Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organic carbonates as green media: from laboratory syntheses to industrial applications
Giacomo
Trapasso
 * and
Fabio
Aricò
* and
Fabio
Aricò
 *
*
Department of Environmental Sciences, Informatics and Statistics, Ca’ Foscari University of Venice, Via Torino155, 30172 Venezia Mestre, Italy. E-mail: fabio.arico@unive.it; giacomo.trapasso@unive.it
First published on 5th May 2025
Abstract
The research on greener solvents is of paramount importance for achieving sustainable processes. To replace traditional hazardous media, green solvents must display negligible environmental effects and biological degradability while being available on a large scale and exhibiting comparable or even superior performances than the currently employed media. In this scenario, organic carbonates (OCs) are among the most prominent candidates as they are commercially available at a reasonable price and offer a broad range of tunable proprieties, making them usable in a wide range of applications. Based on this premise, this review focuses on the use of OCs as green media ranging from laboratory synthetic approaches to industrial applications. According to our literature screening covering the last 40 years, organic carbonates have mostly been investigated as electrolyte solvents (23%), media in organic synthesis (21%) and solvents for the extraction of compounds from different biological and non-biological matrices (13%). Besides, OCs have applications in several other fields spanning from analytical chemistry and biological/biochemical fields to the restoration of ancient artifacts. Most of the OCs used in these applications are dialkyl carbonates (DACs), such as dimethyl carbonate (DMC), propylene carbonate (PC) and ethylene carbonate (EC), which are commercially available at low cost. However, owing to their simple synthetic procedures, new custom-made organic carbonates have been synthetized and used for membrane casting, preparation of polymers and plasticizers, surface modification of materials and as electrolytes in Li-ion batteries. Organic carbonates go beyond simply replacing toxic solvents; they offer an opportunity to transform a variety of processes into sustainable processes. From enhancing the performance of batteries and advancing materials science to driving innovations in green chemistry and improving industrial sustainability, their potential is vast. The adoption of organic carbonates as green media is likely to have far-reaching effects, making them valuable tools for researchers and industries aiming to develop more sustainable processes.
Green foundation1. To the best of our knowledge, this is the first comprehensive review specifically focused on organic carbonates as green media ranging from laboratory organic syntheses to industrial applications. As a result, this work is based on an extensive bibliographic research covering the past 40 years.2. The use of DACs as green solvents is appealing to a broad spectrum of scientific communities, industrial sectors, and policymakers committed to advancing sustainability, reducing environmental impact, and promoting safer chemical practices. 3. DACs offer more than just alternatives to toxic solvents; they present an opportunity to revolutionize various scientific fields towards more sustainable practices. The widespread adoption of DACs as green solvents is likely to have far-reaching effects, making them valuable resources for researchers and industries focused on developing more sustainable processes. |
1. Introduction
Following the worldwide increase in environmental awareness, traditional chemical methods are being replaced with new and more sustainable methods, which is also highlighted in the recent European Green Deal (EGD).1,2 For this, the first and foremost focus should be on the selection and use of solvents. In fact, solvents are responsible for the consumption of more than 60% of the energy required in industrial plants, and they are the most discarded substances in industrial processes, making them one of the major contributors for environmental damage.3–6 Thus, solvent selection is crucial as it impacts the economics and operation time of the production process in plants.7In this case, several pharmaceutical companies, i.e., Pfizer, GSK, Astra Zeneca and Sanofi, have combined the major solvent selection guides developed thus far.8,9 The criteria considered are safety (S), occupational health (H), environment (E), quality (risk of impurities in the drug substance), industrial constraints (i.e., boiling point, freezing temperature, density, recyclability) and cost.9,10
This resulted in a “traffic light” colour code system, where green represents preferred solvents, yellow for compounds showing some health or environmental issues and red for toxic solvents that need to be substituted. Numerous commercially available solvents commonly used in organic synthesis are evidently not recommended owing to their high volatility, flammability, hazardousness and many other health risks.
Considering this, it is necessary to explore more environmentally friendly and tunable solvents that have a negligible environmental effect, are biologically degradable3,11 and can replace traditional hazardous media in chemical processes. Solvents fulfilling these requirements are called “green” solvents.3,6,12 A green solvent must possess the characteristics of low vapor pressure, high boiling point, low price, recyclability, non-toxicity, chemical and thermal stability and non-flammability, while possibly being a bio-derived product.13–15 In addition, a green solvent should be available on a large scale, thus ensuring a stable market production. It must be prepared through energy-saving processes with high atom economy synthetic procedures and should demonstrate comparable or even superior performances to the currently used solvents.15 A wide-range of green solvents derived from renewable feedstocks are currently accessible including (bio)ethanol, ethyl lactate, 2-methyl-tetrahydrofuran (2-Me-THF), cyclopentyl methyl ether (CPME), glycerol, dimethyl isosorbide (DMI), γ-valerolactone (GVL) and Cyrene are some of the most common examples, which can be also defined as bio-derived solvents. Furthermore, other types of compounds are also commonly employed as green solvents, i.e., organic carbonates (OCs), CO2 and supercritical CO2 (scCO2), ionic liquids (ILs) derived from amino acids, proteins, lignin and polysaccharides,16 deep eutectic solvents (DES) and natural DES.17–19
Among them, this review will focus on OCs, exploring their use as media in different fields.
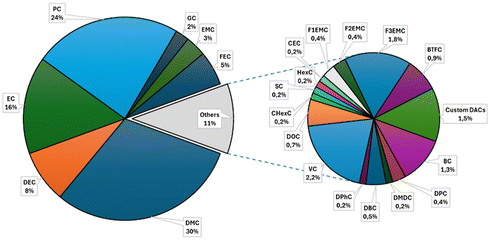
These compounds offer a broad range of proprieties, which are tunable according to their chemical structures. In the literature, numerous organic carbonates, both commercially available and custom-made, have been employed in various fields, as illustrated in Fig. 1 (OC applications) and Fig. 2 (most used OCs). According to our extensive literature screening covering the last 40 years, OCs have been mostly investigated as electrolyte solvents, media in organic synthesis and for the extraction of compounds from different biological and non-biological matrices.
Nevertheless, OCs have found applications in several other fields ranging from analytical chemistry, principally as mobile phases for the separation and identification of products, to the biological/biochemical area and for the restoration of ancient artifacts (Fig. 1). Most of the OCs used are dialkyl carbonates (DACs), which can be easily purchased on the market at a low cost, i.e., dimethyl carbonate (DMC), diethyl carbonate (DEC), propylene carbonate (PC) and ethylene carbonate (EC) (Fig. 2). It must be mentioned that PC and EC are well-known electrolyte solvents, which represent the most studied application area for OCs (Fig. 1). Despite this predominance, custom-made OCs have also been employed in different areas to meet polarity or solubility criteria required for specific applications (Fig. 2).
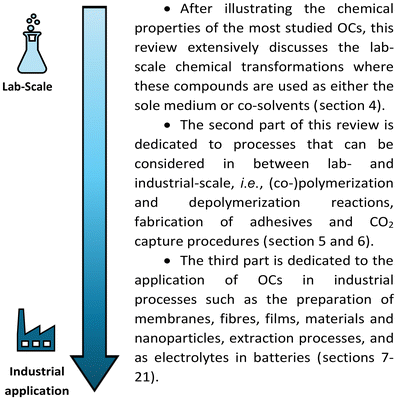
Based on this premise, the present work aims to present an overview focused exclusively on the applications of OCs as solvents, highlighting their use in lab-scale chemical transformations, large-scale industrial processes and end-of-use products. Numerous reviews have been published on OCs as green reagents. However, to the best of our knowledge, this is the first comprehensive review solely focusing on their use as green media.
In particular, the rationale of this work has been organized as follows:
2. Properties and stability of organic carbonates
OCs display completely different proprieties depending on their chemical structure. The characteristics of the most studied OCs (Fig. 1 and 2), DMC, DEC, EC and PC, together with some of the most employed traditional toxic media (see also Table 2) are reported in Table 1.| DMC | DEC | EC | PC | CH2Cl2 | CH3CN | Toluene | DMF | |
|---|---|---|---|---|---|---|---|---|
| LEL: lower explosive limit; UEL: upper explosive limit; n.r.: information not reported, V = volume.a Values available in Sigma-Merck safety data sheet (SDS) of the corresponding compound.b Values available at https://pubchem.ncbi.nlm.nih.gov/.c Values available at https://depts.washington.edu/eooptic/linkfiles/dielectric_chart%5B1%5D.pdf. | ||||||||
| Molecular weight (g mol−1) | 90.08 | 118.13 | 88.06 | 102.09 | 84.93 | 41.05 | 92.14 | 73.09 |
| Melting point (°C) | 4.620 | −4320 | 34–3721 | −48.83 | −97a | −48a | −93a | −61a |
| Boiling point (°C) | 90.320 | 12620 | 24822 | 2403 | 40a | 81a | 110a | 153a |
| Density (g cm−3) | 1.06920 | 0.97520 | 1.32123 | 1.2013 | 1.325a | 0.786a | 0.865a | 0.944a |
| Water solubility (g L−1) | 13920 | Insoluble20 | 77824 | 20024 | 0.013b | >800b | 0.53b | 1000b |
| Dielectric constant (ε) | 3.2025 | 2.8226 | 89.7827 | 66.628 | 8.93c | 37.5c | 2.38c | 36.7c |
| Dipole moment (μ, D) | 0.9129 | 1.0730 | 4.8127 | 5.3628 | 1.55c | 3.45c | 0.43c | 3.86c |
| Explosion limita | ||||||||
| LEL (% V) | 4.2 | 1.4 | 3.6 | 1.8 | 13.0 | 4.4 | 1.2 | 2.2 |
| UEL (% V) | 12.9 | 11.0 | 16.1 | 14.3 | 22.0 | 16.0 | 7.1 | 16.0 |
| Flash pointa | ||||||||
| Closed cup (°C) | 16.0 | 25.0 | 143 | 132 | Does not flash | 2.0 | 4.4 | 57.5 |
| Ignition temperaturea (°C) | n.r. | 445 | n.r. | n.r. | 605 | n.r. | n.r. | 435 |
| Vapour pressurea (hPa at 20.0–25.0 °C) | 24 | 13 | <1 | 0.06 | 584 | 98.64 | 30.88 | 3.77 |
| Solvent polarity (ET(30), kcal mol−1) | 41.131 | 36.231 | 48.632 | 46.631 | 40.731 | 45.631 | 33.931 | 43.231 |
| General application | Specific application | Organic carbonate solvents | Toxic solvents | Ref. |
|---|---|---|---|---|
| n.r.: information not reported; DMA: dimethyl acetamide, GPC: glycerol carbonate propionate, GCB: glycerol carbonate butyrate, GC: glycerol carbonate, DPC: dipropyl carbonate, BC: butylene carbonate, Gly2C: bis(2-methoxyethyl) carbonate, GlyMC: 2-methoxyethyl methyl carbonate, DGlyMC: 2-(2-methoxyethoxy)ethyl methyl carbonate, DGly2C: bis(2-(2-methoxyethoxy)ethyl) carbonate, DPhC: diphenyl carbonate, EMC: ethyl methyl carbonate, DAllC: diallyl carbonate. | ||||
| Simple chemical reactions | Etherification | PC | CH2Cl2, CH3CN, benzene, toluene | 88 and 91 |
| Esterification | DMC | n.r. | 87 | |
| Chlorination and bromination | DMC, PC, DEC | CHCl3, chlorobenzene, CCl4 | 89 and 90 | |
| Oxidation and epoxidation | DMC, DEC, PC, DPC, BC | CH3CN, ionic liquids, DMF, NMF, NMP, DMA, THF | 93–104 | |
| Amine synthesis | DMC, PC | CHCl3, Toluene | 105–107 | |
| Amide synthesis | DMC | CH2Cl2 | 108 | |
| Wolff rearrangement/acylation | DMC, DEC | DMF, CH3CN, toluene, DMSO, DCE | 109 | |
| Dehydration | DMC | THF | 110–116 | |
| Rh-catalysed reactions | Hydroformylation | DMC, DEC, PC | Toluene, benzene | 117–120 |
| Co-oligomerization | PC | n.r. | 117 | |
| ortho-Diarylation | DEC | NMP, 1,4-dioxane | 121 | |
| Hydroacylation | PC | DCE | 122 | |
| Pd-catalysed reactions | Asymmetric allylic substitution | PC, BC, DEC | CH2Cl2 | 123 |
| Telomerization | DMC, DEC, EC, PC, BC, GCP, GCB | CH3CN | 78 | |
| Allylation of heteroarenes | DMC | DMF, 1,4-dioxane, CH3CN | 124 | |
| Synthesis of lactones | DMC | Toluene, CH2Cl2 | 125 | |
| Hydrogenation | DMC | n-Heptane | 126 | |
| Phenoxycarbonylation of aryl iodides | PC, EC | DMF, 1,4-dioxane | 127 | |
| Coupling | DMC, DEC, EC, PC, GC | THF, toluene, anisole, 1,4 dioxane, DMA, DMF | 128–134 | |
| Synthesis of oxime ether derivatives | DMC | n.r. | 135 | |
| Ni-catalysed reactions | Hydrodeacetoxylation | DMC | Toluene | 136 |
| Pt-catalysed reactions | Hydrosilylation | PC | Toluene, cyclohexane | 137 and 138 |
| Ru-catalysed reactions | Olefin metathesis | DMC, PC | Benzene, toluene, CH2Cl2, chlorobenzene | 139–142 |
| Other applications as media in organic synthesis | Organic carbonates and ionic liquids as media in organic synthesis | DEC, PC | n.r. | 143–145 |
| Phase transfer catalysis | DMC | Toluene, chlorobenzene | 146 | |
| Enantioselective reactions | PC, EC | DMF, CH2Cl2 | 147–151 | |
| Photocatalytic reactions | DMC | Toluene, CH3CN, BTF, DMF, benzonitrile, propionitrile, butyronitrile | 152–158 | |
| Other reactions | Condensation/rehydration | DMC | cyclohexane | 159 |
| Radziszewski reaction | PC | DMSO, DMF | 160 | |
| Synthesis of cyclodextrin-based supramolecular assemblies | DEC | n.r. | 161 | |
| Complexation | PC | DMF | 162 | |
| Claisen rearrangement | PC | DCB | 163 | |
| Ring opening | DMC | CH2Cl2, Et2O, CH3CN | 164 | |
Generally, cyclic OCs display a higher dipole moment and dielectric constant compared to linear OCs. Therefore, PC in particular is well-suited for anhydrous electrochemical applications.33,34 Moreover, the polarity and hydrogen-bond acceptor properties (basicity, β) of PC (0.39) match perfectly with that of acetonitrile (0.38).35 Linear DACs such as DMC and DEC, although displaying similar basicity, possess lower polarities, which resemble methylene chloride and THF.36–39 Only scant data are available for other organic carbonates even if modelling predictions of these parameters may be a powerful tool to overcome this limitation.40–44
Concerning the solubility of OCs, most of them only show limited or no miscibility with water,45,46 despite displaying characteristics of highly dipolar solvents similar to other compounds such as dimethyl sulfoxide (DMSO) and dimethyl formamide (DMF).46 In a recent study, it was demonstrated that the substitution of hydrocarbon chains with more polar glycol-based moieties led to a drastic increase in the water solubility of OCs.24,47 Thus, the tuning of the side chains of OCs may allow the wider application of these compounds both as solvents and reagents.47,48
OCs can be considered chemically stable under normal conditions. However, at elevated temperature, these compounds can decompose, releasing mainly CO2. In particular, DMC can release CO2 and dimethyl ether at T > 150 °C in an inert atmosphere, while methanol can originate from DMC hydrolysis in the presence of water.49 Similarly, DEC can decompose into ethylene, ethanol and CO2.49 Also, longer chain DACs have been shown to decarboxylate in the presence of hydrotalcites, producing the corresponding ethers.50 In general, linear OCs degrade at lower temperatures than cyclic OCs, i.e., EC degrades at 335 °C, whereas PC at 316 °C.49
It should be mentioned that the reactivity of DMC and its derivatives was thoroughly investigated in the work of Tundo et al., according to which it is evident that these compounds can easily react with a variety of nucleophiles, yielding either the corresponding alkoxycarbonylated or alkylated derivatives according to the reaction conditions.51
Therefore, the proprieties of OCs as ambident electrophiles may hamper their applications as media, with possible side reaction occurring due to their reactivity. Similarly, cyclic OCs may undergo ring opening in the presence of a suitable nucleophile, thus leading to the formation of side products. Some examples of these issues are reported throughout section 4.
3. Global market, production costs and safety aspects of organic carbonates
3.1 Global market production of the principal organic carbonates
According to ChemAnalyst and KBV reports, the global EC production was estimated to be between 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 460
000 and 460![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2022 with a projection to reach 865
000 tonnes in 2022 with a projection to reach 865![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes by 2032 (compound annual growth rate (CAGR) of 6.5% from 2023 to 2032).52,53 Different reports forecasted the global EC market size to reach a value between 970.4 million USD and 1.16 billion USD by 2030, with a CAGR from 8.6% to 13.7% during the forecast period.53,54
000 tonnes by 2032 (compound annual growth rate (CAGR) of 6.5% from 2023 to 2032).52,53 Different reports forecasted the global EC market size to reach a value between 970.4 million USD and 1.16 billion USD by 2030, with a CAGR from 8.6% to 13.7% during the forecast period.53,54
The global market of PC was approximately 470![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year in 2022, which is anticipated to grow at a CAGR of 5.6% until 2032, reaching 800
000 t per year in 2022, which is anticipated to grow at a CAGR of 5.6% until 2032, reaching 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year (ChemAnalyst report). The increasing demand for electric vehicles and portable electronic devices is also driving the PC market, together with its utilization in skincare formulation products, coatings, paints, sealants, adhesives and degreasers.55,56
000 t per year (ChemAnalyst report). The increasing demand for electric vehicles and portable electronic devices is also driving the PC market, together with its utilization in skincare formulation products, coatings, paints, sealants, adhesives and degreasers.55,56
According to a report by Grand View Research, the global PC market was valued at approximately 393.1 million USD in 2023, which is projected to reach 610.9 million USD by 2030.56
The global DMC production is the highest among the commercially available OCs, with a value estimated around 926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2022 and an expected CAGR of 5.4% until 2032,57 with its major application being polycarbonate production.58 The DMC market was valued between 1.10 and 1.17 billion USD in 2023, which is projected to reach 1.9 billion USD by 2028.59
000 tonnes in 2022 and an expected CAGR of 5.4% until 2032,57 with its major application being polycarbonate production.58 The DMC market was valued between 1.10 and 1.17 billion USD in 2023, which is projected to reach 1.9 billion USD by 2028.59
3.2 Techno-economic analysis of organic carbonate production
One of the most used processes for the production of DMC, patented by Asahi Kasei in 2006, involves CO2 insertion into epoxides, with the formation of EC or PC as intermediates. Subsequently, they are converted to DMC via a transesterification reaction with methanol.60,61 Other well-known procedures for the production of rely on the oxidative carbonylation of methanol (EniChem, Bayer and UBE processes)62–65 and on the alcoholysis of urea.66,67In this aspect, various techno-economic assessments were performed over the years to evaluate the economic feasibility of DMC production through different routes, mainly involving CO2 as the starting reagent.68–70 Considering the oxidative carbonylation of methanol and direct methanolysis of urea as synthetic methods, the steps of methanol and urea syntheses appear to be the major capital investment contributors, rather than the DMC synthesis step.70 In this scenario, the DMC production cost was calculated to be around 520 € per t.68
The Asahi Kasei route was found to give the best performance in terms of energy consumption (11.4% improvement), net CO2 emission (13.4% improvement), global warming potential (58.6% improvement) and human toxicity-carcinogenic effects (99.9% improvement) compared to the Bayer process.69 It is worth mentioning that in the process forming EC as an intermediate to DMC, high-purity ethylene glycol (EG) is also produced as a by-product.69 EG can then be employed in numerous other industrial processes, such as in the synthesis of polyethylene terephthalate (PET) and polyethylene furanoate (PEF) as well as for energy, automobile, and chemical applications.
In addition, the techno-economic assessment performed by Kontou and co-workers in 2022 analysed the DMC/CO2 ratio of different DMC production concepts based on the initial formation of EC from the reaction of ethylene oxide (EO) with CO2, followed by its transesterification with methanol to produce DMC and EG as side products.71
Syntheses carried out with fossil-derived methanol from the market and CO2 procured from a pipeline network were compared to that in which MeOH was produced on-site using externally procured green hydrogen and CO2 was captured from a coal-fired power plant. The DMC/CO2 ratio of the scenarios considered ranged from 1.38 kg kg−1 to 0.53 kg kg−1, which can be further lowered to negative CO2 emission values (−105.48 kt a−1) when grid electricity and natural gas are used for covering the electricity and heating needs of the plant.71
According to these evaluations, the DMC minimum selling price of the different scenarios ranged from 634 to 1263 € per t, which could be reduced (659–707 € per t) assuming a future decrease in the price of green hydrogen. In this case, the minimum cost of DMC would range between 659 and 707 € per t, which is below the current market price of 849 € per t.71
Concerning the other most used cyclic OCs, i.e., EC and PC, they can be obtained using the same procedures previously described for the synthesis of DMC, given that they are formed as intermediates from the reaction of CO2 with the corresponding epoxide (namely, EO and propylene oxide (PO)). Consequently, it is safe to assume that their production cost and CO2 emissions would be lower compared to DMC.
Despite this consideration, other studies focused on the techno-economic analysis of alternative EC and PC syntheses, either starting from EG instead of the petroleum-derived EO73 or from CO2 and PO in the presence of different ILs as catalysts (namely, [P66614][Br] and 1-n-ethyl-3-methylimidazolium chloride), respectively.74,75 In the case of the synthesis of PC, the optimal configuration for the former system displayed an energy consumption of approximately 0.6 kW h kgPC−1 and utility costs of 6.6 USD per tPC.74 Alternatively, in the latter case, the net cost of duty was calculated to be 4.26 USD per tPC considering the production of 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year of PC using 4.5 t h−1 of CO2.75
000 t per year of PC using 4.5 t h−1 of CO2.75
In the production of EC, none of the cases tested were shown to be feasible for commercialization at an annual EC production of 5 ktonnes. The minimum selling price of EC would be in the range of 6.44 USD per kg to 16.73 USD per kg against the current market price fluctuating between 0.88 and 1.16 USD per kg.73,76
Data about the market production and costs of other more complex organic carbonates are still lacking. However, various processes for the synthesis of linear OCs rely on the use of DMC as the starting reagent, which is then subjected to transesterification in the presence of the desired alcohol.24,77 The custom-made cyclic OCs reported in this review were instead synthesized either by reacting GC with the corresponding acyl chloride78 or by reacting a suitable triol with urea in the presence of a catalyst.79
3.3 Safety aspects of organic carbonates
Organic carbonates are generally considered non-toxic compounds, with most of the toxicological studies performed on DMC. DEC is listed as “an experimental tumorigen and teratogen” and “mildly toxic by subcutaneous route”. Specific toxicity data obtained through animal testing were collected by Pacheco and co-workers.80A safety assessment regarding DACs used in cosmetics, namely, dicaprylyl carbonate, bis-propylheptyl carbonate, C14–C15 dialkyl carbonate, diethylhexyl carbonate, DMC and DPC, was published by The Cosmetic Ingredient Review (CIR) Expert Panel established with the support of the U.S. Food and Drug Administration (FDA) and the Consumer Federation of America.72,81 This report presented an overview of the use and toxicokinetic and toxicological effects of the studied DACs with experiments conducted on rats, cavies and human skin samples, concluding that the studied DACs “are safe in the present practices of use and concentration […] when formulated to be non-irritating”.81
The Environmental Protection Agency (EPA) indicated PC as a low concern chemical based on experimental and modelled data.83 Acute toxicity tests were performed by dermally administering PC to rats and rabbits at 5000 mg per kg-bw and 3000 mg per kg-bw, and no mortality was seen after 14 days (LD50 > 5000 and LD50 > 3000), respectively. Rats were also exposed to PC aerosol via inhalation at 0.1, 0.5 and 1.0 mg L−1 for 6 h day−1, 5 days per week, for 14 weeks. Ocular irritation and periocular swelling were seen at 0.5 and 1.0 mg L−1. Alternatively, the rats exposed to PC did not develop any chromosomal aberrations after intraperitoneal injection of PC at 1666 mg per kg-bw.84
In addition, cytotoxicity tests were conducted via the colorimetric MTT assay on a selection of custom-made alkyl methyl carbonates and 2-(2-methoxyethoxy) carbonates, (DGly)2C and DGlyMC, respectively. None of them showed any cytotoxicity effect at all the tested concentrations, both after 24 h and 48 h after the treatment of the cells. Moreover, computational analyses employing different software (CASE Ultra by MultiCASE Inc., USA; Model Applier by LeadScope, USA) and models based on quantitative structure–activity relationships (QSAR) predicted that all the tested DACs were not mutagenic, in compliance with ICH guideline M7.24,82
4. Organic carbonates as media in organic syntheses
OCs have been broadly investigated as green reaction media for many chemical transformations. In 1942, Wallingfod, Thorpe and Homeyer firstly reported DACs as solvents in the alkylation of malonic esters, β-keto esters and α-cyano esters (Scheme 1).85 They discovered that using DACs as media, the formation of the sodium derivative of these substrates went to completion and cleavage by alcoholysis was avoided. Several malonic esters, not achievable by the usual methods, were prepared operating in DEC solution. For instance, alkyl chains such as Et, Bu, iso-amil, n-amyl, allyl, benzyl, and sec-butyl were introduced with yields ranging from 70% to 95% (Scheme 1).85 | ||
| Scheme 1 (a) First examples of organic carbonates as reaction media–alkylation of malonic esters in DEC and (b) alkylation of β-keto and α-cyano esters in organic carbonates.86 | ||
Since the 1960s, the application of OCs as solvents has spread to nearly every field of chemistry.46 Moreover, cyclic carbonates, especially EC and PC, are well known for their high solvency.86 Thus, organic cyclic and linear carbonates started gaining attention given that they might partially or totally replace more expensive or more toxic solvents such as N-methyl pyrrolidone (NMP), dichloromethane, DMF, and isophorone46 in various synthetic approaches (Table 2 and Fig. 3).
 | ||
| Fig. 3 Chemical structures of cyclic and acyclic organic carbonates that have applications as reaction media. | ||
Linear and cyclic carbonates have been widely used as green solvents due to their non-hazardous nature, replacing the more toxic and fossil-derived compounds normally used in different organic synthesis processes.
