Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ambient and green processing of lead-free double perovskite Cs2AgBiBr6 films†
Qian
Chen
 *a,
Abhinav K.
Singh
*a,
Abhinav K.
Singh
 ab,
Hissah
Alghathami
ab,
Hissah
Alghathami
 ab,
Xuzhao
Liu
cd,
Kun
Huang
e,
Andrew G.
Thomas
cd,
Richard J.
Curry
ab,
Xuzhao
Liu
cd,
Kun
Huang
e,
Andrew G.
Thomas
cd,
Richard J.
Curry
 df,
Laurie J.
Phillips
df,
Laurie J.
Phillips
 b and
Amanda J.
Hughes
b and
Amanda J.
Hughes
 *a
*a
aDepartment of Materials, Design & Manufacturing Engineering, School of Engineering, University of Liverpool, UK. E-mail: qian.chen@liverpool.ac.uk; amanda.hughes2@liverpool.ac.uk
bStephenson Institute for Renewable Energy, Department of Physics, University of Liverpool, Liverpool, L69 7ZF, UK
cDepartment of Materials, School of Natural Science, The University of Manchester, Oxford Road, Manchester M13 9PL, UK
dDepartment of Electrical and Electronic Engineering, Photon Science Institute, University of Manchester, Manchester, M13 9PL, UK
eNational Graphene Institute & Department of Chemical Engineering, University of Manchester, Manchester, M13 9PL, UK
fDepartment of Electrical and Electronic Engineering, University of Manchester, Manchester M13 9PL, UK
First published on 29th April 2025
Abstract
Lead-free inorganic perovskite Cs2AgBiBr6 has emerged as a promising alternative to lead-based halide perovskites (LHPs), addressing concerns over toxicity and stability. However, fabricating high-quality Cs2AgBiBr6 films remains significantly more challenging than LHPs. Here, we investigate the impact of processing environments on film morphology, comparing deposition in ambient air and under a nitrogen environment. We find that moisture levels play a critical role in determining Cs2AgBiBr6 film quality, with significant differences observed between the controlled glovebox environment (∼1 ppm moisture) and ambient air with a relative humidity of 60–70%. Pristine films processed in ambient air show large aggregates and poor surface coverage, whereas dense and uniform films are achieved in a nitrogen glovebox. However, achieving cost-effective and scalable manufacturing requires ambient processing methods. To overcome this challenge, we introduce an ethyl acetate (EA) antisolvent treatment that enables the ambient deposition of dense and uniform Cs2AgBiBr6 films, even at a high relative humidity (60–70%). This approach enhances film morphology, crystallinity, and device performance, leading to a five-fold improvement in power conversion efficiency (PCE). EA-treated films achieving a PCE of 1.08%, compared to 0.21% for pristine films are reported. Recognizing the importance of solvent toxicity in ambient and scalable production, we assess the environmental impact using the CHEM21 framework, finding both the precursor solvent and antisolvent in this work to be environmentally favorable. Our findings offer a green, simple, and ambient fabrication strategy for high-quality, lead-free Cs2AgBiBr6 films, advancing their potential for future optoelectronic applications such as solar cells, photodetectors, and memristors.
Green foundation1. This work introduces a green and cost-effective method for processing high-quality lead-free perovskite Cs2AgBiBr6 films under ambient conditions, reducing dependence on harmful solvents and energy-intensive nitrogen environment.2. Using the CHEM21 framework, we confirm the environmental favorability of ethyl acetate (EA) as the antisolvent and dimethyl sulfoxide (DMSO) as the precursor solvent used in this work. The EA antisolvent treatment enables the formation of dense and uniform Cs2AgBiBr6 films even under a high relative humidity condition (60–70%), resulting in a fivefold improvement in device power conversion efficiency (PCE)—from 0.21% for pristine films to 1.08% for EA-treated films. 3. Further research will focus on adapting this method for scalable manufacturing, such as antisolvent bathing to enhance scalability, sustainability, and industrial viability. |
1. Introduction
Lead-based halide perovskites (LHPs) have attracted significant attention over the past decade due to their outstanding intrinsic properties, including adjustable bandgap, high absorption coefficient, long charge-carrier lifetime, and strong defect tolerance.1,2 These remarkable optoelectronic characteristics have positioned LHP as a promising material for diverse applications such as solar cells, photodetectors, X-ray detectors, photocatalysis, solar concentrators, and light-emitting diodes.3,4 Notably, LHP solar cells have achieved a record power conversion efficiency (PCE) of 26.7%.5 Despite their potential, the toxicity of water-soluble lead compounds in LHP raises serious concerns for human health and the environment.6 Research suggests that lead leakage from LHP solar cells poses a significant environmental hazard, as research shows that lead from LHP solar cells is more easily absorbed by plants compared to other sources of lead contamination, ultimately entering the food chain.7 If encapsulation fails, rainwater can accelerate lead leaching, threatening both human health and ecosystems. Furthermore, studies indicate that end-of-life LHP solar cells should be treated as hazardous waste rather than disposed of in landfills, which substantially increases life-cycle costs.8Additionally, conventional LHP fabrication relies on toxic or hazardous solvents such as N,N-dimethylformamide (DMF), N-methylpyrrolidone (NMP), chlorobenzene (CB), toluene, and diethyl either further increasing potential health and environmental concerns.9 Given these risks, raising awareness of these challenges while actively pursuing safer alternatives is crucial. The development of lead-free perovskites with green processing methods is essential for ensuring both environmental sustainability and human safety. Furthermore, LHPs are susceptible to moisture- and oxygen-induced degradation, particularly under photoexcitation and high-temperature conditions, presenting significant barriers to their commercialization.2 Tin (Sn) has been considered a promising alternative candidate for replacing lead in forming non-toxic perovskite materials. Nevertheless, the ease of oxidation of Sn2+ to Sn4+ presents a significant challenge to making ambient stable lead-free perovskite for practical applications.10
The lead-free double perovskite cesium silver bismuth bromide (Cs2AgBiBr6) has gained significant attention due to its favorable optoelectronic properties and crystal structure, closely resembling those of LHPs.11 More importantly, Cs2AgBiBr6 exhibits excellent ambient stability and negligible toxicity,12 making it an attractive candidate for various applications.13 It has been extensively studied for use in solar cells, photodetectors, memristors, X-ray detectors, photocatalysis, and gas and humidity sensors.14 In perovskite solar cells (PSCs), hydrogenated Cs2AgBiBr6-based light absorbers have achieved a record power conversion efficiency (PCE) of 6.37%.11 In photodetectors, Cs2AgBiBr6 has demonstrated a high responsivity (R) of 54.6 A W−1 and a specific detection rate (D*) of 7.4 × 1014 Jones.13 Additionally, flexible and durable memristors using Cs2AgBiBr6 thin films have been successfully fabricated, further demonstrating their potential for future optoelectronic applications.15
A key advantage of perovskite technology is its solution processability, enabling cost-effective and scalable coating or printing techniques for manufacturing. However, forming high-quality solution-processed Cs2AgBiBr6 films presents more significant challenges than LHPs.16 It has been widely reported that solution-processed Cs2AgBiBr6via the spin-coating process leads to poor film morphology with large agglomerates and excessive pinholes due to the rapid crystallization and high annealing temperature.17–19 Therefore, it is essential to achieve high-quality Cs2AgBiBr6 films with dense morphology, smooth surfaces, large grain sizes, and minimal pinholes to optimize optoelectronic device performance.16
Antisolvent treatment is an effective strategy for regulating perovskite crystallization to achieve optimal film morphology. Zhao et al. used CB as an antisolvent during a one-step spin-coating process in a glovebox, resulting in the formation of dense and uniform Cs2AgBiBr6 films with a PCE of 1.33%.20 Pantaler et al. also utilized CB as an antisolvent and achieved a PCE of 1.26% for PSCs.21 Zeng et al. used methyl acetate (MA) as the antisolvent to obtain the high-quality Cs2AgBiBr6 film in the glovebox for memristors.15 Gao et al. used isopropanol (IPA) as the antisolvent and achieved a PCE of 2.2% in their PSCs.22 The antisolvent treatment can also be developed into a scalable bathing process that is compatible with the up-scaling printing process for industrial-scale and roll-to-roll manufacturing.23,24
Recently, Abdelsamie et al. employed in situ spectroscopy to investigate the formation of Cs2AgBiBr6 films using IPA as the antisolvent in the glovebox.25 Their findings confirmed the critical role of antisolvents in improving film morphology. Additionally, they performed data mining across 56 publications to summarize the recent recipes for preparing Cs2AgBiBr6. Among these, CB is identified as the most commonly used antisolvent, appearing in 39% of cases (17% of the total studies with or without antisolvents), followed by IPA (10%), ethanol (7%), and toluene (5%). Furthermore, they noted that 88% of the studies on Cs2AgBiBr6 films were conducted in controlled environments such as gloveboxes or in conditions not specified, with only 12% in ambient conditions. The impact of different processing conditions (e.g. in ambient air or a glovebox) on the film morphology of Cs2AgBiBr6 still remains unclear. Most high-quality Cs2AgBiBr6 films for high-performance devices have been fabricated under controlled conditions within a glovebox.20,25,26
Nevertheless, developing ambient fabrication processes for high-quality Cs2AgBiBr6 films is crucial to reducing manufacturing costs and enabling industrial-scale printing and roll-to-roll manufacturing. For such an ambient process with antisolvent treatment, the careful selection of antisolvents and solvents is vital, not only to ensure film quality but also to mitigate health risks and align with environmental sustainability standards. Antisolvents such as chlorobenzene (CB) or toluene, used for Cs2AgBiBr6 film fabrication under ambient conditions, pose potential health and environmental risks due to their low threshold limit values—the maximum airborne concentration permissible during an 8-hour workday without adverse health effects.27,28 Green solvent and antisolvent strategies, guided by the CHEM21 framework, have been extensively studied for LHPs.9 However, the research of green solvent engineering to Cs2AgBiBr6 films remains in its early stages, particularly for ambient processing. Advancing research in this area is essential to support the sustainable and scalable production of Cs2AgBiBr6-based devices.
In this study, we demonstrate an ambient fabrication process for producing high-quality Cs2AgBiBr6 films with dense and smooth morphology, even under relative humidity (RH) conditions of 60–70%. This process employs ethyl acetate (EA) as the antisolvent in a one-step deposition method. EA antisolvent treatment significantly improves the surface coverage, film morphology, and crystallinity of Cs2AgBiBr6 films, resulting in a high-quality film with minimal pinholes and defects compared to pristine films. Given that our method operates under ambient conditions, we also evaluated the toxicity of the antisolvent (EA) and solvent (dimethyl sulfoxide, DMSO) using the CHEM21 framework and compared it with recent studies. Both EA and DMSO are categorized as “recommended” solvents. Consequently, ambient-processed PSCs based on EA-treated films exhibit a five-fold increase in PCE compared to devices fabricated without EA treatment. This simple and glovebox-free processing strategy enables the fabrication of high-quality Cs2AgBiBr6 films under ambient air conditions. It broadens accessibility and supports the development of lead-free perovskite materials for various future optoelectronic applications such as solar cells, photodetectors, and memristors.
2. Results and discussion
As illustrated in Fig. 1a, we utilized a one-step spin coating process to deposit the Cs2AgBiBr6 film entirely in ambient air under an RH of 60–70% or in a glovebox under a low moisture level (∼1 ppm) nitrogen atmosphere. The perovskite precursor solution was prepared by directly dissolving CsBr, BiBr3, and AgBr salts in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the dimethyl sulfoxide (DMSO). After magnetic stirring the precursor solution at 70 for 1 hour, a homogeneous and clear precursor solution was obtained, as shown in Fig. S1 in ESI.† The fluorine-doped tin oxide (FTO) coated glass or indium tin oxide (ITO) substrates were preheated to 70 °C before the deposition of the precursor solution. Previous studies suggested that a preheating treatment can enhance the quality and surface coverage of the Cs2AgBiBr6 films.17 The pristine Cs2AgBiBr6 film was prepared by directly spin-coating the precursor solution onto FTO- or ITO-glass substrates, followed by annealing at 280 °C for 5 minutes to complete the fabrication process. For the EA-treated film, an antisolvent technique was applied, as illustrated in Fig. 1b. In this method, EA was deposited during the spin-coating of the precursor solution, with subsequent procedures identical to those used for the pristine film. Detailed fabrication processes are provided in ESI.†
1 in the dimethyl sulfoxide (DMSO). After magnetic stirring the precursor solution at 70 for 1 hour, a homogeneous and clear precursor solution was obtained, as shown in Fig. S1 in ESI.† The fluorine-doped tin oxide (FTO) coated glass or indium tin oxide (ITO) substrates were preheated to 70 °C before the deposition of the precursor solution. Previous studies suggested that a preheating treatment can enhance the quality and surface coverage of the Cs2AgBiBr6 films.17 The pristine Cs2AgBiBr6 film was prepared by directly spin-coating the precursor solution onto FTO- or ITO-glass substrates, followed by annealing at 280 °C for 5 minutes to complete the fabrication process. For the EA-treated film, an antisolvent technique was applied, as illustrated in Fig. 1b. In this method, EA was deposited during the spin-coating of the precursor solution, with subsequent procedures identical to those used for the pristine film. Detailed fabrication processes are provided in ESI.†
Fig. 1c displays actual images of the glovebox-processed and ambient-processed pristine and EA-treated Cs2AgBiBr6 films deposited on the FTO-glass substrates. The glovebox-processed pristine film demonstrated good film coverage with a uniform appearance. In contrast, the ambient-processed pristine film showed poor coverage, with visible holes and an island-like morphology. On the other hand, both the glovebox-processed and ambient-processed EA-treated films showed a smooth and yellow surface with excellent film coverage on both FTO- and ITO-glass, as shown in Fig. 1c and S2 (ESI†). These results reveal the critical role of processing conditions and EA treatment in determining the surface coverage and quality of the Cs2AgBiBr6 films.
We then use field emission scanning electron microscopy (FEG-SEM) to further study the morphology of the pristine and EA-treated Cs2AgBiBr6 films prepared in both glove box and ambient air. As shown in Fig. 2a, the glovebox-processed pristine film showed a dense and smooth morphology. In contrast, the ambient-processed pristine films showed large agglomerates with a size of several μm that are loosely distributed on the substrate, as shown in Fig. 2b and c. Previous studies have attributed the formation of these large agglomerates to the rapid crystallization process induced by the high solvent evaporation rate during preheating treatment and the relatively high annealing temperature for obtaining the pure phase of Cs2AgBiBr6.17,19 However, our results suggest that variations in moisture levels resulting from glove box (∼1 ppm) and ambient air (RH of 60–70%) also play a significant role in influencing the quality of Cs2AgBiBr6 films.
Previous studies on LHP films have shown that the solvent evaporation rate during the spin-coating process plays a crucial role in determining film quality.29 A fast evaporation rate promotes rapid supersaturation of the perovskite precursor solution, leading to accelerated nucleation and a higher density of nucleation sites. This results in improved film coverage and more uniform morphology.30,31 In our study, DMSO was used as the precursor solvent for Cs2AgBiBr6. When processed inside a nitrogen-filled glovebox with low humidity (approximately 1 ppm moisture), DMSO evaporated relatively quickly. This facilitated rapid supersaturation and nucleation, yielding a film with good coverage and uniformity, as shown in Fig. 2a. In contrast, under ambient conditions, DMSO tends to absorb moisture from the surrounding air due to its hygroscopic nature. The absorbed moisture slows down the evaporation rate of the solvent, which delays supersaturation and reduces the nucleation rate. According to the perovskite nucleation mechanism, this leads to a lower density of nucleation sites and a larger critical nucleus radius.29 Upon annealing, these factors contribute to the growth of fewer but larger crystalline domains, resulting in an island-like film morphology with large particle features, as presented in Fig. 2b and c.
The glovebox-processed EA-treated film also showed a dense and smooth morphology, as shown in Fig. 2d. However, in contrast to the ambient-processed pristine film, the ambient-processed EA-treated film exhibited a dense and smooth morphology across various magnifications, as shown in Fig. 2e and f. As EA and DMSO are miscible, EA can effectively extract DMSO from the Cs2AgBiBr6 precursor solution during spin-coating. This rapid removal of the DMSO accelerates solvent evaporation, leading to quick supersaturation of the precursor. As a result, nucleation occurs rapidly with a high density of nucleation sites, which improves the uniformity and coverage of the resulting film. More importantly, EA possesses a significantly higher moisture absorption capacity compared to commonly used antisolvents such as toluene, CB, and diethyl ether.32,33 During spin-coating in ambient conditions, EA can absorb moisture both from the precursor solution and the surrounding air. This ability helps regulate the local microenvironment at the film surface in two ways: (1) it accelerates solvent evaporation by drawing moisture away from the ambient deposited precursor solution, enhancing supersaturation and promoting fast nucleation, and (2) it reduces the direct interaction between water molecules from ambient air and the forming perovskite nuclei during the spin-coating process, which otherwise could disrupt the crystallization process. Together, these effects contribute to a more controlled and uniform crystallization pathway. This ultimately results in the smooth and compact film morphology observed in Fig. 2e and f.
To investigate the film composition and elemental distribution of the ambient-processed pristine and EA-treated Cs2AgBiBr6 films, we performed SEM energy-dispersive X-ray (EDX) elemental mapping analysis on the film surfaces. As shown in Fig. 3a and S3 (ESI†), the ambient-processed pristine film showed large Cs2AgBiBr6 aggregates that were loosely distributed across the substrate. Elemental mapping (highlighted in pink) for indium (In) in Fig. 3b revealed large areas of uncoated ITO substrate. The EDX spectra in Fig. 3c further confirmed the poor surface coverage of the pristine film, as evidenced by strong In peaks (circled in red). This poor coverage is likely detrimental to device performance, as it can lead to severe charge recombination resulting from direct contact between different functional layers. In contrast, the EA-treated films demonstrated a uniform distribution of Cs, Ag, Bi, and Br across the film surface, as shown in Fig. 3d, e and Fig. S3 (ESI†), with minimal exposure of In, as confirmed by the EDX spectra in Fig. 3f. The compact and uniform morphology of these films promotes efficient light absorption while suppressing charge recombination, thereby significantly enhancing the performance of devices such as PSCs and photodetectors.34,35
To investigate the film thickness, cross-sectional morphology, and composition of the ambient-processed pristine and EA-treated Cs2AgBiBr6 films, we performed cross-sectional SEM and EDX analyses. As shown in Fig. 4a–c, the ambient-processed pristine film exhibited isolated large aggregates with a maximum height reaching up to 710 nm, consistent with the surface SEM observations. In contrast, the ambient-processed EA-treated film displayed a relatively uniform and smooth morphology over a large area, as shown in Fig. 4d–g. This uniform cross-sectional structure is critical for light absorbers in PSCs, as it reduces the charge recombination sites and thereby enhances photovoltaic performance.36,37 The average thickness of the ambient-processed EA-treated film was approximately 210 nm.
To investigate the crystallinity of the ambient-processed pristine and EA-treated Cs2AgBiBr6 films, we performed XRD analysis, as shown in Fig. 5. Lebail refinement of the XRD patterns was conducted using the FullProf Suite to fit the data to a simulated profile. The XRD patterns for both sample types were indexed to the cubic perovskite phase of Cs2AgBiBr6, with a space group of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and a unit cell parameter of 11.28 Å, in agreement with previous studies.38,39 The EA-treated film exhibited significantly stronger relative intensities for the XRD peaks corresponding to Cs2AgBiBr6 compared to the pristine film, while maintaining similar full width at half maximum (FWHM) values, as summarized in Table S1 (ESI†). This indicates improved crystallinity of the antisolvent-treated Cs2AgBiBr6 films relative to the pristine samples, consistent with the SEM results. A minor impurity phase of Cs3Bi2Br9 was detected in both the pristine and EA-treated film, possibly due to annealing the Cs2AgBiBr6 films in ambient air above 250 °C.40
m and a unit cell parameter of 11.28 Å, in agreement with previous studies.38,39 The EA-treated film exhibited significantly stronger relative intensities for the XRD peaks corresponding to Cs2AgBiBr6 compared to the pristine film, while maintaining similar full width at half maximum (FWHM) values, as summarized in Table S1 (ESI†). This indicates improved crystallinity of the antisolvent-treated Cs2AgBiBr6 films relative to the pristine samples, consistent with the SEM results. A minor impurity phase of Cs3Bi2Br9 was detected in both the pristine and EA-treated film, possibly due to annealing the Cs2AgBiBr6 films in ambient air above 250 °C.40
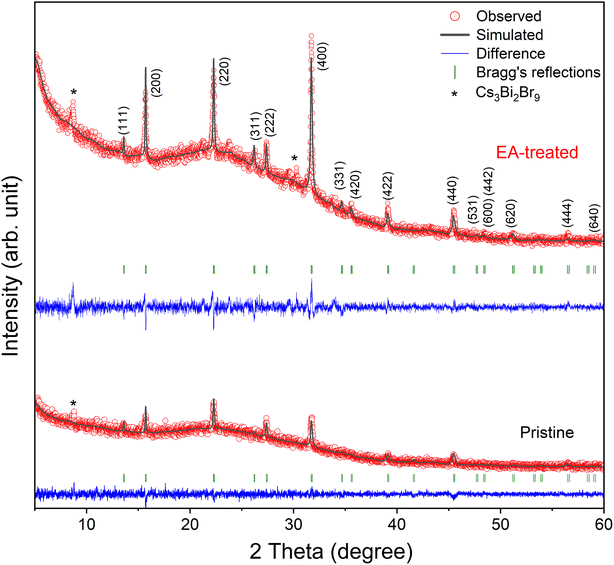 | ||
| Fig. 5 XRD patterns for the ambient-processed EA-treated (top) and pristine (bottom) Cs2AgBiBr6 films. | ||
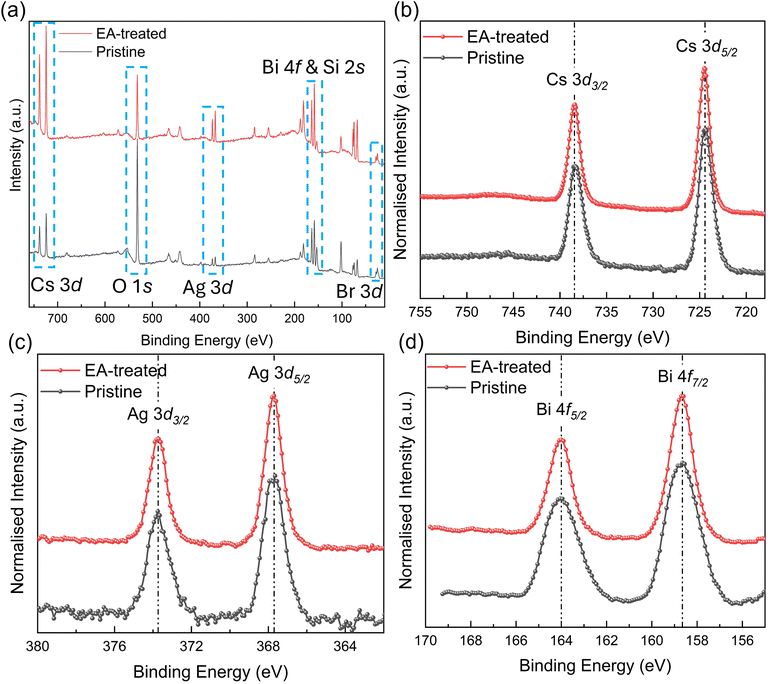
X-ray photoelectron spectroscopy (XPS) is a powerful tool for analyzing the surface chemistry of thin-film materials.41 We conducted the XPS analysis to probe the surface chemistry of the ambient-processed pristine and EA-treated Cs2AgBiBr6 films. As shown in Fig. 6a, the XPS survey spectra confirm the presence of Cs, Ag, Bi, Br, silicon (Si), and oxygen (O) elements. The atomic concentrations of these elements are summarized in Fig. S4.† Notably, the relative atomic concentrations of Si and O were significantly higher in the ambient-processed pristine film (41.7 at% and 44.9 at%, respectively) compared to the EA-treated film (23.57 at% and 35.3 at%, respectively). This elevated Si and O content in the pristine film is attributed to its poor surface coverage, which leaves substantial portions of the underlying quartz glass substrate exposed. In contrast, the EA-treated film exhibits enhanced surface coverage, effectively reducing substrate exposure and lowering oxygen content, consistent with SEM and EDX findings. High-resolution XPS spectra for Cs 3d, Ag 3d, Bi 4f, and Br 3d are presented in Fig. 6b–d and Fig. S5 (ESI†). The peak positions corresponding to Cs 3d3/2, Cs 3d5/2, Ag 3d3/2, Ag 3d5/2, Bi 4f5/2, Bi 4f7/2, Br 3d3/2 and Br 3d5/2 in both ambient-processed pristine and EA-treated films are summarized in Table S2 (ESI†), showing good agreement with previous studies.42,43 No significant peak shifts were observed between the ambient-processed pristine and EA-treated Cs2AgBiBr6 films.
To examine the optical properties of the ambient-processed pristine and EA-treated Cs2AgBiBr6 films, we performed the ultraviolet-visible-near-infrared (UV-vis-NIR) spectroscopy. As shown in Fig. 7a, the EA-treated film exhibits an absorption peak at 438 nm and an absorption edge around 600 nm, consistent with previous studies on high-quality Cs2AgBiBr6 films.18 In contrast, the pristine film demonstrates significantly lower absorption. This is attributed to the poor surface coverage with large uncovered areas, as evident from Fig. 1c and Fig. S2 (ESI†). Given that Cs2AgBiBr6 is considered an indirect semiconductor, its Tauc plot was constructed using the equation:
| (ahv)1/2 = A (hv − Eg) | (1) |
To further explore the optical properties, steady-state photoluminescence (PL) spectroscopy was conducted as shown in Fig. 7b. Since both the pristine and EA-treated films were deposited onto quartz glass for PL measurements, the significantly enhanced PL intensity observed in the EA-treated films suggests a notable improvement in spontaneous radiative recombination with a higher generation of free charge carriers and suppressed non-radiative recombination. We attribute this enhancement to three main factors. First, the EA-treated film exhibits improved light absorption, as confirmed by UV-vis-NIR results. This is due to their enhanced surface coverage and film morphology compared to pristine film, as evidenced by SEM surface analysis. Second, unlike the pristine films, which contain large aggregates, the EA-treated films are more compact and uniform, reducing the density of pinholes and grain boundaries that commonly serve as non-radiative recombination centres.45 SEM cross-sectional analysis also reveals a smoother interface between the EA-treated film and the substrate, further minimizing interface recombination losses.46 Third, XRD analysis exhibits enhanced crystallinity for EA-treated film, which facilitates charge carrier transport and suppresses defect-mediated recombination. We believe these combined effects lead to the significantly enhanced PL intensity observed in the EA-treated films.
To evaluate the photovoltaic performance of the ambient-processed pristine and EA-treated Cs2AgBiBr6 films, we assembled the PSCs with an architecture of ITO-glass/compact and mesoporous TiO2/Cs2AgBiBr6/spiro-OMeTAD/Au, as illustrated in Fig. 8a. Except for the thermal evaporation of the Au electrode, the entire device fabrication process was carried out in ambient air with an RH of 60–70%. Fig. 8b shows the current density–voltage (J–V) curves under AM1.5 sunlight and dark conditions for the champion cells based on the pristine and EA-treated films. The champion cell based on the EA-treated film achieved a champion PCE of 1.08%, representing a more than five-fold improvement compared to the pristine film-based PSCs, which achieved a champion PCE of 0.21%. The PCE for the PSCs based on the EA-treated Cs2AgBiBr6 films is comparable to several devices fabricated in inert glovebox environments with chlorobenzene or IPA as the antisolvents, as reported in recent literature.20,47 The distribution and summary of photovoltaic parameters measured across multiple cells for both pristine and EA-treated films are presented in Fig. 8a, b, S7 (ESI), and Table S3 (ESI).† The improved PCE of the PSCs based on the EA-treated films is primarily attributed to the significantly higher short-circuit current density (JSC) compared to the PSCs based on the pristine films. This enhancement arises from the dense and uniform morphology of the EA-treated film, which facilitates improved light absorption, as evidenced by UV-vis-NIR, PL, and SEM analyses. Furthermore, PSCs based on pristine films exhibited lower open-circuit voltages (VOC) than those based on EA-treated films. This is due to poor surface coverage and the presence of large aggregates in the pristine film, which lead to direct contact between the electron transport layer (TiO2) and the hole transport layer (spiro-OMeTAD), resulting in increased charge recombination. Additionally, PSCs fabricated with EA-treated films demonstrated relatively stable performance under varying scan rates and scan cycles, as shown in Fig. S8 (ESI†). The forward and reverse J–V scans for the EA-treated PSCs are presented in Fig. S9 (ESI†).
Sustainable and cost-effective approaches are essential for developing new materials processing methods while minimizing environmental impact.48,49 Beyond achieving a high-quality Cs2AgBiBr6 film, it is essential to consider the toxicity of the solvents and antisolvents used during fabrication, as well as the complexity of the fabrication process, particularly for ambient processing and industrial-scale production. Here, we summarize recent studies on the preparation of Cs2AgBiBr6 films using various methods, comparing them with our approach, as shown in Table 1. The CHEM21 solvent guide is a widely used tool for assessing the environmental sustainability of solvents and antisolvents used to fabricate perovskite films and devices.9,50,51 Herein, the toxicity and environmental impact of solvents and antisolvents employed in recent studies on the fabrication of Cs2AgBiBr6 were assessed using the CHEM21 framework, which scores solvents based on safety, health, and environmental (SHE) criteria. These scores consider key physical properties and hazard statements, as summarized in Table S4 (ESI†). Based on the combined SHE scores, solvents and antisolvents were categorized into three levels: recommended (R), problematic (P), and hazardous (H). In our work, DMSO was used as the precursor solvent and EA as the antisolvent, both of which have an overall rating of R. By contrast, some studies have employed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DMF and DMSO as the precursor solvent.20,52 However, DMF is classified as H due to its high health score of 9, due to its high risk to human health through inhalation or skin exposure.9 CB, another commonly used antisolvent, is categorized as P because of its high environmental score of 7, due to its ozone-depleting potential and water pollution risk.53 IPA is another widely used antisolvent for Cs2AgBiBr6 films with a rating of R. However, most studies utilizing IPA have been performed in glove boxes, leaving its suitability for ambient processing unclear. Notably, MA has recently been used as an antisolvent for preparing Cs2AgBiBr6 films in the glovebox. While MA shares a similar chemical structure with EA, previous studies on using MA as the antisolvent for LHPs also suggested that MA has a higher water solubility than EA for the ambient processing of LHPs.54 However, MA is categorized as P due to its higher environmental score and lower boiling point (57–58 °C) compared to EA (76.5–77.5 °C).55 A lower boiling point increases the possibility of harmful volatile organic compound emissions. The ideal boiling point range for antisolvents is typically between 70 and 139 °C.27
1 mixture of DMF and DMSO as the precursor solvent.20,52 However, DMF is classified as H due to its high health score of 9, due to its high risk to human health through inhalation or skin exposure.9 CB, another commonly used antisolvent, is categorized as P because of its high environmental score of 7, due to its ozone-depleting potential and water pollution risk.53 IPA is another widely used antisolvent for Cs2AgBiBr6 films with a rating of R. However, most studies utilizing IPA have been performed in glove boxes, leaving its suitability for ambient processing unclear. Notably, MA has recently been used as an antisolvent for preparing Cs2AgBiBr6 films in the glovebox. While MA shares a similar chemical structure with EA, previous studies on using MA as the antisolvent for LHPs also suggested that MA has a higher water solubility than EA for the ambient processing of LHPs.54 However, MA is categorized as P due to its higher environmental score and lower boiling point (57–58 °C) compared to EA (76.5–77.5 °C).55 A lower boiling point increases the possibility of harmful volatile organic compound emissions. The ideal boiling point range for antisolvents is typically between 70 and 139 °C.27
| Processing conditions | Precursor preparation | Solvent | Antisolvent | Film morphology | Ref. |
|---|---|---|---|---|---|
| Ambient RH ≈ 60–70% | Direct dissolution | DMSO (R) | EA (R) | Smooth | This work |
| Unspecified | Polycrystal powder | DMSO (R) | CB (P) | Smooth | 21 |
| Glovebox | Direct dissolution | DMF (H) | CB (P) | Smooth | 20 |
| DMSO (R) | |||||
| Glovebox | Direct dissolution | DMF (H) | CB (P) | Smooth | 52 |
| DMSO (R) | |||||
| Unspecified | Polycrystal powder | DMSO (R) | IPA (R) | Smooth | 22 |
| Glovebox | Direct dissolution | DMSO (R) | IPA (R) | Smooth | 11 |
| Glovebox | Direct dissolution | DMSO (R) | IPA (R) | Smooth | 47 |
| Glovebox | Direct dissolution | DMSO (R) | MA (P) | Smooth | 15 |
| Glovebox | Single crystal powder | DMSO (R) | — | Smooth | 56 |
| Ambient unspecified RH% | Direct dissolution | DMSO (R) | — | Aggregates | 17 |
| Ambient unspecified RH% | Direct dissolution | DMSO (R) | — | Aggregates | 57 |
| Ambient RH ≈ 50% | Polycrystal powder | DMSO (R) | — | Aggregates | 18 |
| Ambient RH ≈ 50% low-pressure annealing | Polycrystal powder | DMSO (R) | — | Smooth | 18 |
We also compared the precursor preparation methods and resulting film morphologies achieved using different approaches under various processing conditions. A number of existing studies on high-quality Cs2AgBiBr6 film fabrication require the prior synthesis of Cs2AgBiBr6 crystalline powders, which are then dissolved in a solvent to prepare the perovskite precursor. For example, Pantaler et al. synthesized Cs2AgBiBr6 crystalline powder by dissolving CsBr, AgBr, and BiBr3 salts in HBr at 150 °C for 2 hours, followed by cooling to room temperature.21 The resulting orange powder was then washed with ethanol and dried overnight, yielding 59.7%, and subsequently dissolved in DMSO for spin-coating the films, using CB as the antisolvent. These requirements increase manufacturing costs and lead times, making the process less sustainable and cost-effective for practical production. In contrast, our method employs a direct dissolution approach, eliminating the need for an intermediate crystallization step. By removing this process, our approach effectively reduces chemical and energy consumption as well as processing time. Moreover, by integrating a green antisolvent EA, our method offers a greener, more efficient, and sustainable approach for Cs2AgBiBr6 film fabrication. Additionally, as shown in Table 1, multiple studies on Cs2AgBiBr6 film fabrication report the formation of aggregates, highlighting the challenge of achieving uniform and compact films. The antisolvent technique we developed addresses this issue by enabling the production of high-quality Cs2AgBiBr6 films with smooth and dense morphologies under high relative humidity (RH) conditions of 60–70%. This reduces the dependence on expensive facilities such as glove boxes and humidity-controlled equipment, further reducing production costs and enhancing scalability.
3. Conclusion
In conclusion, we investigated the impact of processing conditions on the quality of Cs2AgBiBr6 films by comparing fabrication in ambient air and a nitrogen glovebox environment. This revealed the significant challenge of producing high-quality Cs2AgBiBr6 films under a high moisture level in ambient air. To overcome this challenge, we successfully demonstrated EA antisolvent treatment to fabricate dense and uniform Cs2AgBiBr6 films in ambient air, even under an RH of 60–70%. This approach led to a substantial improvement in film morphology and photovoltaic performance, with ambient-processed PSCs based on EA-treated films achieving a PCE of 1.08%, compared to 0.21% for pristine films. We believe this is due to the high water solubility of EA that can withdraw moisture from the precursor solution and facilitate supersaturation that promotes the formation of dense and uniform films. Additionally, we evaluated the environmental impact of the solvents and antisolvents used in this study through the CHEM21 framework. Both EA as the antisolvent and DMSO as the precursor solvent received a “recommended” rating, highlighting their suitability for green and sustainable fabrication of Cs2AgBiBr6 films. Our findings demonstrate a cost-effective, sustainable, and environmentally friendly strategy for producing high-quality, non-toxic double perovskite Cs2AgBiBr6 films, offering promising potential for future optoelectronic applications.Data availability
The data supporting this article have been included as part of the ESI.† Additional data are available from the authors upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the Engineering and Physical Sciences Research Council (EPSRC) funding under grant no. EP/X03660X/1 and EP/V008188/1. The authors would like to thank Dr Matthew Bilton from the SEM Shared Research Facility, University of Liverpool, for his help with SEM characterization.References
- J. Min, Y. Choi, D. Kim and T. Park, Adv. Energy Mater., 2024, 14, 2302659 CrossRef CAS.
- Y. Rong, Y. Hu, A. Mei, H. Tan, M. I. Saidaminov, S. Il Seok, M. D. McGehee, E. H. Sargent and H. Han, Science, 2018, 361, eaat8235 CrossRef PubMed.
- Y. Zhao and K. Zhu, Chem. Soc. Rev., 2016, 45, 655–689 RSC.
- Z. Li, A. Johnston, M. Wei, M. I. Saidaminov, J. M. de Pina, X. Zheng, J. Liu, Y. Liu, O. M. Bakr and E. H. Sargent, Joule, 2020, 4, 631–643 CrossRef CAS.
- NREL, Best Research-Cell Efficiences chart, https://www.nrel.gov/pv/cell-efficiency.html.
- M. Ren, X. Qian, Y. Chen, T. Wang and Y. Zhao, J. Hazard. Mater., 2022, 426, 127848 CrossRef CAS PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 310 CrossRef CAS PubMed.
- N. Moody, S. Sesena, D. W. deQuilettes, B. D. Dou, R. Swartwout, J. T. Buchman, A. Johnson, U. Eze, R. Brenes, M. Johnston, C. L. Haynes, V. Bulović and M. G. Bawendi, Joule, 2020, 4, 970–974 CrossRef.
- H. J. Kim, Y. J. Kim, G. S. Han and H. S. Jung, Sol. RRL, 2024, 8, 2300910 CrossRef CAS.
- L. H. Hernandez, L. Lanzetta, S. Jang, J. Troughton, M. A. Haque and D. Baran, ACS Energy Lett., 2023, 8, 259–273 CrossRef.
- Z. Zhang, Q. Sun, Y. Lu, F. Lu, X. Mu, S.-H. Wei and M. Sui, Nat. Commun., 2022, 13, 3397 CrossRef CAS PubMed.
- M. Zhai, C. Chen and M. Cheng, Sol. Energy, 2023, 253, 563–583 CrossRef CAS.
- H. Lei, D. Hardy and F. Gao, Adv. Funct. Mater., 2021, 31, 2105898 CrossRef CAS.
- S. C. Yadav, A. Srivastava, V. Manjunath, A. Kanwade, R. S. Devan and P. M. Shirage, Mater. Today Phys., 2022, 26, 100731 CrossRef.
- F. Zeng, Y. Tan, W. Hu, X. Tang, H. Yin, T. Jing, L. Huang, Y. Yang, J. Liao and C. Zhou, Appl. Phys. Lett., 2024, 124, 162101 CrossRef CAS.
- J. Huang, H. Xiang, R. Ran, W. Zhou, W. Wang and Z. Shao, Renewable Sustainable Energy Rev., 2024, 191, 114187 CrossRef CAS.
- E. Greul, M. L. Petrus, A. Binek, P. Docampo and T. Bein, J. Mater. Chem. A, 2017, 5, 19972–19981 RSC.
- C. Wu, Q. Zhang, Y. Liu, W. Luo, X. Guo, Z. Huang, H. Ting, W. Sun, X. Zhong, S. Wei, S. Wang, Z. Chen and L. Xiao, Adv. Sci., 2018, 5, 1700759 CrossRef PubMed.
- M. S. Shadabroo, H. Abdizadeh and M. R. Golobostanfard, ACS Appl. Energy Mater., 2021, 4, 6797–6805 CrossRef CAS.
- D. Zhao, C. Liang, B. Wang, T. Liu, Q. Wei, K. Wang, H. Gu, S. Wang, S. Mei and G. Xing, Energy Environ. Mater., 2022, 5, 1317–1322 CrossRef CAS.
- M. Pantaler, K. T. Cho, V. I. E. Queloz, I. García-Benito, C. Fettkenhauer, I. Anusca, M. K. Nazeeruddin, D. C. Lupascu and G. Grancini, ACS Energy Lett., 2018, 3, 1781–1786 CrossRef CAS.
- W. Gao, C. Ran, J. Xi, B. Jiao, W. Zhang, M. Wu, X. Hou and Z. Wu, ChemPhysChem, 2018, 19, 1696–1700 CrossRef CAS PubMed.
- D. S. Ham, W. J. Choi, H. Yun, M. Kim, D.-H. Yeo, S. Lee, B. J. Kim and J. H. Lee, ACS Appl. Energy Mater., 2021, 4, 7611–7621 CrossRef CAS.
- Y. Y. Kim, T.-Y. Yang, R. Suhonen, A. Kemppainen, K. Hwang, N. J. Jeon and J. Seo, Nat. Commun., 2020, 11, 5146 CrossRef CAS PubMed.
- M. Abdelsamie, K. Cruse, N. Tamura, G. Ceder and C. M. Sutter-Fella, J. Mater. Chem. A, 2022, 10, 19868–19880 RSC.
- T. Luo, Y. Zhang, X. Chang, J. Fang, T. Niu, J. Lu, Y. Fan, Z. Ding, K. Zhao and S. (Frank) Liu, J. Energy Chem., 2021, 53, 372–378 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- Q. Chen, J. C. R. Ke, D. Wang, M. Z. Mokhtar, A. G. Thomas and Z. Liu, Appl. Surf. Sci., 2021, 536, 147949 CrossRef CAS.
- H. Gao, C. Bao, F. Li, T. Yu, J. Yang, W. Zhu, X. Zhou, G. Fu and Z. Zou, ACS Appl. Mater. Interfaces, 2015, 7, 9110–9117 CrossRef CAS PubMed.
- S. Sánchez, L. Pfeifer, N. Vlachopoulos and A. Hagfeldt, Chem. Soc. Rev., 2021, 50, 7108–7131 RSC.
- G. Wang, D. Liu, J. Xiang, D. Zhou, K. Alameh, B. Ding and Q. Song, RSC Adv., 2016, 6, 43299–43303 RSC.
- J. Troughton, K. Hooper and T. M. Watson, Nano Energy, 2017, 39, 60–68 CrossRef CAS.
- Q. Chen, M. Z. Mokhtar, J. C.-R. Ke, A. G. Thomas, A. Hadi, E. Whittaker, M. Curioni and Z. Liu, Sustainable Energy Fuels, 2018, 2, 1216–1224 RSC.
- D. Shen, X. Yu, X. Cai, M. Peng, Y. Ma, X. Su, L. Xiao and D. Zou, J. Mater. Chem. A, 2014, 2, 20454–20461 RSC.
- W. Shen, U. Jung, Z. Xian, B. Jung and J. Park, J. Alloys Compd., 2022, 929, 167329 CrossRef CAS.
- P. Jia, L. Qin, D. Zhao, Y. Tang, B. Song, J. Guo, X. Li, L. Li, Q. Cui, Y. Hu, Z. Lou, F. Teng and Y. Hou, Adv. Funct. Mater., 2021, 31, 2107125 CrossRef CAS.
- S. Takahashi, S. Uchida, P. V. V. Jayaweera, S. Kaneko and H. Segawa, Sci. Rep., 2023, 13, 16068 CrossRef CAS PubMed.
- N. K. Tailor, N. Parikh, P. Yadav and S. Satapathi, J. Phys. Chem. C, 2022, 126, 10199–10208 CrossRef CAS.
- W. Yuan, G. Niu, Y. Xian, H. Wu, H. Wang, H. Yin, P. Liu, W. Li and J. Fan, Adv. Funct. Mater., 2019, 29, 1900234 CrossRef.
- P. Pistor, M. Meyns, M. Guc, H.-C. Wang, M. A. L. Marques, X. Alcobé, A. Cabot and V. Izquierdo-Roca, Scr. Mater., 2020, 184, 24–29 CrossRef CAS.
- X. Wu, Q. Yan, H. Wang, D. Wu, H. Zhou, H. Li, S. Yang, T. Ma and H. Zhang, Adv. Funct. Mater., 2024, 34, 2404535 CrossRef CAS.
- G. Yan, B. Jiang, Y. Yuan, M. Kuang, X. Liu, Z. Zeng, C. Zhao, J.-H. He and W. Mai, ACS Appl. Mater. Interfaces, 2020, 12, 6064–6073 CrossRef CAS PubMed.
- F. Igbari, R. Wang, Z.-K. Wang, X.-J. Ma, Q. Wang, K.-L. Wang, Y. Zhang, L.-S. Liao and Y. Yang, Nano Lett., 2019, 19, 2066–2073 CrossRef CAS PubMed.
- Z. Li, S. R. Kavanagh, M. Napari, R. G. Palgrave, M. Abdi-Jalebi, Z. Andaji-Garmaroudi, D. W. Davies, M. Laitinen, J. Julin, M. A. Isaacs, R. H. Friend, D. O. Scanlon, A. Walsh and R. L. Z. Hoye, J. Mater. Chem. A, 2020, 8, 21780–21788 RSC.
- J. Tao, C. Zhao, Z. Wang, Y. Chen, L. Zang, G. Yang, Y. Bai and J. Chu, Energy Environ. Sci., 2025, 18, 509–544 RSC.
- C. M. Wolff, P. Caprioglio, M. Stolterfoht and D. Neher, Adv. Mater., 2019, 31, 1902762 CrossRef CAS PubMed.
- M. T. Sirtl, M. Armer, L. K. Reb, R. Hooijer, P. Dörflinger, M. A. Scheel, K. Tvingstedt, P. Rieder, N. Glück, P. Pandit, S. V. Roth, P. Müller-Buschbaum, V. Dyakonov and T. Bein, ACS Appl. Energy Mater., 2020, 3, 11597–11609 CrossRef CAS.
- Y. Zhou, Y. Zhang, Y. Nie, D. Sun, D. Wu, L. Ban, H. Zhang, S. Yang, J. Chen, H. Du and X. Pan, Prog. Mater. Sci., 2025, 152, 101460 CrossRef CAS.
- Y. Nie, Y. Zhou, Y. Zhang, D. Sun, D. Wu, L. Ban, S. Nanda, C. Xu and H. Zhang, Adv. Funct. Mater., 2025, 2418957, DOI:10.1002/adfm.202418957.
- C. Duan, H. Gao, K. Xiao, V. Yeddu, B. Wang, R. Lin, H. Sun, P. Wu, Y. Ahmed, A. D. Bui, X. Zheng, Y. Wang, J. Wen, Y. Wang, W. Ou, C. Liu, Y. Zhang, H. Nguyen, H. Luo, L. Li, Y. Liu, X. Luo, M. I. Saidaminov and H. Tan, Nat. Energy, 2025, 10, 318–328 CrossRef CAS.
- F. Meng, L. Cheng, F. Wang, K. Chen, Z. Sun and G. Wang, Adv. Sustainable Syst., 2025, 9, 2400695 CrossRef CAS.
- D. Zhao, B. Wang, C. Liang, T. Liu, Q. Wei, S. Wang, K. Wang, Z. Zhang, X. Li, S. Peng and G. Xing, Sci. China Mater., 2020, 63, 1518–1525 CrossRef CAS.
- S. K. Podapangi, F. Jafarzadeh, S. Mattiello, T. B. Korukonda, A. Singh, L. Beverina and T. M. Brown, RSC Adv., 2023, 13, 18165–18206 RSC.
- F. Yang, G. Kapil, P. Zhang, Z. Hu, M. A. Kamarudin, T. Ma and S. Hayase, ACS Appl. Mater. Interfaces, 2018, 10, 16482–16489 CrossRef CAS PubMed.
- N. A. Mali, S. S. Yadav, P. D. Ghuge and S. S. Joshi, J. Chem. Eng. Data, 2017, 62, 4356–4363 CrossRef CAS.
- W. Ning, F. Wang, B. Wu, J. Lu, Z. Yan, X. Liu, Y. Tao, J.-M. Liu, W. Huang, M. Fahlman, L. Hultman, T. C. Sum and F. Gao, Adv. Mater., 2018, 30, 1706246 CrossRef PubMed.
- B. Kumaar Swamy Reddy, A. S. Kumar, R. Akash, E. Ramasamy, S. Badhulika, G. Veerappan and P. H. Borse, Sol. Energy, 2024, 283, 112989 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00722d |
| This journal is © The Royal Society of Chemistry 2025 |