 Open Access Article
Open Access ArticleHydrothermal liquefaction vs. fast/flash pyrolysis for biomass-to-biofuel conversion: new insights and comparative review of liquid biofuel yield, composition, and properties†
Farid
Alizad Oghyanous
 a and
Cigdem
Eskicioglu‡
a and
Cigdem
Eskicioglu‡
 *ab
*ab
aUBC Bioreactor Technology Group, School of Engineering, University of British Columbia, Okanagan Campus, 3333 University Way, Kelowna, BC V1 V 1 V7, Canada. E-mail: cigdem.eskicioglu@ubc.ca; Tel: +1 250 807 8544
bICREA – Catalan Institution for Research and Advanced Studies, Pg. Lluís Companys 23, Barcelona, Spain
First published on 2nd June 2025
Abstract
Hydrothermal liquefaction (HTL) and fast/flash pyrolysis are thermochemical processes (TPs) with proven potential to convert biomass into liquid biofuel, which can be comparable to crude oil. HTL is generally preferred for wet biomass, while fast/flash pyrolysis is more suitable for dried biomass, as moisture content plays a crucial role in determining the appropriate conversion method. Beyond moisture content, the biochemical and elemental composition of biomass significantly impacts the physical and chemical characteristics of the resulting liquid biofuels, often increasing the need for upgrading. This review provides a comprehensive comparison of HTL and fast/flash pyrolysis for converting five biomass types—lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste—into liquid biofuels, highlighting the impact of biomass composition on biofuel yield and quality. By linking biomass type, process severity, and liquid biofuel quantity, this study offers a structured framework for selecting the optimal conversion process and severity range to maximize biofuel yield in large-scale applications. Additionally, this review identifies various organic compounds and their concentrations in liquid biofuels produced through HTL and fast/flash pyrolysis from different biomass sources, serving as a valuable resource for developing novel multistage and selective upgrading processes.
Green foundation1. HTL and fast/flash pyrolysis are key thermochemical processes for converting biomass into liquid biofuels. Traditionally, moisture content has guided process selection, but this review highlights how both biomass composition and process type influence biofuel yield and quality. Expanding selection criteria beyond moisture improves efficiency, reduces waste, and optimizes resource use.2. With growing demand for sustainable energy, choosing the right biomass and conversion method is crucial. This review identifies optimal biomass types for HTL and fast/flash pyrolysis, minimizing upgrading needs. By linking biomass composition to conversion efficiency, it supports efficient biofuel production. 3. By analyzing biomass composition and its impact on biofuel quality, this review aids in developing hybrid, selective, and multistage upgrading processes. These insights will enhance biofuel efficiency, minimize environmental impact, and drive scalable renewable energy solutions, advancing sustainable fuel production. |
1. Introduction
The continuous rise in the global population is driving industries to operate at full capacity to meet increasing human needs. Even in the 21st century, fossil fuels remain the dominant energy source, supplying more than three-quarters of the world's energy demand.1 Crude oil forms from organic materials over millions of years under specific temperature and pressure conditions in aquatic environments. This slow natural process, however, cannot keep up with the growing demands of industries in the near future. Instead, organics can be processed using efficient techniques for biofuel production, providing a sustainable solution to meet global energy needs.2 Biomass, a renewable energy source of organic origin, is one of the primary candidates for biofuel production.3With the use of heat and complicated chemical reactions, thermochemical processes (TPs) can convert biomass into biofuels.4 Among TPs, fast/flash pyrolysis and hydrothermal liquefaction (HTL) show significant potential for producing liquid biofuels from biomass. The elemental characteristics of biomass—carbon (C), hydrogen (H), nitrogen (N), oxygen (O), sulfur (S), along with its proximate features, moisture and ash contents, and biochemical composition (proteins, carbohydrates, and lipids)—are crucial in determining the operational conditions for HTL and fast/flash pyrolysis, as these factors can influence both the yield and quality of the resulting liquid biofuels.5,6 Each biomass type has distinct characteristics; therefore, HTL and fast/flash pyrolysis should be optimized according to these features to maximize liquid biofuel yield and enhance its quality. For biomass to serve as an effective feedstock for biofuel production, it must be renewable, cost-effective, efficient, and a reduced environmental impact compared to conventional fossil fuels.7,8 This study reviews five commonly used biomass feedstocks for biofuel production via fast/flash pyrolysis and HTL: lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste, as they are widely recognized as renewable organic resources with significant potential for biofuel production.
Lignocellulosic biomass, the most abundant source of biomass for fuel production, ranks as the fourth most significant energy source after coal, petroleum, and natural gas.9 Composed primarily of cellulose, hemicellulose, and lignin, it offers substantial potential for biofuel production. While lignin is highly resistant to biodegradation, cellulose and hemicellulose can be hydrolyzed into various sugars, such as glucose, xylose, arabinose, and galactose.10 The distinctive composition of lignocellulosic biomass has positioned it as a key subject in renewable energy research, especially for biofuel production through TPs.
Algal biomass, including both microalgae and macroalgae, stands out as a highly valuable renewable energy source due to its exceptional photosynthetic efficiency, high biomass productivity, and rapid growth rates compared to terrestrial plants. Algae also excel in carbon dioxide (CO2) fixation and oxygen (O2) production. They thrive in liquid media across diverse climates, including marginal areas like deserts and coastal regions, utilizing non-potable water or even wastewater. Unlike traditional crops, algae require minimal water, do not compete with food crops for land, and enable year-round production with the advantage of daily harvesting.11,12 Microalgae are unicellular organisms with simple cellular structures that can rapidly reproduce, making them ideal for biofuel production due to their high lipid content. In contrast, macroalgae are multicellular, structurally complex organisms with specialized parts like blades and holdfasts, mainly accumulating carbohydrates, which makes them suitable for biogas and bioethanol production. Additionally, microalgae's metabolic pathways can be easily engineered for higher biomass yield, while macroalgae grow slower and rely on more complex nutrient absorption systems.13 As a result of its unique properties, algal biomass is a prominent feedstock for producing liquid biofuels through fast/flash pyrolysis and HTL.
Municipal sludge is increasingly recognized as a renewable energy source rather than waste due to its biochemical composition, offering significant potential for profitable biofuel production.14,15 Wastewater treatment plants typically produce two types of sludge: primary and secondary. Primary sludge, generated in the primary clarifier, contains solid materials such as feces, biodegradable organic matter, and grease, making it rich in lipids. Secondary sludge, or activated sludge, is produced in the biological processes and consists mainly of microbial cells and suspended solids, making it protein-rich. These sludge types are often mixed and treated by anaerobic digestion (AD) to produce biogas and digested sludge.16 However, municipal sludge can also be processed directly via TPs to be converted to value-added products or used as digested sludge for biofuel production through TPs after AD.
Food waste, which accounts for nearly one-third of all food produced for human consumption, contributes approximately 1.3 billion tonnes of waste annually, generating around 3.3 billion tonnes of CO2-equivalent greenhouse gas emissions. Rich in organic materials, food waste is seen as an ideal resource for bioenergy conversion and effective waste management.17 It contains a wide range of organic components, including starches, proteins, oils, fats, phosphates, nutrients, amino acids, and natural acids.18 Given its rich content of proteins, carbohydrates, and lipids, food waste is considered as one of the suitable feedstocks for TPs to be converted to biofuels.
From an economic perspective, liquid biofuel production from biomass involves various cost components, including biomass supply, dewatering or drying, pumping, catalysts, phase separation, and by-product management. Among these, biomass supply represents the largest share of the total production cost for the resulting liquid biofuel.19 The production costs range from US$ 20 to US$ 100 per dry tonne for lignocellulosic biomass,20 between US$ 1253 and US$ 2016 per dry ash-free tonne for microalgae cultivation,21 and from US$ 200 to US$ 300 per dry tonne for macroalgae production.22 In this regard, municipal sludge and food waste are cost-free feedstocks since they are produced as waste and their production has a direct correlation with population. As a result, the conversion of municipal sludge and food waste into liquid biofuels through fast/flash pyrolysis and HTL offers a more economical pathway compared to the use of lignocellulosic or algal biomass. The characteristics of liquid biofuels derived from different biomass types can vary significantly, necessitating varying degrees of upgrading with high costs. Therefore, decisions should factor in feedstock supply, pretreatment, and the costs associated with upgrading the liquid biofuels. This study does not focus on the economic aspects of feedstock and upgrading process costs, as large-scale applications of HTL and fast/flash pyrolysis are still in their early stages. For an accurate cost analysis, further research is needed to evaluate the quality of liquid biofuels derived from different biomass types through HTL and fast/flash pyrolysis, and to develop the corresponding upgrading processes before scaling up these processes.
To the best of our knowledge, no comprehensive review currently provides biomass-specific optimal process temperature and reaction time ranges for both HTL and fast/flash pyrolysis to maximize liquid biofuel yield. Furthermore, no study systematically identifies and compares the biomass-specific physical and chemical quality of liquid biofuels obtained from these processes. Moreover, a significant gap exists in the literature regarding the levels and types of organic compounds present in liquid biofuels derived from various biomass types through fast/flash pyrolysis and HTL. Therefore, the first objective of this review is to compare temperature, reaction time, and efficiency in fast/flash pyrolysis and HTL across various biomass types, focusing on liquid biofuel yield while considering the biomass's elemental and proximate characteristics. The second objective is to assess the physical and chemical quality of liquid biofuels in relation to biomass biochemical composition, identifying key organic compounds and their concentrations in biofuels. Addressing these gaps will enhance the understanding of biofuel production via TPs. Additionally, analyzing the composition of organic compounds in liquid biofuels derived from different biomass types through HTL and fast/flash pyrolysis will pave the way for the development of novel, more efficient, selective, scalable, and multistage biofuel upgrading strategies. Ultimately, this comparative review determines the most suitable biomass types for each thermochemical pathway based on liquid biofuel characteristics, rather than solely relying on biomass moisture as the key factor for processing via HTL or fast/flash pyrolysis.
The structure of this review is as follows: Section 2 introduces the liquid biofuels produced from fast/flash pyrolysis (pyrolysis oil) and HTL (bio-crude oil). It also discusses the chemical reactions occurring in fast/flash pyrolysis and HTL as the temperature increases. Section 3 presents the expected liquid biofuel yield ranges for HTL and fast/flash pyrolysis, along with the corresponding temperature and reaction time ranges for each biomass type. Subsequently, Section 4 compares the physical and chemical characteristics of biofuels derived from different biomass types. By identifying the typical biochemical composition of each biomass type, Section 4 also examines the organic compounds present in biofuels produced through HTL and fast/flash pyrolysis, along with their respective levels. Section 5 discusses the biomass-specific advantages and disadvantages of HTL and fast/flash pyrolysis, along with the existing research gaps.
2. Thermochemical processes for liquid biofuel production
Thermochemical processes are typically conducted under harsh operational conditions, with temperatures ranging from 180 to 1000 °C (ref. 23 and 24) and pressure varying between 1.013 bar (in pyrolysis) and 20–400 bar (in HTL).25,26 The choice between HTL and fast/flash pyrolysis is currently based on the moisture content of the biomass,27 without considering the impact of biomass biochemical composition on the chemical characteristics of the liquid biofuels. The specific temperature, reaction time, and pressure levels in these processes are applied based on the feedstock's composition to maximize the liquid biofuel yield.2.1 Pyrolysis
With pyrolysis, biomass is thermally decomposed without oxygen into liquid (pyrolysis oil), solid (biochar), and gases. The pyrolysis processes yield different proportions of these products based on the chemical reactions within feedstocks at varying temperatures.28,29 Pyrolysis commonly occurs at temperatures surpassing 300 °C, initiating intricate reactions within biomass. The initial phase, cracking, ranging from ambient temperature to 200 °C, is associated with the evaporation of moisture and light volatiles. During this phase, the breakage of bonds and the formation of hydroperoxide, –COOH, and –CO groups occur due to moisture evaporation.30 The subsequent stage, spanning from 200 to 500 °C, involves the rapid devolatilization and decomposition of specific kinds of carbohydrates, proteins, and lipids. At this stage, the generation of oxygen-rich functional groups and repolymerization of decomposed compounds also occur.31,32 The final stage, occurring above 500 °C, is characterized by the degradation of lignin and other organic matter with stronger chemical bonds and gasification.30 Lignin degradation does not occur exclusively at temperatures exceeding 500 °C; however, its degradation is significantly accelerated at higher temperatures compared to that of carbohydrates, proteins, and lipids. At all pyrolysis temperatures, the degradation of all organic compounds occurs to varying extents. During the degradation processes, all pyrolysis products are formed; however, the yields of pyrolysis oil, biochar, and gases are influenced by biomass characteristics, temperature, reaction time, and heating rate.33 Since pyrolysis oil is the primary product of fast/flash pyrolysis, this study focuses on its yield and characteristics derived from five different biomass types.Biomass particle size is another crucial parameter in fast/flash pyrolysis, as it influences both the yield of liquid biofuel and the selection of appropriate reactor types.44 In a study conducted by Shen et al.,45 the effect of particle size on pyrolysis oil yield was investigated using oil mallee biomass with a composition of 48.40 wt% C, 6.30 wt% H, 0.10 wt% N, 45.20 wt% O, and 0.50 wt% ash. Fast pyrolysis in a fluidized-bed reactor at 500 °C revealed that increasing the particle size from 0.30 mm to approximately 1.50 mm led to a reduction in pyrolysis oil yield. Similarly, Guizani et al.46 conducted fast pyrolysis of beech wood with 15.20 wt% FC, 84.30 wt% VM, 8.70 wt% moisture, and 0.50 wt% ash in a drop-tube reactor at temperatures ranging from 450 to 600 °C. Using biomass particle sizes of 370, 490, and 640 μm, they found that gas residence time had minimal influence on oil yield, while the highest yield was achieved with 370 μm particles at 500 °C. These studies highlight the importance of optimizing both particle size and reactor configuration. Reactor type plays a pivotal role in fast/flash pyrolysis, as it governs heat transfer efficiency and reaction rates, directly impacting the extent of cracking, devolatilization, and decomposition reactions. Consequently, reactor type significantly influences pyrolysis oil yield and product distribution yield.47,48 As pyrolysis technology has advanced, various reactor designs have been explored to improve pyrolysis oil production from biomass. Each reactor type has its own properties, potential for oil yield, and distinct advantages and disadvantages. Various reactors have specific requirements for biomass particle size, which are essential for efficient heat transfer and smooth operation. For instance, fluidized bed pyrolysis reactors typically require biomass particles ranging from 2 to 6 mm, which necessitate preprocessing steps such as cutting and grinding. Raza et al.49 and Campuzano et al.50 provide a comprehensive review of the reactors used in pyrolysis processes for biomass processing. Therefore, this study does not delve further into the effects of reactor types on pyrolysis oil production via fast/flash pyrolysis.
In fast/flash pyrolysis, biomass must be dried to a moisture content below 15 wt% to prevent negative effects on pyrolysis product properties. While grinding and drying improve pyrolysis oil yields, they also increase costs. After proper preparation, the biomass is introduced into the reactor for pyrolysis. The resulting char serves as a catalyst for vapor cracking, and specialized cyclones are employed to separate the char, effectively removing it from the reactor immediately after pyrolysis. However, some small char particles may still mix with the liquid product. To prevent further decomposition, vapors and gases are quickly cooled using pyrolysis liquid condensers, which cool the vapors rapidly by direct contact with pyrolysis oil or hydrocarbon liquid.51–53 While VM is crucial for pyrolysis oil production, a high ash content in biomass can limit pyrolysis oil yield in fast/flash pyrolysis processes by promoting char formation.54 Therefore, before applying fast/flash pyrolysis, it is essential to analyze the biomass's VM, ash content and composition, and moisture level to ensure it meets the necessary prerequisites for efficient processing and optimal liquid biofuel production. To improve the feasibility of this process for biofuel production, further research is needed to address energy efficiency and economic aspects of reducing particle size. Given the high costs of milling, grinding, and sieving biomass on an industrial scale, additional studies are crucial to design pyrolysis reactors that effectively minimize the impact of particle size during the conversion process.
Biomass characteristics, including elemental composition, ash content, and particle size, along with pyrolysis parameters such as reactor type, temperature, and reaction time, can significantly affect pyrolysis oil yield. Since different biomass types vary in elemental composition and ash content, these differences influence the overall pyrolysis process and pyrolysis oil yield. Table 1 presents the key characteristics of biomass and operational conditions used in fast/flash pyrolysis for maximizing the pyrolysis oil yield derived from lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste. Table 1 clearly indicates that temperature is a critical factor in pyrolysis oil yield. Contrary to common assumptions, higher temperatures in fast/flash pyrolysis do not always reduce pyrolysis oil yield. Instead, increasing the temperature can enhance oil yield up to the point where gasification becomes the dominant reaction. The optimal temperature varies depending on biomass characteristics, heating rate, and reactor type. Table 1 highlights that each type of biomass needs specific temperature and residence time conditions to maximize pyrolysis oil yield. For scaling up fast/flash pyrolysis processes, in addition to biomass characteristics, reaction temperature, and residence time, special attention must be given to biomass particle size and reactor type. With numerous variables at play, optimizing fast/flash pyrolysis at large scales can be challenging. However, when the process is carefully fine-tuned to match biomass characteristics, it becomes a promising method for efficiently producing pyrolysis oil from a diverse range of feedstocks.
| Biomass | T (°C) | Residence time (sec) | Particle size (mm) | Reactor type | Max. pyrolysis oil yield (wt%) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | C (wt%) | H (wt%) | N (wt%) | O (wt%) | Ash (wt%) | ||||||
| Lignocellulosic biomass | |||||||||||
| Kraft lignin | — | — | — | — | — | 399.85 | — | — | Fixed bed | 24.30 | 55 |
| 499.85 | 30.20 | ||||||||||
| 599.85 | 26.00 | ||||||||||
| Wood chips | — | — | — | — | — | 399.85 | — | — | 57.40 | ||
| 499.85 | 65.40 | ||||||||||
| 599.85 | 71.10 | ||||||||||
| Oat straw | 44.11 | 5.98 | 0.62 | 43.65 | 5.64 | 500 | 0.75–2.50 | 0.25–0.75 | Drop tube | 51.16 | 56 |
| 600 | 28.51 | ||||||||||
| 700 | 8.47 | ||||||||||
| Corn straw | 43.12 | 6.19 | 1.44 | 35.68 | 13.57 | 500 | 0.75–2.75 | 52.09 | |||
| 600 | 26.30 | ||||||||||
| 700 | 9.41 | ||||||||||
| Palm kernel shell | 48.82 | 5.68 | 0.42 | 45.08 | 3.87 | 600 | 5.00–6.00 | 0.075–0.125 | Entrained flow | 73.74 | 6 |
| 700 | 68.21 | ||||||||||
| 800 | 65.88 | ||||||||||
| 900 | 53.33 | ||||||||||
| Microalgae | |||||||||||
| Chlorella vulgaris | 43.75 | 6.07 | 7.86 | 41.61 | 5.54 | 600 | 5.00–6.00 | <0.105 | Entrained flow | 45.51 | 6 |
| 700 | 58.35 | ||||||||||
| 800 | 60.22 | ||||||||||
| 900 | 52.60 | ||||||||||
| Scenedesmus sp. | 32.10 | 4.80 | 5.30 | 22.10 | 35.20 | 480 | 2.00 | 2.00 | Fluidized bed | 55.00 | 57 |
| Chlorella vulgaris remnant | 45.04 | 6.88 | 6.64 | 29.42 | 8.34 | 500 | — | 0.42–0.70 | Fluidized bed | 53.00 | 41 |
| Macroalgae | |||||||||||
| Saccharina japonica | 32.89 | 6.17 | 0.93 | 60.01 | 20.21 | 350 | <3.00 | 0.30–0.50 | Bubbling fluidized-bed | 44.99 | 58 |
| 375 | 40.21 | ||||||||||
| 400 | 37.41 | ||||||||||
| 425 | 30.75 | ||||||||||
| 450 | 28.40 | ||||||||||
| 500 | 26.67 | ||||||||||
| Ulva lactuca | 33.60 | 5.10 | 3.30 | 28.20 | 29.10 | 550 | 0.50–2.00 | <1.00 | Centrifugal | 65.00 | 59 |
| Seaweed powder | 36.44 | 5.14 | 3.72 | 39.36 | 14.71 | 400 | 60.00 | — | Thermogravimetric analyzer | 23.57 | 60 |
| 500 | 31.87 | ||||||||||
| 600 | 36.87 | ||||||||||
| 700 | 37.99 | ||||||||||
| Municipal sludge | |||||||||||
| Mixed activated and primary sludge | 38.30 | 5.00 | 3.40 | 37.30 | 16.00 | 400 | 1.70 | 1 | Fluidized bubbling bed | 48.00 | 61 |
| 500 | 53.00 | ||||||||||
| Digested sewage sludge | 25.50 | 4.50 | 4.90 | 25.80 | 37.20 | 450 | <1.00 | 0.50–3.00 | Conical spouted bed | 45.00 | 43 |
| 500 | 48.00 | ||||||||||
| 600 | 47.00 | ||||||||||
| Sewage sludge | 40.60 | 7.10 | 7.70 | 41.20 | 37.20 | 450 | <100.00 ms | 0.50–3.00 | Conical spouted bed reactor | 44.80 | 62 |
| 500 | 48.50 | ||||||||||
| 600 | 45.40 | ||||||||||
| Food waste | |||||||||||
| Waste fish oil | — | — | — | — | — | 525 | 17.00 | — | Continuous pilot plant tubular | 72.83 | 63 |
| Potato peel waste | 43.80 | 6.00 | 4.10 | 46.20 | 9.30 | 450 | 8.00 | 1 | Laboratory auger | 22.70 | 64 |
| Potato peel waste residue | 47.80 | 6.40 | 4.00 | 41.80 | 6.50 | 25.60 | |||||
| Grape seeds powder | 50.90 | 5.40 | 2.50 | 36.90 | 4.10 | 750 | 600.00 | — | Oven | 28.92 | 65 |
| 850 | 32.56 | ||||||||||
2.2 Hydrothermal processes
Hydrothermal processes (HTPs) employ water as a pivotal solvent and reactant to convert biomass into value-added products. This process may occur in subcritical water (T < 374.15 °C and P < 220.64 bar) and/or supercritical water (T > 374.15 °C and P > 220.64 bar), sometimes with organic co-solvents, in an oxygen-free environment. In HTPs, four main products are aqueous phase, hydrochar, bio-crude, and gases. It should be noted that in HTPs, four main products are generated; however, similar to pyrolysis, the yield for each product varies in each process.66 A key advantage of HTPs over pyrolysis is their ability to directly use water as a reaction medium, which removes the need to remove moisture from biomass. As a result, the energy consumption of HTPs compared to pyrolysis can be significantly lower. In HTPs, it is typically practical to manage feedstock with solids making up to 30 wt% of the total weight. The maximum solid loading is determined by the ease of preparing and pumping the feed material. Consequently, feedstock with high moisture content still requires dewatering but does not necessitate the extensive drying typically needed for pyrolysis.67There are three main reactions in HTPs: (1) hydrolysis or depolymerization: the temperature needed for depolymerization in HTPs generally falls within the range of 150–250 °C, and the temperature choice is related to the particular characteristics of the feedstock being processed;68 (2) decomposition: within HTPs, the smaller molecules produced through hydrolysis undergo a sequence of thermal decomposition reactions. These reactions include dehydration, decarboxylation, decarbonylation, deamination, dehydrogenation, and the selective bond cleavage caused by high temperatures. The decomposition of different groups of compounds in HTPs typically takes place within a temperature span of 180–374 °C. Beyond 374 °C, in supercritical conditions, gasification can become the predominant process;69 (3) repolymerization: at elevated temperatures within HTPs, the assorted reactive fragments generated during decomposition reactions begin to recombine, giving rise to the formation of compounds present in bio-crude oil. These reactions happen simultaneously, making it impossible to pinpoint the exact start and end times of each reaction. Large molecules produced through these recombination reactions are particularly instrumental in the formation of bio-crude. In contrast, smaller molecules tend to remain in the aqueous phase. Numerous organic molecule groups found in bio-crude oil are the outcomes of recombination reactions involving long-chain fatty acids.70 The products of HTP reactions are affected by factors like temperature, residence time, and the characteristics of the feedstock. As a result, the mechanisms governing various aspects of HTPs are not yet fully understood. As bio-crude oil is the primary product of HTL, this study examines the impact of temperature and residence time on the yield and quality of bio-crude oil produced from various biomass types using the HTL process.
 | ||
| Fig. 1 Organic compounds that may exist in pyrolysis oil and biocrude oil derived from different biomass.4,38,40,77 | ||
Instead of utilizing the moisture of biomass as the solvent, organic solvents are also investigated in HTL as they play a key role in bio-crude oil yield. The selection of solvent affects both the temperature needed to achieve the maximum bio-crude yield in the HTL process and the overall yield of bio-crude oil. Numerous studies have demonstrated that using biomass moisture or water as the sole solvent typically results in lower yields of water-insoluble oily products compared to organic solvents such as methanol, ethanol, isopropanol, and acetone.78–81 By using alcohol or acetone as solvent, it is possible to reach supercritical conditions at lower temperatures. This is primarily attributed to the high critical temperature and dielectric constant of water, which limits its ability to effectively dissolve hydrophobic, high-molecular-weight decomposition products from biomass components like cellulose, hemicellulose, and lignin.82 Organic solvents—particularly alcohols—exhibit several advantageous properties for the HTL process. Alcoholic solvents possess lower critical temperatures and pressures compared to water, enabling liquefaction under milder reaction conditions. Moreover, their lower dielectric constants enhance the solubility of non-polar, fragmented biomass products, thereby improving the efficiency of the liquefaction process and increasing biocrude oil yields.82–84 In this context, Li et al.85 assessed the effect of using acetone, ethanol, and methanol as solvents on the bio-crude oil yield from HTL of sewage sludge at temperatures ranging from 280 to 360 °C. The highest yields were 42.3 wt% at 360 °C with ethanol, 43.1 wt% at 360 °C with acetone, and 26.3 wt% at 280 °C with methanol. Singh et al.86 evaluated the impact of water, ethanol, and methanol as solvents for HTL of Ulva fasciata at 300 °C for 15 minutes. Methanol produced the highest bio-crude yield.
Additionally, organic solvents can act as hydrogen donors during HTL, stabilizing reactive intermediates and suppressing repolymerization reactions, which would otherwise reduce the biocrude yield and increase the formation of char and gaseous byproducts.87,88 It should be noted that while organic solvents enhance liquefaction conditions, they do not provide the catalytic H+ and OH− ions that water generates at high temperatures, which play a crucial role in hydrolysis reactions during HTL. To address this, several studies have explored the use of mixed solvent systems, combining water with organic solvents to simultaneously leverage the catalytic benefits of water and the solubilizing, stabilizing effects of alcohols and other organics. This co-solvent strategy has shown promise in optimizing biocrude yield and quality by balancing hydrolysis and stabilization pathways during HTL.89 In this regard, Li et al.90 applied HTL to secondary sludge with a moisture content of 83.05 wt% using three different solvents: pure water, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and n-hexane, and a 1
1 mixture of water and n-hexane, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and methanol. The experiments were conducted at temperatures ranging from 300 °C to 380 °C and reaction times of 0–60 minutes. They found that the maximum bio-crude yields were 37.1 wt% at 340 °C for 20 minutes with pure water, 46.5 wt% at 340 °C for 40 minutes with the water/methanol mixture, and 39.3 wt% at 340 °C for 0 minutes using the water/n-hexane mixture. Han et al.91 used HTL to convert microalgae with 90 wt% moisture in the reactor into bio-crude oil. The reaction was carried out in a reactor at temperatures of 275 °C, 300 °C, and 350 °C for 30 minutes, using pure water, 10% isopropyl alcohol, and 10% ethylene glycol as co-solvents. The highest bio-crude yields reported were 29.9 wt% at 325 °C with water, 35.4 wt% at 350 °C with isopropyl alcohol, and 30.4 wt% at 350 °C with ethylene glycol. Yan et al.92 used kitchen food waste as the feedstock for the HTL process, conducted at temperatures ranging from 240 to 280 °C for 30 minutes with varying percentages of ethanol as the co-solvent. They found that up to 50% (v/v) of ethanol had no significant effect on the bio-crude yield. However, when 62.5% (v/v) ethanol was used, the bio-crude oil yield increased by about 115% compared to using pure water as the solvent.
1 mixture of water and methanol. The experiments were conducted at temperatures ranging from 300 °C to 380 °C and reaction times of 0–60 minutes. They found that the maximum bio-crude yields were 37.1 wt% at 340 °C for 20 minutes with pure water, 46.5 wt% at 340 °C for 40 minutes with the water/methanol mixture, and 39.3 wt% at 340 °C for 0 minutes using the water/n-hexane mixture. Han et al.91 used HTL to convert microalgae with 90 wt% moisture in the reactor into bio-crude oil. The reaction was carried out in a reactor at temperatures of 275 °C, 300 °C, and 350 °C for 30 minutes, using pure water, 10% isopropyl alcohol, and 10% ethylene glycol as co-solvents. The highest bio-crude yields reported were 29.9 wt% at 325 °C with water, 35.4 wt% at 350 °C with isopropyl alcohol, and 30.4 wt% at 350 °C with ethylene glycol. Yan et al.92 used kitchen food waste as the feedstock for the HTL process, conducted at temperatures ranging from 240 to 280 °C for 30 minutes with varying percentages of ethanol as the co-solvent. They found that up to 50% (v/v) of ethanol had no significant effect on the bio-crude yield. However, when 62.5% (v/v) ethanol was used, the bio-crude oil yield increased by about 115% compared to using pure water as the solvent.
It can be argued that the reaction environment plays a key role in optimizing bio-crude yield during the HTL process. For lignocellulosic biomass, food waste, and macroalgae, an alkaline solvent may be more advantageous compared to microalgae and municipal sludge when producing bio-crude oil. For instance, Biller and Ross93 conducted HTL on microalgae and cyanobacteria with varying compositions: two rich in protein and lipids and two rich in protein and carbohydrates. The process was performed at 350 °C using water, 1 M sodium carbonate, and 1 M formic acid as solvents. They found that lipids and proteins were efficiently converted into bio-crude with water, while carbohydrates showed improved conversion with Na2CO3. Indeed, different types of biomass exhibit varying behaviors during HTL with different solvents. Solvents not only influence the bio-crude yield but can also alter the characteristics of the liquid products. Therefore, solvent selection is highly dependent on the biochemical composition of the biomass as well as the temperature and pressure conditions of the HTL process. While the use of appropriate solvents can positively influence bio-crude oil yield, selecting an unsuitable solvent may merely increase the overall cost of the HTL conversion without providing any significant improvement in bio-crude oil yield. Further research into developing biomass-specific solvent selection strategies could help identify the most appropriate solvents for each biomass type in the HTL process, while also considering the potential additional costs to overall liquid biofuel production.
The conversion of lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste along with their elemental and proximate composition at different HTL conditions is presented in Table 2. Each biomass type requires specific HTL operational conditions to be converted to the maximum amount of bio-crude oil. Further, the maximum bio-crude oil yield varies based on biomass composition. The HTL process is generally less complex than fast/flash pyrolysis in terms of biomass particle size and reactor type for liquid biofuel production. However, similar to fast/flash pyrolysis, the presence of ash in biomass may limit bio-crude yield, as ash tends to promote the formation of more hydrochar during the HTL process.94 It is worth mentioning that a higher ash content in biomass does not necessarily result in a lower bio-crude oil yield, as the ash composition and specific operational conditions can have a significant impact on maximizing bio-crude oil yield in the HTL process, which is discussed in Section 3. Table 2 indicates that the solvent, along with temperature and reaction time, can be key parameters for bio-crude oil yield in HTL. For large-scale applications, optimizing temperature and reaction time based on biomass characteristics is crucial. In the context of solvents, it is important to note that they are biomass-specific in the HTL process. While using co-solvents or alternative solvents instead of biomass moisture may improve the bio-crude oil yield, it is essential to evaluate their long-term costs and impact on HTL product characteristics. The HTL process produces a substantial amount of aqueous phase (HTL-AP) alongside bio-crude oil, and managing HTL-AP remains a significant bottleneck in the process.95 While there is a comprehensive review on valorization techniques for HTL-AP,96 much remains unknown about the effects of solvents on HTL-AP characteristics for valorization. Therefore, further studies are essential to investigate the impact of solvents on the characteristics of HTL-AP and to explore potential valorization techniques, all while maximizing bio-crude oil yield and analyzing its quality.
| Biomass | Solvent | T (°C) | Residence time (min) | Pressure (bar) | Max. bio-crude yield (wt%) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | C (wt%) | H (wt%) | N (wt%) | O (wt%) | Ash (wt%) | ||||||
| Lignocellulosic biomass | |||||||||||
| Corn stover | 43.57 | 5.84 | 0.56 | 49.98 | 6.96 | Water | 250 | 0.00–60.00 | 75.84–234.42 | 22.20 at 15 min and 110.31 bar | 97 |
| 300 | 29.25 at 0 min and pressure of 151.68 | ||||||||||
| 350 | 17.70 at 15 min and 179.26 and 217.18 bar | ||||||||||
| 375 | 14.25 at 15 min and pressure of 196.5 and 241.31 bar | ||||||||||
| Wheat straw | 42.15 | 6.21 | 0.82 | 50.82 | 6.92 | K2CO3 | 400 | 15.00 | 320.00 | 22.00 | 98 |
| Eucalyptus | 47.85 | 5.81 | 0.10 | 46.23 | 1.15 | 28.00 | |||||
| Pinewood | 49.90 | 6.30 | 0.30 | 42.80 | 0.59 | 27.00 | |||||
| Rice straw | 36.20 | 5.20 | 0.70 | 40.30 | — | Milli-Q water, tap water, seawater, recycled wastewater, industrial wastewater | 350 | 30.00 | 180.00 | 36.40 in industrial wastewater | 99 |
| Microalgae | |||||||||||
| Chlorella vulgaris | 52.60 | 7.10 | 8.20 | 32.20 | 7.00 | Water, Na2CO3, HCOOH | 350 | 60.00 | — | 38.00 in water | 100 |
| Nannochloropsis occulta | 57.80 | 8.00 | 8.60 | 25.70 | 26.40 | 37.50 in water | |||||
| Spirulina | 55.70 | 6.80 | 11.20 | 26.40 | 7.60 | 31.00 in water | |||||
| Porphyridium creuntum | 51.30 | 7.60 | 8.00 | 33.10 | 24.40 | 22.00 in water | |||||
| Scenedesmus obliquus | 33.40 | 4.70 | 4.40 | 16.50 | 40.80 | Water | 250 | 7.00–30.00 | 175–225 | 21.50 | 101 |
| 300 | 225–270 | 31.00 | |||||||||
| 350 | 260–280 | 35.05 | |||||||||
| Chlorella sp. | 56.20 | 6.90 | 7.70 | 28.70 | 11.70 | Water | 350 | 1.40 | 180.00 | 39.70 | 102 |
| 5.80 | 36.80 | ||||||||||
| Macroalgae | |||||||||||
| Sargassum tenerrimum | 32.10 | 4.70 | 0.93 | 60.72 | 26.50 | Water, C2H5OH | 260 | 15.00 | 45.00–120.00 | 18.50 in C2H5OH | 103 |
| 280 | 25.20 in C2H5OH | ||||||||||
| 300 | 20.00 in C2H5OH | ||||||||||
| Ulva fasciata | — | — | — | — | 25.40 | Water | 280 | 15.00 | — | 12.00 | 104 |
| Enteromorpha sp | — | — | — | — | 23.20 | 280 | 7.00 | ||||
| Sargassum tenerrimum | — | — | — | — | 32.00 | 280 | 9.00 | ||||
| Enteromorpha prolifera | 28.75 | 5.22 | 3.65 | 32.28 | 30.10 | Water, 5% Na2CO3 | 220 | 5.00–60.00 | — | 9.60 at 30 min in water | 105 |
| 240 | 12.50 at 30 min in water | ||||||||||
| 260 | 18.24 in 5% Na2CO3 at 30 min | ||||||||||
| 280 | 19.80 in 5% Na2CO3 at 30 min | ||||||||||
| 300 | 23 in 5% Na2CO3 at 30 min | ||||||||||
| 320 | 17.65 at 30 min in water | ||||||||||
| Municipal sludge | |||||||||||
| Swine manure | 46.02 | 6.10 | 2.57 | 45.31 | 11.45 | Water | 350 | 15.00 | — | 33 | 106 |
| Sewage sludge | 51.94 | 7.28 | 8.33 | 32.44 | 25.10 | 37 | |||||
| Mixed primary and secondary sludge | 47.90 | 5.70 | 3.70 | 32.30 | 9.80 | Water | 350 | 15.00 | 170.00 | 38.5 | 107 |
| Primary sludge | 47.80 | 6.50 | 3.60 | 34.10 | 7.50 | 34.7 | |||||
| Secondary sludge | 43.60 | 6.60 | 7.90 | 25.00 | 16.20 | 20 | |||||
| Dehydrated sewage sludge | 15.60 | 2.30 | 1.00 | 13.70 | 67.40 | Water, 2.28 aqueous phase: 1 water | 330 | 30.00 | 250.00 | 30.50 in second run with 17.90 aqueous phase in water | 108 |
| Food waste | |||||||||||
| Food waste | 48.18 | 7.3 | 4.52 | 39.73 | 5.40 | Water | 280 | 30.00 | — | 29 | 109 |
| 310 | 30 | ||||||||||
| 340 | 38 | ||||||||||
| Food waste | 47.80 | 5.11 | 4.78 | 42.10 | 3.30 | Water | 200 | 30.00 | 353 | 12 | 110 |
| 300 | 30.00 | 353 | 28 | ||||||||
| 350 | 30.00 | 138–357 | 36 at 169 bar | ||||||||
| 400 | 30.00 | 353 | 30 | ||||||||
| 500 | 1.00 and 30.00 | 353 | 23 at 1 min | ||||||||
| 600 | 1.00 and 30.00 | 353 | 30.5 at 1 min | ||||||||
| Mixed synthetic food waste | 56.16 | 8.05 | 2.61 | 33.19 | 4.68 | Water | 280–360 | 10.00–60.00 | 12–110 | 46.9 at 360 °C and 40 min | 111 |
As biomass contains carbohydrates and proteins, the Maillard reaction occurs in HTL and it has significant effects on products’ yields and quality.112 The Maillard reaction between hydrolyzed carbohydrates and hydrolyzed proteins occurs during HTL and/or pyrolysis processing of biomass, generating N-containing compounds.113 In a study conducted by Fan et al.,114 the impact of the Maillard reaction on bio-crude yield at 250 °C, 300 °C, and 350 °C was examined. At 350 °C, bio-crude yields were 15.20 wt% for lactose, 13.00 wt% for maltose, and 19.70 wt% for lysine. However, combining lysine with sugars significantly increased yields to 58.00 wt% for lactose and lysine, and 59.90 wt% for maltose and lysine. These findings highlight the role of the Maillard reaction in enhancing bio-crude yield. Additionally, Tang et al.115 found that the highest bio-crude yield was achieved at 280 °C with a 60-minute retention time using a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 protein-to-glucose ratio, suggesting that optimizing this ratio enhances bio-crude production. Studies on model compounds in HTL consistently reveal that a combination of lipids, proteins, and carbohydrates can improve bio-crude oil yield. In this regard, Lu et al.116 explored the effect of substrate composition on HTL products by analyzing individual and mixed model compounds, including soybean oil (lipids), soy protein (proteins), cellulose, xylose, and lignin, at 350 °C for 30 minutes. For individual model compounds, the bio-crude yields were 82.00 wt% for lipids, 21.10 wt% for proteins, 4.60 wt% for cellulose, 6.60 wt% for xylose, and 1.40 wt% for lignin. In dual mixtures, combining proteins with carbohydrates or proteins with lignin resulted in higher bio-crude yields, while cellulose and xylose did not show significant effects. A counterproductive interaction was found when lipids were mixed with lignin, suggesting mutual hindrance in conversion. This underscores the significance of maintaining a well-balanced elemental composition influence in the HTL process to leverage synergistic effects and optimize the conversion of biomass into bio-crude oil. Therefore, it becomes apparent that the biomass composition significantly influences the production of bio-crude oil. Optimizing the conversion of carbohydrates and proteins into bio-crude relies on specific ratios to leverage the Maillard reaction. The literature is limited in providing insights into the optimal carbohydrate-to-protein ratio in HTL studies. Further research is needed to determine the ideal carbohydrate-to-protein-to-lipid ratio in the HTL process. This will facilitate the development of cost-effective pretreatment methods to adjust the biochemical composition of biomass, especially waste biomass, leading to optimized bio-crude oil production. The effects of the biochemical composition of biomass on liquid biofuel characteristics are discussed further in Section 4.
1 protein-to-glucose ratio, suggesting that optimizing this ratio enhances bio-crude production. Studies on model compounds in HTL consistently reveal that a combination of lipids, proteins, and carbohydrates can improve bio-crude oil yield. In this regard, Lu et al.116 explored the effect of substrate composition on HTL products by analyzing individual and mixed model compounds, including soybean oil (lipids), soy protein (proteins), cellulose, xylose, and lignin, at 350 °C for 30 minutes. For individual model compounds, the bio-crude yields were 82.00 wt% for lipids, 21.10 wt% for proteins, 4.60 wt% for cellulose, 6.60 wt% for xylose, and 1.40 wt% for lignin. In dual mixtures, combining proteins with carbohydrates or proteins with lignin resulted in higher bio-crude yields, while cellulose and xylose did not show significant effects. A counterproductive interaction was found when lipids were mixed with lignin, suggesting mutual hindrance in conversion. This underscores the significance of maintaining a well-balanced elemental composition influence in the HTL process to leverage synergistic effects and optimize the conversion of biomass into bio-crude oil. Therefore, it becomes apparent that the biomass composition significantly influences the production of bio-crude oil. Optimizing the conversion of carbohydrates and proteins into bio-crude relies on specific ratios to leverage the Maillard reaction. The literature is limited in providing insights into the optimal carbohydrate-to-protein ratio in HTL studies. Further research is needed to determine the ideal carbohydrate-to-protein-to-lipid ratio in the HTL process. This will facilitate the development of cost-effective pretreatment methods to adjust the biochemical composition of biomass, especially waste biomass, leading to optimized bio-crude oil production. The effects of the biochemical composition of biomass on liquid biofuel characteristics are discussed further in Section 4.
3. Comparison of thermochemical process severity for maximizing biofuel yield
Tables 1 and 2 show that lignocellulosic and algal biomass, municipal sludge, and food waste have been extensively explored for liquid biofuel production using both HTL and fast/flash pyrolysis, regardless of biomass moisture levels. The varying levels of C, H, N, O, S, and ash in biomass influence pyrolysis oil and bio-crude oil yields. Fig. 2 illustrates the average distribution of these elements and ash in different biomass types. Table 3 summarizes the yields of liquid biofuels obtained from fast/flash pyrolysis and HTL of lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste, without the use of catalysts or co-solvents in either process. Based on Fig. 2 and Table 3, C and H levels are directly linked to liquid biofuel yields, meaning that higher C and H content in biomass results in greater biofuel production. However, the unavoidable presence of O and N significantly affects biofuel quality, as discussed in Section 4. | ||
| Fig. 2 Distribution of (a) C, (b) H, (c) N, (d) O, (e) S, and (f) ash in different biomass used for biofuel production through pyrolysis and HTPs.40,41,57,61–65,93,111,116–169 | ||
| Biomass | Thermochemical process | Yield of pyrolysis oil (wt%) | Yield of bio-crude (wt%) |
|---|---|---|---|
| Lignocellulosic biomass | |||
| Fast/flash pyrolysis | 14.5–54.7 | — | |
| HTL | — | 15.6–31.1 | |
| Microalgae | |||
| Fast/flash pyrolysis | 39.6–65.4 | — | |
| HTL | — | 24.4–44.6 | |
| Macroalga | |||
| Fast/flash pyrolysis | 25.3–52.9 | - | |
| HTL | — | 9.4–28.1 | |
| Municipal sludge | |||
| Fast/flash pyrolysis | 21.5–43.5 | — | |
| HTL | — | 18.1–40.5 | |
| Food waste | |||
| Fast/flash pyrolysis | 13.0–49.2 | — | |
| HTL | — | 15.2–52.1 | |
In the context of TPs that predominantly generate liquid biofuels, as summarized in Table 3, microalgae exhibit higher yields compared to other biomass types in both fast/flash pyrolysis and HTL. This high yield from microalgae is likely due to its relatively higher C content compared to other biomass sources as demonstrated in Fig. 2. While lignocellulosic biomass and macroalgae yield more liquid biofuel in fast/flash pyrolysis than in HTL, municipal sludge and food waste generate similar liquid biofuel yields in both processes.
Table 3 and Fig. 2 suggest that although a high carbon content in biomass can lead to increased liquid biofuel production, a high ash content does not necessarily have a negative effect on liquid biofuel yields in fast/flash pyrolysis and HTL. This is evidenced by municipal sludge, which has a high ash content, and food waste, which has a low ash content, producing comparable amounts of liquid biofuels. The ash in biomass is predominantly composed of inorganic matter, with its composition being strongly influenced by both the source of the biomass and the environmental conditions under which it is produced.170 While these inorganics are largely retained in the solid products of HTL and fast/flash pyrolysis, they can also influence the yield of liquid biofuels.4
To gain deeper insights into the role of inorganics in liquid biofuel production, the optimal strategy involves supplementing the feedstock with additional inorganic materials and conducting HTL and/or fast/flash pyrolysis to evaluate their synergistic or antagonistic effects on biofuel yield.171–174 In this context, iron (Fe) serves as an effective transition metal catalyst, substantially enhancing the yield of liquid biofuels. Kandasamy et al.175 reported that adding 0.45 g of Fe3O4 to 33 mL of slurry containing 10–11 wt% dried microalgae increased the bio-crude oil yield in the HTL process by approximately 9% compared to a run without Fe3O4. In another study, Malins et al.176 investigated the effects of three metal catalysts—RANEY® nickel, FeSO4, and MoS2—on bio-crude oil production from municipal sludge via HTL at temperatures ranging from 200 to 350 °C and reaction times between 10 and 100 minutes. All catalysts were applied at a loading of 5 wt% relative to the dried sludge mass. The findings indicated that FeSO4 produced the greatest improvement in bio-crude oil yield. Xu et al.177 similarly observed a notable enhancement in bio-crude oil yield from lignocellulosic biomass when employing an Fe-based catalyst during the HTL process conducted at 349.85 °C for 40 minutes. Huang et al.178 reported that the addition of an Fe-based catalyst at 5 wt% of the dried sludge mass during fast pyrolysis of municipal sludge enhanced the pyrolysis oil yield by about 6% relative to the catalyst-free process. Chen et al.179 confirmed that incorporating a cobalt (Co)-based catalyst, a transition metal, at 10 wt% of the microalgae mass markedly enhanced the bio-crude oil yield in HTL conducted at 320 °C for 30 minutes. The study further revealed that the Co-based catalyst promoted the conversion of carbohydrates. Furthermore, Qian et al.180 reported that Zn2+ facilitated the hydrolysis of carbohydrates and lignin while also promoting deoxygenation reactions. Likewise, a range of transition metals—including Cr3+, Co2+, Cu2+, Fe2+, Fe3+, Mn2+, Ni2+, and Zn2+—have exhibited similar catalytic activities. These effects involve accelerating carbohydrate hydrolysis, facilitating the dehydration of monosaccharides (e.g., glucose and xylose) into furfural derivatives, promoting the formation of organic acids such as lactic, levulinic, and formic acids, and breaking down heavy compounds into low-molecular-weight products within liquid biofuels produced through HTL and fast/flash pyrolysis.181–186 It should be emphasized that the catalytic efficiency of transition metals is strongly influenced by both their specific type and concentration. From the literature, it can be concluded that when biomass contains up to 10 wt% of transition metals based on its total dried weight, the ash content is more likely to have a beneficial effect on liquid biofuel yield in both HTL and fast/flash pyrolysis.
Post-transition metals such as aluminum (Al) and lead (Pb) have demonstrated catalytic effects comparable to transition metals, promoting the depolymerization, decomposition, and repolymerization of carbohydrates and lignin, which contributes to enhanced liquid biofuel yields during HTL and fast/flash pyrolysis of biomass.187,188 Conversely, alkali and alkaline earth metals—including Na+, K+, Li+, Ca2+, Ba2+, and Mg2+—have been shown to either enhance or reduce liquid biofuel yield by affecting repolymerization reactions in the HTL and fast/flash pyrolysis of biomass.189,190 The alkali and alkaline earth metals found in biomass ash predominantly promote the hydrolysis of lipids, carbohydrates, and lignin, enhance fatty acid decarboxylation, facilitate dehydration and hydrogenation reactions, and contribute to Maillard reactions. Nevertheless, their effectiveness in cleaving peptide bonds within proteins is relatively limited.90,123,191 As a result, the presence of alkali metals can have both beneficial and adverse impacts on liquid biofuel yield during HTL and/or fast/flash pyrolysis, depending on their concentration and interaction with biomass components. In summary, the ash composition of biomass is highly influential in either promoting or hindering key reactions—such as depolymerization, decomposition, and repolymerization—that affect liquid biofuel yield in fast/flash pyrolysis and HTL processes. Nonetheless, it is important to recognize that the organic fraction of biomass, along with its specific composition, remains the primary factor governing liquid biofuel production.
Among effective operating parameters in liquid biofuel yield, temperature and residence time are the most practical parameters to be optimized in the HTL and fast/flash pyrolysis processes.192–198 Temperature and reaction time can affect pressure, reactor design, and the amount of energy required for TPs. The correlation between temperature and reaction time can be defined as the severity of TPs. Severity can be calculated by eqn (1).4,199
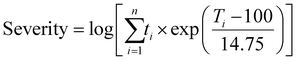 | (1) |
In eqn (1), n represents the number of treatment stages applied in TPs, ti is the reaction time in minutes, excluding ramping and cooling time, and Ti is the reaction temperature in degrees Celsius (°C). For example, conducting an HTL run at 350 °C for 15 minutes would result in a severity factor of  .
.
Fig. 3 illustrates the temperature range and reaction time for fast/flash pyrolysis and HTL for maximizing liquid biofuel yield for each type of biomass. It is generally accepted that fast/flash pyrolysis requires harsher process conditions than HTL for liquid biofuel production from biomass.27,200,201 Presence of water in HTL may facilitate organics conversion at lower process severity. However, as shown in Fig. 3, the severity of the process is biomass-specific. To maximize liquid biofuel yield from lignocellulosic biomass, microalgae, municipal sludge, and food waste, more severe conditions are required in fast/flash pyrolysis compared to HTL. However, macroalgae can be converted to pyrolysis oil under conditions similar to those required for HTL to produce bio-crude oil. Interestingly, municipal sludge requires harsher conditions in both fast/flash pyrolysis and HTL compared to lignocellulosic biomass, algal biomass, and food waste to achieve optimal liquid biofuel yields. Therefore, to optimize the HTL and/or fast/flash pyrolysis processes for maximizing liquid biofuel production from biomass, a specific range of severity given in Fig. 3, can be tested based on the biomass type. As discussed in Section 4.4, proteins, carbohydrates, and lipids are decomposed at different temperatures, and the biomass’ biochemical composition, particularly the ratio of carbohydrates, proteins, and lipids may have a significant impact on the optimum process severity for maximizing liquid biofuels yield in TPs.
 | ||
| Fig. 3 Temperature (T), reaction time (RT), and severity range for achieving maximum liquid biofuel yield via fast/flash pyrolysis and HTL from (a) lignocellulosic biomass;75,97,128,157,159,202–214 (b) microalgae;6,57,160,215–229 (c) macroalgae;58–60,103,104,230–241 (d) municipal sludge;62,76,107,130,161,242–257 (e) food waste.109,111,164,258–270 | ||
In conclusion, in this section, the analysis of literature results suggests that the optimal process severity in fast/flash pyrolysis and HTL depends largely on the biomass types. Therefore, recommendations have been made for the temperature, reaction time, and severity ranges suitable for each biomass in fast/flash pyrolysis and HTL for maximizing liquid biofuel yield for each biomass type (lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste). Another important consideration is that achieving the highest biofuel yield does not always equate to the best biofuel quality. Therefore, the following sections evaluate the biofuel physical and chemical quality produced from the five biomass types via fast/flash pyrolysis and HTL.
4. Physical and chemical characteristics comparison of bio-crude oil and pyrolysis oil
Higher heating value (HHV) is one of the most significant parameters for the quality analysis of biofuels. HHV is the quantity of heat released by a unit mass or volume of fuel (mainly at 25 °C) upon combustion, with the resulting products subsequently cooling back to a temperature of 25 °C. This value includes the latent heat of the vaporization of water. HHV can be measured using a bomb calorimeter according to the American society for testing and materials (ASTM) standard D-2015 (which was withdrawn by ASTM in 2000 and not replaced) or calculated using the Dulong formula or the Milne formula.271,272 While the presence of H and C is critical for liquid biofuels, the presence of O and N can adversely affect the liquid biofuel quality by causing corrosion, NOx emission, and catalyst fouling during upgrading processes.273 Therefore, hydrogen-to-carbon ratio (H/C), oxygen-to-carbon ratio (O/C), carbon-to-nitrogen ratio (C/N), HHV, density, pH, levels of organic compounds, and viscosity of liquid biofuels are among the main parameters that should be evaluated regarding the quality of the liquid biofuel.Temperature and the reaction time are highly effective on the deoxygenation and denitrogenation efficiency. By increasing the process temperature, the H content changes slightly, but the O percentage decreases due to enhanced deoxygenation. Consequently, the C percentage increases at higher temperatures. This results in a reduction of the H/C and O/C ratios in the bio-crude with increasing temperature.274 However, liquid biofuel production decreases at high temperatures, resulting in less liquid biofuel, as indicated in Table 1 for fast/flash pyrolysis and Table 2 for HTL. Feedstock type also plays a key role in deoxygenation and denitrogenation during HTL and fast/flash pyrolysis. Since the feedstock for the HTL and pyrolysis processes is biomass, the presence of N-containing and oxygenated compounds is unavoidable in the liquid biofuel, affecting the density, thermal stability, acidity, HHV, and viscosity of the liquid biofuels.275,276 Based on the van Krevelen diagram depicted in Fig. 4, both fast/flash pyrolysis and HTL can decrease the H/C and O/C ratios of the feedstocks if appropriate operational conditions are applied. Therefore, it can be said that it is worth applying fast/flash pyrolysis or HTL to biomass as they can produce liquid biofuels with much higher energy potential compared to biomass itself.
 | ||
| Fig. 4 van Krevelen diagram of various biomass and their (a) pyrolysis oil40,41,55,57,59,62,64,65,93,158–163,213,218,233,263,264,277,278 and (b) bio-crude oil.97,98,101–103,105,107,109–111,128,130,169,190,237,254,279–283 P-oil-Biomass name: pyrolysis oil derived from that biomass; B-oil-Biomass name: Bio-crude oil derived from that biomass. | ||
In Table 4, HHV, density, and moisture of pyrolysis oil obtained from the fast/flash pyrolysis and bio-crude oil generated by the HTL process of lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste are compared. As can be seen, pyrolysis oil has lower HVV compared to bio-crude oil in all types of feedstocks. High ash content may limit the liquid biofuel yield—as shown in Tables 1 and 2 for HTL and fast/flash pyrolysis, respectively—by promoting char formation. However, the presence of ash in biomass does not significantly affect the energy potential of the resulting biofuels, as indicated in Table 4. Therefore, biomass with high ash content, such as municipal sludge and macroalgae, can still be utilized for biofuel production via fast/flash pyrolysis and HTL without negatively impacting the energy quality of the biofuels.
| Biomass | Operational conditions | Pyrolysis oil or Bio-crude oil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | HHV (MJ kg−1) | Ash (wt%) | H/Ca | O/Ca | C/Na | HHV (MJ kg−1) | Moisture (wt%) | Density (g ml−1) | Ref. | ||
a H/C, O/C, and C/N are calculated using the formula of  , ,  , and , and  , respectively.
b
T: temperature; RT: residence time. , respectively.
b
T: temperature; RT: residence time.
|
|||||||||||
| Lignocellulosic biomass | |||||||||||
| Pyrolysis oil | Corn cobs | 17.80 | 1.98 | T = 500 °C | 1.63 | 0.50 | 115 | 26.20 | 25.30 | — | 158 |
| Corn stover | 18.30 | 4.88 | 1.53 | 0.52 | 53.30 | 24.30 | 9.20 | — | |||
| Maize-cob | 18.06 | 3.90 | T: 600 °C; RTb: 3–30 min | 1.52 | 0.27 | 279 | 30.59 | — | 1.18 at 25 °C | 159 | |
| Wood | 21.50 | 2.70 | T: 550 °C; RT: 0.8 s | 1.51 | 0.46 | 133 | 24.00 | 25.30 | 1.12 at 40 °C | 284 | |
| Straw | 21.30 | 5.80 | 1.24 | 0.46 | 39 | 23.70 | 25.70 | 1.15 at 40 °C | |||
| Lignin | 27.40 | 12.10 | 1.45 | 0.28 | 45 | 29.70 | 27.50 | 1.09 at 40 °C | |||
| Craft lignin | — | — | T: 399.85–599.85 °C | 1.15 | 0.25 | — | 20.00–31.00 | 5.30–7.20 | — | 55 | |
| Wood chips | — | — | 1.35 | 0.50 | — | 14.00–23.00 | 26.50–32.5 | — | |||
| Rice husk | 16.02 | 17.03 | T: 500 °C; RT: 45 s | 2.28 | 3.06 | 24.40 | 16.30 | — | 1.06 at 20 °C | 263 | |
| Beech wood | — | 0.80 | T: 450–600 °C; RT: 16.6 s | 2.28 | 1.21 | — | 21.70–25.10 | 7.80–21.00 | 1.10 at 22 °C | 213 | |
| Bio-crude oil Bio-crude oil | Corn stover | 14.14 | 6.96 | T: 250–375 °C; RT: 0–60 min | 1.29 | 0.33 | 47.10 | 27.47–35.13 | — | — | 97 |
| Wheat straw | 16.53 | 6.92 | T: 400 °C; RT: 15 min | 1.32 | 0.17 | 52.00 | 35.50 | — | — | 98 | |
| Eucalyptus | 18.14 | 1.15 | 1.24 | 0.16 | 93.70 | 35.79 | — | — | |||
| Pinewood | 19.50 | 0.59 | 1.30 | 0.16 | 67.50 | 33.66 | — | — | |||
| Natural hay | 13.20 | 7.80 | T: 240–320 °C; RT: 30 min | 1.22 | 0.20 | — | 25.30–31.50 | — | — | 128 | |
| Oak wood | 21.40 | 2.00 | 1.08 | 0.40 | — | 22.70–27.50 | — | — | |||
| Walnut shell | 14.70 | 1.50 | 1.05 | 0.30 | — | 23.60–28.50 | — | — | |||
| Cellulose | 17.00 | 0.00 | 0.94 | 0.40 | — | 21.10–24.60 | — | — | |||
| Beech wood | — | 0.80 | T: 300 °C; RT: 60 min | 1.19 | 0.30 | — | 27.00–30.10 | <1 | 1.14 at 22 °C | 213 | |
| Rice husk | 14.93 | 14.77 | T: 260–340 °C; RT: 20 min | 1.36 | 0.37 | 30.96 | 25.03 | — | — | 285 | |
| Microalgae | |||||||||||
| Pyrolysis oil | Chlorella vulgaris remnant | 19.44 | 8.34 | T: 500 °C | 1.93 | 0.40 | 4.68 | 24.57 | 15.89 | — | 41 |
| Chlorella vulgaris | 23.00 | 4.30 | T: 450–550 °C | — | — | — | 27.00–32.00 | 55.00–64.0 | — | 160 | |
| Synthetic microalgae | 16.40 | 24.20 | T: 550 °C | — | — | — | 24.00 | 61.00 | — | ||
| Chlorella vulgaris | 17.72 | 4.70 | T: 400 °C; RT: 2 s | 1.51 | 0.24 | 8.64 | 27.90 | 2.00 | — | 218 | |
| N starved Chlorella vulgaris | 20.23 | 2.50 | 1.67 | 0.18 | 16.30 | 28.90 | 2.10 | — | |||
| Chlorella vulgaris | — | 5.54 | T: 600–900 °C; RT: 5–6 s | — | — | — | — | 7.23–47.21 | — | 6 | |
| Bio-crude oil | Chlorella vulgaris | 23.20 | 7.00 | T: 350 °C; RT: 60 min | 1.73 | 0.11 | 17.50 | 35.10 | — | — | 93 |
| Nannochloropsis occulta | 17.90 | 26.4 | 1.69 | 0.105 | 20.30 | 34.50 | — | — | |||
| Spirulina | 21.20 | 7.60 | 1.50 | 0.11 | 12.20 | 36.80 | — | — | |||
| Porphyridium creuntum | 14.70 | 24.40 | 150 | 0.14 | 14.80 | 35.70 | — | — | |||
| Chlorella sp. | 19.90 | 11.70 | T: 350 °C; RT: 1.40 and 5.80 min | 1.68 | 0.009 | 36.50 | 32.90 and 36.10 | — | — | 102 | |
| Spirulina | 19.95 | 9.54 | T: 260 °C; RT: 60 min | 1.97 | 0.42 | 8.30 | 27.49–29.76 | — | — | 281 | |
| Scenedesmus sp. | 15.70 | 40.80 | T: 250–350 °C; RT: 7–30 min | 1.50 | 0.13 | 15.40 | 31.20–35.00 | — | — | 101 | |
| Macroalgae | |||||||||||
| Pyrolysis oil | Saccharina japonica | — | 20.21 | T: 350–550 °C; RT: 60 min | 1.45 | 0.07 | 22.40 | 32.97–33.36 | — | — | 233 |
| Kappaphycus alvarezii | 15.70 | 12.10 | T: 500 °C; RT: 15 min | — | — | — | 24.50 | — | — | 278 | |
| Sargassum wightii | 11.20 | 13.20 | — | — | — | 30.90 | — | — | |||
| Turbinaria ornata | 13.80 | 22.37 | — | — | — | 30.20 | — | — | |||
| Saccharina japonica | 12.11 | 20.21 | T: 350–550 °C; RT: < 3 s | 1.43 | 0.30 | 27.80 | 24.80–28.27 | — | — | 58 | |
| Ulva lactuca | 12.90 | 29.10 | T: 550 °C; RT: 0.8 s | 1.44 | 0.36 | 17.3 | 25.70 | 26.60 | 1.14 at 40 °C | 59 | |
| Bio-crude oil | Sargassum tenerrimum | — | 26.50 | T: 260–300 °C; RT: 15 min | 1.61 | 0.43 | 15.40 | 22.40 | — | — | 103 |
| Enteromorpha prolifera | — | 30.10 | T: 300 °C; RT: 30 min | 1.42 | 0.26 | 13.90 | 28.74 | — | — | 105 | |
| Sargassum angustifolium | 13.25 | 22.90 | T: 250–350 °C; RT: 20–60 min | 1.37 | 0.16 | 34.40 | 33.91 | — | — | 282 | |
| Laminaria digitata | 13.10 | 23.90 | T: 350 °C; RT: 15 min | 1.32 | 0.18 | 20.60 | 32.00 | — | — | 237 | |
| Laminaria saccharina | 12.20 | 21.80 | 1.26 | 0.14 | 29.00 | 33.00 | — | — | |||
| Laminaria hyperborea | 14.20 | 16.60 | 1.26 | 0.15 | 22.90 | 33.90 | — | — | |||
| Alaria Esculenta | 13.90 | 25.20 | 1.29 | 0.14 | 22.60 | 33.80 | — | — | |||
| Derbesia tenuissima | 12.40 | 34.70 | T: 330–341 °C; RT: 8 min | 1.22 | 0.11 | 13.10 | 33.20 | — | — | 286 | |
| Ulva ohnoi | 11.70 | 30.70 | 1.35 | 0.11 | 14.60 | 33.80 | — | — | |||
| Chaetomorpha linum | 10.30 | 36.60 | 1.29 | 0.12 | 12.20 | 32.50 | — | — | |||
| Cladophora coelothrix | 12.70 | 25.50 | 1.33 | 0.11 | 11.80 | 33.30 | — | — | |||
| Oedogonium sp. | 15.80 | 20.60 | 1.34 | 1.08 | 13.30 | 33.70 | — | — | |||
| Cladophora vagabunda | 16.40 | 17.80 | 1.39 | 0.11 | 12.20 | 33.50 | — | — | |||
| Municipal sludge | |||||||||||
| Pyrolysis oil | Mixed sludge | 17.35 | 29.23 | T: 300–500 °C | 3.04 | 0.90 | 8.59 | — | — | — | 161 |
| Anaerobically digested sludge | 14.40 | 32.87 | 1.87 | 0.27 | 10.40 | — | — | — | |||
| Aerobically digested sludge | 12.31 | 29.94 | 1.92 | 0.27 | 11.40 | — | — | — | |||
| Dehydrated aerobically digested sludge | — | 56.8 | T: 400–700 °C | 1.55 | 0.24 | — | — | — | — | 40 | |
| Anaerobically digested and dried sludge | 11.10 | 37.20 | T: 400–600 °C | 2.33 | 0.65 | 7.95 | — | 23.00 | 1.05 | 62 | |
| Thickened excess activated sludge | 16.50 | 25.00 | T: 500 °C | 1.52 | 0.11 | 8.14 | 24.70 | 10.30 | — | 162 | |
| Dewatered digested sludge | 8.60 | 47.50 | 1.54 | 0.06 | 10.70 | 27.90 | 78.70 | — | |||
| Dried excessive activated sludge | 15.00 | 27.30 | 1.78 | 0.26 | 8.26 | 23.90 | 17.00 | — | |||
| Bio-crude oil | Secondary sludge | 16.10 | 35.50 | T: 260–350 °C; RT: 10 min | 1.50 | 0.12 | 19.20 | 34.84–35.94 | — | — | 130 |
| Primary sludge cake | 21.10 | 7.00 | T: 300–350 °C | 1.45 | 0.13 | 27.20 | 30.20–34.60 | — | — | 254 | |
| Secondary sludge | 22.15 | 40.63 | T: 350 and 400 °C; RT: 15 min | 1.67 | 0.09 | 18.80 | 35.30 and 35.95 | — | — | 190 | |
| Mixed sludge | 19.90 | 9.80 | T: 350 °C; RT: 15 min | 1.34 | 0.006 | 20.50 | 33.10 | — | — | 107 | |
| Primary sludge | 20.70 | 7.50 | 1.57 | 0.006 | 20.70 | 37.80 | — | — | |||
| Secondary sludge | 20.00 | 16.20 | 1.43 | 0.009 | 16.60 | 34.80 | — | — | |||
| Bio-degassed sewage sludge | 21.27 | 34.02 | T: 350 and 400 °C; RT: 15 min | 1.62 and 1.63 | 0.12 and 0.11 | 18.40 and 19.60 | 35.22 and 35.42 | — | — | 76 | |
| Food waste | |||||||||||
| Pyrolysis oil | Waste cereals | 19.86 | 1.90 | T: 800 °C; RT: 10 min | 1.63 | — | 21.40 | 27.40 | 79.90 | — | 163 |
| Waste peanuts crisps | 24.75 | 2.20 | 3.17 | — | 41.70 | 14.10 | 73.90 | — | |||
| Potato peel waste | 17.40 | 9.30 | T: 450 °C; RT: 8 s | — | — | — | — | 86.80 | — | 64 | |
| Potato peel waste residue | 19.20 | 6.50 | — | — | — | — | 77.20 | — | |||
| Grape seeds powder | 20.50 | 4.10 | T: 750 and 850 °C; RT: 10 min | 0.84 | 0.10 | 19.20 | 27.54–32.06 | — | — | 65 | |
| Chestnut shel | 19.00 | 0.80 | 0.91 | 0.15 | 42.60 | 20.80–31.41 | — | — | |||
| Mixed food waste | 18.43 | 11.07 | T: 475 °C; RT: 60 min | 1.71 | 0.19 | 12.90 | 29.69 | — | — | 264 | |
| Bio-crude oil | Food waste | 20.64 | 0.51 | T: 280–340 °C RT: 30 min | 1.52 | 0.10 | 21.90 | 37.33 | — | — | 109 |
| Simulated food waste | 22.30 | 5.40 | T: 200–600 °C RT: 1–30 min | 1.07 | 0.09 | 14.40 | 35.00 | — | — | 110 | |
| Food waste | 20.90 | 3.30 | T: 240–295 °C RT: 0–30 min | 1.65 | 0.07 | 69.40 | 35.50–40.20 | — | — | 169 | |
| Food waste mixture | 24.51 | 4.68 | T: 280–360 °C RT: 10–60 min | 1.68 | 0.11 | 24.63 | 38.15 | — | — | 111 | |
| Salad dressing | 30.76 | 4.89 | 1.83 | 0.12 | 282.96 | 39.68 | — | — | |||
| Cream cheese | 31.16 | 3.10 | 1.92 | 0.12 | 380.21 | 40.38 | — | — | |||
| Beef | 29.31 | 2.30 | 1.92 | 0.13 | 51.09 | 39.56 | — | — | |||
| Chicken | 22.61 | 4.68 | 1.69 | 0.15 | 9.13 | 34.41 | — | — | |||
| Hamburger bun | 18.79 | 2.37 | 1.82 | 0.14 | 77.43 | 38.55 | — | — | |||
| Vegetable | 17.16 | 13.11 | 1.41 | 0.17 | 13.37 | 32.48 | — | — | |||
| Fruit peels | 17.82 | 5.00 | 1.27 | 0.17 | 37.22 | 32.71 | — | — | |||
4.1 Hydrogen-to-carbon and oxygen-to-carbon ratios of liquid biofuels
According to the van Krevelen diagram depicted in Fig. 4, while dehydrogenation is limited in both fast/flash pyrolysis and HTL, deoxygenation is much more intense in HTL compared to fast/flash pyrolysis. In this regard, Mullen et al.158 applied fast pyrolysis at 500 °C to corn stover with H/C, O/C, and HHV of 1.27, 0.64, and 18.3 MJ kg−1, respectively, and obtained pyrolysis oil with H/C, O/C and HHV of 1.52, 0.52, and 24.3 MJ kg−1, respectively. On the other hand, Mathanker et al.97 applied HTL at 375 °C to corn stover with H/C, O/C ratios, and HHV of 1.59, 0.86, and 14.14 MJ kg−1, respectively, and produced bio-crude oil with H/C, O/C, and HHV of 1.29, 0.13, and 35.13 MJ kg−1. Similarly, Merdun et al.161 tested fast pyrolysis on mixed primary and secondary sludge with H/C, O/C, and HHV of 0.15, 1.34, and 17.35 MJ kg−1, respectively, and generated pyrolysis oil with H/C and O/C of 3.04 and 0.89, respectively. On the contrary, Liu et al.107 examined the effect of HTL on mixed sludge with H/C, O/C, and HHV of 1.41, 0.51, and 19.9 MJ kg−1, respectively, and produced bio-crude oil with H/C, O/C, and HHV of 1.33, 0.006, and 33.1 MJ kg−1, respectively. These studies have supported the fact that liquid oil produced by the HTL process has higher HHV and lower H/C and O/C ratios compared to fast/flash pyrolysis, requiring less intense post-upgrading processes. Higher deoxygenation of biomass during the HTL process can be beneficial for the upgrading processes.According to Fig. 4, municipal sludge and food waste have a lower O/C ratio than lignocellulosic biomass, microalgae, and macroalgae in the pyrolysis and HTL processes. The HTL process can produce bio-crude oil from municipal sludge that is comparable to crude oil regarding H/C and O/C values,107 from food waste111 and microalgae281 regarding H/C value. Notably, pyrolysis of macroalgae generally produces higher-quality liquid biofuel compared to HTL in terms of H/C and O/C values. Fast/flash pyrolysis of municipal sludge and lignocellulosic biomass can produce liquid biofuels with lower H/C and higher O/C ratios than feedstock, highlighting the influence of process severity on these biomass types. In contrast, HTL consistently improves H/C and O/C ratios across various biomass types and operational conditions. Thus, the choice between fast/flash pyrolysis and HTL significantly affects the H/C and O/C ratios of the resulting biofuel. Trying to optimize many variables in fast/flash pyrolysis, including biomass particle size, reactor type, and process severity, can make it challenging to improve the H/C and O/C ratios of liquid biofuels. In contrast, HTL offers more consistent improvements in these ratios across various biomass sources as it has limited variables.
As shown in Table 5, bio-crude oil derived from municipal sludge and food waste has an O/C value closest to that of crude oil among the different feedstocks. This suggests that these biomass types can be processed via HTL with minimal need for upgrading. In contrast, liquid biofuels derived from lignocellulosic biomass, microalgae, and macroalgae through both fast/flash pyrolysis and HTL require significant upgrading to remove oxygenated compounds, as their O/C ratios are considerably higher than that of crude oil.
| Properties | Crude oil287,288 |
|---|---|
| O/C | 0.001 |
| H/C | 1.68 |
| C/N | 507–967 |
| HHV (MJ kg−1) | 41.7–47 |
| pH | N/A |
| Moisture (wt%) | <1 |
| Density (g ml−1) | 0.85–0.90 |
| Pyrolysis oila | Bio-crude oilb | |
|---|---|---|
| Lignocellulosic biomass | ||
| a Data are gathered and analyzed from ref. 40, 41, 55, 57–59, 62, 64, 65, 158–163, 213, 218, 233, 263, 264, 278, 284, 289–295. b See ref. 89, 93, 97, 98, 101–103, 105, 107, 109–111, 128, 130, 169, 190, 213, 234, 237, 254, 281, 282, 296–300. | ||
| O/C | 0.20–1.10 | 0.15–0.45 |
| H/C | 1.20–2.00 | 1.00–1.36 |
| C/N | 20–205 | 50–300 |
| HHV (MJ kg−1) | 13–32 | 19–36 |
| pH | 2.5–4.9 | N/A |
| Moisture (wt%) | 9–29 | 1–5 |
| Density (g ml−1) | 1.05–1.18 | 1.01–1.15 |
| Microalgae | ||
| O/C | 0.20–0.35 | 0.10–0.30 |
| H/C | 1.50–1.80 | 1.35–1.70 |
| C/N | 6.10–16.40 | 11.00–17.15 |
| HHV (MJ kg−1) | 23–33 | 24–37 |
| pH | 9.3–10.2 | N/A |
| Moisture (wt%) | 15–60 | 1–5 |
| Density (g ml−1) | N/A | N/A |
| Macroalgae | ||
| O/C | 0.15–0.40 | 0.10–0.35 |
| H/C | 1.35–1.63 | 1.20–1.40 |
| C/N | 18.20–40.20 | 14.60–45.00 |
| HHV (MJ kg−1) | 24–34 | 21–34 |
| pH | 3.3–6 | N/A |
| Moisture (wt%) | 25–35 | 2–10 |
| Density (g ml−1) | ∼1 | ∼1 |
| Municipal sludge | ||
| O/C | 0.15–0.70 | 0.005–0.15 |
| H/C | 1.35–2.60 | 1.40–1.65 |
| C/N | 6.50–11.50 | 15.00–30.05 |
| HHV (MJ kg−1) | 16–28 | 30–38 |
| pH | 3.5–8.5 | N/A |
| Moisture (wt%) | 15–79 | N/A |
| Density (g ml−1) | ∼1 | ∼1 |
| Food waste | ||
| O/C | 0.10–0.35 | 0.09–0.20 |
| H/C | 0.90–1.70 | 1.01–1.90 |
| C/N | 12.35–45.30 | 15–380 |
| HHV (MJ kg−1) | 15–32 | 32–42 |
| pH | 3–9 | N/A |
| Moisture (wt%) | 50–80 | 0.3–4 |
| Density (g ml−1) | 1–1.50 | <1 |
4.2 Carbon-to-nitrogen ratio of liquid biofuels
The N content of the produced liquid biofuels is another critical parameter affecting their quality. When biomass contains a high amount of protein, the resulting liquid biofuel will have a high N content.301 A greater C/N ratio of the liquid fuel is favorable as the C/N ratio of crude oil varies between 507–967.302 Lower C/N ratios can result in NOx emissions when the liquid biofuel is burned,303 as well as several other issues, such as increased viscosity, instability leading to cross-linking or oligomerization, and catalyst poisoning in standard refining processes.304 Therefore, appropriate post-upgrading processes should be applied when the N content of the liquid biofuel is high. As can be seen from Table 5, among five reviewed feedstocks, pyrolysis oil and bio-crude oil derived from microalgae and municipal sludge contains high N content compared to lignocellulosic, macroalgae, and food waste derived liquid biofuels. Compared to fast/flash pyrolysis, the C/N ratio of bio-crude oil produced from the same feedstocks in the HTL process is significantly higher, indicating that HTL is more effective at mitigating the impact of high N content in biomass on the resulting liquid biofuel.In conclusion, in this section, the analysis of the literature confirms that microalgae and municipal sludge consistently produce liquid biofuels with higher N content compared to other feedstocks. Some lignocellulosic biomass and food waste can have a high C/N ratio, depending on their source, but still not comparable to crude oil. The HTL process is notably more effective at reducing N content than fast/flash pyrolysis, leading to higher C/N ratios for bio-crude oils from all biomass. This is likely due to the fact that more than 55% of the N is recovered in the aqueous by-product (HTL-AP), rather than remaining in the bio-crude oil during the HTL process.252 The high C/N ratio of liquid biofuels in Table 5 suggests that denitrogenation upgrading processes are essential for liquid biofuels. While fast/flash pyrolysis and HTL can produce biofuels with H/C ratio close to crude oil under certain operational conditions, their C/N ratio remains significantly lower. This highlights the need for additional strategies during or after TPs to achieve more favorable O/C and C/N ratios. Identifying oxygenated and N-containing compounds will be crucial in developing hybrid upgrading processes. The levels of organic compounds in bio-crude and pyrolysis oil are discussed in detail in Section 4.4.
4.3 Physical characteristics of liquid biofuels derived from different biomass
The amount of biofuel entering the fuel injector of a combustion engine is determined by its density, which is influenced by various factors, including the type of feedstock used for its production. Biofuel density is crucial in nozzle design and can impact the engine's operational feasibility.305,306 It directly affects fuel atomization, which in turn influences the engine's thermal efficiency.307 The viscosity of the biofuel is of great importance as it influences the biofuel's pumpability, atomization, and penetration.308 The acidity of biofuel is another key factor regarding the quality of the produced biofuel, and it is a measure that indicates the amount of free fatty acids and acids produced from the degradation reactions of biomass. According to the ASTM D6751-07b standard, the total acid number (TAN) for crude oil should not exceed 0.5 mg KOH per g of oil.309 Moisture in biofuel is another concern, as it can lead to water accumulation, microbial growth, and corrosion in storage and transportation equipment.310 The following subsections summarize biomass-type specific information.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mPa s at 40 °C, which can be problematic and requires further upgrading to enhance pumpability. Bio-crude oil contains less than 1 wt% moisture, whereas the moisture content of pyrolysis oil ranges from 7.8 to 21 wt%. TAN of pyrolysis oil and bio-crude oil derived from beech wood is 45–109 and 32–67 mg KOH per g, respectively. Pittman et al.311 applied fast pyrolysis to corn stalks and found that increasing the pyrolysis temperature and the water content slightly in the biomass decreased the moisture content of the produced pyrolysis oil from 54.7 to 27 wt%. However, this also increased the viscosity of the oil from 1.60 to 41.4 cSt and its HHV from 17.40 to 25.84 MJ kg−1. TAN and pH of the pyrolysis oil varied between 81.7–85.8 mg KOH per g and 2.66–3.29, respectively. Nizamuddin et al.312 analyzed the bio-crude oil derived from the HTL process of corn stalk using ethanol as the solvent. They found that the bio-crude oil had only 4.76 wt% moisture, and its HHV was 30.52 MJ kg−1. From the literature, it can be concluded that the acidity of pyrolysis oil is generally higher compared to bio-crude oil derived from lignocellulosic biomass. The pH of pyrolysis oil obtained from lignocellulosic biomass ranges from 2.5 to 4.9.313–317 In addition, pyrolysis oil derived from lignocellulosic biomass contains a significant amount of water (more than 20 wt%), whereas the water content of bio-crude oil from lignocellulosic biomass is less than 5 wt%. Despite these differences, both pyrolysis oil and bio-crude oil have densities within the same range for lignocellulosic biomass.
000 mPa s at 40 °C, which can be problematic and requires further upgrading to enhance pumpability. Bio-crude oil contains less than 1 wt% moisture, whereas the moisture content of pyrolysis oil ranges from 7.8 to 21 wt%. TAN of pyrolysis oil and bio-crude oil derived from beech wood is 45–109 and 32–67 mg KOH per g, respectively. Pittman et al.311 applied fast pyrolysis to corn stalks and found that increasing the pyrolysis temperature and the water content slightly in the biomass decreased the moisture content of the produced pyrolysis oil from 54.7 to 27 wt%. However, this also increased the viscosity of the oil from 1.60 to 41.4 cSt and its HHV from 17.40 to 25.84 MJ kg−1. TAN and pH of the pyrolysis oil varied between 81.7–85.8 mg KOH per g and 2.66–3.29, respectively. Nizamuddin et al.312 analyzed the bio-crude oil derived from the HTL process of corn stalk using ethanol as the solvent. They found that the bio-crude oil had only 4.76 wt% moisture, and its HHV was 30.52 MJ kg−1. From the literature, it can be concluded that the acidity of pyrolysis oil is generally higher compared to bio-crude oil derived from lignocellulosic biomass. The pH of pyrolysis oil obtained from lignocellulosic biomass ranges from 2.5 to 4.9.313–317 In addition, pyrolysis oil derived from lignocellulosic biomass contains a significant amount of water (more than 20 wt%), whereas the water content of bio-crude oil from lignocellulosic biomass is less than 5 wt%. Despite these differences, both pyrolysis oil and bio-crude oil have densities within the same range for lignocellulosic biomass.
The wide range of results reported for municipal sludge has the problem of lack of specific feedstock information. Municipal sludge characteristics can vary depending on the source of unit processes (primary, secondary, mixed, or digested sludges), process operation (separate versus combined sewer, hydraulic and sludge retention times, conventional versus advanced treatment processes, chemical dosing for dewatering), location of the plant as well as sampling season, especially for secondary sludge from biological treatment processes. Often, literature reports results for different types of municipal sludge without any specifics leading to varied results in the characteristics of liquid biofuel derived from municipal sludge across studies. Generally, due to municipal sludge's high moisture content even after digestion, HTL is considered more suitable than pyrolysis for treating municipal sludge. However, it is important to note that the ash content of municipal sludge can negatively impact bio-crude yield, making hydrothermal carbonization (HTC) a preferable treatment option in such cases.
4.4. Chemical characteristics of bio-crude oil and pyrolysis oil derived from different biomass
The organic components of biomass, including carbohydrates, proteins, lipids, and lignin, are primarily converted into liquid biofuels during fast/flash pyrolysis and HTL processes. In contrast, the inorganic fraction of biomass predominantly accumulates in the solid by-products.256,328 The relative composition of these components significantly influences both the yield of liquid biofuels and the concentrations of organic compounds present in bio-crude and pyrolysis oil. Fig. 5 illustrates the distribution of carbohydrates, proteins, lipids, and lignin in lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste.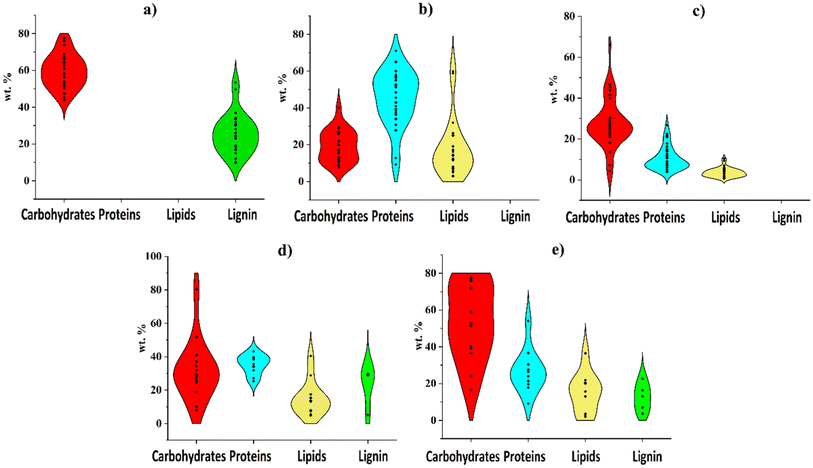 | ||
| Fig. 5 Distribution of carbohydrates, proteins, lipids, and lignin in (a) lignocellulosic biomass;6,75,97,202,204,207,209,210,212,329 (b) microalgae;6,57,93,160,215,217–220,222,224–227,330,331 (c) macroalgae;59,60,104,232,234,235,240 (d) municipal sludge;76,161,249–251,332,333 (e) food waste.109–111,169,261,262,267,268,270,283,334 Note: protein and lipid distribution in lignocellulosic biomass and lignin distribution in microalgae and macroalgae are not reported due to the lack of references that reported these compounds. | ||
The breakdown of carbohydrates in biomass leads to the formation of alcohols, ketones, and carboxylic acids. Aromatic hydrocarbons and phenols primarily originate from the protein fraction in biomass, and their production increases as the temperature increases. The primary decomposition of phenylalanine and tyrosine involves decarboxylation, deamination, and the simultaneous breaking of C–C bonds through concerted rupturing (resulting in radical formation) pathways.335 The Maillard reaction affects the presence of N-containing compounds in liquid biofuels.336 The increased temperature in pyrolysis promoted the Maillard reaction, which involves the incorporation of N by transforming quaternary-N into a more stable N formation. Consequently, the Maillard reaction leads to the formation of heterocyclic N-containing compounds.337 Long-chain fatty acids are obtained through the pyrolysis of lipids in biomass. Despite the thermal cracking of triglycerides leading to the production of short-chain hydrocarbons, long-chain fatty acids can persist if the residence time is short.338 Additionally, aromatic hydrocarbons can be formed from lipids at elevated temperatures, during which cyclization and aromatization reactions take place.339 Phenols can be generated from proteins, carbohydrates, and lignin.138,339Fig. 6 indicates the detailed pathways of alcohols, acids, phenols, ketones, aliphatic and aromatic hydrocarbons, N-containing compounds, and oxygenated compounds formation from carbohydrates, proteins, lipids, and lignin through fast/flash pyrolysis.
 | ||
| Fig. 6 Plausible reaction pathways of pyrolysis of carbohydrates, proteins, lipids, and lignin.138,340–344 | ||
The presence of water in HTL introduces a higher level of complexity to the reactions compared to fast/flash pyrolysis. As shown in Fig. 7, HTL involves a variety of reactions, including hydrolysis, dehydration, hydration, deoxygenation, deamination, and hydrogenation, affecting carbohydrates, proteins, lipids, and lignin. Unlike pyrolysis, the water in HTL facilitates the formation of more complex compounds, which are ultimately incorporated into bio-crude oil. Fig. 8 illustrates the pathways leading to the formation of various alcohols, acids, phenols, ketones, aliphatic and aromatic hydrocarbons, as well as N and oxygenated compounds, all derived from the biomass components during HTL. The concentration of organic compounds in liquid biofuels plays a crucial role in determining the quality of the resulting bio-oils. While some information is available regarding the effects of specific chemicals in biofuels, there remains a significant gap in understanding how each chemical affects the fuel properties of bio-crude oil and pyrolysis oil. Certain compounds may lead to storage and transportation challenges, while others can contribute to pollutant emissions during combustion or lower the heating value of the biofuels. N-heterocyclic compounds, in particular, are a critical concern. Due to the N content in biomass, originating from its cellular structure and protein composition, the presence of N-containing compounds in liquid biofuels is inevitable.345 The presence of N-heterocyclic compounds in biofuels can lead to increased NOx emissions during combustion. Additionally, these compounds can affect the odor of biofuels and pose challenges during the upgrading process. They tend to adhere to acidic catalysts, potentially deactivating them and reducing their effectiveness.346
 | ||
| Fig. 7 Plausible pathways of hydrothermal reactions of carbohydrates, proteins, lipids, and lignin.4,120,347–352 | ||
 | ||
| Fig. 8 Comparing relative area (%) of organic compounds in bio-crude oil and pyrolysis oil after the fast/flash pyrolysis and HTL processes of (a) lignocellulosic biomass;6,75,97,128,159,203,206,210,211,360 (b) microalgae;6,57,215,216,218,222,224,225,229 (c) macroalgae;58,60,103,231–233,236,238,239 (d) municipal sludge;62,76,130,161,243,246,250,252,256 (e) food waste.109–111,164,260–262,264,265,267,269 | ||
Aromatics reduce the fuel quality of liquid biofuels due to their lower H/C ratio, which decreases energy density. Their presence also raises environmental concerns by promoting the formation of polycyclic aromatic hydrocarbons (PAHs) during combustion.353 Wang et al.354 reported that the presence of C8–C15 cyclic alkanes instead of high-carbon aromatics can improve the HHV, H/C, and O content of pyrolysis oil obtained from lignocellulosic biomass via fast pyrolysis.
Alcohols can influence liquid biofuels positively or negatively, depending on the position of the hydroxyl (–OH) group. Long-chain alcohols offer higher energy density than short-chain ones due to their lower O/C ratio. However, both alcohols and acids can cause storage problems, such as corrosion.355 Esters are key components of liquid biofuels and can enhance fuel quality. However, their presence may increase the O/C ratio, reducing the heating value of biofuels.356 Aldehydes and ketones are present in liquid biofuels. While certain aldehydes enhance fuel properties, most contribute to instability, unpleasant odors during combustion, and storage issues due to their corrosiveness. Similarly, ketones can improve some fuel properties but may also alter the O/C ratio, leading to biofuel instability and corrosion.357
Phenols, primarily derived from lignin in biomass, are commonly found in liquid biofuels. They pose challenges during upgrading processes due to coke formation and their corrosive nature, which can also affect the storage of liquid biofuels.358 Additionally, phenols can originate from cellulose and hemicellulose during TPs.359
Fig. 8 presents the comparative relative areas (%) of alcohols, acids, phenols, ketones, aliphatics, aromatics, N-containing compounds, and oxygenated compounds in bio-crude oil and pyrolysis oil derived from five different types of biomass, as analyzed by gas chromatography-mass spectrometry (GC-MS). The list of organic compounds used to generate Fig. 8 is provided in Table S1.† As shown, oxygenated compounds make up a smaller relative area (%) in bio-crude oil compared to pyrolysis oil in lignocellulosic biomass, algal biomass, municipal sludge, and food waste. It means that the HTL process is more effective in deoxygenation than fast/flash pyrolysis regardless of the biomass characteristics. Biomass's biochemical composition plays a crucial role in the distribution of organic compounds. Lignin in lignocellulosic biomass results in bio-crude oil with phenols (12–38%) and pyrolysis oil with phenols (2–25%). Microalgae, with high protein content, produce bio-crude oil (14–66%) and pyrolysis oil (16–27%) with higher levels of N-containing compounds. Aromatics in microalgae-derived bio-crude oil and pyrolysis oil are present in similar relative amounts to N-containing compounds. Macroalgae, with higher carbohydrates and lower proteins and lipids, yields bio-crude oil (9–23%) and pyrolysis oil (9–42%) with significant ketones. Municipal sludge, containing high levels of proteins, carbohydrates, and lignin, produces bio-crude oil and pyrolysis oil with high N-containing compounds (bio-crude oil: 18–48%, pyrolysis oil: 13–30%) and phenols (bio-crude oil: 2–9%, pyrolysis oil: 8–16%). Biomass with higher carbohydrates and lipids, like food waste, leads to liquid biofuels with high acidity. Food waste-derived bio-crude oil and pyrolysis oil contain 35–70% and 5–30% acids, respectively. Therefore, the common belief about the acidity of pyrolysis oil is inaccurate. The acidity of both bio-crude oil and pyrolysis oil is primarily influenced by the carbohydrate content in the biomass used as feedstock for fast/flash pyrolysis or HTL. The HTL and pyrolysis processes have minimal impact on the acidity of the produced liquid biofuels.
Although adjusting the severity of fast/flash pyrolysis and HTL can affect the concentration of organic compounds in liquid biofuels,58,252 it is insufficient to achieve the optimal acidity, HHV, and desired levels of O and N-containing compounds necessary for proper storage, transport, and combustion. Therefore, post-treatment upgrading processes are essential to enhancing liquid biofuels for use as viable fossil fuel substitutes.
Physical methods, chemical processes, steam reforming, and supercritical fluids can all be used to upgrade bio-crude oil and pyrolysis oil. Physical methods like filtration, solvent addition, and emulsification can enhance properties such as reducing impurities, improving viscosity, density, and HHV. However, these methods alone are not enough to make liquid biofuels a viable alternative to fossil fuels.361 Supercritical fluids are effective in removing O-containing compounds, such as carboxylic acids, aldehydes, and levoglucosan, while improving the HHV of liquid biofuels. However, their application typically requires significant energy input and may lead to equipment corrosion during the process.362 Steam reforming operates at high temperatures (700–1000 °C) with the help of a catalyst. By combining catalytic steam reforming with the water–gas shift reaction, it efficiently produces hydrogen (H2) as a clean energy resource. This method also has its own limitations and cannot remove many of the organic compounds in liquid biofuels to make them comparable to crude oil.363
Chemical methods also offer the potential for removing certain organic compounds in liquid biofuels. Esterification, which involves adding alcohol under mild conditions, can improve the HHV and viscosity of liquid biofuels by removing some O-containing compounds. However, this method is ineffective in removing N-containing compounds.364 Hydrodeoxygenation (HDO) is a widely used method for eliminating O-containing compounds in bio-crude oil and pyrolysis oil, with the goal of increasing the H/C ratio and reducing the O/C ratio. Recent studies have concentrated on developing catalysts to improve the HDO upgrading process.365,366 Since several studies have already reviewed liquid biofuel upgrading processes,363,367,368 this article does not provide a detailed description of each upgrading method. However, it should be noted that while these upgrading processes can improve the characteristics of bio-crude oil and pyrolysis oil to some extent, none has emerged as a definitive solution. Liquid biofuels derived from different biomass types contain varying levels of organic compounds and impurities. Therefore, selecting the appropriate upgrading process is crucial to enhancing liquid biofuels quality while minimizing costs.
The choice of upgrading processes should be guided by the specific chemical and physical properties of liquid biofuels, with the levels of organic compounds playing a key role in determining suitable upgrading methods. As shown in Fig. 8, the composition of organic compounds can guide the identification of appropriate processes for bio-crude oil and pyrolysis oil derived from various biomass types, including lignocellulosic biomass, microalgae, macroalgae, municipal sludge, and food waste. For example, the removal of N-containing compounds is less critical for bio-crude oil from lignocellulosic biomass than for that derived from microalgae. This comparative review highlights a significant research gap in the field of liquid biofuels: understanding how different organic compounds impact biofuel quality and the need for selective or multistage upgrading processes. Liquid biofuels derived from HTL and pyrolysis exhibit varying levels of N- and O-containing compounds, aromatics, and aliphatics, depending on the biochemical composition of the biomass. A single upgrading process may indiscriminately remove O from all organic compounds, but certain compounds like aldehydes and alcohols, despite both being oxygenated, may have distinct effects on biofuel quality and require different removal strategies. A deeper understanding of the specific impacts of various organic compound categories is essential for developing effective multistage upgrading processes that produce high-quality liquid biofuels from HTL and fast/flash pyrolysis.
5. Advantages and disadvantages of HTL and fast/flash pyrolysis and prospects toward application of thermochemical processes
Fast/flash pyrolysis processes are compared to the HTL process thoroughly in this review article for their potential to recover liquid biofuels from different biomass. The results reveal that biomass type plays a key role in the efficiency of the TPs as well as operational conditions for biofuel production. Therefore, neither fast/flash pyrolysis nor HTL can produce high-quality liquid biofuel solely by optimizing operational conditions without considering the biomass type. However, each process has its own advantages and drawbacks. Several of these points are tabulated in Table 6. By comparing the advantages and disadvantages of HTL and fast/flash pyrolysis, it can be concluded that the choice of process for converting biomass into liquid biofuel should not rely solely on biomass moisture. Instead, the composition and properties of the resulting liquid biofuels, particularly the concentration of organic compounds, should be considered, as they will determine the extent of upgrading required. Both biomass pretreatment and liquid biofuel post-treatment processes can influence the economic feasibility of the overall process. In general, for industrial-scale applications, the moisture content of biomass, biofuel yield, and biofuel's characteristics can be the key factors in choosing between HTL and fast/flash pyrolysis. In terms of yield and quality of liquid biofuels, HTL can produce bio-crude oil with higher HHV compared to fast/flash pyrolysis processes for all five types of biomass reviewed in this study. However, the yield of liquid biofuels from fast/flash pyrolysis can be higher than that from HTL for specific biomass types. The level of organic compounds in liquid biofuels is highly affected by biomass's biochemical characteristics. Therefore, it is not possible to definitively determine which process is superior in all aspects. In Fig. 9, the recommended TPs for five types of feedstocks reviewed in this study are summarized based on biofuel yield and quality.| Process | Hydrothermal processes (HTL) | Pyrolysis (fast/flash pyrolysis) |
|---|---|---|
| Advantages and (consequences) | - Comparable liquid biofuel yields for municipal sludge and food waste to fast/flash pyrolysis. (Lower capital costs.) | - Greater liquid biofuel yield for lignocellulosic biomass and macroalgae than HTL. (Lower capital costs.) |
| - Better quality of liquid biofuel in terms of HHV, O/C, and moisture. (Decreasing required upgrading processes.) | - There is a limited production of aqueous by-product. (No further management is necessary for by-products.) | |
| - Excessive size reduction of biomass is unnecessary, and wet biomass can be processed. (Reducing pre-treatment costs.) | - Commercialized process. (Minimum unknown parameters.) | |
| Disadvantages and (consequences) | - Production of significant amount of aqueous phase. (Needs further management of by-products.) | - Drying biomass is necessary. (High pre-treatment cost if biomass has a lot of moisture.) |
| - Literally a novel process with a lot of unknown effective parameters. (Scaling up is risky.) | - Poor liquid biofuel quality in terms of HHV, O/C, C/N, and moisture. (Increasing required upgrading processes.) | |
| - Needs water to operate. (Significant amount of water consumption if biomass does not have enough moisture.) | - Reactor design and particle size can affect the efficiency of the process. (Making the process complicated.) | |
| - Inefficient in producing bio-crude oil with acceptable levels of organic compounds (Novel multistage upgrading processes are necessary to address storage, shipment, and combustion.) | - Inefficient in producing pyrolysis oil with acceptable levels of organic compounds. (Novel multistage upgrading processes are necessary to address storage, shipment, and combustion.) | |
| Research gaps | - Investigating the effects of various organic compounds present in bio-crude oil and pyrolysis oil on their storage stability and fuel properties | |
| - Developing biomass-specific pretreatment strategies to minimize feedstock heterogeneity and achieve uniform composition for each biomass type | ||
| - Designing cost-effective, multistep, and selective upgrading processes targeting key organic compounds that negatively impact the quality and stability of liquid biofuels | ||
| - Exploring integrated process configurations and reactor designs to reduce the sensitivity of pyrolysis oil yield and quality to biomass particle size variations | ||
| - Developing biomass-specific resource recovery approaches from HTL-AP to enable the industrial implementation of HTL technology | ||
6. Conclusions
Fast/flash pyrolysis and HTL are two efficient methods for producing liquid biofuels from biomass, comparable to crude oil. The purpose of this review is to equip researchers with new insights into the anticipated liquid biofuel yield from various biomass types through HTL and fast/flash pyrolysis. Additionally, it seeks to assist scholars and industry professionals in selecting between HTL and fast/flash pyrolysis based on the physical and chemical characteristics of liquid biofuels derived from different biomass types, rather than relying solely on biomass moisture. The key findings of this review are summarized below:• Although high C and H content in biomass can enhance liquid biofuel yield, the presence of N and O can negatively impact the quality of the resulting biofuels.
• The high ash content of biomass limits the liquid biofuel yield, but it does not affect the quality of the resulting biofuels.
• Microalgae show higher yields of liquid biofuels compared to other biomass types in both fast/flash pyrolysis and HTL.
• Lignocellulosic biomass and macroalgae generally exhibit higher conversion yields of liquid biofuel in fast/flash pyrolysis compared to HTL. In contrast, municipal sludge and food waste produce similar ranges of liquid biofuel yields with both fast/flash pyrolysis and HTL.
• The solvent choice in HTL is biomass specific. Generally, an alkaline environment in HTL can enhance bio-crude oil production. For carbohydrate-rich biomass, food waste and lignocellulosic biomass, using an alkaline catalyst is advantageous.
• The severity of HTL and fast/flash pyrolysis processes for maximizing liquid biofuel yield is biomass-specific; however, HTL generally requires less severe conditions for most biomass types.
• The HTL process can produce bio-crude oil from municipal sludge and food waste with H/C and O/C values comparable to crude oil.
• Fast/flash pyrolysis of macroalgae typically produces higher-quality liquid biofuel than HTL in terms of H/C and O/C values.
• The C/N ratio of bio-crude oil is much higher than pyrolysis oil from fast/flash pyrolysis, indicating that HTL is more effective at removing N.
• The HHV of bio-crude oil is generally much higher than that of pyrolysis oil for all feedstocks except macroalgae.
• While bio-crude oil has low moisture compared to pyrolysis oil, the densities of pyrolysis oil and bio-crude oil are within the same range for all biomass types.
• Biomass biochemical composition as well as process type can significantly affect the concentration of organic compounds in liquid biofuels.
• Identifying the carbohydrate, protein, and lipid content of biomass provides limited but useful insight into predicting the concentrations of organic compounds in liquid biofuels. Process selection should be based on each method's ability to reduce undesirable organic compounds, thereby minimizing the need for extensive upgrading.
• Alcohols, acids, ketones, phenols, aliphatics, aromatics, N-containing compounds, and oxygenated compounds in liquid biofuels serve as a driving force for developing multistage and/or selective upgrading processes. By addressing these organic compounds, high-quality liquid biofuels can be produced with minimal issues in storage, transport, and combustion.
Data availability
This review does not include any software, code, or primary research results. Only secondary data were analyzed.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Metro Vancouver Industrial Research Chair Program in Advanced Resource Recovery from Wastewater (IRCPJ 548816-18).References
- M. K. G. Deshmukh, M. Sameeroddin, D. Abdul and M. Abdul Sattar, Mater. Today: Proc., 2023, 80, 1756–1759 CAS.
- A. A. Vertes, N. Qureshi, H. P. Blaschek and H. Yukawa, Green energy to sustainability: Strategies for global industries, John Wiley & Sons, 2020 Search PubMed.
- L. Petrus and M. A. Noordermeer, Green Chem., 2006, 8, 861–867 RSC.
- I. A. Basar, H. Liu, H. Carrere, E. Trably and C. Eskicioglu, Green Chem., 2021, 23, 1404–1446 RSC.
- M. Wilk, A. Magdziarz, K. Jayaraman, M. Szymańska-Chargot and I. Gökalp, Biomass Bioenergy, 2019, 120, 166–175 Search PubMed.
- K. Maliutina, A. Tahmasebi, J. Yu and S. N. Saltykov, Energy Convers. Manage., 2017, 151, 426–438 CrossRef CAS.
- A. Singh, D. Pant, N. E. Korres, A.-S. Nizami, S. Prasad and J. D. Murphy, Bioresour. Technol., 2010, 101, 5003–5012 CrossRef CAS PubMed.
- P. S. Nigam and A. Singh, Prog. Energy Combust. Sci., 2011, 37, 52–68 Search PubMed.
- J. Wang and Y. Yin, Renewable Sustainable Energy Rev., 2018, 92, 284–306 CrossRef CAS.
- J. Wang and Y. Yin, Int. J. Hydrogen Energy, 2021, 46, 34599–34625 CrossRef CAS.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 Search PubMed.
- Y. Chisti, Trends Biotechnol., 2008, 26, 126–131 CrossRef CAS PubMed.
- T. Suganya, M. Varman, H. H. Masjuki and S. Renganathan, Renewable Sustainable Energy Rev., 2016, 55, 909–941 CrossRef CAS.
- E. Jardé, L. Mansuy and P. Faure, Water Res., 2005, 39, 1215–1232 CrossRef PubMed.
- P. Manara and A. Zabaniotou, Renewable Sustainable Energy Rev., 2012, 16, 2566–2582 CrossRef CAS.
- R. L. Droste and R. L. Gehr, Theory and practice of water and wastewater treatment, John Wiley & Sons, 2018 Search PubMed.
- O. Parthiba Karthikeyan, E. Trably, S. Mehariya, N. Bernet, J. W. C. Wong and H. Carrere, Bioresour. Technol., 2018, 249, 1025–1039 CrossRef CAS PubMed.
- S. Dhiman and G. Mukherjee, J. Food Process Eng., 2021, 44, e13594 CrossRef.
- B. T. U. S. Department of Energy and Office , Multi-Year Program Plan, 2016.
- M. E. Walsh, Biomass Bioenergy, 1998, 14, 341–350 CrossRef.
- Y. Zhu, S. B. Jones and D. B. Anderson, Algae farm cost model: Considerations for photobioreactors, Pacific Northwest National Lab.(PNNL), Richland, WA (United States), 2018 Search PubMed.
- H. L. Kite-Powell, E. Ask, S. Augyte, D. Bailey, J. Decker, C. A. Goudey, G. Grebe, Y. Li, S. Lindell and D. Manganelli, J. Appl. Phycol., 2022, 3, 435–445 CrossRef.
- J. O. Ighalo, F. U. Iwuchukwu, O. E. Eyankware, K. O. Iwuozor, K. Olotu, O. C. Bright and C. A. Igwegbe, Clean Technol. Environ. Policy, 2022, 24, 2349–2363 CrossRef CAS.
- Y. Marcus, Fluid Phase Equilib., 1999, 164, 131–142 CrossRef CAS.
- P. S. Marathe, R. J. M. Westerhof and S. R. A. Kersten, Energy Fuels, 2020, 34, 1773–1780 CrossRef CAS.
- H. Shahbeik, W. Peng, H. K. S. Panahi, M. Dehhaghi, G. J. Guillemin, A. Fallahi, H. Amiri, M. Rehan, D. Raikwar and H. Latine, Renewable Sustainable Energy Rev., 2022, 167, 112833 Search PubMed.
- C. Xia, A. Pathy, B. Paramasivan, P. Ganeshan, K. Dhamodharan, A. Juneja, D. Kumar, K. Brindhadevi, S.-H. Kim and K. Rajendran, Fuel, 2022, 311, 121932 CrossRef CAS.
- S. Ge and P. Champagne, Environ. Sci. Technol., 2017, 51, 3558–3566 CrossRef CAS PubMed.
- J. A. Magdalena, M. Ballesteros and C. González-Fernandez, Molecules, 2018, 23, 1098 CrossRef PubMed.
- E. Cárdenas-Aguiar, G. Gascó, J. Paz-Ferreiro and A. Méndez, Int. Biodeterior. Biodegrad., 2017, 124, 223–232 CrossRef.
- W. Ding, X. Dong, I. M. Ime, B. Gao and L. Q. Ma, Chemosphere, 2014, 105, 68–74 CrossRef CAS PubMed.
- Q. Wang, H. Song, S. Pan, N. Dong, X. Wang and S. Sun, Sci. Rep., 2020, 10, 3626 CrossRef CAS PubMed.
- M. I. Jahirul, M. G. Rasul, A. A. Chowdhury and N. Ashwath, Energies, 2012, 5, 4952–5001 CrossRef CAS.
- R. Agrawal, M. Agrawal and N. R. Singh, US Pat., US8217211B2, 2012 Search PubMed.
- A. R. K. Gollakota, M. Reddy, M. D. Subramanyam and N. Kishore, Renewable Sustainable Energy Rev., 2016, 58, 1543–1568 CrossRef CAS.
- Z. Yang, A. Kumar and R. L. Huhnke, Renewable Sustainable Energy Rev., 2015, 50, 859–870 CrossRef CAS.
- D. A. Ruddy, J. A. Schaidle, J. R. Ferrell III, J. Wang, L. Moens and J. E. Hensley, Green Chem., 2014, 16, 454–490 RSC.
- M. Gholizadeh, X. Hu and Q. Liu, Renewable Sustainable Energy Rev., 2019, 114, 109313 CrossRef CAS.
- A. Hilal DemirbaŞ, Energy Sources, 2005, 27, 1367–1373 CrossRef.
- X. Huang, J.-P. Cao, P. Shi, X.-Y. Zhao, X.-B. Feng, Y.-P. Zhao, X. Fan, X.-Y. Wei and T. Takarada, J. Anal. Appl. Pyrolysis, 2014, 110, 353–362 CrossRef CAS.
- K. Wang, R. C. Brown, S. Homsy, L. Martinez and S. S. Sidhu, Bioresour. Technol., 2013, 127, 494–499 CrossRef CAS PubMed.
- J. Makibar, A. R. Fernandez-Akarregi, M. Amutio, G. Lopez and M. Olazar, Fuel Process. Technol., 2015, 137, 283–289 CrossRef CAS.
- J. Alvarez, M. Amutio, G. Lopez, I. Barbarias, J. Bilbao and M. Olazar, Chem. Eng. J., 2015, 273, 173–183 CrossRef CAS.
- J. Yu, L. Sun, C. Berrueco, B. Fidalgo, N. Paterson and M. Millan, J. Anal. Appl. Pyrolysis, 2018, 130, 127–134 CrossRef CAS.
- J. Shen, X.-S. Wang, M. Garcia-Perez, D. Mourant, M. J. Rhodes and C.-Z. Li, Fuel, 2009, 88, 1810–1817 CrossRef CAS.
- C. Guizani, S. Valin, J. Billaud, M. Peyrot and S. Salvador, Fuel, 2017, 207, 71–84 CrossRef CAS.
- M. Van de Velden, J. Baeyens, A. Brems, B. Janssens and R. Dewil, Renewable Energy, 2010, 35, 232–242 CrossRef CAS.
- M. R. Barr, R. Volpe and R. Kandiyoti, ACS Sustainable Chem. Eng., 2019, 7, 13734–13745 CrossRef CAS.
- M. Raza, A. Inayat, A. Ahmed, F. Jamil, C. Ghenai, S. R. Naqvi, A. Shanableh, M. Ayoub, A. Waris and Y.-K. Park, Sustainability, 2021, 13, 11061 CrossRef CAS.
- F. Campuzano, R. C. Brown and J. D. Martínez, Renewable Sustainable Energy Rev., 2019, 102, 372–409 CrossRef CAS.
- D. Mohan, C. U. Pittman Jr and P. H. Steele, Energy Fuels, 2006, 20, 848–889 CrossRef CAS.
- S. Czernik and A. V. Bridgwater, Energy Fuels, 2004, 18, 590–598 CrossRef CAS.
- G. Kumar, A. P. Eswari, S. Kavitha, M. D. Kumar, R. Y. Kannah, L. C. How, G. Muthukaruppan and J. R. Banu, Biomass Convers. Biorefin., 2020, 1–26 Search PubMed.
- W. Li, Q. Dang, R. C. Brown, D. Laird and M. M. Wright, Bioresour. Technol., 2017, 241, 959–968 CrossRef CAS PubMed.
- A. Abdulkhani, Z. E. Zadeh, S. G. Bawa, F. Sun, M. Madadi, X. Zhang and B. Saha, Energies, 2023, 16, 2715 CrossRef CAS.
- A. Bieniek, M. Sieradzka, W. Jerzak and A. Magdziarz, J. Anal. Appl. Pyrolysis, 2023, 176, 106241 CrossRef CAS.
- A. E. Harman-Ware, T. Morgan, M. Wilson, M. Crocker, J. Zhang, K. Liu, J. Stork and S. Debolt, Renewable Energy, 2013, 60, 625–632 CrossRef CAS.
- H. V. Ly, S.-S. Kim, H. C. Woo, J. H. Choi, D. J. Suh and J. Kim, Energy, 2015, 93, 1436–1446 CrossRef CAS.
- T. N. Trinh, P. A. Jensen, K. Dam-Johansen, N. O. Knudsen, H. R. Sørensen and S. Hvilsted, Energy Fuels, 2013, 27, 1399–1409 CrossRef CAS.
- M. Li, Y. S. Zhang, S. Cheng, B. Qu, A. Li, F. Meng and G. Ji, Chem. Eng. J., 2023, 457, 141368 CrossRef CAS.
- D. Barry, C. Barbiero, C. Briens and F. Berruti, Biomass Bioenergy, 2019, 122, 472–480 CrossRef CAS.
- J. Alvarez, G. Lopez, M. Amutio, M. Artetxe, I. Barbarias, A. Arregi, J. Bilbao and M. Olazar, Fuel Process. Technol., 2016, 149, 169–175 CrossRef CAS.
- V. R. Wiggers, A. Wisniewski, L. A. S. Madureira, A. A. C. Barros and H. F. Meier, Fuel, 2009, 88, 2135–2141 CrossRef CAS.
- S. Liang, Y. Han, L. Wei and A. G. McDonald, Biomass Convers. Biorefin., 2015, 5, 237–246 CrossRef CAS.
- R. Pardo, L. Taboada-Ruiz, E. Fuente, B. Ruiz, M. Díaz-Somoano, L. F. Calvo and S. Paniagua, Biomass Bioenergy, 2023, 177, 106942 CrossRef CAS.
- P. Biller and A. B. Ross, in Handbook of Biofuels Production (Second Edition), ed. R. Luque, C. S. K. Lin, K. Wilson and J. Clark, Woodhead Publishing, 2016, pp. 509–547 Search PubMed.
- P. Adams, T. Bridgwater, A. Lea-Langton, A. Ross and I. Watson, in Greenhouse Gas Balances of Bioenergy Systems, ed. P. Thornley and P. Adams, Academic Press, 2018, pp. 107–139 Search PubMed.
- F. F. de Menezes, D. B. Martim, L. Y. Ling, A. T. N. Mulato, E. Crespim, J. V. de Castro Oliveira, C. E. Driemeier, P. O. de Giuseppe and G. J. de Moraes Rocha, Int. J. Biol. Macromol., 2022, 223, 223–230 CrossRef CAS PubMed.
- S. M. Changi, J. L. Faeth, N. Mo and P. E. Savage, Ind. Eng. Chem. Res., 2015, 54, 11733–11758 CrossRef CAS.
- M. Déniel, G. Haarlemmer, A. Roubaud, E. Weiss-Hortala and J. Fages, Renewable Sustainable Energy Rev., 2016, 54, 1632–1652 CrossRef.
- M. Sharma, J. Singh, C. Baskar and A. Kumar, BioTechnologia, 2019, 100, 179–194 CrossRef CAS.
- M. Lavanya, A. Meenakshisundaram, S. Renganathan, S. Chinnasamy, D. M. Lewis, J. Nallasivam and S. Bhaskar, Bioresour. Technol., 2016, 203, 228–235 CrossRef CAS PubMed.
- S. Badoga, A. Alvarez-Majmutov, T. Xing, R. Gieleciak and J. Chen, Energy Fuels, 2020, 34, 7160–7169 CrossRef CAS.
- R. Carpio, C.-T. Kuo, R. De Leon, L. C. Schideman and Y. Zhang, Int. J. Smart Grid Clean Energy, 2018, 7, 13–23 CrossRef.
- Z. Zhu, L. Rosendahl, S. S. Toor, D. Yu and G. Chen, Appl. Energy, 2015, 137, 183–192 CrossRef CAS.
- A. A. Shah, S. S. Toor, T. H. Seehar, R. S. Nielsen, A. H. Nielsen, T. H. Pedersen and L. A. Rosendahl, Energies, 2020, 13, 493 CrossRef CAS.
- J. Samanya, A. Hornung, A. Apfelbacher and P. Vale, J. Anal. Appl. Pyrolysis, 2012, 94, 120–125 CrossRef CAS.
- J. Miller, L. Evans, A. Littlewolf and D. Trudell, Fuel, 1999, 78, 1363–1366 CrossRef CAS.
- M. Cemek and M. M. Küçük, Energy Convers. Manage., 2001, 42, 125–130 CrossRef CAS.
- Z. Liu and F.-S. Zhang, Energy Convers. Manage., 2008, 49, 3498–3504 CrossRef CAS.
- J. Chumpoo and P. Prasassarakich, Energy Fuels, 2010, 24, 2071–2077 CrossRef CAS.
- H. Mazaheri, K. T. Lee, S. Bhatia and A. R. Mohamed, Bioresour. Technol., 2010, 101, 7641–7647 CrossRef CAS PubMed.
- I. Smallwood, Handbook of organic solvent properties, Butterworth-Heinemann, 2012 Search PubMed.
- C. L. Yaws, Chemical properties handbook: physical, thermodynamic, environmental, transport, safety, and health related properties for organic and inorganic chemicals, McGraw-Hill, New York, 1999 Search PubMed.
- R. Li, W. Teng, Y. Li and E. Liu, Energy Fuels, 2019, 33, 7415–7423 CrossRef CAS.
- R. Singh, T. Bhaskar and B. Balagurumurthy, Process Saf. Environ. Prot., 2015, 93, 154–160 CrossRef CAS.
- K. M. Isa, T. A. T. Abdullah and U. F. M. Ali, Renewable Sustainable Energy Rev., 2018, 81, 1259–1268 CrossRef CAS.
- S. Brand, R. F. Susanti, S. K. Kim, H.-s. Lee, J. Kim and B.-I. Sang, Energy, 2013, 59, 173–182 CrossRef CAS.
- S. Zhang, S. Zhou, X. Yang, W. Xi, K. Zheng, C. Chu, M. Ju and L. Liu, Environ. Sci. Pollut. Res., 2020, 27, 6362–6374 CrossRef CAS PubMed.
- R. Li, Z. Ma, T. Yang, B. Li, L. Wei and Y. Sun, J. Supercrit. Fluids, 2018, 138, 115–123 CrossRef CAS.
- Y. Han, S. K. Hoekman, Z. Cui, U. Jena and P. Das, Algal Res., 2019, 38, 101421 CrossRef.
- M. Yan, Y. Liu, X. Wen, Y. Yang, J. Cui, F. Chen and D. Hantoko, Renewable Energy, 2023, 215, 118949 CrossRef CAS.
- P. Biller and A. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- H. Liu, I. A. Basar, A. Nzihou and C. Eskicioglu, Water Res., 2021, 199, 117186 CrossRef CAS PubMed.
- P. SundarRajan, K. P. Gopinath, J. Arun, K. GracePavithra, A. Adithya Joseph and S. Manasa, Renewable Sustainable Energy Rev., 2021, 144, 111019 CrossRef CAS.
- J. Watson, T. Wang, B. Si, W.-T. Chen, A. Aierzhati and Y. Zhang, Prog. Energy Combust. Sci., 2020, 77, 100819 CrossRef.
- A. Mathanker, D. Pudasainee, A. Kumar and R. Gupta, Fuel, 2020, 271, 117534 CrossRef CAS.
- K. Sharma, A. A. Shah, S. S. Toor, T. H. Seehar, T. H. Pedersen and L. A. Rosendahl, Energies, 2021, 14, 1708 CrossRef CAS.
- S. Harisankar, R. Vishnu Mohan, V. Choudhary and R. Vinu, Bioresour. Technol., 2022, 351, 127031 CrossRef CAS PubMed.
- P. Biller and A. B. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- M. Wądrzyk, R. Janus, M. P. Vos and D. W. F. Brilman, J. Anal. Appl. Pyrolysis, 2018, 134, 415–426 CrossRef.
- P. Biller, B. K. Sharma, B. Kunwar and A. B. Ross, Fuel, 2015, 159, 197–205 CrossRef CAS.
- B. Biswas, A. Kumar, A. C. Fernandes, K. Saini, S. Negi, U. D. Muraleedharan and T. Bhaskar, Bioresour. Technol., 2020, 307, 123232 CrossRef CAS PubMed.
- R. Singh, B. Balagurumurthy and T. Bhaskar, Fuel, 2015, 146, 69–74 CrossRef CAS.
- D. Zhou, L. Zhang, S. Zhang, H. Fu and J. Chen, Energy Fuels, 2010, 24, 4054–4061 CrossRef CAS.
- A. A. Shah, S. S. Toor, T. H. Seehar, K. K. Sadetmahaleh, T. H. Pedersen, A. H. Nielsen and L. A. Rosendahl, Fuel, 2021, 288, 119407 CrossRef.
- H. Liu, I. A. Basar and C. Eskicioglu, Energy, 2023, 281, 128268 CrossRef CAS.
- H. Song, T. Yang, B. Li, Y. Tong and R. Li, Water Res., 2022, 226, 119278 CrossRef CAS PubMed.
- R. Saengsuriwong, T. Onsree, S. Phromphithak and N. Tippayawong, Bioresour. Technol., 2021, 341, 125750 CrossRef CAS PubMed.
- B. Motavaf and P. E. Savage, ACS ES&T Eng., 2021, 1, 363–374 Search PubMed.
- A. Aierzhati, M. J. Stablein, N. E. Wu, C.-T. Kuo, B. Si, X. Kang and Y. Zhang, Bioresour. Technol., 2019, 284, 139–147 CrossRef CAS PubMed.
- J. Bai, Y. Li, Z. Sun, W. Chen, J. Hu, C. Chang, S. Pang and P. Li, J. Energy Inst., 2024, 116, 101758 CrossRef CAS.
- A. s. D. Chacón-Parra, P. A. Hall, D. M. Lewis, M. Glasius and P. J. van Eyk, ACS Sustainable Chem. Eng., 2022, 10, 10989–11003 CrossRef.
- Y. Fan, U. Hornung, N. Dahmen and A. Kruse, Biomass Convers. Biorefin., 2018, 8, 909–923 CrossRef CAS.
- X. Tang, C. Zhang and X. Yang, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 012026 Search PubMed.
- J. Lu, Z. Liu, Y. Zhang and P. E. Savage, ACS Sustainable Chem. Eng., 2018, 6, 14501–14509 CrossRef CAS.
- K. Anastasakis, P. Biller, R. B. Madsen, M. Glasius and I. Johannsen, Energies, 2018, 11, 2695 CrossRef.
- F. Cheng, J. M. Jarvis, J. Yu, U. Jena, N. Nirmalakhandan, T. M. Schaub and C. E. Brewer, Bioresour. Technol., 2019, 294, 122184 CrossRef CAS PubMed.
- S. Kandasamy, B. Zhang, Z. He, H. Chen, H. Feng, Q. Wang, B. Wang, V. Ashokkumar, S. Siva and N. Bhuvanendran, Energy, 2020, 190, 116236 CrossRef CAS.
- W.-T. Chen, Y. Zhang, J. Zhang, G. Yu, L. C. Schideman, P. Zhang and M. Minarick, Bioresour. Technol., 2014, 152, 130–139 CrossRef CAS PubMed.
- Y. Qiu, A. Aierzhati, J. Cheng, H. Guo, W. Yang and Y. Zhang, Energy Fuels, 2019, 33, 8758–8765 CrossRef CAS.
- W. Song, S. Wang, D. Xu, Y. Guo, C. Yang, J. Zhang and Y. Li, Korean J. Chem. Eng., 2019, 36, 1604–1618 CrossRef CAS.
- R. Shakya, J. Whelen, S. Adhikari, R. Mahadevan and S. Neupane, Algal Res., 2015, 12, 80–90 CrossRef.
- D. R. Vardon, B. Sharma, J. Scott, G. Yu, Z. Wang, L. Schideman, Y. Zhang and T. J. Strathmann, Bioresour. Technol., 2011, 102, 8295–8303 CrossRef CAS PubMed.
- H.-j. Huang, X.-z. Yuan, H.-n. Zhu, H. Li, Y. Liu, X.-l. Wang and G.-m. Zeng, Energy, 2013, 56, 52–60 CrossRef CAS.
- T. Aysu, A. Demirbaş, A. Ş. Bengü and M. M. Küçük, Process Saf. Environ. Prot., 2015, 94, 339–349 CrossRef CAS.
- T. M. Brown, P. Duan and P. E. Savage, Energy Fuels, 2010, 24, 3639–3646 CrossRef CAS.
- B. De Caprariis, P. De Filippis, A. Petrullo and M. Scarsella, Fuel, 2017, 208, 618–625 CrossRef CAS.
- L. Hadhoum, M. Balistrou, G. Burnens, K. Loubar and M. Tazerout, Bioresour. Technol., 2016, 218, 9–17 CrossRef CAS PubMed.
- D. Xu, G. Lin, L. Liu, Y. Wang, Z. Jing and S. Wang, Energy, 2018, 159, 686–695 CrossRef CAS.
- W. Yang, X. Li, S. Liu and L. Feng, Energy Convers. Manage., 2014, 87, 938–945 CrossRef CAS.
- C. Yuan, S. Wang, L. Qian, B. Barati, X. Gong, A. E. F. Abomohra, X. Wang, S. Esakkimuthu, Y. Hu and L. Liu, Int. J. Energy Res., 2019, 43, 8841–8851 CAS.
- B. Zhang, J. Chen, S. Kandasamy and Z. He, Energy, 2020, 193, 116645 CrossRef CAS.
- H. Cong, O. Masek, L. Zhao, Z. Yao, H. Meng, E. Hu and T. Ma, Energy Fuels, 2018, 32, 3743–3750 CrossRef CAS.
- L. Wang, M. N. Olsen, C. Moni, A. Dieguez-Alonso, J. M. de la Rosa, M. Stenrød, X. Liu and L. Mao, Appl. Energy Combus. Sci., 2022, 12, 100090 Search PubMed.
- K. L. Yu, P. L. Show, H. C. Ong, T. C. Ling, W.-H. Chen and M. A. M. Salleh, Clean Technol. Environ. Policy, 2018, 20, 2047–2055 CrossRef CAS.
- A. Amrullah, O. Farobie, A. Bayu, N. Syaftika, E. Hartulistiyoso, N. R. Moheimani, S. Karnjanakom and Y. Matsumura, Sustainability, 2022, 14, 3233 CrossRef CAS.
- O. Farobie, A. Amrullah, A. Bayu, N. Syaftika, L. A. Anis and E. Hartulistiyoso, RSC Adv., 2022, 12, 9567–9578 RSC.
- F. Suo, X. You, S. Yin, H. Wu, C. Zhang, X. Yu, R. Sun and Y. Li, Sci. Total Environ., 2021, 798, 149167 CrossRef CAS PubMed.
- K.-M. Poo, E.-B. Son, J.-S. Chang, X. Ren, Y.-J. Choi and K.-J. Chae, J. Environ. Manage., 2018, 206, 364–372 CrossRef CAS PubMed.
- A. Bhatnagar, A. Singhal, H. Tolvanen, K. Valtonen, T. Joronen and J. Konttinen, Waste Manage., 2022, 138, 298–307 CrossRef CAS PubMed.
- A. K. Sakhiya, A. Anand, I. Aier, V. K. Vijay and P. Kaushal, Bioresour. Technol. Rep., 2021, 15, 100818 CrossRef CAS.
- R. Muzyka, E. Misztal, J. Hrabak, S. W. Banks and M. Sajdak, Energy, 2023, 263, 126128 CrossRef CAS.
- R. Kaur, V. Tarun Kumar, B. B. Krishna and T. Bhaskar, Bioresour. Technol., 2023, 376, 128859 CrossRef CAS PubMed.
- Y.-M. Chang, W.-T. Tsai and M.-H. Li, J. Anal. Appl. Pyrolysis, 2015, 111, 88–93 CrossRef CAS.
- K. Chaiwong, T. Kiatsiriroat, N. Vorayos and C. Thararax, Biomass Bioenergy, 2013, 56, 600–606 CrossRef CAS.
- G. Binda, D. Spanu, R. Bettinetti, L. Magagnin, A. Pozzi and C. Dossi, Algal Res., 2020, 52, 102103 CrossRef.
- A. C. Vilas-Boas, L. A. Tarelho, M. Kamali, T. Hauschild, D. T. Pio, D. Jahanianfard, A. P. D. Gomes and M. A. Matos, Biofuels, Bioprod. Biorefin., 2021, 15, 1054–1072 CrossRef CAS.
- Y. Tian, L. Cui, Q. Lin, G. Li and X. Zhao, Agronomy, 2019, 9, 156 CrossRef CAS.
- A. Khan, I. Ali, S. R. Naqvi, H. AlMohamadi, M. Shahbaz, A. M. Ali and K. Shahzad, Chemosphere, 2023, 337, 139226 CrossRef CAS PubMed.
- I. Gbouri, F. Yu, X. Wang, J. Wang, X. Cui, Y. Hu, B. Yan and G. Chen, Int. J. Environ. Res. Public Health, 2022, 19, 2818 CrossRef CAS PubMed.
- B. R. Patra, S. Nanda, A. K. Dalai and V. Meda, Chemosphere, 2021, 285, 131431 CrossRef CAS PubMed.
- P. Premchand, F. Demichelis, D. Chiaramonti, S. Bensaid and D. Fino, Waste Manage., 2023, 172, 308–319 CrossRef CAS PubMed.
- M. Maniscalco, G. Infurna, G. Caputo, L. Botta and N. T. Dintcheva, Energies, 2021, 14, 8457 CrossRef CAS.
- S. Kordoghli, E. Fassatoui, J. F. Largeau and B. Khiari, C. R. Chim., 2023, 26, 1–15 CrossRef CAS.
- H. S. Choi, Y. S. Choi and H. C. Park, Renewable Energy, 2012, 42, 131–135 CrossRef CAS.
- S. Yang, L. Shi, Q. Zhou, B. Qian, A. De Girolamo and L. Zhang, Chem. Eng. J., 2022, 432, 134372 CrossRef CAS.
- C. A. Mullen, A. A. Boateng, N. M. Goldberg, I. M. Lima, D. A. Laird and K. B. Hicks, Biomass Bioenergy, 2010, 34, 67–74 CrossRef CAS.
- B. Adelawon, G. Latinwo, B. Eboibi, O. Agbede and S. Agarry, Chem. Eng. Commun., 2022, 209, 1246–1276 CrossRef CAS.
- F. Sotoudehniakarani, A. Alayat and A. G. McDonald, J. Anal. Appl. Pyrolysis, 2019, 139, 258–273 CrossRef CAS.
- H. Merdun, Z. Boubacar Laougé, İ. V. Sezgin and A. S. Çığgın, Waste Manage., 2023, 157, 149–158 CrossRef CAS PubMed.
- E. Pokorna, N. Postelmans, P. Jenicek, S. Schreurs, R. Carleer and J. Yperman, Fuel, 2009, 88, 1344–1350 CrossRef CAS.
- B. Grycová, I. Koutník and A. Pryszcz, Bioresour. Technol., 2016, 218, 1203–1207 CrossRef PubMed.
- F. Xu, X. Ming, R. Jia, M. Zhao, B. Wang, Y. Qiao and Y. Tian, Fuel Process. Technol., 2020, 210, 106558 CrossRef CAS.
- S. R. Periyavaram, B. K. L. Uppala and P. H. P. Reddy, J. Environ. Manage., 2023, 347, 119132 CrossRef CAS PubMed.
- I. Idowu, L. Li, J. R. V. Flora, P. J. Pellechia, S. A. Darko, K. S. Ro and N. D. Berge, Waste Manage., 2017, 69, 480–491 CrossRef CAS PubMed.
- N. U. Saqib, S. Baroutian and A. K. Sarmah, Bioresour. Technol., 2018, 266, 357–363 CrossRef CAS PubMed.
- H. B. Sharma and B. K. Dubey, Waste Manage., 2020, 118, 521–533 CrossRef CAS PubMed.
- H. Bayat, M. Dehghanizadeh, J. M. Jarvis, C. E. Brewer and U. Jena, Front. Sustainable Food Syst., 2021, 5, 658592 Search PubMed.
- S. V. Vassilev, D. Baxter, L. K. Andersen and C. G. Vassileva, Fuel, 2013, 105, 40–76 CrossRef CAS.
- F. A. Kucherov, L. V. Romashov, K. I. Galkin and V. P. Ananikov, ACS Sustainable Chem. Eng., 2018, 6, 8064–8092 CrossRef CAS.
- D. Chen, Q. Ma, L. Wei, N. Li, Q. Shen, W. Tian, J. Zhou and J. Long, J. Anal. Appl. Pyrolysis, 2018, 130, 169–180 CrossRef CAS.
- V. Anand, R. Gautam and R. Vinu, Fuel, 2017, 205, 1–10 CrossRef CAS.
- M. Chen, S. Zhang, Y. Su, X. Niu, S. Zhu and X. Liu, Renewable Energy, 2022, 186, 105–114 CrossRef CAS.
- S. Kandasamy, B. Zhang, Z. He, H. Chen, H. Feng, Q. Wang, B. Wang, N. Bhuvanendran, S. Esakkimuthu, V. Ashokkumar and M. Krishnamoorthi, Biomass Bioenergy, 2019, 131, 105417 CrossRef CAS.
- K. Malins, V. Kampars, J. Brinks, I. Neibolte, R. Murnieks and R. Kampare, Bioresour. Technol., 2015, 187, 23–29 CrossRef CAS PubMed.
- C. Xu and T. Etcheverry, Fuel, 2008, 87, 335–345 CrossRef CAS.
- Z. Huang, L. Qin, Z. Xu, W. Chen, F. Xing and J. Han, J. Energy Inst., 2019, 92, 835–842 CrossRef CAS.
- Y. Chen, R. Mu, M. Yang, L. Fang, Y. Wu, K. Wu, Y. Liu and J. Gong, Chem. Eng. Sci., 2017, 161, 299–307 CrossRef CAS.
- F. Qian, X. Zhu, Y. Liu, Q. Shi, L. Wu, S. Zhang, J. Chen and Z. J. Ren, Environ. Sci. Technol., 2018, 52, 2225–2234 CrossRef CAS PubMed.
- X. Cao, X. Peng, S. Sun, L. Zhong, W. Chen, S. Wang and R.-C. Sun, Carbohydr. Polym., 2015, 118, 44–51 CrossRef CAS PubMed.
- E. Z. Hoşgün, Biomass Convers. Biorefin., 2021, 11, 2703–2710 CrossRef.
- L. Kong, G. Li, H. Wang, W. He and F. Ling, J. Chem. Technol. Biotechnol., Biotechnol., 2008, 83, 383–388 CrossRef CAS.
- J. H. Lee, H. Hwang and J. W. Choi, Energy, 2018, 162, 1–9 CrossRef CAS.
- A. H. Hubble, E. M. Ryan and J. L. Goldfarb, Fuel, 2022, 308, 121900 CrossRef CAS.
- K. Kumar, F. Parveen, T. Patra and S. Upadhyayula, New J. Chem., 2018, 42, 228–236 RSC.
- G. Wang, J. Zhang, J. Yu, Z. Zhu, X. Guo, G. Chen, T. Pedersen, L. Rosendahl, X. Yu and H. Wang, Fuel, 2022, 323, 124329 CrossRef CAS.
- S. S. N. S. Zahari, N. A. Zulastry and H. H. Azman, J. Phys.:Conf. Ser., 2020, 1551, 012014 CrossRef CAS.
- Y. Hu, W. Yu, H. Wibowo, Y. Xia, Y. Lu and M. Yan, Sci. Total Environ., 2019, 667, 263–270 CrossRef CAS PubMed.
- A. A. Shah, S. S. Toor, F. Conti, A. H. Nielsen and L. A. Rosendahl, Biomass Bioenergy, 2020, 135, 105504 CrossRef CAS.
- Z. Zhu, S. S. Toor, L. Rosendahl, D. Yu and G. Chen, Energy, 2015, 80, 284–292 CrossRef CAS.
- R. Xiao and W. Yang, Renewable Energy, 2013, 50, 136–141 CrossRef CAS.
- J. Liu, X. Chen, W. Chen, M. Xia, Y. Chen, H. Chen, K. Zeng and H. Yang, Proc. Combust. Inst., 2023, 39, 3157–3181 CrossRef CAS.
- M. I. Din, A. Amanat, Z. Hussain, R. Khalid and A. Rauf, Int. J. Environ. Anal. Chem., 2023, 103, 2827–2840 CrossRef CAS.
- P. Wang, J. Yu, X. Liu and M. Millan, Fuel, 2023, 354, 129191 CrossRef CAS.
- S. Yu, X. Yang, Q. Li, Y. Zhang and H. Zhou, Green Energy Environ., 2023, 8, 1216–1227 CrossRef CAS.
- Q. Fan, P. Fu, C. Song and Y. Fan, Ind. Crops Prod., 2023, 191, 116017 CrossRef CAS.
- F. Güleç, L. M. G. Riesco, O. Williams, E. T. Kostas, A. Samson and E. Lester, Fuel, 2021, 302, 121166 CrossRef.
- R. P. Overend and E. Chornet, Philos. Trans. R. Soc., A, 1987, 321, 523–536 CrossRef CAS.
- Y. Hu, M. Attia, E. Tsabet, A. Mohaddespour, M. T. Munir and S. Farag, J. Mater. Cycles Waste Manage., 2021, 23, 1737–1750 CrossRef CAS.
- M. K. Awasthi, T. Sar, S. C. Gowd, K. Rajendran, V. Kumar, S. Sarsaiya, Y. Li, R. Sindhu, P. Binod, Z. Zhang, A. Pandey and M. J. Taherzadeh, Fuel, 2023, 342, 127790 CrossRef CAS.
- E. Pienihäkkinen, C. Lindfors, T. Ohra-Aho and A. Oasmaa, Energy Fuels, 2022, 36, 3654–3664 CrossRef PubMed.
- J. Fermoso, H. Hernando, S. Jiménez-Sánchez, A. A. Lappas, E. Heracleous, P. Pizarro, J. M. Coronado and D. P. Serrano, Fuel Process. Technol., 2017, 167, 563–574 CrossRef CAS.
- N. Tröger, D. Richter and R. Stahl, J. Anal. Appl. Pyrolysis, 2013, 100, 158–165 CrossRef.
- C. Pfitzer, N. Dahmen, N. Troger, F. Weirich, J. Sauer, A. Gunther and M. Muller-Hagedorn, Energy Fuels, 2016, 30, 8047–8054 CrossRef CAS.
- S. Conrad, C. Blajin, T. Schulzke and G. Deerberg, Environ. Prog. Sustainable Energy, 2019, 38, e13287 CrossRef CAS.
- Y. H. Chan, S. Yusup, A. T. Quitain, R. R. Tan, M. Sasaki, H. L. Lam and Y. Uemura, Energy Convers. Manage., 2015, 104, 180–188 CrossRef CAS.
- S. Feng, Z. Yuan, M. Leitch and C. C. Xu, Fuel, 2014, 116, 214–220 CrossRef CAS.
- Y. Tian, F. Wang, J. O. Djandja, S.-L. Zhang, Y.-P. Xu and P.-G. Duan, Fuel, 2020, 265, 116946 CrossRef CAS.
- S. Harisankar, R. V. Mohan, V. Choudhary and R. Vinu, Bioresour. Technol., 2022, 351, 127031 CrossRef CAS PubMed.
- R. Singh, K. Chaudhary, B. Biswas, B. Balagurumurthy and T. Bhaskar, J. Supercrit. Fluids, 2015, 104, 70–75 CrossRef CAS.
- M. Wadrzyk, M. Berdel, R. Janus and D. W. F. Brilman, E3S Web Conf., 2019, 108, 02004 CrossRef CAS.
- G. Haarlemmer, C. Guizani, S. Anouti, M. Déniel, A. Roubaud and S. Valin, Fuel, 2016, 174, 180–188 CrossRef CAS.
- T. H. Seehar, S. S. Toor, A. A. Shah, T. H. Pedersen and L. A. Rosendahl, Energies, 2020, 13, 3114 CrossRef CAS.
- I. Babich, M. Van der Hulst, L. Lefferts, J. Moulijn, P. O′connor and K. Seshan, Biomass Bioenergy, 2011, 35, 3199–3207 CrossRef CAS.
- A. Campanella and M. P. Harold, Biomass Bioenergy, 2012, 46, 218–232 CrossRef CAS.
- X. Miao, Q. Wu and C. Yang, J. Anal. Appl. Pyrolysis, 2004, 71, 855–863 CrossRef CAS.
- G. Belotti, B. de Caprariis, P. De Filippis, M. Scarsella and N. Verdone, Biomass Bioenergy, 2014, 61, 187–195 CrossRef CAS.
- R. V. Piloni, I. C. Daga, C. Urcelay and E. L. Moyano, Algal Res., 2021, 54, 102206 CrossRef.
- Z. He, D. Xu, L. Liu, Y. Wang, S. Wang, Y. Guo and Z. Jing, Algal Res., 2018, 33, 8–15 CrossRef.
- Y. Han, K. Hoekman, U. Jena and P. Das, Energies, 2019, 13, 124 CrossRef.
- H. Li, Z. Liu, Y. Zhang, B. Li, H. Lu, N. Duan, M. Liu, Z. Zhu and B. Si, Bioresour. Technol., 2014, 154, 322–329 CrossRef CAS PubMed.
- D. L. Barreiro, B. R. Gómez, F. Ronsse, U. Hornung, A. Kruse and W. Prins, Fuel Process. Technol., 2016, 148, 117–127 CrossRef.
- J. Arun, P. Varshini, P. K. Prithvinath, V. Priyadarshini and K. P. Gopinath, Bioresour. Technol., 2018, 261, 182–187 CrossRef CAS PubMed.
- X. Tang, C. Zhang, Z. Li and X. Yang, Bioresour. Technol., 2016, 202, 8–14 CrossRef CAS PubMed.
- C. Gai, Y. Zhang, W.-T. Chen, P. Zhang and Y. Dong, RSC Adv., 2014, 4, 16958–16967 RSC.
- B. Eboibi, D. Lewis, P. Ashman and S. Chinnasamy, Bioresour. Technol., 2014, 170, 20–29 CrossRef CAS PubMed.
- A. Palomino, R. D. Godoy-Silva, S. Raikova and C. J. Chuck, Renewable Energy, 2020, 145, 1720–1729 CrossRef CAS.
- J. Arun, K. Gopinath, S. Shreekanth, R. Sahana, M. Raghavi and D. Gnanaprakash, Pet. Chem., 2019, 59, 194–200 CrossRef CAS.
- R. Zhang, Y. Zhou and C. Hu, J. Appl. Phycol., 2021, 33, 91–99 CrossRef CAS.
- C. Ma, J. Geng, D. Zhang and X. Ning, J. Energy Inst., 2020, 93, 303–311 CrossRef CAS.
- G. Wang, B. Fan, H. Chen and Y. Li, J. Energy Inst., 2020, 93, 1892–1900 CrossRef CAS.
- H. V. Ly, S.-S. Kim, J. H. Choi, H. C. Woo and J. Kim, Energy Convers. Manage., 2016, 122, 526–534 CrossRef CAS.
- N. Neveux, A. Yuen, C. Jazrawi, M. Magnusson, B. Haynes, A. Masters, A. Montoya, N. A. Paul, T. Maschmeyer and R. De Nys, Bioresour. Technol., 2014, 155, 334–341 CrossRef CAS PubMed.
- D. L. Barreiro, M. Beck, U. Hornung, F. Ronsse, A. Kruse and W. Prins, Algal Res., 2015, 11, 234–241 CrossRef.
- Y.-P. Xu, P.-G. Duan and F. Wang, Fuel Process. Technol., 2015, 130, 268–274 CrossRef CAS.
- K. Anastasakis and A. Ross, Fuel, 2015, 139, 546–553 CrossRef CAS.
- J. Xu, X. Dong and Y. Wang, Ind. Crops Prod., 2020, 151, 112458 CrossRef CAS.
- L. Yan, Y. Wang, J. Li, Y. Zhang, L. Ma, F. Fu, B. Chen and H. Liu, Bioresour. Technol., 2019, 292, 121286 CrossRef CAS PubMed.
- S. Raikova, J. Olsson, J. J. Mayers, G. r. M. Nylund, E. Albers and C. J. Chuck, Energy Fuels, 2018, 34, 368–378 CrossRef.
- K. Anastasakis and A. Ross, Bioresour. Technol., 2011, 102, 4876–4883 CrossRef CAS PubMed.
- M. Halalsheh, K. Shatanawi, R. Shawabkeh, G. Kassab, H. Mohammad, M. Adawi, S. Ababneh, A. Abdullah, N. Ghantous and N. Balah, Heliyon, 2024, 10, e28030 CrossRef CAS PubMed.
- R. O. Arazo, D. A. D. Genuino, M. D. G. de Luna and S. C. Capareda, Sustainable Environ. Res., 2017, 27, 7–14 CrossRef CAS.
- T. N. Trinh, P. A. Jensen, K. Dam-Johansen, N. O. Knudsen and H. R. Sørensen, Energy Fuels, 2013, 27, 1419–1427 CrossRef CAS.
- F. Huang, Y. Yu and H. Huang, J. Anal. Appl. Pyrolysis, 2018, 130, 36–42 CrossRef CAS.
- H. Chen, Y. Zhai, B. Xu, B. Xiang, L. Zhu, L. Qiu, X. Liu, C. Li and G. Zeng, Environ. Technol., 2015, 36, 470–478 CrossRef CAS PubMed.
- H. J. Park, H. S. Heo, Y.-K. Park, J.-H. Yim, J.-K. Jeon, J. Park, C. Ryu and S.-S. Kim, Bioresour. Technol., 2010, 101, S83–S85 CrossRef CAS PubMed.
- M. Atienza-Martínez, I. Fonts, L. Lázaro, J. Ceamanos and G. Gea, Chem. Eng. J., 2015, 259, 467–480 CrossRef.
- R. Lin, L. Wang, J. Zhang, X. Li, W. Zheng and L. Zhang, J. Anal. Appl. Pyrolysis, 2023, 173, 106097 CrossRef CAS.
- J. M. Reckamp, R. A. Garrido and J. A. Satrio, Biomass Bioenergy, 2014, 71, 235–244 CrossRef CAS.
- I. A. Basar, H. Liu and C. Eskicioglu, Bioresour. Technol., 2024, 400, 130671 CrossRef CAS PubMed.
- H. Liu, I. A. Basar, N. Lyczko, A. Nzihou and C. Eskicioglu, Chem. Eng. J., 2022, 449, 137838 CrossRef CAS.
- H. Zeb, M. A. Hussain, M. Javed, T. Qureshi, H. Dawood, R. Abbas and M. H. Siddiqi, Therm. Sci., 2023, 262–262 Search PubMed.
- L. B. Silva Thomsen, P. N. Carvalho, J. S. dos Passos, K. Anastasakis, K. Bester and P. Biller, Water Res., 2020, 183, 116101 CrossRef CAS PubMed.
- B. Wu, S. M. Berg, C. K. Remucal and T. J. Strathmann, ACS Sustainable Chem. Eng., 2020, 8, 18303–18313 CrossRef CAS.
- R. Badrolnizam, O. Elham, S. Hadzifah, M. Husain, A. Hidayu, N. Mohammad and A. M. Daud, J. Phys.:Conf. Ser., 2019, 1349, 012108 CrossRef CAS.
- L. Qian, S. Wang and P. E. Savage, Bioresour. Technol., 2017, 232, 27–34 CrossRef CAS PubMed.
- V. Wiggers, A. Wisniewski Jr, L. Madureira, A. C. Barros and H. Meier, Fuel, 2009, 88, 2135–2141 CrossRef CAS.
- J. M. Londoño Feria, G. A. Nausa Galeano and D. H. Malagón-Romero, Chem. Eng. Technol., 2021, 44, 2341–2346 CrossRef.
- K. C. Sembiring, N. Rinaldi and S. P. Simanungkalit, Energy Procedia, 2015, 65, 162–169 CrossRef CAS.
- M. Qing, Y. Long, L. Liu, Y. Yi, W. Li, R. He, Y. Yin, H. Tian, J. He and S. Cheng, J. Anal. Appl. Pyrolysis, 2022, 164, 105543 CrossRef CAS.
- Z. B. Laougé, C. Çorbacıoğlu and H. Merdun, Biomass Convers. Biorefin., 2023, 13, 9807–9819 CrossRef.
- P. Li, X. Shi, X. Wang, J. Song, S. Fang, J. Bai, G. Zhang, C. Chang and S. Pang, J. Cleaner Prod., 2021, 328, 129613 CrossRef CAS.
- H. V. Ly, B. Kwon, J. Kim, C. Oh, H. T. Hwang, J. S. Lee and S.-S. Kim, Waste Manage., 2022, 141, 16–26 CrossRef CAS PubMed.
- A. Aierzhati, J. Watson, B. Si, M. Stablein, T. Wang and Y. Zhang, Energy Convers. Manage.:X, 2021, 10, 100076 CAS.
- A. Gollakota and P. E. Savage, ACS Sustainable Chem. Eng., 2018, 6, 9018–9027 CrossRef CAS.
- E. Tito, C. A. Marcolongo, G. Pipitone, A. H. Monteverde, S. Bensaid and R. Pirone, Bioresour. Technol., 2024, 396, 130446 CrossRef CAS PubMed.
- W.-H. Chen, Y.-Y. Lin, H.-C. Liu, T.-C. Chen, H.-C. Hung and C.-H. Chen, Energy Procedia, 2019, 158, 61–66 CrossRef CAS.
- W.-H. Chen, Y.-Y. Lin, H.-C. Liu, T.-C. Chen, C.-H. Hung, C.-H. Chen and H. C. Ong, Appl. Energy, 2019, 237, 283–291 CrossRef CAS.
- Z. Y. Mahssin, M. M. Zainol, N. A. Hassan, H. Yaacob, M. H. Puteh and N. A. S. Amin, J. Cleaner Prod., 2021, 282, 125422 CrossRef CAS.
- J. Chen, L. Ding, P. Wang, W. Zhang, J. Li, B. A. Mohamed, J. Chen, S. Leng, T. Liu and L. Leng, J. Renewable Mater, 2022, 10, 1555–1574 CAS.
- P. Basu, in Biomass Gasification and Pyrolysis, ed. P. Basu, Academic Press, Boston, 2010, pp. 27–63 Search PubMed.
- L. R. Snyder, Acc. Chem. Res., 1970, 3, 290–299 CrossRef CAS.
- L. Grande, I. Pedroarena, S. A. Korili and A. Gil, Materials, 2021, 14, 5286 CrossRef CAS PubMed.
- E. D. Christensen, G. M. Chupka, J. Luecke, T. Smurthwaite, T. L. Alleman, K. Iisa, J. A. Franz, D. C. Elliott and R. L. McCormick, Energy Fuels, 2011, 25, 5462–5471 CrossRef CAS.
- J. A. Ramirez, R. J. Brown and T. J. Rainey, Energies, 2015, 8, 6765–6794 CrossRef CAS.
- H. S. Heo, H. J. Park, J.-I. Dong, S. H. Park, S. Kim, D. J. Suh, Y.-W. Suh, S.-S. Kim and Y.-K. Park, J. Ind. Eng. Chem., 2010, 16, 27–31 CrossRef CAS.
- R. Gautam, S. Shyam, B. R. Reddy, K. Govindaraju and R. Vinu, Sustainable Energy Fuels, 2019, 3, 3009–3020 RSC.
- R. Younas, S. Hao, L. Zhang and S. Zhang, Renewable Energy, 2017, 113, 532–545 CrossRef CAS.
- G. Haarlemmer, M. Briand, A. Roubaud, J. Roussely and M. Déniel, Detritus, 2018, 3, 84–92 Search PubMed.
- H. Li, Z. Dong, B. Wang, W. Wu, M. Cao, Y. Zhang and Z. Liu, J. Anal. Appl. Pyrolysis, 2023, 173, 106057 CrossRef CAS.
- F. Bayat Mastalinezhad, S. Osfouri and R. Azin, Biomass Bioenergy, 2023, 178, 106963 CrossRef CAS.
- F. Cheng, G. A. Tompsett, C. M. Murphy, A. R. Maag, N. Carabillo, M. Bailey, J. J. Hemingway, C. I. Romo, A. D. Paulsen and P. E. Yelvington, ACS Sustainable Chem. Eng., 2020, 8, 6877–6886 CrossRef CAS.
- N. T. Trinh, P. A. Jensen, H. R. Sørensen, K. Dam-Johansen and S. Hvilsted, Eur. Biomass Conf. Exhib 2012 Search PubMed.
- Y. Liu, X.-z. Yuan, H.-j. Huang, X.-l. Wang, H. Wang and G.-m. Zeng, Fuel Process. Technol., 2013, 112, 93–99 CrossRef CAS.
- N. Neveux, A. K. L. Yuen, C. Jazrawi, M. Magnusson, B. S. Haynes, A. F. Masters, A. Montoya, N. A. Paul, T. Maschmeyer and R. de Nys, Bioresour. Technol., 2014, 155, 334–341 CrossRef CAS PubMed.
- E. H. de Paulo, F. D. dos Santos, G. S. Folli, L. P. Santos, M. H. C. Nascimento, M. K. Moro, P. H. P. da Cunha, E. V. R. Castro, A. Cunha Neto and P. R. Filgueiras, Fuel, 2022, 311, 122527 CrossRef CAS.
- J. G. Speight, in Heavy Oil Recovery and Upgrading, ed. J. G. Speight, Gulf Professional Publishing, 2019, pp. 3–47 Search PubMed.
- N. Ali, M. Ashraf, K. Shahzad, M. Saleem and A. Chughtai, Energy Sources, Part A, 2024, 46, 4244–4256 CrossRef CAS.
- A. K. Sharma, P. Ghodke, P. K. Sharma, S. Manna, A. Pugazhendhi, L. Matsakas and A. Patel, Biomass Convers. Biorefin., 2024, 14, 5261–5274 CrossRef CAS.
- S. Modak, P. Katiyar and S. Yadav, J. Anal. Appl. Pyrolysis, 2024, 182, 106660 CrossRef CAS.
- Y. Makkawi, F. H. Pour, Y. Elsayed, M. Khan, O. Moussa, O. Masek, M. Badrelzaman and W. El Tahir, Energy Convers. Manage., 2022, 272, 116348 CrossRef CAS.
- J. Zhao, Z. Wang, J. Li, B. Yan and G. Chen, Bioresour. Technol., 2022, 354, 127191 CrossRef CAS PubMed.
- J. Chen, Y. Zheng, M. Lv, T. Zhao, Q. Lin, Z. Ni, Z. Zhang and R. Qiu, J. Cleaner Prod., 2024, 473, 143540 CrossRef CAS.
- P. K. Ghodke, A. K. Sharma, J. Pandey, W.-H. Chen, A. Patel and V. Ashokkumar, J. Environ. Manage., 2021, 298, 113450 CrossRef CAS PubMed.
- I. Nava-Bravo, C. Escamilla-Alvarado, J. J. Cano-Gómez, R. Valencia-Vázquez, U. Galván-Arzola and R. Cuevas-García, Biomass Convers. Biorefin., 2024, 1–16 Search PubMed.
- T. A. Silva, E. d. A. do Couto, P. P. Assemany, P. A. C. Costa, P. A. Marques, F. Paradela, A. J. D. Dos Reis and M. L. Calijuri, J. Environ. Manage., 2024, 368, 122091 CrossRef CAS PubMed.
- Z. Wang, C. Yin, J. Su and P. Duan, Ind. Eng. Chem. Res., 2024, 63, 20462–20473 CrossRef CAS.
- B. Browning, N. Batalha, M. Costa Gomes, D. Laurenti, N. Lebaz, C. Geantet and M. Tayakout-Fayolle, Energy Fuels, 2024, 38, 11793–11804 CrossRef CAS.
- L. Nazari, H. Wang, M. B. Ray and C. Xu, Biomass Bioenergy, 2025, 194, 107671 CrossRef CAS.
- C. Jazrawi, P. Biller, Y. He, A. Montoya, A. B. Ross, T. Maschmeyer and B. S. Haynes, Algal Res., 2015, 8, 15–22 CrossRef.
- J. G. Speight, The chemistry and technology of petroleum, CRC press, 2006 Search PubMed.
- S. Clarke and F. Preto, Biomass burn characteristics, Ministry of Agriculture, Food and Rural Affairs Guelph, ON, Canada, 2011 Search PubMed.
- D. C. Elliott, T. R. Hart, A. J. Schmidt, G. G. Neuenschwander, L. J. Rotness, M. V. Olarte, A. H. Zacher, K. O. Albrecht, R. T. Hallen and J. E. Holladay, Algal Res., 2013, 2, 445–454 CrossRef.
- S. Vellaiyan and C. A. Partheeban, Renewable Energy, 2020, 149, 1157–1166 CrossRef CAS.
- S. Vellaiyan, A. Subbiah and P. Chockalingam, Pet. Sci. Technol., 2020, 38, 194–202 CrossRef CAS.
- S. Vellaiyan, Therm. Sci., 2020, 24, 231–241 CrossRef.
- K. Ramalingam, S. Vellaiyan, E. P. Venkatesan, S. A. Khan, Z. Mahmoud and C. A. Saleel, ACS Omega, 2023, 8, 16545–16560 CrossRef CAS PubMed.
- ASTM D6751-10, Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels, ASTM International, Pennsylvania, 2010 Search PubMed.
- B. He, J. Thompson, D. Routt and J. Van Gerpen, Appl. Eng. Agric., 2007, 23, 71–76 Search PubMed.
- C. U. Pittman Jr, D. Mohan, A. Eseyin, Q. Li, L. Ingram, E.-B. M. Hassan, B. Mitchell, H. Guo and P. H. Steele, Energy Fuels, 2012, 26, 3816–3825 CrossRef.
- S. Nizamuddin, H. A. Baloch, N. M. Mubarak, S. Riaz, M. T. H. Siddiqui, P. Takkalkar, M. Tunio, S. Mazari and A. W. Bhutto, Waste Biomass Valorization, 2019, 10, 1957–1968 CrossRef CAS.
- V. Sundaram, K. Muthukumarappan and S. Gent, Appl. Biochem. Biotechnol., 2017, 181, 1060–1079 CrossRef CAS PubMed.
- I. Y. Mohammed, Y. A. Abakr, F. K. Kazi and S. Yusuf, Waste Biomass Valorization, 2017, 8, 755–773 CrossRef CAS.
- L. Zhang, R. Liu, R. Yin, Y. Mei and J. Cai, Chem. Eng. Technol., 2014, 37, 1181–1190 CrossRef CAS.
- M. Zhang and H. Wu, Energy Fuels, 2014, 28, 4650–4656 CrossRef CAS.
- L. Zhu, K. Li, H. Ding and X. Zhu, Fuel, 2018, 211, 704–711 CrossRef CAS.
- C. Hognon, F. Delrue, J. Texier, M. Grateau, S. Thiery, H. Miller and A. Roubaud, Biomass Bioenergy, 2015, 73, 23–31 CrossRef CAS.
- Z. Du, Y. Li, X. Wang, Y. Wan, Q. Chen, C. Wang, X. Lin, Y. Liu, P. Chen and R. Ruan, Bioresour. Technol., 2011, 102, 4890–4896 CrossRef CAS PubMed.
- F. C. Borges, Q. Xie, M. Min, L. A. R. Muniz, M. Farenzena, J. O. Trierweiler, P. Chen and R. Ruan, Bioresour. Technol., 2014, 166, 518–526 CrossRef CAS PubMed.
- S. W. Kim, B. S. Koo and D. H. Lee, Bioresour. Technol., 2014, 162, 96–102 CrossRef CAS PubMed.
- U. Jena and K. Das, Energy Fuels, 2011, 25, 5472–5482 CrossRef CAS.
- H. Zhao, H.-X. Yan, M. Liu, B.-B. Sun, Y. Zhang, S.-S. Dong, L.-B. Qi and S. Qin, Energy Sources, Part A, 2013, 35, 859–867 CrossRef CAS.
- W. Zuo, B. Jin, Y. Huang and Y. Sun, Environ. Sci. Pollut. Res., 2014, 21, 9717–9726 CrossRef CAS PubMed.
- J. M. Jarvis, K. O. Albrecht, J. M. Billing, A. J. Schmidt, R. T. Hallen and T. M. Schaub, Energy Fuels, 2018, 32, 8483–8493 CrossRef CAS.
- H. Kadlimatti, B. R. Mohan and M. Saidutta, Biomass Bioenergy, 2019, 123, 25–33 CrossRef CAS.
- S. Anouti, G. Haarlemmer, M. Déniel and A. Roubaud, Energy Fuels, 2016, 30, 398–406 CrossRef CAS.
- Ü. Ağbulut, R. Sirohi, E. Lichtfouse, W.-H. Chen, C. Len, P. L. Show, A. T. Le, X. P. Nguyen and A. T. Hoang, Bioresour. Technol., 2023, 128860 CrossRef PubMed.
- S. Y. Yang, C. Y. Wu and K. H. Chen, Adv. Mater. Res., 2011, 328, 881–886 Search PubMed.
- P. Biller, R. Riley and A. B. Ross, Bioresour. Technol., 2011, 102, 4841–4848 CrossRef CAS PubMed.
- P. Biller, A. B. Ross, S. C. Skill, A. Lea-Langton, B. Balasundaram, C. Hall, R. Riley and C. A. Llewellyn, Algal Res., 2012, 1, 70–76 CrossRef CAS.
- S. Mishra and K. Mohanty, Energy Convers. Manage., 2020, 204, 112312 CrossRef CAS.
- M. Ellersdorfer, Biomass Bioenergy, 2020, 142, 105796 CrossRef CAS.
- B. Biswas, T. Rahman and S. Adhikari, J. Cleaner Prod., 2024, 471, 143398 CrossRef CAS.
- L. Jie, L. Yuwen, S. Jingyan, W. Zhiyong, H. Ling, Y. Xi and W. Cunxin, Thermochim. Acta, 2008, 467, 20–29 CrossRef.
- H. Liu, Z. Wang, D. Zhang, Q. Shen, T. Hui and J. Ma, Food Res. Int., 2020, 136, 109328 CrossRef CAS PubMed.
- T. Wang, Y. Zhai, Y. Zhu, C. Peng, B. Xu, T. Wang, C. Li and G. Zeng, Bioresour. Technol., 2018, 247, 182–189 CrossRef CAS PubMed.
- K. Maher and D. Bressler, Bioresour. Technol., 2007, 98, 2351–2368 CrossRef CAS PubMed.
- Z. Du, B. Hu, X. Ma, Y. Cheng, Y. Liu, X. Lin, Y. Wan, H. Lei, P. Chen and R. Ruan, Bioresour. Technol., 2013, 130, 777–782 CrossRef CAS PubMed.
- J. H. Grabber, J. Ralph, C. Lapierre and Y. Barrière, C. R. Biol., 2004, 327, 455–465 CrossRef CAS PubMed.
- P. Sarika, P. Nancarrow, A. Khansaheb and T. Ibrahim, Polymers, 2020, 12, 2237 CrossRef CAS PubMed.
- S. Yu, L. Wang, Q. Li, Y. Zhang and H. Zhou, Materials Today Sustainability, 2022, 100209 CrossRef.
- Y.-M. Kim, T. U. Han, B. Lee, A. Watanabe, N. Teramae, J.-H. Kim, Y.-K. Park, H. Park and S. Kim, Algal Res., 2018, 32, 60–69 CrossRef.
- S. Wang, P. Mandfloen, P. Jönsson and W. Yang, Appl. Energy, 2021, 289, 116687 CrossRef CAS.
- D. Chiaramonti, M. Prussi, M. Buffi, A. M. Rizzo and L. Pari, Appl. Energy, 2017, 185, 963–972 CrossRef CAS.
- L. Sheng, X. Wang and X. Yang, Bioresour. Technol., 2018, 247, 14–20 CrossRef CAS PubMed.
- H. B. Sharma, A. K. Sarmah and B. Dubey, Renewable Sustainable Energy Rev., 2020, 123, 109761 CrossRef CAS.
- T. G. Madenoğlu, S. Kurt, M. Sağlam, M. Yüksel, D. Gökkaya and L. Ballice, J. Supercrit. Fluids, 2012, 67, 22–28 CrossRef.
- T. M. Aida, K. Tajima, M. Watanabe, Y. Saito, K. Kuroda, T. Nonaka, H. Hattori, R. L. Smith Jr and K. Arai, J. Supercrit. Fluids, 2007, 42, 110–119 CrossRef CAS.
- D. López Barreiro, M. Beck, U. Hornung, F. Ronsse, A. Kruse and W. Prins, Algal Res., 2015, 11, 234–241 CrossRef.
- Y. Liu, H. Tüysüz, C.-J. Jia, M. Schwickardi, R. Rinaldi, A.-H. Lu, W. Schmidt and F. Schüth, Chem. Commun., 2010, 46, 1238–1240 RSC.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef CAS.
- N. Yilmaz, F. M. Vigil and B. Donaldson, Sci. Total Environ., 2022, 849, 157839 CrossRef CAS PubMed.
- J. Wang, P. Bi, Y. Zhang, H. Xue, P. Jiang, X. Wu, J. Liu, T. Wang and Q. Li, Energy, 2015, 86, 488–499 CrossRef CAS.
- R. W. Jenkins, C. M. Moore, T. A. Semelsberger, C. J. Chuck, J. C. Gordon and A. D. Sutton, ChemSusChem, 2016, 9, 922–931 CrossRef CAS PubMed.
- B. da Costa Magalhães, R. Checa, C. Lorentz, P. Afanasiev, D. Laurenti and C. Geantet, Algal Res., 2023, 71, 103012 CrossRef.
- A. Masudi, O. Muraza, N. W. C. Jusoh and U. Ubaidillah, Environ. Sci. Pollut. Res., 2023, 30, 14104–14125 CrossRef CAS PubMed.
- S. Badoga, R. Gieleciak, A. Alvarez-Majmutov, T. Xing and J. Chen, Fuel, 2022, 324, 124608 CrossRef CAS.
- T. Ohra-aho, M. Tenkanen and T. Tamminen, J. Anal. Appl. Pyrolysis, 2005, 74, 123–128 CrossRef CAS.
- A. E. Pütün, Energy Sources, 2002, 24, 275–285 CrossRef.
- D. K. S. Ng, K. S. Ng and R. T. L. Ng, in Encyclopedia of Sustainable Technologies, ed. M. A. Abraham, Elsevier, Oxford, 2017, pp. 299–314 Search PubMed.
- J.-H. Lee, I.-G. Lee, J.-Y. Park and K.-Y. Lee, Fuel, 2019, 241, 207–217 CrossRef CAS.
- M. Saber, B. Nakhshiniev and K. Yoshikawa, Renewable Sustainable Energy Rev., 2016, 58, 918–930 CrossRef CAS.
- T. Shan Ahamed, S. Anto, T. Mathimani, K. Brindhadevi and A. Pugazhendhi, Fuel, 2021, 287, 119329 CrossRef CAS.
- Q. Peng, X. Jiang, G. Cao, T. Xie, Z. Jin, L. Xie, F. Gan, S. Ma and M. Peng, Bioresour. Technol., 2024, 406, 131059 CrossRef CAS PubMed.
- S. Gea, Y. A. Hutapea, A. F. R. Piliang, A. N. Pulungan, R. Rahayu, J. Layla, A. D. Tikoalu, K. Wijaya and W. D. Saputri, BioEnergy Res., 2023, 16, 325–347 CrossRef CAS.
- A. R. K. Gollakota, C.-M. Shu, P. K. Sarangi, K. P. Shadangi, S. Rakshit, J. F. Kennedy, V. K. Gupta and M. Sharma, Renewable Sustainable Energy Rev., 2023, 187, 113700 CrossRef CAS.
- M. Zhang, Y. Hu, H. Wang, H. Li, X. Han, Y. Zeng and C. C. Xu, Mol. Catal., 2021, 504, 111438 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01314c |
| ‡ Current address: GEMMA – Group of Environmental Engineering and Microbiology, Department of Civil and Environmental Engineering, Universitat Politècnica de Catalunya – Barcelona Tech., c/Jordi Girona 1-3, Building D1, E-08034 Barcelona, Spain. |
| This journal is © The Royal Society of Chemistry 2025 |