In many cases, OCs have been shown to be viable alternative solvents that can compete, and in some cases, even outperform traditional solvents. In the following sections, we present some of the most prominent works where cyclic and linear OCs were used as solvents in different organic synthesis reactions, as summarized in Table 2.
4.1 Etherification and esterification reactions
To the best of our knowledge, only one example was reported in the literature employing OCs as solvents in esterification reactions. In this study, the synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol was performed in DMC. The reactions were carried out at room temperature in the presence of the selected C2–C18 acyl chloride added in slight excess. The final products were isolated in yields in the range of 90% to 98% in the case of tyrosyl and homovanillyl-derived esters, while yields of 60% to 68% for hydroxytyrosyl esters.87Similarly, only scant works focused on the application of OCs as solvents in etherification reactions. In particular, the symmetrical and non-symmetrical etherification of benzyl alcohols was performed in PC, replacing CH2Cl2 and CH3CN. The symmetrical etherification reaction was carried out in the presence of FeCl3·6H2O (5 mol%) as the catalyst and led to the corresponding symmetrical ethers in 53% to 91% yields.91 Recently, DMC was employed to replace benzene and toluene in the self-etherification of the bio-based platform chemical 5-hydroxymethyl furfural (HMF) to obtain 5,5′-[oxybis(methylene)]bis-2-furfural (OBMF). The reaction was carried out in the presence of a heterogeneous Lewis acidic catalyst, Fe2(SO4)3, allowing the recovery of OBMF in ca. 80% yield.88
4.2 Chlorination and bromination
Appel chlorination and bromination mediated by triphenylphosphine oxide (PPh3O) were performed using DMC as the solvent instead of chloroform (Scheme 2).89 The Appel reaction allows the conversion of alcohols to the respective activated alkyl halide promoted by PPh3O. Triphenylphosphine oxide is converted to its respective chlorophosphonium salt (CPS) by reaction with oxalyl chloride (COCl)2. Finally, CPS reacts with an alcohol, leading to the corresponding alkyl chloride.89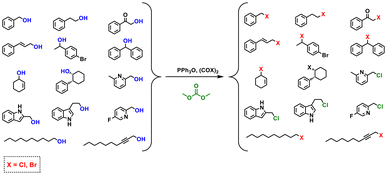 | ||
| Scheme 2 Appel chlorination and bromination reactions of different alcohols in DMC.89 | ||
The substitution of DMC with chloroform led to a slightly longer reaction time (15 min instead of 5 min) to achieve the total conversion of benzyl alcohol into its chloride. This suggests that the generation of CPS in DMC is a slower process than in chloroform. In addition, CPS needed a higher amount of DMC to be completely dissolved.89 The isolated yield values for the chlorination and bromination reactions of different alcohols in DMC varied between 21% and 83% in the former case, and 43% to 89% in the latter case.89
Another example is the Wohl–Ziegler bromination of 2-cyano-4′-methylbiphenyl conducted in PC (conversion and yield of 83% and 76%, respectively) and DEC (conversion and yield of 89% and 71%, respectively), replacing the commonly employed chlorinated solvents such as chlorobenzene and carbon tetrachloride. The obtained product, 4′-(bromomethyl)-2-cyanobiphenyl (BCB), is a key building block to various sartans, which are valuable nonpeptide angiotensin II antagonists (Scheme 3).90
 | ||
| Scheme 3 Bromination of 2-cyano-4′-methylbiphenyl using N-bromosuccinimide (NBS) to yield 4′-(bromomethyl)-2-cyanobiphenyl (BCB).90 | ||
4.3 Oxidation reactions
DACs were investigated as alternative green solvents for the oxidation of aryl–alkyl ketones101 and various aromatic compounds, i.e., styrene, naphthalene derivatives, sulfides, alcohols and phenolic molecules, in the presence of different catalysts.93,96,97 Bernini and co-workers relied on a H2O2/methyltrioxorhenium (MeReO3, MTO) catalytic system using DMC as the medium. The studied oxidations proceeded with good conversions (>98% via GC-MS) and yields of up to 98%.92DMC showed good performances as a green medium for the chemoselective and regioselective oxidation and demethylation of phenolic compounds in the presence of polymer-supported 2-iodoxybenzoic acid (IBX) to obtain bioactive catechol derivatives (Scheme 4A).93,96 DMC was also applied for the oxidation of aromatic alcohols to the corresponding carbonyl compounds employing zinc peroxide (ZnO2) nanoparticles (Scheme 4B; yields varying from 79% to 98%).97
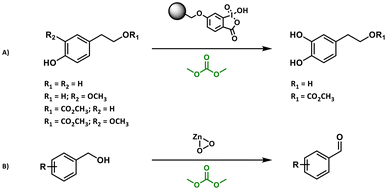 | ||
| Scheme 4 (A) Chemoselective and regioselective oxidation of phenolic compounds in the presence of polymer-supported IBX93 and (B) oxidation of aromatic alcohols to the corresponding carbonyl compounds in DMC.97 | ||
DMC was utilized as a solvent for the epoxidation of alkenes (Scheme 5) in substitution of the commonly employed acetonitrile. The reactions were performed in the presence of different heterogeneous catalysts, i.e., [PO4{WO(O2)2}4] (PW4), nitrogen-free or nitrogen-doped carbon nanotubes (CNTs or N-CNTs)102 and silica-supported cobalt-based (Co/SiO2) materials.94
 | ||
| Scheme 5 (A) Cyclooctene (CyO) epoxidation with H2O2 in DMC and (B) epoxidation of bio-renewable terpenes in DMC.94 | ||
In particular, cyclooctene (CyO) epoxidation with H2O2 in DMC showed better performances in the presence of PW4/CNTs and a tetrahexylammonium salt of PW4 (THA-PW4) as catalysts (conversion: 93% and 80; epoxide selectivity: 97% and 100%, respectively) compared to when PW4/N-CNTs was employed (conversion: 37%; epoxide selectivity: 89%) despite similar initial reaction rates (Scheme 5A).102 Encouraging results were also achieved for the epoxidation of a series of bio-renewable terpenes in DMC, i.e., β-pinene, camphene, 3-carene, limonene, valencene and β-caryophyllene (yield varying between 46% and 99%) as well as terpenes containing an alcohol functionality, i.e., myrtenol, nopol, (S)-(−)-perillyl alcohol and linalool (yield varying between 45% and 92%) using molecular oxygen, isobutyraldehyde (IBA) as a sacrificial reductant and a Co/SiO2 catalyst (Scheme 5B).94
In addition, the catalytic performance of PW4/CNT catalysts was assessed in the selective oxidation of organic sulfides with H2O2 using methyl phenyl sulfide (MPS) as the model substrate (Scheme 6) and compared with that of homogeneous PW4. In this case, the reactions carried out in acetonitrile in the presence of homogeneous THA-PW4 showed a higher sulfide conversion (93%) and selectivity to sulfoxide (83%) compared to that employing DMC (86% and 70%, respectively).102
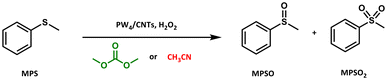 | ||
| Scheme 6 Oxidation of organic sulfides in DMC and acetonitrile as media in the presence of H2O2 and PW4/CNTs as the catalyst.102 | ||
Synthetic protocols for the synthesis of guanidines and amides using DMC as a green medium were reported in the studies by Baeten and Ramarao, respectively. Particularly, guanidines can be obtained via the oxidative rearrangement of amidines into carbodiimides, followed by an in situ reaction with amines (Scheme 7A).104 Amides can be isolated from the corresponding imines using molecular oxygen in air as the sole oxidant (Scheme 7B).103
 | ||
| Scheme 7 DMC as the solvent for the (A) synthesis of guanidines from amidines and (B) synthesis of amides from imines. NHC: N-heterocyclic carbene.103,104 | ||
PC was found to significantly enhance the oxidation reaction of cyclohexane over the Au/SiO2 catalyst with 22% conversion and 83% selectivity towards a cyclohexanone/cyclohexanol mixture (K/A-oil; 65% and 18%, respectively), which can be used for the production of Nylon-6 and Nylon-6,6.98 The conversions (18% to 22%) and selectivity (about 81%) using the cyclic carbonates towards K/A-oil were much higher than that of linear organic carbonates, i.e., DMC, DEC, DPC, EC and BC (conversions between 3% and 5%; selectivity between 44% and 56%).98
Another relevant example is the iron-catalysed aerobic oxidation of 2-benzylpyridines to their corresponding ketones, which was performed in continuous flow using PC instead of more toxic dipolar aprotic solvents, i.e., CH3CN, DMF, NMF, NMP, DMA and DMSO (Scheme 8).99 The reaction time was significantly reduced from hours to minutes and molecular oxygen was replaced by synthetic air as the oxygen source.99
 | ||
| Scheme 8 Iron-catalysed aerobic oxidation of 2-benzylpyridines in continuous flow using PC as the solvent.99 | ||
4.4 Synthesis of amines and amides
DACs were demonstrated to be suitable reaction media for the synthesis of both linear and cyclic amines. For example, primary amines were obtained via the Delépine reaction165 (Scheme 9) by reacting hexamethylenetetramine (HMTA) with an alkyl or benzyl halide in the presence of DMC instead of the commonly employed solvent CHCl3. The product was obtained in comparable yields.105 | ||
| Scheme 9 Synthesis of primary amines via the Delépine reaction.105 | ||
Moreover, DMC was used as the solvent for the synthesis of several pharmaceutically relevant building blocks, i.e., N-Boc-3-pyrroline, with a yield similar to the previously reported synthetic procedures (86% vs. 84.9%).105
DMC also displayed encouraging results as a solvent replacing 1,4-dioxane and toluene for the iron-catalysed one-pot hydrosilylation reaction of a wide range of N-alkylated and arylated cyclic amine derivatives including the pharmaceuticals fenpiprane and prozapine (Scheme 10). The test reactions conducted with glutaric acid, aniline and DMC under visible light irradiation showed similar yield values to that performed using 1,4-dioxane and toluene (70%, 75% and 72% yield, respectively).106 Moreover, the use of Fe(OTf)2 as an additive in some cases further increased the reaction yield of the desired cyclic amines up to 96%.106
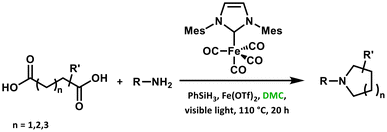 | ||
| Scheme 10 Synthesis of N-substituted cyclic amines using DMC as the solvent.106 | ||
α-Substituted homoallylamines can be obtained using DMC and PC as solvents via the cationic 2-aza-Cope rearrangement of aldimines generated in situ via the condensation of commercially available aldehydes and 1,1-diphenylhomoallylamines (81–98% product yield).107
DMC was also employed as a green substitute for dichloromethane in the microwave-assisted synthesis of peptidomimetic arylamides, compounds that can inhibit cysteine and serine-like proteases.108 Overall, the yields obtained with the use of DMC (32–47%) were slightly lower compared to that obtained when CH2Cl2 was used as the solvent (44–70%), regardless of the coupling reagent employed (Scheme 11). Nevertheless, the isolation of the compounds when the reaction was performed in CH2Cl2 was hampered due to the enhanced solubility of the byproducts in this solvent.108
 | ||
| Scheme 11 Microwave-assisted synthesis of peptidomimetic arylamides in DMC. COMU: third generation uronium-type coupling reagent [(1-cyano-2-ethoxy-2-oxoethylidenaminooxy) dimethylamino-morpholino carbenium hexafluorophosphate].108 | ||
4.5 Wolff rearrangement/acylation reaction
DEC was employed as a green solvent for the chemoselective cascade Wolff rearrangement/acylation reaction between 5-aminopyrazoles and diazo compounds (Scheme 12).109 Among the different solvents tested, i.e., DCE, DMF, CH3CN, dioxane, DMA, EtOH, tBuOH, BuOH, toluene, DMC and DMSO, carbonate media seemed to be particularly beneficial for in this reaction, with DEC and DMC giving yields of up to 95% and 70%, respectively.109 | ||
| Scheme 12 Wolff rearrangement/acylation reaction between 5-aminopyrazoles and diazo compounds.109 | ||
4.6 Dehydration reaction: synthesis of 5-(hydroxymethyl)furfural
DACs such as DMC, DEC, PC and diallyl carbonate (DAllC) have been tested as co-solvents in several synthetic approaches to 5-(hydroxymethyl)furfural (HMF), a well-known bio-based platform chemical, starting from D-fructose or D-glucose (Table 3). DMC was used in the reaction mixture as the extraction solvent for HMF; its use was found to reduce the formation of humins and numerous byproducts (#1, Table 3).111,112| # | Sugar | Solvent | Catalyst | T (°C) | t (h) | Conv. (%) | Selectivity HMF (%) | Yield HMF (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| n.r.: value not reported; n.d. compound not detected. | |||||||||
| 1 | D-Fructose | DMC/H2O | CeP3 (35 wt%) | 150 | 6 | 73 | 93 | 68 | 111 |
| DEC/H2O | 49 | 88 | 45 | ||||||
| PC/H2O | 46 | 85 | 40 | ||||||
| DAllC/H2O | 43 | 80 | 36 | ||||||
| 2 | D-Glucose | DMC/EMIMBr | SnCl4 (10 mol%) | 100 | 2 | n.r. | n.r. | 58 | 112 |
| 3 | D-Fructose | DMC/TEAB | Amberlyst-15 (10 wt%) | 90 | 5 | 99 | n.r. | 77 | 114 |
| DMC/TEAB | Purolite CT275DR (5 wt%) | 110 | 2 | n.r. | 98 | 73 | 116 | ||
| 4 | Corn-cob | DMC | AlCl3 (30 wt%)/HCl (4 N) | 180 | 6 | n.r. | n.r. | 35 | 113 |
| Sugarcane bagasse | n.r. | n.r. | 60 | ||||||
| Rice-straw | n.r. | n.r. | 37 | ||||||
| Corn-straw | n.r. | n.r. | 47 | ||||||
| 5 | D-Glucose | DMC | Sulfonated graphitic carbon nitride (S-GCN, 10–50 wt%) | 200 | 5 | n.r. | 99 | 23 | 115 |
| D-Fructose | 200 | 5 | n.r. | 99 | 17 | ||||
| Cellobiose | 200 | 5 | n.r. | 99 | 16 | ||||
| Sucrose | 200 | 5 | n.r. | 99 | 30 | ||||
| Starch | 200 | 5 | n.d. | n.d. | n.d. | ||||
DMC was also employed as the main reaction medium to achieve HMF either from D-fructose (#3, Table 3)114,116 or from more complex mixtures, i.e., cellobiose, sucrose, starch, corn-cob, sugarcane bagasse, rice-straw and corn-straw (#4 and 5, Table 3) even if in the latter cases with lower HMF yields.113,115 The reason for the utilization of DMC as the medium for sugar dehydration into furan-based molecules may be the enhanced stabilization capacity of compounds containing a carbonyl moiety, i.e., methyl isobutyl ketone (MIBK), OCs and DMF towards furanics.166,167 On this topic, a comprehensive study on the stability of different furan-based compounds both in acidic and basic media in the presence of various solvents was recently carried out by Ananikov and co-workers.166
4.7 Rh-catalysed reactions
The Rh-catalysed hydroformylation (oxo synthesis) of caryophyllene oxide and β-caryophyllene was carried out employing DMC and DEC among other green solvents, replacing the conventionally used hydrocarbons such as toluene and benzene (Scheme 13A).119 The reactions showed high selectivity and complete substrate conversion despite a slightly lower reaction rate than the procedure conducted in toluene, whereas p-cymene performed with the same efficiency.119 | ||
| Scheme 13 (A) Hydroformylation of caryophyllene oxide and β-caryophyllene.119 (B) Isomerizing hydroformylation of trans-4-octene to n-nonanal in a two-phase catalytic reaction system.118 (C) Rh-catalysed hydroformylation of higher olefins (C > 6), with a mixture of PC and n-heptane as the solvent system.120 (D) Rh-catalysed co-oligomerization of fatty acid derivatives.117 (E) Rh-catalysed ortho-diarylation of various arylheteroaryl substrates with N-ligand employing DEC as the green solvent.121 (F) Intermolecular alkyne hydroacylations using PC as the solvent.122 | ||
Another suitable solvent for regioselective, Rh-catalysed hydroformylation reactions is PC, which was shown to effectively mediate the isomerizing hydroformylation of trans-4-octene to n-nonanal in a two-phase catalytic reaction system (Scheme 13B). PC could increase the catalyst activity, leading to 95% conversion and up to 95% selectivity for the linear aldehyde.117,118 Alternatively, tests performed employing PC/dodecane and PC/dodecane/p-xylene solvent mixtures showed that the higher the PC concentration, the higher the selectivity of n-nonanal. This significant influence of PC on the selectivity can be explained by the electron-withdrawing effect of the carbonate group, which may be able to interact with the β-hydride atoms of the σ-rhodium-complex, leading to faster isomerization.118
Similar results were achieved by Tijani and co-workers in the Rh-catalysed hydroformylation of higher olefins (C > 6), where a mixture of PC and n-heptane was reported as the most suitable solvent system (Scheme 13C).120
A solvent mixture of PC/conjugated sunflower fatty acid methyl ester (SFAME)/1,4-dioxane was successfully employed in the Rh-catalysed co-oligomerization of fatty acid derivatives with ethylene, leading to the formation of internal branched fatty substances (Scheme 13D).117 The total yield of the process be increased to 98% in this solvent system under mild conditions (70 °C, 3.0 MPa) and the turnover frequency was enhanced by a factor of 100 (2 vs. 220 h−1).117
In addition, the selective ortho-diarylation of various arylheteroaryl substrates could be achieved via the N-ligand directed Rh-catalysed coupling of highly functionalized aryl phenolate derivatives employing DEC as a green solvent to replace NMP and 1,4 dioxane (Scheme 13E).121 Overall, using DEC as the medium yielded the desired products even with bulkier, more hindered reagents as well as with electron-donating substituents in the para-, meta- and ortho-position.121
Finally, PC was employed by Lenden et al. as a green solvent for intermolecular alkyne hydroacylations, behaving as a valid alternative to DCE and acetone (Scheme 13F).122 The results showed that these reactions could be carried out in PC using [Rh(nbd)2]BF4 as the catalyst in combination with 1,2-bis(diphenylphosphino)ethane (dppe) as the ligand, obtaining yields in the range of 73% to 95%.122
4.8 Pd-catalysed reactions
Linear and cyclic OCs were also shown to be viable alternatives to chlorinated solvents in Pd-catalysed transformations. For example, PC, BC, and DEC were tested as substitutes for CH2Cl2 in the Pd-catalysed asymmetric allylic substitution reactions of rac-1,3-diphenyl-3-acetoxy-prop-1-ene with dimethyl malonate or benzylamine as nucleophiles (enantioselectivities ranging from 83% to 98%, Scheme 14). The use of these green solvents in several cases led to enhanced yields and enantioselectivities compared to CH2Cl2.123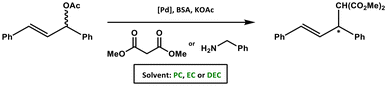 | ||
| Scheme 14 Pd-catalysed asymmetric allylic alkylation of rac-1,3-diphenyl-3-acetoxy-prop-1-ene with dimethyl malonate or benzylamine using DACs as green solvents.123 | ||
Behr et al. investigated the substitution of CH3CN as the solvent with different linear DACs (DMC and DEC), cyclic DACs (EC, PC and BC), custom-made glycerol carbonate esters (glycerol carbonate propionate (GCP) and glycerol carbonate butyrate (GCB)) as well as DAC mixtures in the Pd-catalysed telomerisation of butadiene with carbon dioxide, leading to the formation of δ-lactone 2-ethylidene-6-heptene-5-olide (Scheme 15).78
 | ||
| Scheme 15 Pd-catalysed telomerization of butadiene with carbon dioxide with DACs as the solvent.78 | ||
Among the DACs tested, reactions conducted in PC and EC showed higher selectivity (from 65% to ∼90%, respectively) towards lactone formation compared to linear carbonates and CH3CN (ca. 40% selectivity in the latter case). The reactions carried out in GCP and GCB showed lower yields of δ-lactone compared to EC, PC and BC, leading to the hypothesis that the selectivity of the lactone may depend on the size of the additional substituent in the carbonate solvent.78
DMC was proven to be the optimal green solvent for the chemo- and regio-selective Pd-catalysed allylation of biologically relevant heteroarenes (Scheme 16).124 The reaction displayed enhanced yields of the allylated compound compared to that employing DMF, 1,4-dioxane and CH3CN (92%, 82%, 84% and 80% isolated yield, respectively).124
 | ||
| Scheme 16 Pd-catalysed allylation of biologically relevant heteroarenes with allyl alcohols.124 | ||
In addition, DMC was found to be a suitable replacement for toluene and CH2Cl2 in the selective cyclocarbonylation of allyl phenol derivatives for the synthesis of lactones (Scheme 17).125 However it must be mentioned that in some cases, DMC behaved as a ring-opening reagent, producing methoxycarbonyl compounds when the reactions were conducted for longer periods (48 h) and at higher temperatures (120 °C).125
 | ||
| Scheme 17 Cyclocarbonylation of allyl phenol derivatives for the synthesis of lactones with DMC as the solvent.125 | ||
Partial Pd/C hydrogenation of a fatty acid methyl ester (FAME) mixture was performed in DMC as the medium by Quaranta and co-workers. However, in this case study, lower conversion (37%) and selectivity values were obtained compared to when n-heptane was employed under mild conditions (conversion and selectivity of 97.8% and 81.1%, respectively).126
Gautam et al. demonstrated that PC and EC can be used as green solvents for the Pd/C-catalysed phenoxycarbonylation of aryl iodides in the presence of N-formylsaccharin as a CO surrogate, yielding a library of different phenyl esters (Scheme 18).127 PC displayed higher substrate conversion compared to EC (76% and 67%, respectively), while complete selectivity towards the desired product was achieved in both cases.127
 | ||
| Scheme 18 Pd/C-catalysed phenoxycarbonylation of aryl iodides using DACs as the solvent.127 | ||
 | ||
Scheme 19 Pd-catalysed (A) carbonylative couplings of m-tolylboronic acid and 3-bromoanisole; (B) aminocarbonylation reaction of aryl bromides; and (C) alkoxycarbonylation reactions using DACs as the media. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reactions conducted in DMC led to the formation of the methoxycarbonylated analogue isolated in 93% yield.128 Reactions conducted in DMC led to the formation of the methoxycarbonylated analogue isolated in 93% yield.128 | ||
Pd-catalysed alkoxycarbonylation reactions gave the best results when performed in 2-Me-THF (91% yield), while DEC, EC and PC only led to moderate yields (45%, 30%, and 60%, respectively; Scheme 19C). Alternatively, the reaction conducted in the presence of DMC gave the methoxycarbonylated analogue in 93% isolated yield,128 highlighting the capabilities of OCs as both reactants and reagents.51
 | ||
| Scheme 20 (A) Suzuki–Miyaura cross coupling of amides with DACs as the media;129 (B) Suzuki–Miyaura coupling with different heterocyclic compounds using PC as the solvent;130 (C) carbonylative Suzuki–Miyaura and Sonogashira cross-coupling reactions in PC;134 and (D) Sonogashira cross-coupling reaction of aryl chlorides in PC.133 | ||
Several researchers employed PC as the solvent in the Suzuki–Miyaura and Sonogashira coupling reactions. Czompa et al. reported that different heterocyclic compounds, i.e., 2-iodopyridine, 4-iodopyridine and 6-iodopyridazin-3(2H)-one and various boronic acids can be used as starting materials (Scheme 20B) both under microwave conditions and conventional oil bath heating.130 All the reactions proceeded with good to excellent yields (from 43% to 92% under conventional heating and from 50% to 93% under microwave irradiation) of the corresponding coupling products. However, in the case of pyridazinones, 2-hydroxypropyl-chain-containing side-products were observed due to the ring opening of the cyclic OC.130
In another example, PC was used as the medium in carbonylative Suzuki–Miyaura and Sonogashira cross-coupling reactions catalysed by the aminophosphine pincer complex {[C6H3-2,6-(NHP{piperidinyl}2)2]Pd(Cl)}(III) (Scheme 20C).134 In fact, carbonylation reactions are known to proceed efficiently in cyclic OCs, and thus can be effectively employed for the substitution of other toxic solvents, i.e., anisole, toluene, dioxane, DMA, MTBE, and DMF.134 The reactions proceeded with yields in the range of 70%–80% for both Sonogashira and Suzuki–Miyaura carbonylative cross-coupling.134
Finally, Torborg et al. employed PC as the solvent for the Pd-catalysed Sonogashira cross-coupling reaction of aryl chlorides in the presence of N-substituted heteroaryl phosphines without copper co-catalysts (Scheme 20D).133 The reaction of 3-chlorothiophene and 1-octyne was tested using PC as the solvent at 90 °C, leading to 76% yield of the cross-coupling product. It should be mentioned that PC can also partially displace the ligand, and thus the reaction required a higher Pd/ligand ratio. In contrast, the Sonogashira coupling in toluene with sodium carbonate as the base yielded the desired 3-octinylthiophene in good yield at a lower ligand concentration.133
 | ||
| Scheme 21 Pd-catalysed Heck coupling reaction in EC as the solvent.132 | ||
The same reaction was also tested in DMC, DEC and GC without obtaining comparable results (30%, 30% and 10% yield, respectively).
The highest performance for EC was ascribed to its degradation and release of CO2 upon heating, whose dissolution in the reaction media facilitated Pd solubilization, as previously reported in the literature.168,169 This resulted in an increase in the reaction rate because the Heck reaction occurs homogeneously in the organic phase. However, due to the thermal degradation of EC, its recovery and reuse could not be performed.132
Other studies also reported the ability of PC to act as a colloidal palladium stabilizer. Subsequently, the colloidal solution can catalyse Heck reactions in the absence of phosphine ligands. Given that catalysis is likely to occur on the surface of the clusters, these processes are probably more related to heterogeneous than to homogeneous catalysis.170
4.9 Ni-catalysed reactions
DMC was successfully employed as a green medium substitute for toluene in the hydrodeacetoxylation of aryl acetates mediated by pinacolborane (HBpin) and a nickel-N-heterocyclic carbene (NHC) catalytic system, yielding the corresponding deoxygenated arenes (yields between 46% and 90%).1364.10 Pt-catalysed reactions
PC was applied as a co-solvent in the platinum-catalysed hydrosilylation of unsaturated fatty acids.137,138 In particular, the introduction of a ternary solvent system formed by cyclohexane/toluene/PC allowed the recycle and reuse of the catalyst even if hydrogenation and double bond isomerization of the starting reagent were reported to occur as side reactions.137Moreover, a mixture of cyclohexane/PC and n-hexane/PC could be employed for the recycling of the catalyst in these reactions.137,138
4.11 Ru-catalysed olefin metathesis
Olefin metathesis is almost exclusively carried out in dichloromethane and aromatic solvents (benzene, toluene, and chlorobenzene). However, efficient metathesis transformations can be performed in DMC and PC, given that they were shown to be compatible with ruthenium-catalysed olefin metathesis reactions.139–141Miao et al. reported a series of olefin metathesis transformations, i.e., ring-closing metathesis (RCM), cross-metathesis and ethenolysis of methyl oleate, which could be performed in dimethyl carbonate and CH2Cl2 (or aromatic solvents) with comparable results (Scheme 22). In some cases, the RCM reactions proceeded faster in DMC than in CH2Cl2, despite displaying similar yields.139
 | ||
| Scheme 22 Olefin (A) ring-closing metathesis; (B) cross-metathesis and (C) ethenolysis in DMC.139 | ||
Huang et al. reported the use of PC as a suitable solvent for the ruthenium RCM and cross-metathesis transformations of a variety of substrates including renewable fatty esters (Scheme 23).140
 | ||
| Scheme 23 Olefin (A) ring-closing metathesis and (B) cross-metathesis in PC.140 | ||
Ru-catalysed enyne cross-metathesis of several alkyne derivatives with terminal olefins could also be performed under mild conditions in DMC, substituting dichloromethane and toluene.
A one-pot reaction based on an ethenolysis step followed by an enyne cross-metathesis allowed the efficient transformation of renewable unsaturated fatty esters into valuable conjugated 1,3-dienes.141 This new reaction sequence provides a useful method in oleochemistry for the conversion of natural oils into functional compounds or intermediates of interest for further transformations.141
DMC was also employed as the solvent, substituting toluene, in both ethenolysis and cross-metathesis reactions using different renewable fatty esters, i.e., methyl oleate (Scheme 24A), dimethyl octadec-9-enedioate (Scheme 24B) and methyl ricinoleate (Scheme 24C), yielding conjugated 1,3-dienes of interest for further transformations. This protocol allowed the ene–yne cross-metathesis reaction to be carried out with long-chain terminal olefins and in one-pot with internal olefins after shortening by ethenolysis.142
 | ||
| Scheme 24 Two-step ethenolysis/ene–yne cross-metathesis starting from (A) methyl oleate, (B) dimethyl octadec-9-enedioate and (C) methyl ricinoleate in DMC as the solvent. Yields calculated using GC.142 | ||
In addition, cross-metathesis reactions of dec-1-ene and methyl undec-10-enoate with various terminal and internal propargylic acetates and carbonates were tested in toluene and DMC (yield values between 70%–95% in the former case and 54%–74% in the latter).142
4.12 Organic carbonates and ionic liquids as media in organic synthesis
The combination of ionic liquids and OCs as solvent systems was shown to perform a wide variety of different organic transformations. Particularly, cyclic DACs showed appreciable results for the synthesis of organic compounds in combination with ionic liquids. In fact, PC and supercritical CO2 (scCO2) were applied as solvents for the continuous synthesis of D,L-α-tocopherol using a sulfonic acid-functionalized ionic liquid as the catalyst (yields up to 90%). D,L-α-Tocopherol is the main composition of vitamin E and plays an important role in human health due to its antioxidative capacity and ability to act as a free radical scavenger.144Moreover, the cycloisomerization of N-(prop-2-yn-yl)benzamide to 2-phenyl-5-vinylidene-2-oxazoline in the presence of NHC–Au–X [NHC = (1,3-bis(2,6-di-isopropylphenyl)-imidazol-2-ylidene), X− = BF4−, OTf−, OTs−, and TFA−] as catalysts was carried out in PC, among others, as the medium (Scheme 25).145 However, it must be mentioned that in this case, the green solvents showed, on average, slower conversion with respect to volatile organic solvents (VOS) (conversionPC: 49%, TOFPC: 180 h−1vs. conversionCH2Cl2: 89%, and TOFCH2Cl2: 406 h−1).145
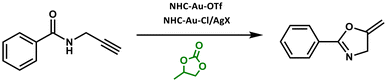 | ||
| Scheme 25 Cycloisomerization of N-(prop-2-yn-yl)benzamide to 2-phenyl-5-vinylidene-2-oxazoline in the presence of NHC–Au–X in PC.145 | ||
The vitamin B1 like-derived acidic ionic liquid [HMTH]2H2[SiW12O40] coupled with DMC, PC and EC as solvents was found to be an efficient heterogeneous catalyst for the direct dehydrative coupling of alcohols with alcohols or alkenes to synthesize various polysubstituted olefins. Excellent yields of the desired compounds were obtained (93% yield at 120 °C for 15 min, 3% catalyst loading) with DMC as a green solvent, while the reaction proceeded with good yields in PC and EC (66% and 37%, respectively).131
4.13 Phase transfer catalysis
DMC was tested together with methyl-tert-amyl ether (MTAE), 5-methyl-2-hexanone (MIAK) and MIBK as alternative media to toluene and chlorobenzene for phase-transfer catalysed (PTC) reactions in organic media. The experiments were conducted by dissolving different quaternary ammonium salts (Q+Y−), i.e., MeBu3N+Cl−, MeBu3N+, p-NO2C6H4O−, Bu4N+Cl−, Bu4N+Br−, Bu4N+p-NO2C6H4O−, Hexyl4N+Cl−, Hexyl4N+Br−, Hexyl4N+p-NO2C6H4O−, Octyl3MeN+Cl−, Octyl4N+Br−, and Bu3P+C16H33Br− in the selected organic media and a water solution containing the corresponding sodium salt (Na+Y−). The data showed that in these media, the partition of the catalyst in the organic phase is comparable to or higher than that in chlorobenzene.146 In particular, the solubility in DMC was similar with that in chlorobenzene under both homogeneous and heterogeneous conditions, and hence DMC represents a valid greener alternative to the solvents traditionally used in PTC processes.1464.14 Enantioselective reactions
EC and PC have been shown to be excellent media for several asymmetric reactions, i.e., aldol reactions,150,171,172 hydrogenation of non-functionalized olefins,147,148 cyanohydrin trimethylsilyl ether synthesis,149 and α-hydrazination of aldehydes and ketones,151 substituting the most employed toxic solvents such as DMF, DMSO, CH2Cl2 and CH3CN (Scheme 26). In all cases, chemical yields of up to 99%, diastereoselectivity of up to 100% and enantioselectivity up to 99% were obtained.1504.15 Photocatalytic reactions
Photocatalytic reactions were reported in the literature using DMC as the medium under both UV and visible light irradiation (Table 4 and Scheme 27). | ||
| Scheme 27 Examples of 3-alkylation of 2-aryl-2H-indazoles in DMC.152 | ||
| # | Reaction type | Organic carbonate | Reagent(s) | Product(s) | Catalysts | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a Conversion values. b Selectivity values. | |||||||
| 1 | Decarboxylative 3-alkylation | DMC |

|

|
4CzIPN, blue led | 41–91 | 152 |
| 2 | CH bond arylation | DMC |

|

|
Ru(bpy)3Cl2, Pd(OAc)2, visible light | 67–94 | 153 |
| 3 | Oxidative hydroxylation | DMC |

|
R–OH | 7H-Benzo[c]thioxanthen-7-one, visible light | 81–97 | 157 |
| 4 | Alcohol oxidation | DMC | R–OH |

|
TiO2(C/T), visible light | 64–95a, 92–99b | 156 |
| 5 | 1,3-Diene derived quinolinone compounds | DMC |

|

|
4CzIPN, blue led | 13–93 | 158 |
| 6 | Aroylated heterocycles | DMC |

|
|
Catalyst free, visible light, blue led | 40–95 | 155 |
A transition-metal-free photocatalytic decarboxylative 3-alkylation reaction of 2-aryl-2H-indazoles was developed under visible-light irradiation (#1, Table 4 and Scheme 27). By employing 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) as the photocatalyst, and alkyl N-hydroxyphthalimide esters as the alkylating reagents, various primary, secondary, and tertiary alkylated 2-aryl-2H-indazoles were synthesized in moderate to good yields (41%–91%). 2-Aryl-2H-indazoles containing strong electron-withdrawing substituents (4-NO2 and 4-CN) showed no reactivity in these conditions. Moreover, the protocol was successfully applied to the late-stage modification of drug molecules such as Pazopanib, ER-16b, Niraparib, and WAY-214950.152
Furthermore, DMC was shown to be a suitable solvent for (i) the direct CH bond arylation of anilides later applied for the gram-scale synthesis of the fungicide Boscalid (81% isolated yield; #2, Table 4);153 (ii) the aerobic oxidative hydroxylation of boronic acids (#3, Table 4);157 (iii) the oxidation of diverse alcohols (#4, Table 4)156 and (iv) the synthesis of 1,3-diene-derived quinolinone compounds (#5, Table 4).158
Additionally, Zeng and co-workers developed a visible-light-induced strategy for the construction of various aroylated heterocycles, including the modification of pharmaceuticals and natural products, i.e., thioflavones, benzimidazo[2,1-a]isoquinolin-6(5H)-ones, indolo[2,1-a]isoquinolin-6(5H)ones, quaternary 3,3-dialkyl 2-oxindoles, inoxaline-2(1H)-ones, and benzo[e][1,2,3] oxathiazine 2,2-dioxides in DMC (#6, Table 4).155
4.16 Other reactions/applications of organic carbonates as media in organic synthesis
 | ||
| Scheme 28 Synthesis of 2,4,5-triaryl imidazoles via the Radziszewski reaction with PC as the medium.160 | ||
All the reactions were performed at room temperature, and the only noticeable difference was in the reaction times (10 h DMC vs. 2–4 h Et2O; 1–2 h DMC vs. 5 h CH3CN), while the isolated product yields varied from 70% to 99%.164
5. Organic carbonates in polymerization and depolymerization reactions
OCs such as PC, EC, DMC have been efficiently employed for different types of polymerizations,173–177 including electroploymerization,178–187 photopolymerization,188,189 as well as depolymerization reactions. Regarding the latter topic, studies have been reported on the depolymerization of cellulosic paper towels,190 solvolysis of cellulose,191 liquefaction of newspaper192 and lignin depolymerization.1935.1 Radical polymerizations
An example of radical polymerization conducted in OCs is the copolymerization of polyethylenes with a low ketone content, which was carried out using DMC or under aqueous conditions at pressures <350 atm.176DAC/water biphasic systems composed of DEC, DMC, PC and EC were also shown to be suitable for single electron transfer Living radical polymerization (SET-LRP) as substitutes for THF and 1,4-dioxane.175 The SET process involves the transfer of a single electron from an electron donor (i.e., Cu(0) and Cu(I)X catalysts) to an electron acceptor, which can be situated in two different molecules or in two sites of a single compound.194 This mechanism can be exploited for the promotion of different chemical transformations in biology, electrochemistry and polymer chemistry.194 DEC as well as other nonpolar solvents i.e., ethyl acetate, toluene, anisole, and cyclohexane, were shown to mediate the SET-LRP of n-butyl acrylate (BA) in ethanol/water mixtures at a 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0
4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 volume ratio instead of hexane.174
2.0 volume ratio instead of hexane.174
Similarly, EC was investigated as the solvent in atom transfer radical polymerization (ATRP).173 In ATRP, the radical species are generated through a reversible red-ox process catalysed by a transition metal complex (Mtn–Y/Ligand, Scheme 29, top side). This process is regulated by the activation (kact) and deactivation (kdeact) constants. Similar to radical polymerization mechanisms, polymer chains propagate by the addition of the intermediate radicals to monomers (propagation constant, kp). Moreover, in ATRP, termination reactions (termination constant, kt) rarely occur majorly through radical coupling and disproportionation.195
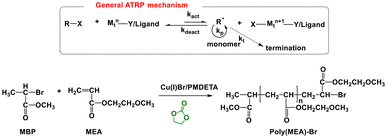 | ||
| Scheme 29 General mechanism for ATRP (top) and ATRP of MEA in EC as the medium.173,195 | ||
For example, the ATRP of 2-methoxy ethyl acrylate (MEA) was carried out in the presence of methyl 2-bromopropionate (MBP) as the initiator and CuBr/N,N,N0,N0,N00-pentamethyldiethylenetriamine (PMDETA) as the catalyst system (Scheme 29, bottom side).173 The resulting polymers were compared to that obtained using toluene as the medium, showing similar results in terms of Mn and Mw/Mn. In addition, both polymers displayed a narrow molecular weight distribution.173
5.2 Electropolymerization
Electropolymerization techniques allow the production of polymeric or inorganic films with precise spatial resolution using the standard three-electrode configuration (working electrode, reference electrode, and counter electrode) in an electrochemical cell dipped in an electrolytic solution (Scheme 30). After the potential is applied, a polymeric thin film starts growing on the surface of the working electrode.196,197 | ||
| Scheme 30 Electropolymerization using DACs as the media. (A) Pyrrole electropolymerization;199 (B) thiophene electropolymerization;200 and (C) N-methylaniline (NMA) and N-butylaniline (NBA) electropolymerizations; mechanism modified from.183 ITO: indium tin oxide, TO: tin oxide. | ||
The obtained polymers can be applied in batteries, conductive textiles and fabrics, antistatic coatings, supercapacitors and special sensors.178 It should be mentioned that the solvent affects the electrochemical activity, conductivity, and morphology of the resulting polymer.198 Therefore, the solvent choice is particularly important given that it provides an ionic conducting medium. It must possess a high relative permittivity, and it must be stable at the oxidation potential of the monomer. PC was reported to be an ideal solvent given that it fulfils all these characteristics.178
Moreover, electropolymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) and tetrafluoroborate was carried out in PC as the medium to create coatings for metal electrodes, which possessed similar proprieties to that obtained in CH3CN. The coatings prepared with PC displayed excellent electrochemical stability and survived autoclave sterilization, prolonged soaking, and electrical stimulation without major changes in their electrochemical properties.187
5.3 Photopolymerization
DMC/CH3CN solvent system favoured the photoinduced polyaddition reaction between α,ω-diiodoperfluoroalkanes and α,ω-unconjugated dienes instead of chain transfer reactions, generating a high yield (76.5%) and high molecular weight (Mn,GPC = 9400 g mol−1) perfluorocarbon-containing alternating copolymers. The polymerization was performed under irradiation with blue light emitting diodes (LEDs) at room temperature (25 °C, Scheme 31).188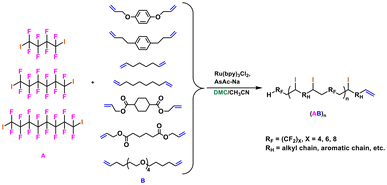 | ||
| Scheme 31 Photoinduced step transfer-addition and radical-termination (START) polymerization between α,ω-diiodoperfluoroalkanes and α,ω-unconjugated dienes in a mixture of DMC and CH3CN as the media.188 | ||
Kim et al. reported DMC as the most efficient solvent among more than 40 solvents for the free radical photopolymerization of alkenes, including vinylidene fluoride (VDF), vinyl acetate, methyl methacrylate, styrene, and butadiene.189
5.4 Depolymerization
The degradation and decomposition of cellulose were studied in the acid-catalysed solvolysis treatment of biomass using polyethylene glycol (PEG) and EC. EC was shown to promote the faster degradation of cellulose compared to PEG, leading to the formation of glucosides, which then decomposed, resulting in a levulinic acid structure (Scheme 32).191 DMC was instead employed as a trapping agent for EG in the depolymerization of polyester fibres from textile products.201 | ||
| Scheme 32 Acid-catalysed solvolysis for the decomposition of cellulose in an EC-PEG system.191 | ||
Furthermore, PC, EC, DMC and their mixtures with water were investigated as green co-solvents for the MW-assisted depolymerization of cellulosic paper towel waste catalysed by dilute sulfuric acid, replacing DMSO and THF. PC/H2O and EC/H2O enhanced the depolymerization of paper towel waste and improved the total sugar yield (up to ca. 25 C mol%) compared to H2O only (up to ca. 11 C mol%) under mild reaction conditions (130 °C, 20 min). The higher performance of PC/H2O and EC/H2O can be attributed to the higher availability of reactive protons in the catalytic system, which facilitates efficient acid hydrolysis of the recalcitrant cellulosic fibres.190 However, the high boiling point (242–248 °C) of these solvents can be challenging for product separation and solvent recovery by distillation.190
5.5 Resin preparation
DMC proved to be useful as a solvent in the synthesis of a bio-based phthalonitrile (PN) resin via the nucleophilic substitution reaction between 4-nitrophthalonitrile and Schiff base bisphenol (VTBP, obtained by reacting vanillin and tyramine in ethanol) (Scheme 33). To further increase the sustainability of the procedure, DMC was recovered and reused.202 | ||
| Scheme 33 Synthesis of a bio-based phthalonitrile (PN) resin (VTPN) via the nucleophilic substitution reaction between 4-nitrophthalonitrile and Schiff base bisphenol (VTBP). VTPB was obtained by reacting vanillin and tyramine in ethanol.202 | ||
5.6 RAFT/MADIX co-polymerization
DMC was used as the medium for the co-polymerization reactions between tetrafluoroethylene (TFE) and isobutyl vinyl ether (iBuVE) via both conventional radical and reversible addition–fragmentation chain transfer polymerization/macromolecular design via the interchange of xanthates (RAFT/MADIX) method.203 The RAFT/MADIX method enables control of the chain growth in radical polymerization through the dynamic equilibrium between the growing chains and dormant chains based on reversible transfer or termination reactions (Scheme 34). This technology can be used to design complex functional architectures in the bulk, organic solvents and water.204 | ||
| Scheme 34 RAFT/MADIX general mechanism (framed reactions); co-polymerization between tetrafluoroethylene (TFE) and isobutyl vinyl ether (iBuVE) via RAFT/MADIX in DMC as the medium.203,204 | ||
In particular, O-ethyl-S-(1-methyloxycarbonyl)ethyl xanthate and benzoyl peroxide (BPO) were used as the RAFT chain transfer agent and initiator, respectively, yielding alternating copolymers (poly(TFE-alt-iBuVE)) (Scheme 34). The molar masses varied between 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 4400 g mol−1 with a broad dispersity (Đ = 2) with the conventional method and from 1200 to 2000 g mol−1 and narrower Đ (1.08–1.11) via RAFT/MADIX.203
000 and 4400 g mol−1 with a broad dispersity (Đ = 2) with the conventional method and from 1200 to 2000 g mol−1 and narrower Đ (1.08–1.11) via RAFT/MADIX.203
Furthermore, Guerre et al.205 demonstrated that radical DMC fragments were generated during the polymerization of vinylidene fluoride (VDF) through a proton transfer process. This may initiate further polymerization or termination of other macroradicals by recombination.203 Proton transfer from DMC or the vinyl ether monomer to the macroradical was observed in uncontrolled copolymerization, whereas much less proton transfer from DMC was noted in RAFT copolymerization.203
5.7 Polymer crystallization
In a reported study, poly(2-isopropyl-2-oxazoline) (PIPOx) crystallization was performed in either DMSO or PC, generating polymers with a higher crystalline content than PIPOx crystallized in CH3CN.206In addition, porous structures were prepared by extrusion-based 3D printing of biodegradable PCL-b-PTMC-b-PCL triblock copolymers based on trimethylene carbonate (TMC) and ε-caprolactone (CL) using EC as a crystallizable and water-extractable solvent.207
5.8 Post-polymerization modifications
Woodfield and co-workers presented a post-polymerization modification to prepare sulfobetaine co-polymers by employing an activated ester precursor, poly-(pentafluorophenyl acrylate), a zwitterionic amine, 3-((3-aminopropyl)dimethylammonio) propane-1-sulfonate, and ADPS (and ADPS mixtures with other amines) with PC as the solvent (Scheme 35).208 The scope of activated esters was also investigated, thus providing synthetic access to a library of well-defined hydrophobically modified zwitterionic co-polymers.208 | ||
| Scheme 35 Sulfobetaine co-polymers via poly(pentafluorophenyl acrylate), pPFPA as activated ester in combination with ADPS and other amines in PC as the solvent.208 | ||
5.9 Lignin-derived polymers
DMC was employed as the solvent instead of dichloromethane for the curing of lignin and plant oils via olefin metathesis to produce thermosetting polymer films. The resulting materials displayed similar Young's moduli and tensile strength, but that produced with DMC showed a lower degree of cross-linking compared to the films produced in CH2Cl2.193 Diallyl carbonate (DAllC) was also used as media to suspend organosolv lignin (OL) and prepare allylated lignin.1935.10 Preparation of polyurethane (PU) adhesives
Polyols, which are important compounds of polyurethane adhesives (PU), were prepared by liquefying beech wood sawdust209 and hardwood residue (HR)210 with EC and sulfuric acid. The obtained bio-polyol was used for the preparation of two types of PU adhesive by blending two types of isocyanates, poly4,4′-diphenyl methane diisocyanate (PMDI) and toluene diisocyanate (TDI), in different NCO/OH ratios.209 EC can restrain the free radical produced by the lignin fragments, and then stop the recondensation in the liquefaction process.210 On this topic, Yamada and Liang211,212 reported that the rate of liquefaction of cellulose and hardwood in EC and PC is almost 30-times faster than other solvents such as polyhydric alcohols, which is probably due to the high permittivity of cyclic carbonates.2095.11 Microencapsulation
DMC and PC were used as solvents to fabricate poly(D,L-lactide-co-glycolide) (PLGA) microspheres213 and nanoparticles, respectively.214 In the former case, DMC was employed as a green dispersion solvent, creating an oil-in-water emulsion made by PLGA/Nile red/progesterone/DMC in the aqueous phase. The subsequent addition of an NaOH solution to the emulsion led to the decomposition of DMC, which partitioned to the water phase, thus allowing the continuous diffusion of DMC existing in emulsion droplets into the aqueous phase and its complete removal. This process allowed the uniform distribution of Nile red across the microsphere matrix. In addition, the drug crystallization phenomenon commonly observed in conventional emulsion-templated processes was inhibited by increasing the hydrolysis rate of DMC. The green solvent hydrolysis-based microencapsulation technique can be a promising alternative to conventional microencapsulation methods using toxic halogenated organic solvents.213In addition, small transparent PLGA nanoparticles (below 70 nm) were obtained by an emulsification-diffusion method employing PC as the medium. Alternatively, larger PLGA nanoparticles (above 290 nm) were obtained using acetone and CH2Cl2.214 The small particle sizes for PC were attributed to both the adequacy of the stabilizer protection against coalescence and the low interfacial tension between the aqueous and organic phases, resulting from their partially water-soluble nature.214
6. Organic carbonates in CO2 capture
The combination of DMC as a solvent and either polydimethylsiloxane (PDMS)/TiO2 or (PDMTS)–SiO2 nanocomposites can be used as a CO2 capture method due to the high solubility of CO2 in DMC and its low desorption.215–217 In this process, CO2 is initially absorbed in DMC, and then CO2 is desorbed by a pervaporation (PV) membrane from the rich liquid solvent, allowing ca. 72% of energy savings compared to conventional CO2 capture methods.218 In addition, diethylenetriamine (DETA) dissolved in different solvents, including ethanol, diethylene glycol dimethyl ether, NMP or DMC, can be effectively used as a CO2 absorber. Single crystals of DETA-carbamate indicated that one mole DETA can absorb one mole of CO2 to form precipitates in organic solvents.219DEC was also shown to perform as a good CO2 absorbent with even better results in terms of liquid–gas ratio, absorption temperature, desorption temperature and N2 flow on CO2 absorptivity compared to DMC.220
Therefore, DMC and DEC were tested as additives to dimethyl ethers of polyethylene glycol (DEPG) for the removal of acid gas such as CO2 and H2S in the Selexol™ process due to their strong CO2 adsorbing characteristics.221–223 The results showed that the addition of DMC and DEC led to reduced net utility costs compared to the normal Selexol™ process. However, a major drawback of DMC and DEC is the vast solvent loss during the solvent regeneration stage. This increases the solvent make-up cost by too much for them to be economically competitive.223 Alternatively, PC showed promising performances as a physical absorbent for biogas upgrading, with a 30% specific cost reduction compared to when water was employed.224
7. Organic carbonates as solvents for the preparation of membranes, films and fibres
The intrinsic proprieties of membrane-based processes make them simple, flexible, selective and an environmentally friendly technology, which require low energy consumption as well as simple scale-up and operational conditions.225–227 Membrane processes are effectively employed in a wide variety of industrial applications including the separation of complex mixtures, hydrogen isolation,228 CO2 removal,229 wastewater treatment230 and water desalination,231,232 allowing up to 50% of energy savings in the production cost compared to other traditional separation technologies.229 Nevertheless, most of the commonly employed solvents in this field such as NMP, DMF and DMA display cancerogenic and teratogenic effects,233 present high volatility, and thus represent a threat to the ecosystem and human beings.234,235Replacing traditional toxic solvents with greener alternatives is not an easy task, given that they have a particular set of properties that play a crucial role in determining the final membrane morphology and performance.236 Solvent properties such as viscosity, dielectric constant, polarity and boiling point greatly affect the characteristics imparted to membranes during their formation.235
In this scenario, traditional solvents need to be replaced with greener solvents possibly synthesized in a sustainable way.
One of the possible solutions for these issues is the employment of OCs.47,237 In fact, it has been reported that replacing NMP with EC in the preparation of PVDF membranes significantly reduced the overall environmental impact by up to 35% according to life cycle assessment analyses.236
Rasool and co-workers employed commercially available DMC, DEC, PC, 1,2-butylene carbonate, GC and the custom-made 1,2 hexylene carbonate and styrene carbonate (SC) as green solvents for membrane preparation using different polymers such as polyethersulphone (PES) and polyacrylonitrile (PAN) polyvinilydene fluoride (PVDF).237 Due to the solubility issues of most polymers in OCs, several mixtures were investigated to obtain suitable polymer–solvent compatibility for membrane casting (homogeneous polymeric solution). The investigated examples include OC/OC (i.e., SC/PC), OC/NMP and OC/methyl lactate.
7.1 Polyvinylidene fluoride membranes
Linear water-soluble OCs can be employed as green media for the preparation of polyvinylidene fluoride (PVDF) membranes both via non-solvent-induced phase separation (NIPS) and a combination of vapor-induced phase separation (VIPS)-NIPS techniques (Scheme 36). Phase inversion techniques involve the transformation of a polymeric solution into a solid polymeric matrix, which, in the case of NIPS, is achieved by the immersion of a cast polymeric solution in a non-solvent bath, while in VIPS, by the non-solvent vapor present in a climatic chamber.225,238 Phase inversion methods are some of the most employed processes to prepare commercially available membranes. These methods are very versatile and allow the production of membranes with different morphologies.238 | ||
| Scheme 36 Synthesis of non-commercial (methoxy ethyl) carbonates as solvents for the preparation of PVDF membranes.47 | ||
The membranes obtained with custom-made DACs displayed greater structural resistance and a smaller pore size compared to that achieved using commercially available cyclic DACs. The collected data showed that it was possible to achieve a wide variety of dense and porous membranes by using a single family of compounds.47
PC and diphenyl carbonate (DPC) were instead employed as diluents to prepare PVDF hollow fibre membranes through a triple-orifice spinneret in thermally induced phase separation (TIPS). In the TIPS technique, polymer precipitation is caused by a decrease in temperature, which can occur steadily or abruptly by immersion in a coagulation bath.225 Different concentrations of DPC and PC generated different membrane structures, showing significant effects on the permeability, rejection, and mechanical strength of the membrane.239 In particular, PC endowed all the membranes with a porous inner and outer surface (ca. 45% porosity and 5.5 μm average pore size), while dense structures were detected in the absence of PC.240 The mechanical strength of the PVDF membrane remained unchanged because of the negligible impact of the solvents used as the bore liquids on the membrane bulk structure.240 Similarly, PC was used as a co-extrusion solvent in the outer layer of a PVDF doping solution in the TIPS process, leading to significant improvements in the pure water permeability stability of the membrane.241 The penetration of PC inside the membrane considerably changed its surface and sublayer structure.
In addition, the effects of the solvent and temperature on the crystal formation were investigated for vinylidene fluoride/trifluoroethylene copolymer (P(VDF-TrFE)). Highly crystalline vinylidene fluoride/trifluoroethylene copolymer P(VDF-TrFE) thin films were fabricated by spin casting using DEC as a polar solvent.242
7.2 Polylactic acid membranes
DMC can be also employed for the preparation of different types of polylactic acid (PLA) membranes as follows:• PLA fibres via solution blow spinning (SBS), a technique allowing the production of micro- and nano-scale fibres from polymeric solutions through pressurized air using a specialized nozzle;243
• PLA porous bioactive nanofibers through SBS combined with thermally induced phase separation;244
• Electrospun nanofibrous supports made of PLA and gelatin.245 Then, the support was used for the production of green thin-film composite (TFC) membranes, which can offer a sustainable solution for the separation of complex mixtures in aqueous and organic solvent nanofiltration (OSN).245 The electrospinning of PLA from DMC solutions produced ultrafine nanofibers in the presence of ammonium salt as an additive.245
PLA was also used in combination with bamboo fibres in the presence of DMC to produce a bio-based membrane applicable as a membrane backing material. The bio-based membrane supports exhibited a porous structure (porosity of 0.719 ± 0.132) with tensile strength (32.7–73.3 MPa) comparable to conventional materials, such as polypropylene. The synthesized supports were found to be stable in green polar aprotic solvents including Cyrene, 2-Me-THF, γ-valerolactone, and PC.246
Poly(glycolic-co-lactic acid) (PLGA) and poly(ε-caprolactone) microspheres were produced under ambient conditions using one-step electrohydrodynamic jetting (atomisation) and TIPS in DMC. The presence of DMC generated microspheres with a diameter in the range of 150–300 μm, suitable for use in a minimally invasive, in situ forming scaffolds.247
7.3 Polyamide membranes
Another example of OCs as green solvents in membrane preparation was presented by Shi and co-workers, in which DMC and tannic acid (TA) were used to prepare high-performance polyamide (PA) reverse osmosis (RO) membranes. RO membranes are used to separate low molecular weight solutes, i.e., inorganic salts or small organic molecules, from a solvent.248 Therefore, these membranes have been applied in the purification of water (desalination) and in the concentration step in the food industry (concentration of fruit juice, sugar, coffee), and the dairy industry (concentration of milk prior to cheese manufacture).238The characterization of the membrane achieved from TA showed that the presence of DMC changed the membrane structure, creating a more pronounced leaf-like architecture, thus demonstrating the significant contribution of the solvent in the final membrane morphology and characteristics. TA and DMC endowed the RO membranes with a high flux and a high salt rejection (99.03% ± 0.02%).248 DMC can also be used as a co-solvent to modify the interfacial polymerization (IP) process of polyamide membranes. The DMC-modified membrane rejection improved, while maintaining an excellent flux.249
In addition, DMC promoted the miscibility of aqueous and organic phases, thus enhancing the diffusion rate of m-phenylenediamine (MPD) from the aqueous phase into the organic phase. The accelerated diffusion of MPD due to DMC affected the structures and performance of the RO membrane, i.e., increasing the overall thickness of their skin layer.248
7.4 Polycarbonate membranes
Poly(trimethylene carbonate)-dimethylamine (PTMC-dMA) porous membrane-based scaffolds were produced via air–water interfacial phase separation using PC as the swelling agent. The formed membrane can find possible applications in tissue engineering.2507.5 Cellulose acetate membranes
The formation of cellulose acetate nanofibers via electrospinning was performed using DMC and cyclopentanone (CPO) as the solvent system. The solvent composition affected the fibre diameter, morphology and porosity. DMC, due to its higher volatility, was responsible for pore formation.2517.6 Ion-exchange membranes
Ion exchange membranes made of highly porous polytriazole and functionalized with sulfonic acid were prepared by solution casting, followed by immersion in a non-solvent bath and applied for selective protein adsorption using a mixture of 1-ethyl-3-methylimidazolium acetate ([C2mim]OAc) and DMC as the medium.252 These types of membranes may be useful for protein separation and purification, offering higher flow rates compared to chromatographic techniques, and thus reducing the processing time.2527.7 Ionomer membranes
EC was used in combination with sulfolane (SL) as a plasticizer to produce lithiated Nafion ionomer membranes, applicable both as electrolytes and separators. The conductivity of these membranes saturated with EC/SL is promising for various practical applications such as a polymer electrolyte in the development of a new generation of lithium-ion batteries with enhanced safety.2537.8 Poly(hydroxybutyrate)-based membranes
Papchenko et al. compared several solvents for the casting of a poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV) random copolymer-based membrane with potential applications in gas separation and CO2 capture.254 Among them, DMC allowed the production of polymer films with transport properties similar to that obtained with chloroform, and it also led to stable crystallinity in the samples over time.254 These results demonstrate that DMC is a green alternative to CHCl3 without compromising the separation performance.2548. Organic carbonates in the preparation of materials and nanoparticles
OCs have shown interesting proprieties as polar media for the preparation of materials and nanoparticles and the substitution of DMF, NMP and DMSO.255DMC was employed as the solvent for the fabrication of 3D porous PLGA-biomimetic carbonated apatite composite scaffolds,256 the liquid phase exfoliation (LPE) of pristine biochars as a substitute for NMP,257 in the production of porous alumina ceramics258 and in debinding assistance in stereolithography-based 3D-printed alumina green bodies.259 The latter materials subsequently underwent thermal debinding and sintering to obtain alumina ceramics. The application of DMC in this process was shown to positively affect the microstructure and properties of the 3D-printed alumina ceramics.259 The obtained materials could be used as ceramic cores for hollow blades in aircraft engines.259
DMC, DEC and EMC have been applied as anti-solvents to improve the efficiency of quasi-two-dimensional (quasi-2D) perovskites259 and perovskite solar cells (PSCs).260,261 The former compounds have been identified as promising emitters for the fabrication of high-efficiency blue PeLEDs. In particular, the residual DMC in the perovskite can impede the grain coarsening during the heating process and preserve a smaller grain size than that of the commonly used anti-solvent chloroform. Furthermore, its better miscibility with the precursor solvent (DMSO) and higher boiling point are beneficial to achieve a more homogeneous morphology.262 Additionally, DMC solvent molecules have been shown to act as a template in the production of methylammonium (CH3NH3+ or MA)-based 2D Ruddlesden-Popper perovskites.263
The combination of PC as a binder and DMC as a solvent was employed for the development of an ultra-low-temperature co-fireable Li2WO4 substrate through the tape-casting technique. The sintered substrate displayed a relatively high thermal expansion coefficient (ca. 16 ppm °C−1) and excellent microwave dielectric properties with a relative permittivity of 5.4 and a very low dielectric loss of 9.21 × 10−5 at 5 GHz, making it suitable for microelectronic applications.264
Grafting organic functionalities on inorganic supports is one of the most used methods for the preparation of composites. Although toluene usually is the solvent of choice for the grafting reaction, it can be substituted with greener media, i.e., (+)-α-pinene, (−)-β-pinene, DMC, (+)-limonene, and Me-THF, even if only in the latter case no residual solvent molecules could be detected.265
Highly porous biocompatible composites made of polycaprolactone (PCL) and 45S5 Bioglass (BG) were prepared via the solid–liquid phase separation method (SLPS) using either DMC or dioxane as the solvent. The mechanical properties of the resulting composite showed a dependence on the type of solvent used for its preparation. The composites prepared with dioxane showed enhanced stress at deformation and higher elastic modulus with respect to that prepared with DMC.266
Mixtures of castor oil (CO) and DMC were used as the media in a microfluidic-assisted solvent extraction process, resulting in the formation of hollow silica microspheres with a hole on their surface, showing potential application as catalyst supports, microreactors or capturers for cells.267 Increasing the DMC content led to the formation of filbert-like silica solid microspheres instead.267
PCL electrospun structures for tissue engineering were prepared using a combination of glacial acetic acid as the solvent and EC as a co-solvent. The concentration of EC in the mixture could influence the diameter of the ultrafine PCL fibres, which decreased with an increase in the EC concentration. Therefore, this stable and low toxic solution electrospinning system may provide a valid strategy in the field of tissue engineering.268
EC was also used to dissolve low molecular weight methacrylate end-functionalized polymers, i.e., poly(trimethylene carbonate-dimethacrylate), poly(D,L-lactide-dimethacrylate), and poly(ethylene glycol-dimethacrylate), to produce porous crosslinked polymer networks applicable in the biomedical field.269
EC, PC and GC were shown to be optimal for the repulsive osmotic delamination of 2D materials as alternatives to N-methylformamide and N-methylacetamide.270 This technique is useful to achieve delamination into monolayers of ionic-layered compounds with quantitative yield.270
Concerning the production of nanomaterials, ruthenium nanoparticles could be deposited on thermally reduced graphite oxide using PC. These Ru@graphene nanomaterials acted as active catalysts for the solvent-free hydrogenation of benzene to cyclohexane under mild conditions (100 °C, 10 bar) with activity of 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (mol cyclohexane) (mol Ru)−1 (h−1) and over 90% conversion in at least ten consecutive runs.271 Additionally, esterified cellulose nanocrystals could be obtained by solution blow spinning (SBS) using DMC as the solvent.272
000 (mol cyclohexane) (mol Ru)−1 (h−1) and over 90% conversion in at least ten consecutive runs.271 Additionally, esterified cellulose nanocrystals could be obtained by solution blow spinning (SBS) using DMC as the solvent.272
Finally, a two-dimensional titanium carbide (Ti3C2Tx) MXene was effectively dispersed in PC, expanding the opportunities for processing techniques, such as mixing MXenes with other nanomaterials or polymers to form composites and preparing inks for printing.255
GC can be used as a suitable green solvent for the synthesis of metal–organic frameworks (MOFs). In particular, Itatani and co-workers reported the synthesis of a zinc-based zeolitic imidazolate framework-8 (ZIF-8) using GC, which could then be recycled and reused for several cycles.273
9. Organic carbonates in surface modification
The enhanced molecular transport together with the good dissolution proprieties of PC makes it a suitable solvent for the preparation of alcohol-based monolayers on the surface of silicon oxides.274 Monolayers prepared from alcohol-based reagents have been previously introduced as an alternative approach to covalently modify the surfaces of silicon oxides. This strategy can be utilized to create silicon oxide surfaces with hydrophobic, oleophobic, or charged functionalities.274 Similarly, the surface modification of smectites was performed using five-membered cyclic OCs, i.e., GC, 4-(2-hydroxyethyl)-1,3-dioxolan-2-one (HED), 4-(4-hydroxybutyl)-1,3-dioxolan-2-one (HBD), 4-((benzyloxy)methyl)-1,3-dioxolan-2-one (BMD), and 4′-(oxybis(methylene))bis(1,3-dioxolan-2-one) (OMD) and hexahydrobenzo[d][1,3]dioxol-2-one (HDD), as the media (Fig. 4).79DMC and EC–PC mixtures were found to be promising solvents for the adsorption of the dye Bixin onto acid- and alkali-treated kaolinite275 and for the adsorption of triblock Pluronic surfactants bearing poly(ethylene oxide) (PEO) chains of different lengths on silica.276
10. Organic carbonates as extracting solvents
Several examples of OCs employed as extracting solvents in a wide variety of applications are available in the literature including liquefaction processes, compound recovery and the determination of pollutants. In the latter case, dispersive liquid–liquid microextraction (DLLME) for the determination of lead content in water was performed using DMC as the extraction solvent.277 The analysis of DMC extracts was also used for the determination of the volatile fatty acid (VFA) concentration in digestates.278 In addition, DEC in combination with ionic liquids showed enhanced performances for the determination of metallic impurities in Arnica montana L. infusions via DLLME.279 DEC can be also used as an extractant for the analysis of highly substituted hydrophobic chlorophenols in wines using liquid-phase microextraction (LPME) and capillary electrophoresis (CE).28010.1 Organic carbonates in liquefaction processes
Pinewood shaves could be liquefied using PC and GC as substitutes for 2-ethylhexanol with a biomass conversion of 96%, 98% and 71%, respectively. The bio-oils obtained led to significantly better calorific properties than that from the biomass itself.281 Alternatively, the liquefaction of recycled newspaper can be carried out in the presence of polyhydric alcohols and EC under acidic conditions.28210.2 Organic carbonates in the extraction of oils and fatty acids
DMC was employed in the extraction of diglycerides (DAGs) and free fatty acids (FFAs) from salmon oil283 and of kernel oils from Litsea cubeba (LC).284 In the latter case, DMC displayed enhanced performances compared to alcoholic solvents (yield values ca. 96%) and with values similar to n-hexane (ca. 96% yield). In addition, the micronutrients in oils extracted by these green solvents were quantified to be much higher than that extracted by n-hexane.284 DMC can also be employed as kernel oil extracting solvent through a controllable blender extractor (CBE).285The application of DMC and DMC–EtOH mixtures as solvents in the pressurized liquid extraction (PLE) of Crambe seed oil showed greater oil removal from the seeds under pressurized conditions.286,287
Tommasi and co-workers developed a new lipid extraction protocol for obtaining a fatty-acid-rich extract from the diatom Phaeodactylum tricornutum.288 Choline chloride-based deep eutectic solvents (DESs) and microwave (MW) pretreatments combined with DMC and scCO2 as the extraction solvents resulted in an increase both the selectivity and the total fatty acid (TFA) extraction yield of DMC. In particular, the TFA yield and fatty acid profile results were comparable to that of the traditional Bligh and Dyer extraction method289 with a much better selectivity (88% vs. 35%). This pretreatment was also demonstrated to significantly improve the extraction efficiency of scCO2, increasing the TFA yield by a factor of 20 and providing highly purified triglyceride extracts.288 In addition, milk fat extraction could be performed from ghee residue using DMC as the solvent.290
An ultrasonic-microwave-assisted extraction (UMAE) method with DMC was developed for the extraction of Manchurian walnut kernel oil (MWKO) with a maximum extraction yield of 59%.291
10.3 Organic carbonates for the recovery of compounds
EC, PC and DMC were shown to be suitable media for the recovery of polyhydroxybutyrate (PHB), biosynthesized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)292 and polyhydroxyalkanoates (PHA) from municipal waste activated sludge.293–295 The same solvents could be used to recover PHA from the bacterial cytoplasm, i.e., Cupriavidus necator cells,296–298 genetically modified E. coli cell cultures299 and from mixed microbial cultures.300–303 With genetically modified E. coli cell cultures, the PHB yield values from DMC-based extraction were similar to or higher than that achieved using chloroform (≥67%).299 Particularly, EC-assisted PHB extraction from Cupriavidus necator cells was obtained with a recovery percentage of 98% and product purity up to 98%, which were the highest among the solvents tested (DMSO, DMF, hexane, propanol, methanol, and acetic acid).297EC was also tested for the industrial separation of acetone and diisopropyl ether employing extractive distillation, even if DL-limonene showed higher performances.304
Acidified GC and EC can be useful for the pretreatment of sugarcane bagasse with a glucose yield of 80% and 15%, respectively.305 The usage of GC is also preferred because its decomposition produces glycerol, while EC generates EG, which is generally harmful.306
PC was found to be a suitable solvent for the isolation of PO during the epoxidation of propylene307 and for the extraction of aromatics in naphtha.308–311 Specifically, a mixture of PC and diethylene glycol as the solvent system led to an increase in the utilization efficiency of naphtha,311 while DMC/n-butyl acetate mixtures were used as extracting media for the separation of coal gasification tar residue (CGTR).312 PC and BC were also used for the extraction of artemisinin from A. annua with high efficiency (90%–95%).313
DMC could be also used to extract β-carotene from Rhodotorula glutinis yeast,314 6-methoxypodophyllotoxin from Linum tissues via ultrasound-assisted extraction315 and peroxidase from bitter gourd (Momordica charantia); the latter one via a three-phase partitioning technique, yielding a peroxidase recovery and fold purity of 177% and 4.84, respectively.316
DMC-based binary azeotropic mixtures showed good performances according to a computer-aided product design in extracting volatile aroma molecules widely used in the perfume and cosmetic industries, i.e., α-pinene, DL-limonene, α-terpinene, terpinolene, and many more.317
DMC has been applied as extracting media for the biomonitoring of nicotine in aqueous samples318 and it can be employed as a precipitating agent to isolate lignin from rice straws with 89% purity after a fractionation step.319
Finally, a three-phase partitioning system with DMC as the organic phase and sodium citrate as the salt phase was used for the partitioning of exopolysaccharide (EPS), namely, EPS-D, from fermentation broth of Phellinus baumii. This procedure could also be applied for the efficient partitioning of natural biomolecules.320
DEC was positively tested as a possible entrainer for separating 1-hexene and n-hexane by extractive distillation,321 as well as a green extraction solvent for the recovery of gold(III) from copper-rich sources322 and chlorophenol determination in water samples with dispersive liquid–liquid microextraction.323 In the latter application, DEC can be employed as a substitute for more toxic or hazardous solvents i.e., hexane, chloroform, toluene and diethyl ether.323
In addition, DEC can be employed as a solvent for the determination of polycyclic aromatic hydrocarbons (PAHs) in different environmental matrices through GC–MS.324
Mixtures of water, propionic acid and DEC can be used for the recovery of propionic acid from aqueous solutions, i.e., fermentation broth and wastewaters.325
11. Organic carbonates in analytical chemistry
DMC as well as mixtures of PC and ethanol were effectively employed as the eluent phase in inductively coupled plasma mass spectrometry (ICPMS),326 HPLC327 and liquid chromatography,328 respectively. Mixtures of PC and ethanol may be considered a greener approach for pharmaceutical applications compared to CH3CN. This replacement is achievable without any major compromise in terms of elution order, chromatographic retention, efficiency and peak symmetry, even if due to the reduced mass transfer of analytes in PC-based mobile phases, the optimal flow rates (necessary for reaching the maximum efficiency) are lower compared to CH3CN-based mobile phases.328 Concerning ICPMS applications, the employment of DMC may facilitate the elution and detection of novel hydrophobic compounds and improve the column recovery under standard ICPMS conditions and instrumental set-up without a compromise in the detection limits.326An assay method incorporating PC as the solvent was also developed to determine chlorthalidone (CLD) and cilnidipine (CIL) in bulk and tablet dosage form using four different UV spectrophotometric methods. Due to the solubility of most drugs in PC, this method can be adapted for the analysis of CIL and CLD drugs and it can be adopted by quality departments for regular research and sustainable development.329
In another example, the addition of supercharging reagents, i.e., PC, EC and BC, in electrospray ionization coupled mass spectrometry (ESI-MS) was demonstrated to increase the protein ion charge as well as narrow the protein charge-state distributions without impacting the obtained drug-to-antibody (DAR) values.330–332 Particularly, 5% (v/v) concentration of BC and 4-vinyl-1,3-dioxolan-2-one could be added to ESI solutions to form higher charge states of cytochrome c and myoglobin ions than by using more traditional additives i.e., sulfolane and m-nitrobenzyl alcohol.331
DMC was also used as an eluent for the chromatographic purification of a 10-amino acid-long peptide (purity of 98.5%)333 and for the separation of two small molecules, i.e., caffeine and paracetamol.334 The results indicated that a small amount (7% v/v) of DMC has the same efficiency as a 2.5-times larger volume of CH3CN (18% v/v), and higher efficiency than alcohols, i.e., ethanol and isopropanol, in small molecule separation.334
12. Organic carbonates in biological/biochemical applications
Linear and cyclic OCs have been employed in some interesting applications as media in biochemical processes and assays. EC was shown to be an appealing alternative to formamide and formaldehyde in fluorescence in situ hybridization (FISH) designed for double-stranded DNA probes in plants. Adding EC to the hybridization solution not only allowed successful overnight hybridization but also enabled the possibility to reduce the hybridization time. The method was reproducible in all the DNA of the plants studied (Allium, Nigella, Tradescantia, and Vicia), giving a positive stimulus for improving gene-mapping approaches in plants.335PC was employed as the medium for a colorimetric pyrophosphate assay used for the determination of the P2O74− anionic species (PPi) and based on the formation and reduction of the 18-molybdopyrophosphate ([(P2O7)Mo18O54]4−) anion. This process decreased the interference by ATP and prevented the yellow coloration of the reducing agent (ascorbic acid) due to the presence of excess Mo(VI) species. Thus, this method was shown to be useful for the assay of AMP + PPi forming enzymes, including adenylation enzymes.336
Solvent systems composed of isosorbide dimethyl ether and PC (also with DMSO in some cases) were used to prepare oral non-steroidal anti-inflammatory drugs (NSAIDs). These compounds have applications in the management of inflammatory diseases, including arthritis, bursitis and tendonitis.337 On this topic, PC and other moderately hydrophobic solvents in combination with phase-sensitive polymers can be utilized for modifying drug release from injectable implant systems for 21 days.338
Finally, the enzyme-catalysed transesterification of ethyl butyrate with n-butanol339,340 and microalgae biomass (Scenedesmus sp.) to produce bioethanol and biodiesel341 was performed using GC and DMC as the solvent, respectively.
12.1 Solid-phase peptide synthesis (SPPS)
Researchers are focused on the development of greener protocols for the production of pharmaceutical-grade peptides via solid-phase peptide synthesis (SPPS) by introducing more sustainable alternatives to the most common reagents and solvents.342 On this topic, Ferrazzano and co-workers demonstrated that the traditional DMF-based protocol for industrial fluorenylmethoxycarbonyl (Fmoc) SPPS could be replaced by a greener one using combinations of Cyrene, sulfolane, or anisole with DMC or DEC, in different proportions.343 This method showed applicability for a wide range of oligopeptides, i.e., Aib-enkephalin and Aib-ACP. Finally, this procedure was applied to the synthesis of the reduced form of the active pharmaceutical ingredient (API) octreotide, isolating it in comparable yield and purity compared to that obtained with DMF.343Also, the deprotection of the Fmoc group can be performed in a sustainable way by employing 3-(diethylamino)propylamine (DEAPA) as an alternative to piperidine in an N-octyl pyrrolidone/DMC 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v solvent system. DMC allowed a decrease in solvent viscosity, making this mixture suitable for the automated solid-phase protocol.344 This approach was proven to be able to minimize the formation of side products, while achieving comparable results to that obtained with piperidine.342
2 v/v solvent system. DMC allowed a decrease in solvent viscosity, making this mixture suitable for the automated solid-phase protocol.344 This approach was proven to be able to minimize the formation of side products, while achieving comparable results to that obtained with piperidine.342
13. Organic carbonates in cultural heritage
Several investigations aimed to replace toxic solvents with greener ones in different aspects of cultural heritage preservation and restoration, i.e., old varnishes, paints and tape removal.345For instance, the cleaning of wax-based coatings applied on indoor 1460s bronzes was performed with a gel made by PHB as a thickening agent, biodiesel and DMC. DMC acts as a solubilizing agent for PHB, forming a jelly phase. This gel was then applied for the removal of fresh and aged beeswax coatings, avoiding problems related to solvent residues and ensuring safety for the artworks, the operators and the environment.346 The polymeric gel poly(ethylmethacrylate)-diethylcarbonate (PEMA-DEC) was also able to remove pressure-sensitive tape (PST) components without damaging the painting underneath.347,348
Graffiti and mural removal from historic buildings, masonries and stone artworks can be achieved with a series of two-component systems, which combines silica sol–gel chemistry and DMC as a green solvent to be loaded into the gel. The efficiency of this system in adsorbing/trapping commercial red aerosol spray paint from Istrian stones was investigated, showing to be a promising cleaning agent.349 Moreover, DMC was also studied as a swelling solvent to produce thiol–ene photocured organogels by combining five different thiol or allyl functionalized bio-based monomers, namely isosorbide, pyrogallol, and limonene. The DMC swollen gels were found to be effective in removing the varnish from the surface of artwork, while avoiding adhesion to the surface layer of paintings,350 while DMC alone was employed for the removal of thermally aged oil-painted mock-ups.351
A ternary mixture of water, PC and C9-11E6 (a non-ionic alcohol ethoxylate surfactant) was employed for the dewetting of a methacrylate/acrylate co-polymer film. The surfactant favours the loss of adhesion of the polymer, which may be found on works of art because of previous restoration interventions, thus needing to be removed.352
PC was also employed as a green medium in magnetic nano gel microemulsions, providing a drastic improvement in the cleaning efficiency of archaeological cartonnage.353
Finally, PC, DMC, DEC and DBC were used in combination with a biodegradable non-ionic surfactant in water to formulate a novel nanostructured cleaning system. This system was loaded in highly retentive hydrogels and effectively applied in the selective removal of over-paintings from laboratory mock-ups and from real pieces of street art.354
14. Organic carbonates as cleaning co-solvents
OCs were demonstrated to be excellent co-solvents as cleaning and de-painting products for carpets, rugs, and fabrics. PC can be used as a sequestering agent in environmentally benign cleaning formulations.355 In high concentrations (up to 25%), PC can be employed as a solvent for cleaning processes involving human contact.356 With its softening and swelling effect on paint, PC was found to be an appropriate solvent in aqueous mixtures for the removal of paints from skin. In this application, it was added as a co-solvent in up to 40%.357The U.S. EPA evaluated the use of PC as a solvent in de-painting operations in air logistics centres.358 Furthermore, alkylene carbonates can be used to reduce the odor of amine-containing compounds such as urine. An advantage is the high biodegradability of OCs. Therefore, odor-reducing agents containing EC, PC or BC can be applied in open environments such as zoos, wool plants, and fish canneries.359 The reduction in odor is achieved by reaction of the respective carbonate with the amine. Furthermore, DACs such as GC are starting materials to synthesize non-ionic surfactants, which can be used in cleaning products.360
15. Organic carbonates in cosmetics and as sunscreen
Examples of DACs displaying long alkyl chains employed in cosmetics can be found in the literature; most of them are reported in patented formulations.Natural make-up primers were prepared using dioctyl carbonate (DOC) as the oil phase solvent, in which plant and mineral-derived compounds are dissolved.361 DOC was applied as an emollient in anhydrous cosmetic sunscreens together with diisopropyl sebacate, isononyl isononanoate and diisopropyl adipate, a UV filter system and silicone blends (selected from the group of dimethicone, dimethicone/vinyl dimethicone co-polymer and polydimethyl siloxane). The obtained cosmetic sunscreen displayed enhanced stability and provided a high sun protection factor (SPF).362 The emollients (namely, alkyl benzoate, dibutyl adipate, caprylic/capric triglyceride, coco-caprylate, isopropyl myristate and dioctyl carbonate) showed impact on the UV-filter performances in terms of the SPF and UVB protection, while the UVA shielding decreased with a decrease in the emollient polarity (dicapryl carbonate being the least polar). Therefore, polar emollients are advocated to optimize the UVA protection.363,364
16. Organic carbonates in varnish and paints
OCs can be used for the dispersion of nonaqueous liquid pigments due to their high boiling and flash points.365 Usually, 50–75 wt% of the chemicals in the lacquer wire-coating process are organic solvents. In particular, thin wires need a higher amount of organic solvents. In addition, cresol could be replaced by PC after the comparison of the complete life cycle of both solvents including production, application, and waste removal in the copper wire-coating process.366Opportunely modified OCs have been employed in new water-based varnish formulations.367 Among the OCs tested, 2-(2-methoxyethoxy)ethyl methyl carbonate (DGlyMC) was found to be the best one in terms of toxicological evaluation.367
17. Organic carbonates in coatings
Miller et al. investigated the use of paint blends thinned by mixtures of DMC and tert-butylbenzene to create low-stress films to be used as solar absorber coatings. These coatings exhibited a strong optical performance with figure of merit (FOM) and solar absorbance values of 91% and 97%, respectively, making them ideal coatings for next-generation concentrated solar power plants.368 The utilization of these solvents also helps reducing the environmental impact of paints, specifically by decreasing the VOC content and MIR value to 395 g L−1 and 1.04, respectively.368 PC was also tested for the solution-processable deposition of CuSCN as the hole transport layer (HTL) in bulk heterojunction solar cells, although with lower efficiencies compared to DMF and DMSO (2.5%, 4.5% and 4.2%, respectively).36918. Organic carbonates in oil and natural gas processing industry
The FLUOR process is one of the oldest industrial applications of OCs (especially PC). This process uses PC as a physical solvent to remove CO2 and H2S. PC also removes C2+ hydrocarbons, COS, SO2, CS2, and H2O from the natural gas stream.370 In fact, PC has an equilibrium capacity for absorbing carbon dioxide several times higher than water and does not absorb high amounts of natural gas and hydrogen. Owing to its low viscosity, low vapor pressure, and noncorrosive behaviour, it is an excellent choice as an absorbing solvent.371Other cyclic OCs, namely EC, PC, BC, hexylene carbonate (HexC), cyclohexene carbonate (CHexC), styrene carbonate (SC), GC and (chloromethyl)ethylene carbonate (CEC), were applied as substitutes for sulfolane to mitigate the residual aromatic content (dearomatization, desulfurization and denitrogenation) in liquid fuels.372 Among them PC, EC, BC and SC showed competitive results compared to sulfolane, with PC providing a promising process performance at a very competitive solvent to feed (S/F) ratio and specific energy consumption.372
19. Organic carbonates and electronics
PC can be used as the solvent in the formation process of alignment films for the development of liquid crystal devices. It is necessary that the solvent can be modified by a second solvent to control the surface tension during the process.373 It was found that PC can be used in both functions, with the best results obtained in combination with glycol ethers. Organic carbonates can be also used for the fabrication of sensors, i.e., a PC-based ammonia sensor was developed.374Furthermore, PC was demonstrated to be an excellent solvent for capillary electrophoresis for the investigation of the mobility and ionization constants of various aliphatic amines.375 This technique allows the separation of ionic compounds based on their electrophoretic mobility, which is dependent on the charge, viscosity and radius of the ions involved.376
The detection of thallium(III) and other inorganic salts has been accomplished by polarographic methods in PC as part of extractive mixtures with water377 for ‘salting-out’ extractions.378 Finally, neutral substances such as phenanthrene could be separated by nonaqueous capillary electrophoresis using cationic additives in PC.379
20. Organic carbonates as electrolytes
20.1 Organic carbonates in lithium batteries
The development of rechargeable lithium batteries based on electrolyte solvents is considered a milestone in the field of energy storage and supply for electrical and electronic devices (Fig. 5).380,381The role of electrolytes in batteries is to serve as the medium for the transfer of charges, which are in the form of ions, between a pair of electrodes.
In lithium-ion batteries, lithium ions are solvated by an organic solvent and they diffuse freely between the two half-cells (anode and cathode compartments), which are physically isolated from each other by a separator membrane.382–384 Thus, an ideal electrolyte solvent must have a high dielectric constant to dissolve a high electrolyte salt concentration, have low viscosity to facilitate ion transport, be chemically inert to all cell components to improve the battery lifetime, be liquid over a wide temperature range (i.e., have low melting point and high boiling point), and have low flammability (high flash point).385
Thus, PC is considered the preferred electrolyte in lithium batteries due to its wide liquid range, high dielectric constant and static stability with lithium. Mixtures of EC and PC are considered the most suitable solvent system for common lithium salts to be used as the electrolyte liquid carrier in lithium-ion batteries386–390 as well as EC and EMC blends,391 thus representing a standard for the evaluation of new salts and electrochemical systems.392–395
On this matter, general investigations on the conductivity of organic electrolyte solutions were published by Petrowsky et al.396 The mass transport and conductivity in DAC electrolytes (PC, EC, and DEC and mixture thereof) were determined for LiClO4,397 KPF6 and LiPF6,398 and for LiBr in mixtures with iodine.399
EC and PC can be also combined with other compounds, i.e., lithium(fluorosulfonyl)(trifluoromethanesulfonyl)imide (LiFTFSI) and lithium bis(fluorosulfonyl)imide (LiFSI), to improve the stability and safety of Li-ion batteries400–402 or with deep eutectic solvents (DES), such as choline chloride/ethylene glycol and choline chloride/malonic acid, to improve the thermodynamic and transport proprieties of LiNO3.403
In addition, DMC is finding increasing application as a non-aqueous electrolyte component in the field of lithium rechargeable batteries, as attested by the number of patents in this area.46,62,404 Hybrid aqueous-DMC electrolytes were also reported.405
Moreover, GC has a higher dielectric constant compared to other carbonate solvents used in lithium cell electrolytes. This enables larger quantities of Li salts, i.e., LiF2BC2O4, LiPF6, LiBF4 and/or LiB(C2O4)2, to be dissolved in GC.406
Finally, non-polar electrolyte solvents such as DMC and EMC can be selectively extracted from spent Li-ion batteries using sub-critical or scCO2, while the recovery of the polar EC seems to be more challenging.407 However, through a low temperature thermal treatment process (<150 °C), EC can also be successfully recovered.408
20.2 Fluorinated organic carbonates as electrolytes
Fluorinated DACs, such as fluoroethylene carbonate (FEC),409–412 monofluoroethyl methyl carbonate (F1EMC), difluoroethyl methyl carbonate (F2EMC), methyl (2,2,2-trifluoroethyl) carbonate (F3EMC),413,414 bis(2,2,2-trifluoroethyl) carbonate (BTFC),415 trifluoropropylene carbonate (TFPC) and their mixtures,416 have been extensively studied as electrolytes. These compounds showed promising performances (i) as high-voltage electrolytes for Li-ion batteries,417–419 (ii) for localized high concentration electrolytes (LHCE),420 (iii) to make highly concentrated electrolyte solutions for Si nano-flake powder negative electrodes421 and (iv) for enabling long-term operation of Li-metal batteries at low temperatures.422FEC was also added to the classical electrolyte mixture of EC and EMC to improve the thermal properties of the solid electrolyte interphases (SEI).423 FEC has been applied as a co-solvent in sodium metal anodes (SMEs), overcoming the low reversibility of SMEs in carbonate-based electrolytes.424
Furthermore, better cathode performances were noted when vinylene carbonate (VC) was added to an electrolyte EC solution.425–428
One major problem for all organic solvent electrolytes is their flammability. This problem can be overcome by adding F3EMC429–431 or tris(2-chloropropyl) phosphate432 to the electrolyte, although this flame-retardant characteristic affects the viscosity and capacity ratio during discharge.433 Other studies evaluated the use of fluorinated ionic liquids and DMC as a co-solvent.434
20.3 Organic carbonates as electrolytes in other applications
Cyclic DACs were employed as media in the electrocatalytic reduction of 1,3-dibromopropane (DB3) at metallic interfaces such as Au, Pt, Pd, and Rh.435 This process permits both the dissolution of precious metals and their deposition onto glassy carbon and graphite, applicable for the fabrication of composite materials.435 Moreover, the mechanical properties of the SEI can be improved by polymer species generated from solvent decomposition, i.e., EC, PC, DEC, FEC and VC.436–439VC has also been shown to decrease the formation of potentially toxic organofluorophosphates (OFPs) within the electrolyte during cycling at conventional upper cut-off voltages (UCVs), while triggering OFP formation at higher UCVs.440 Moreover, VC has been applied as a filmogen to realize a stable solid–solid cyclic process in lithium–sulfur batteries (LSBs).441
EC/DEC blends, in the presence of potassium salts, resulted in superior cycling stability and kinetic performance of a hard carbon (HC) anode in potassium-ion batteries (PIBs).442 EC has been shown to stabilize DEC by weak intermolecular interactions, enhancing the energy difference between the orbitals of the Li+(EC)x(DEC)y complex, demonstrating strong capability against reduction.443 LiPF6-methyl acetate/DEC solution systems can be applied as electrolytes in dual-ion batteries.444 Dimethyl dicarbonate (DMDC)445 as well as mixtures of DMC and co-solvents such as PC, 1,1,1,3,3-pentafluorobutane (PFB) and other fluorinated aromatic hydrocarbons have also been investigated.446–448
A colloid liquid electrolyte (CLE) was designed using the EC/DMC solvent system and trace amounts of lithium thiocarbonate (LTC) colloids. This combination was shown to improve the Li+ transfer kinetics at the cathode/electrolyte interface.449
Additionally, gel polymer electrolyte (GPE) and solid polymer electrolyte (SPE) technologies use organic carbonates in combination with Li salts to obtain high conductivity, cohesion and adhesion.450,451 An effective lithium–air GPE system could be applied with a 50% epoxidized natural rubber polymer with 35% LiCF3SO3 and 10% PC as a plasticizer. Employing different mixtures of EC or PC, lower conductivities were observed.452 In contrast to a liquid electrolyte system of 1.0 M LiClO4/PC, the polymer electrolyte was more stable against corrosion.453
Moreover, non-commercial OCs, i.e., bis(2-methoxyethyl) carbonate (Gly2C)454 and chlorinated EMC showed promise as electrolytes, exhibiting considerable oxidative/reductive stability, relatively weak solvation ability and low flammability.455,456
21. Other applications
In this section, we present the other applications of organic carbonates that were not discussed beforehand.EC and PC were applied as solvents for the synthesis of dielectric gels with 2-ethylhexyl acrylate (2-EHA) and 4-acryloylmorpholine (ACMO) as polymer networks. The sensitivity of the capacitive sensor made of the new dielectric gel increased by about 6 times compared to the sensors made of VHB, polydimethylsiloxane (PDMS), or Ecoflex, making it suitable for application as the transparent cover layer of a cell phone.457
PC, EC and GC can be added to peroxide solutions to improve their stability over an accelerated aging period. This result can be exploited to improve the stability of ready-to-use disinfectants, regardless of the other the ingredients included in their formulations.458
Kupareva et al. investigated the removal of silicon and its chemical species from oil under alkaline conditions by adding DMC to the reaction mixture. DMC favoured the reduction of solid products in the reaction mixture, thus fostering the oil recycling process.459 According to Okamoto et al.,460–462 the siloxane bond is efficiently cleaved with DMC over solid-base catalysts to afford methoxy-terminated linear siloxane and carbon dioxide.
Scanning electrochemical microscopy (SECM) used in the feedback mode is one of the most powerful versatile analytical tools used in the field of battery research. However, the application of SECM in the field of lithium-ion batteries (LIBs) faces challenges associated with the selection of a suitable redox mediator due to its high reactivity at low potentials at lithium metal or lithiated graphite electrodes. In this regard, the electrochemical/chemical stability of 2,5-di-tert-butyl-1,4-dimethoxybenzene (DBDMB) was evaluated and benchmarked with ferrocene. This investigation was systematically carried out in electrolytes containing both linear and cyclic OCs. Measurements of the bulk current with a microelectrode proved that while DBDMB decomposed in the EMC-containing electrolyte, the bulk current remained stable in the cyclic carbonates, EC and PC.
Ferrocene was studied as an alternative redox mediator, showing a superior electrochemical performance in EMC-containing electrolytes in terms of degradation.463
22. Conclusions and future perspectives
As stated above, to the best of our knowledge, this is the first review focusing on the application of OCs as media. In this view, herein we presented a comprehensive analysis of the applications of OCs as green solvents, progressing from lab-scale to industrial applications.OCs are valued for their diverse physical and chemical properties, making them excellent alternatives not only in organic synthesis but also across a range of other applications. Research into the use of OCs as solvents has increased significantly over time (Fig. 6), reflecting the growing recognition of their potential within a sustainability framework.
According to the analysis on the type of OCs employed, numerous works focused on the exploitation of commercially available DACs, i.e., DMC, EC and PC; however, many studies also focused on the development of custom-made OCs to meet the specific chemical and physical criteria required for a precise transformation. This trend opens exciting possibilities for new applications, given that these novel OCs can be synthesized in large quantities owing to mature synthetic methods.
Alternatively, it should be mentioned that despite the well-documented low toxicity and hazardousness of most OCs, a comprehensive evaluation of the greenness of their synthetic processes is still lacking. Although tools such as green metrics and life cycle assessments (LCA) are available, further research is needed on the end-of-life disposal and biodegradability of OCs to fully understand their environmental impact.
As highlighted in this review, OCs offer more than just a replacement for toxic solvents; they present an opportunity to revolutionize various scientific fields towards more sustainable practices. From improving the performance of batteries and advancing materials science to driving innovations in green chemistry and enhancing industrial sustainability, their potential is vast. The widespread adoption of OCs as green solvents is likely to have far-reaching effects, making them a valuable resource for researchers and industries focused on developing more sustainable processes.
Abbreviations
| [C2mim]OAc | 1-Ethyl-3-methylimidazolium acetate |
| 2-EHA | 2-Ethylhexyl acrylate |
| 2-Me-THF | 2-Methyl-tetrahydrofuran |
| 2-Me-β-CD | 2-O-Methylated B-cyclodextrin |
| 4CzIPN | 1,2,3,5-Tetrakis(carbazol-9-Yl)-4,6-dicyanobenzene |
| ACMO | 4-Acryloylmorpholine |
| ADPS | 3-((3-Aminopropyl)dimethylammonio) propane-1-sulfonate |
| API | Active pharmaceutical ingredient |
| ATRP | Atom transfer radical polymerization |
| BA | N-Butyl acrylate |
| BC | Butylene carbonate |
| BCB | 4′-(Bromomethyl)-2-cyanobiphenyl |
| BMD | 4-((Benzyloxy)methyl)-1,3-dioxolan-2-one |
| BPO | Benzoyl peroxide |
| BTF | Benzotrifluoride |
| BTFC | Bis(2,2,2-trifluoroethyl) carbonate |
| CAGR | Compound annual growth rate |
| CBE | Controllable blender extractor |
| CE | Capillary electrophoresis |
| CEC | (Chloromethyl)ethylene carbonate |
| CHexC | Cyclohexene carbonate |
| CIL | Cilnidipine |
| CIR | Cosmetic ingredient review |
| CL | E-caprolactone |
| CLD | Chlorthalidone |
| CLE | Colloid liquid electrolyte |
| CNTs | Carbon nanotubes |
| CO | Castor oil |
| CPME | Cyclopentyl methyl ether |
| CPO | Cyclopentanone |
| CPS | Chlorophosphonium salt |
| CyO | Cyclooctene |
| DA15C5 | Diaza-15-crown-5 |
| DAC | Dialkyl carbonate |
| DAGs | Diglycerides |
| DAllC | Diallyl carbonate |
| DAR | Drug-to-antibody |
| DB3 | 1,3-Dibromopropane |
| DBC | Dibutyl carbonate |
| DBDMB | Dimethoxybenzene |
| DCB | Dichlorobenzene |
| DCE | Dichloroethane |
| DCM | Dichloromethane |
| DEAPA | 3-(Diethylamino)propylamine |
| DEC | Diethyl carbonate |
| DEPG | Dimethyl ethers Of polyethylene glycol |
| DES | Deep eutectic solvent |
| DETA | Diethylenetriamine |
| DGly2C | Bis(2-(2-methoxyethoxy)ethyl) carbonate |
| DGlyMC | 2-(2-Methoxyethoxy)ethyl methyl carbonate |
| DHMF | 2,5-Bis(hydroxymethyl)furan |
| DLLME | Dispersive liquid–liquid microextraction |
| DMA | Dimethyl acetamide |
| DMC | Dimethyl carbonate |
| DMDC | Dimethyl dicarbonate |
| DMF | Dimethyl formamide |
| DMI | Dimethyl Isosorbide |
| DMSO | Dimethyl sulfoxide |
| DOC | Dioctyl carbonate |
| DPC | Dipropyl carbonate |
| DPhC | Diphenyl carbonate |
| dppe | Bis(diphenylphosphino)ethane |
| EC | Ethylene carbonate |
| EG | Ethylene glycol |
| EGD | European green deal |
| EMC | Ethyl methyl carbonate |
| EMIMBr | 1-Ethyl-3-methylimidazolium bromide |
| EO | Ethylene oxide |
| EPA | Environmental protection agency |
| EPS | Exopolysaccharide |
| ESI-MS | Electrospray ionization-coupled mass spectrometry |
| FDA | Food and drug administration |
| F1EMC | Monofluoroethyl methyl carbonate |
| F2EMC | Difluoroethyl methyl carbonate |
| F3EMC | Trifluoroethyl methyl carbonate |
| FEC | Fluoroethylene carbonate |
| FFAs | Free fatty acids |
| FISH | Fluorescence in situ hybridization |
| Fmoc | Fluorenylmethoxycarbonyl |
| FOM | Optical performance |
| GC | Glycerol carbonate |
| GCB | Glycerol carbonate butyrate |
| GCP | Glycerol carbonate propionate |
| Gly2C | Bis(2-methoxyethyl) carbonate |
| GPE | Gel polymer electrolyte |
| GVL | γ-Valerolactone |
| HBD | 4-(4-Hydroxybutyl)-1,3-dioxolan-2-one |
| HBpin | Pinacolborane |
| HC | Hard carbon |
| HDD | Hexahydrobenzo[d][1,3]dioxol-2-one |
| HED | 4-(2-Hydroxyethyl)-1,3-dioxolan-2-one |
| HexC | Hexylene carbonate |
| HMF | 5-Hydroxymethyl Furfural |
| HMTA | Hexamethylenetetramine |
| HR | Hardwood residue |
| HTL | Hole transport layer |
| IBA | Isobutyraldehyde |
| iBuVE | Isobutyl vinyl ether |
| IBX | 2-Iodoxybenzoic acid |
| ICPMS | Inductively coupled plasma mass spectrometry |
| ILs | Ionic liquids |
| ITO | Indium tin oxide |
| LC | Litsea cubeba |
| LEDs | Light emitting diodes |
| LHCE | Localized high concentration electrolytes |
| LIBs | Lithium-ion batteries |
| LiFSI | Lithium bis(fluorosulfonyl)imide |
| LiFTFSI | Lithium (fluorosulfonyl) (trifluoromethanesulfonyl) imide |
| LPE | Liquid phase exfoliation |
| LPME | Liquid-phase microextraction |
| LSBs | Lithium–sulfur batteries |
| LTC | Lithium thiocarbonate |
| MBP | Methyl 2-bromopropionate |
| MEA | 2-Methoxy ethyl acrylate |
| MIAK | 5-Methyl-2-hexanone |
| MIBK | Methyl isobutyl ketone |
| MOF | Metal–organic framework |
| MPD | m-Phenylenediamine |
| MPS | Methyl phenyl sulfide |
| MTAE | Methyl-tert-amyl ether |
| MTBE | Methyl tert-butyl ether |
| MTO | Methyltrioxorhenium |
| MW | Microwave |
| MWKO | Manchurian walnut kernel oil |
| NBA | N-Butylaniline |
| NBS | N-Bromosuccinimide |
| N-CNT | Nitrogen-doped carbon nanotubes |
| NHC | Nickel-N-heterocyclic carbene |
| NIPS | Non-solvent induced phase separation |
| NMA | N-Methylaniline |
| NMF | N-Methylformamide |
| NMP | N-Methyl pyrrolidone |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OBMF | 5,5′-[Oxybis(methylene)]bis-2-furfural |
| OC | Organic carbonate |
| OFPs | Organofluorophosphates |
| OL | Organosolv lignin |
| OMD | 4′-(Oxybis(methylene))bis(1,3-dioxolan-2-one) |
| OSN | Organic solvent nanofiltration |
| PA | Polyamide |
| PAHs | Polycyclic aromatic hydrocarbons |
| PAN | Polyacrylonitrile |
| PC | Propylene carbonate |
| PCL | Polycaprolactone |
| PDMS | Polydimethylsiloxane |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEF | Polyethylene furanoate |
| PEG | Polyethylene glycol |
| PEMA-DEC | Poly(ethylmethacrylate)-diethylcarbonate |
| PEO | Poly(ethylene oxide) |
| PES | Polyethersulphone |
| PET | Polyethylene terephthalate |
| PFB | Pentafluorobutane |
| PHA | Polyhydroxyalkanoates |
| PHB | Polyhydroxybutyrate |
| PHBV | Poly(hydroxybutyrate-co-hydroxyvalerate) |
| PIBs | Potassium-ion batteries |
| PIPOx | Poly(2-isopropyl-2-oxazoline) |
| PLA | Polylactic acid |
| PLE | Pressurized liquid extraction |
| PLGA | Poly(D,L-lactide-co-glycolide) |
| PMDETA | Cubr/N,N,N0,N0,N00-pentamethyldiethylenetriamine |
| PMDI | Poly4,4′-diphenyl methane diisocyanate |
| PO | Propylene oxide |
| PSCs | Perovskites solar cells |
| PST | Pressure sensitive tapes |
| PTC | Phase transfer catalysis |
| PTMC-dMA | Poly(trimethylene carbonate)-dimethylamine |
| PU | Polyurethane |
| PV | Pervaporation |
| PVDF | Polyvinilydene fluoride |
| RAFT/MADIX | Reversible addition–fragmentation chain transfer polymerization/macromolecular design via the interchange of xanthates |
| RCM | Ring-closing metathesis |
| RO | Reverse osmosis |
| SBS | Solution blow spinning |
| SC | Styrene carbonate |
| scCO2 | Supercritical CO2 |
| SECM | Scanning electrochemical microscopy |
| SEI | Solid electrolyte interphases |
| SET-LRP | Single electron transfer living radical polymerization |
| SFAME | Sunflower fatty acid methyl ester |
| SL | Sulfolane |
| SLPS | Solid–liquid phase separation method |
| SMEs | Sodium metal anodes |
| SPE | Solid polymer electrolyte |
| SPF | Sun protection factor |
| SPPS | Solid phase peptide synthesis |
| TA | Tannic acid |
| TDI | Toluene diisocyanate |
| TEAB | Tetraethylammonium bromide |
| TFA | Total fatty acid |
| TFC | Thin-film composite |
| TFE | Tetrafluoroethylene |
| TFPC | Trifluoropropylene carbonate |
| TIPS | Thermally induced phase separation |
| TMC | Trimethylene carbonate |
| TO | Tin oxide |
| UCVs | Upper cut-off voltages |
| UMAE | Ultrasonic-microwave-assisted extraction |
| VC | Vinylene carbonate |
| VDF | Vinylidene fluoride |
| VFAs | Volatile fatty acids |
| VIPS | Vapor induced phase separation |
| VOS | Volatile organic solvents |
| ZIF | Zinc-based zeolitic imidazolate framework |
Author contributions
G. Trapasso: investigation, data curation, visualization, writing – original draft; F. Aricò: conceptualization, supervision, writing – review and editing.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the DoE 2023–2027 (MUR, AIS.DIP.ECCELLENZA2023_27.FF project).References
- European Commission, The European Green Deal, Brussels, Belgium, 2019 Search PubMed.
- United Nations, General Assembly, Res 70/1, UN Doc. A/RES/70/1, 2015.
- P. Shah, S. Parikh, M. Shah and S. Dharaskar, Biomass Convers. Biorefin., 2022, 12, 1985–1999 CrossRef.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369–1379 CrossRef CAS PubMed.
- P. G. Jessop, Green Chem., 2011, 13, 1391 RSC.
- D. F. Aycock, Org. Process Res. Dev., 2007, 11, 156–159 Search PubMed.
- V. J. Barwick, Trends Anal. Chem., 1997, 16, 293–309 CrossRef CAS.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- Regulation (EC) no. 1907/2006 of the European Parliament and of the Council of 18 December 2006.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927 RSC.
- V. M. Parsana, Int. J. Eng. Res. Ind. Appl., 2015, 5, 55–62 Search PubMed.
- Green Solvents I, ed. A. Mohammad, Springer Netherlands, Dordrecht, 2012 Search PubMed.
- K. Häckl and W. Kunz, C. R. Chim., 2018, 21, 572–580 CrossRef.
- Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550 RSC.
- J. Hulsbosch, D. E. De Vos, K. Binnemans and R. Ameloot, ACS Sustainable Chem. Eng., 2016, 4, 2917–2931 Search PubMed.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- V. Hessel, N. N. Tran, M. Razi Asrami, Q. D. Tran, N. Van Duc Long, M. Escribà-Gelonch, J. Osorio Tejada, S. Linke and K. Sundmacher, Green Chem., 2022, 24, 410–437 RSC.
- F. Chemat, M. A. Vian, H. K. Ravi, B. Khadhraoui, S. Hilali, S. Perino and A.-S. Fabiano Tixier, Molecules, 2019, 24, 3007 CrossRef CAS PubMed.
- S. Huang, B. Yan, S. Wang and X. Ma, Chem. Soc. Rev., 2015, 44, 3079–3116 RSC.
- T. Kasakado, T. Fukuyama, T. Nakagawa, S. Taguchi and I. Ryu, Beilstein J. Org. Chem., 2022, 18, 152–158 CrossRef CAS PubMed.
- C. Beattie, M. North and P. Villuendas, Molecules, 2011, 16, 3420–3432 CrossRef CAS PubMed.
- D. J. Schroeder, A. A. Hubaud and J. T. Vaughey, Mater. Res. Bull., 2014, 49, 614–617 CrossRef CAS.
- G. Trapasso, C. Salaris, M. Reich, E. Logunova, C. Salata, K. Kümmerer, A. Figoli and F. Aricò, Sustainable Chem. Pharm., 2022, 26, 100639 CrossRef CAS.
- I. N. Daniels, Z. Wang and B. B. Laird, J. Phys. Chem. C, 2017, 121, 1025–1031 Search PubMed.
- D. S. Hall, J. Self and J. R. Dahn, J. Phys. Chem. C, 2015, 119, 22322–22330 Search PubMed.
- N. Peruzzi, B. W. Ninham, P. Lo Nostro and P. Baglioni, J. Phys. Chem. B, 2012, 116, 14398–14405 CrossRef CAS PubMed.
- Y. Chernyak, J. Chem. Eng. Data, 2006, 51, 416–418 CrossRef CAS.
- S. K. Reddy and S. Balasubramanian, J. Phys. Chem. B, 2012, 116, 14892–14902 CrossRef CAS PubMed.
- J.-L. M. Abboud and R. Notari, Pure Appl. Chem., 1999, 71, 645–718 Search PubMed.
- C. Reichardt, Angew. Chem., Int. Ed. Engl., 1979, 18, 98–110 CrossRef.
- C. Reichardt and T. Welton, Solvents and solvent effects in organic chemistry, John Wiley & Sons, 2011 Search PubMed.
- C. M. Hansen, The three dimensional solubility parameter, Danish Technical, Copenhagen, 1967, vol. 14 Search PubMed.
- C. M. Hansen, Choice Rev. Online, 2000, vol. 37(07) Search PubMed.
- G. Ritzoulis and A. Fidantsi, J. Chem. Eng. Data, 2000, 45, 207–209 CrossRef CAS.
- M. Wu, F. Wu, H. Luan and R. Chen, Acta Chim. Sin. (Chin. Ed.), 2005, 63, 787 CAS.
- C. Reichardt, E. Harbusch-Görnert and G. Schäfer, Liebigs Ann./Recl., 1995, 1579–1582 CrossRef CAS.
- C. Reichardt, Pure Appl. Chem., 2008, 80, 1415–1432 Search PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- J. Schwöbel, R.-U. Ebert, R. Kühne and G. Schüürmann, J. Comput. Chem., 2009, 30, 1454–1464 CrossRef PubMed.
- A. Klamt, Chemosphere, 1996, 32, 717–726 CrossRef CAS.
- A. Klamt, Chemosphere, 1993, 26, 1273–1289 CrossRef CAS.
- J. Schwöbel, R.-U. Ebert, R. Kühne and G. Schüürmann, J. Chem. Inf. Model., 2009, 49, 956–962 Search PubMed.
- M. J. Kamlet, J.-L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 Search PubMed.
- H. Itoh and Y. Shinbori, Bunseki Kagaku, 1977, 26, 134–136 Search PubMed.
- B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554–4581 CrossRef PubMed.
- G. Trapasso, F. Russo, F. Galiano, C. R. McElroy, J. Sherwood, A. Figoli and F. Aricò, ACS Sustainable Chem. Eng., 2023, 11, 3390–3404 CrossRef CAS.
- M. Annatelli, G. Trapasso, C. Salaris, C. Salata, S. Castellano and F. Aricò, Eur. J. Org. Chem., 2021, 3459–3464 Search PubMed.
- Y. Fernandes, A. Bry and S. de Persis, J. Power Sources, 2019, 414, 250–261 Search PubMed.
- P. Tundo, F. Aricò, A. E. Rosamilia and S. Memoli, Green Chem., 2008, 10, 1182–1189 RSC.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706–716 Search PubMed.
- ChemAnalyst, Decode the Future of Ethylene Carbonate (EC), 2023. https://www.chemanalyst.com/industry-report/ethylene-carbonate-market-1827.
- KBV, The Ethylene Carbonate Market is Predict to reach $970.4 Million by 2030, at a CAGR of 8.6%, 2023. https://www.kbvresearch.com/press-release/ethylene-carbonate-market/.
- Maximize Market Research Pvt Ltd, Ethylene Carbonate Market Analysis: Strong Segment Insights Revealed, 2023. https://www.linkedin.com/pulse/ethylene-carbonate-market-analysis-strong.
- ChemAnalyst, Decode the Future of Propylene Carbonate, 2023. https://www.chemanalyst.com/industry-report/propylene-carbonate-market-2882.
- GVR, Propylene Carbonate Market Size, Share & Trends Analysis Report By End Use (Paints & Coatings, Pharmaceuticals, Cosmetics & Personal Care), By Application (Solvent, Electrolyte, Catalyst), By Region, And By Segment Forecasts, 2024–2030, 2023. https://www.grandviewresearch.com/industry-analysis/propylene-carbonate-market-report#.
- ChemAnalyst, Decode the Future of Dimethyl Carbonate, 2023. https://www.chemanalyst.com/industry-report/dimethyl-carbonate-market-1829.
- A. Maleki and F. Bahadori, Sci. Rep., 2023, 13, 16900 CrossRef CAS PubMed.
- Markets and Markets, Dimethyl Carbonate Market, 2024. https://www.marketsandmarkets.com/Market-Reports/dimethyl-carbonate-market-24544228.html?, https://www.marketresearchfuture.com/reports/dimethyl-carbonate-market-5486.
- H. Miyaji, S. Fukuoka and H. Hachiya, Asahi Kasei Chemicals Corporation, WO2007034669A1, 2006.
- M. Tojo and H. Miyaji, Asahi Kasei Chemicals Corporation, EP1760059B1, 2005.
- D. Delledonne, F. Rivetti and U. Romano, Appl. Catal., A, 2001, 221, 241–251 CrossRef CAS.
- U. Romano, R. Tesel, M. M. Mauri and P. Rebora, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19, 396–403 CrossRef CAS.
- T. Matsuzaki, Novel method for dimethyl carbonate synthesis using methyl nitrite, Elsevier, 2003, pp. 447–450 Search PubMed.
- Z. Kricsfalussy, H. Steude, H. Waldmann, K. Hallenberger, W. Wagner and H. Traenckner, Bayer AG, US5523452A, 1996 Search PubMed.
- M. Wang, N. Zhao, G. Wei and Y. Sun, Ind. Eng. Chem. Res., 2005, 44, 7596–7599 CrossRef CAS.
- P. Ball, H. Füllmann and W. Heitz, Angew. Chem., Int. Ed. Engl., 1980, 19, 718–720 Search PubMed.
- A. Sánchez, L. M. Gil and M. Martín, J. CO2 Util., 2019, 33, 521–531 CrossRef.
- P. Kongpanna, V. Pavarajarn, R. Gani and S. Assabumrungrat, Chem. Eng. Res. Des., 2015, 93, 496–510 CrossRef CAS.
- H. Huang, R. C. Samsun, R. Peters and D. Stolten, Green Chem., 2021, 23, 1734–1747 RSC.
- V. Kontou, D. Grimekis, K. Braimakis and S. Karellas, Renewable Sustainable Energy Rev., 2022, 157, 112006 CrossRef CAS.
- W. F. Bergfeld, F. A. C. P. Donald, V. Belsito, R. A. Hill, C. D. Klaassen, D. C. Liebler, J. G. Marks, R. C. Shank, T. J. Slaga, P. W. Snyder, W. Johnson, I. Boyer and B. Heldreth, Safety Assessment of Dialkyl Carbonates as Used in Cosmetics, 2016 Search PubMed.
- W. L. Ng, Z. X. Er, D. Chandrakumar, A. C. M. Loy, J. Vongsvivut and S. Bhattacharya, ACS Sustainable Chem. Eng., 2024, 12, 18320–18334 Search PubMed.
- E. Hernández, A. Belinchón, R. Santiago, C. Moya, P. Navarro and J. Palomar, J. CO2 Util., 2023, 69, 102417 CrossRef.
- Y. Demirel, J. Chem. Eng. Process Technol., 2015, 6, 1000236 Search PubMed.
- IMARC, Ethylene Carbonate (EC) Prices, Trend, Chart, Demand, Market Analysis, News, Historical and Forecast Data Report 2024 Edition, 2024. https://www.imarcgroup.com/ethylene-carbonate-pricing-report.
- H. Mutlu, J. Ruiz, S. C. Solledera and M. A. R. Meier, Green Chem., 2012, 14, 1728–1735 RSC.
- A. Behr, P. Bahke, B. Klinger and M. Becker, J. Mol. Catal. A: Chem., 2007, 267, 149–156 CrossRef CAS.
- W. P. Gates, U. Shaheen, T. W. Turney and A. F. Patti, Appl. Clay Sci., 2016, 124–125, 94–101 CrossRef CAS.
- M. A. Pacheco and C. L. Marshall, Energy Fuels, 1997, 11, 2–29 CrossRef CAS.
- A. Triolo, V. V. Chaban, F. Lo Celso, F. Leonelli, M. Vogel, E. Steinrücken, A. Del Giudice, C. Ottaviani, J. A. Kenar and O. Russina, J. Mol. Liq., 2023, 369, 120854 CrossRef CAS.
- Guideline, ICH Harmonised, Assessment and control of dna reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7, International conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH): Geneva, 2014.
- Data available on the US EPA website. https://www.epa.gov/saferchoice/safer-ingredients#pop108327.
- Data available on the US EPA website. https://chemview.epa.gov/chemview/#.
- V. H. Wallingford, M. A. Thorpe and A. H. Homeyer, J. Am. Chem. Soc., 1942, 64, 580–582 CrossRef CAS.
- J. H. Clements, Ind. Eng. Chem. Res., 2003, 42, 663–674 CrossRef CAS.
- R. Bernini, I. Carastro, F. Santoni and M. Clemente, Antioxidants, 2019, 8, 174 CrossRef CAS PubMed.
- M. Annatelli, G. Trapasso, D. D. Torre, L. Pietrobon, D. Redolfi-Bristol and F. Aricò, Adv. Sustainable Syst., 2022, 6, 2200297 CrossRef CAS.
- A. Jordan, R. M. Denton and H. F. Sneddon, ACS Sustainable Chem. Eng., 2020, 8, 2300–2309 CrossRef CAS.
- S. G. Sveegaard and R. Kristiansen, Org. Process Res. Dev., 2021, 25, 68–74 CrossRef CAS.
- H. Slimi, Z. Litim, T. Ollevier and J. Kraïem, ACS Omega, 2023, 8, 44558–44570 CrossRef CAS PubMed.
- R. Bernini, E. Mincione, M. Barontini, F. Crisante, G. Fabrizi and A. Gambacorta, Tetrahedron, 2007, 63, 6895–6900 CrossRef CAS.
- R. Bernini, E. Mincione, F. Crisante, M. Barontini and G. Fabrizi, Tetrahedron Lett., 2009, 50, 1307–1310 CrossRef CAS.
- L. D. Almeida, F. G. Delolo, A. P. S. Costa, E. V. Gusevskaya and P. A. Robles-Azocar, Mol. Catal., 2022, 527, 112400 CrossRef CAS.
- R. Bernini, E. Mincione, M. Barontini, F. Crisante, G. Fabrizi and A. Gambacorta, Tetrahedron, 2007, 63, 6895–6900 CrossRef CAS.
- R. Bernini, E. Mincione, F. Crisante, M. Barontini and G. Fabrizi, Synfacts, 2009, 0571–0571 Search PubMed.
- S. Verma and S. L. Jain, Inorg. Chem. Front., 2014, 1, 534–539 Search PubMed.
- Z. Gui, W. Cao, L. Chen and Z. Qi, Catal. Commun., 2015, 64, 58–61 Search PubMed.
- B. Pieber and C. O. Kappe, Green Chem., 2013, 15, 320 RSC.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854 RSC.
- L. Huang, L. Zheng, Z. Zhou and Y. Chen, Chem. Commun., 2022, 58, 3342–3345 RSC.
- V. Y. Evtushok, I. D. Ivanchikova, O. Y. Podyacheva, O. A. Stonkus, A. N. Suboch, Y. A. Chesalov, O. V. Zalomaeva and O. A. Kholdeeva, Front. Chem., 2019, 7, 858 Search PubMed.
- J. Ramarao, S. Yadav, K. Satyam and S. Suresh, RSC Adv., 2022, 12, 7621–7625 RSC.
- M. Baeten and B. U. W. Maes, Adv. Synth. Catal., 2016, 358, 826–833 Search PubMed.
- A. Jordan, S. Huang, H. F. Sneddon and A. Nortcliffe, ACS Sustainable Chem. Eng., 2020, 8, 12746–12754 CrossRef CAS.
- D. Wei, C. Netkaew, J. Wu and C. Darcel, ChemCatChem, 2020, 12, 5449–5455 CrossRef CAS.
- K. Gadde, J. Daelemans, B. U. W. Maes and K. Abbaspour Tehrani, RSC Adv., 2019, 9, 18013–18017 Search PubMed.
- A. K. C. A. Reis, A. S. Serralbo, D. D. C. Santos, M. A. V. Silveira, D. N. Okamoto, M. A. Juliano and S. P. De Vasconcellos, Orbital: Electron. J. Chem., 2020, 12, 258–266 Search PubMed.
- X. J. Zhang, J. Zhang, Y.-N. Xu, Y.-M. Li, M. Chi, Y. Yan, R.-X. Wu, H.-R. Zhang and Y.-P. Zhu, J. Org. Chem., 2021, 86, 17471–17481 CrossRef CAS PubMed.
- A. Dibenedetto, M. Aresta, C. Pastore, L. di Bitonto, A. Angelini and E. Quaranta, RSC Adv., 2015, 5, 26941–26948 Search PubMed.
- A. Dibenedetto, M. Aresta, L. di Bitonto and C. Pastore, ChemSusChem, 2016, 9, 118–125 CrossRef CAS PubMed.
- Q. Hou, W. Li, M. Zhen, L. Liu, Y. Chen, Q. Yang, F. Huang, S. Zhang and M. Ju, RSC Adv., 2017, 7, 47288–47296 Search PubMed.
- R. Bains, A. Kumar, A. S. Chauhan and P. Das, Renewable Energy, 2022, 197, 237–243 CrossRef CAS.
- M. Musolino, J. Andraos and F. Aricò, ChemistrySelect, 2018, 3, 2359–2365 Search PubMed.
- T. Chhabra, A. Bahuguna, S. S. Dhankhar, C. M. Nagaraja and V. Krishnan, Green Chem., 2019, 21, 6012–6026 RSC.
- G. Trapasso, G. Mazzi, B. Chícharo, M. Annatelli, D. Dalla Torre and F. Aricò, Org. Process Res. Dev., 2022, 26, 2830–2838 CrossRef CAS PubMed.
- A. Behr and C. Fängewisch, J. Mol. Catal. A: Chem., 2003, 197, 115–126 CrossRef CAS.
- A. Behr, D. Obst and B. Turkowski, J. Mol. Catal. A: Chem., 2005, 226, 215–219 CrossRef CAS.
- A. de Camargo Faria, K. C. B. Oliveira, A. C. Monteiro, E. N. dos Santos and E. V. Gusevskaya, Catal. Today, 2020, 344, 24–31 CrossRef CAS.
- J. Tijani and B. El Ali, Appl. Catal., A, 2006, 303, 158–165 CrossRef CAS.
- J. Roger and J.-C. Hierso, Eur. J. Org. Chem., 2018, 4953–4958 CrossRef CAS.
- P. Lenden, P. M. Ylioja, C. González-Rodríguez, D. A. Entwistle and M. C. Willis, Green Chem., 2011, 13, 1980 RSC.
- B. Schäffner, J. Holz, S. P. Verevkin and A. Börner, ChemSusChem, 2008, 1, 249–253 CrossRef PubMed.
- D. Kumar, S. R. Vemula and G. R. Cook, Green Chem., 2015, 17, 4300–4306 RSC.
- G. Vasapollo, G. Mele, A. Maffei and R. Del Sole, Appl. Organomet. Chem., 2003, 17, 835–839 CrossRef CAS.
- E. Quaranta and D. Cornacchia, Renewable Energy, 2020, 157, 33–42 CrossRef CAS.
- P. Gautam, P. Kathe and B. M. Bhanage, Green Chem., 2017, 19, 823–830 Search PubMed.
- A. Ismael, A. Gevorgyan, T. Skrydstrup and A. Bayer, Org. Process Res. Dev., 2020, 24, 2665–2675 CrossRef CAS.
- P. Lei, Y. Mu, Y. Wang, Y. Wang, Z. Ma, J. Feng, X. Liu and M. Szostak, ACS Sustainable Chem. Eng., 2021, 9, 552–559 CrossRef CAS.
- A. Czompa, B. L. Pásztor, J. A. Sahar, Z. Mucsi, D. Bogdán, K. Ludányi, Z. Varga and I. M. Mándity, RSC Adv., 2019, 9, 37818–37824 RSC.
- X.-Y. Li, S.-S. Zheng, X.-F. Liu, Z.-W. Yang, T.-Y. Tan, A. Yu and L.-N. He, ACS Sustainable Chem. Eng., 2018, 6, 8130–8135 CrossRef CAS.
- R. S. Galaverna, L. P. Fernandes, V. H. Menezes da Silva, A. de Siervo and J. C. Pastre, Eur. J. Org. Chem., 2022, e202200376 CrossRef CAS.
- C. Torborg, J. Huang, T. Schulz, B. Schäffner, A. Zapf, A. Spannenberg, A. Börner and M. Beller, Chem. – Eur. J., 2009, 15, 1329–1336 CrossRef CAS PubMed.
- P. Gautam, N. J. Tiwari and B. M. Bhanage, ACS Omega, 2019, 4, 1560–1574 CrossRef CAS PubMed.
- C. Miao, H. Zhuang, F. Han, H. Lyu and Q. Liu, Shandong Agricultural University, CN114394913A, 2021.
- G. De Smet, X. Bai, C. Mensch, S. Sergeyev, G. Evano and B. U. W. Maes, Angew. Chem., Int. Ed., 2022, 61, e202201751 CrossRef CAS PubMed.
- A. Behr, F. Naendrup and D. Obst, Adv. Synth. Catal., 2002, 344, 1142–1145 CrossRef CAS.
- A. Behr and N. Toslu, Chem. Eng. Technol., 2000, 23, 122–125 CrossRef CAS.
- X. Miao, C. Fischmeister, C. Bruneau and P. H. Dixneuf, ChemSusChem, 2008, 1, 813–816 CrossRef CAS PubMed.
- S. Huang, H. Bilel, F. Zagrouba, N. Hamdi, C. Bruneau and C. Fischmeister, Catal. Commun., 2015, 63, 31–34 CrossRef CAS.
- V. Le Ravalec, C. Fischmeister and C. Bruneau, Adv. Synth. Catal., 2009, 351, 1115–1122 Search PubMed.
- V. Le Ravalec, A. Dupé, C. Fischmeister and C. Bruneau, ChemSusChem, 2010, 3, 1291–1297 Search PubMed.
- S. Hui, L. Zhao, Q. Liu and D. Song, Acta Phys.-Chim. Sin., 2020, 36, 1910067–1910060 Search PubMed.
- H. Xing, T. Wang and Y. Dai, J. Supercrit. Fluids, 2009, 49, 52–58 CrossRef.
- J. Segato, W. Baratta, P. Belanzoni, L. Belpassi, A. Del Zotto and D. Zuccaccia, Inorg. Chim. Acta, 2021, 522, 120372 CrossRef CAS.
- D. Landini, J. Mol. Catal. A: Chem., 2003, 204–205, 235–243 CrossRef CAS.
- B. Schäffner, V. Andrushko, J. Holz, S. P. Verevkin and A. Börner, ChemSusChem, 2008, 1, 934–940 CrossRef PubMed.
- J. Bayardon, J. Holz, B. Schäffner, V. Andrushko, S. Verevkin, A. Preetz and A. Börner, Angew. Chem., Int. Ed., 2007, 46, 5971–5974 CrossRef CAS PubMed.
- M. North and M. Omedes-Pujol, Beilstein J. Org. Chem., 2010, 6, 1043–1055 CrossRef CAS PubMed.
- W. Clegg, R. W. Harrington, M. North, F. Pizzato and P. Villuendas, Tetrahedron: Asymmetry, 2010, 21, 1262–1271 CrossRef CAS.
- C. Beattie, M. North and P. Villuendas, Molecules, 2011, 16, 3420–3432 Search PubMed.
- C. Ma, Z. Feng, J. Li, D. Zhang, W. Li, Y. Jiang and B. Yu, Org. Chem. Front., 2021, 8, 3286–3291 RSC.
- M. K. Sahoo, J. Rana, M. Subaramanian and E. Balaraman, ChemistrySelect, 2017, 2, 7565–7569 CrossRef CAS.
- G. Grampp, C. Mureşanu and S. Landgraf, J. Electroanal. Chem., 2005, 582, 171–178 Search PubMed.
- F.-L. Zeng, K.-C. Xie, Y.-T. Liu, H. Wang, P.-C. Yin, L.-B. Qu, X.-L. Chen and B. Yu, Green Chem., 2022, 24, 1732–1737 RSC.
- L. Yu, Y. Lin and D. Li, Appl. Catal., B, 2017, 216, 88–94 CrossRef CAS.
- A. Ding, Y. Zhang, Y. Chen, R. Rios, J. Hu and H. Guo, Tetrahedron Lett., 2019, 60, 660–663 Search PubMed.
- W. C. de Souza, J. T. M. Correia, P. M. Matos, C. M. Kisukuri, P. S. Carneiro and M. W. Paixão, Eur. J. Org. Chem., 2022, e202101376 CrossRef CAS.
- B. Lasne, P. Mäki-Arvela, A. Aho, Z. Vajglova, K. Eränen, N. Kumar, J. E. Sánchez-Velandia, M. Peurla, C. Mondelli, J. Pérez-Ramírez and D. Y. Murzin, J. Catal., 2022, 405, 288–302 CrossRef CAS.
- J. Hernández Muñoz, M. E. de Cavalho, J. Jones Junior and M. F. da Silva, Curr. Org. Synth., 2016, 13, 432–439 CrossRef.
- J. M. Kalaw, H. Shigemitsu and T. Kida, Langmuir, 2022, 38, 8407–8415 CrossRef CAS PubMed.
- M. Ansarifard, G. H. Rounaghi, M. Chamsaz and K. Taheri, Asian J. Chem., 2009, 21, 2799–2806 Search PubMed.
- K. N. Silgado-Gómez and V. V. Kouznetsov, Mediterr. J. Chem., 2017, 6, 208–214 CrossRef.
- G. Righi, P. Bovicelli, M. Barontini and I. Tirotta, Green Chem., 2012, 14, 495 RSC.
- M. Delépine, Bull. Soc. Chim. Fr., 1895, 3, 352–361 Search PubMed.
- D. A. Kolykhalov, A. N. Golysheva, K. S. Erokhin, B. Ya. Karlinskii and V. P. Ananikov, ChemSusChem, 2025, 18, e202401849 Search PubMed.
