Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Investigating ellagitannins from strawberry (Fragaria × ananassa Duch.) as a new strategy to counteract H. pylori infection and inflammation†
Giulia Martinelli a,
Stefano Piazza
a,
Stefano Piazza a,
Marco Fumagallia,
Nicole Maranta
a,
Marco Fumagallia,
Nicole Maranta a,
Carola Pozzoli
a,
Carola Pozzoli a,
Elisa Sonzognia,
Safwa Moheb El Haddada,
Urska Vrhovsekb,
Fulvio Mattivib,
Enrico Sangiovanni
a,
Elisa Sonzognia,
Safwa Moheb El Haddada,
Urska Vrhovsekb,
Fulvio Mattivib,
Enrico Sangiovanni *a,
Emma De Fabiania and
Mario Dell'Agli
*a,
Emma De Fabiania and
Mario Dell'Agli a
a
aDepartment of Pharmacological and Biomolecular Sciences “Rodolfo Paoletti” (DiSFeB), Università degli Studi di Milano, 20133 Milan, Italy. E-mail: enrico.sangiovanni@unimi.it
bDepartment of Food Quality and Nutrition, Research and Innovation Centre, Fondazione Edmund Mach (FEM), 38098 S. Michele all'Adige, Italy
First published on 2nd June 2025
Abstract
Helicobacter pylori colonises the gastric mucosa of at least 50% of the world's human population, causing a variety of gastric diseases, including chronic gastritis, peptic ulcers, and gastric cancer. The ability of the bacterium to colonise and induce inflammation is achieved through a combination of different virulence factors. Besides the conventional pharmacological treatment of H. pylori infection based on bacterial eradication, natural compounds could function as an adjuvant to existing therapies, potentially mitigating the consequences of H. pylori infection by impairing bacterial adhesion and subsequent gastric inflammation. This study focuses on Fragaria × ananassa extract and its ellagitannins, agrimoniin and casuarictin, as novel strategies to counteract H. pylori-related gastritis. Strawberry tannins exhibited anti-inflammatory properties in both TNFα-challenged and H. pylori-infected models, inhibiting the release of IL-8 and IL-6 by GES-1 cells. This effect was, at least in part, ascribed to the impairment of NF-κB signalling. The study compares the infection of epithelial cells with two different H. pylori strains (cagA+ and cagA−), demonstrating the different capacities of the extract and ellagitannins to impair the release of IL-6, while the effect on IL-8 release was independent of the different virulence potentials of the strains. Moreover, the extract and ellagitannins demonstrated antibacterial activity against cagA+ H. pylori growth, but exhibited reduced activity against the cagA− strain. The results of this study indicate the possible use of Fragaria × ananassa as a pharmacological and nutritional source in the prevention and/or treatment of gastritis induced by H. pylori.
1. Introduction
Helicobacter pylori is a microaerophilic and Gram-negative bacterium, able to colonise the human gastric mucosa, despite the acidic environment, due to the action of various virulence factors.1 The severity of H. pylori infection outcomes, including gastric ulcers and cancer, is the consequence of a complex interaction between virulence factors, bacterial strains, the host's immune response, and environmental factors.2 Colonisation induces a pro-inflammatory response in gastric epithelial cells, leading to the recruitment of several immune cells to the submucosa at the site of infection.3 The cagPAI gene, a key virulence factor, enables H. pylori to attach to host cells and, through the type IV secretion system (T4SS), deliver virulent proteins like CagA into the host cytoplasm.4 Infection activates multiple pathways (e.g., NF-κB, MAPK and STAT3), leading to the production of inflammatory cytokines, such as IL-1, IL-6, IL-8, and TNFα.5,6 In particular, the NF-κB pathway increases the expression and release of IL-8, the main chemokine involved in gastritis.7,8Current treatment of H. pylori infection consists of triple therapy with a proton pump inhibitor and at least two antibiotics, selected according to recent guidelines.9 Although eradication treatment reduces gastric cancer incidence and mortality,10 antibiotic therapy has adverse effects and disrupts the host's bacterial flora.11 Furthermore, the increasing development of antibiotic resistance leads to treatment failure.12 Consequently, there is an urgent need to identify innovative non-antibiotic treatments for H. pylori infections. Natural substances have shown considerable potential in eradicating H. pylori and preventing related gastric diseases,13 such as proanthocyanidins from Peumus boldus14 and EGCG.15
Strawberry (Fragaria × ananassa Duch.) is rich in beneficial nutrients including fibre, vitamins, minerals, and polyphenols.16 Among these, the tannin content, particularly the ellagitannins agrimoniin and casuarictin, has been characterised and quantified.17 Ellagitannins are a class of hydrolysable tannins found in many seeds and fruits (e.g. pomegranates, berries and walnuts), are stable in the gastric environment and are metabolized by the gut microbiota to produce urolithins.18 Ellagitannins contained in berries have exhibited anti-inflammatory19,20 and anti-bacterial activities,21 as reviewed by Golovinskaia et al. and Piazza et al.22,23 Promising results have been obtained by our group investigating other sources of ellagitannins, such as chestnut leaves and the pure compound castalagin.24 The biological activity of strawberry includes anticancer, anti-inflammatory, neuroprotective, cardiovascular, and antioxidant properties, in both in vivo and in vitro studies.16,25,26
Our previous research suggested that tannin-enriched strawberry extract possesses anti-inflammatory properties in AGS cells challenged with TNFα, by dampening the NF-κB pathway at nutritionally relevant concentrations (1–10 μg mL−1). Isolated compounds revealed that casuarictin may preferentially inhibit NF-κB, while agrimoniin inhibits IL-8 secretion by also acting on other biological targets at low μM concentrations.17 Despite promising gastric-level results, ellagitannins are still poorly investigated in H. pylori infection models due to the limitations of in vitro and in vivo analyses. Recently, GES-1 cells, derived from non-tumoral human epithelium, were characterized as a valuable alternative to AGS cells for studying the action of natural compounds against H. pylori-related gastritis.27
Based on these premises, the present work aims to (i) confirm the anti-inflammatory activity of strawberry extract in GES-1 cells challenged by TNFα; (ii) assess the anti-inflammatory activity of strawberry extract in H. pylori-infected GES-1 cells, and the antibacterial activity directly on H. pylori; and (iii) ascribe the biological activity to the pure ellagitannins agrimoniin and casuarictin. To gain an insight into the mode of action of strawberry tannins, the cells were infected with two different H. pylori strains, expressing or not the virulence factor cagA.
2. Materials and methods
2.1 Materials
RPMI 1640 medium, penicillin/streptomycin 100 units per mL solution, L-glutamine (2 mM), and trypsin–EDTA 0.25% solution were purchased from Gibco™ (Thermo Fisher Scientific, Rodano MI, Italy), while the Fetal Bovine Serum (FBS) and all disposable materials for cells were from Euroclone (Euroclone S.p.A., Pero, MI, Italy). The U-bottom plates were from Greiner Bio-One (Euroclone S.p.A., Pero MI, Italy). Mueller Hinton-Agar–5% sheep blood Petri dishes, defibrinated sheep blood, and Brucella broth were purchased from Thermo Fisher Diagnostics (Thermo Fisher Scientific, Rodano, MI, Italy), and the microaerophilic gas pack was from Thermo Scientific™ CampyGen™ (Thermo Fisher Scientific, Rodano MI, Italy). Lipofectamine™ 3000, CarboxyFluorescein Succinimidyl Ester (CFSE) 5 mM (CellTrace™, cell proliferation kits), and ActinRed™ 555 ReadyProbe™ Reagent were purchased from Invitrogen™ (Thermo Fisher Scientific, Rodano MI, Italy). Britelite™ Plus was from PerkinElmer (PerkinElmer, Milan, Italy). The human TNFα, human IL8 and IL6 ELISA development kits were obtained from Peprotech Inc. (Peprotech Inc., London, UK), while the human MMP9 ELISA kit was purchased from Tebu-Bio (Tebu-Bio SRL, Magenta, MI, Italy). The rabbit antibody for p-65, the secondary anti-rabbit antibody conjugated with Alexa Fluor 647, and ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (#8961) were from Cell Signaling Technology (Euroclone, S.p.A., Pero, MI, Italy). DMSO, isopropanol, glycerol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent, ABTS™ solution for ELISA, and tetracycline were provided by Merck (Merck Life Science S.r.l., Milan, Italy). Epigallocatechin 3-gallate (EGCG) and apigenin were provided by Phytolab (Phytolab GmbH & Co. KG, Vestenbergsgreuth, Germany).2.2 Strawberry extract and ellagitannin purification
Tannin-enriched extract from strawberry (Fragaria × ananassa Duch.) and purified ellagitannins were provided by The Edmund Mach Foundation (San Michele all'Adige, TN, Italy). As described in Fumagalli et al.,17 strawberries were cultivated in Vigalzano (Trento, Italy) under controlled conditions to minimize the impact of environmental and agronomic factors. At the time of harvest, the fruits were healthy, and the ripening stage was evaluated as described by Gasperotti et al.28 The extraction of polyphenols from the fruits was evaluated as described by the same authors,29 and the polyphenol fraction was purified using an established method.30 Agrimoniin and casuarictin were isolated as described by Vrhovsek et al.31 and Gasperotti et al.,28 respectively. The phytochemical composition of the tannin-enriched extract used in this study has been previously described.172.3 GES-1 cell culture
The GES-1 cell line (RRID: CVCL_EQ22), a non-tumoral human gastric epithelial cell line immortalized by simian virus 40 (SV-40), was kindly provided by Dr Kidane-Mulat (Howard University, College of Medicine, Washington, DC, USA). The cells were cultured in RPMI 1640 medium containing 1% penicillin/streptomycin 100 units per mL, 1% L-glutamine (2 mM), and 10% heat-inactivated FBS. Every 72 hours of incubation at 37 °C and 5% CO2, the cells were detached from a 75 cm2 flask using trypsin–EDTA, and 5 × 105 cells were cultured in a new flask with fresh medium.2.4 H. pylori culture
The H. pylori 26695 cagA+ strain was purchased from ATCC (ATCC 700392™, Virginia, USA), while the H. pylori cagA− strain (named H. pylori #6) was obtained from Sant'Orsola Hospital (Sant'Orsola Hospital, Bologna, Italy). Both strains were characterized in terms of virulence, antibiotic resistance, and their ability to induce infection and inflammation in the GES-1 cell line, as described in Martinelli et al.27 In brief, both strains are resistant to clarithromycin and metronidazole, while they are sensitive to levofloxacin and amoxicillin. H. pylori 26695 cagA+ strain activates a broad spectrum of inflammatory genes in GES-1 cells, including the NF-κB pathway. Conversely, the pro-inflammatory effect of H. pylori #6 is NF-κB-independent. Both strains were cultured on agar–sheep blood Petri dishes at 37 °C and 100% humidity, under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) obtained by GasPak™ generators (BD, USA). After 72 hours, the bacteria were collected in 1× PBS using a loop and quantified by optical density (O.D.) at 600 nm. A value of O.D. = 5 corresponds to 2 × 108 bacteria. The bacteria were preserved in a medium containing 50% sheep blood, 30% Brucella broth, and 20% glycerol.2.5 Cell treatment
Cells within 20 and 40 sub-culture passages were seeded in 24-well plates at a density of 3 × 104 cells per well and treated after 72 hours of incubation at 37 °C and under 5% CO2. The day before the treatment, a serum starvation process was performed, using an RPMI medium with 0.5% FBS, 1% L-glutamine and 1% penicillin/streptomycin solution. Cells were challenged with TNFα (10 ng mL−1) or infected with H. pylori (MOI of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, cell
50, cell![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bacteria) for 1 or 6 hours depending on the biological assay and co-treated with strawberry extract (range: 0.1–100 μg mL−1, previously dissolved in 50
bacteria) for 1 or 6 hours depending on the biological assay and co-treated with strawberry extract (range: 0.1–100 μg mL−1, previously dissolved in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 H2O
50 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO at a concentration of 50 mg mL−1) and pure ellagitannins (range 0.1–50 μM, previously dissolved in DMSO at a concentration of 10 mM). Stocks of compounds under investigation were aliquoted and stored at −20 °C until the day of treatment. The treatments were conducted using serum- and antibiotic-free medium. EGCG (20 or 50 μM) and apigenin (50 μM) were used as reference anti-inflammatory compounds, as previously published.32 The plates were maintained under an aerobic atmosphere at 37 °C and under 5% CO2 during the treatment.
DMSO at a concentration of 50 mg mL−1) and pure ellagitannins (range 0.1–50 μM, previously dissolved in DMSO at a concentration of 10 mM). Stocks of compounds under investigation were aliquoted and stored at −20 °C until the day of treatment. The treatments were conducted using serum- and antibiotic-free medium. EGCG (20 or 50 μM) and apigenin (50 μM) were used as reference anti-inflammatory compounds, as previously published.32 The plates were maintained under an aerobic atmosphere at 37 °C and under 5% CO2 during the treatment.
2.6 Cell viability
Cell viability was measured after 6 hours of co-treatment with the extract and ellagitannins using a 3,4,5-dimethylthiazol-2-yl-2-5-diphenyltetrazolium bromide (MTT) assay, as previously described.24 Briefly, the medium was discarded after checking its morphological integrity using a light microscope, and 200 μL of MTT solution (0.1 mg mL−1 dissolved in 1× PBS) was added to each well. After incubating for 30 minutes at 37 °C in the absence of light, the MTT solution was discarded and 200 μL of isopropanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO, 90
DMSO, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v, solution was added. The absorbance was then read at 590 nm (Victor™ X3, PerkinElmer, Waltham, MA, USA). Data were calculated as % viability with respect to an unstimulated control, which was arbitrarily assigned the value of 100%. The extract and the ellagitannins, agrimoniin and casuarictin, were found to have no toxic effect on GES-1 cell viability at the concentration used (see Fig. S1 and S2†). The interference of the vehicle (DMSO) with cell viability, either human or bacterial, was excluded within the same experiments.
10 v/v, solution was added. The absorbance was then read at 590 nm (Victor™ X3, PerkinElmer, Waltham, MA, USA). Data were calculated as % viability with respect to an unstimulated control, which was arbitrarily assigned the value of 100%. The extract and the ellagitannins, agrimoniin and casuarictin, were found to have no toxic effect on GES-1 cell viability at the concentration used (see Fig. S1 and S2†). The interference of the vehicle (DMSO) with cell viability, either human or bacterial, was excluded within the same experiments.
2.7 Measurement of NF-κB activation
NF-κB activation was measured through plasmid driven transcription after stimulation with TNFα (10 ng mL−1), and through immunofluorescence techniques when cells were infected with H. pylori (MOI of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, cell
50, cell![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bacteria). Our group previously demonstrated that the H. pylori 26695 cagA+ strain was able to induce the translocation of the p-65 subunit into cell nuclei, contrary to the cagA− strain.27
bacteria). Our group previously demonstrated that the H. pylori 26695 cagA+ strain was able to induce the translocation of the p-65 subunit into cell nuclei, contrary to the cagA− strain.27
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 v/v, overnight at 4 °C. After three washes with 1× PBS, the cells were incubated with a secondary antibody (anti-rabbit IgG conjugated with Alexa Fluor 647, #4414) diluted at 1
400 v/v, overnight at 4 °C. After three washes with 1× PBS, the cells were incubated with a secondary antibody (anti-rabbit IgG conjugated with Alexa Fluor 647, #4414) diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 v/v. A 1
1000 v/v. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dilution of the ActinRed™ reagent in 1× PBS was added 30 minutes before the end of the incubation period. After 2 hours, the coverslips were washed three times with 1× PBS and mounted on slides with a decrease in DAPI (ProLong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole, #8961). The samples were then imaged using a confocal laser scanning microscope (LSM 900, Zeiss, Oberkochen, Germany).
5 dilution of the ActinRed™ reagent in 1× PBS was added 30 minutes before the end of the incubation period. After 2 hours, the coverslips were washed three times with 1× PBS and mounted on slides with a decrease in DAPI (ProLong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole, #8961). The samples were then imaged using a confocal laser scanning microscope (LSM 900, Zeiss, Oberkochen, Germany).2.8 Measurement of IL-8, IL-6 and MMP-9 release
IL-8, IL-6, and MMP-9 released in the medium were quantified by an ELISA assay after 6 hours of cell treatment with TNFα or H. pylori strains and extract or ellagitannins, following the manufacturer's instructions as previously reported.27 The absorbance of the samples was measured at 405 nm (Victor™ X3, PerkinElmer, Waltham, MA, USA) at the end of the assay, and compared with a standard curve made with human recombinant IL-8 (0–1000 pg mL−1), IL-6 (0–1500 pg mL−1), and MMP-9 (0–6000 pg mL−1).2.9 Antibacterial activity
The minimum inhibitory concentration (MIC) assay was used to measure the antibacterial activity of the extract and ellagitannins against both H. pylori strains, following the method previously described by Piazza et al.24 Briefly, the extract and the ellagitannins were diluted in Brucella broth supplemented with 5% FBS, and then 100 μL was added to a U-bottom plate. After performing a serial dilution, 100 μL of the bacterial suspension with O.D. = 0.1 (4 × 106 cells) was added, thus obtaining a final volume of 200 μL. The plate was incubated under static conditions, for 72 hours at 37 °C under a microaerophilic environment (5% O2, 10% CO2, and 85% N2), and was read at 600 nm (Victor™ X3, PerkinElmer, Waltham, MA, USA). Data were calculated as % viability with respect to the untreated bacterium, which was arbitrarily assigned the value of 100%. Tetracycline was used as a reference antibiotic compound (MIC = 0.125 μg mL−1).2.10 Statistical analysis
All biological results are expressed as the mean ± SEM of three independent experiments. Data were analysed by an unpaired ANOVA test followed by Bonferroni post-hoc analysis. Statistical evaluation and IC50 calculation were performed using GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). Values at p < 0.05 were considered statistically significant.3. Results
3.1 Anti-inflammatory activity of Fragaria × ananassa Duch. extract and ellagitannins in GES-1 cells challenged with TNFα
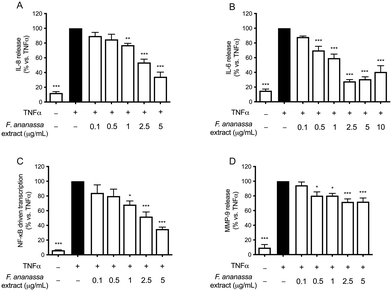
Initially, we tested strawberry tannins on TNFα-treated GES-1 cells, measuring IL-8, IL-6, MMP-9 secretion and NF-κB activity to assess anti-inflammatory effects.The strawberry extract inhibited the secretion of IL-8 and IL-6 in a concentration-dependent manner in the TNFα-treated GES-1 cells (Fig. 1A and B, respectively), with IC50 values of 2.09 μg mL−1 and 0.89 μg mL−1, respectively. Furthermore, the extract inhibited the NF-κB driven transcription (Fig. 1C) with an IC50 value of 2.15 μg mL−1, confirming the impairment of the release of these cytokines via the NF-κB pathway, especially for IL-8 release. The IC50 values for MMP-9 secretion could not be determined based on the tested extract concentrations (highest concentration tested: 5 μg mL−1); however, a slight significant inhibition was observed at higher concentrations (Fig. 1D).
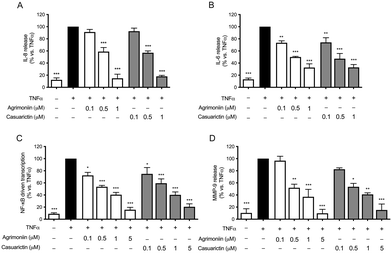
Pure ellagitannins were equally effective in inhibiting all inflammatory parameters assessed in TNFα-treated GES-1 cells. Agrimoniin and casuarictin reduced the secretion of the cytokines IL-8 and IL-6 (Fig. 2A and B, respectively), with lower IC50 values for the inhibition of IL-6 release, confirming the results observed in the strawberry extract. The ellagitannins inhibited NF-κB driven transcription (Fig. 2C), with IC50 values of 0.39 μM and 0.49 μM for agrimoniin and casuarictin, respectively. These results demonstrated that IL-8 release is mostly dependent on NF-κB activation, and both the extract and pure ellagitannins inhibited IL-8 release in an NF-κB dependent manner. The pure molecules also inhibited the MMP-9 release (Fig. 2D), with IC50 values of 0.50 μM and 0.47 μM for agrimoniin and casuarictin, respectively.
For all inflammatory parameters induced by TNFα in GES-1 cells, the IC50 values of the extract and the pure molecules are reported in Table 1.
| IL-8 release | IL-6 release | MMP-9 release | NF-κB driven transcription | |||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μg mL−1) | CI (95%) | IC50 (μg mL−1) | CI (95%) | IC50 (μg mL−1) | CI (95%) | IC50 (μg mL−1) | CI (95%) | |
| Strawberry extract | 2.09 | 1.67 to 2.63 | 0.89 | 0.60 to 1.32 | >5a | — | 2.15 | 1.31 to 3.51 |
| IC50 (μM) | CI (95%) | IC50 (μM) | CI (95%) | IC50 (μM) | CI (95%) | IC50 (μM) | CI (95%) | |
|---|---|---|---|---|---|---|---|---|
| IC50: half-maximal inhibitory concentration, CI (95%): confidence interval 95%.a 5 μg mL−1 is the maximum concentration tested. | ||||||||
| Agrimoniin | 0.51 | 0.45 to 0.58 | 0.28 | 0.19 to 0.42 | 0.50 | 0.25 to 0.87 | 0.39 | 0.27 to 0.55 |
| Casuarictin | 0.50 | 0.44 to 0.57 | 0.28 | 0.16 to 0.48 | 0.47 | 0.25 to 0.87 | 0.49 | 0.28 to 0.83 |
3.2 Anti-inflammatory activity of the Fragaria × ananassa Duch. extract and ellagitannins in GES-1 cells infected with two different strains of H. pylori
Having observed the anti-inflammatory effects of strawberry tannins against TNFα, we tested their impact on IL-8, IL-6, and NF-κB in GES-1 cells infected with cagA+ or cagA− H. pylori strains.The strawberry extract inhibited in a concentration-dependent manner the release of cytokines IL-8 and IL-6 induced by H. pylori-infected GES-1 cells. This effect was observed regardless of the bacterial strain evaluated, and the IC50 range of 28–68 μg mL−1 can be considered easily achievable in vivo.
The extract inhibited the release of IL-8 more effectively than IL-6 release, as evident from the IC50 values of 38.81 μg mL−1 and 28.83 μg mL−1 for the cagA+ and cagA− strains, respectively (Fig. 3A and B, respectively), with respect to the IC50 values measured for the latter, namely 68.64 μg mL−1 and 63.08 μg mL−1 for the cagA+ and cagA− strains, respectively (Fig. 3C and D, respectively). These results were in contrast to those observed before in the TNFα-treated GES-1 cells, where the impact of the extract on IL-6 was more pronounced (see Fig. 1 and Table 1). However, the results may reflect the major relevance of IL-8 impairment during H. pylori infection.
In our previous experiments, agrimoniin and casuarictin had similar inhibitory activities in IL-8 and IL-6 release in TNFα-treated GES-1 cells (see Fig. 2 and Table 1). In H. pylori infection, agrimoniin and casuarictin inhibited IL-8 release with comparable IC50 values. Agrimoniin showed greater activity than casuarictin, with IC50 values of 10.04 μM and 6.57 μM for the cagA+ and cagA− strains, respectively (Fig. 4A and B).
Casuarictin had IC50 values around 16 μM in both strains. Agrimoniin also inhibited IL-6 release, more effectively in the cagA− strain (Fig. 4C and D). Casuarictin demonstrated lower activity on IL-6, with significant inhibition only at 50 μM in cagA+ and inactivity in cagA−.
For the inflammatory parameters induced by H. pylori strains in GES-1 cells, the IC50 values of the extract and the pure molecules are reported in Table 2. To gain further insight into the mechanisms of action of strawberry tannins during cagA+ H. pylori infection, it was hypothesized that they might impair the NF-κB pathway. In this study, only the cagA+ H. pylori strain was used to investigate the activation and translocation of the p-65 subunit inside the GES-1 cell nuclei.
| H. pylori strain | IL-8 release | IL-6 release | |||
|---|---|---|---|---|---|
| IC50 (μg mL−1) | CI (95%) | IC50 (μg mL−1) | CI (95%) | ||
| Strawberry extract | cagA+ 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 695 |
38.81 | 31.55 to 47.74 | 68.64 | 31.62 to 149.0 |
| cagA− #6 | 28.83 | 22.01 to 37.76 | 63.08 | 44.45 to 89.52 | |
| IC50 (μM) | CI (95%) | IC50 (μM) | CI (95%) | ||
|---|---|---|---|---|---|
| IC50: half-maximal inhibitory concentration, CI (95%): confidence interval 95%.a 50 μM is the maximum concentration tested. | |||||
| Agrimoniin | cagA+ 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 695 |
10.04 | 8.15 to 12.37 | 32.36 | 21.88 to 47.87 |
| cagA− #6 | 6.57 | 5.42 to 7.97 | 7.22 | 1.92 to 27.08 | |
| Casuarictin | cagA+ 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 695 |
16.64 | 13.43 to 20.62 | 49.77 | 35.08 to 70.60 |
| cagA− #6 | 16.3 | 12.87 to 20.63 | Not activea | Not activea | |
The strawberry extract at 100 μg mL−1, the highest concentration that inhibits IL-8 release, was investigated for NF-κB pathway impairment. Agrimoniin and casuarictin at 5 μM, reflecting their proportion in the extract, were also tested. As shown in Fig. 5, these treatments inhibited the translocation of the p-65 subunit into the nuclei of H. pylori-infected GES-1 cells, corroborating the involvement of the NF-κB pathway in the anti-inflammatory activities.
3.3 Anti-bacterial activity of Fragaria × ananassa Duch. extract and pure ellagitannins, agrimoniin and casuarictin
In human gastric epithelial cells, strawberry extract and ellagitannins inhibited selected inflammatory mediators induced by H. pylori infection. The anti-inflammatory activity varied depending on the bacterial virulence, as shown in Table 2. We investigated whether the strawberry extract and pure ellagitannins could inhibit the growth of the two H. pylori strains and whether their antibacterial activity was affected by virulence factors.Our experiments confirm the antibacterial activities of strawberry and ellagitannins against our cagA+ H. pylori strain. The extract showed a MIC of 125 μg mL−1, while agrimoniin and casuarictin had MICs of 25 μM and 50 μM, respectively (Fig. 6A and C). Against the cagA− strain, the extract and agrimoniin were only active at the highest concentrations tested (500 μg mL−1 and 100 μM), whereas casuarictin showed no activity (Fig. 6B and D). This indicates that the antibacterial activity may be influenced by bacterial virulence.
4. Discussion
Fragaria × ananassa is one of the most consumed fruits in Europe; it has been studied for health benefits and preventive effects on inflammation, oxidative stress, cardiovascular disease, obesity, type 2 diabetes, and neurodegenerative diseases.16 Beyond vitamins and minerals, strawberries are rich in polyphenols, including flavonoids, phenolic acids, and tannins.16 The tannin content, particularly the ellagitannins agrimoniin and casuarictin, has been characterised.17,28,31 A tannin-enriched strawberry extract, with anthocyanins removed, containing 4.29% agrimoniin and 4.65% casuarictin, was previously shown to counteract inflammation in AGS cells challenged with TNFα, by dampening the NF-κB pathway at nutritionally relevant concentrations (1–10 μg mL−1).17 Recently, GES-1 cells, a non-tumoral gastric epithelial model, were characterized and compared to the AGS cell line.27 The present study employs this model to study the anti-inflammatory effects of a tannin-enriched strawberry extract and pure ellagitannins. The extract demonstrated a concentration-dependent inhibitory effect on the secretion of IL-8 and IL-6 in TNFα-treated GES-1 cells, with IC50 values of approximately 2.0 μg mL−1 and 0.90 μg mL−1, respectively. Furthermore, the NF-κB-driven transcription was inhibited with IC50 values comparable to those found active on IL-8 secretion, indicating that the mechanism of action may be ascribed to the impairment of this pathway. In contrast, the secretion of IL-6 was inhibited at a lower concentration, potentially due to the involvement of additional pathways beyond NF-κB-mediated IL-6 secretion (Table 1 and Fig. 1). Similarly, agrimoniin and casuarictin exhibited comparable inhibition patterns on IL-8, MMP-9, and NF-κB-driven transcription (IC50 values around 0.5 μM), and inhibited IL-6 secretion at lower concentrations (Table 1 and Fig. 2).We then employed the same model (GES-1) infected with two distinct strains of H. pylori, one cagA+ and one cagA−, to assess the anti-inflammatory and anti-bacterial activities of strawberry tannins. The cagA gene is a bacterial virulence factor that plays a pivotal role in the development of inflammation, and the subsequent probability of gastric cancer.4,12 The strawberry extract demonstrated the capacity to inhibit the release of cytokines IL-8 and IL-6 by H. pylori-infected GES-1 cells in a concentration-dependent manner, regardless of strain virulence (the IC50 values are indicated in Table 2). It also inhibited the NF-κB pathway at 100 μg mL−1, corroborating results with TNFα challenge. However, agrimoniin and casuarictin exhibited different activities depending on the strain. Both inhibited IL-8 release in both strains (IC50 value: 10–15 μM) and NF-κB activation induced by cagA+ H. pylori27 at 5 μM, which reflects the percentage of pure molecules present in the strawberry extract. This suggests that ellagitannins may partly account for pathway inhibition (Fig. 5). Agrimoniin was the only compound inhibiting IL-6 release in both strains. Casuarictin slightly inhibited IL-6 secretion in cagA+ infection, but was inactive in cagA− infection (Fig. 4), indicating that it may not affect inflammation activated by cagA− bacteria.
Overall, in TNFα-stimulated GES-1 cells, which predominantly activate NF-κB, both ellagitannins exhibit similar anti-inflammatory profiles. However, in H. pylori infection, which triggers a more complex response, agrimoniin displays a more comprehensive profile by inhibiting inflammation independently of cagA presence.
Previous studies have demonstrated the anti-bacterial activity of certain berries, including strawberries, and certain ellagitannins against H. pylori, with MIC values ranging from 6.5 μM to 50 μM, but using different strains than ours.21,33 We confirmed antibacterial activity against the cagA+ strain by the strawberry extract (MIC: 125 μg mL−1) and ellagitannins (MICs: 25 μM for agrimoniin and 50 μM for casuarictin). Against the cagA− strain, the extract and agrimoniin were active only at the highest concentration tested; casuarictin was ineffective (Fig. 6).
5. Conclusions
In conclusion, these results suggest that the antibacterial activity of strawberry extract and ellagitannins may depend on H. pylori strain virulence. They may be employed in addition to eradication therapy to control virulent H. pylori strains and the related inflammation, at concentrations achievable in the stomach.This work reports, for the first time, an evaluation of the anti-inflammatory activity of Fragaria × ananassa in the context of two different H. pylori infections. Furthermore, the results confirm the extract's antibacterial activity, more pronounced against the virulent bacterial strain (cagA+). Thus, strawberries may be considered a potential natural product to complement eradication therapy, controlling bacterial infection and inflammation at the nutritional and pharmacological levels. The activities observed can be partly attributed to the ellagitannins agrimoniin and casuarictin. Future studies will focus on evaluating the effects of strawberry extract and its key ellagitannins in more complex systems, such as gastric organoids and in vivo models of H. pylori infection. These approaches will help to better understand their potential translational application in gastrointestinal health.
Abbreviations
| AGS | Human gastric adenocarcinoma epithelial cell line |
| ANOVA | Analysis of variance |
| ATCC | American Type Culture Collection |
| cagA | Cytotoxin-associated gene A |
| cagPAI | Cytotoxin-associated gene pathogenicity island |
| CFSE | Carboxy fluorescein succinimidyl ester |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| EGCG | Epigallocatechin-3-gallate |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| GES-1 | Non-tumoral human gastric epithelial cell line immortalized by SV-40 |
| H. pylori | Helicobacter pylori |
| IC50 | Half maximal inhibitory concentration |
| IL | Interleukin |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| MAPK | Mitogen-activated protein kinase |
| MIC | Minimum inhibitory concentration |
| MMP-9 | Matrix metalloproteinase-9 |
| MOI | Multiplicity of infection |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| O.D. | Optical density |
| RPMI | Roswell Park Memorial Institute (medium) |
| SEM | Standard error of the mean |
| STAT3 | Signal transducer and activator of transcription 3 |
| SV-40 | Simian virus 40 |
| T4SS | Type IV secretion system |
| TNFα | Tumor necrosis factor alpha |
Author contributions
G. M.: conceptualization, investigation, formal analysis, and writing – original draft preparation; S. P.: investigation, formal analysis, data curation, and writing – review & editing; M. F.: data curation and methodology; N. M.: investigation and formal analysis; C. P.: investigation; E. S. O.: investigation; S. M. E. H.: investigation; U. V.: investigation and methodology; F. M.: investigation and methodology; E. S. A.: conceptualization, supervision, and writing – review & editing; E. D. F.: conceptualization and writing – review & editing; and M. D. A.: conceptualization, supervision, writing – original draft preparation, funding acquisition, and project administration.Data availability
The data supporting this article have been included as part of the paper and/or the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank Dr Dawit Kidane-Mulat (Howard University, College of Medicine, Washington, DC, USA) for providing the GES-1 cells. This research was supported by grants from MIUR "Progetto Eccellenza" 2023-2027.References
- S. Suerbaum and P. Michetti, Helicobacter pylori infection, N. Engl. J. Med., 2002, 347, 1175–1186 CrossRef CAS PubMed.
- K. Robinson, R. Kenefeck, E. L. Pidgeon, S. Shakib, S. Patel, R. J. Polson, A. M. Zaitoun and J. C. Atherton, Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses, Gut, 2008, 57, 1375–1385 CrossRef CAS PubMed.
- K. W. Cook, D. P. Letley, R. J. Ingram, E. Staples, H. Skjoldmose, J. C. Atherton and K. Robinson, CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa, Gut, 2014, 63, 1550–1559 CrossRef CAS PubMed.
- S. Backert, R. Haas, M. Gerhard and M. Naumann, The Helicobacter pylori type IV secretion system encoded by the cag pathogenicity island: Architecture, function, and signaling, Curr. Top. Microbiol. Immunol., 2017, 413, 187–220 CAS.
- S. Z. Ding, J. B. Goldberg and M. Hatakeyama, Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis, Future Oncol., 2010, 6, 851–862 CrossRef CAS PubMed.
- A. Lamb and L. F. Chen, Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer, J. Cell. Biochem., 2013, 114, 491–497 CrossRef CAS PubMed.
- J. E. Crabtree, J. I. Wyatt, L. K. Trejdosiewicz, P. Peichl, P. H. Nichols, N. Ramsay, J. N. Primrose and I. J. Lindley, Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa, J. Clin. Pathol., 1994, 47, 61–66 CrossRef CAS.
- S. A. Sharma, M. K. Tummuru, M. J. Blaser and L. D. Kerr, Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells, J. Immunol., 1998, 160, 2401–2407 CrossRef CAS.
- C. A. Fallone, S. F. Moss and P. Malfertheiner, Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics, Gastroenterology, 2019, 157(1), 44–53 CrossRef PubMed.
- A. C. Ford, Y. Yuan and P. Moayyedi, Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis, Gut, 2020, 69(12), 2113–2121 CrossRef.
- J. M. Liou, X. T. Jiang, C. C. Chen, J. C. Luo, M. J. Bair, P. Y. Chen, C. K. Chou, Y. J. Fang, M. J. Chen, C. C. Chen, J. Y. Lee, T. H. Yang, C. C. Yu, C. C. Kuo, M. C. Chiu, C. Y. Chen, C. T. Shun, W. H. Hu, M. H. Tsai, Y. C. Hsu, C. H. Tseng, C. Y. Chang, J. T. Lin, E. M. El-Omar and M. S. Wu, Taiwan Gastrointestinal Disease and Helicobacter Consortium, Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: A multicentre, open-label, randomised controlled trial., Lancet Gastroenterol. Hepatol., 2023, 8(3), 228–241 CrossRef CAS.
- P. Malfertheiner, M. C. Camargo, E. El-Omar, J. M. Liou, R. Peek, C. Schulz, S. I. Smith and S. Suerbaum, Helicobacter pylori infection, Nat. Rev. Dis. Primers, 2023, 9(1), 19 CrossRef PubMed.
- C. Sousa, R. Ferreira, N. F. Azevedo, M. Oleastro, J. Azeredo, C. Figueiredo and L. D. R. Melo, Helicobacter pylori infection: From standard to alternative treatment strategies., Crit. Rev. Microbiol., 2022, 48(3), 376–396 CrossRef CAS.
- E. Pastene, V. Parada, M. Avello, A. Ruiz and A. Garcia, Catechin-based procyanidins from Peumus boldus Mol. aqueous extract inhibit Helicobacter pylori urease and adherence to adenocarcinoma gastric cells, Phytother. Res., 2014, 28, 1637–1645 CrossRef CAS PubMed.
- K. M. Lee, M. Yeo, J. S. Choue, J. H. Jin, S. J. Park, J. Y. Cheong, K. J. Lee, J. H. Kim and K. B. Hahm, Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling, Helicobacter, 2004, 9, 632–642 CrossRef CAS PubMed.
- F. Giampieri, S. Tulipani, J. M. Alvarez-Suarez, J. L. Quiles, B. Mezzetti and M. Battino, The strawberry: Composition, nutritional quality, and impact on human health, Nutrition, 2012, 28, 9–19 CrossRef CAS PubMed.
- M. Fumagalli, E. Sangiovanni, U. Vrhovsek, S. Piazza, E. Colombo, M. Gasperotti, F. Mattivi, E. De Fabiani and M. Dell'Agli, Strawberry tannins inhibit IL-8 secretion in a cell model of gastric inflammation., Pharmacol. Res., 2016, 111, 703–712 CrossRef CAS PubMed.
- F. A. Tomás-Barberán, A. González-Sarrías, R. García-Villalba, M. A. Núñez-Sánchez, M. V. Selma, M. T. García-Conesa and J. C. Espín, Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status, Mol. Nutr. Food Res., 2017, 61(1) DOI:10.1002/mnfr.201500901.
- D. M. Grochowski, K. Skalicka-Woźniak, I. E. Orhan, J. Xiao, M. Locatelli, J. P. Piwowarski, S. Granica and M. Tomczyk, A comprehensive review of agrimoniin, Ann. N. Y. Acad. Sci., 2017, 1401, 166–180 CrossRef CAS PubMed.
- E. Sangiovanni, U. Vrhovsek, G. Rossoni, E. Colombo, C. Brunelli, L. Brembati, S. Trivulzio, M. Gasperotti, F. Mattivi, E. Bosisio and M. Dell'Agli, Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies, PLoS One, 2013, 8, e71762 CrossRef CAS PubMed.
- K. Funatogawa, S. Hayashi, H. Shimomura, T. Yoshida, T. Hatano, H. Ito and Y. Hirai, Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori, Microbiol. Immunol., 2004, 48(4), 251–261 CrossRef CAS PubMed.
- O. Golovinskaia and C. K. Wang, Review of functional and pharmacological activities of berries, Molecules, 2021, 26(13), 3904 CrossRef CAS PubMed.
- S. Piazza, M. Fumagalli, S. Khalilpour, G. Martinelli, A. Magnavacca, M. Dell’Agli and E. Sangiovanni, A review of the potential benefits of plants producing berries in skin disorders, Antioxidants, 2020, 9(6), 542 CrossRef CAS PubMed.
- S. Piazza, G. Martinelli, M. Fumagalli, C. Pozzoli, N. Maranta, F. Giavarini, L. Colombo, G. Nicotra, S. F. Vicentini, F. Genova, E. De Fabiani, E. Sangiovanni and M. Dell'Agli, Ellagitannins from Castanea sativa Mill. leaf extracts impair H. pylori viability and infection-induced inflammation in human gastric epithelial cells, Nutrients, 2023, 15(6), 1504 CrossRef CAS PubMed.
- J. M. Alvarez-Suarez, D. Dekanski, S. Ristić, N. V. Radonjić, N. D. Petronijević, F. Giampieri, P. Astolfi, A. M. González-Paramás, C. Santos-Buelga, S. Tulipani, J. L. Quiles, B. Mezzetti and M. Battino, Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase, PLoS One, 2011, 6, e25878 CrossRef CAS PubMed.
- F. Giampieri, T. Y. Forbes-Hernandez, M. Gasparrini, S. Afrin, D. Cianciosi, P. Reboredo-Rodriguez, A. Varela-Lopez, J. L. Quiles, B. Mezzetti and M. Battino, The healthy effects of strawberry bioactive compounds on molecular pathways related to chronic diseases, Ann. N. Y. Acad. Sci., 2017, 1398, 62–71 CrossRef CAS PubMed.
- G. Martinelli, M. Fumagalli, S. Piazza, N. Maranta, F. Genova, P. Sperandeo, E. Sangiovanni, A. Polissi, M. Dell'Agli and E. De Fabiani, Investigating the molecular mechanisms underlying early response to inflammation and Helicobacter pylori infection in human gastric epithelial cells, Int. J. Mol. Sci., 2023, 24(20), 15147 CrossRef CAS PubMed.
- M. Gasperotti, D. Masuero, G. Guella, L. Palmieri, P. Martinatti, E. Pojer, F. Mattivi and U. Vrhovsek, Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp, J. Agric. Food Chem., 2013, 61, 8597–8607 CrossRef CAS.
- M. Gasperotti, D. Masuero, F. Mattivi and U. Vrhovsek, Overall dietary polyphenol intake in a bowl of strawberries: The influence of Fragaria spp in nutritional studies, J. Funct. Foods, 2015, 18, 1057–1069 CrossRef CAS.
- M. Gasperotti, D. Masuero, U. Vrhovsek, G. Guella and F. Mattivi, Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis, J. Agric. Food Chem., 2010, 58(8), 4602–4616 CrossRef CAS PubMed.
- U. Vrhovsek, G. Guella, M. Gasperotti, E. Pojer, M. Zancato and F. Mattivi, Clarifying the identity of the main ellagitannin in the fruit of the strawberry, Fragaria vesca and Fragaria ananassa Duch, J. Agric. Food Chem., 2012, 60, 2507–2516 CrossRef CAS PubMed.
- M. Bottoni, G. Martinelli, N. Maranta, E. Sabato, F. Milani, L. Colombo, P. S. Colombo, S. Piazza, E. Sangiovanni, C. Giuliani, P. Bruschi, G. Vistoli, M. Dell'Agli and G. Fico, From primary data to ethnopharmacological investigations on Achillea erba-rotta subsp. moschata (Wulfen) I.Richardson as a remedy against gastric ailments in Valmalenco (Italy), Plants, 2024, 13(4), 539 CrossRef CAS PubMed.
- A. Chatterjee, T. Yasmin, D. Bagchi and S. J. Stohs, Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin, Mol. Cell. Biochem., 2004, 265(1–2), 19–26 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5fo01022e |
| This journal is © The Royal Society of Chemistry 2025 |