Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Re-wiring petrochemical clusters: impact of using alternative carbon sources for ethylene production†
James Tonny
Manalal
 *a,
Mar
Pérez-Fortes
*a,
Mar
Pérez-Fortes
 a and
Andrea
Ramírez
b
a and
Andrea
Ramírez
b
aDepartment of Engineering, Systems and Services, Faculty of Technology Policy and Management, Delft University of Technology, Jaffalaan 5, 2628BX, Delft, Netherlands. E-mail: j.t.manalal@tudelft.nl
bDepartment of Chemical Engineering, Faculty of Applied Sciences, Delft University of Technology, Van der Maasweg 9, 2629 HZ, Delft, Netherlands
First published on 14th May 2025
Abstract
To achieve climate change mitigation targets, defossilising the production of bulk chemicals like ethylene will be critical. These high-volume petrochemicals are typically produced from fossil-based feedstocks in industrial clusters, which are highly integrated in terms of mass and energy. Replacing fossil-based processes in interconnected industrial clusters can, therefore, impact such interactions and decrease performance or cause lock-in situations at the cluster level. This has, however, been overlooked in the literature. This paper addresses this knowledge gap by evaluating the impacts of replacing fossil-based ethylene production in an existing industrial cluster with processes that use Alternative Carbon Sources (ACS) such as biomass, CO2 and plastic waste. This study explicitly evaluates the performance of the ACS-based production routes at process and cluster levels by assessing changes in mass, energy, prices, CO2 emissions and water demand. The results show that due to the notable difference in product distribution, energy needs and waste generation, a complete re-wiring of the petrochemical cluster in terms of mass, energy and revenue will be required. The results also indicate that defossilising ethylene production in existing industrial clusters can result in a shifting of burden outside the cluster for byproduct production, which can lead to increasing fossil-fuel use outside the cluster. At process level, the main challenges to defossilise ethylene are access to large quantities of clean energy and the large investment costs. Under current market conditions, among the different options examined, plastic pyrolysis is the most competitive ACS-based technology with the lowest impact at the cluster level. However, this requires a large availability of plastic waste, which will be challenging given current recycling rates. Further improvements in waste valorisation and integration of renewable energy-based heating will also be required to make this technology environmentally appealing.
Green foundation1. This work evaluates the broader systemic or cluster level impacts on mass, energy, and price flows for defossilising ethylene production and advances the topic of green chemistry by providing actionable insights for policymakers and industry stakeholders.2. The results of this work indicate that in the case of ethylene, it won't be a plug-and-play situation for defossilising the production of ethylene in an existing petrochemical cluster, as the alternative carbon source (ACS) based processes have highly different product distribution and need around 5 to 10 times higher area, electricity or water. 3. In future studies, the work can be expanded by exploring the systemic impacts of other promising technologies like bio-ethanol or bio-naphtha to ethylene, and studying the potential of valorising by-products to high value chemicals and integration of renewable energy for heating needs in the ACS-based processes. |
1. Introduction
Defossilising1 petrochemical feedstocks by using alternative carbon sources (ACSs) such as CO2, biomass, and waste plastic is key to achieving climate neutrality targets.2,3 Today, about 85% of all chemicals produced globally are made from fossil sources.4 Fossil-based feedstocks like methane and naphtha are currently used for producing chemical building blocks (CBBs) like methanol, ethylene, propylene, benzene, toluene and xylene. These CBB chemicals can, however, also be produced from ACS-based feedstocks, which is expected to not only avoid fossil-fuel usage but also benefit the environment through carbon recycling.5–8Several studies have looked into pathways, enablers and impacts of defossilising the chemical sector. For instance, Bazzanella et al.,5 explored the transition of the European chemical industry to carbon neutrality. They identified several challenges, including the availability of low carbon energy and ACS feedstocks, high investment cost of ACS technologies, uncompetitive production costs of ACS-based chemicals and uncertainty regarding future policies.5,6,9 They estimated the production costs of olefins from ACS-based processes to be two to five times higher than their fossil-based counterparts at current conditions.5 They also concluded that for the European chemical industry to be carbon neutral by 2050, it will require around 4900 TW h low-carbon power, 50 to 300 Mt feedstock CO2, 200 to 250 Mt dry lignocellulosic biomass, and an extensive additional investment of 800 to 900 billion Euro.5 The range in the quantities of CO2 and dry biomass is due to the differences in the intermediate (up to 59% reduction in CO2 emissions) and ambitious (more than 95% reduction in CO2 emissions) scenarios for the chemical industries in Europe.5 Vogt et al.,10 analysed possible pathways to make sustainable fuels (like diesel, kerosene) and chemicals (like olefins, aromatics) by defossilising all refineries by 2050 at global-level. They concluded that about 1400 Mt biomass (with a molecular weight of 30 g mol−1 C) and 630 Mt plastic waste (with a molecular weight of 14 g mol−1 C) will be needed to defossilise the 615 refineries worldwide. Kähler et al.7 also pointed out that recycling (including chemical and mechanical recycling) will be the most important carbon source by 2050 and could contribute up to 55% of the global required carbon in the chemical industry. Their study acknowledged that the main type of ACS can change throughout regions due to feedstock availability, energy availability, access to technology, market conditions and that there will not be a one-size-fits-all solution.
Although these type of global level studies provide broad perspectives regarding the global impact of defossilising the chemical industry, location specific studies have also been conducted to understand the techno-economic and environmental impact of ACS at a regional level. As an example, Stork et al.,11 analysed potential pathways to reduce 80–95% of greenhouse gas emissions in the Dutch chemical industry. They showed that using ACS feedstocks will be vital to reduce non-energy related (scope-1 and scope-3) emissions by 2050 and that around 26 Billion Euro will be required.11 Schijndel et al.12 examined a portfolio of technologies that included electrolysis, pyrolysis, gasification, fermentation, Fischer–Tropsch and methanol synthesis for the Dutch chemical production. They showed that by 2050, around 35 Mt of biomass and recycled plastic will be needed. The study also highlighted that even if the current renewable energy ambitions of the Netherlands would be achieved (70 GW wind and 100 GW solar energy by 2050), this would not be enough, as the required renewable energy demand by a defossilised chemical industry was estimated at 300 TW h. In a more site-specific study, Samadi et al.13 explored defossilisation options for chemical industries in the Port of Rotterdam (PoR). They considered water electrolysis, gasification, syngas hydrogenation and pyrolysis to produce base chemicals. The study showed that by 2050, about 9 Mt of dry biomass and 20–50 TW h of low carbon electricity are required to achieve carbon neutrality.13 Even though these studies provide valuable insights regarding the pathways and bottlenecks for the transition of the Dutch chemical sector; they do not provide insights regarding the impacts of transitioning CBB chemicals in existing industrial clusters.
Among the CBB chemicals, ethylene has the largest global production14 and the highest CO2 emissions during production, due to the energy-intensive process of naphtha or ethane steam cracking.15 Manuel et al.,15 analysed the defossilisation of the Dutch CBBs, including ethylene, and found that synthetic naphtha from waste plastic was the preferred choice for high-value chemical production due to the significantly lower levelised cost of production compared to bio-based or hydrogen-based routes. However, currently, only 9% to 14% of plastics are globally recycled, and studies suggest that by 2050, a circular economy approach could only meet 20% to 30% of the synthetic naphtha demand.9,16,17 Hence, other technologies such as methanol to olefins are gaining interest to fulfil future defossilisation goals for ethylene production.16,18,19 Those studies have also shown that, without integrating ACS feedstock change along with renewable energy, the ACS technologies could result in a net increase in emissions and have unintended environmental impacts.8
There are, however, several important considerations present in state-of-the-art studies,12,17,20 including those discussed before, which require further attention. First, simplified models of the production processes, including black box and linear models are often used, partly due to the low technology readiness level (TRL) of most ACS-based processes. A low TRL level implies high uncertainties in data, leading to large variations in the techno-economic and environmental impacts. For example, pyrolysis oil from waste plastic has different hydrocarbon fractions21 and the current naphtha steam crackers can handle only a part of this hydrocarbon fraction (i.e., hydrocarbon in the boiling range of 35 to 180 °C).22 Studies such as by van Schijndel et al.,12 assumed that the whole pyrolysis oil can be used as feedstock for ethylene production, which will lead to lower estimates of waste plastic needs or CO2 emissions.
Second, chemical production plants are not stand-alone systems, as they produce by-products which are generally used as a feedstock or a source of energy by other units or plants (as shown in Fig. 1). Replacing the fossil feedstock will not only have techno-economic and environmental impacts for the process that is replaced, but might also cause cluster-level impacts due to the high system integration in the chemical sector.10 Chemical production plants are generally allocated in industrial clusters, where mass and energy flows are exchanged with other plants.23 Third, for the proper functioning of a chemical production plant, auxiliary units such as combined heat and power (CHP) plants, steam systems, and cooling water systems are critical.24 Any change in production processes due to the use of ACS feedstock can impact equipment land footprint, scope-2 CO2 emissions and secondary water demand. To date, these impacts have not been well studied in the literature13,25–36
This paper aims to address these three knowledge gaps, by evaluating the techno-economic impacts of displacing fossil-based ethylene production in an existing industrial cluster with processes that use ACS feedstocks. This study explicitly evaluates the performance of the production routes at process and cluster levels by assessing changes in (1) the dependency on import and/or export (changes in mass and energy flows in the cluster), (2) cascading impact of changes in ethylene price throughout different value-chains, (3) changes in CO2 emissions and water demand.
2. Methodology
To study the impacts of defossilising ethylene production in an existing petrochemical cluster, the current situation in the Port of Rotterdam (PoR) was used as a point of departure (reference case cluster). Potential ACS-based processes to produce ethylene were identified and modelled, mimicking industrial scale capacities. Impacts were evaluated both at process and cluster levels. Further details are provided below.2.1 Reference case: fossil-based ethylene cluster
Material, energy and economic data for the reference case were obtained from an in-house model of a petrochemical cluster in the PoR as described in Tan et al.23 Each individual process was modelled at plant level in Aspen Plus v12. In total, the in-house model includes 57 chemical processes and their energy islands. In this study, we focus on the ethylene cluster, as shown in Fig. 2 (the red, blue and green lines represent ethylbenzene, ethylene oxide and ethyl dichloride value-chains).The Naphtha steam cracker in the reference case produces about 900 kt per year ethylene, 500 kt per year propylene and 700 kt per year benzene. Steam cracking involves the high-temperature pyrolysis (at 850 °C) of saturated hydrocarbons in the presence of steam.37 During steam cracking, a wide range of lower hydrocarbons are produced, mainly methane, ethylene, ethane, acetylene, propylene, propane, butene, butadiene, benzene, toluene, xylene, ethylbenzene, methyl hexane and methyl heptane.37 After the steam cracking (as shown in Fig. 3), the hydrocarbon mixture undergoes downstream purification to remove impurities (e.g. CO2, H2S, H2O) followed by complex separation processes (e.g., high pressure cryogenic distillation and extractive distillation) to separate the individual components into high purity products.37Fig. 3 shows the simplified block flow diagram of the model used in this study. The detailed process flow diagram (PFD), modelling assumptions, mass balance, energy balance, economic calculations and data sources is provided in Appendix A.
 | ||
| Fig. 3 Block flow diagram of Naphtha steam cracker process (abbreviation: BTX-benzene, toluene and xylene). | ||
In the in-house petrochemical cluster, we explicitly model the energy islands mimicking existing conditions in the PoR. Note that in many cases the existing energy islands provide steam and electricity to more than one process. Fig. 4 shows the energy island of the reference olefin plant, which includes the electricity, heating and cooling flows/needs, the associated flows of energy to different processes, and the CHP plant. Further detailed of energy and mass balances of the different processes can be seen in Tan et al.23
2.2 Ex-ante ACS process modelling
To defossilise ethylene production, ACS-based ethylene production processes were modelled to match the 900 kt per year ethylene demand in the cluster. Table 1 shows the six ACS-based ethylene production routes that were included in this study together with the TRL of the different steps. For the ACS-based processes, due to time and resource constrains, only two promising technologies from each feedstock were selected using the screening methodology which considered the indicators TRL, number of reaction steps, ideal heat and electricity needs; based on our previous work.38| Alternative carbon source technologies and their TRLs | ||
|---|---|---|
| CO2 routes | Biomass routes | Waste plastic routes |
| Direct electrochemical | Fischer Tropsch ± methanol to olefin | Pyrolysis ± cracking |
| a The Fischer Tropsch and methanol to olefin based production processes occur in parallel and the process configuration is detailed in ESI section S2.† | ||
| - Direct electrochemical reduction of CO2-H2O to ethylene [TRL = 4] | - Biomass steam gasification to syngas [TRL = 7] | - Plastic low temperature pyrolysis [TRL = 7] |
| - Water electrolysis [TRL = 8] | - Water electrolysis [TRL = 8] | |
| - Syngas to Fischer Tropscha [TRL = 9] | - Pyrolysis oil steam cracking [TRL = 7] | |
| - CO2 hydrogenation to methanola [TRL = 7] | ||
| - Methanol to olefina [TRL = 8] | ||
| Methanol to olefin | Methanol to olefin | Methanol to olefin |
| - CO2 hydrogenation to methanol [TRL = 7] | - Biomass steam gasification to syngas [TRL = 7] | - Plastic steam gasification to syngas [TRL = 8] |
| - Water electrolysis [TRL = 8] | - Water electrolysis [TRL = 8] | - Syngas to methanol [TRL = 9] |
| - Methanol to olefin [TRL = 8] | - Syngas to methanol [TRL = 9] | - Methanol to olefin [TRL = 8] |
| - Methanol to olefin [TRL = 8] | ||
The technologies in the ACS-based processes were modelled using Aspen Plus and Aspen Economic Analyzer V12. All models reached an ethylene purity of 99.9 wt% and have a production capacity of about 300 kt per year, equivalent to 1/3rd of the demand in the PoR, which is ambitious for ACS-based technologies while still being industrially relevant capacities.23 The performance of the different routes were evaluated at process and cluster level for 300 kt and 900 kt ethylene per year, respectively, using the indicators explained in section 2.3. A short description of the different technologies in each route is provided in ESI section S2† and the detailed assumptions, mass balance, energy balance, economics and data sources for each technology can be found in the Appendix A.
2.3 Process and cluster level impacts
ACS processes for ethylene production were compared using techno-economic and environmental key performance indicators (KPIs). At process level, KPIs were based on process complexity, process performance, utility needs, economics, CO2 emissions and water demand (as shown in Table 2). The ACS-based processes to produce ethylene were first analysed as stand-alone using process level KPIs. In a second step, they were introduced into the reference case cluster by substituting the fossil-based plant. At cluster level, the KPIs used were based on changes in mass flow, energy flow, price, CO2 emissions and water demand with respect to the reference case (as shown in Table 2).| Assessment level | Key performance indicators | ||
|---|---|---|---|
| Technical | Economic | Environmental | |
| a Change with respect to the reference case. | |||
| Process level | • Process complexity (process temperature range, process pressure range, single pass conversion, bare equipment area) | • CAPEX | • Scope-1 and scope-2 CO2 emissions |
| • Product yields and product purity | • OPEX | • Primary and secondary water demand | |
| • Heating, cooling and electricity (utility needs) | • Minimum selling price (MSP) | • Carbon utilisation efficiency (CUE) | |
| Cluster level | Import or export dependency impacts (feed, products, utility): | • CAPEX | • Scope-1 and scope-2 CO2 emission |
| • Change in mass flowsa | • Change in MSPa | • Primary and secondary water demand | |
| • Change in energy flows | |||
All processes were modelled in Aspen Plus considering the heating and cooling utilities as given in Table 3. The heat integration strategy used in this study was to heat or cool the process streams step-wise using the different utilities, to calculate the net utility need or production.
| Heating utilities | Cooling utilities | ||
|---|---|---|---|
| Type | Conditions | Type | Conditions |
| HT utility– High temperature utility is used when the heat need is greater than 350 °C and in this study natural gas with a lower heating value of 37.8 MJ kg−1 was used. | |||
| R50 generation | Saturated vapor to liquid methane at 1.02 bar | HHPS generation | Saturated liquid to steam at 500 °C and 100 bar |
| R1150 generation | Saturated vapor to liquid ethylene at 1.02 bar | HPS generation | Saturated liquid to steam at 51 bar |
| R134a generation | Saturated vapor to liquid 1,1,1,2-tetrafluoroethane at 1.02 bar | MPS generation | Saturated liquid to steam at 21 bar |
| Chilled water generation | 16 wt% propylene glycol-water mixture from 7.5 °C to 5 °C at 1.02 bar | LPS generation | Saturated liquid to steam at 5.5 bar |
| Low-low pressure steam (LLPS) | Saturated steam to liquid at 3.9 bar | LLPS generation | Saturated liquid to steam at 3.9 bar |
| Low pressure steam (LPS) | Saturated steam to liquid at 5.5 bar | Cooling water | 25 °C to 40 °C at 1.02 bar |
| Medium pressure steam (MPS) | Saturated steam to liquid at 21 bar | Chilled water | 16 wt% propylene glycol-water mixture from 5 °C to 7.5 °C at 1.02 bar |
| High pressure steam (HPS) | Saturated steam to liquid at 51 bar | R134a | Saturated liquid to vapor 1,1,1,2-tetrafluoroethane at 1.02 bar |
| High-high pressure steam (HHPS) | Steam at 500 °C and 100 bar to saturated liquid | R1150 | Saturated liquid to vapor ethylene at 1.02 bar |
| Natural gas or high temperature (HT) utility | 81.4 wt% methane, 1 wt% CO2, 14.4 wt% N2, 3 wt% ethane, 0.2 wt% propane at 15 °C and 1.02 bar | R50 | Saturated liquid to vapor methane at 1.02 bar |
CAPEX was calculated departing from the bare equipment costs obtained from Aspen Economic Analyzer for the different unit models and using estimates from Towler & Sinnott24 (as shown in ESI Table S6†). The fixed OPEX estimate was based on Towler & Sinnott24 (as shown in ESI Table S6†). The variable OPEX was calculated based on mass and energy flows obtained from the Aspen Plus models. Price data39,40 was adjusted to the base year 2018 by using the chemical producer price index41 (as given in ESI Table S7†). The equipment life was assumed 25 years except in the case of proton exchange membrane (PEM) water electrolyzers (9 years)42 and CO2 electrolyzers (assumed to be 5 years based on past industrial scale PEM electrolyzer equipment life).43 Only the electrolyser stack was replaced during the plant life and both the electrolyser stack cost was assumed to be 30% of total electrolyzer cost.44 The minimum selling price (MSP) was calculated for 8% return on investment (ROI) with CAPEX, OPEX and revenue. For multi-product processes, the MSP was calculated by using revenue allocation38 as explained in our previous work.38 The price calculation using the revenue allocation was used only for the hydrocarbon product streams.
To calculate the scope-2 emissions of both the fossil-based and ACS-based processes, a natural gas based combined heat and power (CHP) plant with a CO2 intensity of 0.076 ktonne CO2 per TJ for electricity and 0.073 ktonne CO2 per TJ for steam were used. Furthermore, a renewable electricity source was assumed in addition to the CHP plant, to assess its impact on the CO2 emission results. The assessment of this study does not include scope-3 emissions.
The primary water demand was calculated for each process by summing up the process water needs, for instance, as gasifying agent, stripping steam, dilution water and electrolysis water. To calculate the secondary water demand (defined in this study as the sum of make-up water for utilities); the cooling water and boiler feed water make-up needs were calculated. The cooling water make-up need was calculated by assuming a 2% make-up due to evaporation and blowdown losses.45 The boiler feed water (BFW) make-up need was calculated by assuming 25% make-up due to blowdown and condensate losses.46 Other water demand associated with feedstocks (like water need for biomass cultivation) were not considered in this study.
The carbon utilisation efficiency definition was introduced on previous work.38 It is defined as the ratio between the amount of carbon in the products and the amount of carbon in the feedstock (eqn (1)).
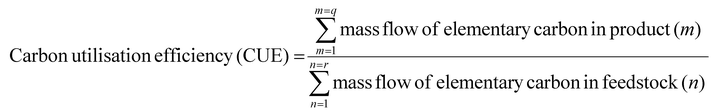 | (1) |
Note that the change in MSP in the cluster looks at the cascading impact on the prices of downstream products in the value chain as a consequence of changes in the price of ethylene. This type of assessment was introduced on previous work38 (eqn (2)) and assumes that for the downstream value-chains, the price increase in ethylene or its derivatives is compensated by a price increase in their respective products, to maintain the same gross margin.
 | (2) |
2.4 Sensitivity analysis
As most ACS-based routes are not yet commercial (as given in Table 1), data is highly uncertain. To explore the impact of uncertainty due to the data inputs and modelling assumptions on the calculated ethylene price of ACS-based processes, a sensitivity analysis was done. Only the uncertainty of ethylene price is discussed in this paper, as the main focus of this study was on ethylene production and because the prices of other by-products vary in the same percentage due to the use of the revenue allocation approach. For the sensitivity analysis, the main contributing factors from OPEX and CAPEX analysis were identified. Based on these major contributing factors and the main underlying assumption behind these factors, the sensitivity analysis on ethylene MSP was conducted by varying these assumptions within the state-of the-art upper and lower limit values (Table 4).| Variables used | Upper limit | Lower limit |
|---|---|---|
| Return on investment | 12% | 4% |
| Food grade CO2 price | 300 EUR per t | 80 EUR per t |
| Biomass pellet price | 400 EUR per t | 50 EUR per t |
| Sorted waste plastic price | 800 EURper t | 400 EUR per t |
| Unsorted waste plastic price | 500 EUR per t | 100 EUR per t |
| RME solvent price | 1000 EUR per t | 350 EUR per t |
| Direct CO2-H2O to ethylene reduction electrochemical cell operating voltage | 3.65 V | 2 V |
| Direct CO2-H2O to ethylene reduction electrochemical cell operational life | 25 years | 5 years (with 50% component replacement) |
| Direct CO2-H2O to ethylene reduction electrochemical cell cost | 1500 EURper kW | 580 EUR perkW |
| PEM water electrolyser operating life | 25 years | 9 years (with 50% component replacement) |
| PEM water electrolyser cost | 1060 EUR per kW | 580 EUR per kW |
| Electricity price | 84 EUR per MW h | 11 EUR per MW h |
| EDTA solvent price | 1340 EUR per t | 500 EUR per t |
3. Results and discussion
3.1 Impacts of using ACS in the ethylene process
The ACS-based routes show considerable differences in techno-economic and environmental performances compared to the reference case (as shown in Tables 5 and 6) and indicates that the defossilisation of ethylene production, is more than just equipment change.| Parameter | Carbon Source | |||||||
|---|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | |||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | ||
| Raw materials | Carbon feedstock | 1034 kt (naphtha) | 1510 kt (CO2) | 4800 kt (CO2) | 6900 kt (biomass) | 5160 kt (biomass) | 5000 kt (sorted plastic waste) | 1860 kt (municipal plastic waste) |
| Water or steam | 568 kt | 2241 kt | 5886 kt | 6907 kt | 5286 kt | 813 kt | 1320 kt | |
| Other feedstocks | 279 kt | — | — | 35 kt | 18 kt | 278 kt | — | |
| Products | Ethylene | 303 kt | 329 kt | 314 kt | 301 kt | 314 kt | 303 kt | 314 kt |
| Propylene | 172 kt | — | 398 kt | 298 kt | 398 kt | 172 kt | 398 kt | |
| Benzene | 237 kt | — | 80 kt | 136 kt | 80 kt | 237 kt | 80 kt | |
| Other hydrocarbon products | 363 kt | 7 kt | 335 kt | 716 kt | 647 kt | 3173 kt | 347 kt | |
| Oxygen | — | 1955 kt | 5226 kt | 2533 kt | 2004 kt | 128 kt | — | |
| Waste | Wastewater | 573 kt | 1338 kt | 2735 kt | 7056 kt | 5315 kt | 573 kt | 1325 kt |
| Hydrocarbon waste | — | — | 1488 kt | 112 kt | — | — | — | |
| Purge or off-gas | 233 kt | 122 kt | 100 kt | 1360 kt | 712 kt | 1035 kt | 256 kt | |
| Char or ash | — | — | 10 kt | 1158 kt | 868 kt | 470 kt | 88 kt | |
| Tar | — | — | — | 172 kt | 126 kt | — | 372 kt | |
| Process carbon utilisation efficiency | 84% | 69% | 68% | 39% | 48% | 74% | 67% | |
| Ethylene yield (ethylene to carbon feedstock mass %) | 29% | 22% | 7% | 4% | 3% | 6% | 18% | |
| Product mass flow | Carbon source | ||||||
|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | ||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | |
| Methane | 143 kt | — | 10 kt | 248 kt | 274 kt | 143 kt | 10 kt |
| Acetylene | 6 kt | — | — | — | — | 6 kt | — |
| Ethane | 43 kt | — | 9 kt | — | 9 kt | 43 kt | 9 kt |
| Ethylene | 303 kt | 329 kt | 314 kt | 301 kt | 314 kt | 303 kt | 314 kt |
| Ethanol | — | 7 kt | — | — | — | — | — |
| Propane | 4 kt | — | 74 kt | 55 kt | 74 kt | 4 kt | 74 kt |
| Propylene | 172 kt | — | 398 kt | 298 kt | 398 kt | 172 kt | 398 kt |
| C4 mixture | 74 kt | — | 106 kt | 154 kt | 106 kt | 74 kt | 106 kt |
| Benzene | 237 kt | — | 80 kt | 136 kt | 80 kt | 237 kt | 80 kt |
| Diesel | — | — | — | — | — | 1456 kt | — |
| VGO | — | — | — | — | — | 1333 kt | — |
| C7+ | 101 kt | — | — | 161 kt | — | 101 kt | — |
| C9+ | 77 kt | — | — | — | — | 77 kt | — |
| Non-aromatics | 101 kt | — | — | 98 kt | — | 101 kt | — |
| Oxygen | — | 1955 kt | 5226 kt | 2533 kt | 2004 kt | — | — |
In terms of mass flow, the ACS-based processes show significantly different raw-material needs, product distribution and waste generation due to the difference in feedstock elemental composition (as shown in Tables S1, S2, S3 and S4 in ESI†) and process efficiencies (Table 5). The differences also exist when looking at the same ACS feedstock. For instance, to produce the same quantity of ethylene, plastic waste pyrolysis requires almost 2.7 times higher feedstock than the plastic based methanol to olefin route. This is because even though the carbon utilisation efficiency of pyrolysis route (74%) is higher than that of the plastic methanol to olefin process (67%), the ethylene yield in the former case (6%) is considerably lower than the methanol to olefin-based process (18%). This can be explained by the differences in by-product production as shown in Table 6.
In terms of product distribution, the plastic based pyrolysis process is most similar to the reference case and the CO2 based direct electrochemical process has the least similarity, as the CO2 based direct electrochemical process is a single product technology while the others are multi-product based technologies. This difference in product distribution can impact downstream units in a highly interconnected cluster, which is discussed in section 3.2.
In terms of energy requirements (Table 7), most ACS-based processes produce sufficient heat energy from off-gas combustion and are energy self-sufficient in terms of heat, except for the plastic based methanol to olefin process. This process requires around 15 PJ per year additional high-temperature heat energy (>900 °C) and 17 PJ per year low-pressure steam, which are almost 8 to 10 times higher than the reference case. The high heat requirements are mostly due to two reasons: the high specific energy need for gasification and the low calorific value of off-gases. For example, the plastic based methanol to olefin process has a reaction energy of 11 MJ kg−1 ethylene, and the off-gas of the process has a lower net calorific value of 27 MJ kg−1; compared to the reaction energy of 13 MJ kg−1 ethylene and lower net calorific value of 52 MJ kg−1 off-gas for the naphtha cracker. This low calorific value for the off-gas from plastic based methanol to olefin process is due to the presence of partially oxidised molecules like CO and CO2 from gasification in the off-gas. To compensate for the low heating value, additional external heat or gas is thus needed in the plastic based methanol to olefin process. In the biomass-based process, even though the heat need is high (∼100 MJ kg−1 ethylene or 30–40 PJ per year), there are significant amounts of off-gases and waste hydrocarbons, which can be internally combusted for energy. Consequently, the biomass-based processes are net heat producers.
| Utility need or production | Carbon source | ||||||
|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | ||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | |
| Negative sign refers to heat production and positive sign refers to cooling, heating or electricity need respectively | |||||||
| Electricity | 2.5 PJ per year | 83.5 PJ per year | 156.6 PJ per year | 117.1 PJ per year | 79.3 PJ per year | 6.1 PJ per year | 6.4 PJ per year |
| LLPS | — | — | −0.5 PJ per year | 0.7 PJ per year | −0.5 PJ per year | — | — |
| LPS | 1.8 PJ per year | −0.6 PJ per year | −4.9 PJ per year | −23.4 PJ per year | −4.9 PJ per year | −3.1 PJ per year | 17.3 PJ per year |
| MPS | −1.2 PJ per year | −1.2 PJ per year | −2.7 PJ per year | −2.4 PJ per year | −7.9 PJ per year | −4.2 PJ per year | −9.4 PJ per year |
| HPS and HHPS | −2.7 PJ per year | — | −0.01 PJ per year | −30.3 PJ per year | −19.3 PJ per year | −33.6 PJ per year | −3.8 PJ per year |
| HT utility | — | — | — | — | — | — | 15 PJ per year |
| Cooling water | 9.1 PJ per year | 15.7 PJ per year | 46.1 PJ per year | 10 PJ per year | 73.5 PJ per year | 12.8 PJ per year | 25.5 PJ per year |
The CO2 based methanol to olefin route has the highest electricity need among all processes, which is almost 62 times higher than the reference case. Of this amount, about 92% of the requirement is for hydrogen generation through water electrolysis (as shown in Fig. 5). In the case of the biomass-based steam gasification route, it requires less electricity for hydrogen production through water electrolysis compared to the CO2 based routes as shown in Fig. 5. However, due to presence of impurities (like methane) in the syngas, the biomass-based processes required more electricity for cryogenic distillation. As a consequence, the CO2 and biomass-based routes show similar electricity needs of around 80–150 PJ per year.
An aspect that is often overlooked is the physical area that new processes would require. This is particularly important when considering their implementation in existing industrial clusters. In the ESI (Table S9†), the bare equipment areas of the different routes are shown. To produce about 300 kt per year ethylene, the biomass-based route has the largest land footprint while CO2 based direct electrochemical process has the lowest. Note that compared to the reference case, all the ACS-based processes require more bare equipment area. In densely populated clusters, such as the PoR, this indicate that physical constrains can affect the potential of technologies.
It is also worth mentioning that even though all the process routes produced 99.9 wt% pure ethylene, their impurities are different (as shown in Table S10 ESI†). For example, in the reference case ethylene have impurities like acetylene, methane and ethane at ppm levels, while the CO2 based direct electrochemical route have CO2 and ethanol. These changes in impurities could potentially affect downstream units, and further research into these impacts needs to be conducted.
The results from the economic analysis show that with exception of plastic pyrolysis, all other ACS-based processes are not competitive under current market conditions (as seen in Table 8). The CO2 based route shows the highest MSP, followed by biomass and plastic. In fact, plastic pyrolysis shows a similar price to the reference case (1075 EUR per t) due to higher CUE and lower CAPEX. A key reason for the higher MSP of ethylene in most ACS processes is that they tend to be more CAPEX and OPEX intensive than the reference route. In absolute terms, the CAPEX investment can be as high as 45 times (CO2 based methanol to olefin route) higher than a conventional naphtha cracker. In all the CAPEX intensive ACS-based routes, hydrogen production accounts for a major share of the costs (as seen in ESI Fig. S25–S38†). For example, in the CO2 based direct electrochemical process, the electrolyzer alone accounts for 54% and in the methanol to olefin-based process the water electrolyser accounts for ∼30% of the total CAPEX.
| Economic indicators | Carbon source | ||||||
|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | ||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | |
| Ethylene MSP (EUR per t) | 1075 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588 588 |
7504 | 4752 | 5268 | 1075 | 2511 |
| CAPEX (million EUR) | 390 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803 803 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 743 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 766 766 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619 619 |
1532 | 2737 |
| OPEX (million EUR per year) | 850 | 3719 | 5872 | 5998 | 5330 | 3616 | 2400 |
The OPEX intensity of the ACS-based routes is due to feedstock price and or utility costs (as seen in ESI Fig. S25–S38†). In the reference case, raw material cost accounts for about 84% of the OPEX, while utilities account for only 12% of the OPEX. For the CO2-based process, the cost of raw material becomes comparatively minor (5% of the OPEX), while utilities account for 53% to 63% of the total OPEX. For the biomass-based process, both raw material and utilities show a similar contribution (31%–40% and 36%–47%, respectively). Finally, for the plastic waste-based routes, raw material cost accounts for 90% and 64% of the OPEX for pyrolysis and plastic based methanol to olefin processes, respectively. The results indicate that the processes which are utility dominant (like CO2 and biomass-based routes) tend to have the highest OPEX intensity compared to those that are feedstock dominant processes (like plastic and fossil-based processes). It is important to note that this can change if feedstock availability is constrained.
It is also important to highlight that for the ACS-based processes, the revenues from by-products are higher than the reference case except for the CO2 based direct electrochemical route (as given in ESI Table S11†). In the reference case, for instance, while ethylene revenue has the highest contribution 33% to the total revenue; for the plastic based pyrolysis route, ethylene contributed only 9% of the total revenue. For the CO2 based direct electrochemical process, the exception is due to the limited by-products. For methanol to olefin-based processes due to the higher propylene to ethylene production ratio compared to the reference case, the propylene contribution to revenue increased and became significantly higher than ethylene (∼30% in methanol to olefin-based vs. 17% in reference case).
In terms of process level CO2 emissions (scope-1), the CO2 based direct electrochemical emits the least CO2 of all routes (95 kt per year) as seen in Fig. 6. Notably, all the other processes emit more direct CO2 than the reference case (633 kt CO2 per year). This higher emission is due to off-gas, char and fuel combustion for high temperature heat or waste to energy recovery in these processes. Thus, for the ACS-based processes, valorisation of off-gas and char to valuable products rather than waste to energy recovery, along with availability of high-temperature renewable heat will be critical for improving their environmental impact. It is important to note that there is a difference in the origin of emissions, while in the reference case, those are of fossil origin, in the ACS routes the emissions have different origins as seen in (Fig. 7). In the case of biogenic emissions, whether they are considered “neutral” will depend on the type and value chain of the biomass. Emissions from waste plastic are so far considered “free” of environmental footprint as they are a waste (following gate-to-gate life cycle analysis guidelines), whether the same principle can be applied to waste to chemical concepts is still under debate. In the case of CO2, the origin will play a key role in how the emissions are considered as they could originated from fossil processes, biogenic processes or the atmosphere (direct air capture).
 | ||
| Fig. 6 Scope-1 CO2 emissions of different ACS-based processes for 300 kt per year ethylene production. | ||
 | ||
| Fig. 7 Scope-1 CO2 emission of different ACS-based technologies for 900 kt per year ethylene production. | ||
In terms of water consumption, in all cases, the ACS-based processes need more process water than the reference case. The biomass-based processes require a larger amount than CO2-based processes (Fig. 8). This is because in this study, a steam dilution ratio (kg steamper kg biomass) of 0.59 is used in the simulation based on GoBiGas gasification technology,47 where 46% to 54% of the process water is needed as gasifying medium. Based on this assumption, biomass steam gasification uses about 13 to 11 times higher process water than a naphtha cracker, while the CO2 based process needs around 10 to 4 times higher process water. The plastic-based methanol to olefin process has around 3.7 times higher water requirement, while the plastic-based pyrolysis process needs the least quantity of process water (1.5 times).
 | ||
| Fig. 8 Primary water demand of different ACS based processes for 300 kt per year ethylene production. | ||
3.2 Cluster level impacts of defossilising the ethylene production
The results indicated that a notable number of flows within the cluster will be significantly affected due to the introductions of ACS processes (e.g., up to 22 mass flows change by more than 100%). This affects both upstream and downstream units interconnected to the olefin plant. The magnitude of this change varies based on the ACS process used, as seen in Tables 9, 10 and Fig. S39–S44 in the ESI.† Hence, defossilising ethylene production in an existing petrochemical cluster is not just limited to changes in process technology but will have larger consequences in terms of mass flow and infrastructure investments.| Mass flow per unit ethylene (kg kg−1) | Carbon source | |||||||
|---|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | |||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | ||
| In | Naphtha | 3.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pygas/reformate | 5.6 | 4.1 | 4.3 | 4.5 | 4.3 | 5.4 | 4.3 | |
| Water | 1.7 | 6.8 | 18.7 | 22.9 | 16.8 | 2.7 | 4.2 | |
| CO2 | 0.0 | 4.6 | 15.3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Biomass | 0.0 | 0.0 | 0.0 | 22.9 | 16.4 | 0.0 | 0.0 | |
| Plastic | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.5 | 5.9 | |
| Other input | 0.4 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | |
| Refinery (propylene) | 0.1 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Refinery (C4s) | 0.4 | 0.6 | 0.3 | 0.1 | 0.3 | 0.4 | 0.3 | |
| Refinery (benzene) | 0.0 | 0.7 | 0.5 | 0.3 | 0.5 | 0.0 | 0.5 | |
| Refinery import (C7+) | 0.0 | 0.3 | 0.3 | 0.0 | 0.3 | 0.0 | 0.3 | |
| Oxygen | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.6 | |
| Mass flow per unit ethylene (kg kg−1) | Carbon source | |||||||
|---|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | |||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | ||
| Out | Ethylene | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Propylene | 0.6 | 0.0 | 1.3 | 1.0 | 1.3 | 0.6 | 1.3 | |
| Refinery (propylene) | 0.1 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| C4s | 0.2 | 0.0 | 0.3 | 0.5 | 0.3 | 0.2 | 0.3 | |
| Refinery (C4s) | 0.4 | 0.6 | 0.3 | 0.1 | 0.3 | 0.4 | 0.3 | |
| Benzene | 0.8 | 0.0 | 0.3 | 0.5 | 0.3 | 0.8 | 0.3 | |
| Refinery (benzene) | 0.0 | 0.7 | 0.5 | 0.3 | 0.5 | 0.0 | 0.5 | |
| Benzene (from aromatics plant) | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | |
| P-xylene | 0.8 | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | |
| O-xylene | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |
| Other products | 0.6 | 0.0 | 0.0 | 1.3 | 0.0 | 9.9 | 0.0 | |
| Other products (from aromatics plant) | 3.1 | 2.8 | 2.9 | 3.1 | 2.9 | 3.0 | 2.9 | |
| C7 + excess | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | |
| Wastewater | 1.9 | 4.1 | 8.7 | 23.4 | 16.9 | 1.9 | 6.5 | |
| Other waste | 0.0 | 0.0 | 5.1 | 9.3 | 5.4 | 5.0 | 0.0 | |
| Ethanol | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Oxygen | 0.7 | 5.9 | 16.6 | 8.4 | 6.4 | 0.7 | 0.6 | |
| Other gases | 0.8 | 0.4 | 0.7 | 0.0 | 1.7 | 0.0 | 0.8 | |
Similar to the results at process level, Table 9 shows considerable variations of ACS feedstock needs to produce similar quantities of ethylene, as the carbon utilisation efficiency and ethylene yield of these technologies are different. For example, to produce 1 kg ethylene almost 15 kg CO2 are needed in the methanol to olefin-based route compared to ca. 5 kg CO2 in the direct electrochemical based process. This significant variation in CO2 need is due to the material inefficiencies in the CO2 hydrogenation to methanol and methanol to olefin process (i.e., high recycle purge needs in methanol reactor (10%) and lower ethylene yield in methanol to olefin process (31%)). However, this does not mean all methanol to olefin-based routes will need higher feedstock. In contrast to the results of the CO2 based routes, to produce 1 kg ethylene; the plastic based methanol to olefin process needs around 6 kg plastic compared to 16 kg plastic in the pyrolysis-based process. This is mainly because, in the plastic based pyrolysis route, the plastic to ethylene yield is 6% compared to 18% in the methanol to olefin-based process, which is due to the high yield of by-products in the pyrolysis process. Therefore, based on the ACS process used, the upstream material infrastructure in the cluster has to be specifically designed.
In addition to these changes, replacing the naphtha cracker for ACS-based ethylene production can also have an unintended effect on imports of other (fossil-based) chemicals in the cluster (as seen in Table 9 and Table S13 ESI†). For instance, the direct electrochemical route does not produce propylene, benzene and C7 + by-products, all of which are important chemical building blocks (CBB) in the cluster. Hence, if these building blocks are not sourced from a sustainable feedstock, it can lead to an increase in fossil feedstock use outside the cluster, to compensate for their lack of production in the cluster, leading to shifting of burden from inside the cluster to outside the cluster. In terms of climate change, it would mean that while the cluster itself could reduce its dependence on fossil feedstocks, the total emissions (estimated as the sum of emissions in and outside the cluster) could increase, thereby taking us farther away of reaching climate targets.
Table 10 shows the changes in mass flow downstream of the olefin plant in the cluster due to ethylene defossilisation. The most affected downstream mass flows in the cluster are wastewater, off-gases, heavy hydrocarbon and oxygen. Except for plastic based pyrolysis, in all other processes, the wastewater production increases from as low as 100% to as high as 1000%. Hence, new wastewater treatment facilities will be required in the cluster. Regarding other utilities, with exception of the waste plastic routes, in all other processes, oxygen production increases between 1000% to 2500%. Therefore, further studies need to explore potential avenues to exploit the high quantity of oxygen. In most processes, except for the CO2 based direct electrochemical and waste plastic-based methanol to olefin, the amount of off-gas and/or heavy hydrocarbons increases in the cluster. This will require additional infrastructure to utilise these hydrocarbon streams for waste to heat recovery, waste to power or as new sources of sustainable feedstock for fuels or chemicals.
The ACS routes significantly affected the energy flows in the cluster. The reference case naphtha cracker has 6 inlet and outlet energy streams, and all these energy streams are affected when the unit is replaced with an ACS-based technology (as seen in Table 11 and Fig. S45–S50 ESI†). These impacts vary from non-use of existing energy infrastructure, to changes in the amount of energy, which can be as large as 650 times in energy flows into the cluster. The most impacted energy streams are cooling water flow, electricity flow and high pressure steam generation flow. The high cooling water flow, for example in the biomass route, is due to the high temperature reactions and distillation-based separation used in these processes. Noted that cooling water requirements could be further decreased by more exhaustive heat integration.
| Utility flow per unit ethylene (MJ kg−1) | Carbon source | |||||||
|---|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | |||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | ||
| Negative sign refers to heat production and positive sign refers to cooling, heating or electricity need respectively | ||||||||
| In | LLPS | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| LPS | 6 | 0 | 0 | 0 | 0 | 0 | 55 | |
| MPS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| HPS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| HHPS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CW | 30 | 48 | 147 | 332 | 234 | 42 | 81 | |
| HT utility | 0 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Electricity | 8 | 254 | 498 | 389 | 253 | 20 | 20 | |
| Out | LLPS | 0 | 0 | −1 | 2 | −2 | 0 | 0 |
| LPS | 0 | −2 | −16 | −78 | −15 | −10 | 55 | |
| MPS | −4 | −4 | −9 | −8 | −25 | −14 | −30 | |
| HPS | −7 | 0 | 0 | −101 | −61 | −109 | −12 | |
| HHPS | −2 | 0 | 0 | 0 | 0 | −2 | 0 | |
The change in ethylene production from fossil naphtha to ACS-based processes will also have economic impacts (as seen in Tabel S14 ESI†) as changes in price of products or byproducts propagate in a cluster through value-chains. Among the ACS routes, the CO2 based ethylene is the most expensive followed by biomass-based ethylene and waste plastic-based ethylene showing the lowest price increase.
Table 12 shows the propagation of the change in cost of ethylene in the value chains. For instance, ethylene is used to produce EDC, which in turn is the main raw material for VCM and VCM is then used to produce PVC (as shown in ESI Table S15†). In this route, CO2 based direct electrochemical process results in an increase in MSP of ethylene of about 1350% which then cascades throughout the chain resulting in an increase on PVC MSP of about 982% (assuming all other products and utilities do not change in price). The impact is highly depending on the importance of ethylene as carbon source in the chain. The CO2 based direct electrochemical process, for instance, affects only the ethylene-based value chains due to the lack of carbon by-products while the CO2 based methanol to olefin route affects the ethylene, propylene and partially benzene value-chains. The partial impact in benzene value-chain is due to the insufficient production of benzene in the ACS-based processes with respect to the demand in the cluster. Among the ethylene value-chains, PVC is impacted the most based on the CO2 process used. This large impact is due to ethylene being the only carbon source for PVC while in other value-chains this is not the case. Among the biomass-based process, the Fischer Tropsch + methanol to olefin route has a slightly higher impact on the PO-SM value-chain than the methanol to olefin-based process as the yield of benzene is slightly higher for Fischer Tropsch + methanol to olefin-based process than the methanol-based process. Compared to CO2, the price impact of biomass-based process is lower. The plastic-based pyrolysis process shows no price impact on the value-chains, as it could produce naphtha at the market price of fossil-based naphtha (∼560 EUR per t). The plastic-based methanol to olefin process creates an increase between 30% to 130% in the value-chains (as shown in Table 12 and ESI Table S15†).
| Price impact in the value-chain (EUR per t) | Carbon source | ||||||
|---|---|---|---|---|---|---|---|
| Naphtha | CO2 based | Biomass based | Plastic based | ||||
| Reference case | Direct electrochemical | Methanol to olefin | Fischer Tropsch + methanol to olefin | Methanol to olefin | Pyrolysis + cracking | Methanol to olefin | |
| In brackets the percentage change in price with respect to the reference case is shown | |||||||
| Polyvinyl chloride (PVC) | 740 | 8009 (+982%) | 3959 (+435%) | 2581 (+249%) | 2840 (+284%) | 740 (No price change) | 1459 (+97%) |
| Polyethylene terephthalate (PET) | 1284 | 5691 (+343%) | 3236 (+152%) | 2401 (+87%) | 2557 (+99%) | 1284 (No price change) | 1720 (+34%) |
| Styrene monomer (SM) | 1328 | 4439 (+234%) | 5293 (+299%) | 4006 (+202%) | 3913 (+195%) | 1328 (No price change) | 2214 (+67%) |
| Propylene oxide (PO) | 1450 | 4847 (+234%) | 5780 (+299%) | 4375 (+202%) | 4275 (+195%) | 1450 (No price change) | 2417 (+67%) |
| Propylene glycol methyl ether (PGME) | 800 | No propylene production | 1666 (+108%) | 1295 (+62%) | 1364 (+70%) | 800 (No price change) | 994 (+24%) |
| Acetone | 856 | No propylene production | 5888 (+588%) | 3734 (+336%) | 4137 (+383%) | 856 (No price change) | 1980 (+131%) |
Regarding CO2 emissions, the indirect (scope-2) emission analysis of the ACS-based processes shows that, as these processes are highly energy intense, scope-2 emissions will be substantially high if existing (fossil) based energy mix is used (as seen in Fig. 9). The major share of scope-2 CO2 emissions is due to the electricity carbon footprint used and if renewable electricity is used, scope-2 emissions are considerably lowered. Replacing fossil-based feedstocks with ACS-based feedstocks requires therefore not only changing technology at plant level, but also major changes in utility infrastructure for the ACS-based processes to be environmentally appealing. It is important to note that this is not the case for waste plastic-based processes as these routes do not need external hydrogen production due to the better C/H ratio found in plastic feedstock.
 | ||
| Fig. 9 Scope-2 CO2 emission of different ACS-based technologies for 900 kt per year ethylene production. | ||
The analysis of the impact on water demand of the cluster due to the ACS-based processes shows that secondary water demand by the cluster also increases, similar to what is observed for primary water demand at process level discussed in the previous section. The highest demand is observed for biomass-based processes in terms of cooling water due to their low-temperature cooling demand (cooling demand between 40 °C and 140 °C). Substantial cooling water and boiler feedwater demand is observed for all ACS-based processes compared to the reference case (as seen in Fig. 10). Hence major infrastructural change in terms of cooling water and boiler feed water will be required to accommodate the new ACS-based processes.
 | ||
| Fig. 10 Primary and secondary water demand of different ACS-based technologies for 900 kt per year ethylene production. | ||
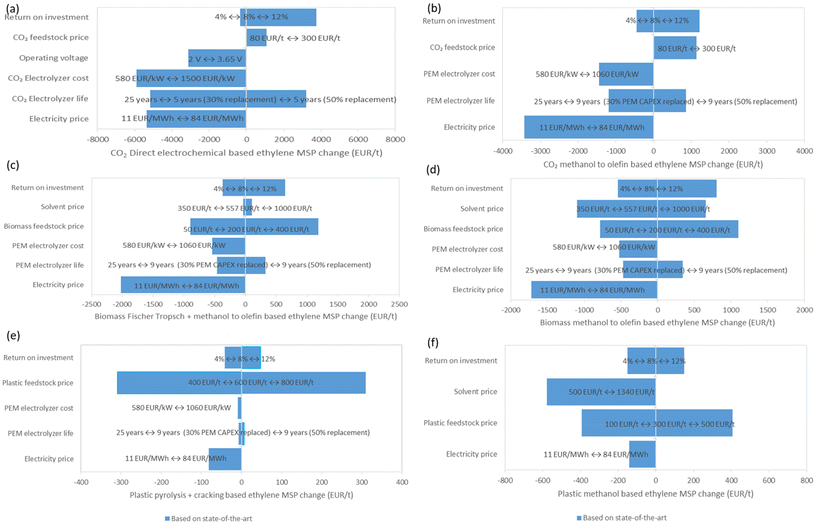
3.3 Sensitivity analysis
The OPEX and CAPEX analysis of the ACS-based processes, shows that the major factors contributing towards the OPEX and CAPEX of these processes are: electrolyser cost, utility cost and raw material cost (as explained in section 3.1 and seen in ESI Fig. S25–S38†). Based on this analysis, a sensitivity analysis is conducted for the following parameters: feedstock price, solvent price, operating voltage, electrolyzer cost, electrolyzer life, electricity price and ROI (as given in Table 4).The sensitivity analysis for CO2 based direct electrochemical process shows that the technology is highly sensitive to electrolyser cost, electrolyser life and electricity price (Fig. 11(a)). For the CO2 based direct electrochemical process (as observed in Fig. S52 ESI†), in the worst-case scenario, the ethylene MSP can be as high as 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 EUR per t. And in the best-case scenario, the ethylene MSP can be as low as 1204 EUR per t. The CO2 based methanol to olefin process is also most sensitive to electricity price (as seen in Fig. 11(b)) and shows an ethylene MSP between 1436 EUR per t to 10
650 EUR per t. And in the best-case scenario, the ethylene MSP can be as low as 1204 EUR per t. The CO2 based methanol to olefin process is also most sensitive to electricity price (as seen in Fig. 11(b)) and shows an ethylene MSP between 1436 EUR per t to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 769 EUR per t in the respective best- and worst-case scenarios (as seen in ESI Fig. S53†).
769 EUR per t in the respective best- and worst-case scenarios (as seen in ESI Fig. S53†).
In the case of biomass-based Fischer Tropsch + methanol to olefin process, it is observed as seen in Fig. 11(c), that the process is most sensitive not only to electricity price but also to biomass feedstock price. For the best-case scenario, the ethylene MSP is as low as 667 EUR per t and for the worst-case scenario, the ethylene MSP is 7041 EUR per t for the biomass-based Fischer Tropsch + methanol to olefin process (as shown in ESI Fig. S54†). In the case of biomass the methanol to olefin route, Fig. 11(d), the process is most sensitive to electricity price, syngas cleaning solvent price and biomass feedstock price. The analysis shows that the biomass-based methanol to olefin process does not have high variability for ethylene MSP (i.e., 1586 EUR per t to 8675 EUR per t in ESI Fig. S55†), as seen in CO2 based processes.
For plastic based pyrolysis process (Fig. 11(e)), the process is highly sensitive to the waste plastic price. The sensitivity analysis (Fig. S56 and S57 in ESI†) shows that the plastic based pyrolysis process has the least variation of 632 EUR per t to 1445 EURper t, compared to the other technologies and potentially could have an MSP comparable to that of the current ethylene MSP (1075 EUR per t). In the case of plastic based methanol to olefin process, Fig. 11(f), the process is most sensitive to the syngas cleaning solvent price and plastic feedstock price. The analysis shows that the plastic based methanol to olefin process has an MSP variation between 1263 EUR per t to 3080 EUR per t (as shown in Fig. S58 in ESI†).
4. Conclusion
Defossilising the production of high-volume petrochemicals like ethylene will be vital to reducing the carbon footprint of our society and meet the ambitious CO2 emissions targets needed to mitigate climate change. However, changing feedstock in a highly interconnected existing cluster can have unintended cascading effects on upstream supply-chains, downstream units and energy islands. The results indicate that in the case of ethylene, it won't be a plug-and-play situation, as the ACS-based processes have highly different product distribution, energy needs and waste generation. The process that has the most similar product distribution and the least impact in the existing petrochemical cluster is the waste plastic pyrolysis process. A significant challenge for other ACS-based technologies is the sizeable amount of renewable electricity at cheap price that will be needed for the hydrogen production required in the routes. The results at cluster level show that for the existing cluster it will not be easy to replace the naphtha cracker with ACS-based technologies due to limitation in area and additional requirements (like electricity supply, water supply); as the ACS-based processes need around 5 to 10 times higher area, electricity or water.The paper also highlights the importance of studying ACS technologies in the context of multiple products in highly symbiotic industrial clusters, as defossilising ethylene can create shifting of burden to outside the cluster and may result in a net increase of the fossil fuel consumption. The unintended major environmental consequences of defossilising ethylene production were the large water consumption and CO2 emissions (scope-1 and scope-2), which could nullify the purpose of defossilisation chemical building blocks. The CO2 emissions analysis shows that ACS-based technologies should be deployed in tandem with renewable energy-based utilities for the system to be low-carbon. Future studies should look into the potential of recycling wastewater, valorising waste and integration of renewable energy for heating needs in ACS-based processes to make it more environmentally appealing. However, to achieve cost competitiveness with existing fossil-based counterparts at current conditions, availability of low-priced renewable energy, feedstocks as well as efficient and durable electrolysers are critical.
Abbreviations
Nomenclature
| ACS | Alternative carbon source |
| BFW | Boiler feed water |
| BTX | Benzene, toluene and xylene |
| CAPEX | Capital expenditure |
| CBB | Chemical building blocks |
| CHP | Combined heat and power |
| CKI | Chloor kringloop installatie (or chlorine incineration) |
| CUE | Carbon utilisation efficiency |
| CW | Cooling water |
| DEG | Diethylene glycol |
| EB | Ethylbenzene |
| EDC | Ethylene dichloride |
| EDTA | Ethylenediamine tetra-acetic acid |
| EG | Ethylene glycol |
| EO | Ethylene oxide |
| HHPS | High-high pressure steam |
| HPS | High pressure steam |
| HT | High temperature |
| IPA | Isopropyl alcohol |
| KPI | Key performance indicator |
| LLPS | Low-low pressure steam |
| LPS | Low pressure steam |
| MDI | Methylene diphenyl diisocyanate |
| MEG | Monoethylene glycol |
| MPS | Medium pressure steam |
| MSP | Minimum selling price |
| MTBE | Methyl tert-butyl ether |
| OPEX | Operational expenditure |
| PEM | Proton exchange membrane |
| PET | Polyethylene terephthalate |
| PFD | Process flow diagram |
| PGME | Propylene glycol methyl ether |
| PO | Propylene oxide |
| PoR | Port of Rotterdam |
| PTA | Purified terephthalic acid |
| PVC | Polyvinyl chloride |
| RME | Rapeseed methyl ester |
| ROI | Return on investment |
| SM | Styrene monomer |
| TBA | Tert-butyl alcohol |
| TEG | Triethylene glycol |
| TEEG | Tetra-ethylene glycol |
| TRL | Technology readiness level |
| VCM | Vinyl chloride monomer |
| VGO | Vacuum gas oil |
Symbols
| EUR per t | Euro per tonne |
| EUR per year | Euro per year |
| GW | Gigawatt |
| kt per year | Kilotonne per year |
| kW | Kilowatt |
| MJ kg−1 | Megajoule per kilogram |
| Mt | Million tonnes |
| MWh | Megawatt-hour |
| PJ per year | Petajoule |
| TJ per year | Tera Joule per year |
| TW h | Terawatt-hour |
| V | Volt |
| wt% | Weight percentage |
Author contributions
J. T. M, M. P.-F. and A. R. R. conceptualised the paper. M. P.-F and A. R. R. supervised the project. J. T. M. wrote the original draft. M. P.-F and A. R. R. reviewed and edited the manuscript. All authors discussed the results and commented on the manuscript.Data availability
The data supporting this article have been included as part of the ESI† and Appendix A.Conflicts of interest
There are no conflicts to declare.Appendix A
The dataset of the process models which contains the detailed assumptions, mass balance, energy balance, economics and sources that was used in the analysis for the manuscript is provided below:• Naphtha steam cracker: https://doi.org/10.5281/zenodo.14825234.
• CO2–H2O direct electrochemical reduction to ethylene: https://doi.org/10.5281/zenodo.14894342.
• CO2 hydrogenation to methanol followed by methanol to olefin conversion: https://doi.org/10.5281/zenodo.14894523.
• Biomass steam gasification followed by methanol to olefin: https://doi.org/10.5281/zenodo.14894523.
• Biomass steam gasification followed by Fischer Tropsch and methanol to olefin: https://doi.org/10.5281/zenodo.14893888.
• Plastic pyrolysis followed by naphtha steam cracking: https://doi.org/10.5281/zenodo.14894590.
• Plastic steam gasification followed by methanol to olefin: https://doi.org/10.5281/zenodo.14894523.
Acknowledgements
This publication is part of the project Unravelling the impacts of using alternative raw materials in industrial clusters (with project number VI.C.183.010) of the research programme Vici DO which is financed by the Dutch Research Council (NWO). We also thank Michael Tan, Inna Stepchuk for sharing the reference case and Sebastian Quevedo for sharing the plastic to methanol Aspen Plus models which were used for the assessment in this study.References
- Renewable Carbon Initiative , Glossary. https://renewable-carbon-initiative.com/renewable-carbon/glossary/, (accessed 2025-02-12).
- Ministry of economic affairs & climate policy (NL), Ministry of infrastructure & water management (NL). The Importance of Sustainable Carbon: Strong Policies Needed to Promote the Uptake of Sustainable Carbon for a Climate-Neutral and Circular Chemical Sector, 2020. https://www.rijksoverheid.nl/documenten/rapporten/2023/09/19/bijlage-3-non-paper-importance-sustainable-carbon.
- European Commission, Towards an Ambitious Industrial Carbon Management for the EU, 2024. https://energy.ec.Europa.eu/document/download/6b89e732-fea4-480b-9d2e-cf64de90247e_en?filename=Communication_-_Industrial_Carbon_Management.pdf.
- F. Kähler and M. Carus, CO2 Reduction Potential of the Chemical Industry through CCU; 2022. https://www.renewable-carbon-initiative.com.
- A. M. Bazzanella and F. Ausfelder, Low Carbon Energy and Feedstock for the European Chemical Industry; 2017. https://dechema.de/dechema_media/Downloads/Positionspapiere/Technology_study_Low_carbon_energy_and_feedstock_for_the_European_chemical_industry.pdf.
-
OECD, The Future of Petrochemicals, 2018. DOI:10.1787/9789264307414-en
.
- F. Kähler, M. Carus, O. Porc and C. vom Berg, Turning off the Tap for Fossil Carbon: Future Prospects for a Global Chemical and Derived Material Sector Based on Renewable Carbon, Ind. Biotechnol., 2021, 17(5), 245–258, DOI:10.1089/ind.2021.29261.fka
.
- The Royal Society, Catalysing Change: Defossilising the Chemical Industry; 2024. https://royalsociety.org/-/media/policy/projects/defossilising-chemicals/defossilising-chemical-industry-report.pdf.
- Center for Global Commons, Systemiq. Planet Positive Chemicals; 2022. https://www.systemiq.earth/wp-content/uploads/2022/09/Main-report-v1.20-2.pdf.
- E. T. C. Vogt and B. M. Weckhuysen, The Refinery of the Future, Nature, 2024, 629(8011), 295–306, DOI:10.1038/s41586-024-07322-2
.
-
M. Stork, J. de Beer, N. Lintmeijer and B. den Ouden, in Chemistry for Climate: Acting on the Need for Speed. Roadmap for the Dutch Chemical Industry towards 2050, 2018. https://task42.ieabioenergy.com/wp-content/uploads/sites/10/2018/10/VNCI_Routekaart-2050.pdf Search PubMed
.
-
J. van Schijndel, R. de Mare, N. Thijssen and J. van der Valk Bouman, TDES:Transformation of the Dutch Energy System, in Proceedings of the 34th European Symposium on Computer Aided Process Engineering/15th International Symposium on Process Systems Engineering (ESCAPE34/PSE24), ed. F. Manenti and G. V. Reklaitis, Florence, Italy, 2024, pp. 2029–2034. DOI:10.1016/B978-0-443-28824-1.50339-2
.
-
S. Samadi, C. Schneider and S. Lechtenböhmer, Deep Decarbonisation Pathways for the Industrial Cluster of the Port of Rotterdam, in Eceee Industrial Summer Study Proceedings, 2018, vol. 2018, pp. 399–409 Search PubMed
.
-
H. Falcke, S. Holbrook, I. Clenahan, A. L. Carretero, T. Sanalan, T. Brinkmann, J. Roth, B. Zerger, S. Roudier and L. D. Sancho, in Best Available Techniques (BAT) Reference Document for the Production of Large Volume Organic Chemicals, 2017. https://tinyurl.com/m6me4rfs Search PubMed
.
- S. D. Manuel, T. Floris, W. Kira, S. Jos and F. André, High Technical and Temporal Resolution Integrated Energy System Modelling of Industrial Decarbonisation, Adv. Appl. Energy, 2022, 7, 100105, DOI:10.1016/j.adapen.2022.100105
.
- F. Kullmann, J. Linßen and D. Stolten, The Role of Hydrogen for the Defossilization of the German Chemical Industry, Int. J. Hydrogen Energy, 2023, 48(99), 38936–38952, DOI:10.1016/j.ijhydene.2023.04.191
.
- G. Lopez, D. Keiner, M. Fasihi, T. Koiranen and C. Breyer, From Fossil to Green Chemicals: Sustainable Pathways and New Carbon Feedstocks for the Global Chemical Industry, Energy Environ. Sci., 2023, 16(7), 2879–2909, 10.1039/D3EE00478C
.
- P. Gabrielli, L. Rosa, M. Gazzani, R. Meys, A. Bardow, M. Mazzotti and G. Sansavini, Net-Zero Emissions Chemical Industry in a World of Limited Resources, One Earth, 2023, 6(6), 682–704, DOI:10.1016/j.oneear.2023.05.006
.
- X. Rixhon, M. Colla, D. Tonelli, K. Verleysen, G. Limpens, H. Jeanmart and F. Contino, Comprehensive Integration of the Non-Energy Demand within a Whole-Energy System: Towards a Defossilisation of the Chemical Industry in Belgium, in 34th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems (ECOS 2021), ECOS 2021 Program Organizers, Tokyo, Japan, 2022, pp. 152–163. DOI:10.52202/062738-0014.
- D. Saygin and D. Gielen, Zero-Emission Pathway for the Global Chemical and Petrochemical Sector, Energies, 2021, 14(13), 3772, DOI:10.3390/en14133772
.
- B. Erkmen, A. Ozdogan, A. Ezdesir and G. Celik, Can Pyrolysis Oil Be Used as a Feedstock to Close the Gap in the Circular Economy of Polyolefins?, Polymers, 2023, 15(4), 859, DOI:10.3390/polym15040859
.
-
H. Zimmermann and R. Walzl, Ethylene, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2009. DOI:10.1002/14356007.a10_045.pub3
.
- M. Tan, P. Ibarra-González, I. Nikolic and A. Ramírez Ramírez, Understanding the Level of Integration in Existing Chemical Clusters: Case Study in the Port of Rotterdam, Circ. Econ. Sustainability, 2025, 5(1), 125–146, DOI:10.1007/s43615-024-00410-5
.
-
G. Towler and R. Sinnott, Chemical Engineering Design, Elsevier, 2nd ed., 2022. DOI:10.1016/C2019-0-02025-0
.
- G. Al-Sharrah, A. Elkamel and A. Almanssoor, Sustainability Indicators for Decision-Making and Optimisation in the Process Industry: The Case of the Petrochemical Industry, Chem. Eng. Sci., 2010, 65(4), 1452–1461, DOI:10.1016/j.ces.2009.10.015
.
- S. Bungener, R. Hackl, G. Van Eetvelde, S. Harvey and F. Marechal, Multi-Period Analysis of Heat Integration Measures in Industrial Clusters, Energy, 2015, 93, 220–234, DOI:10.1016/j.energy.2015.09.023
.
- E. Cuppen, I. Nikolic, J. Kwakkel and J. Quist, Participatory Multi-Modelling as the Creation of a Boundary Object Ecology: The Case of Future Energy Infrastructures in the Rotterdam Port Industrial Cluster, Sustainability Sci., 2021, 16(3), 901–918, DOI:10.1007/s11625-020-00873-z
.
- G. P. J. Dijkema, E. M. Steenkamp and P. J. T. Verheijen, Implementation of Novel Processes in Existing Process Networks: Evaluation of the Industrial Production of C4 Chemicals by Optimization, Comput. Chem. Eng., 1997, 21, S493–S498, DOI:10.1016/S0098-1354(97)87550-3
.
-
R. Hackl and S. Harvey, Opportunities for Process Integrated Biorefinery Concepts in the Chemical Cluster in Stenungsund, Chalmers University of Technology, 2010. https://research.chalmers.se/en/publication/131485 Search PubMed
.
- P. M. Herder and R. M. Stikkelman, Methanol-Based Industrial Cluster Design: A Study of Design Options and the Design Process, Ind. Eng. Chem. Res., 2004, 43(14), 3879–3885, DOI:10.1021/ie030655j
.
- K. M. Holmgren, E. Andersson, T. Berntsson and T. Rydberg, Gasification-Based Methanol Production from Biomass in Industrial Clusters: Characterisation of Energy Balances and Greenhouse Gas Emissions, Energy, 2014, 69, 622–637, DOI:10.1016/j.energy.2014.03.058
.
- M. Pan, J. Sikorski, J. Akroyd, S. Mosbach, R. Lau and M. Kraft, Design Technologies for Eco-Industrial Parks: From Unit Operations to Processes, Plants and Industrial Networks, Appl. Energy, 2016, 175, 305–323, DOI:10.1016/j.apenergy.2016.05.019
.
- T. Ren, M. Patel and K. Blok, Steam Cracking and Methane to Olefins: Energy Use, CO2 Emissions and Production Costs, Energy, 2008, 33(5), 817–833, DOI:10.1016/j.energy.2008.01.002
.
- T. Ren and M. K. Patel, Basic Petrochemicals from Natural Gas, Coal and Biomass: Energy Use and CO2 Emissions, Resour., Conserv. Recycl., 2009, 53(9), 513–528, DOI:10.1016/j.resconrec.2009.04.005
.
- C. Schneider, S. Lechtenböhmer and S. Samadi, Risks and Opportunities Associated with Decarbonising Rotterdam's Industrial Cluster, Environ. Innov. Soc. Trans., 2020, 35, 414–428, DOI:10.1016/j.eist.2019.05.004
.
-
T. Wurth, I. Nikolic, J. Kwakkel, M. Sloot, E. Cuppen and J. Quist, in Eindrapportage Project Windmaster De Weg Naar Een Adaptief Investeringsbeleid, 2019. DOI:10.4233/uuid:122661d9-65eb-4d3a-b91a-2721dcacaaba
.
-
L. Wong and T. Van Dril, Decarbonisation Options for Large Volume Organic Chemicals Production, Shell Moerdijk, 2020. https://www.pbl.nl/en Search PubMed
.
-
J. T. Manalal, M. Pérez-Fortes, P. I. Gonzalez and A. R. Ramirez, Evaluation of Alternative Carbon Based Ethylene Production in a Petrochemical Cluster: Technology Screening & Value Chain Impact Assessment, in Computer Aided Chemical Engineering; Elsevier Masson SAS, 2023, 52, 2453–2458. DOI:10.1016/B978-0-443-15274-0.50390-5
.
- Intratec Solutions LLC , Strategic Commodity Market Information. https://www.intratec.us/, (accessed 2022-11-27).
- ICIS , Resource hub. https://www.icis.com/explore/resources/(accessed 2023-11-27).
- The chemical enginnering plant cost index. Chemical Engineering Magazine. https://www.chemengonline.com/pci-home (accessed 2024-10-20).
- A. Achitaev, A. Suvorov, P. Ilyushin, I. Volkova, K. Kan and K. Suslov, Life Extension of AC-DC Converters for Hydrogen Electrolysers Operating as Part of Offshore Wind Turbines, Int. J. Hydrogen Energy, 2024, 51, 137–159, DOI:10.1016/j.ijhydene.2023.07.283
.
- K. Bareiß, C. de la Rua, M. Möckl and T. Hamacher, Life Cycle Assessment of Hydrogen from Proton Exchange Membrane Water Electrolysis in Future Energy Systems, Appl. Energy, 2019, 237, 862–872, DOI:10.1016/j.apenergy.2019.01.001
.
-
A. Badgett, J. Brauch, A. Thatte, R. Rubin, C. Skangos, X. Wang, R. Ahluwalia, B. Pivovar and M. Ruth, Updated Manufactured Cost Analysis for Proton Exchange Membrane Water Electrolyzers, 2023. https://www.nrel.gov/publications Search PubMed
.
-
E. G. Hertwich, J. A. de Larderel, A. Arvesen, P. Bayer, J. Bergesen, E. Bouman, T. Gibon, G. Heath, C. Peña, P. Purohit, A. Ramirez and S. Suh, Green Energy Choices : The Benefits, Risks and Trade-Offs of Low-Carbon Technologies for Electricity Production. Report of the International Resources Panel, 2016. https://www.resourcepanel.org/reports/green-energy-choices-benefits-risks-and-trade-offs-low-carbon-technologies-electricity Search PubMed
.
-
ARI- Application Techhnology. A Practical Guide to Steam and Condensate Engineering. Technological Concepts for an Efficient and Effective Steam Plant, 2018. https://bermo.com.br/arquivos/filemanager/downloads/informativos/A-Practical-Guide-to-Steam-and-Condensate-Enginnering_Bermo_Ari-Armaturen.pdf Search PubMed
.
- A. Larsson, M. Kuba, T. Berdugo Vilches, M. Seemann, H. Hofbauer and H. Thunman, Steam Gasification of Biomass – Typical Gas Quality and Operational Strategies Derived from Industrial-Scale Plants, Fuel Process. Technol., 2021, 212, 106609, DOI:10.1016/j.fuproc.2020.106609
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc06042c |
| This journal is © The Royal Society of Chemistry 2025 |