- V. A. Klushin, K. I. Galkin, V. P. Kashparova, E. A. Krivodaeva, O. A. Kravchenko, N. V. Smirnova, V. M. Chernyshev and V. P. Ananikov, Russ. J. Org. Chem., 2016, 52, 767–771 CrossRef CAS.
- Y. Akiyama, X. Meng, S. Fujita, Y.-C. Chen, N. Lu, H. Cheng, F. Zhao and M. Arai, J. Supercrit. Fluids, 2009, 51, 209–216 CrossRef CAS.
- S. Fujita, T. Tanaka, Y. Akiyama, K. Asai, J. Hao, F. Zhao and M. Arai, Adv. Synth. Catal., 2008, 350, 1615–1625 CrossRef CAS.
- M. T. Reetz and G. Lohmer, Chem. Commun., 1996, 1921 RSC.
- M. North and P. Villuendas, Org. Lett., 2010, 12, 2378–2381 Search PubMed.
- M. North, F. Pizzato and P. Villuendas, ChemSusChem, 2009, 2, 862–865 CrossRef CAS PubMed.
- A. S. Brar and T. Saini, Eur. Polym. J., 2007, 43, 1046–1054 Search PubMed.
- M. Enayati, R. B. Smail, S. Grama, R. L. Jezorek, M. J. Monteiro and V. Percec, Polym. Chem., 2016, 7, 7230–7241 RSC.
- S. Grama, J. Lejnieks, M. Enayati, R. B. Smail, L. Ding, G. Lligadas, M. J. Monteiro and V. Percec, Polym. Chem., 2017, 8, 5865–5874 Search PubMed.
- T. O. Morgen, M. Baur, I. Göttker-Schnetmann and S. Mecking, Nat. Commun., 2020, 11, 3693 Search PubMed.
- R. J. Ross, G. Ya. Mazo and J. Mazo, New Methods in the Synthesis of Thermal Poly(aspartates), 2001, pp. 172–181.
- A. J. Bard, L. R. Faulkner and H. S. White, Electrochemical methods: fundamentals and applications, John Wiley & Sons, 2022 Search PubMed.
- B. Zaid, S. Aeiyach and P. C. Lacaze, Synth. Met., 1994, 65, 27–34 CrossRef CAS.
- M. Bazzaoui, E. A. Bazzaoui, L. Martins and J. I. Martins, Synth. Met., 2002, 130, 73–83 CrossRef CAS.
- Y. Tachibana, Y. Sakurai and M. Yokoyama, Chem. Lett., 1994, 23, 1119–1122 Search PubMed.
- W. Zhang, W. Plieth and G. Koβmehl, Electrochim. Acta, 1997, 42, 1653–1661 CrossRef CAS.
- M. Blomquist, T. Lindfors, L. Vähäsalo, A. Pivrikas and A. Ivaska, Synth. Met., 2006, 156, 549–557 CrossRef CAS.
- G. Pistoia, J. Polym. Sci., Part B, 1972, 10, 787–790 Search PubMed.
- J. G. Cañadas, A. Lafuente and G. Rodríguez, Port. Electrochim. Acta, 2004, 22, 411–431 CrossRef.
- J. García-Cañadas, A. Lafuente, G. Rodríguez, M. L. Marcos and J. G. Velasco, J. Electroanal. Chem., 2004, 565, 57–64 Search PubMed.
- C. Bodart, N. Rossetti, J. Hagler, P. Chevreau, D. Chhin, F. Soavi, S. B. Schougaard, F. Amzica and F. Cicoira, ACS Appl. Mater. Interfaces, 2019, 11, 17226–17233 Search PubMed.
- T. Xu, L. Zhang, Z. Cheng and X. Zhu, Polym. Chem., 2017, 8, 3910–3920 RSC.
- J.-S. Kim, A. Dutta, V. Vasu, O. I. Adebolu and A. D. Asandei, Macromolecules, 2019, 52, 8895–8909 CrossRef CAS.
- S. Dutta, I. K. M. Yu, D. C. W. Tsang, J. Fan, J. H. Clark, Z. Jiang, Z. Su, C. Hu and C. S. Poon, ACS Sustainable Chem. Eng., 2020, 8, 13100–13110 CrossRef CAS.
- T. Yamada, M. Aratani, S. Kubo and H. Ono, J. Wood Sci., 2007, 53, 487–493 Search PubMed.
- H. J. Shin, C.-J. Kim and S. B. Kim, Biotechnol. Bioprocess Eng., 2009, 14, 349–353 CrossRef CAS.
- L. C. Over, M. Hergert and M. A. R. Meier, Macromol. Chem. Phys., 2017, 218, 1700177 CrossRef.
- G. Lligadas, S. Grama and V. Percec, Biomacromolecules, 2017, 18, 2981–3008 CrossRef CAS PubMed.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- B. Maji, B. Barik and P. Dash, in Nanosensors for Smart Manufacturing, Elsevier, 2021, pp. 3–18 Search PubMed.
- S. Cosnier and K. Arkady, Electropolymerization: concepts, materials and applications, John Wiley & Sons, 2011 Search PubMed.
- M. D. Imisides, R. John, P. J. Riley and G. G. Wallace, Electroanalysis, 1991, 3, 879–889 CrossRef CAS.
- G. Sabouraud, S. Sadki and N. Brodie, Chem. Soc. Rev., 2000, 29, 283–293 RSC.
- R. B. Ambade, S. B. Ambade, N. K. Shrestha, R. R. Salunkhe, W. Lee, S. S. Bagde, J. H. Kim, F. J. Stadler, Y. Yamauchi and S.-H. Lee, J. Mater. Chem. A, 2017, 5, 172–180 RSC.
- S. Tanaka, M. Koga, T. Kuragano, A. Ogawa, H. Ogiwara, K. Sato and Y. Nakajima, ACS Mater. Au, 2024, 4, 335–345 CrossRef CAS PubMed.
- X. He, J. Qi, M. Chen, J. Lv, H. Xiao, J. Hu, K. Zeng and G. Yang, Polymer, 2022, 253, 124973 CrossRef CAS.
- G. Puts, V. Venner, B. Améduri and P. Crouse, Macromolecules, 2018, 51, 6724–6739 CrossRef CAS.
- M. Beija, J.-D. Marty and M. Destarac, Prog. Polym. Sci., 2011, 36, 845–886 CrossRef CAS.
- M. Guerre, B. Campagne, O. Gimello, K. Parra, B. Ameduri and V. Ladmiral, Macromolecules, 2015, 48, 7810–7822 CrossRef CAS.
- N. Oleszko, A. Utrata-Wesołek, W. Wałach, M. Libera, A. Hercog, U. Szeluga, M. Domański, B. Trzebicka and A. Dworak, Macromolecules, 2015, 48, 1852–1859 Search PubMed.
- A. Güney, J. Malda, W. J. A. Dhert and D. W. Grijpma, Int. J. Artif. Organs, 2017, 40, 176–184 CrossRef PubMed.
- P. A. Woodfield, Y. Zhu, Y. Pei and P. J. Roth, Macromolecules, 2014, 47, 750–762 CrossRef CAS.
- S. Daneshvar, R. Behrooz, S. Kazemi Najafi and G. Mir Mohamad Sadeghi, BioResources, 2018, 14, 796–815 Search PubMed.
- X. Lu, Y. Wang, Y. Zhang, X. Cheng, Y. Yu and Y. Jin, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2016, 31, 918–924 Search PubMed.
- L. Liang, Z. Mao, Y. Li, C. Wan, T. Wang, L. Zhang and L. Zhang, BioResources, 2006, 1, 248–256 Search PubMed.
- T. Yamada and H. Ono, Bioresour. Technol., 1999, 70, 61–67 CrossRef CAS.
- H. Kim, S. Kim and H. Sah, J. Biomater. Sci., Polym. Ed., 2018, 29, 35–56 CrossRef CAS PubMed.
- K. C. Song, H. S. Lee, I. Y. Choung, K. I. Cho, Y. Ahn and E. J. Choi, Colloids Surf., A, 2006, 276, 162–167 Search PubMed.
- X. Gui, Z. Tang and W. Fei, J. Chem. Eng. Data, 2010, 55, 3736–3741 Search PubMed.
- E. Ataeivarjovi, Z. Tang and J. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 28992–29002 CrossRef CAS PubMed.
- Z. Tang, H. Li, W. Fei, J. Chen, J. Cui, D. Guo and Z. He, Int. J. Greenhouse Gas Control, 2016, 44, 140–151 Search PubMed.
- E. Ataeivarjovi, Z. Tang, J. Chen, Z. Zhao and G. Dong, ACS Sustainable Chem. Eng., 2019, 7, 12125–12137 CAS.
- Z. Zhang, W. Zhao, J. Nong, D. Feng, Y. Li, Y. Chen and J. Chen, Energy Technol., 2017, 5, 461–468 Search PubMed.
- Z.-G. Tang, H.-W. Li, Z.-M. He, J.-J. Cui, D. Guo and Z.-J. Zhao, J. Chem. Eng. Chin. Univ., 2016, 30, 276–285 Search PubMed.
- W. Fei, Z. Tang, J. Chen, G. Luo, X. Gui, Z. Li and D. Guo, Tsinghua University, CN102151457A, 2011.
- J. Chen, W. Fei, Z. Jiang, G. Luo, Z. Tang and L. Yu, Tsinghua University, CN101830462A, 2010.
- D. Im, K. Roh, J. Kim, Y. Eom and J. H. Lee, Int. J. Greenhouse Gas Control, 2015, 42, 109–116 CrossRef.
- C. Ma, C. Liu, X. Lu and X. Ji, Appl. Energy, 2018, 225, 437–447 Search PubMed.
- A. Figoli, T. Marino, S. Simone, E. Di Nicolò, X. M. Li, T. He, S. Tornaghi and E. Drioli, Green Chem., 2014, 16, 4034–4059 Search PubMed.
- R. Abedini and A. Nezhadmoghadam, Pet. Coal, 2010, 52, 69–80 CAS.
- E. Drioli, A. Brunetti, G. Di Profio and G. Barbieri, Green Chem., 2012, 14, 1561–1572 RSC.
- A. Behr, M. Halama and L. Domke, Chem. Eng. Res. Des., 2017, 123, 23–34 CrossRef CAS.
- J. Dechnik, C. J. Sumby and C. Janiak, Cryst. Growth Des., 2017, 17, 4467–4488 Search PubMed.
- E. Obotey Ezugbe and S. Rathilal, Membranes, 2020, 10, 89 CrossRef PubMed.
- C. Charcosset, Desalination, 2009, 245, 214–231 CrossRef CAS.
- A. M. Alklaibi and N. Lior, Desalination, 2005, 171, 111–131 CrossRef CAS.
- W. H. Organization, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Lyon, France, 2012 Search PubMed.
- N. Ismail, M. Essalhi, M. Rahmati, Z. Cui, M. Khayet and N. Tavajohi, Green Chem., 2021, 23, 2130–2147 RSC.
- F. Russo, F. Galiano, F. Pedace, F. Aricò and A. Figoli, ACS Sustainable Chem. Eng., 2020, 8, 659–668 CrossRef CAS.
- P. Yadav, N. Ismail, M. Essalhi, M. Tysklind, D. Athanassiadis and N. Tavajohi, J. Membr. Sci., 2021, 622, 118987 CrossRef CAS.
- M. A. Rasool, P. P. Pescarmona and I. F. J. Vankelecom, ACS Sustainable Chem. Eng., 2019, 7, 13774–13785 CrossRef CAS.
- M. Mulder, Basic Principles of Membrane Technology, Springer Netherlands, Dordrecht, 1996 Search PubMed.
- C. Fang, S. Rajabzadeh, W. Liu, H.-C. Wu, N. Kato, Y. Sun, S. Jeon and H. Matsuyama, J. Membr. Sci., 2020, 596, 117715 CrossRef CAS.
- C. Fang, W. Liu, P. Zhang, M. Yao, S. Rajabzadeh, N. Kato, H. Kyong Shon and H. Matsuyama, Sep. Purif. Technol., 2021, 258, 117988 CrossRef CAS.
- P. Zhang, C. Fang, S. Rajabzadeh, W. Liu, Y. Jia, Q. Shen, L. Zhang, S. Wang, N. Kato and H. Matsuyama, J. Membr. Sci., 2021, 620, 118854 CrossRef CAS.
- C. Y. B. Ng, W. C. Gan, T. S. Velayutham, B. T. Goh and R. Hashim, Phys. Chem. Chem. Phys., 2020, 22, 2414–2423 RSC.
- D. D. da Silva Parize, J. E. de Oliveira, M. M. Foschini, J. M. Marconcini and L. H. C. Mattoso, J. Appl. Polym. Sci., 2016, 133, 43379 CrossRef.
- E. L. G. Medeiros, A. L. Braz, I. J. Porto, A. Menner, A. Bismarck, A. R. Boccaccini, W. C. Lepry, S. N. Nazhat, E. S. Medeiros and J. J. Blaker, ACS Biomater. Sci. Eng., 2016, 2, 1442–1449 CrossRef CAS PubMed.
- C. Yang, F. Topuz, S.-H. Park and G. Szekely, Green Chem., 2022, 24, 5291–5303 RSC.
- H. A. Le Phuong, N. A. Izzati Ayob, C. F. Blanford, N. F. Mohammad Rawi and G. Szekely, ACS Sustainable Chem. Eng., 2019, 7, 11885–11893 CrossRef CAS.
- H. Ghanbar, C. J. Luo, P. Bakhshi, R. Day and M. Edirisinghe, Mater. Sci. Eng., C, 2013, 33, 2488–2498 CrossRef CAS PubMed.
- M. Shi, W. Yan, Y. Zhou, Z. Wang, L. Liu, S. Zhao, Y. Ji, J. Wang, C. Gao, P. Zhang and X. Cao, J. Membr. Sci., 2020, 595, 117474 CrossRef.
- Y. Liu, H. Wu, S. Guo, C. Cong, J. Du, Z. Xin, H. Zhang, J. Wang and Z. Wang, J. Membr. Sci., 2023, 665, 121123 CrossRef CAS.
- I. Allijn, N. du Preez, M. Tasior, R. Bansal and D. Stamatialis, Membranes, 2022, 12, 453 CrossRef CAS PubMed.
- D. G. Oldal, F. Topuz, T. Holtzl and G. Szekely, ACS Sustainable Chem. Eng., 2023, 11, 994–1005 CrossRef CAS.
- S. Chisca, M. Torsello, M. Avanzato, Y. Xie, C. Boi and S. P. Nunes, Polymer, 2017, 126, 446–454 CrossRef CAS.
- A. S. Istomina, T. V. Yaroslavtseva, O. G. Reznitskikh, R. R. Kayumov, L. V. Shmygleva, E. A. Sanginov, Y. A. Dobrovolsky and O. V. Bushkova, Polymers, 2021, 13, 1150 CrossRef CAS PubMed.
- K. Papchenko, M. Degli Esposti, M. Minelli, P. Fabbri, D. Morselli and M. G. De Angelis, J. Membr. Sci., 2022, 660, 120847 CrossRef CAS.
- K. Maleski, V. N. Mochalin and Y. Gogotsi, Chem. Mater., 2017, 29, 1632–1640 CrossRef CAS.
- M. Schardosim, J. Soulié, D. Poquillon, S. Cazalbou, B. Duployer, C. Tenailleau, C. Rey, R. Hübler and C. Combes, Mater. Sci. Eng., C, 2017, 77, 731–738 CrossRef CAS PubMed.
- J. L. Vidal, S. M. V. Gallant, E. P. Connors, D. D. Richards, S. L. MacQuarrie and F. M. Kerton, ACS Sustainable Chem. Eng., 2021, 9, 9114–9125 CrossRef CAS.
- M. Naviroj, P. W. Voorhees and K. T. Faber, J. Mater. Res., 2017, 32, 3372–3382 CrossRef CAS.
- H. Li, Y. Liu, Y. Liu, K. Hu, Z. Lu and J. Liang, ACS Omega, 2020, 5, 27455–27462 CrossRef CAS PubMed.
- C. Chen, Y. Jiang, Y. Feng, Z. Li, N. Cao, G. Zhou, J.-M. Liu, K. Kempa, S.-P. Feng and J. Gao, Mater. Today Phys., 2021, 21, 100565 CrossRef CAS.
- N. Zhang, Z. Zhang, T. Liu, T. He, P. Liu, J. Li, F. Yang, G. Song, Z. Liu and M. Yuan, Org. Electron., 2023, 113, 106709 CrossRef CAS.
- Y.-W. Zhang, Z.-L. Diao, J.-Y. Chen, W.-Y. Tan, Y.-N. Qian, L.-G. Xiao and Y. Min, J. Mater. Chem. C, 2021, 9, 8939–8946 RSC.
- A. A. Zhumekenov, Y. Li, Y. Zhou, N. Yantara, A. Kanwat, B. Febriansyah, D. J. J. Tay, H. R. Abuzeid, Y. B. Tay, E. B. Miftahullatif, K. Hippalgaonkar, S. A. Pullarkat, J. Yin and N. Mathews, J. Am. Chem. Soc., 2024, 146, 6706–6720 CrossRef CAS PubMed.
- A. Sasidharanpillai, C. H. Kim, C. H. Lee, M. T. Sebastian and H. T. Kim, ACS Sustainable Chem. Eng., 2018, 6, 6849–6855 CrossRef CAS.
- J. C. Fernandes, P. Brito, F. Travagin, I. Miletto, G. B. Giovenzana and E. Gianotti, J. Phys. Chem. B, 2022, 126, 7166–7171 CrossRef PubMed.
- P. Fabbri, V. Cannillo, A. Sola, A. Dorigato and F. Chiellini, Compos. Sci. Technol., 2010, 70, 1869–1878 CrossRef CAS.
- M. Ju, X. Ji, C. Wang, R. Shen and L. Zhang, Chem. Eng. J., 2014, 250, 112–118 Search PubMed.
- Y. Zhang, I. Ullah, W. Zhang, H. Ou, M. Domingos, A. Gloria, J. Zhou, W. Li and X. Zhang, J. Appl. Polym. Sci., 2020, 137, 48387 CrossRef CAS.
- E. Zant, M. M. Blokzijl and D. W. Grijpma, Macromol. Rapid. Commun., 2015, 36, 1902–1909 CrossRef CAS PubMed.
- V. Dudko, K. Ottermann, S. Rosenfeldt, G. Papastavrou and J. Breu, Langmuir, 2021, 37, 461–468 CrossRef CAS PubMed.
- R. Marcos Esteban, K. Schütte, D. Marquardt, J. Barthel, F. Beckert, R. Mülhaupt and C. Janiak, Nano-Struct. Nano-Objects, 2015, 2, 28–34 CrossRef CAS.
- D. D. da S. Parize, J. E. de Oliveira, T. Williams, D. Wood, R. de J. Avena-Bustillos, A. P. Klamczynski, G. M. Glenn, J. M. Marconcini and L. H. C. Mattoso, Carbohydr. Polym., 2017, 174, 923–932 CrossRef CAS PubMed.
- M. Itatani, N. Német, N. Valletti, G. Schuszter, P. Prete, P. Lo Nostro, R. Cucciniello, F. Rossi and I. Lagzi, ACS Sustainable Chem. Eng., 2023, 11, 13043–13049 CrossRef CAS PubMed.
- A. W. H. Lee and B. D. Gates, Langmuir, 2017, 33, 8707–8715 CrossRef CAS PubMed.
- W. Rahmalia, J.-F. Fabre, T. Usman and Z. Mouloungui, Bioinorg. Chem. Appl., 2018, 2018, 1–9 CrossRef PubMed.
- M. Hanzawa, H. Oohinata, S. Kawano, M. Akamatsu, K. Sakai and H. Sakai, Langmuir, 2018, 34, 14180–14185 CrossRef CAS PubMed.
- M. Lorêdo de França, L. Separovic, L. S. Longo Junior, D. C. de Oliveira, F. Rebello Lourenço and L. A. Calixto, Measurement, 2021, 173, 108581 CrossRef.
- M. Ghidotti, D. Fabbri, C. Torri and S. Piccinini, Anal. Chim. Acta, 2018, 1034, 92–101 Search PubMed.
- G. B. Grecco, K. F. Albini, L. S. Longo, M. A. Andreo, B. L. Batista, F. R. Lourenço and L. A. Calixto, J. Iran. Chem. Soc., 2023, 20, 371–380 Search PubMed.
- J. Sun, J. Feng, L. Shi, L. Liu, H. He, Y. Fan, S. Hu and S. Liu, J. Chromatogr. A, 2016, 1461, 161–170 Search PubMed.
- M. Amado, D. Bastos, D. Gaspar, S. Matos, S. Vieira, J. M. Bordado and R. Galhano dos Santos, J. Cleaner Prod., 2021, 304, 127088 Search PubMed.
- H. J. Shin, C.-J. Kim and S. B. Kim, Biotechnol. Bioprocess Eng., 2009, 14, 349–353 Search PubMed.
- M. M. Cascant, C. Breil, S. Garrigues, M. de la Guardia, A. S. Fabiano-Tixier and F. Chemat, Anal. Bioanal. Chem., 2017, 409, 3527–3539 Search PubMed.
- X. Zhuang, Z. Zhang, Y. Wang and Y. Li, Ind. Crops Prod., 2018, 126, 340–346 Search PubMed.
- E. K. Sitepu, A. Candra, E. F. Zaidar, A. Vika, F. Sebayang, F. R. Dewi, J. A. Karo-karo and J. Br. Tarigan, Rasayan J. Chem., 2022, 15, 1063–1070 Search PubMed.
- C. Portilho Trentini, B. T. F. de Mello, V. Ferreira Cabral and C. da Silva, J. Supercrit. Fluids, 2020, 159, 104780 Search PubMed.
- N. Postaue, C. Eduardo Borba and C. da Silva, Fuel, 2022, 324, 124827 Search PubMed.
- E. Tommasi, G. Cravotto, P. Galletti, G. Grillo, M. Mazzotti, G. Sacchetti, C. Samorì, S. Tabasso, M. Tacchini and E. Tagliavini, ACS Sustainable Chem. Eng., 2017, 5, 8316–8322 CrossRef CAS.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 Search PubMed.
- A. D. Wani, W. Prasad, K. Khamrui, S. A. Hussain and A. Deep, Int. J. Food Sci. Technol., 2023, 58, 2085–2091 Search PubMed.
- C. Liu, H. Ni, Y. Chang, Z. Wang, N. Wan, L. Cao, Z. Liu and Y. Fu, J. Food Process. Preserv., 2022, 46, e16603 Search PubMed.
- M. Abbasi, E. R. Coats and A. G. McDonald, Bioresour. Technol. Rep., 2022, 18, 101065 Search PubMed.
- C. M. Vermeer, M. Nielsen, V. Eckhardt, M. Hortensius, J. Tamis, S. J. Picken, G. M. H. Meesters and R. Kleerebezem, J. Environ. Chem. Eng., 2022, 10, 108573 CrossRef CAS.
- G. Montiel-Jarillo, D. A. Morales-Urrea, E. M. Contreras, A. López-Córdoba, E. Y. Gómez-Pachón, J. Carrera and M. E. Suárez-Ojeda, Polymers, 2022, 14, 3938 Search PubMed.
- R. Pei, N. Tarek-Bahgat, M. C. M. Van Loosdrecht, R. Kleerebezem and A. G. Werker, Water Res., 2023, 232, 119653 CrossRef CAS PubMed.
- L. K. M. Quines, J. L. Ienczak, M. Schmidt, K. Zanfonato, M. I. Rodrigues, W. Schmidell and G. M. F. Aragão, Quim. Nova, 2014, 38, 214–220 Search PubMed.
- A. Aramvash, F. Moazzeni Zavareh and N. Gholami Banadkuki, Eng. Life Sci., 2018, 18, 20–28 CrossRef CAS PubMed.
- V. Elhami, N. van de Beek, L. Wang, S. J. Picken, J. Tamis, J. A. B. Sousa, M. A. Hempenius and B. Schuur, Sep. Purif. Technol., 2022, 299, 121773 CrossRef CAS.
- B. Mongili, A. Abdel Azim, S. Fraterrigo Garofalo, E. Batuecas, A. Re, S. Bocchini and D. Fino, Biotechnol. Biofuels, 2021, 14, 13 CrossRef CAS PubMed.
- C. Samorì, F. Abbondanzi, P. Galletti, L. Giorgini, L. Mazzocchetti, C. Torri and E. Tagliavini, Bioresour. Technol., 2015, 189, 195–202 CrossRef PubMed.
- G. A. de Souza Reis, M. H. A. Michels, G. L. Fajardo, I. Lamot and J. H. de Best, Water, 2020, 12, 1185 CrossRef.
- G. Pagliano, P. Galletti, C. Samorì, A. Zaghini and C. Torri, Front. Bioeng. Biotechnol., 2021, 9, 624021 CrossRef PubMed.
- A. Werker, R. Pei, K. Kim, G. Moretto, A. Estevez-Alonso, C. Vermeer, M. Arcos-Hernandez, J. Dijkstra and E. de Vries, Polym. Degrad. Stab., 2023, 209, 110277 Search PubMed.
- T. Brouwer and B. Schuur, Sep. Purif. Technol., 2021, 270, 118749 Search PubMed.
- Z. Zhang, D. W. Rackemann, W. O. S. Doherty and I. M. O'Hara, Biotechnol. Biofuels, 2013, 6, 153 CrossRef CAS PubMed.
- S. K. Karmee, Biocatal. Biotransform., 2024, 42, 286–307 CrossRef CAS.
- M. Lu, X. Zhao, J. Zhou, G. Qian, X. Duan, W. Yuan and X. Zhou, Ind. Eng. Chem. Res., 2019, 58, 395–402 CrossRef CAS.
- H. M. S. Lababidi, S. H. Ali and M. A. Fahim, Ind. Eng. Chem. Res., 2006, 45, 5086–5097 CrossRef CAS.
- M. A. Fahim and S. Q. Merchant, J. Chem. Eng. Data, 1998, 43, 884–888 CrossRef CAS.
- S. H. Ali, H. M. S. Lababidi, S. Q. Merchant and M. A. Fahim, Fluid Phase Equilib., 2003, 214, 25–38 CrossRef CAS.
- T. Wang, B. Shen and H. Sun, China Pet. Process. Petrochem. Technol., 2016, 18, 91–101 CAS.
- Y. Niu, X. Wang, J. Shen, Q. Sheng, G. Liu, C. Li and Y. Wang, Sep. Purif. Technol., 2017, 188, 98–104 CrossRef CAS.
- A. A. Lapkin, M. Peters, L. Greiner, S. Chemat, K. Leonhard, M. A. Liauw and W. Leitner, Green Chem., 2010, 12, 241–251 RSC.
- L. Hladnik, F. A. Vicente, A. Košir, M. Grilc and B. Likozar, Sep. Purif. Technol., 2023, 311, 123293 CrossRef CAS.
- M. Alfieri, I. Mascheretti, R. A. Dougué Kentsop, M. Mattana, M. Laura and G. Ottolina, Molecules, 2022, 27, 2732 CrossRef CAS PubMed.
- D. C. Panadare and V. K. Rathod, Process Biochem., 2017, 61, 195–201 CrossRef CAS.
- I. Rodriguez-Donis, S. Thiebaud-Roux, S. Lavoine and V. Gerbaud, C. R. Chim., 2018, 21, 606–621 CrossRef CAS.
- S. Gurrani, K. Prakasham, J. L. Zii Ying, J. Shiea, Y.-J. Ku, Y.-C. Lin, P.-C. Huang, G. Andaluri, K.-C. Lee and V. K. Ponnusamy, Environ. Res., 2023, 217, 114787 CrossRef CAS PubMed.
- Q. Zhang, C. Dai, J. Zhang, X. He, X. Tan, K. Zhang, X. Xu and X. Zhuang, Int. J. Biol. Macromol., 2023, 230, 123249 CrossRef CAS PubMed.
- Y.-Y. Wang, H. Ma, J.-K. Yan, K.-D. Wang, Y. Yang, W.-H. Wang and H.-N. Zhang, Int. J. Biol. Macromol., 2019, 131, 941–948 CrossRef CAS PubMed.
- B. Marrufo, J. Pla-Franco, E. Lladosa and S. Loras, J. Chem. Eng. Data, 2017, 62, 1355–1364 CrossRef CAS.
- S. Raiguel, L. Gijsemans, A. Van den Bossche, B. Onghena and K. Binnemans, ACS Sustainable Chem. Eng., 2020, 8, 13713–13723 CrossRef CAS.
- M. Tobiszewski, W. Zabrocka and M. Bystrzanowska, Anal. Methods, 2019, 11, 844–850 RSC.
- A. Kamal El-Deen and K. Shimizu, J. Chromatogr. B, 2021, 1171, 122555 CAS.
- M. Bilgin and İ. Birman, Fluid Phase Equilib., 2010, 292, 13–19 CrossRef CAS.
- B. Lajin and W. Goessler, J. Anal. At. Spectrom., 2021, 36, 1272–1279 CAS.
- M. Jurin, D. Kontrec, T. Dražić and M. Roje, Separations, 2022, 9, 157 CrossRef CAS.
- F. Tache, S. Udrescu, F. Albu, F. Micăle and A. Medvedovici, J. Pharm. Biomed. Anal., 2013, 75, 230–238 CAS.
- H. Kumar Chanduluru, A. Sugumaran and K. P. Kannaiah, Anal. Biochem., 2022, 657, 114890 CrossRef PubMed.
- M. Källsten, D. Visanu, M. Pijnappel, F. Lehmann, J. Bergquist, S. B. Lind and L. Kovac, J. Am. Soc. Mass Spectrom., 2022, 33, 1161–1167 CrossRef PubMed.
- M. A. Zenaidee and W. A. Donald, Analyst, 2015, 140, 1894–1905 RSC.
- C. A. Teo and W. A. Donald, Anal. Chem., 2014, 86, 4455–4462 CAS.
- D. Bozza, C. De Luca, S. Felletti, M. Spedicato, F. Presini, P. P. Giovannini, M. Carraro, M. Macis, A. Cavazzini, M. Catani, A. Ricci and W. Cabri, J. Chromatogr. A, 2024, 1713, 464530 CAS.
- S. Felletti, M. Spedicato, D. Bozza, C. De Luca, F. Presini, P. P. Giovannini, M. Carraro, M. Macis, A. Cavazzini, M. Catani, A. Ricci and W. Cabri, J. Chromatogr. A, 2023, 1712, 464477 CrossRef CAS PubMed.
- H. Golczyk, Protoplasma, 2019, 256, 873–880 CrossRef CAS PubMed.
- H. Katano, H. Watanabe, M. Takakuwa, C. Maruyama and Y. Hamano, Anal. Sci., 2013, 29, 1095–1098 CrossRef CAS PubMed.
- R. B. Register, Jf Pharma Tech, US11260023B2, 2021.
- A. Yapar, E. Baykara and T. Tamer, Ankara Univ. Eczacilik Fak. Derg., 2008, 37, 101–109 CrossRef.
- G. Ou, B. He, X. Li and J. Lei, Sci. World J., 2012, 2012, 1–6 CrossRef PubMed.
- G. Ou, B. He and Y. Yuan, Enzyme Microb. Technol., 2011, 49, 167–170 CrossRef CAS PubMed.
- R. Sivaramakrishnan and A. Incharoensakdi, Fuel, 2018, 217, 458–466 CrossRef CAS.
- G. Martelli, P. Cantelmi, C. Palladino, A. Mattellone, D. Corbisiero, T. Fantoni, A. Tolomelli, M. Macis, A. Ricci, W. Cabri and L. Ferrazzano, Green Chem., 2021, 23, 8096–8107 RSC.
- L. Ferrazzano, D. Corbisiero, G. Martelli, A. Tolomelli, A. Viola, A. Ricci and W. Cabri, ACS Sustainable Chem. Eng., 2019, 7, 12867–12877 CrossRef CAS.
- G. Martelli, P. Cantelmi, A. Tolomelli, D. Corbisiero, A. Mattellone, A. Ricci, T. Fantoni, W. Cabri, F. Vacondio, F. Ferlenghi, M. Mor and L. Ferrazzano, Green Chem., 2021, 23, 4095–4106 RSC.
- A. Macchia, L. Rivaroli and B. Gianfreda, Nat. Prod. Res., 2021, 35, 2335–2345 CrossRef CAS PubMed.
- J. Yiming, G. Sciutto, S. Prati, E. Catelli, M. Galeotti, S. Porcinai, L. Mazzocchetti, C. Samorì, P. Galletti, L. Giorgini, E. Tagliavini and R. Mazzeo, Heritage Sci., 2019, 7, 34 CrossRef.
- P. Ferrari, D. Chelazzi, N. Bonelli, A. Mirabile, R. Giorgi and P. Baglioni, J. Cult. Herit., 2018, 34, 227–236 CrossRef.
- A. Mirabile, D. Chelazzi, P. Ferrari, C. Montis, D. Berti, N. Bonelli, R. Giorgi and P. Baglioni, Heritage Sci., 2020, 8, 42 CrossRef CAS.
- M. Musolino, F. Aricò and P. Tundo, J. Cult. Herit., 2019, 36, 268–274 CrossRef.
- Y. Çakmak, E. Çakmakçi, N. K. Apohan and R. Karadag, J. Cult. Herit., 2022, 55, 391–398 CrossRef.
- S. Beskhyroun, S. Darwish and A. Elserogy, Egypt. J. Chem., 2022, 65, 571–583 Search PubMed.
- M. Baglioni, C. Montis, F. Brandi, T. Guaragnone, I. Meazzini, P. Baglioni and D. Berti, Phys. Chem. Chem. Phys., 2017, 19, 23723–23732 RSC.
- H. Sayed, H. Sadek, M. Abdel-Aziz, N. Mahmoud, W. Sabry, G. Genidy and M. Maher, Egypt. J. Archeol. Restor. Stud., 2021, 11, 129–145 Search PubMed.
- M. Baglioni, G. Poggi, R. Giorgi, P. Rivella, T. Ogura and P. Baglioni, J. Colloid Interface Sci., 2021, 595, 187–201 CrossRef CAS PubMed.
- O. D. Chretien, A. M. Delaite and B. G. Papavoine, US20090005284A1, 2006.
- J. R. Machac, S. A. Woodrum, H. P. Klein and E. T. Marquis, JPMorgan Chase Bank NA Indorama Ventures Oxide and Glycols LLC, US6596677B1, 2000.
- N. P. Elepano, W. H. Schnur and J. L. Jorgensen, 3M Co, US4508634A, 1983.
- S. Rosenthal and A. Hooper, Evaluation Of Propylene Carbonate In Air Logistics Center (Alc) Depainting Operations, Washington, 1994 Search PubMed.
- E. T. Marquis, Huntsman Petrochemical LLC, Huntsman Petrochemical LLC, US6015550A, 1998.
- M. Weuthen and U. Hees, Henkel AG and Co KGaA, DE4335947A1, 1993.
- Y. Tian, J. Shen, J. Yuan, Y. Tian, B. Shin, Z. Jiang, T. Yu, G. Xu and J. Zhang, Shanghai Zhenchen Cosmetics Co Ltd, CN115068399A, 2022.
- B. T. Ferreira and A. Zambon Gardolinski, WO2022020913A1, 2020.
- M. Sohn, L. Amorós-Galicia, S. Krus, K. Martin and B. Herzog, J. Photochem. Photobiol., B, 2020, 205, 111818 CrossRef CAS PubMed.
- O. Aubrun, R. Cavazzuti, A. Metivier, L. Mineau, L. Lukyanova and M. Manzola Mazeka, WO2022136106A1, 2020.
- J. Xia, M. E. Ragsdale and E. B. Stephens, Milliken and Co, US6607591B1, 2000.
- S. Silke and N. Hansjörg, Farbe Lack, 2004, 110, 60–62 Search PubMed.
- L. Riva, R. Mangano and P. R. Tundo, WO2009147469A1, 2008.
- J. Miller, K. Nwe, Y. Youn, K. Hwang, C. Choi, P. W. Mola, Y. Kim and S. Jin, Korean J. Chem. Eng., 2019, 36, 996–1003 CrossRef CAS.
- N. Chaudhary, S. Naqvi, D. Rathore, S. Rathi and A. Patra, Mater. Chem. Phys., 2022, 282, 125898 CrossRef CAS.
- A. L. Kohl and P. A. Buckingham, Oil Gas J., 1960, 58, 158–160 Search PubMed.
- G. Murlidhar, I. Coyle and K. Thambimuthu, 1st Canadian CC&S Technology Roadmap Workshop.
- P. Navarro, E. Hernández, D. Rodríguez-Llorente, I. Maldonado-López, R. Santiago, C. Moya, A. Belinchón, M. Larriba and J. Palomar, Fuel, 2022, 321, 124005 CrossRef CAS.
- K. Ishida, Seiko Epson Corp, US20090068343A1, 2008.
- X. Ji, C. E. Banks, D. S. Silvester, A. J. Wain and R. G. Compton, J. Phys. Chem. C, 2007, 111, 1496–1504 CrossRef CAS.
- J. Muzikar, T. van de Goor, B. Gaš and E. Kenndler, Anal. Chem., 2002, 74, 428–433 CrossRef CAS PubMed.
- S. F. Y. Li, Capillary electrophoresis: principles, practice and applications, Elsevier, 1992, vol. 52 Search PubMed.
- Y. Nagaosa and K. Horita, Mikrochim. Acta, 1992, 108, 151–156 CrossRef CAS.
- Y. Nagaosa, N. Yoshida, Y. Maegawa and T. Fuwa, Mikrochim. Acta, 1984, 83, 39–46 CrossRef.
- J. Tjørnelund and S. H. Hansen, J. Chromatogr. A, 1997, 792, 475–482 CrossRef.
- J. Britz, W. H. Meyer and G. Wegner, Macromolecules, 2007, 40, 7558–7565 CrossRef CAS.
- W. Lin, M. Zhu, Y. Fan, H. Wang, G. Tao, M. Ding, N. Liu, H. Yang, J. Wu, J. Fang and Y. Tang, J. Alloys Compd., 2022, 905, 164163 CrossRef CAS.
- Lithium Ion Batteries, ed. M. Wakihara and O. Yamamoto, Wiley, 1998 Search PubMed.
- S. S. Zhang, J. Power Sources, 2007, 164, 351–364 CrossRef CAS.
- D. Baril, Solid State Ionics, 1997, 94, 35–47 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4418 CrossRef CAS PubMed.
- J. M. Whelan and R. J. Cotter, Multiple cyclic carbonate polymers, US 3072613, 1957.
- I. Frischinger, J. Cotting, J. Finter and J. François, Huntsman Advanced Materials Switzerland GmbH, EP0881262A2, 1998.
- K. Izutsu, T. Nakamura, K. Miyoshi and K. Kurita, Electrochim. Acta, 1996, 41, 2523–2527 CrossRef CAS.
- M. R. Wagner, J. H. Albering, K.-C. Moeller, J. O. Besenhard and M. Winter, Electrochem. Commun., 2005, 7, 947–952 CrossRef CAS.
- O. Borodin and G. D. Smith, J. Phys. Chem. B, 2006, 110, 4971–4977 CrossRef CAS PubMed.
- H. Cheng, Z. Ma, P. Kumar, H. Liang, Z. Cao, H. Xie, L. Cavallo, Q. Li and J. Ming, ACS Energy Lett., 2024, 9, 1604–1616 CrossRef CAS.
- N.-S. Choi, S.-W. Ryu and J.-K. Park, Electrochim. Acta, 2008, 53, 6575–6579 CrossRef CAS.
- L. F. Li, H. S. Lee, H. Li, X. Q. Yang, K. W. Nam, W. S. Yoon, J. McBreen and X. J. Huang, J. Power Sources, 2008, 184, 517–521 CrossRef CAS.
- A. Abouimrane, J. Ding and I. J. Davidson, J. Power Sources, 2009, 189, 693–696 CrossRef CAS.
- M. H. Fu, K. L. Huang, S. Q. Liu, J. S. Liu and Y. K. Li, J. Power Sources, 2010, 195, 862–866 CrossRef CAS.
- M. Petrowsky and R. Frech, J. Phys. Chem. B, 2008, 112, 8285–8290 CrossRef CAS PubMed.
- M. W. Verbrugge, B. J. Koch and E. W. Schneider, J. Appl. Electrochem., 2000, 30, 269–275 CrossRef CAS.
- J. R. Rodriguez, B. Seo, B. M. Savoie and V. G. Pol, Batteries Supercaps, 2022, 5, e202100223 CrossRef CAS.
- J. Barthel, R. Neueder, P. Rawytsch and H. Roch, J. Electroanal. Chem., 1999, 471, 78–87 CrossRef CAS.
- J. Chidiac, L. Timperman and M. Anouti, Electrochim. Acta, 2022, 408, 139944 CrossRef CAS.
- A. Kesküla, A.-L. Peikolainen, P. A. Kilmartin and R. Kiefer, Polymers, 2021, 13, 3466 CrossRef PubMed.
- Y. Peng, K. Nishikawa and K. Kanamura, J. Electrochem. Soc., 2022, 169, 060548 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and A. Sadrmousavi-Dizaj, J. Chem. Thermodyn., 2022, 165, 106642 CrossRef CAS.
- B. A. V. Santos, V. M. T. M. Silva, J. M. Loureiro and A. E. Rodrigues, ChemBioEng Rev., 2014, 1, 214–229 CrossRef CAS.
- M.-L. Saboungi, O. Borodin, D. L. Price, B. Farago, M. A. González, S. Kohara, L. Mangin-Thro, A. Wildes and O. Yamamuro, J. Chem. Phys., 2023, 158, 124502 CrossRef CAS PubMed.
- D. P. Abraham, UChicago Argonne LLC, US2011/0117445A1, 2011.
- N. Zachmann, R. V. Fox, M. Petranikova and B. Ebin, J. CO2 Util., 2024, 81, 102703 CrossRef CAS.
- N. Zachmann, M. Petranikova and B. Ebin, J. Ind. Eng. Chem., 2023, 118, 351–361 CrossRef CAS.
- S. Hu, H. Zhao, Y. Qian, S. Xiang, G. Zhang, W. Huang, G. Luo, J. Wang, Y. Deng and C. Wang, J. Energy Storage, 2023, 57, 106266 Search PubMed.
- G. B. Berhe, W.-N. Su, T. T. Hagos, H. K. Bezabh, T. M. Hagos and B. J. Hwang, J. Power Sources, 2023, 558, 232567 CrossRef CAS.
- S. Wang, Z. Xue, F. Chu, Z. Guan, J. Lei and F. Wu, J. Energy Chem., 2023, 79, 201–210 CAS.
- L. Jiang, Y. Cheng, S. Wang, Y. Cheng, K. Jin, J. Sun, M. Winter, I. Cekic-Laskovic and Q. Wang, J. Power Sources, 2023, 570, 233051 CrossRef CAS.
- W. Cai, Y. Deng, Z. Deng, Y. Jia, Z. Li, X. Zhang, C. Xu, X. Zhang, Y. Zhang and Q. Zhang, Adv. Energy Mater., 2023, 13, 2301396 CrossRef CAS.
- Z. Yu, W. Yu, Y. Chen, L. Mondonico, X. Xiao, Y. Zheng, F. Liu, S. T. Hung, Y. Cui and Z. Bao, J. Electrochem. Soc., 2022, 169, 040555 CrossRef CAS.
- S. Zhang, S. Li, X. Wang, C. Li, Y. Liu, H. Cheng, S. Mao, Q. Wu, Z. Shen, J. Mao, H. Pan and Y. Lu, Nano Energy, 2023, 114, 108639 CrossRef CAS.
- E. Adams, M. Parekh, D. Gribble, T. Adams and V. G. Pol, Sustainable Energy Fuels, 2023, 7, 3134–3141 RSC.
- A. K. Kushwaha, S. S. Jena, M. R. Sahoo and S. K. Nayak, J. Electron. Mater., 2021, 50, 1807–1816 CrossRef CAS.
- Z. Wang, T. Lyu, L. Ma, X. Xu, H. Pan and S. Fang, ACS Appl. Energy Mater., 2024, 7, 230–238 CrossRef CAS.
- D. Ouyang, K. Wang, Y. Pang and Z. Wang, ACS Appl. Energy Mater., 2023, 6, 2063–2071 CrossRef CAS.
- Q. Liu, Y.-H. Feng, X. Zhu, M. Liu, L. Yu, G.-X. Wei, X.-Y. Fan, X. Ji, P.-F. Wang and H. Xin, Nano Energy, 2024, 123, 109389 CrossRef CAS.
- R. Okada, Y. Aoki, M. Oda, M. Nakazawa, M. Inaba and T. Doi, ACS Appl. Energy Mater., 2023, 6, 546–553 CrossRef CAS.
- C. Tao, T. Zheng, P. Jia, W. Gong, G. Yila, L. Wang and T. Liu, ACS Appl. Mater. Interfaces, 2024, 16(18), 23325–23333 CAS.
- I. A. Profatilova, S.-S. Kim and N.-S. Choi, Electrochim. Acta, 2009, 54, 4445–4450 CrossRef CAS.
- X. Zheng, S. Weng, W. Luo, B. Chen, X. Zhang, Z. Gu, H. Wang, X. Ye, X. Liu, L. Huang, X. Wu, X. Wang and Y. Huang, Research, 2022, 2022, 9754612 CAS.
- H. Ota, Y. Sakata, A. Inoue and S. Yamaguchi, J. Electrochem. Soc., 2004, 151, A1659 CrossRef CAS.
- D. Aurbach, K. Gamolsky, B. Markovsky, Y. Gofer, M. Schmidt and U. Heider, Electrochim. Acta, 2002, 47, 1423–1439 CrossRef CAS.
- A. Guerfi, M. Dontigny, P. Charest, M. Petitclerc, M. Lagacé, A. Vijh and K. Zaghib, J. Power Sources, 2010, 195, 845–852 CrossRef CAS.
- J. Li, W. Yao, Y. S. Meng and Y. Yang, J. Phys. Chem. C, 2008, 112, 12550–12556 CrossRef CAS.
- P. Liu, Y. Rao, H. Wang, X. Li, X. Wang, M. Yu, Y. Li, Z. Yue, F. Wu and S. Fang, Batteries Supercaps, 2024, 7, e202300353 CrossRef CAS.
- S. He, S. Huang, X. Liu, X. Zeng, H. Chen, L. Zhao, H. Noor and X. Hou, Chem. Eng. J., 2024, 489, 150620 CrossRef CAS.
- C. Xu, L. Chen, X. Zhou and Z. Liu, J. Phys. Chem. C, 2024, 128, 7884–7891 CrossRef CAS.
- M. Chen, J. Mei, S. Wang, Q. Chen, L. Zhao, Q. Kong and X. Wu, J. Energy Storage, 2022, 47, 103642 CrossRef.
- S. Chen, Z. Wang, H. Zhao, H. Qiao, H. Luan and L. Chen, J. Power Sources, 2009, 187, 229–232 CrossRef CAS.
- Y. Li, F. Ding, Y. Shao, H. Wang, X. Guo, C. Liu, X. Sui, G. Sun, J. Zhou and Z. Wang, Angew. Chem., Int. Ed., 2024, 63, e202317148 CrossRef CAS PubMed.
- J. Simonet, Electrochem. Commun., 2012, 19, 93–96 CrossRef CAS.
- D. Kuai and P. B. Balbuena, ACS Appl. Mater. Interfaces, 2022, 14, 2817–2824 CrossRef CAS PubMed.
- P. Shi, Z.-Y. Liu, X.-Q. Zhang, X. Chen, N. Yao, J. Xie, C.-B. Jin, Y.-X. Zhan, G. Ye, J.-Q. Huang, S. Ifan E L, T. Maria-Magdalena and Q. Zhang, J. Energy Chem., 2022, 64, 172–178 CrossRef CAS.
- Y. Aoki, M. Oda, S. Kojima, T. Ishihama, T. Nagashima, T. Doi and M. Inaba, ACS Appl. Energy Mater., 2022, 5, 1085–1094 CrossRef CAS.
- F. Jiang, X. Cheng, S. Yang, J. Xie, H. Yuan, L. Liu, J. Huang and Q. Zhang, Adv. Mater., 2023, 35, 2209114 CrossRef CAS PubMed.
- M. Kubot, L. Balke, J. Scholz, S. Wiemers-Meyer, U. Karst, H. Hayen, H. Hur, M. Winter, J. Kasnatscheew and S. Nowak, Adv. Sci., 2024, 11, 2305282 CrossRef CAS PubMed.
- J. Huang, T. Yan, M. Tao, W. Zhang, W. Li, G. Zheng, L. Du, Z. Cui, X. Wang, S. Liao and H. Song, J. Power Sources, 2023, 563, 232783 CrossRef CAS.
- Z. Wu, J. Zou, S. Shabanian, K. Golovin and J. Liu, Chem. Eng. J., 2022, 427, 130972 CrossRef CAS.
- Y. Wang, Z. Cao, Z. Ma, G. Liu, H. Cheng, Y. Zou, L. Cavallo, Q. Li and J. Ming, ACS Energy Lett., 2023, 8, 1477–1484 CrossRef CAS.
- B. Wang, Y. Huang, Y. Wang and H. Wang, Adv. Funct. Mater., 2023, 33, 2212287 CrossRef CAS.
- C.-C. Su and K. Amine, ACS Energy Lett., 2024, 9, 118–125 CrossRef CAS.
- J. C. Rushing, C. M. Stern, N. Elgrishi and D. G. Kuroda, J. Phys. Chem. C, 2022, 126, 2141–2150 CrossRef CAS PubMed.
- M. Väärtnõu and E. Lust, J. Electroanal. Chem., 2022, 920, 116618 CrossRef.
- H. Zhang, Z. Zeng, F. Ma, X. Wang, Y. Wu, M. Liu, R. He, S. Cheng and J. Xie, Adv. Funct. Mater., 2023, 33, 2212000 CrossRef CAS.
- X. Wang, L. Yang, N. Ahmad, L. Ran, R. Shao and W. Yang, Adv. Mater., 2023, 35, 2209140 CrossRef CAS PubMed.
- R. Braun, T. Meisel, T. Kränzler and W. Scherber, Dornier GmbH, EP0499115A1, 1992.
- Y. Liu and Y. Xu, Chem. Eng. J., 2022, 433, 134471 CrossRef CAS.
- S. N. Mohamed, N. A. Johari, A. M. M. Ali, M. K. Harun and M. Z. A. Yahya, J. Power Sources, 2008, 183, 351–354 CrossRef CAS.
- T. Kuboki, T. Okuyama, T. Ohsaki and N. Takami, J. Power Sources, 2005, 146, 766–769 CrossRef CAS.
- J. Lee, A.-R. Jeon, H. J. Lee, U. Shin, Y. Yoo, H.-D. Lim, C. Han, H. Lee, Y. J. Kim, J. Baek, D.-H. Seo and M. Lee, Energy Environ. Sci., 2023, 16, 2924–2933 RSC.
- J. Chen, D. Zhang, L. Zhu, M. Liu, T. Zheng, J. Xu, J. Li, F. Wang, Y. Wang, X. Dong and Y. Xia, Nat. Commun., 2024, 15, 3217 CrossRef CAS PubMed.
- Y. Lin, J. Shang, Y. Liu, Z. Wang, Z. Bai, X. Ou and Y. Tang, Adv. Mater., 2024, 36, 2402702 CrossRef CAS PubMed.
- H. Guo, L. Shi, M. Yang, J. Wang, R. Yang, S. Qu and T. Lu, Smart Mater. Struct., 2019, 28, 024003 CrossRef CAS.
- F. Ahmadpour, Diversey Inc., US20210061659A1, 2017.
- A. Kupareva, P. Mäki-Arvela, H. Grénman, K. Eränen and D. Yu. Murzin, J. Chem. Technol. Biotechnol., 2015, 90, 34–43 CrossRef CAS.
- M. Okamoto, Catal. Lett., 2003, 88, 115–118 CrossRef CAS.
- M. Okamoto, K. Miyazaki, A. Kado and E. Suzuki, Chem. Commun., 2001, 1838–1839 RSC.
- M. Okamoto, S. Suzuki and E. Suzuki, Appl. Catal., A, 2004, 261, 239–245 CrossRef CAS.
- F. M. Weber, I. Kohlhaas and E. Figgemeier, Molecules, 2022, 27, 1737 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |