Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Adding value to terpenes: copper-catalyzed oxidation of α-pinene in water under micellar conditions†
Gilvan A.
Correia
 a,
Chris H. J.
Franco
a,
Chris H. J.
Franco
 a,
Marina V.
Kirillova
a,
Marina V.
Kirillova
 a,
Fabrice
Gallou
a,
Fabrice
Gallou
 b and
Alexander M.
Kirillov
b and
Alexander M.
Kirillov
 *a
*a
aMINDlab: Molecular Design & Innovation Laboratory, Centro de Química Estrutural, Institute of Molecular Sciences, Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001, Lisboa, Portugal. E-mail: kirillov@tecnico.ulisboa.pt
bChemical & Analytical Development, Novartis Pharma AG, 4056, Basel, Switzerland
First published on 14th February 2025
Abstract
The development of sustainable protocols for the conversion of renewables such as terpenes into value-added products is currently in high demand, which motivates the search for inexpensive and environmentally tolerable catalytic systems and reaction conditions. In the present study, three new prospective catalysts featuring mono-, di-, and tricopper(II) cores were easily assembled in aqueous ethanol medium from copper(II) ions, amino alcohol and carboxylic acid ligands. Their catalytic performance was evaluated in water under micellar conditions with 1% of PS-750-M surfactant, while studying the mild oxidation of α-pinene as an abundant, low-cost, and renewable feedstock. A water-soluble monocopper(II) complex proved to be the most promising catalyst for the oxidation of α-pinene with tert-butyl hydroperoxide under micellar conditions, leading to high substrate conversion (87%) and good yields of the main products (tert-butylperoxy-2-pinene, verbenone, and pinene oxide). A partially water-soluble 1D coordination polymer based on dicopper(II) units also showed a notable catalytic behavior. The effects of different reaction parameters and mechanistic features were investigated. This work opens up the use of micellar catalysis systems and aqueous-medium conditions for the oxidative functionalization of α-pinene and other terpene feedstocks into value-added products.
Green foundation1. A sustainable protocol for catalytic oxidation of α-pinene under aqueous micellar conditions was developed. This approach minimizes the environmental risks associated with organic solvents, creating a green platform for oxidation of low-cost terpene hydrocarbons in water. This work is aligned with the goals of reducing waste, eliminating organic solvents, and using renewable feedstocks.2. The study achieved high conversions of α-pinene, resulting in good yields of value-added oxygenated products. Unlike conventional systems that rely on organic solvents, this work employed a micellar aqueous system with a reduced environmental impact. 3. Further optimization of micellar systems by exploring renewable surfactants and catalysts with improved water solubility may allow for lower catalyst loadings and milder reaction conditions. Broadening the substrate scope toward other terpenes and scaling up these transformations can demonstrate the versatility of the developed protocol. |
Introduction
The generation of added-value chemical products using renewable feedstocks has been one of the objectives of sustainable chemistry, wherein the substitution of harmful organic solvents by benign alternatives is also considered.1–3 In this context, water stands out as the most promising solvent.2,4–6 However, many challenges associated with the solubility issues may need to be overcome for the use of water as a solvent in organic transformations.7–9 One of the promising directions includes the addition surfactants.10–13Drawing inspiration from nature,11 surfactants have a proven efficiency in mitigating low solubility of organic reagents. When introduced into water, surfactants can self-assemble into the nano-sized micelles. Within these micelles, the hydrophobic core readily accommodates organic species, while the hydrophilic portion acts as the reaction medium, surrounding water. This arrangement can be visualized in a schematic representation shown in Fig. 1.4,12,13 As a promising proline-based surfactant,9,14–16 PS-750-M was designed for micellar catalysis. In water, it self-assembles into spherical micelles of ∼110 nm in diameter, with the proline core being the most polar segment of the molecule,17 which enhances the capacity of micelles to incorporate organic molecules.18,19
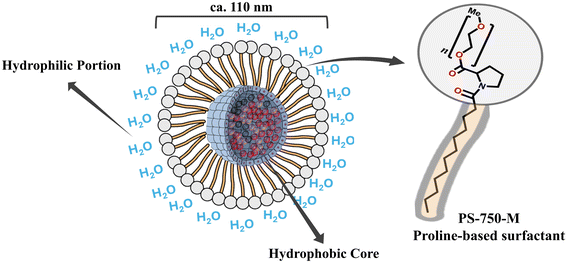 | ||
| Fig. 1 Schematic representation of common morphologies in aqueous system of self-assembled PS-750-M, (C2H4O)nC18H33NO3. | ||
The use of copper coordination compounds as catalysts has constantly increased due to their low cost and eco-tolerance, presence in the active sites of many oxidation enzymes, and significant potential for chemical transformations,20–22 particularly in the context of sustainability toward the valorization of renewable feedstocks.23–25 From the perspective of green chemistry, on the other hand, terpenes represent a broad platform of renewable and widely abundant substrates that offer vast potential as building blocks for the pharmaceutical, fragrance, and flavoring industries.26–28 α-Pinene is a particularly interesting terpene due to its natural abundance, relatively simple structure, and well-understood reactivity.25,29 Its oxygenated derivatives are known for their biologically active properties, such as antibacterial, antimicrobial, and anti-inflammatory.30–32 Regarding the catalytic systems for the oxidative transformations of terpenes, copper coordination compounds offer advantages due to a reduced cost, broad versatility, multiple redox sites, and eco-friendliness.33
There are some examples in the literature regarding the oxidation of hydrocarbons and terpenes, employing copper-based compounds.34–42 For instance, a nanoscaled copper(II) MOF can act as efficient catalyst for the epoxidation of α-pinene (1 bar O2 in CH3CN).43 A catalytic system based on CuCl2 supported on an activated carbon was described for the conversion of (+)-3-carene into (−)-3-carene-2,5-dione.44 Besides, a copper–aluminum mixed oxide was employed in the allylic oxidation of (+)-valencene into nootkatone.45 It is important to note that all these catalytic systems share the use of acetonitrile as an organic solvent. In the assessment method used to rank the standard organic solvent-associated risks (EHS), Capello46 classified acetonitrile as a middle-risk solvent (4.5 points) on a scale ranging from 0 to 9 points, where 0 means no associated risk like in the case of H2O. Hence, the optimization of oxidative catalytic processes is necessary to improve the sustainability parameters of terpene functionalization reactions. Furthermore, the use of copper(II) coordination compounds and micellar conditions to promote hydrocarbon oxidation reactions is limited47 and has not yet been explored with regard to terpenes. Several examples of oxidation reactions under micellar conditions have been reported over different transition-metal catalysts.48–52
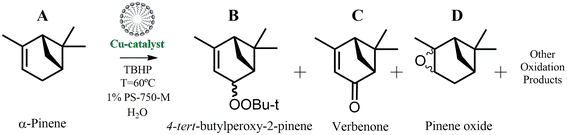
Following the above discussion and our interest in the field of oxidative Cu-catalyzed transformations, we describe herein the self-assembly synthesis and structural characterization of three new copper(II) coordination compounds. The obtained products were formulated as [Cu(H3tea)(H2O)2](nipa)·H2O (1), {[Cu2(μ3-dap)(μ-Hnipa)(μ3-nipa)(H2O)]·H2O}n (2), and {[Cu3(μ3-Hmdea)2(μ4-nipa)(nipa)(H2O)3]}n (3) {H3tea, trietanolamine; Hdap, 1,3-diamino-2-propanol; H2mdea, methyldiethanolamine}. These compounds were employed as prospective catalysts for the oxidation of α-pinene with t-BuOOH in water under micellar conditions using PS-750-M as a surfactant. The conversion and selectivity results are influenced by the solubility of the catalysts, leading to the formation of tert-butylperoxy-2-pinene, verbenone, and pinene oxide as main products that are significantly more expensive than the starting α-pinene. These findings pave the way for a more sustainable approach to enhancing the value of α-pinene. The developed approach minimizes the environmental risks associated with organic solvents, creating a green platform for oxidation of low-cost terpene hydrocarbons in water. This work is aligned with the goals of reducing waste, eliminating organic solvents, and using renewable feedstocks.
Experimental
Synthesis of copper compounds 1–3
5-Nitroisophthalic acid (H2nipa, 0.5 mmol, 105 mg) was dissolved in ethanol (8 mL) and aqueous NH4OH (4 mmol, 1 mL, 4 M) to produce a reaction solution A (Fig. S1†). In parallel, a solution B was prepared by mixing an aqueous solution of Cu(NO3)2·3H2O (1.0 mmol, 1.0 mL, 1 M) with triethanolamine (H3tea, 1 mmol, 149 mg) for compound 1, 1,3-diamino-2-propanol (Hdap, 1 mmol, 91 mg) for compound 2, or N-methyldiethanolamine (H2mdea, 1 mmol, 119 mg) for compound 3, in ethanol (2 mL) under stirring in air at 70 °C. Afterwards, the reaction solution B was slowly added, under constant stirring, into a flask containing solution A. The obtained reaction mixture was stirred for 30 min at 70 °C and then filtered and transferred to an open glass vial to slowly evaporate in the air. Blue crystals of compounds 1, 2, and 3 were formed in a week after partial evaporation of the filtrate. These were isolated manually and dried in air to give the products 1–3 in 65%, 78%, and 69% yields, respectively (yields based on copper(II) nitrate).Analytical data for [Cu(H3tea)(H2O)2](nipa)·H2O (1). Elemental analysis calculated for CuC14H22N2O11 (MW 473.89): C, 35.45%; H, 4.64%; N, 5.91%. Found: C, 35.52%; H, 4.66%; N, 6.07%. FTIR-ATR (KBr, cm−1): 3110 (m br) ν(OH/H2O), 2842 (w) ν(CH), 1632 (s) νas(COO), ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1538 (s), 1439 (m), νs(COO), 1340 (s) ν(N
O) 1538 (s), 1439 (m), νs(COO), 1340 (s) ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1060 (m), 896 (w), 780 (w), 724 (m). Analytical data for {[Cu2(μ3-dap)(μ-Hnipa)(μ3-nipa)(H2O)]·H2O}n (2). Elemental analysis calculated for C19H18N4O15 (MW 669.0): (C, 34.10%; H, 2.69%; N, 8.37%. Found: C, 34.50%; H, 2.74%; N, 8.47%). FTIR-ATR (KBr, cm−1): 3354 (m br) ν(OH/H2O), 2828 (w) ν(CH), 1611 (s) νas(COO), ν(N
O), 1060 (m), 896 (w), 780 (w), 724 (m). Analytical data for {[Cu2(μ3-dap)(μ-Hnipa)(μ3-nipa)(H2O)]·H2O}n (2). Elemental analysis calculated for C19H18N4O15 (MW 669.0): (C, 34.10%; H, 2.69%; N, 8.37%. Found: C, 34.50%; H, 2.74%; N, 8.47%). FTIR-ATR (KBr, cm−1): 3354 (m br) ν(OH/H2O), 2828 (w) ν(CH), 1611 (s) νas(COO), ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1558 (s), 1522 (m), 1381 (vs) νs(COO), 1341 (s) ν(N
O) 1558 (s), 1522 (m), 1381 (vs) νs(COO), 1341 (s) ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1064 (m), 934 (w), 788 (w), 725 (m). Analytical data for {[Cu3(μ3-Hmdea)2(μ4-nipa)(nipa)(H2O)3]}n (3). Elemental analysis calculated for Cu3C26H36N4O19·H2O (MW 896.2): (C: 34.05%; H, 4.18%; N, 6.11%. Found: C, 33.52%; H, 4.06%; N, 6.13%). FTIR-ATR (cm−1): 3393 (m br) ν(OH/H2O), 2864 (w) ν(CH), 1618 (s) νas(COO), ν(N
O), 1064 (m), 934 (w), 788 (w), 725 (m). Analytical data for {[Cu3(μ3-Hmdea)2(μ4-nipa)(nipa)(H2O)3]}n (3). Elemental analysis calculated for Cu3C26H36N4O19·H2O (MW 896.2): (C: 34.05%; H, 4.18%; N, 6.11%. Found: C, 33.52%; H, 4.06%; N, 6.13%). FTIR-ATR (cm−1): 3393 (m br) ν(OH/H2O), 2864 (w) ν(CH), 1618 (s) νas(COO), ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1574 (m), 1531 (m), 1459 (m) 1384 (s) νs(COO), 1349 (s) ν(N
O) 1574 (m), 1531 (m), 1459 (m) 1384 (s) νs(COO), 1349 (s) ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1061 (m), 650 (w).
O), 1061 (m), 650 (w).
General procedure for the oxidation of α-pinene
An aqueous 1% solution of surfactant was prepared by dissolving PS-750-M (0.1 mg) in distilled water (10 mL), which was then stored at 7 °C for further studies. In a typical experiment, an aqueous solution of PS-750-M (1%, 1 mL) was added into a 2 mL GC vial, equipped with a 11 mm stirring bar, followed by the addition of α-pinene (96 μL, 0.6 mmol) and TBHP (70% in H2O, 167 μL, 1.2 mmol). The obtained reaction mixture was stirred at 1500 rpm at different temperatures for a required time. To perform the product analysis after completing the reaction, the crude mixture was first diluted with EtOAc (3 mL), dried over MgSO4, and filtered off. The obtained mixture was further diluted with a 1 mL of acetonitrile, and nitromethane (71 μL, 1.32 mmol) was introduced as an external standard. From the obtained solution, 100 μL was extracted and additionally diluted with acetonitrile (300 μL) before undergoing the analysis by gas chromatography (GC). GC analyses were carried out on an Agilent Technologies 7820A series gas chromatograph (detector: FID; capillary column: BP20/SGE; carrier gas: He). In some cases, additional GC-MS analyses were performed on a Scion 436-GC equipment. For the quantitative monitoring of products by GC, all the molecules were previously confirmed by the NMR spectroscopic elucidation (Fig. S20–22, ESI†).Results and discussion
Synthesis and structures of 1–3
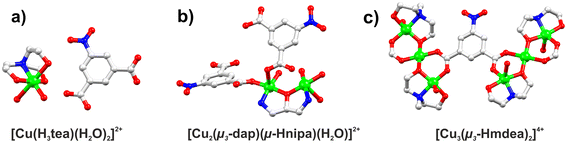
Three new copper(II) compounds with mono-, di- and tricopper(II) cores were generated using a sustainable self-assembly approach in aqueous ethanol medium, which provides high yields and inexpensive catalysts, as summarized in Fig. S1 (ESI).† A series of reactions between copper(II) nitrate, an amino alcohol (H3tea, Hdap, or H2mdea), and 5-nitroisophthalic acid was performed in aqueous ethanol medium at a basic pH that was maintained using an aqueous NH4OH. The obtained products include a mononuclear complex [Cu(H3tea)(H2O)2](nipa)·H2O (1) as well as 1D coordination polymers, {[Cu2(μ3-dap)(μ-Hnipa)(μ3-nipa)(H2O)]·H2O}n (2), and {[Cu3(μ3-Hmdea)2(μ4-nipa)(nipa)(H2O)3]}n (3), which feature dicopper(II) or tricopper(II) cores, respectively. The obtained compounds were characterized by standard methods including infrared spectroscopy (FTIR, Fig. S2–S5, ESI†), elemental (CHN) and thermal analyses (Fig. S9–S11, ESI†), powder and single-crystal X-ray crystallography (Fig. S6–S8 and Table S1; see ESI† for detailed structural description).The structure of (1) reveals a [Cu(H3tea)(H2O)2]2+ cation and a nipa2− anion (Fig. 2a). In the cation, the Cu(II) center is 6-coordinated in a distorted octahedral {CuO5N} environment, which is filled with one H3tea ligand and two coordinated water molecules in a non-centrosymmetric relation. The structure of 2 shows the formation of a 1D coordination polymer composed of dicopper(II) [Cu2(μ3-dap)(μ-Hnipa)(H2O)]2+ secondary building units (SBUs) and μ4-nipa2− linkers (Fig. 2b). One copper(II) center is 5-coordinated and adopts an almost ideal square-pyramidal {CuNO4} geometry, while another one is 6-coordinated in a {CuNO5} environment. The SBUs are extended by the μ4-nipa2− linkers into a 1D coordination polymer structure (Fig. S13, ESI†). The compound 3 also features a 1D coordination network, driven by the presence of two different trinuclear copper(II) SBUs (Fig. 2), namely [Cu3(μ3-Hmdea)2(H2O)4]4+ and [Cu3(μ3-Hmdea)2(nipa)2]. Within these SBUs, the central copper(II) atoms are 6-coordinated and exhibit octahedral {CuO6} environments, while the lateral copper(II) centers a five-coordinated in a distorted {CuO4N} fashion (Fig. S14, ESI†).
Copper-catalyzed oxidation of α-pinene
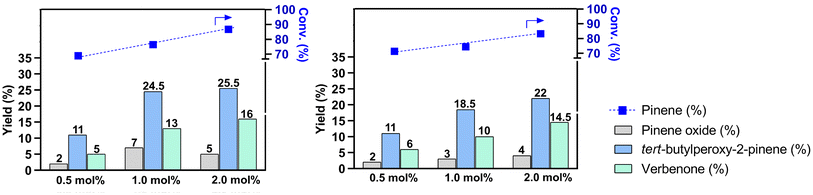
The catalytic activity of the obtained compounds was explored in the mild oxidation of α-pinene with TBHP in aqueous micellar medium, using PS-750-M as a surfactant. For comparison, control experiments in acetonitrile medium were also performed. In particular, the influence of a solvent (aqueous 1% solution of PS-750-M) and catalyst type was investigated. The main products of this reaction were quantified using GC and identified by NMR spectroscopy (Scheme 1). A typical chromatogram of the reaction mixture is shown in Fig. S16, ESI.†Fig. 3 illustrates the data on α-pinene oxidation in the presence of compounds 1 and 2 as catalysts under micellar conditions. The conversion of α-pinene increased proportionally with the concentration of compounds 1 and 2. The optimal concentration of 1 is 1.0 mol%. Under these conditions, 78.5% of α-pinene is converted mainly to tert-butylperoxy-2-pinene, verbenone, and pinene oxide (24.5, 13.5, and 7.0% yields, respectively). At the same concentration of 2, a similar conversion (77.5%) was achieved, although with a lower yield of the main identified products, including tert-butylperoxy-2-pinene, verbenone, and pinene oxide (18.5, 10.0, and 3.0%, respectively). Because of low solubility of 3, the α-pinene oxidation reaction with this catalyst resulted in an inferior conversion (68%) and moderate total product yield (18%; Fig. S17 and Table S8†).
In contrast to micellar conditions, the catalytic performance of 1, 2, and 3 in the α-pinene oxidation in CH3CN medium is different (Fig. S25, ESI†). The coordination polymers 2 and 3 are more active, yielding ca. 29% of tert-butylperoxy-2-pinene, 11% of verbenone and 3% of pinene oxide after 9 h. Lower product yields were achieved in the presence of 1 (Table S14, ESI†).
To investigate the impact of temperature on the oxidation of α-pinene under micellar conditions, the reaction was performed with 1 mol% of 1 and 2 for 9 h, with the temperature ranging from 25 to 70 °C. Fig. S18† presents an overview of this temperature-dependent study, highlighting that compound 1 demonstrates a higher catalytic activity than that of 2 in all scenarios. The optimal reaction conditions for both 1 and 2 were achieved at 60 °C, namely 87% of α-pinene conversion, with the formation of tert-butylperoxy-2-pinene (31% for 1 and 24.5% for 2) and verbenone (19% for 1 and 16% for 2). A noticeable positive effect of the temperature is observed for both catalysts. However, compound 2 led to a higher substrate conversion and yield of tert-butylperoxy-2-pinene, reaching 57% and 16%, respectively, in the temperature range from 25 to 40 °C. This behavior might be associated with a decreased stability of 2 at higher temperatures, leading to disruption of its 1D structure and a release of copper species, thereby increasing the rate of the allylic oxidation of α-pinene, according to proposed mechanism (Scheme S1†). However, compound 2 does not surpass the catalytic activity of 1 at different temperatures tested. As a relatively small mononuclear complex, 1 may interact with the hydrophobic core and accommodate to the environment of nano-sized micelles, promoting more efficient catalytic behavior. Furthermore, lower temperatures (25–50 °C) favored the formation of pinene epoxide (7–8% yields) in the presence of both catalysts. Table S9† displays the complete temperature-dependent studies in the presence of 1 and 2.
Since compound 1 exhibited higher catalytic activity under aqueous micellar conditions, it was selected for further detailed investigation in the oxidation of α-pinene. Fig. S19† demonstrates that the variation of the concentration of PS-750-M in H2O does not influence the conversion of α-pinene, which remains almost constant in the 85–88% range. However, an increase in the surfactant concentration resulted in a slight yield drop. For instance, in the presence of 1.0% PS-750-M, the total yield of identified products is 54%, while at higher loadings of PS-750-M (2.0 and 3.0%) the total yields are lower (49 and 47.5%, respectively; Table S10†). This indicates that even minor changes in the local concentration of micelles can affect the oxidation reaction rate.53,54 Moreover, the optimal oxidant-to-pinene molar ratio in the reaction with 1.0% PS-750-M is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with no significant variations compared to the ratio 3
1, with no significant variations compared to the ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S11†). A gradual addition of aqueous H2O2 solution (25 or 35% in H2O) instead of TBHP showed a destructive effect on the catalyst and micellar system due to catalase activity. Attempts to use acid additives (trifluoracetic or acetic acid) to prevent the catalase activity of 1 were not successful.
1 (Table S11†). A gradual addition of aqueous H2O2 solution (25 or 35% in H2O) instead of TBHP showed a destructive effect on the catalyst and micellar system due to catalase activity. Attempts to use acid additives (trifluoracetic or acetic acid) to prevent the catalase activity of 1 were not successful.
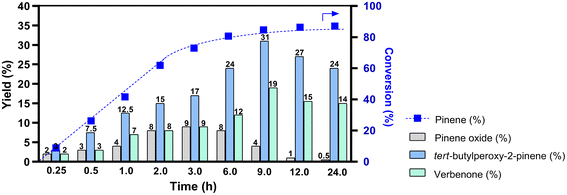
A time-course study of the α-pinene oxidation under micellar conditions at 60 °C is shown in Fig. 4. Throughout the monitoring period, the main product was tert-butylperoxy-2-pinene, reaching a maximum yield of 31% after 9 h, which aligns with the results discussed in the temperature-dependent study (Table S12†). This product, obtained as a result of the allylic oxidation of α-pinene, is a highly reactive intermediate with potential application in organic synthesis.55 Additionally, verbenone was progressively formed over the reaction time with the maximum yield of 19% between 6 and 9 h. Pinene epoxide was predominantly obtained within the first 3 h of the oxidation reaction (9% of yield), followed by its further oxidation.
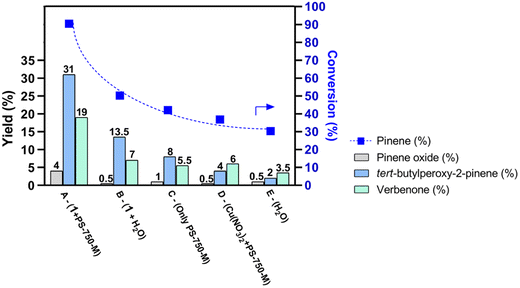
A comparative study was conducted considering the optimal conditions for the allylic oxidation of α-pinene under micellar catalysis (Fig. 5). When comparing the data from micellar catalysis with 1 (Fig. 5, A) to the reaction without surfactant (Fig. 5, B), the conversion dropped by 33%, impacting the total yield of all identified products (21.0% vs. 54% under micellar conditions). These facts indicate that the nano-aggregated micelles of PS-750-M play a crucial role in the copper-catalyzed oxidation of α-pinene in water. PS-750-M surfactant exhibits a prolonged stability and high solubility for certain organic solvents, exponentially increasing the size of micelles to accommodate reaction components for catalysis.9,14–16 Therefore, the copper(II) complex 1 can integrate itself into the nano-sized micelles, acting as a nanoreactor for chemical transformations, thereby improving the reaction rates and yields.56 A higher lability of fully protonated triethanolamine (H3tea) ligand in the structure of mononuclear copper(II) complex 1, compared to more rigid 1D coordination polymers 2 and 3, can explain its superior catalytic performance. As a well-known emulsifier and dispersing agent,57 H3tea likely played an effective, albeit not fully understood role within the micellar medium. As a result, a more homogeneous emulsion (Fig. S27, ESI†) of the reaction medium could be obtained, which enhanced the catalytic performance of the system.
Additionally, a control test was performed with a simple copper salt, Cu(NO3)2, which does not act as a micellar catalyst due to its purely inorganic nature, preventing an interaction with the hydrophobic core where the organic reagents reside. Consequently, the conversion of α-pinene and yield of the main products in this simple Cu-based system drop down to 31.5 and 11.0%, respectively (Fig. 5, D). Additionally, the reactions performed without copper-based catalyst do not exhibit significant catalytic performance (Fig. 5, C and E), thus reinforcing the hypothesis that a copper complex is needed to activate TBHP in micelles for efficient oxidation of α-pinene (Table S13†).
For α-pinene oxidation, more sophisticated Cu-based catalytic systems have been developed in different organic solvents. For example, a bimetallic AuCu/TiO2 catalyst in acetonitrile led to a high α-pinene conversion (94%) and good verbenone selectivity (65%).58 Additionally, an in situ formed Cu(I) complex was reported to yield tert-butylperoxy-2-pinene (39%) in an acetonitrile–acetone system, in the reaction occurring for 10 days at 0 °C.59 In contrast, our work demonstrated distinct advantages over other copper catalytic systems, not only in terms of achieving better yields of the main products [tert-butylperoxy-2-pinene (31%) and verbenone (19%)] but also opening up the use of new cooper coordination compounds as micellar catalysts. In addition, these copper(II) catalysts can be self-assembled in aqueous ethanol medium as pure crystalline solids using relatively simple and low-cost reagents.
The production costs of catalysts used in industrial processes are influenced by various factors, such as the cost of raw materials, catalyst preparation methods, scalability, and reuse potential.60–62 When coordination compounds are involved, these estimations become particularly challenging due to their complex synthesis. Considering compound 1 (65% yield) as example, an analysis of catalyst synthesis costs, based on average values of reagents obtained from chemical industry suppliers, revealed that the production cost of this catalyst can be close to USD 60.0 per kg. An example of estimated cost-effectiveness was reported in the synthesis of nickel nanoparticle catalyst (Ni NPs), which was obtained in a reaction yield of 80–85% with costs ranging from USD 49.2 to 116.25 per kg.63 In comparison, there are even more expensive catalysts, such as immobilized enzymes, which are priced in the range USD 520–1040 per kg.64
In addition, feedstock consumption and product generation in a process can serve as valuable indicators for assessing the overall efficiency and feasibility of the process. Considering the reaction data, the cost of α-pinene used as a raw material, and at least tenfold superior costs of its oxidation products, we could estimate that ∼20 kg of value-added products can be obtained per kg of catalyst used. This very preliminary estimation suggests that the process could potentially be applied in the industrial scale production of small molecules (e.g., fragrance and cosmetic industry), which typically require between 5–100 kg of final product per kg of catalyst.64 Furthermore, technical and economic forecasting studies could certainly be considered in future research.62,65
Conclusions
In summary, this work describes the successful synthesis and characterization of three new coordination compounds, assembled under green conditions from copper(II) ions, amino alcohol chelators, and nitroisophthalic acid as a linker. A water-soluble amino alcohol chelator plays a main structure-guiding role in the assembly process of the products, which include a monocopper(II) complex [Cu(H3tea)(H2O)2](nipa)·H2O (1) and two different 1D coordination polymers, {[Cu2(μ3-dap)(μ-Hnipa)(μ3-nipa)(H2O)]·H2O}n (2) and {[Cu3(μ3-Hmdea)2(μ4-nipa)(nipa)(H2O)3]}n (3), which feature distinct dicopper(II) or tricopper(II) cores.All the obtained products were screened as catalysts for the oxidation of α-pinene under aqueous micellar conditions in the presence of the PS-750-M surfactant. Compound 1 emerged as the most promising catalyst, leading to high substrate conversion (87%), and yielding mainly tert-butylperoxy-2-pinene (31% yield) and verbenone (19% yield), among other products. Catalyst 1 incorporates triethanolamine (H3tea) as a classical industrial emulsifier. This feature likely contributed to the formation of a more homogeneous emulsion, thus resulting in improved catalytic performance. Such a protocol for the catalytic oxidation of α-pinene under aqueous micellar conditions highlights a more sustainable approach for upgrading this abundant terpene into oxygenated value-added products.46
Apart from extending the family of self-assembled coordination compounds with mono-, di-, and tricopper(II) cores, this study has opened up the use of such copper compounds in the area of micellar catalysis, along with broadening the scope of catalytic transformations to terpenes. Ongoing research on examining the factors affecting the self-assembly of PS-750-M micelles and their interactions with substrates and catalytically active species is currently in progress to develop catalytic systems that show enhanced efficiency and product selectivity.
This work inspires further advancements in environmentally friendly oxidation reactions under micellar conditions, using copper-coordination compounds as cost-effective catalysts to afford oxyfunctionalized products with added value. Further optimization of micellar systems by exploring renewable surfactants and catalysts with improved water solubility may allow for lower catalyst loadings and milder reaction conditions. Broadening the substrate scope toward other terpenes and scaling up these transformations can demonstrate the versatility of the developed protocol. These research lines are currently in progress in our laboratory.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received funding from EU's Horizon 2020 research and innovation programme under grant agreement no. 860762 (CHAIR project). We also thank the Foundation for Science and Technology (FCT, Portugal: PTDC/QUI-QIN/3898/2020, UIDB/00100/2020, LA/P/0056/2020). C.H.J. Franco acknowledges the research contracts within the PTDC/QUI-QIN/29697/2017 and PTDC/QUI-QIN/3898/2020 projects (2021-2024). This work was partially carried out by G. Correia at Novartis Pharma (Switzerland) during his secondment within the CHAIR project. The authors also thank Dr Pascal Hauk for the productive discussion on micellar catalysis.References
- P. Shah, S. Parikh, M. Shah and S. Dharaskar, Biomass Convers. Biorefin., 2022, 12, 1985–1999 CrossRef.
- F. Gao, H. Chang, J. Li, R. Wang and Y. Gu, Curr. Opin. Green Sustainable Chem., 2023, 40, 100774 CrossRef CAS.
- G. Wypych, Databook of Green Aolvents, Elsevier, 2024 Search PubMed.
- M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237–4266 RSC.
- S. Hazra, F. Gallou and S. Handa, ACS Sustainable Chem. Eng., 2022, 10, 5299–5306 CrossRef CAS.
- L. Lajoie, A.-S. Fabiano-Tixier and F. Chemat, Pharmaceuticals, 2022, 15, 1507 CrossRef CAS PubMed.
- J. Becker, C. Manske and S. Randl, Curr. Opin. Green Sustainable Chem., 2022, 33, 100562 CrossRef CAS.
- L. Chen, S. Zhang, X. Liu and X. Ge, Curr. Opin. Colloid Interface Sci., 2023, 65, 101691 CrossRef CAS.
- G. Hedouin, D. Ogulu, G. Kaur and S. Handa, Chem. Commun., 2023, 59, 2842–2853 RSC.
- M. Banerjee, P. C. Panjikar, Z. T. Bhutia, A. A. Bhosle and A. Chatterjee, Tetrahedron, 2021, 88, 132142 CrossRef CAS.
- M. Cortes-Clerget, J. R. A. Kincaid, N. Akporji and B. H. Lipshutz, in Supramolecular Catalysis, 2022, pp. 467–487, DOI:10.1002/9783527832033.ch32.
- F. Eisenreich and A. R. A. Palmans, in Supramolecular Catalysis, 2022, pp. 489–506, DOI:10.1002/9783527832033.ch33.
- G. Strukul, F. Fabris and A. Scarso, in Supramolecular Catalysis, 2022, pp. 451–466, DOI:10.1002/9783527832033.ch31.
- S. Handa, F. Ibrahim, T. N. Ansari and F. Gallou, ChemCatChem, 2018, 10, 4229–4233 CrossRef CAS.
- M. Bihani, P. P. Bora, M. Nachtegaal, J. B. Jasinski, S. Plummer, F. Gallou and S. Handa, ACS Catal., 2019, 9, 7520–7526 CrossRef CAS.
- G. Kaur, K. Kaur and S. Handa, Curr. Opin. Green Sustainable Chem., 2022, 38, 100690 CrossRef CAS.
- F. Gallou, Chimia, 2020, 74, 538–548 CrossRef CAS PubMed.
- S. Handa, Y. Wang, F. Gallou and B. H. Lipshutz, Science, 2015, 349, 1087–1091 CrossRef CAS PubMed.
- J. Brals, J. D. Smith, F. Ibrahim, F. Gallou and S. Handa, ACS Catal., 2017, 7, 7245–7250 CrossRef CAS.
- Q. Mei, H. Liu, Y. Yang, H. Liu, S. Li, P. Zhang and B. Han, ACS Sustainable Chem. Eng., 2018, 6, 2362–2369 CrossRef CAS.
- D. Podstawczyk, A. Pawłowska, A. Bastrzyk, M. Czeryba and J. Oszmiański, ACS Sustainable Chem. Eng., 2019, 7, 17535–17543 CrossRef CAS.
- Y. Shao, K. Sun, M. Fan, G. Gao, J. Wang, L. Zhang, S. Zhang and X. Hu, ACS Sustainable Chem. Eng., 2022, 10, 8763–8777 CrossRef CAS.
- M. Hosseini and D. M. Pereira, Pharmaceuticals, 2023, 16, 202 CrossRef CAS PubMed.
- M. A. Upshur, A. G. Bé, J. Luo, J. G. Varelas, F. M. Geiger and R. J. Thomson, Nat. Prod. Rep., 2023, 40, 890–921 RSC.
- N. Semmar, in Secondary Metabolites in Plant Stress Adaptation: Analytic Space of Secondary Metabolites, ed. N. Semmar, Springer International Publishing, Cham, 2024, pp. 71–109, DOI:10.1007/978-3-031-52595-7_5.
- A. Denicourt-Nowicki, M. Rauchdi, M. A. Ali and A. Roucoux, Catalysts, 2019, 9, 893 CrossRef.
- G. Goktepeli, A. Ozgan, V. Onen, G. Ahmetli, M. Kalem and E. Yel, Int. J. Environ. Sci. Technol., 2024, 21, 7981–7998 CrossRef CAS.
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed.
- M. M. Karimkhani, M. Nasrollahzadeh, M. Maham, A. Jamshidi, M. S. Kharazmi, D. Dehnad and S. M. Jafari, Crit. Rev. Food Sci. Nutr., 2024, 64, 4286–4311 CrossRef CAS PubMed.
- H. E. González-Velasco, M. S. Pérez-Gutiérrez, Á. J. Alonso-Castro, J. R. Zapata-Morales, P. d. C. Niño-Moreno, N. Campos-Xolalpa and M. M. González-Chávez, Molecules, 2022, 27, 2612 CrossRef PubMed.
- J. Petrović, V. Kovalenko, A. Svirid, D. Stojković, M. Ivanov and M. Kostić, J. Mol. Struct., 2022, 1251, 131999 CrossRef.
- I. Oualdi, K. Elfazazi, H. Azzouzi, A. Oussaid and R. Touzani, Mater. Today: Proc., 2023, 72, 3768–3774 CAS.
- M. Karuppasamy, B. S. Vachan and V. Sridharan, in Copper in N-Heterocyclic Chemistry, ed. A. Srivastava, Elsevier, 2021, pp. 249–288, DOI:10.1016/B978-0-12-821263-9.00007-2.
- A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062–11087 CrossRef CAS PubMed.
- A. M. Kirillov, M. V. Kirillova and A. J. L. Pombeiro, in Advances in Inorganic Chemistry, ed. R. van Eldik and C. D. Hubbard, Academic Press, 2013, vol. 65, pp. 1–31 Search PubMed.
- A. N. Bilyachenko, M. S. Dronova, A. I. Yalymov, F. Lamaty, X. Bantreil, J. Martinez, C. Bizet, L. S. Shul'pina, A. A. Korlyukov, D. E. Arkhipov, M. M. Levitsky, E. S. Shubina, A. M. Kirillov and G. B. Shul'pin, Chem. – Eur. J., 2015, 21, 8758–8770 CrossRef CAS PubMed.
- E. Loukopoulos, A. Abdul-Sada, G. Csire, C. Kállay, A. Brookfield, G. J. Tizzard, S. J. Coles, I. N. Lykakis and G. E. Kostakis, Dalton Trans., 2018, 47, 10491–10508 RSC.
- J. Gu, M. Wen, Y. Cai, Z. Shi, A. S. Arol, M. V. Kirillova and A. M. Kirillov, Inorg. Chem., 2019, 58, 2403–2412 CrossRef CAS PubMed.
- A. Hossain, A. Bhattacharyya and O. Reiser, Science, 2019, 364, eaav9713 CrossRef PubMed.
- I. F. M. Costa, M. V. Kirillova, V. André, T. A. Fernandes and A. M. Kirillov, Inorg. Chem., 2021, 60, 14491–14503 CrossRef CAS PubMed.
- L. Marais, H. C. M. Vosloo and A. J. Swarts, Coord. Chem. Rev., 2021, 440, 213958 CrossRef CAS.
- B. Xu, Q. Xu, Q. Wang, Z. Liu, R. Zhao, D. Li, P. Ma, J. Wang and J. Niu, Inorg. Chem., 2021, 60, 4792–4799 CrossRef CAS PubMed.
- Y. Qi, Y. Luan, J. Yu, X. Peng and G. Wang, Chem. – Eur. J., 2015, 21, 1589–1597 CrossRef CAS PubMed.
- X. Sun, X. Zhao, Y. Jiang and B. Xu, J. Chin. Chem. Soc., 2013, 60, 103–107 CrossRef CAS.
- A. L. García-Cabeza, R. n. Marín-Barrios, F. J. Moreno-Dorado, M. J. Ortega, G. M. Massanet and F. M. Guerra, Org. Lett., 2014, 16, 1598–1601 CrossRef PubMed.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- X.-H. Li, C. Mi, X.-H. Liao and X.-G. Meng, Catal. Lett., 2017, 147, 2508–2514 CrossRef CAS.
- D. J. Lippincott, P. J. Trejo-Soto, F. Gallou and B. H. Lipshutz, Org. Lett., 2018, 20, 5094–5097 CrossRef CAS PubMed.
- S. Chowdhury, A. Rakshit, A. Acharjee, A. Ghosh, K. Mahali and B. Saha, J. Mol. Liq., 2019, 290, 111247 CrossRef.
- U. Duong, T. N. Ansari, S. Parmar, S. Sharma, P. M. Kozlowski, J. B. Jasinski, S. Plummer, F. Gallou and S. Handa, ACS Sustainable Chem. Eng., 2021, 9, 2854–2860 CrossRef CAS.
- T. Shen, S. Zhou, J. Ruan, X. Chen, X. Liu, X. Ge and C. Qian, Adv. Colloid Interface Sci., 2021, 287, 102299 CrossRef CAS PubMed.
- A. Tomar, U. Arora and J. V. Singh, J. Indian Chem. Soc., 2024, 101, 101267 CrossRef CAS.
- S. Gangwar and M. Rafiquee, Int. J. Chem. Kinet., 2007, 39, 638–644 CrossRef CAS.
- A. Shrivastava and K. K. Ghosh, J. Dispersion Sci. Technol., 2008, 29, 1381–1384 CrossRef CAS.
- Q. Yang, Z. Wang, T. Kato, Y. Liu and K. Maruoka, Org. Lett., 2023, 25, 2958–2963 CrossRef CAS PubMed.
- F. Fabris, M. Illner, J.-U. Repke, A. Scarso and M. Schwarze, Molecules, 2023, 28, 4809 CrossRef CAS PubMed.
- H. Lessmann, W. Uter, A. Schnuch and J. Geier, Contact Dermatitis, 2009, 60, 243–255 CrossRef PubMed.
- S. Ajaikumar, J. Ahlkvist, W. Larsson, A. Shchukarev, A.-R. Leino, K. Kordas and J.-P. Mikkola, Appl. Catal., A, 2011, 392, 11–18 CrossRef CAS.
- M. Schulz, R. Kluge and F. G. Gelalcha, Tetrahedron: Asymmetry, 1998, 9, 4341–4360 CrossRef CAS.
- F. G. Baddour, L. Snowden-Swan, J. D. Super and K. M. Van Allsburg, Org. Process Res. Dev., 2018, 22, 1599–1605 CrossRef CAS.
- C. H. Franco, P. Chagas, G. S. Caldeira, L. C. Oliveira, P. P. de Souza, A. A. Leitão, G. W. Amarante and R. Diniz, Dalton Trans., 2018, 47, 10976–10988 RSC.
- H. Kim, J. Choi, J. Park and W. Won, Green Chem., 2020, 22, 7070–7079 RSC.
- B. E. Petel, K. M. Van Allsburg and F. G. Baddour, Adv. Sustainable Syst., 2024, 8, 2300030 CAS.
- P. r. Tufvesson, J. Lima-Ramos, M. Nordblad and J. M. Woodley, Org. Process Res. Dev., 2011, 15, 266–274 CrossRef CAS.
- B. Velaga and N. R. Peela, Green Chem., 2022, 24, 3326–3343 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data on the synthesis, characterization, and catalytic application of 1–3 (Scheme S1, Fig. S1–S25, and Tables S1–S14). CCDC 2386078–2386080. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4gc06525e |
| This journal is © The Royal Society of Chemistry 2025 |