Open Access Article
Open Access Article“On water” metal-free direct C–H amination and imination of olefins via tandem SNAr, click chemistry, and molecular nitrogen release†
Sudripet
Sharma
a,
Seyedesahar
Miraghaee
a and
Sachin
Handa
 *ab
*ab
aDepartment of Chemistry, 2320 S. Brook Street, University of Louisville, Louisville, Kentucky 40292, USA. E-mail: sachin.handa@missouri.edu
bDepartment of Chemistry, 601 S College Ave, University of Missouri, Columbia, MO 65211, USA
First published on 20th May 2025
Abstract
We report a metal-free, sustainable C–H amination method conducted in an aqueous environment. This method leverages a combination of tandem nucleophilic aromatic substitution, metal-free click reactions, and de-nitrogenation processes, all facilitated by the hydrophobic effect of water. Specifically, perfluo(hetero)aryl compounds undergo azidation, followed by a 1,3-cycloaddition with olefins and nitrogen evolution reactions on the hydrophobic surface of water, yielding the desired amine or imine products. The resulting structures hold significant potential in medicinal chemistry and agrochemicals. This approach obviates the need to isolate unstable and potentially explosive perfluoro-azides and circumvents the pre-functionalization of olefins. Utilizing water as a solvent replaces toxic organic solvents and mitigates safety concerns associated with handling azides. Our method exhibits high selectivity and efficacy across a broad spectrum of perfluoroarenes and olefins. Detailed mechanistic studies reveal the formation of a 1,2,3-triazoline adduct as a reactive intermediate, which subsequently converts into the final product via the elimination of nitrogen gas.
Green foundation1. It advances green chemistry by introducing a metal-free C–H amination and imination of olefins in water, eliminating toxic solvents and hazardous materials. By leveraging water's hydrophobic properties, the method enhances reaction rates and selectivity while simplifying product isolation. It also addresses safety concerns related to perfluoroazides, demonstrating high selectivity and efficiency for safer, more sustainable chemical synthesis. Currently, perfluoroaryls are not classified as PFAS, but there is ongoing debate about whether they may fall into this category in the future. Even if such a change occurs, the production of these compounds may remain necessary, as their benefits are deemed to outweigh the associated risks.2. A significant advancement in green chemistry is the creation of a metal-free synthetic domino process. This approach eliminates the need for isolated unstable azides, resulting in high yields of products that are valuable in medicinal chemistry and agrochemicals. Additionally, it reduces environmental impact and safety risks. 3. Future research could focus on further developing novel micelles derived from biodegradable surfactants, which can enable this chemistry at room temperature. |
Introduction
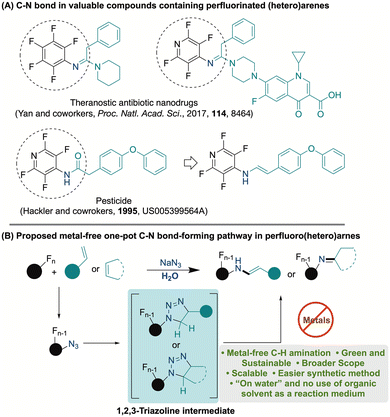
Compounds containing carbon–nitrogen (C–N) and carbon–fluorine (C–F) bonds are important in synthetic chemistry. These bonds are commonly found in various bioactive compounds, natural products, and active pharmaceutical ingredients.1–6 One effective method for accessing molecules containing C–F and C–N bonds involves incorporating C–N bonds into perfluoroarenes. This strategy facilitates the creation of intricate organic frameworks and also enables the precise positioning of fluorine atoms, which can significantly influence the physical and chemical properties of the resulting compounds.7–10 The presence of these fluorine atoms can serve as a strategic chemical handle, enabling additional transformations and modifications of the compound, thereby expanding the potential applications in medicinal chemistry and materials science.8,9,11–13a Furthermore, the compounds containing a perfluoro(hetero)aryl ring, directly attached to imine or amidic nitrogen, can be found in theranostic antibiotic nano-drugs and agrochemicals (Fig. 1A).13b,c Consequently, the reactions that form C–N bonds in perfluoro compounds hold significant importance in both the pharmaceutical and agrochemical fields.The conventional sustainable method for forming C–N bonds primarily relies on nucleophilic substitution reactions. In this process, strong nucleophilic amines engaged with an activated fluorinated site, ultimately produce the desired fluorinated amines.11,14–16 Less activated substrates necessitate the use of strong bases such as sodium hydride, butyllithium, or lithium bis(trimethylsilyl)amide, alongside often unstable amine sources in excess at elevated temperatures, posing major limitations.14–19 Furthermore, the sensitivity of the employed bases to air and moisture exacerbates the difficulties associated with this reaction landscape. An alternative strategy involves transition metal-catalyzed amination—this approach is associated with harsh conditions, the use of expensive transition metal complexes, strong bases, and often toxic organic solvents.20–22 This reliance on expensive materials raises concerns regarding the overall sustainability and practicality of the process. Moreover, achieving high selectivity and yield remains a significant hurdle, particularly when dealing with highly electron-deficient or strongly chelating amines. This complicates the reaction further and limits the method's applicability in synthetic chemistry.
Nitrene- or nitrenoid-mediated chemistry has emerged as a promising alternative for efficient C–H aminations of perfluoro arenes.23–25 A key aspect of this process involves the use of perfluoroaromatic azides, which undergo decomposition through either photolysis or thermolysis, and have been widely applied in nitrene chemistry.23,25 Notably, nitrenes are unique neutral monovalent species characterized by their six valence electrons, which endow them with a high degree of reactivity. However, this reactivity comes at the cost of selectivity, making it challenging to direct the reaction outcomes.9,23,24,26 To address this limitation, various transition metals such as copper (Cu), manganese (Mn), iridium (Ir), cobalt (Co), rhodium (Rh), and ruthenium (Ru) are often employed as stabilizing agents for the nitrene intermediates. These metals play a crucial role in enhancing both the reactivity and selectivity of the reactions, allowing for more controlled outcomes.9,23,24,27,28 Furthermore, these reactions require anhydrous organic solvents, as the presence of water can have detrimental effects, leading to unwanted side reactions and reduced yields.23,24 Thus, careful consideration of the reaction environment is pivotal for the successful application of nitrene-mediated C–H amination.
As an alternative strategy, we propose “on water” tandem nucleophilic azidation of arenes, followed by 1,3-cycloaddition of resulting azide with olefins, with subsequent release of nitrogen gas, which can facilitate C–H amination in a single-step manner (Fig. 1B). The hydrophobic effect of water presents a promising avenue to tackle selectivity challenges that may arise during these reactions. Alternatively, aryl azides can be synthesized in toxic organic solvents, and then isolated azide can be exposed to cycloaddition in traditional organic solvents.29,30 The synthesis and isolation of perfluoroazides can be particularly challenging due to their instability under both photochemical and thermal conditions, further complicated by their inherently explosive nature. This presents significant limitations for their practical applications in synthetic chemistry. Additionally, the reliance on toxic organic solvents raises considerable concerns about the environmental sustainability of these methods, highlighting the urgent need for more eco-friendly alternatives.31–39
Considering the significant environmental concerns associated with the use of transition metals and organic solvents, adopting a metal-free protocol that relies solely on water—Nature's preferred solvent—emerges as an exceptionally favorable strategy for constructing carbon–nitrogen (C–N) bonds.31–34 The approach of conducting reactions “on water” harnesses the distinctive hydrophobic effect. This phenomenon takes advantage of water's polar characteristics, which serve to concentrate non-polar reactants at the liquid surface. As a result, this higher concentration leads to an increased frequency of collisions among the reactants, thereby accelerating reaction rates.31,35–37 Additionally, the unique microenvironment at the water interface can foster highly selective reaction pathways, optimizing product formation.35,36 This method potentially also simplifies the process of isolating the reaction products; since many organic compounds are water-insoluble, they can be effortlessly separated from the aqueous phase due to the inherent phase separation. This separation process often requires minimal to no additional organic solvents, further reducing the environmental footprint of the synthesis.38,39 Ultimately, by significantly curtailing the reliance on harmful organic solvents, the transformation that occurs “on water” stands to make a meaningful contribution to the development of greener chemical synthesis practices.
Results and discussion
By harnessing the substantial hydrophobic effect of water surface, we have developed a sustainable tandem protocol that facilitates the in situ formation of perfluoroazides, which then undergo 1,3-cycloaddition with olefins to yield the desired amine and imine products after nitrogen evolution under mild aqueous conditions. This method eliminates the need to isolate unstable and potentially explosive perfluoroazides, thereby addressing important safety concerns by utilizing water as the solvent. This metal- and organic solvent-free methodology has been successfully applied to a range of perfluoroarenes and olefins, demonstrating high selectivity.To evaluate the viability of our approach, we selected pentafluoropyridine 1 and cyclopentene 2 as benchmark substrates for the one-pot reaction. The corresponding perfluoroazide intermediate was generated in situ by the reaction of compound 1 with sodium azide (NaN3), which subsequently underwent cycloaddition with compound 2, followed by nitrogen evolution, resulting in the formation of the imine product 3. Remarkably, conducting the reaction using neat water as a medium at 60 °C yielded 92% of the desired imine 3 (Table 1, entry 1). Based on the GC-MS analysis of the crude reaction mixture, no perfluoroaniline byproduct formation was observed. To further investigate the “on water” effect and eliminate the “in water” phenomenon, we employed a variety of designer surfactants. The reaction conducted in the micelles of PS-750-M exhibited inferior reactivity, resulting in only 72% of the product formation (entry 2), thus highlighting the benefits of the “on water” effect. In this instance, corresponding anilines and unidentifiable byproducts were produced. Other surfactants, such as SDS and Tween 20, were also ineffective compared to the “on water” reaction, yielding only 17% and 67% of the desired product, respectively (entries 3 and 4). The reaction in THF resulted in 75% of 3, while only 12% of product formation was observed in DMF (entries 5 and 6). Similarly, the reaction conducted in DMSO provided only 14% of the desired product (entry 7). Reaction in the absence of any solvent (water) afforded only the product in traces (entry 8). No perfluoroazide was detected; only the starting material and traces of the product were observed. The reaction temperature played a crucial role; at room temperature, only 11% of 3 was obtained (entry 9). Mild heating at 45 °C afforded a lower yield of 3, i.e., 60% (entry 10). Increasing the global concentration from 0.25 M to 1 M slightly decreased the yield to 86% (entry 11). Thus, the optimal conditions include 1.5 equivalents NaN3, 0.25 M global concentration, neat water as the reaction medium, and 60 °C reaction temperature (for details, see ESI, page S2-S4†). In all reaction conditions other than described in entry 8, no unreacted starting material 1 was observed; other byproducts were most likely formed.
| Entry | Deviation from standard conditions | 3 (%) |
|---|---|---|
| a Conditions. 1 (0.25 mmol), 2 (0.50 mmol), NaN3 (0.75 mmol, 1.5 equiv.), 0.25 M H2O, 24 h. b All yields are based on GC-MS conversions using (0.25 mmol) mesitylene as an internal standard. Perfluoroazide was formed within 5 minutes of reaction, which either converted to the product, remained unreacted, or decomposed to an amine, depending on the reaction conditions. | ||
| 1 | No deviation | 92 |
| 2 | 3 wt% aq. PS-750-M instead of neat water | 72 |
| 3 | 3 wt% aq. SDS instead of neat water | 17 |
| 4 | 3 wt% aq. Tween 20 instead of neat water | 67 |
| 5 | THF as a reaction medium | 75 |
| 6 | DMF as a reaction medium | 12 |
| 7 | DMSO as a reaction medium | 14 |
| 8 | Neat reaction, no water or solvent | Traces |
| 9 | rt. instead of 60 °C | 11 |
| 10 | 45 °C instead of 60 °C | 60 |
| 11 | 1 M global conc. instead of 0.25 M | 86 |
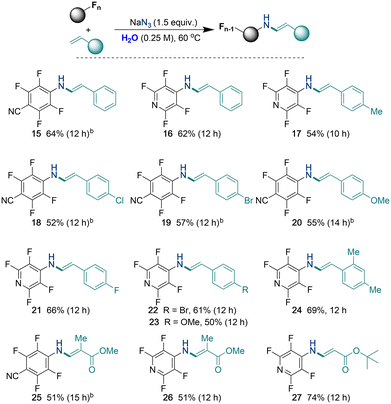
After identifying the optimal reaction conditions, we evaluated the substrate scope using various perfluoroarenes and cycloalkenes to synthesize imine products (Table 2, 3–14). In substrates other than perfluoropyridine, catalytic tetra-n-butylammonium chloride (TBAC) served as a phase transfer catalyst that facilitated the formation of perfluoroazide. Without TBAC, a lower conversion of perfluorarene to azide was observed, resulting in diminished reaction yields. Perfluoropyridine demonstrated remarkable reactivity with cyclopentene (3), cyclohexene (7), 2,3-dihydrofuran (10), and 1,5-cyclooctadiene (12), resulting in the formation of the desired imine products with impressive yields, ranging from good-to-excellent. In addition, perfluoroarenes possessing various functional groups, such as cyano (6, 9, 13), ester (14) trifluoromethyl (4, 8, 11), and ketone (5) functional groups, exhibited excellent reactivity, yielding the desired products. In example 5, we noted a complete absence of byproducts typically associated with aldol reactions, indicating a highly specific transformation. Furthermore, norbornene was shown to react favorably under the optimized conditions, leading to the successful formation of the desired imine product with high yields (13, 14). Particularly, the performance of 2,3-dihydrofuran (9–11) demonstrated remarkable regioselectivity throughout the reactions, without byproduct formation.
Next, both styrenes and acrylates also exhibited a remarkable tolerance in this one-pot transformation, as highlighted in Table 3 (15–27). Instead of anticipated imines, the reactions produced corresponding enamine products. This preference for enamines over imines can be attributed to their thermodynamic stability, which arises from the extended conjugation present in enamine molecules. Furthermore, the results demonstrated the compatibility of various substituents on the aromatic rings. Electron-donating groups in examples 17, 20, 23, and 24, as well as electron-withdrawing groups—such as chloro (18), bromo (19 and 22), and fluoro (21)—all participated effectively in the reaction, showcasing the robustness of the transformation. Additionally, methyl methacrylate (25 and 26) and t-butyl acrylate (27) underwent the reaction smoothly, producing the desired enamines in good yields. Thus, the reactivity showcased in Tables 2 and 3 demonstrates the effectiveness of the one-pot procedure across various substrates.
Subsequently, the scalability of the on-water protocol was evaluated for gram-scale reactions, as illustrated in Scheme 1A. The reaction between substrates 1 and 2 was conducted under the standard reaction conditions and a 62% isolated yield of product 3 was achieved, which was comparable to the yield obtained in small-scale experiments. Likewise, when substrates 28 and 29 were subjected to the same standard conditions, 84% of product 9 was obtained. These results illustrate the methodology's effectiveness, validating its scalability for large-scale applications.
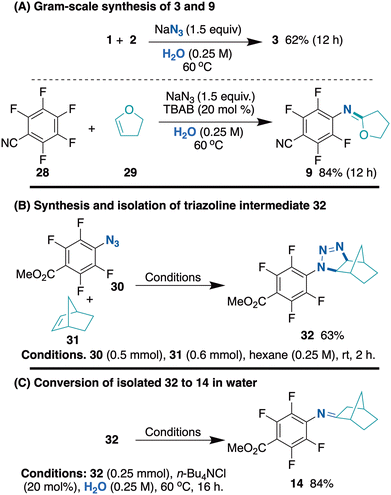 | ||
| Scheme 1 Scalability test and mechanistic investigation. (A) Gram-scale synthesis of 3 and 9. (B) isolation of triazoline reaction intermediate 32.29 (C) Isolated intermediate 32 under on-water conditions. | ||
The mechanistic investigation was also conducted to identify the reaction intermediates involved (Scheme 1B). In the reaction, if a triazoline intermediate is formed and subsequently transformed into the imine product after nitrogen evolution, the desired imine product should also be obtained from the reaction of the isolated triazoline intermediate under aqueous conditions. Thus, triazoline product 32 was synthesized for further exposure to the reaction conditions. The chosen hindered triazoline 32 proved to be stable enough for isolation and characterization. When 32 was subjected to standard reaction conditions in an aqueous environment, it provided 84% of 14 in 16 h (Scheme 1C). This yield was comparable to the isolated yield when the reaction was performed in a one-pot fashion (85%, Table 3). This confirms that the reaction proceeds through a triazoline intermediate.
To validate the findings discussed above, a control 1H NMR analysis of the reaction mixtures was conducted (Scheme 2). Three reactions were performed using isolated azide 30 and norbornene 31 under standard reaction conditions. The first reaction mixture was extracted with CDCl3 after 10 minutes, and the analyte was subsequently analyzed by 1H NMR spectroscopy. Similarly, the second reaction was quenched and analyzed after 60 minutes, while the third was analyzed after 120 minutes. After 10 minutes, the formation of triazoline intermediate 32 was confirmed through 1H NMR, indicated by the appearance of doublets at 4.67 and 4.65 ppm (2B)—the doublets resembled those from the 1H NMR spectrum of isolated 32 (2A). Additionally, a mixture of enamine 14a and imine 14 was detected. The presence of an NH signal around 5.96 ppm supported the formation of 14a, while the signal for the CH3 of the ester at approximately 3.92 ppm further indicated the formation of 14. Enamine 14a was identified as the major product after 10 minutes, likely a kinetic product. After 60 minutes, the triazoline intermediate 32 was completely consumed, as demonstrated by the disappearance of the doublets at 4.67 and 4.65 ppm (2C); the reaction mixture now predominantly contained imine 14, with enamine 14a as the minor product. Within 2 hours, the kinetic product 14a was fully converted into the thermodynamic product 14, as shown in the 1H NMR analysis of the isolated product (2D). Therefore, the control experiment confirms that the reaction pathways involve the formation of a triazoline adduct, which subsequently transforms into a kinetic enamine product before ultimately converting into a more thermodynamically stable imine product.
General procedure for synthesis of compounds
Perfluoroarene (0.5 mmol, 1 equiv.), alkene (1.0 mmol, 2 equiv.), and NaN3 (0.75 mmol, 1.5 equiv.) were taken in a 4 mL reaction vial equipped with a PTFE-coated magnetic stir bar. The reaction vial was closed with a rubber septum, and 1 mL water was added to the reaction mixture. The septum was wrapped with a parafilm. The reaction mixture was then stirred at 60 °C until complete consumption of the starting materials. Initially, the mixture was biphasic, but it became monophasic upon completion of the reaction. After reaction completion, as monitored by TLC or GC-MS for the complete consumption of perfluoroazide intermediate, the reaction mixture was cooled to rt. 1 mL EtOAc was added to the reaction mixture and the mixture was stirred for a minute. Stirring was stopped and the organic layer was withdrawn with the aid of a syringe needle. The same protocol was repeated twice (2 × 1 mL EtOAc). The combined organic layers were dried over anhydrous sodium sulfate. Finally, volatiles were removed under reduced pressure to obtain a crude product, which was further purified by flash chromatography (if needed) using EtOAc/hexanes as eluent.Conclusions
In this study, we developed an alternative for C–H amination that was free from metals and organic solvents. We utilized perfluoroarenes and alkenes as coupling partners, with water serving as the sole reaction medium. This method leveraged the hydrophobic effect at the interface to enhance reaction rates and selectivity, eliminating the need for metal catalysts and organic solvents. Our approach addressed significant safety concerns associated with perfluoroazides and simplified the reaction process by avoiding the need for pre-functionalized olefins. The high selectivity and efficiency demonstrated across various substrates highlighted the potential of this methodology for greener chemical synthesis, contributing to the advancement of environmentally friendly organic chemistry. Mechanistically, the reaction involved the formation of a 1,2,3-triazoline adduct that ultimately converted to the final imine or enamine product.Author contributions
The final manuscript was collaboratively written and approved by all authors. Sudripet Sharma developed and conducted the bulk of experiments and wrote the initial manuscript. Sahar Miraghaee contributed to the substrate scope and NMR experiments, while Sachin Handa led the project, mentored the team, and verified the data and hypotheses.Data availability
Materials and Methods, Supplementary Figures, Supplementary Tables, Supplementary Schemes, Detailed Experimental Procedures, Analytical Data, and Scanned NMR and HRMS Data are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. H. is grateful to the U.S. National Science Foundation for financial support (CHE 2044778, 2345856). We thank Karanjeet Kaur for their support in revising the manuscript through conducting a few experiments.References
- N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- T. Fujiwara and D. O'Hagan, J. Fluor. Chem., 2014, 167, 16 CrossRef CAS.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed.
- Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- N. Matsuda, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2011, 13, 2860 CrossRef CAS PubMed.
- T. Ahrens, J. Kohlmann, M. Ahrens and T. Braun, Chem. Rev., 2015, 115, 93 CrossRef PubMed.
- L.-M. Jin, X. Xu, H. Lu, X. Cui, L. Wojtas and X. P. Zhang, Angew. Chem., Int. Ed., 2013, 52, 5309 CrossRef CAS PubMed.
- S. S. Laev, V. U. Evtefeev and V. D. A. Shteingarts, J. Fluor. Chem., 2001, 110, 43 CrossRef CAS.
- F. Diness and D. P. Fairlie, Angew. Chem., Int. Ed., 2012, 51, 8012 CrossRef CAS PubMed.
- X. Kong, H. Zhang, Y. Xiao, C. Cao, Y. Shi and G. Pang, RSC Adv., 2015, 5, 7035 RSC.
- (a) A. Singh, J. J. Kubik and J. D. Weaver, Chem. Sci., 2015, 6, 7206 RSC; (b) S. Xie, S. Manuguri, G. Proietti, J. Romson, Y. Fu, A. K. Inge, B. Wu, Y. Zhang, D. Häll, O. Ramström and M. Yan, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 8464 CrossRef CAS PubMed; (c) R. E. Hackler, G. P. Jourdan, P. L. Johnson, B. R. Thoreen and J. G. Samaritoni, 1995, US0005399564A.
- F. Diness and D. P. Fairlie, Angew. Chem., Int. Ed., 2012, 51, 8012 CrossRef CAS PubMed.
- S. Fujii, Y. Maki and H. Kimoto, J. Fluor. Chem., 1989, 43, 131 CrossRef CAS.
- J. U. Engelhart, B. D. Lindner, O. Tverskoy, F. Rominger and U. H. F. Bunz, J. Org. Chem., 2013, 78, 10832 CrossRef CAS PubMed.
- W. S. Chow and T. H. Chan, Tetrahedron Lett., 2009, 50, 1286 CrossRef CAS.
- M. W. Cartwright, L. Convery, T. Kraynck, G. Sandford, D. S. Yufit, J. A. K. Howard, J. A. Christopher and D. D. Miller, Tetrahedron, 2010, 66, 519 CrossRef CAS.
- R. Cano, D. J. Ramón and M. Yus, J. Org. Chem., 2011, 76, 654 CrossRef CAS PubMed.
- T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed.
- J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS PubMed.
- A. R. Muci, S. L. Buchwald and S. L. Practical, Palladium Catalysts for C-N and C-O Bond Formation BT - Cross-Coupling Reactions: A Practical Guide, ed. N. Miyaura, Springer Berlin Heidelberg, Berlin, Heidelberg, 2002; pp. 131–209. DOI:10.1007/3-540-45313-X_5.
- M. Ju and J. M. Schomaker, Nat. Rev. Chem., 2021, 5, 580 CrossRef CAS PubMed.
- C. Empel and R. M. Koenigs, Chem. Catal., 2022, 2, 2506 CrossRef CAS.
- K. M. Carsch, I. M. DiMucci, D. A. Iovan, A. Li, S.-L. Zheng, C. J. Titus, S. J. Lee, K. D. Irwin, D. Nordlund, K. M. Lancaster and T. A. Betley, Science, 2019, 365, 1138 CrossRef CAS PubMed.
- A. Singh, J. J. Kubik and J. D. Weaver, Chem. Sci., 2015, 6, 7206 RSC.
- D. M. Sawant, G. Joshi and A. J. Ansari, iScience, 2024, 27, 109311 CrossRef CAS PubMed.
- N. Baris, M. Dračínský, J. Tarábek, J. Filgas, P. Slavíček, L. Ludvíková, S. Boháčová, T. Slanina, B. Klepetářová and P. Beier, Angew. Chem., Int. Ed., 2024, 63, e202315162 CrossRef CAS PubMed.
- S. Xie, S. A. Lopez, O. Ramström, M. Yan and K. N. Houk, J. Am. Chem. Soc., 2015, 137, 2958 CrossRef CAS PubMed.
- G. O. Jones and K. N. Houk, J. Org. Chem., 2008, 73, 1333 CrossRef CAS PubMed.
- F. Zhou, Z. Hearne and C.-J. Li, Curr. Opin. Green Sustainable Chem., 2019, 18, 118 CrossRef.
- T. N. Ansari, A. Taussat, A. H. Clark, M. Nachtegaal, S. Plummer, F. Gallou and S. Handa, ACS Catal., 2019, 9, 10389 CrossRef CAS.
- D. Petkova, N. Borlinghaus, S. Sharma, J. Kaschel, T. Lindner, J. Klee, A. Jolit, V. Haller, S. Heitz, K. Britze, J. Dietrich, W. M. Braje and S. Handa, ACS Sustainable Chem. Eng., 2020, 8, 12612 CrossRef CAS.
- N. Borlinghaus, T. A. Ansari, L. H. Braje, D. Ogulu, S. Handa, V. Wittmann and W. M. Braje, Green Chem., 2021, 23, 3955 RSC.
- T. Kitanosono and S. Kobayashi, Chem. – Eur. J., 2020, 26, 9408 CrossRef CAS PubMed.
- J. E. Klijn and J. B. F. N. Engberts, Nature, 2005, 435, 746 CrossRef CAS PubMed.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed.
- S. Sharma, J. B. Jasinski, W. M. Braje and S. Handa, ChemSusChem, 2023, 16, e2022201826 Search PubMed.
- S. Sharma, N. W. Buchbinder, W. M. Braje and S. Handa, Org. Lett., 2020, 22, 5737 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01076d |
| This journal is © The Royal Society of Chemistry 2025 |