Dietary strategies for appetite regulation: satiety and obesity management
Pengkui
Xia
abc,
Bin
Li
 abc,
Yudie
Yu
abc,
Wanxu
Yu
abc,
Mahmoud
Youssef
abc,
Yudie
Yu
abc,
Wanxu
Yu
abc,
Mahmoud
Youssef
 ad,
Tao
Hou
ad,
Tao
Hou
 *abc and
Jing
Li
*abc and
Jing
Li
 *abc
*abc
aCollege of Food Science and Technology, Huazhong Agricultural University, Wuhan, 430070, China. E-mail: houtao@mail.hzau.edu.cn; lijingfood@mail.hzau.edu.cn
bShenzhen Institute of Nutrition and Health, Huazhong Agricultural University, Shenzhen, 518000, China
cShenzhen Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Genome Analysis Laboratory of the Ministry of Agriculture, Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen, 518000, China
dFood Science and Technology Department, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt
First published on 17th June 2025
Abstract
Increased prevalence of diseases associated with obesity has driven research into appetite suppression to reduce high-calorie intake. Dietary modulation of appetite is recognized as one of the most significant and effective ways to reduce the risk of obesity-related diseases. This review evaluates the roles of dietary nutrients and their metabolites in satiety and proposes dietary strategies for appetite regulation. Brain circuits of hunger, hormones and organs that directly control the appetite, and the role of gut microbiota in indirect appetite modulation are discussed in detail. We explored the impact of dietary nutrients and their metabolites on appetite, based on the basic mechanics of hunger. Additionally, based on the impact of different dietary factors on satiety, we outlined three strategies for appetite regulation: systems for controlled nutrient delivery to decelerate digestion, alteration of dietary physicochemical characteristics, and establishment of dietary rhythms. This review presents a theoretical framework for examining the influence of dietary nutrition on appetite regulation.
1. Introduction
Overweight and obesity are significant risk factors for non-communicable diseases and have escalated into global concerns with their prevalence increasing dramatically.1 In 2022, approximately 43% of adults globally were classified as overweight, while 16% were deemed obese, a figure that had more than doubled since 1990.2 The health consequences linked to obesity and overweight are substantial, resulting in about 2.8 million fatalities each year and 35.8 million disability-adjusted life years. Moreover, they are serious risk factors for several non-communicable diseases, including hypertension, diabetes, stroke, and other chronic conditions.2 Individuals with obesity encounter societal stigma and discrimination, leading to mental health issues that prompt them to seek pharmaceutical and surgical weight loss treatments.3 Nevertheless, the negative consequences of pharmaceutical and surgical weight loss methods have caused some consumers to be reluctant to use them, and accordingly, the appeal of nutritional approaches that diminish appetite and improve fullness has augmented.Appetite, in the absolute sense, is described as the desire to feed. The hunger appetite neural pathways are activated or inhibited by key nutrients, water and sodium.4 Hunger and appetite regulate host's energy balance, satiety (the feeling of fullness that persists after eating), and food intake behavior and are closely related to body fat accumulation and obesity.5,6 Obesity induced by overeating is a burden on overall health. Compared with that in thin people, the response to the nutrient infusion is significantly reduced in the obese, and the response to peptide YY (PYY), pancreatic polypeptide, glucose inhibitory peptide, and glucagon-like peptide-1 (GLP-1) is significantly delayed.7 The combined intake of fat and sugar is the main factor contributing to hyperphagia, and it contributes to the development of obesity. A high-fat diet, even for a short term, activates prepronociceptin (PNOC)-expressing neurons (PNOCARC) in the arcuate nucleus of the hypothalamus (ARC), which is an area that contains multiple neurons involved in maintaining energy balance, causing overeating.8 A high-sugar or high-fat food intake triggers the taste neurons to desire more, improves pleasure and stimulates the intake of sugar and fat.9,10 Both high-sugar and high-fat diets reversibly dampen the rapid agouti-related protein (AgRP) neuron inhibition following food presentation and promote the intake of more food, exacerbating obesity.11 Conversely, obesity leads to increased food desire. In obese individuals, visceral fat accumulates under the influence of external nutrients. Adipocyte O-linked beta-D-N-acetylglucosamine (O-GlcNAc) transferase (OGT) stimulates hyperphagia by the transcriptional activation of de-novo lipid desaturation and the accumulation of N-arachidonyl ethanolamine (an endogenous appetite-inducing cannabinoid).12 Meanwhile, adipose OGT overexpression inhibits lipolysis and exacerbates diet-induced obesity.13 High-fat diets can rapidly upregulate the gene expression of uncoupling protein 2 (Ucp2) in the microglia and alter mitochondrial function and morphology, while specific Ucp2 knockouts in microglia reduce feeding and resist obesity via changes in synaptic input organization and activation of the POMC neurons and astrogliosis.14 Thus, reasonable management of hunger (appetite) is important for obesity regulation.
At present, various appetite-influencing factors controlling food intake, such as emotion, age, exercise, genes, environment, and nutrients, are investigated.15–17 Undoubtedly, dietary intervention is the direct way to achieve satiety. Notably, gut microbiota and metabolites of nutrients, such as γ-aminobutyric acid (GABA), bile acids (BAs), and short-chain fatty acids (SCFAs), also participate in the neural regulation of appetite and host metabolism.18 This review examines the categorization of appetite and its related neuroregulatory pathways, emphasizing the direct influence of prevalent food components on hunger appetite signals and the indirect augmentation of host satiety via compounds generated by the intestinal flora. The functions of gut-secreted factors, such as GLP-1, cholecystokinin (CCK), and PYY, with respect to macronutrient (proteins, carbohydrates, and fats) metabolism are examined. Additionally, the impact of AgRP neurons and associated hypothalamic circuits on appetite regulation is examined. Dietary options designed based on these pathways to enhance appetite regulation are presented, serving as a comprehensive reference for obese patients.
2. Appetite types and related neural pathways
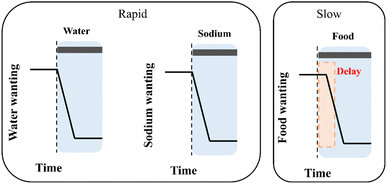
Daily intake of adequate amounts of various nutrients is essential to maintain the stability of the internal environment in the body. Nutritional imbalance is associated with a variety of diseases. For example, a high-sodium diet increases the risk of cognitive and cardiovascular diseases,19,20 water homeostasis disorders induce osmolality-related diseases,21,22 and overfeeding leads to obesity.23 Therefore, optimizing and managing the intake of nutrients is essential for optimal health. Thirst, sodium appetite, and hunger are the main categories related to managing food intake.4 The neural circuits of thirst and sodium appetite act rapidly, and their neural activities can quickly drive and terminate the water and sodium intake behavior,24,25 while the hunger circuit acts more slowly and takes longer to respond to feeding behavior (Fig. 1).26,27 The sensory regulation of appetite circuits relevant to different nutrients (dietary energy, water, and sodium) is governed by other body parts.28,29 These regulatory signals, including chemosensory, hormonal, and gut-brain afferent pathways, play a key role in appetite induction and satiety.2.1 Neuroregulation of thirst
Thirst, sodium appetite, and hunger circuits receive pre-absorption feed-forward satiety signals through multiple sensory systems after nutrient intake. Different internal receptor neurons in the lamina terminalis (LT), solitary-related nucleus, and hypothalamic arcuate nucleus in brain structure regulate thirst, sodium appetite and hunger, respectively.4 The subfornical organ (SFO) and organum vasculosum lamina terminalis (OVLT), situated beyond the blood–brain barrier, are capable of monitoring blood osmotic pressure and detecting various hormonal signals, thereby regulating fluid homeostasis.30 In contrast, the median preoptic nucleus (MnPO) synthesizes information to modulate vasopressin secretion through excitatory neurons that express nitric oxide synthase.31 These three together constitute the LT in the forebrain, stimulating or inhibiting thirst in humans.32 Additionally, the thirst neurons in LT can be rapidly suppressed by liquid gulping signals and gastrointestinal osmolality information.33,34 Therefore, the gut hypotonic responses mediated in vivo by intestinal water injection stimulate the vagal afferents innervating the hepatic portal area (HPA) and regulate thirst and drinking behavior in a very short time.35,362.2 Neuroregulation of sodium appetite
Specific neurons in the nucleus of the solitary tract (NTS) respond to internal sodium depletion through aldosterone and angiotensin.37,38 The stimulation signal is transmitted to the downstream brain region where the pre-locus coeruleus (pre-LC) is located, driving salt intake behavior.39 Meanwhile, increased plasma secretin levels caused by sodium deficiency enter the brain to activate secretin-responsive neurons in NTS, further activating the paraventricular nucleus (PVN) of the hypothalamus and promoting sodium uptake.40 Moreover, sodium depletion activates neurons in the sensory regions of the forebrain LT.41,42 The activation of SFO-prostaglandin E2 and its receptor (Ptger3) neurons could increase the intake of even an aversively high-concentration salt solution.392.3 Neuroregulation of hunger
Animal feeding behavior can be categorized into (1) homeostatic feeding, which is the consumption of food required to sustain normal weight and metabolic function, and (2) hedonic feeding, which is food intake motivated by sensory perception or pleasure.43,44 Hedonic feeding arises from reward and cognitive functions, involves orexins and interactions among dopaminergic, opioidergic and glutamatergic signaling, and can potentially override homeostasis regulation.45,46 Some of the nuclei in the basal part of the hypothalamus are considered key in regulating dietary energy balance and hunger signals.47 ARC and the lateral hypothalamic area (LHA) are the regions associated with appetite signal synthesis and release; PVN is the region of appetite signal interaction, while the suprachiasmatic nucleus (SCN), ventromedial nucleus (VMN), and dorsomedial nucleus (DMN) are regions involved in hunger regulation.48 The neurons that co-express agouti-related peptide and neuropeptide-Y (AgRP/NPY) signal hunger and stimulate food intake, while anorexic pro-opiomelanocortin/cocaine-and amphetamine-related transcript (POMC/CART) neurons signal satiety and reduce food intake (Fig. 2).49 These neural circuits controlling hunger and appetite are modulated by gastrointestinal hormones, including GLP-1, CCK, PYY, and oxyntomodulin (OXM). These hormones exert their effects through the vagus nerve pathway or other hormones released by associated organs, such as the stomach, pancreas, and adipose tissue, which activate specific receptors. The neuronal activity of AgRP is responsive, and its base levels decline rapidly when feeding begins.50 By interacting with melanocortin 4 receptors (MC4R), AgRP can delay chronic feeding response.51,52 The AgRP neurons inhibit proopiomelanocortin (POMC) neurons by the concerted antagonistic action of GABA and NPY while sending collateral projections to many different brain areas corresponding to POMC projections.53 POMC neurons, which are located in the ARC and nucleus tractus solitarius (NTS), are considered the main mediators of satiety signaling, along with CART peptides that inhibit food intake and increase energy expenditure.54 Typically, POMC neurons integrate long-term obesity signals from the hypothalamus and short-term satiation signals from the brain stem, releasing α-melanocyte stimulating hormone (α-MSH) and β-melanocyte stimulating hormone (β-MSH) to activate the expression of melanocortin-3-receptor (MC3R) and MC4R in PVN. It decreases food consumption and enhances energy expenditure, thereby signaling mechanisms that promote thermogenesis and anorexia.55,56 Conversely, the ablation of POMC neurons enhances anxiety-like behavior and increases the risk of obesity.57In addition to these two main appetite-regulating neural pathways, other neurons and glial cells also play an important role in the regulation of feeding. The palatability of food can drive feeding independent of AgRP neurons.58 Obesity leads to the dysregulation of hypothalamic hunger neurons. A high-fat diet attenuates the response of AgRP neurons to an array of other nutritionally relevant stimuli.59 Conversely, activating the ventrolateral periaqueductal gray GABAergic cells can mitigate obesity by restoring the miniature postsynaptic inhibitory currents and calcium responses.60 Inhibition of the non-AGRP GABAergic neurons in the ARC can also reverse leptin-deficient obesity.61 Besides neurons, some non-neuronal cells in the ARC are also involved in the regulation of dietary energy balance, for instance, astrocytes are involved in the transport of nutrients and hormones from the circulation to the brain, glycogen storage, glucose sensing, synaptic plasticity, uptake and metabolism of neurotransmitters.62,63 Glial cells in the ARC reduce ghrelin-induced food intake by regulating extracellular adenosine levels.64,65 Insulin signaling in astrocytes co-control glucose sensing in the central nervous system and systemic glucose metabolism by regulating glucose uptake across the blood–brain barrier and participating in the integration of peripheral satiety signals.66,67 Thus, astrocytes are expected to be a new target for appetite regulation in the future.
3. Appetite and the gut microbiota
The intestine is another organ, besides the brain, involved in appetite regulation. The vagus nerve provides parasympathetic innervation to the gastrointestinal tract, coordinating the complex interactions between the central and peripheral neural control mechanisms.68 Satiety signals from nutrient stimulation or flora metabolism in the gastrointestinal tract are transmitted directly to the brain via the vagus nerve, driving or terminating host feeding behavior.69,70 The gut microbiota influences the communication between the gastrointestinal tract and the brain (gut-brain axis) in a bidirectional manner, which might play a key role in the regulation of energy intake and satiety signaling.71 Intestinal endocrine cells (EEC) sense the gut luminal content and secrete hormones that modulate glucose and lipid metabolism, which ultimately affect satiety. Moreover, metabolites of the intestinal flora significantly affect the function of EEC.72 Nutrient infusion to the colon stimulates immediate bacterial growth, which usually lasts 20 minutes. Bacterial molecules and metabolites that depend on the growth phase are known to regulate the intestinal release of satiety hormones and inhibit feeding behavior.73One of the bacterial proteins, the caseinolytic peptidase B protein homolog (ClpB), which is a conformational antigen-mimetic of α-melanocyte-stimulating hormone (α-MSH), inhibits appetite through direct and indirect mechanisms. The ClpB-like gene function in gut microbiota, which is negatively associated with body mass index, waist circumference, and fat mass, is detected in lower abundance in subjects with obesity.74 However, the injection of Escherichia coli protein in the stationary phase (increased ClpB expression) reduces food intake by activating the ARC of the anorexic arcuate nucleus and the central amygdala.75 Muramyl dipeptide, another peptide produced by intestinal bacteria, can also cross the blood–brain barrier and activate Nod2 in the brain to inhibit the activity of GABaergic neurons directly in the ARC to reduce appetite.76 Intestinal flora is an important factor in mediating host appetite. Significant differences in host feed intake and feeding behavior can be caused by different flora structures.77 The deficiency of intestinal Bacteroides vulgatus and its metabolite pantothenate might lead to dietary sugar preference,78 whereas probiotics administration leads to a positive improvement in feed intake and lipid and glucose metabolism.79
4. Dietary nutrients and appetite regulation
Dietary nutrients play an important role in the process of appetite regulation. Mammals, birds, and insects have different appetite systems for macro-nutrients (carbohydrates, fats and proteins).80 The influence of various nutrients on appetite is summarized in Table 1.| Nutrient | Type | Influence on appetite | Ref. | |
|---|---|---|---|---|
| Protein-associated nutrient | Protein | Whey protein | Slow gastric emptying and altered gut hormone secretion | 87 |
| Casein | Increases satiety but does not affect subsequent energy intake | 88 | ||
| Peptides | Fish protein hydrolysates | Anti-hyperglycemic and satiating effects | 96 | |
| Whey protein-derived peptides | Promote cholecystokinin secretion | 97 and 98 | ||
| Soybean hydrolyzed peptides | Decrease leptin concentration and inhibit cholecystokinin release | 99 and 100 | ||
| D3 | Ameliorates leptin resistance and upregulates the expression of uroguanylin | 133 | ||
| Amino acid | L-Glutamate | Reduces NPF secretion | 105 | |
| Cysteine | Promotes energy expenditure and suppresses food intake | 106 | ||
| BCAAs | Stimulate leptin activity and GLP-1 levels and inhibit ghrelin levels | 109 | ||
| Essential amino acids | Protein deprivation stimulates compensatory appetite for essential amino acids | 111 | ||
| Non-essential amino acids | Reduce the portion size of intake but not the frequency | 112 | ||
| Carbohydrate | Resistant starch | Improves satiety and reduces food intake, but has no significant effect on appetite hormones | 113–116 | |
| Glycyrrhiza polysaccharide | Promotes hedonic eating behavior | 117 | ||
| Ginseng polysaccharide | Promotes hedonic eating behavior | 118 | ||
| Flaxseed polysaccharide | Suppresses appetite | 119 | ||
| Lipid | MUFA | Does not change any measures of appetite | 134 | |
| PUFA | Increases the desire to feed | 128 and 129 | ||
| SFA | Promotes CCK secretion and increases peak PYY levels | 130 and 131 | ||
4.1 Dietary protein and hydrolysates regulate host appetite
4.2 Dietary carbohydrates and appetite regulation
Digestible carbohydrates (mainly starch) are usually absorbed after hydrolysis into glucose. Thus, this section mainly focuses on the effects of indigestible carbohydrates on appetite. By structural modification, starch can be converted to resistant starch with crystalline properties to inhibit digestion by gastrointestinal amylase. The role of resistant starch in insulin sensitivity improvement is well-established. However, the regulation of satiety by resistant starch is still controversial. Some studies have found that resistant starch can improve satiety and reduce food intake.113,114 Meanwhile, other studies have found no significant effect of resistant starch on appetite hormones.115,116 These contradictory results may be related to the type and intake of resistant starch. Moreover, non-starch polysaccharides (NSPs) may have a dual effect on appetite. Some NSPs are known to promote hedonic eating behavior, activate AgRp/NPY and inhibit POMC/CART neural circuits.117,118 Most NSPs suppress appetite via physical effects in the gastrointestinal tract and mechanical and humoral stimulation to secrete satiety hormones.119–121 The targeted regulation of intestinal flora by NSPs to promote changes in the SCFA and BA profiles, is also a potential mechanism of appetite regulation.122,1234.3 Dietary lipids and appetite regulation
High intake of dietary lipids is one of the important causes of obesity and overweight. The structure and form of fatty acids, such as chain length, saturation, and double bond position, affect host energy intake, body weight, and appetite balance.124,125 Total intake of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) is associated with less weight gain, while trans-fat and saturated fatty acids (SFA) promote weight gain.126 However, saturated fatty acids contribute more to satiety,127 while ω-3 PUFA might moderately increase the desire to feed.128,129 Compared with MUFA or PUFA, consumption of SFA promotes CCK secretion and increases peak PYY levels.130,131 Paradoxically, other studies have shown that differences in fatty acid composition in high-fat diets do not lead to differences in appetite and intake.132 The types and specific mechanisms of fatty acids that affect appetite have not been fully revealed, demanding their further studies.5. Dietary metabolites that regulate appetite
Various gut microbiota-associated metabolites are involved in the regulation of appetite (Fig. 3).5.1 Tryptophan metabolites
Tryptophan (Trp), which is metabolized into tryptamine and indole derivates by gut microbiota, can affect gut hormone secretion and cross the blood–brain barrier to directly activate satiety circuits in the brain.135 Intragastric or intraduodenal administration of Trp inhibits subjective appetite and promotes the secretion of CCK, GLP-1, and PYY in a dose-dependent manner in healthy men but not in obese men.136–138 Similarly, supplementation with 5% Trp increases satiety and reduces food intake in healthy rats.139 However, several studies have shown inconsistent and contradictory results. Trp supplementation might promote appetite regulation via a serotonin pathway, enhancing feed efficiency, protein efficiency ratio, and reproduction performance.140,1415.2 GABA
GABA, a neurotransmitter produced by the metabolism of dietary glutamate, connects the gastrointestinal tract to the brain.142 The disruption of GABA signaling pathways inhibits postweaning feeding, NPY-induced hyperphagia and hunger associated with gastrointestinal motility.143 Additionally, GABAergic inputs inhibit AgRP neurons and feeding, impairing sensory learning and food cue acquisition greatly.144,145 However, research on gut microbial-derived GABA and appetite control is limited. Thus, further studies are needed to investigate the role of GABA in host metabolic health and its effects on the central nervous system to regulate appetite.5.3 Dopamine
Dopamine (DA) is involved in normal and pathological food intake in humans.146 Appetite-regulating hormones, such as insulin, leptin, and ghrelin, directly affect the dopamine reward pathway. These hormones activate receptors on dopamine-secreting neurons in the ventral tegmental area (VTA), which either stimulate (Ghrelin) or inhibit (leptin and insulin) dopamine signaling in the nucleus accumbens (NAc), thereby affecting the motivation to feed.147,148 During food intake, immediate and delayed dopamine release occurs in distinct areas of the human brain. Food consumption triggers orosensory and post-ingestive dopamine release in humans, involving different neural pathways: orosensory integrative and higher cognitive centers.149 Likewise, in the brain of a bee model, the need for food has been shown to elevate dopamine levels upon first arrival at the feeder and while departing from the food source and initiating a dance to communicate information about the food source.150 Obesity and a high-fat diet alter dopamine transmission, leading to brain reward dysfunction and overeating. Within the reward system, dopaminergic and dopaminoceptive neurons are the key players involved in the transport, sensing, and metabolism of dietary lipids.151 The Roux-en-Y gastric bypass can also alter fat intake, promote the production of fat-satiety molecule oleoylethanolamide (OEA) in the lower small intestine to induce satiety, and increase dorsal striatal dopamine release via the vagus nerve.1525.4 SCFAs
SCFAs, such as acetate, propionate, and butyrate, are the main products of intestinal microbial fermentation of indigestible carbohydrates, and they are involved in host immune regulation, energy homeostasis and disease treatment.153 The elevation of SCFAs in the colon stimulates the production of large amounts of hormones because their relevant receptors in different organs and tissues, such as G-protein-coupled receptors, free fatty acid receptor (FFAR) 2 and FFAR3, transmit neural signals, cumulatively inhibiting short-term appetite and energy intake.154,155 Meanwhile, SCFAs reduce fat accumulation through appetite-related gene expression.156 Acetate stimulates hypothalamic neurons by crossing the blood–brain barrier, directly suppresses appetite, and controls weight and insulin sensitivity.157,158 Consumption of foods rich in propionate reduces blood glucose response and gastric emptying rates, increasing subjective satiety and reducing hunger, which is important for obesity management.159,160 Colon delivery of propionate stimulates the release of PYY and GLP-1, reducing energy intake and preventing weight gain in overweight subjects.161 Butyric acid is an important regulator of energy homeostasis and has a significant impact on the regulation of thermogenesis and lipid and glucose metabolism.162 Butyrate supplementation inhibits the expression of neuropeptide Y by the appetite neurons in the hypothalamus, decreases neuronal activity in the NTS and dorsal vagal complex, and activates brown fat to enhance fat oxidation and improve energy metabolism.163,1645.5 Bile acid
Bile acid is the product of cholesterol modified by intestinal microbiota. It regulates nutrient absorption, mitochondrial function, inflammation response, appetite regulation, and energy homeostasis.165 Knockout of the Cyp8b1 gene, an enzyme necessary for the production of 12α-hydroxylated bile acids, results in significant alterations in the composition of bile acids and lowers the levels of satiation-enhancing OEA in the jejunum.166 Farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5), which are receptors of bile acids, regulate the secretion of gastrointestinal hormones, including GLP-1, neurotensin and PYY, hepatic gluconeogenesis, glycogen synthesis, and energy expenditure.167 Bile acids stimulate the secretion of appetite-regulating hormones, such as GLP-1, neurotensin, and PYY, by mediating basolateral intestinal TGR5, reducing AgRP/NPY release, and collaborating with CCK to enhance satiety.168–1706. Appetite regulation strategies
Diverse techniques, including dietary modulation to postpone gastrointestinal digestion and environmental modulation to improve the production of satiety hormones in consumers, have been implemented to manage the activity of appetite-related neurons and impact both food intake quantity and frequency.6.1 Construction of slow-digestion nutrient delivery systems
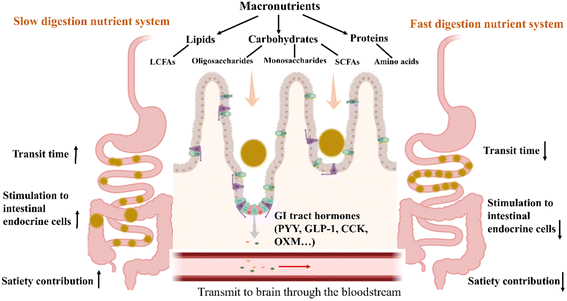
A significant number of intestinal endocrine cells, including L cells, enterochromaffin cells, and P cells, are present near the terminal portion of the small intestine and colon. Undigested chyme moving to the distal colon may efficiently activate these intestinal endocrine cells to secrete satiation peptides (GLP-1 and PYY), hence diminishing hunger.171–173 Nutrient delivery via a slow-digestion strategy can inhibit digestive enzyme activity, allowing nutrients to be transported to the distal ileum and colon slowly, promoting the secretion of appetite hormones and reducing food intake.174,175 Compared with fast-digestible foods, slow-digestible foods allow for more frequent nutrient interaction with the gastrointestinal tract and longer transport time, thereby increasing the stimulation of endocrine cells at the terminal end of the small intestine and promoting the secretion of satiety hormones (Fig. 4). The consumption of slow-digested starch blunts post-meal fluctuations in blood glucose and insulin, thereby prolonging energy supply and satiety and suppressing the expression of appetite-stimulating neuropeptide genes associated with the gut-brain axis.174,176,1776.2 Influence of chyme physicochemical properties on appetite
The physicochemical characteristics of chyme significantly influence satiety (Fig. 5). Food milling and cooking disrupt the microstructure of fibers and alter the physicochemical characteristics, hence enhancing the digestibility of starch and the breakdown of plant-derived chemicals.178 The physicochemical properties of dietary fiber (solubility, viscosity, fermentability, etc.) determine its function in the gastrointestinal tract and can impact nutrient utilization, intestinal motility, fecal formation, and microbial specificity.179 Plant-derived dietary fibers with a relatively dense and orderly physical structure lead to slow digestion and fermentation in the gut via a prolonged fermentation process and facilitate the production of butyrate.180 The viscosity of the dietary fiber impedes the gastric emptying rate of chyme and nutrient absorption in the small intestine while also augmenting perceived fullness.181,182 For example, different structures and physicochemical properties of konjac glucomannan (KGM) have distinct satiety-enhancing effects. The viscous and gel properties of KGM significantly delay the rate of gastric emptying and stimulate satiety via the vagus nerve.183–185 The change in appetite caused by the effects of the physical and chemical properties of dietary fiber profoundly affects host glucose and lipid metabolism.186 It is an effective measure to modify the physical and chemical properties of food, promote the interaction between chyme and the host gastrointestinal tract, increase the secretion of appetite hormones, and enhance satiety.6.3 Appetite regulation based on dietary rhythm
The circadian rhythm significantly affects the temporal regulation of food intake and metabolism, and the related feeding habits regulate the appetite level of the subjects.187 Unusual feed timings might disrupt the circadian system, resulting in digestive enzyme expression disorders. High caloric intake at breakfast could cause significantly more weight loss than those assigned to dinner.188 The thermogenic effect of breakfast foods exceeds that of dinner, whereas dinner foods have a greater probability of elevating blood glucose and insulin levels. A low-calorie breakfast may result in increased hunger and cravings for sweets during the day.189 Conversely, compared with evening energy intake, morning energy intake significantly inhibits food craving and improves satiety and hunger management.190,191 A high-calorie breakfast significantly reduces mean hunger scores in overweight and obese women. Thus, it is helpful for the management of obesity and metabolic syndrome.192 Similarly, eating breakfast and avoiding late-evening snacking sustains lipid oxidation and potentially improves metabolic outcomes.193 It is suggested that people with irregular diets due to long-term overtime work schedule their meal times reasonably to manage obesity and maintain normal glycolipid metabolism. Therefore, maintaining a suitable feeding schedule and energy metabolism is also necessary for appetite regulation. Constructing slow-digestion nutrient delivery systems, regulating chyme physicochemical properties and increasing morning calorie intake are effective strategies to improve appetite, hormone levels and satiety in obese subjects (Table 2).| Dietary strategy | Target | Effect on satiety |
|---|---|---|
| Slow-digestion nutrient delivery systems | Distal colon | Stimulate the secretion of appetite hormones |
| Chyme physicochemical properties | Mouth, stomach, and intestine | Delay gastric emptying and slow down the digestion and absorption of nutrients |
| Dietary rhythm | Brain | Influences digestive enzyme activity |
7. Conclusion
At present, the incidence of obesity-related chronic metabolic diseases is rising at an alarming rate. Dietary management methods to improve satiety and control appetite are widely recommended and considered safe options. AgRp/NPY that promote feeding and POMC/CART that inhibit appetite are two homeostasis neural circuits in the arcuate nucleus regulating hunger. Food palatability can drive the hedonic feeding mechanism of the AgRp neurons. Furthermore, this study also reviews the role of intestinal flora in appetite regulation and satiety contribution. Dietary nutrients are the main driving forces that regulate appetite signals. The contribution of protein nutrients for the stimulation of satiety hormones and appetite neural circuits has important guiding significance for satiating foods. In contrast, the satiety effects of different fatty acid types produced from lipid foods and carbohydrates are still controversial. The small-molecular-weight metabolites of dietary nutrients in the digestive tract, such as dopamine, short-chain fatty acids, and bile acids, are considered potential appetite regulators as they combine with corresponding receptors and participate in the regulation of appetite neural circuits. Additionally, dietary modifications to prolong chyme transport time and change its physical and chemical properties, as well as dietary rhythm regulation, are also highlighted as effective appetite regulation strategies. However, there are still many deficiencies for subsequent research. Many conclusions presented in this review are derived from animal experiments, and there is a lack of necessary evidence for their translation in human studies. Secondly, this review focuses primarily on obesity induced by gluttony, without considering the influence of various factors such as region, religion, dietary culture and living habits. Therefore, to address the need for precision nutrition in the future, designing distinct dietary plans and appetite regulation strategies suitable for different populations is crucial for the prevention of chronic metabolic diseases.Author contributions
Pengkui Xia, Tao Hou, and Jing Li conceived the study and designed the search strategy, synthetized information and wrote the manuscript. Yudie Yu, Wanxu Yu, Mahmoud Youssef, and Bin Li revised the manuscript. All authors read and approved the final version of the manuscript.Data availability
All relevant data are included within the manuscript.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was financially supported by Hubei Provincial Natural Science Foundation for Distinguished Young Scholars (2022CFA085) and HZAU-AGIS Cooperation Fund (SZYJY2022020).References
- S. Djalalinia, S. Saeedi Moghaddam, A. Sheidaei, N. Rezaei, S. S. Naghibi Iravani, M. Modirian, H. Zokaei, M. Yoosefi, K. Gohari, A. Kousha, Z. Abdi, S. Naderimagham, A. R. Soroush, B. Larijani and F. Farzadfar, Patterns of Obesity and Overweight in the Iranian Population: Findings of STEPs 2016, Front. Endocrinol., 2020, 11, 42 CrossRef PubMed.
- A. N. M. S. Islam, H. Sultana, M. Nazmul Hassan Refat, Z. Farhana, A. Abdulbasah Kamil and M. Meshbahur Rahman, The global burden of overweight-obesity and its association with economic status, benefiting from STEPs survey of WHO member states: A meta-analysis, Prev. Med. Rep., 2024, 46, 102882 CrossRef PubMed.
- R. M. Puhl and C. A. Heuer, The Stigma of Obesity: A Review and Update, Obesity, 2009, 17, 941–964 CrossRef PubMed.
- V. Augustine, S. Lee and Y. Oka, Neural Control and Modulation of Thirst, Sodium Appetite, and Hunger, Cell, 2020, 180, 25–32 CrossRef CAS PubMed.
- P. J. Rogers and J. M. Brunstrom, Appetite and energy balancing, Physiol. Behav., 2016, 164, 465–471 CrossRef CAS PubMed.
- T. Amin and J. G. Mercer, Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake, Curr. Obes. Rep., 2016, 5, 106–112 CrossRef PubMed.
- S. Sundaresan, C. Johnson, K. B. Dixon, M. Dole, D. Kilkelly, J. Antoun, C. R. Flynn, N. N. Abumrad and R. Tamboli, Intraduodenal nutrient infusion differentially alters intestinal nutrient sensing, appetite, and satiety responses in lean and obese subjects, Am. J. Clin. Nutr., 2023, 118, 646–656 CrossRef PubMed.
- A. Jais, L. Paeger, T. Sotelo-Hitschfeld, S. Bremser, M. Prinzensteiner, P. Klemm, V. Mykytiuk, P. J. M. Widdershooven, A. J. Vesting, K. Grzelka, M. Minère, A. L. Cremer, J. Xu, T. Korotkova, B. B. Lowell, H. U. Zeilhofer, H. Backes, H. Fenselau, F. T. Wunderlich, P. Kloppenburg and J. C. Brüning, PNOCARC Neurons Promote Hyperphagia and Obesity upon High-Fat-Diet Feeding, Neuron, 2020, 106, 1009–1025 CrossRef CAS PubMed.
- Y. P. Zhao, E. Johansson, J. L. Duan, Z. Han and M. Alenius, Fat- and sugar-induced signals regulate sweet and fat taste perception in Drosophila, Cell Rep., 2023, 42, 113387 CrossRef CAS PubMed.
- C. M. Tenk and T. Felfeli, Sucrose and fat content significantly affects palatable food consumption in adolescent male and female rats, Appetite, 2017, 118, 49–59 CrossRef PubMed.
- C. M. Lorch, N. W. Hayes, J. L. Xia, S. W. Fleps, H. E. McMorrow, H. S. Province, J. A. Frydman, J. G. Parker and L. R. Beutler, Sucrose overconsumption impairs AgRP neuron dynamics and promotes palatable food intake, Cell Rep., 2024, 43, 113675–113675 CrossRef CAS PubMed.
- M.-D. Li, N. B. Vera, Y. Yang, B. Zhang, W. Ni, E. Ziso-Qejvanaj, S. Ding, K. Zhang, R. Yin, S. Wang, X. Zhou, E. X. Fang, T. Xu, D. M. Erion and X. Yang, Adipocyte OGT governs diet-induced hyperphagia and obesity, Nat. Commun., 2018, 9, 5103 CrossRef PubMed.
- Y. Yang, M. Fu, M.-D. Li, K. Zhang, B. Zhang, S. Wang, Y. Liu, W. Ni, Q. Ong, J. Mi and X. Yang, O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity, Nat. Commun., 2020, 11, 181 CrossRef CAS PubMed.
- J. D. Kim, N. A. Yoon, S. Jin and S. Diano, Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding, Cell Metab., 2019, 30, 952–962.e5 CrossRef CAS PubMed.
- S. Akimoto and K. Miyasaka, Age-associated changes of appetite-regulating peptides, Geriatr. Gerontol. Int., 2010, 10, S107–S119 CrossRef PubMed.
- V. L. Li, Y. He, K. Contrepois, H. Liu, J. T. Kim, A. L. Wiggenhorn, J. T. Tanzo, A. S.-H. Tung, X. Lyu, P.-J. H. Zushin, R. S. Jansen, B. Michael, K. Y. Loh, A. C. Yang, C. S. Carl, C. T. Voldstedlund, W. Wei, S. M. Terrell, B. C. Moeller, R. M. Arthur, G. A. Wallis, K. van de Wetering, A. Stahl, B. Kiens, E. A. Richter, S. M. Banik, M. P. Snyder, Y. Xu and J. Z. Long, An exercise-inducible metabolite that suppresses feeding and obesity, Nature, 2022, 606, 785–790 CrossRef CAS PubMed.
- J. M. Moris, C. Heinold, A. Blades and Y. Koh, Nutrient-Based Appetite Regulation, J. Obes. Metab. Syndr., 2022, 31, 161–168 CrossRef PubMed.
- M. van de Wouw, H. Schellekens, T. G. Dinan and J. F. Cryan, Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite, J. Nutr., 2017, 147, 727–745 CrossRef CAS PubMed.
- M. Liu, Z. Ye, P. He, S. Yang, Y. Zhang, C. Zhou, Y. Zhang, F. F. Hou and X. Qin, Adding salt to foods and hazards of microvascular, cerebrovascular and cardiovascular diseases, Eur. J. Clin. Nutr., 2024, 78, 141–148 CrossRef CAS PubMed.
- D. Mohan, K. H. Yap, D. Reidpath, Y. C. Soh, A. McGrattan, B. C. M. Stephan, L. Robinson, N. Chaiyakunapruk, M. Siervo and P. E. C. t. on behalf of De, Link Between Dietary Sodium Intake, Cognitive Function, and Dementia Risk in Middle-Aged and Older Adults: A Systematic Review, J. Alzheimer's Dis., 2020, 76, 1347–1373 Search PubMed.
- J. G. Verbalis, Disorders of body water homeostasis, Best Pract. Res., Clin. Endocrinol. Metab., 2003, 17, 471–503 CrossRef CAS PubMed.
- L. L. Wong and J. G. Verbalis, Systemic diseases associated with disorders of water homeostasis, Endocrinol. Metab. Clin. North Am., 2002, 31, 121–140 CrossRef CAS PubMed.
- A. N. Gearhardt and J. Hebebrand, The concept of “food addiction” helps inform the understanding of overeating and obesity: Debate Consensus, Am. J. Clin. Nutr., 2021, 113, 274–276 CrossRef PubMed.
- S. Lee, V. Augustine, Y. Zhao, H. Ebisu, B. Ho, D. Kong and Y. Oka, Chemosensory modulation of neural circuits for sodium appetite, Nature, 2019, 568, 93–97 CrossRef CAS PubMed.
- C. A. Zimmerman, Y.-C. Lin, D. E. Leib, L. Guo, E. L. Huey, G. E. Daly, Y. Chen and Z. A. Knight, Thirst neurons anticipate the homeostatic consequences of eating and drinking, Nature, 2016, 537, 680–684 CrossRef CAS PubMed.
- Y. Chen, Y.-C. Lin, C. A. Zimmerman, R. A. Essner and Z. A. Knight, Hunger neurons drive feeding through a sustained, positive reinforcement signal, eLife, 2016, 5, e18640 CrossRef PubMed.
- A. T. Goh, J. Y. M. Choy, X. H. Chua, S. Ponnalagu, C. M. Khoo, C. Whitton, R. M. van Dam and C. G. Forde, Increased oral processing and a slower eating rate increase glycaemic, insulin and satiety responses to a mixed meal tolerance test, Eur. J. Nutr., 2021, 60, 2719–2733 CrossRef CAS PubMed.
- V. Augustine, S. K. Gokce and Y. Oka, Peripheral and Central Nutrient Sensing Underlying Appetite Regulation, Trends Neurosci., 2018, 41, 526–539 CrossRef CAS PubMed.
- M. L. Andermann and B. B. Lowell, Toward a Wiring Diagram Understanding of Appetite Control, Neuron, 2017, 95, 757–778 CrossRef CAS PubMed.
- J. Wang, F. Lv, W. Yin, Z. Gao, H. Liu, Z. Wang and J. Sun, The organum vasculosum of the lamina terminalis and subfornical organ: regulation of thirst, Front. Neurosci., 2023, 17, 1223836 CrossRef PubMed.
- V. Augustine, S. K. Gokce, S. Lee, B. Wang, T. J. Davidson, F. Reimann, F. Gribble, K. Deisseroth, C. Lois and Y. Oka, Hierarchical neural architecture underlying thirst regulation, Nature, 2018, 555, 204–209 CrossRef CAS PubMed.
- M. J. McKinley, D. A. Denton, P. J. Ryan, S. T. Yao, A. Stefanidis and B. J. Oldfield, From sensory circumventricular organs to cerebral cortex: Neural pathways controlling thirst and hunger, J. Neuroendocrinol., 2019, 31, e12689 CrossRef PubMed.
- C. A. Zimmerman, E. L. Huey, J. S. Ahn, L. R. Beutler, C. L. Tan, S. Kosar, L. Bai, Y. Chen, T. V. Corpuz, L. Madisen, H. Zeng and Z. A. Knight, A gut-to-brain signal of fluid osmolarity controls thirst satiation, Nature, 2019, 568, 98–102 CrossRef CAS PubMed.
- V. Augustine, H. Ebisu, Y. Zhao, S. Lee, B. Ho, G. O. Mizuno, L. Tian and Y. Oka, Temporally and Spatially Distinct Thirst Satiation Signals, Neuron, 2019, 103, 242–249 CrossRef CAS PubMed.
- K. Ray, Detecting gut osmolality changes to quench thirst, Nat. Rev. Gastroenterol. Hepatol., 2022, 19, 215–215 CrossRef PubMed.
- T. Ichiki, T. Wang, A. Kennedy, A.-H. Pool, H. Ebisu, D. J. Anderson and Y. Oka, Sensory representation and detection mechanisms of gut osmolality change, Nature, 2022, 602, 468–474 CrossRef CAS PubMed.
- J. M. Resch, H. Fenselau, J. C. Madara, C. Wu, J. N. Campbell, A. Lyubetskaya, B. A. Dawes, L. T. Tsai, M. M. Li, Y. Livneh, Q. G. Ke, P. M. Kang, G. Fejes-Tóth, A. Náray-Fejes-Tóth, J. C. Geerling and B. B. Lowell, Aldosterone-Sensing Neurons in the NTS Exhibit State-Dependent Pacemaker Activity and Drive Sodium Appetite via Synergy with Angiotensin II Signaling, Neuron, 2017, 96, 190–206.e7 CrossRef CAS PubMed.
- J. C. Geerling and A. D. Loewy, Aldosterone-sensitive neurons in the nucleus of the solitary tract: Efferent projections, J. Comp. Neurol., 2006, 497, 223–250 CrossRef CAS PubMed.
- Y. Zhang, A.-H. Pool, T. Wang, L. Liu, E. Kang, B. Zhang, L. Ding, K. Frieda, R. Palmiter and Y. Oka, Parallel neural pathways control sodium consumption and taste valence, Cell, 2023, 186, 5751–5765 CrossRef CAS PubMed.
- Y. Liu, J.-A. Wei, Z. Luo, J. Cui, Y. Luo, S. O. K. Mak, S. Wang, F. Zhang, Y. Yang, K.-F. So, L. Shi, L. Zhang and B. K. C. Chow, A gut-brain axis mediates sodium appetite via gastrointestinal peptide regulation on a medulla-hypothalamic circuit, Sci. Adv., 2023, 9, eadd5330 CrossRef CAS PubMed.
- D. A. Fitts, J. A. Freece, J. E. Van Bebber, D. K. Zierath and J. E. Bassett, Effects of forebrain circumventricular organ ablation on drinking or salt appetite after sodium depletion or hypernatremia, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2004, 287, R1325–R1334 CrossRef CAS PubMed.
- S. S. Ch'ng and A. J. Lawrence, The subfornical organ in sodium appetite: Recent insights, Neuropharmacology, 2019, 154, 107–113 CrossRef PubMed.
- M. Lutter and E. J. Nestler, Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake, J. Nutr., 2009, 139, 629–632 CrossRef CAS PubMed.
- M. A. Rossi and G. D. Stuber, Overlapping Brain Circuits for Homeostatic and Hedonic Feeding, Cell Metab., 2018, 27, 42–56 CrossRef CAS PubMed.
- H.-R. Berthoud, Metabolic and hedonic drives in the neural control of appetite: who is the boss?, Curr. Opin. Neurobiol., 2011, 21, 888–896 CrossRef CAS PubMed.
- M. Muthmainah, A. Gogos, P. Sumithran and R. M. Brown, Orexins (hypocretins): The intersection between homeostatic and hedonic feeding, J. Neurochem., 2021, 157, 1473–1494 CrossRef CAS PubMed.
- R. S. Ahima and D. A. Antwi, Brain Regulation of Appetite and Satiety, Endocrinol. Metab. Clin. North Am., 2008, 37, 811–823 CrossRef CAS PubMed.
- K. A. Simpson, N. M. Martin and S. R. Bloom, Hypothalamic regulation of appetite, Expert Rev. Endocrinol. Metab., 2008, 3, 577–592 CrossRef CAS PubMed.
- M. S. Vohra, K. Benchoula, C. J. Serpell and W. E. Hwa, AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity, Eur. J. Pharmacol., 2022, 915, 174611 CrossRef CAS PubMed.
- Y. M. Chen, Y. C. Lin, T. W. Kuo and Z. A. Knight, Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits, Cell, 2015, 160, 829–841 CrossRef CAS PubMed.
- N. M. Semjonous, K. L. Smith, J. R. C. Parkinson, D. J. L. Gunner, Y. L. Liu, K. G. Murphy, M. A. Ghatei, S. R. Bloom and C. J. Small, Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance, Int. J. Obes., 2009, 33, 775–785 CrossRef CAS PubMed.
- M. J. Krashes, B. P. Shah, S. Koda and B. B. Lowell, Rapid versus Delayed Stimulation of Feeding by the Endogenously Released AgRP Neuron Mediators GABA, NPY, and AgRP, Cell Metab., 2013, 18, 588–595 CrossRef CAS PubMed.
- Q. Wu and R. D. Palmiter, GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism, Eur. J. Pharmacol., 2011, 660, 21–27 CrossRef CAS PubMed.
- T. Candler, P. Kühnen, A. M. Prentice and M. Silver, Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease, Front. Neuroendocrinol., 2019, 54, 100773 CrossRef CAS PubMed.
- M. J. Krashes, B. B. Lowell and A. S. Garfield, Melanocortin-4 receptor-regulated energy homeostasis, Nat. Neurosci., 2016, 19, 206–219 CrossRef CAS PubMed.
- R. D. Cone, Studies on the physiological functions of the melanocortin system, Endocr. Rev., 2006, 27, 736–749 CrossRef CAS PubMed.
- Y. Greenman, Y. Kuperman, Y. Drori, S. L. Asa, I. Navon, O. Forkosh, S. Gil, N. Stern and A. Chen, Postnatal Ablation of POMC Neurons Induces an Obese Phenotype Characterized by Decreased Food Intake and Enhanced Anxiety-Like Behavior, Mol. Endocrinol., 2013, 27, 1091–1102 CrossRef CAS PubMed.
- R. G. P. Denis, A. Joly-Amado, E. Webber, F. Langlet, M. Schaeffer, S. L. Padilla, C. Cansell, B. Dehouck, J. Castel, A.-S. Delbès, S. Martinez, A. Lacombe, C. Rouch, N. Kassis, J.-A. Fehrentz, J. Martinez, P. Verdié, T. S. Hnasko, R. D. Palmiter, M. J. Krashes, A. D. Güler, C. Magnan and S. Luquet, Palatability Can Drive Feeding Independent of AgRP Neurons, Cell Metab., 2015, 22, 646–657 CrossRef CAS PubMed.
- L. R. Beutler, T. V. Corpuz, J. S. Ahn, S. Kosar, W. Song, Y. Chen and Z. A. Knight, Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat, eLife, 2020, 9, e55909 CrossRef CAS PubMed.
- X. M. Wang, X. T. Wu, H. Wu, H. Y. Xiao, S. J. Hao, B. W. Wang, C. Li, K. Bleymehl, S. G. Kauschke, V. Mack, B. Ferger, H. Klein, R. M. Zheng, S. M. Duan and H. Wang, Neural adaption in midbrain GABAergic cells contributes to high-fat diet-induced obesity, Sci. Adv., 2023, 9, eadh2884 CrossRef CAS PubMed.
- C. Zhu, Z. Jiang, Y. Xu, Z.-L. Cai, Q. Jiang, Y. Xu, M. Xue, B. R. Arenkiel, Q. Wu, G. Shu and Q. Tong, Profound and redundant functions of arcuate neurons in obesity development, Nat. Metab., 2020, 2, 763–774 CrossRef CAS PubMed.
- M. Obara-Michlewska, The contribution of astrocytes to obesity-associated metabolic disturbances, J. Biomed. Res., 2022, 36, 299–311 CrossRef CAS PubMed.
- J. A. Chowen, L. M. Frago and M. S. Fernández-Alfonso, Physiological and pathophysiological roles of hypothalamic astrocytes in metabolism, J. Neuroendocrinol., 2019, 31, e12671 CrossRef PubMed.
- P. Sweeney, Y. Qi, Z. P. Xu and Y. L. Yang, Activation of Hypothalamic Astrocytes Suppresses Feeding Without Altering Emotional States, Glia, 2016, 64, 2263–2273 CrossRef PubMed.
- L. Yang, Y. Qi and Y. Yang, Astrocytes Control Food Intake by Inhibiting AGRP Neuron Activity via Adenosine A1 Receptors, Cell Rep., 2015, 11, 798–807 CrossRef CAS PubMed.
- C. García-Cáceres, C. Quarta, L. Varela, Y. Gao, T. Gruber, B. Legutko, M. Jastroch, P. Johansson, J. Ninkovic, C.-X. Yi, O. Le Thuc, K. Szigeti-Buck, W. Cai, C. W. Meyer, P. T. Pfluger, A. M. Fernandez, S. Luquet, S. C. Woods, I. Torres-Alemán, C. R. Kahn, M. Götz, T. L. Horvath and M. H. Tschöp, Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability, Cell, 2016, 166, 867–880 CrossRef PubMed.
- A. J. MacDonald, F. E. Holmes, C. Beall, A. E. Pickering and K. L. J. Ellacott, Regulation of food intake by astrocytes in the brainstem dorsal vagal complex, Glia, 2020, 68, 1241–1254 CrossRef PubMed.
- K. N. Browning, S. Verheijden and G. E. Boeckxstaens, The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation, Gastroenterology, 2017, 152, 730–744 CrossRef PubMed.
- H.-R. Berthoud, V. L. Albaugh and W. L. Neuhuber, Gut-brain communication and obesity: understanding functions of the vagus nerve, J. Clin. Invest., 2021, 131, e143770 CrossRef CAS PubMed.
- S. C. Cork, The role of the vagus nerve in appetite control: Implications for the pathogenesis of obesity, J. Neuroendocrinol., 2018, 30, e12643 CrossRef PubMed.
- J. J. A. J. Bastings, K. Venema, E. E. Blaak and T. C. Adam, Influence of the gut microbiota on satiety signaling, Trends Endocrinol. Metab., 2023, 34, 243–255 CrossRef CAS PubMed.
- A. V. Hartstra, M. Nieuwdorp and H. Herrema, Interplay between gut microbiota, its metabolites and human metabolism: Dissecting cause from consequence, Trends Food Sci. Technol., 2016, 57, 233–243 CrossRef CAS.
- S. O. Fetissov, Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour, Nat. Rev. Endocrinol., 2017, 13, 11–25 CrossRef CAS PubMed.
- M. Arnoriaga-Rodríguez, J. Mayneris-Perxachs, A. Burokas, V. Pérez-Brocal, A. Moya, M. Portero-Otin, W. Ricart, R. Maldonado and J.-M. Fernández-Real, Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile, Microbiome, 2020, 8, 59 CrossRef PubMed.
- J. Breton, N. Tennoune, N. Lucas, M. Francois, R. Legrand, J. Jacquemot, A. Goichon, C. Guérin, J. Peltier, M. Pestel-Caron, P. Chan, D. Vaudry, J.-C. do Rego, F. Liénard, L. Pénicaud, X. Fioramonti, I. S. Ebenezer, T. Hökfelt, P. Déchelotte and S. O. Fetissov, Gut Commensal E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth, Cell Metab., 2016, 23, 324–334 CrossRef CAS PubMed.
- I. Gabanyi, G. Lepousez, R. Wheeler, A. Vieites-Prado, A. Nissant, G. Chevalier, S. Wagner, C. Moigneu, S. Dulauroy, S. Hicham, B. Polomack, F. Verny, P. Rosenstiel, N. Renier, I. G. Boneca, G. Eberl and P.-M. Lledo, Bacterial sensing via neuronal Nod2 regulates appetite and body temperature, Science, 2022, 376, eabj3986 CrossRef CAS PubMed.
- H. Yang, M. Yang, S. Fang, X. Huang, M. He, S. Ke, J. Gao, J. Wu, Y. Zhou, H. Fu, C. Chen and L. Huang, Evaluating the profound effect of gut microbiome on host appetite in pigs, BMC Microbiol., 2018, 18, 215 CrossRef CAS PubMed.
- T. Zhang, W. Wang, J. Li, X. Ye, Z. Wang, S. Cui, S. Shen, X. Liang, Y. Q. Chen and S. Zhu, Free fatty acid receptor 4 modulates dietary sugar preference via the gut microbiota, Nat. Microbiol., 2025, 10, 348–361 CrossRef CAS PubMed.
- S. Falcinelli, A. Rodiles, A. Hatef, S. Picchietti, L. Cossignani, D. L. Merrifield, S. Unniappan and O. Carnevali, Influence of Probiotics Administration on Gut Microbiota Core: A Review on the Effects on Appetite Control, Glucose, and Lipid Metabolism, J. Clin. Gastroenterol., 2018, 52, S50–S56 CrossRef CAS PubMed.
- A. L. Carreiro, J. Dhillon, S. Gordon, K. A. Higgins, A. G. Jacobs, B. M. McArthur, B. W. Redan, R. L. Rivera, L. R. Schmidt and R. D. Mattes, The Macronutrients, Appetite, and Energy Intake, Annu. Rev. Nutr., 2016, 36, 73–103 CrossRef CAS PubMed.
- M. Lonnie, E. Hooker, J. M. Brunstrom, B. M. Corfe, M. A. Green, A. W. Watson, E. A. Williams, E. J. Stevenson, S. Penson and A. M. Johnstone, Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults, Nutrients, 2018, 10, 360 CrossRef PubMed.
- P. Morell and S. Fiszman, Revisiting the role of protein-induced satiation and satiety, Food Hydrocolloids, 2017, 68, 199–210 CrossRef CAS.
- A. Kohanmoo, S. Faghih and M. Akhlaghi, Effect of short- and long-term protein consumption on appetite and appetite-regulating gastrointestinal hormones, a systematic review and meta-analysis of randomized controlled trials, Physiol. Behav., 2020, 226, 113123 CrossRef CAS PubMed.
- M. S. Westerterp-Plantenga, S. G. Lemmens and K. R. Westerterp, Dietary protein – its role in satiety, energetics, weight loss and health, Br. J. Nutr., 2012, 108, S105–S112 CrossRef CAS PubMed.
- H. J. Leidy, M. Tang, C. L. H. Armstrong, C. B. Martin and W. W. Campbell, The Effects of Consuming Frequent, Higher Protein Meals on Appetite and Satiety During Weight Loss in Overweight/Obese Men, Obesity, 2011, 19, 818–824 CrossRef CAS PubMed.
- L. Q. Bendtsen, J. K. Lorenzen, N. T. Bendsen, C. Rasmussen and A. Astrup, Effect of Dairy Proteins on Appetite, Energy Expenditure, Body Weight, and Composition: a Review of the Evidence from Controlled Clinical Trials, Adv. Nutr., 2013, 4, 418–438 CrossRef CAS PubMed.
- C. Giezenaar, L. G. Trahair, N. D. Luscombe-Marsh, T. Hausken, S. Standfield, K. L. Jones, K. Lange, M. Horowitz, I. Chapman and S. Soenen, Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women, Am. J. Clin. Nutr., 2017, 106, 865–877 CrossRef CAS PubMed.
- M. A. B. Veldhorst, A. G. Nieuwenhuizen, A. Hochstenbach-Waelen, K. R. Westerterp, M. P. K. J. Engelen, R.-J. M. Brummer, N. E. P. Deutz and M. S. Westerterp-Plantenga, Comparison of the effects of a high- and normal-casein breakfast on satiety, ‘satiety’ hormones, plasma amino acids and subsequent energy intake, Br. J. Nutr., 2008, 101, 295–303 CrossRef PubMed.
- J. Lorenzen, R. Frederiksen, C. Hoppe, R. Hvid and A. Astrup, The effect of milk proteins on appetite regulation and diet-induced thermogenesis, Eur. J. Clin. Nutr., 2012, 66, 622–627 CrossRef CAS PubMed.
- D. Raubenheimer and S. J. Simpson, Protein appetite as an integrator in the obesity system: the protein leverage hypothesis, Philos. Trans. R. Soc., B, 2023, 378, 20220212 CrossRef CAS PubMed.
- A. Grech, Z. Sui, A. Rangan, S. J. Simpson, S. C. P. Coogan and D. Raubenheimer, Macronutrient (im)balance drives energy intake in an obesogenic food environment: An ecological analysis, Obesity, 2022, 30, 2156–2166 CrossRef CAS PubMed.
- A. K. Gosby, A. D. Conigrave, D. Raubenheimer and S. J. Simpson, Protein leverage and energy intake, Obes. Rev., 2014, 15, 183–191 CrossRef CAS PubMed.
- K. M. B. de Carvalho, N. Pizato, P. B. Botelho, E. S. Dutra and V. S. S. Gonçalves, Dietary protein and appetite sensations in individuals with overweight and obesity: a systematic review, Eur. J. Nutr., 2020, 59, 2317–2332 CrossRef CAS PubMed.
- K. Kaneko, Appetite regulation by plant-derived bioactive peptides for promoting health, Peptides, 2021, 144, 170608 CrossRef CAS PubMed.
- I. T. Suryaningtyas and J.-Y. Je, Bioactive peptides from food proteins as potential anti-obesity agents: Mechanisms of action and future perspectives, Trends Food Sci. Technol., 2023, 138, 141–152 CrossRef CAS.
- S. J. Sharkey, P. A. Harnedy-Rothwell, P. J. Allsopp, L. E. Hollywood, R. J. FitzGerald and F. P. M. O'Harte, A Narrative Review of the Anti-Hyperglycemic and Satiating Effects of Fish Protein Hydrolysates and Their Bioactive Peptides, Mol. Nutr. Food Res., 2020, 64, 2000403 CrossRef CAS PubMed.
- S. M. S. Chungchunlam, C. A. Montoya, N. Stroebinger and P. J. Moughan, Effects of the maize-derived protein zein, and the milk proteins casein, whey, and α-lactalbumin, on subjective measures of satiety and food intake in normal-weight young men, Appetite, 2023, 180, 106339 CrossRef PubMed.
- A. E. Rigamonti, R. Leoncini, C. Casnici, O. Marelli, A. De Col, S. Tamini, E. Lucchetti, S. Cicolini, L. Abbruzzese, S. G. Cella and A. Sartorio, Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women, Nutrients, 2019, 11, 247 CrossRef CAS PubMed.
- T. Hira, N. Mori, T. Nakamori, H. Furuta, K. Asano, H. Chiba and H. Hara, Acute effect of soybean beta-conglycinin hydrolysate ingestion on appetite sensations in healthy humans, Appetite, 2011, 57, 765–768 CrossRef CAS PubMed.
- L. Wang, L. Ding, W. Zhu and S. Hang, Soybean protein hydrolysate stimulated cholecystokinin secretion and inhibited feed intake through calcium-sensing receptors and intracellular calcium signalling in pigs, Food Funct., 2021, 12, 9286–9299 RSC.
- Y. Xie, L. Cai, M. Ding, K. Shan, D. Zhao, G. Zhou and C. Li, Plant-based meat analogues enhance the gastrointestinal motility function and appetite of mice by specific volatile compounds and peptides, Food Res. Int., 2023, 174, 113551 CrossRef CAS PubMed.
- W. L. Hall, D. J. Millward, S. J. Long and L. M. Morgan, Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite, Br. J. Nutr., 2003, 89, 239–248 CrossRef CAS PubMed.
- A. E. Rigamonti, R. Leoncini, A. De Col, S. Tamini, S. Cicolini, L. Abbruzzese, S. G. Cella and A. Sartorio, The Appetite–Suppressant and GLP-1-Stimulating Effects of Whey Proteins in Obese Subjects are Associated with Increased Circulating Levels of Specific Amino Acids, Nutrients, 2020, 12, 775 CrossRef CAS PubMed.
- R. A. Elovaris, A. T. Hutchison, K. Lange, M. Horowitz, C. Feinle-Bisset and N. D. Luscombe-Marsh, Plasma Free Amino Acid Responses to Whey Protein and Their Relationships with Gastric Emptying, Blood Glucose- and Appetite-Regulatory Hormones and Energy Intake in Lean Healthy Men, Nutrients, 2019, 11, 2465 CrossRef CAS PubMed.
- J. Gao, S. Zhang, P. Deng, Z. Wu, B. Lemaitre, Z. Zhai and Z. Guo, Dietary L-Glu sensing by enteroendocrine cells adjusts food intake via modulating gut PYY/NPF secretion, Nat. Commun., 2024, 15, 3514 CrossRef CAS PubMed.
- T. Song, W. Qin, Z. Lai, H. Li, D. Li, B. Wang, W. Deng, T. Wang, L. Wang and R. Huang, Dietary cysteine drives body fat loss via FMRFamide signaling in Drosophila and mouse, Cell Res., 2023, 33, 434–447 CrossRef CAS PubMed.
- D. G. Le Couteur, S. M. Solon-Biet, V. C. Cogger, R. Ribeiro, R. de Cabo, D. Raubenheimer, G. J. Cooney and S. J. Simpson, Branched chain amino acids, aging and age-related health, Ageing Res. Rev., 2020, 64, 101198 CrossRef CAS PubMed.
- E. Supruniuk, E. Żebrowska and A. Chabowski, Branched chain amino acids—friend or foe in the control of energy substrate turnover and insulin sensitivity?, Crit. Rev. Food Sci. Nutr., 2023, 63, 2559–2597 CrossRef CAS PubMed.
- B. Lueders, B. C. Kanney, M. J. Krone, N. P. Gannon and R. A. Vaughan, Effect of branched-chain amino acids on food intake and indicators of hunger and satiety- a narrative summary, Hum. Nutr. Metab., 2022, 30, 200168 CrossRef CAS.
- S. M. Solon-Biet, V. C. Cogger, T. Pulpitel, D. Wahl, X. Clark, E. E. Bagley, G. C. Gregoriou, A. M. Senior, Q. P. Wang, A. E. Brandon, R. Perks, J. O'Sullivan, Y. C. Koay, K. Bell-Anderson, M. Kebede, B. Yau, C. Atkinson, G. Svineng, T. Dodgson, J. A. Wali, M. D. W. Piper, P. Juricic, L. Partridge, A. J. Rose, D. Raubenheimer, G. J. Cooney, D. G. Le Couteur and S. J. Simpson, Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control, Nat. Metab., 2019, 1, 532–545 CrossRef CAS PubMed.
- B. Kim, M. I. Kanai, Y. Oh, M. Kyung, E.-K. Kim, I.-H. Jang, J.-H. Lee, S.-G. Kim, G. S. B. Suh and W.-J. Lee, Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit, Nature, 2021, 593, 570–574 CrossRef CAS PubMed.
- P. Viskaitis, M. Arnold, C. Garau, L. T. Jensen, L. Fugger, D. Peleg-Raibstein and D. Burdakov, Ingested non-essential amino acids recruit brain orexin cells to suppress eating in mice, Curr. Biol., 2022, 32, 1812–1821 CrossRef CAS PubMed.
- J. L. Ble-Castillo, I. E. Juárez-Rojop, C. A. Tovilla-Zárate, C. Garcia-Vázquez, M. Z. Servin-Cruz, A. Rodríguez-Hernández, C. I. Araiza-Saldaña, A. M. Nolasco-Coleman and J. C. Díaz-Zagoya, Acute Consumption of Resistant Starch Reduces Food Intake but Has No Effect on Appetite Ratings in Healthy Subjects, Nutrients, 2017, 9, 696 CrossRef PubMed.
- S. Amini, A. Mansoori and L. Maghsumi-Norouzabad, The effect of acute consumption of resistant starch on appetite in healthy adults; a systematic review and meta-analysis of the controlled clinical trials, Clin. Nutr. ESPEN, 2021, 41, 42–48 CrossRef PubMed.
- C. H. Emilien, W. H. Hsu and J. H. Hollis, Effect of resistant wheat starch on subjective appetite and food intake in healthy adults, Nutrition, 2017, 43–44, 69–74 CrossRef CAS PubMed.
- U. White, C. M. Peterson, R. A. Beyl, C. K. Martin and E. Ravussin, Resistant Starch Has No Effect on Appetite and Food Intake in Individuals with Prediabetes, J. Acad. Nutr. Diet., 2020, 120, 1034–1041 CrossRef PubMed.
- Y. Zhao, C. Li, X. Wang, Z. Wang, J. Wang, W. Zhen, S. Huang, T. Li, H. Fan, Y. Ma and C. Zhang, Effects of Glycyrrhiza polysaccharide on growth performance, appetite, and hypothalamic inflammation in broilers, J. Anim. Sci., 2023, 101, skad027 CrossRef PubMed.
- J. Wang, Y. Li, P. Luo, Y. Chen, Q. Xi, H. Wu, W. Zhao, G. Shu, S. Wang, P. Gao, X. Zhu, Y. Zhang, Q. Jiang and L. Wang, Oral supplementation with ginseng polysaccharide promotes food intake in mice, Brain Behav., 2019, 9, e01340 CrossRef PubMed.
- J. Luo, J. Qi, W. Wang, Z. Luo, L. Liu, G. Zhang, Q. Zhou, J. Liu and X. Peng, Antiobesity Effect of Flaxseed Polysaccharide via Inducing Satiety due to Leptin Resistance Removal and Promoting Lipid Metabolism through the AMP-Activated Protein Kinase (AMPK) Signaling Pathway, J. Agric. Food Chem., 2019, 67, 7040–7049 CrossRef CAS PubMed.
- Y. Zhang, Q. Xie, L. You, P. C.-K. Cheung and Z. Zhao, Behavior of Non-Digestible Polysaccharides in Gastrointestinal Tract: A Mechanistic Review of its Anti-Obesity Effect, eFood, 2021, 2, 59–72 CrossRef.
- K. Olli, K. Salli, E. Alhoniemi, M. Saarinen, A. Ibarra, T. Vasankari, N. Rautonen and K. Tiihonen, Postprandial effects of polydextrose on satiety hormone responses and subjective feelings of appetite in obese participants, Nutr. J., 2015, 14, 2 CrossRef PubMed.
- I. S. Waddell and C. Orfila, Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms, Crit. Rev. Food Sci. Nutr., 2023, 63, 8752–8767 CrossRef CAS PubMed.
- M. Akhlaghi, The role of dietary fibers in regulating appetite, an overview of mechanisms and weight consequences, Crit. Rev. Food Sci. Nutr., 2024, 64, 3139–3150 CrossRef CAS PubMed.
- C. L. Lawton, H. J. Delargy, J. Brockman, F. C. Smith and J. E. Blundell, The degree of saturation of fatty acids influences post-ingestive satiety, Br. J. Nutr., 2000, 83, 473–482 CrossRef CAS PubMed.
- C. M. Strik, F. E. Lithander, A.-T. McGill, A. K. MacGibbon, B. H. McArdle and S. D. Poppitt, No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: a randomised trial of fatty acid saturation, Nutr. J., 2010, 9, 24 CrossRef PubMed.
- X. Liu, Y. Li, D. K. Tobias, D. D. Wang, J. E. Manson, W. C. Willett and F. B. Hu, Changes in Types of Dietary Fats Influence Long-term Weight Change in US Women and Men, J. Nutr., 2018, 148, 1821–1829 CrossRef PubMed.
- V. Behrouz and Z. Yari, A review on differential effects of dietary fatty acids on weight, appetite and energy expenditure, Crit. Rev. Food Sci. Nutr., 2022, 62, 2235–2249 CrossRef CAS PubMed.
- S. Moradi, M. Alivand, Y. KhajeBishak, M. AsghariJafarabadi, M. Alipour, P. D. Chilibeck and B. Alipour, The effect of short-term omega-3 fatty acids supplementation on appetite in healthy men: A randomized double-blinded controlled clinical trial, Nutr. Clin. Metab., 2022, 36, 46–53 CrossRef.
- B. Sasanfar, F. Toorang and A. Salehi-Abarghouei, Effects of n-3 polyunsaturated fatty acid supplementation on appetite: a systematic review and meta-analysis of controlled clinical trials, Syst. Rev. Pharm., 2024, 13, 44 Search PubMed.
- M. D. Robertson, K. G. Jackson, B. A. Fielding, L. M. Morgan, C. M. Williams and K. N. Frayn, Acute ingestion of a meal rich in n-3 polyunsaturated fatty acids results in rapid gastric emptying in humans1,2,3, Am. J. Clin. Nutr., 2002, 76, 232–238 CrossRef CAS PubMed.
- A. Kozimor, H. Chang and J. A. Cooper, Effects of dietary fatty acid composition from a high fat meal on satiety, Appetite, 2013, 69, 39–45 CrossRef PubMed.
- A. J. Graybeal, S. Meena and J. L. Willis, Manipulation of fatty acid composition in a high-fat meal does not result in differential alterations in appetite or food intake in normal weight females: A single-blind randomized crossover study, Appetite, 2021, 160, 105085 CrossRef PubMed.
- Z. Li, B. Zhang, N. Wang, Z. Zuo, H. Wei and F. Zhao, A novel peptide protects against diet-induced obesity by suppressing appetite and modulating the gut microbiota, Gut, 2023, 72, 686 CrossRef CAS PubMed.
- K. R. Polley, F. Kamal, C. M. Paton and J. A. Cooper, Appetite responses to high-fat diets rich in mono-unsaturated versus poly-unsaturated fats, Appetite, 2019, 134, 172–181 CrossRef PubMed.
- S. N. Gartner, F. Aidney, A. Klockars, C. Prosser, E. A. Carpenter, K. Isgrove, A. S. Levine and P. K. Olszewski, Intragastric preloads of l-tryptophan reduce ingestive behavior via oxytocinergic neural mechanisms in male mice, Appetite, 2018, 125, 278–286 CrossRef PubMed.
- C. McVeay, P. C. E. Fitzgerald, S. S. Ullrich, R. E. Steinert, M. Horowitz and C. Feinle-Bisset, Effects of intraduodenal administration of lauric acid and L-tryptophan, alone and combined, on gut hormones, pyloric pressures, and energy intake in healthy men, Am. J. Clin. Nutr., 2019, 109, 1335–1343 CrossRef PubMed.
- R. E. Steinert, N. D. Luscombe-Marsh, T. J. Little, S. Standfield, B. Otto, M. Horowitz and C. Feinle-Bisset, Effects of Intraduodenal Infusion of L-Tryptophan on ad Libitum Eating, Antropyloroduodenal Motility, Glycemia, Insulinemia, and Gut Peptide Secretion in Healthy Men, J. Clin. Endocrinol. Metab., 2014, 99, 3275–3284 CrossRef CAS PubMed.
- S. S. Ullrich, P. C. E. Fitzgerald, P. Giesbertz, R. E. Steinert, M. Horowitz and C. Feinle-Bisset, Effects of Intragastric Administration of Tryptophan on the Blood Glucose Response to a Nutrient Drink and Energy Intake, in Lean and Obese Men, Nutrients, 2018, 10, 463 CrossRef PubMed.
- R. Ayaso, H. Ghattas, M. Abiad and O. Obeid, Meal Pattern of Male Rats Maintained on Amino Acid Supplemented Diets: The Effect of Tryptophan, Lysine, Arginine, Proline and Threonine, Nutrients, 2014, 6, 2509–2522 CrossRef CAS PubMed.
- Y. Zhao, X.-Y. Wu, S.-X. Xu, J.-Y. Xie, K.-W. Xiang, L. Feng, Y. Liu, W.-D. Jiang, P. Wu, J. Zhao, X.-Q. Zhou and J. Jiang, Dietary tryptophan affects growth performance, digestive and absorptive enzyme activities, intestinal antioxidant capacity, and appetite and GH–IGF axis-related gene expression of hybrid catfish (Pelteobagrus vachelli♀ × Leiocassis longirostris♂), Fish Physiol. Biochem., 2019, 45, 1627–1647 CrossRef CAS PubMed.
- J. Miao, D. Adewole, S. Liu, P. Xi, C. Yang and Y. Yin, Tryptophan Supplementation Increases Reproduction Performance, Milk Yield, and Milk Composition in Lactating Sows and Growth Performance of Their Piglets, J. Agric. Food Chem., 2019, 67, 5096–5104 CrossRef CAS PubMed.
- M. Valles-Colomer, G. Falony, Y. Darzi, E. F. Tigchelaar, J. Wang, R. Y. Tito, C. Schiweck, A. Kurilshikov, M. Joossens, C. Wijmenga, S. Claes, L. Van Oudenhove, A. Zhernakova, S. Vieira-Silva and J. Raes, The neuroactive potential of the human gut microbiota in quality of life and depression, Nat. Microbiol., 2019, 4, 623–632 CrossRef CAS PubMed.
- H. Han, B. Yi, R. Zhong, M. Wang, S. Zhang, J. Ma, Y. Yin, J. Yin, L. Chen and H. Zhang, From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators, Microbiome, 2021, 9, 162 CrossRef PubMed.
- M. A. Smith, A. I. Choudhury, J. A. Glegola, P. Viskaitis, E. E. Irvine, P. Silva, S. Khadayate, H. U. Zeilhofer and D. J. Withers, Extrahypothalamic GABAergic nociceptin-expressing neurons regulate AgRP neuron activity to control feeding behavior, J. Clin. Invest., 2020, 130, 126–142 CrossRef CAS PubMed.
- J. Berrios, C. Li, J. C. Madara, A. S. Garfield, J. S. Steger, M. J. Krashes and B. B. Lowell, Food cue regulation of AGRP hunger neurons guides learning, Nature, 2021, 595, 695–700 CrossRef CAS PubMed.
- G.-J. Wang, N. D. Volkow and J. S. Fowler, The role of dopamine in motivation for food in humans: implications for obesity, Expert Opin. Ther. Targets, 2002, 6, 601–609 CrossRef CAS PubMed.
- A. Abizaid, Ghrelin and Dopamine: New Insights on the Peripheral Regulation of Appetite, J. Neuroendocrinol., 2009, 21, 787–793 CrossRef CAS PubMed.
- R. D. Palmiter, Is dopamine a physiologically relevant mediator of feeding behavior?, Trends Neurosci., 2007, 30, 375–381 CrossRef CAS PubMed.
- S. E. Thanarajah, H. Backes, A. G. DiFeliceantonio, K. Albus, A. L. Cremer, R. Hanssen, R. N. Lippert, O. A. Cornely, D. M. Small and J. C. Brüning, Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans, Cell Metab., 2019, 29, 695–706 CrossRef CAS PubMed.
- J. Huang, Z. Zhang, W. Feng, Y. Zhao, A. Aldanondo, M. G. de Brito Sanchez, M. Paoli, A. Rolland, Z. Li, H. Nie, Y. Lin, S. Zhang, M. Giurfa and S. Su, Food wanting is mediated by transient activation of dopaminergic signaling in the honey bee brain, Science, 2022, 376, 508–512 CrossRef CAS PubMed.
- C. Berland, D. M. Small, S. Luquet and G. Gangarossa, Dietary lipids as regulators of reward processes: multimodal integration matters, Trends Endocrinol. Metab., 2021, 32, 693–705 CrossRef CAS PubMed.
- M. K. Hankir, F. Seyfried, C. A. Hintschich, T.-A. Diep, K. Kleberg, M. Kranz, W. Deuther-Conrad, L. A. Tellez, M. Rullmann, M. Patt, J. Teichert, S. Hesse, O. Sabri, P. Brust, H. S. Hansen, I. E. de Araujo, U. Krügel and W. K. Fenske, Gastric Bypass Surgery Recruits a Gut PPAR-α-Striatal D1R Pathway to Reduce Fat Appetite in Obese Rats, Cell Metab., 2017, 25, 335–344 CrossRef CAS PubMed.
- Y. Yao, X. Cai, W. Fei, Y. Ye, M. Zhao and C. Zheng, The role of short-chain fatty acids in immunity, inflammation and metabolism, Crit. Rev. Food Sci. Nutr., 2022, 62, 1–12 CrossRef CAS PubMed.
- E. S. Chambers, D. J. Morrison and G. Frost, Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms?, Proc. Nutr. Soc., 2015, 74, 328–336 CrossRef CAS PubMed.
- C. S. Byrne, E. S. Chambers, D. J. Morrison and G. Frost, The role of short chain fatty acids in appetite regulation and energy homeostasis, Int. J. Obes., 2015, 39, 1331–1338 CrossRef CAS PubMed.
- A. Jiao, B. Yu, J. He, J. Yu, P. Zheng, Y. Luo, J. Luo, H. Yan, Q. Wang, H. Wang, X. Mao and D. Chen, Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes, Nutrition, 2021, 87–88, 111198 CrossRef CAS PubMed.
- M. A. González Hernández, E. E. Canfora, J. W. E. Jocken and E. E. Blaak, The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity, Nutrients, 2019, 11, 1943 CrossRef PubMed.
- G. Frost, M. L. Sleeth, M. Sahuri-Arisoylu, B. Lizarbe, S. Cerdan, L. Brody, J. Anastasovska, S. Ghourab, M. Hankir, S. Zhang, D. Carling, J. R. Swann, G. Gibson, A. Viardot, D. Morrison, E. Louise Thomas and J. D. Bell, The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism, Nat. Commun., 2014, 5, 3611 CrossRef CAS PubMed.
- T. Arora, R. Sharma and G. Frost, Propionate. Anti-obesity and satiety enhancing factor?, Appetite, 2011, 56, 511–515 CrossRef PubMed.
- R. M. A. J. Ruijschop, A. E. M. Boelrijk and M. C. te Giffel, Satiety effects of a dairy beverage fermented with propionic acid bacteria, Int. Dairy J., 2008, 18, 945–950 CrossRef CAS.
- E. S. Chambers, A. Viardot, A. Psichas, D. J. Morrison, K. G. Murphy, S. E. K. Zac-Varghese, K. MacDougall, T. Preston, C. Tedford, G. S. Finlayson, J. E. Blundell, J. D. Bell, E. L. Thomas, S. Mt-Isa, D. Ashby, G. R. Gibson, S. Kolida, W. S. Dhillo, S. R. Bloom, W. Morley, S. Clegg and G. Frost, Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults, Gut, 2015, 64, 1744 CrossRef CAS PubMed.
- L. Zhang, C. Liu, Q. Jiang and Y. Yin, Butyrate in Energy Metabolism: There Is Still More to Learn, Trends Endocrinol. Metab., 2021, 32, 159–169 CrossRef CAS PubMed.
- Z. Li, C.-X. Yi, S. Katiraei, S. Kooijman, E. Zhou, C. K. Chung, Y. Gao, J. K. van den Heuvel, O. C. Meijer, J. F. P. Berbée, M. Heijink, M. Giera, K. Willems van Dijk, A. K. Groen, P. C. N. Rensen and Y. Wang, Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit, Gut, 2018, 67, 1269 CrossRef CAS PubMed.
- Y. Wang, Z. Li, C. X. Yi, S. Katiraei, S. Kooijman, E. Zhou, C. Chung, Y. Gao, J. K. van den Heuvel, O. C. Meijer, J. F. P. Berbée, M. Heijink, M. Giera, J. A. P. Willems van Dijk, A. K. Groen and P. C. N. Rensen, Butyrate via the gut-brain neural circuit reduces appetite and activates brown adipose tissue, Atherosclerosis, 2018, 275, e15–e16 CrossRef.
- I. Mohanty, C. Allaband, H. Mannochio-Russo, Y. El Abiead, L. R. Hagey, R. Knight and P. C. Dorrestein, The changing metabolic landscape of bile acids – keys to metabolism and immune regulation, Nat. Rev. Gastroenterol. Hepatol., 2024, 21, 493–516 CrossRef PubMed.
- S. Higuchi, T. R. Ahmad, D. A. Argueta, P. A. Perez, C. Zhao, G. J. Schwartz, N. V. DiPatrizio and R. A. Haeusler, Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice, Gut, 2020, 69, 1620 CrossRef PubMed.
- C. Xie, W. Huang, R. L. Young, K. L. Jones, M. Horowitz, C. K. Rayner and T. Wu, Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease, Nutrients, 2021, 13, 1104 CrossRef CAS PubMed.
- R. E. Kuhre, N. J. Wewer Albrechtsen, O. Larsen, S. L. Jepsen, E. Balk-Møller, D. B. Andersen, C. F. Deacon, K. Schoonjans, F. Reimann, F. M. Gribble, R. Albrechtsen, B. Hartmann, M. M. Rosenkilde and J. J. Holst, Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas, Mol. Metab., 2018, 11, 84–95 CrossRef CAS PubMed.
- A. Perino, L. A. Velázquez-Villegas, N. Bresciani, Y. Sun, Q. Huang, V. S. Fénelon, A. Castellanos-Jankiewicz, P. Zizzari, G. Bruschetta, S. Jin, A. Baleisyte, A. Gioiello, R. Pellicciari, J. Ivanisevic, B. L. Schneider, S. Diano, D. Cota and K. Schoonjans, Central anorexigenic actions of bile acids are mediated by TGR5, Nat. Metab., 2021, 3, 595–603 CrossRef CAS PubMed.
- X. Wu, J. Y. Li, A. Lee, Y. X. Lu, S. Y. Zhou and C. Owyang, Satiety induced by bile acids is mediated via vagal afferent pathways, JCI Insight, 2020, 5, e132400 CrossRef PubMed.
- R. Kamakura, G. S. Raza, N. Sodum, V.-P. Lehto, M. Kovalainen and K.-H. Herzig, Colonic Delivery of Nutrients for Sustained and Prolonged Release of Gut Peptides: A Novel Strategy for Appetite Management, Mol. Nutr. Food Res., 2022, 66, 2200192 CrossRef CAS PubMed.
- C. Beglinger and L. Degen, Gastrointestinal satiety signals in humans—Physiologic roles for GLP-1 and PYY ?, Physiol. Behav., 2006, 89, 460–464 CrossRef CAS PubMed.
- F. Xie, J. Shen, T. Liu, M. Zhou, L. J. Johnston, J. Zhao, H. Zhang and X. Ma, Sensation of dietary nutrients by gut taste receptors and its mechanisms, Crit. Rev. Food Sci. Nutr., 2023, 63, 5594–5607 CrossRef CAS PubMed.
- L. Y. Hasek, R. J. Phillips, G. Zhang, K. P. Kinzig, C. Y. Kim, T. L. Powley and B. R. Hamaker, Dietary Slowly Digestible Starch Triggers the Gut–Brain Axis in Obese Rats with Accompanied Reduced Food Intake, Mol. Nutr. Food Res., 2018, 62, 1700117 CrossRef PubMed.
- W. Qin, W. Ying, B. Hamaker and G. Zhang, Slow digestion-oriented dietary strategy to sustain the secretion of GLP-1 for improved glucose homeostasis, Compr. Rev. Food Sci. Food Saf., 2021, 20, 5173–5196 CrossRef CAS PubMed.
- A. L. Sands, H. J. Leidy, B. R. Hamaker, P. Maguire and W. W. Campbell, Consumption of the slow digesting starch waxy maize leads to blunted and sustained carbohydrate utilization but does not influence energy expenditure or appetite, FASEB J., 2008, 22, 1089 Search PubMed.
- A. L. Sands, H. J. Leidy, B. R. Hamaker, P. Maguire and W. W. Campbell, Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans, Nutr. Res., 2009, 29, 383–390 CrossRef CAS PubMed.
- R. N. Carmody, J. E. Bisanz, B. P. Bowen, C. F. Maurice, S. Lyalina, K. B. Louie, D. Treen, K. S. Chadaideh, V. Maini Rekdal, E. N. Bess, P. Spanogiannopoulos, Q. Y. Ang, K. C. Bauer, T. W. Balon, K. S. Pollard, T. R. Northen and P. J. Turnbaugh, Cooking shapes the structure and function of the gut microbiome, Nat. Microbiol., 2019, 4, 2052–2063 CrossRef PubMed.
- S. K. Gill, M. Rossi, B. Bajka and K. Whelan, Dietary fibre in gastrointestinal health and disease, Nat. Rev. Gastroenterol. Hepatol., 2021, 18, 101–116 CrossRef CAS PubMed.
- W. Qin, L. Sun, M. Miao and G. Zhang, Plant-sourced intrinsic dietary fiber: Physical structure and health function, Trends Food Sci. Technol., 2021, 118, 341–355 CrossRef CAS.
- J. Slavin and H. Green, Dietary fibre and satiety, Nutr. Bull., 2007, 32, 32–42 CrossRef.
- M. Kristensen and M. G. Jensen, Dietary fibres in the regulation of appetite and food intake. Importance of viscosity, Appetite, 2011, 56, 65–70 CrossRef CAS PubMed.
- L. Shang, T. Ai, J. Li and B. Li, Correlations between sol viscosity of the partially degraded konjac glucomannan and appetite response of rats, Food Hydrocolloids Health, 2021, 1, 100026 CrossRef CAS.
- L. P. Guo, D. Goff, M. S. Chen and F. Zhong, The hydration rate of konjac glucomannan after consumption affects its in vivo glycemic response and appetite sensation and in vitro digestion characteristics, Food Hydrocolloids, 2022, 122, 107102 CrossRef CAS.
- L. P. Guo, W. Yokoyama, M. S. Chen and F. Zhong, Konjac glucomannan molecular and rheological properties that delay gastric emptying and improve the regulation of appetite, Food Hydrocolloids, 2021, 120, 106894 CrossRef CAS.
- P. Xia, Y. Zheng, L. Sun, W. Chen, L. Shang, J. Li, T. Hou and B. Li, Regulation of glycose and lipid metabolism and application based on the colloidal nutrition science properties of konjac glucomannan: A comprehensive review, Carbohydr. Polym., 2024, 331, 121849 CrossRef CAS PubMed.
- A. J. Page, S. Christie, E. Symonds and H. Li, Circadian regulation of appetite and time restricted feeding, Physiol. Behav., 2020, 220, 112873 CrossRef CAS PubMed.
- M. Garaulet and P. Gómez-Abellán, Timing of food intake and obesity: A novel association, Physiol. Behav., 2014, 134, 44–50 CrossRef CAS PubMed.
- J. Richter, N. Herzog, S. Janka, T. Baumann, A. Kistenmacher and K. M. Oltmanns, Twice as High Diet-Induced Thermogenesis After Breakfast vs Dinner On High-Calorie as Well as Low-Calorie Meals, J. Clin. Endocrinol. Metab., 2020, 105, E211–E221 CrossRef PubMed.
- I. E. Young, A. Poobalan, K. Steinbeck, H. T. O'Connor and H. M. Parker, Distribution of energy intake across the day and weight loss: A systematic review and meta-analysis, Obes. Rev., 2023, 24, e13537 CrossRef CAS PubMed.
- L. C. Ruddick-Collins, P. J. Morgan, C. L. Fyfe, J. A. N. Filipe, G. W. Horgan, K. R. Westerterp, J. D. Johnston and A. M. Johnstone, Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity, Cell Metab., 2022, 34, 1472–1485 CrossRef CAS PubMed.
- D. Jakubowicz, M. Barnea, J. Wainstein and O. Froy, High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women, Obesity, 2013, 21, 2504–2512 CrossRef CAS PubMed.
- K. P. Kelly, O. P. McGuinness, M. Buchowski, J. J. Hughey, H. Chen, J. Powers, T. Page and C. H. Johnson, Eating breakfast and avoiding late-evening snacking sustains lipid oxidation, PLoS Biol., 2020, 18, e3000622 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |