Polym., 2021, 251, 117118 CrossRef PubMed.
- K. L. Ríos-Ríos, C. Rémond, W. Dejonghe, S. Van Roy, S. Vangeel and W. Van Hecke, Production of tailored xylo-oligosaccharides from beechwood xylan by different enzyme membrane reactors and evaluation of their prebiotic activity, Biochem. Eng. J., 2022, 185, 108494 CrossRef.
- A. Moure, P. Gullón, H. Domínguez and J. C. Parajó, Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals, Process Biochem., 2006, 41(9), 1913–1923 CrossRef CAS.
- P. Ligero, J. C. Van Der Kolk, A. De Vega and J. E. G. Van Dam, Production of xylo-oligosaccharides from Miscanthus x giganteus by autohydrolysis, BioResources, 2011, 6(4), 4417–4429 CAS.
- M. Martins, P. F. Ávila, C. C. Paim de Andrade and R. Goldbeck, Synergic recombinant enzyme association to optimize xylo-oligosaccharides production from agricultural waste, Biocatal. Agric. Biotechnol., 2020, 28, 101747 CrossRef.
- N. R. Choudhury, Xylooligosaccharide Market Size, Share, Trends by 2033, Food Beverages, 2023 Search PubMed . Available from: https://www.futuremarketinsights.com/reports/xylooligosaccharide-market.
- A. Palaniappan, U. Antony and M. N. Emmambux, Current status of xylooligosaccharides: Production, characterization, health benefits and food application, Trends Food Sci. Technol., 2021, 111, 506–519 CrossRef CAS.
- R. D. Singh, J. Banerjee and A. Arora, Prebiotic potential of oligosaccharides: A focus on xylan derived oligosaccharides, Bioact. Carbohydr. Diet. Fibre, 2015, 5(1), 19–30 CrossRef CAS.
- M. A. Kabel, L. Kortenoeven, H. A. Schols and A. G. J. Voragen, In Vitro Fermentability of Differently Substituted Xylo-oligosaccharides, J. Agric. Food Chem., 2002, 50(21), 6205–6210 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/abs/10.1021/jf020220r.
- S. Mathew, A. Aronsson, E. N. Karlsson and P. Adlercreutz, Xylo- and arabinoxylooligosaccharides from wheat bran by endoxylanases, utilisation by probiotic bacteria, and structural studies of the enzymes, Appl. Microbiol. Biotechnol., 2018, 102(7), 3105–3120 CrossRef CAS PubMed.
- P. Immerzeel, P. Falck, M. Galbe, P. Adlercreutz, E. Nordberg Karlsson and H. Stålbrand, Extraction of water-soluble xylan from wheat bran and utilization of enzymatically produced xylooligosaccharides by Lactobacillus, Bifidobacterium and Weissella spp, LWT–Food Sci. Technol., 2014, 56(2), 321–327 CrossRef CAS.
- P. Falck, S. Precha-Atsawanan, C. Grey, P. Immerzeel, H. Staìšlbrand and P. Adlercreutz, et al., Xylooligosaccharides from hardwood and cereal xylans produced by a thermostable xylanase as carbon sources for Lactobacillus brevis and Bifidobacterium adolescentis, J. Agric. Food Chem., 2013, 61(30), 7333–7340, DOI:10.1021/jf401249g.
- J. Holck, A. Lorentzen, L. K. Vigsnæs, T. R. Licht, J. D. Mikkelsen and A. S. Meyer, Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of bifidobacterium spp. In human fecal in vitro fermentations, J. Agric. Food Chem., 2011, 59(12), 6511–6519 CrossRef CAS PubMed.
- E. Nordberg Karlsson, E. Schmitz, J. A. Linares-Pastén and P. Adlercreutz, Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties, Appl. Microbiol. Biotechnol., 2018, 102(21), 9081–9088 CrossRef CAS PubMed.
- N. Schropp, V. Stanislas, K. B. Michels and K. Thriene, How Do Prebiotics Affect Human Intestinal Bacteria?—Assessment of Bacterial Growth with Inulin and XOS In Vitro, Int. J. Mol. Sci., 2023, 24(16), 12796 CrossRef CAS PubMed.
- A. Ghosh, A. Chandra, A. Dhar, P. Shukla and D. Baishya, Multi-efficient thermostable endoxylanase from Bacillus velezensis AG20 and its production of xylooligosaccharides as efficient prebiotics with anticancer activity, Process Biochem., 2021, 109, 59–71 CrossRef CAS.
- F. C. de Figueiredo, F. F. de Barros Rankea and P. de Oliva-Neto, Evaluation of xylooligosaccharides and fructooligosaccharides on digestive enzymes hydrolysis and as a nutrient for different probiotics and Salmonella typhimurium, LWT, 2020, 118, 108761 CrossRef CAS.
- P. Gullón, N. Salazar, M. J. G. Muñoz, M. Gueimonde, P. Ruas-Madiedo and C. G. de los Reyes-Gavilán, et al., Assessment on the fermentability of xylooligosaccharides from rice husks, BioResources, 2011, 6(3), 3096–3114 Search PubMed.
- A. A. I. Ratnadewi, M. T. Rahma, N. Nurhayati, A. B. Santoso, K. Senjarini and A. Labes, et al., Production of Xylooligosaccharide from Cassava Pulp’s Waste by Endo-β-1,4-D-Xylanase and Characterization of Its Prebiotic Effect by Fermentation of Lactobacillus acidophilus, Fermentation, 2022, 8(10), 488 CrossRef CAS.
- N. Zeybek, R. A. Rastall and A. O. Buyukkileci, Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species, Carbohydr. Polym., 2020, 236, 116076 Search PubMed.
- M. Chen, S. Liu, K. M. S. U. Imam, L. Sun, Y. Wang and T. Gu, et al., The Effect of Xylooligosaccharide, Xylan, and Whole Wheat Bran on the Human Gut Bacteria, Front. Microbiol., 2020, 11, 1–12 CrossRef PubMed.
- G. Victoria Gautério, C. Amorim, S. C. Silvério, B. B. Cardoso, L. F. Ballesteros and J. I. Alves, et al., Hydrolysates containing xylooligosaccharides produced by different strategies: Structural characterization, antioxidant and prebiotic activities, Food Chem., 2022, 391, 133231 Search PubMed.
- D. H. Lee, H. Seong, D. Chang, V. K. Gupta, J. Kim and S. Cheon, et al., Evaluating the prebiotic effect of oligosaccharides on gut microbiome wellness using in vitro fecal fermentation, npj Sci. Food, 2023, 7, 18 Search PubMed.
- Z. Li, P. H. Summanen, T. Komoriya and S. M. Finegold, In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp, Int. J. Food Sci. Nutr., 2015, 66(8), 919–922 Search PubMed.
- P. Gullón, M. J. González-Muñoz and J. C. Parajó, Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes, Bioresour. Technol., 2011, 102(10), 6112–6119 CrossRef PubMed.
- K. P. Scott, J. C. Martin, S. H. Duncan and H. J. Flint, Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro, FEMS Microbiol. Ecol., 2014, 87(1), 30–40 Search PubMed.
- P. Moura, R. Barata, F. Carvalheiro, F. Gírio, M. C. Loureiro-Dias and M. P. Esteves, In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains, LWT–Food Sci. Technol., 2007, 40(6), 963–972 CrossRef CAS.
- A. Amaretti, T. Bernardi, A. Leonardi, S. Raimondi, S. Zanoni and M. Rossi, Fermentation of xylo-oligosaccharides by Bifidobacterium adolescentis DSMZ 18350: Kinetics, metabolism, and β-xylosidase activities, Appl. Microbiol. Biotechnol., 2013, 97(7), 3109–3117 Search PubMed.
- D. M. Salas-Veizaga, A. Bhattacharya, P. Adlercreutz, H. Stålbrand and E. N. Karlsson, Glucuronosylated and linear xylooligosaccharides from Quinoa stalks xylan as potential prebiotic source for growth of Bifidobacterium adolescentis and Weissella cibaria, LWT–Food Sci. Technol., 2021, 152, 112348 CrossRef CAS.
- D. Driss, A. Haddar, R. Ghorbel and S. E. Chaabouni, Production of xylooligosaccharides by immobilized his-tagged recombinant xylanase from penicillium occitanis on nickel-chelate Eupergit C, Appl. Biochem. Biotechnol., 2014, 173(6), 1405–1418 CrossRef CAS PubMed.
- Z. Sun, Z. Yue, E. Liu, X. Li and C. Li, Assessment of the bifidogenic and antibacterial activities of xylooligosaccharide, Front. Nutr., 2022, 9, 1–10 Search PubMed.
- K. Kumari, S. Nagar, S. Goyal, S. Maan, M. Sindhu and R. Singh, et al., Production, Characterization and Prebiotic Potential of Xylooligosaccharides Produced from Wheat Bran using Enterobacter hormaechei KS1 Xylanase, Indian J. Microbiol., 2023, 63(3), 352–360 Search PubMed.
- P. Jaichakan, M. Nakphaichit, S. Rungchang, M. Weerawatanakorn, S. Phongthai and W. Klangpetch, Two-stage processing for xylooligosaccharide recovery from rice by-products and evaluation of products: Promotion of lactic acid-producing bacterial growth and food application in a high-pressure process, Food Res. Int., 2021, 147, 110529 Search PubMed.
- C. R. Mousquer, B. B. Thome, W. B. Iserhard, S. M. Callegari-Jacques, A. Della Bona, A. P. Venturini and F. Fornari, Inulin and streptococcus salivarius reduce halitosis associated with tongue coating: a randomized clinical trial, Gastroenterology, 2017, 152(5), S814–S815 CrossRef.
- K. Geetha and P. Gunasekaran, Purification of endoxylanase from bacillus pumilus B20 for production of prebiotic xylooligosaccharide syrup; An in vitro study, Iran. J. Biotechnol., 2017, 15(4), 232–240 CrossRef PubMed.
- C. d. O. Nascimento, L. d. O. Simões, J. d. C. Pereira, R. R. da Silva, E. A. de Lima and G. C. de Almeida, et al., Application of a recombinant GH10 endoxylanase from Thermoascus aurantiacus for xylooligosaccharide production from sugarcane bagasse and probiotic bacterial growth, J. Biotechnol., 2022, 347, 1–8 Search PubMed.
- C. Álvarez, A. González, J. L. Alonso, F. Sáez, M. J. Negro and B. Gullón, Xylooligosaccharides from steam-exploded barley straw: Structural features and assessment of bifidogenic properties, Food Bioprod. Process., 2020, 124, 131–142 Search PubMed.
- A. L. Ho, O. Kosik, A. Lovegrove, D. Charalampopoulos and R. A. Rastall, In vitro fermentability of xylo-oligosaccharide and xylo-polysaccharide fractions with different molecular weights by human faecal bacteria, Carbohydr. Polym., 2018, 179, 50–58 CrossRef CAS PubMed.
- I. Dávila, B. Gullón, J. L. Alonso, J. Labidi and P. Gullón, Vine shoots as new source for the manufacture of prebiotic oligosaccharides, Carbohydr. Polym., 2019, 207, 34–43 CrossRef PubMed.
- P. Moniz, A. L. Ho, L. C. Duarte, S. Kolida, R. A. Rastall and H. Pereira, et al., Assessment of the bifidogenic effect of substituted xylo-oligosaccharides obtained from corn straw, Carbohydr. Polym., 2016, 136, 466–473 CrossRef CAS PubMed.
- V. T. Pham, M. Calatayud, C. Rotsaert, N. Seifert, N. Richard and P. Van Den Abbeele, et al., Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro, Nutrients, 2021, 13(1125), 1–20 Search PubMed.
- S. M. Finegold, Z. Li, P. H. Summanen, J. Downes, G. Thames and K. Corbett, et al., Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota, Food Funct., 2014, 5(3), 436–445 RSC.
- C. E. Childs, H. Röytiö, E. Alhoniemi, A. A. Fekete, S. D. Forssten and N. Hudjec, et al., Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: A double-blind, placebo-controlled, randomised, factorial cross-over study, Br. J. Nutr., 2014, 111(11), 1945–1956 CrossRef CAS PubMed.
- J. Yang, P. H. Summanen, S. M. Henning, M. Hsu, H. Lam and J. Huang, et al., Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: A pilot study, Front. Physiol., 2015, 6, 1–11 Search PubMed.
- S. H. Lin, L. M. Chou, Y. W. Chien, J. S. Chang and C. I. Lin, Prebiotic Effects of Xylooligosaccharides on the Improvement of Microbiota Balance in Human Subjects, Gastroenterol. Res. Pract., 2016, 2016, 5789232 Search PubMed.
- Obesity and overweight [Internet]. 2021 [cited 2024 Feb 28]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- I. S. Larsen, B. S. Y. Choi, B. Föh, N. N. Kristensen, A. Ouellette and R. F. Haller, et al., Experimental diets dictate the metabolic benefits of probiotics in obesity, Gut Microbes, 2023, 15(1) DOI:10.1080/19490976.2023.2192547.
- A. Benítez-Páez, L. Kjølbæk, E. M. Gómez del Pulgar, L. K. Brahe, A. Astrup and S. Matysik, et al., A Multi-omics Approach to Unraveling the Microbiome-Mediated Effects of Arabinoxylan Oligosaccharides in Overweight Humans, mSystems, 2019, 4(4), e00209-19 CrossRef PubMed.
- Z. Li, C. X. Yi, S. Katiraei, S. Kooijman, E. Zhou and C. K. Chung, et al., Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit, Gut, 2018, 67(7), 1269–1279 CrossRef CAS PubMed . Available from: https://gut.bmj.com/lookup/doi/10.1136/gutjnl-2017-314050.
- C. Zhang, A. Abdulaziz Abbod Abdo, B. Kaddour, Q. Wu, L. Xin and X. Li, et al., Xylan-oligosaccharides ameliorate high fat diet induced obesity and glucose intolerance and modulate plasma lipid profile and gut microbiota in mice, J. Funct. Foods, 2020, 64, 103622 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1756464619305468.
- Y. Li, M. Liu, H. Liu, X. Wei, X. Su and M. Li, et al., Oral Supplements of Combined Bacillus licheniformis Zhengchangsheng® and Xylooligosaccharides Improve High-Fat Diet-Induced Obesity and Modulate the Gut Microbiota in Rats, BioMed Res. Int., 2020, 2020, 9067821 CrossRef PubMed.
- D. A. Russell, R. P. Ross, G. F. Fitzgerald and C. Stanton, Metabolic activities and probiotic potential of bifidobacteria, Int. J. Food Microbiol., 2011, 149(1), 88–105 CrossRef CAS PubMed.
- A. Colletti, M. Pellizzato and A. F. Cicero, The Possible Role of Probiotic Supplementation in Inflammation: A Narrative Review, Microorganisms, 2023, 11(9), 2160 CrossRef CAS PubMed . Available from: https://www.mdpi.com/2076-2607/11/9/2160.
- L. B. Thingholm, M. C. Rühlemann, M. Koch, B. Fuqua, G. Laucke and R. Boehm, et al., Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition, Cell Host Microbe, 2019, 26(2), 252–264 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S1931312819303488.
- J. Long, J. Yang, S. M. Henning, S. L. Woo, M. Hsu and B. Chan, et al., Xylooligosaccharide supplementation decreases visceral fat accumulation and modulates cecum microbiome in mice, J. Funct. Foods, 2019, 52, 138–146 CrossRef CAS.
- S. Sriwichaiin, W. Kittichotirat, T. Chunchai, N. Chattipakorn and S. C. Chattipakorn, Profiles of gut microbiota in obese-insulin-resistant rats treated with biotics, Eur. J. Nutr., 2022, 61(5), 2493–2505 CrossRef CAS PubMed . Available from: https://link.springer.com/article/10.1007/s00394-022-02839-6.
- F. Li, Q. Li, Y. Zhang, X. Zhou, R. Yi and X. Zhao, Effects of Xylooligosaccharides on Lipid Metabolism, Inflammation, and Gut Microbiota in C57BL/6J Mice Fed a High-Fat Diet, Front. Pharmacol., 2021, 12, 791614 CrossRef CAS PubMed . Available from: https://www.frontiersin.org.
- Y. Fei, Y. Wang, Y. Pang, W. Wang, D. Zhu and M. Xie, et al., Xylooligosaccharide Modulates Gut Microbiota and Alleviates Colonic Inflammation Caused by High Fat Diet Induced Obesity, Front. Physiol., 2020, 10, 467473 Search PubMed . Available from: https://www.frontiersin.org.
- S. Eaimworawuthikul, W. Tunapong, T. Chunchai, P. Suntornsaratoon, N. Charoenphandhu and P. Thiennimitr, et al., Altered gut microbiota ameliorates bone pathology in the mandible of obese–insulin-resistant rats, Eur. J. Nutr., 2020, 59(4), 1453–1462 CrossRef CAS PubMed . Available from: https://link.springer.com/article/10.1007/s00394-019-02002-8.
- S. Eaimworawuthikul, W. Tunapong, T. Chunchai, S. Yasom, K. Wanchai and P. Suntornsaratoon, et al., Effects of probiotics, prebiotics or synbiotics on jawbone in obese-insulin resistant rats, Eur. J. Nutr., 2019, 58(7), 2801–2810 CAS . Available from: https://link.springer.com/article/10.1007/s00394-018-1829-4.
- S. Eaimworawuthikul, W. Tunapong, T. Chunchai, P. Suntornsaratoon, N. Charoenphandhu and P. Thiennimitr, et al., Lactobacillus paracasei HII01, xylooligosaccharide and synbiotics improve tibial microarchitecture in obese-insulin resistant rats, J. Funct. Foods, 2019, 59, 371–379 CrossRef CAS.
- J. Hintikka, S. Lensu, E. Mäkinen, S. Karvinen, M. Honkanen and J. Lindén, et al., Xylo-Oligosaccharides in Prevention of Hepatic Steatosis and Adipose Tissue Inflammation: Associating Taxonomic and Metabolomic Patterns in Fecal Microbiomes with Biclustering, Public Health, 2021, 18(8), 4049, DOI:10.3390/ijerph18084049.
- S. Lensu, R. Pariyani, E. Mäkinen, B. Yang, W. Saleem and E. Munukka, et al., Prebiotic Xylo-Oligosaccharides Ameliorate High-Fat-Diet-Induced Hepatic Steatosis in Rats, Nutrients, 2020, 12(11), 3225 CrossRef CAS PubMed . Available from: https://www.mdpi.com/2072-6643/12/11/3225/htm.
- T. Li, L. Xu, Q. Yan, J. Liu and Z. Jiang, Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation, glucose and lipid metabolism in liver of mice, Food Sci. Hum. Wellness, 2022, 11(4), 1064–1075 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213453022000374.
- K. Berger, S. Burleigh, M. Lindahl, A. Bhattacharya, P. Patil and H. Stålbrand, et al., Xylooligosaccharides Increase Bifidobacteria and Lachnospiraceae in Mice on a High-Fat Diet, with a Concomitant Increase in Short-Chain Fatty Acids, Especially Butyric Acid, J. Agric. Food Chem., 2021, 69(12), 3617–3625 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/full/10.1021/acs.jafc.0c06279.
- N. Khat-udomkiri, P. Toejing, S. Sirilun, C. Chaiyasut and N. Lailerd, Antihyperglycemic effect of rice husk derived xylooligosaccharides in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rat model, Food Sci. Nutr., 2020, 8(1), 428–444 CrossRef CAS PubMed . Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.1327.
- X. Wu, X. Chen, X. Cai and S. Wang, Lipid-lowering activity and underlying mechanism of glycosylated peptide–calcium chelate prepared by transglutaminase pathway, Food Front., 2024, 5(1), 160–173 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/fft2.320.
- B. K. McFarlin, E. A. Tanner, D. W. Hill and J. L. Vingren, Prebiotic/probiotic supplementation resulted in reduced visceral fat and mRNA expression associated with adipose tissue inflammation, systemic inflammation, and chronic disease risk, Genes Nutr., 2022, 17(1), 1–12 CrossRef PubMed . Available from: https://genesandnutrition.biomedcentral.com/articles/10.1186/s12263-022-00718-7.
- U. K. Jana, N. Kango and B. Pletschke, Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation, Front. Nutr., 2021, 8, 1–13 Search PubMed.
- B. L. Li, D. Y. Zhao, P. L. Du, X. T. Wang, Q. Yang and Y. R. Cai, Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway, Inflammation Res., 2021, 70(6), 705–717 CrossRef CAS PubMed.
- Z. Chen, X. Shen, Q. Zhou, Q. Zhan, X. Xu and Q. Chen, et al., Dietary xylo-oligosaccharide ameliorates colonic mucus microbiota penetration with restored autophagy in interleukin-10 gene–deficient mice, JPEN, J. Parenter. Enteral Nutr., 2022, 46(5), 1130–1140 CrossRef CAS PubMed.
- K. Sheng, S. He, M. Sun, G. Zhang, X. Kong and J. Wang, et al., Synbiotic supplementation containing: Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis, Food Funct., 2020, 11(5), 3964–3974 RSC.
- N. Liu, H. Wang, Z. Yang, K. Zhao, S. Li and N. He, The role of functional oligosaccharides as prebiotics in ulcerative colitis, Food Funct., 2022, 13(13), 6875–6893 RSC.
- V. T. Pham, N. Seifert, N. Richard, D. Raederstorff, R. Steinert and K. Prudence, et al., The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro, PeerJ, 2018, 2018(6), e5288 CrossRef PubMed.
- Z. Li, Z. Li, L. Zhu, N. Dai, G. Sun and L. Peng, et al., Effects of Xylo-Oligosaccharide on the Gut Microbiota of Patients With Ulcerative Colitis in Clinical Remission, Front. Nutr., 2021, 8, 1–11 Search PubMed.
- Y. C. Chung, C. K. Hsu, C. Y. Ko and Y. C. Chan, Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly, Nutr. Res., 2007, 27(12), 756–761 CrossRef CAS.
- A. Trifan, O. Burta, N. Tiuca, D. C. Petrisor, A. Lenghel and J. Santos, Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial, United Eur. Gastroenterol. J., 2019, 7(8), 1093–1101 CrossRef CAS PubMed.
- Crece la carga mundial de cáncer en medio de una creciente necesidad de servicios [Internet]. [cited 2024 Jun 17]. Available from: https://www.who.int/es/news/item/01-02-2024-global-cancer-burden-growing–amidst-mounting-need-for-services.
- P. Mishra, V. M. Badiyani, S. Jain, S. Subramanian, S. V. Maharaj and A. Kumar, et al., Prebiotics: Ignored player in the fight against cancer, Cancer Rep., 2023, 6(11), 1–12 Search PubMed.
- T. Batsalova, Y. Georgiev, D. Moten, I. Teneva and B. Dzhambazov, Natural Xylooligosaccharides Exert Antitumor Activity via Modulation of Cellular Antioxidant State and TLR4, Int. J. Mol. Sci., 2022, 23(18), 10430 CrossRef CAS PubMed.
- A. A. Aachary, D. Gobinath, K. Srinivasan and S. G. Prapulla, Protective effect of xylooligosaccharides from corncob on 1,2-dimethylhydrazine induced colon cancer in rats, Bioact. Carbohydr. Diet. Fibre, 2015, 5(2), 146–152, DOI:10.1016/j.bcdf.2015.03.004.
- Xylooligosaccharides Market | Size, Share, Price, import, export, volume 2024 Forecast to 2031 [Internet]. [cited 2025 Apr 25]. Available from: https://www.24chemicalresearch.com/reports/198722/global-xylooligosaccharides-market-2024-2030.
- C. D. Pinales-Márquez, R. M. Rodríguez-Jasso, R. G. Araújo, A. Loredo-Treviño, D. Nabarlatz and B. Gullón, et al., Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: A review, Ind. Crops Prod., 2021, 162, 113274 CrossRef.
- C. Amorim, S. C. Silvério, K. L. J. Prather and L. R. Rodrigues, From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides, Biotechnol. Adv., 2019, 37(7), 107397 CrossRef CAS PubMed.
- E. Sanz Rodríguez, G. L. Díaz-Arenas, S. Makart, D. Ghosh, A. F. Patti and G. Garnier, et al., Determination of xylooligosaccharides produced from enzymatic hydrolysis of beechwood xylan using high-performance anion-exchange chromatography tandem mass spectrometry, J. Chromatogr. A, 2022, 1666, 462836 CrossRef PubMed.
- K. Ali, N. Niaz, M. Waseem, W. Ashraf, M. Hussain and M. U. Khalid, et al., Xylooligosaccharides: A comprehensive review of production, purification, characterization, and quantification, Food Res. Int., 2025, 201, 115631 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0963996924017022.
- K. K. Valladares-Diestra, L. P. de Souza Vandenberghe, S. Vieira, L. D. Goyzueta-Mamani, P. B. G. de Mattos and M. C. Manzoki, et al., The Potential of Xylooligosaccharides as Prebiotics and Their Sustainable Production from Agro-Industrial by-Products, Foods, 2023, 12(14), 2681 CrossRef CAS PubMed . Available from: https://www.mdpi.com/2304-8158/12/14/2681/htm.
- B. A. Saville and S. Saville, Xylooligosaccharidesand arabinoxylanoligosaccharides and their application as prebiotics, Appl. Food Biotechnol., 2018, 5(3), 121–130 CAS.
- D. Turck, J. L. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait and M. Heinonen, et al., Safety of xylo-oligosaccharides (XOS) as a novel food pursuant to Regulation (EU) 2015/2283, EFSA J., 2018, 16(7), e05361 Search PubMed.
- Comisión Europea and Dirección General de Salud y Seguridad Alimentaria, REGLAMENTO DE EJECUCIÓN (UE) 2020/916 DE LA COMISIÓN de 1 de julio de 2020, Comisión Europea, España, 2020, pp. 62–106 Search PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |